Submitted:
12 May 2023
Posted:
15 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Mechanisms of Sensitivity of Semiconductor Metal Oxide Sensors in the Detection of Reducing Gases
2.1. Mechanisms of Increasing Sensitivity of Two-Component Single-Phase Metal Oxides
2.1.1. Change in lattice of particles during doping – dependence of interaction of doping ions with ions of main lattice on their valence
2.1.2. Effect of doping on adsorption of detected gases
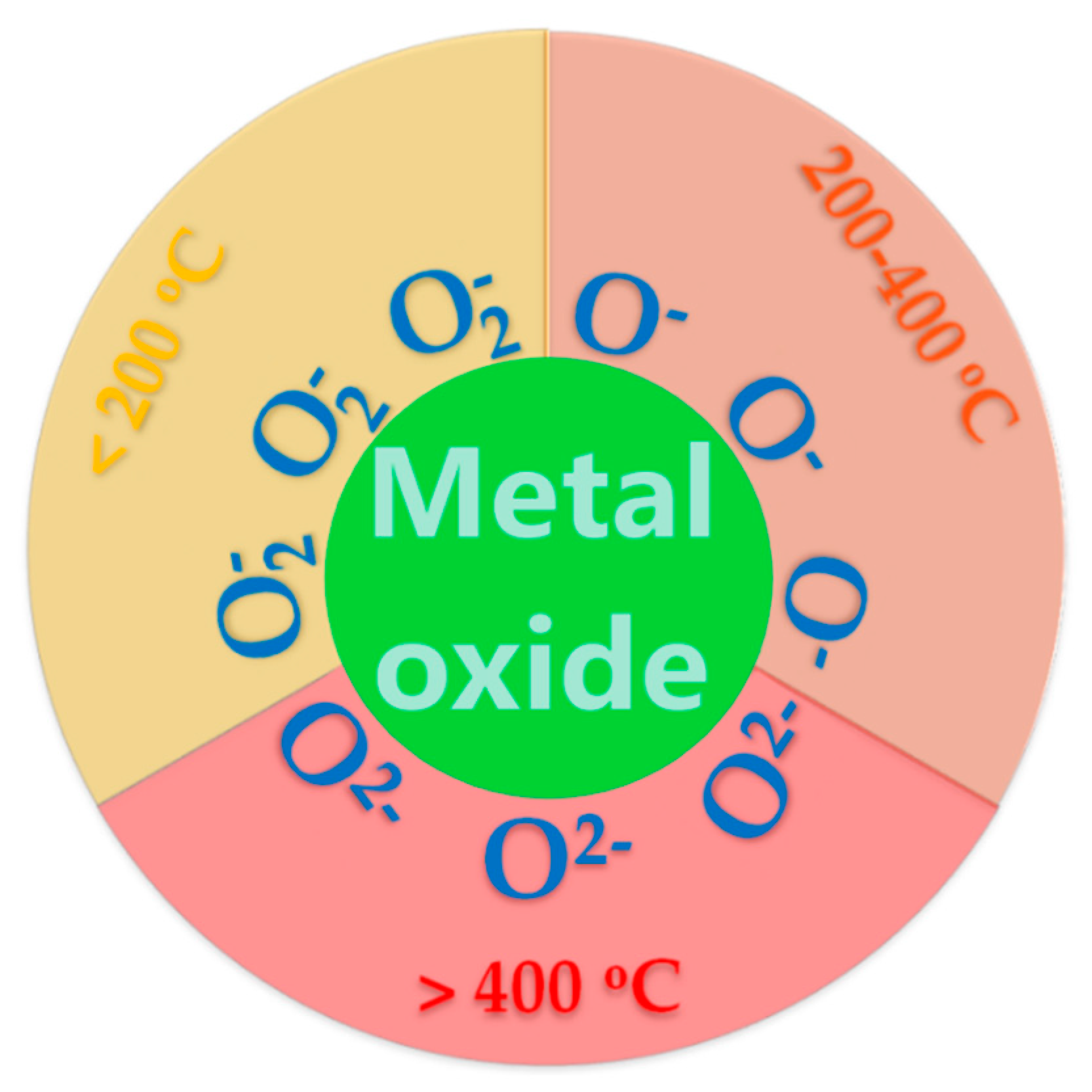
2.1.3. Influence of nature of doping ions on reactivity of oxygen anionic centers
2.2. Mechanisms for Increasing Sensitivity of Two-Component Two-Phase Metal Oxide Composites
2.2.1. Effect of conduction pathways on sensor properties of binary two-phase nanocomposites
2.2.2. Effect of interaction between conducting and modifying nanocrystals on sensor properties of a composite
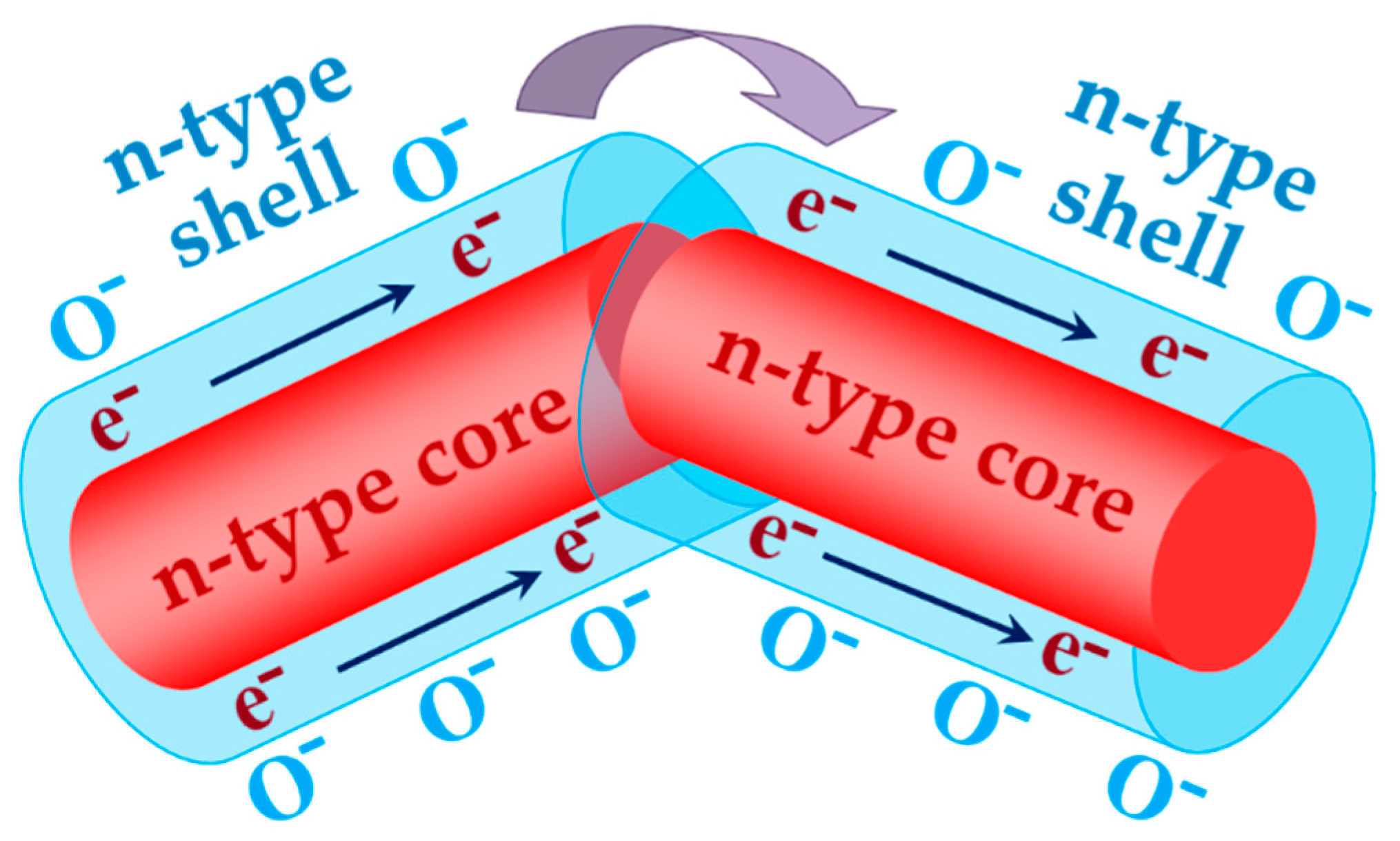
2.3. Interaction of Nanocomponents in Core-Shell Sensor Systems
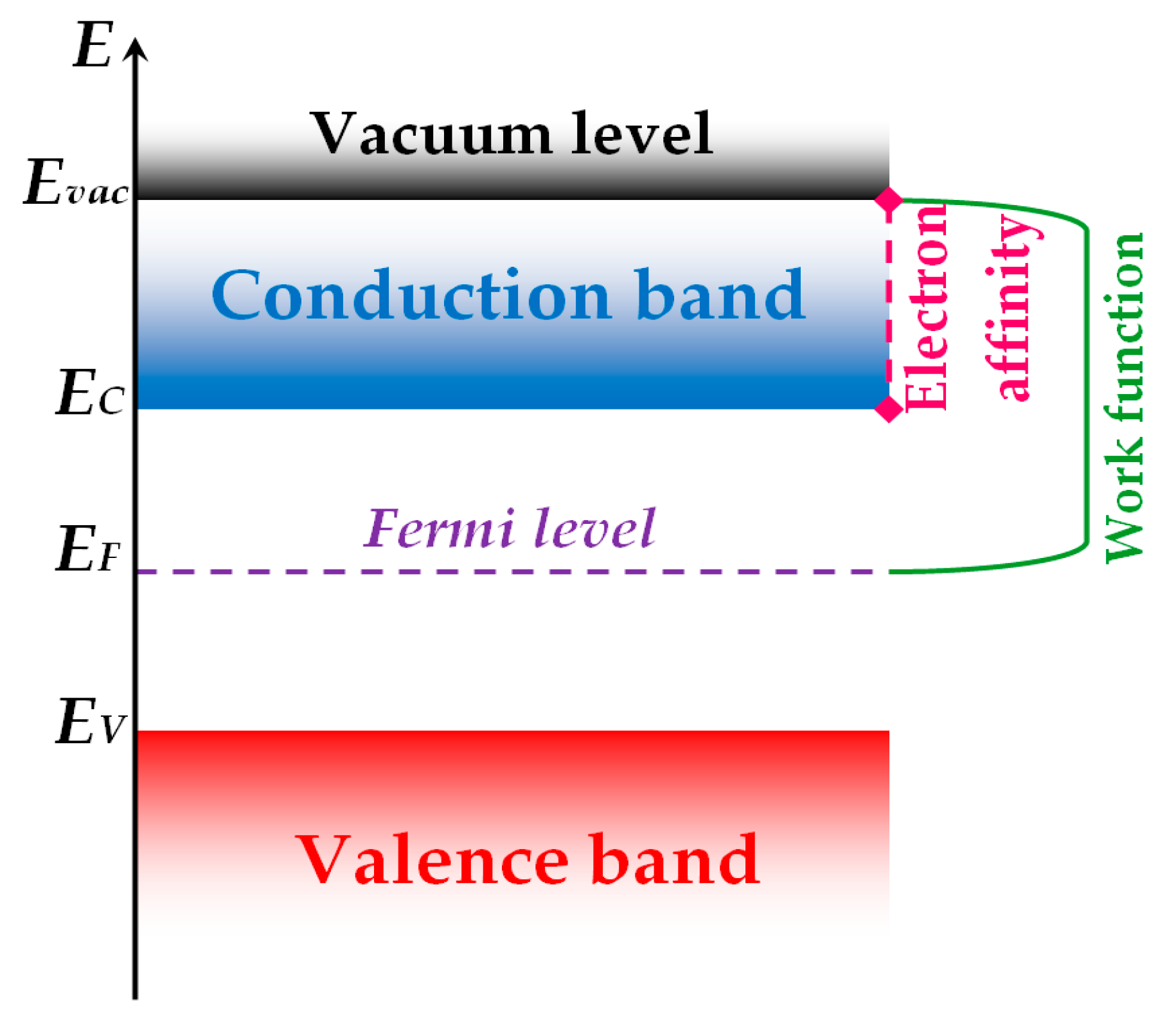
3. Modeling of the Electronic Subsystem of Semiconductor Nanoparticles and Properties of Associated Sensitive Layers
3.1. Distribution of electrons in oxide nanoparticles
3.2. Sensor effect in one-component systems
3.3. Electron distribution and sensor effect in two-component systems
4. Mechanisms of Sensor Selectivity
4.1. Selectivity of Molecule Adsorption on Surface of Sensor Layer
4.2. Selective activity of adsorbed molecules in reaction with oxygen anions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Degler, D.; Weimar, U.; Barsan, N. Current understanding of the fundamental mechanisms of doped and loaded semiconducting metal-oxide-based gas sensing materials. ACS Sens. 2019, 4, 2228–2249. [Google Scholar] [CrossRef] [PubMed]
- Degler, D. Trends and advances in the characterization of gas sensing materials based on semiconducting oxides. Sensors 2018, 18, 3544. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.R.; Akbar, S.A.; Morris, P.A. Nanoscale metal oxide-based heterojunctions for gas sensing: A review. Sens. Actuators B 2014, 204, 250–272. [Google Scholar] [CrossRef]
- Barsan, N.; Koziej, D.; Weimar, U. Metal oxide-based gas sensor research: How to? Sens. Actuators B 2007, 121, 18–35. [Google Scholar] [CrossRef]
- Yamazoe, N.; Sakai, G.; Shimanoe, K. Oxide semiconductor gas sensors. Catal. Surv. Asia 2003, 7, 63–75. [Google Scholar] [CrossRef]
- Liu, J.; Liu, X.; Zhai, Z.; Jin, G.; Jiang, Q.; Zhao, Y.; Luo, C.; Quan, L. Evaluation of depletion layer width and gas-sensing properties of antimony-doped tin oxide thin film sensors. Sens. Actuators B 2015, 220, 1354–1360. [Google Scholar] [CrossRef]
- Sharma, A.; Rout, C.S. Advances in understanding the gas sensing mechanisms by in situ and operando spectroscopy. J. Mat. Chem. A 2021, 9, 18175–18207. [Google Scholar] [CrossRef]
- Gurlo, A.; Riedel, R. In situ and operando spectroscopy for assessing mechanisms of gas sensing. Ang. Chem. Intern. Ed. 2007, 46, 3826–3848. [Google Scholar] [CrossRef]
- Morrison, S.R. The Chemical Physics of Surfaces; Springer Science & Business Media: New York, NY, USA, 2013; Available online: https://scholar.google.com/scholar_lookup?title=The%20Chemical%20Physics%20of%20Surfaces&publication_year=1990&author=S.R.%20Morrison. [Google Scholar]
- Barsan, N.; Weimar, U. Conduction model of metal oxide gas sensors. J. Electroceramics 2001, 7, 143–167. [Google Scholar] [CrossRef]
- Sopiha, K.V.; Malyi, O.I.; Persson, C.; Wu, P. Chemistry of oxygen ionosorption on SnO2 surfaces. Appl. Mater. Interfaces 2021, 13, 33664–33676. [Google Scholar] [CrossRef]
- Yamazoe, N.; Suematsu, K.; Shimano, K. Extention of receptor function theory to include two types of adsorbed oxygen for oxide semiconductor gas sensors. Sens. Actuators B 2012, 163, 128–135. [Google Scholar] [CrossRef]
- Yamazoe, N.; Kurorawa, Y.; Seiyama, T. Effects of additives on semiconductor gas sensors. Sens. Actuators B 1983, 4, 283–289. [Google Scholar] [CrossRef]
- Gerasimov, G.N.; Gromov, V.F.; Ilegbusi, O.J.; Trakhtenberg, L.I. The mechanisms of sensory phenomena in binary metal-oxide nanocomposites. Sens. Actuators B 2017, 240, 613–624. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B.K. Instability of metal oxide-based conductometric gas sensors and approaches to stability improvement (short survey). Sens. Actuators B 2017, 244, 182–210. [Google Scholar] [CrossRef]
- Schwarz, A.; Contescu, C.; Contescu, A. Methods for preparation of catalytic materials. Chem. Rev. 1995, 95, 477–510. [Google Scholar] [CrossRef]
