Submitted:
13 May 2023
Posted:
15 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
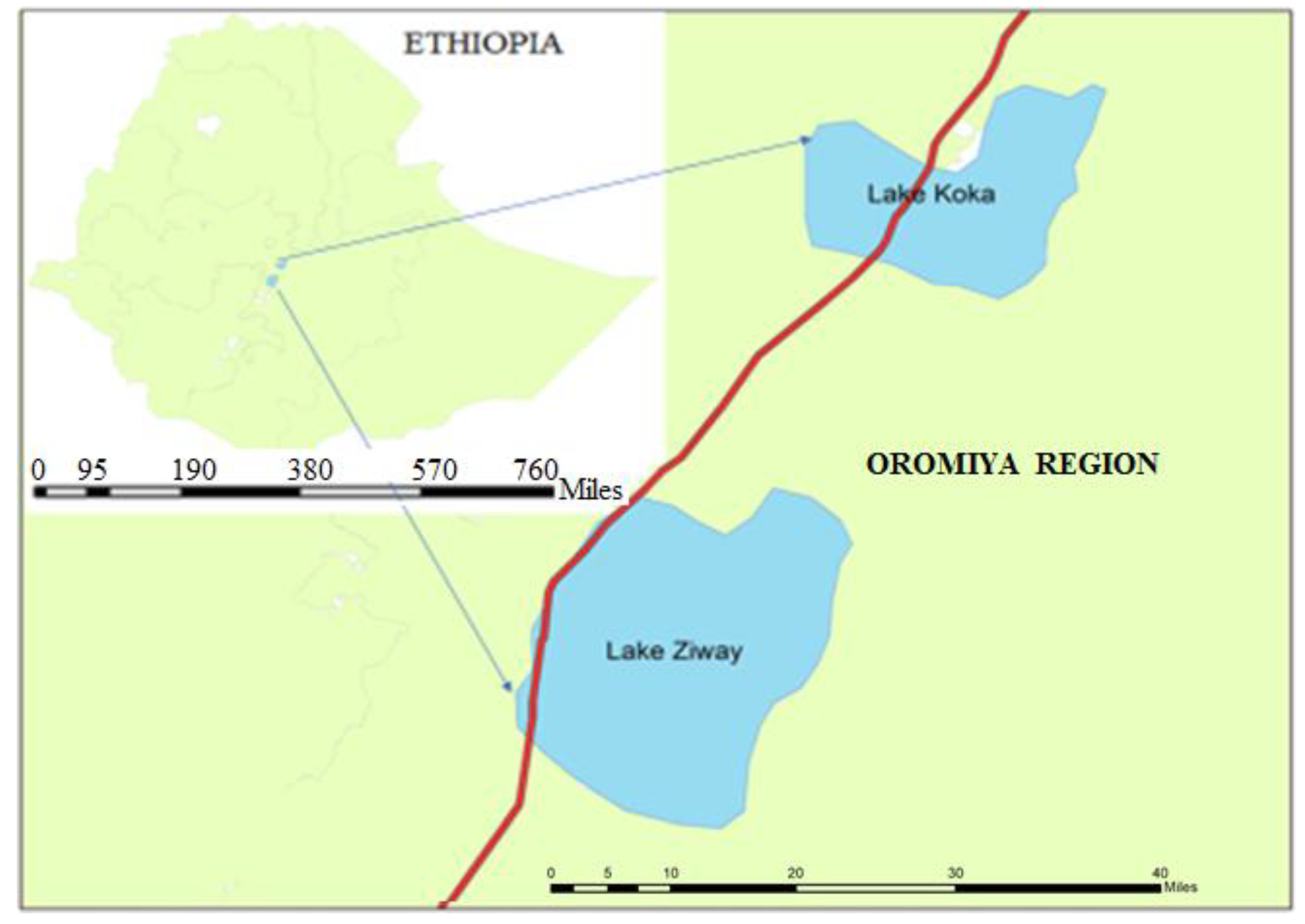
2.1. The study area
2.2. Sample sites
2.3. Lakes’ Water Physico-chemical Variables Test
2.4. Lakes’ Water Quality Measurement by Physico-chemical Character
2.5. Water Sampling
2.6. Sample Analysis
3. Results and Discussion
3.1. Comparison of wet and dry season physico-chemical levels of pollutants in the Lakes
3.2. The effect of water hyacinth on the physico-chemical levels of pollutants in the Lakes
3.3. Comparison of the physico-chemical levels of pollutants in the Lakes at the lake covered by water hyacinth and any other native grasses
4. Conclusion
Acknowledgements
Conflict of Interest
References
- M. Mulugeta, R. M. Mulugeta, R. Sahilu, Z. Kibret, and M. Mersha, ‘Level of Contamination in Lakes and Rivers of Ethiopia: an Overview’, Arabian Journal of Chemical and Environmental Research, vol. 07, no. 2, pp. 158–174, 2020, [Online]. Available: www.mocedes.org.
