1. Introduction
In forest ecosystems, soil extracellular enzymes play essential roles in nutrient cycling and carbon (C) dynamics [
1,
2,
3,
4]. Litters deposited on the forest floor provide nutrients to the soil, but it is the enzymes that break down the organic matter, allowing organisms to reuse the nutrients. Soil extracellular enzymes are predominantly synthesized by soil microorganisms and plant roots, but leaf litter [
5] or throughfall [
6,
7,
8] can also contribute to the pool of soil extracellular enzymes.
In ecosystems where multiple enzymes coexist, the activity of soil extracellular enzymes (i.e.,
Vmax) can serve as an indicator of enzyme abundance [
9,
10]. This enzyme abundance reflects the net result of both enzyme production and degradation, as soil extracellular enzymes can be degraded by proteases [
11,
12]. However, the degradation of these enzymes is rarely measured, despite the fact that the degradation rate of enzymes is often used in ecosystem models. Allison [
13] measured the degradation of enzymes by monitoring changes in enzyme activity using gamma-irradiated soils where enzyme production by microorganisms was halted. Schimel et al. [
11], on the other hand, used chloroform fumigation to halt microbial enzyme production and observed enzyme decay ratios. Both studies reported a significant decrease in enzyme activity during the incubation period, typically within a few weeks, but the degree of decrease varied among different enzymes. Assuming dynamic equilibrium, these results suggest that a similar amount of enzymes is being continuously produced in the field as the amount that is degraded. In field conditions, plants continuously supply resources such as C and nutrients through litters and root exudates. Additionally, plants also provide enzymes to soils through litter layers [
5] or throughfall [
6,
8]. However, the degree to which these resources or enzyme supplies contribute to the soil enzyme pool remains unknown. The first objective of this study is to investigate the extent to which enzyme activity decreases in an incubation conducted under laboratory conditions where these resource supplies are disrupted.
Changes in the availability of nutrients, such as phosphorus (P) and nitrogen (N), strongly influence soil enzyme activity, as well as other ecosystem processes such as primary production [
14,
15], soil respiration [
16,
17,
18], and litter decomposition [
19,
20,
21]. Therefore, numerous studies have investigated the effect of nutrient addition on soil enzyme activity, and multiple meta-analyses have been published demonstrating this impact [
22,
23,
24,
25,
26,
27,
28,
29]. For example, a meta-analysis has shown that N fertilization can stimulate enzymes related to N mineralization [
26], whereas it has also been demonstrated that protein- and chitin-degrading enzymes may be suppressed by N fertilization due to a reduced need for N acquisition [
30,
31]. P addition has generally been found to suppress phosphatase activity [
23,
24]. On the other hand, it has been observed that phosphatase activity is often stimulated by N addition, which is attributed to the increased N supply available for synthesizing phosphatase [
28,
32,
33]. Oxidative enzymes have been shown to be suppressed by N fertilization [
23,
25], but the precise mechanisms are still under discussion [
34,
35]. Thus, the impact of nutrient addition on enzyme activity has been extensively investigated to better understand material dynamics in forest ecosystems. However, it is important to note that the effects of nutrient addition on enzyme activity have often been attributed solely to changes in enzyme production without fully considering degradation [
3], despite the fact that most of the studies have attributed the changes in enzyme activity affected by nutrient addition to changes in enzyme production. The assumption that changes in enzyme activity account for the impacts of nutrient addition on soil enzyme production can lead to a serious misinterpretation. Therefore, the second objective of our study is to demonstrate that the impact of nutrient addition on soil enzyme activity cannot be solely attributed to changes in enzyme production.
To achieve these objectives, we first tested how enzyme activity changes during incubation when there is a disruption in the continuous supply of organic matter or non-soil microbial-derived enzymes. Second, we compared the changes in enzyme activity during incubation in fertilized and unfertilized soils.
2. Materials and Methods
Study site
The study was conducted at the Heshan National Field Research Station of Forest Ecosystems in southern China [
36]. Two 33-year-old plantation forests were chosen for the experiment, namely a leguminous acacia plantation (AA), dominated by
Acacia auriculiformisA.Cunn. ex Beth. with the occasional occurrence of
Acacia mangium Willd., and a non-leguminous eucalyptus plantation (EU), dominated by
Eucalyptus urophylla S.T. Blake. Experimental plots were established in both forests. The soil type of both plantations is acrisols, and the annual temperature and mean annual precipitation are 21.7 °C and 1580.4 mm, respectively [
36]. This site has a high N deposition rate through rainfall, with an average of 43.1 ± 3.9 kg N ha
–1yr
–1 from July 2010 to June 2012 [
37].
Table 1 shows the basic information of the study site.
Experimental design
In July 2010, a fertilization experiment was initiated with seven different treatments that involved the addition of N and/or P as amendments [
37]: control with no fertilization (Con), medium-N fertilized (MN, 50 kg N ha
–1yr
−1), high-N fertilized (HN, 100 kg N ha
–1yr
−1), medium-P fertilized (MP, 50 kg P ha
–1yr
−1), high-P fertilized (HP, 100 kg P ha
–1yr
−1), medium-NP fertilized (MNP, 50 kg P ha
–1yr
−1 and 50 kg N ha
–1yr
−1), and high-NP fertilized (HNP,100 kg P ha
–1yr
−1 and 100 kg N ha
–1yr
−1). Each plot consisted of three replicates and was 10 m × 10 m in size. P-fertilized plots were treated with sodium biphosphate (NaH
2PO
4) spray, and N-fertilized plots were treated with ammonium nitrate (NH
4NO
3). Both NaH
2PO
4 and NH
4NO
3 were dissolved in water and treated bimonthly. Con plot was received commensurable water spray only. Nutrient addition began in August 2010 and is still ongoing. For this study, we focused on three treatments only: Con (hereafter Con-plots), HP (hereafter P-plots), and HNP (hereafter NP-plots). The basic chemical and biological properties in these three plots were shown in
Table 2.
