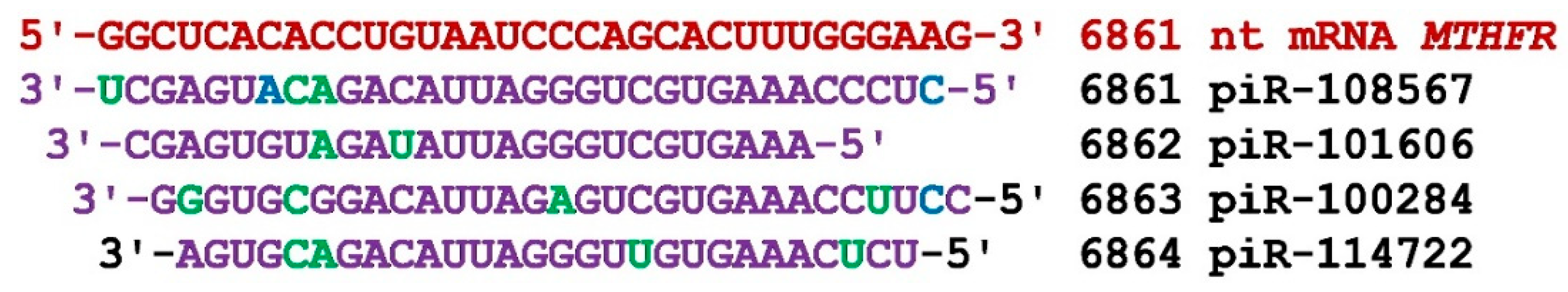1. Introduction
After the sequencing of the human genome, methods of molecular studies of the regulation of gene expression and the human genome began to develop. The piRNAs (Piwi-interacting RNAs) were identified more than twenty years ago [1-2]. In addition to these small RNAs, siRNAs (small interfering RNAs) capable of regulating gene expression were created [3, 4]. For many years, due to a number of misconceptions, miRNAs, and piRNAs have not yet been widely used for practical purposes [5-9]. However, in vivo and in vitro experiments have successfully used siRNAs against undruggable targets for treating cancer and other diseases [10-12]. Therefore, candidate genes responsible for the development of many diseases have been identified [13-17]. This approach allows us to specify disease causes and develop diagnosis and therapies more effectively. The identification of candidate genes further requires the identification of factors regulating their expression. It has been shown that piRNAs found in human and animal cells are associated with gene expression, but it has not been established how these molecules can modify this process [18-20]. In recent years, interest in the study of piRNAs has grown considerably, and results have been obtained demonstrating the essential biological role of these molecules [21-23]. Several publications have established piRNAs' role in carcinogenesis [24-26]. An important fact is a possibility of preserving and transporting small RNA in the human body as part of exosomes and nanosized vesicles through the bloodstream [27-30]. After a database of piRNAs was created [
31], it became possible to study the direct interaction of piRNAs with genes. The more than eight million piRNAs molecules available in the database are difficult and expensive to study in wet experiments, so it is necessary to use bioinformatic approaches to identify the biological function of piRNAs more efficiently. These studies have shown the promise of this approach in determining the properties of piRNAs and their biological role. It was found that piRNAs can interact with mRNA and these interactions can be evaluated by quantitative characteristics [21-23]. This work aimed to identify piRNAs that can interact with candidate esophageal squamous cell carcinoma (ESCC) genes and how this information could be used to diagnose the disease.
2. Materials and Methods
The nucleotide sequence of 66 candidate genes of ESCC (
Table S1) was downloaded from National Center for Biotechnology Information (NCBI) (
https://www.ncbi.nlm.nih.gov, accessed on October 5, 2022). The nucleotide sequences of 480 thousand piRNAs were taken from Wang et al. [
31]. The piRNA binding sites (BSs) in mRNA were predicted using the MirTarget program [
32]. This program predicts the following features of piRNA binding to mRNA: (a) the initiation of piRNA binding to the mRNA from the first nucleotide of the mRNA; (b) the localization of the piRNA BSs in the 5′-untranslated region (5′UTR), coding domain sequence (CDS), and 3′-untranslated region (3′UTR) of the mRNAs; (c) the schemes of nucleotide interactions between piRNAs and mRNA; (d) the free energy of the interaction between piRNAs and the mRNA (ΔG, kJ/mol); and the ratio ΔG/ΔGm (%) is determined for each site. ΔGm equals the free energy of piRNA binding with its fully complementary canonical nucleotide sequence. Only piRNAs whose nucleotides interacted with mRNA using canonical (G-C and A-U) and noncanonical (G-U and A-C) nucleotides with a given ΔG value were selected from the calculated data. The MirTarget program finds hydrogen bonds between piRNAs and mRNA according to the physicochemical characteristics of nucleotide interactions [35-38]. MirTarget differs from other programs in terms of finding piRNA BSs on mRNA in the following: it takes into account the interaction of piRNA with mRNA over the entire piRNA sequence; it considers noncanonical pairs G–U and A–C; and it calculates the free energy of the interaction of the piRNAs with mRNA. Note that the G, A, C, and U nucleotides, which comprise the RNA structure of microorganisms, plants, and animals, interact identically under equal conditions. Therefore, the physicochemical properties of canonical and noncanonical nucleotide pairs given above do not require additional proof of the previously established physicochemical characteristics of their interaction [33-36].
3. Results
In examining the interaction of piRNAs with mRNA genes, criteria were established for the following characteristics. The interaction of piRNAs and mRNA nucleotides must be along the entire length of the piRNAs. The free energy of the interaction must be at least 90% of the maximum ΔG/ΔGm. Splitting piRNAs into three groups based on the value of ΔG and ΔG/ΔGm of the interaction of piRNAs with mRNA will determine whether the values of ΔG and ΔG/ΔGm within the selected limits can reflect the basic properties of the interaction of piRNAs with mRNA. The property of candidate genes to interact with different numbers of piRNAs is acquired by selecting interrelated genes whose expression should be regulated by piRNAs as universal controllers of genome-wide expression. Determine whether piRNAs interact with mRNAs specifically to individual mRNA sites. Clarify the role of clusters of piRNAs BSs as a necessity for coordinated regulation of gene expression with a requirement for compactness of gene regulatory sites. Bioinformatic technologies greatly facilitate the characterization of associations between piRNAs and mRNA interactions.
A study of 480 thousand piRNAs binding to the mRNAs of 66 candidate ESCC genes showed that the mRNAs of only nine genes could interact with piRNAs. The number of interacting piRNAs for each gene differed significantly. To elucidate the interaction of piRNAs with mRNAs, the interacting piRNAs were divided into three groups according to the free energy of interaction: piRNAs interacting with ΔG value of -170 kJ/mol or higher (group 1), with ΔG value of -160 kJ/mol to -169 kJ/mol (group 2), with ΔG value of -150 kJ/mol to -159 kJ/mol (group 3). This approach allowed us to clarify how the interacting groups of piRNAs may vary.
