1. Introduction
The energy dilemma is undeniably one of the most pressing concerns confronting modern society, as it plays a critical role in global and domestic economic development and security [
1,
2]. Due to the overwhelming increase in global population, coupled with the continuous economic growth seen in multiple developed and developing nations, an increase in global energy demand and consumption is unavoidable [
3,
4]. To address these concerns, cost-effective, clean, and renewable energy sources that could be used as viable substitutes for fossil fuels are urgently needed [
5,
6,
7,
8,
9,
10,
11]. Energy storage and conversion devices have received worldwide interest as a key step toward the use of renewable energy resources as primary energy sources, and it has become necessary to thoroughly examine the various promising energy conversion and storage solutions, such as fuel cells (FCs) [
12,
13,
14,
15,
16,
17,
18], and embark on their implementation [
4,
15]. In this regard, the direct alcohol fuel cells, such as the direct 2-propanol FCs (D2PFCs), appeared as excellent candidates [
19,
20].
In fact, 2-propanol is an excellent solvent and is involved in many industry processes such as cosmetics and pharmaceutical industries. Being twice as effective as ethanol, 2-propanol is the widely used disinfectant within pharmaceutics, hospitals, cleanrooms, and electronics or medical device manufacturing [
21]. It’s also used as a gasoline additive to keep carburettors from freezing up in internal combustion vehicles [
22]. The higher gravimetric energy density (8.6 kWh/kg) compared with that of methanol (6.1 kWh/kg) and ethanol (8.0 kWh/kg) is one more advantage for 2-propanol as a liquid fuel [
23].
Recently, interests have been more dedicated to alkaline than acidic D2PFCs because of their higher performance which is likely resulted from the faster kinetics of the alcohol electrooxidation and the oxygen electroreduction in alkaline medium [
24,
25,
26]. As the alkaline 2POR proceeds at low overpotential via the acetone production pathway (CH
3CHOHCH
3 + 2OH
− → CH
3COCH
3 + 2H
2O + 2e
−) [
26,
27,
28], several noble metals, including Pt, Pd, and Au, that exhibited previously excellent catalytic properties for several vital processes, have been recommended for2POR [
5,
12,
29,
30,
31,
32,
33]. However, the obtained oxidation currents (I
p) decayed dramatically at low overpotentials which implies the rapid poisoning of the catalyst surface from the oxidation products [
26,
34,
35]. Acetone was the suspicious poisoning species, as previously proposed for 2POR at Pd surfaces in alkaline medium [
36].
This research addresses the impact of modifying a nanoparticle-based Pd surface with Pt and Au nanoparticles to test their potential to mitigate the activity deterioration of the Pd substrates during 2POR due to acetone poisoning. Three different catalysts (Pd/GC, Pd/Pt/GC, and Pd/Au/GC) will be prepared, characterized, and catalytically evaluated toward 2POR. The relationship between the concentration of electrolyte medium (NaOH) and the potential scan rate on the Ip will be obtained and the mechanism of enhancement toward the 2POR will be discussed.
2. Experimental
A cleaned GC (d = 5.0 mm), an Ag/AgCl/NaCl (3M), a spiral Pt wire were served, respectively, as the working, reference, and counter electrodes. The cleaning of the GC electrode was carried out through the conventional cleaning procedures described previously [
37,
38].
All chemicals in the current study were of high purity and were used without any prior purification. The electrodepositions of Pd, Pt, and Au were all carried out in 0.1 M Na2SO4 solutions containing, respectively, 2.0 mM Pd(CH3COO)2, 2.0 mM H2PtCl6.6H2O, 2.0 mM HAuCl4.3H2O at 0.1 V, allowing the passage of 10 mC.
All electrochemical experiments were conducted at room temperature (25 ± 1 °C) in a two compartments three electrodes glass cell using a Bio-Logic SAS potentiostat (model SP-150) operated with EC-Lab software.
The surface morphology and composition of the proposed catalysts were obtained using a Zeiss Ultra 60 field emission scanning electron microscope (FE-SEM) equipped with an energy-dispersive X-ray spectroscopy (EDX).
3. Results and discussion
3.1. Electrochemical and Materials characterization
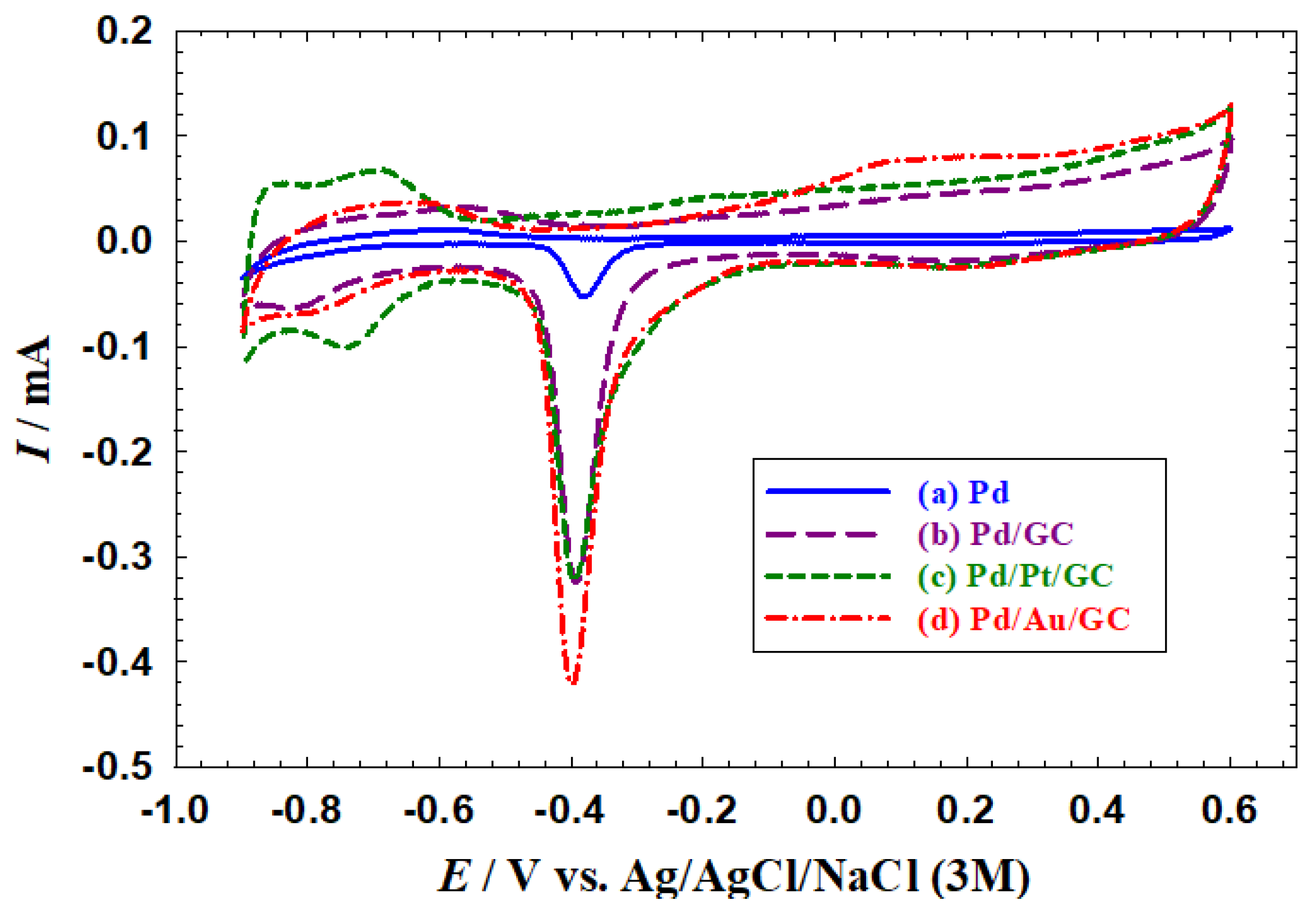
Figure 1 shows the typical behaviour of Pd-based catalysts in alkaline media where the Pd oxidation to PdO which extended from −0.2 to 0.6 V was coupled with the subsequent reduction of PdO to Pd again at −0.4 V. Along with that, the hydrogen adsorption/desorption (H
ads/des) region was observed between −0.6 and −0.9 V. This behaviour was observed for all proposed catalysts (
Figure 1a–d). A deeper inspection of
Figure 1 reveals several other important observations:
Compared to the bulk Pd catalyst (
Figure 1a), a larger surface area (SA) was obtained at the nanoparticles-modified catalysts (
Figure 1b–d). The SA was calculated to be 0.08, 0.52, 0.59, and 0.83 cm
2 for the Pd, Pd/GC, Pd/Pt/GC, and Pd/Au/GC catalysts, respectively, based on the charge associated with the PdO reduction peak using a reference value of 420 µC cm
−2 [
38,
39]. . This trend appeared again in the H
ads/des region because of the large surface area offered by nanoparticles.
