Submitted:
12 May 2023
Posted:
15 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
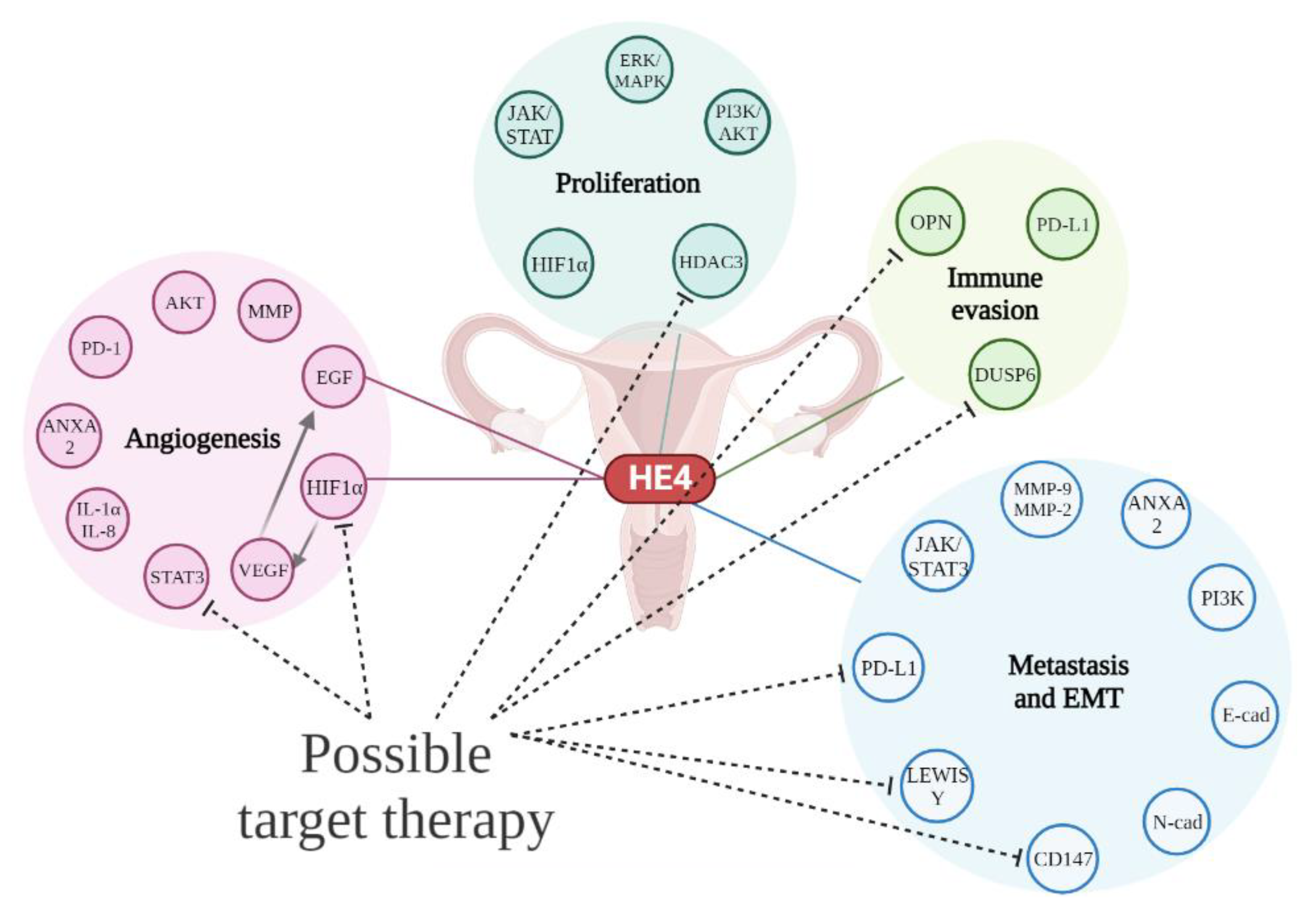
2. HE4 as a Multipurpose Biomarker in OC
3. HE4 in Cell Proliferation and Tumor Growth
4. HE4 and Angiogenesis
5. HE4 and Metastatic Process
6. HE4 and Immune Response
7. HE4 and OC Therapy
8. Conclusions
Acknowledgments
References
- Momenimovahed:, Z. : Tiznobaik, A.; Taheri, S.; Salehiniya, H. Ovarian cancer in the world: epidemiology and risk factors. Int J Womens Health 2019, 11, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wang, C.; Zhou, S. Targeting tumor microenvironment in ovarian cancer: Premise and promise. Biochim Biophys Acta Rev Cancer 2020, 1873, 188361. [Google Scholar] [CrossRef] [PubMed]
- Morand, S.; Devanaboyina, M.; Staats, H.; Stanbery, L.; Nemunaitis, J. Ovarian Cancer Immunotherapy and Personalized Medicine. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Schoutrop, E.; Moyano-Galceran, L.; Lheureux, S.; Mattsson, J.; Lehti, K.; Dahlstrand, H.; Magalhaes, I. Molecular, cellular and systemic aspects of epithelial ovarian cancer and its tumor microenvironment. Semin Cancer Biol 2022, 86, 207–223. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A. Role of Fine Needle Aspiration Cytology in the Diagnosis of Gynecologic Tumors. Acta Cytol 2023, 67, 195–212. [Google Scholar] [CrossRef] [PubMed]
- Menon, U.; Karpinskyj, C.; Gentry-Maharaj, A. Ovarian Cancer Prevention and Screening. Obstet Gynecol 2018, 131, 909–927. [Google Scholar] [CrossRef] [PubMed]
- Brown, Y.; Hua, S.; Tanwar, P.S. Extracellular matrix in high-grade serous ovarian cancer: Advances in understanding of carcinogenesis and cancer biology. Matrix Biol 2023, 118, 16–46. [Google Scholar] [CrossRef]
- Rice, M.S.; Murphy, M.A.; Vitonis, A.F.; Cramer, D.W.; Titus, L.J.; Tworoger, S.S.; Terry, K.L. Tubal ligation, hysterectomy and epithelial ovarian cancer in the New England Case-Control Study. Int J Cancer 2013, 133, 2415–2421. [Google Scholar] [CrossRef]