- Korotcenkov, G.I.; Cornet, B.; Cirera, J.R.; Golovanov, V.; Boris, I.; Lychcovsky, V.; Karkotsry, Y.; Rodriguez, A. The influence of additives on gas sensing and structural properties of In2O3-based ceramics. Sens. Actuators B Chem. 2007, 120, 657–664. [Google Scholar] [CrossRef]
- Lin, C.-Yu.; Fang, Yu.-Yu.; Lin, Ch.-W.; Tunney, J.J.; Ho, K.-C. Fabrication of NOx gas sensors using In2O3-ZnO composite films. Sens. Actuators B 2010, 146, 28–34. [Google Scholar] [CrossRef]
- Gerasimov, G.N.; Ikim, M.I.; Gromov, V.F.; Ilegbusi, O.J.; Trakhtenberg, L.I. Chemical modification of impregnated SnO2-In2O3 nanocomposites due to interaction of sensor components. J. Alloys and Compounds 2021, 883, 160817. [Google Scholar] [CrossRef]
- Trakhtenberg, L.I.; Gerasimov, G.N.; Gromov, V.F.; Belysheva, T.V.; Ilegbusi, O.J. Effect of composition and temperature on conductive and sensing properties of CeO2 + In2O3 nanocomposite films. Sens. Actuators B 2015, 209, 562–569. [Google Scholar] [CrossRef]
- Trakhtenberg, L.I.; Gerasimov, G.N.; Gromov, V.F.; Belysheva, T.V.; Ilegbusi, O.J. Effect of composition on sensing properties of SnO2 + In2O3 mixed nanostructured films. Sens. Actuators B 2012, 169, 32–38. [Google Scholar] [CrossRef]
- Trakhtenberg, L.I.; Gerasimov, G.N.; Gromov, V.F.; Belysheva, T.V.; Ilegbusi, O.J. Conductivity and sensing properties of In2O3 + ZnO mixed nanostructured films: Effect of composition and temperature. Sens. Actuators B 2013, 187, 514–521. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, J.-H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Optimization and gas sensing mechanism of n-SnO2-p-Co3O4 composite nanofibers. Sens. Actuators B 2017, 248, 500–511. [Google Scholar] [CrossRef]
- Yin, X.-T.; Li, J.; Dastan, D.; Zhou, W.-D.; Garmestani, H.; Alamgir, F.M. Ultra-high selectivity of H2 over CO with a p-n nanojunction based gas sensors and its mechanism. Sens. Actuators B 2020, 319, 128330. [Google Scholar] [CrossRef]
- Yamazoe, N. New approaches for improving semiconductor gas sensors. Sens. Actuators B 1991, 5, 7–19. [Google Scholar] [CrossRef]
- Yamazoe, N.; Shimanoe, K. Receptor function and response of semiconductor gas sensor. J. Sensors 2009, 138, 1–21. [Google Scholar] [CrossRef]
- Nagaev, E.L. Small metal particles. Soviet Physics Uspekhi 1992, 35, 747–782. [Google Scholar] [CrossRef]
- Trakhtenberg, L.I.; Gerasimov, G.N.; Grigoriev, E.I.; Zavialov, S.A.; Zagorskaja, O.V.; Zufman, V.Yu.; Smirnov, V.V. Nanoheterogeneous metal-polymer composites as a new type of effective and selective catalysts. Stud. Surf. Sci. Catal. 2000, 130, 941–946. [Google Scholar] [CrossRef]
- Maier, J.; Göpel, W. Investigations of the bulk defect chemistry of polycrystalline tin (IV) oxide. J. Solid State Chemistry 1988, 72, 293–302. [Google Scholar] [CrossRef]
- Kolmakov, A.; Klenov, D.O.; Lilach, Y.; Stemmer, S.; Moskovits, M. Enhanced gas sensing by individual SnO2 nanowires and nanobelts functionalized with Pd catalyst particles. Nano Lett. 2005, 5, 667–673. [Google Scholar] [CrossRef]
- Kozhushner, M.A.; Lidskii, B.V.; Oleynik, I.I.; Posvyanskii, V.S.; Trakhtenberg, L.I. Inhomogeneous charge distribution in semiconductor nanoparticles. J. Phys. Chem. C 2015, 119, 16286–16292. [Google Scholar] [CrossRef]
- Kozhushner, M.A.; Trakhtenberg, L.I.; Landerville, A.C.; Oleynik, I.I. Theory of sensing response of nanostructured tin-dioxide thin films to reducing hydrogen gas. J. Phys. Chem. C 2013, 117, 11562–11568. [Google Scholar] [CrossRef]
- Kozhushner, M.A.; Trakhtenberg, L.I.; Bodneva, V.L.; Belysheva, T.V.; Landerville, A.C.; Oleynik, I.I. Effect of temperature and nanoparticle size on sensor properties of nanostructured tin dioxide films. J. Phys. Chem. C 2014, 118, 11440–11444. [Google Scholar] [CrossRef]
- Kozhushner, M.A.; Bodneva, V.L.; Oleynik, I.I.; Belysheva, T.V.; Ikim, M.I.; Trakhtenberg, L.I. Sensor effect in oxide films with a large concentration of conduction electrons. J. Phys. Chem. C 2017, 121, 6940–6945. [Google Scholar] [CrossRef]
- Bodneva, V.L.; Ilegbusi, O.J.; Kozhushner, M.A.; Kurmangaleev, K.S.; Posvyanskii, V.S.; Trakhtenberg, L.I. Modeling of sensor properties for reducing gases and charge distribution in nanostructured oxides: A comparison of theory with experimental data. Sens. Actuators B 2019, 287, 218–224. [Google Scholar] [CrossRef]