Soil sampling
In December 2016, six years after the commencement of the experiment, 0-5 cm soil samples were collected from ten randomly selected points in each plot (Con-plots, P-plots and NP-plots of AA and EU stands) using a soil corer (inner diameter 3.5 cm). The collected soil samples were then processed by passing them through a 2-mm sieve, with the removal of large organic matter. Soil pH was measured using a pH meter (METTLER TOLEDO FE20) according to a soil:water ratio of 1:2.5, and already reported by Wang et al. (2020) [
36]. Soil pH values in Con-plots, P-plots, and NP-plots were 3.59 ±0.035, 3.68 ±0.082, and 3.59 ±0.037, respectively in AA stands, and 3.56 ±0.044,3.69 ±0.028, and 3.53 ±0.032, respectively in EU stands [
36]. Water contents of the soil samples in Con-plots, P-plots, and NP-plots were 0.23 ±0.004, 0.22 ±0.004, and 0.22 ±0.007, respectively in AA stands, and 0.23 ±0.017, 0.22 ±0.007, and 0.22 ±0.007, respectively in EU stands [
36].
Enzyme assay of the sampled soils
Enzyme assays of β-1,4-glucosidase (BG), β-1,4-xylosidase (XYL), cellobiohydrolase (CBH), and β-1,4-N-acetyl-glucosaminidase (NAG) performed using the fluorescence enzyme assay described by Bell et al. [
40] with minor modifications was already reported by Wang et al. [
36]. Briefly, 1.5 g of fresh soil was added to 91 mL of acetate buffer (50 mM, pH 5.0) and blended for 1 min until well homogenized. Two-hundred microliters of the substrates labeled with 4-methylumbelliferone (MUB) (
Table 3) were added to 800 μL of the homogenized sample solution in deep 96-well plates. The plates were incubated in the dark at 20 °C for 4 hours, followed by centrifugation at 2,090 × g. Then, 250 μL of the supernatant from the incubated mixture of sample and substrates were transferred into black 96-well plates. We measured the fluorescence of the incubated solution using a microplate spectrophotometer (Infinite M200 PRO) with a 365 nm excitation filter and a 450 nm emission filter. Enzyme activity was then calculated using standard lines prepared for each sample.
Incubation and enzyme assay of the incubated soils
Soil samples from Con-plots, P-plots, and NP-plots (3.0 g each) were placed in plastic bottles. After the addition of 0.9 mL deionized water, the bottles were covered with polyethylene rap for preventing the evaporation of water [
41] and put into the incubator. Soils were incubated for 7.5 days at 25 °C in the dark. After the end of the incubation, soils were frozen at -20 °C until the enzyme assay. Enzyme activity of the incubated soils was measured using the same methodology as enzyme assay of the sampled soils (i.e., measurement of soil enzyme activity at the field condition), but a different amount of soil (3.0 g).
Calculation and Statistics
The apparent enzyme decay rate was determined using first-order fitting, as shown by the equation:
where
Eaft represents enzyme activity after incubation,
Ebef represents enzyme activity before incubation,
k is the apparent decay rate constant, and
t is the incubation time (i.e., 7.5 days). It is important to note that the calculated
k does not indicate the true decay rate constant of the enzyme, as enzymes are continually produced during incubation. Therefore,
k should be smaller than the actual decay rate constant of the enzyme. We used a linear mixed-effects model to examine the effect of incubation time and fertilization on enzyme activity, with subplot as a random effect. We calculated the changes in enzyme activities before and after incubation and compared the values among Con-plots, P-plots, and NP-plots using one-way ANOVA, followed by Tukey’s HDS. All statistical analyses were performed using R version 4.2.2 [
42].
3. Results
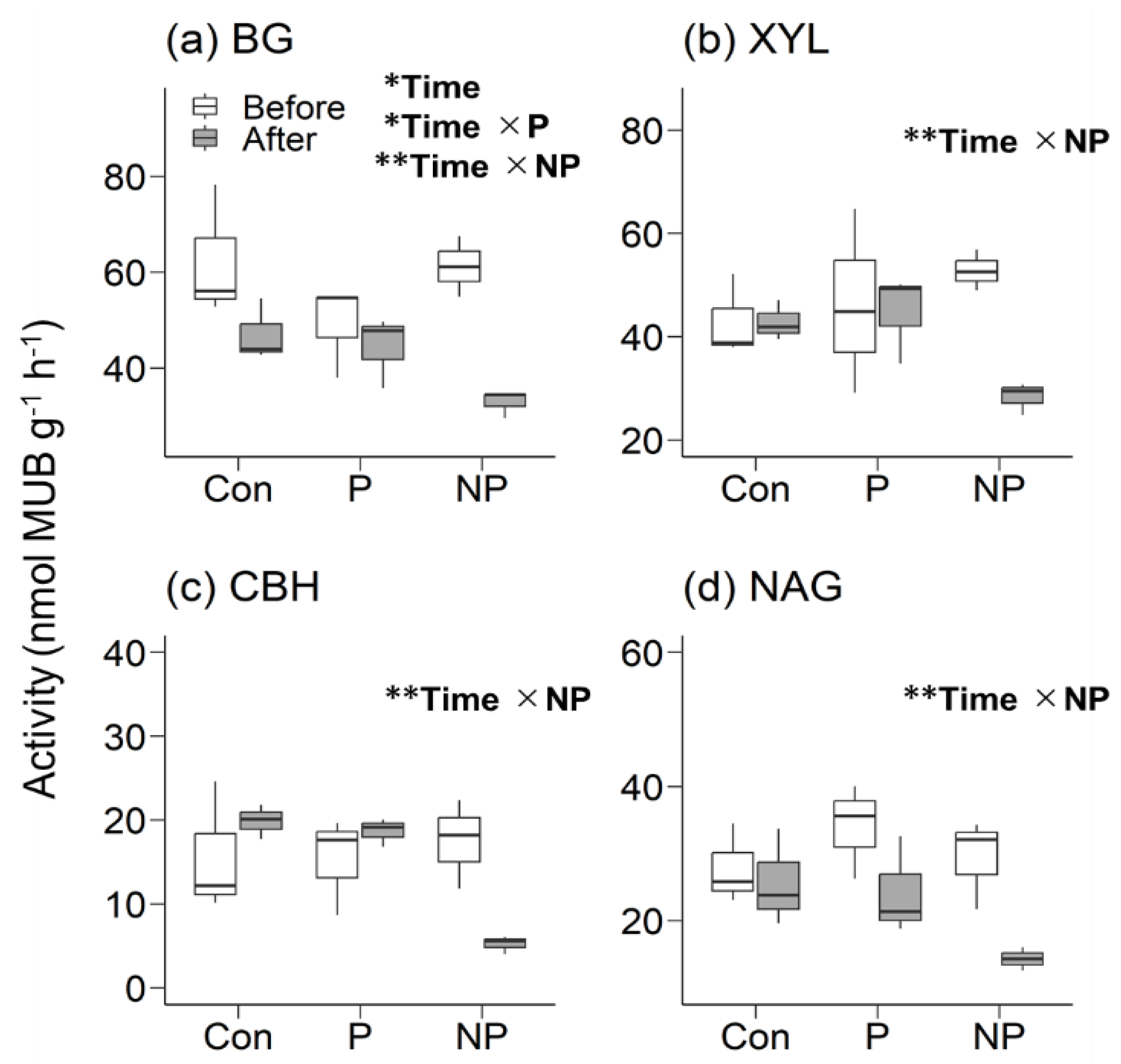
The enzyme activities in AA stands tended to decrease, particularly for BG, as suggested by a linear mixed-effects model analysis (
Figure 1). The apparent enzyme decay rate of BG, XYL, and NAG was 0.038, 0.00038, and 0.010, respectively, in Con-plots of AA stands. Meanwhile, the CBH activity in Con-plots of AA stands was elevated and could not be calculated (
Figure 1). In NP-plots of AA stands, all four enzymes had significantly lower activity than Con-plots or P-plots (
Figure 1), resulting in higher apparent decay rates (0.083, 0.083, 0.16, and 0.12 for BG, XYL, CBH, and NAG, respectively). In contrast, lower enzyme activities compared to Con-plots were not observed in P-plots of AA stands. In P-plots of AA stands, the apparent decay rates were 0.014, 0.0043, and 0.045 for BG, XYL, and NAG, respectively, and CBH activity was elevated and not calculable.
