Submitted:
15 May 2023
Posted:
15 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experiments
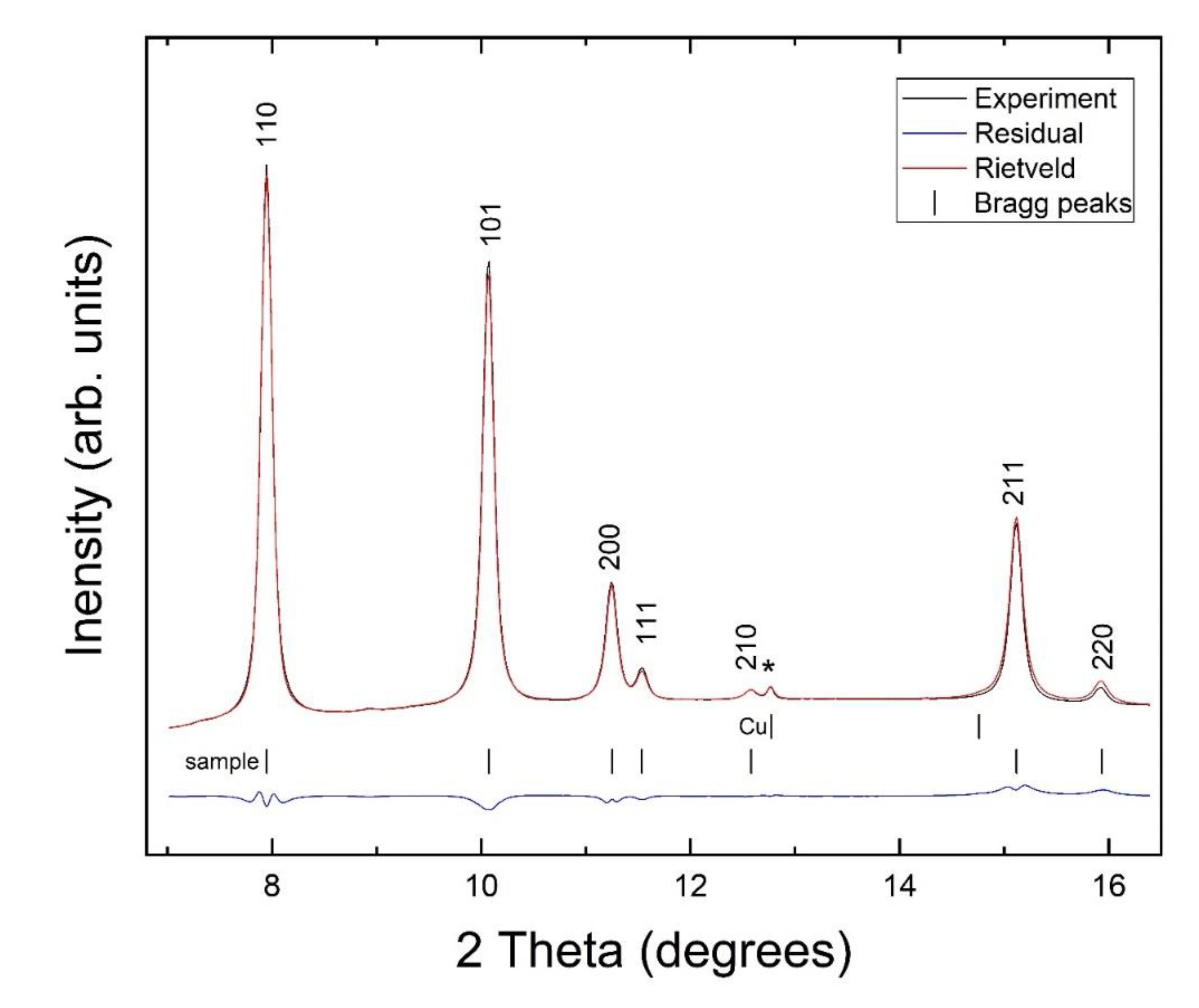
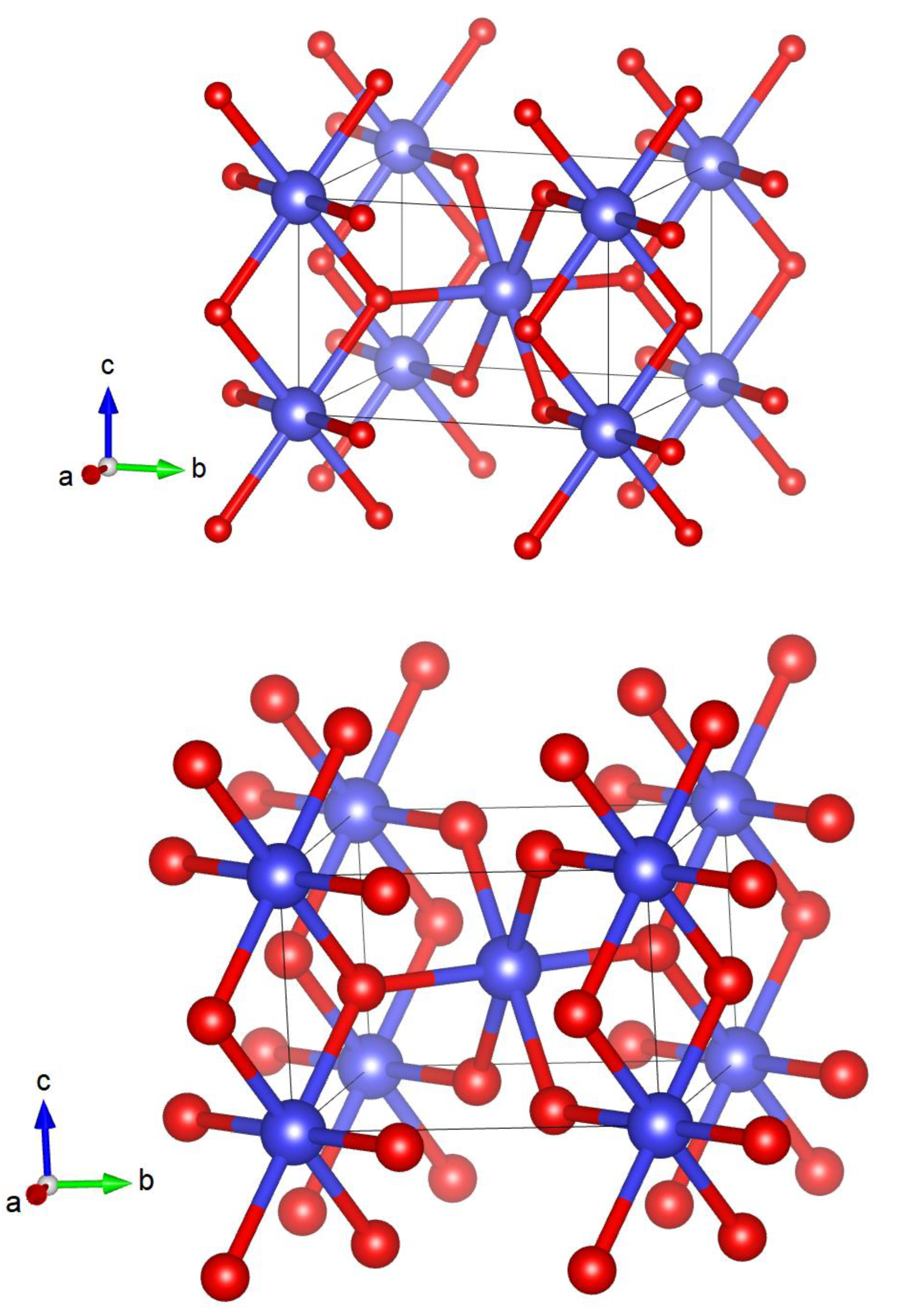
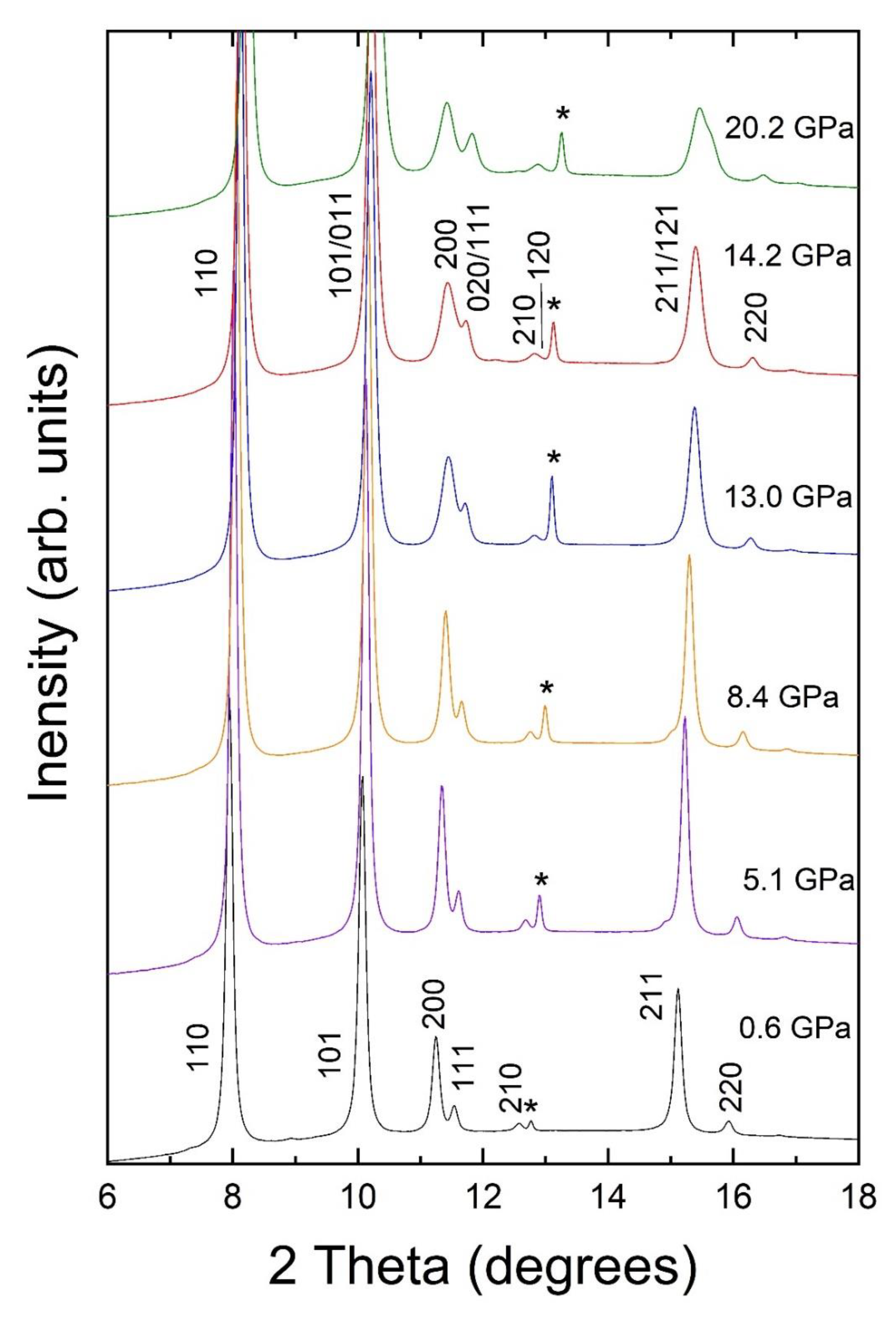
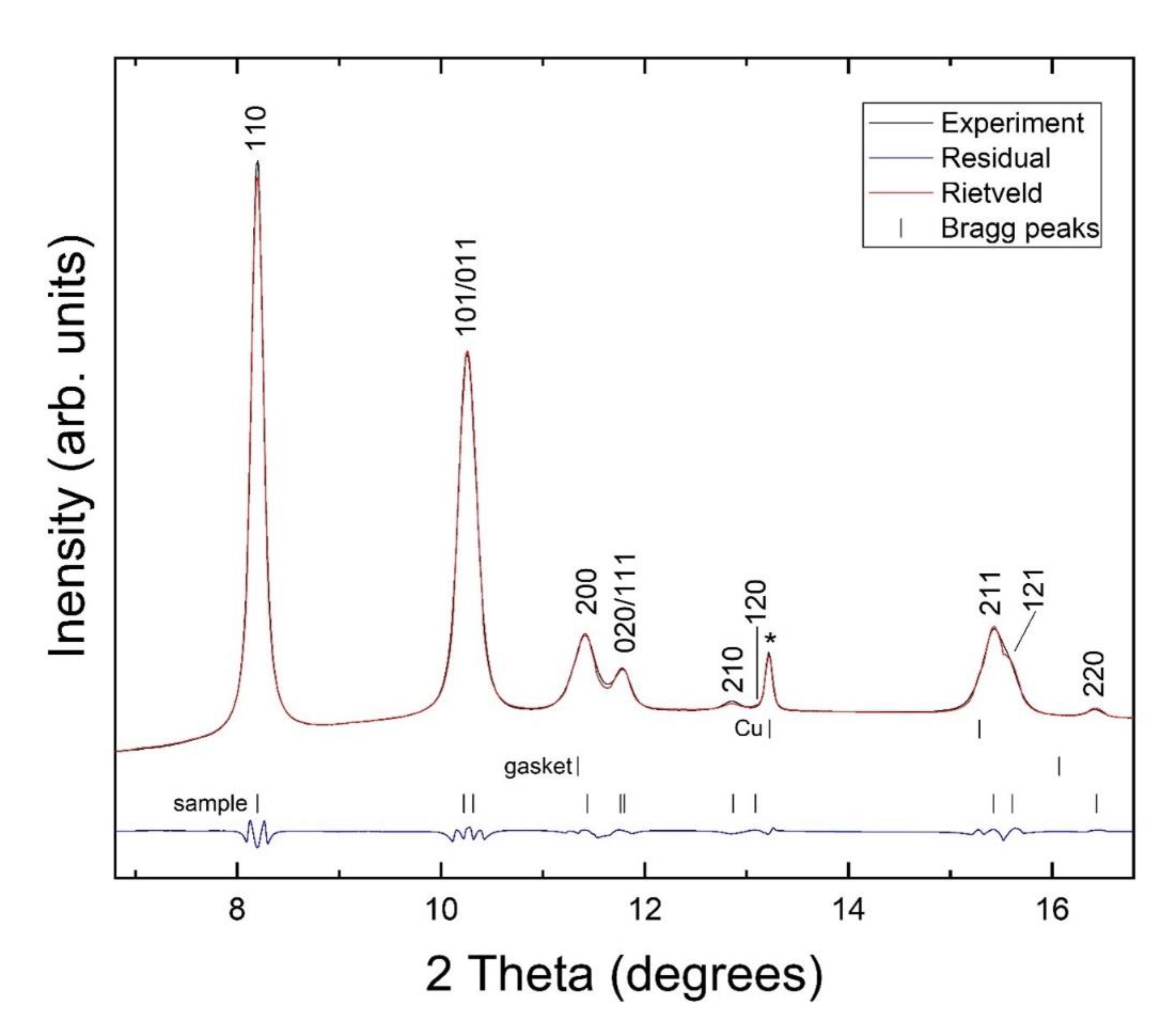
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgements
Conflicts of Interest
References
- Gleiter, H. ; Nanocrystalline materials. Prog. Mat. Science 1989, 33, 223–315. [Google Scholar] [CrossRef]
- Siegel, R. ; Cluster-assembled nanophase materials. Annual Review of Materials Science 1991, 21, 559–578. [Google Scholar] [CrossRef]
- Kodama, R. ; Magnetic Nanoparticles. J. Magn. Magn. Mat. 1999, 200, 359–372. [Google Scholar] [CrossRef]
- Lv, H.; et al. , Effect on grain size on pressure-induced structural transition in Mn3O4. J. Phys. Chem. C 2012, 116, 2165–2171. [Google Scholar] [CrossRef]
- Srihari, V.; Verma, A.; Pandey, K.; Vishwanadh, B.; Panchal, V.; Garg, N.; Errandonea, D. ; Making Yb2Hf2O7 Defect Fluorite Uncompressible by Particle Size Reduction. J. Phys. Chem. C 2021, 125, 27354–27362. [Google Scholar] [CrossRef]