- S. H. Yan, W. S. H. Yan, W. Song, and J. Y. Guo, ‘Advances in management and utilization of invasive water hyacinth (Eichhornia crassipes) in aquatic ecosystems–a review’, Critical Reviews in Biotechnology, vol. 37, no. 2, pp. 218–228, 2017. [CrossRef]
- W. H. T. Ting, I. A. W. W. H. T. Ting, I. A. W. Tan, S. F. Salleh, and N. A. Wahab, ‘Journal of Water Process Engineering Application of water hyacinth ( Eichhornia crassipes ) for phytoremediation of ammoniacal nitrogen : A review’, Journal of Water Process Engineering, vol. 22, no. February, pp. 239–249, 2018. [CrossRef]
- M. F. Zaranyika and T. Ndapwadza, ‘Journal of Environmental Science and Health . Part A : Environmental Science and Engineering and Toxicology : Toxic / Hazardous Substances and Environmental Engineering Uptake of Ni , Zn , Fe , Co , Cr , Pb , Cu and Cd by water hyacinth ( eichhornia crass’, no. June 2013, pp. 157–169.
- L. Navarro and P. George, ‘Water hyacinth in Africa and the Middle East: a survey of problems and solutions’, p. 130 pp., 2000, [Online]. Available: http://books.google.com/books?hl=es&lr=&id=hhXqXNQ0WSQC&pgis=1.
- L. B. Merga, A. A. L. B. Merga, A. A. Mengistie, J. H. Faber, P. J. Van Den Brink, and F. Group, ‘Trends in chemical pollution and ecological status of Lake Ziway , Ethiopia : a review focussing on nutrients , metals and pesticides’, 2020. [CrossRef]
- A. Lagoon, A. Y. Segbefia, E. Honlah, and D. O. Appiah, ‘Effects of water hyacinth invasion on sustainability of fishing livelihoods along the River Tano and Effects of water hyacinth invasion on sustain- ability of fishing livelihoods along the River Tano and Abby-Tano Lagoon , Ghana’, vol. 1932, 2019. [CrossRef]
- E. Honlah, A. Y. E. Honlah, A. Y. Segbefia, D. O. Appiah, and P. O. Atakora, ‘of the communities , and the education of children along River Tano and Abby-Tano Lagoon in Ghana Effects of water hyacinth invasion on the health of the communities , and the education of children along River Tano and Abby-Tano Lagoon in Ghana’, 2019. [CrossRef]
- T. Subash, ‘Study on the benefits and impacts of Water Hyacinth at Pazhayar River Basin in Kanyakumari District, Tamilnadu, India-a Case Review’, 2016. [Online]. Available: www.iaard.net.
- W. Van Oijstaeijen et al., ‘Farmers’ preferences towards water hyacinth control: A contingent valuation study’, Journal of Great Lakes Research, vol. 46, no. 5, 2020. [CrossRef]
- S. Pagad, P. S. Pagad, P. Genovesi, L. Carnevali, R. Scalera, and M. Clout, ‘IUCN SSC Invasive Species Specialist Group : invasive alien species information management supporting practitioners , policy makers and decision takers’, vol. 6, no. 2, pp. 127–135, 2016.