The Pd/Pt/GC (
Figure 1c) and the Pd/Au/GC (
Figure 1d) catalysts acquired a broader PdO reduction peak compared to that obtained at the Pd/GC catalyst (
Figure 1a). This highlighted the role of adding the Pt and Au surface modifiers in providing diverse Pd−Pd and Pd−O bonding and/or facets’ reconstruction for the Pd surface.
The large H
ads/des peaks at the Pd/Pt/GC catalyst referred to the participation of both Pd and Pt in this reaction [
40].
The disappearance of the Au characteristic peaks at the Pd/Au/GC catalyst (
Figure 1d) might refer to the formation of a “core (Au)-shell (Pd)” structure [
40,
41].
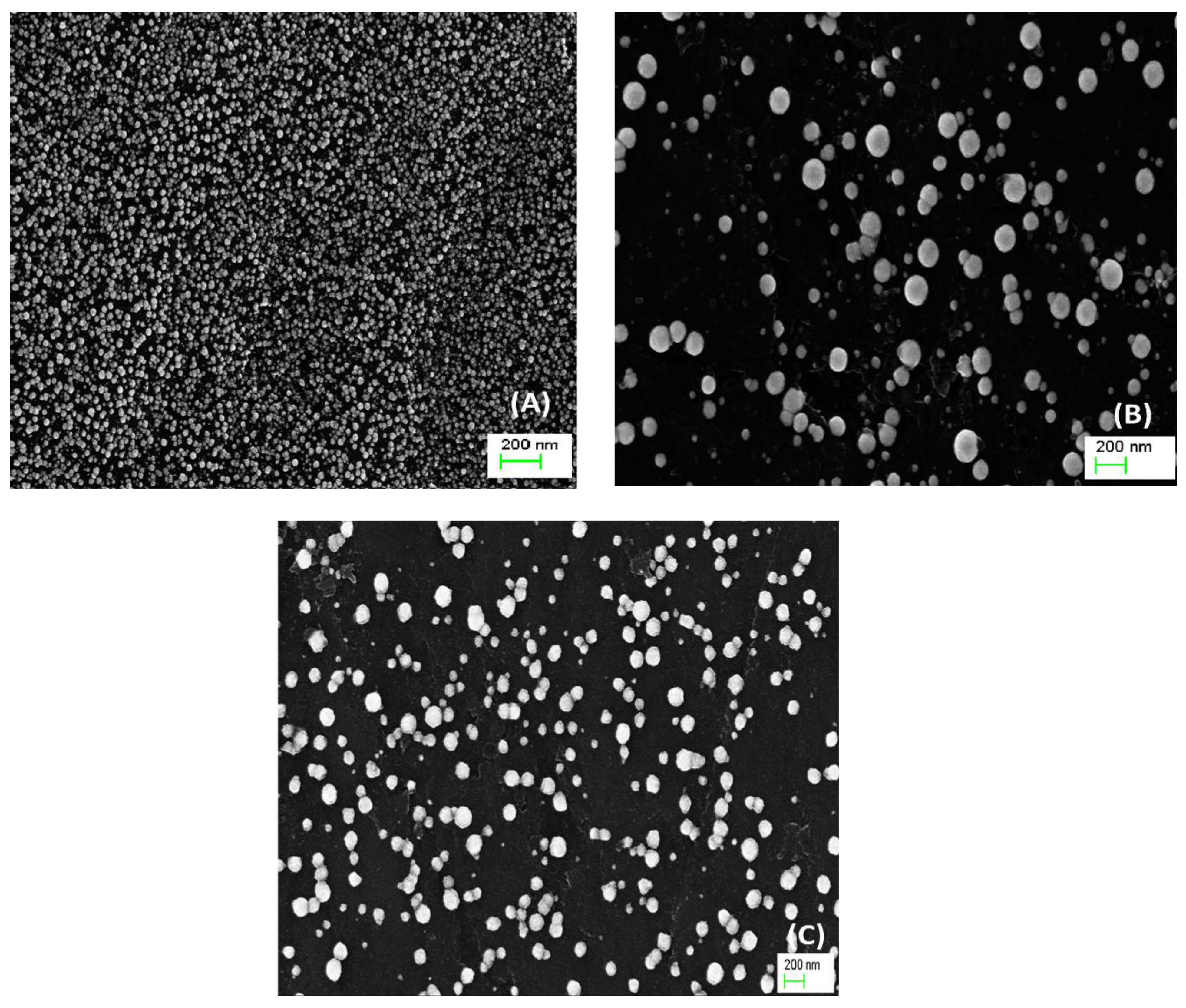
Additionally, by using the stat-of-art technologies, the surface morphology (
Figure 2) of the proposed catalysts; Pd/C (
Figure 2A), Pd/Pt/GC (
Figure 2B), and Pd/Au/GC (
Figure 2C) was obtained using the FE-SEM.
Figure 1A confirmed the successful deposition of Pd in the Pd/GC catalyst as well-distributed spherical nanoparticles having an average diameter of ca. 30 nm. Too larger sizes (100 and 120 nm) of spherical Pd particles were obtained, respectively, at the Pd/Pt/GC (
Figure 2B) and the Pd/Au/GC (
Figure 2C) catalysts. It seems that Pt and Au were simultaneously deposited with Pd in single bigger particles [
37,
42].