- Trakhtenberg, L.I.; Ilegbusi, O.J.; Kozhushner, M.A. Comments on the article “Calculation of the electric potential and surface oxygen ion density for planar and spherical metal oxide grains by numerical solution of the Poisson equation coupled with Boltzmann and Fermi-Dirac statistics” (Sens. Actuators B: Chemical, 2019, 293, 31–40). Sens. Actuators B 2020, 302, 126986. [Google Scholar] [CrossRef]
- Kurmangaleev, K.S.; Ikim, M.I.; Kozhushner, M.A.; Trakhtenberg, L.I. Electron distribution and electrical resistance in nanostructured mixed oxides CeO2-In2O3. Appl. Surf. Sci. 2020, 546, 149011. [Google Scholar] [CrossRef]
- Aragon, F.H.; Coaquira, J.A.H.; Hidalgo, P.; daSilva, S.W.; Brito, S.L.M.; Gouvêa, D.; Morais, P.C. Evidences of the evolution from solid solution to surface segregation in Ni-doped SnO2 nanoparticles using Raman spectroscopy. J. Raman Spectrosc. 2011, 42, 1081–1086. [Google Scholar] [CrossRef]
- Luo, Y.; An, B.; Bai, J.; Wang, Y.; Cheng, X.; Wang, Q.; Li, J.; Yang, Y.; Wu, Z.; Xie, E. Ultrahigh-response hydrogen sensor based on PdO/NiO co-doped In2O3 nanotubes. J. Colloid Interface Sci. 2021, 599, 533–542. [Google Scholar] [CrossRef]
- Gerasimov, G.N.; Gromov, V.F.; Ikim, M.I.; Ilegbusi, O.J.; Ozerin, S.A.; Trakhtenberg, L.I. Structure and gas-sensing properties of SnO2-In2O3 nanocomposites synthesized by impregnation method. Sens. Actuators B 2020, 320, 128406. [Google Scholar] [CrossRef]
- Zhu, X.; Li, Y.; Zhang, H.; Song, L.; Zu, H.; Qin, Y.; Liu, L.; Li, Y.; Wang, F. High-performance field effect transistors based on large ratio metal (Al, Ga, Cr) doped In2O3 nanofibers. J. Alloys Compounds 2020, 830, 154578. [Google Scholar] [CrossRef]
- Sakthiraj, K.; Balachandrakumar, K. Influence of Ti addition on the room temperature ferromagnetism of tin oxide (SnO2) nanocrystal. J. Magn. Magn. Mater. 2015, 395, 205–212. [Google Scholar] [CrossRef]
- Shen, J.; Li, F.; Yin, B.; Sun, L.; Chen, C.; Wen, S.; Chen, Y.; Ruan, S. Enhanced ethyl acetate sensing performance of Al-doped In2O3 microcubes. Sens. Actuators B 2017, 253, 461–469. [Google Scholar] [CrossRef]
- Al-Hashem, M.; Akbar, S.; Morris, P. Role of oxygen vacancies in nanostructured metal-oxide gas sensors: A review. Sens. Actuators B 2019, 301, 126845. [Google Scholar] [CrossRef]
- Zeng, X.; Liu, L.; Lv, Y.; Zhao, B.; Ju, X.; Xu, S.; Zhang, J.; Tian, C.; Sun, D.; Tang, X. Ultra-sensitive and fast response formaldehyde sensor based on La2O3-In2O3 beaded na-notubes at low temperature. Chem. Phys. Lett. 2020, 746, 137289. [Google Scholar] [CrossRef]
- Lemos, S.C.S.; Nossol, E.; Ferrari, J.L.; Gomes, E.O.; Andres, J.; Gracia, L.; Sorribes, I.; Lima, R.C. Joint theoretical and experimental study on the la doping process in In2O3: Phase transition and electrocatalytic activity. Inorganic Chem. 2019, 58, 11738. [Google Scholar] [CrossRef]
- Aragon, F.H.; Coaquira, J.A.H.; Villegas-Lelovsky, L.; da Silva, S.W.; Cesar, D.F.; Nagamine, L.C.C.M.; Cohen, R.; Men´endez-Proupin, E.; Morais, P.C. Evolution of the doping regimes in the Al-doped SnO2 nanoparticles prepared by a polymer precursor method. J. Phys. Condens. Matter 2015, 27, 095301. [Google Scholar] [CrossRef]
- An, Y.; Yang, D.; Ma, G.; Zhu, Y.; Wang, S.; Wu, Z.; Liu, J. Role of Co cluster and oxygen vacancies in the magnetic and transport properties of Co-doped In2O3 films. J. Phys. Chem. C 2014, 118, 10448–10454. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, S.; Wu, Z.; An, Y. Manganese-vacancy complexes induced room temperature ferromagnetism in Mn/Mg co-doped In2O3 diluted magnetic semiconductors. Superlattices Microstruct. 2019, 132, 106174. [Google Scholar] [CrossRef]
- Yamaura, H.; Moriya, K.; Miura, N.; Yamazoe, N. Mechanism of sensitivity promotion in CO sensor using indium oxide and cobalt oxide. Sens. Actuators B 2000, 65, 39–41. [Google Scholar] [CrossRef]
- Bloch, E.D.; Hudson, M.R.; Mason, J.A.; Chavan, S.; Crocellà, V.; Howe, J.D.; Lee, K.; Dzubak, A.L.; Queen, J.W.L.; Zadrozny, M.; et al. Reversible CO binding enables tunable CO/H2 and CO/N2 separations in metal-organic frameworks with exposed divalent metal cations. J. Am. Chem. Soc. 2014, 136, 10752–10761. [Google Scholar] [CrossRef]
- Jinkawa, T.; Sakai, G.; Tamaki, J.; Miura, N.; Yamazoe, N. Relationship between ethanol gas sensitivity and surface catalytic property of tin oxide sensors modified with acidic or basic oxides. J. Mol. Catal. A 2000, 155, 193–200. [Google Scholar] [CrossRef]
- Lupinetti, A.J.; Fau, S.; Frenking, G.; Strauss, S.H. Theoretical analysis of the bonding between CO and positively charged atoms. J. Phys. Chem. A 1997, 101, 9551–9559. [Google Scholar] [CrossRef]
