1. Introduction
In recent years, mathematical modeling using graphs with parameterized theories or invariants has become increasingly popular across various disciplines in the physical sciences. Disciplines such as computer science, physics, and chemistry have utilized these models to solve complex problems. A critical component of these models is the topological index, which provides valuable information about a graph under graph isomorphism conditions. Topological indices are invariant numbers that convey information about the size, symmetry, branching degree, and cyclicity of a graph. In particular, chemical graph theory has emerged as a leading field that combines graph theory with chemistry to study molecular structures. Among molecular descriptors, topological indices have emerged as the most important ones, providing crucial information about graphs that represent chemical compounds. By utilizing these topological indices, researchers can gain valuable insights into the properties and characteristics of various chemical compounds, allowing them to develop more accurate models and predictions. The integration of graph theory and chemistry has opened up new avenues for research in the physical sciences, and it is an exciting area that promises to yield even more insights and breakthroughs in the years to come. (for more details, [
1,
2,
3,
4,
5,
6]). The study of graph theory and its application to chemistry has become a rapidly growing field of research in recent years. In particular, the use of topological graph indexes has proven to be a valuable tool for understanding the structure-property relationships of chemical compounds. The Chemical Data Bases, which contain over 3000 topological graph indexes, demonstrate the vast amount of information that can be extracted from graphs representing chemical structures. Furthermore, the extensive study of dominating problems within graph theory has led to a wealth of knowledge and research in the field. As evidenced by the 1222 papers listed in the 1998 book, dominating problems have been extensively studied and analyzed, providing a solid foundation for the development of new and innovative approaches to solving problems related to chemical structures. With the continued growth and development of graph theory and its applications in chemistry, we can expect to see even more exciting discoveries and advancements in the future [
7,
8,
9,
10].
Highlighted here are some key research studies that demonstrate the significant role of Graph Theory in solving real-world problems. In [
11] the authors present a novel approach to solve fractional boundary value problems on the methylpropane graph. The technique involves utilizing fixed point theorems to demonstrate the existence of solutions, as well as studying stability. To demonstrate the efficacy of the method, an example is provided. Turab et al. [
12] introduce the isobutane graph and investigates the existence of solutions to fractional boundary value problems using fixed point theory. The study includes two examples to support the findings. While [
13], which is Part I of a series of papers, demonstrates that graph theory can be used to provide solutions for problems in distance geometry, potential theory, and theory of metric spaces.
Domination indices are mathematical parameters used in graph theory to study the structural properties of graphs. The concept of domination indices was first introduced in the 1960s, and since then, several types of domination indices have been proposed and investigated [
14]. One of the most commonly used domination indices is the total domination number (
), [
15] which is defined as the minimum number of vertices in a dominating set of a graph. The total domination number has been extensively studied in the literature, with several properties and bounds known. For example, the total domination number of a tree is at most
, where
n is the number of vertices in the tree. Another domination index that has received considerable attention is the connected domination number (
), [
16] which is defined as the minimum number of vertices in a dominating set that induces a connected subgraph. The connected domination number has been studied in various contexts, including its relationship with other graph parameters, such as the vertex cover number and the independent domination number. The domination polynomial [
17] is another important parameter that has been extensively studied in the literature. The domination polynomial of a graph is a polynomial that encodes the number of dominating sets of each size in the graph. Several properties of the domination polynomial have been established, including its relationship with other graph polynomials, such as the chromatic polynomial and the independence polynomial. Other domination indices that have been investigated in the literature include the independent domination number, the bondage number, the game domination number, and the strong domination number [
18]. These parameters have been studied in various contexts, including their relationship with other graph parameters, their computational complexity, and their applicability in real-world problems.
The Sigma index is a topological index introduced by Ivan Gutman in 1978 [
19] and [
20]. It is defined as the square of the difference between the degrees for all pairs of adjacent vertices in a graph. More specifically, let
be a graph with vertex set
V and edge set
E, and let
be the degree of the vertex
u in
. Then, the Sigma index is defined as:
The Sigma index has been extensively studied in the literature due to its applicability in various areas of chemistry, including the prediction of physicochemical properties of molecules [
21]. Several properties and bounds of the Sigma index have been established, including its relationship with other topological indices such as the Wiener index [
22]. Various modifications and generalizations of the Sigma index have also been proposed, such as the modified Sigma index, which takes into account the number of vertices at a given distance from a central vertex [
23]. The degree-based Sigma index has also been introduced, which weights the contributions of each pair of vertices by their respective degrees [
24]. Overall, the Sigma index and its variations have been shown to be valuable tools in the study of molecular structure and properties, as well as in the analysis of various types of networks. Detailed discussions of Sigma index applications can be found in [
25,
26,
27,
28,
29]
Hanan Ahmed introduced the concept of domination topological indices in 2021 [
30]. The domination topological index (DTI) is defined as the sum of the distances between each vertex and its nearest dominating vertex in a graph. The domination number of a graph is the minimum number of vertices required to dominate the graph, and it is a special case of the DTI. The DTI has been shown to be a useful tool for predicting various physicochemical properties of organic compounds. Several variations and extensions have been proposed in the literature. For example, Hosamani et al. proposed a modified version of the DTI called the modified domination topological index (MDTI) in their paper [
31]. The MDTI is defined as the sum of the distances between each vertex and its nearest dominating vertex, where the dominating set is restricted to a subset of vertices with a fixed size. Another variation of the DTI is the connected domination topological index (CDTI), which was introduced by Merrick et al. [
32]. The CDTI is defined as the sum of the distances between each vertex and its nearest dominating vertex in a connected subgraph of the graph. The DTI and its variations have been applied in various fields, including chemistry, biology, and computer science. For example, Yousefi et al. [
33] used the DTI to develop quantitative structure-property relationship (QSPR) models for predicting the boiling points of organic compounds. In another study, Yang et al. used the CDTI to predict the toxicity of polycyclic aromatic hydrocarbons (PAHs) [
34]. Overall, the DTI and its variations have proven to be useful tools for studying the structural properties of graphs and predicting various physicochemical properties of organic compounds. Extensive explanations regarding the applications of topological domination indices can be found in [
35,
36,
37,
38,
39,
40,
41].
