3.1. SPR-Based Biosensors
Surface plasmon resonance (SPR) based assays were successfully used for the detection of numerous biomarkers in plasma and serum [
28]. SPR is very sensitive to the refractive index and can be used to detect any molecule for which a ligand with high affinity such as an antibody or aptamer exists. Usually, the sensor surface is immobilized with a layer of specific antibody or aptamer ligand and the target molecule is injected over the surface. As the target molecules bind to the ligands, the refractive index changes proportionally to the accumulated mass or modifications in the structure of the active layer [
29] and is detected and quantified by SPR. Most of current SPR detection schemes are based on angular or wavelength interrogation (by monitoring the shift of SPR dips), and on intensity interrogation (by monitoring the intensity under a fixed angle of incidence or wavelength). They have a typical resolution of 10
−5–10
−7 refractive index units (RIU)[
30], with the angle resolved-SPR having intrinsic higher sensitivity than single angle SPR [
31]. However, angle-resolved SPR devices generally require expensive equipment, complicated optics, and precise alignment of the components, features that hinder the development of a portable device.
An SPR biosensor immobilized with anti-cardiac troponin I monoclonal antibody was developed by [
32] and used for the detection of cTnI in aqueous solution and patient serum and kinetic analysis. The gold surface of the SPR chip was functionalized with polyacrylic acid and polydiallyldimethylammonium chloride. Using amino-coupling, the anti-cardiac troponin I monoclonal antibody was immobilized and then the SPR biosensor was blocked with BSA. The limit of detection and limit of quantification were calculated as 0.00012 ng/mL and 0.00041 ng/mL, respectively. The SPR measurements were performed using the commercial SPR imaging system GenOptics, SPRiLab (Orsay, France).
A strategy for cTnI detection was developed by constructing a universal biosensing interface composed of zwitterionic peptides and aptamers [
33]. The peptides were self-assembled onto gold chips, some of them being biotinylated. The cTnI-specific biotinylated aptamers were immobilized via streptavidin-biotin system. A custom-made angle-scanning SPR system based on the Kretschmann configuration was used for measurements. The developed aptasensor had a linear detection range of cTnI from 20 ng/ml to 600 ng/ml and a detection limit of 20 ng/ml. Due to the antifouling property of the zwitterionic peptide, the developed aptasensor had a high resistance towards protein fouling.
Long-range surface plasmon-polariton (LRSPP) waveguides were used as biosensors for label-free detection of cTnI [
34]. The sensors consist of 5-µm-wide 35-nm-thick gold stripes embedded in a low-index optical-grade fluoropolymer (CYTOP) with fluidic channels etched to the Au surface of the stripes. Direct and sandwich assays were developed and demonstrated over the concentration range from 1 to 1000 ng/mL, yielding detection limits of 430 pg/mL for the direct assay and 28 pg/mL for the sandwich assay, the latter being physiologically relevant to the early detection or onset of AMI.
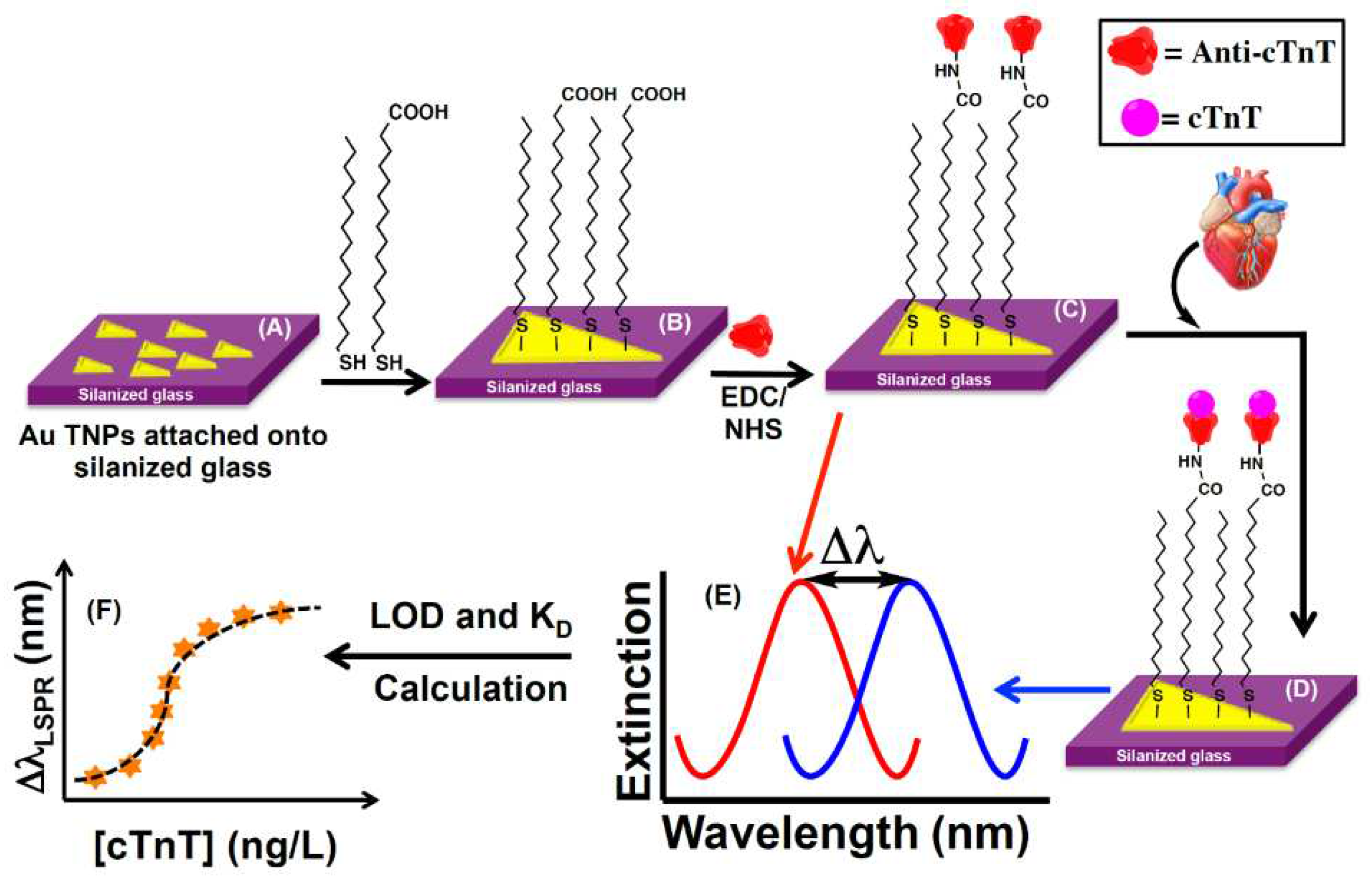
A nanoplasmonic biosensor chip was developed by [
35] to assay cardiac troponin T (cTnT) in human biofluids (plasma, serum, and urine) with high specificity. The sensing mechanism is based on the adsorption model that measures the localized surface plasmon resonance (LSPR) wavelength shift of anti-cTnT functionalized gold triangular nanoprisms (Au TNPs) induced by change of their local dielectric environment upon binding of cTnT (
Figure 1). Controlled manipulation of the sensing volume and decay length of Au TNPs together with the appropriate surface functionalization and immobilization of anti-cTnT onto TNPs allowed to achieve a limit of detection (LOD) of the cTnT assay at attomolar concentration (~15 aM) in human plasma.
A biosensor based on a plasmonic exposed core optical fiber tip was developed for the rapid and label-free detection of the N-Terminal portion of the NT-proBNP [
36]. The biosensor is based on a fiber tip covered with a gold layer, enabling SPR measurements that was functionalized with anti-NTproBNP antibodies. It was capable of monitoring NT-proBNP concentrations from 0.01 to 100 ng/mL, in a concentration range of clinical interest [
36].
An ultrasensitive SPR immunoassay was developed for the specific detection of human cTnI. Based on the classical thin gold layer as the SPR sensing film, the surface was further modified by hollow gold nanoparticles (HGNPs) and polydopamine (PDA) sequentially, and then was immobilized with antibodies for specific recognition of target analyte. The interaction between the localized surface plasmon resonances of HGNPs and the propagating plasmon on the surface of the gold film leads to the amplification of SPR response signal. For additional sensitivity increase the sample was incubated with specific magnetic probes made of PDA-wrapped magnetic multi-walled carbon nanotubes (MMWCNTs-PDA) conjugated with detection antibodies (dAb). The magnetically assisted extraction of the target from the sample overcomes the disadvantage of slow diffusion limited mass transfer and matrix interference, reducing the nonspecific interferences while detecting cTnI in human serum. The combination of the above improvements results in the significant sensitivity enhancement of the SPR immunoassay. The concentration of cTnI with minimum detectable SPR response obtained by the assay was 1.25 ng/mL [
37].
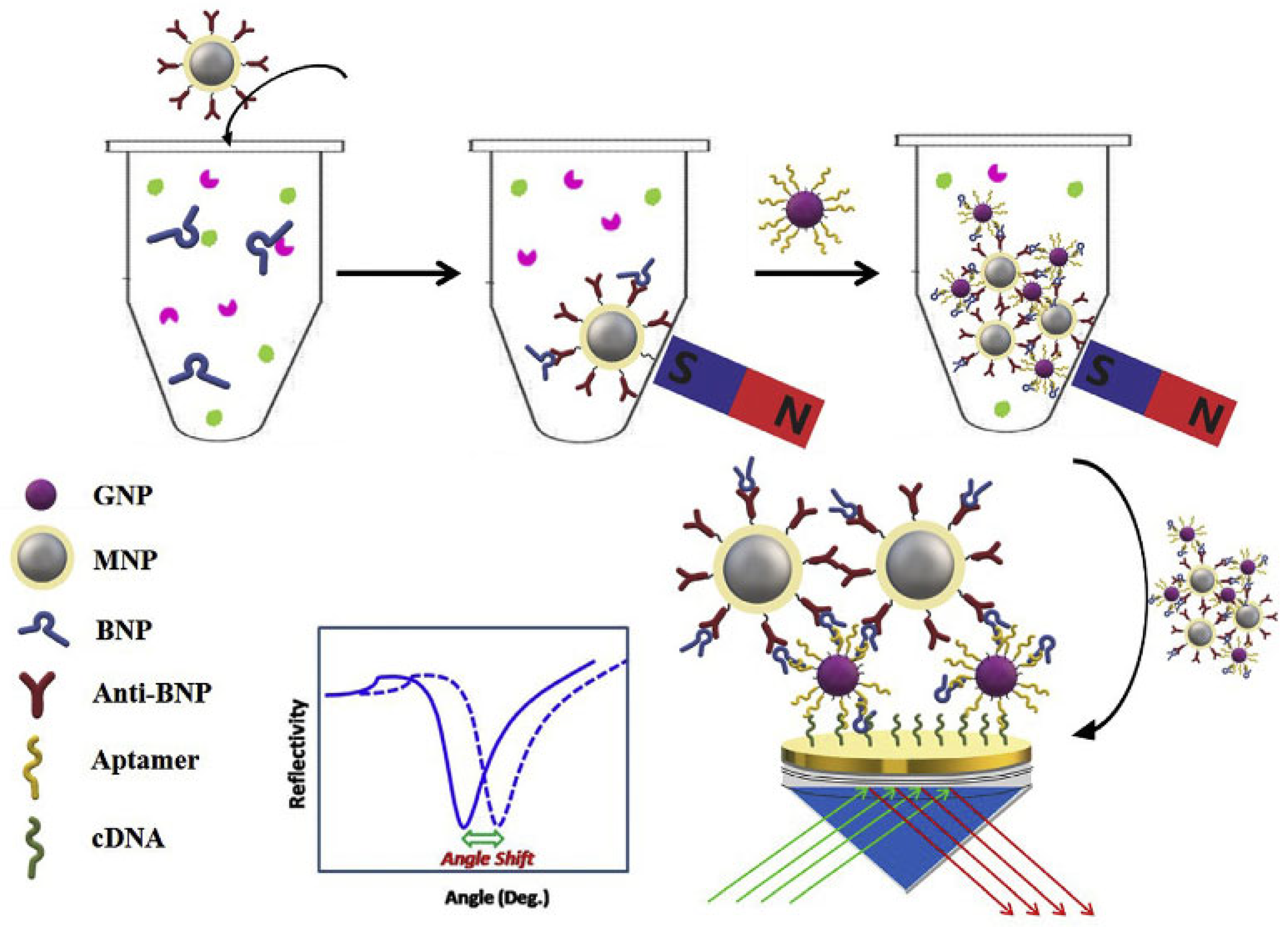
One study lead by Zhao [
38] achieved the detection of BNP in serum samples using aptamer-functionalized Au nanoparticles (GNPs-Apt) and antibody-modified magnetoplasmonic nanoparticles (MNPs-Ab) for dual evaluation. Both types of nanoparticles (NPs) specifically recognize BNP in order to form magnetoplasmonic nanoconjugates (NCs). Avoiding degradation is critical for analysis, so applying an external magnetic field makes possible the separation of the analyte from the complex samples. Next the recognition of NCs is carried out by complementary-DNA (cDNA) of the aptamer immobilized on the gold film of SPR chip (
Figure 2). Therefore, the refractive index of the gold surface significantly modifies due to strong electronic coupling between MNPs and the surface plasmon wave of GNPs. The linear range obtained with this method is from 0.1 pg/mL to 100 pg/mL and the limit of detection equals 28.2 fg/mL. The selectivity matter was addressed using some proteins and molecules as interfering substances, bovine hemoglobin (BHb), ascorbic acid (AA), myoglobin (Myo), ovalbumin (OVA) and bovine serum albumin (BSA). Regarding real sample analysis, the group investigated the feasibility of the SPR biosensor in spiked serum. The recoveries were between 92.5-113.9 %, with RSDs lower than 15 %, indicating great accuracy for BNP sensing by the developed SPR method.
