Submitted:
12 May 2023
Posted:
15 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Dermatological Diseases Directly Associated with Brachycephaly
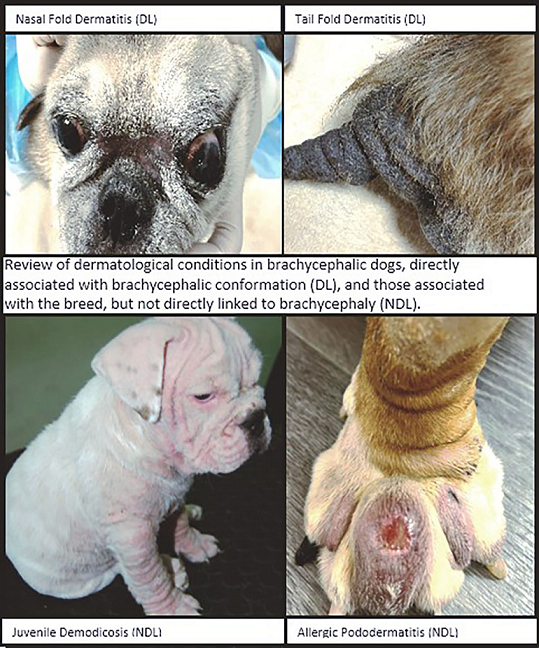
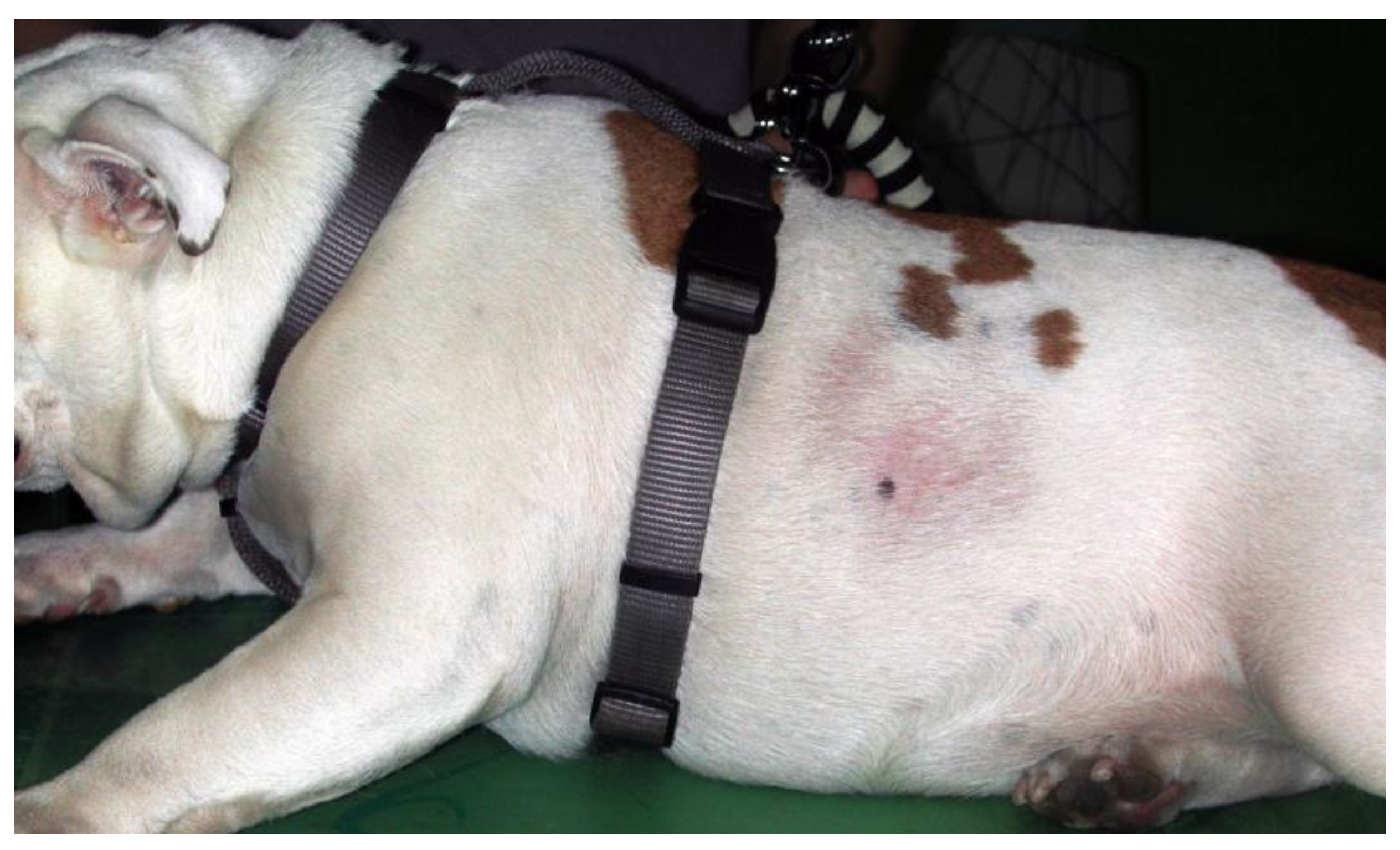
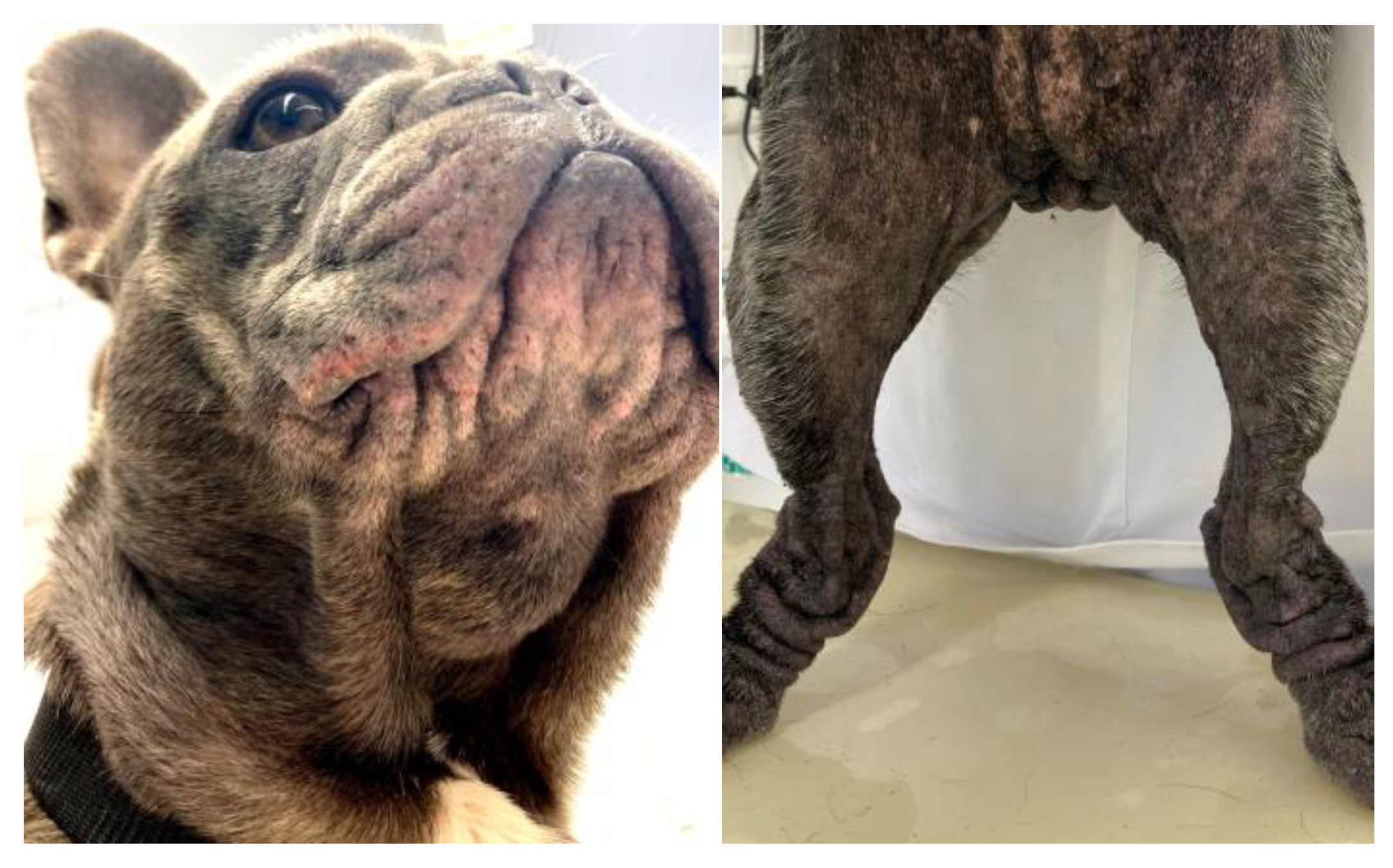
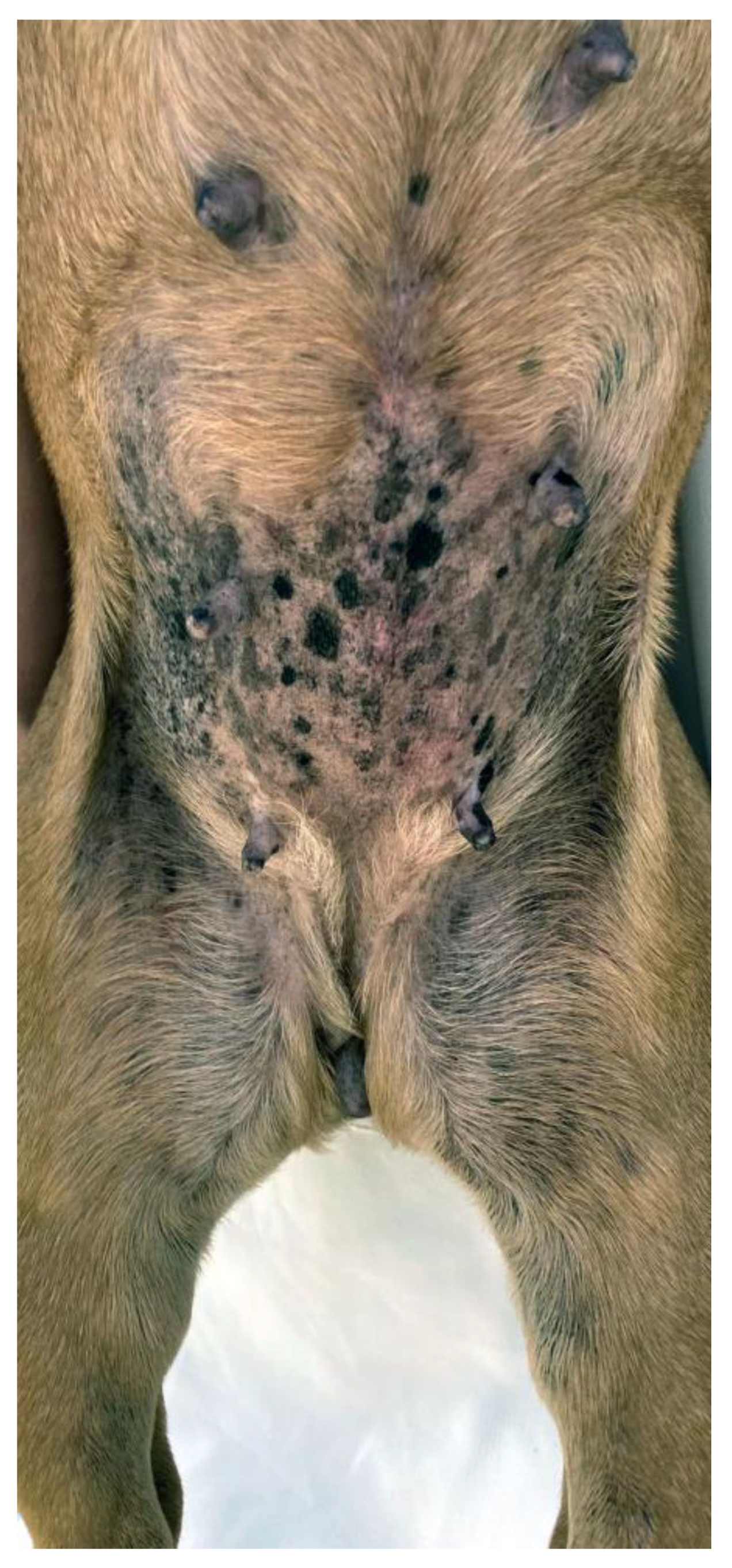
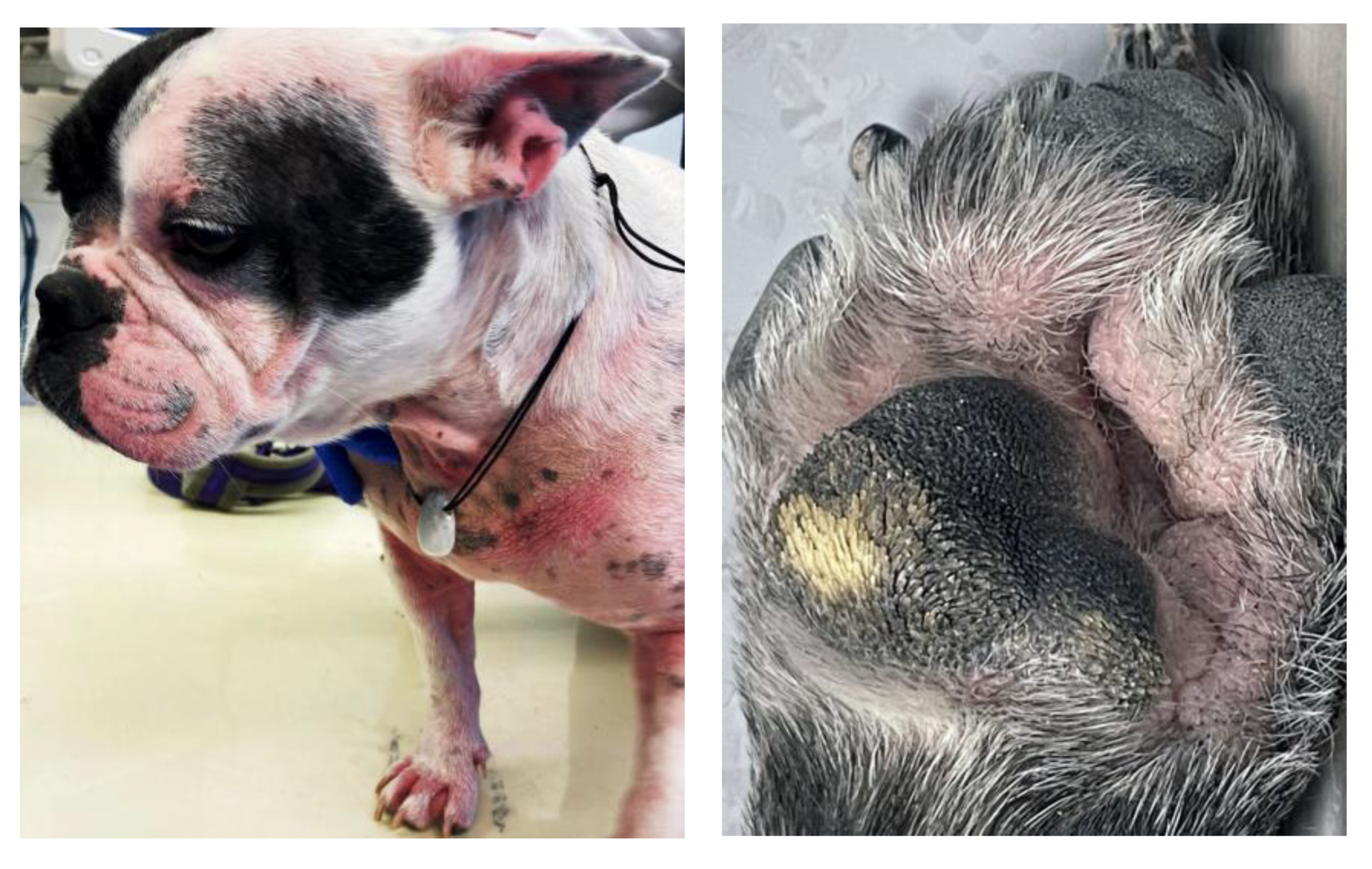
2.1. Skin Fold Dermatitis
2.2. Otitis Externa
2.3. Caudal Occipital Malformation Syndrome/Chiari-like Malformation/Primary Secretory Otitis Media
3. Other Skin Diseases in Brachycephalic Breeds
3.1. Genetic Skin Diseases
3.1.1. Ichthyosis
3.1.2. Tyrosinase Deficiency
3.1.3. Congenital Alopecia
3.1.4. Colour Dilution Alopecia (CDA)/Black Hair Follicular Dysplasia/Follicular Dysplasia
3.1.5. Canine Flank Alopecia/Seasonal Flank Alopecia
3.1.6. Pattern Baldness
3.1.7. Cutaneous Asthenia
3.2. Infectious Skin Diseases
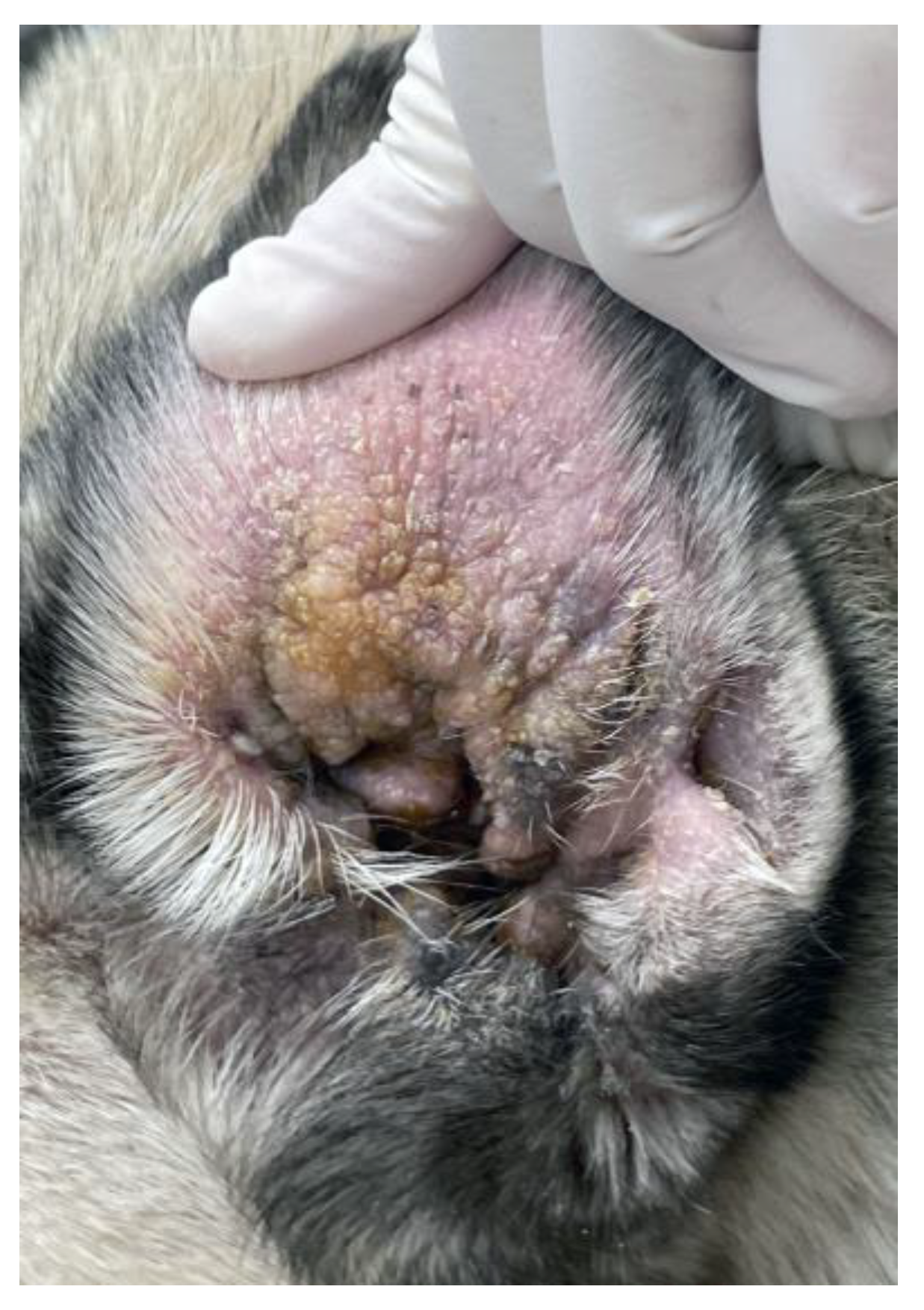
3.2.1. Canine Demodicosis
3.2.2. Malassezia Dermatitis
3.2.3. Viral Pigmented Plaques
3.3. Bacterial Skin Diseases
3.3.1. Bacterial Folliculitis (Superficial Pyoderma)
3.3.2. Pyotraumatic Dermatitis (Hot Spot)
3.3.3. Muzzle Folliculitis and Furunculosis
3.3.4. Canine Leproid Granuloma
3.4. Immunological Skin Diseases
3.4.1. Primary Immune Deficiencies
3.4.2. Hypersensitivities
3.4.3. Pemphigus Foliaceus
3.4.4. Uveodermatologic Syndrome
3.4.5. Sterile Granuloma and Pyogranuloma Syndrome
3.4.6. Acute Febrile Vasculitis
3.5. Miscellaneous Skin Diseases
3.5.1. Anal Sac Disease
3.5.2. Calcinosis Circumscripta
3.5.3. Dermoid Sinus/Cyst
3.6. Other Skin Diseases
4. General Discussion and Ethical Considerations
| Disease Group | Disease | Breeds | References |
|---|---|---|---|
|
Congenital Skin Diseases |
Congenital Alopecia |
Chihuahua French Bulldog Lhasa Apso |
Miller et al. 2012 Ihrke et al. 1993 Marks et al. 1992 O’Neill et al. 1981 |
| Color dilution alopecia Black hair follicular dysplasia Follicular dysplasia |
Blue Chow Chow Boston Terrier Boxer Cavalier King Charles Spaniel Chihuahua Shih Tzu |
Miller et al. 2012 Perego et al. 2009 Kim et al. 2005, 2005 Rachid et al. 2003 Beco et al. 1996 Roperto et al. 1995 |
|
| Flank alopecia | Affenpinscher Boxer Chihuahua English Bulldog Staffordshire Bull Terrier |
Vandenabeele et al. 2014 Mecklenburg et al.2009 Fontaine et al. 1998 Miller et al. 1993 |
|
| Pattern baldness | Boston Terrier Boxer Chihuahua English Bulldog |
Miller et al. 2012 Paradis et al. 2009 |
|
| Ichthyosis | American Bulldog Cavalier King Charles Spaniel |
Mauldin et al. 2013, 2015 Hartley et al. 2012 Barnett et al. 2006 Alhaidari et al. 1994 |
|
| Cutaneous asthenia |
Boxer | Miller et al. 2012 Bellini et al. 2009 |
|
| Tyrosinase deficiency | Chow Chow | Miller et al. 2012 Engstrom et al. 1966 |
|
| Caudal occipital malformation syndrome Chiari-like malformation |
Affenpinscher Boston Terrier Brussels Griffon Cavalier King Charles Spaniel Chihuahua French Bulldog Pomeranian Pug Shih Tzu |
Sanchis-Mora et al. 2016 Lewis et al. 2010 Cagle et al. 2010 Rusbridge et al. 2003, 2004, 2005, 2009 Dewey et al. 2005 |
|
|
Infectious Skin Diseases |
Canine demodicosis |
Boxer Boston Terrier Chihuahua Chow Chow English Bulldog French Bulldog Pugs Shih Tzu Staffordshire Bull Terrier Shar Pei |
O’Neill et al. 2020 Wright et al. 2014 Barrientos et al. 2013 Kuznetsova et al. 2012 Plant et al. 2011 It et al. 2010 Mueller et al. 2009 Holm et al. 2003 Lemaire et al. 1996 Day et al. 1997 Chen et al. 1995 |
| Fungal | Malassezia dermatitis | Boxer Cavalier King Charles Spaniel English Bulldog Lhasa Apso Shih Tzu |
Bajwa et al. 2017 Miller et al. 2012 Mauldin et al. 1997 |
| Bacterial | Superficial pyoderma | Boxer British Bulldog Bullmastiff Pug Shar Pei |
O’Neill et al. 2016, 2019, 2022 Miller et al. 2012 |
| Hot spot | British Bulldogs Pugs |
O’Neill et al. 2016, 2019, 2022 Holm et al. 2004 |
|
| Muzzle folliculitis and furunculosis |
Boxer British Bulldog |
Fawcett et al. 2018 Pedersen et al. 2016 Miller et al. 2012 |
|
| Canine leproid granuloma |
Boxer | Miller et al. 2012 Conceição et al. 2011 Malik et al. 1998 |
|
| Viral | Viral pigmented plaques |
Australian Terrier Boston Terrier Chihuahua French Bulldog Pug |
Nagata et al. 1995, 2013 Luff et al. 2012 Narama et al. 2005 |
| Mixed | Otitis externa | Boxers British Bulldogs Pugs |
O’Neill et al. 2016, 2019, 2021 Sapierzyński et al. 2009 |
| Immunological Diseases | Primary immune deficiencies |
Bull Terrier Chow Chow Pomeranian Shar Pei |
Ellis et al. 2019 Olsson et al. 2015 Miller et al. 2012 Day et al. 1999 Lanevschi et al. 1999 Rivas et al. 1995 |
| Hypersensitivities | American Bulldog Boston Terrier Boxer Chow Chow English Bulldog French Bulldog Lhasa Apso Pug Shar Pei Shih Tzu Staffordshire Bullterrier |
Outerbridge et al. 2021 Mazrier et al. 2016 Miller et al. 2012 Theerawatanasirikul et al. 2012 Jaeger et al. 2010 Picco et al. 2008 Počta et al. 2007 Nødtvedt et al. 2006 Verlinden et al. 2006 Prélaud et al. 1998 Harvey et al. 1993 |
|
| Pemphigus foliaceus | Chow Chow | Goodale et al. 2019 Bizikova et al. 2012 Olivry et al. 2006 Gonsalves-Huber et al. 2005 Kuhl et al. 1994 |
|
| Uveodermatologic syndrome |
Chow Chow | Zarfoss et al. 2018 Miller et al. 2012 Blackwood et al. 2011 |
|
| Acute febrile vasculitis |
Shar Pei | Weingart et al. 2022 Innerå et al. 2013 Malik et al. 2002 Tellier et al. 2001 |
|
| Sterile granuloma and pyogranuloma syndrome |
Boxer English Bulldog French Mastiff |
Miller et al. 2012 Panich et al. 1991 |
|
|
Miscellaneous Skin Diseases |
Skin fold dermatitis | Boston Terriers British Bulldog Pekingese Pug Shar Pei |
O’Neill et al. 2016, 2019, 2022, 2022, 2022 Packer et al. 2021 Fawcett et al. 2018 Beco et al. 2013 Miller et al. 2012 |
| Anal sac disease | Pugs | O’Neill et al. 2020, 2022 Fawcett et al. 2018 Feng et al. 2017 |
|
| Calcinosis circumscripta | Boston Terrier Boxer Shih Tzu |
Doerr et al. 2013 Miller et al. 2012 Tafti et al. 2005 Scott et al. 1988 |
|
| Dermoid sinus/cyst | Boxers Chow Chow English Bull Terrier French Bulldog Shih Tzu Victorian Bulldog |