The combination of the Gutman topological Sigma index and Hanan et al. domination topological index into a single concept, the
domination Sigma index, holds great promise in enhancing our understanding of the structural properties of graphs. This new index combines the mathematical principles of domination topological indices with the concept of Sigma index, creating a distinct index that offers valuable information about the molecular properties. The topological Sigma index provides important information about molecular size, symmetry, branching degree, and cyclicity, while the domination topological index provides insights into dominating sets, subsets of the vertex set such that every vertex outside the set is adjacent to at least one vertex inside the set. The combination of these two indices allows for a more comprehensive analysis of the properties of graphs and has the potential to improve our ability to predict the physicochemical properties of organic compounds. This article presents a novel approach to combining these two indices, providing a framework for further research in the field of mathematical chemistry. Our study presents a novel approach that combines the core principles of the Sigma index with the domination degrees of vertices in a graph to formulate a new composite index. This index is termed as the domination Sigma index and is defined as follows:
In [
30] Hanan Ahmed et al. have introduced new degree-based topological indices called domination topological indices, which are based on the domination degree set defined as: For each vertex
, the domination degree of the vertex
v is denoted by
and defined as the number of minimal dominating set of
which contains
v. The first and second domination Zagreb indices and modified first Zagreb domination indices are defined respectively as:
The forgotten domination, hyper domination, and modified forgotten domination indices of graphs are defined respectively as:
In this study, we aim to investigate the potential of the domination Sigma index and other domination topological indices in predicting the properties of Octanes and its isomers through quantitative structure-property relationship (QSPR) analysis. To achieve this goal, we calculate the domination Sigma index for various families of graphs, including book graphs, compositions of graphs, and special graph classes and find some sharp bounds. By analyzing the domination indices and the new topological index, we hope to gain insights into the properties and structures of these molecules, and apply this knowledge in the design of new chemical compounds with desired properties. However, it is important to acknowledge that the domination Sigma index may not always produce satisfactory results in QSPR analysis. As newly proposed topological indices may not always capture the key features of the chemical structure under investigation, it is not uncommon for a new index to fail to meet expectations. The lack of satisfactory results obtained from the newly introduced topological index may be attributed to its limitations and the nature of the specific property being studied. Nonetheless, it is worth noting that the failure of a new topological index to provide satisfactory results in QSPR analysis does not necessarily imply that the index is of no value. In fact, each new index provides valuable insights into the complex relationship between molecular structure and physical properties. However, the other domination topological indices used in this study have demonstrated good correlation coefficients with the properties of Octanes and its isomers, indicating their effectiveness in predicting such properties. Thus, further research and analysis may be needed to explore the potential of the domination Sigma index in predicting other properties or to modify it to better suit the studied property. Overall, this study contributes to the ongoing process of developing new topological indices and enhancing our understanding of the relationship between molecular structure and physical properties.
2. Preliminaries
In this section, we introduce key concepts, definitions, and assumptions that underpin our research, as well as outline our research questions, methodology, and the structure of our paper.
Let
be connected simple graph with
a set of vertices and
a set of edges. A set
is said to be
a dominating set of a graph
, if for any vertex
, there is a vertex
such that
u and
v are adjacent. A dominating set
is minimal if
is not a dominating set. A dominating set of
of minimum cardinality is said to be a minimum dominating set. Define
, and
Notation
which indicates the total number of minimal dominating set of
and
A graph is said to be full degree graph if the vertices are all of full degree. The
book graph is defined as the graph Cartesian product
where
is a star graph and
is the path graph on two vertices.The Windmill graph
is an undirected graph constructed for
and
by
s copies of the complete graph
at a shared universal vertex. Examples of these two graphs are desiplies in
Figure 1 and
Figure 2. A join of two graphs
and
is denoted by
, with disjoint vertex sets
and
is the graph on the vertex set
and the edge set
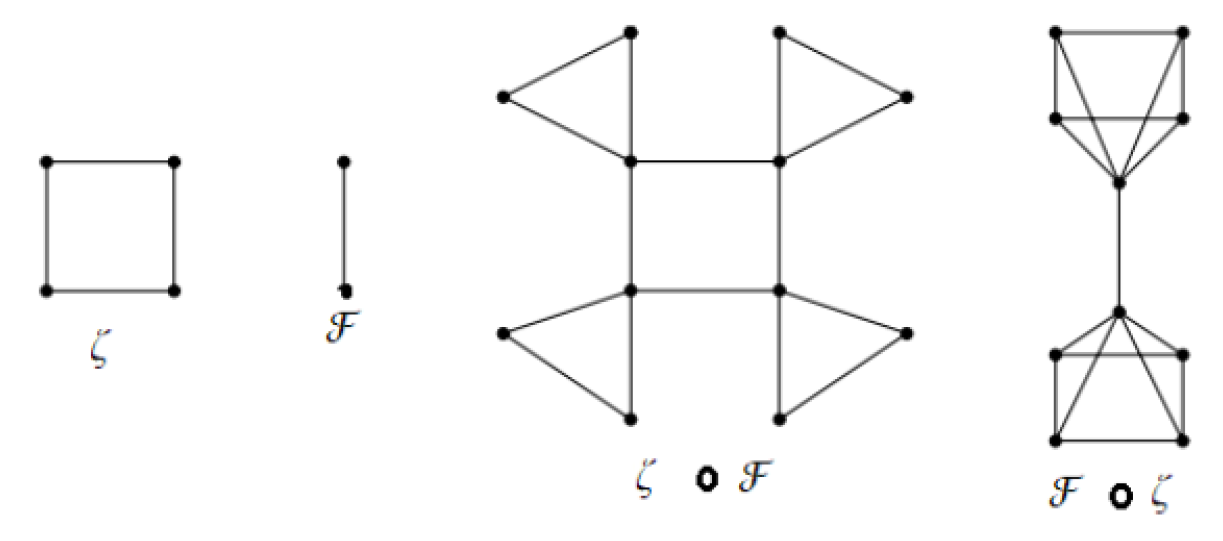
The corona product of two graphs
and
is defined as the graph obtained by taking one copy of
and
copies of
and joining the
vertex of
to every vertex in the
copy of
an example in
Figure 3.