In another study by Harpaz [
39], an SPR chip was designed and used in a novel point-of-care SPR system for the detection of the stroke biomarkers NT-proBNP and S100β in water and plasma samples. The POC system was based on the commercial PhotonicSys SPR H5 from PhotonicSys (Neveh Shalom-Wahat Alsalam, Israel,
www.photonicsys.com). The SPR chip had a bimetallic composition consisting of 30 nm silver and 15 nm gold. The chip was functionalized with thiols and then the specific antibodies for the target biomarkers were immobilized by amino-coupling. NT-proBNP and S100β were detected in a range of clinically relevant concentrations for stroke, from 0.1 ng/mL to 10 ng/mL.
In conclusion, SPR based biosensors are able to detect CVD biomarkers in a label-free and fast manner. Moreover, these biosensors can be implemented in point-of-care (POC) devices due to their versatility, long-term stability, and simple concepts. Amongst the various SPR detection methods, LSPR biosensors proved to be the most sensitive, with a LOD in attomolar range. Various enrichment techniques (adding affine or magnetic tags) may further improve the detection limit and reduce the nonspecific response of complex clinical samples.
3.2. SERS Biosensors for Cardiac Biomarkers
Surface-enhanced Raman spectroscopy (SERS) combines outstanding features for sensing applications, such as specific identification and structural information about the molecular species based on their unique vibrational Raman fingerprint, as well as ultrasensitive detection down to a single molecule. SERS largely relies on collective oscillations of conduction electrons known as surface plasmon resonances that produce drastic amplification of the electromagnetic fields near the surface of noble-metal nanostructures. These, in turn, significantly enhance the Raman signal from the molecules placed in their close vicinity up to 10
8-10
10 orders of magnitude through the so-called electromagnetic mechanism (EM). The molecules directly adsorbed on the nanostructured substrate can experience a charge transfer with the metal surface, leading to an additional enhancement of the Raman signal of 10
1-10
3 orders of magnitude through the so-called chemical charge transfer (CT) mechanism [
40,
41]. The SERS technique has been widely employed as a powerful tool in the development of sensing bioassays for the selective, sensitive and quantitative detection of various cardiac biomarkers. Some of the fabricated SERS bioassays involve the immobilization of the cardiac biomarkers onto nanostructured surfaces, followed by direct analysis and identification of their Raman spectral fingerprint. However, this strategy, called direct SERS detection suffers from low selectivity due to the multiple components found in the biological samples, which can interfere with the SERS signal of the biomarker of interest. This in turn complicates the data analysis and limits accurate biomarker quantification. Indirect SERS detection has been proposed as an alternative to direct detection to improve the selectivity of the assay and simplify the data analysis. For this, the SERS substrate is modified with Raman reporters and receptors to ensure the specific capture of the target biomarkers. SERS nanotags built on noble-metal nanoparticles conjugated with Raman reporters and specific receptors were also used to increase the selectivity of the assay and accomplish simultaneous determination of multiple target cardiac biomarkers.
The recent years witnessed the production and application in sensing of new nanomaterials for enhanced sensitivity of SERS detection, new strategies for the selective and accurate detection in biological samples, in the range relevant for diagnosing CVDs, multiplexed detection and increased use of portable equipment.
For example, nanomaterials like gold or silver nanoaggregates, core-shell plasmonic bimetallic nanoparticles, hybrid plasmonic-magnetic nanoparticles were coupled with specific bioreceptors (mainly antibodies) and Raman reporters like rhodamine-6G (R6G), nile blue A (NBA), malachite green isothiocyanate (MGITC), methylene blue (MB), 4-mercaptobenzoic acid (4-MBA) etc. for selective and ultrasensitive detection of several cardiac biomarkers. Different plasmonic nanoplatforms were fabricated and optimized for high sensitivity for either direct or indirect SERS detection of various cardiac biomarkers, including CRP [
42], cTnI), B-type natriuretic peptide (BNP), CK-MB, Myo,NT-ProBNP, neutrophil gelatinase-associated lipocalin (NGAL), glycogen phosphorylase isoenzyme BB (GPBB), neuropeptide Y (NPY) and heart type fatty acid-binding protein (H-FABP). In the following are summarized several successful SERS biosensors for CVD, with the emphasis on the nanostructures’ designs proposed for efficient sensing.
Benford et al. developed the first SERS assay to qualitatively analyze three cardiac biomarkers in diagnosing acute coronary syndrome [
43]. Specifically, SERS active aggregated gold nanoparticles (AuNPs) trapped at the entrance of a nanofluidic device were used as sensing elements to detect BNP, cTn, and CRP. This sensing platform enables the detection and identification of BNP, cTn, and CRP at physiologically relevant concentrations. Unfortunately, no real sample analysis was reported in the work. A key issue in SERS-based detection bioassays represents the biosensor’s capacity to detect the target analyte with high specificity. Given this, the same group designed an improved SERS bioassay for the specific detection of CRP [
44]. The proposed platform incorporates agarose beads functionalized with an anti-CRP antibody for the specific capture of CRP, aggregated gold nanoparticles as the SERS units, and CRP labeled Rhodamine-6G (R6G) as a target analyte. Besides the specific detection of CRP, a correlation between the amount of CRP and the SERS signal of R6G was also observed. However, there was no validation of the results in clinical samples.
Knowing the concentration of cardiac biomarkers in human blood is essential in diagnosing cardiovascular diseases. Therefore, the efforts in designing SERS assays were focused not only on selective detection but also on the quantification of the biomarkers in blood samples. A good example is a study reported by Cong et al.[
45]. In their work, the SERS technique and an enzyme catalysis bioassay (ELISA) were combined for selective and sensitive detection and quantification of the human cardiac isoform of troponin T, cTnT in human serum. The proposed detection strategy involves the use of citrate-capped spherical AuNPs as a SERS substrate, and the resulting product of the enzyme-catalyzed 3,3′,5,5′-tetramethylbenzidine reaction, TMB
2+ as a SERS probe.
Remarkably, the developed biosensor achieved a broader linear concentration range (2 ~ 320 pg/ml) and an improved sensitivity (limit of detection -LOD of 2 pg/ml) compared with the UV-Vis technique (linear concentration range 4 ~ 80 pg/ml and LOD of 4 pg/ml). The performance of the sensor assay for clinical applications was evaluated with two serum samples containing two concentrations of cTnT, 16 and 8 pg/ml, respectively. The relative standard deviation for these two concentrations was 0.017 and 0.093 and the average recoveries were 100.01% and 86.815%, respectively. Cote and co-authors also exploited AuNPs to develop an optofluidic device for SERS detection of myoglobin [
46]. The fabricated device comprising plastic plates, rubber layers, and a nanoporous membrane was exposed to a mixture containing rhodamine-6G (R6G) labeled myoglobin and colloidal AuNPs. The aggregation of AuNPs on the nanoporous membrane leads to the formation of a robust, sensitive and reproducible SERS active plasmonic substrate. The intensity variation of a characteristic Raman band of R6G was used to quantify myoglobin concentration in solution over a physiologically relevant range (1.2 nM to 30 nM). To further evaluate the performance of the assay in complex samples, bovine serum albumin (BSA) was introduced as a possible interfering compound. Unfortunately, a decrease of the SERS signal was noticed in the presence of BSA for all concentrations of myoglobin tested. Furthermore, no real sample analysis was provided in the work. Later, silver nanoaggregates were exploited by the same authors to fabricate a bioassay for SERS detection of the human cardiac Troponin I (cTnI) in solution [
47]. In this system, silver nanoparticles (AgNPs) were first encoded with the Raman reporter molecule DTNB and then aggregated to give a strong and stable SERS signal. In the second step, the Ag nanoaggregates were encapsulated in a silica shell to stabilize them and facilitate their further bioconjugation. Finally, the core-shell Ag nanoaggregates-silica architecture was coated with polyethylene glycol (PEG) and functionalized with cTnI protein and BSA to endow them with an affinity for the cTnI antibody. Unfortunately, this study did not address the detection of cTnI and instead only examined the SERS signal of the nanoconjugate. 3D silver anisotropic nano-pinetree array modified indium tin oxide (Ag NPT/ITO) was proposed by El-Said and co-authors as an alternative to silver nanoaggregates to develop an ultrasensitive SERS platform for direct, label-free detection of myoglobin [
48]. Another three Ag nanostructures/ITO substrates (Ag nanoaggregates/ITO, Ag nanorods/ITO and Ag nanobranched/ITO) were also prepared and compared with Ag NPT/ITO regarding their SERS performance. The fabricated Ag NPT/ITO substrate showed the best Raman signals, yielding a LOD of 10 × 10
−9 g mL
-1 and a wide working range for myoglobin quantification from 5 × 10
−6 to 10 × 10
−9 g mL
-1. The sensor performance for clinical applications was evaluated by analyzing urine samples spiked with a known amount of myoglobin. A linear relationship between the Raman intensity and the myoglobin concentration in urine over a range of 10 ng/mL to 5 μg/mL was obtained. In addition, the calibration curves for urine and buffer have almost the same slopes, demonstrating the accuracy of the detection without interferences. However, there was no validation of the results by parallel analysis using a standard method.
Ultrasensitivity and high specificity were also reported by Gao et al., who managed to design a novel hybrid microfluidic chip for simultaneous SERS detection of CK-MB and cTnI cardiac markers [
49]. In this system, a SERS substrate fabricated by in-situ synthesis of AuNPs on the patterned paper microchannels was used as a capture platform, while AuNPs labeled with malachite green isothiocyanate (MGITC) Raman reporter molecules were employed as SERS detection nanotags. To achieve specificity toward CK-MB and cTnI, the SERS platform and the Raman probes were conjugated with capture and detection antibodies against CK-MB and cTnI. Selective quantification of CK-MB and cTnI was accomplished by measuring the SERS signal on a sandwich-type nanoarchitecture formed after the immune reaction, yielding a LOD of 7.92 pg mL
-1 and 2.94 pg mL
-1 for CK-MB and cTnI, respectively. However, some interfering SERS signal was noticed in the presence of BSA, thrombin, and PSA spiked in serum samples due to the non-specific adsorption of SERS nanotags on the detection area.
Core-shell plasmonic bimetallic nanoparticles have also been employed in the development of various SERS bioassay as they offer several advantages compared with monometallic nanoparticles, such as high stability and reproducibility of the Raman signal, as well as an increased SERS performance. Both, Raman reporter-labeled and reporter-embedded core-shell nanotags were prepared and exploited as ultrasensitive SERS nanoprobes for selective determination of cardiac biomarkers.