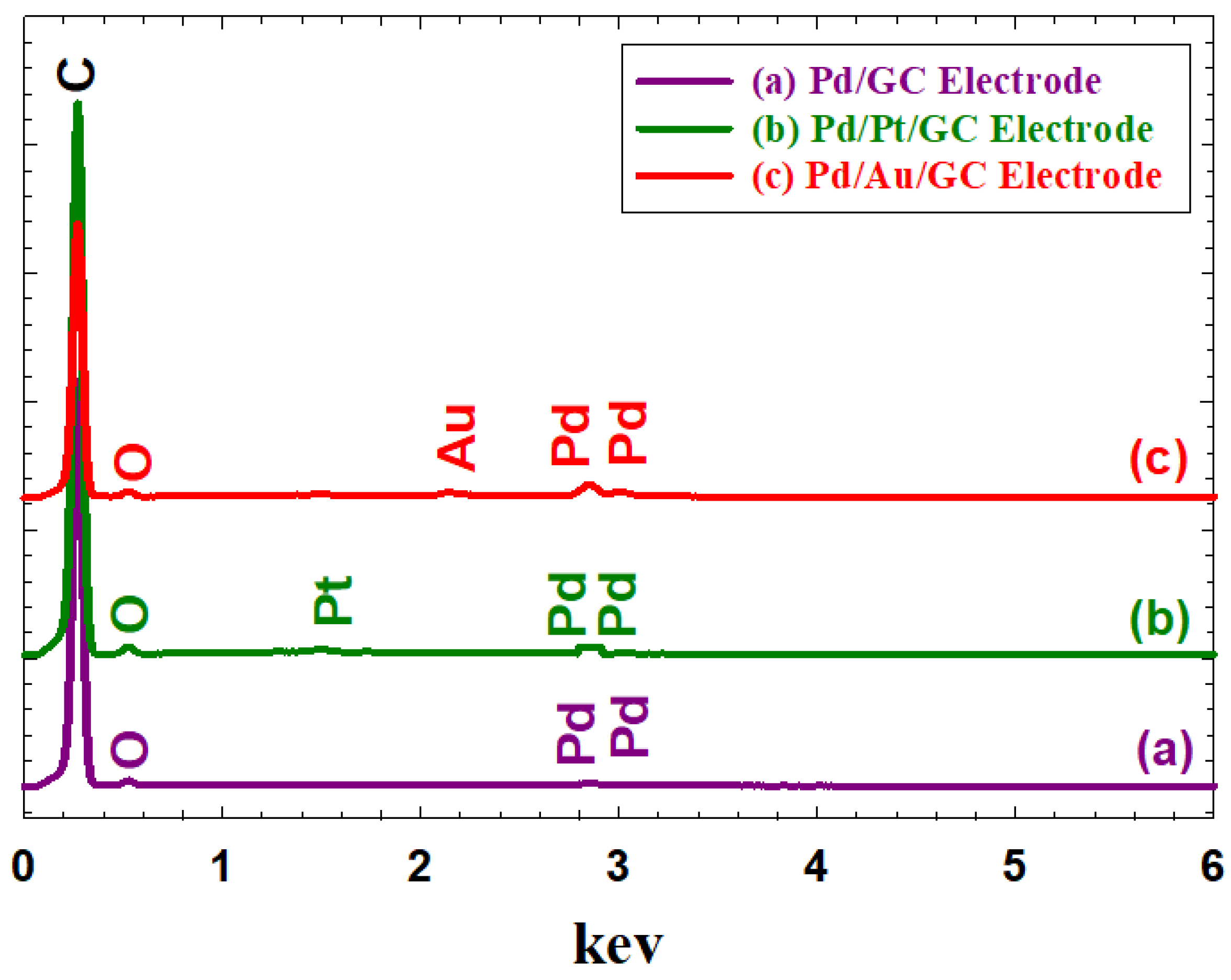
To further confirm the successful deposition of all ingredients of the proposed catalysts, EDX analysis was obtained.
Figure 3 shows the EDX analyses of the Pd/GC (
Figure 3a), Pd/Pt/GC (
Figure 3b), and the Pd/Au/GC (
Figure 3c) catalysts. As clearly shown, all catalysts’ components (C, O, Pd) were existed in the three proposed catalysts additionally with Pt and Au that were observed respectively at the Pd/Pt/GC and the Pd/Au/GC catalysts.
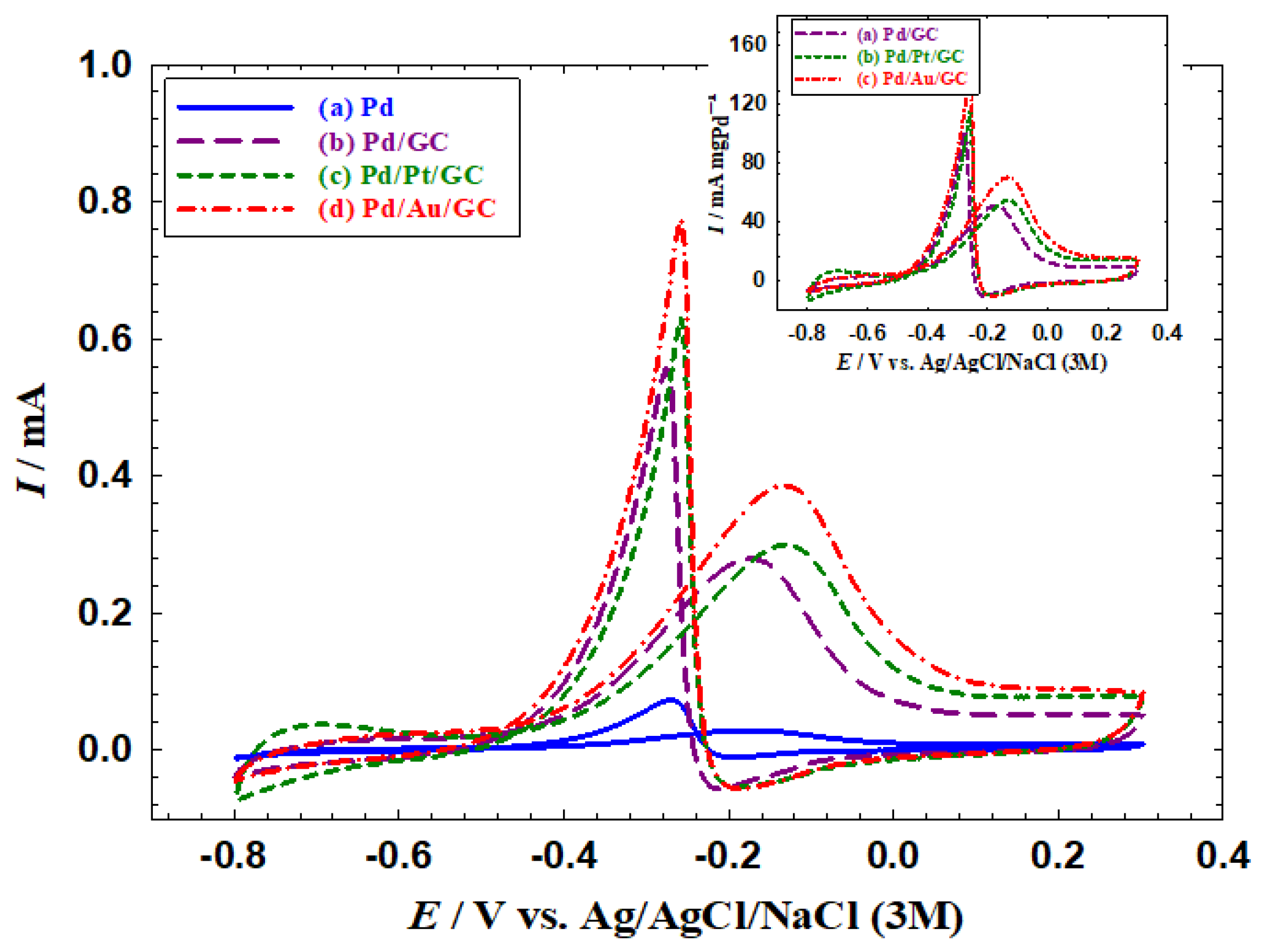
3.2. Electrocatalytic activities of the catalysts toward EOM
Figure 4 shows the alkaline 2POR at the proposed catalysts. All catalysts showed the typical behaviour of the 2POR where two anodic peaks were observed at ca. −0.15 and −0.28 V, respectively, in the positive and the negative going scans, which agreed with previous literature [
27,
31,
34]. The low oxidation current (I
p, ca. 0.029 mA) obtained at the Pd catalyst (
Figure 4a) was attributed to the surface poisoning with the strongly adsorbed oxidation product, acetone, at the Pd surface [
36]. The poisoning with acetone was assumed to block most of the Pd active sites and therefore diminish the overall activity of the 2POR. A better scenario was observed at the Pd/GC (
Figure 4b) catalyst at which the I
p reached ca. 0.279 mA (ca. 10 times higher than that obtained at the Pd catalyst).
