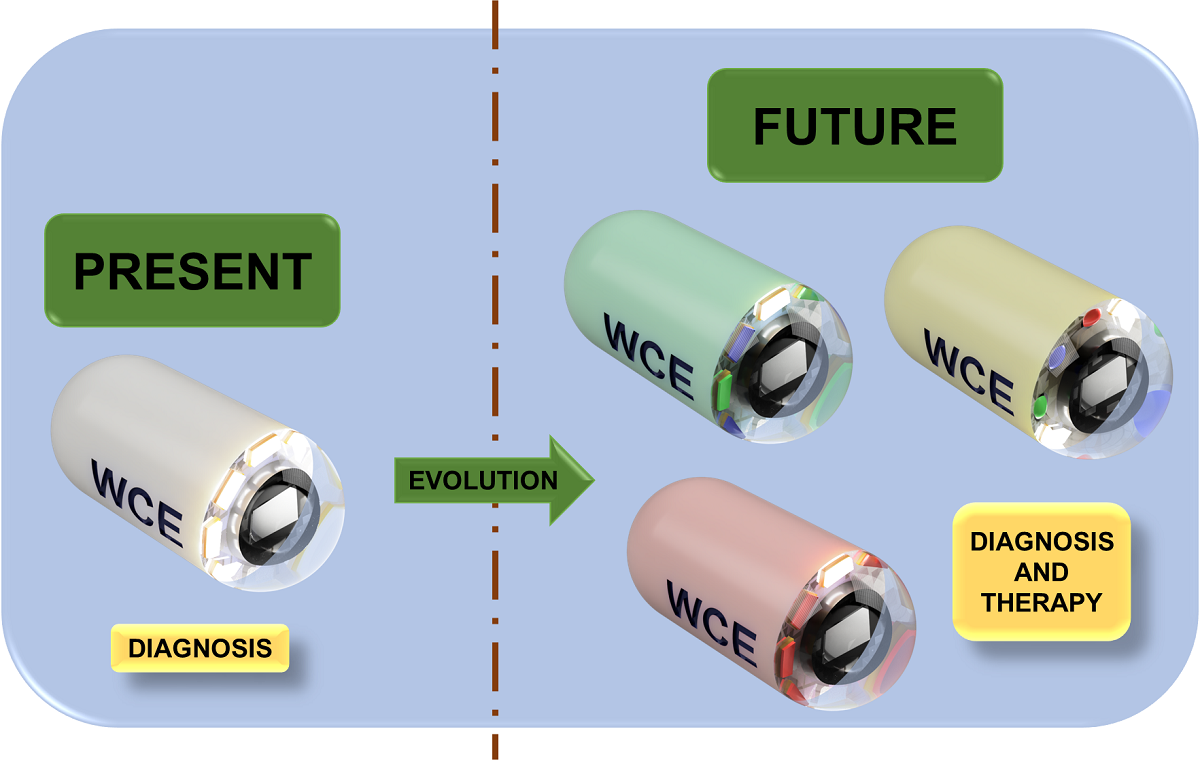
1. Introduction
Since there is no single method to correct identify all types of pathologies of the gastrointestinal tract (some methods are better to detect tumors while others are better to detect bleeding, etc.) we need to combine several techniques in order to achieve the best results using capsule endoscopy technology, so this paper is organized in sections to describe some of these techniques in the start-of-the-art.
1.1. Organization of the Paper
After an introductory section explaining the origins and advent of endoscopic capsules the paper presents the benefits, contraindications and drawbacks of its utilization. Next are presented its use in the main pathologies that affect the gastrointestinal tract, and in the sequence presents some of the commercial devices for capturing images and videos elucidating their technological mechanisms of scientific operation in detail.
1.2. Capsule Endoscopy Origins
Due to the rapid development of medical devices, procedures that were previously invasive and causing discomfort became painless and almost imperceptible to the patients. In this context, so-called “medical images” is intended to provide clinicians with the ability to internally view the human body non-invasively and make the diagnosis more accurate and secure [
1,
2,
3,
4]. To reach this level optical techniques have been very important for researchers and technology developers because it is based in the analyses of light as a result of its interaction with matter, e.g. reflection, transmission, absorption, refraction and diffraction [
5,
6,
7,
8,
9].
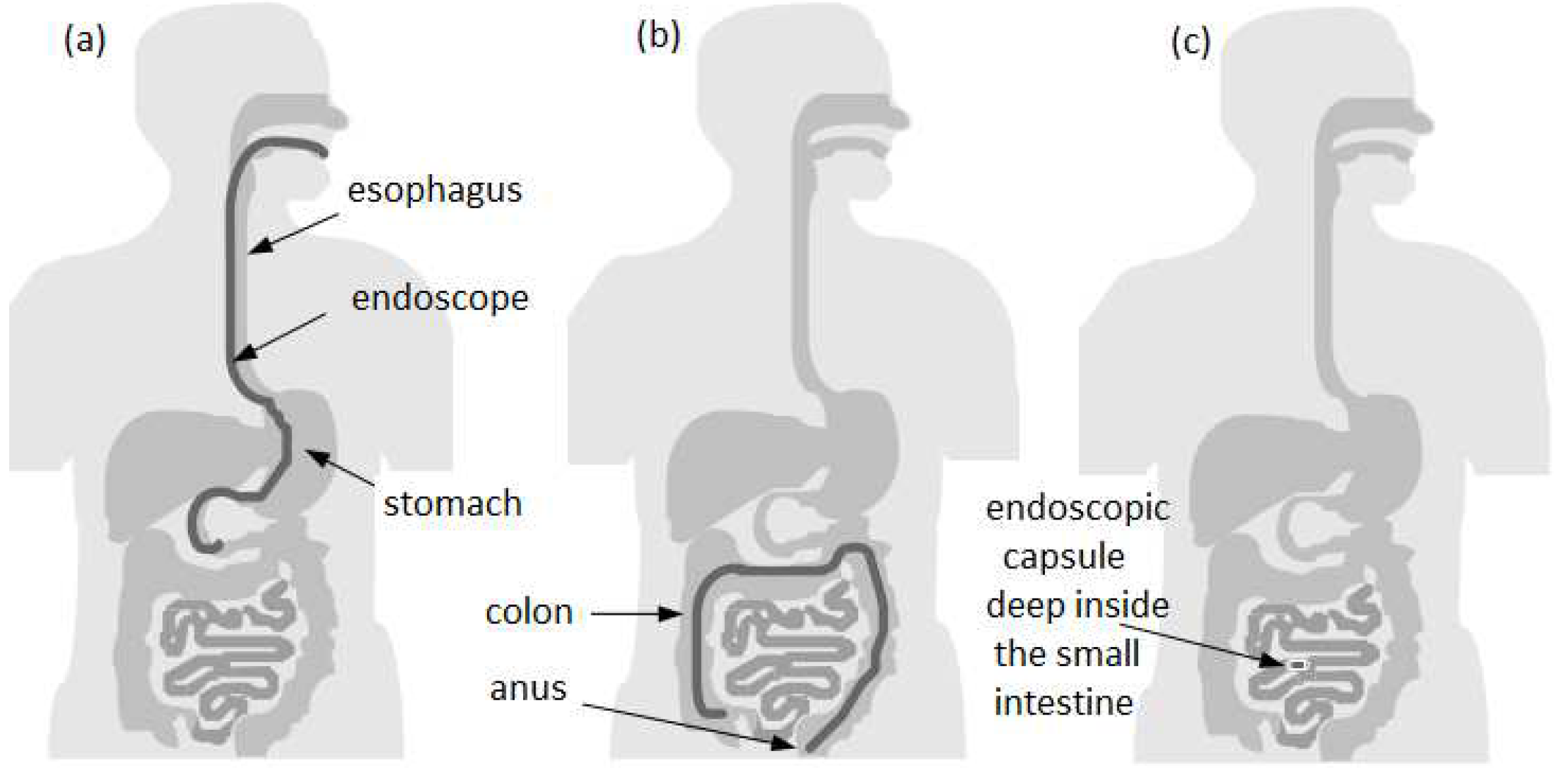
In most cases to perform the observation, diagnosis - and sometimes even treatment of pathologies - in the gastrointestinal (GI) tract, a technique called endoscopy is used. The endoscopy of the GI tract is divided into
upper endoscopy (or esophagogastroduodenoscopy, EGD) [
10], and
lower endoscopy (or colonoscopy) [
11]. The Figures 1(a) and 1(b) illustrates two types of endoscopic procedures, upper and lower, respectively. It can be observed that there are still some areas where the conventional endoscopy is not able to reach. This restriction limits the possibility of detecting common anomalies like bleeding, ulcers, and tumors while they are in the early stages. In this way, the opportunity to control or even cure diseases before the need of complex treatments is lost [
12].
Since the absence of accurate diagnosis often results in degradation of the symptoms and the rapid development of a disease, in order to improve diagnostics a group of researchers from Baltimore invented in 1989 the concept of wireless endoscopic capsule [
13]. Later, in 2000, this concept was introduced by Given Imaging Inc [
14]. This technology reminds the futuristic concept in the 1966’s movie "Fantastic Voyage", where a submarine along with its crew is reduced to a microscopic size and injected into the bloodstream of a terminal patient to travel into a specific part of its body to destroy a malignant tumor with a LASER gun. The technology of wireless endoscopic capsule has revolutionized the gastroenterology and simultaneously promoted a new diagnostic perspective into the GI tract that previously could only be achieved by surgery, such as certain portions of the small intestine [
14].
Figure 1(c) shows the endoscopic capsule exam in the small intestine, where it cannot be accessed with conventional endoscopy in most cases.
This paper focuses on the role of new endoscopic imaging technologies that emerged from the need of better visualization of gastrointestinal tract mucosa. These improvements are expected to provide more precise information for endoscopists, surgeons, physicians, and radiologists.
2. Capsule Endoscopy
2.1. Drawbacks of Capsule Endoscopy
Despite all benefits that the capsule endoscopy may offer the drawbacks must also be considered. These disadvantages are divided into technical and physiological. The first group is related to the capsule itself and the other one is related to the human body, or to be more specific, to the gastrointestinal system.
Sussman and Kulkarni gathered data from different perspectives about risks offered during capsule endoscopy [
15]. The primary topics discussed in this study included capsule retention [
16], patency [
17,
18,
19], difficulty swallowing and aspiration [
16,
20], incomplete examination and suboptimal results [
16,
21,
22], pediatric capsule endoscopy [
23], and bowel preparation, [
16,
24,
25,
26]. Among the technical complications the most common problems were the presence of gaps in the recording, short duration or malfunction of the battery, failure in the capsule activation and incapacity of downloading the images [
16].
Moreover, Penazzio’s study concluded that the endoscopic capsule could not be used to obtain biopsy specimens or to endoscopic treatment and could not be controlled remotely either [
27]. Therefore, the risks of capsule endoscopy should be carefully reviewed, once every patient must be informed about supposed complications that might appear after capsule examination.
2.2. Benefits of Capsule Endoscopy
There are multiple benefits offered by CE. First, the patients do not need sedation to undergo into a CE analysis. The CE can analyze the entire GI tract from the esophagus, passing through the stomach until the small intestine, which could not be properly analyzed by conventional endoscopy. The capsule has a size of a conventional vitamin capsule and it can be easily swallowed, moving naturally through the GI tract until the excretion. This fact indicates a painless procedure, compared to the discomfort suffered by the long endoscopy sessions [
28,[28,
31].
2.3. Indications of the Capsule Endoscopy
The use indication of the capsule endoscopy is mainly for the small bowel diseases evaluation, due to the difficulty of diagnosis in this specific area. According to the Jain [
28] the indications are divided into two subsections:
small bowel and
esophagus.
Related to small bowel analysis the indications are: obscure gastrointestinal (GI) bleeding, occult (positive FOBT), evaluation of iron deficiency anemia, Crohn’s disease, indeterminate colitis, assessment of mucosal healing, abdominal pain, graft-versus-host disease, surveillance of polyposis syndromes, celiac disease, suspected small bowel tumors, follow-up of small bowel tumors, follow-up of small intestine transplantation, evaluation of abnormal small bowel imaging and evaluation of drug-induced injury.
Related to esophagus analysis the indications are: Barrett’s esophagus (BE), esophagitis and variceal evaluation. The main indications of each type of capsule are described in the following section. We denote yet that indications for esophagus analysis are matter of ongoing research studies.
2.4. Contraindications of Capsule Endoscopy
Capsule endoscopy contraindications have been divided into groups to better understand safety issues. The two types of contraindications are the absolute and the relative ones. Absolute use contraindications include the bowel obstruction, extensive and active Crohn’s disease, fistulas and strictures, intestinal pseudo-obstruction, young children [
28]; Relative contraindications are for patients that have dysphagia, previous abdominal surgery, pregnancy, diverticulosis, cardiac pacemakers and implanted electro-medical devices [
28]. The last two contraindications have been excluded from the absolute group since some studies noted no interference between capsule endoscopy and implantable devices functioning [
29,
30].
3. Indications of Esophageal Capsules
3.1. Screening of Barrett’s Esophagus
Barrett’s esophagus consists of a metaplastic change of the esophageal mucosa’s lining, meaning that the columnar epithelium replaces the squamous epithelium that normally overlays the distal esophagus [
32,
33,
34]. Barrett’s esophagus is an important risk factor for indication of esophageal adenocarcinoma, and several studies indicated that its incidence increased rapidly over the years [
35,
36]. The studies comparing the diagnostic yield between CE and conventional EGD demonstrated that CE was feasible, safe, and well tolerated by the patients. Moreover, the patients always preferred CE over unsedated EGD. On the other hand, the sensitivity of the esophageal capsule was variable between the detection of BE and another esophageal disease: 60-100% and 50-89%, respectively [
37,
38]. Although the results of sensitivity are promising, studies suggested that EGD is more cost effective than CE for BE screening [
39].
3.2. Screening for Esophageal Varices
The use of CE in detecting esophageal varices is not well defined, due to the fact that all the studies present considerable heterogeneity between their findings.
Pena
et al. found that esophageal capsule could be used in the assessment of esophageal varices (EV). The sensitivity calculated in this study was 68.4% in detecting EV using CE against 95% using EGD. However, due to the minimal discomfort, lack of sedation, and low risk offered by the CE makes this technology a possible substitute for EGD [
40].
Groce’s study showed a sensitivity of CE in detecting EV around 78%, and that CE may be superior to EGD for identification of small EV [
41]. In the other hand, Einsen’s and Smith’s study indicated a better perspective of CE tests, with sensitivity ranging from 100% [
42,
43]. The same results were showed in Ragunath’s study [
44]. The lowest sensitivity was showed in Jensen’s study, which was only 8.3%, with modest accuracy of the CE in the identification of EVs [
45].
4. Indications of Intestinal Capsules
4.1. Intestinal Tumors
Tumors found in the small bowel (SB) represent 5% of all GI tract tumors and 2% of the cancer rates, despite of very low accuracy of the estimative, once the current methodologies have been proved inadequate [
46]. On the other hand, the investigation of small bowel tumors with CE was established in 2004, an effective diagnostic modality, and the 8.9% of the patients who underwent the procedure was diagnosed with SB tumors. The expectative of the clinicians was that CE may lead to earlier detections and treatment of SB tumors, thereby improve the patients with neoplasms [
47].
4.2. Obscure GI Bleeding
Obscure GI bleeding can be defined by episodes of digestive bleeding, a positive fecal occult blood test, or chronic iron-deficiency anemia [
48,
49]. The complexity of a GI bleeding relates to the fact that the bleeding can occur from multiple lesions at many sites in the GI tract. This pathology is evident to the patient but can be from a source which is not easily identifiable by conventional upper or lower endoscopy [
50]. For that reason, and based on meta-analysis studies, the diagnostic yield of intestinal capsule ranges from to 55% to 81%, which confirmed the superiority of CE diagnosis against other modalities of conventional endoscopy [
51,
52,
53].