Definition 1. A domination regular graph is a type of graph in which every vertex has exactly k neighbors that are also adjacent to each other. In other words, every vertex is adjacent to exactly k vertices that are themselves mutually adjacent.
In this work, the concept of
domination regular graph plays a crucial role, and therefore it is important to provide some literature review on this terminology. In the following, we will provide an overview of the relevant literature on
domination regular graphs, highlighting their key properties and applications. By understanding the background and context of this concept, readers will be better equipped to comprehend the significance of our research and its contribution to the field.
domination regular graphs have been studied in several papers in graph theory. For example, in [
42], the authors investigate the structure of
domination regular graphs and show that these graphs have many interesting properties. They also provide several examples of
domination regular graphs and use them to study the domination number of these graphs. In [
43] the authors studies the
domination number of graphs and provides a characterization of
domination regular graphs. The authors show that a connected graph is
domination regular if and only if it is regular and satisfies a certain condition related to the
domination number. They also investigate the relationship between the
domination number and the total domination number of a graph. In [
44] Hansberg et al. investigate some properties of
domination regular graphs and provide some examples of these graphs. They also study the relationship between the
domination number and the independence number of a graph, and show that for certain families of graphs, the
domination number and the independence number are equal. Finally, in [
45], the authors provide several methods for constructing
domination regular graphs. They show that
domination regular graphs can be constructed from other
domination regular graphs by several operations, including Cartesian product and composition. They also provide some open problems related to
domination regular graphs, such as finding the smallest
k for which a
domination regular graph exists. In summary,
domination regular graphs have been studied extensively in the literature, and they have many interesting properties and applications in graph theory. Further research on these graphs can lead to new insights and solutions in various fields, including computer science, chemistry, and physics.
Our paper aims to address several research questions related to the use of the dominating sigma index in predicting the physicochemical properties of organic compounds. Firstly, we provide an explanation of the dominating sigma index and its relationship with topological indices in mathematical chemistry. Next, we develop linear and non-linear models using the QSPR approach to predict the properties of interest and conduct a comparative analysis to evaluate the effectiveness of the domination sigma index and establish an appropriate domination index that correlates with the physicochemical properties of octane and its isomers. Finally, we explore the relationship between topological indices and molecular properties. The paper is structured as follows:
Section 3 presents the main results of the domination sigma index, while
Section 4 describes the comparative analysis conducted to evaluate its effectiveness and establish an appropriate domination index. Finally, in
Section 5, we provide our conclusions and suggestions for further research into QSAR/QSPR domination indices.
3. Main Results
In this Section, we give results of domination Sigma index for the star, complete bipartite and its complement, book graphs, and for the Windmill graph.
Proposition 1.
Let the star graph of vertices, then
Let the complete graph of r vertices, then
Let the double star graph, then
Let the complete bipartite graph where then
Proof.
Suppose the star graph of r vertices, we have and for all Then
Suppose the complete graph of r vertices, we have and for all Then
Suppose the double star graph, we have and for all Then
-
Suppose
the complete bipartite graph where
, we have
and
This completes the proof.
□
Corollary 1. Any graph ζ is domination regular graph if and only if
Proof. In this case, the sufficient condition is clear. If we consider the necessity condition,
for all
, so if
then
is
domination regular graph. □
Corollary 2. Let ζ be the complete bipartite graph Then
Proposition 2. If then
Proof. Since
hence we have
□
Theorem 1. If where Then
Proof. If
where
we have
and
Which complete the proof. □
Theorem 2. If the book graph for Then
Proof. If
the book graph for
we have
and
Suppose
denote the set of
r edges
with initial and terminal vertices of the same domination degree
Let
denote the set containing only one edge
with initial and terminal vertices of the same domination degree 3. Let
denote the set of
edges of initial vertices of the domination degree 3 and terminal vertices of domination degree
Hence
Which complete the proof. □
Theorem 3. If where . Then
Proof. Suppose that
where
, note that for any vertex
we have
and
where
Hence
□
3.1. Domination Sigma Index of Some Graph Operations
This part, provides the domination Sigma index values for some graph operations such as corona product and join of graphs. In the following Theorem we calculate domination Sigma index for the corona graph of any graph and the complete graph and its complement.
Proof.
- (1)
Let
we note that there are
minimal dominating sets in
and
Also, there are three types of edges in
All edges of
all edges of
and
denote the set of all edges that connect vertex from
and a vertex from
So, we have
- (2)
Let for any vertex we have and Hence is domination regular graph, where which implies that
□
In the following Theorems we calculate domination Sigma index for different cases of join of two graphs. But first we need the following Lemma
Lemma 1.
[35] Let and be any non complete graphs of and vertices respectively, and there is no vertex in or of full degree. Then and
Theorem 5.
Let and be any non complete graphs of and vertices respectively, and there is no vertex in or of full degree. Then
Proof. Let
and
be any non complete graphs of
and
vertices respectively, and there is no vertex in
or
of full degree. Then
Now adding (1), (2) and (3) we conclude our result. □
Using the fact [
35] that for any graph
we have
We conclude the following
Corollary 3.
