Submitted:
15 May 2023
Posted:
15 May 2023
You are already at the latest version
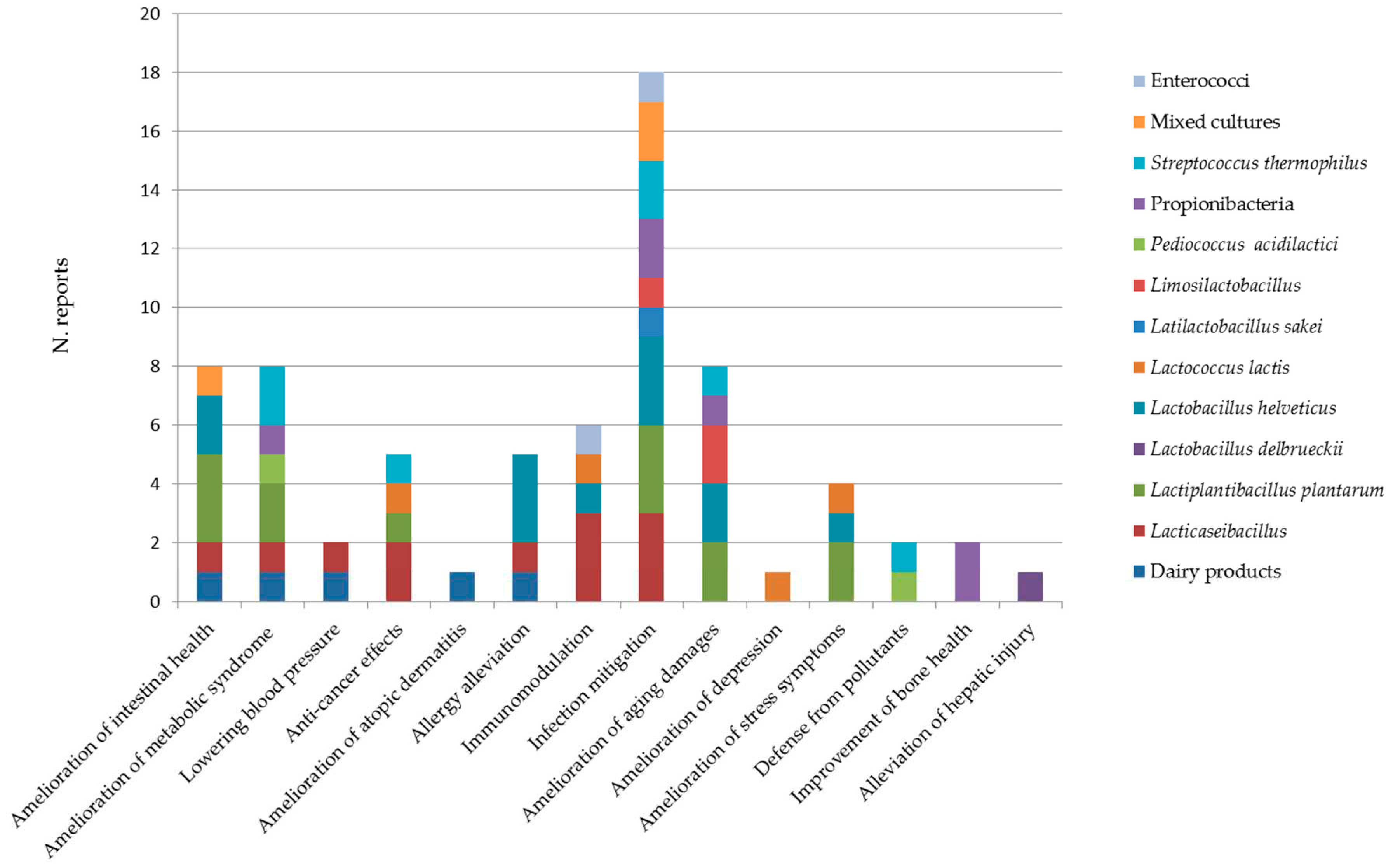
Abstract
Keywords:
1. Introduction
2. Fermented dairy products
2.1. Amelioration of intestinal health
2.2. Amelioration of atopic dermatitis
2.3. Allergy amelioration
2.4. Amelioration of metabolic syndrome
2.5. Antihypertensive effects
3. Lacticaseibacillus
3.1. Improvement of intestinal health
3.2. Anti-cancer effects
3.3. Amelioration of metabolic syndrome
3.4. Immunostimulation
3.5. Infection mitigation
3.6. Allergy alleviation
4. Lactiplanibacillus plantarum
4.1. Amelioration of intestinal health
4.2. Amelioration of metabolic syndrome
4.3. Anti-cancer effects
4.4. Infection mitigation
4.5. Amelioration of aging damages
4.6. Improvement of stress symptoms
5. Lactobacillus delbrueckii subsp. bulgaricus and lactis
5.1. Alleviation of hepatic injury
6. Lactobacillus helveticus
6.1. Amelioration of intestinal health
6.2. Immunomodulation
6.3. Allergy alleviation
6.4. Infection mitigation
6.5. Alleviation of aging damages
6.6. Improvement of stress symptoms
6.7. Benefits in animal production
7. Lactococcus lactis
7.1. Anti-cancer effects
7.2. Immunomodulation
7.3. Improvement of stress symtoms
7.4. Improvement of depressive behaviour
8. Latilactobacillus sakei
8.1. Infection mitigation
9. Limosilactobacillus
9.1. Alleviation of aging damages
9.2. Infection mitigation
10. Pediococcus acidilactici
10.1. Amelioration of metabolic syndrome
10.2. Defense from pollutants
11. Propionibacteria
11.1. Amelioration of metabolic syndrome
11.2. Alleviation of aging damages
11.3. Infection mitigation
11.4. Improvement of bone health
12. Streptococcus thermophilus
12.1. Amelioration of metabolic syndrome
12.2. Anti-cancer effects
12.3. Infection mitigation
12.4. Alleviation of aging damage
12.5. Defense from pollutants
13. Mixed cultures
13.1. Amelioration of intestinal health
13.2. Infection alleviation
14. Enterococci as controversial probiotics
14.1. Immunomodulation
14.2. Infection mitigation
15. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Marco, M.L.; Sanders, M.E.; Gänzle, M.; Arrieta, M.C.; Cotter, P.D.; De Vuyst, L.; Hill, C.; Holzapfel, W.; Lebeer, S.; Merenstein, D.; Reid, G.; Wolfe, B.E.; Hutkins, R. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on fermented foods. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Food and Agricultural Organization. Probiotics in food. Health and nutritional properties and guidelines for evaluation. Available online: https://www.fao.org/3/a0512e/a0512e.pdf (accessed on 24 April 2023).
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli. L.; Canani, R.B.; Flint, H.J.; Salminen, S.; Calder, P.C.; Sanders, M.E. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014, 8, 506–514.