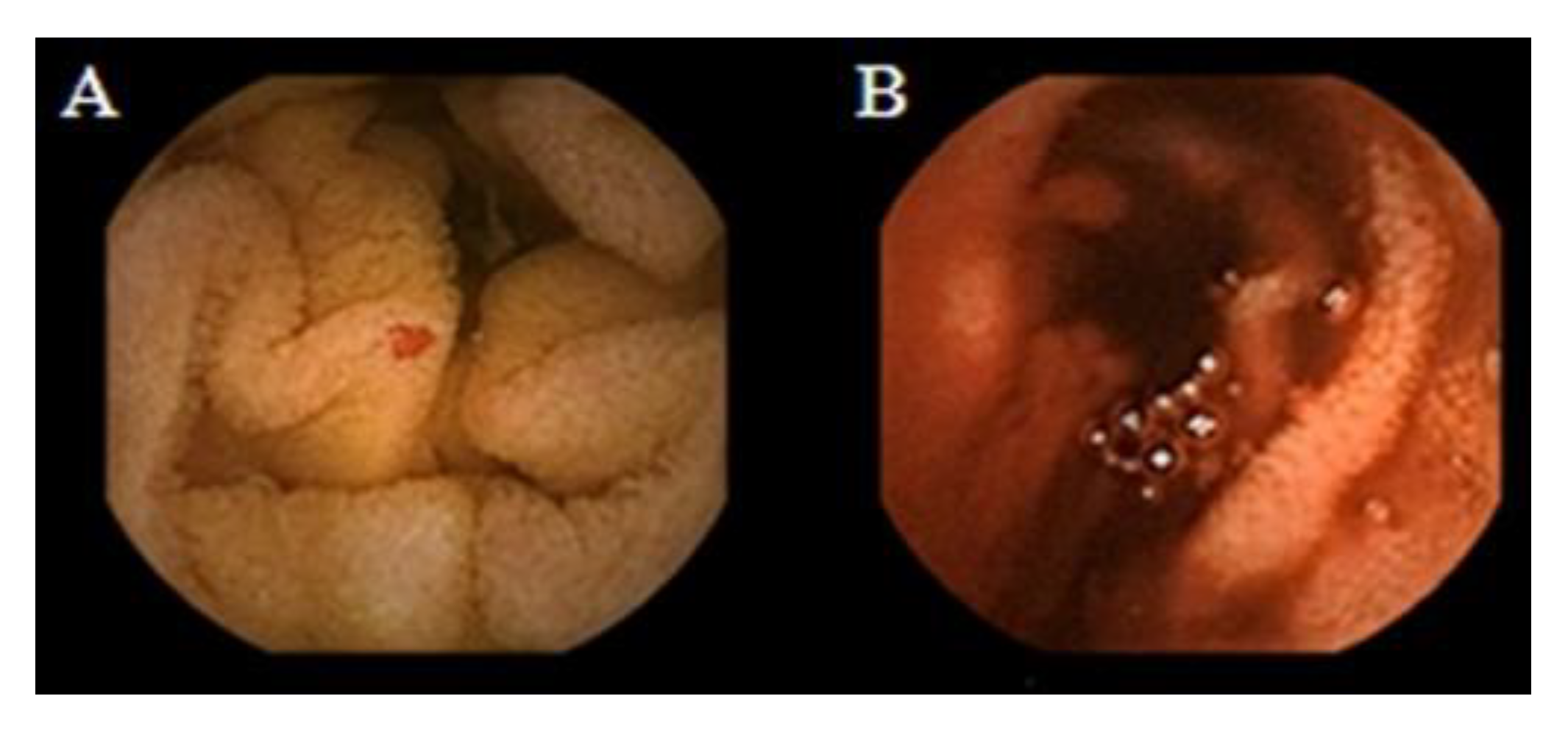
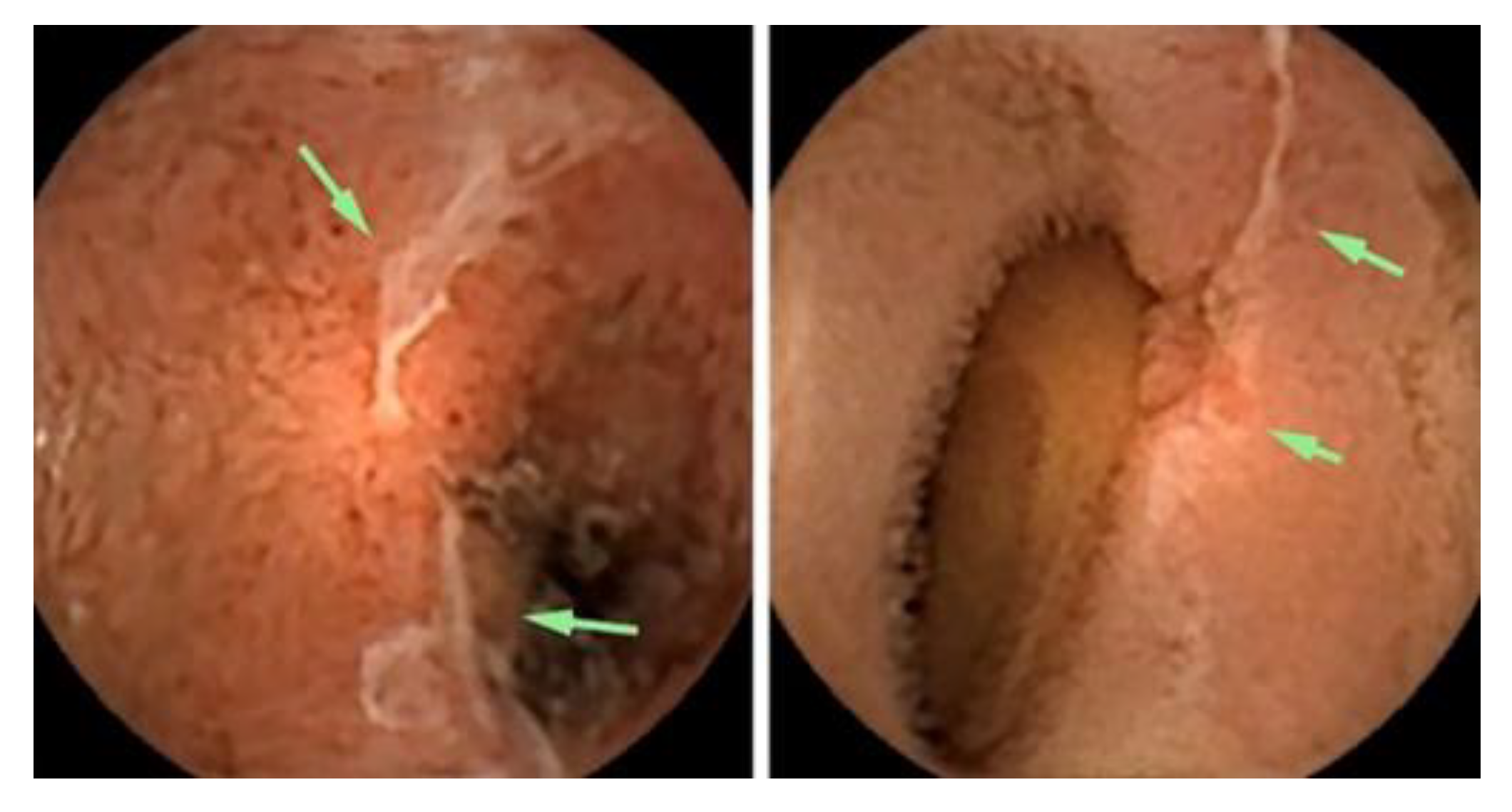
Figure 2 shows the small-bowel findings of obscure GI bleeding made with intestinal CE.
This section may be divided by subheadings. It should provide a concise and precise description of the experimental results, their interpretation, as well as the experimental conclusions that can be drawn.
4.3. Crohn’s Disease
Crohn’s disease consists of an inflammatory disease of the GI tract and often spreads deep into the layers of affected tissue. It can occur from mouth to anus [
55], although the probability of its incidence in the small bowel ranges from 30% to 40% of cases [
56,
57].
Generally, in order to identify a Crohn’s disease occurrence the clinicians must rely on a combination of clinical, endoscopic and histological findings, because there is no single test that can fully diagnose the disease. Imaging studies normally lack sensitivity to identify early lesions [
28].
Schulmann
et al. tested capsule endoscopy for the purpose of finding evidence of Crohn’s disease, and by that time he agreed that full visualization and imaging of the entire length of the SB was unsatisfactory and the CE was considered a promising new approach for the diagnosis of SB diseases [
58].
Albert
et al. compared the CE with magnetic resonance imaging (MRI), and found the CE can detect limited mucosal lesions that may be missed by MRI, and was slightly more sensitive than MRI: 12 versus 10 of 13 in suspected Crohn’s disease and 13 versus 11 of 14 in established Crohn’s disease [
59]. Based on the stated facts, CE proved to be a good complementary method for diagnosing SB Crohn’s disease. More recently, Sange
et al. state that CE innovation reduced reading time, improved diagnostic accuracy, and enhanced image quality [
60].
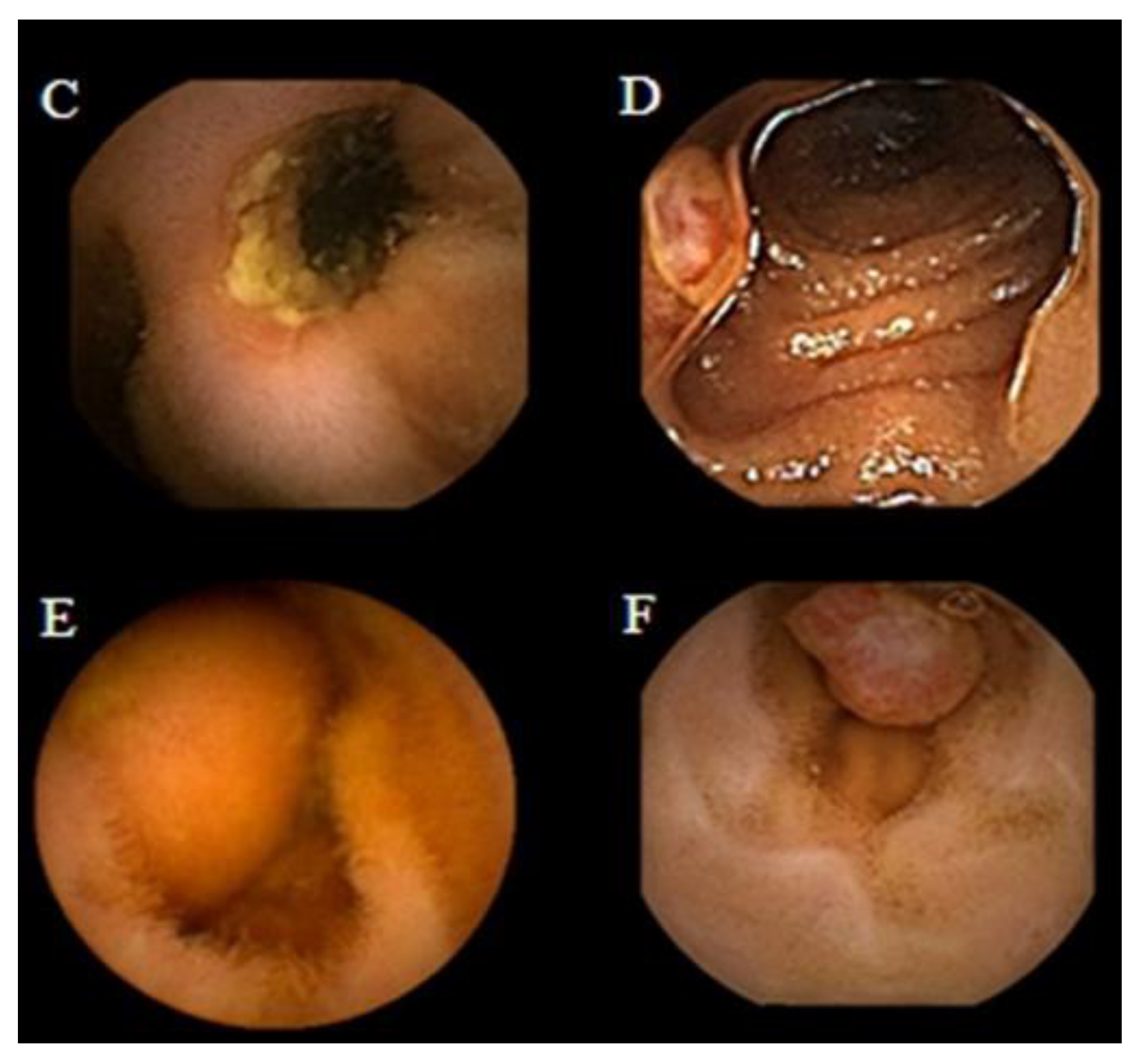
Figure 3 presents findings of active Chron’s disease detected by intestinal CE.
4.4. Celiac Disease
The use of capsule endoscopy in patients with celiac disease consists of finding complications such as unexplained diarrhea, abdominal pain, and small bowel tumors. Fry
et al. found a low yield for capsule endoscopy in patients with abdominal pain or diarrhea, and they recommended this type of evaluation as a first-line test. Moreover, the results showed a yield of 6% for abdominal pain, 14% for diarrhea, and 13% for both [
62]. Based on the experiments conducted by [
63,
64,
65,
66] and presented at the ICCE consensus, the diagnosis of Celiac disease with CE, Cellier
et al. considered that there was enough evidence to support the use of CE in patients who have treated and previously confirmed they have celiac disease [
67].
4.5. Genetic Disorders
Soares
et al. performed a study about Peutz-Jeghers syndrome (PJs) – an inherited gastrointestinal polyposis disorder, most commonly found in the small intestine-. They found that CE offered excellent visualization of the small intestine, and correctly identified all the patients having large polyps, although missed 20% of the total number of them [
68].
5. Commercial Capsules
Since the early years of conventional endoscopic procedures, the small bowel was considered technically difficult to examine for the sake of its length and location. The concept of a capsule indicated for small bowel analysis was developed by two groups. In 1996, a gastroenterologist so-called Dr. Paul Swain demonstrated the first live transmissions of a capsule analysis using a pig’s stomach. In 1997, he decided to collaborate with Dr. Gavriell Iddan, a mechanical engineer [
14,
69,
70]. In 2000 they published successful animal trials [
69], and in 2001 they published human studies on the use of capsule endoscopy in clinical trials [
14,
71]. At this time the Food and Drug Administration (FDA) approved the capsule endoscopy [
49].
Therefore, the small bowel was a difficult organ to explore with the available technologies, such as conventional endoscopy or radiological and nuclear techniques due to anatomical or physiological causes. In 2005, the role of the capsule endoscopy was widely discussed in the International Conference on Capsule Endoscopy (ICCE) [
68,
72,
73,
74,
75,
76,
77], a symposium organized and sponsored by Given Imaging.
Nowadays, there are several brands of capsules which were approved by the FDA, like – PillCam by Given Imaging, OMOM by Jinshan Science & Technology, EndoCapsule by Olympus, and MiroCam by Intromedic-. The following section describes each capsule, its advantages, drawbacks, and main components.
The approval from the FDA for the
PillCam SB capsule was received in 2003, and the market release was indicated for use in pediatric patients, aiming specifically the diagnosis of pathologies of the small bowel [
52,
78].
The
PillCam capsules produced by Given Imaging Ltd. are divided into three categories, - small bowel (SB), esophagus (ESO), and colon (COLON) - which have video cameras designed for imaging the gastrointestinal tract. Each of them is equipped with a battery, LEDs (light-emitting diodes), and a transmitter with an antenna. All these components are enwrapped in a biocompatible plastic casing, and the capsule size is about 26.4 mm length and 11.4 mm diameter [
79].
The
PillCam SB category is subdivided into
SB,
SB2, and
SB3.
PillCam SB capsules incorporate one video camera.
PillCam SB2 consists of a fixed frame rate second-generation capsule, and the
PillCam SB3 has enhanced imaging capabilities with adaptive frame rate (AFR) [
79]. These capsules are indicated for monitoring of lesions that may show Crohn’s disease not detected by upper and lower endoscopy. They are not indicated for patients with GI obstruction, strictures, or fistulas, patients with cardiac pacemakers or any implanted electro-medical devices, and for patients with dysphagia or other swallowing disorders.