Let and be any non complete graphs of and vertices respectively, and there is no vertex in or of full degree. Then
Proposition 3. Let and are both complete graphs of and vertices respectively. Then
Proof. Let and are both complete graphs of and vertices respectively, then and for all which implies that is domination regular graph. Hence □
Theorem 6.
If is a complete graph and is not a complete graphs of and vertices respectively. Then
Proof. Let
be a complete graph and
is not a complete graphs of
and
vertices respectively, then
and
Adding , and we complete the proof. □
Theorem 7.
If is not a complete graph and is a complete graph of and vertices respectively. Then
Proof. Let
be not complete graph and
is a complete graphs of
and
vertices respectively, then
and
Adding , and we complete the proof. □
Lemma 2.
If and are any non complete graphs of and vertices and edges respectively, such that contains all vertices of full degrees and contains all vertices of full degrees. Then and
Theorem 8.
If and are any non complete graphs of and vertices and edges respectively, such that contains all vertices of full degrees and contains all vertices of full degrees. Then
Proof. Suppose
and
are any non complete graphs of
and
vertices and edges respectively, such that
contains all vertices of full degrees and
contains all vertices of full degrees. Then
and
We divide the edge set of
according to the degree domination of the vertices as follows:
Now we calculate the summation for each term as follows:
We notice that the edges of
are
and
. Hence
We notice that the edges of the graph
are the edges of
and
Hence
Now we move to calculate the results on edges between vertices of
and vertices of
Now adding (1),(2), and (3) we obtain our result. □
3.2. Bounds for Domination Sigma Index
To make accurate predictions using topological indices, it is crucial to comprehend the potential range of values that these indices can assume. Determining the bounds of topological indices is a significant task when it comes to predicting the properties of chemical compounds. The bounds refer to the minimum and maximum values that an index can attain. Being aware of the bounds of a topological index can assist researchers in identifying the range of values that are physically relevant and in gaining an understanding of the connection between the topological characteristics of a molecular graph and the properties of a chemical compound. In this section, we will present some lower and upper bounds for the Domination Sigma index.
Theorem 9.
If with order n and size m is not a domination regular graph then
Proof.
In the left side we take the summation over all vertices of
and in the right side we take the summation over all edges of
Hence
Which complete the proof. □
Theorem 10.
If with order n and size m is not a domination regular graph then
Proof. We have
hence
Similarly of Theorem 9 we get
Hence □
Proposition 4. If with order n and size m is not a domination regular graph then
Proof. We have Hence by Theorems 9 and 10 we obtain our results. □
4. Statistical Validity of Domination Indices
Physicochemical and biological properties of molecules are predicted and modeled through QSPR analysis. To extract maximum information from a data set, chemometrics is a powerful tool that incorporates statistical and mathematical methods. Chemical descriptors of a molecule’s chemical structure are used in QSPR to describe how physicochemical properties vary as a consequence of chemometric methods. Hence, calculated descriptors can replace expensive biological tests or experiments concerning a particular physicochemical property. In turn, these descriptors can be used to predict the properties of interest for upcocompounds. QSPR works by finding a quantitative relationship that can be utilized for the prediction of compounds’ properties, even those that can’t be measured. As a matter of fact, QSPR models are primarily affected by the molecular structure parameters employed. Alternative molecular descriptors have been developed that are derived solely from chemical structure information. Researchers have focused much attention on connecting and composing molecules in order to determine "topological indices" that can be used in QSPR analyses. In addition to having the advantage of simplicity, the topological index is also fast to calculate, so scientists are interested in using it. A compound’s physicochemical and biological properties are influenced by its molecular structure, according to several chemical and medical experiments. Quantitative structure property/activity relationship
models are generated by employing mathematical/statistical tools to determine this dependence. Regression models are used for relating physicochemical/biological properties to molecular descriptors. The graph-theoretic topological indices generate
models by converting compounds into chemical graphs. To be accepted by Milan Randic [
46], topological indices must meet certain criteria, the most significant of which is to be positively correlated with at least one physicochemical property. The purpose of this section is to investigate the significance of these newly developed domination topological indices. Octane and some of its isomers are described in
Table 1 according to their experimental data [
47], and also
https://pubchem.ncbi.nlm.nih.gov (accessed on 26 March 2022). Computed domination indices values are shown in
Table 2. Our analysis has shown that these indices play role in evaluating Entropy (E), Acentric factor (AF), Enthalpy of vaporization (HVAP), and Standard enthalpy of vaporization (DHVAP). A correlation coefficient
between these indices and some physicochemical properties can be seen in
Table 3.
4.1. Regression Model
QSPR analysis of domination topological indices will be discussed in this subsection. Furthermore, we demonstrate a positive correlation between the characteristics and the physicochemical characteristics of octane and its isomers. Here we discuss how topological indices can be used to predict physicochemical properties. We calculated six domination topological indices and one physicochemical property using R-software. Based on the below nonlinear regression model, we can derive different nonlinear models for the topological indices of domination:
For the domination Sigma index we use the linear regression model because we have some values of zeros
where
is the physical and chemical properties of octanes and its isomers and
represents the domination topological indices. By using Equations
9 and
10, we can obtain different linear and non-linear models for the domination topological indices as follows:
The domination first Zagreb index
The domination second Zagreb index
The domination modified first Zagreb index
The domination forgotten index
The domination hyper index
The domination modified forgotten index
The domination Sigma index
Table 4.
The (AF) values predicted by domination topological indices
Table 4.
