Preprint
Review
Molecular Mechanisms of the Vicious Cycle between Insulin Resistance and the Inflammatory Response in Obesity
Altmetrics
Downloads
148
Views
46
Comments
0
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Submitted:
13 May 2023
Posted:
15 May 2023
You are already at the latest version
Alerts
Abstract
The comprehensive anabolic effects of insulin throughout the body, in addition to the control of glycemia, also include ensuring lipid homeostasis and anti-inflammatory modulation, especially in adipose tissue (AT). The prevalence of obesity, defined as a body mass index (BMI) ≥ 30 kg/m2, has been increasing worldwide on a pandemic scale with accompanying syndemic health problems, including glucose intolerance, insulin resistance (IR) and diabetes. Impaired tissue sensitivity to insulin or IR paradoxically leads to diseases with an inflammatory component despite hyperinsulinemia. Therefore, an excess of visceral AT in obesity initiates chronic low-grade inflammatory conditions that interfere with insulin signaling via insulin receptor (INSR). Moreover, in response to IR, hyperglycemia itself stimulates a primarily defensive inflammatory response associated with the subsequent release of numerous inflammatory cytokines and a real threat of organ function deterioration. In this review, all components of this vicious cycle are characterized with particular emphasis on the interplay between insulin signaling and both the innate and adaptive immune responses related to obesity. Increased visceral AT accumulation in obesity should be considered the main environmental factor responsible for the disruption in the epigenetic regulatory mechanisms in the immune system, resulting in autoimmunity and inflammation.
Keywords:
Subject: Medicine and Pharmacology - Endocrinology and Metabolism
1. Introductory overview
The Nobel Prize in Physiology or Medicine 1923 was awarded jointly to Frederick Grant Banting and John James Rickard Macleod “for the discovery of insulin”. One hundred years later, knowledge of how insulin works is not just about blood glucose regulation. The comprehensive metabolic effects of this anabolic hormone throughout the body also include lipid and protein homeostasis [1]. Particularly noteworthy is the anti-inflammatory effect of insulin, the only glucose-lowering hormone in the body, manifested by modulation of inflammatory mediators and direct action upon immune cells to enhance immunocompetence [2]. Impaired tissue sensitivity to insulin or insulin resistance (IR) paradoxically leads to diseases with an inflammatory component despite hyperinsulinemia [3]. The prevalence of obesity, defined as a body mass index (BMI) ≥ 30 kg/m2, has been increasing worldwide on a pandemic scale with accompanying syndemic health problems, including IR and diabetes [4]. Moreover, in response to IR, hyperglycemia itself stimulates a primarily defensive inflammatory response associated with the subsequent release of numerous inflammatory cytokines and a real threat of organ function deterioration [5].
1.1. Insulin
Insulin was the first peptide hormone discovered in 1922 during the examination of pancreatic extracts in diabetes research [6,7]. However, it took nearly two and a half decades to achieve the physicochemical characterization of insulin [8]. Finally, after determination of the amino acid sequence of insulin by Sanger sequencing in 1955, it became clear that as a heterodimer composed of 51 amino acids, the insulin molecule consists of two chains: a 21-residue A-chain linked to a 30-residue B chain by two disulfide bonds derived from cysteine residues (A7-B7 and A20-B19). An intrachain disulfide bond also exists within the A-chain (A6-A11) [9] (Figure 1). Thus, insulin produced and secreted by β-cells of the pancreatic islets of Langerhans and encoded in humans by the INS gene became the first protein to have its sequence determined. The molecular formula of human insulin is C257H383N65O77S6, and mature human insulin has a molecular mass of 5808 Da [10]. Although this hormone is a small protein, it contains almost all the structural features typical of proteins, including α-helix, β-sheet, β-turn, high-order assembly, allosteric T (tense) and R (relaxed)-transition, and conformational changes in amyloidal fibrillation [11]. The structure of insulin contains determinants of foldability, trafficking, self-assembly, and receptor binding [12,13].
Insulin is a part of a larger superfamily of evolutionarily sequence- and fold-related active proteins (hormones), which have been found not only in mammals, birds, reptiles, amphibians, fish, and cephalochordates but also in Mollusca, insects, and Caenorhabditis elegans. In addition to insulin, this insulin-like superfamily includes, among others, the relaxin peptides relaxin-1, -2, and -3; the insulin-like growth factors (IGF-1 and IGF-2); the insulin-like peptides [INSL3 - mammalian Leydig cell-specific insulin-like peptide, INSL4 - early placenta insulin-like peptide (EPIL), INSL5, INSL6 and Caenorhabditis elegans insulin-like peptides]; locust insulin-related peptide (LIRP); molluskan insulin-related peptides (MIPs); and insect prothoracicotropic hormone (bombyxin) [14,15,16,17,18,19,20]. All members of the superfamily exhibit the same disulfide-bonding pattern that was determined originally for human insulin. This means that the fold of the protein molecule comprises two polypeptide chains (A and B) linked by two disulfide bonds: all share a conserved arrangement of 4 cysteines in their A chain, the first of which is linked by a disulfide bond to the third, while the second and fourth are linked by interchain disulfide bonds to cysteines in the B chain [21].
1.1.1. Posttranslational modification of the tertiary structure of insulin (Figure 1)
Exclusively produced in pancreatic β-cells, insulin is the biosynthetic end-product of enzymatic decomposition of its single-chain precursor, known as preproinsulin, which is the initial result of the process of insulin mRNA translation. In a preproinsulin molecule, the following are present sequentially from the N-terminus: the signal peptide, insulin B-chain, C-peptide, and insulin A-chain. The signal peptide drives the nascent polypeptide of insulin from the cytosol into the lumen of the endoplasmic reticulum (ER), the starting point of the secretory pathway [22]. Thus, proteolytic processing of preproinsulin is coupled to trafficking between cellular compartments. Cotranslational and posttranslational translocation of preproinsulin into the ER is due to the presence of the translocation-associated protein complex (TRAP), also called signal sequence receptor (SSR), in the ER. TRAP consists of four integral membrane proteins, namely, TRAPα/SSR1, TRAPβ/SSR2, and TRAPδ/SSR4, with their extramembranous portion directed primarily into the ER lumen, whereas the extramembranous portion of TRAPγ/SSR3 is mainly cytosolic [23]. At the level of the ER, the signal peptide is cleaved from preproinsulin by the signal peptidase, allowing the molecule to be converted to proinsulin. Proinsulin folds on the luminal side of the ER, forming three evolutionarily conserved disulfide bonds. Next, folded proinsulin in the form of dimers exits from the ER and traffics through the Golgi complex into immature secretory granules. After excision of the C-peptide by a specialized set of endoproteases and carboxypeptidase activity, fully bioactive two-chain insulin ready for immediate secretion is ultimately stored as microcrystalline arrays of zinc insulin hexamers in mature glucose-regulated granules [13]. Defects in the pancreatic β-cell secretion system are reported in conditions linked to insulin resistance, such as prediabetes and type 2 diabetes (T2D), and include impaired proinsulin processing coexisting with a deficit in mature insulin-containing secretory granules [24].
C-Peptide and insulin enter the bloodstream at the same time and in equal amounts. However, in contrast to insulin, C-peptide is not extracted by the liver and other organs; therefore, it has a longer half-life of approximately 30 min compared with only 6 min for insulin. Thus, C-peptide reflects endogenous insulin secretion more accurately than insulin and – for that reason – is widely used as a clinical marker for pancreatic β-cell function [25,26]. Moreover, many years of research in both humans and animals have shown that C-peptide is much more than a byproduct of insulin biosynthesis [27,28,29]. Although it does not participate in the regulation of glucose levels, C-peptide might play a role in preventing and potentially reversing some of the chronic complications of diabetes, including vascular and nervous damage [30,31]. It remains an open question whether these activities related to the therapeutic potential of C-peptide are mediated by the receptor, the existence of which is still uncertain, or whether an alternative nonreceptor-mediated mechanism is involved [32,33].
1.2. Insulin receptor (INSR)
Because insulin or insulin signaling through INSR plays a key role in the regulation of glucose homeostasis, enabling body cells to access glucose in the blood, virtually all cells express INSR [34,35]. The action of this cell-surface receptor thus translates into whole-body nutrient homeostasis, and INSR malfunction in humans is linked to various disorders, such as type 2 diabetes mellitus (T2DM), obesity, metabolic syndrome, polycystic ovary syndrome (PCOS), atherosclerotic cardiovascular diseases, neurodegenerative disorders including Alzheimer’s disease (AD) and multiple cancers [36,37,38,39,40,41,42,43] (see also: Chapter 2. Insulin resistance).
1.2.1. INSR structure
INSR’s endogenous ligands include insulin, IGF-1 and IGF-2. Similar to the receptors for other protein hormones, INSR belongs to the large class of receptor tyrosine kinases and is located transmembraneously in the plasma membrane of insulin-sensitive cells [44]. INSR is a heterotetrameric protein composed of two entirely extracellular α-subunits (MW 135 kDa) and two penetrating intracellular β-subunits (MW 95 kDa). These subunits are covalently linked by disulfide bonds that determine their α2β2 tetrameric structure. The α-chains of INSR house insulin binding domains that constitute most of the INSR ectodomains, whereas the β-chains constitute the transmembrane and intracellular (tyrosine kinase) domains of INSR [45,46,47]. The receptor is encoded by a single gene that is located on human chromosome 19 and consists of 22 exons (exons 1–11 = α-subunit, exons 12–22 = β-subunit) [48]. The INSR gene encodes a 190-kDa proreceptor that undergoes a number of processing steps. N-Linked glycosylation and amide-linked acylation occur as cotranslational events. Next, the single chain polypeptide precursor is posttranslationally cleaved in the endoplasmic reticulum into the α- and β-subunits. A simplified diagram of the INSR structure is presented in Figure 2. The alternative splicing of exon 11 encodes a 12-amino-acid sequence at the C-terminus of the α-subunit of the IR gene during transcription, resulting in the formation of the isoforms IR-A and IR-B [46,49]. IR-B is a mature isoform because it includes the 12-amino-acid sequence, whereas the fetal isoform IR-A does not [50,51]. Although both isoforms have similar affinity for insulin, IR-A exhibits a higher affinity for IGFs, especially IGF-II, as well as greater fluctuations in its expression than IR-B [52,53]. These isoforms present different functional features and are coexpressed in most cell types, where they can form homodimers (i.e., INSR-A/INSR-A and INSR-B/INSR-B) and heterodimers (i.e., INSR-A/INSR-B), depending on the sorting of the two variants within lipid rafts (lipid microdomains) in the external leaflet of the plasma membrane. However, IR-B possesses more complex and important metabolic functions associated with metabolic and differentiating signals and is the dominant isoform in physiologic conditions [51]. Conversely, IR-A mainly favors cell growth, proliferation, and survival [54].
Subunits α and β then undergo an ester-linked acylation step, N-linked complex glycosylation and O-linked glycosylation (β-subunit), followed by dimerization. The mature insulin receptor is inserted into the plasma membrane and can be further regulated on the cell surface by insulin binding and receptor-mediated endocytosis. Thus, INSR concentration on the cell surface or INSR density in the tissue is a function of the internalization rate and the receptor recycling rate [49,55].
1.2.2. INSR signaling
Following binding of insulin or IGF-1 molecules at the binding site of the ectodomain of INSR within the α-chains, INSR activation (as well as IGFR1 activation) initiates a cascade of phosphorylation events that leads to the activation of enzymes that control many aspects of metabolism and growth [56]. The β-subunit of INSR increases tyrosine kinase activity, resulting in autophosphorylation that promotes the aggregation of heterodimers and stabilizes the receptor tyrosine kinase-activated state [47,57,58]. Next, a complex intracellular signaling network corresponding to the canonical pathway includes the phosphorylation of the docking proteins performing an adaptor function, that is, the insulin receptor substrates (IRSs). The canonical pathway is dichotomized into two branches that correspond to the phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) and mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) pathways [56,59]. These signaling cascades containing phosphorylation products acting as insulin and IGF-1 second messengers are central in mediating the actions of both INSR and IGFR1 in all tissues, including the liver. The specificity of signaling between insulin and IGF via INSR and IGFR1 is probably caused by features intrinsic to the ligands or receptors themselves. It is generally accepted that insulin exerts largely “metabolic” effects, whereas IGFs are responsible mainly for “growth-promoting” effects [59]. As a consequence of INSR activation, glucose transporters translocate from sequestered sites to become exposed on the cell surface [59,60]. The insulin-INSR complex is then internalized into the endosomal apparatus of the cell [61,62]. The main signaling proteins involved in insulin signal transduction within the canonical pathway are presented in Figure 3.
The other known INSR ligands that are not classified as IRSs, such as Grb2-associated binder (Gab) family proteins, β-arrestins, cytohesins, receptor for activated C kinase 1 (RACK1), downstream of kinases, adapter proteins SH2B and Crk, as well as the other pathways (nonreceptor tyrosine kinases, phosphoinositide kinases, and reactive oxygen species), are much less well understood and will not be discussed in detail in this review, except in relation to the IRSs [59].
- IRS proteins
IRSs are a family of six cytoplasmatic proteins (IRS1–6) with no kinase or other enzymatic activity that act as adaptor proteins and are phosphorylated by activated INSR [63]. IRSs have differential tissue distribution and function [64]. IRS1 and IRS2 are ubiquitously expressed and widely distributed in most cell types and are the primary mediators of INSR-related regulation of glucose metabolism and mitogenesis in most cell types [65,66]. IRS3 was identified only in rodent brains and adipocytes, whereas none of the molecular approaches provided evidence for a functional IRS-3 gene in human tissue [67]. IRS4 is characteristic of fetal development, and its expression in humans is restricted mainly to the brain, kidney, thymus and liver [68,69]. IRS-5 and IRS-6 are less related IRS family members (also known as docking proteins 4 and 5 (DOK4 and DOK5, respectively)), which have limited expression and far fewer known functions. Both proteins share homology in their N-termini, and although truncated at their C-termini, preserved functionalities in INSR signaling [70,71,72]. IRS protein sequence alignment analysis revealed high levels of homology in the N-terminal regions, where two conserved domains required for receptor recruitment are located: the pleckstrin homology (PH, also known as IH1) domain and the phosphotyrosine binding (PTB, also known as IH2) domain. The PH domain participates in protein‒protein interactions and facilitates recruitment by receptors and phospholipid proteins located in the plasmatic membrane, whereas PTB contains the tyrosine residues that bind to the phosphorylated NPXY motif (Tyr-960) in the activated insulin receptor, providing a specific mechanism for the interaction between the receptor and IRS-1 [73,74,75,76]. The activation of IRS proteins takes place via the phosphorylation of approximately twenty tyrosine residues within the C-terminal region. Phosphorylated IRSs can bind to tyrosine kinase signaling protein modules of approximately 100 amino acids, so-called Src homology (SH2) domains. The SH2 domains recognize residues C-terminal to the phosphotyrosine in their cognate peptide target ligands, including PI3K (precisely, the PI3K p85 subunit), growth factor receptor-bound protein 2 (GRB2), Src homology region 2-containing protein tyrosine phosphatase 2 (SHP2), and other less extensively studied effector proteins such as Fyn, c-Crk, CrkII and Nck [64,73]. Binding of an SH2 domain to its cognate tyrosine-phosphorylated target links receptor activation to multiple downstream signaling pathways, both to the nucleus to regulate gene expression and throughout the cytoplasm of the cell [77,78,79]. The crucial function of IRSs is therefore the transduction of signals from the extracellular to the intracellular environment via transmembrane receptors. Tyrosine phosphorylation of IRS proteins initiates the canonical pathway, i.e., triggers the activation of both PI3K/AKT/mTOR and MAPK/ERK signaling through IRS binding to the PI3K p85 subunit and GRB2 (as well as Nck, Crk, or the Fyn kinase), respectively. Notably, the release of insulin from pancreatic cells fully relies on this pathway, and IRS-2 is fundamental for insulin signaling in hepatocytes and β-cells [80,81,82]. As the major molecules that mediate the response to insulin and IGF-1 stimulation, as well as responding to other hormonal stimuli and steroids, cytokines and integrins, IRSs regulate many processes, including metabolism, growth, normal and cancer cell proliferation and survival [63,83]. Following activation by cytokine and hormone receptors (e.g., IL-4, leptin, and angiotensin), IRS proteins are able to use Janus kinase 2 (JAK2) and the IRS/JAK2 interaction to activate JAK2/STAT3 with further induction of PI3K/AKT/mTOR and MAPK/ERK signaling [84,85].
It was documented for IRS1 that, considered typical cytosolic proteins, IRSs may contain native nuclear localization signals (NLSs) and can be translocated to the nucleus, probably via association with other NLS-equipped proteins [86,87]. The presence of nuclear IRS-1 was confirmed in cells expressing human JC virus T-antigen, SV40 T-antigen, integrins, estrogen receptor α (ERα) and estrogen receptor β (ERβ) [86,87,88,89]. The role of nuclear IRSs remains unclear. It cannot be ruled out that this IRS location relates exclusively to IGFR1, not INSR [90,91].
The stability of IRS proteins is regulated bidirectionally primarily by ubiquitination through several E3 ubiquitin ligases that increase IRS stability and deubiquitination by deubiquitinating enzymes (DUBs, also known as deubiquitinating peptidases) that precede IRS degradation by the proteasome. Interestingly, prolonged insulin/IGF stimulation induces both ubiquitination and degradation (deubiquitination) of IRSs. This well-documented phenomenon creates one of the major negative-feedback loop mechanisms of insulin/IGF signaling [92,93,94].
Serine phosphorylation is another mechanism of IRS protein downregulation based on the negative feedback mechanism in the insulin/IGF signaling network [56]. Given that IRS1 has over 70 potential phosphorylation sites activated by insulin, IRS kinases such as ERK, ribosomal S6 kinases (S6K1 and S6K2), and c-Jun-N-terminal kinases (JNKs) may act effectively [95]. Moreover, serine phosphorylation of IRS1 via JNKs and inhibitor of NF-κB (IκB) kinases (IKKs) is involved in tumor necrosis factor alpha (TNF-α)/TNF-α receptor 1 (TNFR1) signaling, which is not limited to inflammation and apoptosis [78,96]. It has been shown in human, animal and in vitro studies that such immunometabolic messenger crosstalk between TNFR1- and INSR-related pathways may be responsible for the induction of IR, especially in obesity and T2D [96,97,98,99]. In addition to diabetes, deregulation or metabolic reprogramming of IRSs has been implicated in cancer [63,64,69,100].
2. Insulin resistance (IR)
IR, also known as impaired insulin sensitivity, is defined as an impaired biological response to insulin stimulation of target tissues, including primarily the liver, muscle and adipose tissue (AT) [101,102]. This means that in practice, a known quantity of exogenous or endogenous insulin works much less effectively than in healthy people at increasing glucose uptake and utilization to preserve glucose homeostasis [103]. In a clinical setting, it has been arbitrarily assumed that patients requiring greater than 1 unit/kg/24 h of exogenous insulin to maintain glycemic control and prevent ketosis are insulin resistant [104,105]. Due to IR, the compensatory mechanism is activated, in which pancreatic β-cells increase insulin production, causing hyperinsulinemia. A vicious cycle appears because increased anabolic activity caused by overproduction of endogenous insulin results in weight gain, which, in turn, exacerbates IR [106]. After approximately 10 to 15 years of IR, decompensation leads to relative insulin deficiency with consequent overt diabetes, classified as T2DM. Apart from the dominant result of IR, which is T2DM, the metabolic consequences of IR include a broad spectrum of diseases and pathologic conditions, such as hypertension, dyslipidemia, visceral adiposity, nonalcoholic fatty liver disease (NAFLD), ovarian dysfunction and hyperandrogenism in women, acromegaloid features, hyperuricemia, elevated inflammatory markers, endothelial dysfunction, a prothrombic state, cancer, and neurodegenerative disorders, including AD [107,108,109,110]. Consequently, IR is a permanent component of metabolic syndrome, a cluster of conditions that occur together, increasing the risk of heart disease, stroke and T2DM [111]. In addition, an early and common manifestation of severe IR is skin changes known as acanthosis nigricans that usually develop in skin folds, such as the back of the neck, axilla and groin, and involve darkening and thickening of the skin (velvety overgrowth of the epidermis) [108]. The pathogenesis of acanthosis nigricans is closely related to hyperinsulinemia. High circulating insulin levels cross-react with IGFR1 on keratinocytes and dermal fibroblasts and may displace IGF-1 from IGF binding protein. Increased circulating IGF-1 levels result in the excessive proliferation of keratinocytes and dermal fibroblasts [112,113].
When referring to the causes of IR, abnormalities in INSR function related to defects in receptor structure (i.e., alternative splicing and gene polymorphisms), expression, binding affinity and/or signaling capacity (e.g., the level of tyrosine phosphorylation) should be taken into account. Moreover, the molecular mechanisms of IR also include insulin receptor antibodies, gene polymorphisms and negative regulation of IRSs, as well as negative regulation of PI3K/AKT/mTOR and MAPK/ERK signaling. All of the above mechanisms are extensively reviewed in the cited references [114,115,116,117,118,119,120,121,122].
Hyperglycemia itself also accounts for the development of IR because a coexisting proinflammatory environment leads to the generation of reactive oxygen species (ROS), especially in response to TNF-α, which weakens insulin-induced tyrosine autophosphorylation of INSR [123,124]. As highly oxidant molecules, ROS can oxidize various intracellular components, including membrane phospholipids, proteins, and DNA. Usually, these reactions lead to cellular damage or significant reduction of oxidized biomolecules. In IR, increased ROS production and/or decreased ROS degradation was demonstrated, leading to an oxidative stress condition with subsequent activation of signaling pathways related to stress [124]. Considering the above information, one can speculate that glucose intolerance and IR may serve as protective mechanisms that prevent glucose toxicity.
