Submitted:
13 October 2023
Posted:
16 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Sources and selection criteria
3. Olfactory receptor and odorant detection
4. Viral infection causing olfactory dysfunction
5. Viruses impacting respiratory system
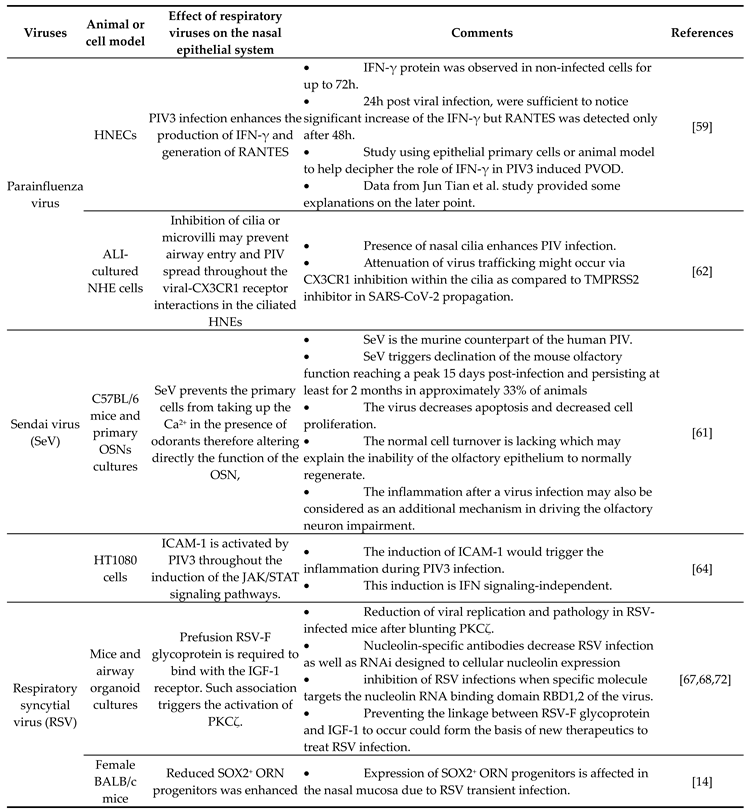 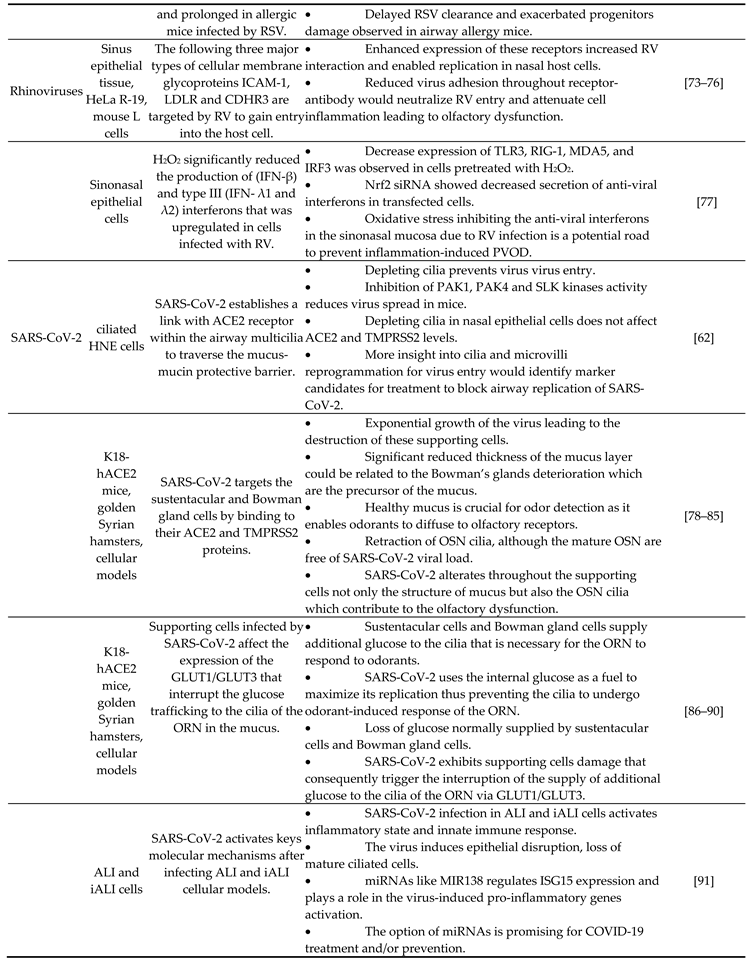
|
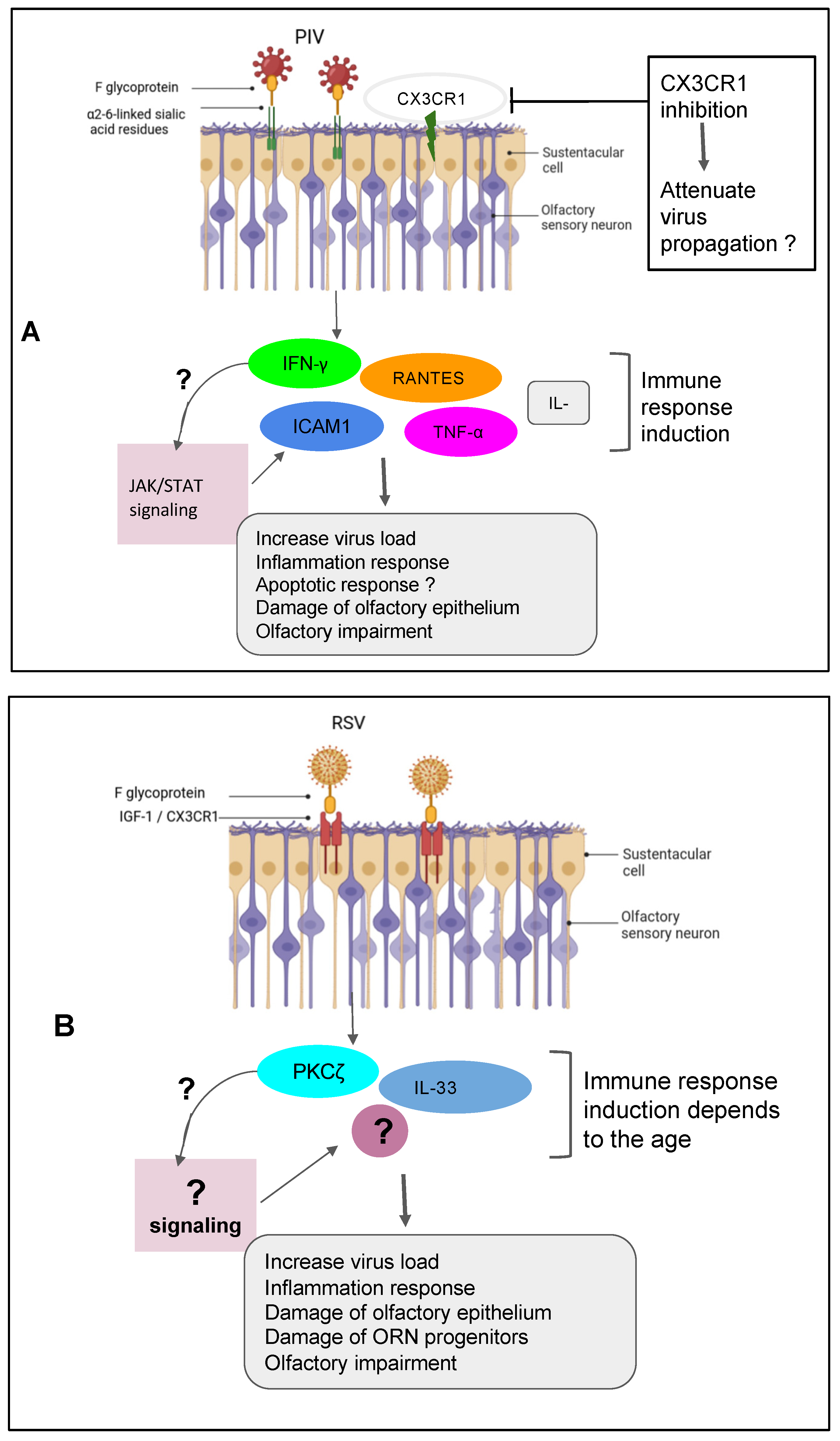
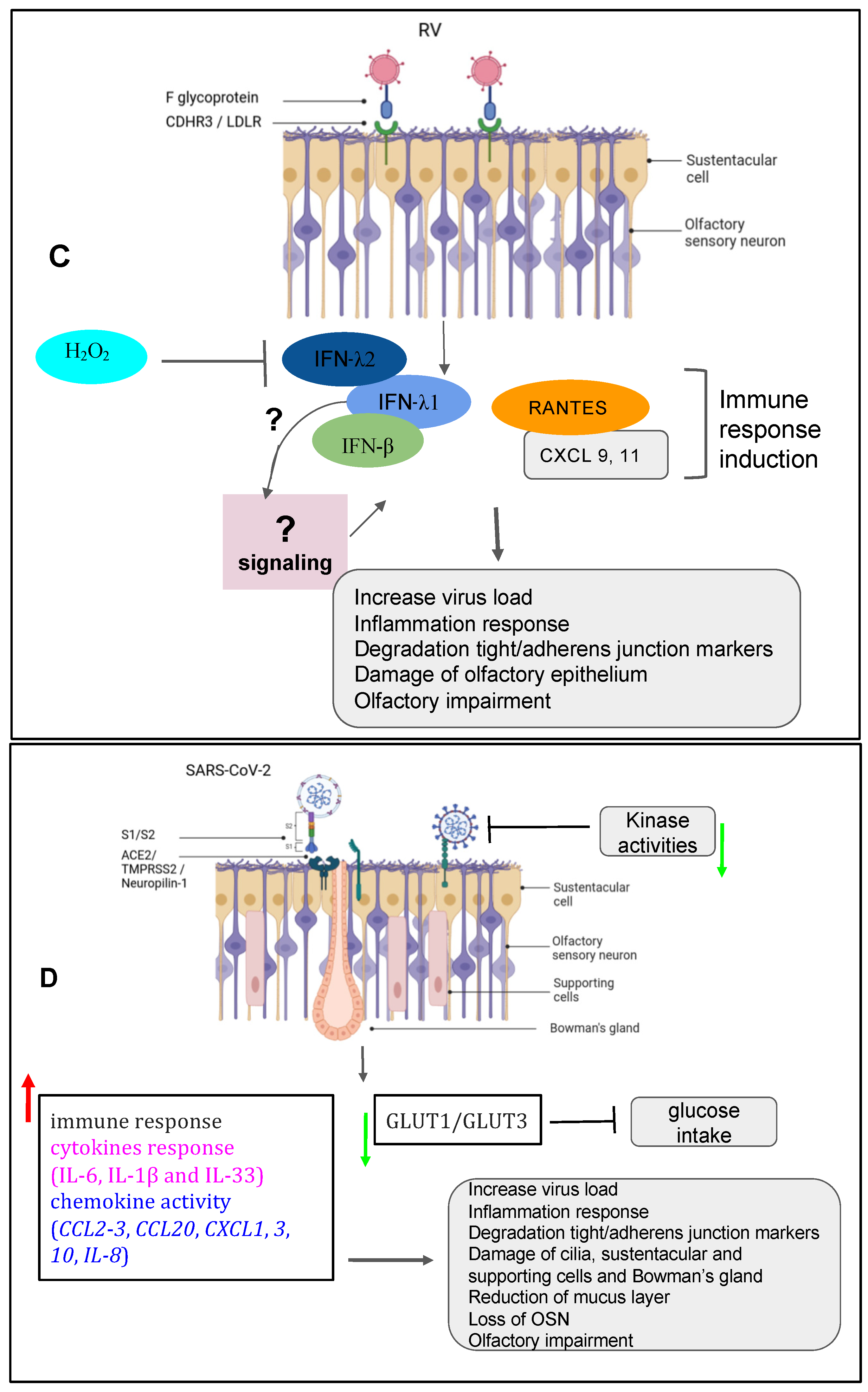
6. Mechanisms of SARS-CoV-2 mediating the loss of smell
6. Conclusion and perspective
Author Contributions
Funding
Conflicts of Interest
References
- Wu, J.T.; Leung, K.; Leung, G.M. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet 2020, 395, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Hui, D.S.; E, I.A.; Madani, T.A.; Ntoumi, F.; Kock, R.; Dar, O.; Ippolito, G.; McHugh, T.D.; Memish, Z.A.; Drosten, C.; et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - The latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis 2020, 91, 264–266. [Google Scholar] [CrossRef]
- Seiden, A.M. Postviral olfactory loss. Otolaryngol Clin North Am 2004, 37, 1159–1166. [Google Scholar] [CrossRef]
- Zhen Yu, L.; Luigi Angelo, V.; Paolo, B.-R.; Abigail, W.; Claire, H. Post-viral olfactory loss and parosmia. BMJ Medicine 2023, 2, e000382. [Google Scholar] [CrossRef]
- Moran, D.T.; Jafek, B.W.; Eller, P.M.; Rowley, J.C. , 3rd. Ultrastructural histopathology of human olfactory dysfunction. Microsc Res Tech 1992, 23, 103–110. [Google Scholar] [CrossRef]
- Welge-Lussen, A.; Wolfensberger, M. Olfactory disorders following upper respiratory tract infections. Adv Otorhinolaryngol 2006, 63, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Dicpinigaitis, P.V. Post-viral Anosmia (Loss of Sensation of Smell) Did Not Begin with COVID-19! Lung 2021, 199, 237–238. [Google Scholar] [CrossRef]
- Khachatryan, V.; Sirunyan, A.M.; Tumasyan, A.; Adam, W.; Bergauer, T.; Dragicevic, M.; Ero, J.; Friedl, M.; Fruhwirth, R.; Ghete, V.M.; et al. Distributions of topological observables in inclusive three- and four-jet events in pp collisions at [Formula: see text][Formula: see text]. Eur Phys J C Part Fields 2015, 75, 302. [Google Scholar] [CrossRef]
- Tian, J.; Pinto, J.M.; Li, L.; Zhang, S.; Sun, Z.; Wei, Y. Identification of Viruses in Patients With Postviral Olfactory Dysfunction by Multiplex Reverse-Transcription Polymerase Chain Reaction. Laryngoscope 2021, 131, 158–164. [Google Scholar] [CrossRef]
- Suzuki, M.; Saito, K.; Min, W.P.; Vladau, C.; Toida, K.; Itoh, H.; Murakami, S. Identification of viruses in patients with postviral olfactory dysfunction. Laryngoscope 2007, 117, 272–277. [Google Scholar] [CrossRef]
- Imam, S.A.; Lao, W.P.; Reddy, P.; Nguyen, S.A.; Schlosser, R.J. Is SARS-CoV-2 (COVID-19) postviral olfactory dysfunction (PVOD) different from other PVOD? World J Otorhinolaryngol Head Neck Surg 2020, 6, S26–S32. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, M.; Aiba, T.; Mori, J.; Nakai, Y. An epidemiological study of postviral olfactory disorder. Acta Otolaryngol Suppl 1998, 538, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Chiguer, D.L.; Tirado-Mendoza, R.; Marquez-Navarro, A.; Ambrosio-Hernandez, J.R.; Ruiz-Fraga, I.; Aguilar-Vargas, R.E.; Lira-Martinez, J.M.; Lopez-Valdes, J.C. Detection and molecular characterization of respiratory viruses that cause acute respiratory infection in the adult population. Gac Med Mex 2019, 155, S7–S12. [Google Scholar] [CrossRef] [PubMed]
- Ueha, R.; Mukherjee, S.; Ueha, S.; de Almeida Nagata, D.E.; Sakamoto, T.; Kondo, K.; Yamasoba, T.; Lukacs, N.W.; Kunkel, S.L. Viral disruption of olfactory progenitors is exacerbated in allergic mice. Int Immunopharmacol 2014, 22, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Heikkinen, T.; Jarvinen, A. The common cold. Lancet 2003, 361, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Potter, M.R.; Chen, J.H.; Lobban, N.S.; Doty, R.L. Olfactory dysfunction from acute upper respiratory infections: relationship to season of onset. Int Forum Allergy Rhinol 2020, 10, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Henrickson, K.J. Parainfluenza viruses. Clin Microbiol Rev 2003, 16, 242–264. [Google Scholar] [CrossRef] [PubMed]
- Rafeek, R.A.M.; Divarathna, M.V.M.; Noordeen, F. A review on disease burden and epidemiology of childhood parainfluenza virus infections in Asian countries. Rev Med Virol 2021, 31, e2164. [Google Scholar] [CrossRef]
