Submitted:
12 December 2023
Posted:
14 December 2023
You are already at the latest version
Abstract
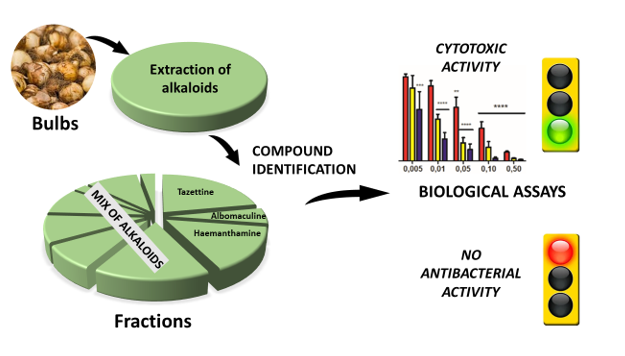
Keywords:
1. Introduction
1.1. Stenomesson miniatum and the Amaryllidaceae alkaloids in Andean traditional medicine
1.2. Dereplication approach for phytochemical characterization
2. Materials and Methods
2.1. Plant material
2.2. Chemicals
2.3. Dereplication approach
2.3.1. Preparation of the alkaloid-enriched extract
2.3.2. Centrifugal Partition Chromatography
2.3.3. UPLC-HRMS
2.3.4. NMR
2.4. Cytotoxic activity
2.4.1. Cell cultures
2.4.2. Cell viability assays
2.4.3. Statistical analysis
2.5. Antibacterial activity
2.5.1. Preparation of extract and fractions for antibacterial activity
2.5.2. Bacterial strains and antibacterial assay
3. Results and Discussion
3.1. Phytochemical characterization by dereplication of S. miniatum bulb extract
3.2. Biological activities of S. miniatum bulb extract
3.2.1. Cytotoxic activities against A431 human epidermoid carcinoma cells
3.2.2. Cytotoxic activities against Jurkat human acute T-leukemia cells
3.2.3. Antibacterial activities
4. Conclusion
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| COCONUT | COlleCtion of Open Natural ProdUcTs |
| CPC | Centrifugal partition chromatography |
| KNApSAcK | Kurokawa Nakamura Asah personal Shinbo Altaf-Ul-Amin computer Kanaya |
| MtBE | Methyl tert-butyl ether |
| NMReDATA | NMR extracted data |
| RPMI | Roswell Park memorial institute |
| SEM | Standard error of the mean |
| TEA | Triethylamine |
| UNPD | Universal natural products database |
| UPLC | Ultra performance liquid chromatography |
References
- Ali, A.A., El Saved, H.M., Abdalliah, O.M., Steglich, W., 1986. Oxocrinine and other alkaloids from Crinum americanum. Phytochemistry 25, 2399–2401. [CrossRef]
- Baldwin, S.W., Debenham, J.S., 2000. Total Syntheses of (−)-Haemanthidine, (+)-Pretazettine, and (+)-Tazettine. Org. Lett. 2, 99–102. [CrossRef]
- Bastida, J., Lavilla, R., Viladomat, F., 2006. Chapter 3 Chemical and Biological Aspects of Narcissus Alkaloids, in: Cordell, G.A. (Ed.), The Alkaloids: Chemistry and Biology. Academic Press, pp. 87–179. [CrossRef]
- Bastien, J.W., 1982. Herbal curing by Qollahuaya Andeans. J. Ethnopharmacol. 6, 13–28. [CrossRef]
- Berkov, S., Osorio, E., Viladomat, F., Bastida, J., 2020. Chemodiversity, chemotaxonomy and chemoecology of Amaryllidaceae alkaloids. Alkaloids Chem. Biol. 83, 113–185. [CrossRef]
- Boit, H.-G., Döpke, W., 1957. Alkaloide aus Urceolina-, Hymenocallis-, Elisena-, Calostemma-, Eustephia- und Hippeastrum-Arten. Chem. Ber. 90, 1827–1830. [CrossRef]
- Cahlíková, L., Kawano, I., Řezáčová, M., Blunden, G., Hulcová, D., Havelek, R., 2021. The Amaryllidaceae alkaloids haemanthamine, haemanthidine and their semisynthetic derivatives as potential drugs. Phytochem. Rev. 20, 303–323. [CrossRef]
- Catanzaro, E., Greco, G., Potenza, L., Calcabrini, C., Fimognari, C., 2018. Natural Products to Fight Cancer: A Focus on Juglans regia. Toxins 10, 469. [CrossRef]
- Chen, H., Lao, Z., Xu, J., Li, Z., Long, H., Li, D., Lin, L., Liu, X., Yu, L., Liu, W., Li, G., Wu, J., 2020. Antiviral activity of lycorine against Zika virus in vivo and in vitro. Virology 546, 88–97. [CrossRef]