The (AF) values predicted by domination topological indices
| octane and its isomers |
|
|
|
|
|
|
| n-octane |
|
|
|
|
|
|
| 2-Methyl-heptane |
|
|
|
|
|
|
| 3-Methyl-heptane |
|
|
|
|
|
|
| 4-Methyl-heptane |
|
|
|
|
|
|
| 3-Ethyl-hexane |
|
|
|
|
|
|
| 2,2-Dimethyl-hexane |
|
|
|
|
|
|
| 2,3-Dimethyl-hexane |
|
|
|
|
|
|
| 2,4-Dimethyl-hexane |
|
|
|
|
|
|
| 2,5-Dimethyl-hexane |
|
|
|
|
|
|
| 3,3-Dimethyl-hexane |
|
|
|
|
|
|
| 3-Ethyl-2-methyl-pentane |
|
|
|
|
|
|
| 2,2,3-Trimethyl-pentane |
|
|
|
|
|
|
| 2,2,4-Trimethyl-pentane |
|
|
|
|
|
|
| 2,3,3-Trimethyl-pentane |
|
|
|
|
|
|
| 2,3,4-Trimethyl-pentane |
|
|
|
|
|
|
| 2,2,3,3-Tetramethylbutane |
|
|
|
|
|
|
Table 5.
The (E) values predicted by domination topological indices
Table 5.
The (E) values predicted by domination topological indices
| octane and its isomers |
|
|
|
|
|
|
| n-octane |
|
|
|
|
|
|
| 2-Methyl-heptane |
|
|
|
|
|
|
| 3-Methyl-heptane |
|
|
|
|
|
|
| 4-Methyl-heptane |
|
|
|
|
|
|
| 3-Ethyl-hexane |
|
|
|
|
|
|
| 2,2-Dimethyl-hexane |
|
|
|
|
|
|
| 2,3-Dimethyl-hexane |
|
|
|
|
|
|
| 2,4-Dimethyl-hexane |
|
|
|
|
|
|
| 2,5-Dimethyl-hexane |
|
|
|
|
|
|
| 3,3-Dimethyl-hexane |
|
|
|
|
|
|
| 3-Ethyl-2-methyl-pentane |
|
|
|
|
|
|
| 2,2,3-Trimethyl-pentane |
|
|
|
|
|
|
| 2,2,4-Trimethyl-pentane |
|
|
|
|
|
|
| 2,3,3-Trimethyl-pentane |
|
|
|
|
|
|
| 2,3,4-Trimethyl-pentane |
|
|
|
|
|
|
| 2,2,3,3-Tetramethylbutane |
|
|
|
|
|
|
Table 6.
The (HVAP) values predicted by domination topological indices
Table 6.
The (HVAP) values predicted by domination topological indices
| octane and its isomers |
|
|
|
|
|
|
| n-octane |
|
|
|
|
|
|
| 2-Methyl-heptane |
|
|
|
|
|
|
| 3-Methyl-heptane |
|
|
|
|
|
|
| 4-Methyl-heptane |
|
|
|
|
|
|
| 3-Ethyl-hexane |
|
|
|
|
|
|
| 2,2-Dimethyl-hexane |
|
|
|
|
|
|
| 2,3-Dimethyl-hexane |
|
|
|
|
|
|
| 2,4-Dimethyl-hexane |
|
|
|
|
|
|
| 2,5-Dimethyl-hexane |
|
|
|
|
|
|
| 3,3-Dimethyl-hexane |
|
|
|
|
|
|
| 3-Ethyl-2-methyl-pentane |
|
|
|
|
|
|
| 2,2,3-Trimethyl-pentane |
|
|
|
|
|
|
| 2,2,4-Trimethyl-pentane |
|
|
|
|
|
|
| 2,3,3-Trimethyl-pentane |
|
|
|
|
|
|
| 2,3,4-Trimethyl-pentane |
|
|
|
|
|
|
| 2,2,3,3-Tetramethylbutane |
|
|
|
|
|
|
Table 7.
The (DHVAP) values predicted by domination topological indices
Table 7.
The (DHVAP) values predicted by domination topological indices
| octane and its isomers |
|
|
|
|
|
|
| n-octane |
|
|
|
|
|
|
| 2-Methyl-heptane |
|
|
|
|
|
|
| 3-Methyl-heptane |
|
|
|
|
|
|
| 4-Methyl-heptane |
|
|
|
|
|
|
| 3-Ethyl-hexane |
|
|
|
|
|
|
| 2,2-Dimethyl-hexane |
|
|
|
|
|
|
| 2,3-Dimethyl-hexane |
|
|
|
|
|
|
| 2,4-Dimethyl-hexane |
|
|
|
|
|
|
| 2,5-Dimethyl-hexane |
|
|
|
|
|
|
| 3,3-Dimethyl-hexane |
|
|
|
|
|
|
| 3-Ethyl-2-methyl-pentane |
|
|
|
|
|
|
| 2,2,3-Trimethyl-pentane |
|
|
|
|
|
|
| 2,2,4-Trimethyl-pentane |
|
|
|
|
|
|
| 2,3,3-Trimethyl-pentane |
|
|
|
|
|
|
| 2,3,4-Trimethyl-pentane |
|
|
|
|
|
|
| 2,2,3,3-Tetramethylbutane |
|
|
|
|
|
|
Table 8.
Statical parameters for the non-linear QSPR model for first Zagreb domination index.
Table 8.
Statical parameters for the non-linear QSPR model for first Zagreb domination index.
| Physical Properties |
|
|
F |
|
|
| Acentric factor
|
|
|
|
|
|
| Entropy
|
|
|
|
|
|
| Enthalpy of vaporization
|
|
|
|
|
|
| Standard enthalpy of vaporization
|
|
|
|
|
|
Table 9.
Statical parameters for the non-linear QSPR model for the second domination Zagreb index.
Table 9.
Statical parameters for the non-linear QSPR model for the second domination Zagreb index.
| Physical Properties |
|
|
F |
|
|
| Acentric factor
|
|
|
|
|
|
| Entropy
|
|
|
|
|
|
| Enthalpy of vaporization
|
|
|
|
|
|
| Standard enthalpy of vaporization
|
|
|
|
|
|
Table 10.
