1. Introduction
The hereditary disease Retinitis pigmentosa (RP) is a frequent cause of blindness among the working population in industrial countries [
1]. There is no effective treatment available at present [
2], with the notable exception of the approved gene therapy for mutations in the RPE65 gene [
3]. RP results in vision loss via fatalities of the light-sensing photoreceptors, which are categorized as rods and cones. The degeneration pattern starts with the death of rods that cause night blindness, which is followed by the degeneration of cones [
4] and hence loss of general vision. The
rd1 mouse model, with the gene mutation of the beta subunit of the enzyme phosphodiesterase-6 (PDE6), is characterized by an abnormally high level of photoreceptor cyclic guanosine monophosphate (cGMP) and a rapid loss of these cells [
5]. The degeneration of photoreceptors in this phenotype has been demonstrated with an increasing decline in outer retinal thickness, and overall amplitude reduction in the waveform via spectral domain optical coherence tomography [
6] and electrophysiological changes [
7], respectively. Although the details on how the high level of cGMP affects the photoreceptors are still not known, previous studies have suggested that at least part of the death-promoting impact on photoreceptors could be exerted via increased activity of cGMP-dependent protein kinase (PKG), probably causing over-phosphorylation within photoreceptors [
8,
9]. It would thus be most helpful if this could be studied more closely.
There are two forms of PKG, PKG1 and PKG2, with PKG1 having two isoforms, PKG1alpha and PKG1beta [
10]. cGMP-PKG functions by phosphorylation of certain serine and threonine residues in a number of biological targets [
10]. The importance of this so-called cGMP-PKG system has been recognized for its essential role in gene regulation in diverse tissues [
11,
12], and also for its involvement in the cell death mechanism [
13]. Despite the deepening insights regarding this system, we are as indicated above still confronted with one unsolved problem, namely, what are the actual details of the downstream signaling of the cGMP-PKG system that could affect the RP progression?
Certain analogues of cGMP, a category of chemically modified versions of the native cGMP [
14,
15,
16], exert considerable inhibition or activation of PKG isoforms. This has been taken advantage of when testing potential treatments, where the systemic administration of a selected liposome-formulated cGMP analogue with PKG inhibitory properties was shown to protect RP retinas from retinal degeneration and to preserve the photoreceptor function [
9]. To reveal the downstream signaling practically, one may thus manipulate the retinal cGMP-PKG system pharmacologically via the addition of relevant cGMP-analogues during organotypic retinal explant culturing, and subsequently, explore the cGMP-PKG downstream pathways with a phosphoproteomic analysis, in analogy with how the effects of PKG manipulation on the retinal transcriptome has been studied [
17]. Together this should be beneficial for studying the cGMP-PKG-dependent targets.
In this study, we have therefore used a PKG inhibitor on
rd1 retinal explants, and via a phosphoproteomic analysis, which combined phosphopeptide enrichment with mass spectrometry (MS) [
18], compared the resulting phosphopeptide pattern with that of the untreated counterparts. The selected lists of altered phosphorylated sites after PKG inhibition were then further analysed via appropriate bioinformatics methods to reveal the downstream pathways that were potentially connected to RP advancement. To study the possible effectors of cGMP-PKG system in RP, we subsequently picked up several phosphorylated proteins for further validation of phosphorylation. Our results show a number of phosphorylated sites that are potentially controlled by cGMP-PKG in the
rd1 retina, and as such provide more insights into the retinal degeneration mechanisms in RP.
2. Materials and methods
2.1. Animals
The C3H/
rd1/
rd1 (
rd1; [
19]) and control C3H wild-type (wt; [
19]) were kept and bred in house under standard white cyclic lighting, with free access to food and water, and used irrespective of sex. Day of birth of the animal was considered as postnatal 0 (P0), with the day following this considered as P1,
etc.
2.2. Organotypic retinal explant culture
Mice were exposed to carbon dioxide (CO
2) to minimize the suffering before Euthanasia. Retinas from P5 mice were used to generate explants according to our standard protocol [
17]. Inserts with the explants were put into six-well culture plates with 1.5 mL serum-free medium in each well. Plates were incubated at 37° C with a 5% CO
2 atmosphere, and the medium was replaced every two days. No additions were made to the cultures for the first 2 days. One group of the cultures, which at this point were at an age corresponding to P7, where then exposed to 50 µM Rp-8-Br-PET-cGMPS (PKG inhibitor; Biolog, Bremen, Germany, Cat. No.: P 007) for the following 4 days, with the end point thus equivalent to P11 (n=4), with their corresponding controls (n = 4) receiving the same amount of distilled water. Another group (n = 4) was exposed to the Rp-8-Br-PET-cGMPS with the same concentration but only for 2 hours before the end of the protocol, with controls (n = 4) similarly exposed to distilled water. All retinal explants were either collected for protein extraction followed by phosphoproteomic analyses. In parallel, another 4 groups of
rd1 explants, reaching a total of 13 explants, were generated, with 4 such in the group of retinas with 4 days of PKG inhibition and 3 each in the other 3 groups. These explants were fixed, sectioned, and used for microscopy-based studies.
Animals were randomly assigned to the experimental groups, and the experimenters were aware of the conditions of the animals during retinal explant culture. No statistical method was applied to predetermine the sample size of the experimental groups. The sample size was chosen based on relevant literature in the field as well as on ethical considerations. The study was not pre-registered. The study was exploratory without exclusion criteria being pre-determined.
2.3. Sample preparation for MS
A total of 16 retinal explants were used for MS measurement, and 4 explants were used per group. The experimenter was unaware of the explant’s group during experimentation. Each explant was homogenized separately in buffer (50 mM Tris-HCl, 50 mM NaCl, 1 mM EDTA, 5 mM NaH2PO4, 1 mM DL-Dithiothreitol (DTT)), supplemented with phosphatase inhibitors (Lot No, 33041800, Roche, Basel, Switzerland, 1 tablet per 10 mL buffer) using a homogenizer (Knotes Glass Company, Vineland, NJ). The homogenate was then centrifuged at 10 000 g for 5 min at 4°C. The soluble fraction was collected after centrifugation, with the concentration measured by Bio-Rad Protein Reagent Assay Kit (Cat. No.: #5000113, #5000114, #5000115, Bio-Rad, Hercules, CA).
For each separated sample, proteins were reduced with DTT to a final concentration of 10 mM and heated at 56 °C for 30 min followed by alkylation with iodoacetamide for 30 min at room temperature in the dark to a final concentration of 20 mM. Subsequently, samples were precipitated with ice cold ethanol for overnight at -20°C followed by centrifugation at 14 000 x g for 10 min. The pellets were resuspended in 100 mM ammonium bicarbonate and sonicated for 20 cycles of 15 sec on, 15 sec off, using a Bioruptor (Diagenode, Denville, NJ). Digestion was performed by adding trypsin (Sequencing Grade Modified Trypsin, Part No. V511A, Promega, Madison, WI) in a ratio of 1:50 to the samples and incubated for overnight at 37 °C. The digestion was stopped by the addition of 5 µL 10% trifluoroacetic acid (TFA).
The Pierce High-Select Fe-NTA Phosphopeptide Enrichment Kit (Cat. No.: A32992; Thermo Fischer Scientific, Waltham, MA) was used to enrich phosphopeptides according to the manufacturer’s protocol. The phosphopeptides were run in a Speed Vac to dryness and resolved in 2% acetonitrile (ACN) and 0.1% TFA to a peptide concentration of 0.25 µg/µL.
2.4. MS acquisition and analysis
The peptide analyses were performed on a Q Exactive HFX mass spectrometer (Thermo Scientific) connected to an EASY-nLC 1200 ultra-high-performance liquid chromatography system (Thermo Scientific). Peptides, 1 µg, were separated on an EASY-Spray column (Thermo Scientific; ID 75μm × 50 cm, column temperature 45°C) operated at a constant pressure of 800 bar. A two-step gradient of buffer B (80% acetonitrile, 0.1% formic acid) in buffer A (aqueous 0.1% formic acid) was applied at a flow rate of 300 nl min-1. In the first step a gradient of 10 to 30% of buffer B was run for 90 min followed by a 30 to 45% gradient of buffer B in 20 min. One full MS scan (resolution 60,000 @ 200 m/z; mass range 350–1,400m/z) was followed by MS/MS scans (resolution 15,000 @ 200 m/z). The precursor ions were isolated with 1.3 m/z isolation width and fragmented using higher-energy collisional-induced dissociation at a normalized collision energy of 28. Charge state screening was enabled, and singly charged ions as well as precursors with a charge state above 6 were rejected. The dynamic exclusion window was set to 10 s. The automatic gain control was set to 3e6 for MS and 1e5 for MS/MS with ion accumulation times of 45 ms and 60 ms, respectively. The intensity threshold for precursor ion selection was set to 1.7e4.