- Karnezis, A.N.; Cho, K.R.; Gilks, C.B.; Pearce, C.L.; Huntsman, D.G. The disparate origins of ovarian cancers: pathogenesis and prevention strategies. Nat Rev Cancer 2017, 17, 65–74. [Google Scholar] [CrossRef]
- Seidman, J.D.; Yemelyanova, A.; Zaino, R.J.; Kurman, R.J. The fallopian tube-peritoneal junction: a potential site of carcinogenesis. Int J Gynecol Pathol 2011, 30, 4–11. [Google Scholar] [CrossRef]
- Budiana, I.N.G.; Angelina, M.; Pemayun, T.G.A. Ovarian cancer: Pathogenesis and current recommendations for prophylactic surgery. J Turk Ger Gynecol Assoc 2019, 20, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Zamwar, U.M.; Anjankar, A.P. Aetiology, Epidemiology, Histopathology, Classification, Detailed Evaluation, and Treatment of Ovarian Cancer. Cureus 2022, 14, e30561. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.; Ralyea, C.; Lockwood, S. Ovarian Cancer: An Integrated Review. Semin Oncol Nurs 2019, 35, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Manganaro, L.; Celli, V.; Viggiani, V.; Berardelli, E.; Granato, T.; Tartaglione, S.; Farina, A.; Catalano, C.; Angeloni, A.; Anastasi, E. CT imaging phenotypes linked to CA125 and HE4 biomarkers are highly predictive in discriminating between hereditary and sporadic ovarian cancer patients. Tumour Biol 2022, 44, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Ramus, S.J.; Song, H.; Dicks, E.; Tyrer, J.P.; Rosenthal, A.N.; Intermaggio, M.P.; Fraser, L.; Gentry-Maharaj, A.; Hayward, J.; Philpott, S.; et al. Germline Mutations in the BRIP1, BARD1, PALB2, and NBN Genes in Women With Ovarian Cancer. J Natl Cancer Inst 2015, 107. [Google Scholar] [CrossRef] [PubMed]
- Dicks, E.; Song, H.; Ramus, S.J.; Oudenhove, E.V.; Tyrer, J.P.; Intermaggio, M.P.; Kar, S.; Harrington, P.; Bowtell, D.D.; Group, A.S.; et al. Germline whole exome sequencing and large-scale replication identifies. Oncotarget 2017, 8, 50930–50940. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Cancer stat facts: ovarian cancer. Available online: https://seer.cancer.gov/statfacts/ html/ovary.