- Illikoud, N.; Mantel, M.; Rolli-Derkinderen, M.; Gagnaire, V.; Jan, G. Dairy starters and fermented dairy products modulate gut mucosal immunity. Immunol. Lett. 2022, 251–252, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Darwish, A.M.G.; Allam, M.G.; Shokery, E.S.; Ayad, E.H.E. Functional products fortified with probiotic LAB isolated from Egyptian dairy sources showed hypolipidemic effects in Albino rats. PLoS One 2022, 17, e0263241. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. EFSA statement on the requirements for whole genome sequence analysis of microorganisms intentionally used in the food chain. EFSA J. 2021, 19, 6506. [Google Scholar]
- Amadoro, C.; Rossi, F.; Pallotta, M.L.; Gasperi, M.; Colavita, G. Traditional dairy products can supply beneficial microorganisms able to survive in the gastrointestinal tract. LWT 2018, 93, 376–383. [Google Scholar] [CrossRef]
- Milani, C.; Duranti, S.; Napoli, S.; Alessandri, G.; Mancabelli, L.; Anzalone, R.; Longhi, G.; Viappiani, A.; Mangifesta, M.; Lugli, G.A.; Bernasconi, S.; Ossiprandi, M.C.; van Sinderen, D.; Ventura, M.; Turroni, F. Colonization of the human gut by bovine bacteria present in Parmesan cheese. Nat Commun. 2019, 10, 1286. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Honme, Y.; Sashihara, T. Lactobacillus delbrueckii subsp. bulgaricus 2038 and Streptococcus thermophilus 1131 Induce the Expression of the REG3 Family in the Small Intestine of Mice via the Stimulation of Dendritic Cells and Type 3 Innate Lymphoid Cells. Nutrients 2019, 11, 2998. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, K.; Kim, W. Cream Cheese-Derived Lactococcus chungangensis CAU 28 Modulates the Gut Microbiota and Alleviates Atopic Dermatitis in BALB/c Mice. Sci Rep. 2019, 9, 446, Erratum in: Sci Rep. 2019, 9, 7001. [Google Scholar] [CrossRef]
- Zhao, L.; Shi, F.; Xie, Q.; Zhang, Y.; Evivie, S.E.; Li, X.; Liang, S.; Chen, Q.; Xin, B.; Li, B.; Huo, G. Co-fermented cow milk protein by Lactobacillus helveticus KLDS 1.8701 and Lactobacillus plantarum KLDS 1.0386 attenuates its allergic immune response in Balb/c mice. J Dairy Sci 2022, 105, 7190–7202. [Google Scholar] [CrossRef]
- Makwana, S.; Prajapati, J.B.; Pipaliya, R.; Hati, S. Effects of probiotic fermented milk on management of obesity studied in high-fat-diet induced obese rat model. Food Prod Process and Nutr 2023, 5, 3. [Google Scholar] [CrossRef]
- Glazunova, O.A.; Moiseenko, K.V.; Savinova, O.S.; Fedorova, T.V. In Vitro and In Vivo Antihypertensive Effect of Milk Fermented with Different Strains of Common Starter Lactic Acid Bacteria. Nutrients 2022, 14, 5357. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Amadoro, C.; Pallotta, M.L.; Colavita, G. Variability of Genetic Characters Associated with Probiotic Functions in Lacticaseibacillus Species. Microorganisms 2022, 10, 1023. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Gatto, V.; Sabattini, G.; Torriani, S. An assessment of factors characterising the microbiology of Grana Trentino cheese, a Grana-type cheese. Int. J. Dairy Techn. 2012, 65, 401–409. [Google Scholar] [CrossRef]
- Del Matto, I.; Rossi, F.; Iannitto, G.; Petrone, D.; Mastrodomenico, M.T.; Alessiani, A.; Sacchini, L.; Amadoro, C.; Tucci, P.; Marino, L. Variability of the microbiota in traditional Caciocavallo, Scamorza and Caciotta cheeses manufactured with raw milk and natural cultures. Int J Dairy Technol. 2021, 74, 564–574. [Google Scholar] [CrossRef]