For instance, Bai et al. fabricated three classes of bimetallic core-shell SERS nanoprobes and one class of monometallic nanoprobe and investigated their SERS activity by experimental measurement and theoretical analysis [
50]. The monometallic class was built on citrate-capped AuNPs-encoded with Nile blue A (NBA) dye. Two classes of the core-shell nanoprobes consist on a metallic core (Au or Ag) with a metallic shell (Ag or Au) and NBA embedded at their interface and also labelled at their surface. The other class of core-shell nanoprobes was built on Au-core labeled with NBA, a silver shell labeled with NBA and then etched with HAuCl
4 to form Au@AgAuNPs with nanometric gaps inside. The obtained Au@Ag-Au NPs were further encoded with NBA. Both experimental and theoretical results showed that Au@Ag-Au NPs exhibited the best SERS performance due to the strong electromagnetic field created in the nanogaps between the core and shell. Therefore, the authors have selected Au@Ag-Au NPs to develop a SERS-based lateral flow assay strips for selective, highly sensitive and quantitative analysis of cardiac troponin I (cTnI). To achieve specificity toward cTnI, the fabricated Au@Ag-AuNPs and test line were conjugated with detection and capture antibodies, respectively. Quantification of cTnI was performed by monitoring the SERS intensity of a characteristic Raman band of NBA in a sandwich immunocomplex formed after exposure of the sample pad to various concentrations of cTnI. The designed SERS-based lateral flow assay strips provide reproducible, selective and high-sensitive detection of cTnI with the LOD of 0.09 ng mL
−1. Even though no interference signal was found when CRP, BNP, Myo, and CK-MB were present, real sample analysis was unfortunately not addressed in this study.
Zhang et al. also exploited the core-shell SERS nanotags to develop a SERS-based lateral flow assay strips for simultaneous detection of Myo, cTnI and CK-MB on three test lines [
51]. In their design, SERS nanotags were built on an Ag core with an Au shell and NBA Raman reporter molecules embedded at their interface. The fabricated SERS nanotags were then conjugated with detection antibodies of three biomarkers to form an immunocomplex with the capture antibodies on a nitrocellulose membrane when the target biomarkers are present. The intensity of the SERS spectra recorded from the three test lines at 785 nm laser excitation was used for quantitative analysis of Myo, cTnI, and CK-MB. The designed SERS-based lateral flow assay provides reproducible, high-sensitive and multiplex detection of Myo, cTnI and CK-MB with a wide linear dynamic range and the LODs of 3.2, 0.44, and 0.55 pg mL
−1, respectively. The diagnostic performance of the assay was evaluated with 50 serum samples collected from hospitalized patients suffering from AMI and the results were compared with the FDA approved clinical chemiluminescence immunoassay (CLIA) method. Passing- Boblok regression and Spearman's rank correlation coefficient were used to examine the linear dependence between the two methods. A good linear correlation of the two methods was obtained. The assay has better sensitivity than CLIA, is low-cost and easy to use and requires 15 min per marker. However, the developed sensor has speed constraints, necessitating the examination of three test lines. Thus, shortly thereafter, the same group reported an improved version of the SERS-based lateral flow assay for rapid, multiplex quantitative detection of CK-MB, cTnI, and Myo using a single test line [
52]. In their design, three different SERS nanotags, namely methylene blue (MB), nile blue A (NBA) and rhodamine 6 G (R6 G) were built on an Ag core with an Au shell and Raman reporters embedded at their interface. The SERS spectra recorded from a single test line at 785 nm laser excitation show distinct features for all corresponding nanotags of biomarkers. The designed sensor yielded a LOD of 0.93, 0.89, and 4.2 pg mL
−1 and linear dynamic range of 0.02−90, 0.01−50, and 0.01−500 ng mL
−1 for CK-MB, cTnI, and Myo, respectively. Five human serum samples from hospitalized patients with AMI were examined to ascertain whether the assay is suitable for usage in clinical settings. The CLIA approach was used to compare the outcomes. Real samples yielded very good recoveries, ranging from 86.7% to 113.5%, demonstrating the accuracy of the assay.
Yu et al. also developed a core-shell SERS nanotag-based sandwich immunoassay for rapid, sensitive and simultaneous detection of cTnI and CK-MB [
53]. The sandwich system was based on Au@Ag core–shell nanoparticles conjugated with malachite green isothiocyanate (MGITC) and polyclonal antibodies as the SERS detection element and a gold-patterned chip functionalized with monoclonal antibodies as the SERS active template. The designed assay enabled quantitative analysis of cTnI and CK-MB with a LOD of 8.9 pg mL
−1 and 9.7 pg mL
−1 for cTnI and CK-MB, respectively. The clinical applicability of the sensor was evaluated with 5 serum samples collected from patients with AMI and the results were compared with those obtained with a commercially available chemiluminescence assay. The concentrations of cTnI and CK-MB determined by the SERS-based assay were comparable to those determined by the chemiluminescence technique, and they were all within the clinically acceptable range. Another approach for ultrasensitive simultaneous detection of cTnI, N-terminal prohormone of brain natriuretic peptide (NT-ProBNP) and neutrophil gelatinase-associated lipocalin (NGAL) was reported by Zhu and co-authors [
54]. Their strategy involves using bimetallic Ag-Au nanostars conjugated with Raman reporters (4-mercaptobenzoic acid (4-MBA), 5′-dithiobis-(2-nitrobenzoic acid) (DTNB), 2-naphthalenethiol (NT)) and detection antibodies as SERS nanotags and a three-dimensional ordered macroporous Au-Ag-Au plasmonic array conjugated with capture antibodies as a substrate. to improve the reproducibility and sensitivity of the assay. The sensitivity of the system is achieved through the formation of Raman hot-spots between nanotags and substrate after the biomolecular recognition. This SERS-based sandwich immunoassay allowed for sensitive and reproducible multiplex detection, yielding a LOD of 0.76, 0.53 and 0.41 fg mL
-1 for cTnI, NT-ProBNP and NGAL, respectively. The suitability of the developed immunoassay for clinical applications was also demonstrated by simultaneous determination of cTnI, NT-ProBNP and NGAL in human serum samples. The results were compared to those obtained using the dot immunogold filtration test (DIGFA) in order to confirm the accuracy of the immunoassay. The two techniques' agreement was found to be reasonable, thus proving the potential of the proposed sensor for clinical applications.
Raman reporter-embedded Au nanorod-core Au-shell nanotags were employed by Khlebtsov et al. for the design of a SERS-based lateral flow immunoassay for fast, sensitive, semiquantitative determination of cTnI [
55]. Detection of cTnI was performed by Raman mapping of the test zone, while quantification was achieved by monitoring the SERS intensity of a specific band of 1,4-nitrobenzenthiole (NBT) Raman reporter following the exposure of NPs to different concentrations of cTnI, reaching a LOD of 0.1 ng mL
-1. However, the selectivity of the assay and analysis of the real samples were not reported in this work. Going one step forward, Tu et al. have recently employed gap-enhanced nanoparticles (GeNPs) as ultrasensitive SERS nanotags to design a paper-based immunoassay for simultaneous quantification of three myocardial infarction biomarkers: cardiac troponin I (cTnI), copeptin, and heart-type fatty acid-binding protein (h-FABP) [
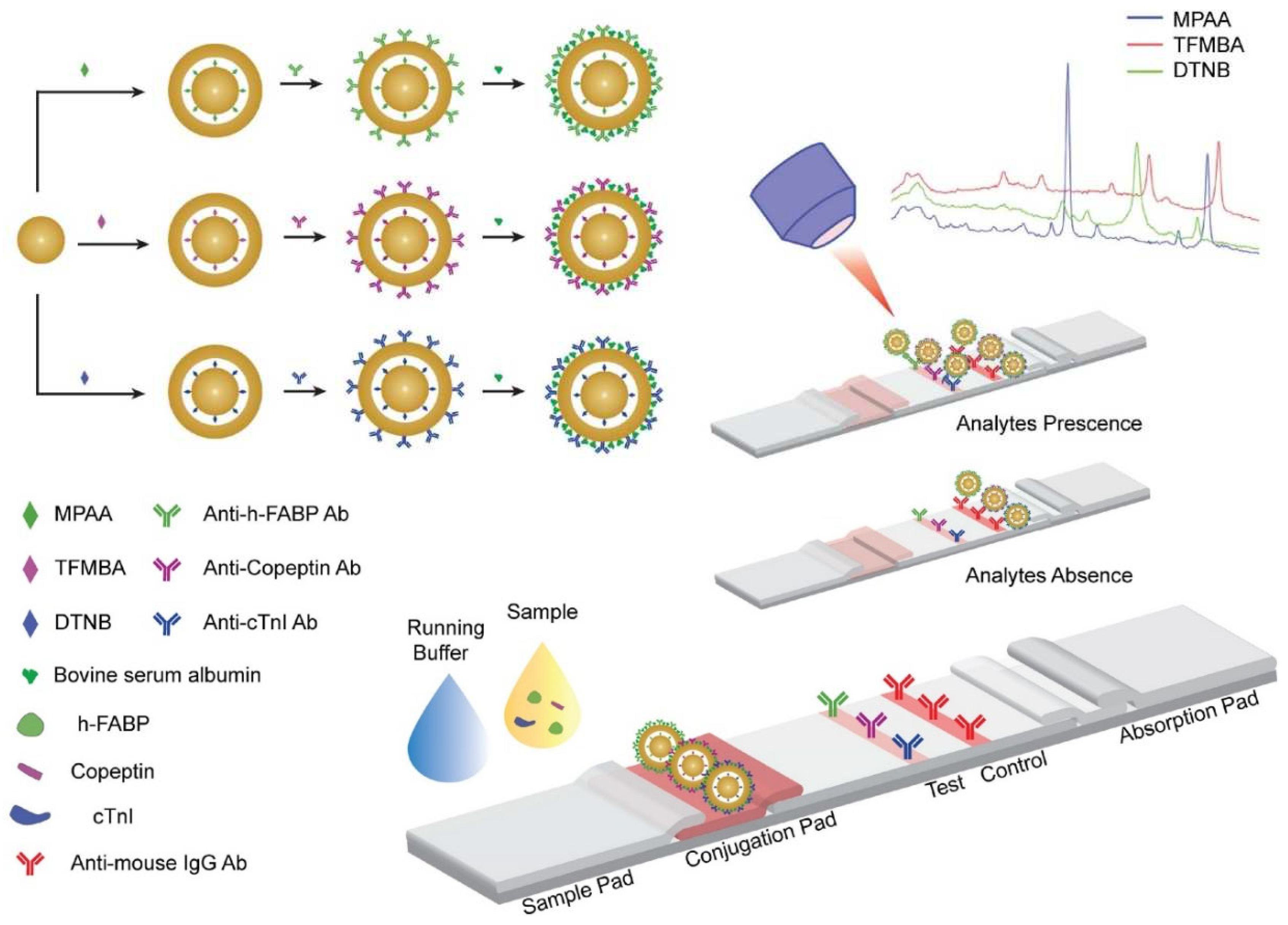
56]. As schematically illustrated in
Figure 3, GeNPs consist of three different SERS tags (4-mercaptophenylacetic acid (MPAA), 2,3,5,6-tetrafluoro-4-mercaptobenzoic acid (TFMBA) and 5,50-dithiobis(2-nitrobenzoic acid) (DTNB)) built on Raman reporter-embedded gold-core gold-shell with nanometric-size gaps of 0.9-1.1 nm created at the interface of the core-shell nanoarchitecture.
Due to the high electromagnetic enhancement of the Raman signal inside the narrow nanogaps, GeNPs enabled an increase of the SERS signal by 105-250 times compared to the Au core. To achieve specificity toward the three target biomarkers, the fabricated GeNPs-based SERS nanotags were conjugated with detection antibodies against cTnI, copeptin and h-FABP (
Figure 3). A single test line consisting of a nitrocellulose membrane conjugated with capture antibodies specific to cTnI, copeptin and h-FABP was used. A sandwich immunocomplex was formed on the test line only when the target biomarkers were present. The SERS spectra recorded from the test line at 780 nm laser excitation exhibited distinct SERS features related to each target biomarker, thus allowing the simultaneous detection, spectral discrimination and quantification of cTnI, copeptin and h-FABP with a LOD of 0.01 ng mL
-1, 0.86 ng mL
-1, 0.004 ng mL
-1 for cTnI, h-FABP, and copeptin, respectively. Notably, the developed paper-based SERS assay enabled quantification of the three biomarkers in human serum samples in a clinically relevant range of concentrations. However, the sensor has some limitations due to the non-specific binding between the three different GeNP/antibody particles, biomarkers, and primary antibodies, which leads to some interference in the multiplex immunoassay.