3.1. Characteristics of the interaction of piRNAs with the mRNA of the AURKA gene
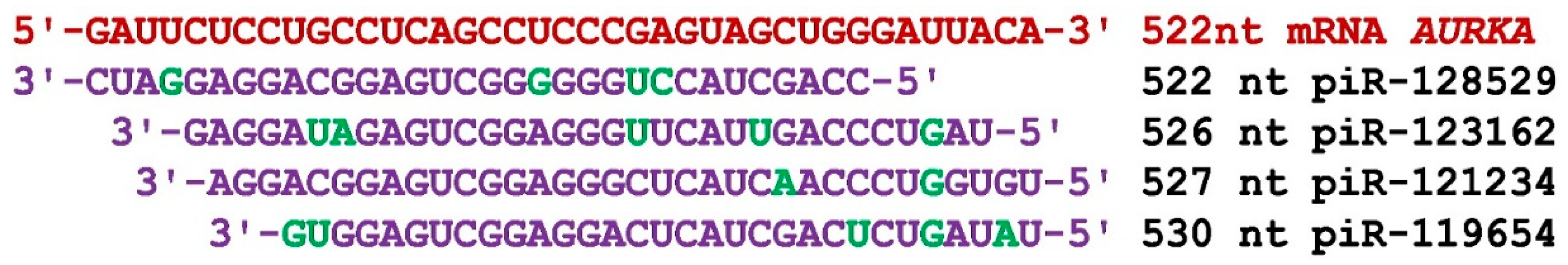
The mRNA of the
AURKA gene consists of 566 nt of 5'UTR, 1212 nt of CDS, and 770 nt of 3'UTR regions. The start of the piRNAs group 1 BSs are localized only in the 5'UTR from 410 nt to 533 nt (
Table S2). The BSs of 43 piRNAs from piR-38326 to piR-479539 were located with overlapping nucleotide sequences, that is, they formed a cluster of BSs and were localized at the end of the 5'UTR. The BSs cluster was 157 nt long and contained BSs piRNAs with lengths of 33 nt and 34 nt. Group 2 consisted of 38 piRNAs whose BSs were localized in the BSs cluster from 414 nt to 534 nt in the length of 153 nt (
Table S2). The piRNAs of group 2 varied in length from 31 nt to 34 nt. In this cluster of BSs located also in the 5'UTR, 38 piRNAs from piR-49581 to piR-363915 were bound. The piRNAs from group 3 were from 27 nt to 33 nt in length. Group 3 consisted of 60 piRNAs whose BSs were localized in a cluster of BSs 410 nt to 536 nt long by 156 nt. Consequently, this cluster of BSs also ended at the 5'UTR before the CDS. The piRNAs from this group were 27 nt to 33 nt long. Group 3 included piRNAs in the range from piR-30739 to piR-480279. The BSs of 141 piRNAs of the three groups were in a cluster 156 nt long and 1.1 nt per piRNA, indicating a high density of BSs for piRNAs.
Figure 1 shows the nucleotide sequences of four piRNAs that interact with a cluster of BSs located in the 5'UTR mRNA of the
AURKA gene with overlapping nucleotide sequences. From these data, we can see how piRNAs will compete for binding in the BS cluster.
3.2. Characteristics of the piRNAs interaction with the mRNA of the BMP7 gene
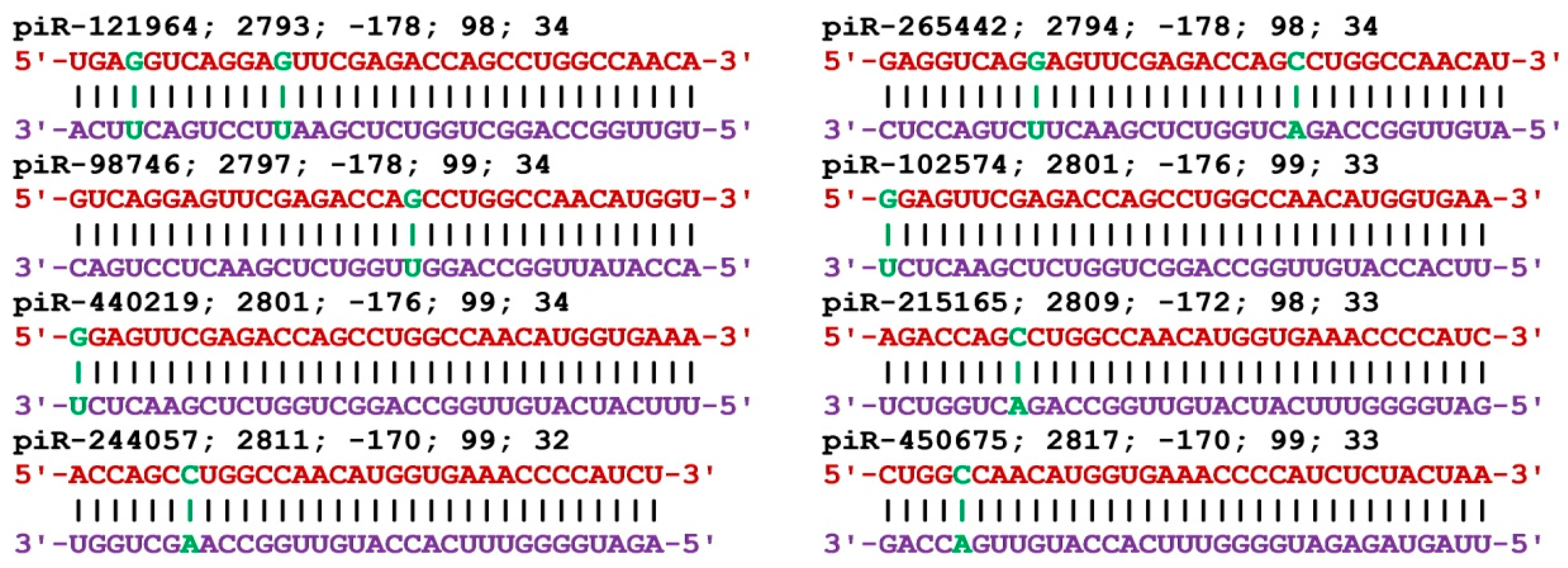
The mRNA of the BMP7 gene includes a 5'UTR of 529 nt, a CDS of 1296 nt, and a 3'UTR of 2207 nt. piRNAs from all three groups bound only in the 3'UTR (
Table S3). In group 1, the BSs of 40 piRNAs started 2756 nt to 2889 nt and the length of the BSs cluster was 166 nt. The length of the binding piRNAs was 33 nt and 34 nt. Forty piRNAs from piR-43404 to piR-440219 were bound in the cluster. Within the large cluster was a 56 nt BSs cluster for eight piRNAs with a ΔG/ΔGm value of 98% to 99% and a free energy value of -170 kJ/mol to -178 kJ/mol (
Figure 2). This corresponds to the formation of predominantly canonical nucleotide pairs except for two noncanonical pairs in the interaction of piR-121964 and piR-265442, and one noncanonical pair each of the remaining piRNAs. Group 2 included 67 piRNAs of length 31 nt to 34 nt that bound in a cluster 2729 nt to 2970 nt of length 273 nt. Group 3 included 111 piRNAs 28 nt - 34 nt long and the beginning of the BSs were located from 2733 nt to 2981 nt and the BSs cluster length was 279 nt. The BSs of the piRNAs were unevenly distributed in the clusters, with a total of 1.3 nt per piRNA in the cluster from 2729 nt to 3012 nt for 218 piRNAs of the three groups.
