Submitted:
13 May 2023
Posted:
15 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
| No. | Authors and year of publication | Country | Number of patients | Period of time analysed | The type of primary cancer/pre-cancer lesion | Median age of participants | Number of AIN/AC | Increased risk | Risk of bias |
|---|---|---|---|---|---|---|---|---|---|
| 1. | Acevedo-Fontánez et al. (2018) [17] | Puerto-Rico | 9,489 | 1987-2013 | 8,039 CC 1,378 VC 773 VaC |
46 CC, 70 VC, 67 VaC |
14 AC after CC 3 AC after VC 1 AC after VaC |
AC after CC: SIR= 1.8 (95% Cl: 0.9- 3.4) AC after VC & VaC: SIR= 2.9 (95% Cl: 0.8-7.5) |
L |
| 2. | Chaturvedi et al. (2007) [18] | USA | 27,466 | 1973–2001 | 27,466 CC* | 48.6 | NI | AC after CC: SIR= 3.12; 95% CI = 1.88-4.88 | L |
| 3. | Ebisch et al. (2017) [19] | Netherlands | 89,018 | 1990-2015 | 89,018 CIN 3 | 36 | 73 AC 80 AIN 3 |
AC after CIN 3: SIR= 3.85 (95% CI: 2.32-6.37) AIN 3 after CIN 3: SIR= 6.68 (95% CI: 3.64- 12.25) |
L |
| 4. | Edgren et al. (2007) [20] | Sweden | 125,292 | 1968-2004 | 125,292 CIN 3 | 35 | 131 AC | AC after CIN 3: SIR= 4.68 (95% Cl: 3.87- 5.62) | L |
| 5. | ElNaggar et al. (2013) [21] | USA | 272 | 2006-2010 | CIN 1 29 CIN 2 16 CIN 3/CIS 41 VIN 1 46 VIN 2 16 VIN 3/CIS 69 VaIN 1 34 VaIN 2 13 VaIN 3/CIS 8 =272 |
39 | 64 AIN (36 stage 1, 6 stage 2, 22 stage 3) | 48 (36.4%) had VIN, 10 (18.2%) had VaIN, and 13 (14.4%) had CIN. | H |
| 6. | Evans et al. (2003) [22] | England | 81,124 | 1960-1999 | 59,519 CIN 3 21,605 CC |
NI | 23 AC after CIN 3 18 AC after CC |
AC after CIN 3: SIR= 5.9 AC after CC: SIR= 6.3 |
L |
| 7. | Gaudet et al. (2014) [23] | Canada | 54,320 | 1985-2005 | 54,320 CIN 2 and CIN 3** |
35 | 4 AC after CIN 2 16 AC after CIN 3 |
AC after CIN 2: SIR= 0.89 (95% Cl: 0.09- 3.35) AC after CIN 3: SIR= 2.28 (95% Cl: 0.71- 5.42) |
L |
| 8. | Hemminki et al. (2001) [24] | Sweden | 17,234 | 1958-1996 | 17,234 CC | NI | 16 AC | AC after CC: SIR= 4.22 (95% Cl: 2.41-6.55) | L |
| 9. | Hemminki et al. (2000) [25] | Sweden | 135,386 | 1958-1996 | 117,830 CIN 3 17,556 CC |
NI | 68 AC after CIN 3 17 AC after CC |
AC after CIN 3: SIR= 3.75 (95% Cl: 2.91-4.69) AC after CC: SIR= 3.92 (95% Cl: 2.28-6.00) |
L |
| 10. | Heráclio et al. (2018) [26] | Brazil | 324 | 2008-2009 | 200 CIN 1, 97 CIN 2 or CIN 3, 27 CC |
33 | 37 AIN | AIN after CIN 1: IR= 7% AIN after CIN 2/3: IR= 18.5% |
L |
| 11. | Jakobsson et al. (2011) [27] | Finland | 26,876 | 1987–2006 | 26,876 CIN (unknown grade) | NI | 3 AC | AC after CIN: SIR= 3.56 (95% Cl: 0.73-10.4) | H |
| 12. | Jiménez et al. (2009) [28] | Canada | 674 | 1992-2005 | 7 CC, 3 VaC and 1 VC | 61 | 674 AC | AC after HPV-RGD: OR= 10.5 (95% CI: 3.6-30.3) AC after CC: OR= 6.84 (95% CI: 2.16-21.61) |
H |
| 13. | Kalliala et al. (2005) [29] | Finland | 7,564 | 1974-2003 | 2,446 CIN 1 1,543 CIN 2 1,334 CIN 3 2,241 CIN “not otherwise specified” |
NI | 3 AC | AC after CIN: SIR= 5.7 (95% Cl: 1.2 to 17.0) | L |
| 14. | Matsuo et al. (2018) [30] | USA | 79,050 | 1973-2013 | 79,050 CC | 63 | 49 AC | 10-, 20-, 30-year cumulative incidence for AC after CC: 0.04%, 0.16%, and 0.38% | H |
| 15. | Neumann et al. (2016) [31] | French | 4,808 | 1989-2007 | 4,234 CC 339 VC 235 VaC |
NI | 5 AC after CC 1 AC after VC 0 AC after VaC |
AC after CC: SIR= 5.42 (95% Cl: 1.75–12.64) AC after VC: SIR= 11.7 (95% Cl: 0.15–65.51) |
L |
| 16. | Pan et al. (2019) [32] | Scotland | NI | 1989-2015 | 69,714 CIN 3 | 30 | 37 AC after CIN 3 | AC after CIN 3: SIR= 2.6 (95% Cl: 1.9–3.6) | L |
| 17. | Papatla et al. (2019) [33] | USA | 21,060 | 1973-2014 | 21,060 CC | 61.73 | 17 AC | AC after CC: SIR= 2.20 (95% Cl: 1.28-3.52) | L |
| 18. | Preti et al. (2020) [34] | Italy | 3,184 | 1992-2004 | 3,184 CIN 2 or 3 | NI | 1 AC | AC after CIN 2 or 3: SIR= 1.8 (95% Cl: 0.04–10.0) | H |
| 19. | Rabkin et al. (1992) [35] | USA | 25,295 | 1935-1988 | 25,295 CC | NI | 12 AC | AC after CC: SIR= 4.6 (95% Cl: 2.4-8.1) | H |
| 20. | Saleem et al. (2011) [36] | USA | 189,206 | 1973-2007 | 124,075 CIN 3 6,792 VIN 3 1,463 VaIN 3 43,669 CC 9,950 VC 3,257 VaC |
NI | 255 AC | AC after CIN 3: SIR= 16.4 (95% CI: 13.7–19.2) AC after CC: SIR= 6.2 (95% CI: 4.1– 8.7) AC after VIN 3: SIR= 22.2 (95% CI: 16.7–28.4) AC after VC: SIR= 17.4 (95% CI: 11.5–24.4) AC after VaIN 3: SIR= 7.6 (95% CI: 2.4–15.6) AC after VaC: SIR= 1.8 (95% CI: 0.2–5.3) |