The main underlying causes of IR are summarized in Figure 4.
Despite a significant increase in knowledge about the various causes of IR, a precise explanation of the molecular mechanisms resulting in the development of this disorder has still not been elucidated [132]. Current research on IR includes, among others, intracellular redox balance, oxidative and nitrative stress, mitochondrial dysfunction, increased plasma-free fatty acid (FFA) levels, especially long-chain saturated fatty acids (SFAs), and the accumulation of intracellular lipid derivatives (diacylglycerol and ceramides) [133,134,135,136]. Mutations and expression (modulation of transcription) of genes involved in lipid and glucose metabolism as well as insulin signaling, inflammation, redox balance, and mitochondrial function are being studied [127]. As gene expression is regulated at various levels and not only in response to DNA modifications, the role of epigenetic mechanisms in IR might be worth noting, including noncoding RNAs (ncRNAs). For example, the long noncoding RNAs (lncRNAs) MEG3 and H19 are involved in obesity and IR in women. The results showed lower mRNA levels of H19 in subcutaneous adipose tissues (SAT) of obese women compared to normal-weight women and a significantly higher expression of MEG3 in the SAT of the obese group vs. lean controls [137].
The relationship between IR and the accumulation of lipids in tissues suggests that these lipids are both markers and mediators of metabolic dysfunction, especially in skeletal muscle, which is the main site of insulin-stimulated glucose disposal [138,139]. Incorrect adjustment of the glucose-fatty acid cycle (also called the Randle cycle) may therefore be the cause of IR. Normally, this biochemical phenomenon is based on the competition between glucose and fatty acids for their oxidation and uptake in muscle and adipose tissue. Briefly, the oxidation of fatty acids yields acetyl-CoA, from which citrate is generated by the action of citrate synthase. Next, increased values of the acetyl-CoA/CoA and NADH/NAD+ ratios stimulate pyruvate dehydrogenase kinase (PDK), which phosphorylates and inactivates pyruvate dehydrogenase (PDH). ATP and citrate inhibit phosphofructokinase, with the accumulation of substrates from previous stages, including glucose-6-phosphate (G-6-P), which has an inhibitory effect on hexokinase, ultimately repressing glycolysis [138]. As a result, a significant reduction in the uptake and utilization of glucose occurs when fatty acid oxidation is intensified. This glucose-fatty acid cross-talk is also observed in AT [140]. It was recently proposed that the starting point for malfunction of the glucose-fatty acid cycle and related IR may be an increase in global protein acetylation from acetyl-CoA. It was demonstrated in cultured adult rat cardiomyocytes that leucine, ketone bodies, palmitate, and oleate promote a rapid increase in protein acetylation, especially in the abundances of the fatty acids that generate high levels of acetyl-CoA. Thus, enhanced protein acetylation initiates fatty acid-mediated inhibition of cardiac glucose transport, resulting in impairment in the translocation of vesicles containing the glucose transporter GLUT4 to the plasma membrane [141].
3. Inflammatory response and IR (see also the summary in Figure 5 at the end of this chapter)
The results of numerous experiments have demonstrated that in normal conditions, insulin exerts glucose homeostatic and anti-inflammatory effects. Pretreatment with insulin inhibits the activation of NF-κB and the expression of proinflammatory cytokines to alleviate the inflammatory response in vitro and in vivo [2,142,143]. The molecular mechanism of this immunomodulation provided by insulin is based on downregulation of the nucleotide-binding oligomerization domain (NOD), leucine-rich repeat (LRR)-containing protein (NLR) family member 3 (NLRP3) inflammasome, a critical component of the innate immune system that mediates caspase-1 activation and the secretion of the proinflammatory cytokines IL-1β/IL-18 in response to microbial infection and cellular damage [144]. In the case of IR, the anti-inflammatory effect of insulin is significantly hampered and insufficient to block low-grade inflammation that typically develops, especially in obese individuals [145]. The mechanism of the vicious cycle is that IR due to insulin signaling inhibition results in a series of immune responses caused by abnormal glucose tolerance that exacerbate the inflammatory state, which in turn leads to an increase in both IR and blood glucose levels.
Abnormally high deposition of visceral AT or increased AT surrounding the intra-abdominal organs is known as visceral obesity. It has been distinctly linked to several pathological conditions, including impaired glucose and lipid metabolism and IR, and is an independent component of metabolic syndrome [146]. Such an excess of fat tissue alters the secretion of FFAs, adipokines, growth factors and proinflammatory cytokines. Moreover, obesity increases macrophage infiltration within the AT, which can increase the level of proinflammatory cytokines. Altered glucose and lipid metabolism is also clearly detectable in hepatic, pancreatic and muscular tissues as the modifications of the inflammatory response spread out. Thus, the contribution of the inflammatory process to IR is not only limited to AT but is also generalized (systemic) [147]. Importantly, among the proinflammatory factors secreted in the AT and by macrophages are those that can directly contribute to IR, including TNF-α, IL-1β, IL-6, IL-18, and angiotensin II (Ang II). For example, it was suggested that Ang II may lead to IR through protein kinase C (PKC) activation in adipocytes [148]. In the case of TNF-α, IL-1β, IL-6, and IL-18, the impaired biologic response to insulin stimulation of target tissues may be caused by multiple mechanisms, such as degradation of IRS1, serine/threonine protein kinase (STK) activation, impaired GLUT4 translocation from intracellular stores to the plasma membrane, decreased expression of peroxisome proliferator-activated receptor gamma (PPAR-γ) or increased expression of suppressor of cytokine signaling protein 3 (SOCS-3) [149,150,151,152,153,154,155].
The innate and adaptive immune systems are involved in the pathomechanism of obesity-induced inflammation and systemic proinflammatory polarization of immune cells, resulting in augmented secretion of proinflammatory cytokines [156].
Considering the involvement of CD4+ T lymphocytes depending on the stimuli/environments, the inflammatory response can be broadly divided into two types: type 1, related to T helper 1 (Th1) lymphocytes, and type 2, related to Th2 cells. In simple terms, Th1 cells promote and coordinate the cell-mediated host inflammatory response to intracellular pathogens by inducing the activation of macrophages, NK cells, B cells, and CD8 T cells, whereas Th2 cells mediate the activation and maintenance of the humoral, or antibody-mediated, immune response against extracellular pathogens. Th1 cells primarily synthesize interferon gamma (IFN-γ), TNF-α, and interleukin-2 (IL-2), while Th2 cells synthesize other cytokines, such as IL-4, IL-5, IL-6, IL-9, IL-13, and IL-17E (IL-25). Suited to the immune challenge, a well-balanced Th1 and Th2 response is required in humans because it was demonstrated that the clear dominance of Th2 responses will counteract the Th1-mediated microbicidal action, and vice versa [157,158].
Given that there is a linking mechanism between obesity, inflammation, and IR, the predominance of the type 1 inflammatory response in AT may be involved in the etiopathogenesis of altered insulin signaling. In contrast to AT type 1 inflammation in obesity, type 2 inflammatory conditions are a part of AT immune homeostasis regulation in lean healthy individuals [111,159]. It has been suggested that the initiation of generalized low-grade inflammation in obesity may be triggered by the production of chemokines by adipose cells, the release of damage-associated molecular patterns (DAMPs) from necrotic AT, augmented rates of lipolysis with a subsequent increase in the flux of nonesterified fatty acids, and hypoxia with activation of hypoxia-induced factor (HIF)-1, which controls the expression of proinflammatory proteins [160].
3.1. Innate immune system and pattern recognition receptors (PRRs)
The innate immune system, or nonspecific immune system, is the body’s first line of defense against germs entering the body. This system includes physical and anatomical barriers as well as effector cells, humoral mediators, antimicrobial peptides, and a limited number of diverse receptors that are encoded by intact genes inherited through the germline, that is, pattern recognition receptors (PRRs) [161]. PRRs are proteins expressed mainly on the effector cells of the innate immune system, such as macrophages, monocytes, granulocytes (especially the most abundant neutrophils), dendritic cells, and epithelial cells, that are capable of identifying two classes of molecules: pathogen-associated molecular patterns (PAMPs), which are frequently found in microbial pathogens, and DAMPs, which are unique molecules displayed on or released from stressed, injured, infected, or transformed cells. There are several subgroups of PRRs. They are classified according to their ligand specificity, function, localization and/or evolutionary relationships. Based on their localization, PRRs may be divided into membrane-bound PRRs, including Toll-like receptors (TLRs) and C-type lecithin receptors (CLRs), and cytoplasmic PRRs, including nucleotide oligomerization domain (NOD)-like receptors (NLRs) and retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs) [162]. Among all the PRRs identified, TLRs are the most ancient class, with the most extensive spectrum of pathogen recognition [163,164].
Ligation of PRRs on immune cells leads to the transmission of a threat signal to the cells and initiates a cascade of responses that direct host defense responses. Typically, the response is based on cytokines or antimicrobial compounds produced by leukocytes after stimulation by PRRs. The second important consequence of PRR detection is the induction of competency of selected cells to present antigen to T cells. Such maturation of antigen-presenting cells (APCs) relies on the stabilization of major histocompatibility complex (MHC) molecules on their surface. Thus, PRRs are also important for the initiation of adaptive immunity because the presentation of antigen by APCs is necessary to initiate adaptive immune responses [165].
As obesity-related insulin resistance develops, adipocyte cell death and innate immune activation provide antigenic stimuli similar to those in microbial or other infections. DAMPs, consisting of endogenous intracellular molecules released by necrotic cells and extracellular matrix (ECM) molecules that are upregulated upon injury, provide a clear signal of vital danger. Then, TLRs are activated, resulting in increased inflammatory gene expression to mediate tissue repair processes [166].
Transmembrane proteins known as TLRs belong to the family of Type I integral membrane glycoproteins, and in humans, they comprise a family of ten members (TLR1–TLR10) responsible for detecting various PAMPs and DAMPs. TLRs are expressed on all innate immune cells (innate leukocytes), such as natural killer (NK), mast cells (MCs), eosinophils, and basophils, as well as on phagocytic cells, including macrophages, neutrophils, and dendritic cells (DCs). Moreover, TLRs can also be detected on adaptive immune cells, including T and B cells, and on a large majority of nonhematopoietic cells, such as mesenchymal stromal cells, fibroblasts and endothelial cells [167]. Thus, molecules released following TLR activation signal to other cells of the immune system, making TLRs key elements of innate immunity and adaptive immunity. The dynamic nature of TLR expression is that modulation of the expression pattern may rapidly change in response to environmental stresses, pathogens or a variety of released cytokines. Furthermore, TLRs may be expressed on the cell surface (extracellularly), as in the case of TLRs 1, 2, 4, 5, and 6, or may be almost exclusively in intracellular compartments such as endosomes and their ligands, primarily nucleic acids. Intracellular localization was confirmed for TLRs 3, 7, 8, and 9.
Although each of the TLRs recognizes different molecular patterns, the structure of all TLRs is generally quite conserved. All TLRs contain extracellular domains containing variable numbers of leucine-rich-repeat (LRR) motifs and a cytoplasmic Toll/interleukin 1 (IL-1) receptor (TIR) homology domain. The LRR domain, responsible for ligand binding, is connected via a transmembrane domain with a TIR domain that is required for signal transduction. The transmembrane and membrane-proximal regions are important for the cellular compartmentalization of these receptors [164,168].
3.1.1. TLR activation
When bound to a specific ligand (PAMP or DAMP), TLRs form homodimers (e.g., TLR4) or heterodimers (e.g., TLR1, TLR2, and TLR6) and initiate signaling pathways that are very similar to the IL-1 receptor-mediated pathway of NF-κB activation [167,169]. TLR signaling is initiated when the cytoplasmic TIR domain interacts with adaptor molecules such as myeloid differentiation primary response gene 88 (MyD88). MyD88 is the canonical adaptor for inflammatory signaling pathways downstream of members of the Toll-like receptor (TLR) and interleukin-1 (IL-1) receptor families. MyD88 attracts the IL-1 receptor-associated kinase 4 (IRAK4) molecule to the TLR family members or IL-1 receptor, which induces the phosphorylation of IRAK1 and IRAK2 and the formation of the signaling complex. Following activation of IRAK family kinases, the signaling complex reacts with TNF receptor associated factor 6 (TRAF6), which leads to TGFβ-activated kinase 1 (TAK1) activation and consequently the induction of inflammatory cytokines via nuclear factor κB (NF-κB), MAPKs, and activator protein 1 (AP-1) [170]. Thus, the adaptor molecule MyD88 serves as a central node of inflammatory pathways [171,172].
In addition to the MyD88-dependent pathway, TLRs may be activated through Toll/IL-1R domain-containing adaptor-inducing IFN-β (TRIF)-dependent signaling. The TRIF-dependent pathway is induced by macrophages and DCs after TLR3 and TLR4 stimulation. This pathway is required for the TLR-mediated production of type-I IFN and several other proinflammatory mediators [173].
TLR signaling in IR, especially coexisting with obesity, may play a pivotal role in the development of so-called trained immunity, a functional state of the innate immune response characterized by long-term epigenetic reprogramming of innate immune cells, which depends on prior exposure to a signal [174,175]. This reprogramming of monocytes, NK cells and DCs is a manifestation of an adaptation of the innate immune system to the metabolic changes related to altered insulin signaling because the factors demonstrated to facilitate trained immunity include, in addition to pathogenic signals (bacteria, viruses, and fungi), initially nonpathogenic signals such as insulin, cytokines, adipokines or hormones [176].
It was demonstrated that TLR4 contributes to the development of IR and inflammation in obesity via its activation by excess enteric lipopolysaccharide (LPS) and SFAs as well as via endogenous ligands, e.g., FFAs. The resulting activation of the proinflammatory kinases JNK, IKK, MAPKs and p38 in insulin target tissues impairs insulin signal transduction directly through inhibitory phosphorylation of IRSs on serine residues [177]. The tissue inflammatory state during obesity may be caused by TLR-4/NF-κB pathway activation in macrophages via FFA action, which promotes the synthesis and secretion of proinflammatory cytokines, including IL-6, TNF-α, IL-1β and IL-18 [178].
Activation of the TLR4 pathway promotes the priming of the NLRP3 inflammasome, a multiprotein complex that assembles in the cytosol after exposure to PAMPs or DAMPs [179] (see the next subchapter 3.1.2).
3.1.2. NLR family pyrin domain containing 3 (NLRP3) activation
Activation of NLRPs results in the formation of many distinct inflammasome complexes, each of which has a unique PRR and activation triggers [179]. The best characterized is the NLRP3 complex, which is composed of NLRP3, apoptosis-associated speck-like protein containing caspase recruitment domain (ASC, also PYCARD), pro-caspase-1, and the serine-threonine kinase NEK7 (NIMA “never in mitosis gene A”-related kinase 7). NLRP3 is a cytoplasmic PRR that acts as a dominant innate immune sensor for tissue damage. For that reason, it plays a crucial role in the development of sterile inflammation during the course of AT metabolic dysfunction caused by IR [180,181]. By sensing self-danger signals (activation by DAMPs), NLRP3 undergoes a conformational change (oligomerization) that results in unfolding and binding to the key adaptor protein ASC through homotypic pyrin–pyrin domain interactions, ultimately leading to ASC nucleation. On this scaffold, recruitment, self-cleavage and autoactivation of the effector pro-caspase-1 generate mature caspase-1 and eventually lead to NLRP3 inflammasome formation. Next, activated caspase-1 processes pro-IL-1β and pro-IL-18 into their bioactive forms to initiate inflammation [182,183]. Thus, inflammasomes are molecular platforms that are activated upon cellular infection or stress, including IR-induced metabolic dysregulation, that trigger the maturation of proinflammatory cytokines [184]. Strong associations between dysregulated inflammasome activity and human heritable and acquired inflammatory diseases highlight the importance of this pathway in tailoring immune responses [179,183].
3.2. Inflammatory response related to B cells and T cells
The adaptive, or acquired, immune response to an antigen is more specific but takes days or even weeks to become established – much longer than the innate immune response. Moreover, without information from the innate immune system, the adaptive response cannot be mobilized [185]. Two main types of adaptive immune responses, humoral and cell-mediated responses, are strictly linked to the function of lymphocyte subtypes called B cells and T cells, respectively. Activated B cells proliferate and differentiate into antibody-secreting effector cells, known as plasma cells. Apart from generating antibodies and an antibody-mediated memory response against pathogens, B cells secrete cytokines and are also capable of generating cell-mediated immunity, serving as professional APCs to activate antigen-specific CD4+ and CD8+ T cells. CD4+ T cells, which mature in the thymus gland, are MHC-II restricted and preprogrammed for helper functions, whereas MHC I-restricted CD8+ T cells are preprogrammed for cytotoxic functions and are capable of detecting immunogenic peptide–MHC class I (pMHCI) complexes presented on nucleated cells [186]. Specific CD4+ T cells are activated and undergo clonal expansion after recognition of antigenic peptide/MHC-II on antigen-presenting cells via a T-cell receptor [187,188]. Following activation by recognized antigenic structures, CD4+ T cells activate B cells and differentiate into distinct subpopulations that produce different cytokines. Similarly, CD8+ T cells mediate their effector functions through the production of cytokines such as IFN-γ and tumor necrosis factor (TNF)-α and/or by cytolytic mechanisms [189].
IR significantly affects the adaptive immune system, although the root cause is unclear. It cannot be excluded that in this interdependence, the IR initiating factor comes from the adaptive immune system and is associated with a low-grade chronic inflammatory state within excessive AT. Although macrophages are the most abundant immune cell type in the AT of both mice and humans, and the M1 phenotype might especially be a major driver of AT inflammation, T cells and B cells also play important roles in modulating AT inflammation [190,191,192,193].
Interestingly, as IR is primarily associated with obesity, prediabetes and T2D, people with type 1 diabetes (T1D) can also become insulin resistant. IR is not a cause of T1D, but people with type 1 insulin resistance will need higher insulin doses to keep their blood glucose under control than those who are more sensitive to insulin. The autoimmune process mediated via T cells is a main part of the etiopathogenesis in T1D, but B cells also clearly participate in pancreatic islets of Langerhans destruction, as autoantibodies recognizing insulin-secreting β cell antigens commonly appear in the circulation before the onset of the disease [194]. The development of IR in T1DM may be triggered by many risk factors, and even if it is distinctively different from the IR in T2DM, obesity and the inflammatory background are consistently present in both types of diabetes [195].
3.2.1. B cells
Immunological B cells are generally divided into two major subsets. B2 cells generate specific antibodies against foreign antigens in secondary lymphoid organs. B1 cells, found predominantly in the peritoneal and pleural cavities, instead produce “natural” antibodies as part of the innate immune system. B cells that reside in nonlymphoid organs, including AT, function to maintain homeostasis at steady state [196,197].
It was demonstrated that B cells can modulate AT function in obesity. Moreover, an increase in the proportion of B cells is also a feature of adipose tissue in obesity. B cells are one of the first immune cells to accumulate in AT in response to a high-fat diet. A 3-fold increase in B-cell number in epididymal visceral AT depots was reported in C57BL6/J mice three weeks after a high-fat diet [198]. Moreover, diet-induced obese (DIO) mice lacking B cells are protected from IR despite weight gain due to the accumulation of AT. Consequently, treatment with a B-cell-depleting CD20 antibody attenuates IR, whereas transfer of IgG from DIO mice rapidly induces IR and glucose intolerance [199]. In visceral AT, B cells are involved in the activation of proinflammatory macrophages and T cells as well as in the production of pathogenic IgG antibodies. The profile of IgG autoantibodies isolated from obese humans with IR is specific and differs from that of nonobese diabetic persons or obese nondiabetic persons [199]. It was suggested that a change in the proportion of B1 to B2 cells in obese AT resulting from the increase in B2 cells may induce IR. The recruitment/chemotaxis of B2 cells to AT and direct activation or stimulation of the B2 cell proinflammatory phenotype are processes mediated by signaling through the chemokine leukotriene B4 (LTB4) and its G protein-coupled receptor, LTB4R1 (also known as BLT1). LTB4 is an endogenous lipid mediator of inflammation derived from arachidonic acid by the sequential action of cytosolic 5-lipoxygenase, 5-lipoxygenase-activating protein, and leukotriene A4 hydrolase. LTB4, via LTBR1, induces recruitment and activation of neutrophils, monocytes and eosinophils. It also stimulates the production of a number of proinflammatory cytokines and mediators, indicating an ability to augment and prolong tissue inflammation [200,201,202].
Loss of LTB4R1 prevents obesity-induced B2 cell recruitment into visceral adipose tissue, mitigating the contribution of B2 cells to the pathogenesis of obesity-induced adipose tissue inflammation and IR. Thus, the LTB4/LTB4R1 axis facilitates the metabolic effects of B2 cells toward glucose intolerance and IR in obese AT. Moreover, an increased number of B2 cells in obese AT exacerbates insulin resistance through Th1 lymphocyte- and M1 macrophage-mediated mechanisms. In addition, previous studies have demonstrated the effects of the LTB4/LTB4R1 axis on the recruitment and activation of macrophages in the context of obesity [203,204,205,206].
Thus, the recruitment of B lymphocytes in obese visceral AT precedes the infiltration of inflammatory CD4+ and CD8+ T cells and macrophages, leading to Th1 polarization and the production of IgG autoantibodies by B2 cells, which are major factors contributing to the development of IR [198,199,206,207].
