1. Introduction
Tuberculosis (TB) continues to remain a global public health problem. It is the second most common cause of death worldwide among infectious diseases, next only to COVID-19. According to the World Health Organization’s (WHO) Global Tuberculosis Report, an estimated 10.6 million people fell ill with TB worldwide in the year 2021 (an increase of 4.5% compared to 2020) and 1.6 million people died due to TB [
1]. This is the first time in many years that the estimated incidence of TB has shown an increase, primarily due to COVID-19 related disruptions to the health systems and services [
1]. For the same reason, the number of TB patients diagnosed and notified has decreased. This suggests that there is an increase in the number of tuberculosis cases which are undiagnosed and untreated, and as per the Global TB report, this accounted for 40% of all incident cases in 2021 [
1]. This has two important consequences: (i) increased deaths among undiagnosed/untreated cases and (ii) continued transmission of infection in the community, which may lead to increased numbers of active TB disease in future.
The situation in Kyrgyzstan, a lower middle-income country in Central Asia and considered a high priority TB country in the European region, is no different. Of the 8500 estimated incident cases of TB in 2021, only 4500 were detected and notified, indicating that about 46% of the incident cases were undiagnosed and untreated [
1]. One of the reasons for this gap is the suboptimal engagement of private health care providers in the management of TB. Till recently, TB was diagnosed and treated only in the public sector. This changed on 31
st December 2020, with an order of the Ministry of Health which permits the private health care providers to identify and refer presumptive tuberculosis patients to the public health facilities for TB diagnosis and treatment.
In the year 2021, a project titled, “Together Against TB” was launched and a model of engagement of private providers was implemented, with funding support from the STOP TB Partnership. Under this project, the private-for-profit health care providers in four regions of Kyrgyzstan and Bishkek city were mapped, trained and incentivized to screen and identify presumptive TB patients as per the diagnostic algorithm of the national TB programme, and refer them to the public health facilities for diagnosis and treatment.
This project provides an excellent opportunity to assess the cascade of care and quantify the gaps at each stage of the TB care cascade. Evidence from other countries indicate two major gaps: pre-diagnostic loss to follow-up (LFU), which indicates the gap between identification as ‘presumptive TB’ and getting tested for TB and pre-treatment LFU, which indicates the gap between diagnosis and treatment [
2]. While there are many studies reporting on pre-treatment loss to follow-up, studies on pre-diagnostic loss to follow-up are limited [
3]. Evidence from the private health sector is scarce [
4].
There is no previous published study on TB care cascade from Kyrgyzstan. Such evidence is useful for two reasons: (i) to identify gaps in the care cascade and take steps for improving the quality of care of TB patients; (ii) to optimize the implementation model of private-provider engagement and take decisions on nationwide scale-up. Hence, we decided to undertake this study. The aim of the study was to describe and quantify the gaps in the cascade of care among presumptive TB patients identified and referred by the private health care providers in Kyrgyzstan. Specific objectives were to determine among patients attending the private health facilities in four selected regions and Bishkek city of Kyrgyzstan, between February 2021 and March 2022: (i) the number of individuals screened for TB and among them, the number (proportion) identified as presumptive TB (ii) among presumptive TB patients, the number (proportion) of individuals tested for TB using sputum microscopy and/or Xpert MTB/RIF and the number (proportion) diagnosed as TB (iii) among diagnosed TB patients, the number (proportion) started on treatment (iv) treatment outcomes and (v) the demographic and clinical factors associated with pre-treatment loss to follow-up and unsuccessful treatment outcomes.
2. Materials and Methods
2.1. Study design
This was a cohort study involving analysis of secondary data collected by the project.
2.2. Study setting
The Kyrgyz Republic is a land locked, lower-middle-income country in Central Asia and has a population of 6.7 million [
5]. The country is divided into seven oblasts or regions (Chuy, Osh, Issyk-Kul, Naryn, Talas, Jalal-Abat, Batken) and two cities (Bishkek and Osh). The Gross Domestic Product per capita in Kyrgyzstan was 1,276 US dollars in 2021 [
6]. The terrain of Kyrgyzstan is dominated by the Tian Shan and Pamir mountains, which together occupy about 65% of national territory.
The private health sector is largely concentrated in the urban areas, especially the two large cities: Bishkek (~1 million population) and Osh (~250,000 population). Private health insurance is generally not prevalent in the country and the individuals have to pay out-of-pocket in order to access health care services from the private health care providers.
2.3. Specific setting
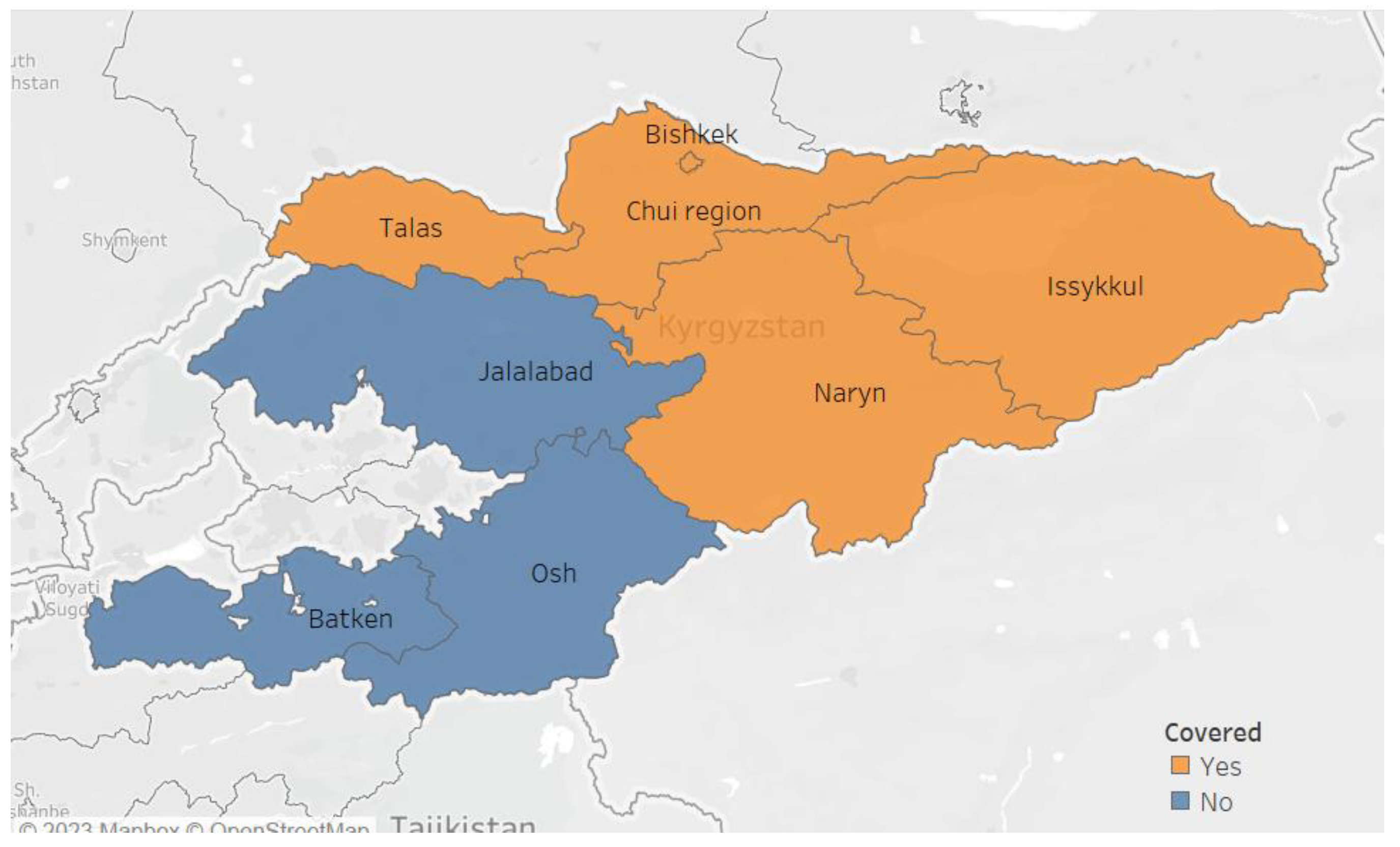
The “Together against TB’ project was implemented in four oblasts (Chuy, Issyk-Kul, Naryn, Talas) and the city of Bishkek (
Figure 1). These regions were selected based on convenience and feasibility of implementing the project. The project was led by AFEW (AIDS Foundation East-West, the principal recipient of the grant) in collaboration with KNCV-KG Tuberculosis Foundation. Before the launch of the project, the project team underwent a training conducted by STOP TB Partnership (the donor) on all the specifics of the project including an overview of the national TB program and requirements of recording, reporting, monitoring and evaluation of project. Within the project area, there were a total of 89 private health facilities, of which 55 (62%) were engaged in the project. A total of 83 providers (which included general physicians, pulmonologists and nurses) underwent a dedicated training for two days on national TB program guidelines and project activities. Providers who could not attend the training were visited by the project staff and sensitized on-the-job.