- Jiang, J.Z.; Gerward, L.; Frost, D.; Secco, R.; Peyronneau, J.; Olsen, J.S. Grain-size effect on pressure-induced semiconductor-to-metal transition in ZnS. J. Appl. Phys. 1999, 86, 6608–6610. [Google Scholar] [CrossRef]
- Wang, Z.; Tait, K.; Zhao, Y.; Schiferl, D.; Zha, C.; Uchida, H.; Downs, R.T. Size-Induced Reduction of Transition Pressure and Enhancement of Bulk Modulus of AlN Nanocrystals. J. Phys. Chem. B 2004, 108, 11506–11508. [Google Scholar] [CrossRef]
- Wang, Z.; Saxena, S.K.; Pischedda, V.; Liermann, H.P.; Zha, C.S. In situx-ray diffraction study of the pressure-induced phase transformation in nanocrystallineCeO2. Phys. Rev. B 2001, 64, 012102. [Google Scholar] [CrossRef]
- Zvoriste-Walters, C.; Heathman, S.; Jovani-Abril, R.; Spino, J.; Janssen, A.; Caciuffo, R. Crystal size effect on the compressibility of nano-crystalline uranium dioxide. J. Nucl. Mater. 2012, 435, 123–127. [Google Scholar] [CrossRef]
- Bouras, K.; Schmerber, G.; Rinnert, H.; Aureau, D.; Park, H.; Ferblantier, G.; Colis, S.; Fix, T.; Park, C.; Kim, W.K.; et al. Structural, optical and electrical properties of Nd-doped SnO2 thin films fabricated by reactive magnetron sputtering for solar cell devices. Sol. Energy Mater. Sol. Cells 2016, 145, 134–141. [Google Scholar] [CrossRef]
- Wu, J.-M.; Kuo, C.-H. Ultraviolet photodetectors made from SnO2 nanowires. Thin Solid Films 2009, 517, 3870–3873. [Google Scholar] [CrossRef]
- Tsai, M.-Y.; Bierwagen, O.; Speck, J.S. Epitaxial Sb-doped SnO2 and Sn-doped In2O3 transparent conducting oxide contacts on GaN-based light emitting diodes. Thin Solid Films 2016, 605, 186–192. [Google Scholar] [CrossRef]
- Ogale, S.B.; Choudhary, R.J.; Buban, J.P.; Lofland, S.E.; Shinde, S.R.; Kale, S.N.; Kulkarni, V.N.; Higgins, J.; Lanci, C.; Simpson, J.R.; et al. High Temperature Ferromagnetism with a Giant Magnetic Moment in Transparent Co-dopedSnO2−δ. Phys. Rev. Lett. 2003, 91, 077205. [Google Scholar] [CrossRef] [PubMed]
- Tadeev, A.; Delabouglise, G.; Labeau, M. Influence of Pd and Pt additives on the microstructural and electrical properties of SnO2-based sensors. Mater. Sci. Eng. B 1998, 57, 76–83. [Google Scholar] [CrossRef]
- Bose, A.C.; Kalpana, D.; Thangadurai, P.; Ramasamy, S. Synthesis and characterization of nanocrystalline SnO2 and fabrication of lithium cell using nano-SnO2. J. Power Sources 2002, 107, 138–141. [Google Scholar] [CrossRef]
- Haines, J.; Léger, J.M. X-ray diffraction study of the phase transitions and structural evolution of tin dioxide at high pressure:ffRelationships between structure types and implications for other rutile-type dioxides. Phys. Rev. B 1997, 55, 11144–11154. [Google Scholar] [CrossRef]
- Shieh, S.; Kubo, A.; Duffy, T.; Prakapenka, V.; Guoyin, G. ; High pressure phases in SnO2 to 117 GPa. Phys. Rev. B 2006, 73, 14105. [Google Scholar] [CrossRef]
- Parlinski, K.; Kawazoe, Y. ; Ab Initio study of phonons in the rutile structure of SnO2 under pressure. Eur. Phys. J. B 2000, 13, 679–683. [Google Scholar] [CrossRef]
- Hassan, F.; Alaeddine, A.; Zoaeter, M.; Rachidi, I. ; First-principles investigation of SnO2 at high pressure. Inter. J. of Mod. Phys. B 2005, 19, 4081–4092. [Google Scholar] [CrossRef]
- Gracia, L.; Beltran, A.; Andres, J.; Characterization of high-pressure structures and phase transformations in SnO2. A density functional theory study. J. Phys. Chem. B 2007, 111, 6479–6485. [Google Scholar] [CrossRef]
- Casali, R.; Lasave, J.; Caravaca, M.; Koval, S.; Ponce, C.; Migoni, R. ; Ab initio and shell model studies of structural, thermoelastic and vibrational properties of SnO2 under pressure. J. Phys.: Condens. Matter 2013, 25, 135404. [Google Scholar] [PubMed]