It is important to note that unlike B2 cells, B1a and B1b cell subtypes are able to improve insulin sensitivity in diet-induced obesity via the secretion of IgM autoantibodies. In addition, distinct B-cell populations, designated regulatory B (Breg) cells, produce the anti-inflammatory cytokine CXCL12 (C-X-C Motif Chemokine Ligand 12), which maintains the balance of the immune response and restrains immune responses associated with autoimmune diseases. Adipose environmental factors, including CXCL12 and free fatty acids, support Breg cell function, and the Breg cell fraction and function were reduced in adipose tissue from obese mice and humans. Therefore, B1a, B1b and Breg are promising therapeutic targets in obesity to overcome IR [208,209,210].
Regardless of high-fat diet consumption, the aging of the body has been linked to AT B-cell mediation via visceral AT accumulation of follicular B2 cells and “age-associated B cells” (ABCs) that can produce proinflammatory IgG and cytokines. ABCs are a heterogeneous B-cell subset (CD19+, CD21−, CD11c+, T-bet+) that is expanded in the elderly but also accumulates prematurely in patients with autoimmune disorders and/or infectious diseases [211,212]. Moreover, the expression of a B-cell-specific coactivator of octamer-binding transcription, Oct coactivator B (OcaB), is increased in visceral AT with age in both humans and mice. OcaB is important for transcription of the variable parts of the kappa light chain of immunoglobulins. Global deletion of OcaB in mice resulted in a reduction in B2 cell numbers in AT and reduced proinflammatory IgG2c antibody levels with subsequent improvement in insulin sensitivity [213].
It was recently demonstrated that phenotypic and functional features of B cells from two different human subcutaneous adipose depots may differ significantly [214]. Analyses of the B cells from the breast and abdominal subcutaneous AT revealed that in obese women, B cells from the abdominal AT are “more inflammatory” than those from the breast. This inflammation manifested itself through higher frequencies of inflammatory B-cell subsets, higher expression of RNA for inflammatory markers associated with senescence and higher secretion of autoimmune antibodies in abdominal AT than in breast AT. Moreover, a higher number of autoimmune B cells with the membrane phenotype CD21lowCD95+ B cells expressing the transcription factor T-bet (Tbx21) was found in the abdominal AT. It is worth noting that T-bet directs T-cell homing to proinflammatory sites by regulating chemokine receptor CXCR3 expression [215,216]. This highlights the pivotal role of abdominal AT in the development of chronic inflammation and IR.
3.2.2. T cells
Chronic inflammation characterized by T-cell and macrophage infiltration of visceral AT is a hallmark of obesity-associated IR and glucose intolerance. The cell-mediated immune response is especially present in obesity, when hypertrophic adipocytes produce proinflammatory cytokines, such as IL-6 and TNF-α, which leads to increased vascular permeability and the recruitment of cytotoxic T cells, including T-cell phenotypes associated with IR [217,218,219]. Indeed, insulin-sensitive (IS) patients with metabolically healthy obesity (MHO) show significant immunological differences in T-cell phenotypes compared to obese patients with IR (metabolically unhealthy). For example, the frequencies of naïve (CD45RA+CCR7+CD27+CD28+) CD4+ and CD8+ T-cell subsets were positively associated with the ISIOGTT [insulin sensitivity index (ISI) obtained from the oral glucose tolerance test (OGTT)] and inversely correlated with HOMA-IR (homeostasis model assessment of insulin resistance) in older participants, whereas the percentage of central memory (CD45RA−CD27+CD28+) CD4+ T cells was negatively associated with the ISIOGTT and positively associated with HOMA-IR. In addition, the percentage of effector memory (CD45RA−CD27−CD28−) CD8+ T cells correlated positively with HOMA-IR [220]. Considering the above results, the percentage of naïve CD4+ and CD8+ T cells may be treated as a predictor of impaired insulin sensitivity. These findings support the hypothesis that parameters of systemic inflammation related to the T-cell subtypes can differentiate IS from IR obese individuals who are at higher risk of cardiometabolic diseases and should be preferentially subjected to obesity treatment.
Significant changes in adaptive immunity have been reported after bariatric surgery, where the most obvious effect, a loss of up to half of total adipose tissue mass within the first year following surgery, is accompanied by a reduction in CD4+ and CD8+ T-cell counts, a decrease in the Th1/Th2 ratio, an increase in B regulatory cells, and a reduction in proinflammatory cytokine secretion [218,221]. Overall, there is a shift in the T-cell subtypes, from the proinflammatory to the less- or anti-inflammatory subtypes [222,223].
Alteration in the Th1/Th2 balance in obese visceral AT that is typically observed in IR may be caused by abnormal expression of the transcription factor T-bet (Tbx21) and related T-cell chemotaxis governed by CXCR3 and its ligands [CXCL9 or monokine induced by IFN-γ (Mig), CXCL10, and CXCL11] [215,216]. The expression of the chemokine receptor CX3CR3 was confirmed on many different cell types, including T cells, B cells, NK cells, renal tubular epithelial cells, fibroblasts, and vascular pericytes [224,225,226,227,228]. Thus, originally described as the master regulator of Th1 development, T-bet performs regulatory functions necessary in both the adaptive and innate immune systems [216]. Quiescent T cells show weak CXCR3 expression, whereas in the process of T-cell activation, the expression of CXCR3 markedly increases. The important role of CXCR3 in supporting the proper function of activated T cells is unambiguously connected with the local inflammatory response. The addition of CXCR3 ligands to normal human T cells expressing CXCR3 led to the tyrosine phosphorylation of multiple proteins. CXCR3-mediated T-cell chemotaxis involves zeta-associated protein of 70,000 molecular weight (ZAP-70) and is regulated by signaling through the T-cell receptor (TCR). This means that essential molecules in TCR signal transduction, such as ZAP-70, linker for the activation of T-cell (LAT), and phospholipase-C-γ1 (PLCγ1), become phosphorylated on tyrosine 319 (ZAP-70), tyrosines 171 and 191 (LAT), and on tyrosine 783 (PLCγ1). Thus, cytoplasmic protein tyrosine kinases play a critical role in the events involved in initiating T-cell responses by the TCR [229,230].
Interestingly, mice deficient in T-bet may display greater visceral AT content but paradoxically more IS than T-bet+/+ mice [231]. This finding indicates that the absence of T-bet can separate obesity from IR. However, the precise mechanism of this potentially crucial role of T-bet in physiological and pathophysiological metabolism remains unclear [216].
- Peroxisome proliferator-activated receptors (PPARs) and T cells
PPARs are a group of three nuclear hormone receptor proteins (PPARα, PPARβ, and PPARγ) that promote ligand-dependent transcription of target genes that regulate energy production, glucose and lipid metabolism, cell proliferation/differentiation, and inflammation [232,233]. The human PPARα gene is located on chromosome 22, PPARβ is located on chromosome 6, and PPARγ, which encodes three isoforms, has been identified on chromosome 3 [234].
The best-known mechanism by which all PPAR subfamilies downregulate inflammation is through transrepression [235]. This activity involves indirect association (tethering) of the PPARs with target genes. There are many mechanisms by which PPARs can transrepress the inflammatory response, including competition for a limiting pool of coactivators, direct interaction with the p65 subunit of NF-κB and c-Jun subunit of AP-1, modulation of p38 mitogen-activated protein kinase (MAPK) activity, and partitioning of corepressor B-cell lymphoma 6 (BCL-6) [235,236].
PPAR natural ligands include lipid-derived metabolites such as fatty acids, acyl-CoAs, glycerol-phospholipids, and eicosanoids. PPAR polymorphisms may be responsible for the risk of being overweight or obese, whereas dominant-negative PPARγ mutations result in T2DM, hypertension and IR [237,238]. PPARs are expressed not only in adipose tissue but also in various tissues and cell types, including pancreatic β cells and T cells, where they regulate insulin secretion and T-cell differentiation, respectively [239]. PPARs have been shown to regulate T-cell survival, activation, and CD4+ T helper cell differentiation into the Th1, Th2, Th17, and Treg lineages [240].
Based on human and animal studies, the anti-inflammatory role of PPARγ has been clearly demonstrated in adaptive immune cells, including T cells. For example, PPARγ activation in mouse CD4+ T cells selectively suppresses Th17 differentiation by inhibiting TGF-β/IL-6-induced expression of retinoic acid receptor-related orphan receptor γt (RORγt) [241]. Consistently, it was also demonstrated that human CD4+ T cells with PPARγ small interfering RNAs (siRNAs) increased IL-17A production [239]. However, as this effect was detected exclusively in female T cells, the results of the study revealed that human T cells exhibit a sex difference in the production of IFN-γ and IL-17A that may be driven by the expression of PPARα and PPARγ [239].
On the other hand, PPARγ is involved in the TCR–mTORC1–PPARγ and TCR–mTORC1–SREBP1 signaling axes, which are required for the activation of the fatty acid metabolic program in activated CD4+ T cells [242]. Therefore, even if PPARγ downregulates the generation of effector cells with Th17 properties, its direct binding to DNA and regulation of the induction of genes associated with fatty acid uptake and metabolism may lead to the development of memory cells related to fatty acid metabolism that may foster IR.
As demonstrated in a murine model of cardiac transplantation, T-cell-specific PPARγ deficiency may lead to T-cell activation and the generation of alloreactive T cells with subsequent chronic allograft rejection. This was associated with higher Thl/Th2 and Th17/Treg ratios among the infiltrating CD4+ T cells [243].
In visceral AT, PPARγ is involved in the control of the inflammatory state of adipose tissue, which translates into IS [244,245]. The control of AT inflammation by PPARγ depends on the molecular regulation mediated by PPARγ Ser273, which results in significant changes in the transcriptional profile driving the differentiation of Tregs in visceral AT [246]. By regulating lipid metabolism and calming the inflammatory response, PPARs may also influence Treg survival [247].
Overall, the improvement/restoration of IS related to PPARγ action on metabolism may be achieved clinically by the use of PPARγ agonists [e.g., thiazolidinediones (TZDs)] or compounds that prevent PPARγ phosphorylation [e.g., influencing ligand-independent activation function 1 (AF-1) within the N-terminal A/B domain of PPARs]. A significant anti-inflammatory reprogramming of T cells is central to both therapeutic strategies [248,249,250]. The activation of PPARγ has also been suggested to regulate microRNA expression to inhibit inflammatory responses. PPARγ could upregulate microRNA (miR)-124 in vitro and in vivo to inhibit the production of proinflammatory cytokines [251].
PPARα and PPARβ are highly expressed and involved in oxidative metabolism by regulating genes that control substrate delivery and oxidative phosphorylation (OXPHOS) and the regulation of energy homeostasis. However, since they are expressed on T cells and other immune cells, PPARα and PPARβ are not as important in the regulation of the adaptive immune response in white AT as PPARγ is. Moreover, the expression levels of PPARα and PPARβ in white AT are considerably lower than the expression of PPARγ [252]. Thus, PPARα and PPARβ have an emerging critical role in immune cell differentiation and fate commitment but are not principal AT components in the context of IR [253]. Even if some of the experimental studies had linked the activation of PPARα in rodents with significant improvement in IS, the effect of a PPARα agonist in humans was much less pronounced, probably due to a lower expression of PPARα in AT relative to rodents and to possibly different mechanisms [254].
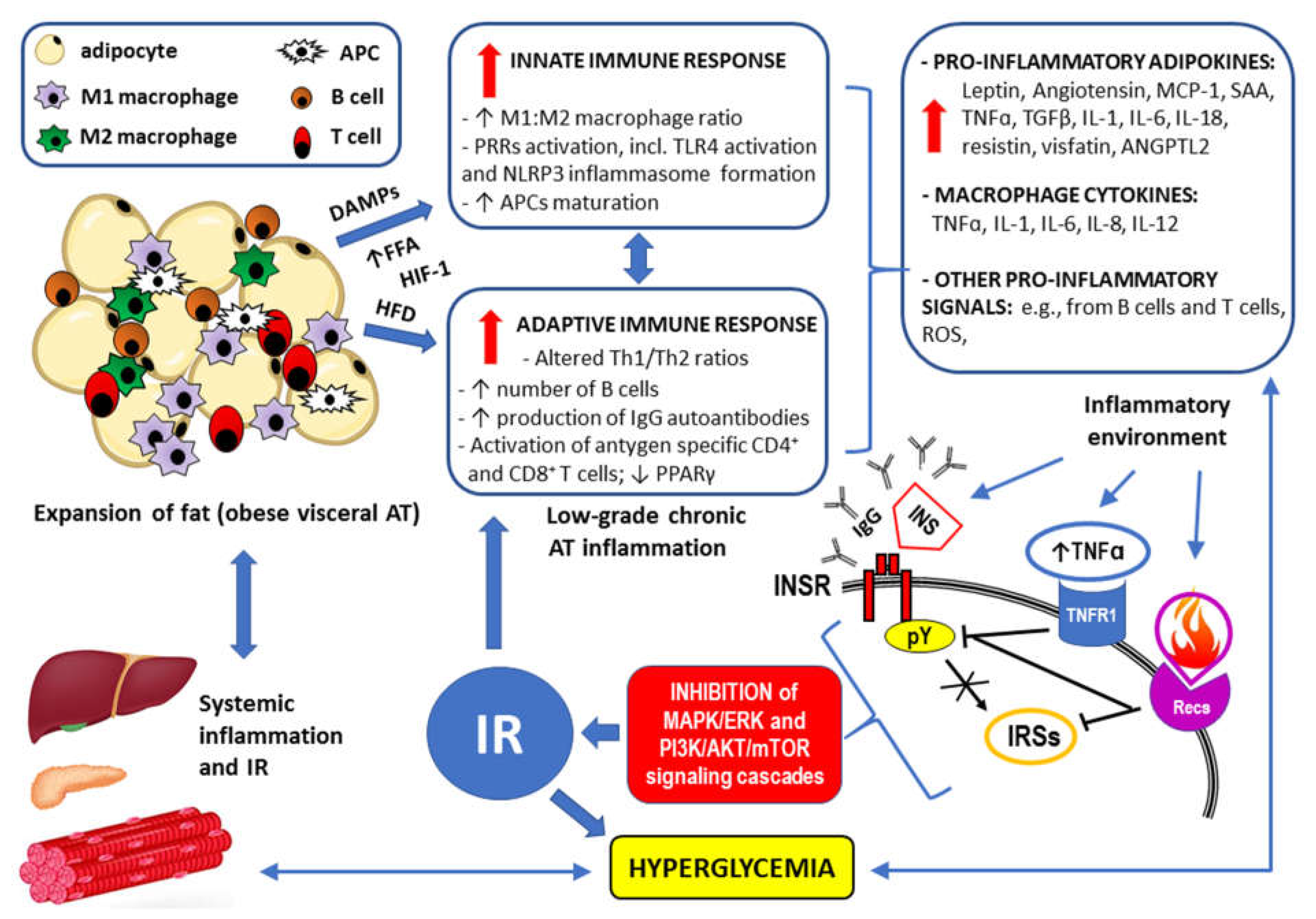
Figure 5.
Summary of mechanisms by which visceral obesity is a triggering factor for both systemic inflammation and insulin resistance (IR). For details see Chapter 3. Strongly enlarged adipocytes of the obese visceral adipose tissue (AT) fail to maintain longer metabolic homeostasis, because the lipid overload leads to endoplasmic reticulum stress, increased expression of the inflammation regulator NF-kB and the production of inflammation-inducing signals [160]. This chronic metabolism-induced inflammation or metaflammation in obese AT activates the resident immune cells, including macrophages, B cells, T cells and antigen presenting cells (APCs) [156]. High fat diet (HFD), increased flux of free fatty acids (FFA) accumulating damage-associated molecular patterns (DAMPs) from necrotic AT, and hypoxia-induced factor 1 (HIF-1) trigger both innate and adaptive immune responses [160]. Resulting low-grade chronic AT inflammation manifests as significantly elevated levels of the proinflammatory adipokines and cytokines as well as through overproduction of reactive oxygen species (ROS) [145,146,147,148,265,266]. Such inflammatory environment interferes with insulin signaling via: insulin receptor (INSR) through antibodies/autoantibodies against both insulin (INS) and INSR [117,125]; tumor necrosis factor receptor (TNFR1) through inhibition of the tyrosine autophosphorylation (pY) within INSR and/or serine/threonine phosphorylation (pS) of the insulin receptor substrates (IRSs) [114,118]; receptors for other proinflammatory cytokines (Recs) through abnormal, similar to observed for TNFR1 signaling, phosphorylation of INSR and IRSs [114,118,133]. Disrupted downstream signaling via mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) and phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) pathways eventually lead to IR. Resulting hyperglycemia may increase the inflammatory response by itself and lead to systemic inflammation [102,103,111]. The remaining abbreviations: ANGPTL2 – angiopoietin Like 2; IL-1, IL-6, IL-8, IL-12, IL-18 – the respective interleukins; MCP-1 – monocyte chemoattractant protein-1 (also known as CCL2); NLRP3 inflammasome – leucine-rich repeat (LRR)-containing proteins (NLR) family member 3 inflammasome; SAA – sulfur amino acids; TGFβ – transforming growth factor β
Figure 5.
Summary of mechanisms by which visceral obesity is a triggering factor for both systemic inflammation and insulin resistance (IR). For details see Chapter 3. Strongly enlarged adipocytes of the obese visceral adipose tissue (AT) fail to maintain longer metabolic homeostasis, because the lipid overload leads to endoplasmic reticulum stress, increased expression of the inflammation regulator NF-kB and the production of inflammation-inducing signals [160]. This chronic metabolism-induced inflammation or metaflammation in obese AT activates the resident immune cells, including macrophages, B cells, T cells and antigen presenting cells (APCs) [156]. High fat diet (HFD), increased flux of free fatty acids (FFA) accumulating damage-associated molecular patterns (DAMPs) from necrotic AT, and hypoxia-induced factor 1 (HIF-1) trigger both innate and adaptive immune responses [160]. Resulting low-grade chronic AT inflammation manifests as significantly elevated levels of the proinflammatory adipokines and cytokines as well as through overproduction of reactive oxygen species (ROS) [145,146,147,148,265,266]. Such inflammatory environment interferes with insulin signaling via: insulin receptor (INSR) through antibodies/autoantibodies against both insulin (INS) and INSR [117,125]; tumor necrosis factor receptor (TNFR1) through inhibition of the tyrosine autophosphorylation (pY) within INSR and/or serine/threonine phosphorylation (pS) of the insulin receptor substrates (IRSs) [114,118]; receptors for other proinflammatory cytokines (Recs) through abnormal, similar to observed for TNFR1 signaling, phosphorylation of INSR and IRSs [114,118,133]. Disrupted downstream signaling via mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) and phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) pathways eventually lead to IR. Resulting hyperglycemia may increase the inflammatory response by itself and lead to systemic inflammation [102,103,111]. The remaining abbreviations: ANGPTL2 – angiopoietin Like 2; IL-1, IL-6, IL-8, IL-12, IL-18 – the respective interleukins; MCP-1 – monocyte chemoattractant protein-1 (also known as CCL2); NLRP3 inflammasome – leucine-rich repeat (LRR)-containing proteins (NLR) family member 3 inflammasome; SAA – sulfur amino acids; TGFβ – transforming growth factor β
4. Concluding remarks
A high prevalence of IR, especially coexisting with obesity, explains the broad clinical spectrum of dysmetabolic conditions (e.g., glucose intolerance, diabetes, and metabolic syndrome) with which the pathomechanisms of IR are associated [255]. In 1998, the WHO proposed a unifying definition for “the syndrome of abnormal metabolism” and chose to call it the metabolic syndrome rather than the IR syndrome [256]. The main reason was that it was not considered established that IR is the cause of all the components of the syndrome (e.g., hypertension, dyslipidemia, visceral obesity or microalbuminuria) [256,257].
According to the current state of knowledge, there is no doubt that the immune response in excessive visceral AT interferes with insulin signaling. Both innate and adaptive responses take part in the development of reduced IS [156]. Given that the chronic low-grade inflammatory process in visceral AT is driven by obesity, the role of environmental factors and epigenetics should be considered [145,146]. Distinct from genetic mutation, epigenetic influences refer to modifications of gene expression (silencing or activation) without permanent changes in the genomic sequence. Epigenetic changes (e.g., DNA methylation or chromatin remodeling) provide a molecular basis for cellular memory in relation to environmental triggers [258]. Epigenetic factors are mediators of inflammation and chronic inflammatory disease. Consistently, a disruption of immune cell epigenetic regulation is thus predicted to be a major contributor to unrestrained immune responses and immune tolerance disruption that lead to diseases such as autoimmunity and inflammation [259,260,261]. These epigenetic changes can be caused by smoking, exposure to chemicals, including endocrine-disrupting compounds (EDCs), in the environment, or diet and other lifestyle factors, including sedentary behavior. Low or decreasing physical activity levels together with unbalanced and high-energy diets, commonly called Western-style diets, abundant in carbohydrates and saturated fats usually lead to increased visceral fat accumulation [135,262]. The high extent to which environmental effects can provoke epigenetic responses in obesity is clearly visible after satisfactory treatment (e.g., bariatric surgery), where the improvement in IS and lowering of the levels of inflammatory markers have been reported [263,264].
Therefore, all therapeutic strategies in IR coexisting with obesity should primarily consider the interruption of the vicious cycle of illness in the system, which includes the following: excess visceral AT, immune response/inflammation, and decreased IS.
Funding Statement
This research received no external funding.