- Hocking, R.K.; Hambley, T.W. Database analysis of transition metal carbonyl bond lengths: Insight into the periodicity of π back-bonding, σ donation, and the factors affecting the electronic structure of the TM-CtO moiety. Organometallics 2007, 26, 2815–2823. [Google Scholar] [CrossRef]
- Liang, Q.; Zou, X.; Chen, H.; Fan, M.; Dong, G.-L. High-performance formaldehyde sensing realized by alkaline-earth metals doped In2O3 nanotubes with optimized surface properties. Sens. Actuators B 2020, 304, 127241–127246. [Google Scholar] [CrossRef]
- Gao, L.; Fu, H.; Zhu, J.; Wang, J.; Chen, Y.; Liu, H. Synthesis of SnO2 nanoparticles for formaldehyde detection with high sensitivity and good selectivity. J. Mat. Res. 2020, 35, 2208–2217. [Google Scholar] [CrossRef]
- Zhao, Y.; Zou, X.; Chen, H.; Chu, X.; Li, G.-D. Tailoring energy level and surface basicity of metal oxide semiconductors by rare-earth incorporation for high-performance formaldehyde detection. Inorg. Chem. Front. 2019, 6, 1767–1774. [Google Scholar] [CrossRef]
- Dimitrov, V.; Komatsu, T. Correlation among electronegativity, cation polarizability, optical basicity and single bond strength of simple oxides. J. Solid State Chemistry 2012, 196, 574–578. [Google Scholar] [CrossRef]
- Mahajan, S.; Jagtap, S. Metal-oxide semiconductors for carbon monoxide (CO) gas sensing: A review. Appl. Mater. Today 2020, 18, 100483. [Google Scholar] [CrossRef]
- Hurlburt, P.K.; Rack, J.J.; Luck, J.S.; Dec, S.F.; Webb, J.D.; Anderson, O.P.; Strauss, S.H. Nonclassical metal carbonyls: [Ag(CO)]+ and [Ag(CO)2]+. J. Am. Chem. Soc. 1994, 116, 10003–10014. [Google Scholar] [CrossRef]
- Kim, S.-J.; Hwang, I.-S.; Kang, Y.C.; Lee, J.-H. Design of selective gas sensors using additive-loaded In2O3 hollow spheres prepared by combinatorial hydrothermal reactions. Sensors 2011, 11, 10603–10614. [Google Scholar] [CrossRef]
- Singh, N.; Comini, E.; Ponzoni, A.; Lee, P.S. Chemical sensing investigation on Zn-In2O3 nanowires. Sens. Actuators B 2012, 171–172, 244–248. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, W.; Xu, L.; Kumar, R.; Gui, Y.; Zhao, Z.; Tang, C.; Zhu, S. Highly sensitive carbon monoxide (CO) gas sensors based on Ni and Zn doped SnO2 nanomaterials. Ceram. Int. 2018, 44, 4392–4399. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, Y.; Shi, L.; Li, G.-D.; Sun, L.; Zou, X. Revealing the relationship between energy level and gas sensing performance in heteroatom-doped semiconducting nanostructures. ACS Appl. Mater. Interfaces 2018, 10, 29795–29804. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Sun, L.; Li, G.-D.; Zou, X. Well-tuned surface oxygen chemistry of cation off-stoichiometric spinel oxides for highly selective and sensitive formaldehyde detection. Chem. Mater. 2018, 30, 2018–2027. [Google Scholar] [CrossRef]
- Shao, G. Work function and electron affinity of semiconductors: Doping effect and complication due to Fermi level pinning. Energy nviron. Mater. 2021, 4, 273–276. [Google Scholar] [CrossRef]
- Bonu, V.; Das, A.; Prasad, A.K.; Krishna, N.G.; Dhara, S.; Tyagi, A.K. Influence of in-plane and bridging oxygen vacancies of SnO2 nanostructures on CH4 sensing at low operating temperatures. Appl. Phys. Lett. 2014, 105, 243102. [Google Scholar] [CrossRef]
- Liu, L.; Shu, S.; Zhang, G.; Liu, S. Highly selective sensing of C2H6O, HCHO, and C3H6O gases by controlling SnO2 nanoparticle vacancies. ACS Appl. Nano Mater. 2018, 1, 31–37. [Google Scholar] [CrossRef]
- Kumar, R.; Jaiswal, M.; Singh, O.; Gupta, A.; Ansari, M.S.; Mittal, J. Selective and reversible sensing of low concentration of carbon monoxide gas using Nb-doped OMS-2 nanofibers at room temperature. IEEE Sensors J. 2019, 19, 7201–7206. [Google Scholar] [CrossRef]
- Genuino, H.C.; Seraji, M.S.; Meng, Y.; Valencia, D.; Suib, S.L. Combined experimental and computational study of CO oxidation promoted by Nb in manganese oxide octahedral molecular sieves. Appl. Catal. B Environ. 2015, 163, 361–369. [Google Scholar] [CrossRef]
- Kuntaiah, K.; Sudarsanam, P.; Reddy, B.M.; Vinu, A. Nanocrystalline Ce1-xSmxO2-δ (x = 0.4) solid solutions: Structural characterization versus CO oxidation. RSC Adv. 2013, 3, 7953–7962. [Google Scholar] [CrossRef]
- Savage, N.; Chwieroth, B.; Ginwalla, A.; Patton, B.R.; Akbar, S.A.; Dutta, P.K. Composite n-p-semiconducting titanium oxide as gas sensors. Sens. Actuators B 2001, 79, 17–27. [Google Scholar] [CrossRef]
- Efros, A.L. Physics and geometry of disorder: Percolation theory, Mir, Moscow (1986). https://archive.org/details/physics-of-disorder.