3.3. Characteristics of the piRNAs interaction with the mRNA of the ERCC1 gene
The mRNA of the ERCC1 gene consists of 146 nt of 5'UTR, 822 nt of CDS, and 2343 nt of 3'UTR in length. The start of the BSs of 47 piRNAs of group 1 with ΔG values varying from -170 kJ/mol to -180 kJ/mol were located in the 3'UTR from 2597 nt to 2794 nt in a 228 nt cluster length (
Table S4). The length of the piRNAs varied from 32 nt to 34 nt. Forty-nine piRNAs of group 2 bind to the mRNA. The BSs of these piRNAs are also localized in the 3'UTR from 2592 nt to 2796 nt. The BSs cluster of 49 piRNAs was 235 nt long, accounting for 10% of the 3'UTR length, in this cluster of BSs, piRNAs with numbers from piR-41630 to piR-365910 bind. The beginning of piRNAs BSs in group 3 were 2595 nt to 2785 nt and the length of the cluster of BSs was 221 nt. Consequently, the clusters of BSs of the three groups of piRNAs were close and each of the 158 piRNAs averaged 1.5 nt. The piR-289349, piR-298414, and piR-436360 bound with mRNA of the ERCC1 gene fully complementary, forming canonical nucleotide pairs only, and the ΔG/ΔG value was 100% (
Table S4). An example of a very compact arrangement of piRNAs BSs to the mRNA of the ERCC1 gene is shown in
Figure 3. In the 35 nt cluster, seven piRNAs were bound through predominantly by canonical nucleotide pairs.
3.4. Characteristics of the interaction of piRNAs with the mRNA of the GCOM1 gene
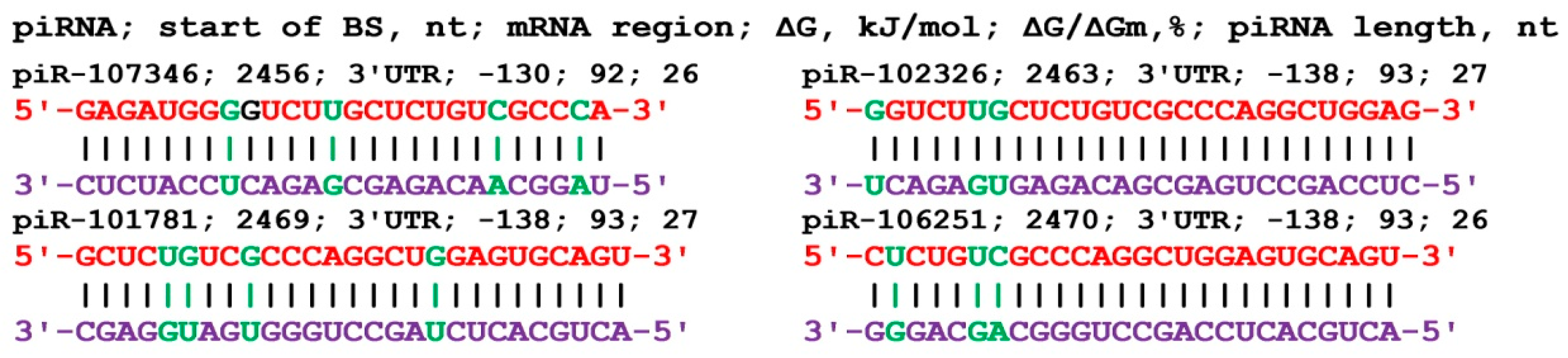
The nucleotide sequence of the GCOM1 gene consists of 131 nt of 5'UTR, 1653 nt of CDS, and 2879 nt of 3'UTR in length. Analysis of the interaction parameters of the 16 piRNAs of group 1 with the mRNA of the GCOM1 gene shows that these piRNAs bind from 2458 nt to 2466 nt in the 3'UTR only (
Table S5). The beginning of the BSs cluster of these piRNAs with a length of 33-34 nt begins at 2458 nt and ends at position 2499 nt, i.e., its length is 41 nt. This cluster of BSs binds piRNAs with numbers from piR-56610 to piR-465348. All of the starts of the BSs of the 20 piRNAs of group 2 are localized by mRNA in the site from 2453 nt to 2486 nt in the 3'UTR with a BSs cluster length of 66 nt. In this cluster of BSs, piRNAs with numbers from piR-34813 to piR-458324 bind. The length of the interacting piRNAs varied from 30 to 34 nt. The 14 piRNAs of group 3 bound from 2456 nt to 2488 nt in a cluster of 61 nt length. In this cluster of BSs piRNAs bind with numbers ranging from piR-117518 to piR-468536. On average, 1.3 nt of the 50 piRNAs interact with the mRNA of the GCOM1 gene in the 66 nt cluster. piRNAs less than 30 nt in length can also interact with the GCOM1 gene (
Figure 4). The ΔG/ΔGm value was above 92%, indicating the preferential formation of canonical nucleotide pairs.
3.5. Characteristics of the interaction of piRNAs with the mRNA of the MTHFR gene
The mRNA of the MTHFR gene has a long 3'UTR of 4951 nt, unlike the mRNA of the other candidate ESCC genes studied. The 5'UTR and CDS consist of 229 nt and 1971 nt, respectively. Group 1 piRNAs bound to the 3'UTR in two clusters of BSs (
Table S6). The first cluster of BSs contained BSs from 6182 nt to 6385 nt, and the second from 6849 nt to 7051nt. The distance between the second cluster's beginning and the first cluster's end was 464 nt. Accordingly, 16 and 29 piRNAs were bound in these clusters of BSs. The length of both clusters was 235 nt. The first cluster bound piRNAs from piR-47672 to piR-456736 and the second cluster from piR-36604 to piR-475473. Group 2 piRNAs are also bound in two clusters of BSs: the beginning BSs of the first cluster were from 6190 nt to 6383 nt and the second cluster was from 6849 nt to 7062 nt. The interval between the end and beginning of the clusters was 466 nt. In the first and second clusters, 12 and 53 piRNAs were bound, respectively. Group 3 piRNAs are also bound in two clusters of BSs (
Table S6). The first cluster of BSs began at 6177 nt and ended with piRNAs binding at 6388 nt position. It bound 25 piRNAs and the second cluster bound 78 piRNAs. The second cluster started at 6848 nt and the last piRNAs were bound at position 7056 nt. As a result, 53 piRNAs of the three groups were bound in the first cluster and 160 piRNAs were bound in the second cluster. The total length of the first cluster for the three groups of piRNAs was 240 nt and the second cluster 246 nt. As a result, the first cluster had 4.5 nt per piRNAs and the second cluster had 1.5 nt. Both clusters of BSs are localized at the end of the 3'UTR mRNA of the MTHFR gene.