Author Contributions
The author confirms sole responsibility for the following: study conception and design, data collection, analysis and interpretation of results, and manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
Abbreviations
| ABCs | age-associated B cells |
| acetyl-CoA | acetyl coenzyme A |
| AD | Alzheimer's disease |
| AF-1 | activation function 1 |
| AKT | protein kinase B |
| Ang II | angiotensin II |
| AP-1 | activator protein 1 |
| APCs | antigen-presenting cells |
| ASC | apoptosis-associated speck-like protein containing caspase recruitment domain (also PYCARD – PYD and CARD domain) |
| AT | adipose tissue |
| ATP | adenosine triphosphate |
| BCL-6 | corepressor B-cell lymphoma 6 |
| BMI | body mass index |
| Breg | regulatory B cells |
| CLRs | C-type lecithin receptors |
| Crk | adapter protein (also known as proto-oncogene c-Crk) |
| CXCR3 | chemokine receptor CXCR3 |
| CXCL10, CXCL11, and CXCL12 | chemokines – C-X-C Motif Chemokine Ligand 10, 11, and 12, respectively |
| DAMPs | damage-associated molecular patterns |
| DCs | dendritic cells |
| DIO mice | diet-induced obese mice |
| DOK4, DOK5 | docking proteins 4 and 5 (also known as IRS-5, IRS-6 – insulin receptor substrates 5 and 6, respectively) |
| DUBs | deubiquitinating enzymes (also known as deubiquitinating peptidases) |
| ECM | extracellular matrix |
| EDCs | endocrine disrupting compounds |
| EPIL | early placenta insulin-like peptide (also known as INSL4 – insulin-like growth factor 4) |
| ERα, ERβ | estrogen receptor alpha, beta, respectively |
| ER | endoplasmic reticulum |
| ERK | extracellular signal-regulated kinase |
| FFAs | free fatty acids |
| G-6-P | glucose-6-phosphate |
| Gab | Grb2-associated binder family proteins |
| GLUT4 | glucose transporter type 4 |
| GRB2 | growth factor receptor-bound protein 2 |
| HIF-1 | hypoxia-induced factor 1 |
| HOMA-IR | homeostasis model assessment (HOMA) of insulin resistance (IR) |
| IFN-γ | interferon gamma |
| IGF-1, IGF-2 | insulin-like growth factor 1 and 2 |
| IGFR1 | insulin-like growth factor receptor 1 |
| IκB | inhibitor of NF-κB |
| IKKs | inhibitor of NF-κB (IκB) kinases |
| IL-1β, IL-2, IL-4, IL-5, IL-6, IL-9, IL-13, IL-17A, IL-17E(IL-25), IL-18 | interleukins |
| INSL3 | mammalian Leydig cell-specific insulin-like peptide |
| INSL4 | insulin-like peptide 4 (also known as EPIL – early placenta insulin-like peptide), |
| INSL5, INSL6 | insulin-like peptides 5 and 6 |
| INSR | insulin receptor |
| IR | insulin resistance |
| IRAK1, IRAK2, IRAK4 | IL-1 receptor-associated kinases 1, 2, and 4, respectively |
| IRS-5, IRS-6 | insulin receptor substrates 5 and 6 (also known as DOK4, DOK5 – docking proteins 4 and 5, respectively) |
| IRSs | insulin receptor substrates |
| ISIOGTT | insulin sensitivity index (ISI) obtained from oral glucose tolerance test (OGTT) |
| JAK2 | Janus kinase 2 |
| JNKs | c-Jun-N-terminal kinases |
| LAT | linker for the activation of T cell |
| LIRP | locust insulin-related peptide |
| lncRNAs | long non-coding RNAs |
| LPS | lipopolysaccharide |
| LRR | leucine-rich-repeat motifs |
| LTB4 | chemokine leukotriene B4 |
| LTB4R1 | leukotriene B4 receptor 1 (also known as BLT1) |
| MAPK | mitogen-activated protein kinase |
| MCs | mast cells |
| MHC | major histocompatibility complex |
| MHO | metabolically healthy obesity |
| MIG | monokine induced by IFN-γ or chemokine CXCL9 |
| MIP | molluscan insulin-related peptides |
| mTOR | mammalian target of rapamycin |
| MW | molecular weight |
| ncRNAs | non-coding RNAs |
| NEK7 | serine-threonine kinase NEK7 (NIMA "never in mitosis gene a"-related kinase 7) |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NK cells | natural killer cells |
| NLRP3 inflammasome | leucine-rich repeat (LRR)-containing proteins (NLR) family member 3 inflammasome |
| NLRs | nucleotide oligomerization domain (NOD)-like receptors |
| NLSs | nuclear localization signals |
| NOD | nucleotide-binding oligomerization domain |
| NPXY (Asn-Pro-x-Tyr) motif (also known as the juxtamembrane Tyr960) | a conserved tyrosine phosphorylation motif in the activated insulin receptor (INSR) |
| OcaB | Oct coactivator B |
| OXPHOS | oxidative phosphorylation |
| PCOS | polycystic ovary syndrome |
| PDH | pyruvate dehydrogenase |
| PDK | pyruvate dehydrogenase kinase |
| PH | pleckstrin homology (also known as IH1) domain of the insulin receptor substrates (IRSs) |
| PI3K | phosphoinositide 3-kinase |
| PKC | protein kinase C |
| PLCγ1 | phospholipase-C-γ1 |
| PPARs | peroxisome proliferator-activated receptors |
| pMHC-I | immunogenic peptide – MHC class I |
| PPARα, PPARβ, and PPARγ | peroxisome proliferator-activated receptor alpha, beta and gamma, respectively |
| PRRs | pattern recognition receptors |
| PTB | phosphotyrosine binding (also known as IH2) domain of the insulin receptor substrates (IRSs) |
| RACK1 | receptor for activated C kinase 1 |
| RLRs | retinoic acid-inducible gene I (RIG-I)-like receptors |
| RORγt | retinoic acid receptor-related orphan receptor γt (also known as RORγ2) |
| ROS | reactive oxygen species |
| SAT | subcutaneous adipose tissues |
| SFAs | saturated fatty acids |
| SH2B | adapter protein containing a SH2 (Src homology 2) and a PH (pleckstrin homology) domains |
| SHP2 | protein tyrosine phosphatase 2 of the Src homology region 2 (SH2) |
| siRNAs | small interfering RNAs (also known as silencing RNAs) |
| SOCS-3 | suppressor of cytokine signaling protein 3 |
| SREBP-1 | sterol regulatory element-binding protein 1 (also known as SREBF1 – sterol regulatory element-binding transcription factor 1) |
| STAT3 | signal transducer and activator of transcription 3 |
| STKs | serine/threonine protein kinases |
| T1D, T2D | type 1, type 2 diabetes mellitus, respectively |
| TAK1 | transforming growth factor (TGF)-β-activated kinase 1 |
| T-bet | transcription factor T-bet (also known as Tbx21) |
| TCR | T-cell receptor |
| Th1, Th2 cells | T helper 1, T helper 2 lymphocytes, respectively |
| TIR | toll/interleukin 1 (IL-1) receptor homology domain |
| TLRs, TLR1-10 | toll-like receptors |
| TNF-α | tumor necrosis factor alpha |
| TNFR1 | tumor necrosis factor alpha (TNF-α) receptor 1 |
| TRAF6 | tumor necrosis factor (TNF) receptor associated factor 6 |
| TRAPα, TRAPβ, TRAPγ, TRAPδ | translocation-associated protein complexes α, β, γ, and δ (also known as SSR1, SSR2, SSR3, and SSR3 – signal sequence receptors, respectively) |
| Treg | regulatory T cells |
| TRIF | toll/interleukin 1 receptor (IL-1R) domain-containing adaptor-inducing interferon beta (IFN-β) |
| TZDs | thiazolidinediones |
| ZAP-70 | zeta-associated protein of 70,000 molecular weight |
References
- Magkos, F.; Wang, X.; Mittendorfer, B. Metabolic actions of insulin in men and women. Nutrition 2010, 26, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Li, J.; Gao, F. New insights into insulin: The anti-inflammatory effect and its clinical relevance. World J. Diabetes 2014, 5, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Rehman, K.; Akash, M.S.H. Mechanisms of inflammatory responses and development of insulin resistance: how are they interlinked? J. Biomed. Sci. 2016, 23, 87. [Google Scholar] [CrossRef] [PubMed]
- Meldrum, D.R.; Morris, M.A.; Gambone, J.C. Obesity pandemic: causes, consequences, and solutions—but do we have the will? Fertil. Steril. 2017, 107, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Ramana, K.V.; Plowman, T.J.; Shah, M.H.; Fernandez, E.; Christensen, H.; Aiges, M. Role of innate immune and inflammatory responses in the development of secondary diabetic complications. Curr. Mol. Med. 2022. [Google Scholar] [CrossRef]
- Banting FG, Best CH, Collip JB, Campbell WR, Fletcher AA, MacLeod JJR, Noble EC. The effect produced on diabetes by extracts of pancreas. Trans. Assoc. Am. Physicians 1922; 37:337-347.
- Banting, F.G.; Best, C.H.; Collip, J.B.; Campbell, W.R.; Fletcher, A. Pancreatic Extracts in the Treatment of Diabetes Mellitus: Preliminary Report. Can. Med. Assoc. J. 1922, 12, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Ward, C.W.; Lawrence, M.C. Landmarks in Insulin Research. Front. Endocrinol. 2011, 2, 76. [Google Scholar] [CrossRef]
- Stretton, A.O.W. The First Sequence: Fred Sanger and Insulin. Genetics 2002, 162, 527–532. [Google Scholar] [CrossRef]
- PubChem [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; 2004-. PubChem Compound Summary for CID 118984375, Insulin Human; [cited 2023 Mar. 21]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Insulin-Human.
- Hua, Q. Insulin: a small protein with a long journey. Protein Cell 2010, 1, 537–551. [Google Scholar] [CrossRef]
- Halban, P.A. Structural domains and molecular lifestyles of insulin and its precursors in the pancreatic Beta cell. Diabetologia 1991, 34, 767–778. [Google Scholar] [CrossRef]
- Weiss M, Steiner DF, Philipson LH. Insulin Biosynthesis, Secretion, Structure, and Structure-Activity Relationships. 2014 Feb 1. In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA, McLachlan R, New M, Purnell J, Sahay R, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000–. 2: PMID, 2590.
- Yegorov, S.; Bogerd, J.; Good, S.V. The relaxin family peptide receptors and their ligands: New developments and paradigms in the evolution from jawless fish to mammals. Gen. Comp. Endocrinol. 2014, 209, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Millar, L.; Streiner, N.; Webster, L.; Yamamoto, S.; Okabe, R.; Kawamata, T.; Shimoda, J.; Büllesbach, E.; Schwabe, C.; Bryant-Greenwood, G. Early placental insulin-like protein (INSL4 or EPIL) in placental and fetal membrane growth. Biol. Reprod. 2005, 73, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Hwa, V.; Oh, Y.; Rosenfeld, R. Insulin-like growth factor binding proteins: a proposed superfamily. Acta Paediatr. 1999, 88, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Nistor, M.; Schmidt, M.; Schiffner, R. The relaxin peptide family – potential future hope for neuroprotective therapy? A short review. Neural Regen. Res. 2018, 13, 402–405. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, Y.; Kawano, T. The C. elegans insulin-like peptides (ILPs). AIMS Biophys. 2018, 5, 217–230. [Google Scholar] [CrossRef]
- Li, K.W.; Geraerts, W.P.; Ebberink, R.H.; Joosse, J. Purification and sequencing of molluscan insulin-related peptide I (MIP I) from the neuroendocrine light green cells of Lymnaea stagnalis. Mol. Cell. Endocrinol. 1992, 85, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Nagata, K.; Hatanaka, H.; Kohda, D.; Kataoka, H.; Nagasawa, H.; Isogai, A.; Ishizaki, H.; Suzuki, A.; Inagaki, F. Three-dimensional Solution Structure of Bombyxin-II an Insulin-like Peptide of the SilkmothBombyx mori: Structural Comparison with Insulin and Relaxin. J. Mol. Biol. 1995, 253, 749–758. [Google Scholar] [CrossRef]
- Guo, Z.-Y.; Qiao, Z.-S.; Feng, Y.-M. TheIn VitroOxidative Folding of the Insulin Superfamily. Antioxidants Redox Signal. 2008, 10, 127–140. [Google Scholar] [CrossRef]
- Liu, M.; Wright, J.; Guo, H.; Xiong, Y.; Arvan, P. Proinsulin Entry and Transit Through the Endoplasmic Reticulum in Pancreatic Beta Cells. 2014, 95, 35–62. [CrossRef]
- Xu, X.; Huang, Y.; Li, X.; Arvan, P.; Liu, M. The Role of TRAPγ/SSR3 in Preproinsulin Translocation Into the Endoplasmic Reticulum. Diabetes 2021, 71, 440–452. [Google Scholar] [CrossRef]
- E Rohli, K.; Boyer, C.K.; Bearrows, S.C.; Moyer, M.R.; Elison, W.S.; Bauchle, C.J.; E Blom, S.; Zhang, J.; Wang, Y.; Stephens, S.B. ER Redox Homeostasis Regulates Proinsulin Trafficking and Insulin Granule Formation in the Pancreatic Islet β-Cell. Function 2022, 3. [Google Scholar] [CrossRef]
- Saisho, Y.; Arai, T. Postprandial C-Peptide to Glucose Ratio as a Marker of β Cell Function: Implication for the Management of Type 2 Diabetes. Int. J. Mol. Sci. 2016, 17, 744. [Google Scholar] [CrossRef] [PubMed]
- Maddaloni, E.; Bolli, G.B.; Frier, B.M.; Little, R.R.; Leslie, R.D.; Pozzilli, P.; Buzzetti, R. C-peptide determination in the diagnosis of type of diabetes and its management: A clinical perspective. Diabetes, Obes. Metab. 2022, 24, 1912–1926. [Google Scholar] [CrossRef] [PubMed]
- Marques, R.G.; Fontaine, M.J.; Rogers, J. C-Peptide. Pancreas 2004, 29, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Wahren, J.; Ekberg, K.; Johansson, J.; Henriksson, M.; Pramanik, A.; Johansson, B.-L.; Rigler, R.; Jörnvall, H. Role of C-peptide in human physiology. Am. J. Physiol. Metab. 2000, 278, E759–E768. [Google Scholar] [CrossRef] [PubMed]
- Vejrazkova, D.; Vankova, M.; Lukasova, P.; Vcelak, J.; Bendlova, B. Insights Into the Physiology of C-peptide. Physiol. Res. 2020, 69, S237–S243. [Google Scholar] [CrossRef] [PubMed]
- Ido, Y.; Vindigni, A.; Chang, K.; Stramm, L.; Chance, R.; Heath, W.F.; DiMarchi, R.D.; Di Cera, E.; Williamson, J.R. Prevention of Vascular and Neural Dysfunction in Diabetic Rats by C-Peptide. Science 1997, 277, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Wallerath, T.; Kunt, T.; Forst, T.; I Closs, E.; Lehmann, R.; Flohr, T.; Gabriel, M.; Schäfer, D.; Göpfert, A.; Pfützner, A.; et al. Stimulation of endothelial nitric oxide synthase by proinsulin C-peptide. Nitric Oxide 2003, 9, 95–102. [Google Scholar] [CrossRef]
- Souto, S.B.; Campos, J.R.; Fangueiro, J.F.; Silva, A.M.; Cicero, N.; Lucarini, M.; Durazzo, A.; Santini, A.; Souto, E.B. Multiple Cell Signalling Pathways of Human Proinsulin C-Peptide in Vasculopathy Protection. Int. J. Mol. Sci. 2020, 21, 645. [Google Scholar] [CrossRef]
- Washburn, R.L.; Mueller, K.; Kaur, G.; Moreno, T.; Moustaid-Moussa, N.; Ramalingam, L.; Dufour, J.M. C-Peptide as a Therapy for Type 1 Diabetes Mellitus. Biomedicines 2021, 9, 270. [Google Scholar] [CrossRef]
- Jensen, M.; De Meyts, P. Chapter 3 Molecular Mechanisms of Differential Intracellular Signaling From the Insulin Receptor. 2009, 80, 51–75. [CrossRef]
- Zhang, X.; Zhu, X.; Bi, X.; Huang, J.; Zhou, L. The Insulin Receptor: An Important Target for the Development of Novel Medicines and Pesticides. Int. J. Mol. Sci. 2022, 23, 7793. [Google Scholar] [CrossRef]
- King, G.L.; Park, K.; Li, Q. Selective Insulin Resistance and the Development of Cardiovascular Diseases in Diabetes: The 2015 Edwin Bierman Award Lecture. Diabetes 2016, 65, 1462–1471. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Yu, M.G.; Li, Q.; Park, K.; King, G.L. Insulin's actions on vascular tissues: Physiological effects and pathophysiological contributions to vascular complications of diabetes. Mol. Metab. 2021, 52, 101236. [Google Scholar] [CrossRef] [PubMed]
- Szukiewicz, D.; Trojanowski, S.; Kociszewska, A.; Szewczyk, G. Modulation of the Inflammatory Response in Polycystic Ovary Syndrome (PCOS)—Searching for Epigenetic Factors. Int. J. Mol. Sci. 2022, 23, 14663. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, K. Role of insulin action in the pathogenesis of diabetic complications. Diabetol. Int. 2022, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, G.G.; Huerta, M.; A González-Usigli, H.; Torres-Sánchez, E.D.; Delgado-Lara, D.L.; Pacheco-Moisés, F.P.; A Mireles-Ramírez, M.; Torres-Mendoza, B.M.; I Moreno-Cih, R.; E Velázquez-Brizuela, I. Cognitive disorder and dementia in type 2 diabetes mellitus. World J. Diabetes 2022, 13, 319–337. [Google Scholar] [CrossRef] [PubMed]
- Ezkurdia, A.; Ramírez, M.J.; Solas, M. Metabolic Syndrome as a Risk Factor for Alzheimer’s Disease: A Focus on Insulin Resistance. Int. J. Mol. Sci. 2023, 24, 4354. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Wang, X.; Wang, Y.; Sun, G.; Zhang, J.; Li, Z. Obesity and endocrine-related cancer: The important role of IGF-1. Front. Endocrinol. 2023, 14. [Google Scholar] [CrossRef]
- Singh, P.; Alex, J.M.; Bast, F. Insulin receptor (IR) and insulin-like growth factor receptor 1 (IGF-1R) signaling systems: novel treatment strategies for cancer. Med Oncol. 2013, 31, 1–14. [Google Scholar] [CrossRef]
- Lee, J.; Pilch, P.F. The insulin receptor: structure, function, and signaling. Am. J. Physiol. Physiol. 1994, 266, C319–C334. [Google Scholar] [CrossRef]
- Hubbard, S.R. The Insulin Receptor: Both a Prototypical and Atypical Receptor Tyrosine Kinase. Cold Spring Harb. Perspect. Biol. 2013, 5, a008946–a008946. [Google Scholar] [CrossRef]
- Ye, L.; Maji, S.; Sanghera, N.; Gopalasingam, P.; Gorbunov, E.; Tarasov, S.; Epstein, O.; Klein-Seetharaman, J. Structure and dynamics of the insulin receptor: implications for receptor activation and drug discovery. Drug Discov. Today 2017, 22, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Scapin, G.; Dandey, V.P.; Zhang, Z.; Prosise, W.; Hruza, A.; Kelly, T.; Mayhood, T.; Strickland, C.; Potter, C.S.; Carragher, B. Structure of the insulin receptor–insulin complex by single-particle cryo-EM analysis. Nature 2018, 556, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Payankaulam, S.; Raicu, A.-M.; Arnosti, D.N. Transcriptional Regulation of INSR, the Insulin Receptor Gene. Genes 2019, 10, 984. [Google Scholar] [CrossRef] [PubMed]
- Gorden, P.; Arakaki, R.; Collier, E.; Carpentier, J.L. Biosynthesis and regulation of the insulin receptor. Yale J. Biol. Med. 1989, 62, 521–31. [Google Scholar] [PubMed]
- Westermeier, F.; Sáez, T.; Arroyo, P.; Toledo, F.; Gutiérrez, J.; Sanhueza, C.; Pardo, F.; Leiva, A.; Sobrevia, L. Insulin receptor isoforms: an integrated view focused on gestational diabetes mellitus. Diabetes/Metabolism Res. Rev. 2015, 32, 350–365. [Google Scholar] [CrossRef] [PubMed]
- Belfiore, A.; Malaguarnera, R.; Vella, V.; Lawrence, M.; Sciacca, L.; Frasca, F.; Morrione, A.; Vigneri, R. Insulin Receptor Isoforms in Physiology and Disease: An Updated View. Endocr. Rev. 2017, 38, 379–431. [Google Scholar] [CrossRef] [PubMed]
- Frasca, F.; Pandini, G.; Scalia, P.; Sciacca, L.; Mineo, R.; Costantino, A.; Goldfine, I.D.; Belfiore, A.; Vigneri, R. Insulin Receptor Isoform A, a Newly Recognized, High-Affinity Insulin-Like Growth Factor II Receptor in Fetal and Cancer Cells. Mol. Cell. Biol. 1999, 19, 3278–3288. [Google Scholar] [CrossRef]
- Vogt, B.; Carrascosa, J.M.; Ermel, B.; Ullrich, A.; Häring, H.-U. The two isotypes of the human insulin receptor (HIR-A and HIR-B) follow different internalization kinetics. Biochem. Biophys. Res. Commun. 1991, 177, 1013–1018. [Google Scholar] [CrossRef]
- Beneit, N.; Fernández-García, C.E.; Martín-Ventura, J.L.; Perdomo, L.; Escribano. ; Michel, J.B.; García-Gómez, G.; Fernández, S.; Díaz-Castroverde, S.; Egido, J.; et al. Expression of insulin receptor (IR) A and B isoforms, IGF-IR, and IR/IGF-IR hybrid receptors in vascular smooth muscle cells and their role in cell migration in atherosclerosis. Cardiovasc. Diabetol. 2016, 15, 1–14. [Google Scholar] [CrossRef]
- Barthel, A.; Joost, H. Insulin Receptor. 2008, 632–636. [CrossRef]
- De Meyts, P. The Insulin Receptor and Its Signal Transduction Network. 2016 Apr 27. In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA, McLachlan R, New M, Purnell J, Sahay R, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000–.
