Submitted:
13 May 2023
Posted:
15 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
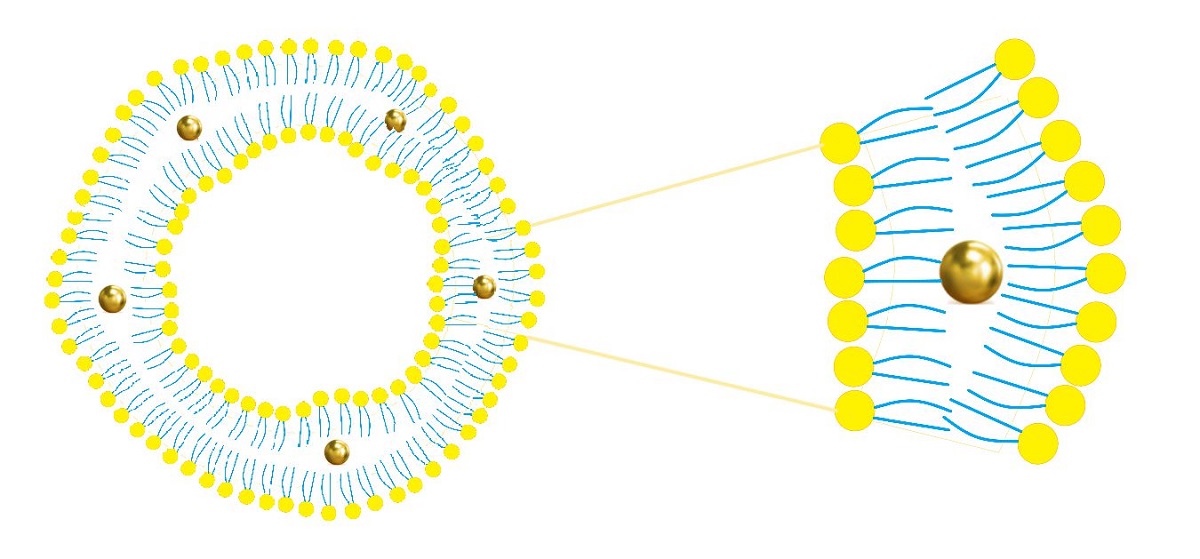
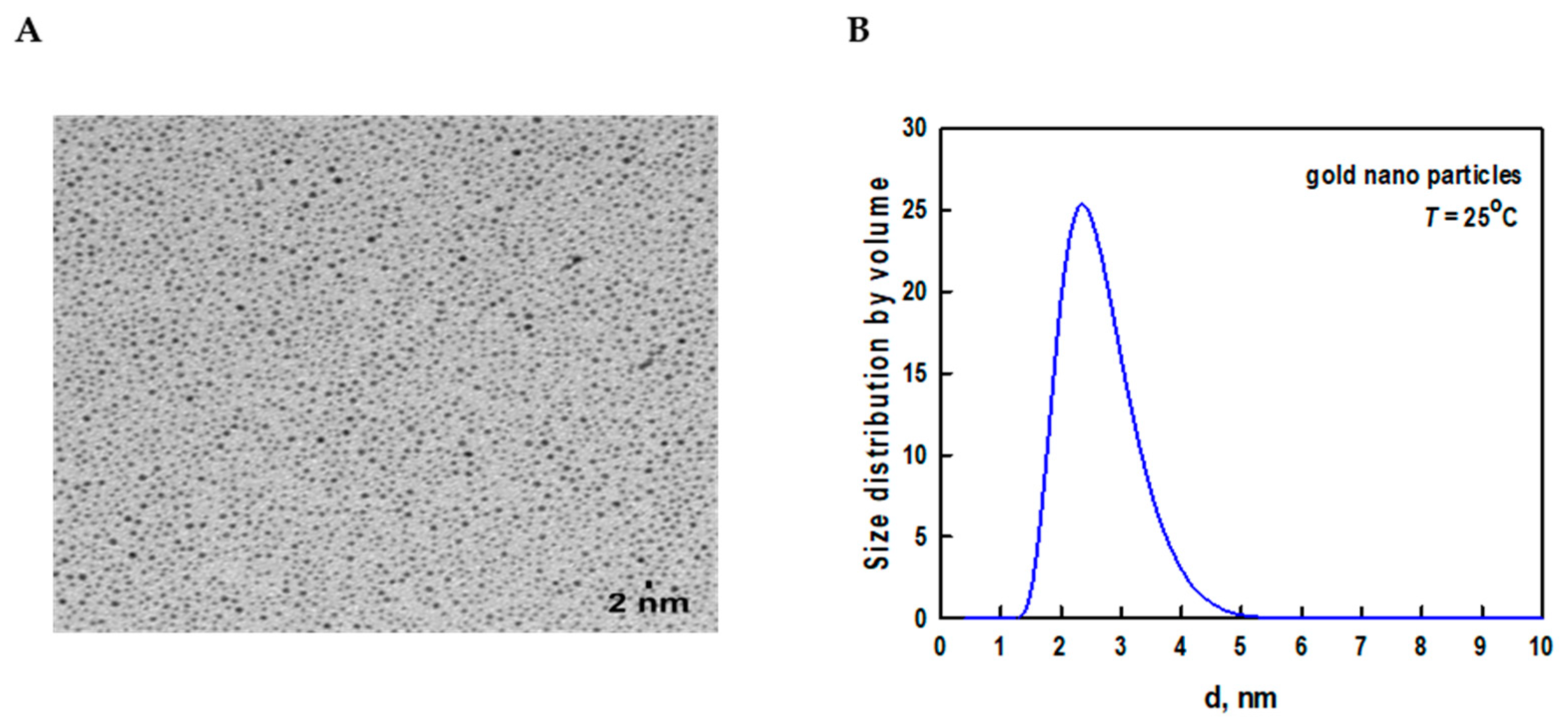
2.1. Nanoparticles Characterization
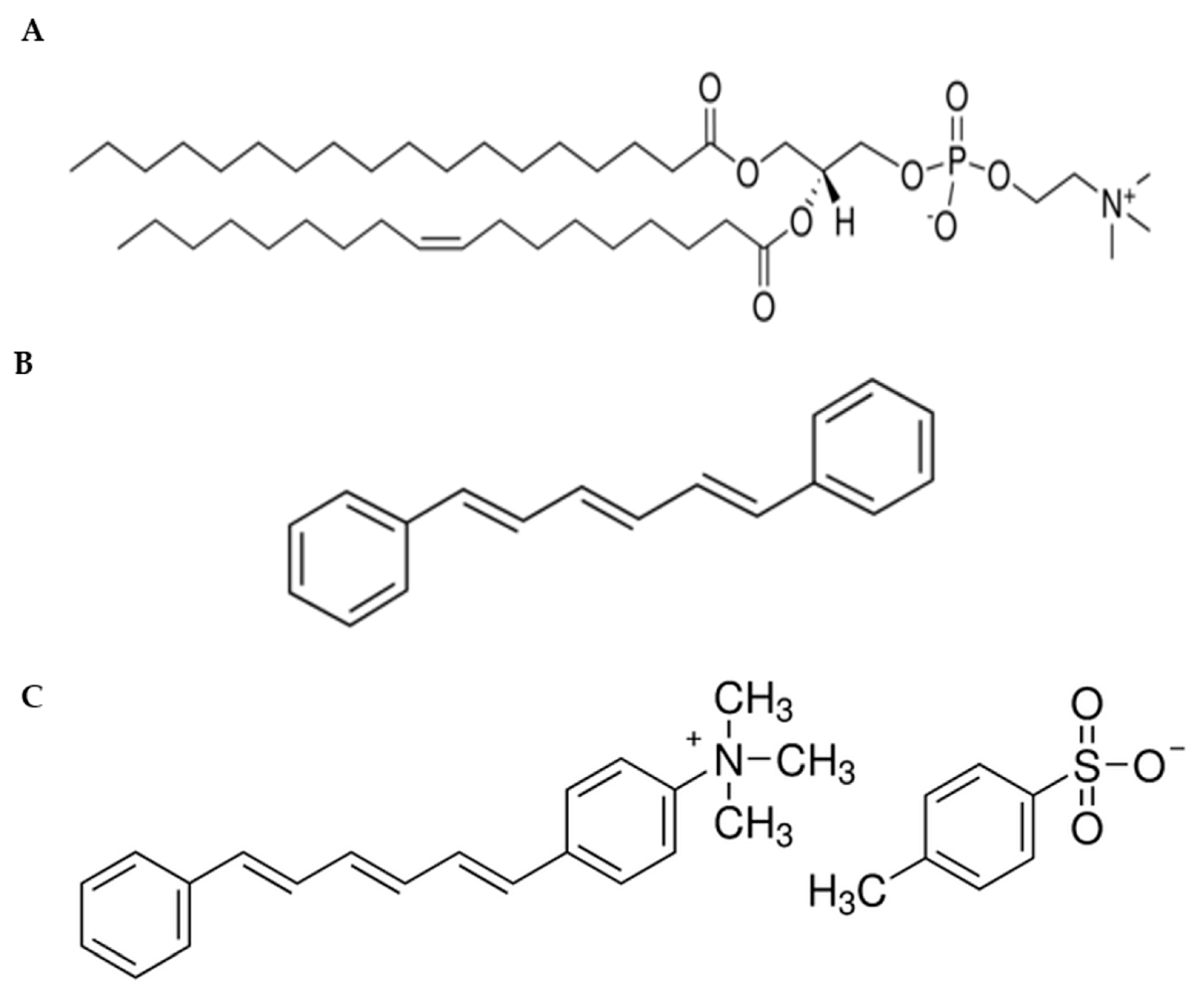
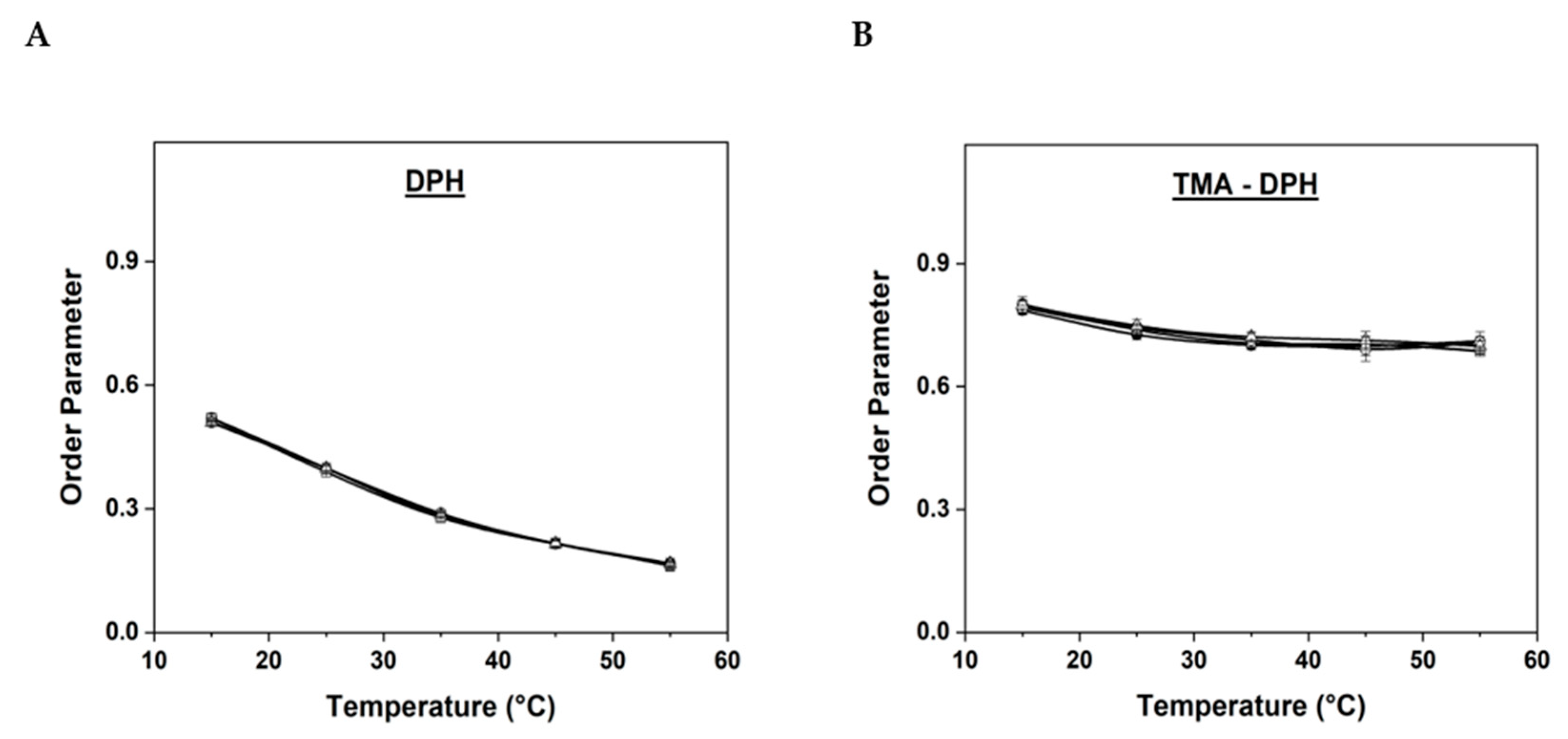
2.2. Membrane Fluidity
2.3. FTIR Spectroscopy
2.3.1. Analysis of Symmetric and Antisymmetric C-H Vibrations
2.3.2. Analysis of Carbonyl and Phosphate Group Vibrations
3. Materials and Methods
3.1. Liposomes Preparation and Nanoparticles Characterization
3.2. Fluorescence Spectroscopy
3.3. FTIR Spectroscopy
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Umapathi, A.; Kumawat, M.; Daima, H.K. Engineered nanomaterials for biomedical applications and their toxicity: A Review. Environ. Chem. Lett. 2021, 20, 445–468. [Google Scholar] [CrossRef]
- Albalawi, F.; Hussein, M.Z.; Fakurazi, S.; Masarudin, M.J. Engineered nanomaterials: The challenges and opportunities for nanomedicines. Int. J. Nanomed. 2021, 16, 161–184. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.A.; Kumar, V.; Karimi, J.; Singh, A.P.; Kumar, S. Role of gold nanoparticles in advanced biomedical applications. Nanoscale. Adv. 2020, 2, 3764–3787. [Google Scholar] [CrossRef] [PubMed]
- Dheyab, M.A.; Aziz, A.A.; Khaniabadi, P.M.; Jameel, M.S.; Oladzadabbasabadi, N.; Rahman, A.A.; Braim, F.S.; Mehrdel, B. Gold nanoparticles-based photothermal therapy for breast cancer. Photodiagnosis. Photodyn. Ther. 2023, 42, 103312. [Google Scholar] [CrossRef]
- Yang, T.; Wang, H.; Zhou, Q.; Huang, W.; Zhang, J.; Yu, Y.; Sun, T. Mild chemo-photothermal synergistic therapy for tumors based on gold-nanoparticles coupled with metformin. ACS Appl. Nano Mater. 2023, 6, 5729–5736. [Google Scholar] [CrossRef]