Figure 5 shows one of several clusters of BSs formed from the BSs of four competing piRNAs located one to three nucleotides apart.
3.6. Characteristics of the interaction of piRNAs with the mRNA of the SASH1 gene
The mRNA of the SASH1 gene in the 5'UTR, CDS, and 3'UTR contained 475 nt, 3744 nt, and 3491 nt, respectively. The 63 piRNAs of group 1 had the beginnings of BSs only in the 3'UTR from 5490 nt to 5741 nt (
Table S7). The piRNA interval from piR-26543 to piR-459543 indicates the participation of piRNAs from the entire studied range of 480 thousand piRNAs. The 61 piRNAs in group 2 had the beginnings of BSs in the 3'UTR from 5507 nt to 5747 nt. The piRNAs from piR-40007 to piR-355919 were associated in this cluster. The piR-50295 bound to the mRNA of the SASH1 gene is fully complementary with a ΔG/ΔG value of 100%. The 73 piRNAs of group 3 had the beginnings of BS in the 3'UTR from 5491 nt to 5741 nt. In this cluster, piRNAs from piR-21000 to piR-466023 were bound. The length of the cluster common to the three groups of piRNAs from 5490 nt to 5777 nt was 287 nt in which 197 piRNAs were bound, hence 1.5 nt per piRNAs.
Figure 6 shows diagrams of the interaction of several piRNAs with the mRNA of the SASH1 gene, indicating their binding using canonical and noncanonical nucleotide pairs.
3.7. Characteristics of the interaction of piRNAs with the mRNA of the SIX4 gene
The mRNAs of the SIX4 gene in 5'UTR, CDS, and 3'UTR contain 60 nt, 2346 nt, and 3870 nt, respectively. There are 86 piRNAs in group 1 whose beginning of the BSs are located only in the 3'UTR from 4026 nt to 4266 nt (
Table S8). In this interval, piRNAs from piR-38669 to piR-479539 bind to mRNAs. The 83 piRNAs of group 2 bound from 4021 nt to 4259 nt. The corresponding piRNAs were between piR-40921 and piR-414045. There are 105 piRNAs in group 3 with the beginning of their BSs also located in the 3'UTR from 4013 nt to 4269 nt. The length of this BSs cluster was 287 nt. The 105 piRNAs included from piR-20572 to piR-480249. The number of three groups of piRNAs was 274, which were bound in a 287 nt cluster with a high density of 1.1 nt per piRNA.
Figure 7 shows the nucleotide sequences of the piRNAs and mRNA of the SIX4 gene in one of the clusters clearly indicating a fully complementary interaction between their nucleotides. These results clearly indicate a high competition of 15 piRNAs for binding in this cluster. If any piRNA is present in concentrations an order of magnitude higher than the others, it will dominate in the suppression of mRNA translation.
3.8. Characteristics of the interaction of piRNAs with the mRNA of the SULT1A1 gene
The nucleotide sequence of the SULT1A1 gene consists of 473 nt 5'UTR, 888 nt CDS, and 231 nt 3'UTR in length. Analysis of the interaction of the eight piRNAs from group 1 with the mRNA of the SULT1A1 gene shows that these piRNAs begins binding from 1483 nt to 1510 nt in the 3'UTR alone (
Table S9). In this cluster of BSs, piRNAs with numbers ranging from piR-10749 to piR-475473 bind. All of the starts of the ten piRNAs BSs of group 2 are localized by mRNA in a site starting from 1482 nt to 1521 nt in the 3'UTR with a BSs cluster length 72 nt. In this cluster of BSs, piRNAs with numbers from piR-200742 to piR-472871 bind. The 11 piRNAs of group 3 were bound from 1489 nt to 1517 nt in a cluster of 69 nt in length. In this cluster of BSs, piRNAs with numbers ranging from piR-104710 to piR-447258 bind. On average, one of the 29 piRNAs interacting with the mRNA of the SULT1A1 gene in the 71 nt cluster is 2.4 nt. The BSs cluster of all piRNAs was located at the end of the 3'UTR. The small number of piRNAs that bind to the mRNA of the SULT1A1 gene is probably due to the short length of the 3'UTR. The low density of piRNAs BSs in the mRNA of the gene revealed only one cluster for the binding of three piRNAs, with a significant shift in the beginning of the BSs (
Figure 8).
3.9. Characteristics of the interaction of piRNAs with the mRNA of the TP53 gene
The mRNAs of the TP53 gene mRNAs in the 5'UTR, CDS, and 3'UTR contain 197 nt, 1041 nt, and 1409 nt, respectively. In group 1 there are 19 piRNAs whose beginning BSs are located only in the 3'UTR from 2293 nt to 2512 nt (
Table S10). In this interval, mRNAs bind piRNAs from piR-44059 to piR-455123. The 30 piRNAs of group 2 bound from 2292 nt to 2516 nt. The corresponding piRNAs were between piR-40367 and piR-291404. Group 3 had 42 piRNAs beginning with their BSs in the 3'UTR from 2290 nt to 2533 nt. The 42 piRNAs included piR-32165 through piR-365438. The number of piRNAs of the three groups was 91, bound in a cluster 274 nt long with a density of 3.0 nt per piRNA. It should be noted that there are portions of the cluster that do not bind piRNAs between 2301 nt and 2382 nt length 81 nt and between 2390 nt and 2456 nt length 66 nt, minus which the cluster is reduced by 147 nt and the working part of the cluster is 127 nt where 1.4 nt are per piRNAs, which is comparable with the results obtained for other candidate genes. Two BSs at 163 nt intervals of ten piRNAs binding at positions 2293 nt and 2456 nt were identified in the mRNA of the TP53 gene. These are piR-101480 and piR-69443 from group 1 piRNAs; piR-106322, piR-53597 and piR-62868 from group 2 piRNAs; piR-32165, piR-37715, piR-52128, piR-57508, piR-32194 from group 3. This is explained by the presence of two sites in the 3'UTR mRNA of the TP53 gene with identical nucleotide sequences binding these piRNAs. The relatively small number of piRNAs interacting with the mRNA of the gene (
Table S10) is reflected in the absence of large clusters of BSs in the mRNA of the TP53 gene. However, the binding of piRNAs to the mRNA is efficient (
Figure 9).
3.10. piRNAs that bind to the mRNA of two or more candidate genes
It is essential to know which piRNAs bind to which genes and how this knowledge can be adequately used to regulate gene expression with piRNAs. We analyzed the interaction of piRNAs in nine candidate ESCC genes. Finding BSs of piRNAs in the mRNAs of alternative candidate genes was considered positive when the ΔG value was -160 kJ/mol or higher and the ΔG/ΔGm value was 90% or higher.
Table S11 shows the search results for piRNAs that bind to the mRNAs of the AURKA, ERCC1, SASH1, and SIX4 genes. Only piR-55670 and piR-93385 are bound to the mRNA of the AURKA and BMP7 genes. Only piR-55670, piR-93385, piR-89432, piR-218175, piR-55670, and piR-93385 bound to mRNA of the AURKA and MTHFR genes. The piR-67883, piR-200086, and piR-306330 bind to mRNAs of the AURKA and TP53 genes. An example of the interaction between piRNAs in AURKA and SIX4 genes is shown in
Figure 10.