- Choi, S.-W.; Katoch, A.; Kim, J.-H.; Kim, S.S. Striking sensing improvement of n-type oxide nanowires by electronic sensitization based on work function difference. J. Mater. Chem. C 2015, 3, 1521–1527. [Google Scholar] [CrossRef]
- Nam, B.; Ko, T.-K.; Hyun, S.-K.; Lee, C. NO2 sensing properties of WO3-decorated In2O3 nanorods and In2O3-decorated WO3 nanorods. Nano Converg. 2019, 6, 40. [Google Scholar] [CrossRef] [PubMed]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mat. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Sowmya, B.; Athira, J.; Panda, P.K. A review on metal-oxide based p-n and n-n heterostructured nanomaterials for gas sensing applications. Sens. International 2021, 2, 100085. [Google Scholar] [CrossRef]
- Mirzaei, A.; Yousefi, H.R.; Falsafi, F.; Bonyani, M.; Lee, J.-H.; Kim, J.-H.; Kim, H.W.; Kim, S.S. An overview on how Pd on resistive-based nanomaterial gas sensors can enhance response toward hydrogen gas. Int. J. Hydrogen Energy 2019, 44, 20522–20571. [Google Scholar] [CrossRef]
- Dhall, S.; Kumar, M.; Bhatnagar, M.; Mehta, B.R. Dual gas sensing properties of graphene-Pd/SnO2 composites for H2 and ethanol: Role of nanoparticles-graphene interface. Int. J. Hydrogen Energy 2018, 43, 17921–17927. [Google Scholar] [CrossRef]
- Feng, Q.; Li, X.; Wang, J. Percolation effect of reduced graphene oxide (rGO) on ammonia sеnsing of rGO-SnO2 composite based sensor. Sens. Actuators B 2017, 243, 1115–1126. [Google Scholar] [CrossRef]
- Sygellou, L.; Paterakis, G.; Galiotis, C.; Tasis, D. Work function tuning of reduced graphene oxide thin films. J. Phys. Chem. C 2016, 120, 281–290. [Google Scholar] [CrossRef]
- Yamazoe, N.; Shimanoe, K. Theory of power laws for semiconductor gas sensors. Sens. Actuators B 2008, 128, 566–573. [Google Scholar] [CrossRef]
- Miller, R.D.; Akbar, A.S.; Morris, P.A. Nanoscale metal oxide-based heterojunctions for gas sensing: A Review. Sens. Actuators B 2014, 204, 250–272. [Google Scholar] [CrossRef]
- Hübner, M.; Simion, C.E.; Tomescu-Stanoiu, A.; Pokhrel, S.; Bârsan, N.; Weimar, U. Influence of humidity on CO sensing with p-type CuO thick film gas sensors. Sens. Actuators B 2011, 153, 347–353. [Google Scholar] [CrossRef]
- Pokhrel, S.; Simion, C.E.; Quemener, V.; Bârsan, N.; Weimar, U. Investigations of conduction mechanism in Cr2O3 gas sensing thick films by ac impedance spectroscopy and work function changes measurements. Sens. Actuators B 2008, 133, 78–83. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, J.-H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. (n)-SnO2 – (p)-NiO composite nanowebs: Gas sensing properties and sensing mechanisms. Sens. Actuators B 2018, 258, 204–214. [Google Scholar] [CrossRef]
- Zhou, W.; Dastan, D.; Yin, X.; Nie, S.; Wu, S.; Wang, Q.; Li, J. Optimization of gas sensing properties of n-SnO2/p-xCuO sensors for homogenous gases and the sensing mechanism. J. Mater. Sci. Mater. Electron. 2020, 31, 18412–18426. [Google Scholar] [CrossRef]
- Zhang, R.; Cao, S.; Zhou, T.; Fei, T.; Wang, R.; Zhang, T. Rational design and tunable synthesis of Co3O4 nanoparticle-incorporating into In2O3 one-dimensional ribbon as effective sensing material for gas detection. Sens. Actuators B 2020, 10, 127695. [Google Scholar] [CrossRef]
- Yamazoe, N. New approaches for improving semiconductor gas sensors. Sens. Actuators B 1991, 5, 7–19. [Google Scholar] [CrossRef]
- Walker, J.M.; Akbar, S.A.; Morris, P.A. Synergistic effects in gas sensing semiconducting oxide nanoheterostructures: A review. Sens. Actuators B 2019, 286, 624–640. [Google Scholar] [CrossRef]
- Kim, J.-H.; Wu, P.; Kim, H.W.; Kim, S.S. Highly selective sensing of CO, C6H6, and C7H8 gases by catalytic functionalization with metal nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 7173–7183. [Google Scholar] [CrossRef]
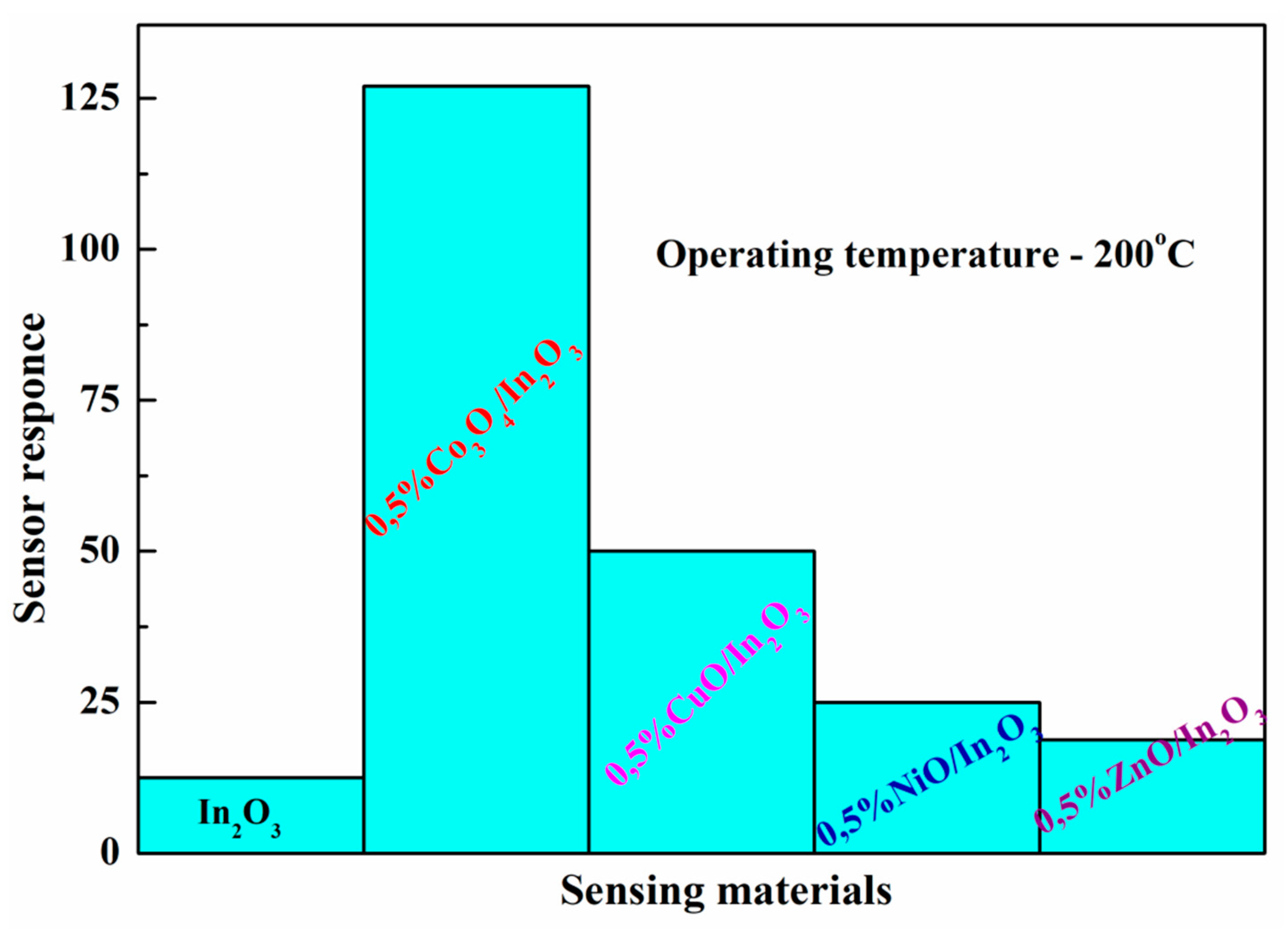
- Gromov, V.F.; Gerasimov, G.N.; Belysheva, T.V.; Ikim, M.I.; Spiridonova, E.Yu.; Grekhov, M.M.; Ali-zade, R.A.; Trakhtenberg, L.I. Sensor properties of nanostructured systems based on indium oxide with Co3O4 or ZrO2 additives. Russian J. Phys. Chem. B 2018, 12, 129–134. [Google Scholar] [CrossRef]
- Nowotny, J.; Sloma, M.; Weppner, W. Surface reactivity of yttria-doped zirconia with oxygen. Solid State Ionics 1989, 32/33, 709–713. [Google Scholar] [CrossRef]
- Zakrzewska, K.; Radecka, M. TiO2-SnO2 composites and solid solutions for chemical nanosensors. Procedia Eng. 2012, 47, 1077–1083. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Han, S.H.; Cho, B.K. Metal Oxide Nanocomposites: Advantages and shortcomings for application in conductometric gas sensors. Materials Science Forum 2016, 872, 223–229. [Google Scholar] [CrossRef]
- Xu, H.; Ju, D.; Li, W.; Zhang, J.; Wang, J.; Cao, B. Superior triethylamine-sensing properties based on TiO2/SnO2 n-n heterojunction nanosheets directly grown on ceramic tubes. Sens. Actuators B 2016, 228, 634–642. [Google Scholar] [CrossRef]
- Zhang, K.; Shen, Y.; Li, Y.; Zhang, W. Synthesis of ZnO@In2O3 heterojunction with unique hexagonal three-dimensional structure for ultra sensitive ethanol detection. Materials Science in Semiconductor Processing 2022, 143, 106523. [Google Scholar] [CrossRef]
- Li, R.; Zhou, Y.; Sun, M.; Gong, Z.; Guo, Y.; Wu, F.; Li, W.; Ding, W. Influence of charge carriers concentration and mobility on the gas sensing behavior of tin dioxide thin films. Coatings 2019, 9, 591–597. [Google Scholar] [CrossRef]