2.3.1. Screening and identification of presumptive TB patients
All patients who visited the private health facilities and consulted the general physician or pulmonologist of the clinic, were screened for symptoms suggestive of TB (cough of ≥ 2 weeks, fever, weight loss, night sweats, chest pain) regardless of the reason for their visit. People with cough were considered ‘presumptive TB’. People with symptoms other than cough were examined by a clinician and based on detailed medical history (such as contact history with a case of TB) and physical examination findings, a decision was made if the patient was a ‘presumptive TB’ or not. All patients considered ‘presumptive TB’ were requested to undergo chest radiography, if not already done. In addition, patients undergoing chest radiography for any reason and found to have shadows suggestive of TB were also considered ‘presumptive TB’ and investigated further.
2.3.2. Sputum collection and transport
Presumptive TB patients were educated to collect two sputum specimens (both collected early morning the next day) in sputum containers and submit it either to the private health facility or the public laboratory, as per the convenience of the patient. Sputum specimens submitted to the private health facility were transported by the nurse (human carrier) or couriered to the public laboratory for testing. Of the two specimens, one was tested using sputum microscopy and another was tested using Xpert MTB/RIF test. There were 10 GeneXpert laboratories and 47 microscopy laboratories in the project areas.
2.3.3. Diagnosis and treatment of TB
All patients positive for acid-fast bacilli on sputum microscopy or who were positive for TB by Xpert MTB/RIF were considered as ‘bacteriologically-confirmed’ tuberculosis cases. People who were not positive on microscopy or Xpert, but with shadows suggestive of TB on chest radiography and persistent symptoms after an unsuccessful course of broad spectrum antibiotics were considered ‘clinically-diagnosed’ tuberculosis cases. The decision of clinical diagnosis was made by a committee of TB doctors. People with symptoms and signs suggestive of extra pulmonary TB were investigated further and a diagnosis was made based on the results of investigations and clinical judgement. Patients found to have rifampicin resistance on Xpert MTB/RIF test were investigated further (using line probe assay and liquid culture and phenotypic drug susceptibility tests) for presence of resistance to other first-line and second-line drugs. These tests were available only at the reference laboratory at Bishkek city. This required collection of fresh sputum samples and transportation to the reference laboratory. Based on the results, the type of resistant TB (isoniazid mono resistant TB, poly resistant TB, multidrug resistant TB, extensively drug-resistant TB) was determined and the appropriate regimen was prescribed. Patients diagnosed with TB were started on treatment at a health facility nearest to their residence. All private health care providers received an incentive of $10 for sputum collection and transportation of each presumptive TB patient (or $5 if patients were identified and referred) and $10 per TB case detected. Diagnosis and treatment of TB was provided only at the public health facilities, free of cost to the patients.
2.3.4. Recording and reporting of the data
At each private health facility, two registers were maintained: (i) TB symptom screening register and (ii) Sputum collection register. These registers were maintained by the nurse, who received an incentive of $20 per month. There were field specialists (n=7) hired by the project to coordinate with the private clinics and visit the clinics once every month. They provided on-the-job training to private providers if needed, conducted data validation, compiled monthly reports and provided supportive supervision and monitoring. The monthly reports included aggregate numbers of people screened for symptoms and number with presumptive TB, number tested for TB using sputum microscopy and Xpert MTB/RIF and test results – all these disaggregated by gender. Once a patient was diagnosed as TB, the details were entered in an individual patient electronic database. This was maintained by the M&E specialist of the project. A WhatsApp group was established and all the project staff and the private providers were included in this group. One dedicated person was in-charge of the WhatsApp group and was responsible for addressing any queries and provide clarifications of the providers. A call center established, shared the test results to private providers via phone followed by transportation of paper based results by a courier.
2.4. Study population
All the patients who presented at private health care facilities for any reason and screened for TB in four selected regions (Chuy, Issyk-Kul, Naryn, Talas) and the city of Bishkek from February 2021 to March 2022 constituted the study population.
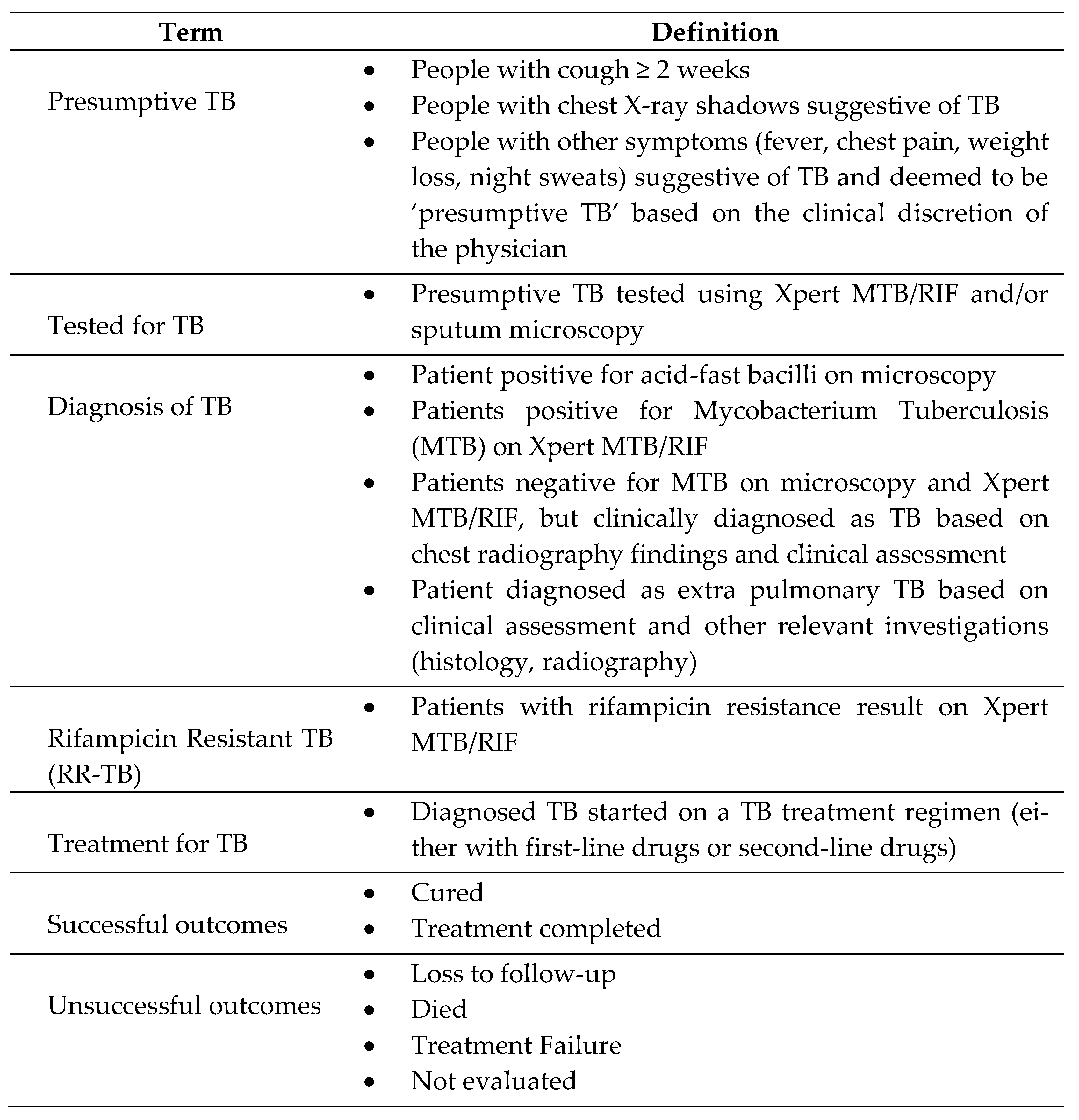
2.5. Operational definitions
2.6. Data collection, variables and sources of data
Data variables included aggregate numbers of patients screened, numbers identified as presumptive TB, numbers tested for TB, diagnosed and treated for TB. These were extracted from the monthly reports submitted by the private health facilities. For all diagnosed TB patients, we extracted patient-wise data on socio-demographic, clinical and risk factors from the electronic patient database managed by National Phthisiology Centre.