- Yang, L.; Fan, W.; Li, Y.; Wei, L.; Zhao, X. Pressure-induced ferroelastic phase transition in SnO2 from density functional theory. J. Chem. Phys. 2014, 140, 164706. [Google Scholar] [CrossRef] [PubMed]
- Hellwig, H.; Goncharov, A.; Gregoryanz, E.; Mao, H.; Hemley, R. ; Brillouin and Raman spectroscopy of the ferroelastic rutile-to-CaCl2 transition in SnO2 at high pressure. Phys. Rev. B 2003, 67, 174110. [Google Scholar] [CrossRef]
- Garg, A.B. Pressure-induced volume anomaly and structural phase transition in nanocrystalline SnO2. Phys. Status solidi (b) 2014, 251, 1380–1385. [Google Scholar] [CrossRef]
- Jiang, J.; Gerward, L.; Olsen, J. Pressure induced phase transformation in nanocrystal SnO2. Scr. Mater. 2001, 44, 1983–1986. [Google Scholar] [CrossRef]
- He, Y.; Liu, J.; Chen, W.; Wang, Y.; Wang, H.; Zeng, Y.; Zhang, G.; Wang, L.; Liu, J.; Hu, T.; Hahn, H.; Gleiter, H.; Jiang, J. ; High pressure behavior of SnO2 nanocrystals. Phys. Rev. B 2005, 72, 212102. [Google Scholar] [CrossRef]
- Grinblat, F.; Ferrari, S.; Pampillo, L.; Saccone, F.; Errandonea, D.; Santamaria-Perez, D.; Segura, A.; Vilaplana, R.; Popescu, C. Compressibility and structural behavior of pure and Fe-doped SnO2 nanocrystals. Solid State Sci. 2017, 64, 91–98. [Google Scholar] [CrossRef]