- Park, S.; Sun, G.-J.; Kheel, H.; Lee, W.I.; Lee, S.; Choi, S.-B.; Lee, C. Synergistic effects of codecoration of oxide nanoparticles on the gas sensing performance of In2O3 nanorods. Sens. Actuators B 2016, 227, 591–599. [Google Scholar] [CrossRef]
- Wei, X.; Xie, T.; Peng, L.; Fu, W.; Chen, J.; Gao, Q.; Hong, G.; Wang, D. Effect of heterojunction on the behavior of photogenerated charges in Fe3O4@Fe2O3 nanoparticle photocatalysts. J. Phys. Chem. C 2011, 115, 8637–8642. [Google Scholar] [CrossRef]
- Feste, P.D.; Crisci, M.; Barbon, F.; Tajoli, F.; Salerno, M.; Drago, F.; Prato, M.; Gross, S.; Gatt, T.; Lamberti, F. Work function tuning in hydrothermally synthesized vanadium-doped MoO3 and Co3O4 mesostructures for energy conversion devices. Appl. Sci. 2021, 11, 2016. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, Z.; Suematsu, K.; Zhang, W.; Zhang, W.; Zhuiykov, S.; Shimanoe, K.; Hu, J. MOF-derived Au-NiO/In2O3 for selective and fast detection of toluene at ppb-level in high humid environments. Sens. Actuators B 2022, 360, 13163. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kim, J.-H.; Kim, H.W.; Kim, S.S. How shell thickness affects the gas sensing properties of nanostructured materials: Survey of literature. Sens. Actuators B 2018, 256, 270–294. [Google Scholar] [CrossRef]
- Wang, S.; Kang, Y.; Wang, L.; Zhang, H.; Wang, Y. Organic/inorganic hybrid sensors: A review. Sens. Actuators B 2013, 182, 467–482. [Google Scholar] [CrossRef]
- Mirzaei, A.; Janghorban, K.; Hashemi, B.; Neri, G. Synthesis, characterization and gas sensing properties of Ag@αFe2O3 core-shell nanocomposited. Nanomaterials 2015, 17, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Karnati, P.; Akbar, S.; Morris, P.A. Conduction mechanisms in one dimensional core-shell nanostructures for gas sensing; A review. Sens. Actuators B 2019, 295, 127–143. [Google Scholar] [CrossRef]
- Majhi, P.; Rai, Y.-T. Facile approach to synthesize Au-ZnO core shell nanoparticles and their application for highly sensitive and selective gas sensors. ACS Appl. Mater. Interfaces 2015, 7, 9462–9468. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-W.; Katoch, A.; Sun, G.-J.; Kim, J.-H.; Kim, S.-H.; Kim, S.-S. Dual functional sensing mechanism in SnO2-ZnO core-shell nanowires. ACS Appl. Mater. Interfaces 2014, 6, 8281. [Google Scholar] [CrossRef]
- Lee, D.-J.; Kim, K.-J.; Kim, S.-H.; Kwon, J.-Y.; Xu, J.; Kim, K.-B. Atomic layer deposition of Ti-doped ZnO films with enhanced electron mobility. J. Mater. Chem. C 2013, 1, 4761. [Google Scholar] [CrossRef]
- Katoch, A.; Choi, S.-W.; Sun, G.-J.; Kim, S.-S. An approach to detecting a reducing gas by radial modulation of electron-depleted shells in core-shell nanofibers. J. Mater. Chem. A 2013, 1, 13588–13596. [Google Scholar] [CrossRef]
- Diao, K.; Xiao, J.; Zheng, Z.; Cui, X. Enhanced sensing performance and mechanism of CuO nanoparticle-loaded. Appl. Surf. Sci. 2018, 459, 630–638. [Google Scholar] [CrossRef]
- Barsan, N.; Simion, C.; Heine, T.; Pokhrel, S.; Weimar, U. Modeling of sensing and transduction for p-type semiconducting metal oxide based gas sensors. J Electroceram. 2010, 25, 11–19. [Google Scholar] [CrossRef]
- Park, S.; Ko, H.; Kim, S.; Lee, C. Role of the interfaces in multiple networked one-dimensional core-shell nanostructured gas sensors. ACS Appl. Mater. Interfaces 2014, 6, 9595–9600. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Extremely sensitive and selective sub-ppm CO detection by the synergistic effect of Au nanoparticles and core-shell nanowires. Sens. Actuators B 2017, 249, 177–188. [Google Scholar] [CrossRef]
- Qin, Y.; Wen, Z.; Zhang, T. Facile realization of Ag functionalized W18O49@PPy core-shell nanorods for multieffect modulation on gas sensing response. J. Mat. Sci. Materials in Electronics 2019, 30, 15031–15041. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, H.W.; Kim, S.S. Ultra-sensitive benzene detection by a novel approach: Core-shell nanowires combined with Pd-functionalization. Sens. Actuators B 2017, 239, 578–585. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, S.S. Realization of ppb-scale toluene-sensing abilities with Pt-functionalized SnO2-ZnO core-shell nanowires. ACS Appl. Mater. Interfaces 2015, 7, 17199–17208. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Variation of shell thickness in ZnO-SnO2 core-shell nanowires for optimizing sensing behaviors to CO, C6H6, and C7H8 gases. Sens. Actuators B 2020, 302, 127150. [Google Scholar] [CrossRef]
- Park, S.; Lee, S.; Kim, H.W.; Lee, C. Hydrogen sensing properties of multiple networked Nb2O5/ZnO core-shell nanorod sensors. Sens. Actuators B 2014, 202, 840–845. [Google Scholar] [CrossRef]
- Singh, N.; Ponzoni, A.; Gupta, R.K.; Lee, P.S.; Comini, E. Synthesis of In2O3-ZnO core-shell nanowires and their application in gas sensing. Sens. Actuators B 2011, 160, 1346–1351. [Google Scholar] [CrossRef]
- Hwang, I.-S.; Kim, S.-J.; Choi, J.-K.; Choi, J.; Ji, H.; Kim, G.-T.; Cao, G.; Lee, J.-H. Synthesis and gas sensing characteristics of highly crystalline ZnO-SnO2 core-shell nanowires. Sens. Actuators B 2010, 148, 595–600. [Google Scholar] [CrossRef]
- Yamazoe, N.; Shimanoe, K. Theory of power laws for semiconductor gas sensors. Sens. Actuators. B 2008, 128, 566–573. [Google Scholar] [CrossRef]