- Aprea, G.; Alessiani, A.; Rossi, F.; Sacchini, L.; Boni, A.; D’Angelantonio, D.; Scattolini, S.; Sperandii, A.F.; Centorotola, G.; Neri, D.; Pomilio, F.; Di Giannatale, E.; Del Matto, I.; Tucci, P.; Migliorati, G. Characterization of Lactic Acid Bacteria in Pecorino di Farindola Cheese and Manufacturing with a Lacticaseibacillus paracasei Autochthonous Culture. Appl. Sci. 2021, 11, 7897. [Google Scholar] [CrossRef]
- Saxami, G.; Ypsilantis, P.; Sidira, M.; Simopoulos, C.; Kourkoutas, Y.; Galanis, A. Distinct adhesion of probiotic strain Lactobacillus casei ATCC 393 to rat intestinal mucosa. Anaerobe 2012, 18, 417–420. [Google Scholar] [CrossRef]
- Bengoa, A.A.; Errea, A.J.; Rumbo, M.; Abraham, A.G.; Garrote, G.L. Modulatory properties of Lactobacillus paracasei fermented milks on gastric inflammatory conditions. Int. Dairy J. 2020, 111, 104839. [Google Scholar] [CrossRef]
- Aindelis, G.; Tiptiri-Kourpeti, A.; Lampri, E.; Spyridopoulou, K.; Lamprianidou, E.; Kotsianidis, I.; Ypsilantis, P.; Pappa, A.; Chlichlia, K. Immune Responses Raised in an Experimental Colon Carcinoma Model Following Oral Administration of Lactobacillus casei. Cancers (Basel) 2020, 12, 368. [Google Scholar] [CrossRef]
- Spyridopoulou, K.; Tryfonopoulou, E.; Aindelis, G.; Ypsilantis, P.; Sarafidis, C.; Kalogirou, O.; Chlichlia, K. Biogenic selenium nanoparticles produced by Lactobacillus casei ATCC 393 inhibit colon cancer cell growth in vitro and in vivo. Nanoscale Adv. 2021, 3, 2516–2528. [Google Scholar] [CrossRef]
- Le Barz, M.; Daniel, N.; Varin, TV.; Naimi, S.; Demers-Mathieu, V.; Pilon, G.; Audy, J.; Laurin, É.; Roy, D.; Urdaci, M.C.; St-Gelais, D.; Fliss, I.; Marette, A. In vivo screening of multiple bacterial strains identifies Lactobacillus rhamnosus Lb102 and Bifidobacterium animalis ssp. lactis Bf141 as probiotics that improve metabolic disorders in a mouse model of obesity. FASEB J. 2019, 33, 4921–4935. [Google Scholar]
- Chondrou, P.; Karapetsas, A.; Kiousi, D.E.; Vasileiadis, S.; Ypsilantis, P.; Botaitis, S.; Alexopoulos, A.; Plessas, S.; Bezirtzoglou, E.; Galanis, A. Assessment of the Immunomodulatory Properties of the Probiotic Strain Lactobacillus paracasei K5 In Vitro and In Vivo. Microorganisms 2020, 8, 709. [Google Scholar] [CrossRef] [PubMed]
- Karaffová, V.; Mudroňová, D.; Mad’Ar, M.; Hrčková, G.; Faixová, D.; Gancarčíková, S.; Ševčíková, Z.; Nemcová, R. Differences in Immune Response and Biochemical Parameters of Mice Fed by Kefir Milk and Lacticaseibacillus paracasei Isolated from the Kefir Grains. Microorganisms 2021, 9, 831. [Google Scholar] [CrossRef] [PubMed]
- Valente, G.L.C, Acurcio, L.B, Freitas, L.P.V, Nicoli, J.R, Silva, A.M, Souza, M.R, Penna, C.F.A.M. Short communication: In vitro and in vivo probiotic potential of Lactobacillus plantarum B7 and Lactobacillus rhamnosus D1 isolated from Minas artisanal cheese. J Dairy Sci. 2019, 102, 5957–5961.