The
PillCam ESO category has two variants –
PillCam ESO 2 with fixed high frame rate, and
PillCam ESO 3 with fixed high frame rate and enhanced imaging capabilities [
79]. The
PillCam ESO capsules are composed of two video cameras, and it can be used for investigation of esophageal disorders, such as esophageal varices, esophagitis and Barret’s esophagus [
80], and in patients complaining about heartburn [
81,
82].
The
PillCam COLON capsules also contain two video cameras, and this category is divided into two variants - COLON 1 with a fixed frame rate capsule, while the COLON 2 has an enhanced imaging capability AFR- [
79]. Both
PillCam ESO and
PillCam COLON are contraindicated for patients with the profile already described in the
PillCam SB section. This capsule is indicated to investigate intestinal disorders and tumors.
After various studies showing the risks of capsule retention, Given Imaging Inc. created the
Patency Capsule (PC) System, an ingestible, dissolvable and disposable capsule, composed by biocompatible materials. The patency capsule is given before a video capsule endoscopy, in order to prevent or minimize the risk of capsule retention [
83]. The body of the capsule is composed by compressed lactose that dissolves in GI liquids and 5% barium sulfate, which makes the capsule radiopaque. The system is composed by a non-video disintegrating capsule, radiofrequency identification (RFID) tag, and a RFID scanner [
84]. The PC is supposed to remain intact in the GI tract for about 80 hours according its design, and after that, if not excreted yet, it disintegrates in a spontaneous way [
85].
In 2005 Jinshan Science and Technology Company (Chongqing, China) released to the market the OMOM CE. This CE is indicated for investigate obscure gastrointestinal bleeding (OGIB), abdominal pain or diarrhea, partial intestinal obstruction, suspected inflammatory bowel disease, and tumors [
86]. A study showed that the visualization of the entire small bowel was achieved in 75% of the patients which had undergone the procedure with the OMOM. In the patients with suspected small bowel (SB) disease, the detection of abnormalities was 70.5%. The diagnostic yield for patients with OGIB was 85.7%, while the detection in cases of abdominal pain or diarrhea was around 53.3% [
86].
The Olympus Medical Systems (Olympus, Tokyo, Japan) has received marketing clearance from the FDA in 2007 for its
EndoCapsule endoscope system [
87]. This capsule was compared with the
PillCam SB and classified with same quality level by Pennazio [
27]
et al. The
EndoCapsule capsule contains a camera, a transmitter, batteries, and a light source. It differs from the
PillCam capsule in the matters of having a high resolution image chip and an external real time viewer [
88,
89].
A prospective randomized comparison between both capsules - Given
PillCam SB and Olympus
EndoCapsule - was carried out by Hartmann
et al. In this study was showed that the Olympus
EndoCapsule could detect more GI bleeding sources than the Given
PillCam SB, although the difference was only numerical, and statistically nonsignificant [
90].
The Korean MiroCam (Intromedic, Seoul, Korea) is another capsule, with similar components of the capsules from Given Imaging and Olympus. The first clinical trial using MiroCam was in 2009, involving 45 patients. The quality of image was rated as good in 91.1% of the cases, and transmission rates of the captured image in the stomach, small bowel, and colon were 99.5%, 99.6%, and 97.2%, respectively. The authors disclosed that MiRo was safe and effective to investigate the entire SB, offering good image quality and real-time feasibility [
88].
Moreover, a study carried out in 2012 showed that the evaluation of the entire SB using MiRoCam was achieved in 96% cases, and relevant lesion findings occured in 58% of the patients. They also considered MiRoCam a safe and effective tool for exploring SB with a high completion rate [
91].
Table 1 summarizes the commercial capsules available, as well as the indications, imaging system, size and respective references with studies about each type of capsule.
6. New Functionalities for Capsule Endoscopy
Although numerous results about clinical studies and experiments of endoscopic capsules are being presented, the unfeasibility of motion control of the capsule makes the diagnostic of the gastrointestinal not accurate enough. Since the impossibility of any motion control of the capsule has arisen, studies about possible solutions for motion control has been described in some patents [
92,
93,
94,
95].
The basic concept behind these patents were capsules being intrinsically controllable by including induction coils, magnetic parts inside an invented capsule structure, in the interest of make it responsive to an external magnetic field. However, as this kind of solutions required characteristic design, like: structure of capsule, geometrical shape, and magnetic properties, they would have their costs raised.
Moreover, a different solution by Carpi
et al. [
96] was disclosed in 2007, which on the contrary, permitted to control a traditional and commercially available endoscopic capsule without any structural modification.
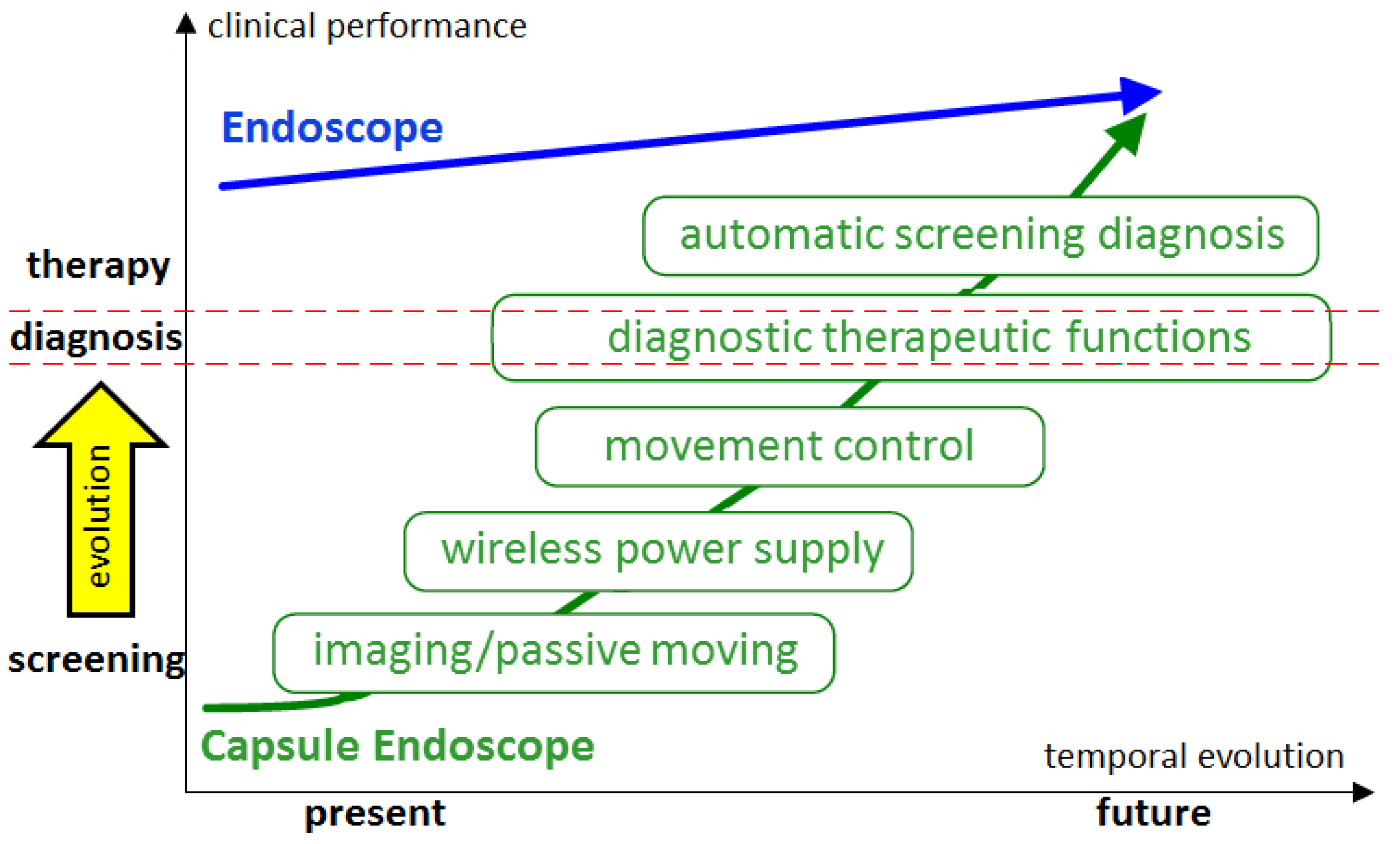
Figure 4 illustrates the personal vision of the President of Olympus, Mr. Shimoyana, during his talk at the MicroMachine Summit 2005 [
97]. From his view, the capsule endoscopy would have a rapid growth compared to the conventional endoscopy. The reason for this increase might be because of all the functionalities that can be integrated into the endoscopic capsule. The growth rate is directly related to the number of possible functions that can be incorporated in the endoscopic capsule.
In this context, it is important to discuss the new technologies which can be integrated to the capsule endoscopy, in order to increase the performance in screening, diagnosis, and therapy. In the following sections, we present the concept of confocal laser endomicroscopy, photodynamic therapy, narrow band imaging, and how those technologies can be integrated into the endoscopic capsules.
7. Photodybamic Therapy
It is well known that light has been used as therapy since the ancient civilizations, but until the last century, it was not known that photodynamic therapy (PDT) was developed. Since then, the applications of PDT have been tested by the clinicians for use in oncology, such as treatment of cancers of the neck, brain, breast, head, lung, pancreas, prostate, skin, and intraperitoneal cavity, and gastrointestinal tract [
98,
99,
100,
101]. In this cont photodyext, PDT represents an encouraging method for treatment of cancer, and even non-malignant conditions [
99].
The technique of PDT combines an administration of some photosensitizing and an exposure of the tissue to visible light, which means, in the range of 400 – 760 nm. When light with appropriate wavelength encounters the photosensitizer, the molecule is excited. This interaction generates a liberation of singlet oxygen, promoted by the series of molecular energy transfers. The singlet oxygen is highly reactive and cytotoxic species, and results in cell death [
102].
The activation wavelength of light differs according to the site we pretend to perform the therapy, usually, the wavelengths between 630-700 nm have been shown better results. As our focus is this review is GI tract, the activation wavelengths for esophageal and gastric cancers are 630 nm and 635 nm [
100,
101].
PDT can be coupled to the conventional endoscopes or even to the endoscopic capsules. Following the same logic of capsule endoscopy with NBI technology, we can also fabricate optical filters with a different wavelength, which can be used for PDT, and then integrate both technologies in the capsule. The problem of coupling PDT to the endoscopic capsules is that they must have integrated motion control, in order to guarantee that the therapy is being delivered in the exact sites of interest.
Before we discuss the clinical application using PDT as a therapy to diverse cancer types, we may first consider the treatment specificities and indications. Primarily, PDT is a local treatment, rather than systemic, and therefore, it is only suitable for localized disease. Secondly, when compared to other types of treatment, such as radiotherapy and chemotherapy, PDT represents a much faster and cost-effective treatment. Lastly, a huge advantage of PDT is that the limited light penetration protects healthy tissue beneath the tumor (or region of interest) from phototoxicity. Moreover, the treatment can be repeated in case of recurrence of the disease in the previously treated area [
103].
The studies of PDT in the treatment of esophageal cancer were firstly done as a palliative for obstructive tumors; McCaughan
et al. stated that the operative risk was minimal, and PDT had the ability to destroy tumor as well as to increase the size of esophageal lumen [
104]. Schweitzer’s, Qumseya’s and Moghissi’s studies confirmed the efficacy of PDT as palliative therapy of dysphagia evidencing the need of development of more tumor-specific photosensitizers [
105,
106,
107]. Nonetheless, side effects of PDT for esophageal cancer were listed in other studies, such as skin photosensitivity, stenosis, fistulas, and perforations (reported in up to 50% of the patients) [
105,
108,
109].
PDT is also suitable for treatment of Barret’s esophagus. There is an estimative that shows that 50% of esophageal cancers develop from Barret’s esophagus. Therefore, effective treatment of Barret’s esophagus is very important [
110]. PDT combined with long-term acid inhibition provided effective endoscopic therapy for elimination of Barrett’s mucosal dysplasia, superficial esophageal cancer, and also reduced/eliminated Barrett’s mucosa [
111]. Later the same group reported a conversion of approximately 80% of treated Barrett’s mucosa to normal squamous epithelium in all 100 patients who had undergone the study [
112].
As shown in
Figure 1, the conventional endoscopy has the particularity and drawback of not being able to observe the entire small intestine, only a small portion of it. This is mainly due to the possibility of increased risk of perforation of the intestine, taking into account its reduced thickness and sinuous structure [
12]. On the basis of the above reasons, the endoscopic capsule (EC) becomes a means of excellence to implement a form of photodynamic therapy (PDT) using light emitting diodes (LEDs) with wavelength in the region of red (e.g., from 620nm to750nm) that can be incorporated into EC, as shown in
Figure 5(a) [
113], allowing this equipment the ability to perform treatment in the GI system. Naturally, it is necessary to carry out preliminary tests on biopsies of the patient’s own cells in order to define the doses of both the photosensitizers and light, as well as, the exposure time in the endoscopic capsule to be applied during the treatment itself. The work developed by Gounella
et al. [
114] presents an platform for PDT, which validates such a procedure with the 5-aminolevulinic acid (ALA) photosensitizer on in-vitro assays of human gastric adenocarcinoma cells. This work also presents other photosensitizers available commercially or in clinical tests and their main characteristics [
114].
Figure 5(b) shows a functional prototype of an evaluation system side-by-side and connected with the smartphone running a host application.
8. Laser Endomicroscopy
Confocal laser endomicroscopy (CLE) is an imaging technique that uses a low-power laser to focus on a single point in a microscopic field of interest. The term confocal comes from the fact that the lens used in this technology allows the illumination and detection systems to be aligned in the same focal plane [
115,
116].
Costa
et al. proposed an integration of an imaging magnification optical microsystem (IMON), including a PDMS lens, which was able to perform in vivo and real-time tissue microscopy (
Figure 6). With total length of 12.164 mm and a lateral lens assembly of 3.894 mm, a paraxial magnification of 4-14 times was achieved with great performance [
113]. In this sequence of ideas, another similar IMON for ECs can be found in the work developed by Ribeiro
et al. [
117]. Such a IMON has a diameter of only 11.2mm a length of 18.6mm and comprises an imaging system with a dedicated IMOM and light emitting diodes (LEDs). Moreover, they fabricated and integrated a microlenses have been fabricated using the “hanging droplet” approach in the IMOM subsystem to provide an image magnificationof 4× with an improvement of 30% in the optical irradiance from the LED illumination [
117].