L |
| 21. | Sand et al. (2016) [37] | Denmark | 156,290 | 1978-2012 | 52,135 CIN 2 104,155 CIN 3 |
33.8 for CIN 2 34.0 for CIN 3 |
32 AC after CIN 2 125 AC after CIN 3 |
AC after CIN 2: SIR= 2.9 (2.0–4.1) AC after CIN 3: SIR= 4.2 (3.4–5.0) |
L |
| 22. | Suk et al. (2018) [38] | USA | 52,589 | 1973-2014 | 44,011 CC 6,905 VC 1,673 VaC |
63 for CC, 61 for VC, 95 for VaC |
34 AC after CC 31 AC after VC 1 AC after VaC |
AC after CC: SIR= 2.3 (95% CI: 1.6-3.2) AC after VC: SIR=13.2 (95% CI: 8.9-18.7) AC after VaC: SIR= 2.3 (95% CI: 0.1-12.8) |
L |
| 23. | Tatti et al. (2012) [39] | Argentina | 481 | 2005-2011 | 121 CIN 1 114 CIN 2/3 188 VIN 1 39 VIN 2/3 70 VaIN 1 22 VaIN 2/3 |
35 | 28 AIN 2/3 106 AIN 1 |
No info (AIN after CIN 2/3 comparted to AIN after CIN 1: OR= 1.91) |
H |
| 24. | Tomassi et al. (2018) [40] | USA | 221,511 | 2005-2015 | 1,168 CC 15,711 CIN 2/3 109,893 CIN 1 94,739 genital warts |
63.8 | 1 AC after CC 5 AC after CIN 2/3 14 AC after CIN 1 14 AC after genital wards |
AC after CC: IR= 0.09% AC after CIN 2/3: IR= 0.03% AC after CIN 1: IR= 0.01% AC after genital wards: IR= 0.01% |
H |
| 25. | Wang et al. (2020) [41] | USA | 56,127 | 2000-2015 | 46,550 CC 7,855 VC 1,722 VaC |
NI | 50 AC after CC 9 AC after VC 1 AC after VaC |
AC after CC: SIR= 1.63 (95% Cl: 1.21- 2.14) AC after VC: SIR= 1.10 (95% Cl: 0.50-2.09) AC after VaC: SIR= 0.62 (95% Cl: 0.01- 3.47) |
L |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations:
| AC | anal cancer |
| AIN | anal intraepithelial neoplasia |
| CC | cervical cancer |
| CIN | cervical intraepithelial neoplasia |
| Cl | confidence level |
| HPV | human papillomavirus |
| HPV-RGD | HPV-related gynecological diseases |
| IR | incidence risk |
| OR | odds ratio |
| PY | person-years |
| SD | standard deviation |
| SIR | standardized incidence ratio |
| VaC | vaginal cancer |
| VaIN | vaginal intraepithelial neoplasia |
| VIN | vulvar intraepithelial neoplasia |
| VC | vulvar cancer |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Kreisel, K.M.; Spicknall, I.H.; Gargano, J.W.; Lewis, F.M.T.; Lewis, R.M.; Markowitz, L.E.; Roberts, H.; Johnson, A.S.; Song, R.; St Cyr, S.B.; et al. Sexually Transmitted Infections Among US Women and Men: Prevalence and Incidence Estimates, 2018. Sex. Transm. Dis. 2021, 48, 208–214. [Google Scholar] [CrossRef] [PubMed]
- de Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide Burden of Cancer Attributable to HPV by Site, Country and HPV Type. Int. J. Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Gheit, T. Mucosal and Cutaneous Human Papillomavirus Infections and Cancer Biology. Front. Oncol. 2019, 9, 355. [Google Scholar] [CrossRef]
- Ibeanu, O.A. Molecular Pathogenesis of Cervical Cancer. Cancer Biol. Ther. 2011, 11, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, B.Y.; McDuffie, K.; Zhu, X.; Wilkens, L.R.; Killeen, J.; Kessel, B.; Wakabayashi, M.T.; Bertram, C.C.; Easa, D.; Ning, L.; et al. Anal Human Papillomavirus Infection in Women and Its Relationship with Cervical Infection. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2005, 14, 2550–2556. [Google Scholar] [CrossRef] [PubMed]
- Jacot-Guillarmod, M.; Balaya, V.; Mathis, J.; Hübner, M.; Grass, F.; Cavassini, M.; Sempoux, C.; Mathevet, P.; Pache, B. Women with Cervical High-Risk Human Papillomavirus: Be Aware of Your Anus! The ANGY Cross-Sectional Clinical Study. Cancers 2022, 14, 5096. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, G.; Wiley, D.J.; Li, X.; Chmiel, J.S.; Margolick, J.B.; Cranston, R.D.; Jacobson, L.P. Incidence and Epidemiology of Anal Cancer in the Multicenter AIDS Cohort Study. J. Acquir. Immune Defic. Syndr. 1999 2008, 48, 491–499. [Google Scholar] [CrossRef]
- Silverberg, M.J.; Lau, B.; Justice, A.C.; Engels, E.; Gill, M.J.; Goedert, J.J.; Kirk, G.D.; D’Souza, G.; Bosch, R.J.; Brooks, J.T.; et al. Risk of Anal Cancer in HIV-Infected and HIV-Uninfected Individuals in North America. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2012, 54, 1026–1034. [Google Scholar] [CrossRef]
- Shiels, M.S.; Pfeiffer, R.M.; Chaturvedi, A.K.; Kreimer, A.R.; Engels, E.A. Impact of the HIV Epidemic on the Incidence Rates of Anal Cancer in the United States. J. Natl. Cancer Inst. 2012, 104, 1591–1598. [Google Scholar] [CrossRef]