Even though core-shell bimetallic (Au@Ag or Ag@Au) nanoparticles were extensively employed in several types of SERS bioassays, Tu et al. have proposed gold-core silica-shell nanoparticles as SERS nanotags to design a stable, reproducible and cost-effective aptamer-based sandwich assay on a paper strip for determination of cTnI
via surface-enhanced resonance Raman spectroscopy (SERRS) [
57]. In the developed design, spherical AuNPs with a diameter of 60 nm were first encoded with the Raman reporter molecule malachite green isothiocyanate (MGITC) and then encapsulated in a silica shell. For specific recognition of cTnI, the core-shell nanoparticles were functionalized with a secondary aptamer of cTnI, while the test line was modified with a primary aptamer of cTnI. The specific molecular interaction of aptamers and cTnI results in a sandwich architecture between the core-shell nanoparticles and the test line. Selective and sensitive detection of cTnI was accomplished by measuring the SERRS signal of the test line under resonant excitation at 638 nm, reaching a detection range of 0.016 to 0.1 ng ml
-1, with a LOD of 0.016 ng ml
-1. The suitability of the developed aptamer-based paper strip assay toward clinical applications was demonstrated by SERRS detection and quantification of cTnI in serum samples. Recovery factors were evaluated by spiking cTnI at 0.03 and 0.05 ng∕ml in human serum without any treatment. The obtained values, in the range of 93.8% to 95.8% indicated the good accuracy of the method. Furthermore, the aptamer-based paper strip assay showed good stability after 10 days of storage at room temperature. However, the assay remains to be confirmed by parallel analysis with a larger sample set and a standardized technique.
Raman-encoded gold or silver nanoparticles enveloped in a silica shell were also employed by Lim et al. as extrinsic SERS probes to design a microfluidic paper-based device (μPAD) for simultaneous quantitative SERS measurement of three cardiac biomarkers: glycogen phosphorylase isoenzyme BB (GPBB), cTnI and creatine kinase-MB isoenzymes (CK-MB) [
58]. The proposed bioassay ensured high reproducibility and ultrasensitivity to detect the three cardiac biomarkers with a LOD of 8, 10, and 1 pg mL
-1 for GPBB, CK -MB and cTnT, respectively, showing high accuracy even in the detection of clinical samples of human serum. The results were further validated by a laboratory reference method, Siemens Centaur XPT Immunoassay System. Additionally, a predictive model was created to estimate the unknown concentration of cardiac biomarkers in serum samples and eliminate the standard calibration curves. A good fit of the predicted and experimental data was obtained.
Combining plasmonic substrates with magnetic nanoparticles has brought new advantages in SERS detection of cardiac biomarkers, enabling an increased sensitivity and a simple analysis procedure after magnetic separation. For example, a fast and sensitive SERS-based competitive immunoassay for the simultaneous detection of cTnI and CK-MB was demonstrated by Choo et al. [
59]. Their method involves the use of magnetic beads functionalized with monoclonal antibodies for cTnI and CK-MB as capture agents and two different SERS nanoprobes based on hollow gold nanospheres conjugated with Raman reporters and cTnI and CK-MB antigens as SERS sensitive platforms for cTnI and CK-MB recognition. The proposed detection strategy involves the competitive interaction of free target antigens and antigen-conjugated SERS nanoprobes with monoclonal antibodies on magnetic beads, followed by magnetic separation and SERS analysis of the supernatant. The results showed that the developed SERS-based competitive immunoassay achieves a 100 to 1000-fold increase in sensitivity compared to electro-chemiluminescent assay, yielding a LOD of 42.5 pg mL
-1 and 33.7 pg mL
-1 for CK-MB and cTnI, respectively. The agreement of the two analytical methods was validated with Bland–Altman analysis and Passing–Bablok regression analysis. Remarkably, the fabricated SERS-based competitive immunoassay enables simultaneous quantitative detection of cTnI and CK-MB in patient serum at a single excitation wavelength.
Another study uses metal-organic frameworks (MOFs)@Au Tetrapods (AuTPs) immobilized toluidine blue as the SERS tag, and Au nanoparticles functionalized CoFe
2O
4 magnetic nanospheres (CoFe
2O
4@AuNPs) as the purification and signal amplification agents to develop a highly efficient SERS-based sandwich immunosensor for ultrasensitive detection of N-terminal pro-brain natriuretic peptide (NT-proBNP) [
60]. The fabricated SERS immunosensor enables specific capture of NT-proBNP
via antigen-antibody immunoreaction, yielding a wide linear range for NT-proBNP quantification from 1 fg mL
-1 to 1 ng mL
-1 and a LOD of 0.75 fg mL
-1. Good recovery factors, in the range of 90.66% to 105.1% were obtained in human real serum samples, confirming the accuracy of the method. However, the results remain to be verified through parallel analysis with a standard commercially available technique.
Recently, Zheng et al. also employed CoFe
2O
4@AuNPs conjugated with specific antibodies to ensure the magnetic purification of the analytes and improve the sensitivity of their microfluidic immunosensor developed for specific SERS detection of brain natriuretic peptide (BNP) cardiac biomarker [
61]. Apart from CoFe2O4@AuNPs, a SERS substrate consisting of metal–organic framework and Au-HEPES coupling nanoparticles (AuHPs) conjugated with toluidine blue and antibody against BNP was also introduced to further enhance the Raman signal of the sensor. Specific quantitative detection of BNP was performed by measuring the SERS signal with a portable Raman spectrometer based on a sandwich approach. The proposed immunosensor exhibited high stability, portability and ultrasensitivity, yielding a LOD at a level of pg mL
-1. However, the performance of the immunoassay in clinical samples was not reported in the study. A highly sensitive SERS-based immunoassay for selective quantitative detection of cTnI has been demonstrated by Fu and co-authors [
62]. The fabricated immunosensor is composed on antibody/Raman reporter conjugated AuNP–functionalized graphene oxide as the SERS nanotags and signal amplification elements, and antibody modified magnetic beads as the capture probe and separation agents. By exploiting the high SERS performance of graphene oxide/AuNPs and strong binding chance between cTnI and the GO/AuNP an ultrasensitive analysis of cTnI is achieved with the LOD of 5 pg mL
−1 and a linear range of detection from 0.01 to 1000 ng mL
−1. The suitability of the designed SERS immunoassay for practical applications was also demonstrated by the determination of TnI in serum substitute media. However, the results remain to be confirmed in clinical samples and compared with those obtained with a standard technique.
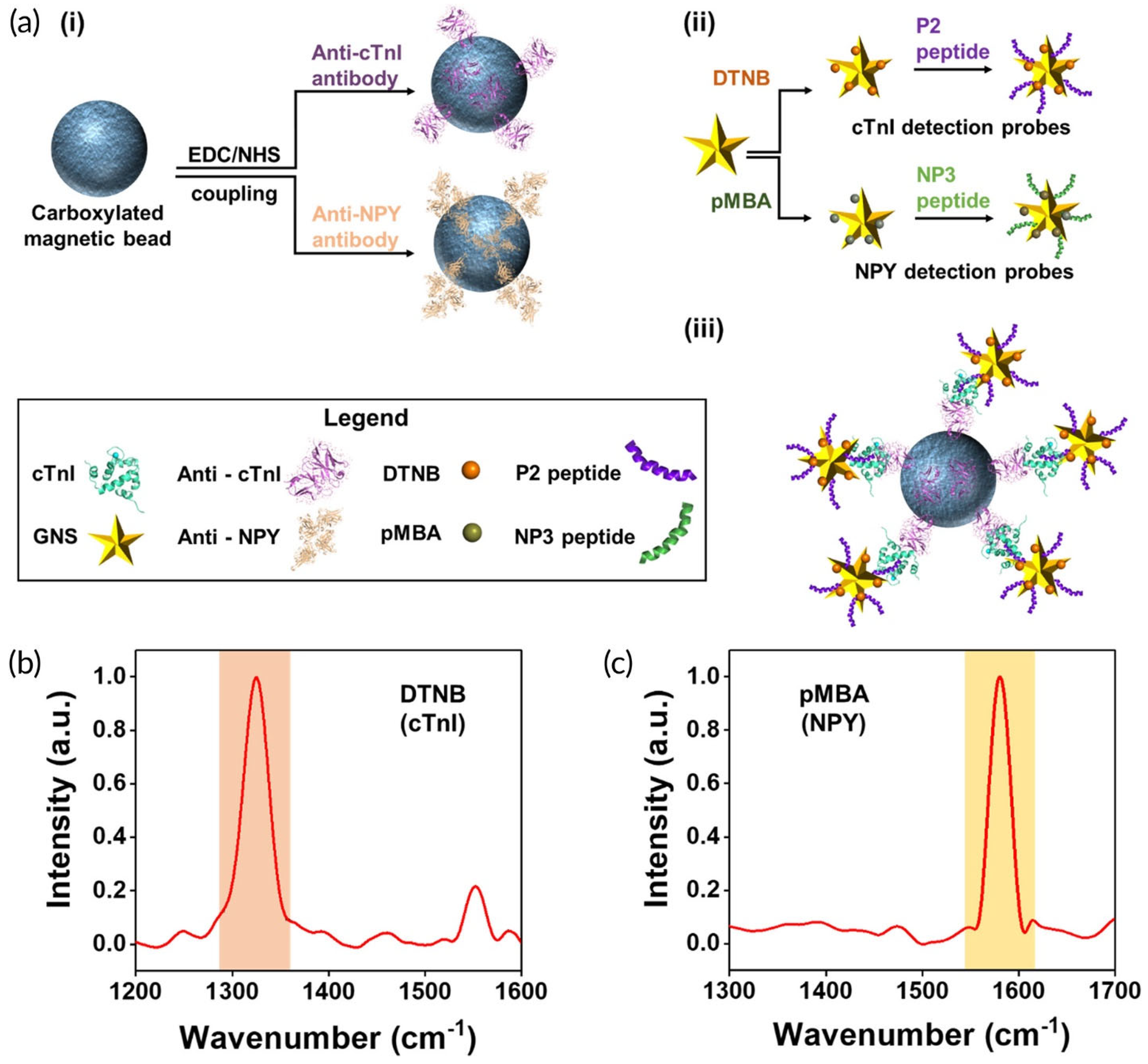
Wen et al. have demonstrated an innovative, portable reusable accurate diagnostics (PRADA) SERS-based immunoassay for simultaneous quantitative detection of troponin I (cTnI) and neuropeptide Y (NPY) in a microfluidic platform [
63].
As illustrated in
Figure 4a, antibody-conjugated magnetic beads were used as the capture platform, while SERS nanotags based on gold nanostars labeled with two different Raman reporters and peptide biorecognition elements were introduced as SERS detection probes. The SERS spectra recorded from the sandwich-type-immunocomplex at 785 nm laser excitation show the distinct features of the two nanotags corresponding to cTnI and NPY (
Figure 4b). Specifically, the SERS intensity of DTNB at 1325 cm
−1 was used for cTnI quantification, while NPY determination was accomplished by monitoring the SERS signal of pMBA at 1580 cm
−1. The PRADA sensing device exhibited high sensitivity, achieving a LOD comparable with the commercially available test kits: 0.0055 ng ml
-1 and 0.12 ng ml
-1 for cTnI NPY, respectively. Moreover, the sensor chip can be regenerated, thus being reusable for multiple detection cycles. Remarkably, the developed PRADA assay showed high accuracy and reproducibility on evaluating cTnI in cardiac patient serum achieving limit of quantification (LOQ) of ≥0.03 ng/ml which is comparable to commercial assay and lower than many troponin immunoassays reported in the literature. Furthermore, the clinical performance of the PRADA assay was validated by parallel measurements with the ABBOTT ARCHITECT chemiluminescence assay system and Passing-Bablok regression analysis.