- Tatulian, S.A. Structural Dynamics of Insulin Receptor and Transmembrane Signaling. Biochemistry 2015, 54, 5523–5532. [Google Scholar] [CrossRef]
- Kuznetsov, A.S.; Zamaletdinov, M.F.; Bershatsky, Y.V.; Urban, A.S.; Bocharova, O.V.; Bennasroune, A.; Maurice, P.; Bocharov, E.V.; Efremov, R.G. Dimeric states of transmembrane domains of insulin and IGF-1R receptors: Structures and possible role in activation. Biochim. et Biophys. Acta (BBA) - Biomembr. 2020, 1862. [Google Scholar] [CrossRef] [PubMed]
- Siddle, K. Signalling by insulin and IGF receptors: supporting acts and new players. J. Mol. Endocrinol. 2011, 47, R1–R10. [Google Scholar] [CrossRef] [PubMed]
- Di Guglielmo GM, Drake PG, Baass PC, Authier F, Posner BI, Bergeron JJ. Insulin receptor internalization and signalling. Mol Cell Biochem. 1998;182(1-2):59-63.
- Chang, L.; Chiang, S.-H.; Saltiel, A.R. Insulin Signaling and the Regulation of Glucose Transport. Mol. Med. 2004, 10, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Posner, B.I. Insulin Signalling: The Inside Story. Can. J. Diabetes 2016, 41, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Machado-Neto, J.A.; Fenerich, B.A.; Alves, A.P.N.R.; Fernandes, J.C.; Scopim-Ribeiro, R.; Coelho-Silva, J.L.; Traina, F. Insulin Substrate Receptor (IRS) proteins in normal and malignant hematopoiesis. Clinics 2018, 73, e566s. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.M. The insulin receptor substrate (IRS) proteins. Cell Cycle 2011, 10, 1750–1756. [Google Scholar] [CrossRef]
- Sun, X.J.; Rothenberg, P.; Kahn, C.R.; Backer, J.M.; Araki, E.; A Wilden, P.; A Cahill, D.; Goldstein, B.J.; White, M.F. Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature 1991, 352, 73–77. [Google Scholar] [CrossRef]
- White, M.F. IRS proteins and the common path to diabetes. Am. J. Physiol. Metab. 2002, 283, E413–E422. [Google Scholar] [CrossRef]
- M. , B.; A., H.; A., A.; S., L.; S., L.; G., L.; S., T.; P., A.; J., Z.; Björnholm, M.; et al. Absence of functional insulin receptor substrate-3 ( IRS-3 ) gene in humans. Diabetologia 2002, 45, 1697–1702. [Google Scholar] [CrossRef]
- Lavan, B.E.; Fantin, V.R.; Chang, E.T.; Lane, W.S.; Keller, S.R.; Lienhard, G.E. A Novel 160-kDa Phosphotyrosine Protein in Insulin-treated Embryonic Kidney Cells Is a New Member of the Insulin Receptor Substrate Family. J. Biol. Chem. 1997, 272, 21403–21407. [Google Scholar] [CrossRef]
- Mardilovich, K.; Pankratz, S.L.; Shaw, L.M. Expression and function of the insulin receptor substrate proteins in cancer. Cell Commun. Signal. 2009, 7, 14–14. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Dhe-Paganon, S.; Melendez, P.A.; Lee, J.; Shoelson, S.E. Two New Substrates in Insulin Signaling, IRS5/DOK4 and IRS6/DOK5. J. Biol. Chem. 2003, 278, 25323–25330. [Google Scholar] [CrossRef] [PubMed]
- Grimm, J.; Sachs, M.; Britsch, S.; Di Cesare, S.; Schwarz-Romond, T.; Alitalo, K.; Birchmeier, W. Novel p62dok family members, dok-4 and dok-5, are substrates of the c-Ret receptor tyrosine kinase and mediate neuronal differentiation. J. Cell Biol. 2001, 154, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Favre, C.; Gérard, A.; Clauzier, E.; Pontarotti, P.; Olive, D.; A Nunès, J. DOK4 and DOK5: new dok-related genes expressed in human T cells. Genes Immun. 2003, 4, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Hanke, S.; Mann, M. The Phosphotyrosine Interactome of the Insulin Receptor Family and Its Substrates IRS-1 and IRS-2. Mol. Cell. Proteom. 2009, 8, 519–534. [Google Scholar] [CrossRef]
- Voliovitch, H.; Schindler, D.G.; Hadari, Y.R.; Taylor, S.I.; Accili, D.; Zick, Y. Tyrosine Phosphorylation of Insulin Receptor Substrate-1 in Vivo Depends upon the Presence of Its Pleckstrin Homology Region. J. Biol. Chem. 1995, 270, 18083–18087. [Google Scholar] [CrossRef]
- Yenush, L.; Makati, K.J.; Smith-Hall, J.; Ishibashi, O.; Myers, M.G.; White, M.F. The Pleckstrin Homology Domain Is the Principle Link between the Insulin Receptor and IRS-1. J. Biol. Chem. 1996, 271, 24300–24306. [Google Scholar] [CrossRef]
- Sawka-Verhelle, D.; Tartare-Deckert, S.; White, M.F.; Van Obberghen, E. Insulin Receptor Substrate-2 Binds to the Insulin Receptor through Its Phosphotyrosine-binding Domain and through a Newly Identified Domain Comprising Amino Acids 591–786. J. Biol. Chem. 1996, 271, 5980–5983. [Google Scholar] [CrossRef]
- Liu, B.A.; Jablonowski, K.; Shah, E.E.; Engelmann, B.W.; Jones, R.B.; Nash, P.D. SH2 Domains Recognize Contextual Peptide Sequence Information to Determine Selectivity. Mol. Cell. Proteom. 2010, 9, 2391–2404. [Google Scholar] [CrossRef]
- Taniguchi, C.M.; Emanuelli, B.; Kahn, C.R. Critical nodes in signalling pathways: Insights into insulin action. Nat. Rev. Mol. Cell Biol. 2006, 7, 85–96. [Google Scholar] [CrossRef]
- Waksman, G.; Kumaran, S.; Lubman, O. SH2 domains: role, structure and implications for molecular medicine. Expert Rev. Mol. Med. 2004, 6, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.J.; Wang, L.-M.; Zhang, Y.; Yenush, L.; Jr, M.G.M.; Glasheen, E.; Lane, W.S.; Pierce, J.H.; White, M.F. Role of IRS-2 in insulin and cytokine signalling. Nature 1995, 377, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Takatani, T.; Shirakawa, J.; Shibue, K.; Gupta, M.K.; Kim, H.; Lu, S.; Hu, J.; White, M.F.; Kennedy, R.T.; Kulkarni, R.N. Insulin receptor substrate 1, but not IRS2, plays a dominant role in regulating pancreatic alpha cell function in mice. J. Biol. Chem. 2021, 296, 100646. [Google Scholar] [CrossRef] [PubMed]
- Valverde. M.; González-Rodríguez,. IRS2 and PTP1B: Two opposite modulators of hepatic insulin signalling. Arch. Physiol. Biochem. 2011, 117, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.T.-Y.; Lee, A.V. Insulin Receptor Substrates (IRSs) and Breast Tumorigenesis. J. Mammary Gland. Biol. Neoplasia 2008, 13, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Du, T.; Li, C.; Yang, G. STAT3 phosphorylation in central leptin resistance. Nutr. Metab. 2021, 18, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Giani, J.F.; Gironacci, M.M.; Muñoz, M.C.; Peña, C.; Turyn, D.; Dominici, F.P. Angiotensin-(1–7) stimulates the phosphorylation of JAK2, IRS-1 and Akt in rat heart in vivo: role of the AT1 and Mas receptors. Am. J. Physiol. Circ. Physiol. 2007, 293, H1154–H1163. [Google Scholar] [CrossRef]
- Lassak, A.; Del Valle, L.; Peruzzi, F.; Wang, J.Y.; Enam, S.; Croul, S.; Khalili, K.; Reiss, K. Insulin Receptor Substrate 1 Translocation to the Nucleus by the Human JC Virus T-antigen. J. Biol. Chem. 2002, 277, 17231–17238. [Google Scholar] [CrossRef]
- Prisco, M.; Santini, F.; Baffa, R.; Liu, M.; Drakas, R.; Wu, A.; Baserga, R. Nuclear Translocation of Insulin Receptor Substrate-1 by the Simian Virus 40 T Antigen and the Activated Type 1 Insulin-like Growth Factor Receptor. J. Biol. Chem. 2002, 277, 32078–32085. [Google Scholar] [CrossRef]
- Vuori, K.; Ruoslahti, E. Association of insulin receptor substrate-1 with integrins. Science 1994, 266, 1576–1578. [Google Scholar] [CrossRef]
- Urbanska, K.; Pannizzo, P.; Lassak, A.; Gualco, E.; Surmacz, E.; Croul, S.; Del Valle, L.; Khalili, K.; Reiss, K. Estrogen receptor β-mediated nuclear interaction between IRS-1 and Rad51 inhibits homologous recombination directed DNA repair in medulloblastoma. J. Cell. Physiol. 2008, 219, 392–401. [Google Scholar] [CrossRef]
- Tu, X.; Batta, P.; Innocent, N.; Prisco, M.; Casaburi, I.; Belletti, B.; Baserga, R. Nuclear Translocation of Insulin Receptor Substrate-1 by Oncogenes And Igf-I. J. Biol. Chem. 2002, 277, 44357–44365. [Google Scholar] [CrossRef] [PubMed]
- Nagao, H.; Cai, W.; Albrechtsen, N.J.W.; Steger, M.; Batista, T.M.; Pan, H.; Dreyfuss, J.M.; Mann, M.; Kahn, C.R. Distinct signaling by insulin and IGF-1 receptors and their extra- and intracellular domains. Proc. Natl. Acad. Sci. 2021, 118. [Google Scholar] [CrossRef] [PubMed]
- Hakuno, F.; Fukushima, T.; Yoneyama, Y.; Kamei, H.; Ozoe, A.; Yoshihara, H.; Yamanaka, D.; Shibano, T.; Sone-Yonezawa, M.; Yu, B.-C.; et al. The Novel Functions of High-Molecular-Mass Complexes Containing Insulin Receptor Substrates in Mediation and Modulation of Insulin-Like Activities: Emerging Concept of Diverse Functions by IRS-Associated Proteins. Front. Endocrinol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Abu Ahmad, Y.; Oknin-Vaisman, A.; Bitman-Lotan, E.; Orian, A. From the Evasion of Degradation to Ubiquitin-Dependent Protein Stabilization. Cells 2021, 10, 2374. [Google Scholar] [CrossRef] [PubMed]
- Girnita, L.; Girnita, A.; Larsson, O. Mdm2-dependent ubiquitination and degradation of the insulin-like growth factor 1 receptor. Proc. Natl. Acad. Sci. 2003, 100, 8247–8252. [Google Scholar] [CrossRef] [PubMed]
- Zick, Y. Ser/Thr Phosphorylation of IRS Proteins: A Molecular Basis for Insulin Resistance. Sci. STKE 2005, 2005, pe4–pe4. [Google Scholar] [CrossRef]
- Sethi, J.K.; Hotamisligil, G.S. Metabolic Messengers: tumour necrosis factor. Nat. Metab. 2021, 3, 1302–1312. [Google Scholar] [CrossRef]
- Hotamisligil, G. Mechanisms of TNF-α-induced insulin resistance. Exp. Clin. Endocrinol. Diabetes 1999, 107, 119–125. [Google Scholar] [CrossRef]
- Da Costa, R.M.; Neves, K.B.; Mestriner, F.L.; Louzada-Junior, P.; Bruder-Nascimento, T.; Tostes, R.C. TNF-α induces vascular insulin resistance via positive modulation of PTEN and decreased Akt/eNOS/NO signaling in high fat diet-fed mice. Cardiovasc. Diabetol. 2016, 15, 119. [Google Scholar] [CrossRef]
- Lavin, D.P.; White, M.F.; Brazil, D.P. IRS proteins and diabetic complications. Diabetologia 2016, 59, 2280–2291. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.A.; Ibrahim, Y.H.; Oh, A.S.; Fagan, D.H.; Byron, S.; Sarver, A.L.; Lee, A.V.; Shaw, L.M.; Fan, C.; Perou, C.; et al. Insulin Receptor Substrate Adaptor Proteins Mediate Prognostic Gene Expression Profiles in Breast Cancer. PLOS ONE 2016, 11, e0150564. [Google Scholar] [CrossRef] [PubMed]
- Lebovitz, H.E. Insulin resistance: definition and consequences. Exp. Clin. Endocrinol. Diabetes 2001, 109, S135–S148. [Google Scholar] [CrossRef] [PubMed]
- Freeman AM, Pennings N. Insulin Resistance. 2022 Sep 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan–. 2: PMID, 2993.
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef] [PubMed]
- Church, T.J.; Haines, S.T. Treatment Approach to Patients With Severe Insulin Resistance. Clin. Diabetes 2016, 34, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.R.; Matta, S.T.; Haymond, M.W.; Chung, S.T. Measuring Insulin Resistance in Humans. Horm. Res. Paediatr. 2020, 93, 577–588. [Google Scholar] [CrossRef]
- Kolb, H.; Kempf, K.; Röhling, M.; Martin, S. Insulin: too much of a good thing is bad. BMC Med. 2020, 18, 1–12. [Google Scholar] [CrossRef]
- Kolb, H.; Stumvoll, M.; Kramer, W.; Kempf, K.; Martin, S. Insulin translates unfavourable lifestyle into obesity. BMC Med. 2018, 16, 1–10. [Google Scholar] [CrossRef]
- Angelidi, A.M.; Filippaios, A.; Mantzoros, C.S. Severe insulin resistance syndromes. J. Clin. Investig. 2021, 131. [Google Scholar] [CrossRef]
- DeHaan KN, Preszler J, Hansen K. Nonalcoholic Fatty Liver Disease in Women with Polycystic Ovarian Syndrome: A Narrative Review. S D Med. 4: 2022;75(9), 2022.
- Yoon, J.H.; Hwang, J.; Son, S.U.; Choi, J.; You, S.-W.; Park, H.; Cha, S.-Y.; Maeng, S. How Can Insulin Resistance Cause Alzheimer’s Disease? Int. J. Mol. Sci. 2023, 24, 3506. [Google Scholar] [CrossRef]
- Zhao, X.; An, X.; Yang, C.; Sun, W.; Ji, H.; Lian, F. The crucial role and mechanism of insulin resistance in metabolic disease. Front. Endocrinol. 2023, 14, 1149239. [Google Scholar] [CrossRef] [PubMed]
- Lause, M.; Kamboj, A.; Faith, E.F. Dermatologic manifestations of endocrine disorders. Transl. Pediatr. 2017, 6, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Brady MF, Rawla P. Acanthosis Nigricans. [Updated 2022 Oct 9]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih. 4310.