- Ferrari, S.; Bilovol, V.; Pampillo, L.G.; Grinblat, F.; Saccone, F.D.; Errandonea, D. Characterization of V-doped SnO2 nanoparticles at ambient and high pressures. Mater. Res. Express 2018, 5, 125005. [Google Scholar] [CrossRef]
- Ferrari, S.; Pampillo, L.; Saccone, F. Magnetic properties and environment sites in Fe doped SnO 2 nanoparticles. Mater. Chem. Phys. 2016, 177, 206–212. [Google Scholar] [CrossRef]
- Hazen, R.M.; Finger, L.W. Bulk moduli and high-pressure crystal structures of rutile-type compounds. J. Phys. Chem. Solids 1981, 42, 143–151. [Google Scholar] [CrossRef]
- Bauer, W.; Rutile type compounds. V. Refinements of MnO2 and MgF2. Acta Crystallogr. B 1976, 32, 2200–2204. [Google Scholar]
- Zhang, Z.; Zhou, F.; Lavernia, E.J. On the analysis of grain size in bulk nanocrystalline materials via x-ray diffraction. Met. Mater. Trans. A 2003, 34, 1349–1355. [Google Scholar] [CrossRef]
- Klotz, S.; Chervin, J.-C.; Munsch, P.; Le Marchand, G. Hydrostatic limits of 11 pressure transmitting media. J. Phys. D: Appl. Phys. 2009, 42. [Google Scholar] [CrossRef]