- Weston, S.; Frieman, M.B. Respiratory Viruses. In Encyclopedia of Microbiology (Fourth Edition); Schmidt, T.M., Ed.; Academic Press: Oxford, 2019; pp. 85–101. [Google Scholar] [CrossRef]
- van Kempen, M.; Bachert, C.; Van Cauwenberge, P. An update on the pathophysiology of rhinovirus upper respiratory tract infections. Rhinology 1999, 37, 97–103. [Google Scholar]
- Xatzipsalti, M.; Kyrana, S.; Tsolia, M.; Psarras, S.; Bossios, A.; Laza-Stanca, V.; Johnston, S.L.; Papadopoulos, N.G. Rhinovirus viremia in children with respiratory infections. Am J Respir Crit Care Med 2005, 172, 1037–1040. [Google Scholar] [CrossRef]
- Loeffelholz, M.J.; Trujillo, R.; Pyles, R.B.; Miller, A.L.; Alvarez-Fernandez, P.; Pong, D.L.; Chonmaitree, T. Duration of rhinovirus shedding in the upper respiratory tract in the first year of life. Pediatrics 2014, 134, 1144–1150. [Google Scholar] [CrossRef]
- WHO. Severe Acute Respiratory Syndrome (SARS).
- de Groot, R.J.; Baker, S.C.; Baric, R.S.; Brown, C.S.; Drosten, C.; Enjuanes, L.; Fouchier, R.A.; Galiano, M.; Gorbalenya, A.E.; Memish, Z.A.; et al. Middle East respiratory syndrome coronavirus (MERS-CoV): announcement of the Coronavirus Study Group. J Virol 2013, 87, 7790–7792. [Google Scholar] [CrossRef]
- Zaki, A.M.; van Boheemen, S.; Bestebroer, T.M.; Osterhaus, A.D.; Fouchier, R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med 2012, 367, 1814–1820. [Google Scholar] [CrossRef]
- de Groot, R.J.; Baker, S.C.; Baric, R.S.; Brown, C.S.; Drosten, C.; Enjuanes, L.; Fouchier, R.A.; Galiano, M.; Gorbalenya, A.E.; Memish, Z.A.; et al. Middle East respiratory syndrome coronavirus (MERS-CoV): announcement of the Coronavirus Study Group. J Virol 2013, 87, 7790–7792. [Google Scholar] [CrossRef]
- Khalafalla, A.I.; Lu, X.; Al-Mubarak, A.I.; Dalab, A.H.; Al-Busadah, K.A.; Erdman, D.D. MERS-CoV in Upper Respiratory Tract and Lungs of Dromedary Camels, Saudi Arabia, 2013-2014. Emerg Infect Dis 2015, 21, 1153–1158. [Google Scholar] [CrossRef]
- Wolfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Muller, M.A.; Niemeyer, D.; Jones, T.C.; Vollmar, P.; Rothe, C.; et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020, 581, 465–469. [Google Scholar] [CrossRef]
- de Melo, G.D.; Lazarini, F.; Levallois, S.; Hautefort, C.; Michel, V.; Larrous, F.; Verillaud, B.; Aparicio, C.; Wagner, S.; Gheusi, G.; et al. COVID-19-related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters. Sci Transl Med 2021, 13. [Google Scholar] [CrossRef]
- Zazhytska, M.; Kodra, A.; Hoagland, D.A.; Frere, J.; Fullard, J.F.; Shayya, H.; McArthur, N.G.; Moeller, R.; Uhl, S.; Omer, A.D.; et al. Non-cell-autonomous disruption of nuclear architecture as a potential cause of COVID-19-induced anosmia. Cell 2022, 185, 1052–1064. [Google Scholar] [CrossRef]
- Bilinska, K.; Butowt, R. Anosmia in COVID-19: A Bumpy Road to Establishing a Cellular Mechanism. ACS Chem Neurosci 2020, 11, 2152–2155. [Google Scholar] [CrossRef]
- Samaranayake, L.P.; Fakhruddin, K.S.; Panduwawala, C. Sudden onset, acute loss of taste and smell in coronavirus disease 2019 (COVID-19): a systematic review. Acta Odontol Scand 2020, 78, 467–473. [Google Scholar] [CrossRef]
- Dammalli, M.; Dey, G.; Madugundu, A.K.; Kumar, M.; Rodrigues, B.; Gowda, H.; Siddaiah, B.G.; Mahadevan, A.; Shankar, S.K.; Prasad, T.S.K. Proteomic Analysis of the Human Olfactory Bulb. OMICS 2017, 21, 440–453. [Google Scholar] [CrossRef]
- Dammalli, M.; Dey, G.; Kumar, M.; Madugundu, A.K.; Gopalakrishnan, L.; Gowrishankar, B.S.; Mahadevan, A.; Shankar, S.K.; Prasad, T.S.K. Proteomics of the Human Olfactory Tract. OMICS 2018, 22, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Oboti, L.; Peretto, P.; Marchis, S.D.; Fasolo, A. From chemical neuroanatomy to an understanding of the olfactory system. Eur J Histochem 2011, 55, e35. [Google Scholar] [CrossRef] [PubMed]
- Barral-Arca, R.; Gomez-Carballa, A.; Cebey-Lopez, M.; Bello, X.; Martinon-Torres, F.; Salas, A. A Meta-Analysis of Multiple Whole Blood Gene Expression Data Unveils a Diagnostic Host-Response Transcript Signature for Respiratory Syncytial Virus. Int J Mol Sci 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Boesveldt, S.; Postma, E.M.; Boak, D.; Welge-Luessen, A.; Schopf, V.; Mainland, J.D.; Martens, J.; Ngai, J.; Duffy, V.B. Anosmia-A Clinical Review. Chem Senses 2017, 42, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Firestein, S. How the olfactory system makes sense of scents. Nature 2001, 413, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Brennan, P.A.; Keverne, E.B. Something in the air? New insights into mammalian pheromones. Curr Biol 2004, 14, R81–R89. [Google Scholar] [CrossRef] [PubMed]