The raw DDA data were analyzed with Proteome Discoverer™ Software (Version 2.3, Thermo Fisher Scientific). Peptides were identified using both SEQUEST HT and Mascot against UniProtKB mouse database (UP000000589 plus isoforms). The search was performed with the following parameters applied: static modification: cysteine carbamidomethylation and dynamic modifications: N-terminal acetylation. Phosphorylation (S, T, Y, for serine, threonine and tyrosine, respectively) was set as variable for the phosphopeptide analysis. Precursor tolerance was set to 10 ppm and fragment tolerance to 0.02 ppm. Up to 2 missed cleavages were allowed and Percolator was used for peptide validation at a q-value of maximum 0.05. Peptides with different amino acid sequences or modifications were considered as unique. Extracted peptides, with a quality q-value lower than 0.05, and modification of S, T, and Y phosphorylation detected were used to identify and quantify them by label-free relative quantification. The extracted chromatographic intensities were used to compare peptide abundance across samples.
The MS results were processed via Perseus software (version 1.6.0.7, Tyanova et al., 2016). The protein intensities were log2 transformed. Selective criteria were defined as: Among 4 replicates of 4 conditions (16 samples totally), only a peptide with the missing value of less than 30% in total (less than 5 samples with missing value) would be selected and the missing values were replaced from a normal distribution was performed though data imputation by using the following settings: width 0.3 and downshift 0. The further bioinformatics analysis of these processed data was done via the web-based tool Phosphomatics (
https://phosphomatics.com/; [
20]). In Phosphomatics, two-Sample Student's t-test (two-tailed) were performed to compare phosphorylated site levels between the
rd1 explants with 2 hours of PKG inhibition vs untreated control, and 4 days of PKG inhibition vs untreated controls. A p-value of 0.05 was defined as the cut-off. Two lists of the phosphorylated sites identified previously were used to perform a further upstream kinase analysis in Phosphomatics. The biological pathways that might be affected between
rd1 untreated and
rd1 with PKG inhibition samples were determined in Enrichr (
https://maayanlab.cloud/Enrichr/), where differentially phosphorylated sites mentioned above were used as input list to perform pathway-based analysis of Kyoto Encyclopedia of Genes and Genomes (KEGG).
2.5. Cryosection, immunohistochemistry and Proximity ligation assay (PLA)
Retinal tissues from rd1 and wt in vivo at P9, as well as P11 cultured explants from rd1, were treated with 4% formaldehyde for 2 hours, washed 3 × 15 min in phosphate-buffered saline (PBS), cryoprotected in PBS + 10% sucrose for overnight at 4 °C and subsequently with PBS + 25% sucrose for 2 hours. After embedding in a medium with 30% bovine serum albumin (BSA; Cat. No.: A5253-250G; Sigma-Aldrich, St. Louis, MO, USA) and 3% gelatine (Cat. No.: 1040781000, Merck Millipore, Burlington, MA, USA) mixed in H2O, 12 μm thick retinal cross-sections were cut and collected from a HM560 cryotome (Microm, Walldorf, Germany). The sections were stored at -20 °C for later usage. Cryosections were then used for immunostaining and PLA. Totally 10 retinal samples were used, with 5 each in either rd1 or wt strains, while 13 rd1 explants were used, with 4 in group of retinas with 4 days of PKG inhibition and 3 each in other 3 groups.
For immunostaining, briefly, the cryosections were dried in room temperature for 15 minutes and rehydrated in PBS. Then they were blocked with 1% BSA + 0.25% Triton X100 + 5% goat serum in PBS at room temperature for 45 min. The primary antibody anti-Raf1 (Cat#: MA5-17162, ThermoFisher) was diluted with 1% BSA and 0.25% Triton X100 in PBS (PTX) and incubated at 4 °C for overnight; a no primary antibody control ran in parallel. Sections were washed 3 × 5 min each in PTX and incubated with a donkey anti-mouse IgG (H+L) highly cross-adsorbed secondary antibody, Alexa Fluor™ Plus 488 (#A32766, ThermoFisher) at 1:800 dilution in PTX. After 3 x 5-min PBS washes the sections were mounted with Vectashield DAPI (Vector, Burlingame, CA, USA).
The PLA, which detects if two antigens are in close proximity (<40 nm) with each other, was performed on cryosections, using Detection Reagents Red kit (DUO92008-100RXN, Merck, Readington Township, NJ), the PLA Probe anti-rabbit PLUS (DUO92002, Merck, Readington Township, NJ) and anti-mouse MINUS (DUO92004, Merck, Readington Township, NJ). The procedure followed the manufacturer's instructions. In short, the retinal sections were blocked with blocking solution (provided in PLA Probe kit) for 45 min at 20°C, and primary antibodies antibody anti-Raf1 (Cat#: MA5-17162, ThermoFisher) and anti-Phosphoserine (ab9332, Abcam, Cambridge, UK) were incubated overnight at 4°C. PLA Probe anti-rabbit PLUS and anti-mouse MINUS were incubated for 1 hr at 37°C. The ligation and amplification steps were performed using the Detection Reagents Red, followed by the addition mounting of Vectashield DAPI (Vector, Burlingame, CA, USA).
2.6. Microscopy and image processing
A Zeiss Imager Z1 Apotome Microscope (Zeiss, Oberkichen, Germany), with a Zeiss Axiocam digital camera was used for microscopy observations. Image generation and contrast enhancement were performed identically for all images via the ZEN2 software (blue edition). The immunostaining was analysed for staining differences via three sections each from three to five animals for each condition, after which the fluorescent intensities of positive cells randomly distributed within in the area of interest (outer nuclear layer, ONL, i.e. the photoreceptor layer) were assessed. Fluorescence intensity was captured and analysed by the ImageJ software (version 1.53a, NIH, Maryland, USA). The freehand selection function was used to target the ONL, after which the fluorescence intensity was calculated with the measure function. The values of all sections from the same animal were averaged. For PLA, the images were also generated with the aid of a Zeiss Imager Z1 Apotome Microscope and the ZEN2 software (blue edition). The punctuations of ONL, which represent reaction products from when the two antigens are in close proximity, was counted manually of three sections each from three to five animals for each condition, with values from the same animal averaged. The experimenter was unaware of the groups of the samples during image processing.
2.7. Statistical analysis
The student’s t-test was used to compare the values of immunostaining intensities and PLA punctuations in different conditions in R, and p value cutoff was defined as 0.05. Visualization of KEGG analysis was also performed in R.
4. Discussion
MS-based phosphoproteomics techniques has been extensively applied in biological research, including in retinal studies [
25]. Technically, one option is to apply immunoprecipitation for phosphoprotein enrichment prior to MS detection. However, due to the limitation of commercially phospho-selective antibodies, this approach has not provided promising results in phosphoprotein enrichment [
26], and is therefore unsuitable for high throughput phosphoproteomic study. Another widely applied strategy is the use of immobilized metal affinity chromatography, as in the current study. Though the non-specificity has been noticed for this method, the continuous optimization of the protocol has made it frequently applied in phosphoproteomic research [
27] including here.
Our study has three clear outcomes: 1) It identified potential retinal PKG substrates that could be involved in retinal degeneration, 2) it gave information on the kinase and pathway networks in operation during the degenerative events, and 3) it showed a correlation between the cGMP-PKG system and the RAS/RAF/MAPK/ERK pathway in this situation.
The upstream kinase and KEGG analyses both picked up PKG or the cGMP-PKG signaling pathway as being affected by the treatment, which corroborated the usefulness of the approach. In addition, the two treatment regimes, i.e. 2 hours or 4 days of PKG inhibition, gave data on proteins whose phosphorylation was decreased as well as such that displayed increased phosphorylation in either or both of these regimes.