- J. Turbelin, B. D. J. Turbelin, B. D. Malamud, and R. A. Francis, ‘Mapping the global state of invasive alien species: patterns of invasion and policy responses’, Global Ecology and Biogeography, vol. 26, no. 1, pp. 78–92, 2017. [CrossRef]
- W. Shiferaw, S. W. Shiferaw, S. Demissew, and T. Bekele, ‘Invasive alien plant species in Ethiopia: ecological impacts on biodiversity a review paper’, International Journal of Molecular Biology, vol. 3, no. 4, pp. 169–176, 2018. [CrossRef]
- H. Getnet, D. H. Getnet, D. Kifle, and T. Fetahi, ‘Water hyacinth ( Eichhornia crassipes ) affects the composition and abundance of zooplankton in the littoral region of Koka Reservoir , Ethiopia’, 2020. [CrossRef]
- D. Tewabe, E. D. Tewabe, E. Asmare, W. Zelalem, and B. Mohamed, ‘Identification of impacts , some biology of water hyacinth ( Eichhornia crassipes ) and its management options in Lake Tana , Ethiopia’, vol. 5, no. February, pp. 8–15, 2017.
- M. C. T. Scholten, C. C. M. C. T. Scholten, C. C. Karman, and S. Huwer, ‘Ecotoxicological risk assessment related to chemicals and pollutants in off-shore oil production’, vol. 113, pp. 283–288, 2000.
- D. A. H. Nash et al., ‘Phytoremediation of nutrients and organic carbon from sago mill effluent using water hyacinth (Eichhornia crassipes)’, Journal of Engineering and Technological Sciences, vol. 51, no. 4, pp. 573–584, 2019. [CrossRef]
- U. F. C. Sayago, ‘Design and development of a biotreatment of E. crassipes for the decontamination of water with Chromium (VI)’, Scientific Reports, vol. 11, no. 1, pp. 1–16, 2021. [CrossRef]
- J. A. Coetzee and M. P. Hill, ‘The role of eutrophication in the biological control of water hyacinth , Eichhornia crassipes , in South Africa’, pp. 247–261, 2012. [CrossRef]
- E. Honlah, A. Y. E. Honlah, A. Y. Segbefia, D. O. Appiah, E. Honlah, A. Y. Segbefia, and D. O. Appiah, ‘Cogent Food & Agriculture The Effects of Water Hyacinth Invasion on Smallholder Farming along River Tano and Tano The Effects of Water Hyacinth Invasion on Smallholder Farming along River Tano and Tano’, Cogent Food & Agriculture, vol. 5, no. 1, 2019. [CrossRef]
- S. World and H. Assembly, ‘Global vector control response : an integrated approach for the control of vector-borne diseases’, no. May, pp. 15–17, 2017.
- M. Paulraj, G. M. Paulraj, G. Prapakorn, T. Santi, C. Kraipat, and C. Sanket, ‘Improvement of Water Hyacinth Bioconversion by Different Organic and Mineral Acid Pretreatment and the Effect of Post - pretreatment Washing’, BioEnergy Research, 2022. [CrossRef]
- T. Huynh, Y. C. T. Huynh, Y. C. Chen, and B. N. T. Tran, ‘A small-scale study on removal of heavy metals from contaminated water using water hyacinth’, Processes, vol. 9, no. 10, 2021. [CrossRef]
- M. G. Dersseh et al., ‘Potential of water hyacinth infestation on Lake Tana, Ethiopia: A prediction using a GIS-based multi-criteria technique’, Water (Switzerland), vol. 11, no. 9, 2019. [CrossRef]
- Minych, G. Minych G. Dersoh, ‘Spatial and Temporal Dynamics of Water Hyacinth and Its Linkage with Lake-Level Fluctuation : Lake’, Eth, pp. 1–15, 2020.