- Coppo, E., Marchese, A., 2014. Antibacterial Activity of Polyphenols. Curr. Pharm. Biotechnol. 15, 380–390. [CrossRef]
- de Andrade, J.P., Guo, Y., Font-Bardia, M., Calvet, T., Dutilh, J., Viladomat, F., Codina, C., Nair, J.J., Zuanazzi, J.A.S., Bastida, J., 2014. Crinine-type alkaloids from Hippeastrum aulicum and H. calyptratum. Phytochemistry 103, 188–195. [CrossRef]
- de Andrade, J.P., Pigni, N.B., Torras-Claveria, L., Berkov, S., Codina, C., Viladomat, F., Bastida, J., 2012. Bioactive alkaloid extracts from Narcissus broussonetii: Mass spectral studies. J. Pharm. Biomed. Anal. 70, 13–25. [CrossRef]
- Ee, E., Gi, S., J, V.S., 2004. Acetylcholinesterase enzyme inhibitory effects of Amaryllidaceae alkaloids. Planta Med. 70. [CrossRef]
- Fimognari, C., Ferruzzi, L., Turrini, E., Carulli, G., Lenzi, M., Hrelia, P., Cantelli-Forti, G., 2012. Metabolic and toxicological considerations of botanicals in anticancer therapy. Expert Opin. Drug Metab. Toxicol. 8, 819–832. [CrossRef]
- Frahm, A.W., Ali, A.A., Ramadan, M.A., 1985. 13C nuclear magnetic resonance spectra of amaryllidaceae alkaloids. I—alkaloids with the crinane skeleton. Magn. Reson. Chem. 23, 804–808. [CrossRef]
- Gaudêncio, S.P., Pereira, F., 2015. Dereplication: racing to speed up the natural products discovery process. Nat. Prod. Rep. 32, 779–810. [CrossRef]
- Girault, L., 2018. Kallawaya, guérisseurs itinérants des Andes: Recherches sur les pratiques médicinales et magiques. IRD Éditions.
- Hohmann, J., Forgo, P., Szabó, P., 2002. A new phenanthridine alkaloid from Hymenocallis × festalis. Fitoterapia 73, 749–751. [CrossRef]
- Hubert, J., Nuzillard, J.-M., Renault, J.-H., 2017. Dereplication strategies in natural product research: How many tools and methodologies behind the same concept? Phytochem. Rev. 16, 55–95. [CrossRef]
- Jerald J. Nair, Johannes Van Staden, Jaume Bastida, 2016. Cytotoxic alkaloid constituents of the Amaryllidaceae.
- Knolker, H., 2020. The Alkaloids. Elsevier.
- Kobayashi, S., Kihara, M., Shingu, T., Shingu, K., 1980. Transformation of Tazettine to Pretazettine. Chem. Pharm. Bull. (Tokyo) 28, 2924–2932. [CrossRef]
- Kotland, A., Chollet, S., Diard, C., Autret, J.-M., Meucci, J., Renault, J.-H., Marchal, L., 2016. Industrial case study on alkaloids purification by pH-zone refining centrifugal partition chromatography. J. Chromatogr. A 1474, 59–70. [CrossRef]
- Kuhn, S., Wieske, L.H.E., Trevorrow, P., Schober, D., Schlörer, N.E., Nuzillard, J.-M., Kessler, P., Junker, J., Herráez, A., Farès, C., Erdélyi, M., Jeannerat, D., 2021. NMReDATA: Tools and applications. Magn. Reson. Chem. 59, 792–803. [CrossRef]
- Lenzi, M., Cocchi, V., Novaković, A., Karaman, M., Sakač, M., Mandić, A., Pojić, M., Barbalace, M.C., Angeloni, C., Hrelia, P., Malaguti, M., Hrelia, S., 2018. Meripilus giganteus ethanolic extract exhibits pro-apoptotic and anti-proliferative effects in leukemic cell lines. BMC Complement. Altern. Med. 18, 300. [CrossRef]
- Lévi-Strauss, C., 1952. The use of wild plants in tropical South America. Econ. Bot. 6, 252–270. [CrossRef]
- Lianza, M., Leroy, R., Machado Rodrigues, C., Borie, N., Sayagh, C., Remy, S., Kuhn, S., Renault, J.-H., Nuzillard, J.-M., 2021. The Three Pillars of Natural Product Dereplication. Alkaloids from the Bulbs of Urceolina peruviana (C. Presl) J.F. Macbr. as a Preliminary Test Case. Molecules 26, 637. [CrossRef]