Sandwich-based immunoassays are constantly developed for SERS quantification of cardiac biomarkers. For instance, a new SERS-based magnetic immunoassay was designed by Hu et al., which combined magnetic beads and Raman reported embedded Au-core Ag-shell nanotags for sensitive and selective determination of cTnI [
64]. In this system, the Raman embedded core-shell nanotags were introduced to increase the stability and sensitivity of the SERS sensor, while magnetic beads ensured the magnetic separation and concentration. Specific recognition of cTnI was accomplished by modifying both SERS tags and magnetic beads with antibodies against cTnI. Once the sandwich-type was formed based on an immune reaction, it was magnetically separated and subjected to SERS analysis using a portable Raman instrument. The developed immunoassay achieved a LOD of 9.80 pg mL
-1. 50 serum samples from AMI patients were analyzed using the SERS assay and the FDA-approved clinical chemiluminescence immunoassay (CLIA) to assess the clinical performance of the proposed sensor and its diagnostic potential. The two approaches had a strong correlation, demonstrating the practical usability of the SERS-based immunoassay. This kind of immunoassay was also demonstrated by the same group of authors for selective, sensitive, accurate simultaneous determination of cTnI and heart type fatty acid-binding protein (H-FABP) [
65]. The obtained LOD was 0.6396 and 0.0044 ng mL
-1 for H-FABP and cTnI, respectively, which is much lower than the clinical cutoff value for the diagnosis of acute myocardial infarction disease. Moreover, the described approach allowed simultaneous determination of H-FABP and cTnI in human serum samples, demonstrating great potential for clinical applicability. High recovery factors in the range of 96.4–110.0% were obtained, indicating the accuracy of the method. However, cTnI and H-FABP were spiked in already diluted serum samples of healthy people. Results remain to be confirmed in undiluted samples collected from AMI patients.
Atomically flat Au nanoplates present great potential as sensing nanoplatforms as Lee et al. exploited such substrates to design an innovative sandwich-based approach for SERS detection of cTnI at the attomolar level [
66]. In this strategy, AuNPs conjugated with a cTnI aptamer modified with a Raman reporter molecule served as a SERS detection probe, while the aptamer conjugated Au nanoplates enabled the specific capture of cTnI. The detection of cTnI was accomplished by measuring the SERS signal of the AuNPs-on-nanoplate architecture formed upon the specific capture of cTnI. By optimizing the immobilization of the aptamer onto Au nanoplate the binding capacity for cTnI was significantly improved. Therefore, the LOD was determined to be 100 aM (2.4 fg mL
-1) in buffer solution and 100 fM (2.4 pg m
-1) in serum samples, respectively, which is much lower than the existing cutoff values. Nine clinical samples from both healthy humans and AMI patients were collected and analyzed in parallel using the developed SERS assay and ELISA. Remarkably, the accuracy of the proposed strategy for cTnI detection is higher than that of the conventional ELISA.
A microcavity-based sandwich immunosensor was designed by Wang et al., who combined the light confinement effect of polystyrene (PS) microcavities and the localized surface plasmon resonance (LSPR) properties of AuNPs to achieve simultaneous ultrasensitive SERS quantification of cTnI and CK-MB [
67]. The sensor is composed of PS microspheres modified with AuNPs deposited on a silicon wafer as a capture substrate, and Raman-encoded AuNPs as SERS signal probes, respectively. For recognition of cTnI and CK-MB, both SERS tags and the capture substrate were modified with antibodies specific to cTnI and CK-MB. Sensitive and selective detection of cTnI and CK-MB was accomplished by SERS measurement based on a sandwich strategy, reaching a LOD of 3.16 pg mL
-1 and 4.27 pg mL
-1 for cTnI and CKMB, respectively. The performance of the method was evaluated in whole blood samples. The obtained recovery factors of cTnI and CK-MB ranged from 94.9 % to 121.6 % while the average coefficient of variance (CV (%)) between replicates was below 15 %, all of these indicating a good accuracy, reproducibility and stability of the developed SERS immunoassay.
In summary, the recent studies concerning SERS-based detection of cardiac biomarkers were focused on sensitivity, selectivity, accuracy, rapidity and portability, with most sensors achieving comparable or superior performance to existing analytical methods in terms of sensitivity and rapidity. While the above described detection approaches picture a rich toolbox available to a scientist wishing to develop new methods for CVD biomarkers detection, many studies are at a preliminary level. Further work is necessary to bring these sensors closer to application, to characterize and validate them fully and to apply them on larger sets of clinical samples in order to prove their utility. Increased application of chemometrics and the use of machine learning/ AI to interpret the complex SERS spectra and extract the specific information pertaining to CVD biomarkers can substantially and rapidly improve the performance of SERS-based detection methods. Aptamers and MIPs appear still underrepresented among the specific receptors used in cardiac biosensors based on SERS. Considering the effervescent research in the field of aptasensors and MIPs for cardiac biomarkers with improved stability and high specificity and reproducibility compared to antibodies, it is anticipated the number of detection strategies based on the combination of these receptors with SERS will significantly grow in the future.
3.3. Fluorescence Based Biosensors
Fluorescence is a preferred detection mode in biomarker analysis, enabling high sensitivity and multiplexing as probes labeled with various fluorescent dyes or nanomaterials become increasingly available and diversified. Examples of fluorescent probes employed in CVDs biomarker detection include fluorochrome dyes and nanomaterials such as lanthanide-doped upconversion nanoparticles (UCNPs) [
68], quantum dots (QDs) [
69] , carbon dots (CDs) [
70], europium chelate-contained silica nanoparticle (EuSiNP) [
71], metal organic frameworks, MOFs, [
72] etc. These probes were linked to antibodies [
68], aptamers [
73] or molecular imprinted polymers (MIPs) [
74].
Fluorophore-marked bioreceptors were used in both homogenous and heterogeneous systems (
Table 1), selectively binding the analyte and thus enabling to correlate its concentration with the fluorescence signal.
Among the various types of tests for the fluorescence -based detection of CVDs, lateral flow assays (LFAs) were a preferred choice and appear particularly promising and when designing as they facilitate simple, rapid, low cost, portable and user-friendly tests [
68].
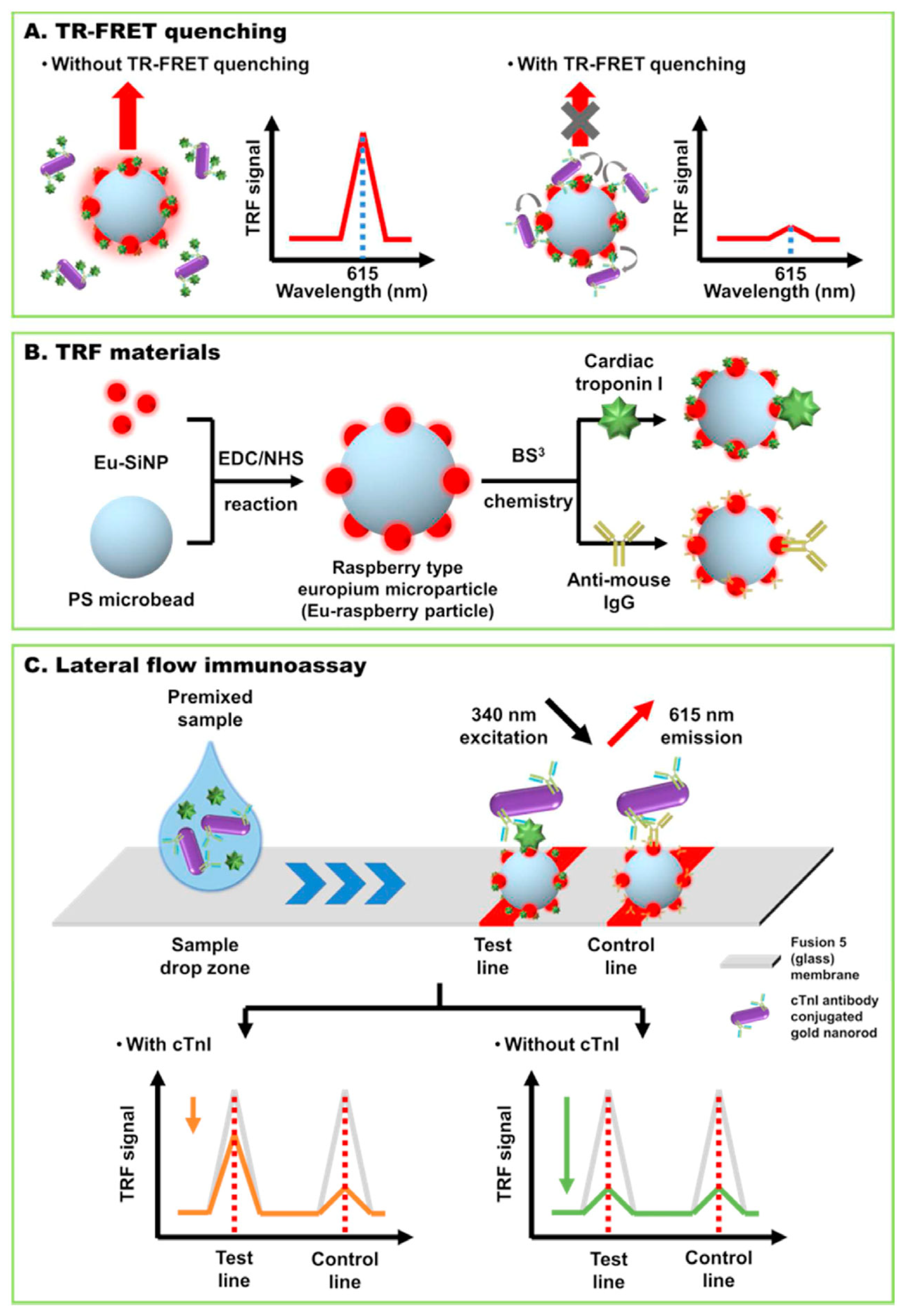
In one example, troponin I detection was achieved by time-resolved fluorescence resonance energy transfer (TR-FRET) using raspberry-type polystyrene microparticles coated with europium chelate-modified silica nanoparticles (EuSiNP) as donor and gold nanorods (GNR) as fluorescence acceptor [
71]. The use of a fusion 5 membrane (a proprietary, commercially available porous and hydrophilic membrane) enabled a simplified lateral flow assay system lacking the sample, conjugate and absorbent pads needed with conventional strips made of nitrocellulose. The test was conceived as a competitive immunoassay whose sensitivity was enhanced thanks to TRF measurements, enabling to remove the background fluorescence by taking advantage also of the long fluorescence decay time of lanthanides. The lateral flow strip has a test line consisting of cTnI conjugated Eu-raspberry particle and a control line with immobilized anti-mouse-antibody-conjugated Eu-raspberry particle (
Figure 5). The quencher particles, GNR conjugated with a specific antibody for cTnI Ab are first mixed with the sample. When placed on the designed sample zone of the strip they advance through capillary force towards the test and control lines. In the absence of cTnI in the sample, the GNR-cTnIAb particles bind to the cTni-EuSiNP particles in the test line, drastically decreasing their fluorescence signal. The excess quencher particles are captured at the control line. In the presence of cTnI, the GNR-cTnI Ab bind to the biomarker in the sample instead of the cTnI at the test line, thus the quenching of the fluorescence signal at the test line is reduced (i.e, the fluorescence is higher). The competitive test was conducted both in buffer and serum samples, featuring LODs of 24 pg/mL and 97 pg/mL, respectively. This LFIA system was compared with standard cTnI ELISA assay and showed good accuracy.
The traditional bench-top detection equipment for fluorescence measurements is bulky and expensive. Portable readers facilitate the adoption of fluorescence methods by users and fully exploite the potential of LFIA systems for POC measurements. A POC platform including a portable detection module and a sample processing module (LFA strip) relied on upconversion nanoparticles (UCNPs) for myoglobin detection [
68]. The UCNPs were NaYF4:Yb,Er@NaLuF4 core-shell nanoparticles and the specificity of the detection was ensured by a classic sandwich immunoassay. The ratiometric approach, where the concentration of myoglobin was proportional to the fluorescence intensity ratio of test and control (T/C ) lines helped minimizing the sensitivity of the assay to possible deviations between different strips. A 10-minute period was optimum for the immune-reaction to take place so the T/C ratio reached saturation. The test is rapid, the time per assay being three times shorter than for the standard method. Interestingly, different types of plasma sample (hemolysis, high-bilirubin, high-lipids) were analyzed to test potential interfering effects. Based on recoveries of 89.0-110.5% from spiked samples and CVs of less than 10% the authors concluded that the type of sample had little effect on the test result. The results obtained with this LFIA method were compared to the Abbott Chemiluminescence assay, typically used in the clinical practice, showing great consistence between them (i.e., coefficient of determination of 0.95, slope of the linear regression =0.92). Intra and inter-assays performed with LFIA were characterized by coefficients of variation (CVs) under 14 %. showing the good precision of the proposed sensor strip.