- Walker, J.L.; Powell, C.B.; Chen, L.M.; Carter, J.; Bae Jump, V.L.; Parker, L.P.; Borowsky, M.E.; Gibb, R.K. Society of Gynecologic Oncology recommendations for the prevention of ovarian cancer. Cancer 2015, 121, 2108–2120. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, X.; Shang, J.; Lin, Y.; Yang, Y.; Song, Y.; Yu, S. Association between dietary fiber intake and risk of ovarian cancer: a meta-analysis of observational studies. J Int Med Res 2018, 46, 3995–4005. [Google Scholar] [CrossRef]
- Cancer Research, UK. Ovarian cancer risk factors. Available online: https://www.cancerresearchuk.
- Schenken, R.S. Endometriosis: Pathogenesis, epidemiology, and clinical impact. Available online: https://www.uptodate.
- Angeloni, A.; De Vito, C.; Farina, A.; Terracciano, D.; Cennamo, M.; Passerini, R.; Bottari, F.; Schirinzi, A.; Vettori, R.; Steffan, A.; et al. New Analytical Approach for the Alignment of Different HE4 Automated Immunometric Systems: An Italian Multicentric Study. J Clin Med 2022, 11. [Google Scholar] [CrossRef]
- Chudecka-Głaz, A.; Strojna, A.; Michalczyk, K.; Wieder-Huszla, S.; Safranow, K.; Skwirczyńska, E.; Jurczak, A. Evaluation of He4 Use in the Diagnosis of Ovarian Cancer: First and Second Recurrence, and an Analysis of HE4 Concentration during Second- and Third-Line Chemotherapy. Diagnostics (Basel) 2023, 13. [Google Scholar] [CrossRef]
- Zalfa, F.; Perrone, M.G.; Ferorelli, S.; Laera, L.; Pierri, C.L.; Tolomeo, A.; Dimiccoli, V.; Perrone, G.; De Grassi, A.; Scilimati, A. Genome-Wide Identification and Validation of Gene Expression Biomarkers in the Diagnosis of Ovarian Serous Cystadenocarcinoma. Cancers (Basel) 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Kirchhoff, C.; Habben, I.; Ivell, R.; Krull, N. A major human epididymis-specific cDNA encodes a protein with sequence homology to extracellular proteinase inhibitors. Biol Reprod 1991, 45, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Qing, X.; Liu, L.; Mao, X. A Clinical Diagnostic Value Analysis of Serum CA125, CA199, and HE4 in Women with Early Ovarian Cancer: Systematic Review and Meta-Analysis. Comput Math Methods Med 2022, 2022, 9339325. [Google Scholar] [CrossRef] [PubMed]
- Hellström, I.; Raycraft, J.; Hayden-Ledbetter, M.; Ledbetter, J.A.; Schummer, M.; McIntosh, M.; Drescher, C.; Urban, N.; Hellström, K.E. The HE4 (WFDC2) protein is a biomarker for ovarian carcinoma. Cancer Res 2003, 63, 3695–3700. [Google Scholar] [PubMed]
- Granato, T.; Porpora, M.G.; Longo, F.; Angeloni, A.; Manganaro, L.; Anastasi, E. HE4 in the differential diagnosis of ovarian masses. Clin Chim Acta 2015, 446, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Anastasi, E.; Granato, T.; Marchei, G.G.; Viggiani, V.; Colaprisca, B.; Comploj, S.; Reale, M.G.; Frati, L.; Midulla, C. Ovarian tumor marker HE4 is differently expressed during the phases of the menstrual cycle in healthy young women. Tumour Biol 2010, 31, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Hamed, E.O.; Ahmed, H.; Sedeek, O.B.; Mohammed, A.M.; Abd-Alla, A.A.; Abdel Ghaffar, H.M. Significance of HE4 estimation in comparison with CA125 in diagnosis of ovarian cancer and assessment of treatment response. Diagn Pathol 2013, 8, 11. [Google Scholar] [CrossRef]