Tabatabaei
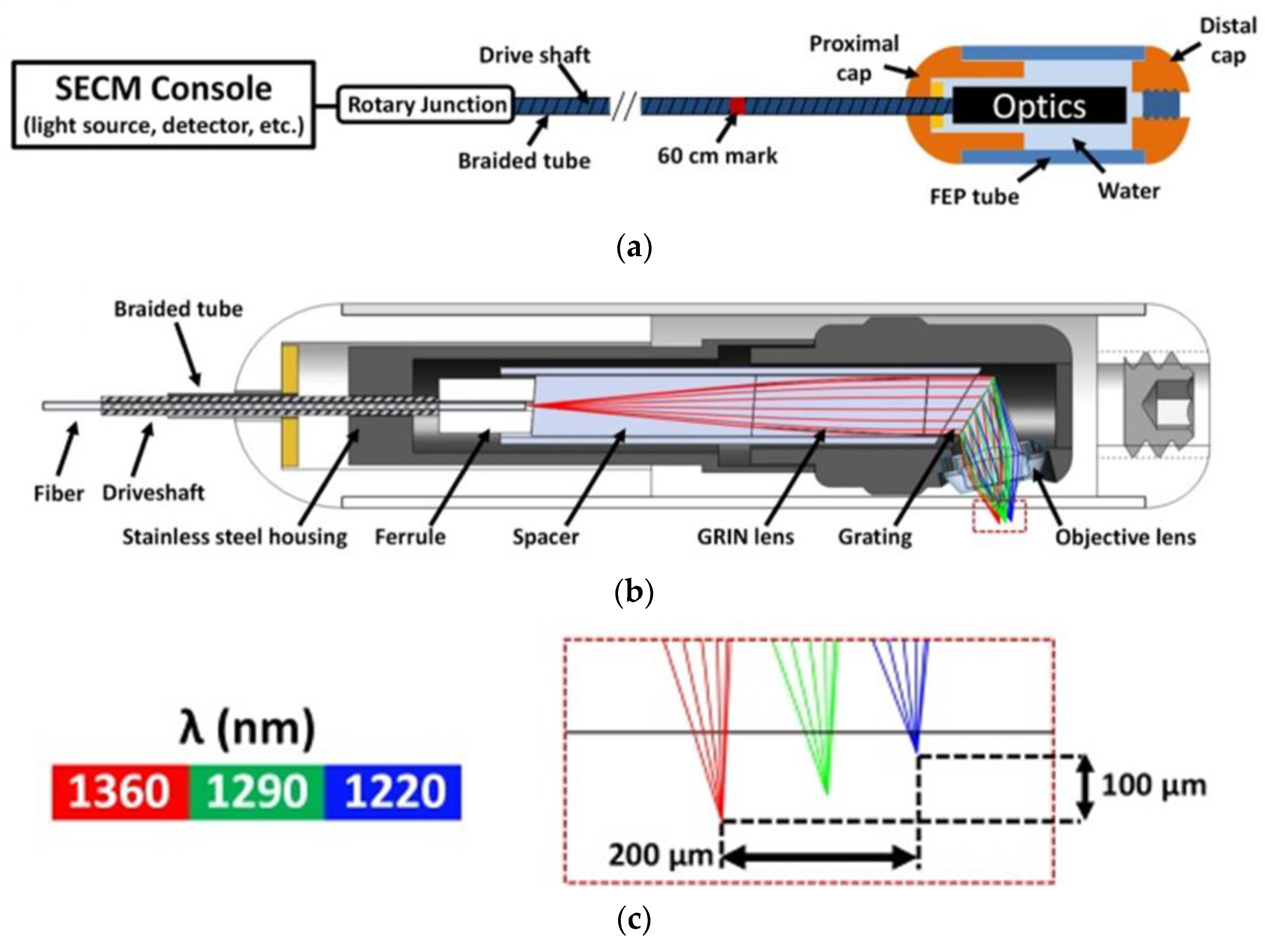
et al. reported a development of a confocal microscopy capsule for diagnosis and monitoring of an esophageal disease, called eosinophilic esophagitis (EoE). The EoE consists in an allergic condition characterized by eosinophils infiltration of the esophageal wall. Previously, treatment offered for the EoE required multiple follow-up sedated endoscopies and even biopsies to confirm the complete elimination of eosinophils. They develop a swallowable capsule which implements a high-speed fiber-based reflectance confocal microscopy, named spectrally encoded confocal microscopy (SECM). They presented imaging of esophageal biopsies from EoE patients ex vivo demonstrating the capability of SECM to visualize individual eosinophils [
118].
Figure 7 shows the schematic of the SECM clinical system.
As we can see the presented studies, CLE integration in the CE is still a new field of study underdevelopment. Nevertheless, there are sufficient indications that this is a promising technology and it can be expanded to the analysis of various disease of the GI tract.
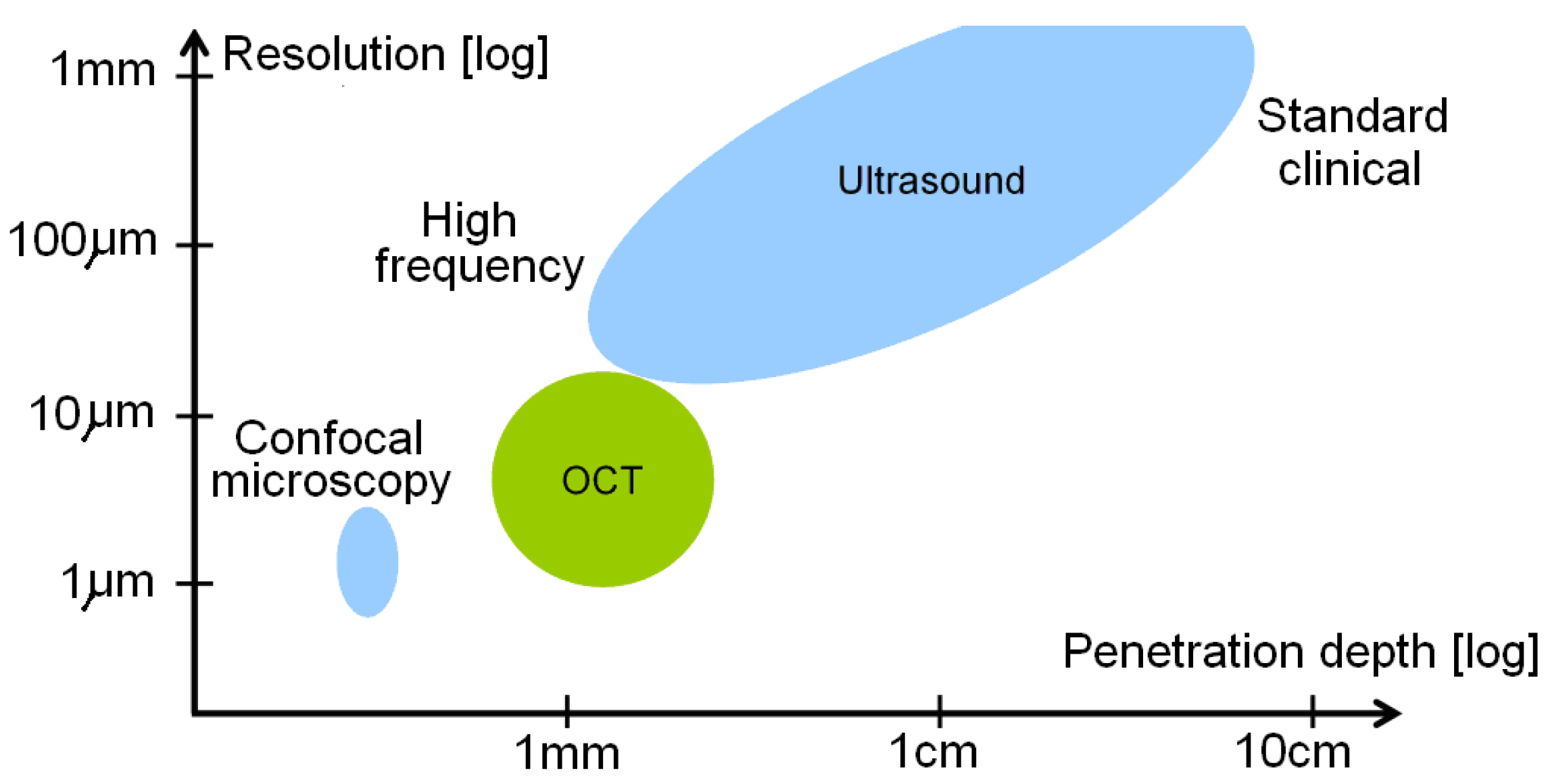
An Optical coherence tomography (OCT, also known as volumetric laser endomicroscopy) is a noninvasive optical diagnostic tool that enables in-vivo cross-sectional tomographic 3D visualization of internal microstructure and functional information in biological systems. OCT is based on measurement of reflected light from tissue optical interfaces and uses the principles of optical interferometry capable of imaging tissue at micron-level resolution [
119]. Another greatest advantage of OCT (see
Figure 8) is a good compromise between the spatial resolution and penetration depth [
120]. Typically, image resolutions of 1μm to 15μm can be achieved with OCT measurement, corresponding to one or two orders of magnitude higher than the ones with conventional ultrasonic scan. Confocal microscopy uses point illumination and a spatial pinhole to eliminate out-of-focus light in specimens to achieve a resolution of submicrometers [
121]. However, the image penetration of confocal microscopy is limited to a few hundred micrometers in general scattering media, which is much lower than those penetrations achieved with OCT that can penetrate 2-3mm. OCT presents additional merits such as contactless measurement, relatively simply setup and computation, fast scan and display.
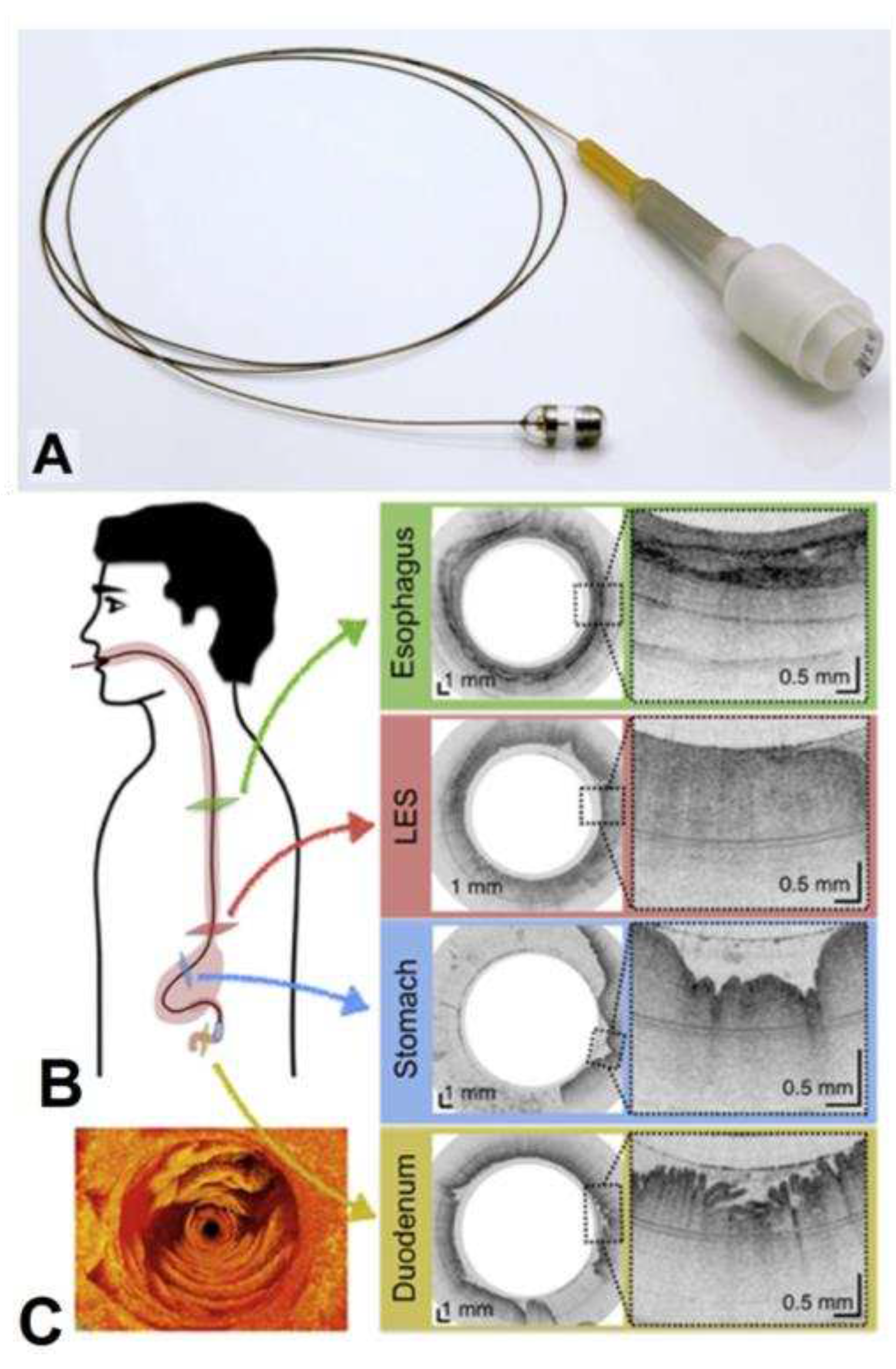
More recently in 2018, the research group of G. J. Tearney [
122] proposed a swallowed tethered capsule endomicroscopy (TCE) for microscopic imaging of the esophagus, stomach and duodenum without sedation in humans by using a ballon catheter and OCT technology. The tethered capsule has a diameter ranging from 11 to 12.8 mm and a length of 24 to 24.8 mm. A 2 meter long tether that connects it to an OCT imaging console allowed the real time acquisition of images at a frame rate of 20 fps, with an axial resolution (penetration depth) of 10 µm, and resolution of 35 µm along the lateral axis in 2-dimensions cross-sections (
Figure 9).
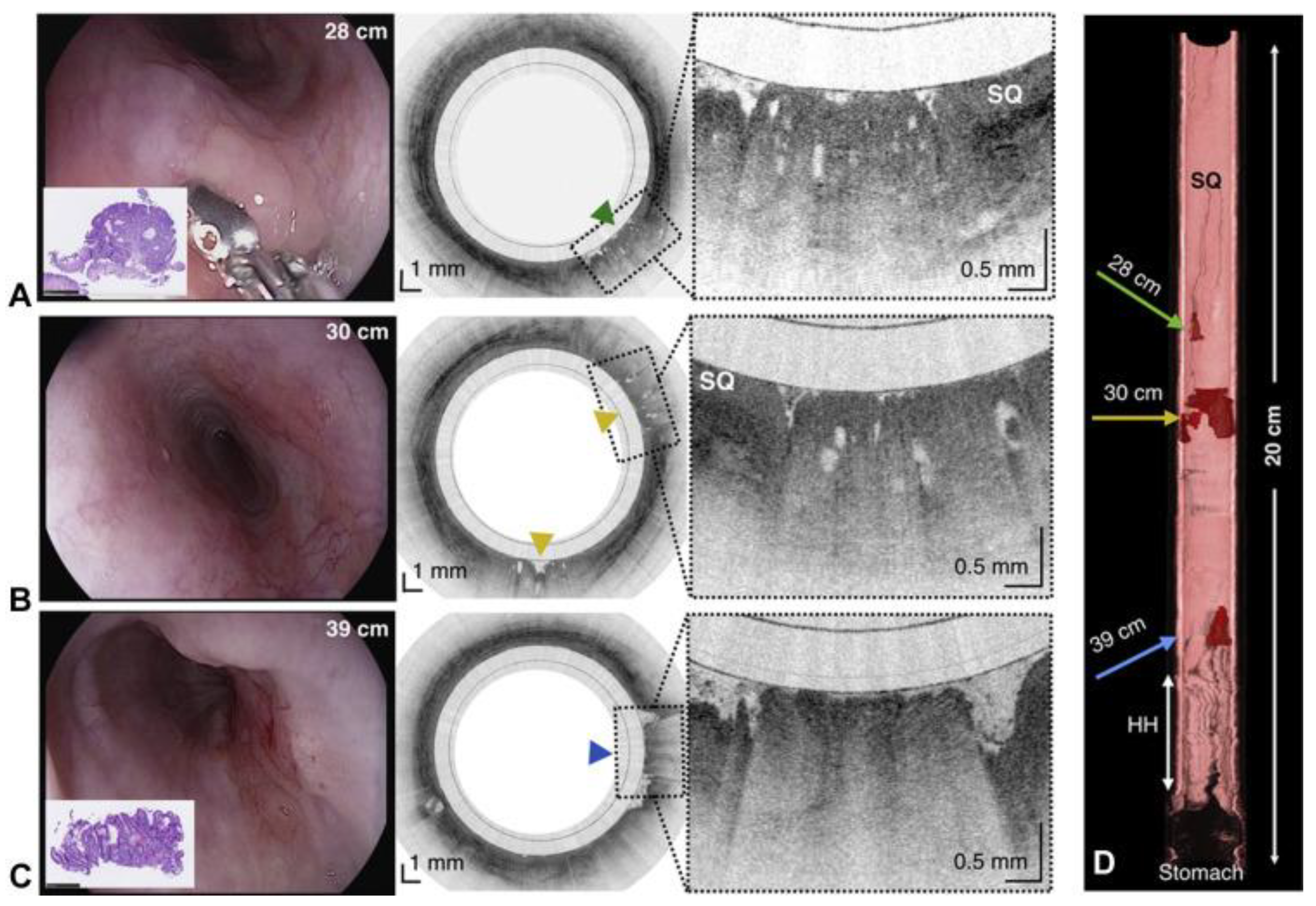
The capsule is re-used by withdrawn it through the mouth and followed by a disinfection. In this work the images acquired by OCT were compared with histopathology findings (when available), and it was proven the safety of the TCE, making it feasible as a procedure for obtaining microscopic images of upper gastrointestinal tract at high resolution without endoscopic assistance and/or the need of sedation as presented in
Figure 10.