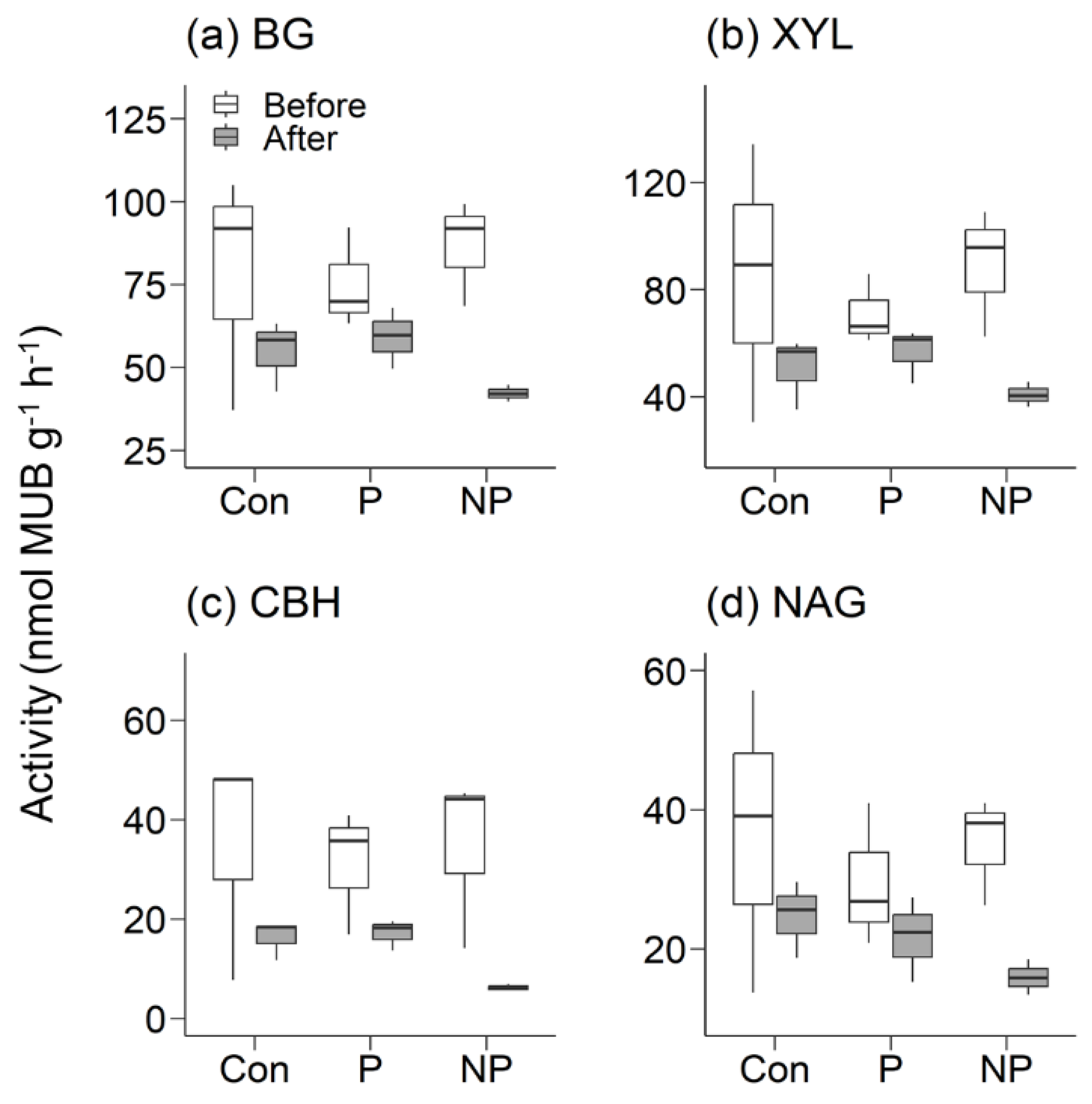
In EU stands, the decreases in enzyme activities tended to be larger compared to AA stands. In Con-plots of EU stands, the apparent enzyme decay rate of BG, XYL, CBH, and NAG was 0.047, 0.069, 0.10, and 0.053, respectively. However, due to the larger variability of data, these decreasing trends did not have statistical significance. Similar to AA stands, enzyme activities tended to be higher in NP-plots, but the results were statistically insignificant due to large variances among the data (
Figure 2). The apparent decay rates in NP-plots of EU stands were 0.096, 0.10, 0.23, and 0.11 for BG, XYL, CBH, and NAG, respectively, and 0.03, 0.03, 0.08, and 0.04, respectively, in P-plots of EU stands.