- Wang, G.; Zhang, Y.; Song, X.; Xia, Y.; Lai, P.F.; Ai, L. Lactobacillus casei LC2W can inhibit the colonization of Escherichia coli O157:H7 in vivo and reduce the severity of colitis. Food Funct. 2019, 10, 5843–5852. [Google Scholar] [CrossRef]
- Zhang, W.; Yu, D.; Sun, Z.; Wu, R.; WChenu, X.; Chen, W.; Meng, H.; Hu, S.; Zhang, H. Complete genome sequence of Lactobacillus casei Zhang, a new probiotic strain isolated from traditional homemade koumiss in Inner Mongolia, China. J Bacteriol. 2010, 192, 5268–5269. [Google Scholar] [CrossRef] [PubMed]
- Kwok, L.Y.; Wang, L.; Zhang, J.; Guo, Z.; Zhang, H. A pilot study on the effect of Lactobacillus casei Zhang on intestinal microbiota parameters in Chinese subjects of different age. Benef. Microbes. 2014, 5, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Liu, G.; Wang, W.; Wang, Y.; Cao, Z.; Yang, H.; Li, S. Lactobacillus casei Zhang Counteracts Blood-Milk Barrier Disruption and Moderates the Inflammatory Response in Escherichia coli-Induced Mastitis. Front Microbiol. 2021, 12. [Google Scholar] [CrossRef]
- Fu, L.; Xie, M.; Wang, C.; Qian, Y.; Huang, J.; Sun, Z.; Zhang, H.; Wang, Y. Lactobacillus Casei Zhang Alleviates Shrimp Tropomyosin-Induced Food Allergy by Switching Antibody Isotypes through the NF-κB-Dependent Immune Tolerance. Mol Nutr Food Res 2020, 64, e1900496. [Google Scholar] [CrossRef]
- Zago, M.; Fornasari, M.E.; Carminati, D.; Burns, P.; Suàrez, V.; Vinderola, G.; Reinheimer, J.; Giraffa, G. Characterization and probiotic potential of Lactobacillus plantarum strains isolated from cheeses. Food Microbiol. 2011, 28, 1033–1040. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Zhang, L.; Zhao, Y.; Niu, C.; Yang, Z.; Li, S. Potential probiotic characterization of Lactobacillus plantarum strains isolated from Inner Mongolia "Hurood" cheese. J Microbiol Biotechnol. 2014, 24, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Ismael, M.; Gu, Y.; Cui, Y.; Wang, T.; Yue, F.; Qin, Y.; Lü, X. Probiotic of Lactiplantibacillus plantarum NWAFU-BIO-BS29 Isolated from Chinese Traditional Fermented Milk and Its Potential Therapeutic Applications Based on Gut Microbiota Regulation. Foods 2022, 11, 3766. [Google Scholar] [CrossRef] [PubMed]
- Nami, Y.; Bakhshayesh, R.V.; Manafi, M.; Hejazi, M.A. Hypocholesterolaemic activity of a novel autochthonous potential probiotic Lactobacillus plantarum YS5 isolated from yogurt. LWT, 2019, 111, 876–882. [Google Scholar] [CrossRef]
- Zambou, N.F.; Barry, R.B.; Bindzi, J.M.; Kenfack, C.H.M.; Bemmo, U.L.K. Viability and in vivo Hypocholesterolemic Effect of Lactobacillus plantarum 29V in Local Honey. J Adv Biol Biotechnol. 2021, 24, 24–33. [Google Scholar]
- Chouikhi, A.; Ktari, N.; Bardaa, S.; Hzami, A.; Ben Slima, S.; Trabelsi, I.; Asehraou, A.; Ben Salah, R. A novel probiotic strain, Lactiplantibacillus plantarum LC38, isolated from Tunisian camel milk promoting wound healing in Wistar diabetic rats. Arch Microbiol. 2021, 204, 24. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, S.; Cui, J.; Guo, T.; Zhang, J. Effect of bile salt hydrolase-active Lactobacillus plantarum Y15 on high cholesterol diet induced hypercholesterolemic mice. CyTA - Journal of Food 2021, 19, 408–417. [Google Scholar] [CrossRef]
- Hu, J.; Wang, C.; Ye, L.; Yang, W.; Huang, H.; Meng, F.; Shi, S.; Ding, Z. Anti-tumour immune effect of oral administration of Lactobacillus plantarum to CT26 tumour-bearing mice. J. Biosci. 2015, 40, 269–279. [Google Scholar] [CrossRef]
- Fayol-Messaoudi, D.; Coconnier-Polter, M.H.; Moal, V.L. .; Atassi, F..; Berger, C.N.; Servin, A.L. The Lactobacillus plantarum strain ACA-DC287 isolated from a Greek cheese demonstrates antagonistic activity in vitro and in vivo against Salmonella enterica serovar Typhimurium. J Appl Microbiol. 2007, 103, 657–665. [Google Scholar] [CrossRef]
- Acurcio, L.B.; Bastos, R.W.; de Cicco Sandes, S.H.; de Carvalho Guimarães, A.C..; Alves, C.G.; dos Reis, D.C.; Wuyts, S.; Cantini Nunes, Á.; Cassali, G.D.; Lebeer, S.; Resende de Souza, M.; Nicoli, J.R. Protective effects of milk fermented by Lactobacillus plantarum B7 from Brazilian artisanal cheese on a Salmonella enterica serovar Typhimurium infection in BALB/c mice. J. Funct. Foods 2017, 33, 436–445.