2.7. Data entry and analysis
The aggregate data were summarized as frequencies and percentages and the care cascade was presented as a flowchart. Individual patient-wise data downloaded in MS Excel was cleaned and exported to EpiData Analysis (version 2.2.2.187, EpiData Association, Odense, Denmark) for further analysis. Continuous variables were summarized using means or medians (along with standard deviation/interquartile range) as appropriate. Categorical variables were summarized using frequencies (and percentages). To determine the demographic and clinical factors associated with pre-treatment loss to follow-up and unsuccessful treatment outcomes, we conducted chi-square tests and calculated risk ratios and 95% confidence intervals as measures of association. We also calculated the delays in testing, diagnosis and treatment and expressed as median (interquartile range). A p value of <0.05 was considered statistically significant. Since the overall numbers and the key events of interest (for example, unsuccessful outcomes and pre-treatment loss to follow-up) were small across several sub-groups, we considered it prudent not to conduct a multivariable analysis.
3. Results
3.1. Cascade of care
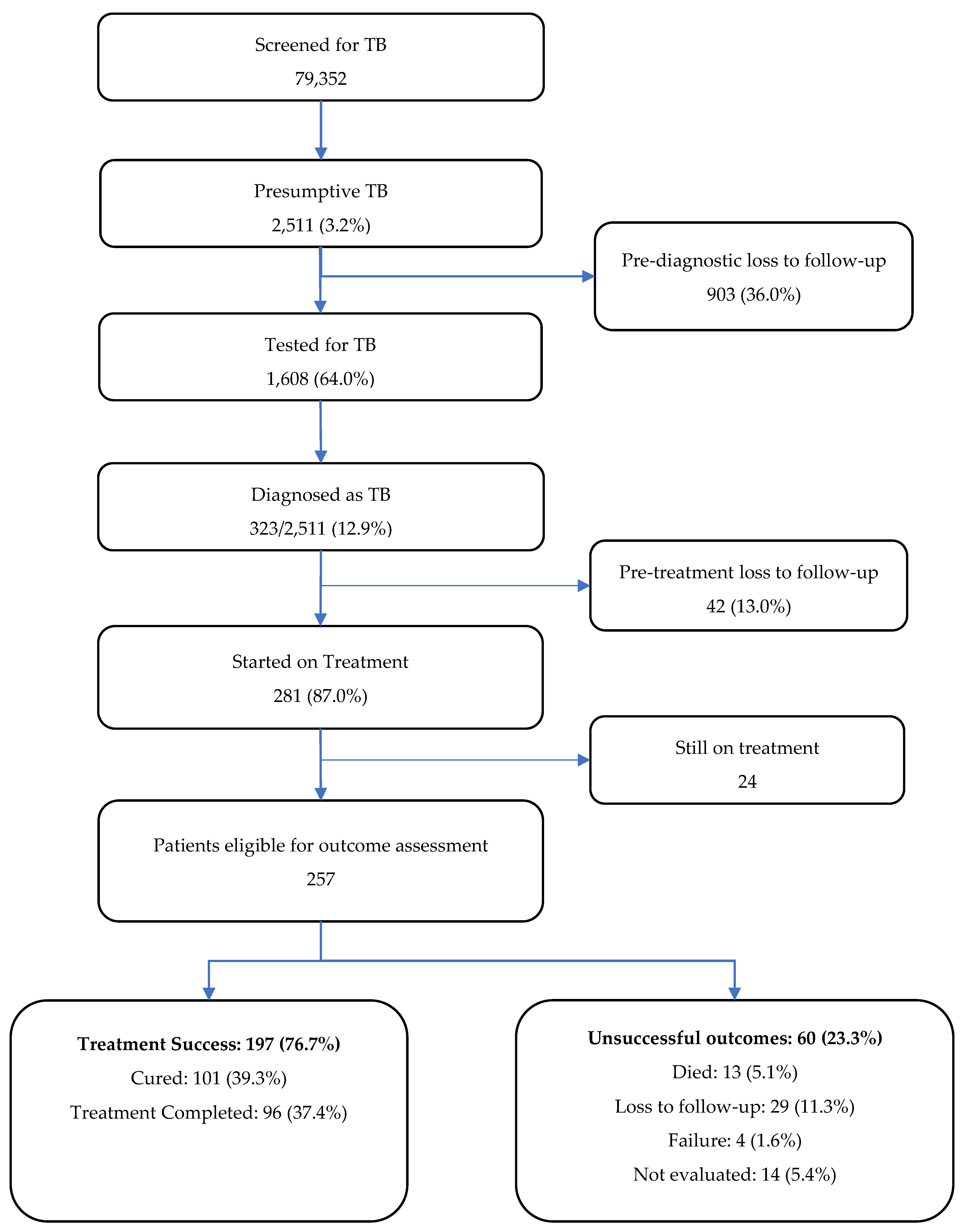
The cascade of care is depicted in
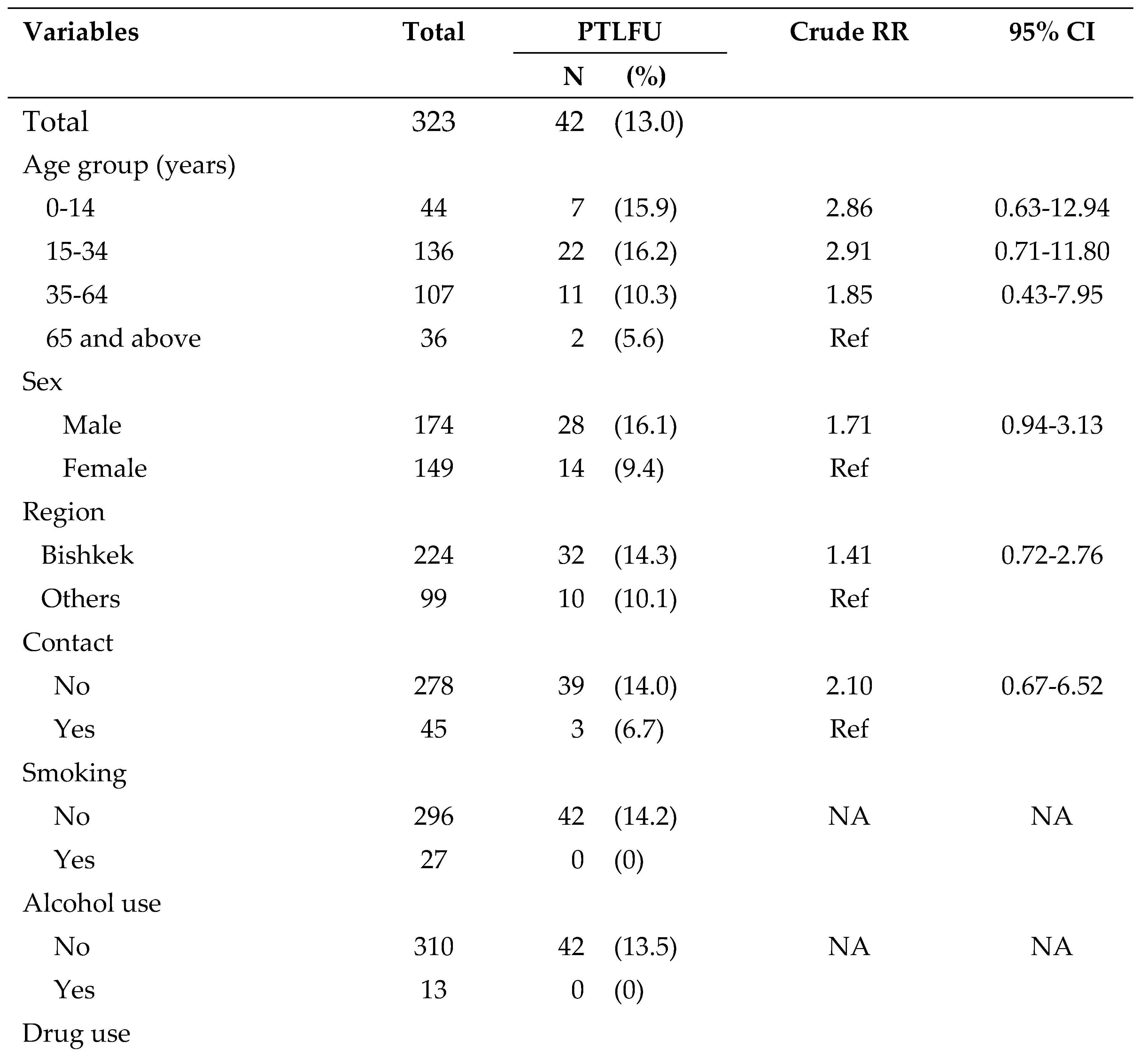
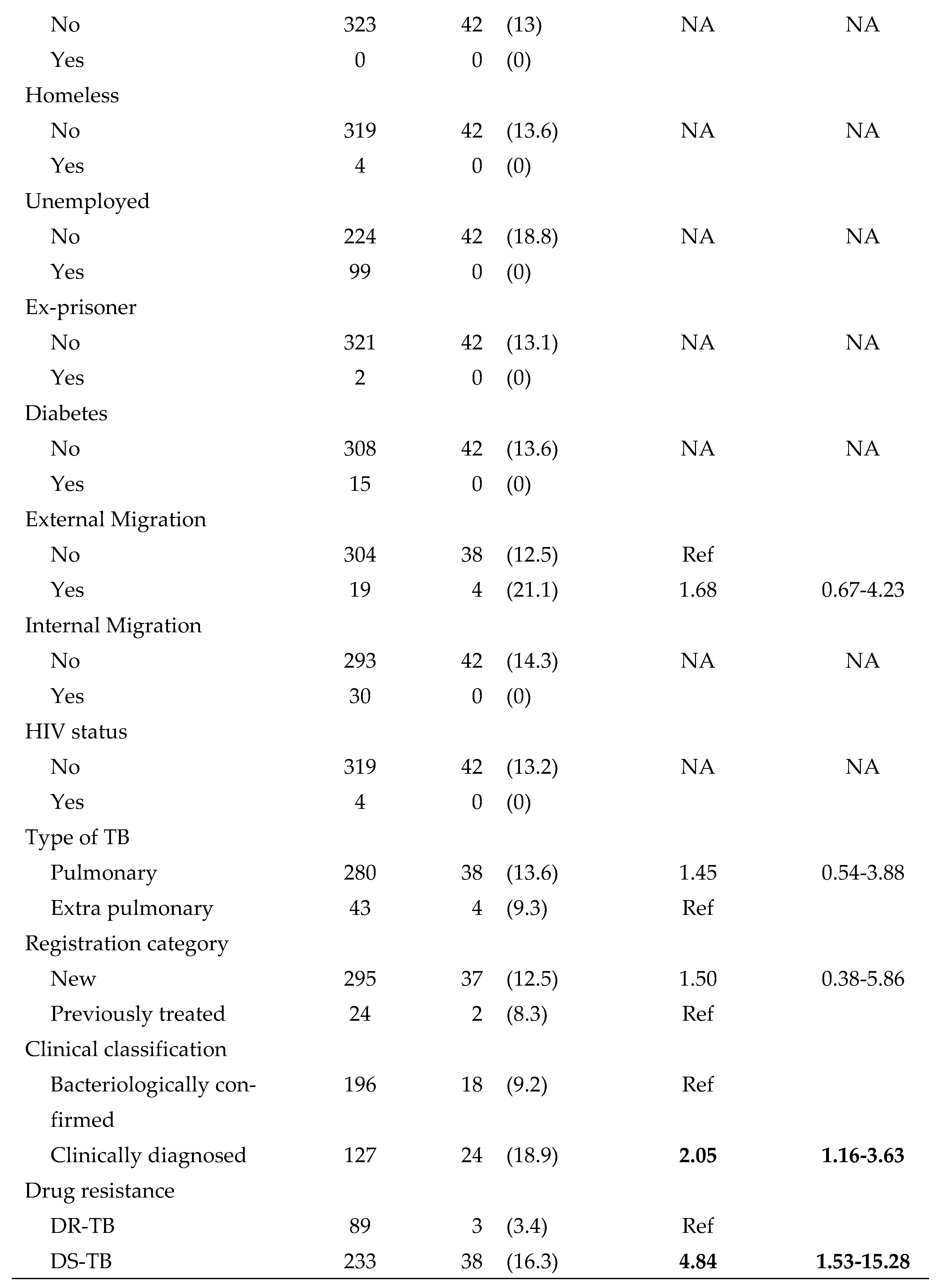
Figure 2. A total of 79,352 patients were screened for TB during the study period and among them, 2,511 (3%) had presumptive TB. Of the latter, 1608 (64%) were tested for TB using sputum microscopy and/or Xpert MTB/RIF assay. The remaining 903 (36%) presumptive TB patients who were not tested for TB were considered as ‘pre-diagnostic loss to follow-up’. A total of 323 (13%) patients were diagnosed as TB and among them, 281 (87%) were started on treatment. The remaining 42 (13%) were considered ‘pre-treatment loss to follow-up’.
At the time of data collection, 24 patients were still on treatment. Among 257 patients eligible for outcome assessment, 197 patients (77%) had treatment success (39% cured and 38% completed treatment). Out of 60 patients (23%) with unsuccessful outcomes, 29 (11%) were lost to follow-up, 14 (5%) were not evaluated, 13 (5%) died and 4 patients (2%) had treatment failure.