- Moore, R.G.; McMeekin, D.S.; Brown, A.K.; DiSilvestro, P.; Miller, M.C.; Allard, W.J.; Gajewski, W.; Kurman, R.; Bast, R.C.; Skates, S.J. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol Oncol 2009, 112, 40–46. [Google Scholar] [CrossRef]
- Cviič, D.; Jagarlamudi, K.; Meglič, L.; Škof, E.; Zore, A.; Lukanović, D.; Eriksson, S.; Osredkar, J. A Dual Biomarker TK1 Protein and CA125 or HE4-Based Algorithm as a Better Diagnostic Tool than ROMA Index in Early Detection of Ovarian Cancer. Cancers (Basel) 2023, 15. [Google Scholar] [CrossRef]
- Manganaro, L.; Anastasi, E.; Porpora, M.G.; Vinci, V.; Saldari, M.; Bernardo, S.; Ballesio, L.; Sollazzo, P.; Pecorella, I.; Recchia, N.; et al. Biparametric Magnetic Resonance Imaging as an Adjunct to CA125 and HE4 to Improve Characterization of Large Ovarian Masses. Anticancer Res 2015, 35, 6341–6351. [Google Scholar]
- Cao, H.; You, D.; Lan, Z.; Ye, H.; Hou, M.; Xi, M. Prognostic value of serum and tissue HE4 expression in ovarian cancer: a systematic review with meta-analysis of 90 studies. Expert Rev Mol Diagn 2018, 18, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Samborski, A.; Miller, M.C.; Blackman, A.; MacLaughlan-David, S.; Jackson, A.; Lambert-Messerlian, G.; Rowswell-Turner, R.; Moore, R.G. HE4 and CA125 serum biomarker monitoring in women with epithelial ovarian cancer. Tumour Biol 2022, 44, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Plotti, F.; Terranova, C.; Guzzo, F.; De Cicco Nardone, C.; Luvero, D.; Bartolone, M.; Dionisi, C.; Benvenuto, D.; Fabris, S.; Ciccozzi, M.; et al. Role of BRCA Mutation and HE4 in Predicting Chemotherapy Response in Ovarian Cancer: A Retrospective Pilot Study. Biomedicines 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- James, N.E.; Chichester, C.; Ribeiro, J.R. Beyond the Biomarker: Understanding the Diverse Roles of Human Epididymis Protein 4 in the Pathogenesis of Epithelial Ovarian Cancer. Front Oncol 2018, 8, 124. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhuang, H.; Wang, H.; Tan, M.; Schwab, C.L.; Deng, L.; Gao, J.; Hao, Y.; Li, X.; Gao, S.; et al. Overexpression of HE4 (human epididymis protein 4) enhances proliferation, invasion and metastasis of ovarian cancer. Oncotarget 2016, 7, 729–744. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.F.; Gao, G.L.; Tang, S.B.; Zhang, Z.D.; Huang, Q.S. Effect of WFDC 2 silencing on the proliferation, motility and invasion of human serous ovarian cancer cells in vitro. Asian Pac J Trop Med 2013, 6, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhu, L.; Gao, J.; Hu, Z.; Lin, B. Promotive role of recombinant HE4 protein in proliferation and carboplatin resistance in ovarian cancer cells. Oncol Rep 2015, 33, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Sun, X.; Xiao, R.; Zhou, L.; Gao, X.; Guo, L. Human epididymis protein 4 (HE4) plays a key role in ovarian cancer cell adhesion and motility. Biochem Biophys Res Commun 2012, 419, 274–280. [Google Scholar] [CrossRef]
- Lee, S.; Choi, S.; Lee, Y.; Chung, D.; Hong, S.; Park, N. Role of human epididymis protein 4 in chemoresistance and prognosis of epithelial ovarian cancer. J Obstet Gynaecol Res 2017, 43, 220–227. [Google Scholar] [CrossRef]