- Chong, H.X.; Yusoff, N.A.A.; Hor, Y.Y.; Lew, L.C.; Jaafar, M.H.; Choi, S.B.; Yusoff, M.S.B.; Wahid, N.; Abdullah, M.F.I.L.; Zakaria, N.; Ong, K.L.; Park, Y.H.; Liong, M.T. Lactobacillus plantarum DR7 improved upper respiratory tract infections via enhancing immune and inflammatory parameters: A randomized, double-blind, placebo-controlled study. J Dairy Sci. 2019, 102, 4783–4797. [Google Scholar] [CrossRef]
- Zhao, X.; Yi, R.; Zhou, X.; Mu, J.; Long, X.; Pan, Y.; Song, J.L.; Park, K.Y. Preventive effect of Lactobacillus plantarum KSFY02 isolated from naturally fermented yogurt from Xinjiang, China, on D-galactose-induced oxidative aging in mice. J Dairy Sci. 2019, 102, 5899–5912. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, X.; Jiang, Y.;, Zhao, W.; Guo, T.; Cao ,Y.; Teng, J.; Hao, X.; Zhao, J.; Yang, Z. Antioxidant status and gut microbiota change in an aging mouse model as influenced by exopolysaccharide produced by Lactobacillus plantarum YW11 isolated from Tibetan kefir. J Dairy Sci. 2017, 100, 6025–6041. [CrossRef] [PubMed]
- Liu, G.; Chong, H.X.; Chung, F.Y.; Li, Y.; Liong, M.T. Lactobacillus plantarum DR7 Modulated Bowel Movement and Gut Microbiota Associated with Dopamine and Serotonin Pathways in Stressed Adults. Int J Mol Sci. 2020, 21, 4608. [Google Scholar] [CrossRef] [PubMed]
- Chong, H.X.; Yusoff, N.A.A.; Hor, Y.Y.; Lew, L.C.; Jaafar, M.H.; Choi, S.B.; Yusoff, M.S.B.; Wahid, N.; Abdullah, M.F.I.L.; Zakaria, N.; Ong, K.L.; Park, Y.H.; Liong, M.T. Lactobacillus plantarum DR7 alleviates stress and anxiety in adults: a randomised, double-blind, placebo-controlled study. Benef Microbes 2019, 10, 355–373. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, M.; Yang, S.; Shen, C.; Wang, X.; Liu, W.; Guo, Y. Fermented milk of cheese-derived Lactobacillus delbrueckii subsp. bulgaricus displays potentials in alleviating alcohol-induced hepatic injury and gut dysbiosis in mice. Food Res Int. 2022, 157. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, S.; Cui, J.; Guo, T.; Zhang, J.; Li, B. Alleviative Effects of Exopolysaccharide Produced by Lactobacillus helveticus KLDS1.8701 on Dextran Sulfate Sodium-Induced Colitis in Mice. Microorganisms 2021, 9, 2086. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.W.; El-Nezami, H.; Corke, H.; Ho, C.S.; Shah, N.P. L-citrulline enriched fermented milk with Lactobacillus helveticus attenuates dextran sulfate sodium (DSS) induced colitis in mice. J Nutr Biochem. 2022, 99. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, S.; Chen, L.; Chen, C.; Ren, X.; Zheng, Z.; Weng, L.; Ge, H.; Wang, J.; Liu, G.; Ye, X. Immunomodulatory effects of L. helveticus WHH2580 fermented milk on an immunosuppressed murine model. J Funct Foods, 2022, 99, 105353. [Google Scholar] [CrossRef]
- Makino, T.; Yamashita, M.; Takeuchi, N.; Kabuki, T.; Hattori, M.; Yoshida, T. Lactobacillus helveticus SBT2171 alleviates allergic symptoms in a murine model for pollen allergy. Biosci Biotechnol Biochem. 2019, 83, 2298–2306. [Google Scholar] [CrossRef]
- Yamashita, M.; Kobatake, E.; Obuchi, S.; Iwai, M.; Ichikawa, K.; Kabuki, T.; Enomoto, T. Intake safety of Lactobacillus helveticus SBT2171 and its effects on nasal and ocular symptoms associated with mites and house dust: An open-label study. Functional Foods in Health and Disease 2019, 9, 52–78. [Google Scholar] [CrossRef]
- Yamashita, M.; Miyoshi, M.; Iwai, M.; Takeda, R.; Ono, T.; Kabuki, T. Lactobacillus helveticus SBT2171 Alleviates Perennial Allergic Rhinitis in Japanese Adults by Suppressing Eosinophils: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2020, 12, 3620. [Google Scholar] [CrossRef] [PubMed]