3.2. Demographic, risk and clinical characteristics of TB patients
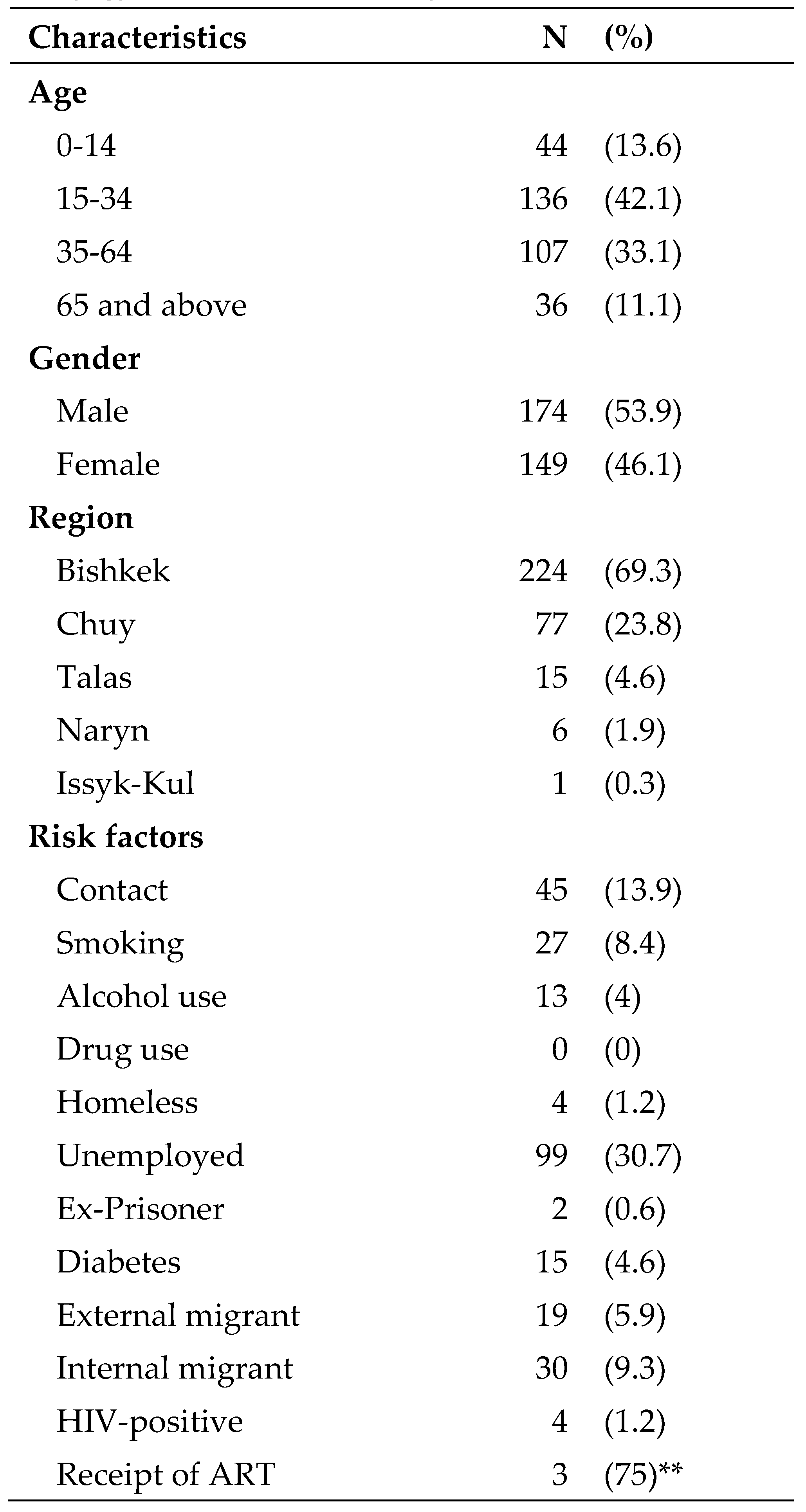
Demographic characteristics and risk factor profile of TB patients are shown in
Table 1.
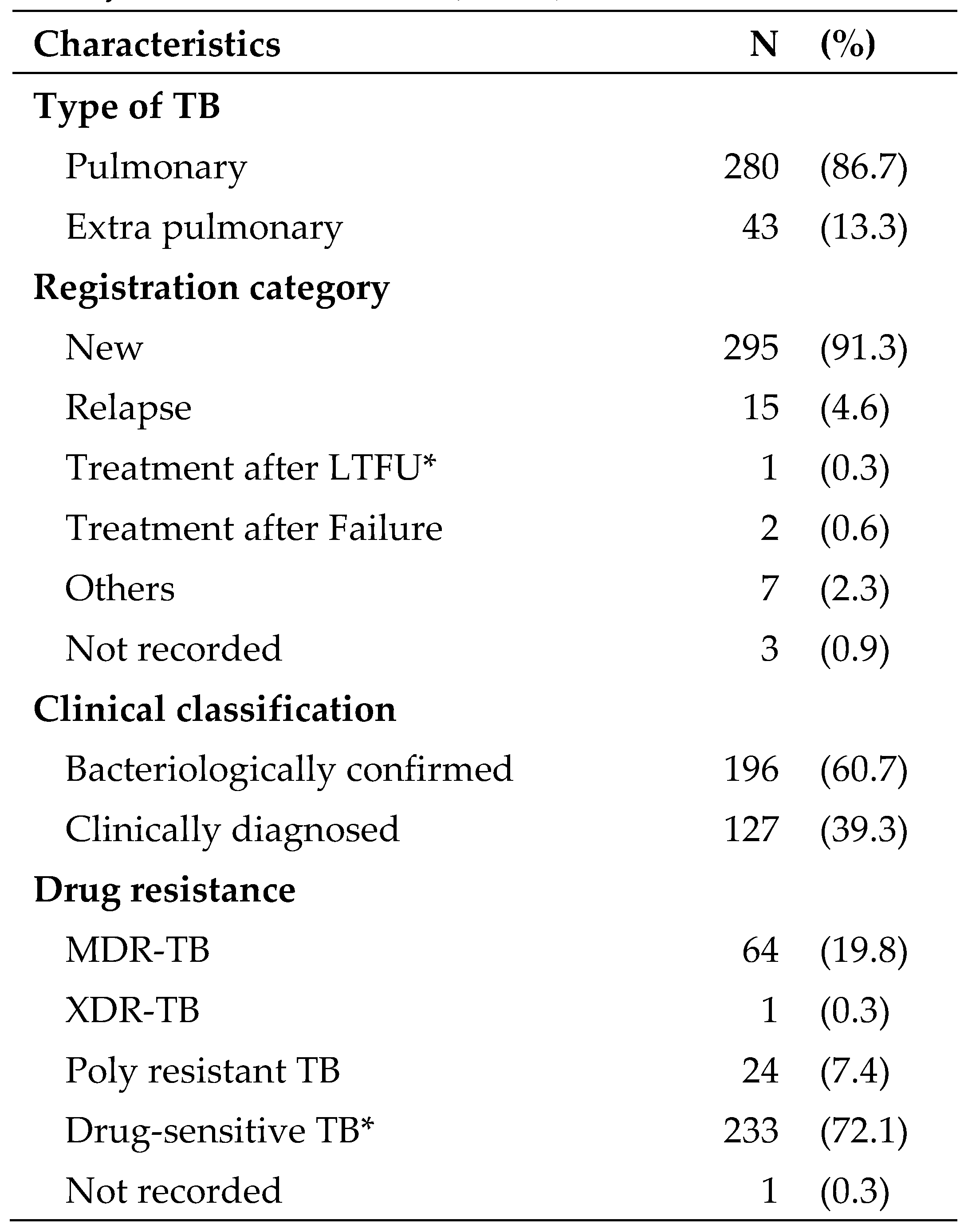
About 54% of patients were males and the mean (SD) age of the patients was 35 (19) years. Majority (69%) of study participants lived in Bishkek city. As for the risk factors, 31% were unemployed, 14% had a history of contact with TB, 9% were internal migrants, 8% were smokers, 6% were external migrants, 5% had known diabetes, 4% used alcohol, 1.2% were homeless, and 0.6% were ex-prisoners. Four patients were HIV-positive and among them, 3 (75%) were started on ART. The clinical characteristics of TB patients are presented in
Table 2.