Barrios et al. 2014 Ployart et at. 2013 Motta et al. 2012 Sturgeon et al. 2008 Bornard et al. 2007 Colón et al. 2007 Bowens et al. 2005 Burrow et al. 2004 Fatone et al. 1995 Booth et al. 1998 Selcer et al. 1984 |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Packer, R.; Murphy, D.; Farnworth, M. Purchasing popular purebreds: investigating the influence of breed-type on the pre-purchase motivations and behaviour of dog owners. Animal Wefare 2017, 26, 191–201. [Google Scholar] [CrossRef]
- Kenny, D.D.; Freemantle, R.; Jeffery, A.; Tivers, M.S. Impact of an educational intervention on public perception of brachycephalic obstructive airway syndrome in brachycephalic dogs. Veterinary Record 2022, 190, no-no. [Google Scholar] [CrossRef]
- Fawcett, A.; Barrs, V.; Awad, M.; Child, G.; Brunel, L.; Mooney, E.; Martinez-Taboada, F.; McDonald, B.; McGreevy, P. Consequences and Management of Canine Brachycephaly in Veterinary Practice: Perspectives from Australian Veterinarians and Veterinary Specialists. Animals (Basel) 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Mitze, S.; Barrs, V.R.; Beatty, J.A.; Hobi, S.; Bęczkowski, P.M. Brachycephalic Obstructive Airway Syndrome: much more than a surgical problem. Veterinary Quarterly 2022, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ekenstedt, K.; Crosse, K.; Risselada, M. Canine brachycephaly: anatomy, pathology, genetics and welfare. Journal of comparative pathology 2020, 176, 109–115. [Google Scholar] [CrossRef] [PubMed]
- O'Neill, D.G.; Pegram, C.; Crocker, P.; Brodbelt, D.C.; Church, D.B.; Packer, R.M.A. Unravelling the health status of brachycephalic dogs in the UK using multivariable analysis. Sci Rep 2020, 10, 17251. [Google Scholar] [CrossRef]
- Schroers, M.; Meyer-Lindenberg, A. [Assessment of clinical signs of brachycephalic obstructive airway syndrome and other breed-specific diseases in pug dogs - an online survey]. Tierarztl Prax Ausg K Kleintiere Heimtiere 2022, 50, 261–268. [Google Scholar] [CrossRef]
- O’Neill, D.G.; Volk, A.V.; Soares, T.; Church, D.B.; Brodbelt, D.C.; Pegram, C. Frequency and predisposing factors for canine otitis externa in the UK–a primary veterinary care epidemiological view. Canine Medicine and Genetics 2021, 8, 1–16. [Google Scholar] [CrossRef]
- O’Neill, D.G.; Skipper, A.; Packer, R.M.A.; Lacey, C.; Brodbelt, D.C.; Church, D.B.; Pegram, C. English Bulldogs in the UK: a VetCompass study of their disorder predispositions and protections. Canine Medicine and Genetics 2022, 9, 5. [Google Scholar] [CrossRef]
- O'Neill, D.G.; Turgoose, E.; Church, D.B.; Brodbelt, D.C.; Hendricks, A. Juvenile-onset and adult-onset demodicosis in dogs in the UK: prevalence and breed associations. J Small Anim Pract 2020, 61, 32–41. [Google Scholar] [CrossRef]
- Nuttall, T.J.; Marsella, R.; Rosenbaum, M.R.; Gonzales, A.J.; Fadok, V.A. Update on pathogenesis, diagnosis, and treatment of atopic dermatitis in dogs. J Am Vet Med Assoc 2019, 254, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- O'Neill, I.D.; Rowe, D.; Brodbelt, D.C.; Pegram, C.; Hendricks, A. Ironing out the wrinkles and folds in the epidemiology of skin fold dermatitis in dog breeds in the UK. Sci Rep 2022, 12, 10553. [Google Scholar] [CrossRef] [PubMed]
- McGreevy, P.D.; Georgevsky, D.; Carrasco, J.; Valenzuela, M.; Duffy, D.L.; Serpell, J.A. Dog behavior co-varies with height, bodyweight and skull shape. PloS one 2013, 8, e80529. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, D.G.; Sahota, J.; Brodbelt, D.C.; Church, D.B.; Packer, R.; Pegram, C. Health of Pug dogs in the UK: disorder predispositions and protections. Canine Medicine and Genetics 2022, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.H.; Griffin, C.E.; Campbell, K.L. Muller and Kirk's small animal dermatology; Elsevier Health Sciences, 2012. [Google Scholar]
- O’Neill, D.G.; Darwent, E.C.; Church, D.B.; Brodbelt, D.C. Demography and health of Pugs under primary veterinary care in England. Canine Genetics and Epidemiology 2016, 3, 1–12. [Google Scholar] [CrossRef]
- O’Neill, D.G.; Skipper, A.M.; Kadhim, J.; Church, D.B.; Brodbelt, D.C.; Packer, R.M. Disorders of Bulldogs under primary veterinary care in the UK in 2013. PLoS One 2019, 14, e0217928. [Google Scholar] [CrossRef]
- O’Neill, D.G.; Skipper, A.; Packer, R.; Lacey, C.; Brodbelt, D.C.; Church, D.B.; Pegram, C. English Bulldogs in the UK: a VetCompass study of their disorder predispositions and protections. Canine Medicine and Genetics 2022, 9, 1–14. [Google Scholar] [CrossRef]
- O’NeillI, D.G.; Rowe, D.; Brodbelt, D.C.; Pegram, C.; Hendricks, A. Ironing out the wrinkles and folds in the epidemiology of skin fold dermatitis in dog breeds in the UK. Scientific Reports 2022, 12, 1–13. [Google Scholar] [CrossRef]
- Packer, R.; O'Neill, D. Health and welfare of brachycephalic (flat-faced) companion animals: a complete guide for veterinary and animal professionals; CRC Press, 2021. [Google Scholar]
- Beco, L.; Guaguère, E.; Lorente Méndez, C.; Noli, C.; Nuttall, T.; Vroom, M. Suggested guidelines for using systemic antimicrobials in bacterial skin infections (1): diagnosis based on clinical presentation, cytology and culture. Vet Rec 2013, 172, 72–78. [Google Scholar] [CrossRef]
- Banovic, F.; Strzok, E. Skin Fold Dermatitis (Intertrigo) in Dogs. Today’s Veterinary Practice 2019. [Google Scholar]
- Zanna, G.; Docampo, M.J.; Fondevila, D.; Bardagí, M.; Bassols, A.; Ferrer, L. Hereditary cutaneous mucinosis in shar pei dogs is associated with increased hyaluronan synthase-2 mRNA transcription by cultured dermal fibroblasts. Veterinary dermatology 2009, 20, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.A.; Saiyad, S.; Rao, N. Common health issues related to brachycephalic dogs. 2022. [Google Scholar]
- Sapierzyński, R. Otitis externa in dogs. Medycyna Weterynaryjna 2009, 65, 552–556. [Google Scholar]
- Töpfer, T.; Köhler, C.; Rösch, S.; Oechtering, G. Brachycephaly in French bulldogs and pugs is associated with narrow ear canals. Veterinary Dermatology 2022, 33, 214–e260. [Google Scholar] [CrossRef] [PubMed]
- Pye, C. Pseudomonas otitis externa in dogs. Can Vet J 2018, 59, 1231–1234. [Google Scholar] [PubMed]
- Gotthelf, L.N. Diagnosis and treatment of otitis media in dogs and cats. Veterinary Clinics: Small Animal Practice 2004, 34, 469–487. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, T. Successful management of otitis externa. In Practice 2016, 38, 17–21. [Google Scholar] [CrossRef]