- Buck, L.B. The molecular architecture of odor and pheromone sensing in mammals. Cell 2000, 100, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Mombaerts, P. Genes and ligands for odorant, vomeronasal and taste receptors. Nat Rev Neurosci 2004, 5, 263–278. [Google Scholar] [CrossRef]
- Restrepo, D.; Arellano, J.; Oliva, A.M.; Schaefer, M.L.; Lin, W. Emerging views on the distinct but related roles of the main and accessory olfactory systems in responsiveness to chemosensory signals in mice. Horm Behav 2004, 46, 247–256. [Google Scholar] [CrossRef]
- Lavoie, J.; Gasso Astorga, P.; Segal-Gavish, H.; Wu, Y.C.; Chung, Y.; Cascella, N.G.; Sawa, A.; Ishizuka, K. The Olfactory Neural Epithelium As a Tool in Neuroscience. Trends Mol Med 2017, 23, 100–103. [Google Scholar] [CrossRef]
- Liang, F.; Wang, Y. COVID-19 Anosmia: High Prevalence, Plural Neuropathogenic Mechanisms, and Scarce Neurotropism of SARS-CoV-2? Viruses 2021, 13. [Google Scholar] [CrossRef]
- Olender, T.; Lancet, D.; Nebert, D.W. Update on the olfactory receptor (OR) gene superfamily. Hum Genomics 2008, 3, 87–97. [Google Scholar] [CrossRef]
- Kurian, S.M.; Gordon, S.; Barrick, B.; Dadlani, M.N.; Fanelli, B.; Cornell, J.B.; Head, S.R.; Marsh, C.L.; Case, J. Feasibility and Comparison Study of Fecal Sample Collection Methods in Healthy Volunteers and Solid Organ Transplant Recipients Using 16S rRNA and Metagenomics Approaches. Biopreserv Biobank 2020, 18, 425–440. [Google Scholar] [CrossRef]
- Glezer, I.; Malnic, B. Olfactory receptor function. Handb Clin Neurol 2019, 164, 67–78. [Google Scholar] [CrossRef]
- Schwob, J.E. Neural regeneration and the peripheral olfactory system. Anat Rec 2002, 269, 33–49. [Google Scholar] [CrossRef]
- Graziadei, P.P.; Graziadei, G.A. Neurogenesis and neuron regeneration in the olfactory system of mammals. I. Morphological aspects of differentiation and structural organization of the olfactory sensory neurons. J Neurocytol 1979, 8, 1–18. [Google Scholar] [CrossRef]
- Dicpinigaitis, P.V. Post-viral Anosmia (Loss of Sensation of Smell) Did Not Begin with COVID-19! Lung 2021, 199, 237–238. [Google Scholar] [CrossRef]
- Urata, S.; Maruyama, J.; Kishimoto-Urata, M.; Sattler, R.A.; Cook, R.; Lin, N.; Yamasoba, T.; Makishima, T.; Paessler, S. Regeneration Profiles of Olfactory Epithelium after SARS-CoV-2 Infection in Golden Syrian Hamsters. ACS Chem Neurosci 2021, 12, 589–595. [Google Scholar] [CrossRef]
- Suzuki, M.; Saito, K.; Min, W.P.; Vladau, C.; Toida, K.; Itoh, H.; Murakami, S. Identification of viruses in patients with postviral olfactory dysfunction. Laryngoscope 2007, 117, 272–277. [Google Scholar] [CrossRef]
- Lee, D.Y.; Lee, W.H.; Wee, J.H.; Kim, J.W. Prognosis of postviral olfactory loss: follow-up study for longer than one year. Am J Rhinol Allergy 2014, 28, 419–422. [Google Scholar] [CrossRef]
- Tian, J.; Pinto, J.M.; Li, L.; Zhang, S.; Sun, Z.; Wei, Y. Identification of Viruses in Patients With Postviral Olfactory Dysfunction by Multiplex Reverse-Transcription Polymerase Chain Reaction. Laryngoscope 2021, 131, 158–164. [Google Scholar] [CrossRef]
- Suzuki, M.; Saito, K.; Min, W.P.; Vladau, C.; Toida, K.; Itoh, H.; Murakami, S. Identification of viruses in patients with postviral olfactory dysfunction. Laryngoscope 2007, 117, 272–277. [Google Scholar] [CrossRef]
- Seiden, A.M. Postviral olfactory loss. Otolaryngol Clin North Am 2004, 37, 1159–1166. [Google Scholar] [CrossRef]
- Doty, R.L.; Hawkes, C.H. Chemosensory dysfunction in neurodegenerative diseases. Handb Clin Neurol 2019, 164, 325–360. [Google Scholar] [CrossRef]
- Wang, J.H.; Kwon, H.J.; Jang, Y.J. Detection of parainfluenza virus 3 in turbinate epithelial cells of postviral olfactory dysfunction patients. Laryngoscope 2007, 117, 1445–1449. [Google Scholar] [CrossRef]
- Lewandowska-Polak, A.; Brauncajs, M.; Paradowska, E.; Jarzebska, M.; Kurowski, M.; Moskwa, S.; Lesnikowski, Z.J.; Kowalski, M.L. Human parainfluenza virus type 3 (HPIV3) induces production of IFNgamma and RANTES in human nasal epithelial cells (HNECs). J Inflamm (Lond) 2015, 12, 16. [Google Scholar] [CrossRef]