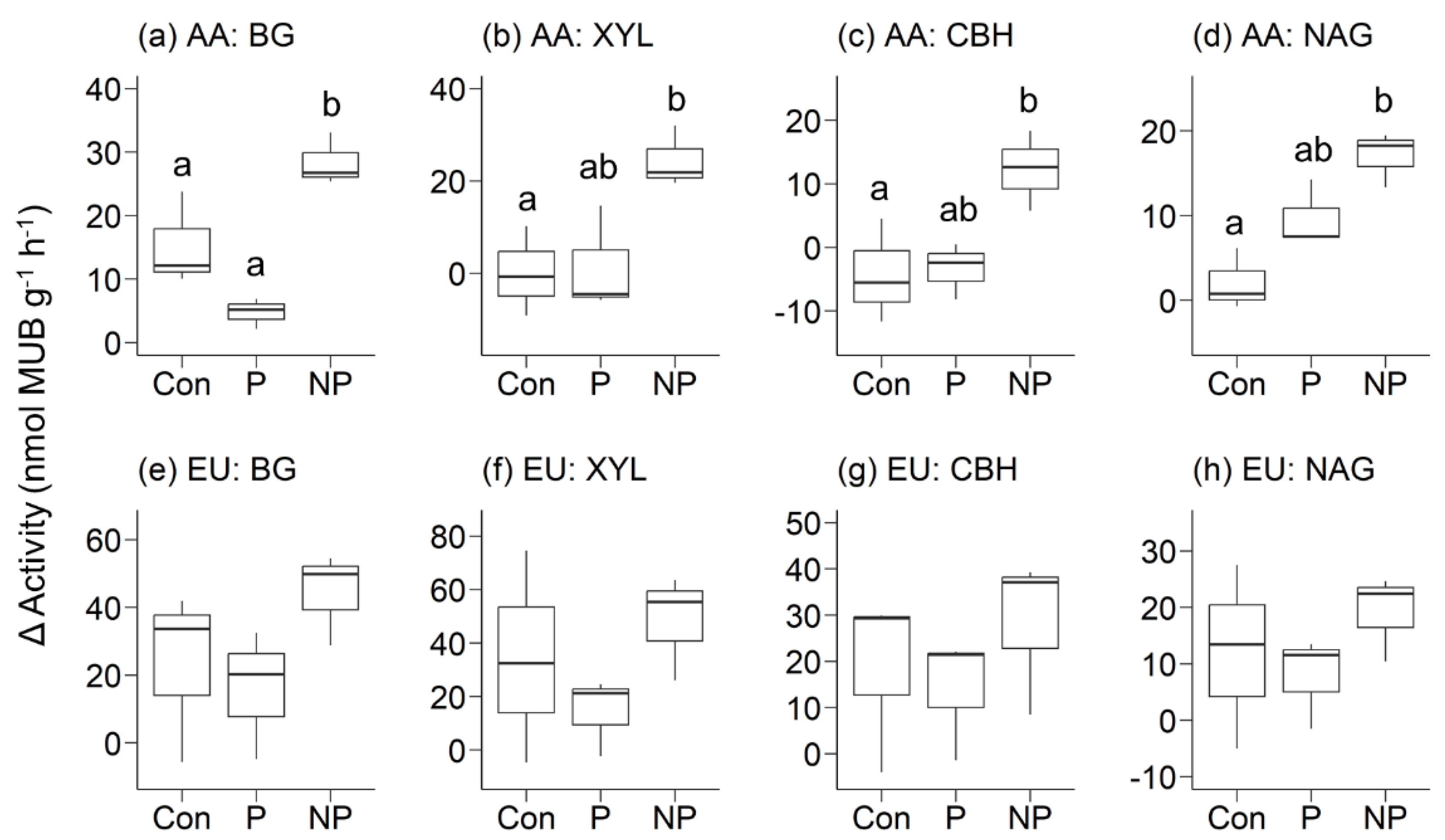
Differences in enzyme activity values before and after incubation were significantly larger in NP-plots in AA stands for all four enzymes: BG, XYL, CBH, and NAG (
Figure 3a-d). In EU stands, the averages of the differences in enzyme activity values before and after incubation were consistently highest in NP-plots, but the differences were not statistically significant (
Figure 3e-h).
4. Discussion
In our study, microorganisms continuously produced enzymes during incubation, because we did not inhibit soil microbial activity through gamma-irradiation [
13] or chloroform fumigation [
11]. However, we observed high decay rates of BG and NAG in Con-plots. The values were 0.038 and 0.047 in AA stands, and 0.010 and 0.053 in EU stands for BG and NAG, respectively, which were higher than those reported for most soils by Schimel et al. [
11], where microbial activity had been excluded by chloroform fumigation. Our findings indicate that the activity of at least some enzymes could decrease significantly under laboratory conditions, despite the continuous production of enzymes by microorganisms during incubation. This decrease in enzyme activity in laboratory conditions could be attributed to the interruption of the natural, continuous supply of organic matter or enzymes provided by microorganisms in areas other than the soil, such as the litter layer[
5] or phyllosphere [
6], which occur in field conditions. This highlights the significance of a continuous supply of organic matter and non-soil microbial-derived enzymes in maintaining enzyme activity in forest soils.
In ecosystems where multiple enzymes coexist, the activity of soil extracellular enzymes (i.e.,
Vmax) can serve as an indicator of enzyme abundance [
9,
10]. Therefore, various studies have utilized the ratios of different enzyme activities as indicators of microbial resource allocation for nutrient acquisition[
33,
43,
44,
45,
46,
47] (it should be noted that several studies questioned this approach [
48,
49,
50,
51,
52,
53]). In these studies, accurately determining enzyme production by microorganisms, rather than enzyme activity, is essential because microbial resource allocation to obtain nutrients should be related to microbial resource allocation to produce enzymes. Our results demonstrate that enzyme activity (or enzyme pools) are maintained by a continuous supply of organic matter and non-soil microbial-derived enzymes in forest soils. Therefore, relying solely on soil enzyme activity measurements may lead to misleading conclusions regarding microbial resource allocation for nutrient acquisition.
Under field conditions in both AA and EU stands (i.e., before incubations as shown in
Figure 1 and 2), the activities of BG, XYL, CBH, and NAG were similar between NP-plots and Con-plots. However, a significant reduction in activity was observed during short-term laboratory incubation in AA stands, with a higher reduction in NP-plots than in Con-plots (
Figure 1, 2a-d). In EU stands, a similar trend was observed, although it was statistically insignificant due to high variability. These results show that the impact of interrupting the natural, continuous supply of organic matter or non-soil microbial-derived enzymes, which occurs under field conditions, on enzyme activities was higher in NP-plot soils compared to Con-plot soils. It is possible that the higher quantity of protease in NP-plot soils, as compared to the Con-plot soils, may have led to faster degradation of enzymes in the NP-plot soils. Because P fertilization alone did not have a similar effect observed in the NP-plot soils, it is likely that N fertilization is the main cause of the substantially larger decreases in enzyme activity during incubation in NP-plots compared to Con-plots. If this is the case, our findings challenge the assumption that the impacts of N fertilization are less crucial than those of P fertilization in tropical forest soils that are often considered N-rich and P-poor [
16,
54] (but see [
55]). Future research should aim to determine the impact of nutrient addition on pure turnover rate of enzymes by excluding enzyme production by soil microorganisms, as previous studies have done [
11,
13].