- E. S. Oliveira Junior et al., ‘Water Hyacinth’s Effect on Greenhouse Gas Fluxes: A Field Study in a Wide Variety of Tropical Water Bodies’, Ecosystems, vol. 24, no. 4, pp. 988–1004, 2021. [CrossRef]
- P. Ilo, M. D. P. Ilo, M. D. Simatele, S. L. Nkomo, N. M. Mkhize, and N. G. Prabhu, ‘The benefits of water hyacinth (Eichhornia crassipes) for Southern Africa: A review’, Sustainability (Switzerland), vol. 12, no. 21, pp. 1–20, 2020. [CrossRef]
- F. Karouach et al., ‘A Comprehensive Evaluation of the Existing Approaches for Controlling and Managing the Proliferation of Water Hyacinth (Eichhornia crassipes): Review’, Frontiers in Environmental Science, vol. 12, no. February, pp. 1–20, 2020. [CrossRef]
- F. Karouach et al., ‘A Comprehensive Evaluation of the Existing Approaches for Controlling and Managing the Proliferation of Water Hyacinth (Eichhornia crassipes): Review’, Frontiers in Environmental Science, vol. 9, no. February, pp. 1–22, 2022. [CrossRef]
- H. Elbasiouny et al., ‘Ecofriendly remediation technologies for wastewater contaminated with heavy metals with special focus on using water hyacinth and black tea wastes: a review’, Environmental Monitoring and Assessment, vol. 193, no. 7, 2021. [CrossRef]
- L. Dsikowitzky, M. Mengesha, E. Dadebo, C. Eduardo, V. De Carvalho, and S. Sindern, ‘Assessment of heavy metals in water samples and tissues of edible fish species from Awassa and Koka Rift Valley’, pp. 3117–3131, 2013. [CrossRef]
- H. S. Ibrahim, N. S. H. S. Ibrahim, N. S. Ammar, M. Soylak, and M. Ibrahim, ‘Spectrochimica Acta Part A : Molecular and Biomolecular Spectroscopy Removal of Cd ( II ) and Pb ( II ) from aqueous solution using dried water hyacinth as a biosorbent’, SPECTROCHIMICA ACTA PART A: MOLECULAR AND BIOMOLECULAR SPECTROSCOPY, vol. 96, pp. 413–420, 2012. [CrossRef]
- J. Tabla-Hernandez, P. F. J. Tabla-Hernandez, P. F. Rodriguez-Espinosa, J. A. Mendoza-Pérez, E. Sánchez-Ortíz, E. Martinez-Tavera, and A. G. Hernandez-Ramirez, ‘Assessment of potential toxic metals in a ramsar wetland, Central Mexico and its self-depuration through Eichhornia crassipes’, Water (Switzerland), vol. 11, no. 6, 2019. [CrossRef]
- S. Liao and W. Chang, ‘Heavy Metal Phytoremediation by Water Hyacinth at Constructed Wetlands in Taiwan’, pp. 110–118, 2004.
- A. Malik, ‘Environmental challenge vis a vis opportunity : The case of water hyacinth’, vol. 33, pp. 122–138, 2007. [CrossRef]
- T. Eliku and S. Leta, ‘Spatial and seasonal variation in physicochemical parameters and heavy metals in Awash River, Ethiopia’, Applied Water Science, vol. 8, no. 6, pp. 1–13, 2018. [CrossRef]
- R. Shift, T. R. Shift, T. Lake, and L. Ziway, ‘Satellite Imageries and Field Data of Macrophytes Reveal a’, 2021.
- T. Alamirew and G. Zeleke, ‘Spatiotemporal Dynamics of Water Quality Indicators in Koka’, 2023.
- E. Gimeno-García, V. E. Gimeno-García, V. Andreu, and R. Boluda, ‘Heavy metals incidence in the application of inorganic fertilizers and pesticides to rice farming soils’, Environmental Pollution, vol. 92, no. 1, pp. 19–25, 1996. [CrossRef]
- A. Teklay and M. Amare, ‘Water quality characteristics and pollution levels of heavy metals in Lake Haiq, Ethiopia’, Ethiopian Journal of Science and Technology, vol. 8, no. 1, p. 15, 2015. [CrossRef]
- T. Nizamutdinov, E. T. Nizamutdinov, E. Abakumov, E. Morgun, R. Loktev, and R. Kolesnikov, ‘Agrochemical and pollution status of urbanized agricultural soils in the central part of yamal region’, Energies, vol. 14, no. 14, pp. 1–18, 2021. [CrossRef]
- Gizaw, F. Gizaw, F. Zewge, A. Kumar, A. Mekonnen, and M. Tesfaye, ‘A comprehensive review on nitrate and phosphate removal and recovery from aqueous solutions by adsorption’, Journal of Water Supply: Research and Technology-Aqua, vol. 00, no. 0, pp. 1–27, 2021. [CrossRef]
- S. W. Liao and W. L. Chang, ‘Heavy metal phytoremediation by water hyacinth at constructed wetlands in Taiwan’, Journal of Aquatic Plant Management, vol. 42, no. JAN., pp. 60–68, 2004.