- Negoda, A.; Liu, Y.; Hou, W.C.; Corredor, C.; Moghadam, B.Y.; Musolff, C.; Li, L.; Walker, W.; Westerhoff, P.; Mason, A.J.; Duxbury, P.; Posner, J.D.; Worden, R.M. Engineered nanomaterial interactions with bilayer lipid membranes: Screening platforms to assess nanoparticle toxicity. IJBNN. 2013, 3, 52. [Google Scholar] [CrossRef]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, composition, types, and clinical applications. Heliyon. 2022, 8, e09394. [Google Scholar] [CrossRef]
- Tseu, G.Y.; Kamaruzaman, K.A. A review of different types of liposomes and their advancements as a form of gene therapy treatment for breast cancer. Molecules. 2023, 28, 1498. [Google Scholar] [CrossRef]
- Genova, J.; Decheva-Zarkova, M.; Pavlic, J.I. Morphological study of lipid vesicles in presence of amphotericin B via modification of the microfluidic CellASIC platform and led Illumination Microscopy. J. Phys. Conf. Ser. 2016, 682, 012029. [Google Scholar] [CrossRef]
- Liu, Y.; Castro Bravo, K.M.; Liu, J. Targeted liposomal drug delivery: A nanoscience and biophysical perspective. Nanoscale. Horiz. 2021, 6, 78–94. [Google Scholar] [CrossRef]
- Leroueil, P.R.; Hong, S.; Mecke, A.; Baker, J.R.; Orr, B.G.; Banaszak Holl, M.M. Nanoparticle interaction with biological membranes: does nanotechnology present a Janus face. Acc. Chem. Res. 2007, 40, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Genova, J.; Vitkova, V.; Bivas, I. Registration and analysis of the shape fluctuations of nearly spherical lipid vesicles. Phys. Rev. E. 2013, 88, 022707. [Google Scholar] [CrossRef] [PubMed]
- Genova, J.; Slavkova, Z.; Chamati, H.; Petrov, M. Gel–liquid crystal phase transition in dry and hydrated SOPC phospholipid studied by differential scanning calorimetry. Phase. Transit. 2019, 92, 323–333. [Google Scholar] [CrossRef]