The assumption that increased phosphorylation by PKG is part of the degeneration mechanism, makes the proteins with reduced phosphorylation at both time points of PKG inhibition particularly interesting with respect to a pathologic involvement. Among the five proteins of this category, we noticed DNA Topoisomerase II Beta (TOP2B) and capicua transcriptional repressor (CIC). Deletion of TOP2B harms photoreceptors [
28], and TOP2B mRNA was in our previous transcriptome study reduced by PKG inhibition in
rd1 explants [
17]. Given that TOP2B phosphorylation affects its functions [
29], one may consider that PKG-dependent over-phosphorylation of TOP2B leads to dysfunctional transcription [
30], which could affect the photoreceptors’ wellbeing. Likewise, our transcriptome study also connected CIC with the cGMP-PKG system [
17]. CIC is a transcriptional repressor, with an involvement in neurodegeneration [
31] and in nervous system differentiation. CIC has a connection also with the ataxin-1 like protein (ATXN1L), in the sense that they in concert with yet other proteins (ETS transcription factors) may act to modulate the sensitivity of the MAPK pathway [
32]. It is in this context interesting that the phosphorylation of ATXN1L was reduced in the 2 hours PKG inhibition experiment (Supplementary Table S1). Taken together, there is reason to keep TOP2B and CIC as candidates for links between PKG activity and events during retinal degeneration.
Proteins that instead showed persistent
increased phosphorylation after PKG inhibition are, by contrast, more likely to be dependent on kinases under direct or indirect negative regulation by PKG, and were represented by as many as 16 proteins. Some deserve to be mentioned here, such as ankyrin 2 (ANK2), with a possible role in maintaining ion balance, via anchoring of ion transporters to the photoreceptor cell membrane [
33]. Proteins of the microtubule-associated protein (MAP) family, including MAP1B, MAP2 and MAP4, were also seen. MAPs could be critical for photoreceptors and at least one of these may associate with retinal degeneration [
34,
35].
The upstream kinase analysis indicated an integration of the cGMP-PKG pathway with other kinases, of which those with potentially lower activity after 2 hours and 4 days of treatment included CDK1 and protein kinase cAMP-activated catalytic subunit alpha (PRKACA). For the CDK1, we recently demonstrated its expression in degenerating
rd1 photoreceptors, as well as that this was reduced by PKG inhibition [
36]. Together with our present observation that lower CDK1 activities were predicted already 2 hours and 4 days after PKG was inhibited, a possible interpretation would be that CDK1 expression is somehow affected by a cGMP-PKG regulated phosphorylation.
Several kinases with potential higher activities after PKG inhibition were from the mitogen-activated protein kinase (MAPK) family. MAPK signaling, particularly via the activation of the p38 MAPK subclass, has been shown to have neuroprotective effects in degenerating photoreceptors [
37]. MAPK14 belongs to this subclass and was suggested to have increased activity after both 2 hours and 4 days of PKG inhibition. The upstream kinase analysis moreover pointed to higher activities of two dual-specificity MAPKs, termed MAP kinase kinases (MAP2K) and mitogen activated protein kinase kinase kinase (MAP3K), and at least MAP2K is known as a p38 MAPK regulator responsible for its activation [
38]. The MAPKs therefore appear connected with the degeneration, including in a PKG related way.
The RAS/RAF1/MAPK/ERK pathway with its diverse biological functions is one of the most extensively studied signal transduction routes [
39] and here we provide insights into how RAF1 may connect with retinal degeneration. In wt retinas the predominant expression of RAF1 was in the segments, pointing to it being a photoreceptor specific kinase. The increased expression and phosphorylation of RAF1 in the
rd1 compared to wt ONL revealed in turn that it may participate in the degeneration of the photoreceptors, as did the above mentioned suggested increased activation of several MAPKs.
However, neither its increased expression nor the increased phosphorylation specifies whether RAF1 works for or against the degeneration. Our results of PKG inhibition in
rd1 explants provided more clues, though, since RAF1 expression increased after both lengths of treatment and more phosphorylated RAF1 was seen after 2 hours of PKG inhibition. Given that retinal degeneration benefits from such PKG inhibition [
8,
9], RAF1 may exert neuroprotective effects during photoreceptor death. Had it been the opposite, that the expression and phosphorylation state of RAF1 drives the degeneration, both these parameters would have been expected to decrease after PKG inhibition. Yet the situation is not straightforward, since phosphorylation of RAF1 may lead to either its inhibition or activation, depending on the actual serine site [
40], which we were not able to discriminate between here. While highly speculative, the suggested activation of RAF1 in the upstream kinase analysis could mean that the degeneration initiates a protective program involving RAF1 activity, but that PKG counteracts the same by blocking the activity, probably via other kinase or even phosphatase activities.
In conclusion, our study shows an intricate pattern of changes with respect to protein phosphorylations after inhibition of PKG. It appears likely that several of these results, including those connected to the RAS/RAF1/MAPK/ERK pathway, relate to the yet unclarified mechanisms of inherited retinal degeneration and as such will aid in designing future investigations in this area.
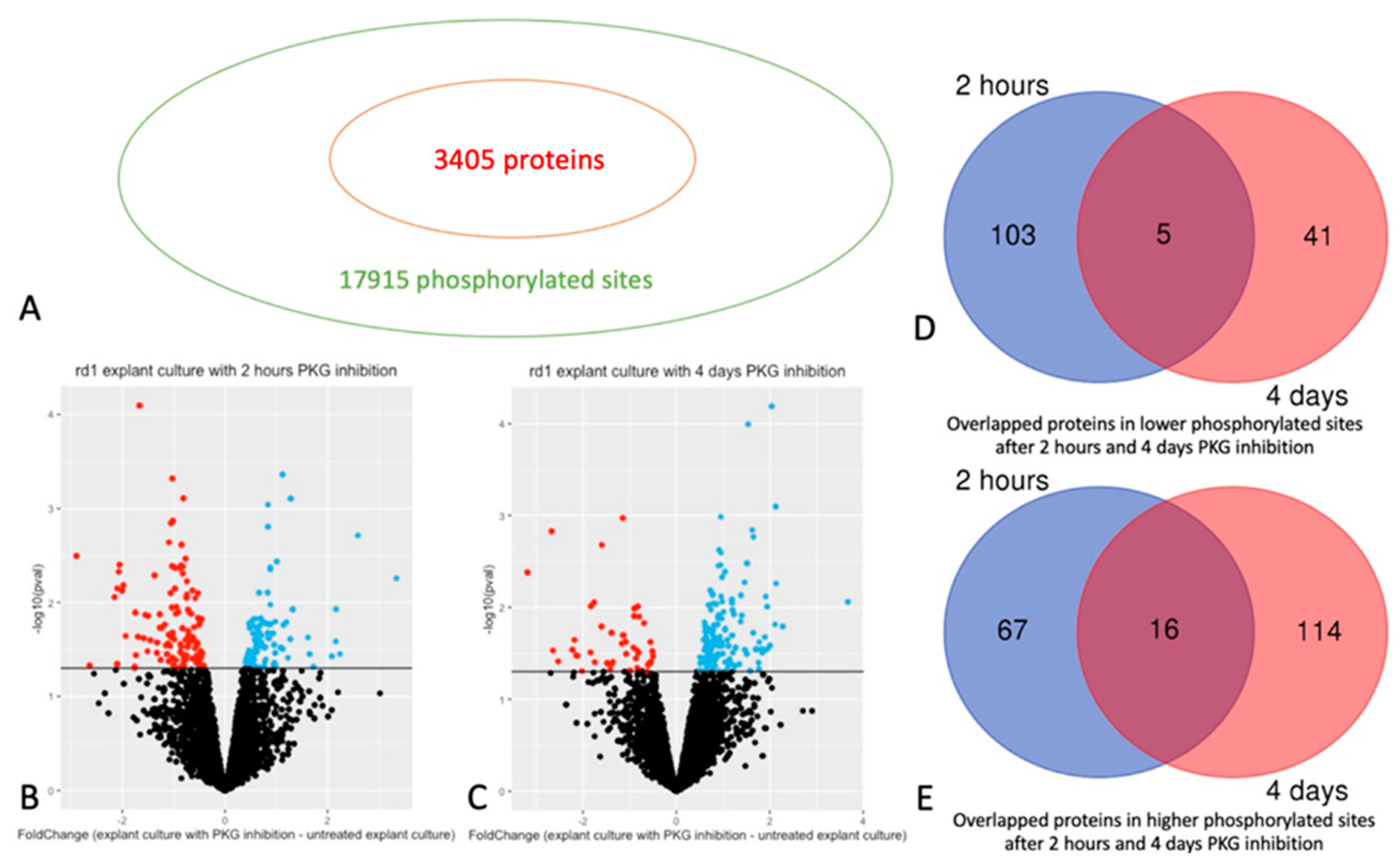
Figure 1.