- N. Sasidharan, T. N. Sasidharan, T. Azim, D. Devi, and S. Mathew, ‘Water hyacinth for heavy metal scavenging and utilization as organic manure’, Indian Journal of Weed Science, vol. 45, no. 3, pp. 204–209, 2013.
- V. Kumar et al., ‘Modeling of water hyacinth growth and its role in heavy metals accumulation from unoperated old Ganga canal at Haridwar, India’, Rendiconti Lincei, vol. 32, no. 4, pp. 805–816, 2021. [CrossRef]
- N. M. Sidek, S. R. S. N. M. Sidek, S. R. S. Abdullah, N. U. Ahmad, S. F. S. Draman, M. M. M. Rosli, and M. F. Sanusi, ‘Phytoremediation of abandoned mining lake by water hyacinth and water lettuces in constructed wetlands’, Jurnal Teknologi, vol. 80, no. 5, pp. 87–93, 2018. [CrossRef]
| Description | Lakes | |||||||
|---|---|---|---|---|---|---|---|---|
| Lake Koka | Lake Ziway | |||||||
| Study sites | Site 1 SK1 |
Site 2 SK2 |
Site 3 SK3 |
Site 4 SK4 |
Site1 SZ1 |
Site 2 SZ2 |
Site 3 SZ3 |
Site 4 SZ4 |
| Water hyacinth invasion or infestation level | Low (L) | Medium (M ) | High (H) | Other grasses (G) | Low (L) | Medium (M ) | High (H) | Other grasses (G) |
| Label | SK1L | SK2M | SK3H | SK4G | SZ1L | SZ2M | SZ3H | SZ4G |
| Lake Ziway | Season | WHO Stand | |||||||
|---|---|---|---|---|---|---|---|---|---|
| wet | Dry | ||||||||
| Parameters | SZ1L | SZ2M | SZ3H | SZ4G | SZ1L | SZ2M | SZ3H | SZ4G | |
| Cr | ND | ND | ND | ND | ND | ND | ND | ND | 0.05 |
| Pb | ND | ND | ND | ND | 0.69 | 0.68 | 0.69 | 0.71 | 0.05 |
| Cd | ND | ND | ND | ND | ND | ND | ND | ND | 0.01 |
| Zn | 0.05 | 0.10 | 0.08 | 0.04 | 0.59 | 0.38 | 0.57 | 0.53 | 0.01 |
| Cu | ND | ND | ND | 0.01 | ND | ND | ND | ND | 2 |
| EC | 347.3 | 315 | 289 | 284.4 | 337.3 | 306 | 283.6 | 280.4 | 300 |
| PO43-P | 24.7 | 17.1 | 13.1 | 28.8 | 0.6 | 0.60 | 0.8 | 0.8 | 5 |
| NO3-N | 15.3 | 27.0 | 36.6 | 18.2 | 8.4 | 9.5 | 9.6 | 7.3 | 50 |
| COD | 312 | 379 | 344 | 330.3 | 260 | 192 | 203 | 229 | 4.5 |
| BOD5 | 6.4 | 7.8 | 9.7 | 11.2 | 18.3 | 11.7 | 15.2 | 16.7 | 2 |
| pH | 6.5 | 6.0 | 6.0 | 7.8 | 6.0 | 5.9 | 5.5 | 7.9 | 6.5-8 |
| T | 25.5 | 25.5 | 26.5 | 23 | 26 | 28.3 | 29 | 23 | 30 |
| Lake Koka | Season | WHO Stand | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Wet | Dry | ||||||||
| Parameters | SK1L | SK2M | SK3H | SK4G | SK1L | SK2M | SK3H | SK4G | |
| Cr | ND | ND | ND | ND | ND | ND | ND | ND | 0.05 |
| Pb | ND | ND | 0.08 | ND | 0.49 | 0.66 | 0.56 | 0.56 | 0.05 |
