Submitted:
12 May 2023
Posted:
12 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Material and Methods
2.1. Plant material collection
2.2. Animal, Diets, and Experimental Design
2.3. Animal housing
2.4. Recorded egg quality
2.5. Blood sampling and analyses
2.6. Determination of cytokines
2.7. Determination of oxidative status
2.8. Intestinal Tissue Sampling and Analyses
2.8.1. Short Chain Fatty Acid (SCFA) Analyses
2.9. Histological Morphometry
2.9.1. Sampling and tissue preparation
2.9.2. Morphometry analysis
2.10. Statistical analysis
3. Results and Discussion
3.1. Egg quality
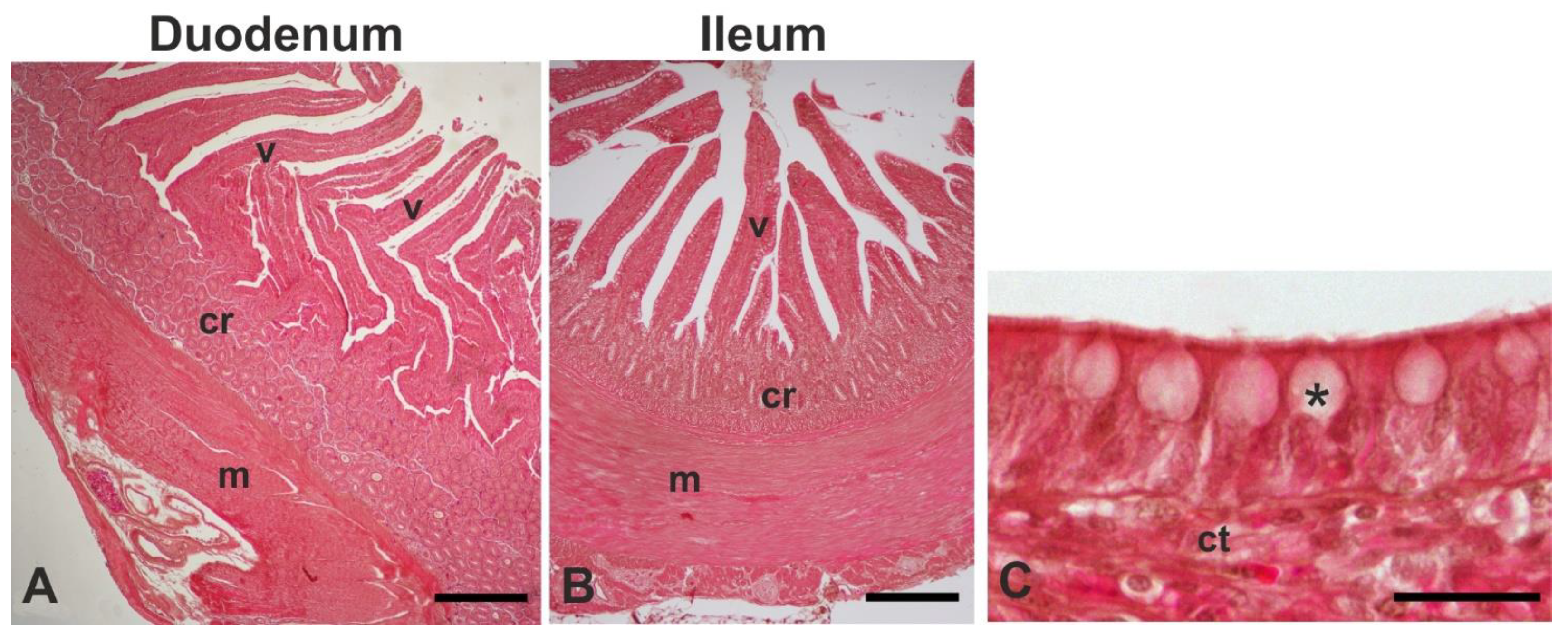
3.2. Histology of the Small Intestine
3.3. Concentration of short chain fatty acids (SCFAs) in caecum contents
3.4. Biochemical parameters
3.5. Oxidative status
3.6. Immunomodulatory effects
3.7. Liver enzymes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdelli, N.; Solà-Oriol, D.; Pérez, J.F. Phytogenic feed additives in poultry: achievements, prospective and challenges. Animals 2021, 11, 3471. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Salem, H.M.; El-Tahan, A.M.; Soliman, M.M.; Youssef, G.B.A.; Taha, A.E-; Soliman, S.M-; Ahmed, A.E-; El-Kott, A.F-; Al Syaad, K.M.; Swelum, A.A. Alternatives to antibiotics for organic poultry production: types, modes of action and impacts on bird's health and production. Poult. Sci. 2022, 101, 101696. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, S.; Banday, M.T.; Shakeel, I.; Adil, S.; Mir, M.S.; Beigh, Y.A.; Amin, U. Histomorphological studies of broiler chicken fed diets supplemented with either raw or enzyme treated dandelion leaves and fenugreek seeds. Vet. World 2016, 9, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Gadde, U.; Kim, W.H; Oh, S.T.; Lillehoj, HS. Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: a review. Anim. Health. Res. Rev. 2017, 18, 26–45. [Google Scholar] [CrossRef] [PubMed]
- Seidavi, A.; Tavakoli, M.; Asroosh, F.; - Scanes, C.G.; Abd El-Hack, M.E.; Naiel, M.A.E.; Taha, A.E.; Aleya, L.; El-Tarabily, K.A.; Swelum, A.A. Antioxidant and antimicrobial activities of phytonutrients as antibiotic substitutes in poultry feed. Environ. Sci. Pollut. Res. Int. 2022, 2, 5006–5031. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Abdel-Moneim, A.E.; Shehata, A.M.; Alzahrani, S.O.; Shafi, M.E.; Mesalam, N.M.; Taha, A.E.; Swelum, A.A.; Arif, M.; Fayyaz, M.; Abd El-Hack, M.E. The role of polyphenols in poultry nutrition. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1851–1866. [Google Scholar] [CrossRef]
- Luo, J.; Song, J.; Liu, L.; Xue, B.; Tian, G.; Yang, Y. Effect of epigallocatechin gallate on growth performance and serum biochemical metabolites in heat-stressed broilers. Poult. Sci. 2018, 97, 599–606. [Google Scholar] [CrossRef]
- Simitzis, P.; Spanou, D.; Glastra, N.; Goliomytis, M. Impact of dietary quercetin on laying hen performance, egg quality and yolk oxidative stability. Anim. Feed. Sci. Technol. 2018, 239, 27–32. [Google Scholar] [CrossRef]
- Surai, P. Polyphenol compounds in the chicken/animal diet: from the past to the future. J. Anim. Physiol. Anim. Nutr. 2014, 98, 19–31. [Google Scholar] [CrossRef]
- Petti, S.; Scully, C. Polyphenols, oral health and disease: A review. J. Dent. 2009, 37, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Galli, G.M.; Da Silva, A.S.; Biazus, A.H.; Reis, J.H.; Boiago, M.M.; Topazio, J.P.; Migliorini, M.J.; Guarda, N.S.; Moresco, R.N.; Ourique, A.F.; Santos, C.G.; Lopes, L.S.; Baldissera, M. D.; Stefani, L.M. Feed addition of curcumin to laying hens showed anticoccidial effect, and improved egg quality and animal health. Res. Vet. Sci. 2018, 118, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Abou-Elkhair, R.; Abdo Basha, H.; Abd El Naby, W.; Ajarem, J.S.; Maodaa, S.N.; Allam, A.A.; Naiel, M.A.E. Effect of a diet supplemented with the Moringa oleifera seed powder on the performance, egg quality, and gene expression in Japanese laying quail under heat stress. Animals 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Oviedo-Rondon, E.O. Holistic view of intestinal health in poultry. Anim. Feed. Sci. Technol. 2019, 250, 1–8. [Google Scholar] [CrossRef]
- Dosoky, WM.; Zeweil, H.S.; Ahmed, M.H.; Zahran, S.M.; Shaalan, M., M.; Abdelsalam, N.R.; Abdel-Moneim, A.E.; Taha. AE.; El-Tarabily, K.A.; Abd El-Hack, M.E. Impacts of onion and cinnamon supplementation as natural additives on the performance, egg quality and immunity in laying Japanese quail. Poult. Sci. 2021, 100. [Google Scholar] [CrossRef] [PubMed]
- Pirman, T.; Rezar, V.; Vrecl, M.; Salobir, J.; Levart, A. Effect of olive leaves or marigold petal extract on oxidative stress, gut fermentative activity, and mucosa morphology in broiler chickens fed a diet rich in n-3 polyunsaturated fats. J. Poult. Sci. 2021, 58, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, R.R.; Omer, A.K.; Yener, Z.; Uyar, A.; Ahmed, A.K. Biomedical effects of Laurus nobilis L. leaf extract on vital organs in streptozotocin-induced diabetic rats: Experimental research. Ann. Med. Surg. 2021, 61, 188–197. [Google Scholar] [CrossRef]
- Delgado Pertinez, M.; Chesson, A.; Provan, G.J.; Garrido, A.; Gomez Cabrera, A. Effect of different drying systems for the conservation of olive leaves on their nutritive value for ruminants. Ann. Zootechn. 1998, 47, 141–150. [Google Scholar] [CrossRef]
- Şahin, S.; Bilgin, M. Olive tree (Olea europaea L.) leaf as a waste by-product of table olive and olive oil industry: a review. J. Sci. Food Agric. 2018, 98, 1271–1279. [Google Scholar] [CrossRef]
- Contreras, M.D.M.; Lama-Muñoz, A.; Espínola, F.; Moya, M.; Romero, I.; Castro, E. Valorization of olive mill leaves through ultrasound-assisted extraction. Food Chem. 2020, 314. [Google Scholar] [CrossRef]
- Bulotta, S.; Corradino, R.; Celano, M.; D’Agostino, M.; Maiuolo, J.; Oliverio, M.; Procopio, A.; Iannone, M.; Rotiroti, D.; Russo, D. Antiproliferative and antioxidant effects on breast cancer cells of oleuropein and its semisynthetic peracetylated derivatives. Food Chem. 2011, 127, 1609–1614. [Google Scholar] [CrossRef]