Phosphorylated sites and proteins in retinal explants affected by PKG inhibition. Retinal explants generated from the rd1 strain were treated with PKG inhibitor for 2 hours or 4 days, and phosphoproteomics was done via mass spectrometry (MS) after phosphopeptide enrichment. A: 17915 phosphorylated sites of 3405 proteins were identified. B: Volcano plot showing Fold Change between and -Log10(p-value) of phosphorylated sites with 2 hours treatment. C: Volcano plot showing Fold Change between and -Log10(p-value) of phosphorylated sites with 4 days treatment. For both B and C red and blue dots represent sites with decreased or increased phosphorylation, respectively (p < 0.05), while black dots represent sites without difference. D: Venn diagram showing the overlapped proteins in decreased phosphorylated sites after 2 hours and 4 days PKG inhibition. E: Venn diagram showing the overlapped proteins in increased phosphorylated sites after 2 hours and 4 days PKG inhibition.
Figure 1.
Phosphorylated sites and proteins in retinal explants affected by PKG inhibition. Retinal explants generated from the rd1 strain were treated with PKG inhibitor for 2 hours or 4 days, and phosphoproteomics was done via mass spectrometry (MS) after phosphopeptide enrichment. A: 17915 phosphorylated sites of 3405 proteins were identified. B: Volcano plot showing Fold Change between and -Log10(p-value) of phosphorylated sites with 2 hours treatment. C: Volcano plot showing Fold Change between and -Log10(p-value) of phosphorylated sites with 4 days treatment. For both B and C red and blue dots represent sites with decreased or increased phosphorylation, respectively (p < 0.05), while black dots represent sites without difference. D: Venn diagram showing the overlapped proteins in decreased phosphorylated sites after 2 hours and 4 days PKG inhibition. E: Venn diagram showing the overlapped proteins in increased phosphorylated sites after 2 hours and 4 days PKG inhibition.
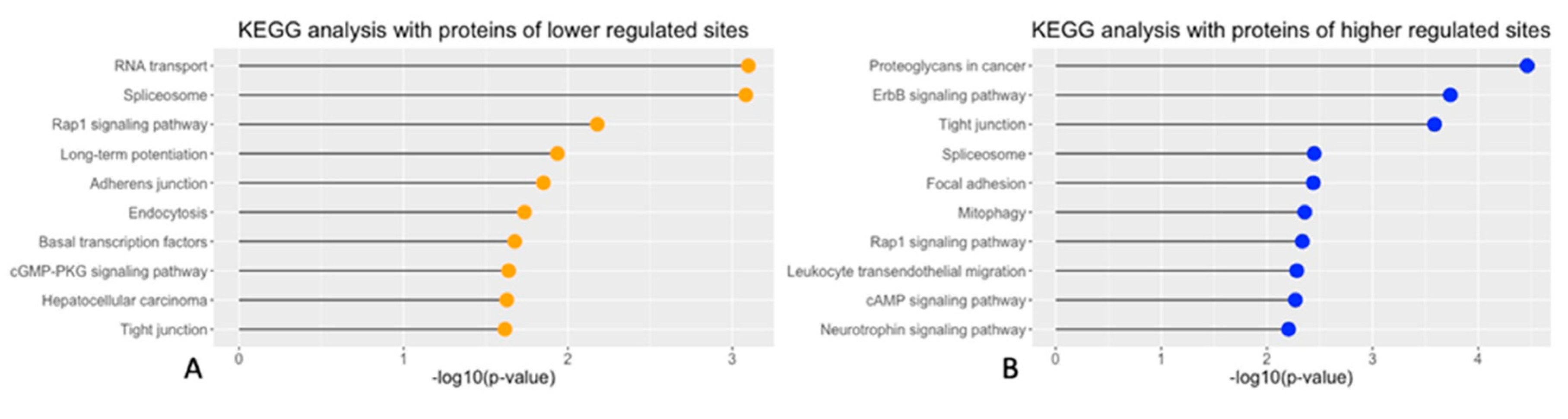
Figure 2.
KEGG analyses of pathways connected with the observed phosphorylation changes. A: Top 10 significant biological pathways enriched in the KEGG analysis and thus connected with proteins having decreased phosphorylated sites. B: Top 10 significant biological pathway for sites with increased phosphorylation.
Figure 2.
KEGG analyses of pathways connected with the observed phosphorylation changes. A: Top 10 significant biological pathways enriched in the KEGG analysis and thus connected with proteins having decreased phosphorylated sites. B: Top 10 significant biological pathway for sites with increased phosphorylation.
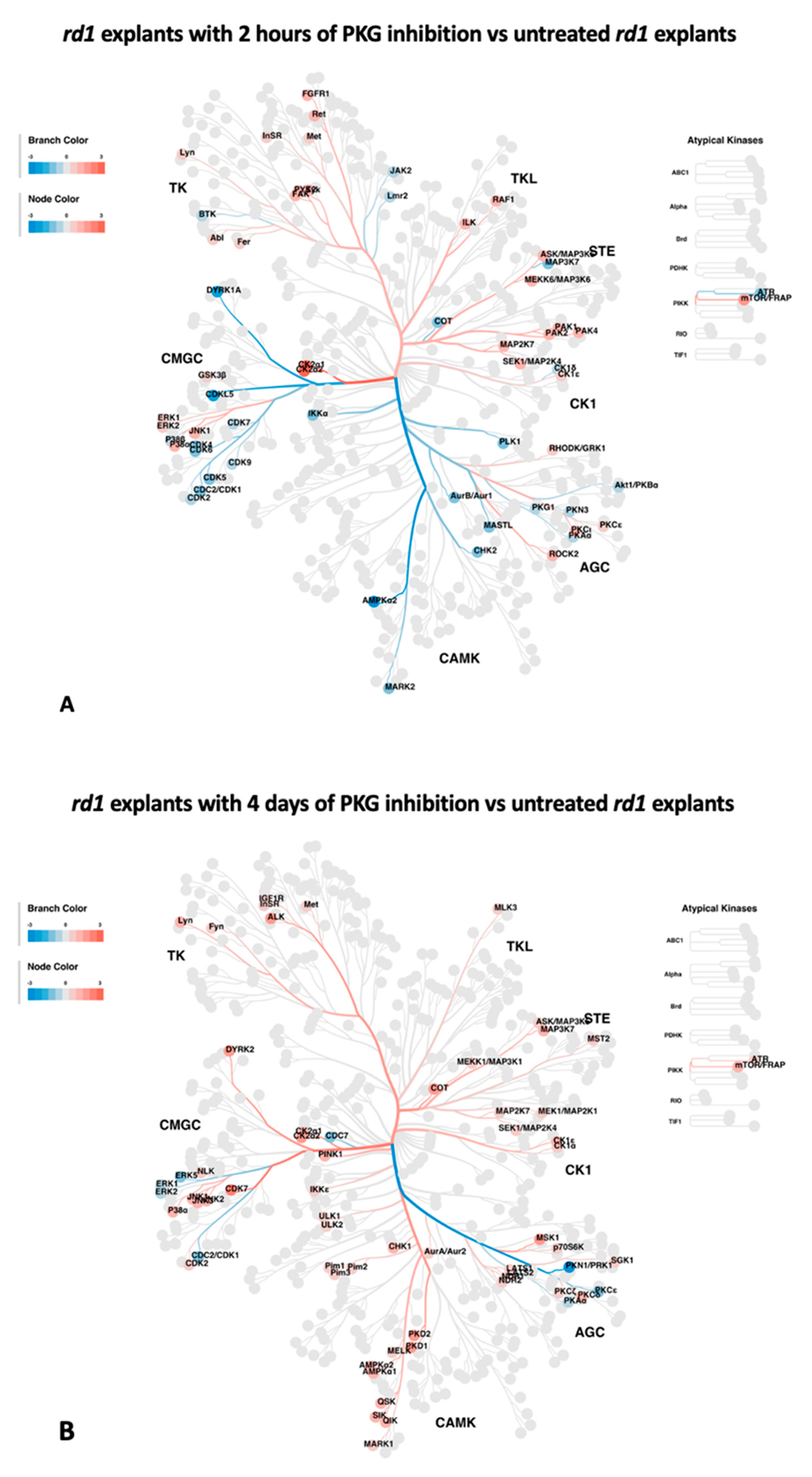
Figure 3.
Potential altered kinases, as indicated by the upstream kinase tool, are visualized as kinome phylogenetic trees. The branch and node colors are encoded by Fold Change, with values < 0 (in blue) and > 0 (in red) representing kinase activity as decreased or increased, respectively, in rd1 explants with PKG inhibition compared to untreated rd1 explants. A: Kinome phylogenetic tree with potential kinases altered in rd1 with PKG inhibition for 2 hours. B. Kinome phylogenetic tree with potential kinases altered in rd1 with PKG inhibition for 4 days.