5. Conclusion
In this study, we demonstrated that the activities of hydrolysable enzymes, specifically BG and NAG, decreased rapidly during incubation when the natural and continuous supply of organic matter or enzymes provided by microorganisms in areas other than the soil were disrupted. This highlights the importance of a continuous supply of organic matter and non-soil microbial-derived enzymes in maintaining enzyme activity in forest soils. Therefore, caution is necessary when assessing microbial resource allocation for nutrient acquisition (or energy) by measuring enzyme activity, as the measured values may not reflect enzyme production accurately.
We also demonstrated that the activities of four hydrolysable enzymes, i.e., BG, XYL, CBH, and NAG, were comparable in N and P fertilized soils and the unfertilized control under field conditions, but decreases in those activities were substantially greater in the fertilized soils during short-term laboratory incubation. Since a similar pattern was not observed in P-fertilized soils, our results suggest that the interruption of the continuous supply of organic matter or non-soil microbial-derived enzymes in the field leads to a more significant reduction in enzyme activities in N-fertilized soils compared to unfertilized control.
In conclusion, the present study highlights the importance of considering enzyme degradation when investigating material dynamics in forest ecosystems, including the impact of nutrient addition on enzyme activity, as soil enzyme activity may not always reflect enzyme production.
Author Contributions
Conceptualization, T.M.; software, T.M. and S.W.; validation, T.M. and S.W.; formal analysis, T.M., C.P. and S.W.; investigation, T.M., C.P., C.W. and S.W.; resources, W.Z. and J.M.; data curation, T.M., C.W., M.Z. and S.W.; writing—original draft preparation, T.M.; writing—review and editing, T.M., C.P., M.Z. and W.Z.; visualization, T.M.; supervision, W.Z. and J.M.; project administration, W.Z.; funding acquisition, W.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the National Natural Science Foundation of China (42077311, 42173077), Grant-in-Aid for JSPS Postdoctoral Fellowships for Research Abroad (28·601), and a grant from The Sumitomo Foundation (153082).
Data Availability Statement
Data are provided by corresponding author when requested.
Acknowledgments
We thank Shengxing Fu and Meifang Hu for their support for our field work.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Wang, S.; Zhou, K.; Mori, T.; Mo, J.; Zhang, W. Effects of Phosphorus and Nitrogen Fertilization on Soil Arylsulfatase Activity and Sulfur Availability of Two Tropical Plantations in Southern China. For. Ecol. Manage. 2019, 453, 117613. [Google Scholar] [CrossRef]
- Burns, R.G.; DeForest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A. Soil Enzymes in a Changing Environment: Current Knowledge and Future Directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Wallenstein, M.D.; Weintraub, M.N. Emerging Tools for Measuring and Modeling the in Situ Activity of Soil Extracellular Enzymes. Soil Biol. Biochem. 2008, 40, 2098–2106. [Google Scholar] [CrossRef]
- Wang, C.; Mori, T.; Mao, Q.; Zhou, K.; Wang, Z. Long-Term Phosphorus Addition Downregulates Microbial Investments on Enzyme Productions in a Mature Tropical Forest. J. Soils Sediments 2020, 20(2), 921–930. [Google Scholar] [CrossRef]
- Zhang, N.; Li, Y.; Wubet, T.; Bruelheide, H.; Liang, Y.; Purahong, W.; Buscot, F.; Ma, K. Tree Species Richness and Fungi in Freshly Fallen Leaf Litter: Unique Patterns of Fungal Species Composition and Their Implications for Enzymatic Decomposition. Soil Biol. Biochem. 2018, 127, 120–126. [Google Scholar] [CrossRef]
- Mori, T.; Wang, S.; Zhang, W.; Mo, J. A Potential Source of Soil Ecoenzymes: From the Phyllosphere to Soil via Throughfall. Appl. Soil Ecol. 2019, 139, 25–28. [Google Scholar] [CrossRef]
- Mori, T.; Wang, S.; Zhang, W.; Mo, J. Ecoenzyme Data in Throughfall and Rainfall in Five Subtropical Forests in Southern China. Data in Brief 2019, 26, 103906. [Google Scholar] [CrossRef]
- Mori, T.; Wang, S.; Zhou, K.; Mo, J.; Zhang, W. Ratios of Phosphatase Activity to Activities of Carbon and Nitrogen-Acquiring Enzymes in Throughfall Were Larger in Tropical Forests than a Temperate Forest. Tropics 2021, 30, 25–29. [Google Scholar] [CrossRef]
- Mori, T.; Wang, S.; Zhang, W.; Mo, J. Microbial Assembly Adapted to Low-P Soils in Three Subtropical Forests by Increasing the Maximum Rate of Substrate Conversion of Acid Phosphatases but Not by Decreasing the Half-saturation Constant. Eur. J. Soil Biol. 2022, 108, 103377. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Belnap, J.; Findlay, S.G.; Shah, J.J.F.; Hill, B.G.; Kuehn, K.A.; Kuske, C.R.; Litvak, M.E.; Martinez, N.G.; Moorhead, D.L.; et al. Extracellular Enzyme Kinetics Scale with Resource Availability. Biogeochemistry 2014, 121, 287–304. [Google Scholar] [CrossRef]
- Schimel, J.; Becerra, C.A.; Blankinship, J. Estimating Decay Dynamics for Enzyme Activities in Soils from Different Ecosystems. Soil Biol. Biochem. 2017, 114, 5–11. [Google Scholar] [CrossRef]