- Cavaca, L.A.; López-Coca, I.M.; Silvero, G.; Afonso, C.A. The olive-tree leaves as a source of high-added value molecules: Oleuropein. Stud. Nat. Prod. Chem. 2020, 64, 131–180. [Google Scholar]
- Jemai, H.; Fki, I.; Bouaziz, M.; Bouallagui, Z.; El Feki, A.; Isoda, H.; Sayadi, S. Lipid-lowering and antioxidant effects of hydroxytyrosol and its triacetylated derivative recovered from olive tree leaves in cholesterol-fed rats. J. Agric. Food Chem. 2008, 56, 2630–2636. [Google Scholar] [CrossRef]
- Benavente-García, O.; Castillo, J.; Lorente, J.; Ortuño, A.D.R.J.; Del Rio, J.A. Antioxidant activity of phenolics extracted from Olea europaea L. leaves. Food Chem. 2000, 68, 457–462. [Google Scholar] [CrossRef]
- Erbay, Z.; Icier, F. The importance and potential uses of olive leaves. Food Rev. Int. 2010, 26, 319–334. [Google Scholar] [CrossRef]
- Huang, Y.L.; Oppong, M.B.; Guo, Y.; Wang, L.Z.; Fang, S.M.; Deng, Y.R.; Gao, X.M. The Oleaceae family: a source of secoiridoids with multiple biological activities. Fitoterapia 2019, 136, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Nediani, C.; Ruzzolini, J.; Romani, A.; Calorini, L. Oleuropein, a bioactive compound from Olea europaea L., as a potential preventive and therapeutic agent in non-communicable diseases. Antioxidants 2019, 8, 1–26. [Google Scholar] [CrossRef]
- Romani, A.; Mulas, S.; Heimler, D. Polyphenols and secoiridoids in raw material (Olea europaea L. leaves) and commercial food supplements. Eur. Food Res. Technol. 2017, 243, 429–435. [Google Scholar] [CrossRef]
- El-Damrawy, S.Z.; Khalifah, M.M.; Fares, W.A. Dietary olive leaf and antioxidative status in chickens “performance, some physiological traits and immunological responses of Mandarah chicks supplemented olive leaves powder in their diets". Egypt. Poult. Sci. J. 2013, 33, 279–287. [Google Scholar]
- Cayan, H.; Erener, G. Effect of olive leaf (Olea europaea) powder on laying hens performance, egg quality and egg yolk cholesterol levels. Asian-Australas J. Anim. Sci. 2015, 28, 538–43. [Google Scholar] [CrossRef]
- Bahşi, M.; Ciftci, M.; Simsek, U.G.; Azman, M.A.; Özdemir, G.; Yilmaz, Ö.; Dalkiliç, B. Effects of olive leaf extract (oleuropein) on performance, fatty acid levels of breast muscle and some blood parameters in Japanese quail (Coturnix coturnix Japonica) reared in different stocking densities. Ankara Univ. Vet. Fak. Derg 2016, 63, 61–68. [Google Scholar]
- Singletary, K. Bay leaf: Potential health benefits. Nutr. Today 2021, 56, 202–208. [Google Scholar] [CrossRef]
- Dias, M.I.; Barros, L.; Dueñas, M.; Alves, R.C.; Oliveira, M, B.; Santos-Buelga, C.; Ferreira, I.C. Nutritional and antioxidant contributions of Laurus nobilis L. leaves: would be more suitable a wild or a cultivated sample? Food Chem. 2014, 156, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Statti, G.; Uzunov, D.; Menichinia, F. Comparative chemical composition and antioxidant activities of wild and cultivated Laurus nobilis L. leaves and Foeniculum vulgare subsp. piperitum (Ucria) Coutinho seeds. Biol. Pharm. Bul. 2006, 29, 2056–2064. [Google Scholar] [CrossRef] [PubMed]
- Carocho, M.; Ferreira, I.C.F.R. A review on antioxidants, prooxidants and related controversy: natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Škerget, M.; Kotnik, P.; Hadolin, M.; Hraš, A.R.; Simonič, A.M.; Knez, Ž. Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chem. 2005, 89, 191–198. [Google Scholar] [CrossRef]
- Lu, M.; Yuan, B.; Zeng, M.; Chen, J. Antioxidant capacity and major phenolic compounds of spices commonly consumed in China. Food Res. Intern. 2011, 44, 530–536. [Google Scholar] [CrossRef]
- Broadhurst, C.L.; Polansky, M.M.; Anderson, R.A. Insulin-like biological activity of culinary and medicinal plant aqueous extracts in vitro. J. Agric. Food Chem. 2000, 48, 849–852. [Google Scholar] [CrossRef]
- Khan, A.; Zaman, G.; Anderson, R.A. Bay leaves improve glucose and lipid profile of people with type 2 diabetes, J. Clin. Biochem. Nutr. 2009, 44, 52–56. [Google Scholar] [CrossRef]
- Kilic, A.; Hafizoglu, H.; Kollmannsberger, H.; Nitz, S. Volatile constituents and key odorants in leaves, buds, flowers and fruits of Laurus nobilis L. J. Agr. Food Chem. 2004, 52, 1601–1606. [Google Scholar] [CrossRef]
- Said, CM.; Hussein, K. Determination of the chemical and genetic differences of Laurus collected from three different geographic and climatic areas in Lebanon. Eur. Sci. J. 2014, 2, 412–419. [Google Scholar]
- Ramos, C.; Teixeira, B.; Batista, I.; Matos, O.; Serrano, C.; Neng, N.R.; Nogueira, J.M.F.; Nunes, M.L.; Marques, M. Antioxidant and antibacterial activity of essential oil and extracts of bay leave Laurus nobilis Linnaeus (Lauraceae) from Portugal. Nat. Prod. Res. 2012, 6, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Taban, A.; Saharkhiz, M.J.; Niakousari, M. Sweet bay (Laurus nobilis L.) essential oil and its chemical composition, antioxidant activity and leaf micromorphology under different extraction methods. Sustain. Chem. Pharm. 2018, 9, 12–18. [Google Scholar] [CrossRef]
- Ulbricht, C.; Abrams, TR.; Brigham, A.; Ceurvels, J.; Clubb, J.; Curtiss, W.; DeFranco Kirkwood, C.; Giese, N.; Hoehn, K.; Iovin, R.; Isaac, R.; Rusie, E.; Grimes Serrano, J.M.; Varghese, M.; Weissner, W.; Windsor, R.C. An evidence-based systematic review of rosemary (Rosmarinus officinalis) by the Natural Standard Research Collaboration. J. Diet. 2010, 7, 351–413. [Google Scholar] [CrossRef] [PubMed]
- Borrás-Linares, I.; Stojanović, Z.; Quirantes-Piné, R.; Arráez-Román, D.; Švarc-Gajić, J.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Rosmarinus officinalis leaves as a natural source of bioactive compounds. Int. J. Mol. Sci. 2014, 15, 20585–20606. [Google Scholar] [CrossRef] [PubMed]