- Chan, W.Y.; Hickey, E.E.; Page, S.W.; Trott, D.J.; Hill, P.B. Biofilm production by pathogens associated with canine otitis externa, and the antibiofilm activity of ionophores and antimicrobial adjuvants. Journal of veterinary pharmacology and therapeutics 2019, 42, 682–692. [Google Scholar] [CrossRef]
- Seo, M.; Oh, T.; Bae, S. Antibiofilm activity of silver nanoparticles against biofilm forming Staphylococcus pseudintermedius isolated from dogs with otitis externa. Veterinary Medicine and Science 2021, 7, 1551–1557. [Google Scholar] [CrossRef]
- Pickrell, J.; Oehme, F.; Cash, W. Ototoxicity in dogs and cats. In Proceedings of the Seminars in Veterinary Medicine and Surgery (Small Animal); 1993; pp. 42–49. [Google Scholar]
- Oishi, N.; Talaska, A.E.; Schacht, J. Ototoxicity in dogs and cats. Veterinary Clinics: Small Animal Practice 2012, 42, 1259–1271. [Google Scholar] [CrossRef]
- Rusbridge, C.; Knowler, P.; Rouleau, G.A.; Minassian, B.A.; Rothuizen, J. Inherited occipital hypoplasia/syringomyelia in the cavalier King Charles spaniel: experiences in setting up a worldwide DNA collection. Journal of Heredity 2005, 96, 745–749. [Google Scholar] [CrossRef]
- Rusbridge, C.; Knowler, S.P. Inheritance of occipital bone hypoplasia (Chiari type I malformation) in Cavalier King Charles Spaniels. Journal of Veterinary Internal Medicine 2004, 18, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Lewis, T.; Rusbridge, C.; Knowler, P.; Blott, S.; Woolliams, J.A. Heritability of syringomyelia in Cavalier King Charles spaniels. The Veterinary Journal 2010, 183, 345–347. [Google Scholar] [CrossRef] [PubMed]
- Rusbridge, C.; Knowler, S. Hereditary aspects of occipital bone hypoplasia and syringomyelia (Chiari type I malformation) in cavalier King Charles spaniels. Veterinary Record 2003, 153, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Cagle, L. Concurrent occipital hypoplasia, occipital dysplasia, syringohydromyelia, and hydrocephalus in a Yorkshire terrier. The Canadian Veterinary Journal 2010, 51, 904. [Google Scholar] [PubMed]
- Sanchis-Mora, S.; Pelligand, L.; Thomas, C.; Volk, H.; Abeyesinghe, S.; Brodbelt, D.; Church, D.; Thomson, P.; McGreevy, P.; O'Neill, D. Dogs attending primary-care practice in England with clinical signs suggestive of Chiari-like malformation/syringomyelia. Veterinary Record 2016, 179, 436–436. [Google Scholar] [CrossRef] [PubMed]
- Rusbridge, C.; Knowler, S.; Pieterse, L.; McFadyen, A. Chiari-like malformation in the Griffon Bruxellois. Journal of Small Animal Practice 2009, 50, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Dewey, C.W.; Berg, J.M.; Barone, G.; Marino, D.J.; Stefanacci, J.D. Foramen magnum decompression for treatment of caudal occipital malformation syndrome in dogs. Journal of the American Veterinary Medical Association 2005, 227, 1270–1275. [Google Scholar] [CrossRef] [PubMed]
- Cerda-Gonzalez, S.; Olby, N.; Pease, T.; McCullough, S.; Massoud, N.; Broadstone, R. Morphology of the caudal fossa in Cavalier King Charles Spaniels. In Proceedings of the Journal of Veterinary Internal Medicine; 2006; pp. 736–736. [Google Scholar]
- Lu, D.; Lamb, C.; Pfeiffer, D.; Targett, M. Neurological signs and results of magnetic resonance imaging in 40 cavalier King Charles spaniels with Chiari type 1-like malformations. Veterinary Record 2003, 153, 260–263. [Google Scholar] [CrossRef]
- Plessas, I.; Rusbridge, C.; Driver, C.; Chandler, K.; Craig, A.; McGonnell, I.; Brodbelt, D.; Volk, H. Long-term outcome of Cavalier King Charles spaniel dogs with clinical signs associated with Chiari-like malformation and syringomyelia. Veterinary Record 2012, 171, 501–501. [Google Scholar] [CrossRef]
- Cole, L.K. Primary secretory otitis media in Cavalier King Charles spaniels. Veterinary Clinics: Small Animal Practice 2012, 42, 1137–1142. [Google Scholar] [CrossRef]
- Kubba, H.; Pearson, J.; Birchall, J. The aetiology of otitis media with effusion: a review. Clinical Otolaryngology & Allied Sciences 2000, 25, 181–194. [Google Scholar]
- Hayes, G.; Friend, E.; Jeffery, N. Relationship between pharyngeal conformation and otitis media with effusion in Cavalier King Charles spaniels. Veterinary Record 2010, 167, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Salazar, V.; Dewey, C.W.; Schwark, W.; Badgley, B.L.; Gleed, R.D.; Horne, W.; Ludders, J.W. Pharmacokinetics of single-dose oral pregabalin administration in normal dogs. Veterinary Anaesthesia and Analgesia 2009, 36, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Grubb, T. Chronic neuropathic pain in veterinary patients. Topics in Companion Animal Medicine 2010, 25, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.; Luna, S.P.L.; Joaquim, J.G.F.; Coutinho, H.D.; Possebon, F.S. Effect of acupuncture on pain and quality of life in canine neurological and musculoskeletal diseases. Can Vet J 2017, 58, 941–951. [Google Scholar] [PubMed]
- Rusbridge, C. Chiari-like malformation with syringomyelia in the Cavalier King Charles spaniel: long-term outcome after surgical management. Veterinary Surgery 2007, 36, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Colverde, A.S.; Nicetto, T.; Falzone, C. Occipital cranioplasty using customized titanium prosthesis yields successful outcome in association with foramen magnum decompression in dogs suffering by Chiari-like malformation. Am J Vet Res 2021, 83, 275–282. [Google Scholar] [CrossRef]
- Loughin, C.A. Chiari-like Malformation. Vet Clin North Am Small Anim Pract 2016, 46, 231–242. [Google Scholar] [CrossRef]
- Ortinau, N.; Vitale, S.; Akin, E.Y.; Beasley, M.; Shores, A. Foramen magnum decompression surgery in 23 Chiari-like malformation patients 2007-2010: outcomes and owner survey results. Can Vet J 2015, 56, 288–291. [Google Scholar]
- Hartley, C.; Donaldson, D.; Smith, K.C.; Henley, W.; Lewis, T.W.; Blott, S.; Mellersh, C.; Barnett, K.C. Congenital keratoconjunctivitis sicca and ichthyosiform dermatosis in 25 Cavalier King Charles spaniel dogs–part I: clinical signs, histopathology, and inheritance. Veterinary ophthalmology 2012, 15, 315–326. [Google Scholar] [CrossRef]