- Mhashal, A.R.; Roy, S. Effect of gold nanoparticle on structure and fluidity of lipid membrane. PLoS ONE. 2014, 9, e114152. [Google Scholar] [CrossRef] [PubMed]
- Contini, C.; Hindley, J.W.; Macdonald, T.J.; Barritt, J.D.; Ces, O.; Quirke, N. Size dependency of gold nanoparticles interacting with model membranes. Commun. Chem. 2020, 3, 130. [Google Scholar] [CrossRef] [PubMed]
- Cardellini, J.; Caselli, L.; Lavagna, E.; Salassi, S.; Amenitsch, H.; Calamai, M.; Montis, C.; Rossi, G.; Berti, D. Membrane phase drives the assembly of gold nanoparticles on biomimetic lipid bilayers. J. Phys. Chem. C. Nanomater. Interfaces. 2022, 126, 4483–4494. [Google Scholar] [CrossRef] [PubMed]
- Mady, M.M.; Fathy, M.M.; Youssef, T.; Khalil, W.M. Biophysical characterization of gold nanoparticles-loaded liposomes. Physica Medica. 2012, 28, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Zolghadr, A.R.; Moosavi, S.S. Interactions of neutral gold nanoparticles with DPPC and POPC lipid bilayers: Simulation and experiment. RSC Advances 2019, 9, 5197–5205. [Google Scholar] [CrossRef]
- Santhosh, P.B.; Thomas, N.; Sudhakar, S.; Chadha, A.; Mani, E. Phospholipid stabilized gold nanorods: towards improved colloidal stability and biocompatibility. Phys. Chem. Chem. Phys. 2017, 19, 18494–18504. [Google Scholar] [CrossRef]
- Mishra, K.P. Fluorescence studies on radiation oxidative damage to membranes with implications to cellular radiosensitivity. Proc. Indian. Acad. Sci. (Chem Sci), 2002, 114, 705–711. [Google Scholar] [CrossRef]
- Hurjui, I.; Neamtu, A.; Dorohoi, D. The interaction of fluorescent DPH probes with unsaturated phospholipid membranes: A molecular dynamics study. J. Mol. Struct. 2013, 1044, 134–139. [Google Scholar] [CrossRef]