- Schimel, J.P.; Weintraub, M.N. The Implications of Exoenzyme Activity on Microbial Carbon and Nitrogen Limitation in Soil: A Theoretical Model. Soil Biol. Biochem. 2003, 35, 549–563. [Google Scholar] [CrossRef]
- Allison, S.D. Soil Minerals and Humic Acids Alter Enzyme Stability: Implications for Ecosystem Processes. Biogeochemistry 2006, 81, 361–373. [Google Scholar] [CrossRef]
- Wright, S.J.; Yavitt, J.B.; Wurzburger, N.; Turner, B.I.; Tanner, E.V.J.; Sayer, E.J.; Santiago, L.S.; Kaspari, M.; Hedin, L.O.; Harms, K.E.; et al. Potassium, Phosphorus, or Nitrogen Limit Root Allocation, Tree Growth, or Litter Production in a Lowland Tropical Forest. Ecology 2011, 92, 1616–1625. [Google Scholar] [CrossRef]
- Alvarez-Clare, S.; Mack, M.C.; Brooks, M. A Direct Test of Nitrogen and Phosphorus Limitation to Net Primary Productivity in a Lowland Tropical Wet Forest. Ecology 2013, 94, 1540–1551. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Townsend, A.R.; Schmidt, S.K. Phosphorus Limitation of Microbial Processes in Moist Tropical Forests : Evidence from Short-Term Laboratory Incubations and Field Studies. Ecosystems 2002, 5, 680–691. [Google Scholar] [CrossRef]
- Mo, J.; Zhang, W.; Zhu, W.; Gundersen, P.; Fang, Y.; Li, D.; Wang, H. Nitrogen Addition Reduces Soil Respiration in a Mature Tropical Forest in Southern China. Glob. Chang. Biol. 2008, 14, 403–412. [Google Scholar] [CrossRef]
- Mori, T.; Ohta, S.; Ishizuka, S.; Konda, R.; Wicaksono, A.; Heriyanto, J.; Hamotani, Y.; Gobara, Y.; Kawabata, C.; Kuwashima, K.; Nakayama, Y.; Hardjono, A. Soil Greenhouse Gas Fluxes and C Ctocks as Affected by Phosphorus Addition in a Newly Established Acacia Mangium Plantation in Indonesia. Forest Ecology and Management 2013, 310, 643–651. [Google Scholar] [CrossRef]
- Kaspari, M.; Garcia, M.N.; Harms, K.E.; Santana, M.; Wright, S.J.; Yavitt, J.B. Multiple Nutrients Limit Litterfall and Decomposition in a Tropical Forest. Ecol. Lett. 2008, 11, 35–43. [Google Scholar] [CrossRef]
- Mori, T.; Ishizuka, S.; Konda, R.; Wicaksono, A.; Heriyanto, J. Phosphorus Addition Reduced Microbial Respiration during the Decomposition of Acacia Mangium Litter in South Sumatra, Indonesia. Tropics 2015, 24(3), 113–118. [Google Scholar] [CrossRef]
- Fang, H.; Mo, J.; Peng, S.; Li, Z.; Wang, H. Cumulative Effects of Nitrogen Additions on Litter Decomposition in Three Tropical Forests in Southern China. Plant Soil 2007, 233–242. [Google Scholar] [CrossRef]
- Mori, T.; Zhou, K.; Wang, C.; Wang, S.; Wang, Y.; Zheng, M.; Lu, X.; Zhang, W.; Mo, J. Effects of 14-Year Continuous Nitrogen Addition on Soil Arylsulfatase and Phosphodiesterase Activities in a Mature Tropical Forest. Global Ecology and Conservation 2020, 22, e00934. [Google Scholar] [CrossRef]
- Xiao, W.; Chen, X.; Jing, X.; Zhu, B. A Meta-Analysis of Soil Extracellular Enzyme Activities in Response to Global Change. Soil Biol. Biochem. 2018, 123, 21–32. [Google Scholar] [CrossRef]
- Mori, T. Greater Impacts of Phosphorus Fertilization on Soil Phosphatase Activity in Tropical Forests than in Non-Tropical Natural Terrestrial Ecosystems: A Meta-Analysis. Pedobiologia 2022, 91–92, 150808. [Google Scholar] [CrossRef]
- Jian, S.; Li, J.; Chen, J.; Wang, G.; Mayes, M.A.; Dzantor, K.E.; Hui, D.; Luo, Y. Soil Extracellular Enzyme Activities, Soil Carbon and Nitrogen Storage under Nitrogen Fertilization: A Meta-Analysis. Soil Biol. Biochem. 2016, 101, 32–43. [Google Scholar] [CrossRef]
- Chen, H.; Li, D.; Zhao, J.; Xiao, K.; Wang, K. Effects of Nitrogen Addition on Activities of Soil Nitrogen Acquisition Enzymes:A Meta-Analysis. Agriculture, Ecosystems and Environment 2018, 252, 126–131. [Google Scholar] [CrossRef]
- Mori, T. The Ratio of β-1,4-Glucosidase Activity to Phosphomonoesterase Activity Remains Low in Phosphorus-Fertilized Tropical Soils: A Meta-Analysis. Appl. Soil Ecol. 2022, 180, 104635. [Google Scholar] [CrossRef]
- Marklein, A.R.; Houlton, B.Z. Nitrogen Inputs Accelerate Phosphorus Cycling Rates across a Wide Variety of Terrestrial Ecosystems. New Phytol. 2012, 193, 696–704. [Google Scholar] [CrossRef]
- Margalef, O.; Sardans, J.; Maspons, J.; Molowny-Horas, R.; Fernández-Martínez, M.; Janssens, I.A.; Richter, A.; Ciais, P.; Obersteiner, M.; Peñuelas, J. The Effect of Global Change on Soil Phosphatase Activity. Glob. Chang. Biol. 2021, 27, 5989–6003. [Google Scholar] [CrossRef]
- Allison, S.D.; Czimczik, C.I.; Treseder, K.K. Microbial Activity and Soil Respiration under Nitrogen Addition in Alaskan Boreal Forest. Glob. Chang. Biol. 2008, 14, 1156–1168. [Google Scholar] [CrossRef]
- Olander, L.P.; Vitousek, P.M. Regulation of Soil Phosphatase and Chitinase Activity by N and P Availability. Biogeochemistry 2000, 49, 175–190. [Google Scholar] [CrossRef]
- Houlton, B.Z.; Wang, Y.-P.; Vitousek, P.M.; Field, C.B. A Unifying Framework for Dinitrogen Fixation in the Terrestrial Biosphere. Nature 2008, 454, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Imai, N.; Yokoyama, D. Effects of Nitrogen and Phosphorus Fertilization on the Ratio of Activities of Carbon-Acquiring to Nitrogen-Acquiring Enzymes in a Primary Lowland Tropical Rainforest in Borneo, Malaysia. Soil Sci. Plant Nutr. 2018, 64(5), 554–557. [Google Scholar] [CrossRef]