- Ribeiro, J.R.; Schorl, C.; Yano, N.; Romano, N.; Kim, K.K.; Singh, R.K.; Moore, R.G. HE4 promotes collateral resistance to cisplatin and paclitaxel in ovarian cancer cells. J Ovarian Res 2016, 9, 28. [Google Scholar] [CrossRef]
- Gao, J.; Li, J.; Ma, L. Regulation of EGF-induced ERK/MAPK activation and EGFR internalization by G protein-coupled receptor kinase 2. Acta Biochim Biophys Sin (Shanghai) 2005, 37, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.G.; Hill, E.K.; Horan, T.; Yano, N.; Kim, K.; MacLaughlan, S.; Lambert-Messerlian, G.; Tseng, Y.D.; Padbury, J.F.; Miller, M.C.; et al. HE4 (WFDC2) gene overexpression promotes ovarian tumor growth. Sci Rep 2014, 4, 3574. [Google Scholar] [CrossRef] [PubMed]
- Lou, T.; Zhuang, H.; Liu, C.; Zhang, Z. HDAC3 positively regulates HE4 expression to promote ovarian carcinoma progression. Arch Biochem Biophys 2019, 675, 108044. [Google Scholar] [CrossRef] [PubMed]
- James, N.E.; Emerson, J.B.; Borgstadt, A.D.; Beffa, L.; Oliver, M.T.; Hovanesian, V.; Urh, A.; Singh, R.K.; Rowswell-Turner, R.; DiSilvestro, P.A.; et al. The biomarker HE4 (WFDC2) promotes a pro-angiogenic and immunosuppressive tumor microenvironment via regulation of STAT3 target genes. Sci Rep 2020, 10, 8558. [Google Scholar] [CrossRef] [PubMed]
- Tam, S.; Fu, S.; Xu, L.; Krause, K.J.; Lairson, D.R.; Miao, H.; Sturgis, E.M.; Dahlstrom, K.R. The epidemiology of oral human papillomavirus infection in healthy populations: A systematic review and meta-analysis. Oral Oncol 2018, 82, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Poddar, A.; Aranha, R.R.; K Muthukaliannan, G.; Nachimuthu, R.; Jayaraj, R. Head and neck cancer risk factors in India: protocol for systematic review and meta-analysis. BMJ Open 2018, 8, e020014. [Google Scholar] [CrossRef]
- Karna, H.; Gonzalez, J.; Radia, H.S.; Sedghizadeh, P.P.; Enciso, R. Risk-reductive dental strategies for medication related osteonecrosis of the jaw among cancer patients: A systematic review with meta-analyses. Oral Oncol 2018, 85, 15–23. [Google Scholar] [CrossRef]
- Dabkeviciene, D.; Sasnauskiene, A.; Leman, E.; Kvietkauskaite, R.; Daugelaviciene, N.; Stankevicius, V.; Jurgelevicius, V.; Juodka, B.; Kirveliene, V. mTHPC-mediated photodynamic treatment up-regulates the cytokines VEGF and IL-1alpha. Photochem Photobiol 2012, 88, 432–439. [Google Scholar] [CrossRef]
- Wang, A.; Jin, C.; Tian, X.; Wang, Y.; Li, H. Knockdown of HE4 suppresses aggressive cell growth and malignant progression of ovarian cancer by inhibiting the JAK/STAT3 pathway. Biol Open 2019, 8. [Google Scholar] [CrossRef]
- Fu, H.; Ma, Y.; Yang, M.; Zhang, C.; Huang, H.; Xia, Y.; Lu, L.; Jin, W.; Cui, D. Persisting and Increasing Neutrophil Infiltration Associates with Gastric Carcinogenesis and E-cadherin Downregulation. Sci Rep 2016, 6, 29762. [Google Scholar] [CrossRef]
- Bekes, E.M.; Schweighofer, B.; Kupriyanova, T.A.; Zajac, E.; Ardi, V.C.; Quigley, J.P.; Deryugina, E.I. Tumor-recruited neutrophils and neutrophil TIMP-free MMP-9 regulate coordinately the levels of tumor angiogenesis and efficiency of malignant cell intravasation. Am J Pathol 2011, 179, 1455–1470. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Liu, S.; Zhang, M.; Chen, S.; Gan, S.; Chen, C.; Chen, W.; Li, L.; Zhu, Z. A dual role of HIF1α in regulating osteogenesis-angiogenesis coupling. Stem Cell Res Ther 2022, 13, 59. [Google Scholar] [CrossRef] [PubMed]