The consecutive modification of the Pd/GC catalyst with Pt and Au could further diminish such a poisoning impact where the I
p value reached 0.299 and 0.386 mA respectively at the Pd/Pt/GC (
Figure 4c) and the Pd/Au/GC (
Figure 4d) catalysts. The inset of
Figure 4 shows the current values normalized to the mass of deposited Pd (specific currents) for the Pd/GC, Pd/Pt/GC, and the Pd/Au/GC catalysts. The obtained values were 51, 55, and 71 mA mg
Pd−1, respectively.
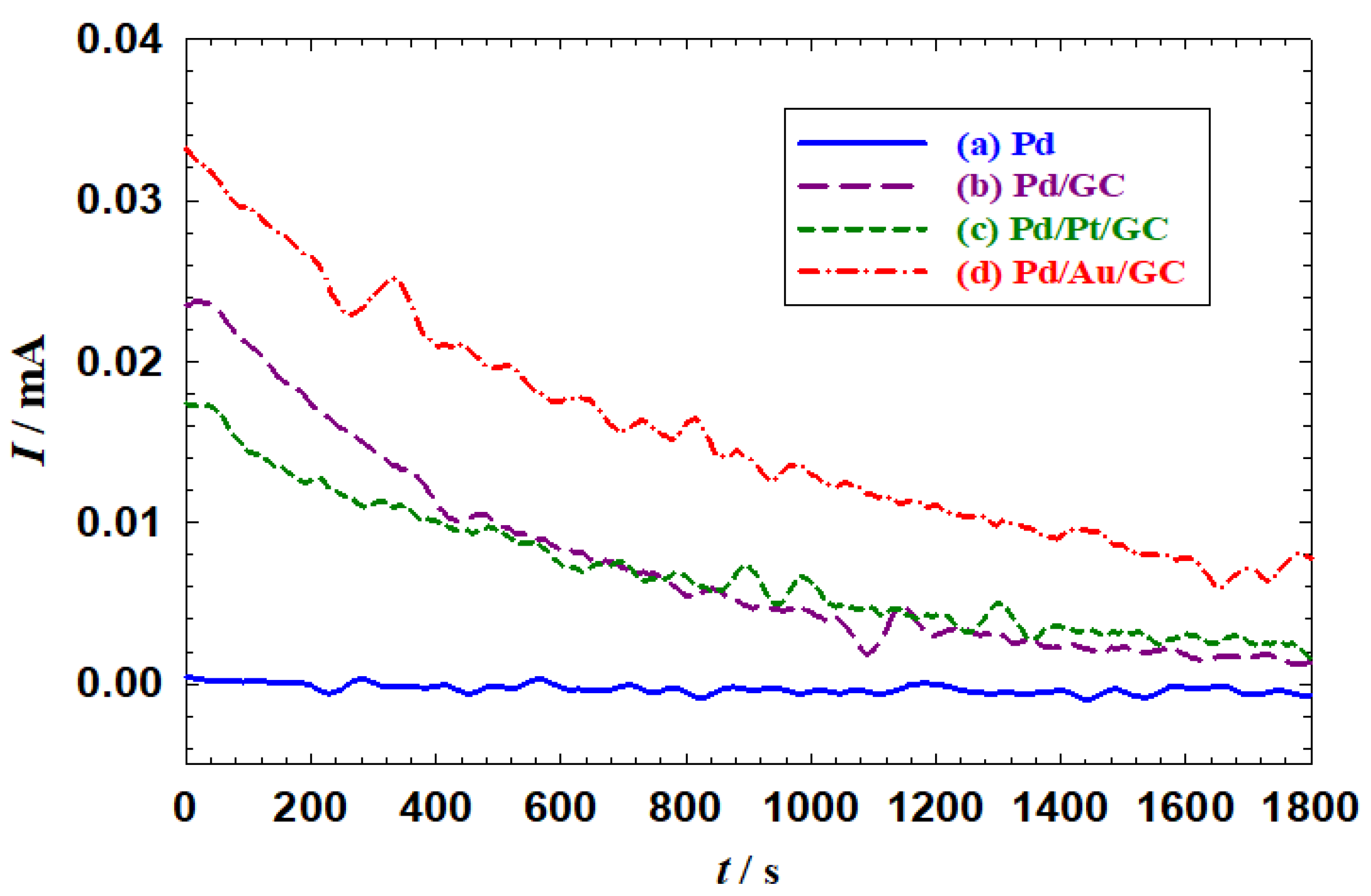
The enhancement behavior in the catalytic activity was also reflected in measuring the catalytic stability.
Figure 5 shows the current transients (i-t) of the proposed catalysts. The enhancement in the catalytic stability in terms of achieving higher currents after 1800s of continuous electrolysis. As observed, the current trend was in the order of Pd (
Figure 5a) < Pd/GC (
Figure 5b) < Pd/Pt/GC (
Figure 5c) < Pd/Au/GC (
Figure 5d).