The piRNAs interacting with mRNAs of AURKA and SIX4 genes bind in the clusters of BSs having similar nucleotide sequences in the mRNA of both genes. In the mRNA of the AURKA gene piRNAs bind in 5'UTR from 523 nt to 534 nt, and in the mRNA of the SIX4 gene piRNAs bind in 3'UTR from 4115 nt to 4126 nt with the same difference of BS beginning is about 11 nt. The value of the free binding energy of each piRNA in both mRNAs was close and high. The results demonstrate that one of the functions of the 5'UTR and 3'UTR is the binding of piRNAs regulating mRNA translation.
As a result, the mRNAs of all candidate genes binding piRNAs had BSs in two or more mRNAs. These data indicate the interconnection of candidate genes through the interaction of piRNAs, which should be considered both in developing diagnostic methods for ESCC and in creating therapeutic drugs using piRNAs. For using piRNAs as markers of ESCC, it is logical to use the genes that are targeted with the highest free energy of interaction between their mRNA and piRNAs, as well as the genes whose mRNAs bind mainly due to canonical nucleotide pairs.
Figure 11 shows the interaction schemes of such associations of piRNAs and mRNA candidate genes. The piR-289349, piR-298414, and piR-436360 can completely block protein synthesis at a concentration equal to or greater than that of the mRNA of ERCC1. Similarly, piR-50295 can completely inhibit the translation of the mRNA of the SASH1 gene.
4. Discussion
The free energy value of the interaction of piRNAs with mRNA determines the probability of its interaction with mRNA. Depending on the free energy of interaction of piRNAs with the mRNA of a gene, groups of piRNAs differ little in the BS interval and their position in the mRNA region, which is due to the selection during the evolution of the site for binding different in length and nucleotide composition of piRNAs. Therefore, changing piRNAs' length and nucleotide composition leads to the selection of optimal piRNAs for the desired effect in regulating the expression of one or more genes. The piRNAs that we identified regulating two or more candidate genes form, respectively, both single bonds in regulating the expression of these genes and associations of several genes regulated by a group of piRNAs. When studying the interaction of piRNAs with mRNA genes, criteria were established for the following characteristics. 1. The interaction of piRNAs and mRNA nucleotides occurs along the entire length of piRNAs. 2. The free interaction energy (ΔG) must be at least 90% of the maximum ΔGm value. 3. The division of piRNAs into three groups according to the value of ΔG and ΔG/ΔGm of the interaction of piRNAs with mRNA allowed us to determine that these characteristics do not fundamentally change the basic properties of the interaction of piRNAs with mRNA within the chosen limits.
The above results indicate non-random binding of piRNAs in a narrow interval of mRNA length only in the 3'UTR. This allows the human genome to control gene expression by many piRNAs, keeping only a small portion of the gene conserved. Another property of piRNAs is the presence of BSs of some piRNAs in the mRNA of more than one gene. This fact indicates the general dependence of a group of candidate genes on the regulatory influence of the same piRNAs. This important property must be known for each piRNA when using them as disease markers and a therapeutic agent. Ignorance of this property can lead to side effects of using piRNAs.
The organization of BSs into clusters reduces the mRNA region responsible for regulating expression by piRNA molecules, which must be kept conservative to preserve the regulation of genome expression balanced during evolution. The binding of piRNAs in clusters leads to competition between them for the ability to exert a regulatory effect on the expression of candidate genes. At the same time, the competition between piRNAs ensures the stability of their regulation of the target gene expression because an increase or decrease in the concentration of any piRNA will have a compensatory effect on other piRNAs. That is, a significant effect of one piRNA requires considerable increases in its concentration to exceed the total impact of other piRNAs.
Using piRNAs for disease diagnosis requires compliance with several criteria that will ensure the validity of the proposed piRNAs as markers. These are as follows: 1. piRNAs must bind to mRNAs with sufficiently high free energy to affect the expression of one or more candidate genes; 2. piRNAs must be expressed ordinarily comparable to the expression of candidate target genes; 3. Measurements of piRNAs and candidate gene concentrations should be made simultaneously, directly indicating which piRNAs correlate with the target gene's expression level. The studies conducted show specific associations of piRNAs and target genes that need to be studied. The findings contribute to determining the expression level of piRNAs and their target genes in normal and disease for the diagnosis and therapy of diseases.
Supplementary Materials
Table S1: List of candidate genes for ESCC; Table S2: Characteristics of piRNA interaction with mRNA of the AURKA gene; Table S3: Characteristics of piRNA interaction with mRNA of the BMP7 gene; Table S4: Characteristics of piRNA interaction with mRNA ERCC1 gene; Table S5: Characteristics of piRNA interaction with mRNA GCOM1 gene; Table S6: Characteristics of piRNA interaction with mRNA MTHFR gene; Table S7: Characteristics of piRNA interaction with mRNA SASH1 gene; Table S8: Characteristics of piRNA interaction with mRNA SIX4 gene; Table S9: Characteristics of piRNA interaction with mRNA SIX4 gene; Table S10: Characteristics of piRNA interaction with mRNA TP53 gene; Table S11: piRNAs that bind to the mRNA of AURKA, ERCC1, SASHA and SIX4 genes.
Author Contributions
Conceptualization, A.I., A.A., and T.N.; methodology, A.I., A.P. and P.Z.; software, A.P. and A.I.; validation, A.A., A.R., T.N. and M.T.; investigation, T.N., A.A., A.R. and M.T.; resources, A.A., A.R., and T.N.; data curation, A.P. and M.T.; writing—original draft preparation, A.I. and P.Z.; writing—review and editing, A.A., A.R., and T.N; visualization, A.A. and A.R.; supervision, A.I. and P.Z.; project administration, P.Z. and A.I.; funding acquisition, P.Z. All authors have read and agreed to the published version of the manuscript.