Most (87%) patients had pulmonary TB and nearly 91% were new cases. About 61% of patients were bacteriologically-confirmed. Majority (72%) of the patients had drug-sensitive TB followed by MDR-TB (20%) and poly-resistant TB (7%). One patient had XDR-TB.
3.3. Delays in testing, diagnosis and initiation of treatment
Table 3 depicts the delays involved at different stages of cascade of care. The proportion of patients with valid dates for the different stages ranged between 65% and 96%. The median duration between screening and registration of TB among those with valid dates was 7 days.
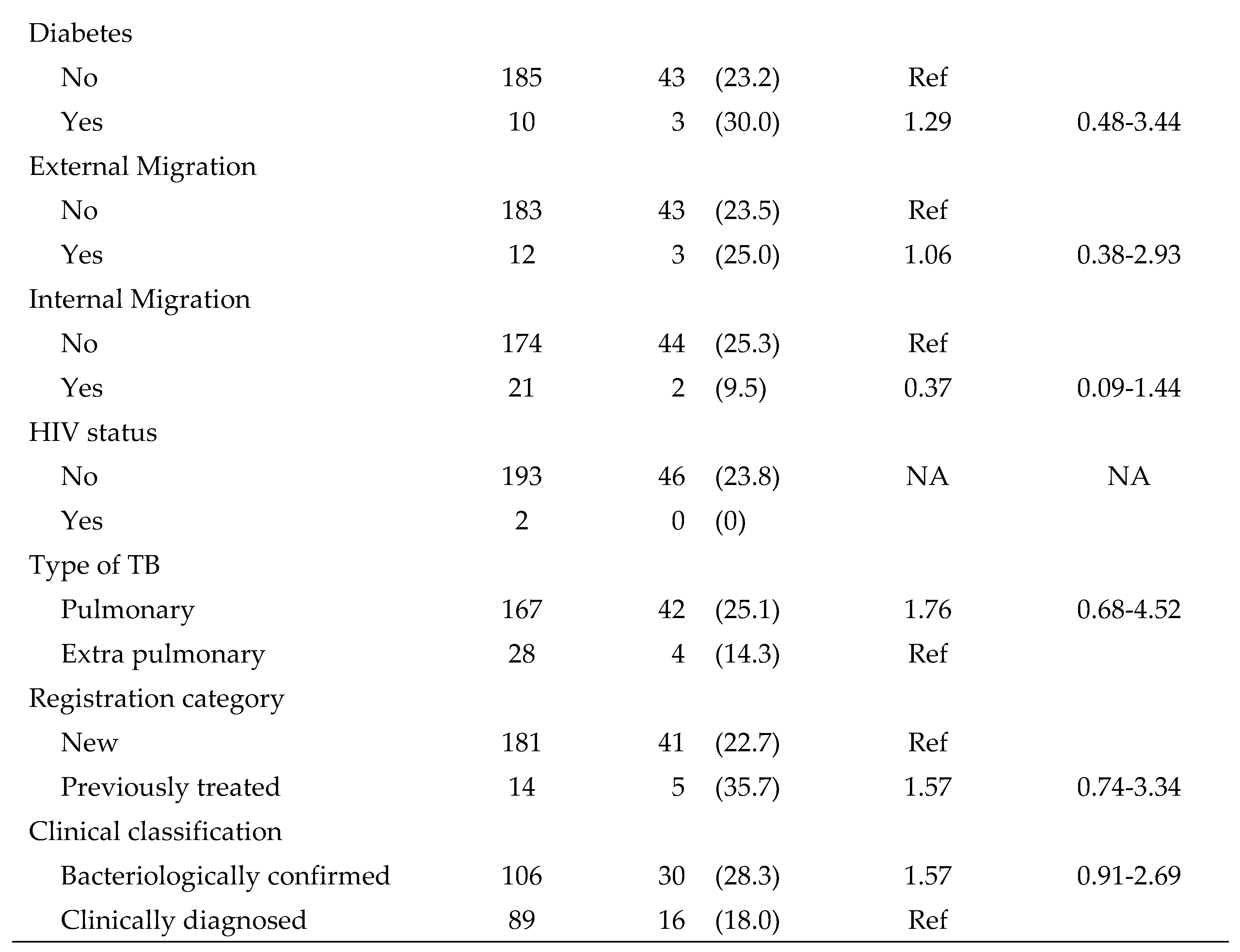
3.4. Factors associated with pre-treatment loss to follow-up
Factors associated with pre-treatment loss to follow-up among diagnosed TB patients are shown in
Table 4. The only statistically significant variables associated with pre-treatment loss to follow-up were clinically diagnosed TB (RR=2.05; 95% CI: 1.16-3.63) and drug sensitive TB (RR=4.84; 95% CI: 1.53-15.28). None of the other socio-demographic characteristics or risk factors were statistically significant.
3.5. Factors associated with unsuccessful treatment outcomes
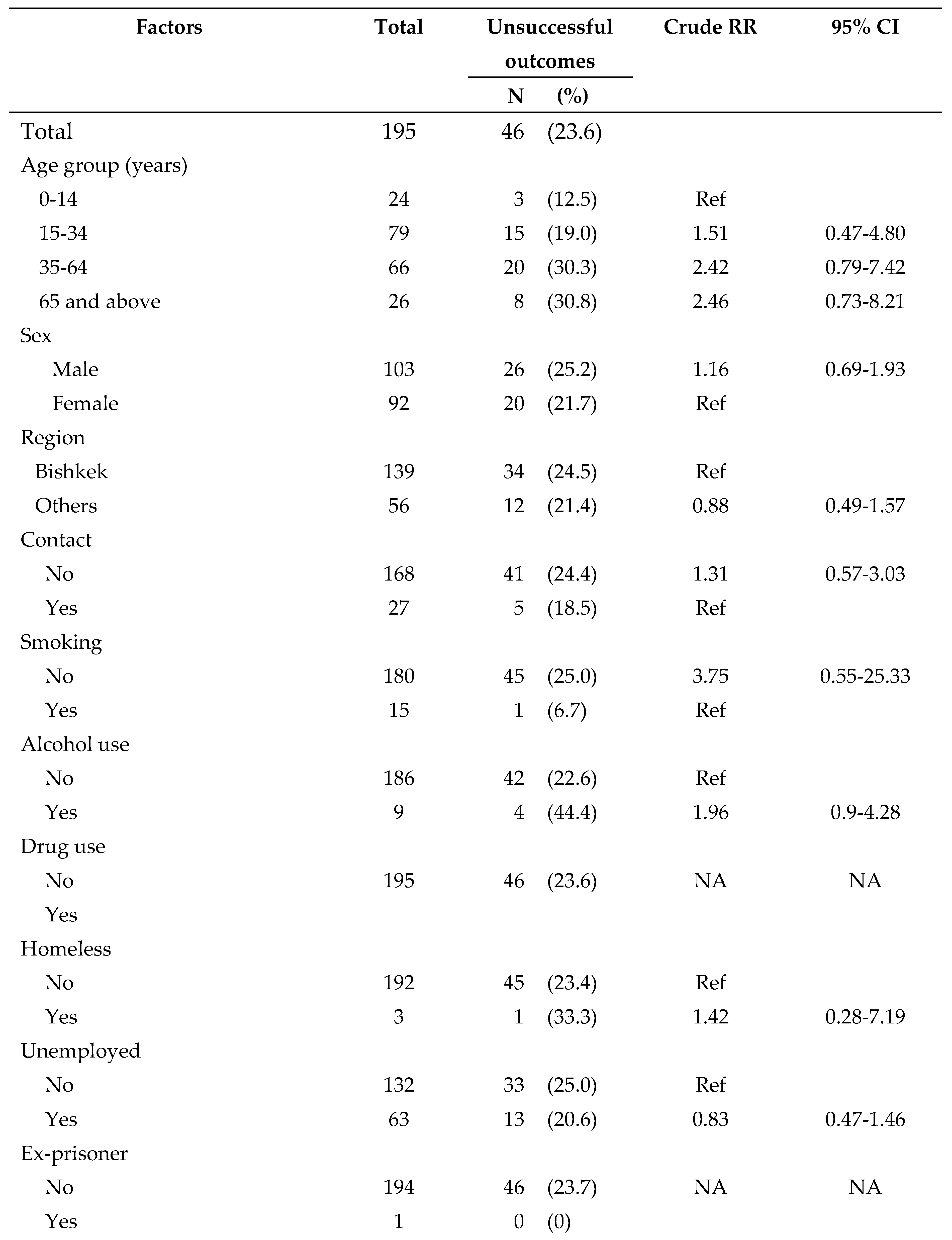
Given the small numbers of drug resistant TB patients, we analyzed factors associated with unsuccessful treatment outcomes only among drug-sensitive TB patients and these are presented in
Table 5. None of the variables showed a statistically significant association.