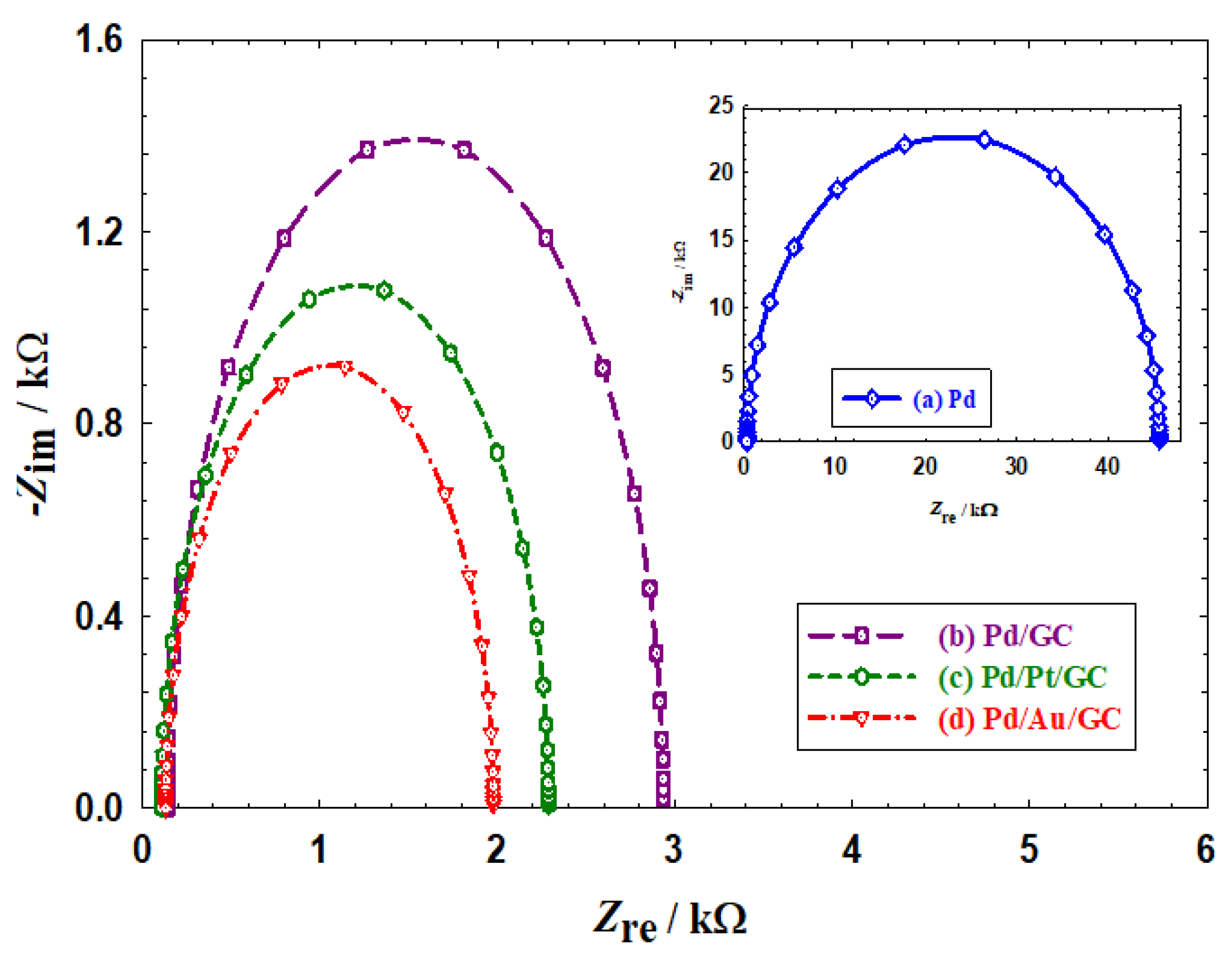
To understand the mechanism of enhancement toward the 2POR, in terms of the charge transfer, Nyquist plots were obtained and analysed (see
Figure 6). As well-known, Nyquist plots assess the charge transfer resistance (R
ct) of the catalyst, which is obtained from the semicircle diameter; evaluating the reaction kinetics [
37,
40]. In agreement with the data of Figure 4a and Figure 5a depicting, respectively, the poor activity and stability of the Pd catalyst,
Figure 6a assigned the highest R
ct value (45.39 kΩ) for the same “pristine” Pd catalyst toward the 2POR. This indicates a sluggish kinetics toward the 2POR at the Pd catalyst.
On the other hand, the Pd/GC (
Figure 6b), Pd/Pt/GC (
Figure 6c), and the Pd/Au/GC catalysts offered much lower R
ct values (2.79, 2.18, and 1.85 kΩ, respectively); indicating faster charge transfer kinetics toward the 2POR. It is thought that such an enhancement came from the weak adsorption of acetone and/or its faster removal at the modified catalysts [
26].
3.3. Parameters affecting 2POR
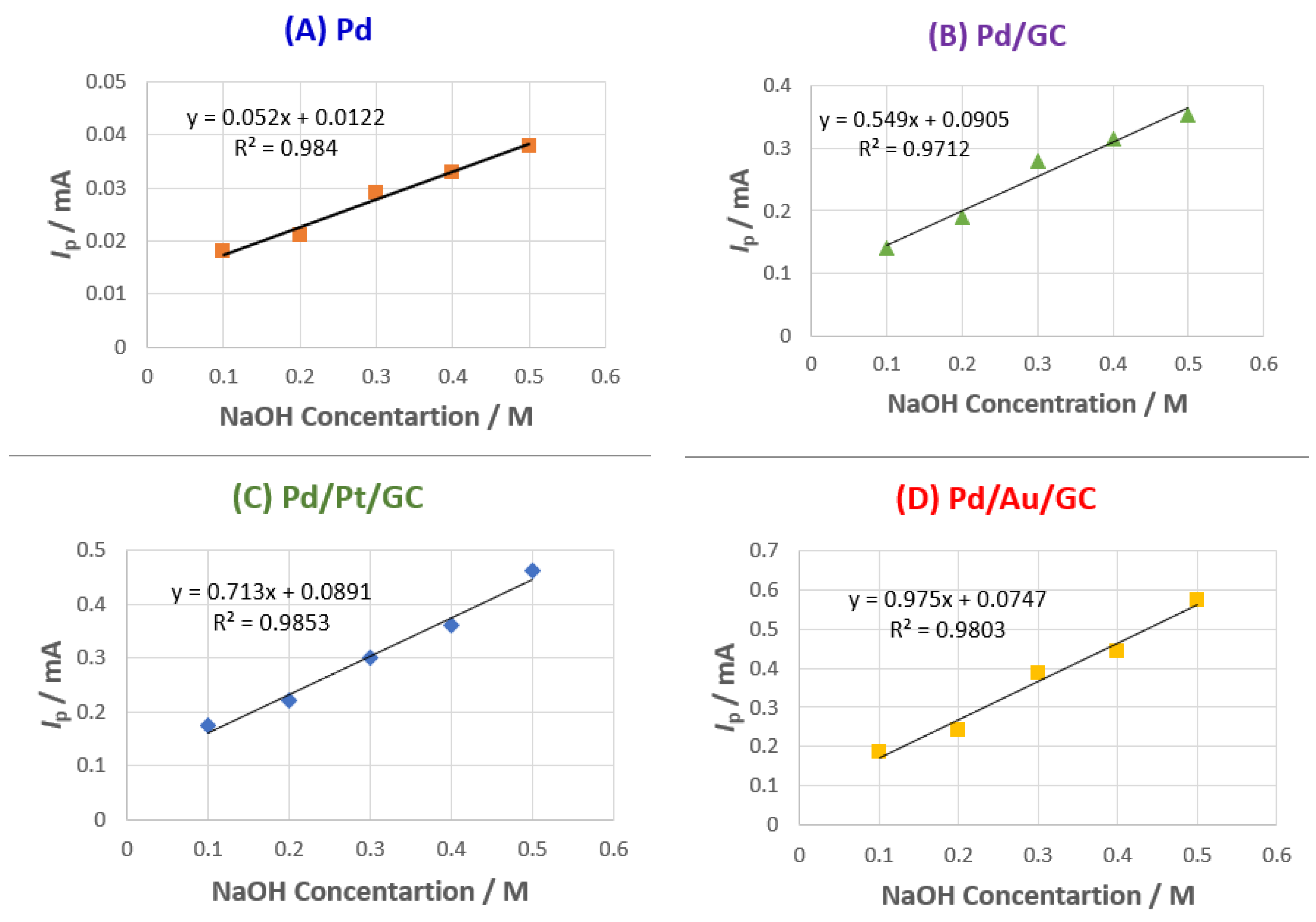
To achieve a better electrocatalytic 2POR, the effect of changing the NaOH concentration and scan rate were examined.