The definitive perspective of one day to found ECs for OCT in the market is expected to be a reality, when analyzing recent works with a panoply of optical devices can be integrated at the wafer level in silicon to implement the required Michelson’s interferometer [
123]. Other materials, which can include the glass, are feasible candidates for heterogeneous integration using Multi-Chip-Modules (MCM) techniques with CMOS imagers [
124]. These kinds of Microsystems are able to pave the way to have OCT in ECs in the near future.
9. Spectroscopy
It is of major importance the detection of cancer at the dysplasia stage, e.g., before the occurrence of visible changes at macroscopic level on the tissues. A dysplasia consists is not more than a pre-cancerous change of the gastrointestinal tissue [
125]. The early detection of dysplasias can increase the chance of successful treatment and full survival of the patient [
125,
126]. These kinds of changes are very difficult to identify and detect with conventional illumination and visual inspection because a large area of changes in the tissue is required to become easily observable [
126,
127]. Spectroscopic techniques are based in the interactions between the light and the tissue. These techniques can be based on the diffuse reflectance or based in the fluorescence. Nonetheless, both have the potential to allow the detection of small changes of the tissue, e.g., macroscopically invisible lesions on the tissue surface [
127,
128]. Moreover, some morphological and biochemical changes on the tissues (related with early cancer progression) can modify the shape and intensity of the signals involved in the diffuse reflected and fluorescence signals. The extraction of the diffuse reflectance signal of a tissue can be used to detect small changes related with cancer progression, since its intensity and shape is affected by absorption and scattering effects [
129]. As a result, an increase of hemoglobin results in a reduction of the diffuse reflectance signal, because this optical effect is associated with angiogenesis during cancer progression. Moreover, as the dysplasia is progressing towards a cancer, the thickness of the epithelial tissue increases, thus, reducing the quantity of light that reaches the deeper tissues. Therefore, less quantity of light reaches the collagen fibers (the main tissue scatterer) in the connective tissue, decreasing scattering and, consequently decreasing the diffuse reflectance signal intensity [
127,
128].
It can be found in the literature several developments and research of systems for spectroscopy signals extraction and detection of gastrointestinal dysplasia [
130,
131,
132,
133]. However, most of the proposed solutions require complex and bulky spectroscopy systems, including, xenon lamps, ultra-violet (UV) LASERs, monochromators, optical fibers and high-quantum efficiency (QE) detectors. The consequence of using such components is the impossibility of their integration with the endoscopic equipment. Additionally, it is referred in the literature few attempts [
134,
135] to potentiate the miniaturization by replacing few of the components with photodiodes and/or Light Emitting Diodes (LEDs). However, it still requires few macroscopic equipments.
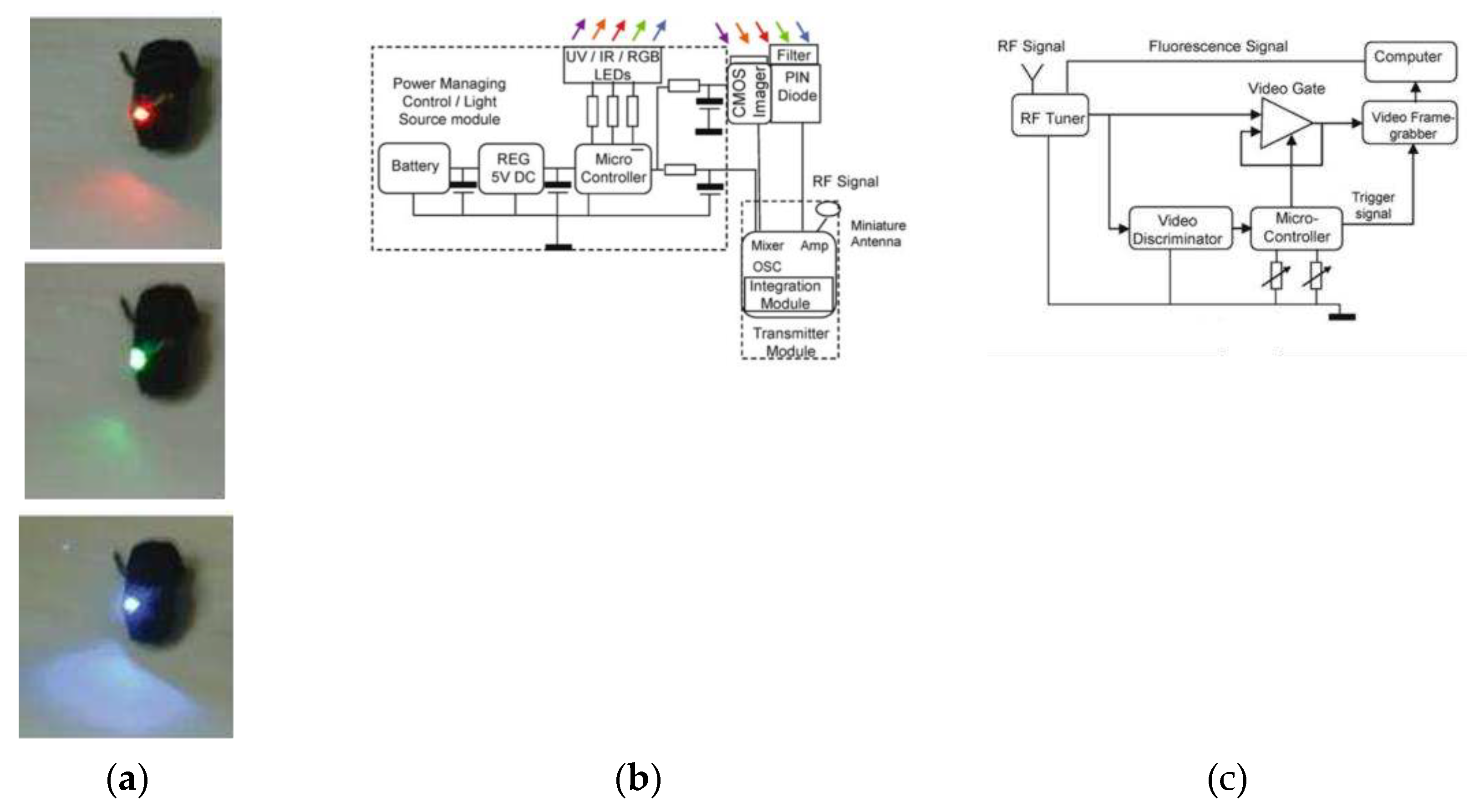
One of the successful attempts to integrate spectroscopic techniques on endoscopic capsules can be tracked to the year of 2005 and credited to the research group of R. R. Alfano, which presents a fully working and tested prototype of a compact endoscopic capsule [
136]. This medical device named Compact Photonics Explorer (CPE) is a further development of their work patented on 2001 [
137] and is a complete endoscopic capsule solution composed by five light sources to cover the ultra-violet (UV), near infra-red (NIR) and the entire visible spectrums (using three RGB LEDs),
Figure 11 shows a photograph and block diagram of the current CPE and also the block diagram of receiving station module. The spanning of these colors across these three bands increases the chance of early detection of lesions in the tissue, improving the chances of cancer treatment. Moreover, this endoscopic capsule is also composed by a CMOS image sensor, a PIN diode with an optical filter on top, a system to manage the energy supplied to the capsule, a radio-frequency transmission module, a microcontroller to perform core and control operations, and by a small-sized battery. The effectiveness of working was proven in the reference [
136] and further consolidated on 2011 through a US patent [
138].
10. Narrow Band Imaging
The idea of the NBI was conceived in 1999 by the Japanese National Cancer Center Hospital, and Olympus Medical Systems (Tokyo, Japan) [
119,
139]. To confirm this idea, a study was conducted using a multispectral camera with a source of high-power light. In this study, Kazuhiro Gono volunteered himself to do the first tests, and they revealed that the use of a narrowband at a wavelength of 415nm could increase the contrast of the images of blood capillaries [
140].
The NBI technology emerged from the need of detecting lesions which were not able to be observed in the white light. Therefore, the technique is used to increase the endoscopic image contrast by capturing real-time images and using a system composed of cameras and optical filters.
The optical filters are positioned under the endoscope light to create narrowband wavelength in the blue (in the range 400-430nm centered at 415nm) and green (in the range of 530-550nm centered at 540nm) [
141].
In 2004, Machida
et al. [
142] described the first clinical utility of NBI for gastrointestinal endoscopy. The first release of the technique was in 2005 with a system developed by Olympus Medical Systems [
140,
143,
144]. Since then, most of the countries that have endoscopy procedures started applying the NBI combined to the conventional techniques of endoscopy in clinical studies.
In 2003 Gono
et al. conducted an experiment observing endoscopic images of the back mucosa of a human tongue and investigated the effect of NBI through preliminary clinical tests in upper and lower endoscopy [
145]. The study showed that NBI could enhance the capillary and the crypt pattern on the mucosa, which are useful features for diagnosing early cancer [
140,
144,
146].
The structures such as blood vessels have high hemoglobin content, that is, hemoglobin index, which can be assessed by adjusting the color of the reflected light that penetrates the mucosa [
147,
148,
149]. In this way, they appear darker, creating a higher contrast between the surrounding mucosa, which appears brighter when it reflects the light.
The blue light, at the wavelength of 415 nm, allows the obtainment of a superficial mucosa image, in which can be observed the superficial capillaries network. While the green light, at the wavelength of 540 nm allows a deeper penetration in the mucosa, thus enabling the observation of subepithelial vessels. The photons at a wavelength of 600 nm, that means, red light, are less scattered and penetrate more deeply. Although their longer wavelength is outside of the hemoglobin absorption band, the red photons reproduce a morphological image of large vessels [
144].
Moreover, the inclusion of a lens with a high magnification factor will improve even more the image quality and detail, the global impact and the importance of NBI [
117].
In the context of NBI technology, there are two options for the implementation, it can be used commercial LEDs and then adapt them with optical filters, or the LEDs can be fabricated with the transmittance peaks at the wanted wavelength. The fabrication of optical filters offers a much cheaper alternative to the idea of fabricating LEDs with exactly transmittance peaks.
Considering the scenario described before, it was decided to go for the option of fabricating the optical filters for adaptation into the commercial endoscopic capsules. For that matter, different methods and processes have been tried until the final functional version of the filter was reached. The optical filters were first calculated, simulated and adjusted, and then fabricated.
10.1. Design and Fabrication of Optical Filters
As a first step, the materials from which the filters will be made of should be chosen and specified. In this case, dielectric materials following the physical principle of Fabry-Perot were chosen, although there was also the possibility of working with metals. The filters consist of a double stack of layers, with a high refractive index material (H) and a low (L) refractive index material, alternately. The stacks of layers are called mirrors, and between both mirrors, there is a resonance cavity. This type of filter consists of a structure in which light is captured in certain wavelengths and it operates as an optical transmission incorporating feedback – the light is repeatedly reflected between the two mirrors, without escaping. The transmitted light in the resonance cavity is the sum of all beams transmitted through the stack of layers, due to a constructive interference, which depends on the wavelength of the incident light and the thickness of the resonance cavity.
The project of a Fabry-Perot filter requires selectivity and low absorption in the mirrors and in the resonance cavity. A slight change in the mirrors spacing can cause a significant change in the pretended wavelength. The optical filter is considered ideal if we assume that there is no loss on both mirrors and that they are perfectly parallel to each other [
150,
151]. Once the materials are chosen, the width of each layer of the mirror and of the resonance cavity should be calculated, based on the wavelength and refractive index we want for the filter. For the computational simulation, we used the software TFcalcTM 3.5 from Software Spectra Inc. TFcalcTM consists of a thin film design software, which enables the analysis and design of multilayer thin-film coatings, such as calculation of transmittance, absorption, optical density, loss, color, and other features. These simulations took into account the spectrum of the optical sources. The optical filters were designed following a stack of seven layers, whose succession of refractive indexes follows the HLH-L-HLH structure. Therefore, the materials must be selected in order to provide high (H) and low (L) indexes of refraction in the visible range. The silicon dioxide (SiO
2) and titanium dioxide (TiO
2) were selected as H and L materials because both can be obtained using physical processes, e.g., both can be deposited by sputtering, both are compatible in the sense that their mutual adhesion is high, and the silicon dioxide presents index of refraction practically constant in the visible range [
148]. The TiO
2 and SiO
2 present typically indexes of refractions of 1.45 and 2.65, respectively. In these filters, the three top layers correspond to the first mirror, and the three bottom layers correspond to the second mirror. Between the mirrors, there is a resonance cavity.
The optical filters were fabricated using a glass substrate B270 from Schott Advanced Optics, measuring 20x20mm and thickness of 1mm. The thin-films of SiO
2 and TiO
2 were deposited by DC-magnetron sputtering, and were previously characterized by ellipsometry, in order to measure the refractive index and thin-film thickness of both materials. Full fabrication details can be found on the work by Gounella
et al. [
152].
10.2. Optical Filters Characterization and Results
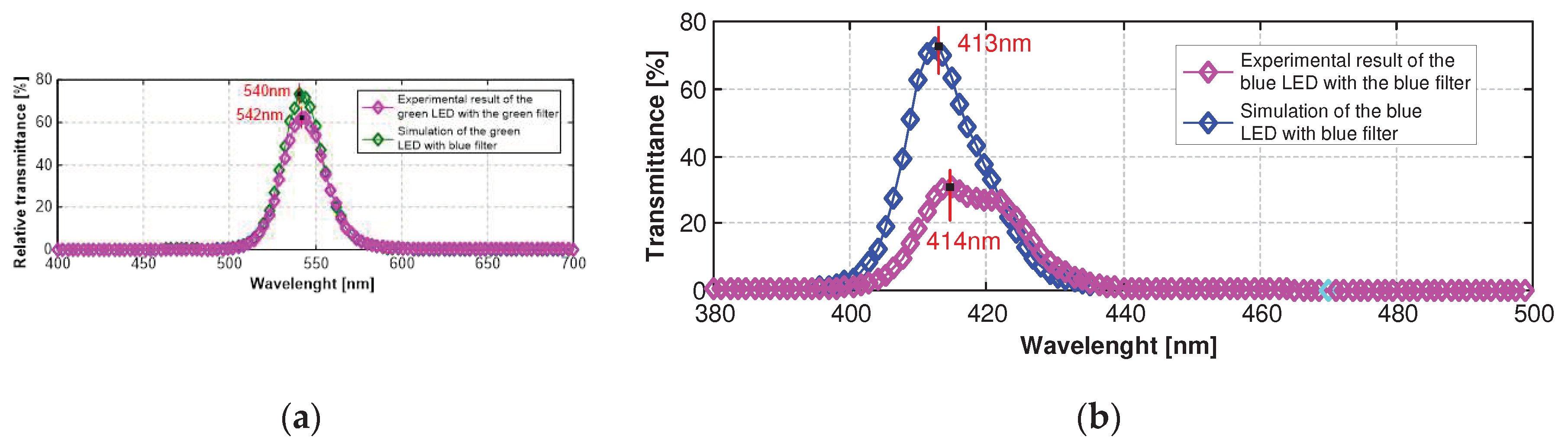
The optical filters were characterized by spectrometry, which allows the evaluation of their optical performance. The characterization of transmittance was performed to know the optical response of the NBI filters to the fringes @415nm (blue region) and @540nm (green region).