- Kuhry, J.G.; Fonteneau, P.; Duportail, G.; Maechling, C.; Laustriat, G. TMA-DPH: A suitable fluorescence polarization probe for specific plasma membrane fluidity studies in intact living cells. Cell. Biophys. 1983, 5, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Barraza, K.M.; Beauchamp, J.L. Cholesterol provides nonsacrificial protection of membrane lipids from chemical damage at air–water interface. PNAS. 2018, 115, 3255–3260. [Google Scholar] [CrossRef] [PubMed]
- Schachter, I.; Paananen, R.O.; Fabian, B.; Jurkiewicz, P.; Javanainen, M. The two faces of the liquid ordered phase. J. Phys. Chem. Lett. 2022, 13, 307–1313. [Google Scholar] [CrossRef] [PubMed]
- Santhosh, P.B.; Drasler, B.; Drobne, D.; Kreft, M.E; Kralj, S.; Makovec, D.; Ulrih, N.P. Effect of superparamagnetic iron oxide nanoparticles on fluidity and phase transition of phosphatidylcholine liposomal membranes. Int. J. Nanomed. 2015, 10, 6089–103. [Google Scholar]
- Park, S.H.; Oh, S.G.; Mun, J.Y.; Han, S.S. Loading of gold nanoparticles inside the DPPC bilayers of liposome and their effects on membrane fluidities. Colloids. Surf. B. Biointerfaces. 2006, 48, 112–118. [Google Scholar] [CrossRef]
- Ibrahim, B.; Akere, T.H.; Chakraborty, S.; Valsami-Jones, E.; Ali-Boucetta, H. Gold Nanoparticles Induced Size Dependent Cytotoxicity on Human Alveolar Adenocarcinoma Cells by Inhibiting the Ubiquitin Proteasome System. Pharmaceutics. 2023, 15, 432. [Google Scholar] [CrossRef]
- Ozcicek, I.; Aysit, N.; Cakici, C.; Aydeger, A. The effects of surface functionality and size of gold nanoparticles on neuronal toxicity, apoptosis, ROS production and cellular/suborgan biodistribution. Mater. Sci. Eng. C. 2021, 128, 112308. [Google Scholar] [CrossRef]