- Dewaele, A.; Loubeyre, P.; Mezouar, M. ; Equation of state of six metals above 94 GPa. Phys. Rev. B 2004, 70, 094112. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B Condens. Matter 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Liu, X.; Gong, W.; Iqbal, J.; He, B.; Yu, R. Structural defects-mediated room-temperature ferromagnetism in Co-doped SnO2 insulating films. Thin Solid Films 2009, 517, 6091–6095. [Google Scholar] [CrossRef]
- Gao, Y.; He, J.; Guo, J. ; Effect of co-doping and defects on electronic, magnetic, and optical properties in SnO2: A first-principles study. Physica B 2022, 639, 413924. [Google Scholar]
- Gonzalez-Platas, J.; Alvaro, M.; Nestola, F.; Angel, R. ; EosFit7-GUI: a new graphical user interface for equation of state calculations, analyses and teaching. J. Appl. Cryst. 2016, 49, 1377–1382. [Google Scholar] [CrossRef]
- Haines, J.; Leger, J.; Schulte, O.; The high-pressure phase transition sequence from the rutile-type through to the cotunnite-type structure in PbO2. J. Phys. Condens. Matter 1996, 8, 1631. [Google Scholar] [CrossRef]
- Ross, N.; Shu, J.; Hazen, R.; Gasparik, T. ; High-pressure crystal chemistry of stishovite. Am. Mineral. 1990, 75, 739–747. [Google Scholar]