- de Sanjosé, S.; Bruni, L.; Alemany, L. HPV in Genital Cancers (at the Exception of Cervical Cancer) and Anal Cancers. Presse Medicale Paris Fr. 1983 2014, 43, e423–e428. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.A.; Wentzensen, N. Strategies for Screening and Early Detection of Anal Cancers: A Narrative and Systematic Review and Meta-Analysis of Cytology, HPV Testing, and Other Biomarkers. Cancer Cytopathol. 2018, 126, 447–460. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Slama, J.; Gonzalez, P.; Goodman, M.T.; Xia, N.; Kreimer, A.R.; Wu, T.; Hessol, N.A.; Shvetsov, Y.; Ortiz, A.P.; et al. Cervical Determinants of Anal HPV Infection and High-Grade Anal Lesions in Women: A Collaborative Pooled Analysis. Lancet Infect. Dis. 2019, 19, 880–891. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Waxman, A.G.; Chelmow, D.; Darragh, T.M.; Lawson, H.; Moscicki, A.-B. Revised Terminology for Cervical Histopathology and Its Implications for Management of High-Grade Squamous Intraepithelial Lesions of the Cervix. Obstet. Gynecol. 2012, 120, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.F.; Rutjes, A.W.S.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M.M. QUADAS-2 Group QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Acevedo-Fontánez, A.I.; Suárez, E.; Torres Cintrón, C.R.; Ortiz, A.P. Risk of Anal Cancer in Women With a Human Papillomavirus–Related Gynecological Neoplasm: Puerto Rico 1987–2013. J. Low. Genit. Tract Dis. 2018, 22, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, A.K.; Engels, E.A.; Gilbert, E.S.; Chen, B.E.; Storm, H.; Lynch, C.F.; Hall, P.; Langmark, F.; Pukkala, E.; Kaijser, M.; et al. Second Cancers Among 104760 Survivors of Cervical Cancer: Evaluation of Long-Term Risk. JNCI J. Natl. Cancer Inst. 2007, 99, 1634–1643. [Google Scholar] [CrossRef]
- Ebisch, R.M.F.; Rutten, D.W.E.; IntHout, J.; Melchers, W.J.G.; Massuger, L.F.A.G.; Bulten, J.; Bekkers, R.L.M.; Siebers, A.G. Long-Lasting Increased Risk of Human Papillomavirus–Related Carcinomas and Premalignancies After Cervical Intraepithelial Neoplasia Grade 3: A Population-Based Cohort Study. J. Clin. Oncol. 2017, 35, 2542–2550. [Google Scholar] [CrossRef]
- Edgren, G.; Sparén, P. Risk of Anogenital Cancer after Diagnosis of Cervical Intraepithelial Neoplasia: A Prospective Population-Based Study. Lancet Oncol. 2007, 8, 311–316. [Google Scholar] [CrossRef]
- ElNaggar, A.C.; Santoso, J.T. Risk Factors for Anal Intraepithelial Neoplasia in Women With Genital Dysplasia. Obstet. Gynecol. 2013, 122, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Evans, H.S.; Newnham, A.; Hodgson, S.V.; Møller, H. Second Primary Cancers after Cervical Intraepithelial Neoplasia III and Invasive Cervical Cancer in Southeast England. Gynecol. Oncol. 2003, 90, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, M.; Hamm, J.; Aquino-Parsons, C. Incidence of Ano-Genital and Head and Neck Malignancies in Women with a Previous Diagnosis of Cervical Intraepithelial Neoplasia. Gynecol. Oncol. 2014, 134, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Hemminki, K.; Jiang, Y.; Dong, C. Second Primary Cancers after Anogenital, Skin, Oral, Esophageal and Rectal Cancers: Etiological Links? Int. J. Cancer 2001, 93, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Hemminki, K.; Dong, C.; Vaittinen, P. Second Primary Cancer after in Situ and Invasive Cervical Cancer. Epidemiol. Camb. Mass 2000, 11, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Heráclio, S.A.; De Souza, A.S.R.; De Souza, P.R.E.; Katz, L.; Lima Junior, S.F.; Amorim, M.M.R. Cross-Sectional Study of Anal Intraepithelial Lesions in Women with Cervical Neoplasia without HIV. Int. J. Gynecol. Obstet. 2018, 140, 233–240. [Google Scholar] [CrossRef]
- Jakobsson, M.; Pukkala, E.; Paavonen, J.; Tapper, A.; Gissler, M. Cancer Incidence among Finnish Women with Surgical Treatment for Cervical Intraepithelial Neoplasia, 1987–2006. Int. J. Cancer 2011, 128, 1187–1191. [Google Scholar] [CrossRef]
- Jiménez, W.; Paszat, L.; Kupets, R.; Wilton, A.; Tinmouth, J. Presumed Previous Human Papillomavirus (HPV) Related Gynecological Cancer in Women Diagnosed with Anal Cancer in the Province of Ontario. Gynecol. Oncol. 2009, 114, 395–398. [Google Scholar] [CrossRef]