Figure 7A–D shows the effect of changing the NaOH concentration on the I
p value of the 2POR at all proposed catalysts. It was obvious that increasing the NaOH concentration increases the I
p value in a linear way with a correlation coefficient of ca. 0.98. It seems that increasing the OH
− concentration facilitated the removal of the adsorbed intermediates that increased the availability of more active sites for the 2POR [
43].
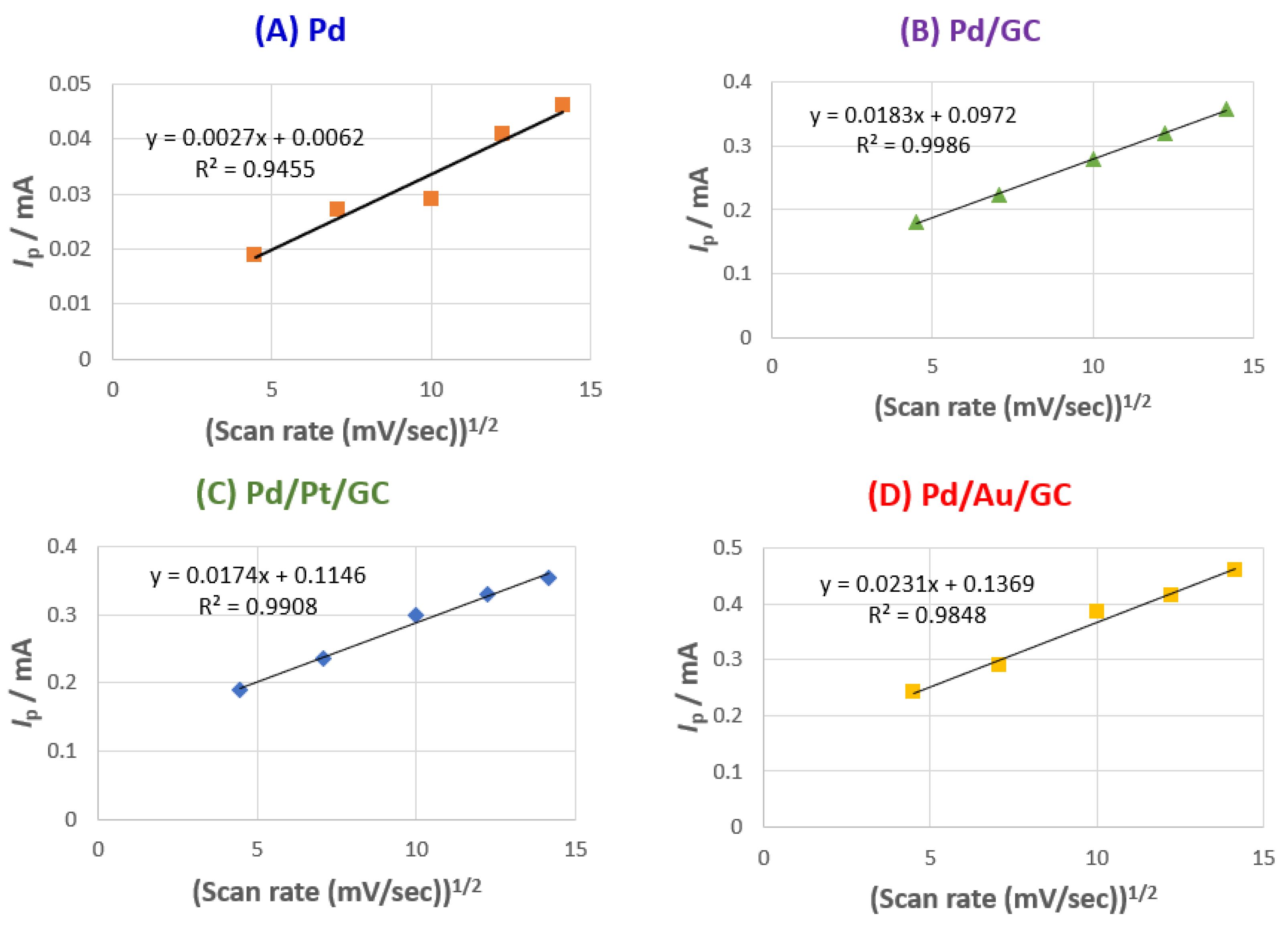
Additionally,
Figure 8A–D shows the effect of changing the scan rate on the
Ip value of the 2POR at all proposed catalysts. It was clear that a slight shift of the
Ip to more positive values was observed with increasing the potential scan rates, suggesting a kinetic limitation [
44]. Moreover, as the scan rate increases, the
Ip value increases and a linear dependence of the square root of the scan rate and the
Ip was observed with a correlation coefficient ranging from 0.95 to 0.99, which confirmed the diffusion-controlled process [
45].
4. Conclusion
This research aimed at examining the electro-catalytic performance toward 2POR in an alkaline medium at several proposed (Pd/GC, Pd/Pt/GC, and Pd/Pt/GC) nanocatalysts. The Pd/Au/GC catalyst showed the highest catalytic activity and stability in terms of the highest current values. Nyquist plots assessed the charge transfer enhancement where the Pd/Au/GC catalyst showed the fastest charge transfer kinetics (the lowest Rct value) toward the 2POR. From another perspective, the effects of changing the potential scan rate and the NaOH concentration on the oxidation currents were monitored and several mechanistic correlation were deduced in view of the recommended modifications at all the proposed catalysts.