- Rašković, A.; Milanović, I.; Pavlović, N.; Ćebović, T.; Vukmirović, S.; Mikov, M. Antioxidant activity of rosemary (Rosmarinus officinalis L.) essential oil and its hepatoprotective potential. BMC Complement Altern. Med. 2014, 14, 225. [Google Scholar] [CrossRef] [PubMed]
- Rizwana, H.; Bokahri, N.A.; Rashed, S.A.; Shehri, S.A.; Awad, M.A.; Merghani, N.; Tabasuum, H. Characterizing silver nanoparticles biosynthesized from Salvia rosmarinus and assessing their in vitro antifungal and cytotoxic activities against phytopathogens and cervical cells. J. Anim. Plant Sci. 2022, 32, 764–774. [Google Scholar]
- Aguilar, F.; Autrup, H.; Barlow, S.; Castle, L.; Crebelli, R.; Dekant, W.; Engel, Kh.; Gonard, N.; Gott, D.; Grilli, S. Use of rosemary extracts as a scientific food additive of the panel on food additives, aromas, technological and material adjuvants in contact with food. Efsa. J. 2008, 721, 1–29. [Google Scholar]
- Rice-Evans, C.A.; Miller, N.J.; Bolwell, P.G.; Bramley, P.M.; Pridham, J.B. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radical Res. 1995, 22, 375–383. [Google Scholar] [CrossRef]
- Angioni, A.; Barra, A.; Cereti, E.; Barile, D.; Coïsson, J. D.; Arlorio, M.; Dessi, S.; Coroneo, V.; Cabras, P.C. Chemical composition, plant genetic differences, antimicrobial and antifungal activity investigation of the essential oil of Rosmarinus officinalis L. J. Agric. Food. Chem. 2004, 52, 3530–3535. [Google Scholar] [CrossRef]
- Aruoma, OI.; Halliwell, B.; Aeschbach, R.; Löligers, J. Antioxidant and pro-oxidant properties of active rosemary constituents: carnosol and carnosic acid. Xenobiotica 1992, 22, 257–68. [Google Scholar] [CrossRef] [PubMed]
- Wijeratne, S.S.; Cuppett, S.L. Potential of rosemary (Rosemarinus officinalis L.) diterpenes in preventing lipid hydroperoxide-mediated oxidative stress in Caco-2 cells. J. Agric. Food Chem. 2007, 55, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Shan, B.; Cai, YZ.; Sun, M.; Corke, H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J. Agric. Food Chem. 2005, 53, 7749–7759. [Google Scholar] [CrossRef]
- de Falco, B.; Grauso, L.; Fiore, A.; Bonanomi, G.; Lanzotti, V. Metabolomics and chemometrics of seven aromatic plants: carob, eucalyptus, laurel, mint, myrtle, rosemary and strawberry tree. Phytochem. Anal. 2022, 33, 696–709. [Google Scholar] [CrossRef] [PubMed]
- Nieto, G.; Ros, G.; Castillo, J. Antioxidant and antimicrobial properties of rosemary (Rosmarinus officinalis, L.): A review. Medicines (Basel) 2018, 5, 98. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, J.R.; Camargo, S.E.A.; De Oliveira, L.D. Rosmarinus officinalis L.(rosemary) as therapeutic and prophylactic agent. J. Biomed. Sci. 2019, 26, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.C.; Zhang, H.Y.; Deng, J.S.; Wu, S.X.; Cui, Y.; Yang, M. Chemical constituents and pharmacological activities of Rosmarinus officinalis herba. Chin. J. Exper. Tradit. Medic. Form. 2019, 211–218. [Google Scholar]
- Afonso, MS.; de O Silva, AM.; Carvalho, EB.; Rivelli, DP.; Barros, SB.; Rogero, MM.; Lottenberg, AM.; Torres, RP.; Mancini-Filho, J. Phenolic compounds from Rosemary (Rosmarinus officinalis L.) attenuate oxidative stress and reduce blood cholesterol concentrations in diet-induced hypercholesterolemic rats. Nutr. Metab. (Lond) 2013, 10, 19. [Google Scholar] [CrossRef]
- El-Demerdash, F.M.; El-Sayed, R.A.; Abdel-Daim, M.M. Hepatoprotective potential of Rosmarinus officinalis essential oil against hexavalent chromium-induced hematotoxicity, biochemical, histological, and immunohistochemical changes in male rats. Environ. Sci. Pollut. Res. 2021, 28, 17445–17456. [Google Scholar] [CrossRef]
- Cimrin, T. Thyme (Thymbra spicata L.), rosemary (Rosmarinus officinalis L.) and vitamin E supplementation of laying hens. S. Afr. J. Anim. Sci. 2019, 49, 912–919. [Google Scholar] [CrossRef]
- Christaki, E.; Giannenas, I.; Bonos, E.; Florou-Paneri, P. Innovative uses of aromatic plants as natural supplements in nutrition. In Feed Additives; Florou-Paneri, P., Christaki, E., Giannenas, I., Eds.; Academic Press: London, UK, 2020; pp. 19–34. [Google Scholar]
- Alagawany, M.; Abd El-Hack, M.A. The effect of rosemary herb as a dietary supplement on performance, egg quality, serum biochemical parameters, and oxidative status in laying hens. J. Anim Feed Sci. 2015, 24, 341–347. [Google Scholar] [CrossRef]
- Celi, P.; Cowieson, A.J.; Fru-Nji, F.; Steinert, R.E.; Kluenter, A.-M.; Verlha, V. Gastrointestinal functionality in animal nutrition and health: new opportunities for sustainable animal production. Anim. Feed Sci. Technol. 2017, 234, 88–100. [Google Scholar] [CrossRef]
- Mahfuz, S.; Shang, Q.; Piao, X. Phenolic compounds as natural feed additives in poultry and swine diets: a review. J. Anim. Sci. Biotechnol. 2021, 12, 48. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, C. 2015. Some strategies for the stabilization of long chain n-3 PUFA-enriched foods: a review. Eur. J. Lipid Sci. Tecnol. 2015, 117, 1853–1866. [Google Scholar] [CrossRef]
- Haak, L.; Raes, K.; Van Dyck, S.; De Smet, S. Effect of dietary rosemary and alpha-tocopheryl acetate on the oxidative stability of raw and cooked pork following oxidized linseed oil administration. Meat Sci. 2008, 78, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Hashemzadeh-Cigari, F.; Khorvash, M.; Ghorbani, GR.; Kadivar, M.; Riasi, A.; Zebeli, Q. Effects of supplementation with a phytobiotics-rich herbal mixture on performance, udder health, and metabolic status of Holstein cows with various levels of milk somatic cell counts. J. Dairy Sci. 2014, 97, 7487–97. [Google Scholar] [CrossRef]
- Guo, F.C.; Kwakkel, R.P.; Soede, J.; Williams, B.A.; Verstegen, M.W.A. Effect of a Chinese herb medicine formulation, as an alternative for antibiotics, on performance of broilers. Br. Poult. Sci. 2004, 45, 793–797. [Google Scholar] [CrossRef]
- AOAC international, 2005. Official Methods of Analysis. 18th ed. Association of Analytical Chemists, AOAC International, Arlington Virginia, USA, 2005.