- Kalliala, I.; Anttila, A.; Pukkala, E.; Nieminen, P. Risk of Cervical and Other Cancers after Treatment of Cervical Intraepithelial Neoplasia: Retrospective Cohort Study. BMJ 2005, 331, 1183–1185. [Google Scholar] [CrossRef]
- Matsuo, K.; Blake, E.A.; Machida, H.; Mandelbaum, R.S.; Roman, L.D.; Wright, J.D. Incidences and Risk Factors of Metachronous Vulvar, Vaginal, and Anal Cancers after Cervical Cancer Diagnosis. Gynecol. Oncol. 2018, 150, 501–508. [Google Scholar] [CrossRef]
- Neumann, F.; Jégu, J.; Mougin, C.; Prétet, J.-L.; Guizard, A.-V.; Lapôtre-Ledoux, B.; Bara, S.; Bouvier, V.; Colonna, M.; Troussard, X.; et al. Risk of Second Primary Cancer after a First Potentially-Human Papillomavirus-Related Cancer: A Population-Based Study. Prev. Med. 2016, 90, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Kavanagh, K.; Cuschieri, K.; Pollock, K.G.; Gilbert, D.C.; Millan, D.; Bell, S.; Graham, S.V.; Williams, A.R.W.; Cruickshank, M.E.; et al. Increased Risk of HPV-Associated Genital Cancers in Men and Women as a Consequence of Pre-Invasive Disease. Int. J. Cancer 2019, 145, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Papatla, K.; Halpern, M.T.; Hernandez, E.; Brown, J.; Benrubi, D.; Houck, K.; Chu, C.; Rubin, S. Second Primary Anal and Oropharyngeal Cancers in Cervical Cancer Survivors. Am. J. Obstet. Gynecol. 2019, 221, 478.e1. [Google Scholar] [CrossRef] [PubMed]
- Preti, M.; Rosso, S.; Micheletti, L.; Libero, C.; Sobrato, I.; Giordano, L.; Busso, P.; Gallio, N.; Cosma, S.; Bevilacqua, F.; et al. Risk of HPV-Related Extra-Cervical Cancers in Women Treated for Cervical Intraepithelial Neoplasia. BMC Cancer 2020, 20, 972. [Google Scholar] [CrossRef] [PubMed]
- Rabkin, C.S.; Biggar, R.J.; Melbye, M.; Curtis, R.E. Second Primary Cancers Following Anal and Cervical Carcinoma: Evidence of Shared Etiologic Factors. Am. J. Epidemiol. 1992, 136, 54–58. [Google Scholar] [CrossRef]
- Saleem, A.M.; Paulus, J.K.; Shapter, A.P.; Baxter, N.N.; Roberts, P.L.; Ricciardi, R. Risk of Anal Cancer in a Cohort With Human Papillomavirus–Related Gynecologic Neoplasm. Obstet. Gynecol. 2011, 117, 643–649. [Google Scholar] [CrossRef]
- Sand, F.L.; Munk, C.; Jensen, S.M.; Svahn, M.F.; Frederiksen, K.; Kjær, S.K. Long-Term Risk for Noncervical Anogenital Cancer in Women with Previously Diagnosed High-Grade Cervical Intraepithelial Neoplasia: A Danish Nationwide Cohort Study. Cancer Epidemiol. Biomarkers Prev. 2016, 25, 1090–1097. [Google Scholar] [CrossRef]
- Suk, R.; Mahale, P.; Sonawane, K.; Sikora, A.G.; Chhatwal, J.; Schmeler, K.M.; Sigel, K.; Cantor, S.B.; Chiao, E.Y.; Deshmukh, A.A. Trends in Risks for Second Primary Cancers Associated With Index Human Papillomavirus–Associated Cancers. JAMA Netw. Open 2018, 1, e181999. [Google Scholar] [CrossRef]
- Tatti, S.; Suzuki, V.; Fleider, L.; Maldonado, V.; Caruso, R. Anal Intraepithelial Lesions in Women With Human PapillomavirusYRelated Disease. J Low Genit Tract Dis 2012, 16, 454–459. [Google Scholar] [CrossRef]
- Tomassi, M.J.; Abbas, M.A.; Klaristenfeld, D.D. Expectant Management Surveillance for Patients at Risk for Invasive Squamous Cell Carcinoma of the Anus: A Large US Healthcare System Experience. Int. J. Colorectal Dis. 2019, 34, 47–54. [Google Scholar] [CrossRef]
- Wang, M.; Sharma, A.; Osazuwa-Peters, N.; Simpson, M.C.; Schootman, M.; Piccirillo, J.F.; Huh, W.K.; Adjei Boakye, E. Risk of Subsequent Malignant Neoplasms after an Index Potentially-Human Papillomavirus (HPV)-Associated Cancers. Cancer Epidemiol. 2020, 64, 101649. [Google Scholar] [CrossRef] [PubMed]
- Santoso, J.T.; Long, M.; Crigger, M.; Wan, J.Y.; Haefner, H.K. Anal Intraepithelial Neoplasia in Women with Genital Intraepithelial Neoplasia. Obstet. Gynecol. 2010, 116, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.A.; Deshmukh, A.A.; Suk, R.; Roberts, J.; Gilson, R.; Jay, N.; Stier, E.A.; Wentzensen, N. A Systematic Review and Meta-analysis of Cytology and HPV-related Biomarkers for Anal Cancer Screening among Different Risk Groups. Int. J. Cancer 2022, 151, 1889–1901. [Google Scholar] [CrossRef] [PubMed]
- IANS - IANS Committees. Available online: https://iansoc.org/IANS-Committees (accessed on 11 May 2023).