Statical parameters for the linear QSPR model for modified zagreb domination index.
Table 10.
Statical parameters for the linear QSPR model for modified zagreb domination index.
| Physical Properties |
|
|
F |
|
|
| Acentric factor
|
|
|
|
|
|
| Entropy
|
|
|
|
|
|
| Enthalpy of vaporization
|
|
|
|
|
|
| Standard enthalpy of vaporization
|
|
|
|
|
|
Table 11.
Statical parameters for the non-linear QSPR model for forgotten domination index.
Table 11.
Statical parameters for the non-linear QSPR model for forgotten domination index.
| Physical Properties |
|
|
F |
|
|
| Acentric factor
|
|
|
|
|
|
| Entropy
|
|
|
|
|
|
| Enthalpy of vaporization
|
|
|
|
|
|
| Standard enthalpy of vaporization
|
|
|
|
|
|
Table 12.
Statical parameters for the linear QSPR model for hyper domination index.
Table 12.
Statical parameters for the linear QSPR model for hyper domination index.
| Physical Properties |
|
|
F |
|
|
| Acentric factor
|
|
|
|
|
|
| Entropy
|
|
|
|
|
|
| Enthalpy of vaporization
|
|
|
|
|
|
| Standard enthalpy of vaporization
|
|
|
|
|
|
Table 13.
Statical parameters for the non-linear QSPR model for modified forgotten domination index.
Table 13.
Statical parameters for the non-linear QSPR model for modified forgotten domination index.
| Physical Properties |
|
|
F |
|
|
| Acentric factor
|
|
|
|
|
|
| Entropy
|
|
|
|
|
|
| Enthalpy of vaporization
|
|
|
|
|
|
| Standard enthalpy of vaporization
|
|
|
|
|
|
Table 14.
Statical parameters for the linear QSPR model for sigma domination index.
Table 14.
Statical parameters for the linear QSPR model for sigma domination index.
| Physical Properties |
|
|
F |
|
|
| Acentric factor
|
|
|
|
|
|
| Entropy
|
|
|
|
|
|
| Enthalpy of vaporization
|
|
|
|
|
|
| Standard enthalpy of vaporization
|
|
|
|
|
|
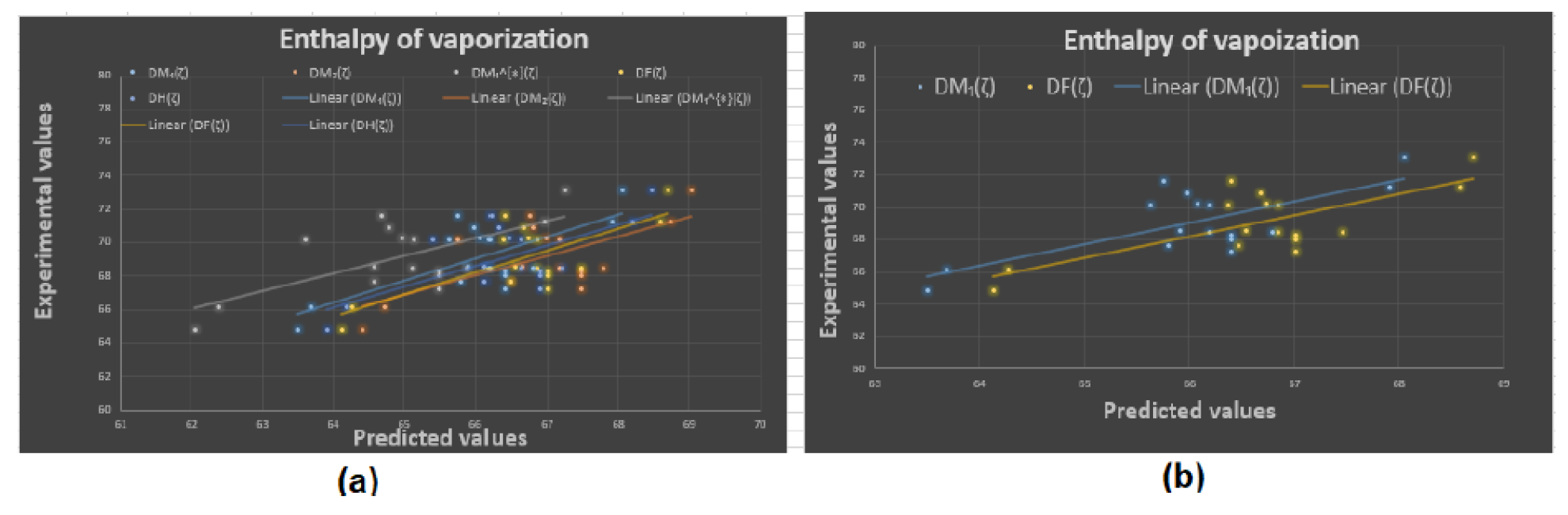
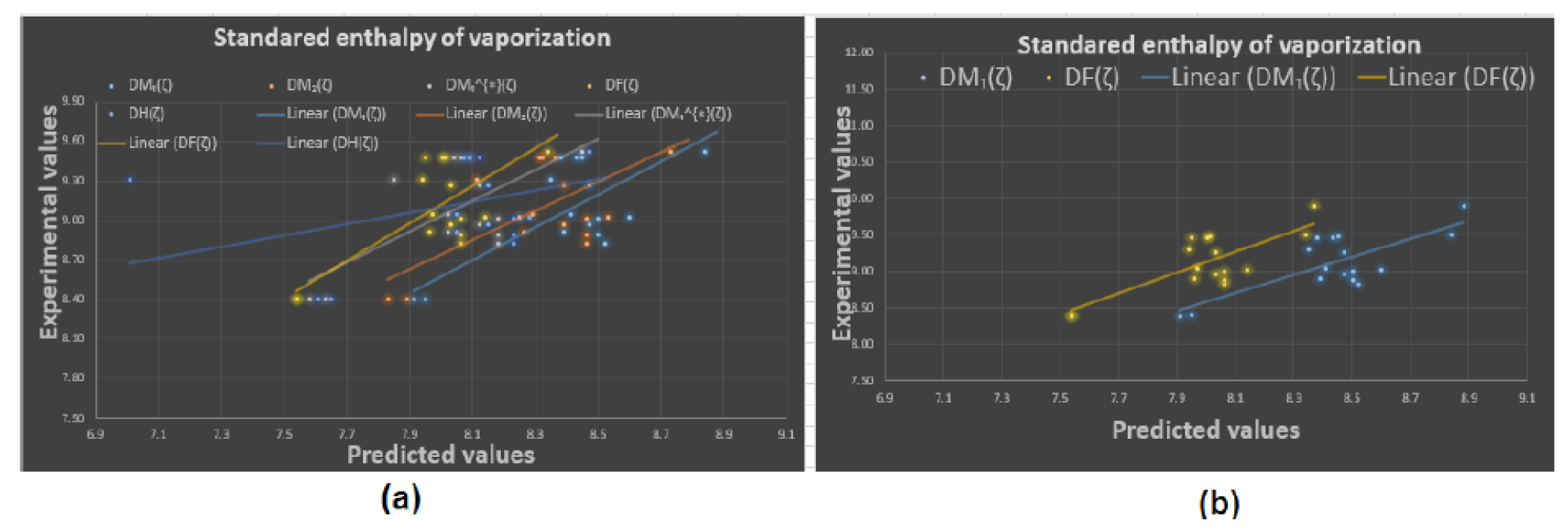
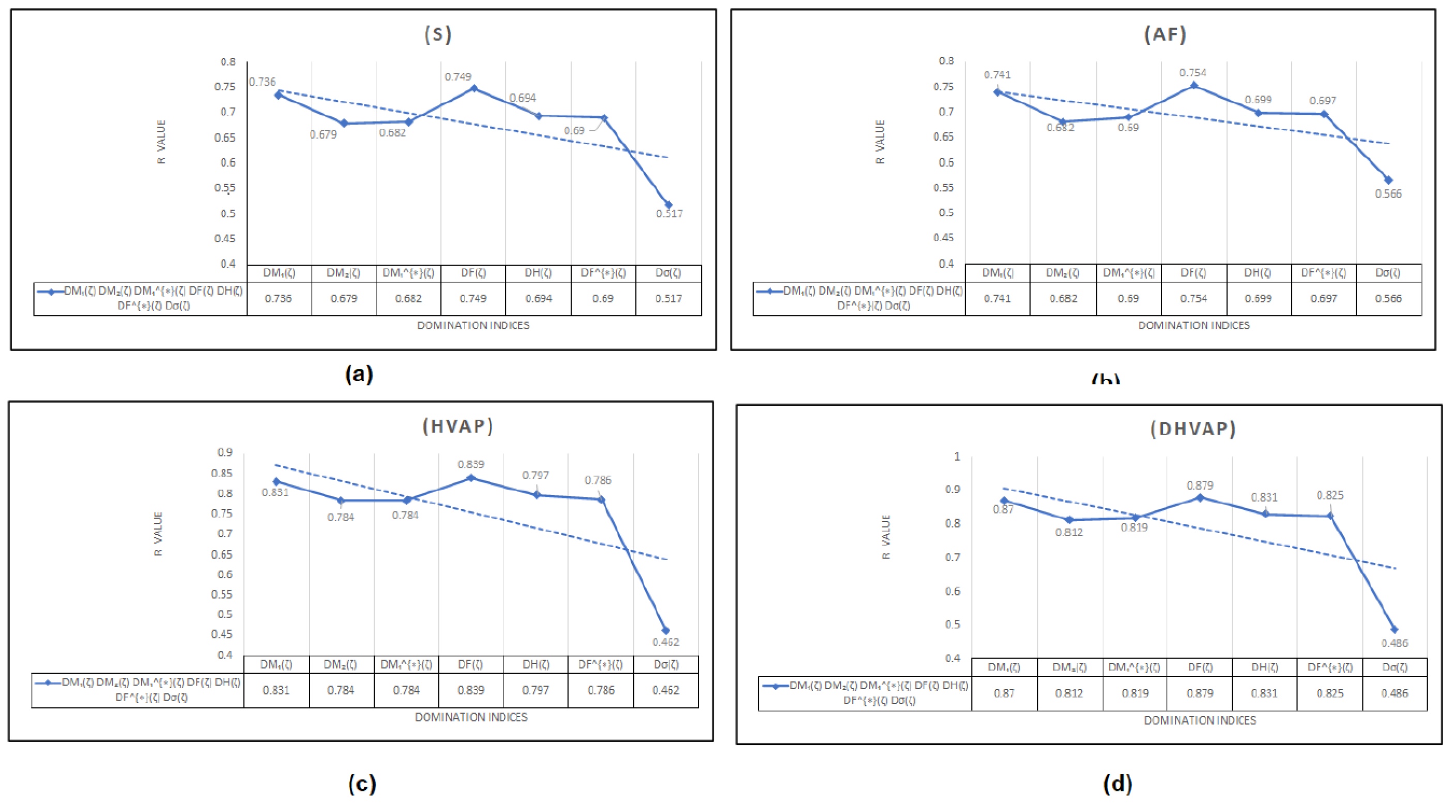
4.2. Results and Discussion
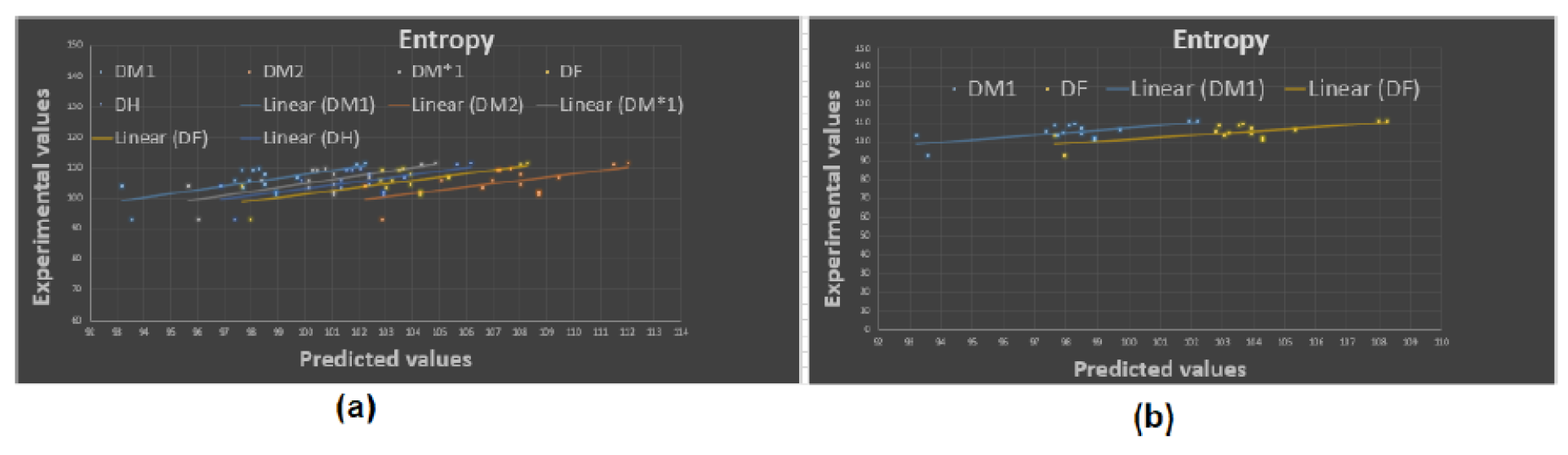
To begin with, it was discovered see
Figure 8, that any structure-property relationship could be achieved using the domination forgotten index
. Based on
Table 3 we can determine that the domination forgotten index
is the most appropriate index to model Standard enthalpy of vaporization
