1. Introduction
The red palm weevil, sago palm weevil, or the Asian palm weevil, also known scientifically as
Rhynchophorus ferrugineus (Olivier) is a species of insect from the family Curculionidae [
1]. This insect species is widely recognized as a significantly destructive pest to plants belonging to the family Arecaceae (palms), including but not limited to date palms, sago palms, talipot palms, coconut palms, and oil palms, as they feed and nest within these trees. The larvae of RPWs develop within the host's young shoots, while adults create a nest to lay eggs, which often leads to the death of the plant. The insect species is commonly found in Southern Asia and Melanesia, though they can be found causing damage in countries such as Saudi Arabia [
2,
3].
The adult female RPW is capable of laying approximately 30 white, elongated, ovoid-shaped eggs per day. After 2-5 days in this stage, the egg will hatch and emerge as a first instar larva [
4]. The body of the newly hatched larvae displays a white, segmented, and legless appearance with reddish-brown coloration on the heads. In their natural habitat as a pest, the larvae undergo approximately 10-11 molting cycles while nesting and feeding inside the trunks of their host plant until they are fully developed after 1-3 months and ready to construct a cocoon and enter pupation. As they develop, the coloration of the larvae will gradually change to a darker hue, with the body becoming brownish-yellow, and weighing approximately 5 grams. The larval stage is when weevil farmers collect their cultivated weevils for the market. If the RPWs are not collected during this stage for commercial purposes, the fully developed larvae will utilize available surrounding fibers from their environment, such as coconut fibers, fibers from their host plant, or fibers from their feed to construct a cocoon. The ovoid-shaped cocoon will encase the entire body of the larva, allowing it to undergo metamorphosis and develop into a dark brown pupa with physical characteristics similar to that of an adult RPW [
1]. The pupal stage of RPWs lasts for a duration of 14-21 days, after which the insect emerges from the cocoon as an imago [
4]. The RPW imago displays wide variations in their coloration and patterns among individuals, with base colors ranging from reddish-brown to brownish-black. The male RPWs possess shorter snouts than their female counterparts, and both sexes have hairy snouts. The morphological difference between RPW males and females are crucial for identifying the sex and play an essential role in the reproductive process, as they enable the female weevils to use their elongated snouts bore into the soft tissue of the host plant for oviposition [
1]. The average time period a RPW will stay in the imago stage is around 98 days [
4].
Since the RPW is a devastating pest to the palm industry, in coconut palms (
Cocos nucifera), RPWs start their infestation when the adults bore into the top parts or the stem of the host tree, where they undergo their entire life cycle until they are mature imagoes and are ready to spread to other trees. The female RPWs use their elongated snouts to bore into the stem of the host plant and lay their eggs in cracks and scarred parts of the stem, or in parts that have already been bored by other insects that are also pests to palm plants such as the coconut rhinoceros beetle (
Oryctes rhinoceros). Once the RPW eggs hatch, the larvae will tunnel into the heart of the palm plant, causing major damage inside the trunk of the tree and cutting off the connection between the roots and the tops of the infested tree. This damage disrupts the plant’s transport of water and nutrients to the tops, causing them to rot and fall. This damage could be fatal to the plant since these RPW damage cases eventually lead to the death of the host plant causing major damage to the palm industry. In Thailand, a total of two species of RPW are found, namely the species in the study,
Rhynchophorus ferrugineus, and the other RPW species of
Rhynchophorus vulneratus, both of which are considered major pests to the palm industry [
1]. Since they are destructive pests, the farmers found a way to manage the RPW infestation problem by consuming and cultivating the larvae of the red palm weevil as an alternative protein source. By consuming the larvae, farmers are not only able to manage the RPW pest problem but also create a sustainable and profitable business benefiting them.
The larvae of RPWs are recognized as a nutritious food source and cultivated for human consumption due to their high nutritional value [
5]. They serve as an eco-friendly and alternative source of protein that can help alleviate the world's food shortage problem [
6,
7]. In comparison to farm animals, cultivating RPWs has numerous benefits, including reducing greenhouse gas emissions that contribute to global warming, such as reducing carbon dioxide by up to 730,000 million kilograms per year and reducing methane by up to 30 trillion kilograms per year [
8,
9]. It also reduces the space needed, since insects can be raised in plastic basins [
10]. However, the increase in RPW cultivation has led to the release of adults into the environment, resulting in pest problems that cannot be controlled in numerous economic plants, namely plants in the family Arecaceae [
11].