- Muñoz, N.; Manalastas, R.; Pitisuttithum, P.; Tresukosol, D.; Monsonego, J.; Ault, K.; Clavel, C.; Luna, J.; Myers, E.; Hood, S.; et al. Safety, Immunogenicity, and Efficacy of Quadrivalent Human Papillomavirus (Types 6, 11, 16, 18) Recombinant Vaccine in Women Aged 24-45 Years: A Randomised, Double-Blind Trial. Lancet Lond. Engl. 2009, 373, 1949–1957. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Xie, X.; Liu, J.; Zhao, Y.; Chen, W.; Zhao, C.; Wang, S.; Liao, X.; Shou, Q.; Qiu, Y.; et al. Efficacy of Quadrivalent Human Papillomavirus Vaccine against Persistent Infection and Genital Disease in Chinese Women: A Randomized, Placebo-Controlled Trial with 78-Month Follow-Up. Vaccine 2019, 37, 3617–3624. [Google Scholar] [CrossRef] [PubMed]
- Bartels, H.C.; Postle, J.; Rogers, A.C.; Brennan, D. Prophylactic Human Papillomavirus Vaccination to Prevent Recurrence of Cervical Intraepithelial Neoplasia: A Meta-Analysis. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2020, 30, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Jentschke, M.; Kampers, J.; Becker, J.; Sibbertsen, P.; Hillemanns, P. Prophylactic HPV Vaccination after Conization: A Systematic Review and Meta-Analysis. Vaccine 2020, 38, 6402–6409. [Google Scholar] [CrossRef] [PubMed]
- Kechagias, K.S.; Kalliala, I.; Bowden, S.J.; Athanasiou, A.; Paraskevaidi, M.; Paraskevaidis, E.; Dillner, J.; Nieminen, P.; Strander, B.; Sasieni, P.; et al. Role of Human Papillomavirus (HPV) Vaccination on HPV Infection and Recurrence of HPV Related Disease after Local Surgical Treatment: Systematic Review and Meta-Analysis. BMJ 2022, 378, e070135. [Google Scholar] [CrossRef]
- Goodman, E.; Reuschenbach, M.; Kaminski, A.; Ronnebaum, S. Human Papillomavirus Vaccine Impact and Effectiveness in Six High-Risk Populations: A Systematic Literature Review. Vaccines 2022, 10, 1543. [Google Scholar] [CrossRef]
- Drolet, M.; Bénard, É.; Pérez, N.; Brisson, M. HPV Vaccination Impact Study Group Population-Level Impact and Herd Effects Following the Introduction of Human Papillomavirus Vaccination Programmes: Updated Systematic Review and Meta-Analysis. Lancet Lond. Engl. 2019, 394, 497–509. [Google Scholar] [CrossRef]
- Kjaer, S.K.; Nygård, M.; Sundström, K.; Dillner, J.; Tryggvadottir, L.; Munk, C.; Berger, S.; Enerly, E.; Hortlund, M.; Ágústsson, Á.I.; et al. Final Analysis of a 14-Year Long-Term Follow-up Study of the Effectiveness and Immunogenicity of the Quadrivalent Human Papillomavirus Vaccine in Women from Four Nordic Countries. EClinicalMedicine 2020, 23, 100401. [Google Scholar] [CrossRef] [PubMed]
- Olsson, S.-E.; Restrepo, J.A.; Reina, J.C.; Pitisuttithum, P.; Ulied, A.; Varman, M.; Van Damme, P.; Moreira, E.D.; Ferris, D.; Block, S.; et al. Long-Term Immunogenicity, Effectiveness, and Safety of Nine-Valent Human Papillomavirus Vaccine in Girls and Boys 9 to 15 Years of Age: Interim Analysis after 8 Years of Follow-Up. Papillomavirus Res. Amst. Neth. 2020, 10, 100203. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Ploner, A.; Elfström, K.M.; Wang, J.; Roth, A.; Fang, F.; Sundström, K.; Dillner, J.; Sparén, P. HPV Vaccination and the Risk of Invasive Cervical Cancer. N. Engl. J. Med. 2020, 383, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Falcaro, M.; Castañon, A.; Ndlela, B.; Checchi, M.; Soldan, K.; Lopez-Bernal, J.; Elliss-Brookes, L.; Sasieni, P. The Effects of the National HPV Vaccination Programme in England, UK, on Cervical Cancer and Grade 3 Cervical Intraepithelial Neoplasia Incidence: A Register-Based Observational Study. Lancet Lond. Engl. 2021, 398, 2084–2092. [Google Scholar] [CrossRef] [PubMed]
- Kjaer, S.K.; Dehlendorff, C.; Belmonte, F.; Baandrup, L. Real-World Effectiveness of Human Papillomavirus Vaccination Against Cervical Cancer. J. Natl. Cancer Inst. 2021, 113, 1329–1335. [Google Scholar] [CrossRef]
- Dehlendorff, C.; Baandrup, L.; Kjaer, S.K. Real-World Effectiveness of Human Papillomavirus Vaccination Against Vulvovaginal High-Grade Precancerous Lesions and Cancers. J. Natl. Cancer Inst. 2021, 113, 869–874. [Google Scholar] [CrossRef]
- Zhang, L.; Hemminki, O.; Chen, T.; Zheng, G.; Försti, A.; Sundquist, K.; Sundquist, J.; Hemminki, K. Familial Clustering, Second Primary Cancers and Causes of Death in Penile, Vulvar and Vaginal Cancers. Sci. Rep. 2019, 9, 11804. [Google Scholar] [CrossRef]
- Herrero, R.; Quint, W.; Hildesheim, A.; Gonzalez, P.; Struijk, L.; Katki, H.A.; Porras, C.; Schiffman, M.; Rodriguez, A.C.; Solomon, D.; et al. Reduced Prevalence of Oral Human Papillomavirus (HPV) 4 Years after Bivalent HPV Vaccination in a Randomized Clinical Trial in Costa Rica. PLoS ONE 2013, 8, e68329. [Google Scholar] [CrossRef]
- Lehtinen, M.; Apter, D.; Eriksson, T.; Harjula, K.; Hokkanen, M.; Lehtinen, T.; Natunen, K.; Damaso, S.; Soila, M.; Bi, D.; et al. Effectiveness of the AS04-Adjuvanted HPV-16/18 Vaccine in Reducing Oropharyngeal HPV Infections in Young Females-Results from a Community-Randomized Trial. Int. J. Cancer 2020, 147, 170–174. [Google Scholar] [CrossRef]