- Errandonea, D.; Muñoz, A.; Gonzalez-Platas, J. ; Comment on high pressure x-ray diffraction study of YBO3/ Eu3+, GdBO3, and EuBO3, : Pressure induced amorphization in GdBO3. J. Applied Physics 2014, 115, 216101. [Google Scholar] [CrossRef]
- Anzellini, S.; Errandonea, D.; MacLeod, S.G.; Botella, P.; Daisenberger, D.; De’ath, J.M.; Gonzalez-Platas, J.; Ibáñez, J.; McMahon, M.I.; Munro, K.A.; et al. Phase diagram of calcium at high pressure and high temperature. Phys. Rev. Mater. 2018, 2, 083608. [Google Scholar] [CrossRef]
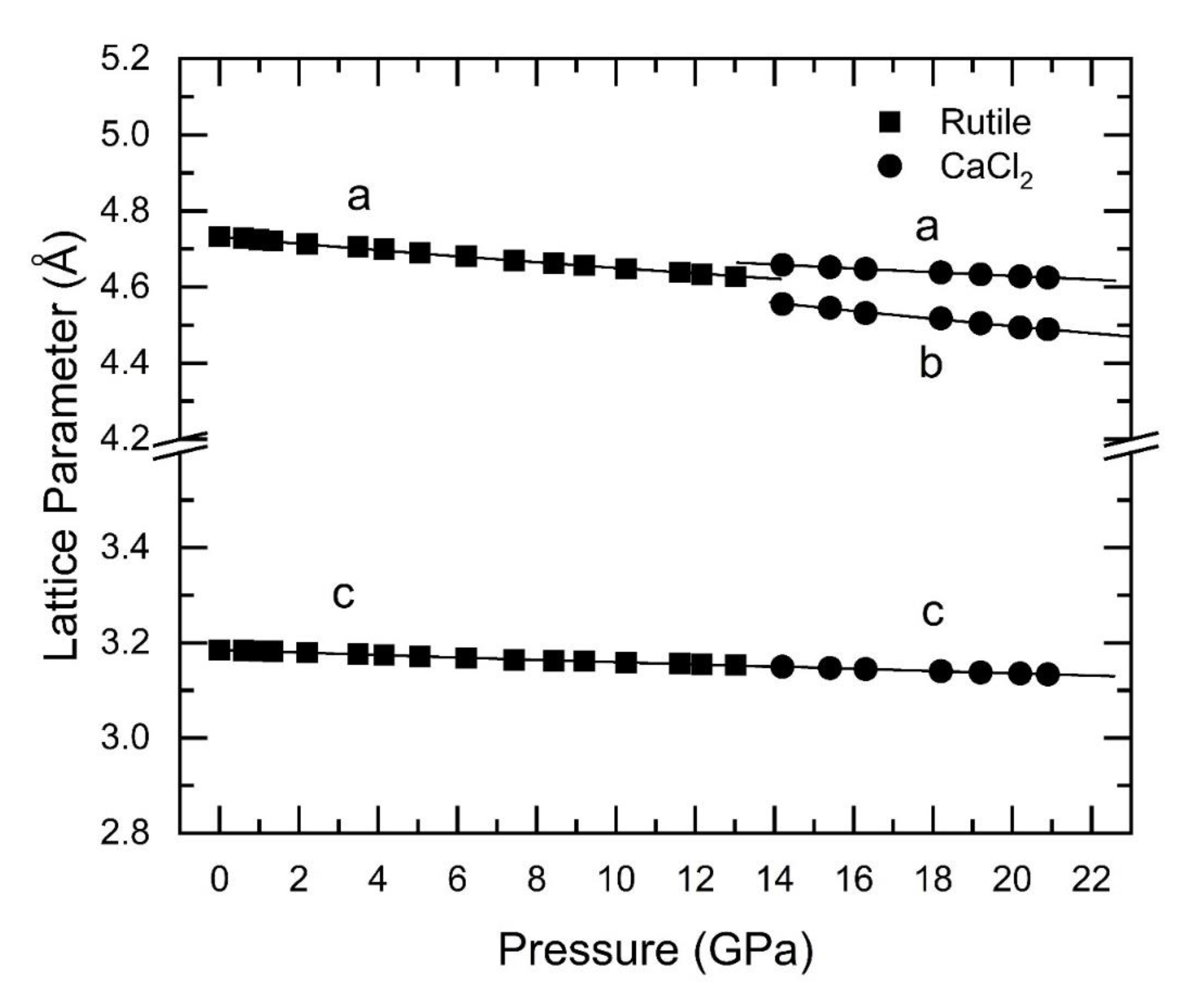
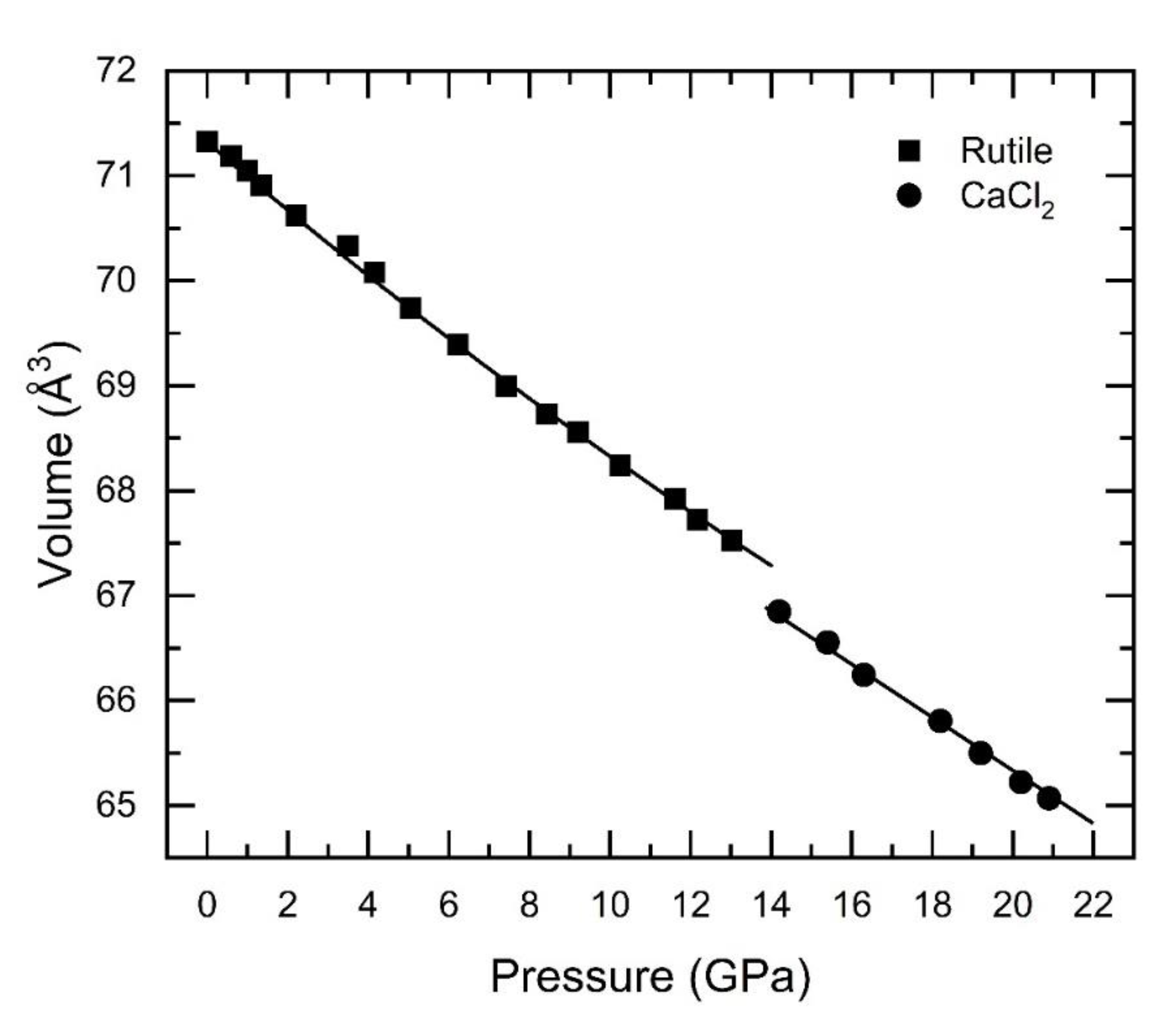
| Sample | Bulk Modulus (GPa) Rutile-type SnO2 |
Bulk Modulus (GPa) CaCl2-type SnO2 |
Ref. |
|---|---|---|---|
| Bulk | 205 | 204 | 17 |
| Bulk | 252 | ---- | 26 |
| Bulk | 205 | 204 | 16 |
| Nanocrystalline (5nm) | 217 | --- | 24 |
| Nanocrystalline (3nm) |
233 | --- | 26 |
| Nanocrystalline (30nm) |
210 | 252 | 27 |
| Nanocrystalline Fe-doped (18nm) | 213 | 256 | 27 |
| Nanocrystalline V-doped (13nm) | 185 | -- | 28 |
| Nanocrystalline Co-doped (15nm) | 213(9) | 228(9) | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