| Cd | ND | ND | ND | ND | ND | ND | ND | ND | 0.01 |
| Zn | 0.18 | 0.03 | 0.02 | 0.19 | 0.34 | 0.45 | 0.37 | 0.72 | 0.01 |
| Cu | 0.07 | 0.04 | 0.03 | 0.07 | ND | ND | ND | ND | 2 |
| EC | 335.1 | 309 | 290.9 | 276.3 | 330.1 | 291 | 281.9 | 274.3 | 300 |
| PO43-P | 16.1 | 16.2 | 24.8 | 29.1 | 3.2 | 1.5 | 0.9 | 4.5 | 5 |
| NO3-N | 22.2 | 23.6 | 14.1 | 21.1 | 8.3 | 10.8 | 10.6 | 7.6 | 50 |
| COD | 291.0 | 263 | 333.7 | 323 | 246 | 536 | 264 | 305 | 4.5 |
| BOD5 | 8.4 | 7.5 | 9.6 | 8.1 | 26.25 | 37.5 | 22.5 | 31.5 | 2 |
| pH | 6.9 | 5.9 | 5.1 | 7.5 | 6.9 | 5.5 | 5.6 | 7.6 | 6.5-8 |
| T | 28.5 | 28.5 | 29.5 | 25 | 27 | 28 | 29 | 26 | 30 |
| Lakes | Parameters | Wet | Dry | ANOVA Seasonal | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Koka | Cr | NA | NA | NA | NA | NA |
| Pb | 0.02 | 0.045 | 0.567 | 0.071 | *0.000 | |
| Cd | NA | NA | NA | NA | NA | |
| Zn | 0.105 | 0.095 | 0.47 | 0.173 | *0.01 | |
| Cu | 0.07 | 0.077 | 0 | 0.000 | 0.132 | |
| EC | 302.85 | 25.319 | 294.35 | 24.791 | 0.648 | |
| PO43-P | 21.55 | 6.478 | 2.52 | 1.637 | *0.001 | |
| NO3-N | 20.25 | 4.226 | 9.32 | 1.616 | *0.003 | |
| COD | 302.67 | 32.073 | 337.75 | 134.453 | 0.630 | |
| BOD5 | 8.4 | 0.883 | 29.44 | 6.520 | *0.001 | |
| pH | 6.35 | 1.063 | 6.4 | 1.025 | 0.948 | |
| T | 27.87 | 1.97 | 27.5 | 1.29 | 0.761 | |
| Ziway | Cr | NA | NA | NA | NA | NA |
| Pb | 0 | 0 | 0.69 | 0.01 | *0.000 | |
| Cd | NA | NA | NA | NA | NA | |
| Zn | 0.067 | 0.032 | 0.52 | 0.095 | *0.000 | |
| Cu | 0.01 | 0.020 | 0 | 0 | 0.356 | |
| EC | 308.92 | 28.91 | 301.85 | 26.23 | 0.729 | |
| PO43-P | 20.925 | 7.121 | 0.7 | 0.114 | *0.001 | |
| NO3-N | 24.275 | 9.605 | 8.7 | 1.080 | *0.018 | |
| COD | 341.32 | 28.33 | 221 | 30.28 | *0.001 | |
| BOD5 | 8.77 | 2.11 | 15.47 | 2.82 | *0.01 | |
| pH | 6.57 | 0.85 | 6.32 | 1.07 | 0.727 | |
| T | 25.12 | 1.49 | 26.32 | 2.53 | 0.45 | |
| Lake | Parameter | SK1L | SK2M | SK3H | ANOVA spatial | |||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |||
| Koka | CrW | NA | NA | NA | NA | NA | NA | NA |
| CrD | NA | NA | NA | NA | NA | NA | NA | |
| PbW | 0 | 0 | 0 | 0 | 0.083 | 0.101 | 0.211 | |
| PbD | 0.497 | 0.108 | 0.66 | 0.044 | 0.56 | 0.036 | 0.075 | |
| CdW | NA | NA | NA | NA | NA | NA | NA | |
| CdD | NA | NA | NA | NA | NA | NA | NA | |
| ZnW | 0.173 | 0.006 | 0.02 | 0.000 | 0.027 | 0.006 | *0.000 | |
| ZnD | 0.347 | 0.006 | 0.457 | 0.012 | 0.37 | 0.030 | *0.001 | |
| CuW | 0.04 | 0.010 | 0.037 | 0.006 | 0.04 | 0.000 | 0.786 | |
| CuD | NA | NA | NA | NA | NA | NA | NA | |
| ECW | 335.00 | 4.58 | 309.00 | 3.61 | 291.00 | 2.000 | *0.000 | |
| ECD | 330.00 | 3.46 | 291.00 | 1.73 | 282.00 | 1.000 | *0.000 | |
| PO43-PW | 3.17 | 0.18 | 0.93 | 0.01 | 1.47 | 0.017 | *0.000 | |
| PO43-PD | 16.13 | 0.06 | 16.20 | 0.17 | 24.73 | 0.058 | *0.000 | |
| NO3-NW | 22.17 | 0.15 | 23.60 | 0.20 | 14.13 | 0.404 | *0.000 | |
| NO3-ND | 8.30 | 0.20 | 10.80 | 0.00 | 10.57 | 0.058 | *0.000 | |
| CODW | 291.00 | 2.00 | 263.00 | 1.00 | 333.67 | 3.215 | *0.000 | |
| CODD | 246.00 | 0.00 | 535.67 | 0.58 | 263.67 | 0.577 | *0.000 | |
| BOD5W | 8.33 | 0.15 | 7.50 | 0.10 | 9.60 | 0.361 | *0.0001 | |
| BOD5D | 26.25 | 0.25 | 37.50 | 0.10 | 22.50 | 0.100 | *0.000 | |
| pHW | 6.43 | 0.40 | 5.87 | 0.12 | 5.10 | 0.100 | *0.002 | |
| pHD | 6.83 | 0.06 | 5.37 | 0.15 | 5.40 | 0.346 | *0.0002 | |
| TW | 28 | 1.000 | 28.5 | 0.500 | 29.33 | 0.577 | 0.155 | |
| TD | 27 | 1.000 | 28 | 0.000 | 28.67 | 1.155 | 0.145 | |
| Parameter | SZ1L | SZ2M | SZ3H | ANOVA spatial | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| CrW | NA | NA | NA | NA | NA | NA | NA |
| CrD | NA | NA | NA | NA | NA | NA | NA |
| PbW | NA | NA | NA | NA | NA | NA | NA |
| PbD | 0.693 | 0.067 | 0.687 | 0.045 | 0.697 | 0.025 | 0.968 |
| CdW | NA | NA | NA | NA | NA | NA | NA |
| CdD | NA | NA | NA | NA | NA | NA | NA |
| ZnW | 0.05 | 0.000 | 0.107 | 0.001 | 0.07 | 2E-02 | *0.0017 |
| ZnD | 0.59 | 0.000 | 0.377 | 0.001 | 0.57 | 2E-02 | *0.000 |
| CuW | NA | NA | NA | NA | NA | NA | NA |
| CuD | NA | NA | NA | NA | NA | NA | NA |
| ECW | 347.33 | 4.16 | 315.33 | 4.04 | 289.00 | 1.000 | *0.000 |
| ECD | 337.33 | 4.16 | 306.33 | 5.51 | 283.67 | 5.508 | *0.000 |
| PO43-PW | 0.64 | 0.02 | 0.60 | 0.01 | 0.79 | 0.020 | *0.000 |
| PO43-PD | 24.73 | 0.06 | 17.10 | 0.00 | 13.13 | 0.058 | *0.000 |
| NO3-NW | 15.27 | 0.38 | 26.97 | 0.35 | 36.63 | 1.159 | *0.000 |
| NO3-ND | 8.40 | 0.20 | 9.53 | 0.06 | 9.60 | 0.000 | *0.000 |
| CODW | 312.33 | 1.16 | 378.67 | 2.52 | 344.00 | 53.703 | 0.102 |
| CODD | 260.00 | 2.65 | 192.00 | 2.00 | 203.00 | 0.000 | *0.000 |
| BOD5W | 6.40 | 0.46 | 7.87 | 0.15 | 9.70 | 0.100 | *0.000 |
| BOD5D | 18.30 | 0.20 | 11.70 | 0.00 | 15.13 | 0.058 | *0.000 |
| pHW | 6.83 | 0.29 | 6.00 | 0.50 | 6.00 | 0.866 | 0.226 |
| pHD | 6.07 | 0.31 | 5.87 | 0.12 | 5.33 | 0.416 | 0.061 |
| TW | 25.33 | 0.29 | 25.33 | 1.16 | 26.67 | 0.289 | 0.096 |