- Pugh, C.W.; Ratcliffe, P.J. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med 2003, 9, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Scortegagna, M.; Cataisson, C.; Martin, R.J.; Hicklin, D.J.; Schreiber, R.D.; Yuspa, S.H.; Arbeit, J.M. HIF-1alpha regulates epithelial inflammation by cell autonomous NFkappaB activation and paracrine stromal remodeling. Blood 2008, 111, 3343–3354. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Liu, G.; Huang, K.; Zheng, Q.; Li, Y.; Yu, C. Hypoxia-Induced Upregulation of HE4 Is Responsible for Resistance to Radiation Therapy of Gastric Cancer. Mol Ther Oncolytics 2019, 12, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, P.H.; Wiesener, M.S.; Chang, G.W.; Clifford, S.C.; Vaux, E.C.; Cockman, M.E.; Wykoff, C.C.; Pugh, C.W.; Maher, E.R.; Ratcliffe, P.J. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 1999, 399, 271–275. [Google Scholar] [CrossRef]
- Dang, C.V.; Semenza, G.L. Oncogenic alterations of metabolism. Trends Biochem Sci 1999, 24, 68–72. [Google Scholar] [CrossRef]
- Amer, H.; Kartikasari, A.E.R.; Plebanski, M. Elevated Interleukin-6 Levels in the Circulation and Peritoneal Fluid of Patients with Ovarian Cancer as a Potential Diagnostic Biomarker: A Systematic Review and Meta-Analysis. J Pers Med 2021, 11. [Google Scholar] [CrossRef]
- Sanguinete, M.M.M.; Oliveira, P.H.; Martins-Filho, A.; Micheli, D.C.; Tavares-Murta, B.M.; Murta, E.F.C.; Nomelini, R.S. Serum IL-6 and IL-8 Correlate with Prognostic Factors in Ovarian Cancer. Immunol Invest 2017, 46, 677–688. [Google Scholar] [CrossRef]
- Zhang, T.; Long, H.; Li, J.; Chen, Z.; Wang, F.; Jiang, S.W. WFDC2 gene deletion in mouse led to severe dyspnea and type-I alveolar cell apoptosis. Biochem Biophys Res Commun 2020, 522, 456–462. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, C.; Bai, H.; Cao, G.; Cui, R.; Zhang, Z. Combinatorial therapy of immune checkpoint and cancer pathways provides a novel perspective on ovarian cancer treatment. Oncol Lett 2019, 17, 2583–2591. [Google Scholar] [CrossRef] [PubMed]
- Będkowska, G.E.; Piskór, B.; Gacuta, E.; Zajkowska, M.; Osada, J.; Szmitkowski, M.; Dąbrowska, M.; Ławicki, S. Diagnostic Power of Selected Cytokines, MMPs and TIMPs in Ovarian Cancer Patients - ROC Analysis. Anticancer Res 2019, 39, 2575–2582. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.L.; Yang, Z.Y.; Wu, Q.J.; Li, Y.Z.; Li, X.Y.; Liu, F.H.; Wei, Y.F.; Wen, Z.Y.; Lin, B.; Gong, T.T. The Role of Human Epididymis Protein 4 in the Diagnosis and Prognosis of Diseases: An Umbrella Review of Systematic Reviews and Meta-Analyses of Observational Studies. Front Med (Lausanne) 2022, 9, 842002. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Nie, X.; Gou, R.; Qi, Y.; Liu, J.; Lin, B. Interaction of CD147 and human epididymis protein 4 promotes invasion and metastasis of ovarian cancer. J Cancer 2021, 12, 7422–7435. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gomez, A.; Rodríguez-Ubreva, J.; Ballestar, E. Epigenetic interplay between immune, stromal and cancer cells in the tumor microenvironment. Clin Immunol 2018, 196, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Cheng, H.Y.; Dong, L.; Ye, X.; Liu, Y.N.; Chang, X.H.; Cheng, Y.X.; Chen, J.; Ma, R.Q.; Cui, H. The role of HE4 in ovarian cancer: inhibiting tumour cell proliferation and metastasis. J Int Med Res 2011, 39, 1645–1660. [Google Scholar] [CrossRef]