The growth of RPWs is influenced by various factors. Internal biological factors such as growth hormones and gut microbiota [
12,
13]. According to previous research, gut microbiota located in RPW’s digestive track produces enzymes that help digest cellulose [
13], which affects the metamorphosis of RPWs. To slow down their metamorphosis cycle, we found that roselle (
Hibiscus sabdariffa), garlic (
Allium sativum) and turmeric (
Curcuma longa) can reduce the growth of bacteria found in the RPW digestive track [
14,
15,
16]. Additionally, some plants, namely mulberry (
Morus alba), basil (
Ocimum basilicum), and holy basil (
Ocimum tenuiflorum) contain small amounts of juvenile hormone-like secondary metabolites, called phytojuvenoid, which maintain insects in an immature state until the appropriate developmental stage for reproduction is reached [
17,
18].
In nature, many plants contain certain compounds that can affect the symbiotic relationship between the RPW and its gut microbiota, and some plants can affect the insect's hormone levels. These plants can be used as a biological factor to control the metamorphosis of the weevil larvae. The RPW has a mutualistic relationship with its gut microbiota, which produces enzymes that aid in digesting cellulose, providing the insect with the necessary nutrients for growth and development, thus affecting its metamorphosis [
13]. Plants such as roselle (
Hibiscus sabdariffa), known locally in Thai as “Kra-jeab”, could slow down the RPW’s metamorphosis cycle since it can reduce the growth of RPW gut microbiota, particularly those of the family Enterobacteriaceae, which is a significant percentage of the insect’s gut microbiota [
14,
15]. Garlic (
Allium sativum), and turmeric (
Curcuma longa), known in Thai as “Kra-thiam” and “Kha-min”, respectively, have also been known to be able to reduce the growth of general bacteria found in the RPW’s gut [
16].
In addition, animals in the phylum Arthropoda have been known to produce hormones that regulate their metamorphosis cycle. The juvenile hormone is an example of these hormones, which slow down growth and increases molting, thus regulating metamorphosis. Plants commonly found in Thailand, such as the leaves of mulberry (
Morus alba), basil (
Ocimum basilicum), and holy basil (
Ocimum tenuiflorum), known in Thai as “Bai-Mon”, “Ho-ra-pha”, and “Kra-pao”, respectively, have been reported to contain small amounts of compounds called phytojuvenoid, which are juvenile hormone-like secondary metabolites, having similar properties to the juvenile hormone, thus also able to slow down the metamorphosis of RPWs [
17,
18]. This suggests that these plants may be able to be used as a natural regulator of the RPW metamorphosis cycle.
External factors such as host plants and environmental conditions also influence the growth of RPWs [
19]. According to previous research, different-sized coconut fiber also affects the rate of their pupation, where the length of fiber affects the pupation ability of insects [
20].
As certain physical factors also play a role, the availability of surrounding fibers can affect the construction of the RPW's cocoon when it enters pupation, which is essential for the proper progression of the metamorphosis cycle, and any disruption in this process can have significant consequences on the metamorphosis of the RPW. As previous research has indicated, the fully developed larvae of RPWs rely on available fibers from the surrounding environment to construct a cocoon, and the availability of these fibers can significantly impact their ability to do so [
20]. This behavior of the RPW could potentially slow down its metamorphosis cycle if there is less fiber available.
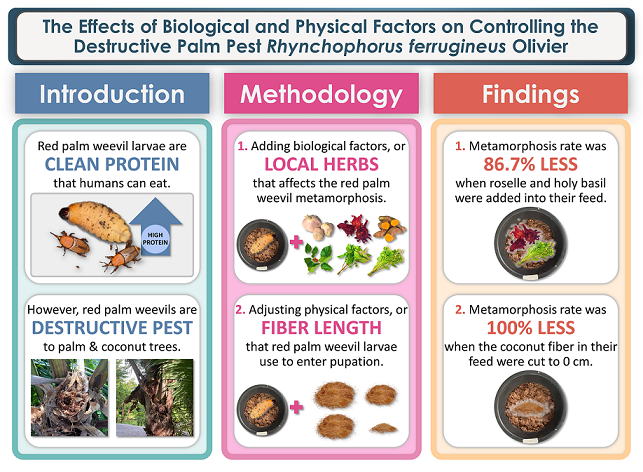
As RPWs become adults, their reproduction rate becomes costly to control, making the development of effective control methods critical. This study highlights the potential for a sustainable, organic, and cost-effective method to control RPW growth by manipulating their gut microbiota, growth hormones, and altering their pupation nature for RPW farmers.
4. Discussions
We conducted an investigation to determine the impact of various biological and physical factors on the metamorphosis rate of RPWs, for the development of a sustainable, organic, and cost-effective approach to control the insect’s metamorphosis rate, with an ultimate goal of reducing RPW invasion problems and major destruction in palm plantations. The current method for controlling RPW pest problems involves the use of harmful and toxic insecticides, which are unsustainable and environmentally damaging. Therefore, we were interested in developing an eco-friendly and sustainable approach to mitigate the RPW problem.