- Jimenez, L.C.; Mendez, H.A.; Paez, B.A.; Ramirez, M.E.; Rodriguez, H. Production and characterization of indium oxide and indium nitride. Brazilian J. Physics 2006, 36, 1017–1024. [Google Scholar] [CrossRef]
- Prathap, P.; Devi, G.G.; Subbaiah, Y.P.V.; Ramakrishna Reddy, K.T.; Ganesan, V. Growth and characterization of indium oxide films. Curr. Appl. Phys. 2008, 8, 120–124. [Google Scholar] [CrossRef]
- Ahlers, S.; Miller, G.; Doll, T. A rate equation approach to the gas sensitivity of thin film metal oxide materials. Sens. Actuators B 2005, 107, 587–599. [Google Scholar] [CrossRef]
- Xu, C.; Tamaki, J.; Miura, N.; Yamazoe, N. Grain size effects on gas sensitivity of porous SnO2-based elements. Sens. Actuators B 1991, 3, 147–155. [Google Scholar] [CrossRef]
- Gerasimov, G.N.; Gromov, V.F.; Ikim, M.I.; Ilegbusi, O.J.; Trakhtenberg, L.I. Effect of interaction between components of In2O3-CeO2 and SnO2-CeO2 nanocomposites on structure and sensing properties. Sens. Actuators B 2019, 279, 22–30. [Google Scholar] [CrossRef]
- Yang, F.; Graciani, J.; Evans, J.; Liu, P.; Hrbek, J.; Sanz, J.F.; Rodriguez, J.A. CO oxidation on inverse CeOx/Cu(111) catalysts: High catalytic activity and ceria-promoted dissociation of O2. J. Am. Chem. Soc. 2011, 133, 3444–3451. [Google Scholar] [CrossRef]
- Xu, L.; Song, H.; Dong, B. Preparation and bifunctional gas sensing properties of porous In2O3-CeO2 binary oxide nanotubes. Inorg. Chem. 2010, 49, 10590–10597. [Google Scholar] [CrossRef]
- Jiang, F.; Zhao, H.; Chen, H.; Xu, C.; Chen, J. Enhancement of photocatalytic decomposition of perfluorooctanoic acid on CeO2/In2O3. RSC Advances 2016, 6, 72015–72021. [Google Scholar] [CrossRef]
- Zappa, D.; Galstyan, V.; Kaur, N.; Munasinge, M.A.H.M.; Sisman, O.; Comini, E. Metal oxide-based heterostructures for gas sensors – A review. Analytica Chimica Acta 2018, 1039, 1–23. [Google Scholar] [CrossRef]
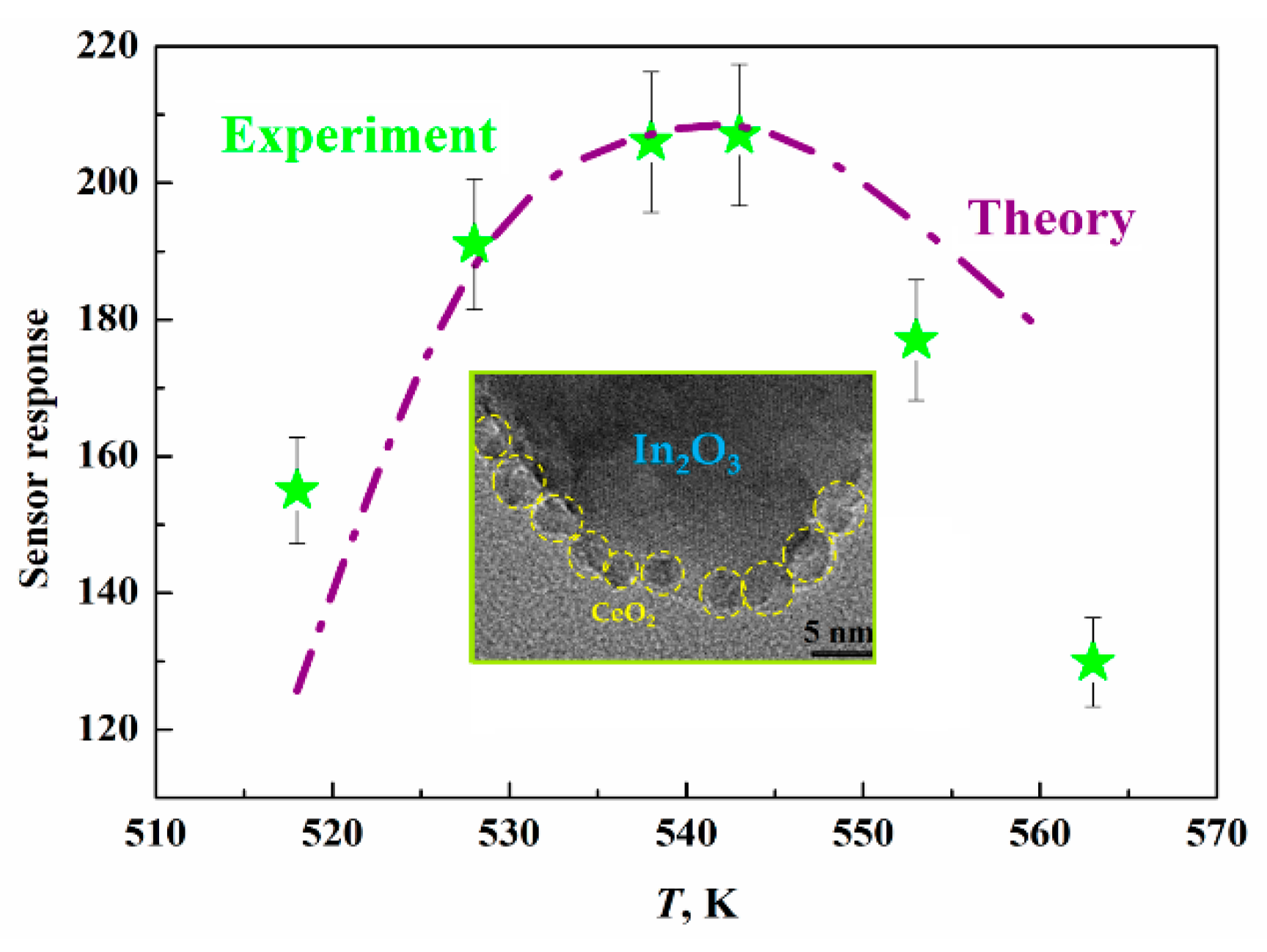
- Kurmangaleev, K.S.; Bodneva, V.L.; Posvyansky, V.S.; Trakhtenberg, L.I. Sensory effect toward hydrogen in a nanostructured CeO2-In2O3 system. Russian J. Phys. Chem. A 2022, 96, 2056–2058. [Google Scholar] [CrossRef]
- Wusiman, M.; Taghipour, F. Methods and mechanisms of gas sensor selectivity. Crit. Rev. Solid State Mater. Sci. 2022, 47, 416–435. [Google Scholar] [CrossRef]
- Walker, J.; Karnati, P.; Akbar, Sh.A.; Morris, P.A. Selectivity mechanisms in resistive-type metal oxide heterostructural gas sensors. Sens. Actuators B 2022, 355, 131242. [Google Scholar] [CrossRef]
- Mirzaei, A.; Leonardi, S.G.; Neri, G. Detection of hazardous volatile organic compounds (VOCs) by metal oxide nanostructures-based gas sensors: A review. Ceram. Int. 2016, 42, 15119–15141. [Google Scholar] [CrossRef]
- Liang, Q.; Qu, X.; Bai, Ni.; Chen, H.; Zou, X.; Li, G.-D. Alkali metal-incorporated spinel oxide nanofibers enable high performance detection of formaldehyde at ppb level. J. Hazardous Materials 2020, 400, 123301. [Google Scholar] [CrossRef]
- San, X.; Li, M.; Liu, D.; Wang, G.; Shen, Y.; Meng, D.; Meng, F. A facile one-step hydrothermal synthesis of NiO/ZnO heterojunction microflowers for the enhanced formaldehyde sensing properties. J. Alloys Compounds 2018, 739, 260–269. [Google Scholar] [CrossRef]
- Zhao, Y.; Zou, X.; Chen, H.; Chu, X.; Li, G.-D. Tailoring energy level and surface basicity of metal oxide semiconductors by rare-earth incorporation for high-performance formaldehyde detection. Inorg. Chem. Front. 2019, 6, 1767–1774. [Google Scholar] [CrossRef]
- Liang, Q.; Zou, X.; Chen, H.; Fan, M.; Li, G.-D. High-performance formaldehyde sensing realized by alkaline-earth metals doped In2O3 nanotubes with optimized surface properties. Sens. Actuators B 2020, 304, 127241. [Google Scholar] [CrossRef]
- Galstyan, V.; Ponzoni, A.; Kholmanov, I.; Natile, M.M.; Comini, E.; Sberveglier, G. Highly sensitive and selective detection of dimethylamine through Nb-doping of TiO2 nanotubes for potential use in seafood quality control. Sens. Actuators B 2019, 303, 127217. [Google Scholar] [CrossRef]
- Zhang, W.-H.; Zhang, W.-D. Fabrication of SnO2-ZnO nanocomposite sensor for selective sensing of trimethylamine and the freshness of fishes. Sens. Actuators B 2008, 134, 403–408. [Google Scholar] [CrossRef]
- Hemmati, S.; Firooz, A.A.; Khodadadi, A.A.; Mortazavi, Y. Nanostructured SnO2-ZnO sensors: Highly sensitive and selective to ethanol. Sens. Actuators B 2011, 160, 1298–1303. [Google Scholar] [CrossRef]
- Park, H.; Kim, J.-H.; Vivod, D.; Kim, S.; Mirzaeie, A.; Zahn, D.; Park, C.; Kim, S.S.; Halik, M. Chemical- recognition-driven selectivity of SnO2-nanowire-based gas sensors. Nano Today 2021, 40, 101265–101266. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kim, J.-H.; Kim, H.W.; Kim, S.S. Resistive-based gas sensors for detection of benzene, toluene and xylene (BTX) gases: A review. J. Mat. Chem. C 2018, 6, 4342–4370. [Google Scholar] [CrossRef]
- Pasti, I.A.; Gavrilov, N.M.; Mentus, S.V. Hydrogen adsorption on palladium and platinum overlayers: DFT study. Hindawi Publ. Corp. Adv. Phys. Chem. 2011, 4–6, 1–8. [Google Scholar] [CrossRef]
- Molina, L.M.; Hammer, B. Active role of oxide support during CO oxidation at Au/MgO. Phys. Rev. Lett. 2003, 90, 206102. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.-T.; Li, J.; Dastan, D.; Zhou, W.-D.; Garmestani, H.; Alamgir, F.M. Ultra-high selectivity of H2 over CO with a p-n nanojunction based gas sensors and its mechanism. Sens. Actuators B 2020, 319, 128330. [Google Scholar] [CrossRef]