- Boucher, J.; Kleinridders, A.; Kahn, C.R. Insulin Receptor Signaling in Normal and Insulin-Resistant States. Cold Spring Harb. Perspect. Biol. 2014, 6, a009191–a009191. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Wei, T. Inputs and outputs of insulin receptor. Protein Cell 2014, 5, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Catalano, K.J.; Maddux, B.A.; Szary, J.; Youngren, J.F.; Goldfine, I.D.; Schaufele, F. Insulin Resistance Induced by Hyperinsulinemia Coincides with a Persistent Alteration at the Insulin Receptor Tyrosine Kinase Domain. PLOS ONE 2014, 9, e108693. [Google Scholar] [CrossRef] [PubMed]
- A Corbin, J.; Bhaskar, V.; Goldfine, I.D.; Issafras, H.; Bedinger, D.H.; Lau, A.; Michelson, K.; Gross, L.M.; A Maddux, B.; Kuan, H.F.; et al. Inhibition of insulin receptor function by a human, allosteric monoclonal antibody. mAbs 2013, 6, 262–272. [Google Scholar] [CrossRef]
- Pei, J.; Wang, B.; Wang, D. Current Studies on Molecular Mechanisms of Insulin Resistance. J. Diabetes Res. 2022, 2022, 1–11. [Google Scholar] [CrossRef]
- Teimouri, M.; Hosseini, H.; ArabSadeghabadi, Z.; Babaei-Khorzoughi, R.; Gorgani-Firuzjaee, S.; Meshkani, R. The role of protein tyrosine phosphatase 1B (PTP1B) in the pathogenesis of type 2 diabetes mellitus and its complications. J. Physiol. Biochem. 2022, 78, 307–322. [Google Scholar] [CrossRef]
- Taguchi, A.; Tanokashira, D.; Fukuokaya, W. Involvement of insulin receptor substrates in cognitive impairment and Alzheimer’s disease. Neural Regen. Res. 2019, 14, 1330–1334. [Google Scholar] [CrossRef]
- Yoon, M.-S. The Role of Mammalian Target of Rapamycin (mTOR) in Insulin Signaling. Nutrients 2017, 9, 1176. [Google Scholar] [CrossRef]
- Sinha, S.; Haque, M. Insulin Resistance and Type 2 Diabetes Mellitus: An Ultimatum to Renal Physiology. Cureus 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Hansen, L.L.; Ikeda, Y.; Olsen, G.S.; Busch, A.K.; Mosthaf, L. Insulin Signaling Is Inhibited by Micromolar Concentrations of H2O2. J. Biol. Chem. 1999, 274, 25078–25084. [Google Scholar] [CrossRef] [PubMed]
- Volpe, C.M.O.; Villar-Delfino, P.H.; Dos Anjos, P.M.F.; Nogueira-Machado, J.A. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Willard, D.L.; Stevenson, M.; Steenkamp, D. Type B insulin resistance syndrome. Curr. Opin. Endocrinol. Diabetes 2016, 23, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Anna, A.; Monika, G. Splicing mutations in human genetic disorders: examples, detection, and confirmation. J. Appl. Genet. 2018, 59, 253–268. [Google Scholar] [CrossRef]
- Horikawa, O.; Ugi, S.; Takayoshi, T.; Omura, Y.; Yonishi, M.; Sato, D.; Fujita, Y.; Fuke, T.; Hirota, Y.; Ogawa, W.; et al. A family with type A insulin resistance syndrome caused by a novel insulin receptor mutation. Endocrinol. Diabetes Metab. Case Rep. 2023, 2023. [Google Scholar] [CrossRef]
- Yang, G.-Q.; Wang, B.-A.; Zhao, W.-R.; Gu, W.-J.; Lui, Z.-H.; Dou, J.-T.; Mu, Y.-M.; Lu, J.-M. Clinical and genetic analysis of the insulin receptor gene in a Chinese patient with extreme insulin resistance. Diabetes Res. Clin. Pr. 2010, 89, e56–e58. [Google Scholar] [CrossRef]
- Rasool, S.U.A.; Nabi, M.; Ashraf, S.; Amin, S. Insulin Receptor Substrate 1 Gly972Arg (rs1801278) Polymorphism Is Associated with Obesity and Insulin Resistance in Kashmiri Women with Polycystic Ovary Syndrome. Genes 2022, 13, 1463. [Google Scholar] [CrossRef]
- Gehart, H.; Kumpf, S.; Ittner, A.; Ricci, R. MAPK signalling in cellular metabolism: stress or wellness? EMBO Rep. 2010, 11, 834–840. [Google Scholar] [CrossRef]
- Sahakyan, G.; Vejux, A.; Sahakyan, N. The Role of Oxidative Stress-Mediated Inflammation in the Development of T2DM-Induced Diabetic Nephropathy: Possible Preventive Action of Tannins and Other Oligomeric Polyphenols. Molecules 2022, 27, 9035. [Google Scholar] [CrossRef]
- Avram, V.F.; Merce, A.P.; Hâncu, I.M.; Bătrân, A.D.; Kennedy, G.; Rosca, M.G.; Muntean, D.M. Impairment of Mitochondrial Respiration in Metabolic Diseases: An Overview. Int. J. Mol. Sci. 2022, 23, 8852. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Mechanistic Insight into Oxidative Stress-Triggered Signaling Pathways and Type 2 Diabetes. Molecules 2022, 27, 950. [Google Scholar] [CrossRef] [PubMed]
- Black, H.S. A Synopsis of the Associations of Oxidative Stress, ROS, and Antioxidants with Diabetes Mellitus. Antioxidants 2022, 11, 2003. [Google Scholar] [CrossRef] [PubMed]
- Stefan, N. Metabolically Healthy and Unhealthy Normal Weight and Obesity. Endocrinol. Metab. 2020, 35, 487–493. [Google Scholar] [CrossRef]
- Jana, B.A.; Chintamaneni, P.K.; Krishnamurthy, P.T.; Wadhwani, A.; Mohankumar, S.K. Cytosolic lipid excess-induced mitochondrial dysfunction is the cause or effect of high fat diet-induced skeletal muscle insulin resistance: a molecular insight. Mol. Biol. Rep. 2018, 46, 957–963. [Google Scholar] [CrossRef]
- Daneshmoghadam, J.; Omidifar, A.; Dilmaghani, N.A.; Karimi, Z.; Emamgholipour, S.; Shanaki, M. The gene expression of long non-coding RNAs (lncRNAs): MEG3 and H19 in adipose tissues from obese women and its association with insulin resistance and obesity indices. J. Clin. Lab. Anal. 2021, 35, e23741. [Google Scholar] [CrossRef]
- Hue, L.; Taegtmeyer, H. The Randle cycle revisited: a new head for an old hat. Am. J. Physiol. Metab. 2009, 297, E578–E591. [Google Scholar] [CrossRef]
- Guo, Z. Pyruvate dehydrogenase, Randle cycle, and skeletal muscle insulin resistance. Proc. Natl. Acad. Sci. 2015, 112, 201505398–E2854. [Google Scholar] [CrossRef]
- Camastra, S.; Ferrannini, E. Role of anatomical location, cellular phenotype and perfusion of adipose tissue in intermediary metabolism: A narrative review. Rev. Endocr. Metab. Disord. 2022, 23, 43–50. [Google Scholar] [CrossRef]
- De Loof, M.; Renguet, E.; Ginion, A.; Bouzin, C.; Horman, S.; Beauloye, C.; Bertrand, L.; Bultot, L. Enhanced protein acetylation initiates fatty acid-mediated inhibition of cardiac glucose transport. Am. J. Physiol. Circ. Physiol. 2023, 324, H305–H317. [Google Scholar] [CrossRef]
- Chen, Q.; Yu, W.; Shi, J.; Shen, J.; Gao, T.; Zhang, J.; Xi, F.; Li, J.; Li, N. Insulin alleviates the inflammatory response and oxidative stress injury in cerebral tissues in septic rats. J. Inflamm. 2014, 11, 18–18. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-T.; Lue, J.-H.; Cheng, T.-H.; Tsai, Y.-J. Glycemic control with insulin attenuates sepsis-associated encephalopathy by inhibiting glial activation via the suppression of the nuclear factor kappa B and mitogen-activated protein kinase signaling pathways in septic rats. Brain Res. 2020, 1738, 146822. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-W.; Hung, L.-C.; Chen, Y.-C.; Wang, W.-H.; Lin, C.-Y.; Tzeng, H.-H.; Suen, J.-L.; Chen, Y.-H. Insulin Reduces Inflammation by Regulating the Activation of the NLRP3 Inflammasome. Front. Immunol. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Khanna, D.; Khanna, S.; Khanna, P.; Kahar, P.; Patel, B.M. Obesity: A Chronic Low-Grade Inflammation and Its Markers. Cureus 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Santillana, N.; Astudillo-Guerrero, C.; D’espessailles, A.; Cruz, G. White Adipose Tissue Dysfunction: Pathophysiology and Emergent Measurements. Nutrients 2023, 15, 1722. [Google Scholar] [CrossRef] [PubMed]
- Daryabor, G.; Kabelitz, D.; Kalantar, K. An update on immune dysregulation in obesity-related insulin resistance. Scand. J. Immunol. 2018, 89, e12747. [Google Scholar] [CrossRef]
- Gutierrez-Rodelo, C.; Arellano-Plancarte, A.; Hernandez-Aranda, J.; Landa-Galvan, H.V.; Parra-Mercado, G.K.; Moreno-Licona, N.J.; Hernandez-Gonzalez, K.D.; Catt, K.J.; Villalobos-Molina, R.; Olivares-Reyes, J.A. Angiotensin II Inhibits Insulin Receptor Signaling in Adipose Cells. Int. J. Mol. Sci. 2022, 23, 6048. [Google Scholar] [CrossRef]
- Akash, M.S.H.; Rehman, K.; Liaqat, A. Tumor Necrosis Factor-Alpha: Role in Development of Insulin Resistance and Pathogenesis of Type 2 Diabetes Mellitus. J. Cell. Biochem. 2017, 119, 105–110. [Google Scholar] [CrossRef]
- Jager, J.; Grémeaux, T.; Cormont, M.; Le Marchand-Brustel, Y.; Tanti, J.-F. Interleukin-1β-Induced Insulin Resistance in Adipocytes through Down-Regulation of Insulin Receptor Substrate-1 Expression. Endocrinology 2007, 148, 241–251. [Google Scholar] [CrossRef]
- Rehman, K.; Akash, M.S.H.; Liaqat, A.; Kamal, S.; Qadir, M.I.; Rasul, A. Role of Interleukin-6 in Development of Insulin Resistance and Type 2 Diabetes Mellitus. Crit. Rev. Eukaryot. Gene Expr. 2017, 27, 229–236. [Google Scholar] [CrossRef]
- Miura, S.; Kai, Y.; Ono, M.; Ezaki, O. Overexpression of Peroxisome Proliferator-activated Receptor γ Coactivator-1α Down-regulates GLUT4 mRNA in Skeletal Muscles. J. Biol. Chem. 2003, 278, 31385–31390. [Google Scholar] [CrossRef]
- Lebrun, P.; Van Obberghen, E. SOCS proteins causing trouble in insulin action. Acta Physiol. 2007, 192, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, S.B.; O’neill, H.M.; Sylow, L.; Honeyman, J.; Hewitt, K.A.; Palanivel, R.; Fullerton, M.D.; Öberg, L.; Balendran, A.; Galic, S.; et al. Deletion of Skeletal Muscle SOCS3 Prevents Insulin Resistance in Obesity. Diabetes 2012, 62, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, X. Research Advances on Suppressor of Cytokine Signaling 3 (SOCS3) in Animal Carbohydrate and Lipid Metabolism Processes. Pak. J. Biol. Sci. 2022, 25, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, T.; Ackerman, S.E.; Shen, L.; Engleman, E. Role of innate and adaptive immunity in obesity-associated metabolic disease. J. Clin. Investig. 2017, 127, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Li, C.; Zhang, D.; Li, Z.; Xia, P.; Liu, X.; Cai, X.; Yang, P.; Ling, J.; Zhang, J.; et al. Advances in T Cells Based on Inflammation in Metabolic Diseases. Cells 2022, 11, 3554. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Bris, R.; Saez, A.; Herrero-Fernandez, B.; Rius, C.; Sanchez-Martinez, H.; Gonzalez-Granado, J.M. CD4 T-Cell Subsets and the Pathophysiology of Inflammatory Bowel Disease. Int. J. Mol. Sci. 2023, 24, 2696. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ballantyne, C.M. Metabolic Inflammation and Insulin Resistance in Obesity. Circ. Res. 2020, 126, 1549–1564. [Google Scholar] [CrossRef]
- Kolb, H. Obese visceral fat tissue inflammation: from protective to detrimental? . 2022, 20, 1–14. [Google Scholar] [CrossRef]
- Tse, K.M.; Takeuchi, O. Innate immune sensing of pathogens and its post-transcriptional regulations by RNA-binding proteins. Arch. Pharmacal Res. 2023, 46, 65–77. [Google Scholar] [CrossRef]
- Amarante-Mendes, G.P.; Adjemian, S.; Branco, L.M.; Zanetti, L.C.; Weinlich, R.; Bortoluci, K.R. Pattern Recognition Receptors and the Host Cell Death Molecular Machinery. Front. Immunol. 2018, 9, 2379. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Cai, S.-Y.; Shao, J.-Z.; Chen, J. Toll-Like Receptors, Associated Biological Roles, and Signaling Networks in Non-Mammals. Front. Immunol. 2018, 9, 1523. [Google Scholar] [CrossRef] [PubMed]
- Lannoy, V.; Côté-Biron, A.; Asselin, C.; Rivard, N. TIRAP, TRAM, and Toll-Like Receptors: The Untold Story. Mediat. Inflamm. 2023, 2023, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Suresh, R.; Mosser, D.M. Pattern recognition receptors in innate immunity, host defense, and immunopathology. Adv. Physiol. Educ. 2013, 37, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Piccinini, A.M.; Midwood, K.S. DAMPening Inflammation by Modulating TLR Signalling. Mediat. Inflamm. 2010, 2010, 672395. [Google Scholar] [CrossRef] [PubMed]
- Duan, T.; Du, Y.; Xing, C.; Wang, H.Y.; Wang, R.-F. Toll-Like Receptor Signaling and Its Role in Cell-Mediated Immunity. Front. Immunol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Botos, I.; Segal, D.M.; Davies, D.R. The Structural Biology of Toll-like Receptors. Structure 2011, 19, 447–459. [Google Scholar] [CrossRef]
- Gao, D.; Li, W. Structures and recognition modes of toll-like receptors. Proteins: Struct. Funct. Bioinform. 2016, 85, 3–9. [Google Scholar] [CrossRef]
- Landström, M. The TAK1–TRAF6 signalling pathway. Int. J. Biochem. Cell Biol. 2010, 42, 585–589. [Google Scholar] [CrossRef]
- Buchholz, B.M.; Billiar, T.R.; Bauer, A.J. Dominant role of the MyD88-dependent signaling pathway in mediating early endotoxin-induced murine ileus. Am. J. Physiol. Liver Physiol. 2010, 299, G531–G538. [Google Scholar] [CrossRef]
- Deguine, J.; Barton, G.M. MyD88: a central player in innate immune signaling. F1000Prime Rep. 2014, 6, 97–97. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.O.; Sweet, M.J.; Mansell, A.; Kellie, S.; Kobe, B. TRIF-dependent TLR signaling, its functions in host defense and inflammation, and its potential as a therapeutic target. J. Leukoc. Biol. 2016, 100, 27–45. [Google Scholar] [CrossRef] [PubMed]
- Daryabor, G.; Kabelitz, D.; Kalantar, K. An update on immune dysregulation in obesity-related insulin resistance. Scand. J. Immunol. 2018, 89, e12747. [Google Scholar] [CrossRef] [PubMed]
- Ochando, J.; Mulder, W.J.M.; Madsen, J.C.; Netea, M.G.; Duivenvoorden, R. Trained immunity — basic concepts and contributions to immunopathology. Nat. Rev. Nephrol. 2022, 19, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Domínguez-Andrés, J.; Barreiro, L.B.; Chavakis, T.; Divangahi, M.; Fuchs, E.; Joosten, L.A.B.; Van Der Meer, J.W.M.; Mhlanga, M.M.; Mulder, W.J.M.; et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020, 20, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Sears, D.D. TLR4 and Insulin Resistance. Gastroenterol. Res. Pr. 2010, 2010, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ieronymaki, E.; Daskalaki, M.G.; Lyroni, K.; Tsatsanis, C. Insulin Signaling and Insulin Resistance Facilitate Trained Immunity in Macrophages Through Metabolic and Epigenetic Changes. Front. Immunol. 2019, 10, 1330. [Google Scholar] [CrossRef]
- Schroder, K.; Tschopp, J. The Inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef]
- Chait, A.; den Hartigh, L.J. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 22. [Google Scholar] [CrossRef]
- Shimobayashi, M.; Albert, V.; Wölnerhanssen, B.; Frei, I.C.; Weissenberger, D.; Meyer-Gerspach, A.C.; Clement, N.; Moes, S.; Colombi, M.; Meier, J.A.; et al. Insulin resistance causes inflammation in adipose tissue. J. Clin. Investig. 2018, 128, 1538–1550. [Google Scholar] [CrossRef]
- Chen, M.-Y.; Ye, X.-J.; He, X.-H.; Ouyang, D.-Y. The Signaling Pathways Regulating NLRP3 Inflammasome Activation. Inflammation 2021, 44, 1229–1245. [Google Scholar] [CrossRef] [PubMed]
- Blevins, H.M.; Xu, Y.; Biby, S.; Zhang, S. The NLRP3 Inflammasome Pathway: A Review of Mechanisms and Inhibitors for the Treatment of Inflammatory Diseases. Front. Aging Neurosci. 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Xu, S.; Ma, Y.; Liu, G.; Jang, H.; Fang, J. Modulatory Mechanisms of the NLRP3 Inflammasomes in Diabetes. Biomolecules 2019, 9, 850. [Google Scholar] [CrossRef] [PubMed]
- Cronkite, D.A.; Strutt, T.M. The Regulation of Inflammation by Innate and Adaptive Lymphocytes. J. Immunol. Res. 2018, 2018, 1467538. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Bosselut, R. CD4–CD8 differentiation in the thymus: connecting circuits and building memories. Curr. Opin. Immunol. 2012, 24, 139–145. [Google Scholar] [CrossRef] [PubMed]
- O, E.; Lee, Y.-T.; Ko, E.-J.; Kim, K.-H.; Lee, Y.-N.; Song, J.-M.; Kwon, Y.-M.; Kim, M.-C.; Perez, D.R.; Kang, S.-M. Roles of Major Histocompatibility Complex Class II in Inducing Protective Immune Responses to Influenza Vaccination. J. Virol. 2014, 88, 7764–7775. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, I.; Jeon, D.; Moseman, J.E.; Muralidhar, A.; Potluri, H.K.; McNeel, D.G. Role of B cells as antigen presenting cells. Front. Immunol. 2022, 13. [Google Scholar] [CrossRef]
- Marshall, J.S.; Warrington, R.; Watson, W.; Kim, H.L. An introduction to immunology and immunopathology. Allergy, Asthma Clin. Immunol. 2018, 14, 1–10. [Google Scholar] [CrossRef]
- Schipper, H.S.; Prakken, B.; Kalkhoven, E.; Boes, M. Adipose tissue-resident immune cells: key players in immunometabolism. Trends Endocrinol. Metab. 2012, 23, 407–415. [Google Scholar] [CrossRef]
- Lu, J.; Zhao, J.; Meng, H.; Zhang, X. Adipose Tissue-Resident Immune Cells in Obesity and Type 2 Diabetes. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Mraz, M.; Haluzik, M. The role of adipose tissue immune cells in obesity and low-grade inflammation. J. Endocrinol. 2014, 222, R113–R127. [Google Scholar] [CrossRef] [PubMed]
- Boutens, L.; Hooiveld, G.J.; Dhingra, S.; Cramer, R.A.; Netea, M.G.; Stienstra, R. Unique metabolic activation of adipose tissue macrophages in obesity promotes inflammatory responses. Diabetologia 2018, 61, 942–953. [Google Scholar] [CrossRef] [PubMed]
- Vandamme, C.; Kinnunen, T. B-cell helper T cells and type 1 diabetes. Scand. J. Immunol. 2020, 92. [Google Scholar] [CrossRef] [PubMed]
- Wolosowicz, M.; Lukaszuk, B.; Chabowski, A. The Causes of Insulin Resistance in Type 1 Diabetes Mellitus: Is There a Place for Quaternary Prevention? Int. J. Environ. Res. Public Heal. 2020, 17, 8651. [Google Scholar] [CrossRef] [PubMed]
- Sanz, I.; Wei, C.; Jenks, S.A.; Cashman, K.S.; Tipton, C.; Woodruff, M.C.; Hom, J.; Lee, F.E.-H. Challenges and Opportunities for Consistent Classification of Human B Cell and Plasma Cell Populations. Front. Immunol. 2019, 10, 2458. [Google Scholar] [CrossRef]
- Srikakulapu, P.; McNamara, C.A. B Lymphocytes and Adipose Tissue Inflammation. Arter. Thromb. Vasc. Biol. 2020, 40, 1110–1122. [Google Scholar] [CrossRef]
- Duffaut, C.; Galitzky, J.; Lafontan, M.; Bouloumié, A. Unexpected trafficking of immune cells within the adipose tissue during the onset of obesity. Biochem. Biophys. Res. Commun. 2009, 384, 482–485. [Google Scholar] [CrossRef]
- A Winer, D.; Winer, S.; Shen, L.; Wadia, P.P.; Yantha, J.; Paltser, G.; Tsui, H.; Wu, P.; Davidson, M.G.; Alonso, M.N.; et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat. Med. 2011, 17, 610–617. [Google Scholar] [CrossRef]
- Crooks, S.W.; Stockley, R.A. Leukotriene B4. Int. J. Biochem. Cell Biol. 1998, 30, 173–178. [Google Scholar] [CrossRef]
- Asahara, M.; Ito, N.; Hoshino, Y.; Sasaki, T.; Yokomizo, T.; Nakamura, M.; Shimizu, T.; Yamada, Y. Role of leukotriene B4 (LTB4)-LTB4 receptor 1 signaling in post-incisional nociceptive sensitization and local inflammation in mice. PLOS ONE 2022, 17, e0276135. [Google Scholar] [CrossRef]
- He, R.; Chen, Y.; Cai, Q. The role of the LTB4-BLT1 axis in health and disease. Pharmacol. Res. 2020, 158, 104857. [Google Scholar] [CrossRef]
- Horrillo, R.; González-Périz, A.; Martínez-Clemente, M.; López-Parra, M.; Ferré, N.; Titos, E.; Morán-Salvador, E.; Deulofeu, R.; Arroyo, V.; Clària, J. 5-Lipoxygenase Activating Protein Signals Adipose Tissue Inflammation and Lipid Dysfunction in Experimental Obesity. J. Immunol. 2010, 184, 3978–3987. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Oh, D.Y.; Bandyopadhyay, G.; Lagakos, W.S.; Talukdar, S.; Osborn, O.; Johnson, A.; Chung, H.; Mayoral, R.; Maris, M.; et al. LTB4 promotes insulin resistance in obese mice by acting on macrophages, hepatocytes and myocytes. Nat. Med. 2015, 21, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Mothe-Satney, I.; Filloux, C.; Amghar, H.; Pons, C.; Bourlier, V.; Galitzky, J.; Grimaldi, P.A.; Féral, C.C.; Bouloumié, A.; Van Obberghen, E.; et al. Adipocytes Secrete Leukotrienes. Diabetes 2012, 61, 2311–2319. [Google Scholar] [CrossRef] [PubMed]