As a first element of the setup, the illuminants (in this case, the blue and green LEDs) were placed before a collimator. The collimator played the role of narrowing the scattered beams from the LED and aligned them with the spectrometer. This step was necessary since the aperture from the spectrometer was very narrow, which made the alignment difficult. For this experiment, it was used the CCD compact spectrometer CCS200 from Thorlabs, with wavelength range from 200 – 1000nm.
Figure 12(a) shows the transmittance spectrum from the green optical filter, along with the picture of the physical filter fabricated. The green filter presented a maximum relative transmittance of 62% @542nm and Full Width at Half Maximum (FWHM) of 29nm.
Figure 12(b) shows the experimental result of the blue filter with the blue LED as illuminant, along with the picture of the physical filter fabricated. The result showed a maximum relative transmittance of 33% @414nm, and a FWHM of 17nm. The peaks of transmittance were satisfactory, once the goal was a blue filter centered @415nm and a green filter centered @540nm. The blue filter presented only 1nm of deviation from the objective, despite that the experimental transmittance was half of the simulated. The green filter deviated 2nm to the left from the objective, although with also higher transmittance @540nm (~60%).
10.3. Narrow Band Imaging vs CLE
The advantage of CLE lies in its ability to be used with any endoscopy system and visualise at cellular level thus lending to molecular imaging. On the other hand, it is also a limitation since it cannot be used widely in community setting outside of academic centres [
153].
10.4. Narrow Band Imaging vs FICE
Flexible spectral imaging color enhancement (FICE) and narrow band imaging are forms of digital chromoendoscopy and enhance the endoscopic images without the need for a dye. Alis
et al. finds no statistically significant difference between them for
in vivo histologic diagnosis of polyps in a study with 134 patients (72 male / 62 female) [
154].
10.5. Narrow Band Imaging vs BLI
Recently, a new optical imaging technology known as blue-light imaging (BLI; Fujifilm) was developed. The system uses a either a laser or 4 LED multilight technology to produce brighter images. It is the first nonfilter technology producing blue light in a narrow spectrum that is bright enough to identify subtle changes in surface and vessel patterns, which is of relevance in early Barrett’s neoplasia [
155]. BLI magnification images with early gastric cancer are compared with those of NBI magnification images in deeply depressed area, supported by post ESD (scopic submucosal dissection) specimen. NBI magnification cannot focus on the shallow area in cancerous lesion very sharply when the deeply depressed area is observed on good focus. By contrast, BLI magnification can focus on both shallow and deeply depressed areas on the same image because of great depth of field. This case shows a typically fine network pattern of early gastric cancer in the gastric body. BLI bright images show fine network pattern in depressed cancer by exhibiting clearly irregular microvessels surrounding various sizes of white spots corresponding to histologically marginal crypt epithelium [
156].
10.6. Narrow Band Imaging, FICE and BLI Images Enhanced by i-SCAN
Developed by Pentax, i-SCAN is a dynamic, software-based, image enhancement technology that provides the user an enhanced view of the texture of the mucosal surface and blood vessels. A study by Lee
et al. presented results on i-SCAN vs. the more widely studied NBI for the prediction of diminutive colonic polyp pathology and found that both technologies had statistically significant higher sensitivity and accuracy compared to BLI. In addition, no significant differences were evident between NBI and i-SCAN (sensitivity, 88.8% vs. 94.6%; specificity, 86.8% vs. 86.4%; accuracy, 87.8% vs. 90.7%, respectively; p<.05) [
157]. Another study investigated i-SCAN’s role in evaluating duodenal villous patterns [
158].
11. Conclusions
The aim of this research paper is to present a detailed review of the current status of endoscopic techniques and the recent technological advancements that can be employed in conjunction with these techniques, in order to improve the timely diagnosis of gastrointestinal disorders. Furthermore, this study highlights the design and fabrication of two optical filters, which have been created with the specific purpose of enhancing the detection of hemoglobin in the gastrointestinal tract. These filters have been centered at wavelengths of 414 nm and 542 nm, which coincide with the absorption peaks of hemoglobin. In order to create these optical filters, Narrow Band Imaging (NBI) technology has been utilized. The ultimate objective of this effort is to integrate these filters into the endoscopic capsules for practical applications. Despite the challenges associated with the fabrication of these optical filters, the results of the experimental studies have been satisfactory when compared to the simulations. The complexity and difficulty of the manufacturing process have been taken into account while evaluating the outcomes of the study. The future advances of diagnostic endoscopy now lies in combinations of these new optical techniques with improvements from the field of Artificial Intelligence to minimizing human error and maximizing its efficiency, finally enabling the automatic screening diagnosis predict by the companies in the past.
Author Contributions
Conceptualization, J.P.C; methodology, R.H.G., T.C.G., and J.P.C.; software, R.H.G; validation, R.H.G. and T.C.G.; formal analysis, R.H.G. and T.C.G.; investigation, R.H.G., T.C.G. and J.P.C.; resources, O.H.A.J. and J.P.C.; data curation, R.H.G.; writing—original draft preparation, R.H.G. and T.C.G.; writing—review and editing, O.H.A.J., M.G. and J.P.C.; visualization, R.H.G, O.H.A.J. and J.P.C.; supervision, J.P.C.; project administration, J.P.C.; funding acquisition, O.H.A.J. and J.P.C.. All authors have read and agreed to the published version of the manuscript. M.G. and D.L. perform the final revisions.
Funding
his research was partially supported by the FAPESP agency (Fundação de Amparo à Pesquisa do Estado de São Paulo) through the project with the reference 2019/05248-7. Professor João Paulo Carmo was support by a PQ scholarship with the reference CNPq 304312/2020-7. This research was also partially supported by FACEPE agency (Fundação de Amparo a Pesquisa de Pernambuco) throufht the project with references APQ-0616-9.25/21 and APQ-0642-9.25/22. O.H.A.J. was funded by the Brazilian National Council for Scientific and Technological Development (CNPq), grant numbers 407531/2018-1, 303293/2020-9, 405385/2022-6, 405350/2022-8 and 40666/2022-3.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
This work was sponsored by the Brazilian agency National Council of Scientific Research (Conselho Nacional de Desenvolvimento Científico e Tecnológico, CNPq) under the grant 400110/2014-8. Professor João Paulo Carmo was also sponsored by a scholarship PQ 305250/2015-9 from the CNPq. The authors would like to thank the staff at the Center for Semiconductor Components and Nanotechnologies (CCSNano) for the assistance in this work.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Moltzer, E.; Noordman, B.J.; Renken, N.S.; Roos, D. Determination of Tumor Location in Rectosigmoid Carcinomas: Difficulties in Preoperative Diagnostics. Gastrointestinal Disorders 2019, 1, 210–219. [CrossRef]
- Ortega, S.; Fabelo, H.; Iakovidis, D.K.; Koulaouzidis, A.; Callico, G.M. Use of Hyperspectral/Multispectral Imaging in Gastroenterology. Shedding Some–Different–Light into the Dark. J Clin Med 2019, 8.
- Beeley, J.; Melino, G.; Al-Rawahani, M.; Turcanu, M.; Stewart, F.; Cochran, S.; Cumming, D. Imaging Fluorophore-Labelled Intestinal Tissue via Fluorescence Endoscope Capsule. Proc West Mark Ed Assoc Conf 2018, 2, 766–769. [CrossRef]
- Nakamura, M.; Yamamura, T.; Maeda, K.; Sawada, T.; Mizutani, Y.; Ishikawa, T.; Furukawa, K.; Ohno, E.; Kawashima, H.; Miyahara, R.; et al. Validity of Capsule Endoscopy in Monitoring Therapeutic Interventions in Patients with Crohn’s Disease. J Clin Med 2018, 7. [CrossRef]
- Sahoo, G.R.; Singh, P.; Pandey, K.; Kala, C.; Pradhan, A. Improving Diagnosis of Cervical Pre-Cancer: Combination of PCA and SVM Applied on Fluorescence Lifetime Images. Photonics 2018, 5. [CrossRef]
- Askoura, M.L.; Vaudelle, F.; L’Huillier, J.P. Experimental Study of Light Propagation in Apple Tissues Using a Multispectral Imaging System. Photonics 2016, 3. [CrossRef]
- Rohrbach, D.J.; Salem, H.; Aksahin, M.; Sunar, U. Photodynamic Therapy-Induced Microvascular Changes in a Nonmelanoma Skin Cancer Model Assessed by Photoacoustic Microscopy and Diffuse Correlation Spectroscopy. Photonics 2016, 3. [CrossRef]
- Peretti, R.; Braud, F.; Peytavit, E.; Dubois, E.; Lampin, J.F. Broadband Terahertz Light-Matter Interaction Enhancement for Precise Spectroscopy of Thin Films and Micro-Samples. Photonics 2018, 5. [CrossRef]
- Tansu, N. Photonics-Advances in Fundamental Sciences and Engineering Technologies of Light. Photonics 2014, 1.
- Chiu, P.W.Y. Second Look Endoscopy in Acute Non-Variceal Upper Gastrointestinal Bleeding. Best Pract Res Clin Gastroenterol 2013, 27, 905–911.
- Kahi, C.J.; Myers, L.J.; Slaven, J.E.; Haggstrom, D.; Pohl, H.; Robertson, D.J.; Imperiale, T.F. Lower Endoscopy Reduces Colorectal Cancer Incidence in Older Individuals. Gastroenterology 2014, 146. [CrossRef]
- Pan, G.; Wang, L. Swallowable Wireless Capsule Endoscopy: Progress and Technical Challenges. Gastroenterol Res Pract 2012.
- Karargyris, A.; Bourbakis, N. Wireless Capsule Endoscopy and Endoscopy Imaging. IEEE Engineering in Medicine and Biology, 2010;
- Iddan, G.; Meron, G.; Glukhovsky, A.; Swain, P. Wireless Capsule Endoscopy. Nature 2000, 405, 417. [CrossRef]
- Schnoll-Sussman, F.; Kulkarni, K. Risks of Capsule Endoscopy. Tech Gastrointest Endosc 2008, 10, 25–30. [CrossRef]
- Rondonotti, E.; Herrerias, J.M.; Pennazio, M.; Caunedo, A.; Mascarenhas-Saraiva, M.; De Franchis, R. Complications, Limitations, and Failures of Capsule Endoscopy: A Review of 733 Cases. Gastrointest Endosc 2005, 62, 712–716. [CrossRef]
- Spada, C.; Shah, S.K.; Riccioni, M.E.; Spera, G.; Marchese, M.; Iacopini, F.; Familiari, P.; Costamagna, G. Video Capsule Endoscopy in Patients With Known or Suspected Small Bowel Stricture Previously Tested With the Dissolving Patency Capsule. J Clin Gastroenterol 2007, 41, 576–582.
- Boivin, M.L.; Lochs, H.; Voderholzer, W.A. Does Passage of a Patency Capsule Indicate Small−Bowel Patency? A Prospective Clinical Trial? Endoscopy 2005, 37, 808–815. [CrossRef]
- Signorelli, C.; Rondonotti, E.; Villa, F.; Abbiati, C.; Beccari, G.; Avesani, E.C.; Vecchi, M.; de Franchis, R. Use of the Given® Patency System for the Screening of Patients at High Risk for Capsule Retention. Digestive and Liver Disease 2006, 38, 326–330. [CrossRef]
- Selby, W.; Sydney, F. Complete Small-Bowel Transit in Patients Undergoing Capsule Endoscopy: Determining Factors and Improvement with Metoclopramide. Gastrointest Endosc 2005, 61, 80–85.
- Selby, W.; Sydney, F. Complete Small-Bowel Transit in Patients Undergoing Capsule Endoscopy: Determining Factors and Improvement with Metoclopramide. Gastrointest Endosc 2005, 61, 80–85.
- Fireman, Z.; Paz, D.; Elsevier, K.; Kopelman, Y. Capsule Endoscopy: Improving Transit Time and Image View. World J Gastroenterol 2005, 11, 5863–5866.
- Moy, L.; Levine, J. Wireless Capsule Endoscopy in the Pediatric Age Group: Experience and Complications. J Pediatr Gastroenterol Nutr 2007, 44, 516–520.
- Ben-Soussan, E.; Savoye, G.; Antonietti, M.; Ramirez, S.; Ducrotté, P.; Lerebours, E. Is a 2-Liter PEG Preparation Useful Before Capsule Endoscopy? J Clin Gastroenterol 2005, 39, 381–384.
- Dai, N.; Gubler, C.; Hengstler, P.; Meyenberger, C.; Bauerfeind, P.; Gallen, S. Improved Capsule Endoscopy after Bowel Preparation. Gastrointest Endosc 2005, 61, 28–31.
- Shiotani, A.; Opekun, A.R.; Graham, D.Y. Visualization of the Small Intestine Using Capsule Endoscopy in Healthy Subjects. Dig Dis Sci 2007, 52, 1019–1025. [CrossRef]
- Pennazio, M. Capsule Endoscopy: Where Are We after 6 Years of Clinical Use? Digestive and Liver Disease 2006, 38, 867–878.
- Jain, R.K.; Jain, S. Capsule Endoscopy: A Comprehensive Review. In New Techniques in Gastrointestinal Endoscopy; Pascu, O., Seicean, A., Eds.; IntechOpen: Rijeka, 2011; p. Ch. 6.
- Leighton, J.A.; Sharma, V.K.; Srivathsan, K.; Heigh, R.I.; McWane, T.L.; Post, J.K.; Robinson, S.R.; Bazzell, J.L.; Fleischer, D.E. Safety of Capsule Endoscopy in Patients with Pacemakers. Gastrointest Endosc 2004, 59, 567–569. [CrossRef]
- Leighton, J.A.; Srivathsan, K.; Carey, E.J.; Sharma, V.K.; Heigh, R.I.; Post, J.K.; Erickson, P.J.; Robinson, S.R.; Bazzell, J.L.; Fleischer, D.E. Safety of Wireless Capsule Endoscopy in Patients with Implantable Cardiac Defibrillators. Official journal of the American College of Gastroenterology | ACG 2005, 100.
- Li, F.; Leighton, J.A.; Sharma, V.K. Capsule Endoscopy in the Evaluation of Obscure Gastrointestinal Bleeding: A Comprehensive Review; 2007; Vol. 3;
- Shaheen, N.J.; Richter, J.E. Barrett’s Oesophagus. The Lancet 2009, 373, 850–861. [CrossRef]
- Spechler, S.J. Barrett’s Esophagus. New England Journal of Medicine 2002, 346, 836–842. [CrossRef]
- Shaheen, N.J.; Crosby, M.A.; Bozymski, E.M.; Sandler, R.S. Is There Publication Bias in the Reporting of Cancer Risk in Barrett’s Esophagus? Gastroenterology 2000, 119, 333–338. [CrossRef]
- Blot, W.J.; Devesa, S.S.; Kneller, R.W.; Fraumeni Jr, J.F. Rising Incidence of Adenocarcinoma of the Esophagus and NGastric Cardia. JAMA 1991, 265, 1287–1289. [CrossRef]
- Devesa, S.S.; Blot, W.J.; Fraumeni Jr., J.F. Changing Patterns in the Incidence of Esophageal and Gastric Carcinoma in the United States. Cancer 1998, 83, 2049–2053. [CrossRef]
- Galmiche, J.P.; Coron, E.; Sacher-Huvelin, S. Recent Developments in Capsule Endoscopy. Gut 2008, 57, 695. [CrossRef]
- Gerson, L.; Lin, O.S. Cost-Benefit Analysis of Capsule Endoscopy Compared With Standard Upper Endoscopy for the Detection of Barrett’s Esophagus. Clinical Gastroenterology and Hepatology 2007, 5, 319-325.e3. [CrossRef]
- Pena, L.R.; Cox, T.; Koch, A.G.; Bosch, A. Study Comparing Oesophageal Capsule Endoscopy versus EGD in the Detection of Varices. Digestive and Liver Disease 2008, 40, 216–223. [CrossRef]
- Pena, L.R.; Cox, T.; Koch, A.G.; Bosch, A. Study Comparing Oesophageal Capsule Endoscopy versus EGD in the Detection of Varices. Digestive and Liver Disease 2008, 40, 216–223. [CrossRef]
- McCarty, T.R.; Afinogenova, Y.; Njei, B. Use of Wireless Capsule Endoscopy for the Diagnosis and Grading of Esophageal Varices in Patients With Portal Hypertension: A Systematic Review and Meta-Analysis. J Clin Gastroenterol 2017, 51.