- Tsentemeidou, A.; Fyrmpas, G.; Stavrakas, M.; Vlachtsis, K.; Sotiriou, E.; Poutoglidis, A.; Tsetsos, N. Human Papillomavirus Vaccine to End Oropharyngeal Cancer. A Systematic Review and Meta-Analysis. Sex. Transm. Dis. 2021, 48, 700–707. [Google Scholar] [CrossRef]
- Hirsch, B.E.; McGowan, J.P.; Fine, S.M.; Vail, R.; Merrick, S.T.; Radix, A.; Hoffmann, C.J.; Gonzalez, C.J. Screening for Anal Dysplasia and Cancer in Adults With HIV; New York State Department of Health AIDS Institute Clinical Guidelines; Johns Hopkins University: Baltimore (MD), 2022. [Google Scholar]
- Leeds, I.L.; Fang, S.H. Anal Cancer and Intraepithelial Neoplasia Screening: A Review. World J. Gastrointest. Surg. 2016, 8, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Kelly, H.; Chikandiwa, A.; Alemany Vilches, L.; Palefsky, J.M.; de Sanjose, S.; Mayaud, P. Association of Antiretroviral Therapy with Anal High-Risk Human Papillomavirus, Anal Intraepithelial Neoplasia, and Anal Cancer in People Living with HIV: A Systematic Review and Meta-Analysis. Lancet HIV 2020, 7, e262–e278. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, A.; Stirrup, O.; Nathan, M.; Clifford, G.M. Burden of Anal Squamous Cell Carcinoma, Squamous Intraepithelial Lesions and HPV16 Infection in Solid Organ Transplant Recipients: A Systematic Review and Meta-Analysis. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2020, 20, 3520–3528. [Google Scholar] [CrossRef] [PubMed]
- Garland, S.M.; Brotherton, J.M.L.; Moscicki, A.B.; Kaufmann, A.M.; Stanley, M.; Bhatla, N.; Sankaranarayanan, R.; de Sanjosé, S.; Palefsky, J.M. ; IPVS HPV Vaccination of Immunocompromised Hosts. Papillomavirus Res. Amst. Neth. 2017, 4, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Perkins, R.B.; Guido, R.S.; Castle, P.E.; Chelmow, D.; Einstein, M.H.; Garcia, F.; Huh, W.K.; Kim, J.J.; Moscicki, A.-B.; Nayar, R.; et al. 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J. Low. Genit. Tract Dis. 2020, 24, 102–131. [Google Scholar] [CrossRef] [PubMed]
- Mugo, N.R.; Eckert, L.; Magaret, A.S.; Cheng, A.; Mwaniki, L.; Ngure, K.; Celum, C.; Baeten, J.M.; Galloway, D.A.; Wamalwa, D.; et al. Quadrivalent HPV Vaccine in HIV-1-Infected Early Adolescent Girls and Boys in Kenya: Month 7 and 12 Post Vaccine Immunogenicity and Correlation with Immune Status. Vaccine 2018, 36, 7025–7032. [Google Scholar] [CrossRef] [PubMed]
- Mugo, N.; Eckert, L.O.; Odero, L.; Gakuo, S.; Ngure, K.; Celum, C.; Baeten, J.M.; Barnabas, R.V.; Wald, A. Antibody Responses to Prophylactic Quadrivalent Human Papillomavirus Vaccine at 48 Months among HIV-Infected Girls and Boys Ages 9-14 in Kenya, Africa. Vaccine 2021, 39, 4751–4758. [Google Scholar] [CrossRef]
- Staadegaard, L.; Rönn, M.M.; Soni, N.; Bellerose, M.E.; Bloem, P.; Brisson, M.; Maheu-Giroux, M.; Barnabas, R.V.; Drolet, M.; Mayaud, P.; et al. Immunogenicity, Safety, and Efficacy of the HPV Vaccines among People Living with HIV: A Systematic Review and Meta-Analysis. EClinicalMedicine 2022, 52, 101585. [Google Scholar] [CrossRef]
- Winer, R.L.; Kiviat, N.B.; Hughes, J.P.; Adam, D.E.; Lee, S.-K.; Kuypers, J.M.; Koutsky, L.A. Development and Duration of Human Papillomavirus Lesions, after Initial Infection. J. Infect. Dis. 2005, 191, 731–738. [Google Scholar] [CrossRef]
- Zielinski, G.D.; Snijders, P.J.; Rozendaal, L.; Voorhorst, F.J.; van der Linden, H.C.; Runsink, A.P.; de Schipper, F.A.; Meijer, C.J. HPV Presence Precedes Abnormal Cytology in Women Developing Cervical Cancer and Signals False Negative Smears. Br. J. Cancer 2001, 85, 398–404. [Google Scholar] [CrossRef]
- Deshmukh, A.A.; Suk, R.; Shiels, M.S.; Sonawane, K.; Nyitray, A.G.; Liu, Y.; Gaisa, M.M.; Palefsky, J.M.; Sigel, K. Recent Trends in Squamous Cell Carcinoma of the Anus Incidence and Mortality in the United States, 2001-2015. J. Natl. Cancer Inst. 2020, 112, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Grulich, A.E.; Poynten, I.M.; Machalek, D.A.; Jin, F.; Templeton, D.J.; Hillman, R.J. The Epidemiology of Anal Cancer. Sex. Health 2012, 9, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Stier, E.A.; Goldstone, S.E.; Einstein, M.H.; Jay, N.; BERRY, J.M.; Wilkin, T.; LEE, J.Y.; DARRAGH, T.M.; DA COSTA, M.; PANTHER, L.; et al. Safety and Efficacy of Topical Cidofovir to Treat High-Grade Perianal and Vulvar Intraepithelial Neoplasia in HIV-Positive Men and Women. AIDS Lond. Engl. 2013, 27, 545–551. [Google Scholar] [CrossRef]
- Tranoulis, A.; Laios, A.; Mitsopoulos, V.; Lutchman-Singh, K.; Thomakos, N. Efficacy of 5% Imiquimod for the Treatment of Vaginal Intraepithelial Neoplasia-A Systematic Review of the Literature and a Meta-Analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 218, 129–136. [Google Scholar] [CrossRef]
- Palefsky, J.M.; Lee, J.Y.; Jay, N.; Goldstone, S.E.; Darragh, T.M.; Dunlevy, H.A.; Rosa-Cunha, I.; Arons, A.; Pugliese, J.C.; Vena, D.; et al. Treatment of Anal High-Grade Squamous Intraepithelial Lesions to Prevent Anal Cancer. N. Engl. J. Med. 2022, 386, 2273–2282. [Google Scholar] [CrossRef]
- Available online: https://www.cdc.gov/nceh/cancer-environment/pdfs/standardized-incidence-ratio-fact-sheet-508.pdf (accessed on 10 May 2023).
- Cervical Cancer — Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/cervix.html (accessed on 11 May 2023).