- Kobatake, E.; Kobayashi, R.; Kabuki, T.; Kurita-Ochiai, T. Lactobacillus helveticus SBT2171 upregulates the expression of β-defensin and ameliorates periodontal disease caused by Porphyromonas gingivalis. Microbiol Immunol. 2019, 63, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Shi, R.; Guan, D.; Wu, Y.; Qian, W. Lactobacillus helveticus Prevents Periodontitis Induced by Aggregatibacter actinomycetemcomitans in Rats by Regulating β-Defensins. Comput Math Methods Med 2022, 2022, 4968016. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, M.; Naderi Farsani, M.; Ghafarifarsani, H.; Raeeszadeh, M. Dietary Lactobacillus helveticus and Gum Arabic improves growth indices, digestive enzyme activities, intestinal microbiota, innate immunological parameters, antioxidant capacity, and disease resistance in common carp. Fish Shellfish Immunol. 2023, 135, 108652. [Google Scholar] [CrossRef]
- Li, B.; Evivie, S.E.; Lu, J.; Jiao, Y.; Wang, C.; Li, Z , Liu, F.; Huo, G. Lactobacillus helveticus KLDS1.8701 alleviates d-galactose-induced aging by regulating Nrf-2 and gut microbiota in mice. Food Funct. 2018, 9, 6586–6598. [CrossRef]
- Li, B.; Du, P.; Smith, E.E.; Wang, S.; Jiao, Y.; Guo, L.; Huo, G.; Liu, F. In vitro and in vivo evaluation of an exopolysaccharide produced by Lactobacillus helveticus KLDS1.8701 for the alleviative effect on oxidative stress. Food Funct. 2019, 10, 1707–1717. [Google Scholar] [CrossRef]
- Gao K, Chen CL, Ke XQ, Yu YX, Chen S, Liu GC, Wang HF, Li YJ. Ingestion of Lactobacillus helveticus WHH1889 improves depressive and anxiety symptoms induced by chronic unpredictable mild stress in mice. Benef Microbes. 2022, 13, 473–488. [CrossRef]
- Yang, G.; Cui, X.; Liu, S.; Lu, J.; Hou, X.; Meng, W.; Wu, B.; Su, Y.; Zhang, H.; Zheng, W.; Fang, Y. Effects of dietary Lactobacillus helveticus on the growth rate, disease resistance and intestinal health of pond loach (Misgurnus anguillicaudatus). Aquaculture 2021, 544, 737038. [Google Scholar] [CrossRef]
- Saleena, L.A.K.; Teo, M.Y.M.; How, Y.H.; In, L.L.A.; Pui, L.P. Immunomodulatory action of Lactococcus lactis. Journal of Bioscience and Bioengineering, 2022, 135, 1–9. [Google Scholar] [CrossRef]
- Barcellos Jaskulski, I.; Uecker, J.; Bordini, F.; Moura, F.; Gonçalves, T.; Garcia Chaves, N.; Camargo, F.; Borelli Grecco, F.; Fiorentini, Â.M.; Padilha da Silva, W.; Andreazza, R.; Pieniz, S. In vivo action of Lactococcus lactis subsp. lactis isolate (R7) with probiotic potential in the stabilization of cancer cells in the colorectal epithelium. Process Biochemistry, 2020, 91, 165–171. [Google Scholar] [CrossRef]
- Jin, S.W.; Lee, G.H.; Jang, M.J.; Hong, G.E.; Kim, J.Y.; Park, G.D.; Jin, H.; Kim, H.S.; Choi, J.H.; Choi, C.Y.; Lee, S.G.; Jeong, H.G.; Hwang, Y.P. Immunomodulatory Activity of Lactococcus lactis GCWB1176 in Cyclophosphamide-Induced Immunosuppression Model. Microorganisms 2020, 8, 1175. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Farzi, A.; Ke, X.; Yu, Y.; Chen, C.; Chen, S.; Yu, T.; Wang, H.; Li, Y. Oral administration of Lactococcus lactis WHH2078 alleviates depressive and anxiety symptoms in mice with induced chronic stress. Food Funct. 2022, 13, 957–969. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, J.B.; Soares, M.B.; Spiazzi, C.C.; Bicca, D.F.; Soares, V.M.; Pereira, J.G.; da Silva, W.P.; Sehn, C.P.; Cibin, F.W.S. In Vitro Probiotic and Antioxidant Potential of Lactococcus lactis subsp. cremoris LL95 and Its Effect in Mice Behaviour. Nutrients 2019, 11, 901. [Google Scholar] [CrossRef] [PubMed]
- Toukam, L.L.; Tatsinkou Fossi, B.; Taiwe, G.S.; Bila, R.B.; Feugaing Sofeu.; D.D, Ivo.; E.P, Achidi.; E.A. In vivo antimalarial activity of a probiotic bacterium Lactobacillus sakei isolated from traditionally fermented milk in BALB/c mice infected with Plasmodium berghei ANKA. J Ethnopharmacol 2021, 280, 114448.