- Mauldin, E.; Wang, P.; Evans, E.; Cantner, C.; Ferracone, J.; Credille, K.; Casal, M. Autosomal recessive congenital ichthyosis in American Bulldogs is associated with NIPAL4 (ICHTHYIN) deficiency. Veterinary pathology 2015, 52, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Mauldin, E.A. Canine ichthyosis and related disorders of cornification. Veterinary Clinics: Small Animal Practice 2013, 43, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Barnett, K. Congenital keratoconjunctivitis sicca and ichthyosiform dermatosis in the cavalier King Charles spaniel. Journal of small animal practice 2006, 47, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Alhaidari, Z.; Ortonne, J.P.; Pisani, A. Congenital ichthyosis in two cavalier King Charles spaniel littermates. Veterinary dermatology 1994, 5, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Mauldin, E.A.; Elias, P.M. Ichthyosis and hereditary cornification disorders in dogs. Veterinary Dermatology 2021, 32, 567–e154. [Google Scholar] [CrossRef]
- Engstrom, D.; Kirk, R. Tyrosinase deficiency in the chow chow. In Current veterinary therapy small animal practice; WB Saunders: Philadelphia, 1966; p. 350. [Google Scholar]
- Ihrke, P.J.; Mueller, R.S.; Stannard, A.A. Generalized congenital hypotrichosis in a female Rottweiler. Veterinary dermatology 1993, 4, 65–69. [Google Scholar] [CrossRef]
- Marks, A.; van den Broek, A.; Else, R. Congenital hypotrichosis in a French bulldog. Journal of Small Animal Practice 1992, 33, 450–452. [Google Scholar] [CrossRef]
- O'Neill, C. Hereditary skin disease in the dog and the cat. Compendium on Continuing Education for the Practicing Veterinarian 1981, 3, 791–801. [Google Scholar]
- Mecklenburg, L. An overview on congenital alopecia in domestic animals. Veterinary dermatology 2006, 17, 393–410. [Google Scholar] [CrossRef]
- Moura, E.; Daltro, S.; Sás, D.; Engracia Filho, J.; Farias, M.; Pimpão, C. Genetic analysis of a possible case of canine X-linked ectodermal dysplasia. Journal of Small Animal Practice 2021, 62, 1127–1130. [Google Scholar] [CrossRef]
- Perego, R.; Proverbio, D.; Roccabianca, P.; Spada, E. Color dilution alopecia in a blue Doberman pinscher crossbreed. Can Vet J 2009, 50, 511–514. [Google Scholar] [PubMed]
- Kim, S.-r.; Kim, Y.-i.; Seo, J.; Park, J.-w.; Jeong, A.-y.; Lee, K.-w.; Oh, T.-h. Black Hair Follicular Dysplasia in a Shih Tzu. Journal of Veterinary Clinics 2005, 22, 157–159. [Google Scholar]
- Kim, J.H.; Kang, K.I.; Sohn, H.J.; Woo, G.H.; Jean, Y.H.; Hwang, E.K. Color-dilution alopecia in dogs. Journal of veterinary science 2005, 6, 259–261. [Google Scholar] [CrossRef] [PubMed]
- Rachid, M.A.; Demaula, C.D.; Scott, D.W.; Miller, W.H.; Senter, D.A.; Myers, S. Concurrent follicular dysplasia and interface dermatitis in Boxer dogs. Veterinary Dermatology 2003, 14, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Beco, L.; Fontaine, J.; Gross, T.L.; Charlier, G. Colour dilution alopecia in seven Dachshunds. A clinical study and the hereditary, microscopical and ultrastructural aspect of the disease. Veterinary dermatology 1996, 7, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Roperto, F.; Cerundolo, R.; Restucci, B.; Vincensi, M.R.; Caprariis, D.D.; Vico, G.D.; Maiolino, P. Colour dilution alopecia (CDA) in ten Yorkshire Terriers. Veterinary dermatology 1995, 6, 171–178. [Google Scholar] [CrossRef]
- Caramalac, S.M.; Caramalac, S.M.; Babo-Terra, V.J.; Ramos, C.A.; Palumbo, M.I. PCR-RFLP molecular confirmation of color dilution alopecia in dogs in Brazil. Journal of Veterinary Diagnostic Investigation 2021, 33, 984–986. [Google Scholar] [CrossRef]
- Welle, M.; Philipp, U.; Rüfenacht, S.; Roosje, P.; Scharfenstein, M.; Schütz, E.; Brenig, B.; Linek, M.; Mecklenburg, L.; Grest, P. MLPH genotype—melanin phenotype correlation in dilute dogs. Journal of heredity 2009, 100, S75–S79. [Google Scholar] [CrossRef]
- Von Bomhard, W.; Mauldin, E.A.; Schmutz, S.M.; Leeb, T.; Casal, M.L. Black hair follicular dysplasia in Large Münsterländer dogs: clinical, histological and ultrastructural features. Veterinary dermatology 2006, 17, 182–188. [Google Scholar] [CrossRef]
- Antunes, M.I.P.P.; Fabris, V.E.; Machado, L.H.d.A. Carcinoma de células escamosas em um cão com alopecia por diluição de cor. Veterinária e Zootecnia 2012, 507–512. [Google Scholar]
- Mecklenburg, L.; Linek, M.; Tobin, D.J. Hair loss disorders in domestic animals; John Wiley & Sons, 2009. [Google Scholar]
- Vandenabeele, S.; Declercq, J.; De Cock, H.; Daminet, S. Canine recurrent flank alopecia: a synthesis of theory and practice. Vlaams Diergeneeskundig Tijdschrift 2014, 83, 275–283. [Google Scholar] [CrossRef]
- Fontaine, J.; Beco, L.; Paradis, M. Alopécie récidivante des flancs: Étude de douze cas chez le griffon Korthals. Point vétérinaire 1998, 29, 445–449. [Google Scholar]
- Miller, M.; Dunstan, R. Seasonal flank alopecia in boxers and Airedale terriers: 24 cases (1985-1992). Journal of the American Veterinary Medical Association 1993, 203, 1567–1572. [Google Scholar] [PubMed]
- Verschuuren, M.; Schlotter, Y.M.; van Geijlswijk, I.M.; van der Lugt, J.J.; Gehring, R. The efficacy of subcutaneous slow-release melatonin implants in the prevention of canine flank alopecia recurrence is uncertain: A double-blind, randomized, placebo-controlled study. Vet Dermatol 2022, 33, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Paradis, M. An approach to symmetrical alopecia in the dog. In BSAVA Manual of Canine and Feline Dermatology; BSAVA Library, 2012; pp. 91–102. [Google Scholar]
- Paradis, M. 3.3. 8 Canine pattern alopecia. Hair Loss Disorders in Domestic Animals 2009, 164. [Google Scholar]
- Paradis, M. Melatonin in the treatment of canine pattern baldness. 1998. [Google Scholar]
- Bellini, M.; Caldini, E.; Scapinelli, M.; Simões, M.; Machado, D.; Nürmberg, R. Increased elastic microfibrils and thickening of fibroblastic nuclear lamina in canine cutaneous asthenia. Veterinary dermatology 2009, 20, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Freeman, L.; Hegreberg, G.; Robinette, J. Ehlers-Danlos syndrome in dogs and cats. In Proceedings of the Seminars in Veterinary Medicine and Surgery (Small Animal); 1987; pp. 221–227. [Google Scholar]
- Patterson, D.; Minor, R. Hereditary fragility and hyperextensibility of the skin of cats. A defect in collagen fibrillogenesis. Laboratory investigation; a journal of technical methods and pathology 1977, 37, 170–179. [Google Scholar]