Figure 3.
Potential altered kinases, as indicated by the upstream kinase tool, are visualized as kinome phylogenetic trees. The branch and node colors are encoded by Fold Change, with values < 0 (in blue) and > 0 (in red) representing kinase activity as decreased or increased, respectively, in rd1 explants with PKG inhibition compared to untreated rd1 explants. A: Kinome phylogenetic tree with potential kinases altered in rd1 with PKG inhibition for 2 hours. B. Kinome phylogenetic tree with potential kinases altered in rd1 with PKG inhibition for 4 days.
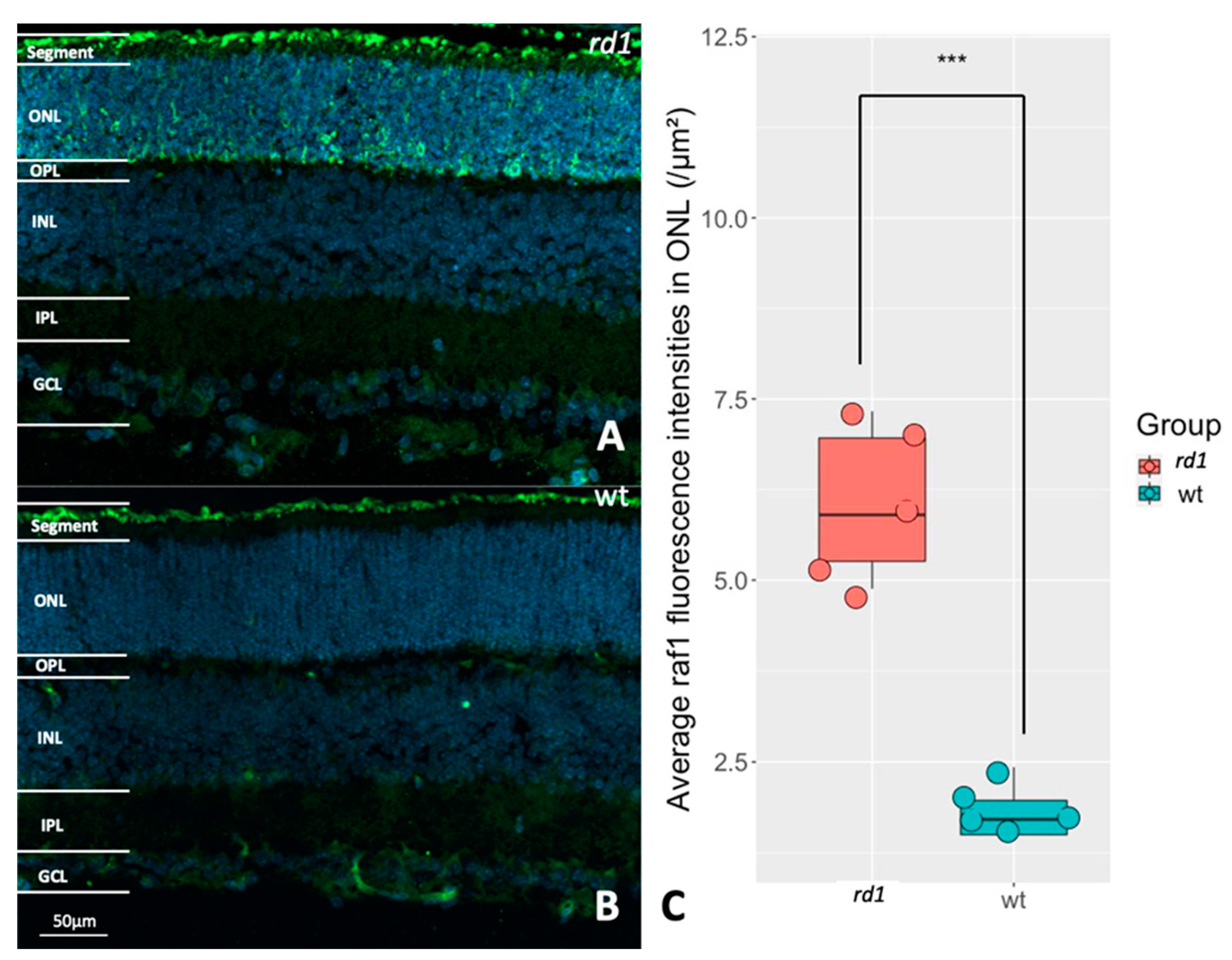
Figure 4.
Evaluation of RAF1 expression in rd1 and wt retinas. A: Immunostaining of RAF1 (green) in a P11 rd1 retina. B: Immunostaining of RAF1 (green) in a P11 wt retina. DAPI (blue) was used as nuclear counterstain. C: The box chart shows the comparison of RAF fluorescence intensities within the outer nuclear layer (ONL) between rd1 (n=5) and wt (n=5), ***p < 0.001.
Figure 4.
Evaluation of RAF1 expression in rd1 and wt retinas. A: Immunostaining of RAF1 (green) in a P11 rd1 retina. B: Immunostaining of RAF1 (green) in a P11 wt retina. DAPI (blue) was used as nuclear counterstain. C: The box chart shows the comparison of RAF fluorescence intensities within the outer nuclear layer (ONL) between rd1 (n=5) and wt (n=5), ***p < 0.001.
Figure 5.
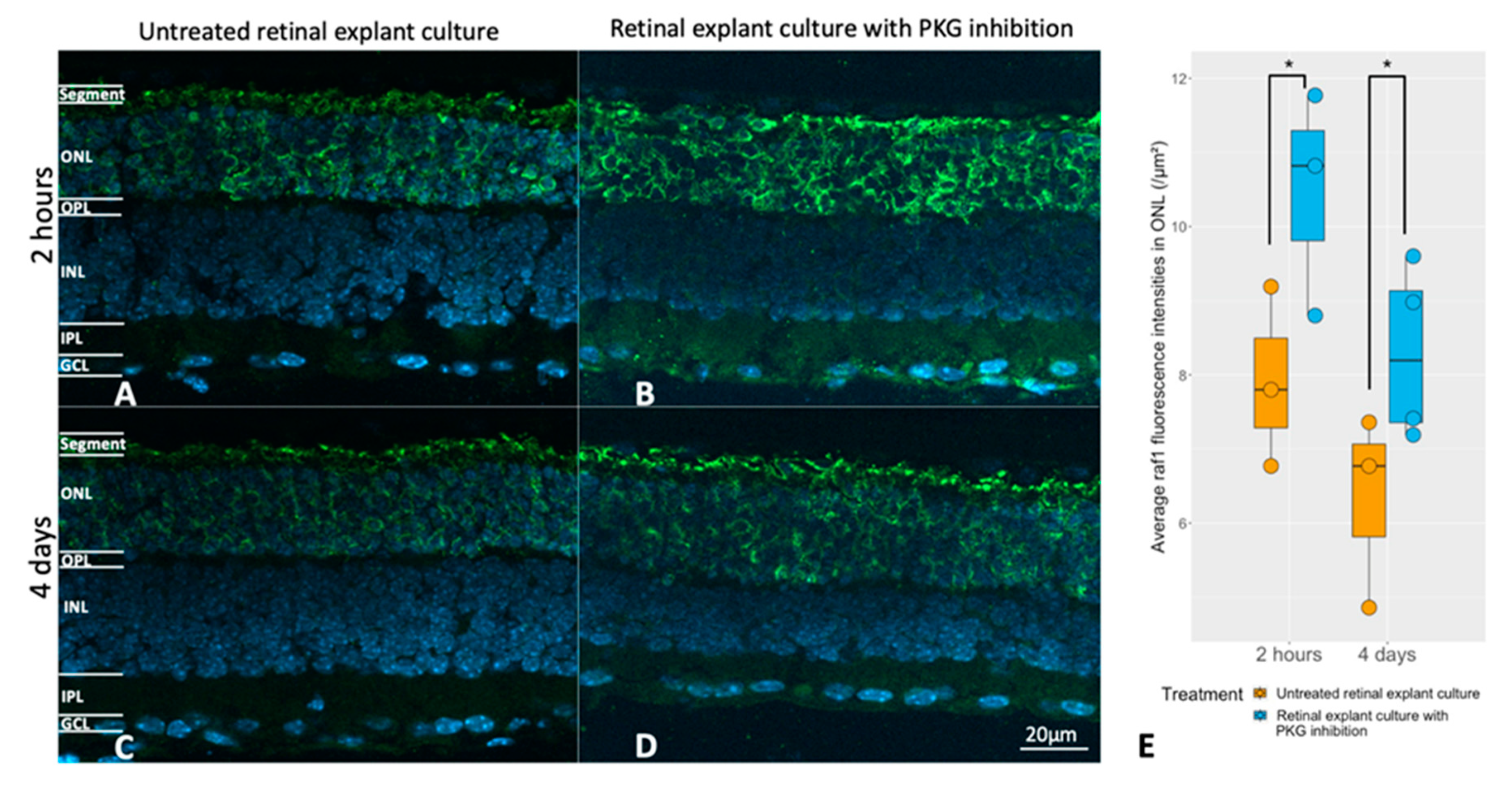
Evaluation of the relation between the cGMP-PKG system and RAF1 expression. Retinal explants from rd1 were treated with 50 μM Rp-8-Br-PET-cGMPS (PKG inhibitor) for 2 hours (n=3) and their untreated counterparts (n=3), or the same compound in the same concentration for 4 days (n=4) and their untreated controls (n=3). A and B represent RAF1 (green) immunostaining in untreated retinal explants and their counterparts with 2 hours of PKG inhibition. C and D represent immunostaining of RAF1 in untreated retinal explants and their counterparts with 4 days of PKG inhibition. DAPI (blue) was used as nuclear counterstain. E: The box chart compares RAF fluorescence intensities within the ONL between untreated explants and peers with PKG inhibitor in different treatment lengths. *p < 0.05.