Funding
Polish Ministry of Science and Higher Education, under the project: DIR/WK/2018/06.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kim, V.N. Small RNAs just got bigger: Piwi-interacting RNAs (piRNAs) in mammalian testes. Genes Dev 2006, 20, 1993–1997. [Google Scholar] [CrossRef] [PubMed]
- Aravin, A.A.; Sachidanandam, R.; Girard, A.; Fejes-Toth, K.; Hannon, G.J. Developmentally regulated piRNA clusters implicate MILI in transposon control. Science 2007, 316, 744–747. [Google Scholar] [CrossRef] [PubMed]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Timmons, L.; Tabara, H.; Mello, C.C.; Fire, A.Z. Inducible Systemic RNA Silencing in Caenorhabditis elegans. Mol Biol Cell 2003, 14, 2972–2983. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Mallick, B. ; Predicting sequence and structural features of effective piRNA target binding sites. J Mol Recognit 2022, 35, e2949. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Zhang, Y.; Li, J.; Zhu, Q. Small Non-Coding RNAs in Human Cancer. Genes 2022, 13, 2072. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gou, L.T.; Liu, M.F. Noncanonical functions of PIWIL1/piRNAs in animal male germ cells and human diseases. Biol Reprod 2022, 107, 101–108. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, S.; Liu, K. Structural insights into piRNA biogenesis. Biochim Biophys Acta Gene Regul Mech 2022, 1865, 194799. [Google Scholar] [CrossRef]
- Priyadarshini, M.; AlHarbi, S.; Frøkjær-Jensen, C. Acute and inherited piRNA-mediated silencing in a rde-3 ribonucleotidyltransferase mutant. MicroPubl Biol 2022, 4. [Google Scholar] [CrossRef]
- Feng, Y.; Lin, Y.; Jiang, Z.; Wu, L.; Zhang, Y.; Wu, H.; Yuan, X. Insulin-like growth factor-2 mRNA-binding protein 3 promotes cell migration, invasion, and epithelial-mesenchymal transition of esophageal squamous cell carcinoma cells by targeting zinc finger E-box-binding homeobox 1 mRNA. Mol Carcinog, 23 January 2023. [Google Scholar] [CrossRef]
- Sinha, A.; Bhattacharjee, R.; Bhattacharya, B.; Nandi, A.; Shekhar, R.; Jana, A.; Saha, K.; Kumar, L.; Patro, S.; Panda, P.K.; Kaushik, N.K.; Suar, M.; Verma, S.K. The paradigm of miRNA and siRNA influence in Oral-biome. Biomed Pharmacother 2023, 159, 114269. [Google Scholar] [CrossRef]
- Lee, Y.R.; Tsai, H.P.; Yeh, C.S.; Fang, C.Y.; Chan, M.W.Y.; Wu, T.Y.; Shen, C.H. RNA Interference Approach Is a Good Strategy against SARS-CoV-2. Viruses 2022, 15, 100. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, T.; Gupta, P.; Nayak, R.; Mallick, B. Genome-wide profiling of dysregulated piRNAs and their target genes implicated in oncogenicity of tongue squamous cell carcinoma. Gene 2023, 849, 146919. [Google Scholar] [CrossRef] [PubMed]
- Belkozhayev, A.; Niyazova, R.; Wilson, C.; Jainakbayev, N.; Pyrkova, A.; Ashirbekov, Y.; Akimniyazova, A.; Sharipov, K.; Ivashchenko, A. Bioinformatics Analysis of the Interaction of miRNAs and piRNAs with Human mRNA Genes Having di- and Trinucleotide Repeats. Genes (Basel) 2022, 13, 800. [Google Scholar] [CrossRef] [PubMed]
- AmeliMojarad, M.; Amelimojarad, M. piRNAs and PIWI proteins as potential biomarkers in Breast cancer. Mol Biol Rep 2022, 49, 9855–9862. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.K.; Mukherjee, S. Piwi-interacting RNAs (piRNAs) and colorectal carcinoma: Emerging non-invasive diagnostic biomarkers with potential therapeutic target based clinical implications. Curr Mol Med. [CrossRef]
- Riquelme, I.; Pérez-Moreno, P.; Letelier, P.; Brebi, P.; Roa, J.C. The Emerging Role of PIWI-Interacting RNAs (piRNAs) in Gastrointestinal Cancers: An Updated Perspective. Cancers (Basel) 2021, 14, 202. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Zhang, Y.; Li, J.; Zhu, Q. Small Non-Coding RNAs in Human Cancer. Genes (Basel) 2022, 13, 2072. [Google Scholar] [CrossRef] [PubMed]
- Heng, B.; Xie, X.; Zeng, W.; Li, H.; Shi, L.; Ye, W.; Wu, F. PIWI-Interacting RNA Pathway Genes: Potential Biomarkers for Clear Cell Renal Cell Carcinoma. Dis Markers 2022, 2022, 3480377. [Google Scholar] [CrossRef]
- Riquelme, I.; Pérez-Moreno, P.; Letelier, P.; Brebi, P.; Roa, J.C. The Emerging Role of PIWI-Interacting RNAs (piRNAs) in Gastrointestinal Cancers: An Updated Perspective. Cancers (Basel) 2021, 14, 202. [Google Scholar] [CrossRef]
- Kamenova, S.; Sharapkhanova, A.; Akimniyazova, A.; Kuzhybayeva, K.; Kondybayeva, A.; Rakhmetullina, A.; Pyrkova, A.; Ivashchenko, A. piRNA and miRNA Can Suppress the Expression of Multiple Sclerosis Candidate Genes. Nanomaterials (Basel) 2022, 13, 22. [Google Scholar] [CrossRef]
- Akimniyazova, A.N.; Niyazova, T.K.; Yurikova, O.Y.; Pyrkova, A.Y.; Zhanuzakov, M.A.; Ivashchenko, A.T. piRNAs may regulate expression of candidate genes of esophageal adenocarcinoma. Front Genet 2022, 13, 1069637. [Google Scholar] [CrossRef]
- Akimniyazova, A.; Yurikova, O.; Pyrkova, A.; Rakhmetullina, A.; Niyazova, T.; Ryskulova, A.G.; Ivashchenko, A. In Silico Study of piRNA Interactions with the SARS-CoV-2 Genome. Int J Mol Sci 2022, 23, 9919. [Google Scholar] [CrossRef] [PubMed]
- Jian, Z.; Han, Y.; Li, H. Potential roles of PIWI-interacting RNAs in lung cancer. Front Oncol 2022, 12, 944403. [Google Scholar] [CrossRef] [PubMed]
- Dabi, Y.; Bendifallah, S.; Suisse, S.; Haury, J.; Touboul, C.; Puchar, A.; Favier, A.; Daraï, E. Overview of non-coding RNAs in breast cancers. Transl Oncol 2022, 101512. [Google Scholar] [CrossRef] [PubMed]
- AmeliMojarad, M.; AmeliMojarad, M.; Wang, J. The function of novel small non-coding RNAs (piRNAs, tRFs) and PIWI protein in colorectal cancer. Cancer Treat Res Commun 2022, 31, 100542. [Google Scholar] [CrossRef] [PubMed]
- Goh, T.X.; Tan, S.L.; Roebuck, M.M.; Teo, S.H.; Kamarul, T. A Systematic Review of Extracellular Vesicle-Derived Piwi-Interacting RNA in Human Body Fluid and Its Role in Disease Progression. Tissue Eng Part C Methods 2022, 28, 511–528. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Zhang, Z.; Wu, J. on-coding RNA delivery for bone tissue engineering: Progress, challenges, and potential solutions. iScience 2022, 25, 104807. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Yang, X.; Wang, T.; Xing, Y.; Chen, H.; Zhu, S.; Zeng, J.; Zhou, Q.; Chen, F.; Zhang, X.; Wang, W.J. Evaluating the Effects of Storage Conditions on Multiple Cell-Free RNAs in Plasma by High-Throughput Sequencing. Biopreserv Biobank, 24 August 2022. [Google Scholar] [CrossRef]
- Cho, O.; Kim, D.W.; Cheong, J.Y. Screening Plasma Exosomal RNAs as Diagnostic Markers for Cervical Cancer: An Analysis of Patients Who Underwent Primary Chemoradiotherapy. Biomolecules 2021, 11, 1691. [Google Scholar] [CrossRef]
- Wang, J.; Shi, Y.; Zhou, H.; Zhang, P.; Song, T.; Ying, Z.; Yu, H.; Li, Y.; Zhao, Y.; Zeng, X.; He, S.; Chen, R. piRBase: integrating piRNA annotation in all aspects. Nucleic Acids Res 2021, 50, 265–272. [Google Scholar] [CrossRef]
- Ivashchenko, A.; Berillo, O.; Pyrkova, A.; Niyazova, R.; Atambayeva, S. MiR-3960 binding sites with mRNA of human genes. Bioinformation 2014, 10, 423–427. [Google Scholar] [CrossRef]
- Friedman, R.A.; Honig, B.A. Free Energy Analysis of Nucleic Acid Base Stacking in Aqueous Solution. Biophys. J. 1995, 69, 1528–1535. [Google Scholar] [CrossRef]
- Garg, A.; Heinemann, U.A. Novel Form of RNA Double Helix Based on G·U and C·A+ Wobble Base Pairing. RNA 2018, 24, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Leontis, N.B.; Stombaugh, J.; Westhof, E. The Non-watson-crick Base Pairs and Their Associated Isostericity Matrices. Nucleic Acids Res 2002, 30, 3497–3531. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.; Caiment, F.; Tordoir, X.; Cavaillé, J.; Ferguson-Smith, A.; Cockett, N.; Georges, M.; Charlier, C. RNAi-Mediated Allelic Trans-interaction at the Imprinted Rtl1/ Peg11 Locus. Curr. Biol 2005, 15, 743–749. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Nucleotide sequences of piRNAs and the AURKA mRNA BS in a region from 522 nt to 560 nt. Note: The mRNA nucleotides are highlighted in red. The piRNA nucleotides that form canonical pairs with mRNA are highlighted in violet and noncanonical pairs are highlighted in green. The names of the piRNAs are followed by the beginning of their BSs.