- Espiau Romera, A.; Cuesta Guardiola, T.; Benito Vielba, M.; De Bonrostro Torralba, C.; Coronado Martín, P.J.; Baquedano Mainar, L. HE4 tumor marker as a predictive factor for lymphatic metastasis in endometrial cancer. Int J Gynaecol Obstet 2020, 149, 265–268. [Google Scholar] [CrossRef]
- Zhuang, H.; Hu, Z.; Tan, M.; Zhu, L.; Liu, J.; Liu, D.; Yan, L.; Lin, B. Overexpression of Lewis y antigen promotes human epididymis protein 4-mediated invasion and metastasis of ovarian cancer cells. Biochimie 2014, 105, 91–98. [Google Scholar] [CrossRef]
- Bingle, L.; Cross, S.S.; High, A.S.; Wallace, W.A.; Rassl, D.; Yuan, G.; Hellstrom, I.; Campos, M.A.; Bingle, C.D. WFDC2 (HE4): a potential role in the innate immunity of the oral cavity and respiratory tract and the development of adenocarcinomas of the lung. Respir Res 2006, 7, 61. [Google Scholar] [CrossRef]
- Labani-Motlagh, A.; Ashja-Mahdavi, M.; Loskog, A. The Tumor Microenvironment: A Milieu Hindering and Obstructing Antitumor Immune Responses. Front Immunol 2020, 11, 940. [Google Scholar] [CrossRef]
- Rowswell-Turner, R.B.; Singh, R.K.; Urh, A.; Yano, N.; Kim, K.K.; Khazan, N.; Pandita, R.; Sivagnanalingam, U.; Hovanesian, V.; James, N.E.; et al. HE4 Overexpression by Ovarian Cancer Promotes a Suppressive Tumor Immune Microenvironment and Enhanced Tumor and Macrophage PD-L1 Expression. J Immunol 2021, 206, 2478–2488. [Google Scholar] [CrossRef] [PubMed]
- Dubey, H.; Modi, M.; Verma, S.; Sinha, R.; Goel, H.; Ranjan, A.; Tanwar, P.; Chopra, A.; Rahul, E.; Ranjan, L.; et al. Role of Human Epididymis Protein 4 in Tumour Angiogenesis. Recent Advances, New Perspectives and Applications in the Treatment of Ovarian Cancer.
- Li, R.; Xu, J.; Wu, M.; Liu, S.; Fu, X.; Shang, W.; Wang, T.; Jia, X.; Wang, F. Circulating CD4. Medicina (Kaunas) 2023, 59. [Google Scholar] [CrossRef]
- Liu, P.; Chen, R.; Zhang, X.; Fu, R.; Tao, L.; Jia, W. Combined PD-1/PD-L1 and tumor-infiltrating immune cells redefined a unique molecular subtype of high-grade serous ovarian carcinoma. BMC Genomics 2022, 23, 51. [Google Scholar] [CrossRef] [PubMed]
- Saffarieh, E.; Nassiri, S.; Mirmohammadkhani, M. Predicting value of HE4 and CA125 markers for optimal cytoreductive surgery in ovarian cancer patients. Eur J Transl Myol 2022, 32. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Shenoy, P.S.; Mehrotra, M.; Phadte, P.; Singh, P.; Rekhi, B.; Ray, P. Through the Looking Glass: Updated Insights on Ovarian Cancer Diagnostics. Diagnostics (Basel) 2023, 13. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Kim, A.; Jang, H. Immunotherapeutic Approaches in Ovarian Cancer. Curr Issues Mol Biol 2023, 45, 1233–1249. [Google Scholar] [CrossRef] [PubMed]
- Angioli, R.; Capriglione, S.; Aloisi, A.; Guzzo, F.; Luvero, D.; Miranda, A.; Damiani, P.; Montera, R.; Terranova, C.; Plotti, F. Can HE4 predict platinum response during first-line chemotherapy in ovarian cancer? Tumour Biol 2014, 35, 7009–7015. [Google Scholar] [CrossRef]