- Lianza, M., Verdan, M.H., de Andrade, J.P., Poli, F., de Almeida, L., Costa-Lotufo, L., Cunha Neto, Á., Oliveira, S., Bastida, J., Batista, A., Batista Jr., J., Borges, W., 2020. Isolation, Absolute Configuration and Cytotoxic Activities of Alkaloids from Hippeastrum goianum (Ravenna) Meerow (Amaryllidaceae). J. Braz. Chem. Soc. [CrossRef]
- Ločárek, M., Nováková, J., Klouček, P., Hošt’álková, A., Kokoška, L., Gábrlová, L., Šafratová, M., Opletal, L., Cahlíková, L., 2015. Antifungal and Antibacterial Activity of Extracts and Alkaloids of Selected Amaryllidaceae Species. Nat. Prod. Commun. 10, 1934578X1501000. [CrossRef]
- Maltese, F., van der Kooy, F., Verpoorte, R., 2009. Solvent Derived Artifacts in Natural Products Chemistry. Nat. Prod. Commun. 4, 1934578X0900400. [CrossRef]
- Mandrone, M., Bonvicini, F., Lianza, M., Sanna, C., Maxia, A., Gentilomi, G.A., Poli, F., 2019. Sardinian plants with antimicrobial potential. Biological screening with multivariate data treatment of thirty-six extracts. Ind. Crops Prod. 137, 557–565. [CrossRef]
- Masi, M., Di Lecce, R., Mérindol, N., Girard, M.-P., Berthoux, L., Desgagné-Penix, I., Calabrò, V., Evidente, A., 2022. Cytotoxicity and Antiviral Properties of Alkaloids Isolated from Pancratium maritimum. Toxins 14, 262. [CrossRef]
- McNulty, J., Nair, J.J., Codina, C., Bastida, J., Pandey, S., Gerasimoff, J., Griffin, C., 2007. Selective apoptosis-inducing activity of crinum-type Amaryllidaceae alkaloids. Phytochemistry 68, 1068–1074. [CrossRef]
- Meerow, A., Jost, L., Oleas, N., 2015. Two new species of endemic Ecuadorean Amaryllidaceae (Asparagales, Amaryllidaceae, Amarylloideae, Eucharideae). PhytoKeys 48, 1–9. [CrossRef]
- Meerow, A.W., 1985. A New Species of Eucrosia and a New Name in Stenomesson (Amaryllidaceae). Brittonia 37, 305. [CrossRef]
- Nair, J.J., 2019. The Plant Family Amaryllidaceae: Special Collection Celebrating the 80th Birthday of Professor Johannes van Staden. Nat. Prod. Commun. 14, 1934578X1987293. [CrossRef]
- Nair, J.J., Van Staden, J., 2021. Cytotoxic tazettine alkaloids of the plant family Amaryllidaceae. South Afr. J. Bot. 136, 147–156. [CrossRef]
- Nair, J.J., van Staden, J., 2013. Pharmacological and toxicological insights to the South African Amaryllidaceae. Food Chem. Toxicol. 62, 262–275. [CrossRef]
- Nair, J. J., Van Staden, J., Bastida, J. 2016. Cytotoxic alkaloid constituents of the Amaryllidaceae. Stud. Nat. Prod. Chem. 49, 107-156. [CrossRef]
- Nair, J.J., Wilhelm, A., Bonnet, S.L., van Staden, J., 2017. Antibacterial constituents of the plant family Amaryllidaceae. Bioorg. Med. Chem. Lett. 27, 4943–4951. [CrossRef]
- Renault, J.-H., Nuzillard, J.-M., Le Crouérour, G., Thépenier, P., Zèches-Hanrot, M., Le Men-Olivier, L., 1999. Isolation of indole alkaloids from Catharanthus roseus by centrifugal partition chromatography in the pH-zone refining mode. J. Chromatogr. A 849, 421–431. [CrossRef]
- Renault, J.-H., Nuzillard, J.-M., Maciuk, A., Zeches-Hanrot, M., 2009. Use of centrifugal partition chromatography for purifying galanthamine. US20090216012A1.
- Ünver, N., Gözler, T., Walch, N., Gözler, B., Hesse, M., 1999. Two novel dinitrogenous alkaloids from Galanthus plicatus subsp. byzantinus (Amaryllidaceae). Phytochemistry 50, 1255–1261. [CrossRef]
- Viet Nguyen, K., Laidmäe, I., Kogermann, K., Lust, A., Meos, A., Viet Ho, D., Raal, A., Heinämäki, J., Thi Nguyen, H., 2019. Preformulation Study of Electrospun Haemanthamine-Loaded Amphiphilic Nanofibers Intended for a Solid Template for Self-Assembled Liposomes. Pharmaceutics 11, 499. [CrossRef]
- Viladomat, F., Bastida, J., Tribo, G., Codina, C., Rubiralta, M., 1990. Alkaloids from Narcissus bicolor. Phytochemistry 29, 1307–1310. [CrossRef]
- Viladomat, F., Codina, C., Bastida, J., Mathee, S., Campbell, W.E., 1995. Further alkaloids from Brunsvigia josephinae. Phytochemistry 40, 961–965. [CrossRef]
- Viladomat, F., Sellés, M., Cordina, C., Bastida, J., 1997. Alkaloids from Narcissus asturiensis. Planta Med. 63, 583–583. [CrossRef]
- Wu, Z., Chen, Y., Xia, B., Wang, M., Dong, Y.-F., Feng, X., 2009. Two Novel Ceramides with a Phytosphingolipid and a Tertiary Amide Structure from Zephyranthes candida. Lipids 44, 63–70. [CrossRef]
- Zupkó, I., Réthy, B., Hohmann, J., Molnár, J., Ocsovszki, I., Falkay, G., 2009. Antitumor Activity of Alkaloids Derived from Amaryllidaceae Species. In Vivo 8.
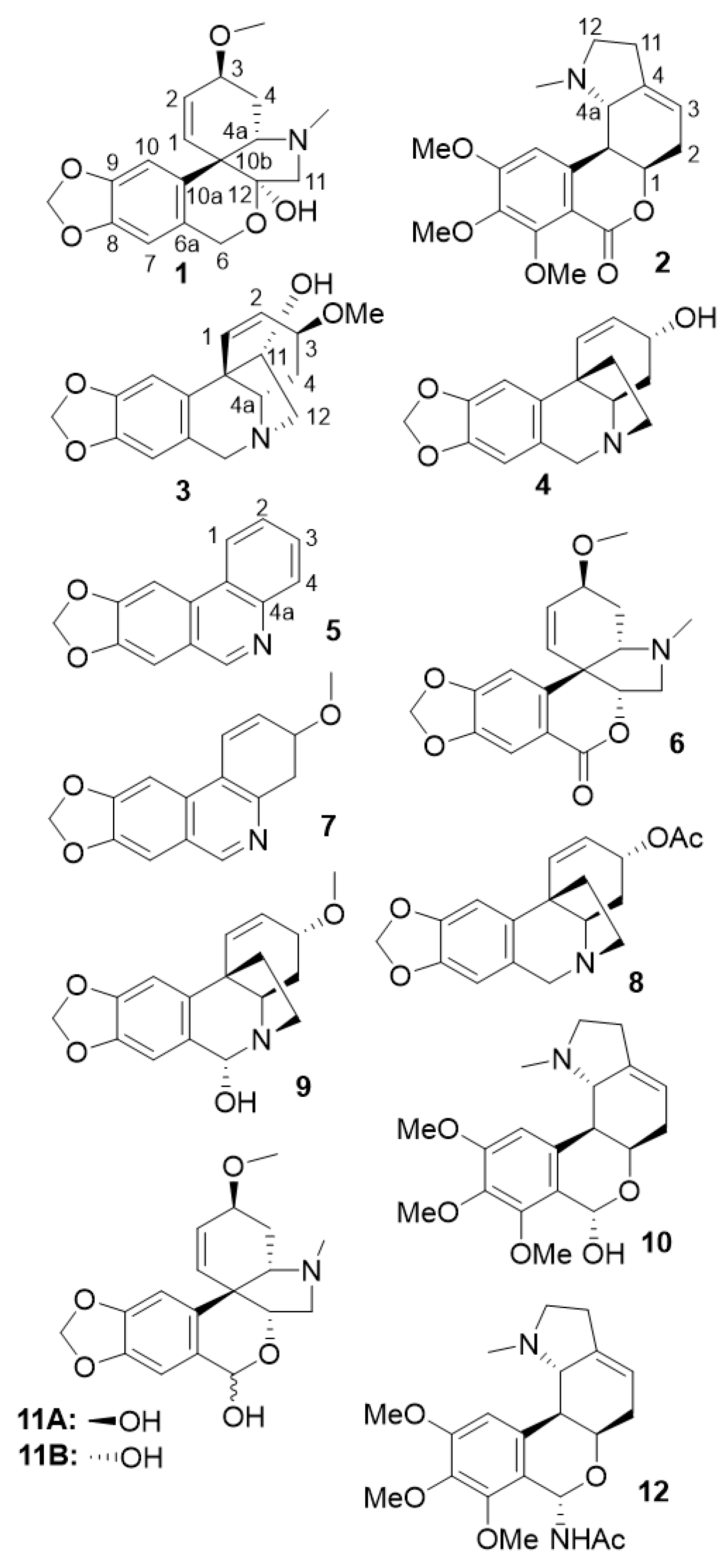
| FRACTION | Fraction composition | Identified Alkaloid | Reference for 13C NMR-based dereplication |
|---|---|---|---|
| A1 | - | - | - |
| A2 | tazettine; trisphaeridine; 3-epimacronine; 3-methoxy-8,9-methylenedioxy-3,4-dihydrophenanthridine |
Trisphaeridine 5; 3-epimacronine 6; 3-methoxy-8,9-methylenedioxy-3,4-dihydrophenanthridine 7 |
(Viladomat et al. 1997) (Viladomat et al. 1990) (Hohmann et al. 2002) |
| A3 | tazettine; trisphaeridine | ||
| A4 | tazettine | tazettine 1 | (Knolker 2020) |
| A5 | tazettine; crinine acetate | ||
| A6 | crinine acetate; albomaculine | crinine acetate 8 | (Ali et al. 1986) |
| A7 | albomaculine | albomaculine 2 | (de Andrade et al. 2014) |
| A8 | albomaculine; 6α-hydroxybuphanisine; haemanthamine | 6α-hydroxybuphanisine 9 | (Frahm et al. 1985) |
| A9 | haemanthamine | haemanthamine 3 | (Viet Nguyen et al. 2019) |
| A10 | haemanthamine; nerinine | nerinine 10 | (de Andrade et al. 2014) |
| A11 | crinine; α-pretazettine | crinine 4 | (Viladomat et al. 1995) |
| A12 | α-pretazettine; β-pretazettine; 6-dehydroxy- 6-acetamido-nerinine |
β-pretazettine 11A α-pretazettine 11B 6-dehydroxy-6-acetamido-nerinine 12 |
(Baldwin and Debenham 2000) (Kobayashi et al. 1980) - |
| A13 | β-pretazettine; α-pretazettine; 6-dehydroxy- 6-acetamido-nerinine |
| Sample | IC50 24h |
IC50 48h |
IC50 72h |
|---|---|---|---|
| extract | 9.1 | 6.7 | 3.3 |
| A2 | 347.1 | 297.5 | 232.1 |
| A4 (tazettine) | 901.3 | 1171.0 | 869.2 |
| A6 | 394.0 | 419.0 | 412.9 |
| A7 (albomaculine) | 201.5 | 251.5 | 168.7 |
| A8 | 10.1 | 7.1 | 5.1 |
| A9 (haemanthamine) | 7.6 | 5.4 | 3.7 |
| A10 | 16.1 | 13.2 | 5.2 |
| A11 | 9.9 | 10.3 | 8.2 |
| A12 | 5.7 | 4.3 | 5.3 |
| A13 | 6.4 | 4.9 | 3.8 |
| Sample | IC50 24h |
IC50 48h |
IC50 72h |
|---|---|---|---|
| extract | 124.6 | 31.4 | 10.9 |
| A2 | 309.9 | 209.5 | 123.8 |
| A4 (tazettine) | 1373.0 | 857.8 | 881.9 |
| A6 | 894.8 | 360.7 | 256.1 |
| A7 (albomaculine) | 1669.0 | 1073.0 | 446.1 |
| A8 | 233.3 | 31.7 | 13.7 |
| A9 (haemanthamine) | 70.4 | 31.2 | 4.5 |
| A10 | 292.3 | 53.7 | 13.9 |
| A11 | 102.4 | 53.7 | 8.6 |
| A12 | 119.3 | 16.4 | 5.1 |
| A13 | 65.6 | 12.4 | 5.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