- Mori, I.; Komatsu, T.; Takeuchi, K.; Nakakuki, K.; Sudo, M.; Kimura, Y. Parainfluenza virus type 1 infects olfactory neurons and establishes long-term persistence in the nerve tissue. J Gen Virol 1995, 76 ( Pt 5) Pt 5, 1251–1254. [Google Scholar] [CrossRef]
- Tian, J.; Pinto, J.M.; Cui, X.; Zhang, H.; Li, L.; Liu, Y.; Wu, C.; Wei, Y. Sendai Virus Induces Persistent Olfactory Dysfunction in a Murine Model of PVOD via Effects on Apoptosis, Cell Proliferation, and Response to Odorants. PLoS One 2016, 11, e0159033. [Google Scholar] [CrossRef]
- Wu, C.T.; Lidsky, P.V.; Xiao, Y.; Cheng, R.; Lee, I.T.; Nakayama, T.; Jiang, S.; He, W.; Demeter, J.; Knight, M.G.; et al. SARS-CoV-2 replication in airway epithelia requires motile cilia and microvillar reprogramming. Cell 2023, 186, 112–130. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Volpe, S.J.; Chang, E.H. The Role of Viruses in the Inception of Chronic Rhinosinusitis. Clin Exp Otorhinolaryngol 2022, 15, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Choudhary, S.; Banerjee, A.K.; De, B.P. Human parainfluenza virus type 3 upregulates ICAM-1 (CD54) expression in a cytokine-independent manner. Gene Expr 2000, 9, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Bryche, B.; Fretaud, M.; Saint-Albin Deliot, A.; Galloux, M.; Sedano, L.; Langevin, C.; Descamps, D.; Rameix-Welti, M.A.; Eleouet, J.F.; Le Goffic, R.; et al. Respiratory syncytial virus tropism for olfactory sensory neurons in mice. J Neurochem 2020, 155, 137–153. [Google Scholar] [CrossRef]
- Griffiths, C.D.; Bilawchuk, L.M.; McDonough, J.E.; Jamieson, K.C.; Elawar, F.; Cen, Y.; Duan, W.; Lin, C.; Song, H.; Casanova, J.L.; et al. IGF1R is an entry receptor for respiratory syncytial virus. Nature 2020, 583, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Tayyari, F.; Marchant, D.; Moraes, T.J.; Duan, W.; Mastrangelo, P.; Hegele, R.G. Identification of nucleolin as a cellular receptor for human respiratory syncytial virus. Nat Med 2011, 17, 1132–1135. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelo, P.; Chin, A.A.; Tan, S.; Jeon, A.H.; Ackerley, C.A.; Siu, K.K.; Lee, J.E.; Hegele, R.G. Identification of RSV Fusion Protein Interaction Domains on the Virus Receptor, Nucleolin. Viruses 2021, 13. [Google Scholar] [CrossRef]
- Sourimant, J.; Lieber, C.M.; Aggarwal, M.; Cox, R.M.; Wolf, J.D.; Yoon, J.J.; Toots, M.; Ye, C.; Sticher, Z.; Kolykhalov, A.A.; et al. 4'-Fluorouridine is an oral antiviral that blocks respiratory syncytial virus and SARS-CoV-2 replication. Science 2022, 375, 161–167. [Google Scholar] [CrossRef]
- Li, H.H.; Xu, J.; He, L.; Denny, L.I.; Rustandi, R.R.; Dornadula, G.; Fiorito, B.; Zhang, Z.Q. Development and qualification of cell-based relative potency assay for a human respiratory syncytial virus (RSV) mRNA vaccine. J Pharm Biomed Anal 2023, 234, 115523. [Google Scholar] [CrossRef]
- Whitaker, J.A.; Sahly, H.M.E.; Healy, C.M. mRNA vaccines against respiratory viruses. Curr Opin Infect Dis 2023, 36, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.D.; Bilawchuk, L.M.; McDonough, J.E.; Jamieson, K.C.; Elawar, F.; Cen, Y.; Duan, W.; Lin, C.; Song, H.; Casanova, J.L.; et al. IGF1R is an entry receptor for respiratory syncytial virus. Nature 2020, 583, 615–619. [Google Scholar] [CrossRef]
- Bochkov, Y.A.; Watters, K.; Ashraf, S.; Griggs, T.F.; Devries, M.K.; Jackson, D.J.; Palmenberg, A.C.; Gern, J.E. Cadherin-related family member 3, a childhood asthma susceptibility gene product, mediates rhinovirus C binding and replication. Proc Natl Acad Sci U S A 2015, 112, 5485–5490. [Google Scholar] [CrossRef]
- Staunton, D.E.; Merluzzi, V.J.; Rothlein, R.; Barton, R.; Marlin, S.D.; Springer, T.A. A cell adhesion molecule, ICAM-1, is the major surface receptor for rhinoviruses. Cell 1989, 56, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Hofer, F.; Gruenberger, M.; Kowalski, H.; Machat, H.; Huettinger, M.; Kuechler, E.; Blaas, D. Members of the low density lipoprotein receptor family mediate cell entry of a minor-group common cold virus. Proc Natl Acad Sci U S A 1994, 91, 1839–1842. [Google Scholar] [CrossRef] [PubMed]