Figure 5.
Evaluation of the relation between the cGMP-PKG system and RAF1 expression. Retinal explants from rd1 were treated with 50 μM Rp-8-Br-PET-cGMPS (PKG inhibitor) for 2 hours (n=3) and their untreated counterparts (n=3), or the same compound in the same concentration for 4 days (n=4) and their untreated controls (n=3). A and B represent RAF1 (green) immunostaining in untreated retinal explants and their counterparts with 2 hours of PKG inhibition. C and D represent immunostaining of RAF1 in untreated retinal explants and their counterparts with 4 days of PKG inhibition. DAPI (blue) was used as nuclear counterstain. E: The box chart compares RAF fluorescence intensities within the ONL between untreated explants and peers with PKG inhibitor in different treatment lengths. *p < 0.05.
Figure 6.
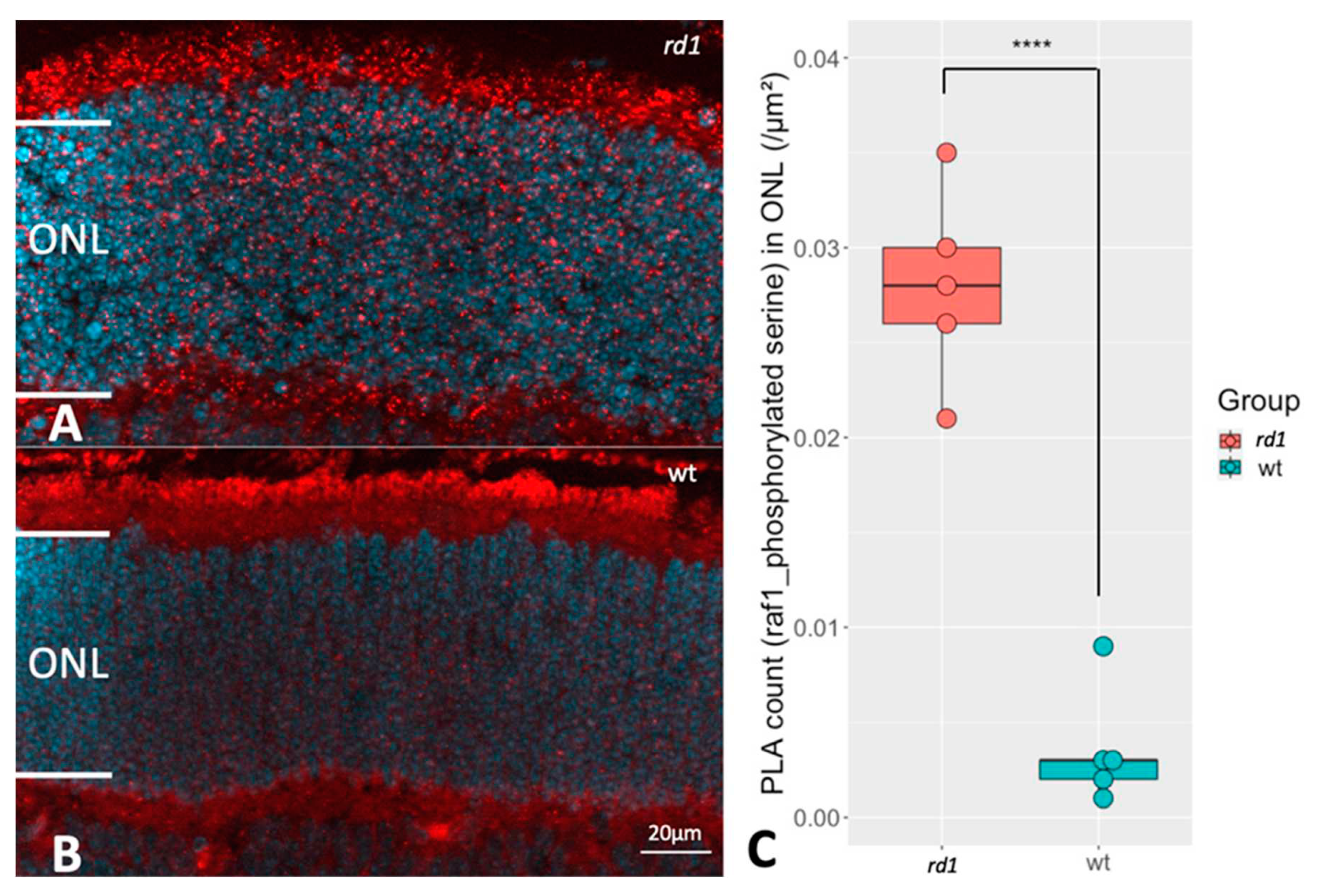
Comparison of RAF1 phosphorylation between rd1 and wt retinas. The proximity ligation assay (PLA) punctuations indicate that RAF1 is in proximity with phosphoserine, which reveals the RAF1 phosphorylation profile. A and B represent PLA punctuations (RAF1 and phosphorylated serine) in ONL from rd1 and wt, respectively. DAPI (blue) was used as nuclear counterstain C: The box chart shows the comparison of PLA counts in ONL of retinas from rd1 (n=5) and wt (n=5). ****p < 0.0001.
Figure 6.
Comparison of RAF1 phosphorylation between rd1 and wt retinas. The proximity ligation assay (PLA) punctuations indicate that RAF1 is in proximity with phosphoserine, which reveals the RAF1 phosphorylation profile. A and B represent PLA punctuations (RAF1 and phosphorylated serine) in ONL from rd1 and wt, respectively. DAPI (blue) was used as nuclear counterstain C: The box chart shows the comparison of PLA counts in ONL of retinas from rd1 (n=5) and wt (n=5). ****p < 0.0001.
Figure 7.