- Ying, W.; Wollam, J.; Ofrecio, J.M.; Bandyopadhyay, G.; El Ouarrat, D.; Lee, Y.S.; Oh, D.Y.; Li, P.; Osborn, O.; Olefsky, J.M. Adipose tissue B2 cells promote insulin resistance through leukotriene LTB4/LTB4R1 signaling. J. Clin. Investig. 2017, 127, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- DeFuria, J.; Belkina, A.C.; Jagannathan-Bogdan, M.; Snyder-Cappione, J.; Carr, J.D.; Nersesova, Y.R.; Markham, D.; Strissel, K.J.; Watkins, A.A.; Zhu, M.; et al. B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proc. Natl. Acad. Sci. 2013, 110, 5133–5138. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Chng, M.H.Y.; Alonso, M.N.; Yuan, R.; Winer, D.A.; Engleman, E.G. B-1a Lymphocytes Attenuate Insulin Resistance. Diabetes 2014, 64, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Harmon, D.B.; Srikakulapu, P.; Kaplan, J.L.; Oldham, S.N.; McSkimming, C.; Garmey, J.C.; Perry, H.M.; Kirby, J.L.; Prohaska, T.A.; Gonen, A.; et al. Protective Role for B-1b B Cells and IgM in Obesity-Associated Inflammation, Glucose Intolerance, and Insulin Resistance. Arter. Thromb. Vasc. Biol. 2016, 36, 682–691. [Google Scholar] [CrossRef]
- Nishimura, S.; Manabe, I.; Takaki, S.; Nagasaki, M.; Otsu, M.; Yamashita, H.; Sugita, J.; Yoshimura, K.; Eto, K.; Komuro, I.; et al. Adipose Natural Regulatory B Cells Negatively Control Adipose Tissue Inflammation. Cell Metab. 2013, 18, 759–766. [Google Scholar] [CrossRef]
- Ma, S.; Wang, C.; Mao, X.; Hao, Y. B Cell Dysfunction Associated With Aging and Autoimmune Diseases. Front. Immunol. 2019, 10, 318. [Google Scholar] [CrossRef]
- Sachinidis, A.; Xanthopoulos, K.; Garyfallos, A. Age-Associated B Cells (ABCs) in the Prognosis, Diagnosis and Therapy of Systemic Lupus Erythematosus (SLE). Mediterr. J. Rheumatol. 2020, 31, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Carter, S.; Miard, S.; Caron, A.; Sallé-Lefort, S.; St-Pierre, P.; Anhê, F.F.; Lavoie-Charland, E.; Blais-Lecours, P.; Drolet, M.-C.; Lefebvre, J.S.; et al. Loss of OcaB Prevents Age-Induced Fat Accretion and Insulin Resistance by Altering B-Lymphocyte Transition and Promoting Energy Expenditure. Diabetes 2018, 67, 1285–1296. [Google Scholar] [CrossRef] [PubMed]
- Frasca, D.; Garcia, D.; Diaz, A.; Romero, M.; Thaller, S.; Blomberg, B.B. Phenotypic and functional features of B cells from two different human subcutaneous adipose depots. PLOS ONE 2023, 18, e0285025. [Google Scholar] [CrossRef]
- Karin, N. CXCR3 Ligands in Cancer and Autoimmunity, Chemoattraction of Effector T Cells, and Beyond. Front. Immunol. 2020, 11, 976. [Google Scholar] [CrossRef] [PubMed]
- Stolarczyk, E.; Lord, G.M.; Howard, J.K. The immune cell transcription factor T-bet: A novel metabolic regulator. Adipocyte 2013, 3, 58–62. [Google Scholar] [CrossRef] [PubMed]
- A Exley, M.; Hand, L.; O'Shea, D.; Lynch, L. Interplay between the immune system and adipose tissue in obesity. J. Endocrinol. 2014, 223, R41–R48. [Google Scholar] [CrossRef] [PubMed]
- Villarreal-Calderón, J.R.; Cuéllar, R.X.; Gonzalez, M.R.R.; Rubio-Infante, N.; Castillo, E.C.; Elizondo-Montemayor, L.; García-Rivas, G. Interplay between the Adaptive Immune System and Insulin Resistance in Weight Loss Induced by Bariatric Surgery. Oxidative Med. Cell. Longev. 2019, 2019, 1–14. [Google Scholar] [CrossRef]
- Bensussen, A.; Torres-Magallanes, J.A.; de Álvarez-Buylla, E.R. Molecular tracking of insulin resistance and inflammation development on visceral adipose tissue. Front. Immunol. 2023, 14. [Google Scholar] [CrossRef]
- Sbierski-Kind, J.; Goldeck, D.; Buchmann, N.; Spranger, J.; Volk, H.-D.; Steinhagen-Thiessen, E.; Pawelec, G.; Demuth, I.; Spira, D. T cell phenotypes associated with insulin resistance: results from the Berlin Aging Study II. Immun. Ageing 2020, 17, 1–11. [Google Scholar] [CrossRef]
- Rakotoarivelo, V.; Lacraz, G.; Mayhue, M.; Brown, C.; Rottembourg, D.; Fradette, J.; Ilangumaran, S.; Menendez, A.; Langlois, M.-F.; Ramanathan, S. Inflammatory Cytokine Profiles in Visceral and Subcutaneous Adipose Tissues of Obese Patients Undergoing Bariatric Surgery Reveal Lack of Correlation With Obesity or Diabetes. Ebiomedicine 2018, 30, 237–247. [Google Scholar] [CrossRef]
- Frikke-Schmidt, H.; O'Rourke, R.W.; Lumeng, C.N.; Sandoval, D.A.; Seeley, R.J. Does bariatric surgery improve adipose tissue function? Obes. Rev. 2016, 17, 795–809. [Google Scholar] [CrossRef] [PubMed]
- Osorio-Conles. ; Vidal, J.; de Hollanda, A. Impact of Bariatric Surgery on Adipose Tissue Biology. J. Clin. Med. 2021, 10, 5516. [Google Scholar] [CrossRef] [PubMed]
- Alonso, G.T.; Fomin, D.S.; Rizzo, L.V. Human follicular helper T lymphocytes critical players in antibody responses. 2021, 19, eRB6077. [CrossRef]
- Altara, R.; Manca, M.; Brandão, R.D.; Zeidan, A.; Booz, G.W.; Zouein, F.A. Emerging importance of chemokine receptor CXCR3 and its ligands in cardiovascular diseases. Clin. Sci. 2016, 130, 463–478. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wu, L.; Wang, S.; Chen, X. Role of Chemokine (C–X–C Motif) Ligand 10 (CXCL10) in Renal Diseases. Mediat. Inflamm. 2020, 2020, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Billottet, C.; Quemener, C.; Bikfalvi, A. CXCR3, a double-edged sword in tumor progression and angiogenesis. Biochim. et Biophys. Acta (BBA) - Rev. Cancer 2013, 1836, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Huen, A.C.; Wells, A. The Beginning of the End: CXCR3 Signaling in Late-Stage Wound Healing. Adv. Wound Care 2012, 1, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Dar, W.A.; Knechtle, S.J. CXCR3-mediated T-cell chemotaxis involves ZAP-70 and is regulated by signalling through the T-cell receptor. Immunology 2007, 120, 467–485. [Google Scholar] [CrossRef]
- Wang, H.; Kadlecek, T.A.; Au-Yeung, B.B.; Goodfellow, H.E.S.; Hsu, L.-Y.; Freedman, T.S.; Weiss, A. ZAP-70: An Essential Kinase in T-cell Signaling. Cold Spring Harb. Perspect. Biol. 2010, 2, a002279–a002279. [Google Scholar] [CrossRef]
- Stolarczyk, E.; Vong, C.T.; Perucha, E.; Jackson, I.; Cawthorne, M.A.; Wargent, E.T.; Powell, N.; Canavan, J.B.; Lord, G.; Howard, J.K. Improved Insulin Sensitivity despite Increased Visceral Adiposity in Mice Deficient for the Immune Cell Transcription Factor T-bet. Cell Metab. 2013, 17, 520–533. [Google Scholar] [CrossRef]
- Varga, T.; Czimmerer, Z.; Nagy, L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim. et Biophys. Acta (BBA) - Mol. Basis Dis. 2011, 1812, 1007–1022. [Google Scholar] [CrossRef]
- Wagner, N.; Wagner, K.-D. The Role of PPARs in Disease. Cells 2020, 9, 2367. [Google Scholar] [CrossRef] [PubMed]
- Christofides, A.; Konstantinidou, E.; Jani, C.; Boussiotis, V.A. The role of peroxisome proliferator-activated receptors (PPAR) in immune responses. Metabolism 2020, 114. [Google Scholar] [CrossRef] [PubMed]
- Ricote, M.; Glass, C.K. PPARs and molecular mechanisms of transrepression. Biochim. et Biophys. Acta (BBA) - Mol. Cell Biol. Lipids 2007, 1771, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Le Menn, G.; Neels, J.G. Regulation of Immune Cell Function by PPARs and the Connection with Metabolic and Neurodegenerative Diseases. Int. J. Mol. Sci. 2018, 19, 1575. [Google Scholar] [CrossRef] [PubMed]
- Maculewicz, E.; Mastalerz, A.; Maciejewska-Skrendo, A.; Cięszczyk, P.; Cywińska, A.; Borecka, A.; Garbacz, A.; Szarska, E.; Dziuda. ; Lorenz, K.; et al. Association between peroxisome proliferator-activated receptor-alpha, -delta and -gamma gene (PPARA, PPARD, PPARG) polymorphisms and overweight parameters in physically active men. Biol. Sport 2021, 38, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Barroso, I.; Gurnell, M.; Crowley, V.E.F.; Agostini, M.; Schwabe, J.W.; Soos, M.A.; Maslen, G.L.; Williams, T.D.M.; Lewis, H.; Schafer, A.J.; et al. Dominant negative mutations in human PPARγ associated with severe insulin resistance, diabetes mellitus and hypertension. Nature 1999, 402, 880–883. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.A.; Rego, D.; Moshkova, M.; Kebir, H.; Chruscinski, A.; Nguyen, H.; Akkermann, R.; Stanczyk, F.Z.; Prat, A.; Steinman, L.; et al. Peroxisome proliferator-activated receptor (PPAR)α and -γ regulate IFNγ and IL-17A production by human T cells in a sex-specific way. Proc. Natl. Acad. Sci. 2012, 109, 9505–9510. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-M.; Bothwell, A.L.M. The nuclear receptor PPARs as important regulators of T-cell functions and autoimmune diseases. Mol. Cells 2012, 33, 217–222. [Google Scholar] [CrossRef]
- Klotz, L.; Burgdorf, S.; Dani, I.; Saijo, K.; Flossdorf, J.; Hucke, S.; Alferink, J.; Novak, N.; Beyer, M.; Mayer, G.; et al. The nuclear receptor PPARγ selectively inhibits Th17 differentiation in a T cell–intrinsic fashion and suppresses CNS autoimmunity. J. Exp. Med. 2009, 206, 2079–2089. [Google Scholar] [CrossRef]
- Angela, M.; Endo, Y.; Asou, H.K.; Yamamoto, T.; Tumes, D.J.; Tokuyama, H.; Yokote, K.; Nakayama, T. Fatty acid metabolic reprogramming via mTOR-mediated inductions of PPARγ directs early activation of T cells. Nat. Commun. 2016, 7, 13683. [Google Scholar] [CrossRef]
- Huang, X.; Ren, L.; Ye, P.; Cheng, C.; Wu, J.; Wang, S.; Sun, Y.; Liu, Z.; Xie, A.; Xia, J. Peroxisome Proliferator-Activated Receptor γ Deficiency in T Cells Accelerates Chronic Rejection by Influencing the Differentiation of CD4+ T Cells and Alternatively Activated Macrophages. PLOS ONE 2014, 9, e112953. [Google Scholar] [CrossRef] [PubMed]
- Cipolletta, D.; Feuerer, M.; Li, A.; Kamei, N.; Lee, J.; Shoelson, S.E.; Benoist, C.; Mathis, D. PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature 2012, 486, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Feuerer, M.; Herrero, L.; Cipolletta, D.; Naaz, A.; Wong, J.; Nayer, A.; Lee, J.; Goldfine, A.B.; Benoist, C.; Shoelson, S.; et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 2009, 15, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Cipolletta, D.; Cohen, P.; Spiegelman, B.M.; Benoist, C.; Mathis, D. Appearance and disappearance of the mRNA signature characteristic of T reg cells in visceral adipose tissue: Age, diet, and PPARγ effects. Proc. Natl. Acad. Sci. 2014, 112, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Michalek, R.D.; Gerriets, V.A.; Jacobs, S.R.; Macintyre, A.N.; MacIver, N.J.; Mason, E.F.; Sullivan, S.A.; Nichols, A.G.; Rathmell, J.C. Cutting Edge: Distinct Glycolytic and Lipid Oxidative Metabolic Programs Are Essential for Effector and Regulatory CD4+ T Cell Subsets. J. Immunol. 2011, 186, 3299–3303. [Google Scholar] [CrossRef] [PubMed]
- Peraldi, P.; Xu, M.; Spiegelman, B.M. Thiazolidinediones block tumor necrosis factor-α-induced inhibition of insulin signaling. J. Clin. Investig. 1997, 100, 1863–1869. [Google Scholar] [CrossRef] [PubMed]
- Leonardini, A.; Laviola, L.; Perrini, S.; Natalicchio, A.; Giorgino, F. Cross-Talk between PPAR�and Insulin Signaling and Modulation of Insulin Sensitivity. PPAR Res. 2009, 2009, 1–12. [Google Scholar] [CrossRef]
- Frkic, R.L.; Richter, K.; Bruning, J.B. The therapeutic potential of inhibiting PPARγ phosphorylation to treat type 2 diabetes. J. Biol. Chem. 2021, 297, 101030. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Luo, S.; Zhan, Y.; Lu, Q. The roles of PPARγ and its agonists in autoimmune diseases: A comprehensive review. J. Autoimmun. 2020, 113, 102510–102510. [Google Scholar] [CrossRef]
- Corrales, P.; Vidal-Puig, A.; Medina-Gómez, G. PPARs and Metabolic Disorders Associated with Challenged Adipose Tissue Plasticity. Int. J. Mol. Sci. 2018, 19, 2124. [Google Scholar] [CrossRef]
- Pour, N.J.A.; Zabihi-Mahmoudabadi, H.; Ebrahimi, R.; Yekaninejad, M.S.; Hashemnia, S.M.R.; Meshkani, R.; Emamgholipour, S. Principal component analysis of adipose tissue gene expression of lipogenic and adipogenic factors in obesity. BMC Endocr. Disord. 2023, 23, 1–12. [Google Scholar] [CrossRef]
- Haluzík, M. PPAR-alpha and insulin sensitivity. Physiol. Res. 2006, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Jokelainen, J.; Auvinen, J.; Puukka, K.; Keinänen-Kiukaanniemi, S.; Järvelin, M.-R.; Kettunen, J.; Mäkinen, V.-P.; Ala-Korpela, M. Insulin resistance and systemic metabolic changes in oral glucose tolerance test in 5340 individuals: an interventional study. BMC Med. 2019, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539-53. [CrossRef]
- Groop, L.; Orho-Melander, M. The dysmetabolic syndrome. J. Intern. Med. 2001, 250, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Jaenisch, R.; Bird, A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat. Genet. 2003, 33, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, A.; Giovenzana, A.; Insalaco, V.; Phillips, B.E.; Pietropaolo, M.; Giannoukakis, N. Autoimmune Inflammation and Insulin Resistance: Hallmarks So Far and Yet So Close to Explain Diabetes Endotypes. Curr. Diabetes Rep. 2021, 21, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Szukiewicz, D. Epigenetic regulation and T-cell responses in endometriosis – something other than autoimmunity. Front. Immunol. 2022, 13. [Google Scholar] [CrossRef]
- Kohil, A.; Al-Asmakh, M.; Al-Shafai, M.; Terranegra, A. The Interplay Between Diet and the Epigenome in the Pathogenesis of Type-1 Diabetes. Front. Nutr. 2021, 7. [Google Scholar] [CrossRef]
- Mahmoud, A.M. An Overview of Epigenetics in Obesity: The Role of Lifestyle and Therapeutic Interventions. Int. J. Mol. Sci. 2022, 23, 1341. [Google Scholar] [CrossRef]
- Biobaku, F.; Ghanim, H.; Monte, S.V.; A Caruana, J.; Dandona, P. Bariatric Surgery: Remission of Inflammation, Cardiometabolic Benefits, and Common Adverse Effects. J. Endocr. Soc. 2020, 4, bvaa049. [Google Scholar] [CrossRef]
- Suárez, R.; Chapela, S.P.; Álvarez-Córdova, L.; Bautista-Valarezo, E.; Sarmiento-Andrade, Y.; Verde, L.; Frias-Toral, E.; Sarno, G. Epigenetics in Obesity and Diabetes Mellitus: New Insights. Nutrients 2023, 15, 811. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Olsen, T.; Vinknes, K.J.; Refsum, H.; Gulseth, H.L.; Birkeland, K.I.; Drevon, C.A. Plasma Sulphur-Containing Amino Acids, Physical Exercise and Insulin Sensitivity in Overweight Dysglycemic and Normal Weight Normoglycemic Men. Nutrients 2018, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, S.K.; Jang, Y.J.; Park, H.S.; Kim, J.-H.; Hong, J.P.; Lee, Y.J.; Heo, Y.-S. Enhanced ANGPTL2 expression in adipose tissues and its association with insulin resistance in obese women. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
The formation of mature insulin by post-translational modifications of tertiary structure of the insulin precursors: preproinsulin and proinsulin.Proteolysis of preproinsulin (110 amino acid chain) with removal of the signal peptide produces proinsulin. Proinsulin has 86 amino acids containing six cysteine residues, and the folded protein contains three intramolecular disulfide bridges between A and B chains. C-peptide is the part of proinsulin which is cleaved prior to co-secretion with insulin (51 amino acids) from pancreatic beta cells. The biologically active insulin molecule therefore contains less than half of the original translation product.
Figure 1.
The formation of mature insulin by post-translational modifications of tertiary structure of the insulin precursors: preproinsulin and proinsulin.Proteolysis of preproinsulin (110 amino acid chain) with removal of the signal peptide produces proinsulin. Proinsulin has 86 amino acids containing six cysteine residues, and the folded protein contains three intramolecular disulfide bridges between A and B chains. C-peptide is the part of proinsulin which is cleaved prior to co-secretion with insulin (51 amino acids) from pancreatic beta cells. The biologically active insulin molecule therefore contains less than half of the original translation product.
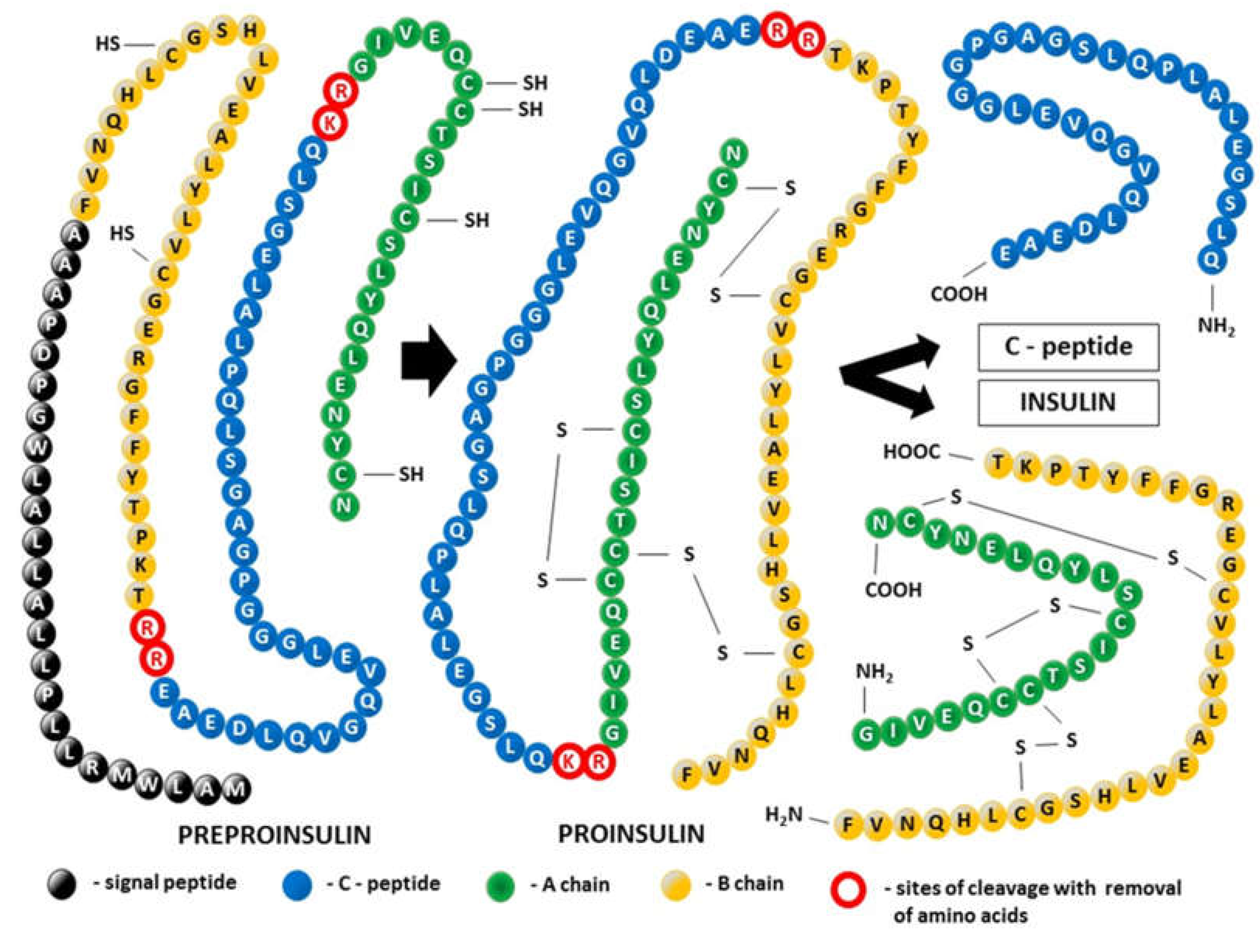
Figure 2.
Modular structure of the insulin receptor (INSR).The INSR is a preformed, covalently linked tetramer with two extracellular α subunits and two cell membrane-penetrating, tyrosine kinase-containing β subunits. The transmembrane localization of the receptor corresponds to the presence of the domains: the ectodomain (ECD), the transmembrane (TMD) and the tyrosine kinase (TKD) domains. Binding of insulin or IGF-1 to the dimeric ECD triggers auto-phosphorylation of the TKD and subsequent activation of downstream signaling molecules. On the left half of INSR, spans of recognized protein modules whose boundaries typically correspond to the boundaries of the 22 exon-encoded sequences detected within the α and β subunits (not shown in this figure). N – NH2-terminus of the polypeptide chain; L1, L2 – two large, leucine-rich repeat domains; CR – cysteine-rich region (domain); FnIII-1, FnIII-2a, FnIII-2b, FnIII-3 – fibronectin type III domains; ID-α, ID-β – α or β subunit insert domain in FnIII-2 (the splitting into FnIII2a and FnIII2b), respectively; α-CT – C-terminus of the α-subunit; JM – juxtamembrane domain; TK – tyrosine kinase domain; C – C-terminal tail. The two α-subunits are linked by a disulfide bond between the two Cys 524 in the FnIII-1. One to three of the triplet Cys at 682, 683 and 685 in the ID-α within the FnIII-2 are also involved in α-α disulfide bridges. There is a single disulfide bridge between α and β subunits between Cys 647 in the ID-α and Cys 872 of the FnIII-3 (nomenclature of the B isoform) [56]. * The alternative splicing of exon 11 encodes a 12-amino-acid sequence at the C-terminus of the α-subunit of the INSR gene during transcription, resulting in the formation of the isoforms INSR-A and INSR-B.
Figure 2.
Modular structure of the insulin receptor (INSR).The INSR is a preformed, covalently linked tetramer with two extracellular α subunits and two cell membrane-penetrating, tyrosine kinase-containing β subunits. The transmembrane localization of the receptor corresponds to the presence of the domains: the ectodomain (ECD), the transmembrane (TMD) and the tyrosine kinase (TKD) domains. Binding of insulin or IGF-1 to the dimeric ECD triggers auto-phosphorylation of the TKD and subsequent activation of downstream signaling molecules. On the left half of INSR, spans of recognized protein modules whose boundaries typically correspond to the boundaries of the 22 exon-encoded sequences detected within the α and β subunits (not shown in this figure). N – NH2-terminus of the polypeptide chain; L1, L2 – two large, leucine-rich repeat domains; CR – cysteine-rich region (domain); FnIII-1, FnIII-2a, FnIII-2b, FnIII-3 – fibronectin type III domains; ID-α, ID-β – α or β subunit insert domain in FnIII-2 (the splitting into FnIII2a and FnIII2b), respectively; α-CT – C-terminus of the α-subunit; JM – juxtamembrane domain; TK – tyrosine kinase domain; C – C-terminal tail. The two α-subunits are linked by a disulfide bond between the two Cys 524 in the FnIII-1. One to three of the triplet Cys at 682, 683 and 685 in the ID-α within the FnIII-2 are also involved in α-α disulfide bridges. There is a single disulfide bridge between α and β subunits between Cys 647 in the ID-α and Cys 872 of the FnIII-3 (nomenclature of the B isoform) [56]. * The alternative splicing of exon 11 encodes a 12-amino-acid sequence at the C-terminus of the α-subunit of the INSR gene during transcription, resulting in the formation of the isoforms INSR-A and INSR-B.