- Bochkov, Y.A.; Gern, J.E. Rhinoviruses and Their Receptors: Implications for Allergic Disease. Curr Allergy Asthma Rep 2016, 16, 30. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Han, M.S.; Lee, T.H.; Lee, D.B.; Park, J.H.; Lee, S.H.; Kim, T.H. Hydrogen peroxide attenuates rhinovirus-induced anti-viral interferon secretion in sinonasal epithelial cells. Front Immunol 2023, 14, 1086381. [Google Scholar] [CrossRef] [PubMed]
- Torabi, A.; Mohammadbagheri, E.; Akbari Dilmaghani, N.; Bayat, A.H.; Fathi, M.; Vakili, K.; Alizadeh, R.; Rezaeimirghaed, O.; Hajiesmaeili, M.; Ramezani, M.; et al. Proinflammatory Cytokines in the Olfactory Mucosa Result in COVID-19 Induced Anosmia. ACS Chem Neurosci 2020, 11, 1909–1913. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wong, L.R.; Li, K.; Verma, A.K.; Ortiz, M.E.; Wohlford-Lenane, C.; Leidinger, M.R.; Knudson, C.M.; Meyerholz, D.K.; McCray, P.B., Jr.; et al. COVID-19 treatments and pathogenesis including anosmia in K18-hACE2 mice. Nature 2021, 589, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Bilinska, K.; Jakubowska, P.; Von Bartheld, C.S.; Butowt, R. Expression of the SARS-CoV-2 Entry Proteins, ACE2 and TMPRSS2, in Cells of the Olfactory Epithelium: Identification of Cell Types and Trends with Age. ACS Chem Neurosci 2020, 11, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Randeva, H.S.; Chatha, K.; Hall, M.; Spandidos, D.A.; Karteris, E.; Kyrou, I. Neuropilin-1 as a new potential SARS-CoV-2 infection mediator implicated in the neurologic features and central nervous system involvement of COVID-19. Mol Med Rep 2020, 22, 4221–4226. [Google Scholar] [CrossRef]
- Kang, Y.L.; Chou, Y.Y.; Rothlauf, P.W.; Liu, Z.; Soh, T.K.; Cureton, D.; Case, J.B.; Chen, R.E.; Diamond, M.S.; Whelan, S.P.J.; et al. Inhibition of PIKfyve kinase prevents infection by Zaire ebolavirus and SARS-CoV-2. Proc Natl Acad Sci U S A 2020, 117, 20803–20813. [Google Scholar] [CrossRef]
- Mayi, B.S.; Leibowitz, J.A.; Woods, A.T.; Ammon, K.A.; Liu, A.E.; Raja, A. The role of Neuropilin-1 in COVID-19. PLoS Pathog 2021, 17, e1009153. [Google Scholar] [CrossRef]
- Bryche, B.; St Albin, A.; Murri, S.; Lacote, S.; Pulido, C.; Ar Gouilh, M.; Lesellier, S.; Servat, A.; Wasniewski, M.; Picard-Meyer, E.; et al. Massive transient damage of the olfactory epithelium associated with infection of sustentacular cells by SARS-CoV-2 in golden Syrian hamsters. Brain Behav Immun 2020, 89, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Cantuti-Castelvetri, L.; Ojha, R.; Pedro, L.D.; Djannatian, M.; Franz, J.; Kuivanen, S.; van der Meer, F.; Kallio, K.; Kaya, T.; Anastasina, M.; et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 2020, 370, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Daly, J.L.; Simonetti, B.; Klein, K.; Chen, K.E.; Williamson, M.K.; Anton-Plagaro, C.; Shoemark, D.K.; Simon-Gracia, L.; Bauer, M.; Hollandi, R.; et al. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science 2020, 370, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, C.; Blanchard, K.; Bacigalupo, J.; Vergara, C. Possible ATP trafficking by ATP-shuttles in the olfactory cilia and glucose transfer across the olfactory mucosa. FEBS Lett 2019, 593, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Villar, P.S.; Vergara, C.; Bacigalupo, J. Energy sources that fuel metabolic processes in protruding finger-like organelles. FEBS J 2021, 288, 3799–3812. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Nordqvist, H.; Ambikan, A.T.; Gupta, S.; Sperk, M.; Svensson-Akusjarvi, S.; Mikaeloff, F.; Benfeitas, R.; Saccon, E.; Ponnan, S.M.; et al. Metabolic Perturbation Associated With COVID-19 Disease Severity and SARS-CoV-2 Replication. Mol Cell Proteomics 2021, 20, 100159. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Yoo, S.J.; Clijsters, M.; Backaert, W.; Vanstapel, A.; Speleman, K.; Lietaer, C.; Choi, S.; Hether, T.D.; Marcelis, L.; et al. Visualizing in deceased COVID-19 patients how SARS-CoV-2 attacks the respiratory and olfactory mucosae but spares the olfactory bulb. Cell 2021, 184, 5932–5949. [Google Scholar] [CrossRef] [PubMed]
- Assou, S.; Ahmed, E.; Morichon, L.; Nasri, A.; Foisset, F.; Bourdais, C.; Gros, N.; Tieo, S.; Petit, A.; Vachier, I.; et al. The Transcriptome Landscape of the In Vitro Human Airway Epithelium Response to SARS-CoV-2. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef]