- Li, C.P.; Weng, M.C.; Huang, S.L. Preparation and characterization of ph sensitive chitosan/3-glycidyloxypropyl trimethoxysilane (GPTMS) hydrogels by sol-gel method. Polymers (Basel). 2020, 12, 1326. [Google Scholar] [CrossRef]
- Ramalho, M.J.; Andrade, S.; Loureiro, J.A.; Pereira, M.C. Interaction of Bortezomib with cell membranes regulates its toxicity and resistance to therapy. Membranes. 2022, 12, 823. [Google Scholar] [CrossRef]
- Ota, A.; Gmajner, D.; Sentjurc, M.; Ulrih, N.P. Effect of growth medium pH of Aeropyrum pernix on structural properties and fluidity of archaeosomes. Archaea. 2012, 2012, 285152. [Google Scholar] [CrossRef] [PubMed]
- Gmajner, D.; Grabnar, P.A.; Znidaric, M.T.; Strus, J.; Sentjurc, M.; Ulrih, N.P. Structural characterization of liposomes made of diether archaeal lipids and dipalmitoyl-L-α- phosphatidylcholine. Biophys. Chem. 2011, 158, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Santhosh, P.B.; Velikonja, A.; Perutkova, S.; Gongadze, E.; Kulkarni, M.; Genova, J.; Elersic, K.; Iglic, A.; Kralj-Iglic, V.; Ulrih, N.P. Influence of nanoparticle–membrane electrostatic interactions on membrane fluidity and bending elasticity. Chem. Phys. Lipids. 2014, 178, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Santhosh, P.B.; Velikonja, A.; Gongadze, E.; Iglic, A.; Kralj-Iglic, V.; Ulrih, N.P. Interactions of divalent calcium ions with head groups of zwitterionic phosphatidylcholine liposomal membranes. Acta. Chim. Slov. 2014, 61, 215–222. [Google Scholar] [PubMed]
- Santhosh, P.B.; Kiryakova, S.I.; Genova, J.L.; Ulrih, N.P. Influence of iron oxide nanoparticles on bending elasticity and bilayer fluidity of phosphatidylcholine liposomal membranes. Colloids. Surf. A: Physicochem. Eng. Asp. 2014, 460, 248–253. [Google Scholar] [CrossRef]
- Velikonja, A.; Santhosh, P.; Gongadze, E.; Kulkarni, M.; Elersic, K.; Perutkova, S.; Kralj-Iglic, V.; Ulrih, N.; Iglic, A. Interaction between dipolar lipid headgroups and charged nanoparticles mediated by water dipoles and ions. IJMS. 2013, 14, 15312–15329. [Google Scholar] [CrossRef]
- Lewis, R.N.A.H.; McElhaney, R.N. Membrane lipid phase transitions and phase organization studied by Fourier transform infrared spectroscopy. Biochim. Biophys. Acta. Biomembr. 2013, 1828, 2347–2358. [Google Scholar] [CrossRef]
- Faramarzi, B.; Moggio, M.; Cardamuro, V.; Portaccio, M.; Diano, N.; Manti, L.; Lepore, M. An FTIR spectroscopy investigation on different methods of lipid extraction from HepG2 cells. Eng. Proc. 2022, 27, 39. [Google Scholar]
- Pakbin, B.; Zolghadr, L.; Rafiei, S.; Bruck, W.M.; Bruck, T.B. FTIR differentiation based on genomic DNA for species identification of Shigella isolates from stool samples. Sci. Rep. 2022, 12, 2780. [Google Scholar] [CrossRef]
- Fadlelmoula, A.; Pinho, D.; Carvalho, V.H.; Catarino, S.O.; Minas, G. Fourier Transform Infrared (FTIR) spectroscopy to analyse human blood over the last 20 years: A review towards lab-on-a-chip devices. Micromachines (Basel). 2022, 13, 187. [Google Scholar] [CrossRef]
- De Meutter, J.; Goormaghtigh, E. Evaluation of protein secondary structure from FTIR spectra improved after partial deuteration. Eur. Biophys. J. 2021, 50, 613–628. [Google Scholar] [CrossRef] [PubMed]
- Biruss, B.; Dietl, R.; Valenta, C. The influence of selected steroid hormones on the physicochemical behaviour of DPPC liposomes. Chem. Phys. Lipids. 2007, 148, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Casal, H.L.; Mantsch, H.H. Polymorphic phase behaviour of phospholipid membranes studied by infrared spectroscopy. Biochim. Biophys. Acta. 1984, 779, 381–401. [Google Scholar] [CrossRef] [PubMed]
- Brumm, T.; Jorgensen, K.; Mouritsen, D.G.; Bayerl, T.M. The effect of increasing membrane curvature on the phase transition and mixing behavior of a dimyristoyl-sn-glycero-3- phosphatidylcholine distearoyl-sn-glycero-3-phosphatidylcholine lipid mixture, as studied by Fourier transform infrared spectroscopy and differential scanning calorimetry. Biophys. J. 1996, 70, 1373–1379. [Google Scholar] [PubMed]
- Akkas, S.B.; Inci, S.; Zorlu, F.; Severcan, F. Melatonin affects the order, dynamics and hydration of brain membrane lipids. J. Mol. Struct. 2007, 836, 207–215. [Google Scholar] [CrossRef]
- Genova, J.; Petrov, M.; Bivas, I.; Rafailov, P.; Naradikian, H.; Katranchev, B. Fourier-transform infrared and Raman characterization of bilayer membranes of the phospholipid SOPC and its mixtures with cholesterol. Colloids. Surf. A: Physicochem. Eng. Asp. 2018, 557, 85–93. [Google Scholar] [CrossRef]
- Santhosh, P.B.; Genova, J.; Slavkova, Z.; Chamati, H. Influence of melatonin on the structural and thermal properties of SOPC lipid membranes. Colloids. Surf. A: Physicochem. Eng. Asp. 2022, 647, 129081. [Google Scholar] [CrossRef]
- Krecisz, M.; Rybka, J.D.; Strugala, A.J.; Skalski, B.; Figlerowicz, M.; Kozak, M.; Giersig, M. Interactions between magnetic nanoparticles and model lipid bilayers-Fourier transformed infrared spectroscopy (FTIR) studies of the molecular basis of nanotoxicity. J. Appl. Phys. 2016, 120, 124701. [Google Scholar] [CrossRef]
- Slavkova, Z.; Genova, J.; Chamati, H.; Koroleva, M.; Yancheva, D. Influence of hydrophobic Au nanoparticles on SOPC lipid model systems. Colloids. Surf. A: Physicochem. Eng. Asp. 2020, 603, 125090. [Google Scholar] [CrossRef]