| TD | 25.50 | 0.50 | 27.33 | 0.58 | 28.83 | 0.764 | *0.002 |
| Lake | Parameter | Hyacinth infested | Other native grasses covered the lake | ANOVA | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Koka | CrW | NA | NA | NA | NA | NA |
| CrD | NA | NA | NA | NA | NA | |
| PbW | NA | NA | NA | NA | NA | |
| PbD | 0.57 | 0.1 | 0.54 | 0 | 0.586 | |
| CdW | NA | NA | NA | NA | NA | |
| CdD | NA | NA | NA | NA | NA | |
| ZnW | 0.08 | 0.1 | 0.19 | 0 | 0.094 | |
| ZnD | 0.39 | 0 | 0.72 | 0 | *0.001 | |
| CuW | 0.05 | 0 | 0.07 | 0 | 0.099 | |
| CuD | NA | NA | NA | NA | NA | |
| ECW | 311.67 | 22.12 | 276.33 | NA | 0.054 | |
| ECD | 301.00 | 25.51 | 274.33 | 3.51 | 0.147 | |
| PO43-PW | 1.87 | 1.19 | 4.51 | 0.14 | *0.019 | |
| PO43-PD | 19.03 | 4.99 | 29.13 | 0.00 | *0.025 | |
| NO3-NW | 19.93 | 5.19 | 21.07 | 0.30 | 0.725 | |
| NO3-ND | 9.90 | 1.39 | 7.60 | 0.00 | *0.046 | |
| CODW | 295.67 | 35.23 | 323.33 | 1.15 | 0.246 | |
| CODD | 348.67 | 162.48 | 303.33 | 3.05 | 0.654 | |
| BOD5W | 8.50 | 1.05 | 8.13 | 0.00 | 0.580 | |
| BOD5D | 28.73 | 7.81 | 31.47 | 0.00 | 0.577 | |
| pHW | 5.97 | 0.90 | 7.43 | 0.00 | *0.048 | |
| pHD | 6 | 0.78 | 7.4 | 0.20 | *0.040 | |
| TW | 28.83 | 0.57 | 25.00 | 1.00 | *0.005 | |
| TD | 28.00 | 1.00 | 25.67 | 0.57 | *0.025 | |
| Ziway | CrW | NA | NA | NA | NA | NA |
| CrD | NA | NA | NA | NA | NA | |
| PbW | NA | NA | NA | NA | NA | |
| PbD | 0.69 | 0 | 0.71 | 0 | 0.101 | |
| CdW | NA | NA | NA | NA | NA | |
| CdD | NA | NA | NA | NA | NA | |
| ZnW | 0.077 | 0.031 | 0.043 | 0 | 0.089 | |
| ZnD | 0.513 | 0.114 | 0.507 | 0 | 0.926 | |
| CuW | NA | NA | NA | NA | NA | |
| CuD | NA | NA | NA | NA | NA | |
| ECW | 317.00 | 29.05 | 284.33 | 0.57 | 0.123 | |
| ECD | 309.00 | 26.63 | 280.33 | 0.57 | 0.136 | |
| PO43-PW | 0.667 | 0.11 | 0.770 | 0.00 | 0.200 | |
| PO43-PD | 18.30 | 5.89 | 28.77 | 0.00 | *0.037 | |
| NO3-NW | 26.3 | 10.67 | 18.2 | 0.30 | 0.259 | |
| NO3-ND | 9.17 | 0.66 | 7.30 | 0.10 | *0.009 | |
| CODW | 345.00 | 33.51 | 330.33 | 1.53 | 0.491 | |
| CODD | 218.33 | 36.50 | 229.00 | 0.00 | 0.639 | |
| BOD5W | 7.97 | 1.66 | 11.20 | 0.10 | *0.028 | |
| BOD5D | 15.07 | 3.30 | 16.67 | 0.00 | 0.449 | |
| pHW | 6.17 | 0.28 | 7.73 | 0.14 | *0.001 | |
| pHD | 5.80 | 0.26 | 7.90 | 0.00 | *0.000 | |
| TW | 25.83 | 0.57 | 22.67 | 0.57 | *0.003 | |
| TD | 27.43 | 1.50 | 23.00 | 1.00 | *0.013 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