- Fernandez, C.J.; Scott, D.W.; Erb, H.N.; Minor, R.R. Staining abnormalities of dermal collagen in cats with cutaneous asthenia or acquired skin fragility as demonstrated with Masson's trichrome stain. Veterinary Dermatology 1998, 9, 49–54. [Google Scholar] [CrossRef]
- Mueller, R.S.; Rosenkrantz, W.; Bensignor, E.; Karaś-Tęcza, J.; Paterson, T.; Shipstone, M.A. Diagnosis and treatment of demodicosis in dogs and cats: Clinical consensus guidelines of the World Association for Veterinary Dermatology. Veterinary dermatology 2020, 31, 4–e2. [Google Scholar] [CrossRef]
- Ferrer, L.; Ravera, I.; Silbermayr, K. Immunology and pathogenesis of canine demodicosis. Veterinary Dermatology 2014, 25, 427–e465. [Google Scholar] [CrossRef]
- Rahman, M.; Bostami, M.B.; Datta, A.; Sabuj, A.A.M.; Rana, E.A.; Mannan, A.; Hossain, M.M.A.; Chowdhury, M.Y.E. Estimation of the prevalence and determination of risk factors associated with demodicosis in dogs. Journal of advanced veterinary and animal research 2021, 8, 116. [Google Scholar] [CrossRef] [PubMed]
- Gazi, U.; Taylan-Ozkan, A.; Mumcuoglu, K.Y. Immune mechanisms in human and canine demodicosis: A review. Parasite immunology 2019, 41, e12673. [Google Scholar] [CrossRef] [PubMed]
- Mueller, R.S.; Meyer, D.; Bensignor, E.; Sauter-Louis, C. Treatment of canine generalized demodicosis with a ‘spot-on’formulation containing 10% moxidectin and 2.5% imidacloprid (Advocate®, Bayer Healthcare). Veterinary Dermatology 2009, 20, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, E.; Bettenay, S.; Nikolaeva, L.; Majzoub, M.; Mueller, R. Influence of systemic antibiotics on the treatment of dogs with generalized demodicosis. Veterinary Parasitology 2012, 188, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Wright, I. Case study: generalised demodicosis in a Chihuahua. Companion Animal 2014, 19, 342–344. [Google Scholar] [CrossRef]
- Barrientos, L.S.; Crespi, J.A.; Peral Garcia, P.; Castellano, M.C.; Giovambattista, G. Prevalence of canine juvenile generalized demodicosis in the Buenos Aires region, Argentina. 2013. [Google Scholar]
- It, V.; Barrientos, L.; López Gappa, J.; Posik, D.; Díaz, S.; Golijow, C.; Giovambattista, G. Association of canine juvenile generalized demodicosis with the dog leukocyte antigen system. Tissue Antigens 2010, 76, 67–70. [Google Scholar] [CrossRef]
- Holm, B.R. Efficacy of milbemycin oxime in the treatment of canine generalized demodicosis: a retrospective study of 99 dogs (1995–2000). Veterinary Dermatology 2003, 14, 189–195. [Google Scholar] [CrossRef]
- Plant, J.D.; Lund, E.M.; Yang, M. A case–control study of the risk factors for canine juvenile-onset generalized demodicosis in the USA. Veterinary Dermatology 2011, 22, 95–99. [Google Scholar] [CrossRef]
- Lemarie, S.; Hosgood, G.; Foil, C. A retrospective study of juvenile-and adult-onset generalized demodicosis in dogs (1986–91). Veterinary Dermatology 1996, 7, 3–10. [Google Scholar] [CrossRef]
- Day, M. An immunohistochemical study of the lesions of demodicosis in the dog. Journal of comparative pathology 1997, 116, 203–216. [Google Scholar] [CrossRef]
- Chen, C. A Short-tailed Demodectic Mite and Demodex canis Infestation in a Chihuahua Dog. Vet Dermatol 1995, 6, 227–229. [Google Scholar] [CrossRef] [PubMed]
- Saridomichelakis, M.N.; Farmaki, R.; Leontides, L.S.; Koutinas, A.F. Aetiology of canine otitis externa: a retrospective study of 100 cases. Veterinary dermatology 2007, 18, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Bowden, D.G.; Outerbridge, C.A.; Kissel, M.B.; Baron, J.N.; White, S.D. Canine demodicosis: a retrospective study of a veterinary hospital population in California, USA (2000–2016). Veterinary Dermatology 2018, 29, 19–e10. [Google Scholar] [CrossRef] [PubMed]
- Mueller, R.; Hastie, K.; Bettenay, S. Daily oral ivermectin for treatment of generalised demodicosis in 23 dogs. Australian Veterinary Practitioner 1999, 29, 132. [Google Scholar]
- Duangkaew, L.; Larsuprom, L.; Anukkul, P.; Lekcharoensuk, C.; Chen, C. A field trial in Thailand of the efficacy of oral fluralaner for the treatment of dogs with generalized demodicosis. Veterinary Dermatology 2018, 29, 208–e274. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; McConnell, C.; O’hara, K.; Chai, J.; Spadafori, G. Brachycephalic Breed Disease Prevalence Study. 2017. [Google Scholar]
- Hobi, S.; Cafarchia, C.; Romano, V.; Barrs, V.R. Malassezia: Zoonotic Implications, Parallels and Differences in Colonization and Disease in Humans and Animals. Journal of Fungi 2022, 8, 708. [Google Scholar] [CrossRef]
- Bajwa, J. Canine Malassezia dermatitis. Can Vet J 2017, 58, 1119–1121. [Google Scholar]
- Mauldin, E.A.; Scott, D.W.; Miller, W.H.; Smith, C.A. Malassezia dermatitis in the dog: a retrospective histopathological and immunopathological study of 86 cases (1990–95). Veterinary Dermatology 1997, 8, 191–202. [Google Scholar] [CrossRef]
- Bond, R.; Morris, D.O.; Guillot, J.; Bensignor, E.J.; Robson, D.; Mason, K.V.; Kano, R.; Hill, P.B. Biology, diagnosis and treatment of Malassezia dermatitis in dogs and cats Clinical Consensus Guidelines of the World Association for Veterinary Dermatology. Vet Dermatol 2020, 31, 28–74. [Google Scholar] [CrossRef]
- Nagata, M.; Rosenkrantz, W. Cutaneous viral dermatoses in dogs and cats. Compendium (Yardley, PA) 2013, 35, E1–E1. [Google Scholar]
- Luff, J.A.; Affolter, V.K.; Yeargan, B.; Moore, P.F. Detection of six novel papillomavirus sequences within canine pigmented plaques. Journal of Veterinary Diagnostic Investigation 2012, 24, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Nagata, M.; Nanko, H.; Moriyama, A.; Washizu, T.; Ishida, T. Pigmented plaques associated with papillomavirus infection in dogs: is this epidermodysplasia verruciformis? Veterinary Dermatology 1995, 6, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Narama, I.; Kobayashi, Y.; Yamagami, T.; Ozaki, K.; Ueda, Y. Pigmented cutaneous papillomatosis (pigmented epidermal nevus) in three pug dogs; histopathology, electron microscopy and analysis of viral DNA by the polymerase chain reaction. J Comp Pathol 2005, 132, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Munday, J.S.; Lam, A.T.; Sakai, M. Extensive progressive pigmented viral plaques in a Chihuahua dog. Veterinary Dermatology 2022, 33, 252–254. [Google Scholar] [CrossRef] [PubMed]