- Hofer, F.; Gruenberger, M.; Kowalski, H.; Machat, H.; Huettinger, M.; Kuechler, E.; Blaas, D. Members of the low density lipoprotein receptor family mediate cell entry of a minor-group common cold virus. Proc Natl Acad Sci U S A 1994, 91, 1839–1842. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, T.B.; Kalie, E.; Crisafulli-Cabatu, S.; Abramovich, R.; DiGioia, G.; Moolchan, K.; Pestka, S.; Schreiber, G. Binding and activity of all human alpha interferon subtypes. Cytokine 2011, 56, 282–289. [Google Scholar] [CrossRef]
- Jaks, E.; Gavutis, M.; Uze, G.; Martal, J.; Piehler, J. Differential receptor subunit affinities of type I interferons govern differential signal activation. J Mol Biol 2007, 366, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.P.; Sheikh, F.; Kotenko, S.V.; Dickensheets, H. The expanded family of class II cytokines that share the IL-10 receptor-2 (IL-10R2) chain. J Leukoc Biol 2004, 76, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ninaber, D.K.; van Schadewijk, A.; Hiemstra, P.S. Tiotropium and Fluticasone Inhibit Rhinovirus-Induced Mucin Production via Multiple Mechanisms in Differentiated Airway Epithelial Cells. Front Cell Infect Microbiol 2020, 10, 278. [Google Scholar] [CrossRef]
- Lo, D.; Kennedy, J.L.; Kurten, R.C.; Panettieri, R.A., Jr.; Koziol-White, C.J. Modulation of airway hyperresponsiveness by rhinovirus exposure. Respir Res 2018, 19, 208. [Google Scholar] [CrossRef]
- Loxham, M.; Smart, D.E.; Bedke, N.J.; Smithers, N.P.; Filippi, I.; Blume, C.; Swindle, E.J.; Tariq, K.; Howarth, P.H.; Holgate, S.T.; et al. Allergenic proteases cleave the chemokine CX3CL1 directly from the surface of airway epithelium and augment the effect of rhinovirus. Mucosal Immunol 2018, 11, 404–414. [Google Scholar] [CrossRef]
- Papi, A.; Papadopoulos, N.G.; Stanciu, L.A.; Bellettato, C.M.; Pinamonti, S.; Degitz, K.; Holgate, S.T.; Johnston, S.L. Reducing agents inhibit rhinovirus-induced up-regulation of the rhinovirus receptor intercellular adhesion molecule-1 (ICAM-1) in respiratory epithelial cells. FASEB J 2002, 16, 1934–1936. [Google Scholar] [CrossRef]
- Song, Y.P.; Tang, M.F.; Leung, A.S.Y.; Tao, K.P.; Chan, O.M.; Wong, G.W.K.; Chan, P.K.S.; Chan, R.W.Y.; Leung, T.F. Interactive effects between CDHR3 genotype and rhinovirus species for diagnosis and severity of respiratory tract infections in hospitalized children. Microbiol Spectr 2023, e0118123. [Google Scholar] [CrossRef]
- Greiller, C.L.; Suri, R.; Jolliffe, D.A.; Kebadze, T.; Hirsman, A.G.; Griffiths, C.J.; Johnston, S.L.; Martineau, A.R. Vitamin D attenuates rhinovirus-induced expression of intercellular adhesion molecule-1 (ICAM-1) and platelet-activating factor receptor (PAFR) in respiratory epithelial cells. J Steroid Biochem Mol Biol 2019, 187, 152–159. [Google Scholar] [CrossRef]
- Khani, E.; Khiali, S.; Beheshtirouy, S.; Entezari-Maleki, T. Potential pharmacologic treatments for COVID-19 smell and taste loss: A comprehensive review. Eur J Pharmacol 2021, 912, 174582. [Google Scholar] [CrossRef]
- Silva Andrade, B.; Siqueira, S.; de Assis Soares, W.R.; de Souza Rangel, F.; Santos, N.O.; Dos Santos Freitas, A.; Ribeiro da Silveira, P.; Tiwari, S.; Alzahrani, K.J.; Goes-Neto, A.; et al. Long-COVID and Post-COVID Health Complications: An Up-to-Date Review on Clinical Conditions and Their Possible Molecular Mechanisms. Viruses 2021, 13. [Google Scholar] [CrossRef]
- Fernandez-de-Las-Penas, C.; Cancela-Cilleruelo, I.; Rodriguez-Jimenez, J.; Gomez-Mayordomo, V.; Pellicer-Valero, O.J.; Martin-Guerrero, J.D.; Hernandez-Barrera, V.; Arendt-Nielsen, L.; Torres-Macho, J. Associated-Onset Symptoms and Post-COVID-19 Symptoms in Hospitalized COVID-19 Survivors Infected with Wuhan, Alpha or Delta SARS-CoV-2 Variant. Pathogens 2022, 11. [Google Scholar] [CrossRef]
- Rodriguez-Sevilla, J.J.; Guerri-Fernadez, R.; Bertran Recasens, B. Is There Less Alteration of Smell Sensation in Patients With Omicron SARS-CoV-2 Variant Infection? Front Med (Lausanne) 2022, 9, 852998. [Google Scholar] [CrossRef]
- Chee, J.; Chern, B.; Loh, W.S.; Mullol, J.; Wang, Y. Pathophysiology of SARS-CoV-2 Infection of Nasal Respiratory and Olfactory Epithelia and Its Clinical Impact. Curr Allergy Asthma Rep 2023, 23, 121–131. [Google Scholar] [CrossRef]
- Mutiawati, E.; Fahriani, M.; Mamada, S.S.; Fajar, J.K.; Frediansyah, A.; Maliga, H.A.; Ilmawan, M.; Emran, T.B.; Ophinni, Y.; Ichsan, I.; et al. Anosmia and dysgeusia in SARS-CoV-2 infection: incidence and effects on COVID-19 severity and mortality, and the possible pathobiology mechanisms - a systematic review and meta-analysis. F1000Res 2021, 10, 40. [Google Scholar] [CrossRef]