- Severcan, F.; Sahin, I.; Kazanci, N. Melatonin strongly interacts with zwitterionic model membranes - evidence from Fourier transform infrared spectroscopy and differential scanning calorimetry. Biochim. Biophys. Acta. 2005, 1668, 215–222. [Google Scholar] [CrossRef]
- Pruchnik, H.; Gliszczynska, A.; Włoch, A. Evaluation of the physico-chemical properties of liposomes assembled from bioconjugates of anisic acid with phosphatidylcholine. Int. J. Mol. Sci. 2021, 22, 13146. [Google Scholar] [CrossRef] [PubMed]
- Santhosh, P.B.; Penic, S.; Genova, J.; Iglic, A.; Kralj-Iglic, V.; Ulrih, N.P. A study on the interaction of nanoparticles with lipid membranes and their influence on membrane fluidity. J. Phys. Conf. Ser. 2012, 398, 012034. [Google Scholar] [CrossRef]
- Pottel, H.; Van der Meer, W.; Herreman, W. Correlation between the order parameter and the steady-state fluorescence anisotropy of 1,6-diphenyl-1,3,5-hexatriene and an evaluation of membrane fluidity. Biochim. Biophys. Acta. 1983, 730, 181–186. [Google Scholar] [CrossRef]
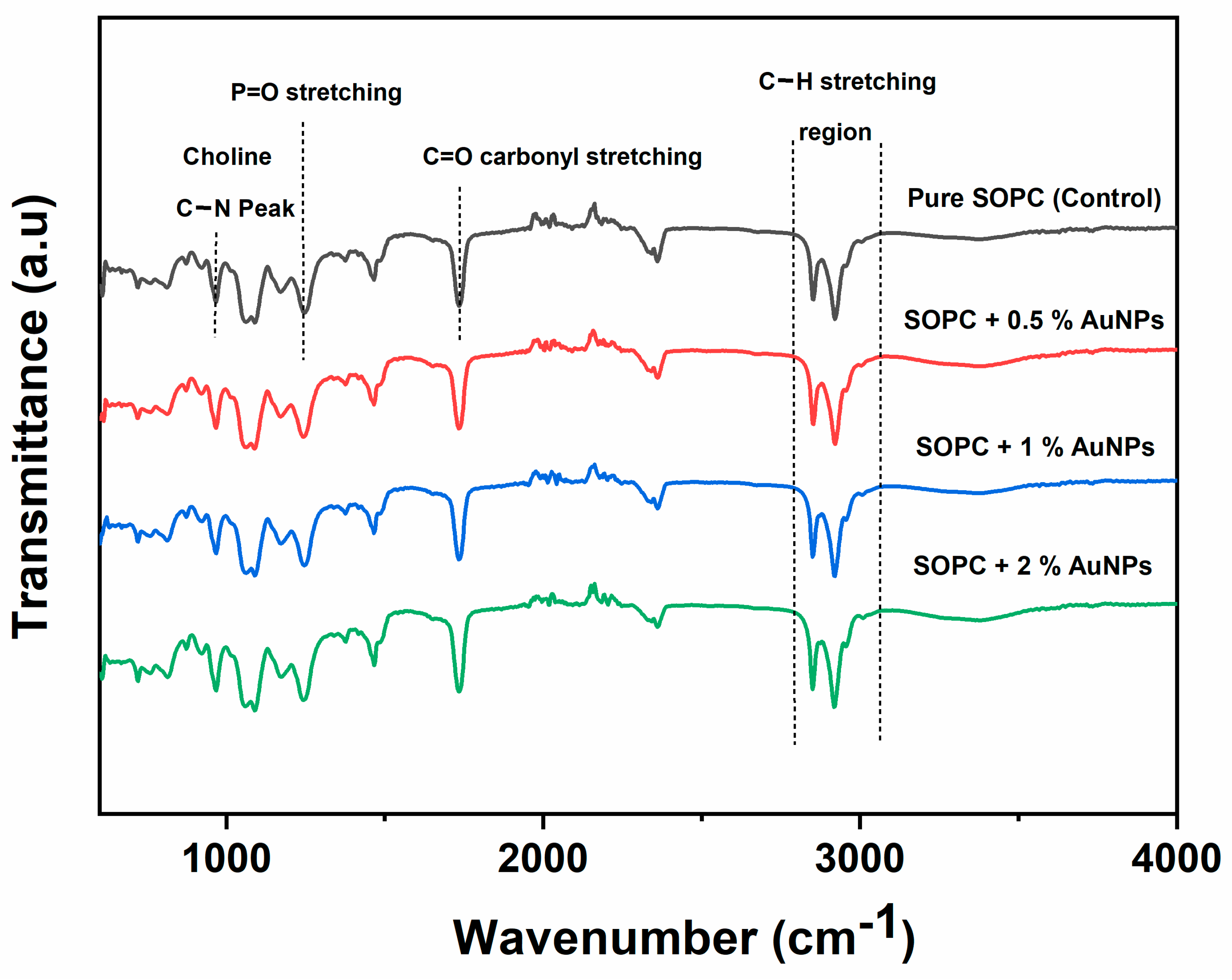
| Samples | Choline C-H stretching (cm−1) |
Methylene C-H antisymmetric stretching (cm−1) |
Methylene C-H symmetric stretching (cm−1) |
C=O stretching (cm−1) |
PO2¯ antisymmetric stretching (cm−1) |
Choline C-N peak height position (cm−1) |
|---|---|---|---|---|---|---|
| Pure SOPC | 3011.0 | 2923 | 2850.0 | 1738.2 | 1250.0 | 969.5 |
| SOPC + 0.5 % AuNPs | 3010.0 | 2922.5 | 2849.7 | 1738.0 | 1249.8 | 969.5 |
| SOPC + 1 % AuNPs | 3009.6 | 2922.0 | 2849.1 | 1737.8 | 1249.5 | 969.3 |
| SOPC + 2 % AuNPs | 3008.2 | 2921.0 | 2848.5 | 1737.3 | 1249.2 | 969.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