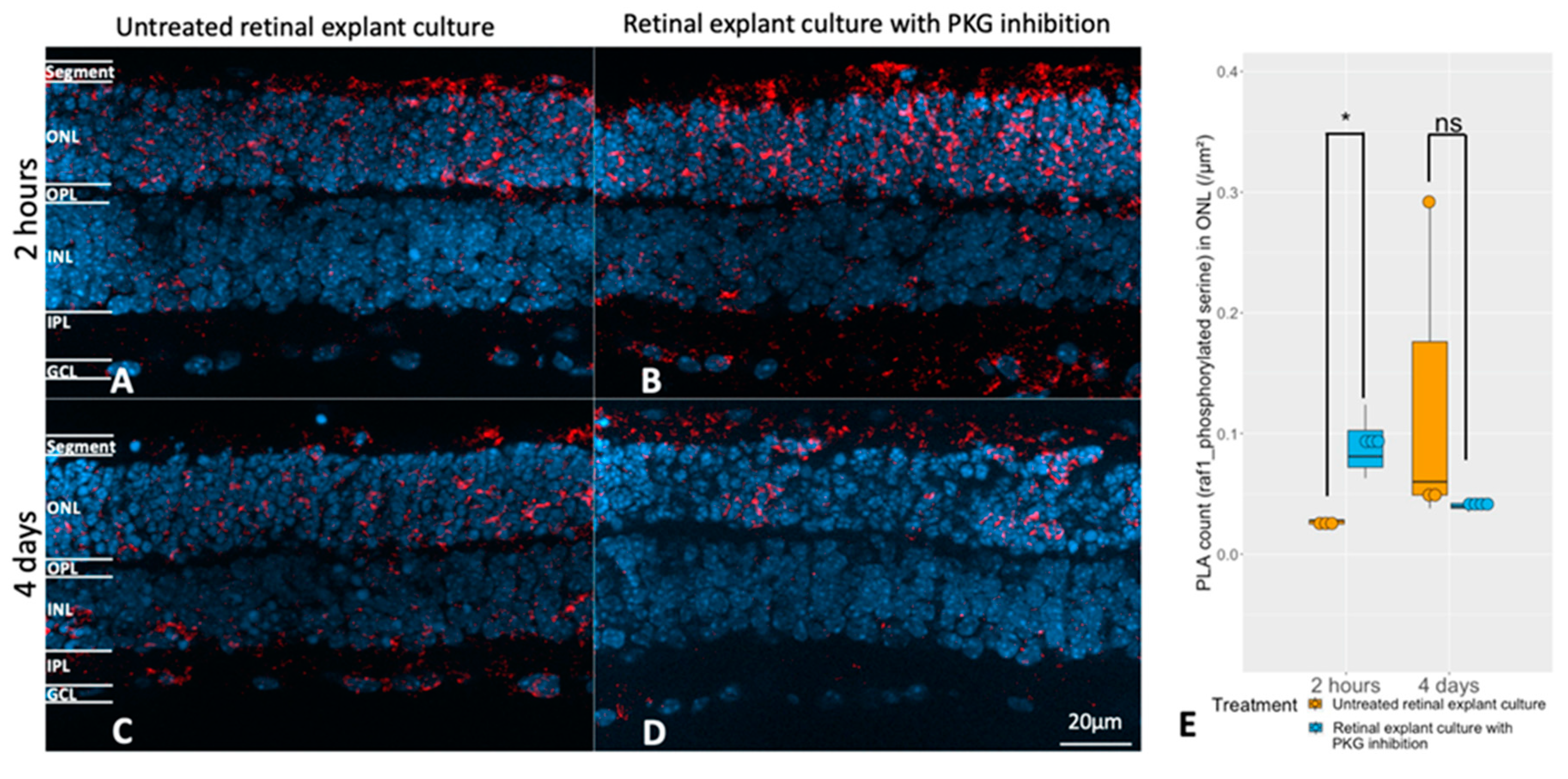
Evaluation of the relation between the cGMP-PKG system and RAF1 phosphorylation. Retinal explants from rd1 were treated with 50 μM Rp-8-Br-PET-cGMPS (PKG inhibitor) for 2 hours (n=3) and their untreated counterparts (n=3), or the same compound in the same concentration for 4 days (n=4) and their untreated controls (n=3). A and B represent PLA (red, RAF1 and phosphorylated serine) in untreated retinal explants and the counterparts with 2 hours PKG inhibition. C and D represent PLA punctuations in untreated retinal explants and the counterparts with 4 days PKG inhibition. DAPI (blue) was used as nuclear counterstain. E: The box chart shows the comparison of PLA counts within ONL between untreated retinal explants controls and peers with PKG inhibitor in different length of treatment (n=3-4). *p < 0.05.
Figure 7.
Evaluation of the relation between the cGMP-PKG system and RAF1 phosphorylation. Retinal explants from rd1 were treated with 50 μM Rp-8-Br-PET-cGMPS (PKG inhibitor) for 2 hours (n=3) and their untreated counterparts (n=3), or the same compound in the same concentration for 4 days (n=4) and their untreated controls (n=3). A and B represent PLA (red, RAF1 and phosphorylated serine) in untreated retinal explants and the counterparts with 2 hours PKG inhibition. C and D represent PLA punctuations in untreated retinal explants and the counterparts with 4 days PKG inhibition. DAPI (blue) was used as nuclear counterstain. E: The box chart shows the comparison of PLA counts within ONL between untreated retinal explants controls and peers with PKG inhibitor in different length of treatment (n=3-4). *p < 0.05.
Table 1.
Lists of proteins showing decreased phosphorylation after PKG inhibition for either 2 hours (left column) or 4 hours (center column), whereas the right column shows proteins that showed reduced phosphorylation at both time points. Note that the left and center columns only show the 20 proteins with highest fold change for 2 hours and 4 days, respectively. The total number of proteins with decreased phosphorylation was 108 and 46 after 2 hours and 4 days PKG inhibition, respectively. Full lists of all proteins/peptides with decreased phosphorylation are found in Supplementary Tables S1 and S2.
Table 1.
Lists of proteins showing decreased phosphorylation after PKG inhibition for either 2 hours (left column) or 4 hours (center column), whereas the right column shows proteins that showed reduced phosphorylation at both time points. Note that the left and center columns only show the 20 proteins with highest fold change for 2 hours and 4 days, respectively. The total number of proteins with decreased phosphorylation was 108 and 46 after 2 hours and 4 days PKG inhibition, respectively. Full lists of all proteins/peptides with decreased phosphorylation are found in Supplementary Tables S1 and S2.
| Proteins with decreased phosphorylation after PKG inhibition |
|---|
| Top 20 after 2 hours |
Top 20 after 4 days |
Appearing in both |
| Gene symbol |
Protein name |
Fold Change |
p-value |
Gene symbol |
Protein name |
Fold Change |
p-value |
Gene symbol |
Protein name |
| CDC27 |
cell division cycle 27 |
-2,894 |
0,003 |
EPN1 |
epsin 1 |
-3,188 |
0,004 |
PKN1 |
protein kinase N1 |
| AUTS2 |
activator of transcription and developmental regulator |
-2,638 |
0,047 |
PKN1 |
protein kinase N1 |
-2,668 |
0,001 |
CIC |
capicua transcriptional repressor |
| DMXL2 |
Dmx like 2 |
-2,150 |
0,009 |
PITPNM1 |
phosphatidylinositol transfer protein membrane associated 1 |
-2,640 |
0,029 |
TOP2B |
DNA topoisomerase II beta |
| PICALM |
phosphatidylinositol binding clathrin assembly protein |
-2,100 |
0,045 |
MAP7D1 |
MAP7 domain containing 1 |
-2,530 |
0,039 |
CCDC88A |
coiled-coil domain containing 88A |
| TRAF7 |
TNF receptor associated factor 7 |
-2,100 |
0,007 |
MTMR2 |
myotubularin related protein 2 |
-2,225 |
0,029 |
PBRM1 |
polybromo 1 |
| PARD3B |
par-3 family cell polarity regulator beta |
-2,069 |
0,005 |
GRK1 |
G protein-coupled receptor kinase 1 |
-2,180 |
0,023 |
|
|
| AMPH |
amphiphysin |
-2,051 |
0,004 |
PCBP1 |
poly(rC) binding protein 1 |
-2,144 |
0,033 |
|
|
| ATG16L1 |
autophagy related 16 like 1 |
-2,006 |
0,008 |
JPT1 |
Jupiter microtubule associated homolog 1 |
-2,115 |
0,034 |
|
|
| RNF34 |
ring finger protein 34 |
-1,980 |
0,007 |
EPB41 |
Protein 4.1 isoform 2 |
-2,023 |
0,049 |
|
|
| ACIN1 |
apoptotic chromatin condensation inducer 1 |
-1,934 |
0,023 |
GPHN |
Gephyrin |
-1,836 |
0,031 |
|
|
| SYN3 |
synapsin III |
-1,759 |
0,048 |
PPP6R2 |
protein phosphatase 6 regulatory subunit 2 |
-1,826 |
0,010 |
|
|
| DPY30 |
dpy-30 histone methyltransferase complex regulatory subunit |
-1,759 |
0,050 |
FLNB |
filamin B |
-1,762 |
0,009 |
|
|
| ZNF687 |
zinc finger protein 687 |
-1,747 |
0,013 |
RNF20 |
ring finger protein 20 |
-1,747 |
0,040 |
|
|
| CTBP2 |
C-terminal binding protein 2 |
-1,736 |
0,036 |
TSC22D1 |
TSC22 domain family member 1 |
-1,602 |
0,016 |
|
|
| CCDC88A |
coiled-coil domain containing 88A |
-1,696 |
0,023 |
LIN37 |
lin-37 DREAM MuvB core complex component |
-1,595 |
0,002 |
|
|
| SMPD3 |
sphingomyelin phosphodiesterase 3 |
-1,666 |
0,000 |
CIC |
capicua transcriptional repressor |
-1,480 |
0,040 |
|
|
| NKTR |
natural killer cell triggering receptor |
-1,583 |
0,024 |
SLC20A2 |
solute carrier family 20 member 2 |
-1,416 |
0,048 |
|
|
| NONO |
non-POU domain containing octamer binding |
-1,554 |
0,014 |
PRKG1 |
protein kinase cGMP-dependent 1 |
-1,387 |
0,019 |
|
|
| RBM8A |
RNA binding motif protein 8A |
-1,509 |
0,033 |
ARHGAP21 |
Rho GTPase activating protein 21 |
-1,385 |
0,041 |
|
|
| SNX2 |
sorting nexin 2 |
-1,500 |
0,014 |
PABIR1 |
PP2A Aalpha (PPP2R1A) and B55A (PPP2R2A) interacting phosphatase regulator 1 |
-1,356 |
0,039 |
|
|
Table 2.