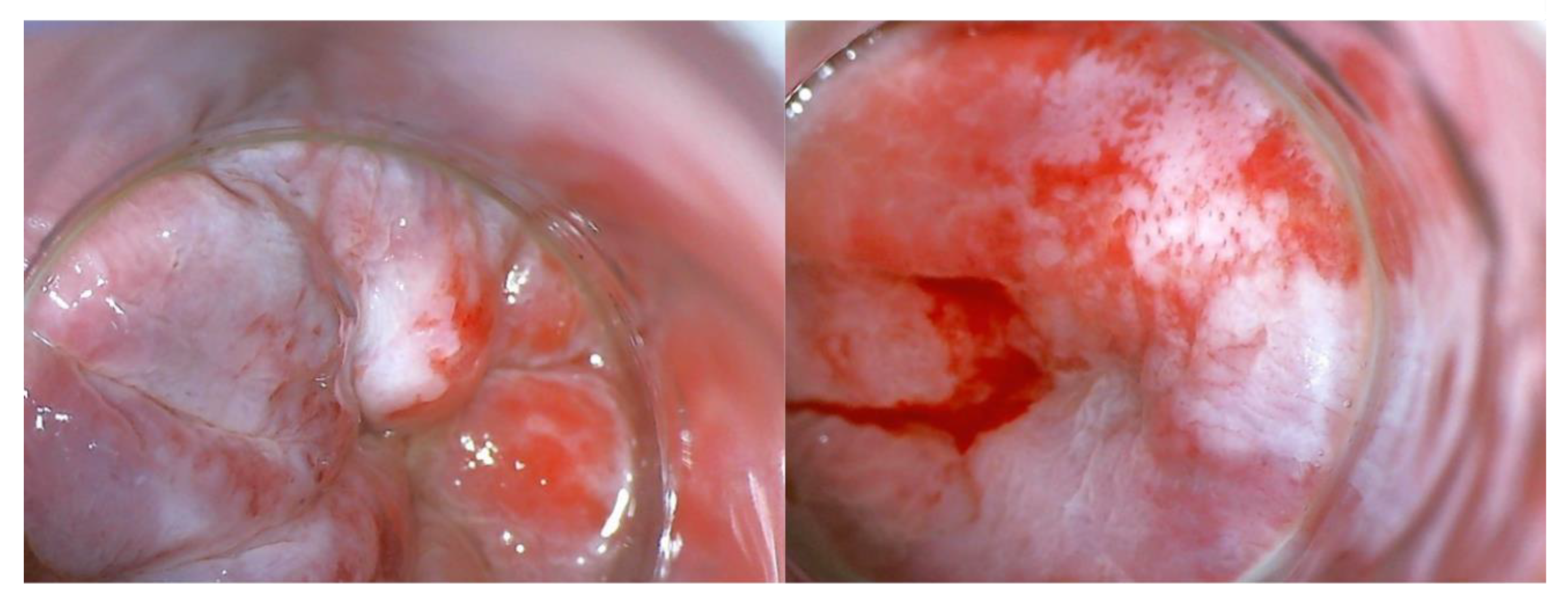
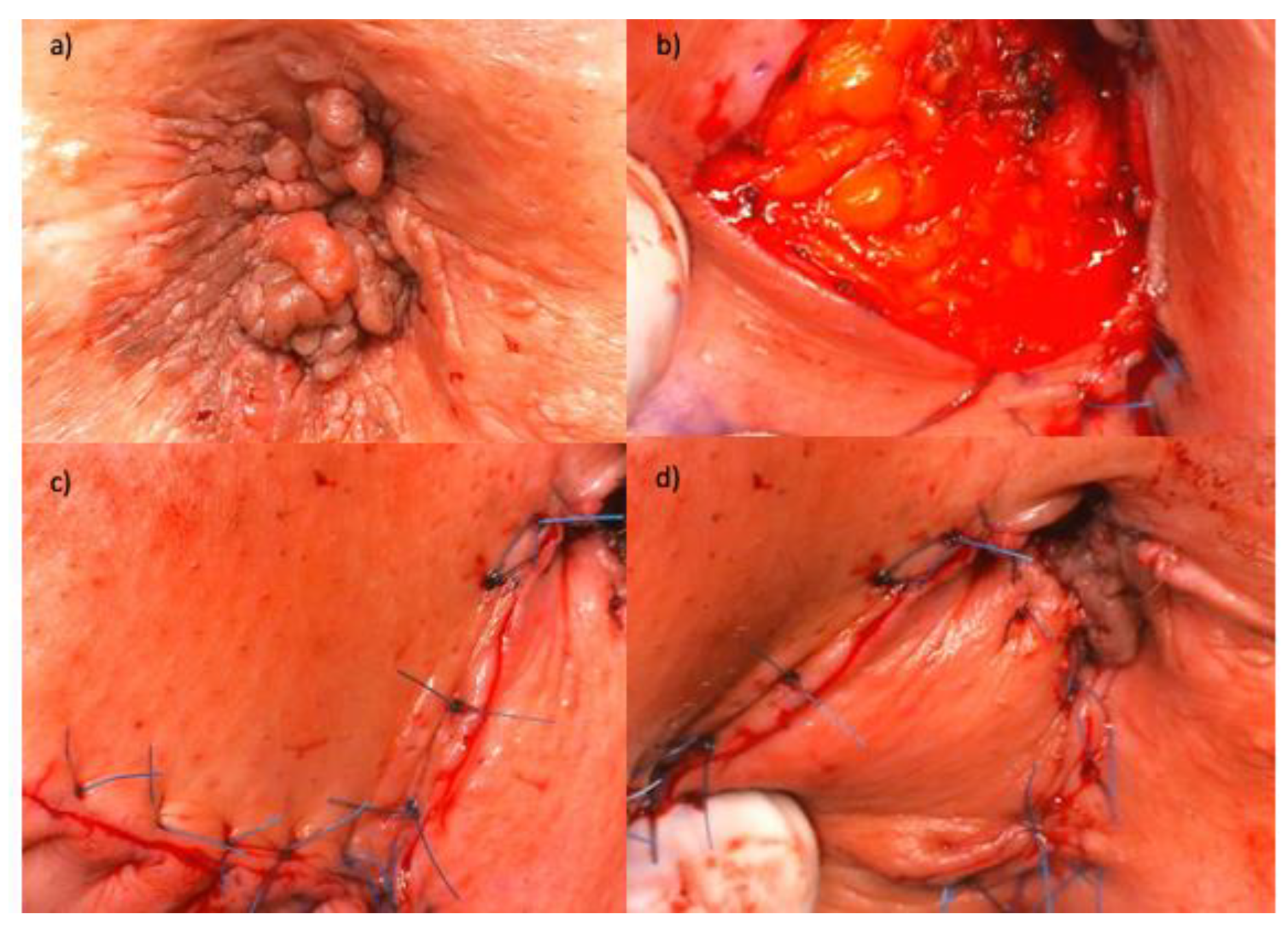
| No. | Authors and year of publication | Number of secondary AIN or AC / number of primary HPV-RGD | Person-years | Incidence rate of AIN or AC | IR per 100,000 person-years | Comment |
|---|---|---|---|---|---|---|
| 1. | Acevedo-Fontánez et al. (2018) [17] | 10 AC / 8,039 CC 3 AC / 1,378 VC 1 AC / 773 VaC |
119,617 14,631 6,554 |
0.124% 0.217% 0.129% |
8.36 20.5 15.26 |
|
| 2. | Chaturvedi et al. (2007) [18] | - | - | - | - | No information about the number of AC cases. |
| 3. | Ebisch et al. (2017) [19] | 73 AC / 89,018 CIN 3 80 AIN 3 / 89,018 CIN 3 |
1,261,804 1,261,804 |
0.082% 0.090% |
5.79 6.34 |
|
| 4. | Edgren et al. (2007) [20] | 131 AC / 125,292 CIN 3 | 2 193 409 | 0.105% | 5.97 | |
| 5. | ElNaggar et al. (2013) [21] | 13 AIN / 90 CIN 48 AIN / 132 VIN 10 AIN / 55 VaIN |
- - - |
14.4% 36.4% 18.2% |
- - - |
1 AIN out of 3 CC but not included because of to small number of cases |
| 6. | Evans et al. (2003) [22] | 23 AC / 59,519 CIN 3 18 AC / 21,605 CC |
477,069 145,621 |
0.039% 0.083% |
4.82 12.36 |
|
| 7. | Gaudet et al. (2014) [23] | 20 AC / 54,320 CIN 2 and CIN 3 | 545,945 | 0.037% | 3.66 | |
| 8. | Hemminki et al. (2001) [24] | 16 AC / 17,234 CC | - | 0.093% | - | |
| 9. | Hemminki et al. (2000) [25] | 68 AC / 117,830 CIN 3 17 AC / 17,556 CC |
- | 0.058% 0.097% |
- | |
| 10. | Heráclio et al. (2018) [26] | 14 AIN / 200 CIN 1 23 AIN / 124 CIN 2 and 3 |
- | 7% 18.5% |
- | |
| 11. | Jakobsson et al. (2011) [27] | 3 AC / 26,876 CIN | 226,510 | 0.011% | 1.32 | |
| 12. | Jiménez et al. (2009) [28] | - | - | - | - | No information about the total number of CC, VC or VaC cases. |
| 13. | Kalliala et al. (2005) [29] | 3 AC / 7,564 CIN | 97,556 | 0.040% | 3.08 | |
| 14. | Matsuo et al. (2018) [30] | 49 AC / 79,050 CC | - | 0.062% | - | |
| 15. | Neumann et al. (2016) [31] | 3 AC / 4,234 CC 1 AC / 339 VC |
28,122 1,533 |
0.071% 0.295% |
10.67 65,23 |
|
| 16. | Pan et al. (2019) [32] | 37 AC / 69,714 CIN 3 | 893,622 | 0.053% | 4.14 | |