- Butowt, R.; von Bartheld, C.S. Anosmia in COVID-19: Underlying Mechanisms and Assessment of an Olfactory Route to Brain Infection. Neuroscientist 2021, 27, 582–603. [Google Scholar] [CrossRef]
- Kalra, R.S.; Dhanjal, J.K.; Meena, A.S.; Kalel, V.C.; Dahiya, S.; Singh, B.; Dewanjee, S.; Kandimalla, R. COVID-19, Neuropathology, and Aging: SARS-CoV-2 Neurological Infection, Mechanism, and Associated Complications. Front Aging Neurosci 2021, 13, 662786. [Google Scholar] [CrossRef] [PubMed]
- Rebholz, H.; Braun, R.J.; Ladage, D.; Knoll, W.; Kleber, C.; Hassel, A.W. Loss of Olfactory Function-Early Indicator for Covid-19, Other Viral Infections and Neurodegenerative Disorders. Front Neurol 2020, 11, 569333. [Google Scholar] [CrossRef] [PubMed]
- Reyna, R.A.; Kishimoto-Urata, M.; Urata, S.; Makishima, T.; Paessler, S.; Maruyama, J. Recovery of anosmia in hamsters infected with SARS-CoV-2 is correlated with repair of the olfactory epithelium. Sci Rep 2022, 12, 628. [Google Scholar] [CrossRef] [PubMed]
- Tanzadehpanah, H.; Lotfian, E.; Avan, A.; Saki, S.; Nobari, S.; Mahmoodian, R.; Sheykhhasan, M.; Froutagh, M.H.S.; Ghotbani, F.; Jamshidi, R.; et al. Role of SARS-COV-2 and ACE2 in the pathophysiology of peripheral vascular diseases. Biomed Pharmacother 2023, 166, 115321. [Google Scholar] [CrossRef]
- Mutiawati, E.; Fahriani, M.; Mamada, S.S.; Fajar, J.K.; Frediansyah, A.; Maliga, H.A.; Ilmawan, M.; Emran, T.B.; Ophinni, Y.; Ichsan, I.; et al. Anosmia and dysgeusia in SARS-CoV-2 infection: incidence and effects on COVID-19 severity and mortality, and the possible pathobiology mechanisms - a systematic review and meta-analysis. F1000Res 2021, 10, 40. [Google Scholar] [CrossRef]
- Butowt, R.; von Bartheld, C.S. Anosmia in COVID-19: Underlying Mechanisms and Assessment of an Olfactory Route to Brain Infection. Neuroscientist 2021, 27, 582–603. [Google Scholar] [CrossRef]
- Butowt, R.; Bilinska, K.; von Bartheld, C.S. Olfactory dysfunction in COVID-19: new insights into the underlying mechanisms. Trends Neurosci 2023, 46, 75–90. [Google Scholar] [CrossRef]
- Buqaileh, R.; Saternos, H.; Ley, S.; Aranda, A.; Forero, K.; AbouAlaiwi, W.A. Can cilia provide an entry gateway for SARS-CoV-2 to human ciliated cells? Physiol Genomics 2021, 53, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Butowt, R.; Meunier, N.; Bryche, B.; von Bartheld, C.S. The olfactory nerve is not a likely route to brain infection in COVID-19: a critical review of data from humans and animal models. Acta Neuropathol 2021, 141, 809–822. [Google Scholar] [CrossRef] [PubMed]
- Bilinska, K.; Jakubowska, P.; Von Bartheld, C.S.; Butowt, R. Expression of the SARS-CoV-2 Entry Proteins, ACE2 and TMPRSS2, in Cells of the Olfactory Epithelium: Identification of Cell Types and Trends with Age. ACS Chem Neurosci 2020, 11, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Bryche, B.; St Albin, A.; Murri, S.; Lacote, S.; Pulido, C.; Ar Gouilh, M.; Lesellier, S.; Servat, A.; Wasniewski, M.; Picard-Meyer, E.; et al. Massive transient damage of the olfactory epithelium associated with infection of sustentacular cells by SARS-CoV-2 in golden Syrian hamsters. Brain Behav Immun 2020, 89, 579–586. [Google Scholar] [CrossRef]
- Sia, S.F.; Yan, L.M.; Chin, A.W.H.; Fung, K.; Choy, K.T.; Wong, A.Y.L.; Kaewpreedee, P.; Perera, R.; Poon, L.L.M.; Nicholls, J.M.; et al. Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature 2020, 583, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Lechien, J.R.; Chiesa-Estomba, C.M.; De Siati, D.R.; Horoi, M.; Le Bon, S.D.; Rodriguez, A.; Dequanter, D.; Blecic, S.; El Afia, F.; Distinguin, L.; et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol 2020, 277, 2251–2261. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Min, P.; Lee, S.; Kim, S.W. Prevalence and Duration of Acute Loss of Smell or Taste in COVID-19 Patients. J Korean Med Sci 2020, 35, e174. [Google Scholar] [CrossRef]
- Fodoulian, L.; Tuberosa, J.; Rossier, D.; Boillat, M.; Kan, C.; Pauli, V.; Egervari, K.; Lobrinus, J.A.; Landis, B.N.; Carleton, A.; et al. SARS-CoV-2 Receptors and Entry Genes Are Expressed in the Human Olfactory Neuroepithelium and Brain. iScience 2020, 23, 101839. [Google Scholar] [CrossRef]