Our findings revealed that plants with inhibitory properties on the mutualistic relationship of RPWs and their gut microbiota can effectively reduce the metamorphosis rate of RPWL. Our results indicate that
Hibiscus sabdariffa, when combined with weevil feed showed the most significant reduction in RPWL metamorphosis rate, with only 23.3% of the treated larvae entering pupation, which is 76.7% lower than the RPWL in the control group, which had a pupation rate of 100%. The metamorphosis rate of the RPWLs was followed by feed containing
Curcuma longa and
Allium sativum as the supplemental plants, respectively. Both of these plants have been proven to also be effective, as the results revealed that they could also reduce the metamorphosis of the RPWL, evidenced by the 26.7% and 32.2% of the treated larvae pupating, which is 73.3% and 67.8% lower than the RPWL in the control group, respectively. This finding is supported by the previous research of Habineza et al. [
13], which demonstrated that disruptions in the mutualistic relationship of RPWs with their gut microbiota could affect their growth, thus slowing their metamorphosis rates due to the decreased production of enzymes.
Additionally, the results of this study revealed that plants containing phytojuvenoid compounds, which have the properties of increasing the juvenile hormone level in RPWs can also reduce the metamorphosis rate of the weevil larvae. Our results showed that
Ocimum tenuiflorum, when combined with weevil feed was the most effective in slowing down the RPWL metamorphosis rate, with only 24.4% of the treated larvae entering pupation, which is 75.6% lower than the control group which had a pupation rate of 100%. The metamorphosis rate of the RPWL were followed by feed containing
Ocimum basilicum and
Morus alba as the supplemental plants, respectively. Both of these plants have been proven to also be effective, as the results revealed that they could also reduce the metamorphosis of the RPWL, evidenced by the 27.8% and 28.9% of the treated larvae pupating, which is 72.2% and 71.1% lower than the RPWL in the control group, respectively. This finding is consistent with the previous studies by Khyade et al. [
17] and Jaiswal and Srivastava [
18], which reported that insects exposed to secondary metabolites with hormonal activity similar to juvenile hormone (phytojuvenoid) would exhibit slower metamorphosis rates.
Furthermore, our results showed that the use of the combination of plants that inhibit the mutualism of gut microbiota and plants containing phytojuvenoid compounds, which have properties of increasing the juvenile hormone level showed the best results in reducing the metamorphosis rate of RPWL. The combination of Hibiscus sabdariffa and Ocimum tenuiflorum in the weevil feed produced the most significant reduction in the RPWL metamorphosis rate, with only 13.3% of the larvae treated with the combination entering pupation, which is 86.7% lower than the control group, which had a pupation rate of 100%.
Moreover, we conducted an investigation to determine the physical factor that affects the metamorphosis rate of red palm weevils. Our findings revealed that reducing the length of coconut fibers in the feed that the RPWL use for constructing their cocoons to enter pupation can effectively slow down their metamorphosis rate. The results of this study showed that weevil feed containing coconut fibers less than 1 centimeter in length can slow down red palm weevil metamorphosis rate the best, as seen by the least number of larvae pupating at 0.0%. The metamorphosis rate of the RPWL were followed by feed containing coconut fiber with lengths of 5, 10, and 15 centimeters at 28.9, 36.7, and 100.0%, respectively. This finding is consistent with the previous studies by Norzainih et al. [
20], which reported that when RPWs receive the ideal length of coconut fibers, they will be able to construct a cocoon and enter pupation, thus having a higher metamorphosis rate.
In conclusion, our findings suggest that the combined use of Hibiscus sabdariffa and Ocimum tenuiflorum in the weevil feed, along with the reduction of the coconut fiber length in the weevil feed to less than 1 centimeter were best to be used in slowing down RPW metamorphosis rate to reduce their pest problems.
Author Contributions
Conceptualization, S.A., T.C., C.K. and W.B.; Data curation, S.A. and T.C.; Formal analysis, S.A., T.C., C.K. and W.B.; Investigation, S.A. and T.C.; Methodology, S.A., T.C., C.K. and W.B.; Project administration, S.A. and T.C.; Supervision, C.K. and W.B.; Visualization, S.A. and T.C.; Writing – original draft, S.A. and T.C.; Writing – review & editing, S.A., T.C., C.K. and W.B. All authors will be informed about each step of manuscript processing including submission, revision, revision reminder, etc. via emails from our system or assigned Assistant Editor.