- Eisen R; Zaman A; Schwartz J; Faigel D; Rondonotti E; Villa F; Weizman E; Yassin K; deFranchis R, G.M.E. The Accuracy of PillCam ESO Capsule Endoscopy Versus Conventional Upper Endoscopy for the Diagnosis of Esophageal Varices: A Prospective Three-Center Pilot Study. Endoscopy 2006, 38, 31–35. [CrossRef]
- Lu, Y.; Gao, R.; Liao, Z.; Hu, L.H.; Li, Z.S. Meta-Analysis of Capsule Endoscopy in Patients Diagnosed or Suspected with Esophageal Varices. World J Gastroenterol 2009, 15, 1254–1258. [CrossRef]
- Beg S.; Card, T.; Warburton S.; Rahman I.; Wilkes E.; White J.; Ragunath K. Diagnosis of Barrett’s esophagus and esophageal varices using a magnetically assisted capsule endoscopy system. Gastrointestinal Endoscopy 2020, 91, 773–781.
- Jensen, D.M.; Singh, B.; Chavalitdhamrong, D.; Kovacs, T.O.; Carrico, M.; Han, S.-H.B.; Durazo, F. a.; Saab, S. Is Capsule Endoscopy Accurate Enough to Screen Cirrhotics for High Risk Varices & Other Lesions? A Blinded Comparison of EGD & PillCam ESO. Gastrointest Endosc 2008, 67, AB122. [CrossRef]
- Smith, R.A.; Cokkinides, V.; von Eschenbach, A.C.; Levin, B.; Cohen, C.; Runowicz, C.D.; Sener, S.; Saslow, D.; Eyre, H.J. American Cancer Society Guidelines for the Early Detection of Cancer. CA Cancer J Clin 2002, 52, 8–22. [CrossRef]
- Cobrin, G.M.; Pittman, R.H.; Lewis, B.S. Increased Diagnostic Yield of Small Bowel Tumors with Capsule Endoscopy. Cancer 2006, 107, 22–27. [CrossRef]
- Delvaux, M.; Gay, G. Capsule Endoscopy: Technique and Indications. Best Pract Res Clin Gastroenterol 2008, 22, 813–837. [CrossRef]
- Alquist, D.; Fennerty, B.; Fleischer, D.; McDonnell, W.M.; McGill, D.B.; Waring, P.; Wilcox, C.M.; Winawer, S. American Gastroenterological Association Medical Position Statement: Evaluation and Management of Occult and Obscure Gastrointestinal Bleeding. Gastroenterology 2000, 118. [CrossRef]
- Rockey, D.C. Occult and Obscure Gastrointestinal Bleeding: Causes and Clinical Management. Nat Rev Gastroenterol Hepatol 2010, 7, 265–279. [CrossRef]
- Pennazio, M.; Santucci, R.; Rondonotti, E.; Abbiati, C.; Beccari, G.; Rossini, F.P.; De Franchis, R. Outcome of Patients with Obscure Gastrointestinal Bleeding after Capsule Endoscopy: Report of 100 Consecutive Cases. Gastroenterology 2004, 126, 643–653. [CrossRef]
- Triester, S.L.; Leighton, J.A.; Leontiadis, G.I.; Gurudu, S.R.; Fleischer, D.E.; Hara, A.K.; Heigh, R.I.; Shiff, A.D.; Sharma, V.K. A Meta-Analysis of the Yield of Capsule Endoscopy Compared to Other Diagnostic Modalities in Patients with Non-Stricturing Small Bowel Crohn’s Disease. Official journal of the American College of Gastroenterology | ACG 2006, 101.
- Yamamoto, H.; Sekine, Y.; Sato, Y.; Higashizawa, T.; Miyata, T.; Iino, S.; Ido, K.; Sugano, K. Total Enteroscopy with a Nonsurgical Steerable Double-Balloon Method. Gastrointest Endosc 2001, 53, 216–220. [CrossRef]
- Rondonotti, E.; Koulaouzidis, A.; Silvia, P.; Franco, R.; Pennazio, M. Obscure Gastrointestinal Bleeding and Iron-Deficiency Anemia-Where Does Capsule Endoscopy Fit? Tech Gastrointest Endosc 2015, 17, 12–18.
- Lennard-Jones, J.E. Classification of Inflammatory Bowel Disease. Scand J Gastroenterol 1989, 24, 2–6. [CrossRef]
- Fornaro, R.; Frascio, M.; Denegri, A.; Stabilini, C.; Imperatore, M.; F, M.; Fabrizio, L.; E., G. Malattia Di Crohn e Cancro. Ann Ital Chir 2009, 80, 119–125.
- Baumgart, D.C.; Sandborn, W.J. Crohn’s Disease. The Lancet 2012, 380, 1590–1605. [CrossRef]
- Schulmann, K.; Hollerbach, S.; Schmiegel, W. Diagnosing Small Bowel Crohn’s Disease with Wireless Capsule Endoscopy. Gut 2003, 52, 1531. [CrossRef]
- Albert, J.G.; Martiny, F.; Krummenerl, A.; Stock, K.; Leßke, J.; Göbel, C.M.; Lotterer, E.; Nietsch, H.H.; Behrmann, C.; Fleig, W.E. Diagnosis of Small Bowel Crohn’s Disease: A Prospective Comparison of Capsule Endoscopy with Magnetic Resonance Imaging and Fluoroscopic Enteroclysis. Gut 2005, 54, 1721. [CrossRef]
- Odeyinka O.; Alhashimi R.; Thoota S. The Role of Capsule Endoscopy in Crohn’s Disease: A Review. Cureus 2022, 14(7). [CrossRef]
- Leighton, J.A.; Helper, D.J.; Gralnek, I.M.; Dotan, I.; Fernandez-Urien, I.; Lahat, A.; Malik, P.; Mullin, G.E.; Rosa, B. Comparing Diagnostic Yield of a Novel Pan-Enteric Video Capsule Endoscope with Ileocolonoscopy in Patients with Active Crohn’s Disease: A Feasibility Study. Gastrointest Endosc 2017, 85, 196-205.e1. [CrossRef]
- Fry E J; Shiff A D; Heigh R I; Sharma V K; Post J K; Hentz J G; Fleischer D E; Leighton J A, L.C.C. The Yield of Capsule Endoscopy in Patients with Abdominal Pain or Diarrhea. Endoscopy 2006, 38, 498–502. [CrossRef]
- Petroniene, R.; Dubcenco, E.; Baker, J.P.; Ottaway, C.A.; Tang, S.-J.; Zanati, S.A.; Streutker, C.J.; Gardiner, G.W.; Warren, R.E.; Jeejeebhoy, K.N. Given® Capsule Endoscopy in Celiac Disease: Evaluation of Diagnostic Accuracy and Interobserver Agreement. Official journal of the American College of Gastroenterology | ACG 2005, 100.
- Green, P.H.R.; Rubin, M. Capsule Endoscopy in Celiac Disease. Gastrointest Endosc 2005, 62, 797–799. [CrossRef]
- Lewis, S.K.; Semrad, C.E. Capsule Endoscopy and Enteroscopy in Celiac Disease. Gastroenterol Clin North Am 2019, 48, 73–84. [CrossRef]
- Luján-Sanchis, M.; Pérez-Cuadrado-Robles, E.; García-Lledó, J.; Fernández, J.F.J.; Elli, L.; Jiménez-García, V.A.; Egea-Valenzuela, J.; Valle-Muñoz, J.; Carretero-Ribón, C.; Fernández-Urién-Sainz, I.; et al. Role of Capsule Endoscopy in Suspected Celiac Disease: A European Multi-Centre Study. World J Gastroenterol 2017, 23, 703–711. [CrossRef]
- Soares L; Vilas Boas G; Pinho† C, J.L. Wireless Capsule Endoscopy for Evaluation of Phenotypic Expression of Small-Bowel Polyps in Patients with Peutz-Jeghers Syndrome and in Symptomatic First-Degree Relatives. Endoscopy 2004, 36, 1060–1066. [CrossRef]
- 68. Soares L; Vilas Boas G; Pinho† C, J.L. Wireless Capsule Endoscopy for Evaluation of Phenotypic Expression of Small-Bowel Polyps in Patients with Peutz-Jeghers Syndrome and in Symptomatic First-Degree Relatives. Endoscopy 2004, 36, 1060–1066. [CrossRef]
- Swain, C.P.; Gong, F.; Mills, T.N. Wireless Transmission of a Colour Television Moving Image from the Stomach Using a Miniature CCD Camera, Light Source and Microwave Transmitter. Gastrointest Endosc 1997, 45, AB40. [CrossRef]
- Appleyard, M.; Glukhovsky, A.; Swain, P. Wireless-Capsule Diagnostic Endoscopy for Recurrent Small-Bowel Bleeding. New England Journal of Medicine 2001, 344, 232–233. [CrossRef]
- Kornbluth, A.; Legnani, P.; Lewis, B.S. Video Capsule Endoscopy in Inflammatory Bowel Disease: Past, Present, and Future. Inflamm Bowel Dis 2004, 10, 278–285. [CrossRef]
- Cave P; de Franchis R; Lewis B S, D.L. ICCE Consensus for Capsule Retention. Endoscopy 2005, 37, 1065–1067. [CrossRef]
- Lewis J F; Seidman E G, B.S.R. Capsule Endoscopy 2005: Results of the 2005 International Consensus Conference - Introduction. Endoscopy 2005, 37, 1038–1039. [CrossRef]
- de Franchis A; Barkin J; Cave D; Filoche B, R.A. ICCE Consensus for Bowel Preparation and Prokinetics. Endoscopy 2005, 37, 1040–1045. [CrossRef]
- Pennazio G; Goldfarb N, M.E. ICCE Consensus for Obscure Gastrointestinal Bleeding. Endoscopy 2005, 37, 1046–1050. [CrossRef]
- Kornbluth J F; Leighton J A; Loftus E, A.C. ICCE Consensus for Inflammatory Bowel Disease. Endoscopy 2005, 37, 1051–1054. [CrossRef]
- Sharma R; Sharma P; Faigel D, V.K.E. ICCE Consensus for Esophageal Capsule Endoscopy. Endoscopy 2005, 37, 1060–1064. [CrossRef]
- Gurudu, S.R.; Vargas, H.E.; Leighton, J.A. New Frontiers in Small-Bowel Imaging: The Expanding Technology of Capsule Endoscopy and Its Impact in Clinical Gastroenterology. Rev Gastroenterol Disord 2008, 8.
- Available online: https://www.medtronic.com/covidien/en-us/products/capsule-endoscopy/pillcam-sb3-system.html (accessed on 1 Sep 2023).
- Galmiche, J.P.; Sacher-Huvelin, S.; Coron, E.; Cholet, F.; Soussan, E. Ben; Sébille, V.; Filoche, B.; D’Abrigeon, G.; Antonietti, M.; Robaszkiewicz, M.; et al. Screening for Esophagitis and Barrett’s Esophagus with Wireless Esophageal Capsule Endoscopy: A Multicenter Prospective Trial in Patients with Reflux Symptoms. American Journal of Gastroenterology 2008, 103, 538–545. [CrossRef]
- Eliakim, R.; Yassin, K.; Shlomi, I.; Suissa, A.; Eisen, G.M. A Novel Diagnostic Tool for Detecting Oesophageal Pathology: The PillCam Oesophageal Video Capsule. Aliment Pharmacol Ther 2004, 20, 1083–1089. [CrossRef]
- Eliakim, R.; Sharma, V.K.; Yassin, K.; Adler, S.N.; Jacob, H.; Cave, D.R.; Sachdev, R.; Mitty, R.D.; Hartmann, D.; Schilling, D.; et al. A Prospective Study of the Diagnostic Accuracy of PillCam ESO Esophageal Capsule Endoscopy versus Conventional Upper Endoscopy in Patients with Chronic Gastroesophageal Reflux Diseases. J Clin Gastroenterol 2005, 39, 572–578.
- Römmele, C.; Brueckner, J.; Messmann, H.; Gölder, S.K. Clinical Experience with the PillCam Patency Capsule Prior to Video Capsule Endoscopy: A Real-World Experience. Gastroenterol Res Pract 2016, 2016. [CrossRef]
- Spada, C.; Spera, G.; Riccioni, M.; Biancone, L.; Petruzziello, L.; Tringali, A.; Familiari, P.; Marchese, M.; Onder, G.; Mutignani, M.; et al. A Novel Diagnostic Tool for Detecting Functional Patency of the Small Bowel: The given Patency Capsule. Endoscopy 2005, 37. [CrossRef]
- Caunedo-Álvarez, Á.; Romero-Vazquez, J.; Herrerias-Gutierrez, J.M. Patency© and Agile© Capsules. World J Gastroenterol 2008, 14. [CrossRef]
- Li, C.Y.; Zhang, B.L.; Chen, C.X.; Li, Y.M. OMOM Capsule Endoscopy in Diagnosis of Small Bowel Disease. J Zhejiang Univ Sci B 2008, 9. [CrossRef]
- Moglia, A.; Menciassi, A.; Schurr, M.O.; Dario, P. Wireless Capsule Endoscopy: From Diagnostic Devices to Multipurpose Robotic Systems. Biomed Microdevices 2007, 9. [CrossRef]
- Bang, S.; Park, J.Y.; Jeong, S.; Kim, Y.H.; Shim, H.B.; Kim, T.S.; Lee, D.H.; Song, S.Y. First Clinical Trial of the “MiRo” Capsule Endoscope by Using a Novel Transmission Technology: Electric-Field Propagation. Gastrointest Endosc 2009, 69, 253–259. [CrossRef]
- Available online: https://www.olympus.co.uk/medical/en/Products-and-solutions/Products/Product/ENDOCAPSULE-10-System.html (accessed on 1 Sep 2023).
- Hartmann, D.; Eickhoff, A.; Damian, U.; Riemann, J.F. Diagnosis of Small-Bowel Pathology Using Paired Capsule Endoscopy with Two Different Devices: A Randomized Study. Endoscopy 2007, 39. [CrossRef]
- Mussetto, A.; Fuccio, L.; Dari, S.; Gasperoni, S.; Cantoni, F.; Brancaccio, M.L.; Triossi, O.; Casetti, T. MiroCam Capsule for Obscure Gastrointestinal Bleeding: A Prospective, Single Centre Experience. Digestive and Liver Disease 2013, 45, 124–128. [CrossRef]
- Esaki, M.; Matsumoto, T. Capsule Endoscopy. In Endoscopy in the Diagnosis of Small Intestine Diseases; Matsui, T., Matsumoto, T., Aoyagi, K., Eds.; Springer Japan: Tokyo, 2014; pp. 19–23 ISBN 978-4-431-54352-7.
- Uchiyama, A.; Takizawa, H.; Yokoi, T.; Mizuno, H. Encapsulated Endoscope System in Which Endoscope Moves in Lumen by Itself and Rotation of Image of Region to Be Observed Is Ceased 2007.
- Hu, C.; Gao, M.; Chen, Z.; Zhang, H.; Liu, S. Magnetic Analysis and Simulations of a Self-Propelled Capsule Endoscope. In Proceedings of the 2010 11th International Conference on Thermal, Mechanical and Multi-Physics Simulation, and Experiments in Microelectronics and Microsystems, EuroSimE 2010; 2010.
- Matsui, T.; Murata, S.; Honda, T. Fabrication of Magnetically Driven Biopsy Mechanism Applicable to Capsule-Type Medical Device. Journal of Robotics and Mechatronics 2018, 30. [CrossRef]
- Carpi, F.; Galbiati, S.; Carpi, A. Controlled Navigation of Endoscopic Capsules: Concept and Preliminary Experimental Investigations. IEEE Trans Biomed Eng 2007, 54. [CrossRef]
- Casanovas, O.A. Enabling Active Locomotion and Advanced Features in Capsule Endoscopy, 2012.