4. Discussion
This is the first study from Kyrgyzstan describing the cascade of care of tuberculosis patients identified in the private health sector. This adds to the limited global evidence on the TB care cascade from the private sector. Traditionally, private health sector has not been involved in TB care in The Kyrgyz Republic. One of the big achievements of this project has been to garner political commitment to create a legal and regulatory framework for engaging the private health sector. There were three key findings and we discuss them below.
First, pre-diagnostic LFU was observed in 36% of the presumptive TB patients. This is relatively higher, when compared to findings from other settings. A study from South Africa reported a pre-diagnostic LFU of 18%, although it is to be noted that this study was conducted in government health facilities [
7,
8]. Another study from Zimbabwe reported an overall pre-diagnostic LFU of 25%, with significantly higher LFU rates observed in patients attending private-for-profit (36%) health facilities, local self-government run council hospitals (35%) and church-run mission hospitals (25%), compared to government health facilities (14%) run by the Ministry of Health [
4]. In our study, we did not have individual patient-wise data on all presumptive patients and hence were unable to assess the factors associated with pre-diagnostic LFU. The Zimbabwe study reported that pre-diagnostic LFU was higher among HIV-infected presumptive TB patients and those residing in rural areas (as compared to urban areas) – reflecting poorer access to diagnostic health facilities and tools, and inefficient specimen collection and transport mechanisms.
Second, pre-treatment LFU was observed in 13% of patients. Pre-treatment LFU is relatively better studied compared to pre-diagnostic LFU. Studies across the globe report that pre-treatment LFU among TB patients varied from 4% to 38% and was more common in settings from Africa (18%, 95% CI: 13-22) compared to Asia (13%, 95% CI: 10-15) [
3]. Most of these studies were conducted in public health sector. There is very limited evidence from the private-for-profit health sector on this issue, barring a study from Pakistan which reported an alarming pre-treatment LFU of 64% among smear-positive tuberculosis patients [
9]. We found that pre-treatment LFU was significantly lower in drug-resistant TB patients compared to drug-sensitive TB patients, possibly reflecting higher priority accorded to drug resistant TB patients by the NTP and better tracking and follow-up. We also found that pre-treatment LFU was higher in clinically diagnosed TB patients compared to bacteriologically-confirmed TB patients. While we did not study the reasons for pre-treatment LFU, we speculate that this might indicate low risk perception among the clinically diagnosed TB patients, especially those with minimal or no symptoms and non-specific, TB-suggestive shadows on chest radiography without any bacteriological confirmation. Traditionally, such patients have received lower priority from the NTPs, given the limited role they play in community transmission. This needs further investigation.
Third, treatment outcomes were poor and nearly one-fourth of the patients started on treatment had an unsuccessful outcome. This is relatively higher compared to the treatment outcomes of patients identified, diagnosed and treated in public health sector of The Kyrgyz Republic. LFU accounted for about half of all unsuccessful outcomes followed by ‘not evaluated’ which accounted for another 25% of unsuccessful outcomes – both indicating suboptimal mechanisms for patient tracking and follow-up and poor supervision and monitoring. We did not find any demographic and clinical factors associated with unsuccessful outcomes.
Our study had some strengths. First, this study addressed a national research priority. Second, we included all the patients cared for as part of the project without any sampling and covered four regions and the city of Bishkek city (with a large private sector). Thus, the findings reflect the ground realities. Third, we conducted and reported the study in line with STROBE (strengthening the reporting of observational studies in epidemiology) guidelines [
10].
We had some limitations too. There were missing data and inconsistencies, especially with regards to variables such as dates of screening, diagnosis and treatment. The data on risk factors were self-reported by the patients. Given the lack of standard definitions of the risk factors (such as alcohol use and smoking) in the national guidelines, ‘misclassification’ of exposure status cannot be ruled out. This may partly explain the lack of associations of risk factors with unsuccessful outcomes in our analyses. The other reason for lack of statistically significant associations was low sample size. Many exposure subgroups had zero outcome events, which precluded us from conducting detailed and robust multivariable analyses. We defined pre-diagnostic LFU based on testing with sputum microscopy and or Xpert MTB/RIF assay. It is possible that many presumptive TB patients would have undergone testing using other tests (such as radiography, histopathology, ultrasound and so on) and we did not have any information on these – this might have marginally overestimated the rates of pre-diagnostic LFU.
Despite these limitations, there were many policy and practice implications. First, donor-funded private sector engagement projects, such as the one described in this study, are challenging to sustain in the long term. Given the crucial role of private sector engagement in the efforts to End TB, the NTP needs to take ownership of this initiative by including it in the national strategic plan with dedicated budgets, activities and plans to monitor the progress. Currently, there is no field in the electronic database to capture if the patient diagnosed was referred from the private sector or not. Having such a field will enable calculation of an indicator, “proportion of TB patients referred by the private sector” on an ongoing basis. This is crucial for monitoring the engagement of private providers in TB care, going forward.
Second, the recording system needs to be strengthened. The definitions of risk factors need to be standardized and communicated to the health providers involved in the care and documentation of TB patients. Measures to monitor the patient database for missing data and validation need to be put in place.
Third, qualitative research needs to be undertaken to understand the root causes of LFU at every stage (pre-diagnostic, pre-treatment and during treatment), from the perspective of both patients and providers. The results should guide future programmatic interventions. Possible reasons for pre-diagnostic and pre-treatment LFU reported in the published literature include (i) deficiencies in documentation (of addresses and phone numbers of patients) in the presumptive TB and laboratory registers, (ii) poor sputum collection and transport mechanisms, (iii) poor access to diagnostic tools and facilities, (iv) lack of awareness among patients about their TB diagnosis, (v) a lack of risk perception about the consequences of not treating TB, (vi) death in severely ill patients before starting treatment, (vii) long distances from the health facility, (viii) transport costs and (ix) dissatisfaction with the health services [
11,
12,
13,
14,
15,
16]. A simple programmatic intervention could be to ensure recording the addresses and phone numbers of the patients in the registers and using them to track and follow-up.
5. Conclusions
In conclusion, we found that there were many gaps in the cascade of care of presumptive TB patients identified and referred by the private healthcare providers in The Kyrgyz Republic. There were losses at each step of the cascade which included pre-diagnostic LFU, pre-treatment LFU and LFU during the treatment. Further qualitative enquiry is urgently needed to understand the reasons for these gaps and instituting corrective measures.
Author Contributions
Conceptualization, D.M., A.D., A.M., A.K., N.S., and A.M.V.K.; methodology, D.M. and A.D.; validation, D.M., A.M. and A.M.V.K.; formal analysis, D.M., A.M. and A.M.V.K.; writing—original draft preparation, D.M., A.M., and A.M.V.K.; writing—review and editing, A.M.V.K.; All authors have read and agreed to the published version of the manuscript.