- Vetter, M.H.; Hays, J.L. Use of Targeted Therapeutics in Epithelial Ovarian Cancer: A Review of Current Literature and Future Directions. Clin Ther 2018, 40, 361–371. [Google Scholar] [CrossRef]
- Lisio, M.A.; Fu, L.; Goyeneche, A.; Gao, Z.H.; Telleria, C. High-Grade Serous Ovarian Cancer: Basic Sciences, Clinical and Therapeutic Standpoints. Int J Mol Sci 2019, 20. [Google Scholar] [CrossRef]
- Algethami, M.; Kulkarni, S.; Sadiq, M.T.; Tang, H.K.C.; Brownlie, J.; Jeyapalan, J.N.; Mongan, N.P.; Rakha, E.A.; Madhusudan, S. Towards Personalized Management of Ovarian Cancer. Cancer Manag Res 2022, 14, 3469–3483. [Google Scholar] [CrossRef]
| Pathway |
Description and functions | HE4 influence |
|---|---|---|
|
ERK/MAPK (extracellular signal-regulated kinases/ mitogen-activated protein kinase) |
▪ pathway usually activated by EGF. ▪ phosphorylation of neighboring proteins by ERK ("on" or "off" switch) ▪ ERK required for activation of genes for entry into the cell cycle. ▪ pathway mutated in many cancers |
▪ regulation of proliferation and invasion of SOC cells ▪ ERK activation with HE4 overexpression ▪ decrease in proliferation when HE4 was silenced in SKOV3 cells. ▪ activation of ERK/MAPK pathway by interaction of HE4 with EGF/EGFR |
|
PI3K/AKT (phosphoinositide 3- kinases/ Protein kinase B) |
▪ PI3K indirectly activates AKT after phosphorylation of phosphatidyl inositol 4,5 bisphosphate (PIP2) and phosphatidyl inositol 3,4,5 trisphosphate (PIP3) ▪ phosphorylation of protein substrates by AKT ▪ activation of biochemical pathways leading to cell growth and resistance to apoptosis ▪ mTOR protein involved in angiogenesis and increase of membrane glucose transporters. |
▪ AKT increase and subsequent cell growth in OVCAR3 cells when HE4 is overexpressed. ▪ AKT decrease and subsequent reduced cell growth in OVCAR3 cells when there is HE4 knockout. |
|
HDAC3 (histone deacetylase 3) |
▪ role in S phase progression, DNA damage control, genomic stability maintenance |
▪ HDAC3 expression or knockdown lead to a corresponding increase or decrease in HE4 expression. ▪ HE4 and HDAC3 binding activates the PI3K/AKT signaling pathway. ▪ inhibition of the interaction between HDAC3 and HE4 may have potential therapeutic value |
|
HIF1α (hypoxia-inducible factor 1-alpha) |
▪ key mediator of cellular adaptation to hypoxia ▪ Involved in processes of proliferation, survival and angiogenesis. ▪ modulated by hydroxylation, acetylation, and phosphorylation. |
▪ HE4-HIF1α interaction is yet not well understood. ▪ decrease in HE4 levels in SKOV3 cells treated with HIF1α siRNA or with HIF1α inhibitors |
|
JAK/STAT (Janus kinases/signal transducer and activator of transcription proteins) |
▪ pathway activated by cytokines and growth factors. ▪ intracellular response triggered by the action of the activated STAT proteins. ▪ alteration of gene expression of proteins involved in proliferation, differentiation and apoptosis |
▪ HE4 knockdown inhibits the activity of the JAK/STAT3 pathway in vitro and in vivo. ▪ HE4 knockdown suppresses cell proliferation and malignant progression of ovarian cancer |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