- Yin, X.-T.; Wu, S.-S.; Dastan, D.; Nie, S.; Liu, Y.; Li, Z.-G.; Zhou, Y.-W.; Li, J.; Faik, A.; Shan, K.; et al. Sensing selectivity of SnO2-Mn3O4 nanocomposite sensors for the detection of H2 and CO gases. Surf. Interfaces 2021, 25, 101190. [Google Scholar] [CrossRef]
- Nakate, U.T.; Ahmad, R.; Patil, P.; Wang, Y.; Bhat, K.S.; Mahmoudi, T.; Yu, Y.T.; Suh, E.-k.; Hahn, Y.-B. Improved selectivity and low concentration hydrogen gas sensor application of Pd sensitized heterojunction n-ZnO/p-NiO nanostructures. J. Alloys and Compounds 2019, 797, 456–464. [Google Scholar] [CrossRef]
| Material | Synthesis Method | Sensor response (gas concentration) | Temperature | References |
|---|---|---|---|---|
| 0.5SnO2-0.5Co3O4 | electrospinning method | 18.7 (1 ppm C6H6) | 350 °C | [23] |
| WO3-SnO2 |
sputtering high-purity Ti or W targets on SnO2 | 140 (1 ppm H2) | 300 °C | [74] |
| TiO-SnO2 | 232 (1 ppm O2) | |||
| WO3-In2O3 | sol-gel method | 27 (200 ppm NO2) | 300 °C | [75] |
| graphene-Pd/SnO2 composites | grapheme by CVD method, SnO2 gas phase synthesis method | 14.8% (1% C2H5OH) | 200 °C | [79] |
| rGO-SnO2 | rGO by hydrothermal treatment of aqueous dispersion of GO, rGO-SnO2 composite by mixing | 1.3 (50 ppm NH3) | 22 °C | [80] |
| 0.5SnO2-0.5NiO | electrospinning process | 36 (10 ppm NO2) | 300 °C | [86] |
| SnO2-2.78CuO | sol-gel route | 200% (400 ppm CO) | 350 °C | [87] |
| In2O3-Co3O4 | Electrospinning method | 39 (200 ppm HCHO) | 260 °C | [88] |
| Pt-SnO2 | photolithographic process and γ-ray radiolysis method | 40 (1 ppm C7H8) | 300 °C | [91] |
| 10%Co3O4-90% In2O3 | Mixing metal oxides | 1300 (1100 ppm H2) | 250 °C | [92] |
| 20%ZrO-80% In2O3 | 280 (1100 ppm H2) | 315 °C | ||
| TiO2/SnO2 | hydrothermal process | 52.3 (100 ppm triethylamine) | 260 °C | [96] |
| ZnO@In2O3 | hydrothermal method | 28.6 (100 ppm C2H5OH) | 160 °C | [97] |
| Au-NiO/In2O3 | solvothermal method | 80.6 (10 ppm toluene) | 250 °C | [102] |
| Material | Synthesis Method | Sensor response (gas concentration) | Temperature | References |
|---|---|---|---|---|
| Ag–α-Fe2O3 core-shell composites |
two-step reduction-sol gel approach, including Ag nanoparticles | 9 (500 ppm) | 250 °C | [105] |
| Au-ZnO core-shell nanoparticles | facile low-temperature solution route | 103.9 (100 ppm H2) | 300 °C | [107] |
| SnO2-ZnO core-shell nanowires | two-step process | 25 (10 ppm NO2) 75 (10 ppm C7H8) 83 (10 ppm C6H6) 77 (10 ppm CO) |
300 °C | [108] |
| SnO2-ZnO core-shell nanofibers | two-step process | 6.5 (1 ppm CO) 48 (1 ppm NO2) |
300 °C | [110] |
| ZnO-CuO core-shell nanowires | facile three-step process | 29 (10 ppm NO2) | 350 °C | [111] |
| In2O3/ZnO core-shell nanowires | thermal evaporation of indium powder in an oxidizing atmosphere followed by the atomic layer deposition of ZnO | 196 (1000 ppm C2H5OH) | 300 °C | [113] |
| SnO2-ZnO core-shell nanowires functionalized by Au nanoparticles | vapor–liquid–solid growth method | 26.6 (100 ppb CO) | 300 °C | [114] |
| Ag functionalized W18O49@PPy core-shell nanorods | polymerizing the uniform PPy shell film on surface of W3 nanorods with AgNO3 as oxidant and DBSA as modifier. | 2.5 (20 ppm NH3) | 40 °C | [115] |
| Pd-functionalized SnO2-ZnO core-shell nanowires | two-step growth technique. Pd functionalized SnO2-ZnO by using the ray radiolysis technique |
71 (100 ppb C6H6) | 300 °C | [116] |
| SnO2-ZnO core-shell nanowires functionalized Pt nanoparticles | two-step growth technique. Pt functionalized SnO2-ZnO by using the ray radiolysis technique |
279 (100 ppb C7H8) | 300 °C | [117] |
| ZnO-SnO2 core-shell nanowires | two-step growth technique | 41.13 (10 ppm CO) 39.48 (10 ppm C7H8) 40.34 (10 ppm C6H6) |
300 °C | [118] |
| Nb2O5/ZnO core-shell nanorod | two-step growth process | 156 (100 ppm H2) | 300 °C | [119] |
| In2O3-ZnO core-shell nanowires | two-step growth process | 265 (400 ppm C2H5OH) 7 (2000 ppm H2 |
350 °C | [120] |
| ZnO-SnO2 core-shell nanowires | two-step vapor growth method | 66.3 (10 ppm NO2) | 200 °C | [121] |
| Material | Synthesis | Gas selectivity | Temperature | References |
|---|---|---|---|---|
| ZnO/NiO | ZnO nanorods by the one-pot chemical method, NiO by hydrothermal method | H2 > CO, NO2, CO2, CH4 | 237 °C | [149] |
| Pd-ZnO/NiO | Pd by chemical reduction method, ZnO nanorods by the one-pot chemical method, NiO by hydrothermal method | H2 > CO, NO2, CO2, CH4 | 225 °C | [149] |
| SnO2-Mn3O4 | sol-gel technique | H2 > CO | 350 °C | [148] |
| SnO2/Co3O4 | sol-gel technique | H2 > CO | 350 °C | [147] |
| SnO2-ZnO | precipitation method | C2H5OH > CO | 300 °C | [142] |
| SnO2 | precipitation method | CO > C2H5OH | 250-300 °C | [142] |
| SnO2-ZnO | hydrothermal method | Trimethylamine > other gases | 330 °C | [141] |
| Nb-doped TiO2 nanotubes | anodic oxidation method | Dimethylamine > NH3, C2H5OH, CO | 300 °C | [140] |
| 5%Ca-In2O3 | electrospun method | Formaldehyde > other volatile organic compounds | 130 °C | [139] |
| 5Y-In2O3 | electrospun method | formaldehyde > other gases | 100-120 °C | [138] |
| NiO/ZnO | hydrothermal method | formaldehyde > other volatile organic compounds | 200 °C | [137] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