- Mori, T.; Imai, N.; Kitayama, K. Does Simultaneous Phosphorus Fertilization Negate the Suppressive Effect of Nitrogen Fertilization on Polyphenol Oxidase Activity? Geoderma 2023, 1–20. [Google Scholar] [CrossRef]
- Bonner, M.T.L.; Castro, D.; Schneider, A.N.; Sundström, G.; Hurry, V.; Street, N.R.; Näsholm, T. Why Does Nitrogen Addition to Forest Soils Inhibit Decomposition? Soil Biol. Biochem. 2019, 137, 107570. [Google Scholar] [CrossRef]
- Wang, S.; Mori, T.; Mo, J.; Zhang, W. The Responses of Carbon-and Nitrogen-Acquiring Enzymes to Nitrogen and Phosphorus Additions in Two Plantations in Southern China. Journal of Forestry Research 2020, 31(4), 1319–1324. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, X.; Luo, Y.; Rafique, R.; Chen, H.; Huang, J.; Mo, J. Responses of Nitrous Oxide Emissions to Nitrogen and Phosphorus Additions in Two Tropical Plantations with N-Fixing vs. Non-N-Fixing Tree Species. Biogeosciences 2014, 11, 4941–4951. [Google Scholar] [CrossRef]
- Mori, T.; Wang, S.; Wang, C.; Mo, J. Is Microbial Biomass Measurement by the Chloroform Fumigation Extraction Method Biased by Experimental Addition of N and P? iForest-Biogeosciences 2021, 14, 408–412. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, X.; Liu, L.; Fu, S.; Chen, H.; Huang, J.; Lu, X.; Liu, Z.; Mo, J. Large Difference of Inhibitive Effect of Nitrogen Deposition on Soil Methane Oxidation between Plantations with N-Fixing Tree Species and Non- N-Fixing Tree Species. J. Geophys. Res. D: Atmos. 2012, 117, G00N16. [Google Scholar] [CrossRef]
- Bell, C.W.; Fricks, B.E.; Rocca, J.D.; Steinweg, J.M.; McMahon, S.K.; Wallenstein, M.D. High-Throughput Fluorometric Measurement of Potential Soil Extracellular Enzyme Activities. J. Vis. Exp. 2013, e50961. [Google Scholar]
- Mori, T.; Ohta, S.; Ishizuka, S.; Konda, R. Effects of Phosphorus Addition on N2O and NO Emissions from Soils of an Acacia Mangium Plantation. Soil Science and Plant Nutrition 2010, 56(5), 782–788. [Google Scholar] [CrossRef]
- R Core Team R: A Language and Environment for Statistical Computing. Statistical, R Foundation for Computing, Vienna, Austria. 2022. Available online: https://www.r-project.org/.
- Sinsabaugh, R.L.; Hill, B.H.; Follstad Shah, J.J. Ecoenzymatic Stoichiometry of Microbial Organic Nutrient Acquisition in Soil and Sediment. Nature 2009, 462, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Sinsabaugh, R.L.; Lauber, C.L.; Weintraub, M.N.; Ahmed, B.; Allison, S.D.; Crenshaw, C.; Contosta, A.R.; Cusack, D.; Frey, S.; Gallo, M.E.; et al. Stoichiometry of Soil Enzyme Activity at Global Scale. Ecol. Lett. 2008, 11, 1252–1264. [Google Scholar] [CrossRef]
- Waring, B.G.; Weintraub, S.R.; Sinsabaugh, R.L. Ecoenzymatic Stoichiometry of Microbial Nutrient Acquisition in Tropical Soils. Biogeochemistry 2014, 117, 101–113. [Google Scholar] [CrossRef]
- Moorhead, D.L.; Sinsabaugh, R.L.; Hill, B.H.; Weintraub, M.N. Vector Analysis of Ecoenzyme Activities Reveal Constraints on Coupled C, N and P Dynamics. Soil Biol. Biochem. 2016, 93, 1–7. [Google Scholar] [CrossRef]
- Mori, T.; Aoyagi, R. Does Anthropogenic Phosphorus Input Reduce Soil Microbial Resource Allocation to Acquire Nitrogen Relative to Carbon ? Eurasian Journal of Soil Science 2019, 8, 54–59. [Google Scholar] [CrossRef]
- Rosinger, C.; Rousk, J.; Sandén, H. Can Enzymatic Stoichiometry Be Used to Determine Growth-Limiting Nutrients for Microorganisms? - A Critical Assessment in Two Subtropical Soils. Soil Biol. Biochem. 2019, 128, 115–126. [Google Scholar] [CrossRef]
- Mori, T. Does Ecoenzymatic Stoichiometry Really Determine Microbial Nutrient Limitations? Soil Biol. Biochem. 2020, 146, 107816. [Google Scholar] [CrossRef]
- Mori, T.; Aoyagi, R.; Kitayama, K.; Mo, J. Does the Ratio of β-1, 4-Glucosidase to β-1, 4-N-Acetylglucosaminidase Indicate the Relative Resource Allocation of Soil Microbes to C and N Acquisition? Soil Biol. Biochem. 2021, 160, 108363. [Google Scholar] [CrossRef]
- Mori, T.; Rosinger, C.; Margenot, A.J. Enzymatic C: N: P Stoichiometry: Questionable Assumptions and Inconsistencies to Infer Soil Microbial Nutrient Limitation. Geoderma 2023. [Google Scholar] [CrossRef]
- Mori, T. Possibly Underestimated Microbial Carbon Limitation Determined by Enzymatic Stoichiometry Approach : Comments on “ Crop Rotation Stage Ha s a Greater Effect than Fertilisation on Soil Microbiome Assembly and Enzymatic Stoichiometry”. Sci. Total Environ. 2022, 846, 157931. [Google Scholar] [CrossRef] [PubMed]
- Mori, T. Microbial Nutrient Limitation in Tropical Forest Soils Determined Using the V-T Model Contradicts the Traditional View That C Is the Major Limiting Element. Tropics 2022, 31, 59–63. [Google Scholar] [CrossRef]
- Camenzind, T.; Hättenschwiler, S.; Treseder, K.K.; Lehmann, A.; Rillig, M.C. Nutrient Limitation of Soil Microbial Processes in Tropical Forests. Ecol. Monogr. 2018, 88, 4–21. [Google Scholar] [CrossRef]
- Mori, T. P Fertilization Experiments Require Reinterpretation: Abiotic Elevation of Available C and N Could Influence Microbial Processes in Soil. Appl. Soil Ecol. 2023, 189, 104899. [Google Scholar] [CrossRef]
Figure 1.