Figure 1.
Nucleotide sequences of piRNAs and the AURKA mRNA BS in a region from 522 nt to 560 nt. Note: The mRNA nucleotides are highlighted in red. The piRNA nucleotides that form canonical pairs with mRNA are highlighted in violet and noncanonical pairs are highlighted in green. The names of the piRNAs are followed by the beginning of their BSs.
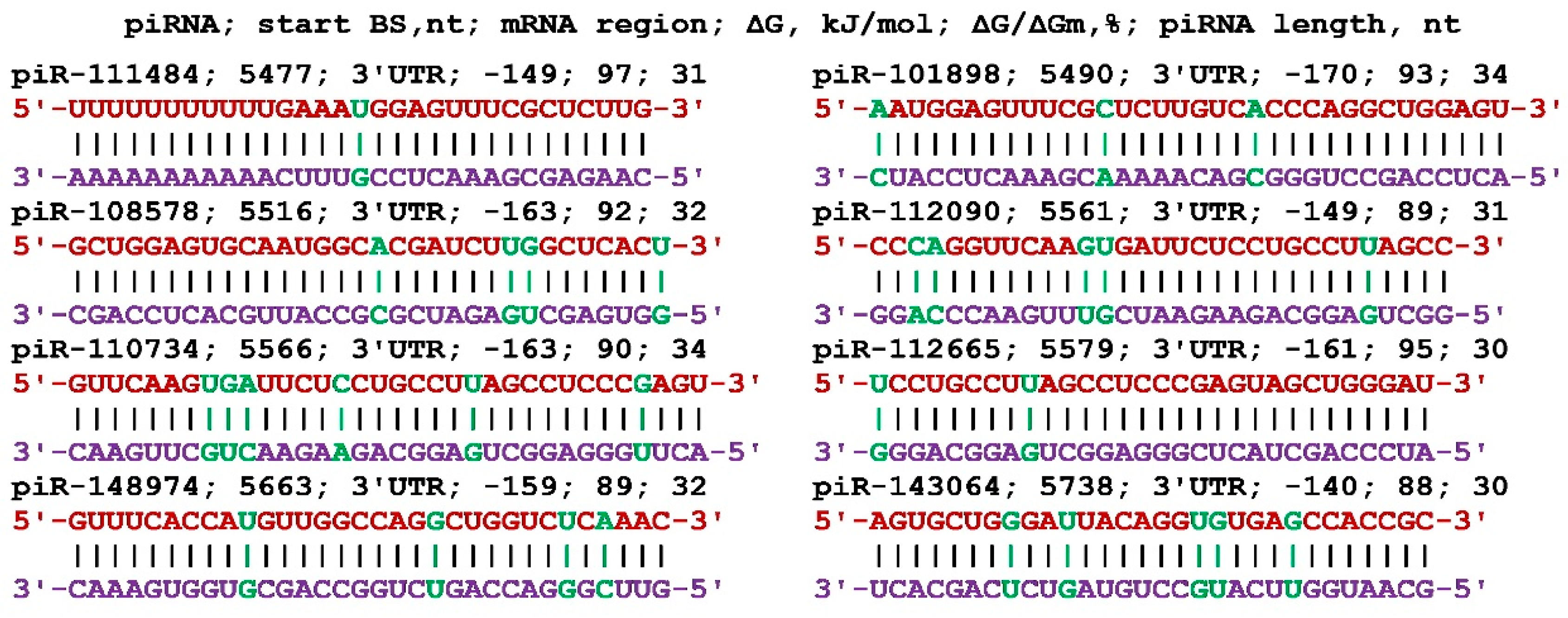
Figure 2.
Nucleotide sequences of piRNAs and characteristics of their interaction with mRNA of BMP7 gene. The mRNA nucleotides are highlighted in red. The piRNA nucleotides that form canonical pairs with mRNA are highlighted in violet and noncanonical pairs are highlighted in green. The names of the piRNAs are followed by the beginning of their BSs.
Figure 2.
Nucleotide sequences of piRNAs and characteristics of their interaction with mRNA of BMP7 gene. The mRNA nucleotides are highlighted in red. The piRNA nucleotides that form canonical pairs with mRNA are highlighted in violet and noncanonical pairs are highlighted in green. The names of the piRNAs are followed by the beginning of their BSs.
Figure 3.
Nucleotide sequences of piRNAs and the ERCC1 mRNA BS in a region from 2674 nt to 2710 nt. Note: The mRNA nucleotides are highlighted in red. The piRNA nucleotides that form canonical pairs with mRNA are highlighted in violet and noncanonical pairs are highlighted in green. The names of the piRNAs are followed by the beginning of their BSs.
Figure 3.
Nucleotide sequences of piRNAs and the ERCC1 mRNA BS in a region from 2674 nt to 2710 nt. Note: The mRNA nucleotides are highlighted in red. The piRNA nucleotides that form canonical pairs with mRNA are highlighted in violet and noncanonical pairs are highlighted in green. The names of the piRNAs are followed by the beginning of their BSs.
Figure 4.