Molecularly imprinted polymers are increasingly researched as antibody replacers in the aim to decrease costs and improve the stability and reproducibility of bioanalytical testing devices. Moreover, significant advances have been made with regards to such “plastic antibodies” with high affinity and specificity for high molecular weight molecules including protein biomarkers. Due to their large surface displaying a variety of functional groups developing MIPs with cavities complementary to such large target molecules is a very difficult task.
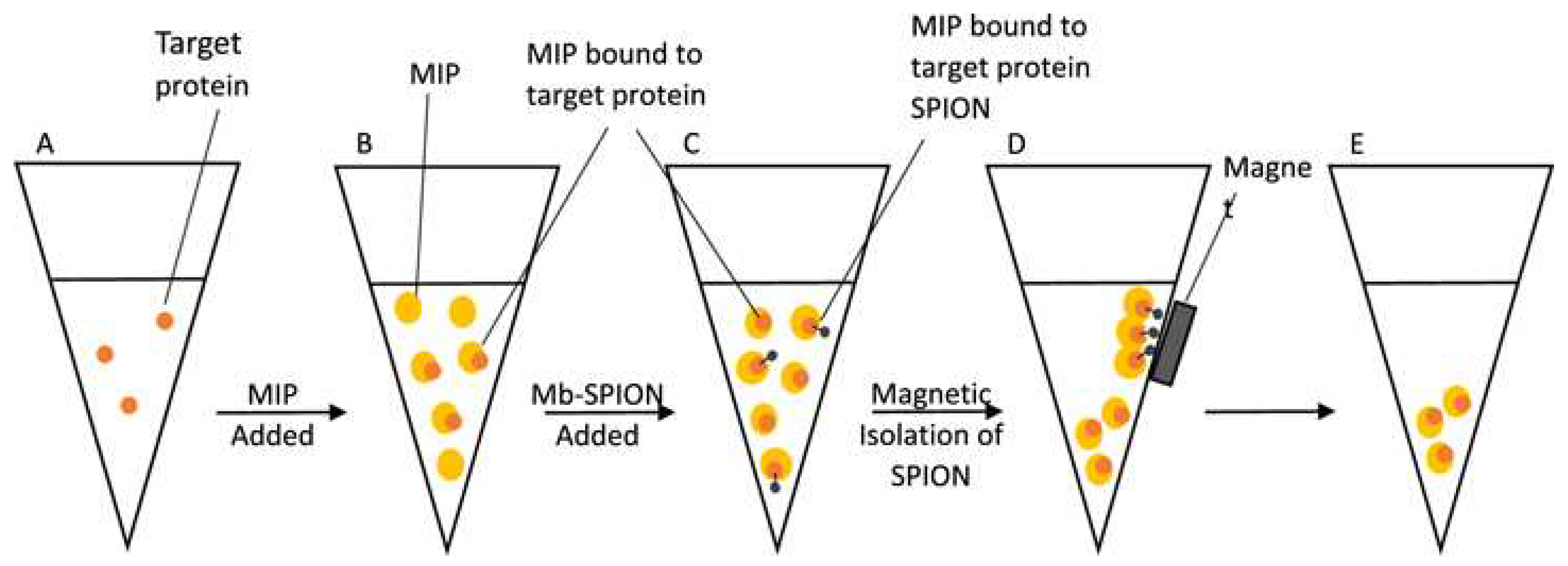
A MIP-based fluorimetric assay was developed to detect myoglobin in biological samples [
74]. The MIP was obtained from fluorescein
O-acrylate and was used to capture myoglobin from test samples in a homogeneous assay. To ensure that the measured fluorescence signal was due exclusively to the myoglobin- bound MIP, supermagnetic iron oxide nanoparticles (SPIONs) functionalized with myoglobin were added to the sample vial, after the MIP-sample binding reached saturation (5-10 minutes). The SPION particles were used to remove by using a magnet the excess, unbound MIP so that only the target protein-bound MIPs was left in suspension in the vial and the fluorescence signal was proportional to the quantity of myoglobin in the sample (
Figure 6). Noteworthy, the magnetic nanoparticles were obtained by a “green” synthesis method. The logarithm of the fluorescence intensity varied linearly with the logarithm of protein concentration in the range from 60 pg/mL to 6 mg/mL. The imprinting factor of the MIP is 1.95 and the non-specific binding, evaluated with BSA, amounted to 42.4%, about half of the response for the same concentration of myoglobin (3mg/mL). In the same time, when analyzing spiked fetal calf serum samples spiked with myoglobin, the average recovery was 93 %, indicating that the technique has adequate accuracy for myoglobin detection in biological samples. Together this data shows a good potential for analyzing clinical samples but also the necessity to include control tests with non-imprinted polymer (NIP) particles in order to account for the non-specific effects. A wider interference study is necessary to prove the selectivity of the assay. By improving the imprinting factor and preventing non-specific adsorption effects, the performances of the assay can be enhanced further.
An alternative to starting from a fluorophore-labeled monomer for obtaining the MIP is to label the MIP with fluorescence producing probes. e.g., with quantum dots ( QD, [
80]). A MIP-imprinted hydroxyethylcellulose membrane where the MIP was tagged with CdTe QD was used for the detection of myoglobin starting from 7.39 pg/mL (the lower limit of the linear range). The imprinted strip had a storage stability of (at least) 15 days. The detection was achieved in human serum that was diluted 1000 times with buffer. The interference study has shown that cardiac troponin T, creatinine, and human serum albumin do not significantly affect the sensor’s response when tested at concentrations ten times higher than the level of myoglobin. Nonetheless, some proteins, e.g., human serum albumin in present in much higher excess compared to myoglobin. Testing a “standard” panel of potentially interfering compounds at ratios reflecting those typical for clinical samples would be a big step forward to enable an objective evaluation of this and all other various sensing concepts proposed.
The challenges associated with measurements in complex samples such as serum are not trivial, as proven in another report on the detection of myoglobin [
81]. The test relies on the conjugation of a dabcyl-labeled aptamer with a FAM-labeled partially complementary DNA sequence, resulting in the quenching of the FAM fluorescence signal in the absence of myoglobin and its specific recovery in the presence of myoglobin. The authors noted that proteins that are present in serum in high concentration, such as human serum albumin, present at 35-50 mg/mL, induce a high fluorescence background. For accurate measurements of cTnI the sample pretreatment consisted in diluting the serum by a factor of 10, and further purification by filtration through a 30 kDa cutoff centrifuge filter.
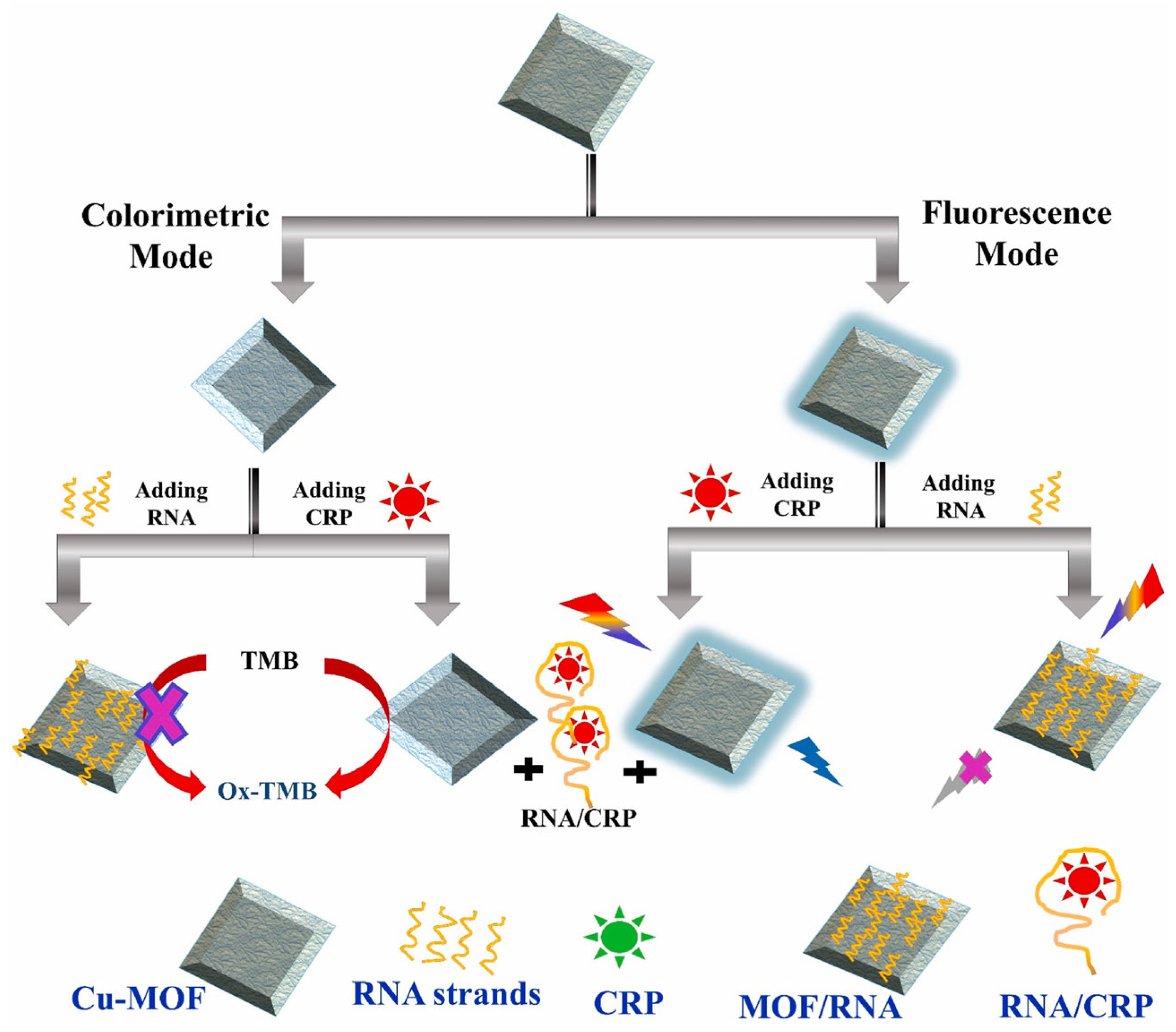
In the recent period the trends of developing multiplexed assays and assays based on dual detection modes continued. With regards to the latter, an illustrative example is a biosensor for the measurement of CRP by both colorimetry and fluorescence [
72]. At the core of the assay stands a Cu-MOF material coated with an RNA aptamer specific for CRP. The Cu-MOF has peroxidase like activity, functioning as catalyst for the classic reaction of TMB and H
2O
2 resulting in the blue colored compound measurable by colorimetry. The Cu-MOF also presents “stimulated fluorescence”, i.e., is not a fluorescent material per se but it is converted into one, following its reaction with H
2O
2; then, if excited at 320 nm it will emit at 410 nm. The catalytic activity and the stimulated fluorescence properties of the Cu-MOF are both inhibited when the material is coated with the aptamer and recovered when the aptamer is desorbed following its interaction with the CRP in the sample. The approach enables to have two reliable results (by fluorescence and colorimetry) with one platform (
Figure 7). The size of Cu-MOF particles is in the sub-micrometer to micrometer range as characterized by FESEM. A selectivity study was conducted for both detection techniques and concluded that the signal for CRP was high and selective when compared to several compounds tested with 100 times more concentrated solutions than CRP. Nonetheless, in real serum fluid the quantity of some biomolecules such as serum albumin are more elevated than the tested amounts. Further investigations are thus necessary and they need to include other cardiac biomarkers as potential interferents in order to definitively prove that the analytical system is adequate as a diagnosis tool. When the method was applied on spiked diluted serum, the recovery percentages were 84-102 %, showing good accuracy for real sample testing. Unfortunately, the analysis of a set of clinical samples and the comparison with a standard method toward comparing the obtained data was not addressed and remains as a next step for advancing the proposed concept.
The multiplexed detection of 8 biomarkers with a single strip was demonstrated by Huang et al [
79], which combined the analysis of Myo, CK-MB, and cTnI by a fluorescence sandwich immunoassay with the determination of cholesterol (TC), triglyceride (TG), high density lipoprotein cholesterol (HDL-C) and uric acid by dry chemistry. Additionally, the content of low-density lipoprotein cholesterol (LDL-C) in the sample was derived by calculation. The strip was intended as a diagnostic tool for acute myocardial infarction (AMI). Towards this goal, the selectivity for cTnI detection was first proven by comparing the signals for cTnI (50 ng mL
−1) with those for CK-MB (500 ng mL
−1), Myo (500 ng mL
−1), TC (50 mmol L
−1), TG(50 mmol L
−1), UA (50 mmol L
−1), and HDL-C (50 mmol L
−1) . The analytical performances of the strip feature among others detection limits for Myo, CK-MB and cTnI of
10 pg /mL, 2pg/mL and 1 pg/mL, respectively. Following detailed characterization, the sensor strip was used to analyze a set of serum samples collected from AMI patients and the results were compared with the current clinical methods based on chemiluminescence immunoassay (CLIA). The good correlation between the two sets of results stands as evidence of the applicability and usefulness of the proposed 8-in 1 test.
In the search to enhance the sensitivity of the measurements and reduce the time per assay, new materials are critical. CdSe/ZnS quantum dots of 14 nm medium size were assembled into nanobeads by encapsulation in CTAB and were subsequently coated with SiO
2 and polyvinylpyrrolidone in order to preserve their luminescence properties in various environmental conditions [
69]. The coated beads (QBs@SiO2-COOH )with an average diameter of 235 nm displayed an enhancement of 1967 times in luminescence and a remarkable stability in complex samples and at different pH and temperatures [
69]. The principle of the test is that classic for a LFIA, where the ratio between the fluorescence signal at the test and control lines are proportional to the biomarker concentration in the sample (
Figure 8).
Nonetheless, the strong luminescence and optimized ratio between the nanobeads and the cTnI antibodies were the key to achieve a detection limit of 0.036 ng mL-1, i.e., about 20 times lower than the smallest concentration detectable by a similar LFIA using SiO2-coated QDs. The stability of the sensor strips was evaluated based on the measurements for four concentrations of cTnI and the results support the stability of the strip stored at either room temperature, 37º C or 45º C for 120 days. By appropriate modification with specific antibodies, the sensor was used for the simultaneous measurement of CK MB, Myo and cTnI. Remarkably, a set of 38 human serum samples were analyzed in parallel with the proposed QBs@SiO2-COOH-based LFIA and by ELISA and it was determined that the concentrations of cTnI, CK-MB and Myo measured by the two methods were linearly correlated, with slopes of the fitting lines very close to 1 and correlation coefficients R =0.980-0.991. The assay time with this strip was 10 minutes and the reproducibility of the test was adequate, i.e., the CV of intraassay and inter-assay tested at 3 concentration levels of cTnI were below 8 %. Noteworthy, a large set of 103 clinical serum samples were analyzed for their level of cTnI in parallel by the LFIA and by a standard chemiluminescence method and the results were in good agreement, supporting the accuracy of the LFIA. This work is a very nice illustration of a detailed, very informative research reporting on all of stability characteristics, analytical performances, feasibility for testing clinical samples and comparison with standard methods. The benefits of the new nanobeads material are clear as they are quantitatively evaluated in comparison with the starting, simple QDs (in terms of luminescence intensity and detection limit achieved for cTnI). This work serves as a model for studies aiming to close the gap between research laboratory and clinical practice.
In summary, fluorescence enables a high flexibility in designing the detection approach due to the variety of fluorochrome probes and dyes with unique properties. Fast simplified POC detection enabled by portable readers combined with LFA appear as the main avenue of research and vector of progress towards commercially available devices. Some of the recent studies focus on new materials and specific receptors for achieving a large linear range and great sensitivity. The search for ultrasensitivity of detection was prompted in the recent works by the desire to depart from the traditional approaches based on invasive blood testing towards patient-friendlier procedures, e.g testing of saliva [
70] or analysis from very low volumes of blood. Thus, efficient signal amplification was ensured e.g by deoxyribonuclease I-aided target recycling (Chen, RSC Advances 2019) and in some of these works issues like selectivity, real sample testing and verifying the accuracy with standard assays remain to be addressed. A glimpse at the data in
Table 1 emphasizes that in many cases the verification of accuracy was limited to spiking and recovery studies. Not all concepts were verified with clinical samples and compared to standard or current methods in clinical laboratories. While the main reason for switching from antibodies to MIP is to gain in stability, this aspect was rarely investigated in detail, for long time periods. Despite the progress in the biomarkers analysis, there are significant challenges related to the complex composition of the serum samples that new assays relying on new materials and recognition mechanism must overcome. Works reporting validation tests and the analysis of large sets of clinical samples, multiplexed detection for analyzing specific CVDs and combining different detection modes in the same analytical platform converge with studies on new materials and hint to a promising future of fluorescence-based tests for the CVD biomarkers.
3.5. Colorimetry-Based Biosensors
Colorimetry is a simple optical method which measures the color change when modifications occur as the result of a reaction in solution [
85] or on a surface ( e.g lateral-flow assay. Colorimetry is very promising in developing point-of-care (POC) testing because it can be rapid, cheap and can be carried out by unskilled personnel.
Consequently, several studies in the recent years used this detection method for the analysis of CVD biomarkers. Data summarized in
Table 2 shows a variety of colorimetry-based approaches aimed at sensitive and accurate detection meeting the clinical cutoff for specific CVD biomarkers in serum samples.
Paper based, microfluidic chips or solution-based assays were reported (
Table 2). Most analytical approaches are derived from sandwich type immunoassays by replacing enzymes with nanozymes [
72,
93,
97] and DNAzymes [
96]. This corresponds to a growing trend compared to the previous period, as is the case also with the development of aptamer based assays, which increased in the context of continuing efforts to select new specific sequences with high affinity for CVD biomarkers (e.g for cTnT, [
95]. Oligonucleotides enable additional sensing strategies compared to antibodies including new approaches for signal generation and amplification e.g. supersandwiches made by DNA hybridization [
85], DNAzymes [
96] and Exo -I assisted amplification [
97]. Increased stability is the major goal driving these changes from enzymes and antibodies to nanozymes/DNAzymes and aptamers, respectively. From this perspective, the lack of stability data of these new materials and sensors is intriguing and disappointing.
The time per assay ranged from 1.5-20 minutes [86,90-92] indicating potential for POC testing) to more than several hours (
Table 2). Undiluted serum was used in several LFIA [
92] and homogeneous assays, however in general, dilution with buffer was found to be an adequate procedure to bring the sample concentration in the linear range of the method and minimize interferences.
Screening several CVD biomarkers simultaneously is time-saving and helps establishing the type of CVD. Ozen et al [
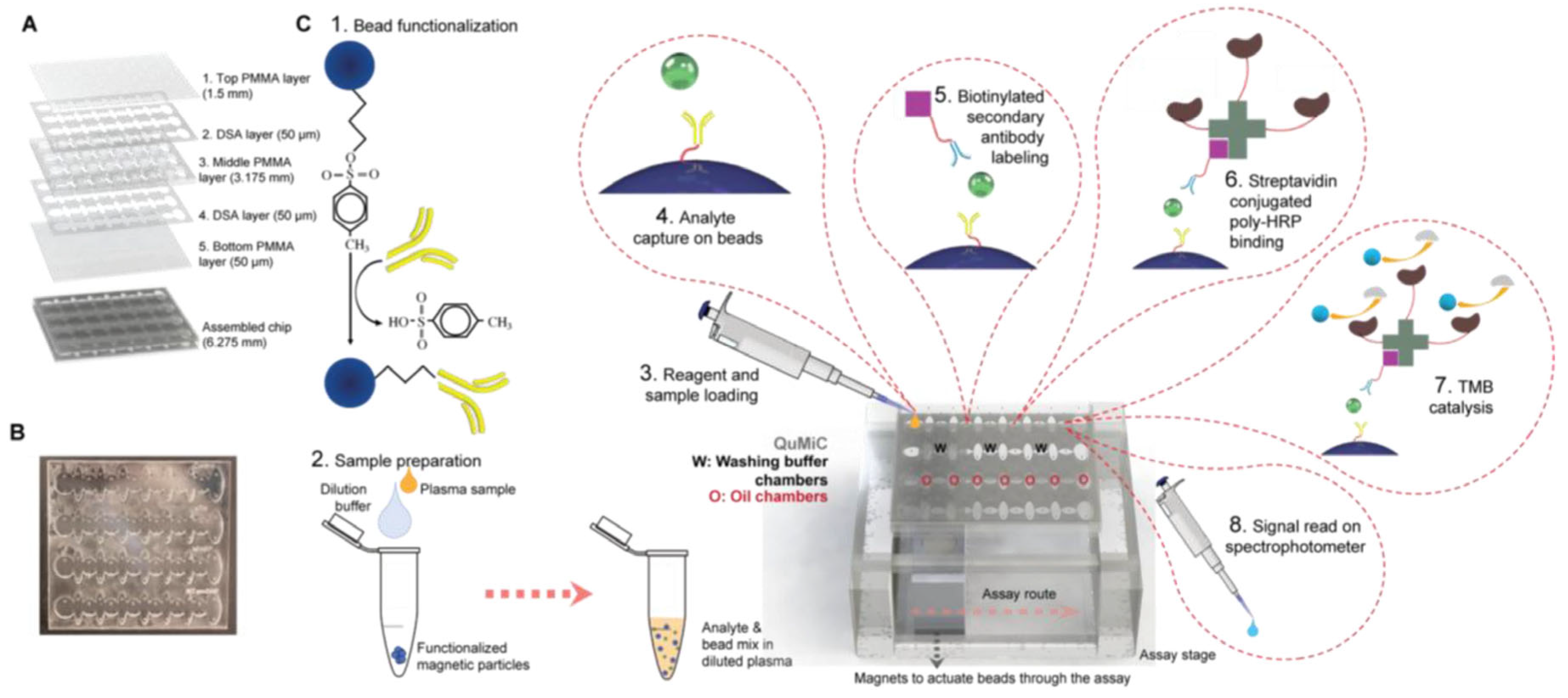
90]. developed a Total Microfluidics platform for Multiplexed diagnostics (ToMMx) for detection of cardiac troponin-I (cTnI), heart-type fatty acid binding protein (hFABP) and N-terminal pro-brain natriuretic peptide (NT-proBNP) (
Figure 9). The assay included similar steps as ELISA, with modifications adapted for ToMMx. The platform is filled iteratively with mineral oil, washing buffer and water-based reagents with the help of surface tension differences so these components did not mix. They were preloaded before sample processing. The magnetic beads employed in the assay are actuated using a magnet. The analytes are specifically captured between Ab immobilized on the beads and a biotinylated secondary Ab, then a complex is formed when adding streptavidin- conjugated poly-HRP. The analytes’ concentration is evaluated by color changes when TMB s added as substrate whose oxidation is catalyzed by HRP. A set of clinical samples was analyzed in parallel by the platform and by standard ELISA and the results have shown that the proposed method is precise and accurate. The sample set included 38 pacient samples corresponding to different type of CVDs and 12 control samples. Using the platform’s results the pacients were diagnosed with an accuracy of 91 % for acute coronary syndrome (via cTnI and hFABP) and 95% for severe symptomatic aortic stenosis (via NT-proBNP), respectively. When NT-proBNP was used as a diagnostic biomarker, its detection with this analytical platform led to the identification of patients suffering from dilated cardiomyopathy with 100 % accuracy.
Even if these results are very promising, a wide selectivity study is mandatory in order to validate the method. The total time of the assay is less than 20 minutes, that is 15-fold reduced compared with ELISA. In the future, the authors predicted that by integrating ToMMx platform with portable detection systems such as smartphones and by mass production of the assay kit this multiplex method can become easy and cheap, accessible for everyone. (Total Microfluidic chip for Multiplexed diagnostics (ToMMx) )
With the same aim of simplified assays for CVD biomarker detection, Wang et al [
96] developed an ingenious instrument-free detection method for cTnI where the length of a “coffee ring” type colored band, developed on a microfluidic paper device -simply measurable with a ruler- was proportional with the concentration of the cTnI in the sample. The making of this microfluidic device involved a pair of antibodies (for the sandwich-type sensing) as well as oligonucleotides (to form a DNAzyme) and other reagents: hemin (for the DNAzyme), iodide and H
2O
2 (DNAzyme substrates) and starch (for the blue color development by reaction with iodine). The work nicely illustrates the versatility of DNA hybridization which in this case served to assemble the DNAzyme. Specifically, the detection antibodies were labeled with oligonucleotides that hybridized to complementary DNA to form a G quadruplex. In the presence of hemin this acts as a peroxidase mimic catalyzing the oxidation of iodide to iodine.
In another report, DNA hybridization was used to (i) attach the aptamer to a capture probe fixed on magnetic beads, (ii) assemble the enzyme-labeled signaling probe [
85] and (iii) bind the signaling probe to the aptamer. The quantitative test for myoglobin was performed in a test tube and an optical amplifier was used as readout instead of a spectrophotometer to simplify the equipment needed for the quantitative detection. Developed as a turnoff assay, the measurement relied on the proportionality between the decreased amount of HRP-labeled probe hybridized to the aptamer (and consequently, lower color intensity following the enzymatic reaction) and the quantity of myoglobin in the sample, bound by the aptamer. This test required almost 3 hours from which the longest step (90 minutes) was the attachment of the DNA-based, HRP- labeled “supersandwich” signaling probe. Consequently, the time per assay remains an aspect to be significantly improved in the future.
New aptamers were recently selected for cTnI [
95], targeting different epitopes of the biomarker and with affinities of KD = 122 ± 14 nM (Apt.1) and 190 ± 20 nM (Apt2). These were used to developed an Enzyme Linked Oligonucleotide Assay (ELONA). The work emphasized one important advantage of the sandwich over the direct detection format, namely it enabled to mitigate the matrix effects and observe the same sensitivity when analyzing cTnI in undiluted serum as in the buffer solution.
Aiming to replace enzymatic labels to increase the stability and reduce costs, while allowing for multiplexed colorimetric sensing, Pu et al [
87] reported the use of phenolphthalein, methyl red, bromothymol blue dyes as labels enabling the specific, simultaneous immunoassay of 3 biomarkers for AMI (NT-proBNP, CK-MB and cTnT). The dyes with pH dependent color were loaded on Au nanovesicles and their loading/release was temperature- controlled. The nanovesicles were functionalized with specific antibodies and the assay was performed similarly to a classic ELISA. A comparative analysis of a set of serum samples via the proposed test and by a standard immunofluorescence assay typically used in a clinical laboratory indicated similar results. The new assay has the benefits of higher sensitivity and wider linear range.
Xie et al [
94] described an antibody free, ELISA like assay for CRP where the biomarker was sandwiched between a conjugate of citicoline and BSA as capture probe and an aptamer as detection probe. The aptamer was labeled with AuNPs acting as HRP-mimicking nanozyme while TMB was used as nanozyme’s substrate. Remarkably, the reproducibility of the materials used in the test, i.e. the citicoline-BSA conjugate and the AuNPs labeled aptamer was assessed by performing the analysis of a serum sample with 5 lots. Low, acceptable variations between batches of materials were found as indicated by the CV of 7.11% for the CRP concentration in the sample. The accuracy of the test was demonstrated by the similarity of results obtained by the proposed assay and classic ELISA, performed in parallel.
While noble metals were increasingly used as colorimetric probes and as nanozyme labels the kit developers authors struggled to minimize the amounts of these expensive and rare materials [
93]. For example, Panferov et al [
93] integrated trimetallic nanoparticles made from Au, Ag and Pt into a lateral flow immunoassay (LFIA) for C-reactive protein (CRP) where Pt atoms were dispersed on the surface of nanoparticles rather than forming a full coating. With this nanozyme, the detection limit was improved to 15 pg/ml CRP, i.e., 65 times compared to using AuNPs alone while the ratio material/catalytic performance was minimized. It is also important to mention the same authors’ preoccupation towards less invasive approaches as besides testing serum sample, the researchers did some preliminary studies with capillary blood. While acknowledging that the background interference found for some samples impose the necessity of sample pre-treatment, the problem might find an easy solution in the future and the use of capillary blood appears as an avenue worth investigating. In view of the adoption as a POC device, the assay will benefit from further simplification to eliminate the need that the enzymatic substrate, 3,3, diaminobenzidine and the H
2O
2 be added separately. Nonetheless, the measurements with the described LFIA were performed in less than 10 minutes.
A very recent report combining aptasensing with nanozymes for the detection of cTnI [
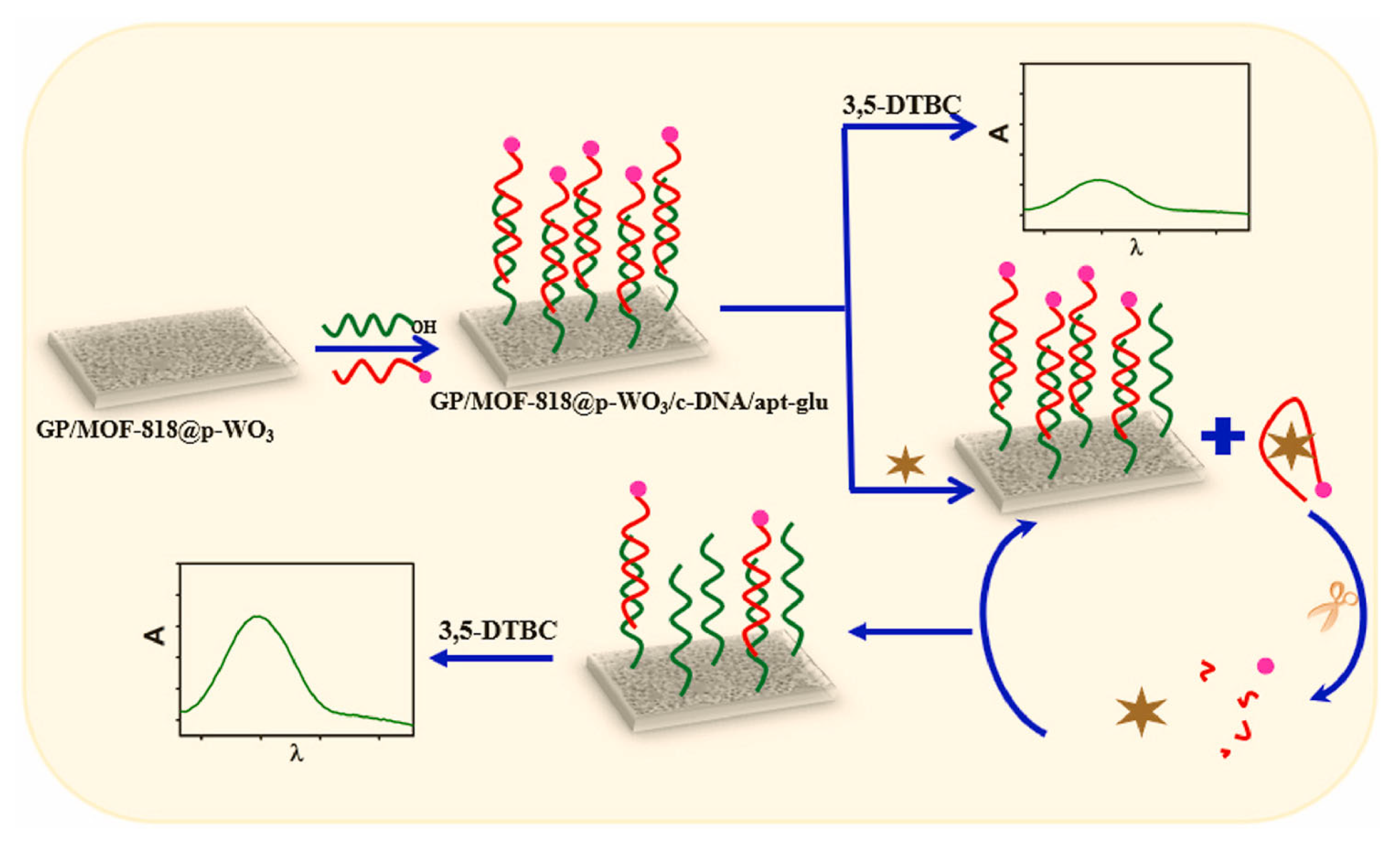
97] included several innovative aspects. For increased efficiency, the nanozyme catalyzed reaction was confined into pores of tungsten trioxide (p-WO3) serving as reactors. The color-producing signal readout was based on “enzymatic” inhibition and a less usual metallic organic framework nanozyme was employed, MOF-818 with catechol oxidase-like activity. The performance of the nanozyme towards the oxidation of 3,5-Di-tert-butylcatechol (3,5-DTBC) drastically improved upon confinement into the 400 nm pores of WO
3, showing a Michaelis Menten constant of 1.42 mM, a catalytic yield of 95.2% and a rate constant of 31.47 s
– 1 compared to 2.49 mM, 26% and 9.94 s
− 1, respectively in solution. Moreover, the test integrated a signal amplification step assisted by Exo-I, as another illustration of the variety of configurations and sensing approaches enabled by the use of oligonucleotides.
In more detail, a glass plate was coated with the nanozyme/p-WO
3 material and a DNA sequence, complementary to the cTnI aptamer was covalently bound to this material (
Figure 10). Next, the cTnI aptamer, labeled with glutathione was anchored to the surface by hybridization to the cDNA. In the presence of 3,5-di-tert-butylcatechol (3,5-DTBC), the assembled sensor produced a low absorbance at 425 nm as the substrate’s conversion was limited due to the presence of glutathione, a known inhibitor of catechol oxidase. The sensor was then incubated with the sample containing cTnI and Exo1. The aptamer, having a high affinity towards cTnI desorbed from the sensor surface while the Exo I in solution cut the aptamer and enabled the recovery of cTnI which bind further to the surface, repeating the cycle for an amplified desorption of the aptamer. In the last step the sensor was incubated again with the substrate and since the inhibition due to glutathione was removed following the desorption of the aptamer, the catalytic activity of the nanozyme was recovered. The conversion of the substrate, expressed through the increase in the absorbance at 425 nm, was proportional to the amount of desorbed aptamer and by consequence to the cTnI in the sample. Altogether this sensing configuration enabled to achieve a detection limit of 18 pg/mL cTnI [
97]. While the method presumes 3 steps totaling more than 30 minutes, it was shown that it can be applied to undiluted serum samples with good accuracy (i.e., 95-107% recovery for 4 spiked samples; the analysis of the unspiked healthy serum was in agreement with results of a parallel ELISA test). Moreover, the optical aptasensor can be reused multiple times, the decrease in response after 30 uses being lower than 15% (estimated based on the data presented in [
97].
To summarize, the colorimetry based approaches discussed above for the detection of CVD biomarkers reflect sustained research efforts towards (i) improving the detection sensitivity -via new nanomaterials or image processing methods, (ii) simplifying the equipment (e.g. using a smartphone as a read-out tool or measuring the width of the colored bands using a ruler, using an optical fiber amplifier), (iii) improving the stability (e.g., by replacing enzymes with nanozymes and DNAzymes), (iv) developing multiplexed platforms for the detection of multiple biomarkers relevant for the diagnosis of specific CVDs and (v) developing dual or multimodal detection strategies. New detection mechanisms (e.g using enzymatic inhibition) new labeled probes (e.g. glutathione labeled aptamer) and new aptamers were proposed. In the same time, disappointingly there was no evaluation of the stability and reproducibility of the new materials, with very few exceptions. Some lateral flow strips were evaluated and found to be stable for months at either 4ºC [
98] or at room temperature[
92].
The progress in the field of nanomaterials lead to increasingly more applications in sensing. In particular, major gains were related to nanoimaterials’ role as catalysts (i.e, nanozymes;[
94,
97] in comparison with the more “traditional” use as high-loading capacity carriers for colorimetric probes or recognition molecules [
87] or as signaling probes themselves [
89,
92].
The vast majority of reports describe the analysis of clinical samples and their comparison with current procedures implemented in clinical laboratories (
Table 2). Some works were limited to the analysis of a few samples of spiked serum, indicative of applicative potential and adequate accuracy of the new methods for CBD diagnosis. Nonetheless, many of the studies went further to include the application of the new methods for testing a few up to 50 patient samples [
90]. Particularly remarkable are reports on multiplexed detection of several biomarkers targeting the diagnostic of specific CVD diseases (see the review of [
9] which include data on their diagnostic accuracy [
90] . This trend is encouraging for the future development of the proposed methods into commercial kits and devices.