Author Contributions
Conceptualization, Islam M. Al-Akraa and Ahmad M. Mohammad; methodology, Kareem Ayman and Yaser M. Asal; Formal analysis, Yaser M. Asal and Islam M. Al-Akraa; Investigation, Islam M. Al-Akraa and Ahmad M. Mohammad; Resources, Islam M. Al-Akraa; Writing—original draft preparation, Kareem Ayman and Islam M. Al-Akraa; Writing—review & editing, Ahmad M. Mohammad; Supervision, Islam M. Al-Akraa. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pang, L.; Zhu, M.N.; Yu, H. Is green finance really a blessing for green technology and carbon efficiency? Energy Economics 2022, 114, 106272. [Google Scholar] [CrossRef]
- Su, C.-W.; Pang, L.-D.; Qin, M.; Lobonţ, O.-R.; Umar, M. The spillover effects among fossil fuel, renewables and carbon markets: Evidence under the dual dilemma of climate change and energy crises. Energy 2023, 274, 127304. [Google Scholar] [CrossRef]
- Molodtsova, T.; Gorshenkov, M.; Kubrin, S.; Saraev, A.; Ulyankina, A.; Smirnova, N. One-step access to bifunctional γ-Fe2O3/δ-FeOOH electrocatalyst for oxygen reduction reaction and acetaminophen sensing. Journal of the Taiwan Institute of Chemical Engineers 2022, 140, 104569. [Google Scholar] [CrossRef]
- Kaya, S. Synthesis, characterization and 1-propanol electrooxidation application of carbon nanotube supported bimetallic catalysts. International Journal of Hydrogen Energy 2023. [Google Scholar] [CrossRef]
- Farrag, H.H.; Al-Akraa, I.M.; Allam, N.K.; Mohammad, A.M. Amendment of palladium nanocubes with iron oxide nanowires for boosted formic acid electro−oxidation. Arabian Journal of Chemistry 2023, 16. [Google Scholar] [CrossRef]
- Hassan, H.E.; Asal, Y.M.; Mohammad, A.M.; Al-Akraa, I.M. BIODIESEL PRODUCTION FROM CASTOR OIL: MIXING OPTIMIZATION DURING TRANSESTERIFICATION. ARPN Journal of Engineering and Applied Sciences 2022, 17, 844–848. [Google Scholar]
- Ayman, R.; Asal, Y.M.; Mohammad, A.M.; Al-Akraa, I.M. CASTOR OIL CONVERSION TO BIODIESEL: A PROCESS SIMULATION STUDY. ARPN Journal of Engineering and Applied Sciences 2022, 17, 964–968. [Google Scholar]
- Al-Akraa, I.M.; Ohsaka, T.; Mohammad, A.M. A promising amendment for water splitters: Boosted oxygen evolution at a platinum, titanium oxide and manganese oxide hybrid catalyst. Arabian Journal of Chemistry 2019, 12, 897–907. [Google Scholar] [CrossRef]
- Al-Akraa, I.M.; Asal, Y.M.; Khamis, S.D. Assembling of NiOx/MWCNTs-GC anodic nanocatalyst for water electrolysis applications. International Journal of Electrochemical Science 2018, 13, 9712–9720. [Google Scholar] [CrossRef]
- Al-Akraa, I.M.; Asal, Y.M.; Arafa, A.M. Fabrication of MnOx/MWCNTs-GC nanocatalyst for oxygen evolution reaction. International Journal of Electrochemical Science 2018, 13, 8775–8783. [Google Scholar] [CrossRef]
- Al-Akraa, I.M.; Mohammad, A.M.; El-Deab, M.S.; El-Anadouli, B.E. Flower-shaped gold nanoparticles: Preparation, characterization, and electrocatalytic application. Arabian Journal of Chemistry 2017, 10, 877–884. [Google Scholar] [CrossRef]
- Asal, Y.M.; Mohammad, A.M.; Abd El Rehim, S.S.; Al-Akraa, I.M. Co-electrodeposited PtPd anodic catalyst for the direct formic acid fuel cells. Energy Reports 2022, 8, 560–564. [Google Scholar] [CrossRef]
- Al-Qodami, B.A.; Alalawy, H.H.; Sayed, S.Y.; Al-Akraa, I.M.; Allam, N.K.; Mohammad, A.M. Tailor-designed nanowire-structured iron and nickel oxides on platinum catalyst for formic acid electro-oxidation. RSC Advances 2022, 12, 20395–20402. [Google Scholar] [CrossRef] [PubMed]
- Al-Qodami, B.A.; Alalawy, H.H.; Al-Akraa, I.M.; Sayed, S.Y.; Allam, N.K.; Mohammad, A.M. Surface engineering of nanotubular ferric oxyhydroxide “goethite” on platinum anodes for durable formic acid fuel cells. International Journal of Hydrogen Energy 2022, 47, 264–275. [Google Scholar] [CrossRef]
- Al-Akraa, I.M.; Mohammad, A.M. A spin-coated TiOx-modified Pt anodic catalyst for the direct methanol fuel cells. Energy Reports 2022, 8, 438–442. [Google Scholar] [CrossRef]
- Al-Akraa, I.M.; Salama, A.E.; Asal, Y.M.; Mohammad, A.M. Boosted performance of NiOx/Pt nanocatalyst for the electro-oxidation of formic acid: A substrate’s functionalization with multi-walled carbon nanotubes. Arabian Journal of Chemistry 2021, 14. [Google Scholar] [CrossRef]
- Rubio, G.A.; Agila, W.E. A Fuzzy Model to Manage Water in Polymer Electrolyte Membrane Fuel Cells. Processes 2021, 9. [Google Scholar] [CrossRef]
- Shyu, J.-C.; Hung, S.-H. Flow Field Effect on the Performance of Direct Formic Acid Membraneless Fuel Cells: A Numerical Study. Processes 2021, 9. [Google Scholar] [CrossRef]
- Siwal, S.S.; Thakur, S.; Zhang, Q.B.; Thakur, V.K. Electrocatalysts for electrooxidation of direct alcohol fuel cell: chemistry and applications. Materials Today Chemistry 2019, 14, 100182. [Google Scholar] [CrossRef]
- Berretti, E.; Longhi, M.; Atanassov, P.; Sebastián, D.; Lo Vecchio, C.; Baglio, V.; Serov, A.; Marchionni, A.; Vizza, F.; Santoro, C.; et al. Platinum group metal-free Fe-based (FeNC) oxygen reduction electrocatalysts for direct alcohol fuel cells. Current Opinion in Electrochemistry 2021, 29, 100756. [Google Scholar] [CrossRef]
- Logsdon, J.E.; Loke, R.A. Isopropyl Alcohol. In Kirk-Othmer Encyclopedia of Chemical Technology; 2000.