Nucleotide sequences of piRNAs and characteristics of their interaction with mRNA of GCOM1 gene. The mRNA nucleotides are highlighted in red. The piRNA nucleotides that form canonical pairs with mRNA are highlighted in violet and noncanonical pairs are highlighted in green. The names of the piRNAs are followed by the beginning of their BSs.
Figure 4.
Nucleotide sequences of piRNAs and characteristics of their interaction with mRNA of GCOM1 gene. The mRNA nucleotides are highlighted in red. The piRNA nucleotides that form canonical pairs with mRNA are highlighted in violet and noncanonical pairs are highlighted in green. The names of the piRNAs are followed by the beginning of their BSs.
Figure 5.
Nucleotide sequences of piRNAs and the MTHFR mRNA BS in a region from 6861 nt to 6893 nt. Note: The mRNA nucleotides are highlighted in red. The piRNA nucleotides that form canonical pairs with mRNA are highlighted in violet and noncanonical pairs are highlighted in green. The names of the piRNAs are followed by the beginning of their BSs.
Figure 5.
Nucleotide sequences of piRNAs and the MTHFR mRNA BS in a region from 6861 nt to 6893 nt. Note: The mRNA nucleotides are highlighted in red. The piRNA nucleotides that form canonical pairs with mRNA are highlighted in violet and noncanonical pairs are highlighted in green. The names of the piRNAs are followed by the beginning of their BSs.
Figure 6.
Nucleotide sequences of piRNAs and characteristics of their interaction with mRNA of SASH1 gene. The mRNA nucleotides are highlighted in red. The piRNA nucleotides that form canonical pairs with mRNA are highlighted in violet and noncanonical pairs are highlighted in green. The names of the piRNAs are followed by the beginning of their BSs.
Figure 6.
Nucleotide sequences of piRNAs and characteristics of their interaction with mRNA of SASH1 gene. The mRNA nucleotides are highlighted in red. The piRNA nucleotides that form canonical pairs with mRNA are highlighted in violet and noncanonical pairs are highlighted in green. The names of the piRNAs are followed by the beginning of their BSs.
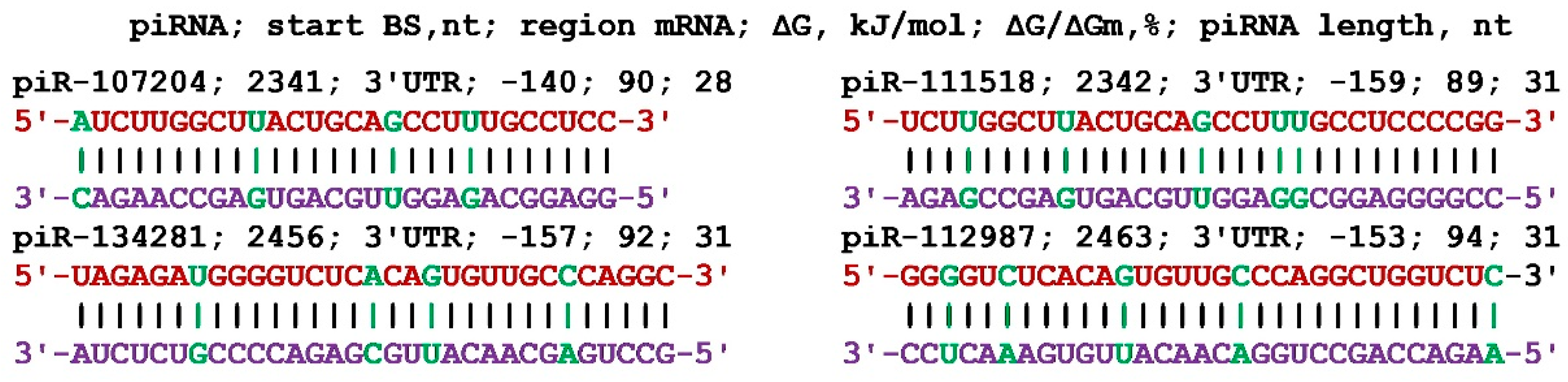
Figure 7.
Interaction nucleotide sequences of BSs cluster in the 3'UTR of mRNA SIX4 gene from 4114 nt and nucleotide sequences of 15 piRNAs. Note: The mRNA nucleotides are highlighted in red. The piRNA nucleotides that form canonical pairs with mRNA are highlighted in violet and noncanonical pairs are highlighted in green. The names of the piRNAs are followed by the beginning of their BSs.
Figure 7.
Interaction nucleotide sequences of BSs cluster in the 3'UTR of mRNA SIX4 gene from 4114 nt and nucleotide sequences of 15 piRNAs. Note: The mRNA nucleotides are highlighted in red. The piRNA nucleotides that form canonical pairs with mRNA are highlighted in violet and noncanonical pairs are highlighted in green. The names of the piRNAs are followed by the beginning of their BSs.
Figure 8.
Nucleotide sequences of piRNAs and the SULT1A1 mRNA BS in a region from 1507 nt to 1543 nt. Note: The mRNA nucleotides are highlighted in red. The piRNA nucleotides that form canonical pairs with mRNA are highlighted in violet and noncanonical pairs are highlighted in green. The names of the piRNAs are followed by the beginning of their BSs.
Figure 8.
Nucleotide sequences of piRNAs and the SULT1A1 mRNA BS in a region from 1507 nt to 1543 nt. Note: The mRNA nucleotides are highlighted in red. The piRNA nucleotides that form canonical pairs with mRNA are highlighted in violet and noncanonical pairs are highlighted in green. The names of the piRNAs are followed by the beginning of their BSs.
Figure 9.
Nucleotide sequences of piRNAs and characteristics of their interaction with mRNA of TP53 gene. Note: The mRNA nucleotides are highlighted in red. The piRNA nucleotides that form canonical pairs with mRNA are highlighted in violet and noncanonical pairs are highlighted in green. The names of the piRNAs are followed by the beginning of their BSs.
Figure 9.
Nucleotide sequences of piRNAs and characteristics of their interaction with mRNA of TP53 gene. Note: The mRNA nucleotides are highlighted in red. The piRNA nucleotides that form canonical pairs with mRNA are highlighted in violet and noncanonical pairs are highlighted in green. The names of the piRNAs are followed by the beginning of their BSs.
Figure 10.
Schemes of the interaction of identical piRNAs with the mRNAs of the AURKA and SIX4 genes. Note: The mRNA nucleotides are highlighted in red. The piRNA nucleotides that form canonical pairs with mRNA are highlighted in violet.
Figure 10.
Schemes of the interaction of identical piRNAs with the mRNAs of the AURKA and SIX4 genes. Note: The mRNA nucleotides are highlighted in red. The piRNA nucleotides that form canonical pairs with mRNA are highlighted in violet.
Figure 11.
Schemes of interaction between piRNAs and mRNAs of candidate genes with ΔG/ΔGm equal from 92% to 100% and ΔG value equal to -180 kJ/mol and more.
Figure 11.
Schemes of interaction between piRNAs and mRNAs of candidate genes with ΔG/ΔGm equal from 92% to 100% and ΔG value equal to -180 kJ/mol and more.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).