- Schifano, E.; Zinno, P.; Guantario, B.; Roselli, M.; Marcoccia, S.; Devirgiliis, C.; Uccelletti, D. The Foodborne Strain Lactobacillus fermentum MBC2 Triggers pept-1-Dependent Pro-Longevity Effects in Caenorhabditis elegans. Microorganisms 2019, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Hor, Y.Y.; Lew, L.C.; Jaafar, M.H.; Lau, A.S.; Ong, J.S.; Kato, T.; Nakanishi, Y.; Azzam, G.; Azlan, A.; Ohno, H.; Liong, M.T. Lactobacillus sp. improved microbiota and metabolite profiles of aging rats. Pharmacol Res. 2019, 146. [Google Scholar] [CrossRef]
- Forooghi Nia, F.; Rahmati, A.; Ariamanesh, M.; Saeidi, J.; Ghasemi, A.; Mohtashami, M. The Anti-Helicobacter pylori effects of Limosilactobacillus reuteri strain 2892 isolated from Camel milk in C57BL/6 mice. World J Microbiol Biotechnol. 2023, 39, 119. [Google Scholar] [CrossRef] [PubMed]
- Al-Emran, H.M.; Moon, J.F.; Miah, M.L.; Meghla, N.S.; Reuben, R.C.; Uddin, M.J.; Ibnat, H.; Sarkar, S.L.; Roy, P.C.; Rahman, M.S.; Alam, A.S.M.R.U.; Islam, O.K.; Jahid, I.K. Genomic analysis and in vivo efficacy of Pediococcus acidilactici as a potential probiotic to prevent hyperglycemia, hypercholesterolemia and gastrointestinal infections. Sci Rep. 2022, 12, 20429. [Google Scholar] [CrossRef]
- Feng, P.; Yang, J.; Zhao, S.; Ling, Z.; Han,.;Wu, Y.; Salama, E-S.; Kakade, A.; Khan, A.; Jin, W.; Zhang, W.; Jeon, B-H.; Fan, J.; Liu, M.; Mamtimin, T.; Liu, P Li, X. Human supplementation with Pediococcus acidilactici GR-1 decreases heavy metals levels through modifying the gut microbiota and metabolome. npj Biofilms and Microbiomes 2022, 8, 1.
- Rossi, F.; Torriani, S.; Dellaglio, F. Identification and clustering of dairy propionibacteria by RAPD-PCR and CGE-REA methods. J Appl Microbiol. 1998, 85, 956–964. [Google Scholar] [CrossRef]
- An, M.; Park, Y.H.; Lim, Y.H. Antiobesity and antidiabetic effects of the dairy bacterium P. freudenreichii MJ2 in high-fat diet-induced obese mice by modulating lipid metabolism. Sci Rep. 2021, 11, 2481. [Google Scholar] [CrossRef] [PubMed]
- Kwon, G.; Lee, J.; Lim, Y.H. Dairy Propionibacterium extends the mean lifespan of Caenorhabditis elegans via activation of the innate immune system. Sci Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Nair, D.V.T.; Vazhakkattu Thomas, J.; Dewi, G.; Noll, S.; Brannon, J.; Kollanoor Johny, A. Reduction of Multidrug-Resistant Salmonella enterica Serovar Heidelberg Using a Dairy-Originated Probiotic Bacterium, Propionibacterium freudenreichii freudenreichii B3523, in Growing Turkeys. Journal of Applied Poultry Research, 2019, 28, 356–363. [Google Scholar] [CrossRef]
- Nair, D.V.T.; Johnson, T.J.; Noll, S.L.; Kollanoor Johny, A. Effect of supplementation of a dairy-originated probiotic bacterium, Propionibacterium freudenreichii subsp. freudenreichii, on the cecal microbiome of turkeys challenged with multidrug-resistant Salmonella Heidelberg. Poult Sci. 2021, 100, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Yeom, J.; Ma, S.; Lim, Y.-H. Probiotic P. freudenreichii MJ2 Enhances Osteoblast Differentiation and Mineralization by Increasing the OPG/RANKL Ratio. Microorganisms 2021, 9, 673. [Google Scholar] [CrossRef] [PubMed]
- Yeom, J.; Yim, D.J.; Ma, S.; Lim, Y.-H. Propionibacterium freudenreichii Inhibits RANKL-Induced Osteoclast Differentiation and Ameliorates Rheumatoid Arthritis in Collagen-Induced Arthritis Mice. Microorganisms 2022, 10, 48. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Liang, H.; Luo, Y.; Li, Z.; He, F.; Han, X.; Zhang, L. Anti-adipogenesis and metabolism-regulating effects of heat-inactivated Streptococcus thermophilus MN-ZLW-002. Lett Appl Microbiol, 2021, 72, 677–687. [Google Scholar] [CrossRef]
- WHO. Cardiovascular Diseases. 2020. Available online: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1 (accessed on 30 April 2023).
- Ishimwe, N.; Daliri, E.B.; Lee, B.H.; Fang, F.; Du, G. The perspective on cholesterol-lowering mechanisms of probiotics. Mol. Nutr. Food Res. 2015, 59, 94–105. [Google Scholar] [CrossRef]
- WHO. Diet, nutrition and the prevention of chronic diseases. World Health Organ. Tech. Rep. Ser. 2003, 916, 1–149. [Google Scholar]
- Li, Q.; Hu, W.; Liu, W.X.; Zhao, L.Y.; Huang, D.; Liu, X.D.; Chan, H.; Zhang, Y.; Zeng, J.D.; Coker, O.O.; Kang, W.; Ng, S.S.M.; Zhang. L.; Wong, S.H.; Gin, T.; Chan, M.T.V.; Wu, J.L.; Yu, J.; Wu, W.K.K. Streptococcus thermophilus Inhibits Colorectal Tumorigenesis Through Secreting β-Galactosidase. Gastroenterology. 2021, 160, 1179–1193.e14.