- Luff, J.; Rowland, P.; Mader, M.; Orr, C.; Yuan, H. Two canine papillomaviruses associated with metastatic squamous cell carcinoma in two related Basenji dogs. Veterinary pathology 2016, 53, 1160–1163. [Google Scholar] [CrossRef] [PubMed]
- Hansen, N.; Nicholas, N.; Pack, G.; Mackie, J.T.; Shipstone, M.; Munday, J.S.; Reddell, P.; Orbell, G.; Malik, R. Progressive cutaneous viral pigmented plaques in three Hungarian Vizslas and the response of lesions to topical tigilanol tiglate gel. Veterinary Medicine and Science 2018, 4, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Banovic, F.; Linder, K.; Olivry, T. Clinical, microscopic and microbial characterization of exfoliative superficial pyoderma-associated epidermal collarettes in dogs. Veterinary Dermatology 2017, 28, 107–e123. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, J. Canine superficial pyoderma and therapeutic considerations. The Canadian veterinary journal 2016, 57, 204. [Google Scholar]
- Hillier, A.; Lloyd, D.H.; Weese, J.S.; Blondeau, J.M.; Boothe, D.; Breitschwerdt, E.; Guardabassi, L.; Papich, M.G.; Rankin, S.; Turnidge, J.D. Guidelines for the diagnosis and antimicrobial therapy of canine superficial bacterial folliculitis (A ntimicrobial G uidelines W orking G roup of the I nternational S ociety for C ompanion A nimal I nfectious D iseases). Veterinary dermatology 2014, 25, 163–e143. [Google Scholar] [CrossRef]
- Holm, B.R.; Rest, J.R.; Seewald, W. A prospective study of the clinical findings, treatment and histopathology of 44 cases of pyotraumatic dermatitis. Veterinary Dermatology 2004, 15, 369–376. [Google Scholar] [CrossRef]
- Pedersen, N.C.; Pooch, A.S.; Liu, H. A genetic assessment of the English bulldog. Canine Genet Epidemiol 2016, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Conceição, L.G.; Acha, L.M.R.; Borges, A.S.; Assis, F.G.; Loures, F.H.; e Silva, F.F. Epidemiology, clinical signs, histopathology and molecular characterization of canine leproid granuloma: a retrospective study of cases from Brazil. Veterinary dermatology 2011, 22, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Malik, R.; Love, D.; Wigney, D.; Martin, P. Mycobacterial nodular granulomas affecting the subcutis and skin of dogs (canine leproid granuloma syndrome). Australian veterinary journal 1998, 76, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Biezus, G.; de Cristo, T.G.; Ikuta, C.Y.; Carniel, F.; Volpato, J.; de Souza Teixeira, M.B.; Neto, J.S.F.; Casagrande, R.A. Canine leproid granuloma (CLG) caused by mycobacterial species closely related to members of Mycobacterium simiae complex in a dog in Brazil. Topics in Companion Animal Medicine 2022, 50, 100672. [Google Scholar] [CrossRef] [PubMed]
- Malik, R.; Martin, P.; Wigney, D.; Swan, D.; Sattler, P.; Cibilic, D.; Allen, J.; Mitchell, D.; Chen, S.; Hughes, M. Treatment of canine leproid granuloma syndrome: preliminary findings in seven dogs. Australian Veterinary Journal 2001, 79, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.A. Canine IgA and IgA deficiency: Implications for immunization against respiratory pathogens. The Canadian Veterinary Journal 2019, 60, 1305. [Google Scholar]
- Olsson, M.; Tengvall, K.; Frankowiack, M.; Kierczak, M.; Bergvall, K.; Axelsson, E.; Tintle, L.; Marti, E.; Roosje, P.; Leeb, T. Genome-wide analyses suggest mechanisms involving early B-cell development in canine IgA deficiency. PloS one 2015, 10, e0133844. [Google Scholar]
- Day, M. Possible immunodeficiency in related rottweiler dogs. Journal of small animal practice 1999, 40, 561–568. [Google Scholar] [CrossRef]
- Lanevschi, A.; Daminet, S.; Niemeyer, G.P.; Lothrop Jr, C.D. Granulocyte Colony-Stimulating Factor Deficiency in a Rottweiler with Chronic Idiopathic Neutropenia. Journal of Veterinary Internal Medicine 1999, 13, 72–75. [Google Scholar]
- Rivas, A.L.; Tintle, L.; Argentieri, D.; Kimball, E.S.; Goodman, M.G.; Anderson, D.W.; Capetola, R.J.; Quimby, F.W. A primary immunodeficiency syndrome in Shar-Pei dogs. Clinical immunology and immunopathology 1995, 74, 243–251. [Google Scholar] [CrossRef]
- Outerbridge, C.A.; Jordan, T.J.M. Current Knowledge on Canine Atopic Dermatitis: Pathogenesis and Treatment. Adv Small Anim Care 2021, 2, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Mazrier, H.; Vogelnest, L.J.; Thomson, P.C.; Taylor, R.M.; Williamson, P. Canine atopic dermatitis: breed risk in Australia and evidence for a susceptible clade. Veterinary Dermatology 2016, 27, 167–e142. [Google Scholar] [CrossRef] [PubMed]
- Theerawatanasirikul, S.; Sailasuta, A.; Thanawongnuwech, R.; Suriyaphol, G. Alterations of keratins, involucrin and filaggrin gene expression in canine atopic dermatitis. Research in veterinary science 2012, 93, 1287–1292. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, K.; Linek, M.; Power, H.; Bettenay, S.; Zabel, S.; Rosychuk, R.; Mueller, R.S. Breed and site predispositions of dogs with atopic dermatitis: a comparison of five locations in three continents. Veterinary dermatology 2010, 21, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Picco, F.; Zini, E.; Nett, C.; Naegeli, C.; Bigler, B.; Rüfenacht, S.; Roosje, P.; Gutzwiller, M.E.; Wilhelm, S.; Pfister, J.; et al. A prospective study on canine atopic dermatitis and food-induced allergic dermatitis in Switzerland. Vet Dermatol 2008, 19, 150–155. [Google Scholar] [CrossRef]
- Počta, S.; Svoboda, M. Approach to the diagnostics of atopic dermatitis in dogs in conditions of clinical practice. Acta Veterinaria Brno 2007, 76, 461–468. [Google Scholar] [CrossRef]
- Nødtvedt, A.; Egenvall, A.; Bergval, K.; Hedhammar, Å. Incidence of and risk factors for atopic dermatitis in a Swedish population of insured dogs. Veterinary Record 2006, 159, 241–246. [Google Scholar] [CrossRef]
- Verlinden, A.; Hesta, M.; Millet, S.; Janssens, G. Food allergy in dogs and cats: a review. Critical reviews in food science and nutrition 2006, 46, 259–273. [Google Scholar] [CrossRef]
- Prélaud, P.; Guaguere, E.; Alhaidari, Z.; Faivre, N.; Heripret, D.; Gayerie, A. Reevaluation of diagnostic criteria of canine atopic dermatitis. Revue de Medecine Veterinaire (France). 1998. [Google Scholar]