- Daniell, M.D.; Hill, J.S. A HISTORY OF PHOTODYNAMIC THERAPY. Australian and New Zealand Journal of Surgery 1991, 61.
- Ackroyd, R.; Kelty, C.; Brown, N.; Reed, M. The History of Photodetection and Photodynamic Therapy¶. Photochem Photobiol 2007, 74. [CrossRef]
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Photodynamic Therapy for Cancer. Nat Rev Cancer 2003, 3, 380–387. [CrossRef]
- Dhaneshwar, S.; Patil, K.; Bulbule, M.; Kinjawadekar, V.; Joshi, D.; Joshi, V. Photodynamic Therapy for Cancer. Int. J Pharm Sci Rev Res 2014, 27.
- Weishaupt, K.R.; Gomer, C.J.; Dougherty, T.J. Identification of Singlet Oxygen as the Cytotoxic Agent in Photo-Inactivation of a Murine Tumor. Cancer Res 1976, 36.
- Triesscheijn, M.; Baas, P.; Schellens, J.H.M.; Stewart, F.A. Photodynamic Therapy in Oncology. Oncologist 2006, 11, 1034–1044. [CrossRef]
- McCaughan, J.S.; Hicks, W.; Laufman, L.; May, E.; Roach, R. Palliation of Esophageal Malignancy with Photoradiation Therapy. Cancer 1984, 54. [CrossRef]
- Schweitzer, V.G.; Bologna, S.; Batra, S.K. Head and Neck and Plastic Surgery A Targeted Problem and Its Solution. Laryngoscope 1993, 103. [CrossRef]
- Qumseya, B.J.; David, W.; Wolfsen, H.C. Photodynamic Therapy for Barrett’s Esophagus and Esophageal Carcinoma. Clin Endosc 2013, 46. [CrossRef]
- Moghissi, K.; Dixon, K.; Thorpe, J.A.C.; Stringer, M.; Moore, P.J. The Role of Photodynamic Therapy (PDT) in Inoperable Oesophageal Cancer. European Journal of Cardio-thoracic Surgery 2000, 17. [CrossRef]
- Sibille, A.; Lambert, R.; Souquet, J.C.; Sabben, G.; Descos, F. Long-Term Survival after Photodynamic Therapy for Esophageal Cancer. Gastroenterology 1995, 108. [CrossRef]
- Grosjean, P.; Savary, J.F.; Mizeret, J.; Wagnieres, G.; Woodtli, A.; Theumann, J.F.; Fontolliet, C.; Van Den Bergh, H.; Monnier, P. Photodynamic Therapy for Cancer of the Upper Aerodigestive Tract Using Tetra(m-Hydroxyphenyl)Chlorin. J Clin Laser Med Surg 1996, 14. [CrossRef]
- Brown, L.M.; Devesa, S.S. Epidemiologic Trends in Esophageal and Gastric Cancer in the United States. Surg Oncol Clin N Am 2002, 11.
- OVERHOLT, B.F.; PANJEHPOUR, M. Photodynamic Therapy in Barrett’s Esophagus. J Clin Laser Med Surg 1996, 14, 245–249. [CrossRef]
- Overholt, B.F.; Panjehpour, M.; Haydek, J.M. Photodynamic Therapy for Barrett’s Esophagus: Follow-up in 100 Patients. Gastrointest Endosc 1999, 49. [CrossRef]
- Costa, C.G.; Gomes, J.M.; Wolffenbuttel, R.F.; Correia, J.H. Optical Microsystem Design and Fabrication for Medical Image Magnification. Microsystem Technologies 2016, 22, 1747–1755. [CrossRef]
- Gounella, R.H.; Leite, I.S.; Inada, N.M.; Do Carmo, J.P.P. Wireless Portable Evaluation Platform for Photodynamic Therapy: In Vitro Assays on Human Gastric Adenocarcinoma Cells. IEEE Sens J 2020, 20, 13950–13958. [CrossRef]
- Teubner, D.; Kiesslich, R.; Matsumoto, T.; Rey, J.W.; Hoffman, A. Beyond Standard Image-Enhanced Endoscopy Confocal Endomicroscopy. Gastrointest Endosc Clin N Am 2014, 24.
- Clark, C.; Turner, J. Diagnostic Modalities for Inflammatory Bowel Disease. Serologic Markers and Endoscopy. Surgical Clinics of North America 2015, 95.
- Ribeiro, J.F.; Costa, A.C.; Gomes, J.M.; Costa, C.G.; Goncalves, S.B.; Wolffenbuttel, R.F.; Correia, J.H. PDMS Microlenses for Optical Biopsy Microsystems. IEEE Transactions on Industrial Electronics 2017, 64. [CrossRef]
- Tabatabaei, N.; Kang, D.; Wu, T.; Kim, M.; Carruth, R.W.; Leung, J.; Sauk, J.S.; Shreffler, W.; Yuan, Q.; Katz, A.; et al. Tethered Confocal Endomicroscopy Capsule for Diagnosis and Monitoring of Eosinophilic Esophagitis. Biomed Opt Express 2014, 5, 197–207. [CrossRef]
- Hou, R.; Le, T.; Murgu, S.D.; Chen, Z.; Brenner, M. Recent Advances in Optical Coherence Tomography for the Diagnoses of Lung Disorders. Expert Rev Respir Med 2011, 5, 711–724. [CrossRef]
- Bouma, B.E. (viaf)26406747; Tearney, G.J. Handbook of Optical Coherence Tomography; New York : Marcel Dekker, 2002; ISBN 0824705580.
- Pawley, J.B.; Masters, B.R. Handbook of Biological Confocal Microscopy, Third Edition. J Biomed Opt 2008, 13. [CrossRef]
- Gora, M.J.; Quénéhervé, L.; Carruth, R.W.; Lu, W.; Rosenberg, M.; Sauk, J.S.; Fasano, A.; Lauwers, G.Y.; Nishioka, N.S.; Tearney, G.J. Tethered Capsule Endomicroscopy for Microscopic Imaging of the Esophagus, Stomach, and Duodenum without Sedation in Humans (with Video). Gastrointest Endosc 2018, 88, 830-840.e3. [CrossRef]
- Maciel, M.J.; Costa, C.G.; Silva, M.F.; Peixoto, A.C.; Wolffenbuttel, R.F.; Correia, J.H. A Wafer-Level Miniaturized Michelson Interferometer on Glass Substrate for Optical Coherence Tomography Applications. Sens Actuators A Phys 2016, 242, 210–216. [CrossRef]
- Maciel, M.J.; Rosa, C.C.; Wolffenbuttel, R.F.; Correia, J.H. Optical Coherence Tomography within a Single Microsystem. J Phys D Appl Phys 2018, 51. [CrossRef]
- Pimenta, S.; Castanheira, E.M.S.; Minas, G. Optical Microsystem for Analysis of Diffuse Reflectance and Fluorescence Signals Applied to Early Gastrointestinal Cancer Detection. Sensors (Switzerland) 2015, 15. [CrossRef]
- Yu, C.-C.; Lau, C.; O’Donoghue, G.; Mirkovic, J.; McGee, S.; Galindo, L.; Elackattu, A.; Stier, E.; Grillone, G.; Badizadegan, K.; et al. Quantitative Spectroscopic Imaging for Non-Invasive Early Cancer Detection. Opt Express 2008, 16. [CrossRef]
- Georgakoudi, I. The Color of Cancer. J Lumin 2006, 119–120, 75–83. [CrossRef]
- Brown, J.Q.; Vishwanath, K.; Palmer, G.M.; Ramanujam, N. Advances in Quantitative UV-Visible Spectroscopy for Clinical and Pre-Clinical Application in Cancer. Curr Opin Biotechnol 2009, 20.
- Pimenta, S.; Carmo, J.P.; Correia, R.G.; Minas, G.; Castanheira, E.M.S. Characterization of Silicon Photodiodes for Diffuse Reflectance Signal Extraction. In Proceedings of the Proceedings - 2015 IEEE 4th Portuguese Meeting on Bioengineering, ENBENG 2015; 2015.
- Ell, C. Improving Endoscopic Resolution and Sampling: Fluorescence Techniques. Gut 2003, 52, iv30. [CrossRef]
- Georgakoudi, I.; Jacobson, B.C.; Van Dam, J.; Backman, V.; Wallace, M.B.; Müller, M.G.; Zhang, Q.; Badizadegan, K.; Sun, D.; Thomas, G.A.; et al. Fluorescence, Reflectance, and Light-Scattering Spectroscopy for Evaluating Dysplasia in Patients with Barrett’s Esophagus. Gastroenterology 2001, 120. [CrossRef]
- Mayinger, B.; Jordan, M.; Horner, P.; Gerlach, C.; Muehldorfer, S.; Bittorf, B.R.; Matzel, K.E.; Hohenberger, W.; Hahn, E.G.; Guenther, K. Endoscopic Light-Induced Autofluorescence Spectroscopy for the Diagnosis of Colorectal Cancer and Adenoma. J Photochem Photobiol B 2003, 70. [CrossRef]
- Liu, N.R.; Chen, G.N.; Wu, S.S.; Chen, R. Distinguishing Human Normal or Cancerous Esophagus Tissue Ex Vivo Using Multiphoton Microscopy. Journal of Optics (United Kingdom) 2014, 16. [CrossRef]
- Yu, B.; Lo, J.Y.; Kuech, T.F.; Palmer, G.M.; Bender, J.E.; Ramanujam, N. Cost-Effective Diffuse Reflectance Spectroscopy Device for Quantifying Tissue Absorption and Scattering in Vivo. J Biomed Opt 2008, 13. [CrossRef]
- 135. Yu, B.; Lo, J.Y.; Kuech, T.F.; Palmer, G.M.; Bender, J.E.; Ramanujam, N. Cost-Effective Diffuse Reflectance Spectroscopy Device for Quantifying Tissue Absorption and Scattering in Vivo. J Biomed Opt 2008, 13. [CrossRef]
- Wang, L.; Zhang, G.; Luo, J.C.; Zeng, F.; Wang, Q.Z.; Alfano, S.A.; Katz, A.; Zevallos, M.; Alfano, R.R. Wireless Spectroscopic Compact Photonic Explorer for Diagnostic Optical Imaging. Biomed Microdevices 2005, 7, 111–115. [CrossRef]
- Alfano, R.R.; Alfano, S.; Wang, Q.; Ho, P.P. Remote-Controllable, Micro-Scale Device for Use in in Vivo Medical Diagnosis and/or Treatment 2001.
- Alfano, R.; Katz, A.; Alfano, S. Micro-Scale Compact Device for in Vivo Medical Diagnosis Combining Optical Imaging and Point Fluorescence Spectroscopy 2005.
- Gono, K.; Obi, T.; Yamaguchi, M.; Oyama, N.; Machida, H.; Sano, Y.; Yoshida, S.; Hamamoto, Y.; Endo, T. Appearance of Enhanced Tissue Features in Narrow-Band Endoscopic Imaging. J Biomed Opt 2004, 9, 568–577. [CrossRef]
- Gono, K. An Introduction to High-Resolution Endoscopy and Narrowband Imaging. In Comprehensive Atlas of High-Resolution Endoscopy and Narrowband Imaging; 2017; pp. 7–15 ISBN 9781118705940.
- VAN DEN BROEK, F.J.C.; FOCKENS, P.; DEKKER, E. Review Article: New Developments in Colonic Imaging. Aliment Pharmacol Ther 2007, 26, 91–99. [CrossRef]
- Machida Y; Hamamoto Y; Muto M; Kozu T; Tajiri H; Yoshida S, H.S. Narrow-Band Imaging in the Diagnosis of Colorectal Mucosal Lesions: A Pilot Study. Endoscopy 2004, 36, 1094–1098. [CrossRef]
- Muto, M.; Horimatsu, T.; Ezoe, Y.; Morita, S.; Miyamoto, S. Improving Visualization Techniques by Narrow Band Imaging and Magnification Endoscopy. J Gastroenterol Hepatol 2009, 24, 1333–1346. [CrossRef]
- Kuznetsov R; Rey J -F, K.L. Narrow-Band Imaging: Potential and Limitations. Endoscopy 2006, 38, 76–81. [CrossRef]
- Gono, K.; Yamazaki, K.; Doguchi, N.; Nonami, T.; Obi, T.; Yamaguchi, M.; Ohyama, N.; Machida, H.; Sano, Y.; Yoshida, S.; et al. Endoscopic Observation of Tissue by Narrowband Illumination. Opt Rev 2003, 10, 211–215. [CrossRef]
- Wong Kee Song, L.M.; Adler, D.G.; Conway, J.D.; Diehl, D.L.; Farraye, F.A.; Kantsevoy, S. V; Kwon, R.; Mamula, P.; Rodriguez, B.; Shah, R.J.; et al. Narrow Band Imaging and Multiband Imaging. Gastrointest Endosc 2008, 67, 581–589. [CrossRef]
- Yao M; Fujisaki J, K.K. Techniques Using the Hemoglobin Index of the Gastric Mucosa. Endoscopy 2005, 37, 479–486. [CrossRef]
- Kato, M.; Nakagawa, S.; Shinmizu, Y.; Sugiyama, T.; Asaka, M. THE EFFICACY OF MAGNIFYING ENDOSCOPY WITH ADAPTIVE INDEX OF HEMOGLOBIN ENHANCEMENT FOR DIAGNOSIS OF HELICOBACTER PYLORI-INDUCED GASTRITIS. Digestive Endoscopy 2002, 14, S72–S75. [CrossRef]
- Toyota, Y.; Honda, H.; Omoya, T.; Inayama, K.; Suzuki, M.; Kubo, K.; Nakasono, M.; Muguruma, N.; Okamura, S.; Shimizu, I.; et al. Usefulness of a Hemoglobin Index Determined by Electronic Endoscopy in the Diagnosis of Helicobacter Pylori Gastritis. Digestive Endoscopy 2002, 14, 156–162. [CrossRef]
- Macleod, H.A. Thin-Film Optical Filters, Fifth Edition; CRC Press, 2017; ISBN 9781351982245.
- Correia, J.H.G. Optical Microsystems in Silicon Based on a Fabry-Perot Resonance Cavity: Application for Spectral Analysis of Visible Light; Delft University Press, 1999;
- Gounella, R.H.; Granado, T.C.; da Costa, J.P.C.; Carmo, J.P. Optical Filters for Narrow Band Light Adaptation on Imaging Devices. IEEE Journal of Selected Topics in Quantum Electronics 2021, 27, 1–8. [CrossRef]
- Ragunath K.; Chiu P. A primer to image enhanced endoscopy. Transl Gastroenterol Hepatol. 2022. [CrossRef]
- Akarsu C.; Sahbaz N.A.; Dural A.C.; Unsal M.G.; Kones O.; Kocatas A.; Halicioglu I.; Alis H. FICE vs Narrow Band Imaging for In Vivo Histologic Diagnosis of Polyps. JSLS 2016. [CrossRef]
- Subramaniam S.; Kandiah K.; Schoon E.; Aepli P.; Hayee B.; Pischel A.; Stefanovic M.; Alkandari A.; Coron E.; Omae M.; Baldaque-Silva F.; Maselli R.; Bisschops R.; Sharma P.; Repici A.; Bhandari P. Development and validation of the international Blue Light Imaging for Barrett’s Neoplasia Classification. Gastrointest Endosc. 2020. [CrossRef]
- Osawa H.; Yamamoto H.; Miura Y.; Sasao W.; Ino Y.; Satoh H.; Satoh K.; Sugano K. Blue Laser Imaging Provides Excellent Endoscopic Images of Upper Gastrointestinal Lesions, Video Journal and Encyclopedia of GI Endoscopy, 2014. [CrossRef]
- Lee C.K.; Lee S.H.; Hwangbo Y. Narrow-band imaging versus I-SCAN for the real-time histological prediction of diminutive colonic polyps: a prospective comparative study by using the simple unified endoscopic classification. Gastrointest Endosc. 2011. [CrossRef]
- Cammarota, G.; Ianiro, G.; Sparano, L. DigDisSci, 1: 58, 1287.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).