, Enthalpy of vaporization
, Entropy
, and Acentric factor
with
, and
, respectively. We have found that our approach using the forgotten domination index
has provided a significant improvement in predicting the physicochemical properties of octane and its isomers compared to some of the recent studies. For example, in a study by Xie et al. [
48], they used machine learning techniques to predict the physicochemical properties of organic compounds, including octane and its isomers, and reported correlation coefficients between
and
for various properties. In contrast, our approach using the
index achieved higher correlation coefficients of
to
for the same properties. It has been demonstrated that the Standard Enthalpy of Vaporization
with correlation coefficient range between
(except the correlation coefficient for the dominion Sigma index
which was
) is best physicochemical property predicted by the new domination indices. We can see from
Table 3 that for
and
the domination first Zagreb index
gives the second highest correlation coefficients
and
respectively. Furthermore, in a recent study by Zhang et al. [
49], they used graph theory-based topological indices to predict the boiling points of organic compounds, including octane and its isomers, and reported correlation coefficients of
to
. In our study, we have also used graph theory-based topological indices, and our approach using the domination first Zagreb index
achieved a correlation coefficient of
for predicting the standard enthalpy of vaporization
of octane and its isomers. Therefore, we believe that our work provides a significant contribution to the field of predicting the physicochemical properties of organic compounds and offers a promising alternative to the existing approaches. It is worth noting that our finding that the domination first Zagreb index
provides a high degree of correlation for predicting various physicochemical properties of octane and its isomers is consistent with several recent studies in the field. For example, a study by Ghorbani et al. [
50] also found that the
index was a reliable predictor of various physicochemical properties of hydrocarbons, including octane. Similarly, a study by Moosavi et al. [