Funding
This research paper was produced as a result of the SORT IT program, which was funded by USAID and supported by TDR and implementing partners. TDR is able to conduct its work thanks to the commitment and support from a variety of funders. A full list of TDR donors is available at:
https://tdr.who.int/about-us/our-donors.
Institutional Review Board Statement
Permission to use the programme data was obtained from the programme manager of the National Tuberculosis Programme in The Kyrgyz Republic. Ethics approval was received from the Ethical Committee of the Research and Production Association "Preventive Medicine" under the Ministry of Health of the Kyrgyz Republic (No. 5/2023, dated 11.04.2023) and the Ethics Advisory Group of the International Union against Tuberculosis and Lung Disease, Paris, France (No. 11/2022, dated 26.01.2023).
Informed Consent Statement
Since the study involved review of existing records without direct interaction with individual patients, the need for informed consent was waived by the ethics committees.
Data Availability Statement
Data available on request from the corresponding author of the study.
Acknowledgments
This research was conducted through the Structured Operational Research and Training Initiative (SORT IT), a global partnership coordinated by TDR, the Special Programme for Research and Training in Tropical Diseases at the World Health Organization (WHO). The specific SORT IT program that led to this publication included a partnership of TDR the European Tuberculosis Research Initiative (ERI-TB) at the WHO Regional Office for Europe the WHO Country office in The Kyrgyz Republic and the National TB Control programme of The Kyrgyz Republic. The SORT IT programme was implemented along with the National TB Control programme of The Kyrgyz Republic, National Center of Phthisiology, Kyrgyz Republic; Tuberculosis Research and Prevention Center Non-Governmental Organization, Armenia; The International Union Against Tuberculosis and Lung Diseases, Paris and South East Asia offices; Turpanjian School of Public Health; Institute of Public Health, United Arab Emirates University (UAEU); ICF Alliance for Public Health, Kyiv, Ukraine; Damien Foundation, Belgium; University of Chester, United Kingdom; All India Institute of Medical Sciences, Nagpur, India.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Open Access Statement and Disclaimer
In accordance with WHO’s open-access publication policy for all work funded by WHO or authored/co-authored by WHO staff members, WHO retains the copyright of this publication through a Creative Commons Attribution IGO license (http:// creativecommons.org/licenses/by/3.0/igo/legalcode (last accessed on 23 February 2022)) which permits unrestricted use, distribution and reproduction in any medium provided the original work is properly cited. There should be no suggestion that WHO endorses any specific organization, products, or services. The views expressed in this article are those of the authors and do not necessarily reflect those of their affiliated institutions. The use of the WHO logo is not permitted. This notice should be preserved along with the article’s original URL.
References
- World Health Organization Global Tuberculosis Report 2022. Available online: https://www.who.int/teams/global-tuberculosis-programme/data (accessed on 8 May 2023).
- Subbaraman, R.; Nathavitharana, R.R.; Satyanarayana, S.; Pai, M.; Thomas, B.E.; Chadha, V.K.; Rade, K.; Swaminathan, S.; Mayer, K.H. The Tuberculosis Cascade of Care in India’s Public Sector: A Systematic Review and Meta-Analysis. PLoS Med 2016, 13, e1002149. [Google Scholar] [CrossRef] [PubMed]
- MacPherson, P.; Houben, R.M.; Glynn, J.R.; Corbett, E.L.; Kranzer, K. Pre-Treatment Loss to Follow-up in Tuberculosis Patients in Low- and Lower-Middle-Income Countries and High-Burden Countries: A Systematic Review and Meta-Analysis. Bull World Health Organ 2014, 92, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Padingani, M.; Kumar, A.; Tripathy, J.P.; Masuka, N.; Khumalo, S. Does Pre-Diagnostic Loss to Follow-up among Presumptive TB Patients Differ by Type of Health Facility? An Operational Research from Hwange, Zimbabwe in 2017. Pan Afr Med J 2018, 31, 196. [Google Scholar] [CrossRef] [PubMed]
- World Bank The World Bank in the Kyrgyz Republic. Available online: https://www.worldbank.org/en/country/kyrgyzrepublic/overview (accessed on 8 May 2023).
- Trading economics Kyrgyzstan GDP per Capita. Available online: https://tradingeconomics.com/kyrgyzstan/gdp-per-capita (accessed on 8 May 2023).
- Naidoo, P.; Theron, G.; Rangaka, M.X.; Chihota, V.N.; Vaughan, L.; Brey, Z.O.; Pillay, Y. The South African Tuberculosis Care Cascade: Estimated Losses and Methodological Challenges. J Infect Dis 2017, 216, S702–S713. [Google Scholar] [CrossRef] [PubMed]
- Botha, E.; den Boon, S.; Lawrence, K.-A.; Reuter, H.; Verver, S.; Lombard, C.J.; Dye, C.; Enarson, D.A.; Beyers, N. From Suspect to Patient: Tuberculosis Diagnosis and Treatment Initiation in Health Facilities in South Africa. Int J Tuberc Lung Dis 2008, 12, 936–941. [Google Scholar] [PubMed]
- Khan, B.J.; Kumar, A.M. V.; Stewart, A.; Khan, N.M.; Selvaraj, K.; Fatima, R.; Samad, Z. Alarming Rates of Attrition among Tuberculosis Patients in Public-Private Facilities in Lahore, Pakistan. Public Health Action 2017, 7, 127–133. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Int J Surg 2014, 12, 1495–1499. [CrossRef] [PubMed]
- Gopi, P.G.; Chandrasekaran, V.; Subramani, R.; Narayanan, P.R. Failure to Initiate Treatment for Tuberculosis Patients Diagnosed in a Community Survey and at Health Facilities under a DOTS Programme in a District of South India. Indian Journal of Tuberculosis5 2005, 52, 153–156. [Google Scholar]
- Thomas, B.E.; Suresh, C.; Lavanya, J.; Lindsley, M.M.; Galivanche, A.T.; Sellappan, S.; Ovung, S.; Aravind, A.; Lincy, S.; Raja, A.L.; et al. Understanding Pretreatment Loss to Follow-up of Tuberculosis Patients: An Explanatory Qualitative Study in Chennai, India. BMJ Glob Health 2020, 5, e001974. [Google Scholar] [CrossRef] [PubMed]
- Squire, S.B.; Belaye, A.K.; Kashoti, A.; Salaniponi, F.M.L.; Mundy, C.J.F.; Theobald, S.; Kemp, J. “Lost” Smear-Positive Pulmonary Tuberculosis Cases: Where Are They and Why Did We Lose Them? Int J Tuberc Lung Dis 2005, 9, 25–31. [Google Scholar] [PubMed]
- Buu, T.N.; Lönnroth, K.; Quy, H.T. Initial Defaulting in the National Tuberculosis Programme in Ho Chi Minh City, Vietnam: A Survey of Extent, Reasons and Alternative Actions Taken Following Default. Int J Tuberc Lung Dis 2003, 7, 735–741. [Google Scholar] [PubMed]
- Botha, E.; Den Boon, S.; Verver, S.; Dunbar, R.; Lawrence, K.-A.; Bosman, M.; Enarson, D.A.; Toms, I.; Beyers, N. Initial Default from Tuberculosis Treatment: How Often Does It Happen and What Are the Reasons? Int J Tuberc Lung Dis 2008, 12, 820–823. [Google Scholar] [PubMed]
- Sai Babu, B.; Satyanarayana, A.V. V; Venkateshwaralu, G.; Ramakrishna, U.; Vikram, P.; Sahu, S.; Wares, F.; Dewan, P.K.; Santosha, K.; Jyoti, J.; et al. Initial Default among Diagnosed Sputum Smear-Positive Pulmonary Tuberculosis Patients in Andhra Pradesh, India. Int J Tuberc Lung Dis 2008, 12, 1055–1058. [Google Scholar] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).