Lists of proteins showing increased phosphorylation after PKG inhibition for either 2 hours (left column) or 4 hours (center column), whereas the right column shows proteins that showed increased phosphorylation at both time points. Note that the left and center columns only show the 20 proteins with highest fold change for 2 hours and 4 days, respectively. The total number of proteins with increased phosphorylation was 83 and 130 after 2 hours and 4 days PKG inhibition, respectively. Full lists of all proteins/peptides with increased phosphorylation are found in Supplementary Tables S1 and S2.
Table 2.
Lists of proteins showing increased phosphorylation after PKG inhibition for either 2 hours (left column) or 4 hours (center column), whereas the right column shows proteins that showed increased phosphorylation at both time points. Note that the left and center columns only show the 20 proteins with highest fold change for 2 hours and 4 days, respectively. The total number of proteins with increased phosphorylation was 83 and 130 after 2 hours and 4 days PKG inhibition, respectively. Full lists of all proteins/peptides with increased phosphorylation are found in Supplementary Tables S1 and S2.
| Proteins with increased phosphorylation after PKG inhibition |
|---|
| Top 20 after 2 hours |
Top 20 after 4 days |
Appearing in both |
| Gene symbol |
Protein name |
Fold Change |
P-value |
Gene symbol |
Protein name |
Fold Change |
P-value |
Gene symbol |
Protein name |
| HNRNPUL1 |
heterogeneous nuclear ribonucleoprotein U like 1 |
3,343 |
0,006 |
MAP1B |
microtubule associated protein 1B |
3,674 |
0,009 |
HNRNPUL1 |
heterogeneous nuclear ribonucleoprotein U like 1 |
| EEF1B2 |
eukaryotic translation elongation factor 1 beta 2 |
2,593 |
0,002 |
LGALS12 |
galectin 12 |
2,279 |
0,016 |
PALM2AKAP2 |
PALM2 and AKAP2 fusion |
| HNRNPU |
heterogeneous nuclear ribonucleoprotein U |
2,242 |
0,035 |
HNRNPUL1 |
heterogeneous nuclear ribonucleoprotein U like 1 |
2,138 |
0,006 |
DLG1 |
discs large MAGUK scaffold protein 1 |
| GTPBP1 |
GTP binding protein 1 |
2,165 |
0,012 |
NR5A1 |
nuclear receptor subfamily 5 group A member 1 |
2,128 |
0,001 |
SEPTIN4 |
septin 4 |
| MAP4 |
microtubule associated protein 4 |
2,161 |
0,026 |
GTF2F1 |
general transcription factor IIF subunit 1 |
2,115 |
0,015 |
MAP1B |
microtubule associated protein 1B |
| VPS53 |
VPS53 subunit of GARP complex |
2,083 |
0,037 |
SRRM2 |
serine/arginine repetitive matrix 2 |
2,041 |
0,000 |
HNRNPU |
heterogeneous nuclear ribonucleoprotein U |
| NES |
nestin |
1,725 |
0,048 |
RBSN |
rabenosyn, RAB effector |
2,006 |
0,026 |
MCM7 |
minichromosome maintenance complex component 7 |
| LARP1 |
La ribonucleoprotein 1, translational regulator |
1,650 |
0,035 |
TPD52L2 |
TPD52 like 2 |
1,946 |
0,010 |
ATF7IP |
activating transcription factor 7 interacting protein |
| CDK13 |
cyclin dependent kinase 13 |
1,621 |
0,024 |
VSX2 |
visual system homeobox 2 |
1,945 |
0,028 |
MAP4 |
microtubule associated protein 4 |
| AFDN |
afadin, adherens junction formation factor |
1,323 |
0,012 |
RBM12 |
RNA binding motif protein 12 |
1,915 |
0,008 |
ANK2 |
ankyrin 2 |
| PXN |
paxillin |
1,284 |
0,001 |
DZIP3 |
DAZ interacting zinc finger protein 3 |
1,897 |
0,021 |
SRRM1 |
serine and arginine repetitive matrix 1 |
| RCOR1 |
REST corepressor 1 |
1,275 |
0,025 |
ABLIM1 |
actin binding LIM protein 1 |
1,842 |
0,031 |
KIAA0930 |
KIAA0930 |
| RUNX1T1 |
RUNX1 partner transcriptional co-repressor 1 |
1,230 |
0,039 |
PITPNM2 |
phosphatidylinositol transfer protein membrane associated 2 |
1,827 |
0,028 |
CCDC88A |
coiled-coil domain containing 88A |
| HTATSF1 |
HIV-1 Tat specific factor 1 |
1,199 |
0,016 |
ELF2 |
E74 like ETS transcription factor 2 |
1,778 |
0,024 |
CTBP2 |
C-terminal binding protein 2 |
| ZC3H6 |
zinc finger CCCH-type containing 6 |
1,143 |
0,017 |
BICRAL |
BRD4 interacting chromatin remodeling complex associated protein like |
1,771 |
0,047 |
MAP2 |
microtubule associated protein 2 |
| BRAF |
B-Raf proto-oncogene, serine/threonine kinase |
1,127 |
0,031 |
PALM2AKAP2 |
PALM2 and AKAP2 fusion |
1,761 |
0,040 |
RERE |
arginine-glutamic acid dipeptide repeats |
| BASP1 |
brain abundant membrane attached signal protein 1 |
1,122 |
0,000 |
DNAJC5 |
DnaJ heat shock protein family (Hsp40) member C5 |
1,707 |
0,028 |
|
|
| PLCL2 |
phospholipase C like 2 |
1,086 |
0,030 |
PACS2 |
phosphofurin acidic cluster sorting protein 2 |
1,705 |
0,029 |
|
|
| SPHK2 |
sphingosine kinase 2 |
1,012 |
0,004 |
CCNL1 |
cyclin L1 |
1,650 |
0,002 |
|
|
| SEPTIN4 |
septin 4 |
0,987 |
0,047 |
EIF4EBP1 |
eukaryotic translation initiation factor 4E binding protein 1 |
1,647 |
0,019 |
|
|
Table 3.
Kinases with proposed altered activities after various lengths of PKG inhibition, as indicated by the upstream kinase tool. The four columns give kinases that appear in both of the two situations indicated at the head of each column. Full lists of all proposed kinase alterations are found in Supplementary Tables S3 and S4.
Table 3.
Kinases with proposed altered activities after various lengths of PKG inhibition, as indicated by the upstream kinase tool. The four columns give kinases that appear in both of the two situations indicated at the head of each column. Full lists of all proposed kinase alterations are found in Supplementary Tables S3 and S4.
| Decreased after 2 hours/ Decreased after 4 days |
Increased after 2 hours /Increased after 4 days |
Decreased after 2 hours /Increased after 4 days |
Increased after 2 hours /Decreased after 4 days |
| CDK1 |
CSNK1E |
CDK2 |
MAPK3 |
| PRKACA |
MAP2K7 |
PRKAA2 |
MAPK1 |
| |
MAP2K4 |
MAP3K7 |
PRKCE |
| |
MAP3K5 |
CDK7 |
|
| |
CSNK2A1 |
|
|
| |
CSNK2A2 |
|
|
| |
CSNK1E |
|
|
| |
BCR |
|
|
| |
MAPK8 |
|
|
| |
MTOR |
|
|
| |
MAPK14 |
|
|