51] also reported that the
index was a useful predictor of the thermodynamic properties of various hydrocarbons, including isomers of octane. Overall, our findings, along with those of other studies, highlight the potential usefulness of the
index in predicting the physicochemical properties of octane and its isomers. However, it is important to note that the effectiveness of this index, like any other index or method, may depend on various factors such as the size and diversity of the dataset, the modeling techniques used, and the specific properties being predicted. Therefore, further investigation is needed to fully understand the potential and limitations of the
index and other related indices in the context of predicting physicochemical properties of octane and its isomers.
It is important to note that the other domination topological indices used in this study have demonstrated good effectiveness in predicting the properties of Octanes and its isomers. However, it is also essential to acknowledge that the domination Sigma index, while providing valuable insights into the complex relationship between molecular structure and physical properties, showed poor correlation in this particular study, since it does not allow modifying the values of it using the non-linear model. The limitations of newly proposed topological indices and the specific property being studied can affect its effectiveness. As such, this study highlights the need for further research to explore modifications to the domination Sigma index for better suitability or to investigate its potential in predicting other properties. Additionally, investigating the effectiveness of the newly proposed index for graphs that are not domination regular graph can be an exciting avenue for future research.
Author Contributions
Conceptualization, S.W. and H.A., methodology, S.W., validation, S.W. and H.A., formal analysis, S.W., investigation, S.W. and H.A., resources, H.A., data curation, H.A., writing— original draft S.W., preparation, S.W., writing— review and editing, S.W.and H.A., supervision, S.W., project administration, S.W., funding acquisition, S.W. All authors have read and agreed to the published version of the manuscript.