| 17. | Papatla et al. (2019) [33] | 17 AC / 21,060 CC | - | 0.081% | - | |
| 18. | Preti et al. (2020) [34] | 1 AC / 3,184 CIN 2 and 3 | 20,022 | 0.031% | 4.99 | |
| 19. | Rabkin et al. (1992) [35] | 12 AC / 25,295 CC | 156,838 | 0.047% | 7.65 | |
| 20. | Saleem et al. (2011) [36] | 137 AC / 124,075 CIN 3 28 AC / 43,669 CC 5 AC / 1,463 VaIN 3 2 AC / 3,257 VaC 55 AC / 6,792 VIN 3 28 AC / 9,950 VC |
- | 0.110% 0.064% 0.342% 0.061% 0.810% 0.281% |
- | |
| 21. | Sand et al. (2016) [37] | 32 AC / 52,135 CIN 2 125 AC / 104,155 CIN 3 |
597,467 1,529,564 |
0.061% 0.120% |
5.36 8.17 |
|
| 22. | Suk et al. (2018) [38] | 34 AC / 44,011 CC 31 AC / 6,905 VC 1 AC / 1,673 VaC |
473,820 48,373 9,057 |
0.077% 0.449% 0.060% |
7.18 64.09 11.04 |
|
| 23. | Tatti et al. (2012) [39] | 20 AIN / 114 CIN 2 and 3 35 AIN / 121 CIN 1 18 AIN / 39 VIN 7 AIN / 22 VaIN 2 and 3 27 AIN / 70 VaIN 1 |
- | 17.544% 28.926% 46.154% 31.818% 38.571% |
- | Results without dividing AIN into HSIL (AIN 2/3) and LSIL (AIN 1). |
| 24. | Tomassi et al. (2018) [40] | 1 AC / 1,168 CC 14 AC / 109,893 CIN 1 5 AC / 15,711 CIN 2 and 3 |
10,359 708,690 114,031 |
0.086% 0.013% 0.032% |
9.65 12.74 4.38 |
|
| 25. | Wang et al. (2020) [41] | 50 AC / 46,550 CC 9 AC / 7,855 VC 1 AC / 1,722 VaC |
- - - |
0.107% 0.115% 0.058% |
7.6 2.1 8.3 |
No information about PY. IR per 100,000 PY as provided by the authors of the publication. |
| Type of HPV-related gynecological disease | Risk of AC mean SIR (range)1 | Risk of AC mean IR2 | Risk of AC mean IR per 100,000 PY3 | Risk of AIN mean SIR (range)1 | Risk of AIN mean IR2 | Risk of AIN mean IR per 100,000 PY3 |
|---|---|---|---|---|---|---|
| Cervical cancer | 3.6 (1.6-6.3) | 0.084% | 9.73 | |||
| Vulvar cancer | 10.8 (1.1-17.4) | 0.286% | 37.98 | |||
| Vaginal cancer | 1.6 (0.6-2.3) | 0.059% | 11.78 | |||
| CIN 3 | 5.6 (2.3-16.4) | 0.075% | 5.78 | 6.7 (3.64- 12.25) | 6.34 | |
| CIN(1-3) | 4.6 (0.9-16.4) | 0.030% | 5.37 | 16.45% | ||
| VIN 3 | 0.810% | |||||
| VIN(1-3) | 36.4% | |||||
| VaIN 3 | 0.342% | |||||
| VaIN(1-3) | 18.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