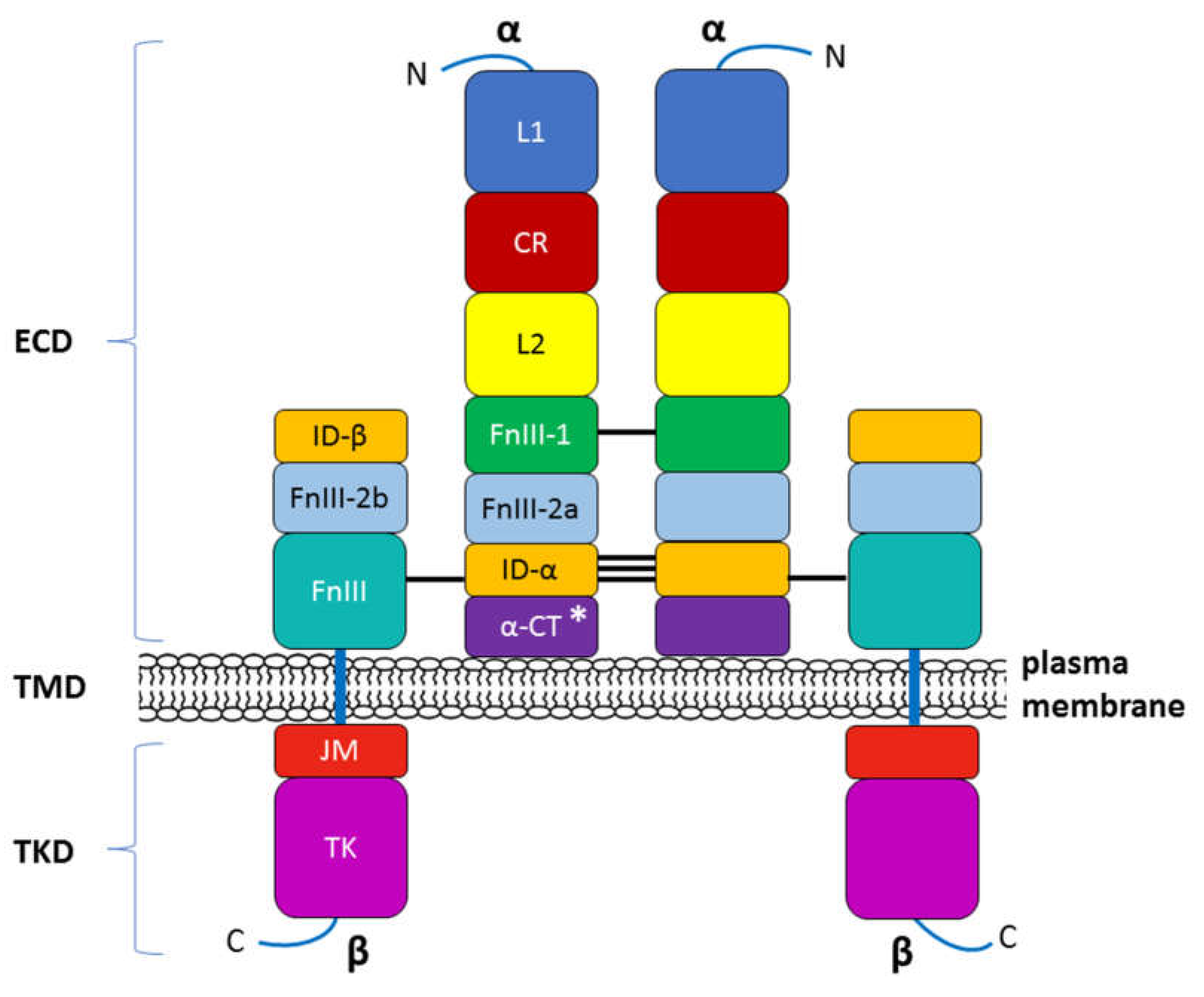
Figure 3.
The canonical pathway of the insulin receptor (INSR) signaling.Binding of insulin (INS) or IGF-1 to INSR or insulin-like growth factor receptor 1 (IGFR1) triggers increased tyrosine kinase activity that initiates a cascade of phosphorylation (marked with a yellow circle containing letter P) events that leads to the activation of enzymes that control many aspects of metabolism and growth. Following phosphorylation of adaptor proteins known as insulin receptor substrates (IRSs), the canonical pathway is dichotomized into the two major signaling cascades that correspond to the phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) and mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) pathways (marked in green and black, respectively). The PI3K/AKT/mTOR pathway is linked exclusively through IRSs and is responsible for most of insulin’s metabolic effects at the cellular level. MAPK/ERK pathway derives from both IRSs and Src homologous and collagen (SHC) protein and, in addition to the functions common with the PI3K/AKT/mTOR pathway, also regulates gene transcription, cell growth and proliferation [56]. The diagram has been simplified for clarity. The critical nodes of both pathways (IRSs, ERK1/2, PI3K, AKT, mTORC1) are highlighted with a yellowish-orange border. The main metabolic (anabolic) effects of INSR signaling are listed in red rounded rectangles. Crosstalk from tumor necrosis factor alpha (TNF-α)/TNF-α receptor 1 (TNFR1) signaling via c-Jun N-terminal kinases (JNKs) and inhibitor of κB (IκB) kinases (IKKs) is indicated (marked in blue), because proinflammatory cytokines (e.g., TNF-α) may produce systemic insulin resistance by phosphorylation of IRS1. The remaining abbreviations: 4EBPI – 4E-binding protein 1, an eukaryotic translation initiation factor; AKT – protein kinase B; DOK4, DOK5 – docking protein 5 and 6, also known as IRS-5 and IRS-6, respectively; ERK1/2 – extracellular signal-regulated kinases; FOXO1 – forkhead homolog in rhabdomyosarcoma 1; GDP, GTP – guanosine diphosphate, guanosine triphosphate; Grb2 – growth factor receptor-bound protein 2; GSK3 – glycogen synthase kinase-3; MEK1/2 – mitogen activated protein kinase (isoforms 1 and 2); mTORC1 – mechanistic (previously referred to as mammalian) target of rapamycin complex 1; PDK1 – phosphoinositide-dependent protein kinase 1; PIP2, PIP3 – phosphatidylinositol 4,5-bisphosphate, phosphatidylinositol 3,4,5-trisphosphate; PRAS40 – proline-rich Akt substrate 40 kDa; Raf-1 – (Rapidly accelerated fibrosarcoma) proto-oncogene serine/threonine-specific protein kinase; Ras – (Rat sarcoma virus)GTPase; S6K – protein S6 kinase; SOS – (Son of sevenless), refers to a set of genes encoding guanine nucleotide exchange factors (GEFs); SREBP1c – sterol regulatory element-binding protein 1C; TBC1D1, TBC1D4 – RabGTPase-activating proteins (RabGAPs); TSC1-TSC2 – tuberous sclerosis complex consisting of the TSC1 (also known as hamartin) and TSC2 (also known as tuberin) proteins.
Figure 3.
The canonical pathway of the insulin receptor (INSR) signaling.Binding of insulin (INS) or IGF-1 to INSR or insulin-like growth factor receptor 1 (IGFR1) triggers increased tyrosine kinase activity that initiates a cascade of phosphorylation (marked with a yellow circle containing letter P) events that leads to the activation of enzymes that control many aspects of metabolism and growth. Following phosphorylation of adaptor proteins known as insulin receptor substrates (IRSs), the canonical pathway is dichotomized into the two major signaling cascades that correspond to the phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) and mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) pathways (marked in green and black, respectively). The PI3K/AKT/mTOR pathway is linked exclusively through IRSs and is responsible for most of insulin’s metabolic effects at the cellular level. MAPK/ERK pathway derives from both IRSs and Src homologous and collagen (SHC) protein and, in addition to the functions common with the PI3K/AKT/mTOR pathway, also regulates gene transcription, cell growth and proliferation [56]. The diagram has been simplified for clarity. The critical nodes of both pathways (IRSs, ERK1/2, PI3K, AKT, mTORC1) are highlighted with a yellowish-orange border. The main metabolic (anabolic) effects of INSR signaling are listed in red rounded rectangles. Crosstalk from tumor necrosis factor alpha (TNF-α)/TNF-α receptor 1 (TNFR1) signaling via c-Jun N-terminal kinases (JNKs) and inhibitor of κB (IκB) kinases (IKKs) is indicated (marked in blue), because proinflammatory cytokines (e.g., TNF-α) may produce systemic insulin resistance by phosphorylation of IRS1. The remaining abbreviations: 4EBPI – 4E-binding protein 1, an eukaryotic translation initiation factor; AKT – protein kinase B; DOK4, DOK5 – docking protein 5 and 6, also known as IRS-5 and IRS-6, respectively; ERK1/2 – extracellular signal-regulated kinases; FOXO1 – forkhead homolog in rhabdomyosarcoma 1; GDP, GTP – guanosine diphosphate, guanosine triphosphate; Grb2 – growth factor receptor-bound protein 2; GSK3 – glycogen synthase kinase-3; MEK1/2 – mitogen activated protein kinase (isoforms 1 and 2); mTORC1 – mechanistic (previously referred to as mammalian) target of rapamycin complex 1; PDK1 – phosphoinositide-dependent protein kinase 1; PIP2, PIP3 – phosphatidylinositol 4,5-bisphosphate, phosphatidylinositol 3,4,5-trisphosphate; PRAS40 – proline-rich Akt substrate 40 kDa; Raf-1 – (Rapidly accelerated fibrosarcoma) proto-oncogene serine/threonine-specific protein kinase; Ras – (Rat sarcoma virus)GTPase; S6K – protein S6 kinase; SOS – (Son of sevenless), refers to a set of genes encoding guanine nucleotide exchange factors (GEFs); SREBP1c – sterol regulatory element-binding protein 1C; TBC1D1, TBC1D4 – RabGTPase-activating proteins (RabGAPs); TSC1-TSC2 – tuberous sclerosis complex consisting of the TSC1 (also known as hamartin) and TSC2 (also known as tuberin) proteins.
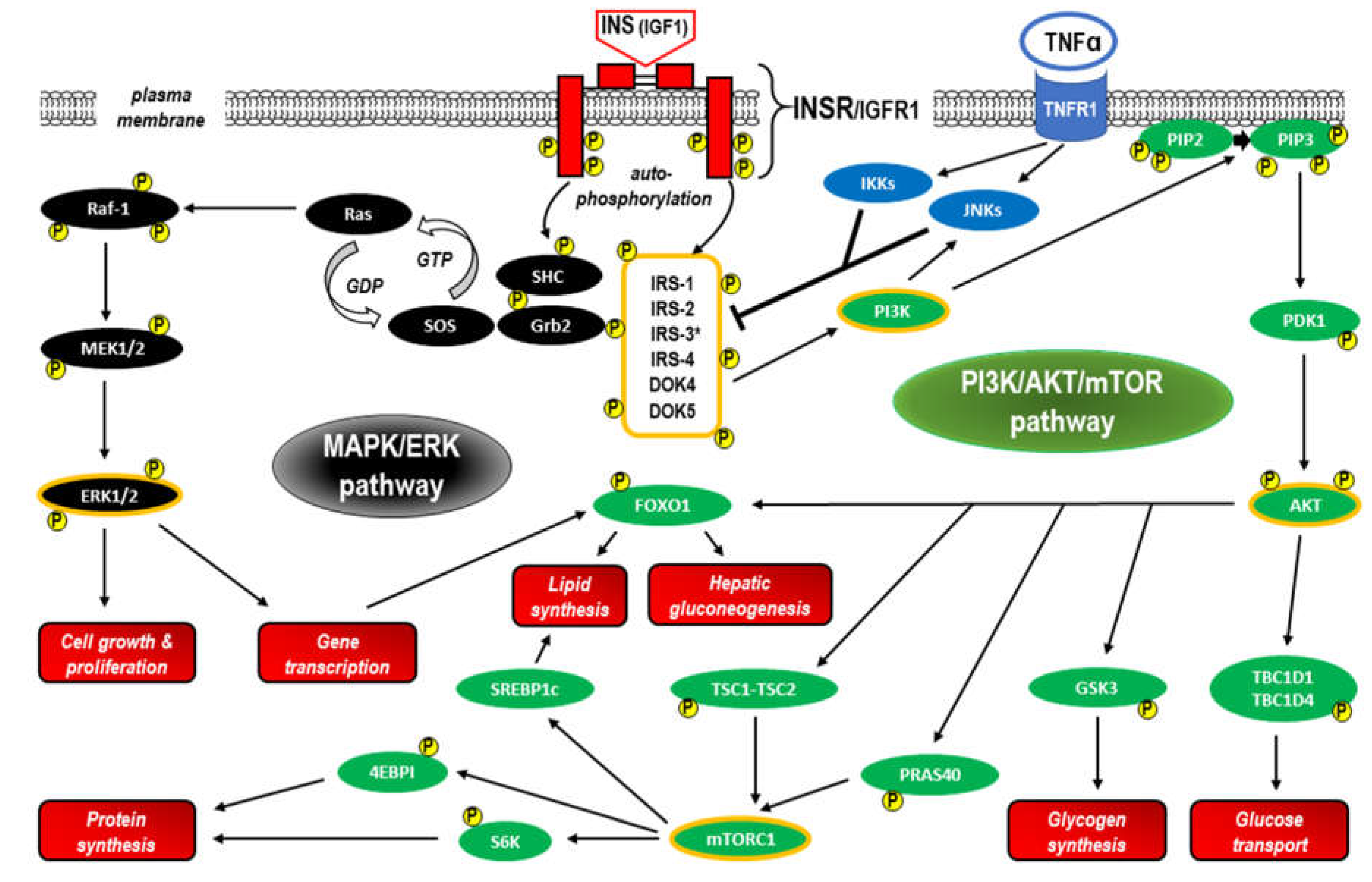
Figure 4.
The main underlying causes/mechanisms of insulin resistance (IR) - (successive boxes going counter-clockwise from the top-left): Antibodies including autoantibodies antibodies can be produced against both insulin (INS) and insulin receptor (INSR). Type B IR syndrome is a rare disorder caused by autoantibodies to the insulin receptor [117,125]; Alternative RNA splicing events including ❶- exon retention, ❷- exon skipping,❸- alternative 5’ donor sites, ❹- alternative 3’ acceptor sites, and ❺- mutually exclusive exons can lead to the splicing mutation that may occur in both introns and exons and disrupt existing splice sites, create new ones, or activate the cryptic ones [126]; Type A IR is caused by a mutation in the INSR gene [127,128]; Defects of INSR structure and function may be caused by the common in type 2 diabetes (T2D) Gly971Arg polymorphism that is responsible for altered tyrosine phosphorylation at a specific site of insulin receptor substrate 1 (IRS-1) [129]; Both alternative RNA splicing and gene polymorphism may significantly affect INSR/IRSs downstream signaling, producing IR-dependent inhibition of MAPK/ERK and PI3K/AKT/mTOR pathways [114,115,121,130]; Proinflammatory environment and hyperglycemia create a vicious cycle of the complex cause-and-effect relationship, causing IR through inhibition of the tyrosine autophosphorylation (pY) within INSR and serine/threonine phosphorylation (pS) of the insulin receptor substrates (IRSs). Reactive oxygen species (ROS) and signaling via membrane-bound receptors for tumor necrosis factor receptor 1 (TNFR1) and the receptors for other proinflammatory cytokines (Recs) play crucial roles [114,115,118,131].
Figure 4.
The main underlying causes/mechanisms of insulin resistance (IR) - (successive boxes going counter-clockwise from the top-left): Antibodies including autoantibodies antibodies can be produced against both insulin (INS) and insulin receptor (INSR). Type B IR syndrome is a rare disorder caused by autoantibodies to the insulin receptor [117,125]; Alternative RNA splicing events including ❶- exon retention, ❷- exon skipping,❸- alternative 5’ donor sites, ❹- alternative 3’ acceptor sites, and ❺- mutually exclusive exons can lead to the splicing mutation that may occur in both introns and exons and disrupt existing splice sites, create new ones, or activate the cryptic ones [126]; Type A IR is caused by a mutation in the INSR gene [127,128]; Defects of INSR structure and function may be caused by the common in type 2 diabetes (T2D) Gly971Arg polymorphism that is responsible for altered tyrosine phosphorylation at a specific site of insulin receptor substrate 1 (IRS-1) [129]; Both alternative RNA splicing and gene polymorphism may significantly affect INSR/IRSs downstream signaling, producing IR-dependent inhibition of MAPK/ERK and PI3K/AKT/mTOR pathways [114,115,121,130]; Proinflammatory environment and hyperglycemia create a vicious cycle of the complex cause-and-effect relationship, causing IR through inhibition of the tyrosine autophosphorylation (pY) within INSR and serine/threonine phosphorylation (pS) of the insulin receptor substrates (IRSs). Reactive oxygen species (ROS) and signaling via membrane-bound receptors for tumor necrosis factor receptor 1 (TNFR1) and the receptors for other proinflammatory cytokines (Recs) play crucial roles [114,115,118,131].
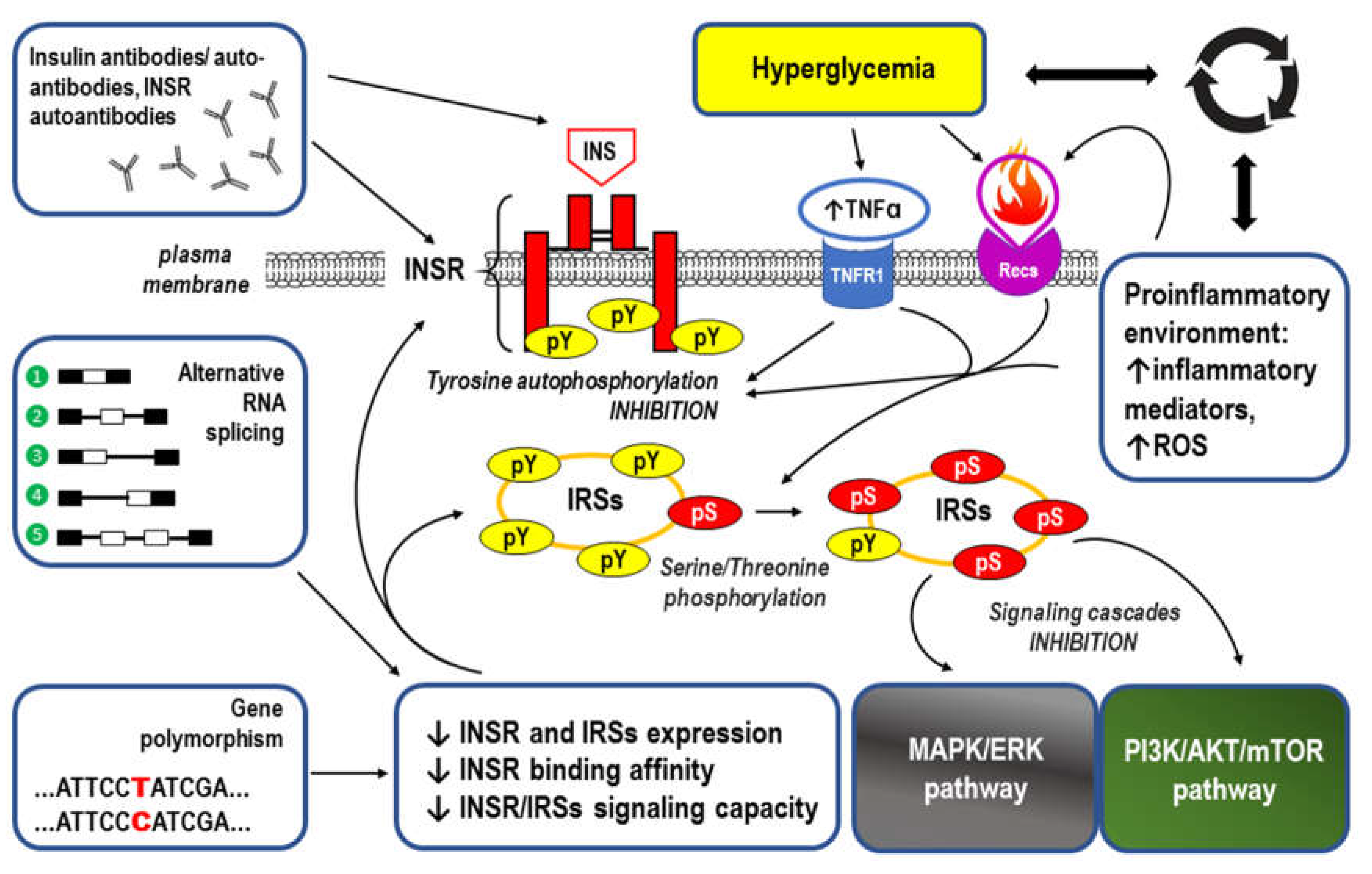
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated