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of the folin-ciocalteu reagent. Meth. Enzymol. 1999, 299, 152–178. [Google Scholar]
- NRC. Nutrient requirements of poultry, 9th ed.; National Academy Press: Washington, DC, USA, 1994. [Google Scholar]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef]
- Lashkari, S.; Taghizadeh, A.; Seifdavati, J.; Salem, A.Z.M. Qualitative characteristics, microbial populations and nutritive values of orange pulp ensiled with nitrogen supplementation. Slovak J. Anim. Sci. 2014, 47, 90–99. [Google Scholar]
- Botsoglou, E.; Govaris, A.; Fletouris, D.; Iliadis, S. Olive leaves (Olea europea L.) and α-tocopheryl acetate as feed antioxidants for improving the oxidative stability of α-linolenic acid-enriched eggs. J. Anim. Physiol. Anim. Nutr. (Berl). 2013, 97, 740–53. [Google Scholar] [CrossRef] [PubMed]
- Christaki, E.V.; Bonos, E.M.; Florou-Paneri, P.C. Comparative evaluation of dietary oregano, anise and olive leaves in laying Japanese quails. Brazil. J. Poult. Sci. 2011, 13, 97–101. [Google Scholar] [CrossRef]
- Ahmed, M.M.; El-Saadany, A.S.; Shreif, E.Y. Effect of dietary olive leaves extract (oleuropein) supplementation on productive, physiological and immunological parameters in bandarah chickens 2- during production period. Egypt. Poult. Sci. J. 2017, 37, 277–292. [Google Scholar]
- Karaalp, M.; Elmastas, M.; Genc, N.; Sezer, M.; Yavuz, M.; Ozkan, M. Bay laurel (Laurus nobilis L.) in Japanese quails feeding 1. Performance and egg quality parameters. J. Anim. Vet. Adv. 2011, 10, 1883–1889. [Google Scholar] [CrossRef]
- Hajiazizi, F.; Torki, M.; Habibian, M. Effects of rosemary essential oil and zinc on performance, egg quality traits, and some serum metabolites in laying hens. J. Livest Sci. Technol. 2016, 4, 1–6. [Google Scholar]
- Botsoglou, N.; Florou-Paneri, P.; Botsoglou, E.; Dotas, V.; Giannenas, I.; Koidis, A.; Mitrakos, P. The effect of feeding rosemary, oregano, saffron and a-tocopheryl acetate on hen performance and oxidative stability of eggs. S. Afr. J. Anim. Sci. 2005, 35, 143–15. [Google Scholar] [CrossRef]
- Erener, G.; Ocak, N.; Ozturk, E.; Cankaya, S.; Ozkanca, R.; Altop, A. Evaluation of olive leaf extract as a growth promoter on the performance, blood biochemical parameters, and caecal microflora of broiler chickens. Rev. Bras. Zootec. 2020, 49, e20180300. [Google Scholar] [CrossRef]
- Ali, N.A.-L.; Al-Shuhaib, M.B.S. Highly effective dietary inclusion of laurel (Laurus nobilis) leaves on productive traits of broiler chickens. Acta Sci. – Anim. Sci. 2021, 43, e52198. [Google Scholar] [CrossRef]
- Abo Ghanima, M.M.; Elsadek, M.F.; Taha, A.E.; Abd El-Hack, M.E.; Alagawany, M.; Ahmed, B.M.; Elshafie, M.M.; El-Sabrout, K. Effect of housing system and rosemary and cinnamon essential eils on layers performance, egg quality, haematological traits, blood chemistry, immunity, and antioxidant. Animals (Basel) 2020, 10, 245. [Google Scholar] [CrossRef]
- Ferdous, M.F.; Arefin, M.S.; Rahman, M.M.; Ripon, M.M.R.; Rashid, M.H.; Sultana, M.R.; Hossain, M.T.; Ahammd, M.U.; Rafiq, K. Beneficial effects of probiotic and phytobiotic as growth promoter alternative to antibiotic for safe broiler production. J. Adv. Vet. Anim. Res. 2019, 6, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Lipiński, K.; Mazur, M.; Antoszkiewicz, Z.; Purwin, C. Polyphenols in monogastric nutrition – A review. Ann. Anim. Sci. 2017, 17, 41–58. [Google Scholar] [CrossRef]
- Martínez, Y.; Yero, O.M.; Liu, G.; Ren, W.; Bertot, R.R.; Yimenez, Y.F.; Gonzalez, C.O.; Del Toro, M.I.; Arocho, R.; Navarro, M.V.; Nyachoti, C.M. Effect of dietary supplementation with Anacardium occidentale on growth performance and immune and visceral organ weights in replacement laying pullets. J. Food. Agric. Environ. 2013, 13, 1352–1357. [Google Scholar]
- Rezaei, M.; KarimiTorshizi, M.A.; Wall, H.; Ivarsson, E. Body growth, intestinal morphology and microflora of quail on diets supplemented with micronised wheat fibre. Brit. Poult. Sci. 2018, 59, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Galosi, L.; Desantis, S.; Roncarati, A.; Robino, P.; Bellato, A.; Ferrocino, I.; Santamaria, N.; Biagini, L.; Filoni, L.; Attili, A.R.; Rossi, G. Positive influence of a probiotic mixture on the intestinal morphology and microbiota of farmed guinea fowls (Numida meleagris). Front. Vet. Sci. 2021, 8, 743899. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhang, H.J.; Yu, S.H.; Wu, S.G.; Yoon, I.; Quigley, J.; Gao, Y.P.; Qi, G.H. Effects of yeast culture in broiler diets on performance and immunomodulatory functions. Poult Sci. 2008, 87, 1377–84. [Google Scholar] [CrossRef] [PubMed]
- Hamedi, S.; Rezaian, M.; Shomali, T. Histological changes of small intestinal mucosa of cocks due to sunflower meal single feeding. Am. J. Anim. Vet. Sci. 2011, 6, 171–175. [Google Scholar]
- Gao, Y.Y.; Zhang, X.L.; Xu, L.H.; Peng, H.; Wang, C.K.; Bi, Y.Z. Encapsulated blends of essential oils and organic acids improved performance, intestinal morphology, cecal microflora, and jejunal enzyme activity of broilers. Czech. J. Anim. Sci. 2019, 64, 189–1898. [Google Scholar] [CrossRef]
- Hampson, D.J. Alterations in piglet small intestinal structure at weaning. Res. Vet. Sci. 1986, 40, 32–40. [Google Scholar] [CrossRef]
- Pearson, J.P.; Brownlee, I.A. The interaction of large bowel microflora with the colonic mucus barrier. Int. J. Inflam. 2010, 2010, 321426. [Google Scholar] [CrossRef]
- Desantis, S.; Galosi, L.; Santamaria, N.; Roncarati, A.; Biagini, L.; Rossi, G. Modulation of morphology and glycan composition of mucins in Guinea fowl (Numida meleagris) intestine by the multistrain probiotic Slab51. Animals (Basel) 2021, 11, 495. [Google Scholar] [CrossRef] [PubMed]
- Meimandipour, A.; Soleimanifarjam, A.; Azhar, K.; Hair-Bejo, M.; Shuhaimi, M.; Nateghi, L.; Yazid, A.M. Age effects on short-chain fatty acids concentrations and pH values in the gastrointestinal tract of broiler chickens. Arch. Fur Geflugelkunde 2011, 75, 164–168. [Google Scholar]
- Herrero-Encinas, J.; Menoyo, D.; Blanch, M.; Pastor, J.J.; Rochell, S.J. Response of broiler chickens fed diets supplemented with a bioactive olive pomace extract from Olea europaea to an experimental coccidial vaccine challenge. Poul. Sci. 2021, 100, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Cross, D.E.; McDevitt, R.M.; Hillman, K.; Acamovic, T. The effect of herbs and their associated essential oils on performance, dietary digestibility and gut microflora in chickens from 7 to 28 days of age. Br. Poult. Sci. 2007, 48, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Yang, H.; Wang, X.; Xia, W.; Lv, W.; Xiao, Y.; Zou, X. Early intervention with cecal fermentation broth regulates the colonization and development of gut microbiota in broiler chickens. Front. Microbiol. 2019, 10, 1422. [Google Scholar] [CrossRef]
- Oost, MJ.; Velkers, F.C.; Kraneveld, A.D.; Venema, K. Development of the in vitro cecal chicken alimentary tract model-2 to study microbiota composition and function. Front. Microbiol. 2021, 12, 726447. [Google Scholar] [CrossRef] [PubMed]
- González-Ortiz, G.; Olukosi, O.A.; Jurgens, G.; Apajalahti, J.; Bedford, M.R. Short-chain fatty acids and ceca microbiota profiles in broilers and turkeys in response to diets supplemented with phytase at varying concentrations, with or without xylanase. Poult. Sci. 2020, 99, 2068–2077. [Google Scholar] [CrossRef]
- Aguirre, M.; Eck, A.; Koenen, M.E.; Savelkoul, P.H.M.; Budding, A.E.; Venema, K. Diet drives quick changes in the metabolic activity and composition of human gut microbiota in a validated in vitro gut model. Res. Microbiol. 2016, 167, 114–125. [Google Scholar] [CrossRef]
- Rios-Covian, D.; Salazar, N.; Gueimonde, M.; de los Reyes-Gavilan, C.G. Shaping the metabolism of intestinal Bacteroides population through diet to improve human health. Front. Microbiol. 2017, 8, 376. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Backhed, F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Salonen, A.; Lahti, L.; Salojarvi, J.; Holtrop, G.; Korpela, K.; Duncan, S.H.; Date, P.; Farquharson, F.; Johnstone, A.M.; Lobley, G.E.; Louis, P.; Flin, H.J.; de Vos, W.M. Impact of diet and individual variation on intestinal microbiota composition and fermentation products in obese men. ISME J. 2014, 8, 2218–2230. [Google Scholar] [CrossRef] [PubMed]
- Baothman, O.A.; Zamzami, M.A.; Taher, I.; Abubaker, J.; Abu-Farha, M. The role of gut microbiota in the development of obesity and diabetes. Lipids Health Dis. 2016, 15, 108. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Pandit, R.J.; Hinsu, A.T.; Patel, N.V.; Koringa, P.; Jakhesara, S.J.; Thakkar, J.R.; Shah, T.M.; Limon, G.; Psifidi, A.; Guitian, J.; Hume, D.A.; Tomley, F.M.; Rank, D.N.; Raman, M.; Tirumurugaan, K.G.; Blake, D.P.; Joshi, C.G. Microbial diversity and community composition of caecal microbiota in commercial and indigenous Indian chickens determined using 16s rDNA amplicon sequencing. Microbiome 2018, 6, 115. [Google Scholar] [CrossRef] [PubMed]
- Besten, G.D.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Awad, W.A.; Dublecz, F.; Hess, C.; Khayal, B.; Aschenbach, J.R.; Hess, M. Campylobacter jejuni colonization promotes the translocation of Escherichia coli to extra-intestinal organs and disturbs the short-chain fatty acids profiles in the chicken gut. Poult. Sci. 2016, 95, 2259–2265. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.K.; Tyagi, A.; Kumar, A.; Panwar, S.; Grover, S.; Saklani, A.C.; Hemalatha, R.; Batish, V.K. Adhesion of Lactobacilli and their anti-infectivity potential. Crit. Rev. Food Sci. Nutr. 2017, 57, 2042–2056. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, J.; Shi, Y.; Su, W.; Chen, J.; Zhang, Z.; Wang, G.; Wang, F. Short chain fatty acid acetate protects against ethanol-induced acute gastric mucosal lesion in mice. Biol. Pharm. Bull. 2017, 40, 1439–1446. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, Y.; Wang, P.; Huang, Y.; Wang, F. Short-chain fatty acids manifest stimulative and protective effects on intestinal barrier function through the inhibition of NLRP3 inflammasome and autophagy. Cell. Physiol. Biochem. 2018, 49, 190–205. [Google Scholar] [CrossRef]
- Macia, L.; Tan, J.; Vieira, A.T.; Leach, K.; Stanley, D.; Luong, S.; Maruya, M.; McKenzie, C.I.; Hijikata, A.; Wong, C.; Binge, L.; Thorburn, A.N.; Chevalier, N.; Ang, C.; Marino, E.; Robert, R.; Offermanns, S.; Teixeira, M.M.; Moore, R.J.; Flavell, R.A.; Fagarasan, S.; Mackay, C.R. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat. Commun. 2015, 6, 6734. [Google Scholar] [CrossRef]
- Nowarski, R.; Jackson, R.; Gagliani, N.; de Zoete, M.R.; Palm, N.W.; Bailis, W.; Low, J.S.; Harman, C.C.D.; Graham, M.; Elinav, E.; Flavell, R.A. Epithelial IL-18 equilibrium controls barrier function in colitis. Cell 2015, 163, 1444–1456. [Google Scholar] [CrossRef] [PubMed]
- Borda-Molina, D.; Mátis, G.; Mackei, M.; Neogrády, Z.; Huber, K.; Seifert, J.; Camarinha-Silva, A. Caeca microbial variation in broiler chickens as a result of dietary combinations using two cereal types, supplementation of crude protein and sodium butyrate. Front. Microbiol. 2021, 11, 617800. [Google Scholar] [CrossRef] [PubMed]
- Mathewson, N.D.; Jenq, R.; Mathew, A.V.; Koenigsknecht, M.; Hanash, A.; Toubai, T.; Oravecz-Wilson, K.; Wu, S.R.; Sun, Y.; Rossi, C. Gut microbiome-derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease. Nat. Immunol. 2016, 17, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Gaudier, E.; Jarry, A.; Blottiere, H.M.; de Coppet, P.; Buisine, M.P.; Aubert, J.P.; Laboisse, C.; Cherbut, C.; Hoebler, C. Butyrate specifically modulates MUC gene expression in intestinal epithelial goblet cells deprived of glucose. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G1168–G1174. [Google Scholar] [CrossRef]
- Tong, L.-C.; Wang, Y.; Wang, Z.-B.; Liu, W.-Y.; Sun, S.; Li, L.; Su, D.-F.; Zhang, L.-C. Propionate ameliorates dextran sodium sulfate-induced colitis by improving intestinal barrier function and reducing inflammation and oxidative stress. Front. Pharmacol. 2016, 7, 253. [Google Scholar] [CrossRef]
- Schoenfeld, P.; Wojtczak, L. Short- and medium-chain fatty acids in energy metabolism: The cellular perspective. J. Lipid Res. 2016, 57, 943–954. [Google Scholar] [CrossRef]
- Brüssow, H.; Parkinson, S.J. Parkinson, S.J. You are what you eat. Nat. Biotechnol. 2014, 32, 243–245. [Google Scholar] [CrossRef]
- Boets, E.; Gomand, S.V.; Deroover, L.; Preston, T.; Vermeulen, K.; De Preter, V.; Hamer, H.M.; Van den Mooter, G.; De Vuyst, L.; Courtin, C.M.; Annaert, P.; Delcour, J.A.; Verbeke, K.A. Systemic availability and metabolism of colonic-derived short-chain fatty acids in healthy subjects: a stable isotope study. J. Physiol. 2017, 595, 541–555. [Google Scholar] [CrossRef]
- Clench, M.; Mathias, J. The avian cecum: a review. Wilson Bull. 1995, 10, 93–121. [Google Scholar]
- Jamroz, D.; Jakobsen, K.; Bach, K.K.; Wiliczkiewicz, A.; Orda, J. Digestibility and energy value of non-starch polysaccharides in young chickens, ducks and geese, fed diets containing high amounts of barley. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2002, 131, 657–668. [Google Scholar] [CrossRef]
- Stanley, D.; Hughes, R.J.; Moore, R.J. Microbiota of the chicken gastrointestinal tract: influence on health, productivity and disease. Appl. Microbiol. Biotechnol. 2014, 98, 4301–4310. [Google Scholar] [CrossRef] [PubMed]
- Waite, D.W.; Taylor, M.W. Characterizing the avian gut microbiota: membership, driving influences, and potential function. Front. Microbiol. 2014, 5, 223. [Google Scholar] [CrossRef] [PubMed]
- Diaz Carrasco, J.M.; Casanova, N.A.; Fernández Miyakawa, M.E. Microbiota, gut health and chicken productivity: what is the connection? Microorganisms 2019, 7, 374. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Wang, J.; Yu, L.; Zhang, Q.; Chen, K.; Liu, B. Modulation of growth performance and intestinal microbiota in chickens fed plant extracts or virginiamycin. Front. Microbiol. 2019, 10, 1333. [Google Scholar] [CrossRef]
- Romo-Vaquero, M.; Selma, M.-V.; Larrosa, M.; Obiol, M.; García-Villalba, R.; González-Barrio, R.; Issaly, N.; Flanagan, J.; Roller, M.; Tomás-Barberán, F.A.; García-Conesa, M.-T. A rosemary extract rich in carnosic acid selectively modulates caecum microbiota and inhibits -glucosidase activity, altering fiber and short-chain fatty acids fecal excretion in lean and obese female rats. PLoS ONE 2014, 9, e94687. [Google Scholar] [CrossRef]
- He, X.; Zhang, M.; Li, S.T.; Li, X.; Huang, Q.; Zhang, K.; Zheng, X.; Xu, XT.; Zhao, DG.; Ma, Y.Y. Alteration of gut microbiota in high-fat diet-induced obese mice using carnosic acid from rosemary. Food Sci. Nutr. 2022, 10, 2325–2332. [Google Scholar] [CrossRef]
- Derwich, E.; Benziane, Z.; Boukir, A. Chemical composition and antibacterial activity of leaves essential oil of Laurus nobilis from Morocco. Aust. J. Basic Appl. Sci. 2009, 3, 3818–3824. [Google Scholar]
- Jabri, J.; Kacem, H.; Yaich, H.; Abid, K.; Kamoun, M.; Rekhis, J.; Malek, A. Effect of olive leaves extract supplementation in drinking water on zootechnical performances and cecal microbiota balance of broiler chickens. J. New Sci. Sustain Livest. Manag. 2017, 4, 69–75. [Google Scholar]
- Xie, P.; Deng, Y.; Huang, L.; Zhang, C. Effect of olive leaf (Olea europaea L.) extract addition to broiler diets on the growth performance, breast meat quality, antioxidant capacity and caecal bacterial populations. Ital. J. Anim. Sci. 2022, 21, 1246–1258. [Google Scholar] [CrossRef]
- Lin, Y.T.; Vattem, D.A.; Labbe, R.G.; Shetty, K. Enhancement of antioxidant activity and inhibition of Helicobacter pylori by phenolic phytochemical enriched alcoholic beverages. Process Biochem. 2005, 40, 2059–2065. [Google Scholar] [CrossRef]
- Cardona, F.; Andres-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–22. [Google Scholar] [CrossRef] [PubMed]
- García-Ruiz, A.; Bartolome, B.; Martínez-Rodríguez, AJ.; Pueyo, E.; Martín-Alvarez, PJ.; Moreno-Arribas, M.V. Potential of phenolic compounds for controlling lactic acid bacteria growth in wine. Food Control. 2008, 19, 835–841. [Google Scholar] [CrossRef]
- Amit-Romach, E.; Sklan, D.; Uni, Z. Microflora ecology of the chicken intestine using 16S ribosomal DNA primers. Poult. Sci. 2004, 83, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Corrier, D.E.; Nisbet, D.J.; Scanlan, C.M.; Hollister, A.G.; Deloach, J.R. Control of Salmonella typhimurium colonization in broiler chicks with a continuous-flow characterized mixed culture of cecal bacteria. Poult. Sci. 1995, 74, 916–24. [Google Scholar] [CrossRef] [PubMed]
- Travers, M.A.; Florent, I.; Kohl, L.; Grellier, P. Probiotics for the control of parasites: an overview. J. Parasitol. Res. 2011, 2011, 610769. [Google Scholar] [CrossRef] [PubMed]
- Van Immerseel, F.; Fievez, V.; De Buck, J.; Pasmans, F.; Martel, A.; Haesebrouck, F.; Ducatelle, R. Microencapsulated short-chain fatty acids in feed modify colonization and invasion early after infection with Salmonella enteritidis in young chickens. Poult. Sci. 2004, 83, 69–74. [Google Scholar] [CrossRef]
- Vermeulen, K.; Verspreet, J.; Courtin, C.M.; Haesebrouck, F.; Ducatelle, R.; Immerseel, F.V. Reduced particle size wheat bran is butyrogenic and lowers Salmonella colonization, when added to poultry feed. Vet. Microbiol. 2017, 198, 64–71. [Google Scholar] [CrossRef]
- Sun, Y.; O’Riordan, M.X.D. Regulation of bacterial pathogenesis by intestinal short-chain fatty acids. Adv. Appl. Microbiol. 2013, 85, 93–118. [Google Scholar]
- Attia, Y.A.; Bovera, F.; El-Tahawy, W.S.; El-Hanoun, A.M.; Al-Harthi, M.A.; Habiba, H.I. Productive and reproductive performance of rabbits does as affected by bee pollen and/or propolis, inulin and/or mannan-oligosaccharides. World Rabbit Sci. 2015, 2, 273–282. [Google Scholar] [CrossRef]
- Sarica, S.; Toptas, S. Effects of dietary oleuropein supplementation on growth performance, serum lipid concentrations and lipid oxidation of Japanese quails. J. Anim. Physiol. Anim. Nutr. 2014, 98, 1176–1186. [Google Scholar] [CrossRef]
- Talhaoui, N.; Taamalli, A.; Gómez-Caravaca, A.M.; Fernández-Gutiérrez, A.; SeguraCarretero, A. Phenolic compounds in olive leaves: analytical determination, biotic and abiotic influence, and health benefits. Food Res. Int. 2015, 77, 92–108. [Google Scholar] [CrossRef]
- Elazab, M.A.; Khalifah, A.M.; Elokil, A.A.; Elkomy, A.E.; Rabie, M.M.; Mansour, A.T.; Morshedy, S.A. Effect of dietary rosemary and ginger essential oils on the growth performance, feed utilization, meat nutritive value, blood biochemicals, and redox status of growing NZW rabbits. Animals 2022, 12, 375. [Google Scholar] [CrossRef] [PubMed]
- Torki, M.; Sedgh-Gooya, S.; Mohammadi, H. Effects of adding essential oils of rosemary, dill and chicory extract to diets on performance, egg quality and some blood parameters of laying hens subjected to heat stress. J. App. Anim. Res. 2018, 46, 1118–1126. [Google Scholar] [CrossRef]
- Radwan, N.L.; Hassan, R.A.; Qota, E.M.; Fayek, H.M. Effect of natural antioxidant on oxidative stability of eggs and productive and reproductive performance of laying hens. Int. J. Poult. Sci. 2008, 7, 134–150. [Google Scholar]
- Bolukbasi, S.C.; Erhan, M.K.; Kaynar, O. The effect of feeding thyme, sage and rosemary oil on laying hen performance, cholesterol and some proteins ratio of egg yolk and E. coli count in feces. Arch. Geflügelk. 2008, 72, 231–237. [Google Scholar]
- Chalabi, M.; Majeed, D.; Jasim, A.; Al-Azzawi, S. Beneficial effects of ethanolic extract of bay leaves (Laurus nobilis) on blood sugar levels in adult diabetic rats induced by alloxan monohydrate. Ann. Trop. Med. Publ. Health. 2020, 23, e175–e184. [Google Scholar] [CrossRef]
- Chiang, J.Y.L.; Ferrell, J.M. Up to date on cholesterol 7 alpha-hydroxylase (CYP7A1) in bile acid synthesis. Liver Res. 2020, 4, 47–63. [Google Scholar] [CrossRef]
- Wasburn, K.W.; Nix, DF. Genetic basis of yolk cholesterol content. Poult. Sci. 1974, 53, 109–15. [Google Scholar] [CrossRef]
- Rahimi, G. Dietary forage legume (Onobrychis altissima grossh.) supplementation on serum/yolk cholesterol, triglycerides and eggshell characteristics in laying hens. Int. J. Poult. Sci. 2005, 4, 772–776. [Google Scholar]
- Hargis, P.S.; Van Elswyk, M.E.; Hargis, B.M. Dietary modification of yolk lipid with savelha oil. Poult. Sci. 1991, 70, 874–83. [Google Scholar] [CrossRef]
- Darmawan, A.; Hermana, W.; Suci, D.M.; Mutia, R.; Sumiati; Jayanegara, A.; Ozturk, E. Dietary phytogenic extracts favorably influence productivity, egg quality, blood constituents, antioxidant and immunological parameters of laying hens: a meta-analysis. Animals (Basel) 2022, 12, 2278.
- Sharma, M.K.; Dinh, T.; Adhikari, P.A. Production performance, egg quality, and small intestine histomorphology of the laying hens supplemented with phytogenic feed additive. J. Appl. Poult. Res. 2020, 29, 362–371. [Google Scholar] [CrossRef]
- Bertechini, A.G. Mitos e verdades sobre o ovo de consumo. In 21th Conferência de Ciência e Tecnologia Avícola; Santos: São Paulo. Brasil, 2003; pp. 19–26. [Google Scholar]
- Shafey, T.M.; Cham, B.E. Altering fatty acid and cholesterol contents of eggs for human consumption. In Egg uses and processing technologies: new developments; Sim, J.S., Nakai, S., Eds.; CAB International: Washington, DC, USA, 1994; pp. 374–385. [Google Scholar]
- Griffin, H.D. Manipulation of egg yolk cholesterol: a physiologist's view. World Poult. Sci. J. 1992, 48, 101–112. [Google Scholar] [CrossRef]
- Fennema, O.R. Química de los alimentos, Zaragoza: Acribia; 1993.
- Wu, R.; Feng, J.; Yang, Y.; Dai, C.; Lu, A.; Li, J.; Liao, Y.; Xiang, M.; Huang, Q.; Wang, D.; Du, X.-B. Significance of serum total oxidant/antioxidant status in patients with colorectal cancer. PLoS ONE 2017, 12, e0170003. [Google Scholar] [CrossRef] [PubMed]
- Bulbul, T.; Ulutas, E. The effects of dietary supplementation of false flax (Camelina sativa L.) meal on performance, egg quality traits, and lipid peroxidation in laying quails. Eurasian J. Vet. Sci. 2015, 31, 8–15. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Regueiro, J.; Martínez-Huélamo, M.; Alvarenga, J.F.; Leal, L.N.; Lamuela-Raventos, R. MA comprehensive study on the phenolic profile of widely used culinary herbs and spices: rosemary, thyme, oregano, cinnamon, cumin and bay. Food Chem. 2014, 154, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Falade, A.O.; Adewole K., E.; Adekola, A.O.; Ikokoh, H.A.; Okaiyeto, K.; Oguntibeju, O.O. Aqueous extract of bay leaf (Laurus nobilis) ameliorates testicular toxicity induced by aluminum chloride in rats. Vet. World 2022, 15, 2525–2534. [Google Scholar] [CrossRef]
- Wenk, C. Herbs, botanicals and other related substances. WPSA-Bremen. Germany, 2002.
- Chahal, K.; Kaur, M.; Bhardwaj, U.; Singla, N.; Kaur, A. A review on chemistry and biological activities of Laurus nobilis L. essential oil. J. Pharm. Phytochem. 2017, 6, 1153–1161. [Google Scholar]
- Loussouarn, M.; Krieger-Liszkay, A.; Svilar, L.; Bily, A.; Birtić, S.; Havaux, M. Carnosic acid and carnosol, two major antioxidants of rosemary, act through different mechanisms. Plant Physiol. 2017, 75, 1381–1394. [Google Scholar] [CrossRef]
- Silva, S.; Gomes, L.; Leitão, F.; Coelho, AV.; Boas, LV. Phenolic compounds and antioxidant activity of Olea europaea L. fruits and leaves. Food Sci. Technol. Int. 2006, 12, 385–395. [Google Scholar] [CrossRef]
- Hayes, J.E.; Allen, P.; Brunton, N.; O’Grady, M. .N.; Kerry, JP. Phenolic composition and in vitro antioxidant capacity of four commercial phytochemical products: olive leaf extract (Olea Europaea L.), lutein, sesamol and ellagic acid. Food Chem. 2011, 126, 948–955. [Google Scholar] [CrossRef]
- Turkez, H.; Togar, B.; Polat, E. Olive leaf extract modulates permethrin induced genetic and oxidative damage in rats. Cytotechnology 2012, 64, 459–64. [Google Scholar] [CrossRef] [PubMed]
- Sarıca, S.; Aydın, H.; Ciftci, G. Effects of dietary supplementation of some antioxidants on liver antioxidant status and plasma biochemistry parameters of heat-stressed quail. Turk. J. Food Agric. Sci. 2017, 5, 773–779. [Google Scholar] [CrossRef]
- Adedayo, B.C.; Oyeleye, S.I.; Okeke, B.M.; Oboh, G. Anti-cholinesterase and antioxidant properties of alkaloid and phenolic-rich extracts from pawpaw (Carica papaya) leaf: A comparative study. Flavour Fragr. J. 2020, 00, 1–8. [Google Scholar] [CrossRef]
- Khayyal, A.; El-Badawy, M.; Ashmawy, T. Effect of rosemary or laurel leaves as feed additives on performance of growing lambs. Egypt. J. Nutr. Feeds 2021, 24, 343–356. [Google Scholar] [CrossRef]
- Liu, Z.; Luo, Z.; Jia, C.; Wang, D.; Li, D. Synergistic effects of Potentilla fruticosa L. leaves combined with green tea polyphenols in a variety of oxidation systems. J. Food Sci. 2016, 81, C1091–C1101. [Google Scholar] [CrossRef]
- Wu, L.; Xiong, X.; Wu, X.; Ye, Y.; Jian, Z.; Zhi, Z.; Gu, L. Targeting oxidative stress and inflammation to prevent ischemia- Reperfusion injury. Front. Mol. Neurosci. 2020, 13, 28. [Google Scholar] [CrossRef]
- Yuan, X.; Zhu, J.; Kang, Q.; He, X.; Guo, D. Protective effect of hesperidin against sepsis-induced lung injury by inducing the heat-stable protein 70 (Hsp70)/Toll-Like Receptor 4 (TLR4)/ Myeloid differentiation primary response 88 (MyD88) pathway. Med. Sci. Monit. 2019, 25, 107–114. [Google Scholar] [CrossRef]
- Estienne, A.; Bongrani, A.; Reverchon, M.; Ramé, C.; Ducluzeau, P.H.; Froment, P.; Dupont, J. Involvement of novel adipokines, chemerin, visfatin, resistin and apelin in reproductive functions in normal and pathological conditions in humans and animal models. Int. J. Mol. Sci. 2019, 20, 4431. [Google Scholar] [CrossRef]
- Barbe, A.; Mellouk, N.; Ramé, C.; Grandhaye, J.; Staub, C.; Venturi, E.; Cirot, M.; Petit, A.; Anger, K.; Chahnamian, M.; Ganier, P.; Callut, O.; Cailleau-Audouin, E.; Metayer-Coustard, S.; Riva, A.; Froment, P.; Dupont, J. A grape seed extract maternal dietary supplementation in reproductive hens reduces oxidative stress associated to modulation of plasma and tissue adipokines expression and improves viability of offsprings. PLoS One 2020, 15, e0231131. [Google Scholar] [CrossRef]
- Miliaraki, M.; Briassoulis, P.; Ilia, S.; Michalakakou, K.; Karakonstantakis, T.; Polonifi, A.; Bastaki, K.; Briassouli, E.; Vardas, K.; Pistiki, A.; Theodorakopoulou, M.; Tavladaki, T.; Spanaki, AM.; Kondili, E.; Dimitriou, H.; Venihaki, M.; Tsiodras, S.; Georgopoulos, D.; Mantzourani, M.; Nanas, S.; Armaganidis, A.; Daikos, GL.; Papassotiriou, I.; Briassoulis, G. Oxidant/antioxidant status is impaired in sepsis and is related to anti-apoptotic, inflammatory, and innate immunity alterations. Antioxidants 2022, 11, 231. [Google Scholar] [CrossRef] [PubMed]
- Vezza, T.; Algieri, F.; Rodríguez-Nogales, A.; Garrido-Mesa, J.; Utrilla, M.P.; Talhaoui, N.; Gómez-Caravaca, A.M.; Segura-Carretero, A.; Rodríguez-Cabezas, M.E.; Monteleone, G.; Gálvez, J. Immunomodulatory properties of Olea europaea leaf extract in intestinal inflammation. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- De Cicco, P.; Maisto, M.; Tenore, G.C.; Ianaro, A. Olive leaf extract, from Olea europaea L., reduces palmitate-induced inflammation via regulation of murine macrophages polarization. Nutrients 2020, 12, 3663. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Sano, M.; Seno, K.; Oogaki, Y.; Takahashi, H.; Ohkuchi, A.; Yokozawa, M.; Yamauchi, K.; Iwata, H.; Kuwayama, T.; Shirasuna, K. Olive Leaf Extract (OleaVita) suppresses inflammatory cytokine production and NLRP3 inflammasomes in human placenta. Nutrients 2019, 11, 970. [Google Scholar] [CrossRef]
- Lee, E.H.; Shin, J.H.; Kim, S.S.; Lee, H.; Yang, S.R.; Seo, S.R. Laurus nobilis leaf extract controls inflammation by suppressing NLRP3 inflammasome activation. J. Cell Physiol. 2019, 234, 6854–6864. [Google Scholar] [CrossRef] [PubMed]
- Farouk, S.M.; Abdel-Rahman, H.G.; Abdallah, O.A.; El-Behidy, N.G. Comparative immunomodulatory efficacy of rosemary and fenugreek against Escherichia coli infection via suppression of inflammation and oxidative stress in broilers. Environ. Sci. Pollut. Res. 2022, 29, 40053–40067. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.H.; Chuang, L.T.; Lien, T.J.; Liing, Y..R.; Chen, W.Y.; Tsai, PJ. Rosmarinus officinalis extract suppresses Propionibacterium acnes-induced inflammatory responses. J. Med. Food. 2013, 16, 324–33. [CrossRef]
- Aroche, R.; Martínez, Y.; Ruan, Z.; Guan, G.; Waititu, S.; Nyachoti, C.M.; Más, D.; Lan, S. Dietary inclusion of a mixed powder of medicinal plant leaves to enhance the feed efficiency and immune function in broiler chickens. J. Chem. 2018, 394, 1–6. [Google Scholar] [CrossRef]
- Diaz, G.J.; Roldan, L.P.; Cortes, A. Intoxication of Crotalaria pallida seeds to growing broiler chicks. Vet. Hum. Toxicol. 2003, 45, 187–189. [Google Scholar]
- Valchev, I.; Kanakov, D.; Hristov, TS.; Lazarov, L.; Binev, R.; Grozeva, N.; Nikolov, Y. Investigations on the liver function of broiler chickens with experimental aflatoxicosis. Bulg. J. Vet. Med. 2014, 17, 302–313. [Google Scholar]
- Khalil, E.A.M. Evaluation of the hepatoprotective activity of an aqueous extract of olive leaves in male albino rats. Egypt. J. Hosp. Med. 2004, 15, 118–123. [Google Scholar] [CrossRef]
- Abdel-Wahhab, K.G.E.; El-Shamy, K.A.; El-Beih, N.A.E.; Morcy, F.A.; Mannaa, F.A.E. Protective effect of a natural herb (Rosmarinus officinalis) against hepatotoxicity in male albino rats. Com. Sci. 2011, 2, 9–17. [Google Scholar]
- Al-Attar, A.M.; Shawush, N.A. Influence of olive and rosemary leaves extracts on chemically induced liver cirrhosis in male rats. Saudi J. Biol. Sci. 2015, 22, 157–63. [Google Scholar] [CrossRef] [PubMed]
- Al Chalabi, S.; Majeed, D.; Jasim, A.; Al-Azzawi, K. Benefit effect of ethanolic extract of Bay leaves (Laura nobilis) on blood sugar level in adult diabetic rats induced by alloxan monohydrate. Ann. Trop. Med. Publ. Health 2020, 23, 16. [Google Scholar] [CrossRef]
- Abdel-Azeem, A.; Fayed, A.; Azoz, A. Physiological response, semen quality and blood biochemical parameters of rabbit bucks supplemented with phytogenic components during summer season of Egypt. Egypt. J. Nutr. Feeds 2018, 21, 711–724. [Google Scholar] [CrossRef]
| Olive | Laurel | Rosemary | |
|---|---|---|---|
| Dry matter (%) | 60.57 ± 0.95 | 30.76 ± 0.83 | 31.91 ± 0.80 |
| Crude protein | 9.66 ± 0.61 | 5.13 ± 0.31 | 6.40 ± 0.02 |
| Ether extract | 2.34 ± 0.51 | 1.42 ± 0.71 | 12.85 ± 0.15 |
| Ash | 7.15 ± 0.36 | 3.75 ± 0.34 | 7.24 ± 0.93 |
| Crude fiber | 24.09 ± 1.25 | 12.67 ± 0.60 | 26.20 ± 1.80 |
| Neutral detergent fiber | 46.12 ± 1.58 | 23.85 ± 3.93 | 45.34 ± 1.01 |
| Acid detergent fiber | 28.52 ±0.87 | 14.69 ± 0.69 | 33.17 ± 0.69 |
| Acid detergent lignin | 19.46 ± 0.81 | 9.63 ± 1.94 | 22.23 ± 0.53 |
| Acid insoluble ash | 0.20 ± 0.02 | 0.11 ± 0.09 | 0.35 ± 0.05 |
| Total phenols (mg GAE/g DM) | 6.10 ± 0.80 | 3.90 ± 1.12 | 5.10 ± 1.11 |
| Ingredients | |
|---|---|
| Corn meal | 57.60 |
| Soybean meal (46% CP) | 22.00 |
| Sunflower flour (36% CP) | 6.00 |
| Limestone granular | 6.00 |
| Limestone | 3.30 |
| Soyabean oil | 2.50 |
| Bicalcium phosphate | 1.50 |
| Vitamin and mineral premix1 | 0.50 |
| Sodium chloride | 0.20 |
| Sodium bicarbonate | 0.15 |
| Methionine (MHA)2 | 0.14 |
| Lisine | 0.09 |
| Magnesium oxide | 0.02 |
| Metabolizable energy (kcal/kg) | 2,700 |
| Crude protein (%) | 17.80 |
| Calcium (%) | 4.10 |
| Phosphorus (available, %) | 0.45 |
| Methionine (%) | 0.31 |
| Lisine | 0.74 |
| Arginine | 0.70 |
| Threonine | 0.37 |
| Leucine | 0.74 |
| Isoleucine | 0.43 |
| Valine | 0.46 |
| Histidine | 0.25 |
| Phenylalanine | 0.48 |
| Tryptophan | 0.13 |
| Dietary treatment | SEM | P-value | ||
| CON | LPM | |||
| Egg weight, g | 60.64 | 61.03 | 0.53 | 0.722 |
| Shell weight, g | 7.73 | 7.79 | 0.09 | 0.783 |
| Yolk weight, g | 15.82 | 15.68 | 0.22 | 0.747 |
| Albumen weight, g | 35.60 | 35.56 | 0.41 | 0.960 |
| Yolk ratio, % | 26.15 | 25.67 | 0.30 | 0.440 |
| Albumen ratio, % | 58.67 | 58.26 | 0.38 | 0.591 |
| Shell ratio, % | 12.76 | 12.78 | 0.14 | 0.933 |
| Yolk albumen ratio, % | 44.75 | 44.21 | 0.66 | 0.690 |
| Egg shell thickness, mm | 0.43 | 0.40 | 0.025 | 0.602 |
| Yolk color fan (1-15 scale)1 | 7.69 | 7.86 | 0.16 | 0.598 |
| Item | CON | LPM | P-value |
|---|---|---|---|
| Duodenum | |||
| Villus height (µm) | 1046.57±199.55 | 1121.73±211.15 | 0.004 |
| Crypt depth (µm) | 245.78±59.35 | 303.44±11.84 | <0.001 |
| Villus height: crypt depth | 4.25±1.39 | 3.69±1.24 | 0.046 |
| Ileum | |||
| Villus height (µm) | 482.44±102.28 | 593.15±148.24 | <0.001 |
| Crypt depth (µm) | 148.24±30.58 | 201.05±77.25 | <0.001 |
| Villus height: crypt depth | 3.25±1.09 | 2.95±0.61 | 0.145 |
| Dietary treatment | SEM | P value | ||
| CON | LPM | |||
| Animals, n. | 8 | 8 | ||
| Acetic acid | 20.71 | 28.11 | 1.34 | 0.022 |
| Propionic acid | 4.79 | 10.85 | 0.62 | 0.046 |
| Isobutyric acid | 0.83 | 2.40 | 0.15 | <0.001 |
| Butyric acid | 0.54 | 1.40 | 0.08 | 0.048 |
| Isocaproic acid | 1.29 | 1.66 | 0.23 | 0.304 |
| Eptanoic acid | 2.26 | 2.35 | 0.31 | 0.867 |
| Dietary treatment | SEM | P value | ||
| CON | LPM | |||
| Animals, n. | 30 | 30 | ||
| Total protein, g/L | 61.52 | 62.61 | 0.23 | 0.015 |
| Tryglicerides, mg/dL | 796.42 | 669.93 | 35.70 | 0.076 |
| Total cholesterol, mg/dL | 151.48 | 125.73 | 6.19 | 0.037 |
| HDL cholesterol, mmol/L | 20.61 | 24.36 | 0.63 | 0.002 |
| LDL cholesterol, mg/dL | 143.69 | 135.12 | 1.33 | 0.001 |
| NEFA, mmol/L | 1.39 | 1.31 | 0.04 | 0.331 |
| Dietary treatment | SEM | P value | ||
| CON | LPM | |||
| Animals, n. | 30 | 30 | ||
| TOS, mmol H2O2 Eq/L | 23.06 | 17.97 | 0.67 | 0.002 |
| TAS, Trolox Eq/L | 393.78 | 469.71 | 24.74 | 0.11 |
| ROMs, UCarr | 39.29 | 28.07 | 1.74 | 0.001 |
| Dietary treatment | SEM | P value | ||
| CON | LPM | |||
| Animals, n. | 30 | 30 | ||
| TNF-a, pg/mL | 25.85 | 21.07 | 1.10 | 0.029 |
| IL-1b, pg/mL | 69.71 | 57.42 | 2.66 | 0.02 |
| IL-6, pg/mL | 21.61 | 15.95 | 1.16 | 0.013 |
| Dietary treatment | SEM | P value | ||
| CON | LPM | |||
| Animals, n. | 30 | 30 | ||
| ALP, IU/L | 275.71 | 261.98 | 2.92 | 0.017 |
| AST, IU/L | 186.27 | 176.10 | 1.22 | <0.001 |
| ALT, IU/L | 17.16 | 16.18 | 0.11 | <0.001 |
| Bilirubin, mmol/L | 0.99 | 1.03 | 0.30 | 0.299 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