- Harvey, R. Food allergy and dietary intolerance in dogs: a report of 25 cases. Journal of Small Animal Practice 1993, 34, 175–179. [Google Scholar] [CrossRef]
- O’Neill, D.G.; Darwent, E.C.; Church, D.B.; Brodbelt, D.C. Demography and health of Pugs under primary veterinary care in England. Canine Genetics and Epidemiology 2016, 3, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, T.J.; Marsella, R.; Rosenbaum, M.R.; Gonzales, A.J.; Fadok, V.A. Update on pathogenesis, diagnosis, and treatment of atopic dermatitis in dogs. Journal of the American Veterinary Medical Association 2019, 254, 1291–1300. [Google Scholar] [CrossRef]
- Jeandel, A.; Garosi, L. Gait abnormalities in brachycephalic breeds: should we be more concerned? The Veterinary Record 2018, 182, 164. [Google Scholar] [CrossRef]
- Nuttall, T. Chronic pododermatitis and interdigital furunculosis in dogs. Companion animal 2019, 24, 194–200. [Google Scholar] [CrossRef]
- Laffort-Dassot, C. Flea allergy in dogs: clinical signs and diagnosis. European Journal of Companion Animal Practice 2009, 19, 242–248. [Google Scholar]
- Zur, G.; Ihrke, P.J.; White, S.D.; Kass, P.H. Canine atopic dermatitis: a retrospective study of 266 cases examined at the University of California, Davis, 1992–1998. Part I. Clinical features and allergy testing results. Veterinary dermatology 2002, 13, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Griffin, C.; DeBoer, D. The ACVD task force on canine atopic dermatitis (XIV): clinical manifestations of canine atopic dermatitis. Veterinary immunology and immunopathology 2001, 81, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Favrot, C. Clinical signs and diagnosis of canine atopic dermatitis. 2015. [Google Scholar]
- Corbee, R.J.; Woldring, H.H.; van den Eijnde, L.M.; Wouters, E.G.H. A Cross-Sectional Study on Canine and Feline Anal Sac Disease. Animals (Basel) 2021, 12. [Google Scholar] [CrossRef]
- Mueller, R.S.; Olivry, T. Critically appraised topic on adverse food reactions of companion animals (6): prevalence of noncutaneous manifestations of adverse food reactions in dogs and cats. BMC Vet Res 2018, 14, 341. [Google Scholar] [CrossRef]
- Goodale, E. Pemphigus foliaceous. Can Vet J 2019, 60, 311–313. [Google Scholar]
- Bizikova, P.; Dean, G.A.; Hashimoto, T.; Olivry, T. Cloning and establishment of canine desmocollin-1 as a major autoantigen in canine pemphigus foliaceus. Veterinary immunology and immunopathology 2012, 149, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Olivry, T. A review of autoimmune skin diseases in domestic animals: I–superficial pemphigus. Veterinary Dermatology 2006, 17, 291–305. [Google Scholar] [CrossRef] [PubMed]
- Gonsalves-Hubers, T. Pemphigus erythematosus in a chow chow. The Canadian Veterinary Journal 2005, 46, 925. [Google Scholar] [PubMed]
- Kuhl, K.; Shofer, F.; Goldschmidt, M. Comparative histopathology of pemphigus foliaceus and superficial folliculitis in the dog. Veterinary pathology 1994, 31, 19–27. [Google Scholar] [CrossRef] [PubMed]
- White, A.; Hicks, K.; Bizikova, P.; Bailey, J.; Linder, K. Probable drug-triggered pemphigus foliaceus in a dog following administration of afoxolaner (NexGard). Veterinary Record Case Reports 2019, 7, e000735. [Google Scholar] [CrossRef]
- Zhou, Z.; Corner, S.; Petersen, A.; Rosser, E.; Noland, E.L. Clinical presentation, treatment and outcome in dogs with pemphigus foliaceus with and without vasculopathic lesions: an evaluation of 41 cases. Veterinary Dermatology 2021, 32, 503–e139. [Google Scholar] [CrossRef] [PubMed]
- Zarfoss, M.K.; Tusler, C.A.; Kass, P.H.; Montgomery, K.; Lim, C.C.; Mowat, F.; Thomasy, S.M. Clinical findings and outcomes for dogs with uveodermatologic syndrome. J Am Vet Med Assoc 2018, 252, 1263–1271. [Google Scholar] [CrossRef]
- Blackwood, S.E.; Barrie, K.P.; Plummer, C.E.; Taylor, D.; Nunnery, C.M.; Seltzer, J.D.; Ben-Shlomo, G.; Brooks, D.E. Uveodermatologic syndrome in a rat terrier. Journal of the American Animal Hospital Association 2011, 47, e56–e63. [Google Scholar] [CrossRef]
- Egbeto, I.A.; Garelli, C.J.; Piedra-Mora, C.; Wong, N.B.; David, C.N.; Robinson, N.A.; Richmond, J.M. Case Series: Gene Expression Analysis in Canine Vogt-Koyanagi-Harada/Uveodermatologic Syndrome and Vitiligo Reveals Conserved Immunopathogenesis Pathways Between Dog and Human Autoimmune Pigmentary Disorders. Front Immunol 2020, 11, 590558. [Google Scholar] [CrossRef]
- Tham, H.L.; Linder, K.E.; Olivry, T. Autoimmune diseases affecting skin melanocytes in dogs, cats and horses: vitiligo and the uveodermatological syndrome: a comprehensive review. BMC veterinary research 2019, 15, 1–17. [Google Scholar] [CrossRef]
- Oliveira, A.T.C.; de Oliveira, A.R.F.; Santiago, I.L.T.; de Lima, Y.B.S.; Ferreira, T.C. Clinical, diagnostic and therapeutic approach of uveodermatologic syndrome in dogs: a review. Revista Brasileira de Higiene e Sanidade Animal 2020, 14, 248–261. [Google Scholar] [CrossRef]
- Panich, R.; Scott, D.; Miller Jr, W. Canine cutaneous sterile pyogranuloma/granuloma syndrome: a retrospective analysis of 29 cases (1976 to 1988). The Journal of the American Animal Hospital Association (USA) 1991. [Google Scholar]
- Santoro, D.; Prisco, M.; Ciaramella, P. Cutaneous sterile granulomas/pyogranulomas, leishmaniasis and mycobacterial infections. Journal of Small Animal Practice 2008, 49, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Diaz, S. Canine Sterile Papular and Nodular Skin Diseases. Clinical Small Animal Internal Medicine 2020, 1441–1448. [Google Scholar]
- Moosavian, H.; Mashayekhi-Goyonlo, V.; Rajayee Mousavi, S.A. Long-term successful management of an idiopathic interstitial pyogranulomatous/granulomatous dermatitis and folliculitis by omega 3 fatty acid in a dog. Comparative Clinical Pathology 2021, 30, 335–339. [Google Scholar] [CrossRef]
- Weingart, C.; Kershaw, O.; Kohn, B.; Rohwedder, T. Life-threatening acute neutrophilic vasculitis in a Shar-Pei puppy. Tierarztliche Praxis. Ausgabe K, Kleintiere/heimtiere 2022, 50, 57–63. [Google Scholar]
- Malik, R.; Foster, S.; Martin, P.; Canfield, P.; Mason, K.; Bosward, K.; Gough, A.; Rippon, G. Acute febrile neutrophilic vasculitis of the skin of young Shar-Pei dogs. Australian veterinary journal 2002, 80, 200–206. [Google Scholar] [CrossRef]