The enzyme activity of β-1,4-glucosidase (BG), β-1,4-xylosidase (XYL), cellobiohydrolase (CBH), and β-1,4-N-acetyl-glucosaminidase (NAG) measured before and after a 7.5-day incubation in soils taken from Con-plots, P-plots, and NP-plots of AA stands. MUB, 4-methylumbelliferone.
Figure 1.
The enzyme activity of β-1,4-glucosidase (BG), β-1,4-xylosidase (XYL), cellobiohydrolase (CBH), and β-1,4-N-acetyl-glucosaminidase (NAG) measured before and after a 7.5-day incubation in soils taken from Con-plots, P-plots, and NP-plots of AA stands. MUB, 4-methylumbelliferone.
Figure 2.
The enzyme activity of β-1,4-glucosidase (BG), β-1,4-xylosidase (XYL), cellobiohydrolase (CBH), and β-1,4-N-acetyl-glucosaminidase (NAG) measured before and after a 7.5 day incubation in soils taken from Con-plots, P-plots, and NP-plots of EU stands. MUB, 4-methylumbelliferone.
Figure 2.
The enzyme activity of β-1,4-glucosidase (BG), β-1,4-xylosidase (XYL), cellobiohydrolase (CBH), and β-1,4-N-acetyl-glucosaminidase (NAG) measured before and after a 7.5 day incubation in soils taken from Con-plots, P-plots, and NP-plots of EU stands. MUB, 4-methylumbelliferone.
Figure 3.
The differences in enzyme activity values before and after the 7.5-day incubation (ΔActivity) of (a) β-1,4-glucosidase (BG), (b) β-1,4-xylosidase (XYL), (c) cellobiohydrolase (CBH), and (d) β-1,4-N-acetyl-glucosaminidase (NAG) in AA stands, and (e) BG, (f) XYL, (g) CBH, and (h) NAG in EU stands. MUB, 4-methylumbelliferone.
Figure 3.
The differences in enzyme activity values before and after the 7.5-day incubation (ΔActivity) of (a) β-1,4-glucosidase (BG), (b) β-1,4-xylosidase (XYL), (c) cellobiohydrolase (CBH), and (d) β-1,4-N-acetyl-glucosaminidase (NAG) in AA stands, and (e) BG, (f) XYL, (g) CBH, and (h) NAG in EU stands. MUB, 4-methylumbelliferone.
Table 1.
Basic information of the study site.
Table 1.
Basic information of the study site.
| Properties |
AA |
EU |
| Soil total organic C (g kg−1)a,c
|
22.1 (2.3) |
15.5 (0.9) |
| Soil total N (g kg−1)a,c
|
1.6 (0.1) |
1.5 (0.1) |
| Soil NH4+ (mg kg−1)a,c
|
16.0 (1.0) |
13.4 (2.0) |
| Soil NO3– (mg kg−1)a,c
|
17.8 (1.4) |
13.6 (2.6) |
| DOC (mg C kg-1)b
|
275.6 (3.5) |
297.9 (3.6) |
| DN (mg N kg-1)b
|
38.5 (1.1) |
41.8 (2.2) |
| Soil available P (mg kg−1)a,c
|
1.8 (0.2) |
1.6 (0.3) |
| Soil pHa,c
|
3.83 (0.03) |
3.91 (0.05) |
| Fine root biomass (g m−2)a,c
|
92.9 (13.6) |
74.4 (3.2) |
| Standing litter-layer mass (g m−2 yr−1)c
|
694.1 (25) |
590.2 (14) |
| Basal area (m2 ha−1)d
|
12.5 |
16.7 |
| Mean height of planted trees (m) e
|
12.2 |
11.5 |
| DBH of planted trees (cm) e
|
15 |
11.1 |
| Stem density (tree ha-1)e
|
2076 |
1962 |
| Litterfall mass (g m-2 yr-1)e
|
841 (58) |
870 (67) |
Table 2.
Impacts of fertilization on soil chemical and biological properties.
Table 2.
Impacts of fertilization on soil chemical and biological properties.
| Stand |
Properties |
Con-plot |
P-plot |
NP-plot |
| |
Soil total organic C (g kg−1) |
40.7(3) |
45.3(4) |
55.8(4) |
| |
Soil total N (g kg−1) |
2.2(0.1) |
2.2(0.2) |
2.0(0.2) |
| |
C:N ratio |
18.5 (1) |
18.9 (3) |
27.9 (3) |
| AA |
Soil NH4+ (mg kg−1) |
9.4(0.5) |
11.9(0.7) |
12.2(0.8) |
| |
Soil NO3– (mg kg−1) |
7.7(0.9) |
6.6(0.4) |
11.3(1.0) |
| |
MBC (µg C g-1) |
330(31) |
634(38) |
446(34) |
| |
MBN (µg N g-1) |
67(12) |
86(17) |
52(14) |
| |
Soil available P (mg kg−1) |
2.9(0.3) |
4.1(0.5) |
4.0(0.1) |
| |
|
|
|
|
| |
Soil total organic C (g kg−1) |
20.9(3) |
33.9(2) |
33.6(3) |
| |
Soil total N (g kg−1) |
1.6(0.1) |
1.6(0.3) |
1.7(0.1) |
| |
C:N ratio |
13.1 (2) |
21.2 (2) |
19.8 (1) |
| EU |
Soil NH4+ (mg kg−1) |
6.7(0.2) |
5.2(0.8) |
6.9(0.7) |
| |
Soil NO3– (mg kg−1) |
5.6(0.5) |
4.2(0.7) |
6.0(0.6) |
| |
MBC (µg C g-1) |
378(33) |
359(26) |
350(20) |
| |
MBN (µg N g-1) |
78(8) |
47(12) |
80(10) |
| |
Soil available P (mg kg−1) |
2.6(0.1) |
4.1(0.4) |
4.0(0.5) |
Table 3.
Extracellular enzymes, abbreviation, and substrate used in the present study.
Table 3.
Extracellular enzymes, abbreviation, and substrate used in the present study.
| Enzyme |
Abbreviation |
Substrate |
| β-1,4-Glucosidase |
BG |
MUB-β-D-glucosid |
| β-1,4-Xylosidase |
XYL |
MUB-β-D-cellobioside |
| Cellobiohydrolase |
CBH |
MUB-β-D-xyloside |
| β-1,4-N-acetylglucosaminidase |
NAG |
MUB-N-acetyl-β-D-glucosaminide |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).