- Chen, M.; Shen, W.; Rowan, N.R.; Kulaga, H.; Hillel, A.; Ramanathan, M., Jr.; Lane, A.P. Elevated ACE-2 expression in the olfactory neuroepithelium: implications for anosmia and upper respiratory SARS-CoV-2 entry and replication. Eur Respir J 2020, 56. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef]
- Ziegler, C.G.K.; Allon, S.J.; Nyquist, S.K.; Mbano, I.M.; Miao, V.N.; Tzouanas, C.N.; Cao, Y.; Yousif, A.S.; Bals, J.; Hauser, B.M.; et al. SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell 2020, 181, 1016–1035. [Google Scholar] [CrossRef]
- Gkogkou, E.; Barnasas, G.; Vougas, K.; Trougakos, I.P. Expression profiling meta-analysis of ACE2 and TMPRSS2, the putative anti-inflammatory receptor and priming protease of SARS-CoV-2 in human cells, and identification of putative modulators. Redox Biol 2020, 36, 101615. [Google Scholar] [CrossRef]
- Sungnak, W.; Huang, N.; Becavin, C.; Berg, M.; Queen, R.; Litvinukova, M.; Talavera-Lopez, C.; Maatz, H.; Reichart, D.; Sampaziotis, F.; et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med 2020, 26, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, M.; Ou, G. COVID-19, cilia, and smell. FEBS J 2020, 287, 3672–3676. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, A.; Naujokat, C. Structural features of coronavirus SARS-CoV-2 spike protein: Targets for vaccination. Life Sci 2020, 257, 118056. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Pekosz, A.; Villano, J.S.; Shen, W.; Zhou, R.; Kulaga, H.; Li, Z.; Beck, S.E.; Witwer, K.W.; Mankowski, J.L.; et al. Evolution of nasal and olfactory infection characteristics of SARS-CoV-2 variants. bioRxiv 2022. [Google Scholar] [CrossRef]
- von Bartheld, C.S.; Wang, L. Prevalence of Olfactory Dysfunction with the Omicron Variant of SARS-CoV-2: A Systematic Review and Meta-Analysis. Cells 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- von Bartheld, C.S.; Hagen, M.M.; Butowt, R. The D614G Virus Mutation Enhances Anosmia in COVID-19 Patients: Evidence from a Systematic Review and Meta-analysis of Studies from South Asia. ACS Chem Neurosci 2021, 12, 3535–3549. [Google Scholar] [CrossRef]
- Schreiner, T.; Allnoch, L.; Beythien, G.; Marek, K.; Becker, K.; Schaudien, D.; Stanelle-Bertram, S.; Schaumburg, B.; Mounogou Kouassi, N.; Beck, S.; et al. SARS-CoV-2 Infection Dysregulates Cilia and Basal Cell Homeostasis in the Respiratory Epithelium of Hamsters. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Liang, F.; Wang, Y. COVID-19 Anosmia: High Prevalence, Plural Neuropathogenic Mechanisms, and Scarce Neurotropism of SARS-CoV-2? Viruses 2021, 13. [Google Scholar] [CrossRef]
- Seehusen, F.; Clark, J.J.; Sharma, P.; Bentley, E.G.; Kirby, A.; Subramaniam, K.; Wunderlin-Giuliani, S.; Hughes, G.L.; Patterson, E.I.; Michael, B.D.; et al. Neuroinvasion and Neurotropism by SARS-CoV-2 Variants in the K18-hACE2 Mouse. Viruses 2022, 14. [Google Scholar] [CrossRef]
- Trobaugh, D.W.; Klimstra, W.B. MicroRNA Regulation of RNA Virus Replication and Pathogenesis. Trends Mol Med 2017, 23, 80–93. [Google Scholar] [CrossRef]
- Hanna, J.; Hossain, G.S.; Kocerha, J. The Potential for microRNA Therapeutics and Clinical Research. Front Genet 2019, 10, 478. [Google Scholar] [CrossRef] [PubMed]
- Osan, J.K.; DeMontigny, B.A.; Mehedi, M. Immunohistochemistry for protein detection in PFA-fixed paraffin-embedded SARS-CoV-2-infected COPD airway epithelium. STAR Protoc 2021, 2, 100663. [Google Scholar] [CrossRef] [PubMed]
- Li, H.H.; Xu, J.; He, L.; Denny, L.I.; Rustandi, R.R.; Dornadula, G.; Fiorito, B.; Zhang, Z.Q. Development and qualification of cell-based relative potency assay for a human respiratory syncytial virus (RSV) mRNA vaccine. J Pharm Biomed Anal 2023, 234, 115523. [Google Scholar] [CrossRef] [PubMed]
- Mayi, B.S.; Leibowitz, J.A.; Woods, A.T.; Ammon, K.A.; Liu, A.E.; Raja, A. The role of Neuropilin-1 in COVID-19. PLoS Pathog 2021, 17, e1009153. [Google Scholar] [CrossRef] [PubMed]
- Iacobucci, G. Covid-19: Runny nose, headache, and fatigue are commonest symptoms of omicron, early data show. BMJ 2021, 375, n3103. [Google Scholar] [CrossRef] [PubMed]
- Ohkubo, K.; Lee, C.H.; Baraniuk, J.N.; Merida, M.; Hausfeld, J.N.; Kaliner, M.A. Angiotensin-converting enzyme in the human nasal mucosa. Am J Respir Cell Mol Biol 1994, 11, 173–180. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