- Tellier, L.A. Immune-mediated vasculitis in a shar-pei with swollen hock syndrome. Can Vet J 2001, 42, 137–139. [Google Scholar]
- Innerå, M. Cutaneous vasculitis in small animals. Vet Clin North Am Small Anim Pract 2013, 43, 113–134. [Google Scholar] [CrossRef]
- O'Neill, D.G.; Sahota, J.; Brodbelt, D.C.; Church, D.B.; Packer, R.M.A.; Pegram, C. Health of Pug dogs in the UK: disorder predispositions and protections. Canine Med Genet 2022, 9, 4. [Google Scholar] [CrossRef]
- O'Neill, D.G.; Hendricks, A.; Phillips, J.A.; Brodbelt, D.C.; Church, D.B.; Loeffler, A. Non-neoplastic anal sac disorders in UK dogs: Epidemiology and management aspects of a research-neglected syndrome. Vet Rec 2021, 189, e203. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; McConnell, C.; O’Hara, K.; Chai, J.; Spadafori, G. Nationwide’s brachycephalic breed disease prevalence study. March 2017. 2017. [Google Scholar]
- Corbee, R.J.; Woldring, H.H.; van den Eijnde, L.M.; Wouters, E.G. A Cross-Sectional Study on Canine and Feline Anal Sac Disease. Animals 2022, 12, 95. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, L.; Lee, K. Anal sac disease in dogs. In Practice 2015, 37, 435–444. [Google Scholar] [CrossRef]
- Lundberg, A.; Koch, S.N.; Torres, S.M.F. Local treatment for canine anal sacculitis: A retrospective study of 33 dogs. Vet Dermatol 2022, 33, 426–434. [Google Scholar] [CrossRef]
- Doerr, K.A.; Outerbridge, C.A.; White, S.D.; Kass, P.H.; Shiraki, R.; Lam, A.T.; Affolter, V.K. Calcinosis cutis in dogs: histopathological and clinical analysis of 46 cases. Veterinary Dermatology 2013, 24, 355–e379. [Google Scholar] [CrossRef]
- Tafti, A.; Hanna, P.; Bourque, A.C. Calcinosis circumscripta in the dog: a retrospective pathological study. Journal of Veterinary Medicine Series A 2005, 52, 13–17. [Google Scholar] [CrossRef]
- Scott, D.; Buerger, R. Idiopathic calcinosis circumscripta in the dog: a retrospective analysis of 130 cases. The Journal of the American Animal Hospital Association (USA) 1988. [Google Scholar]
- Barrios, N.; Gómez, M.; Mieres, M.; Vera, F.; Alvial, G. Spinal dermoid sinus in a Dachshund with vertebral and thoracic limb malformations. BMC Vet Res 2014, 10, 54. [Google Scholar] [CrossRef]
- Salmon Hillbertz, N.H.; Isaksson, M.; Karlsson, E.K.; Hellmén, E.; Pielberg, G.R.; Savolainen, P.; Wade, C.M.; Von Euler, H.; Gustafson, U.; Hedhammar, Å. Duplication of FGF3, FGF4, FGF19 and ORAOV1 causes hair ridge and predisposition to dermoid sinus in Ridgeback dogs. Nature genetics 2007, 39, 1318–1320. [Google Scholar] [CrossRef]
- Motta, L.; Skerritt, G.; Denk, D.; Leeming, G.; Saulnier, F. Dermoid sinus type IV associated with spina bifida in a young Victorian bulldog. Veterinary Record-English Edition 2012, 170, 127. [Google Scholar] [CrossRef]
- Ployart, S.; Doran, I.; Bomassi, E.; Bille, C.; Libermann, S. Myelomeningocoele and a dermoid sinus-like lesion in a French bulldog. Can Vet J 2013, 54, 1133–1136. [Google Scholar] [PubMed]
- Sturgeon, C. Nasal dermoid sinus cyst in a shih tzu. The Veterinary Record 2008, 163, 219. [Google Scholar] [CrossRef] [PubMed]
- Bornard, N.; Pin, D.; Carozzo, C. Bilateral parieto-occipital dermoid sinuses in a Rottweiler. Journal of Small Animal Practice 2007, 48, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Colón, J.A.; Maritato, K.C.; Mauterer, J.V. Dermoid sinus and bone defects of the fifth thoracic vertebrae in a shih-tzu. Journal of Small Animal Practice 2007, 48, 180–180. [Google Scholar] [CrossRef] [PubMed]
- Bowens, A.L.; Ducoté, J.M.; Early, P.J. What is your neurologic diagnosis? Journal of the American Veterinary Medical Association 2005, 227, 713–715. [Google Scholar] [CrossRef] [PubMed]
- Burrow, R. A nasal dermoid sinus in an English bull terrier. Journal of small animal practice 2004, 45, 572–574. [Google Scholar] [CrossRef] [PubMed]
- Fatone, G.; Brunetti, A.; Lamagna, F.; Potena, A. Dermoid sinus and spinal malformations in a Yorkshire terrier: Diagnosis and follow-up. Journal of Small Animal Practice 1995, 36, 178–180. [Google Scholar] [CrossRef]
- Booth, M. Atypical dermoid sinus in a chow chow dog: case report. Journal of the South African Veterinary Association 1998, 69, 102–104. [Google Scholar] [CrossRef]
- Selcer, E. Dermoid sinus in a shih tzu and a boxer. J Am An Hosp Assoc 1984, 20, 634–636. [Google Scholar]
- Scott, D.; Miller Jr, W.H. Idiopathic nasodigital hyperkeratosis in dogs: a retrospective analysis of 35 cases (1988–1998). Jpn J Vet Dermatol 2012, 18, 169–170. [Google Scholar] [CrossRef]
- Lee, F.F.; Bradley, C.W.; Cain, C.L.; White, S.D.; Outerbridge, C.A.; Murphy, L.A.; Mauldin, E.A. Localized parakeratotic hyperkeratosis in sixteen Boston terrier dogs. Veterinary dermatology 2016, 27, 384–e396. [Google Scholar] [CrossRef] [PubMed]
- Lachaume, P.; Hitte, C.; Jouquand, S.; Priat, C.; Galibert, F. Identification and analysis of the dog keratin 9 (KRT9) gene. Animal genetics 1998, 29, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.W.; Miller, W.H. Retrospective record review of canine postclipping hair follicle arrest. Vet Dermatol 2012, 23, 248–249. [Google Scholar] [CrossRef]
- Diamond, J.C.; Schick, R.O.; Savage, M.Y.; Fadok, V.A. A small scale study to evaluate the efficacy of microneedling in the presence or absence of platelet-rich plasma in the treatment of post-clipping alopecia in dogs. Vet Dermatol 2020, 31, 214–e245. [Google Scholar] [CrossRef]
| Affenpinscher |
|---|
|
Bulldog Breeds: Alapaha Blue Blood Bulldog; American Bulldog; British Bulldog; Bulldog; Dorset Olde Tyme Bulldogge; French Bulldog; Victorian Bulldog |
| Boxer; Bull Boxer; German Boxer |
| Brasileiro |
| Brussels Griffon; Griffon |
| Boston Terrier |
| Cavalier King Charles Spaniel |
| Chihuahua; Long-haired Chihuahua; short-Haired Chihuahua; Teacup Chihuahua |
| Chow Chow |
| Dogue de Bordeaux |
| English Toy Spaniel |
| Japanese Chin |
| Lhasa Apso |
| Mastiff Breeds: American Bandogge Mastiff; Bullmastiff; Cane Corso (Italian Mastiff); English Mastiff; Neapolitan Mastiff; Tibetan Mastiff |
| Pekingese |
| Pug |
| Shar Pei |
| Shi Tzu |
| Staffordshire Bull Terrier |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