- Evivie, S.E.; Ogwu, M.C.; Abdelazez, A.; Bian, X.; Liu, F.; Li, B.; Huo, G. Suppressive effects of Streptococcus thermophilus KLDS 3.1003 on some foodborne pathogens revealed through in vitro, in vivo and genomic insights. Food Funct. 2021, 11, 6573–6587. [Google Scholar] [CrossRef]
- Han, F.; Wu, G.; Zhang, Y.; Zheng, H.; Han, S.; Li, X.; Cai, W.; Liu, J.; Zhang, W.; Zhang, X.; Hu, D. Streptococcus thermophilus Attenuates Inflammation in Septic Mice Mediated by Gut Microbiota. Front Microbiol 2020, 11, 598010. [Google Scholar] [CrossRef] [PubMed]
- Desaka, N.; Ota, C.; Nishikawa, H.; Yasuda, K.; Ishii, N.; Bito, T.; Kishinaga, Y.; Naito, Y.; Higashimura, Y. Streptococcus thermophilus extends lifespan through activation of DAF-16-mediated antioxidant pathway in Caenorhabditis elegans. J Clin Biochem Nutr. 2022, 70, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Jeon, M.G.; Kim, T.R.; Lee, J.Y.; Kim, H.S.; Ji, Y.; Holzapfel, W.H.; Bae, D.; Choi, C.Y.; Hwang, Y.P. Hepatoprotective Effects of Streptococcus thermophilus LM1012 in Mice Exposed to Air Pollutants. J Med Food. 2020, 23, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Wasilewska, E.; Zlotkowska, D.; Wroblewska, B. Yogurt starter cultures of Streptococcus thermophilus and Lactobacillus bulgaricus ameliorate symptoms and modulate the immune response in a mouse model of dextran sulfate sodium-induced colitis. J Dairy Sci. 2019, 102, 37–53. [Google Scholar] [CrossRef]
- Evivie, S.E.; Abdelazez, A.; Li, B.; Bian, X.; Li, W.; Du, J.; Huo, G.; Liu, F. In vitro Organic Acid Production and In Vivo Food Pathogen Suppression by Probiotic S. thermophilus and L. bulgaricus. Front Microbiol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Evivie, S.E.; Abdelazez, A.; Li, B.; Lu, S.; Liu, F.; Huo, G. Lactobacillus delbrueckii subsp. bulgaricus KLDS 1.0207 Exerts Antimicrobial and Cytotoxic Effects in vitro and Improves Blood Biochemical Parameters in vivo Against Notable Foodborne Pathogens. Front Microbiol. 2020, 11. [Google Scholar]
- Aljasir, S.F.; D'Amico, D.J. Probiotic potential of commercial dairy-associated protective cultures: In vitro and in vivo protection against Listeria monocytogenes infection. Food Res Int. 2021, 149. [Google Scholar] [CrossRef]
- Baccouri, O.; Boukerb, A.M.; Farhat, L.B.; Zébré, A.; Zimmermann, K.; Domann, E.; Cambronel, M.; Barreau, M.; Maillot, O.; Rincé, I.; Muller, C.; Marzouki, M.N.; Feuilloley, M.; Abidi, F.; Connil, N. Probiotic Potential and Safety Evaluation of Enterococcus faecalis OB14 and OB15, Isolated From Traditional Tunisian Testouri Cheese and Rigouta, Using Physiological and Genomic Analysis. Front Microbiol 2019, 10, 881. [Google Scholar] [CrossRef]
- Lester, C.H.; Frimodt-Møller, N.; Sørensen, T.L.; Monnet, D.L.; Hammerum, A.M. In vivo transfer of the vanA resistance gene from an Enterococcus faecium isolate of animal origin to an E. faecium isolate of human origin in the intestines of human volunteers. Antimicrob Agents Chemother. 2006, 50, 596–9. [Google Scholar] [CrossRef]
- Apostolakos, I.; Tsigkrimani, M.; Paramithiotis, S.; Mataragas, M. Whole-Genome Sequencing and Comparative Genomic Analysis of Enterococcus spp. Isolated from Dairy Products: Genomic Diversity, Functional Characteristics, and Pathogenic Potential. Appl. Sci. 2022, 12, 9620. [Google Scholar] [CrossRef]
- Baños, A.; Ariza, J.J.; Nuñez, C.; Gil-Martínez, L.; García-López, J.D.; Martínez-Bueno, M.; Valdivia, E. Effects of Enterococcus faecalis UGRA10 and the enterocin AS-48 against the fish pathogen Lactococcus garvieae. Studies in vitro and in vivo. Food Microbiol. 2019, 77, 69–77. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