- Nacef, M.; Lelièvre-Desmas, M.; Drider, D.; Flahaut, C.; Chollet, S. Artisanal and industrial Maroilles cheeses: Are they different? Comparison using sensory, physico-chemical and microbiological approaches. International Dairy Journal 2019, 89, 42–52. [Google Scholar] [CrossRef]
- Qi, Z.; Kaufman, A. Performance of 2-propanol in direct-oxidation fuel cells. Journal of Power Sources 2002, 112, 121–129. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Hu, L.; Zhuang, L.; Lu, J.; Xu, B. A feasibility analysis for alkaline membrane direct methanol fuel cell: thermodynamic disadvantages versus kinetic advantages. Electrochemistry Communications 2003, 5, 662–666. [Google Scholar] [CrossRef]
- Lu, J.; Lu, S.; Wang, D.; Yang, M.; Liu, Z.; Xu, C.; Jiang, S.P. Nano-structured PdxPt1−x/Ti anodes prepared by electrodeposition for alcohol electrooxidation. Electrochimica Acta 2009, 54, 5486–5491. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, Y.; Liu, R.; Wu, H.; Wang, G.; Cao, D. Poisoning of acetone to Pt and Au electrodes for electrooxidation of 2-propanol in alkaline medium. Electrochimica Acta 2012, 76, 174–178. [Google Scholar] [CrossRef]
- Markiewicz, M.E.P.; Bergens, S.H. Electro-oxidation of 2-propanol and acetone over platinum, platinum–ruthenium, and ruthenium nanoparticles in alkaline electrolytes. Journal of Power Sources 2008, 185, 222–225. [Google Scholar] [CrossRef]
- Santasalo-Aarnio, A.; Kwon, Y.; Ahlberg, E.; Kontturi, K.; Kallio, T.; Koper, M.T.M. Comparison of methanol, ethanol and iso-propanol oxidation on Pt and Pd electrodes in alkaline media studied by HPLC. Electrochemistry Communications 2011, 13, 466–469. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, C.; Xu, J.; Du, Y.; Yang, P. Enhanced electrocatalytic performance for isopropanol oxidation on Pd–Au nanoparticles dispersed on poly(p-phenylene) prepared from biphenyl. Materials Chemistry and Physics 2010, 123, 390–395. [Google Scholar] [CrossRef]
- Santasalo, A.; Vidal-Iglesias, F.J.; Solla-Gullón, J.; Berná, A.; Kallio, T.; Feliu, J.M. Electrooxidation of methanol and 2-propanol mixtures at platinum single crystal electrodes. Electrochimica Acta 2009, 54, 6576–6583. [Google Scholar] [CrossRef]
- Liu, J.; Ye, J.; Xu, C.; Jiang, S.P.; Tong, Y. Electro-oxidation of methanol, 1-propanol and 2-propanol on Pt and Pd in alkaline medium. Journal of Power Sources 2008, 177, 67–70. [Google Scholar] [CrossRef]
- Asal, Y.M.; Mohammad, A.M.; El Rehim, S.S.A.; Al-Akraa, I.M. Preparation of Co-electrodeposited Pd-Au Nanocatalyst for Methanol Electro-oxidation. International Journal of Electrochemical Science 2021, 16, 1–11. [Google Scholar] [CrossRef]
- Al-Akraa, I.M.; Mohammad, A.M.; El-Deab, M.S.; El-Anadouli, B.E. Self-assembling of gold nanoparticles array for electro-sensing applications. International Journal of Electrochemical Science 2013, 8, 458–466. [Google Scholar] [CrossRef]
- Markiewicz, M.E.P.; Hebert, D.M.; Bergens, S.H. Electro-oxidation of 2-propanol on platinum in alkaline electrolytes. Journal of Power Sources 2006, 161, 761–767. [Google Scholar] [CrossRef]
- Xu, C.; Tian, Z.; Chen, Z.; Jiang, S.P. Pd/C promoted by Au for 2-propanol electrooxidation in alkaline media. Electrochemistry Communications 2008, 10, 246–249. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, Y.; Cao, D.; Wang, G.; Gao, Y. Effects of acetone on electrooxidation of 2-propanol in alkaline medium on the Pd/Ni-foam electrode. Journal of Power Sources 2011, 196, 3124–3128. [Google Scholar] [CrossRef]
- Asal, Y.M.; Mohammad, A.M.; Abd El Rehim, S.S.; Al-Akraa, I.M. Augmented formic acid electro-oxidation at a co-electrodeposited Pd/Au nanoparticle catalyst. Journal of Saudi Chemical Society 2022, 26, 101508. [Google Scholar] [CrossRef]
- Farrag, H.H.; Al-Akraa, I.M.; Allam, N.K.; Mohammad, A.M. Amendment of palladium nanocubes with iron oxide nanowires for boosted formic acid electro−oxidation. Arabian Journal of Chemistry 2023, 16, 104524. [Google Scholar] [CrossRef]
- Al-Akraa, I.M.; Asal, Y.M.; Darwish, S.A.; Fikry, R.M.; Mahmoud, R.H.; Hassan, M.; Mohammad, A.M. Effect of Palladium Loading on Catalytic Properties of Pd/GCE for the Electro-oxidation of Methanol, Formic Acid, and Ethylene Glycol. International Journal of Electrochemical Science 2022, 17, 220455. [Google Scholar] [CrossRef]
- Al-Akraa, I.M.; Asal, Y.M.; Mohammad, A.M. Surface engineering of Pt surfaces with Au and cobalt oxide nanostructures for enhanced formic acid electro-oxidation. Arabian Journal of Chemistry 2022, 15, 103965. [Google Scholar] [CrossRef]
- Mohammad, A.M.; Al-Akraa, I.M.; El-Deab, M.S. Superior electrocatalysis of formic acid electro-oxidation on a platinum, gold and manganese oxide nanoparticle-based ternary catalyst. International Journal of Hydrogen Energy 2018, 43, 139–149. [Google Scholar] [CrossRef]
- Al-Akraa, I.M.; Mohammad, A.M.; El-Deab, M.S.; El-Anadouli, B.E. Electrocatalysis by design: Synergistic catalytic enhancement of formic acid electro-oxidation at core–shell Pd/Pt nanocatalysts. International Journal of Hydrogen Energy 2015, 40, 1789–1794. [Google Scholar] [CrossRef]
- Hassan, K.M.; Hathoot, A.A.; Maher, R.; Abdel Azzem, M. Electrocatalytic oxidation of ethanol at Pd, Pt, Pd/Pt and Pt/Pd nano particles supported on poly 1,8-diaminonaphthalene film in alkaline medium. RSC Advances 2018, 8, 15417–15426. [Google Scholar] [CrossRef] [PubMed]
- Raoof, J.B.; Ojani, R.; Hosseini, S.R. An Electrochemical Investigation of Methanol Oxidation on Nickel Hydroxide Nanoparticles. South African Journal of Chemistry 2013, 66, 47–53. [Google Scholar]
- Danaee, I.; Jafarian, M.; Mirzapoor, A.; Gobal, F.; Mahjani, M.G. Electrooxidation of methanol on NiMn alloy modified graphite electrode. Electrochimica Acta 2010, 55, 2093–2100. [Google Scholar] [CrossRef]
Figure 1.
CVs obtained at the (a) Pd, (b) Pd/GC, (c) Pd/Pt/GC, and (e) Pd/Au/GC catalysts in 0.1 M NaOH. Potential scan rate: 100 mV s−1.
Figure 1.
CVs obtained at the (a) Pd, (b) Pd/GC, (c) Pd/Pt/GC, and (e) Pd/Au/GC catalysts in 0.1 M NaOH. Potential scan rate: 100 mV s−1.
Figure 2.
FE-SEM images of the (A) Pd/GC, (B) Pd/Pt/GC, and (C) Pd/Au/GC catalysts.
Figure 2.
FE-SEM images of the (A) Pd/GC, (B) Pd/Pt/GC, and (C) Pd/Au/GC catalysts.
Figure 3.
EDX analyses of the (a) Pd/GC, (b) Pd/Pt/GC, and (c) Pd/Au/GC catalysts.
Figure 3.
EDX analyses of the (a) Pd/GC, (b) Pd/Pt/GC, and (c) Pd/Au/GC catalysts.
Figure 4.
CVs obtained at the (a) Pd, (b) Pd/GC, (c) Pd/Pt/GC, and (d) Pd/Au/GC catalysts in 0.1 M NaOH solution containing 0.3 M 2-propanol. Potential scan rate: 100 mV s−1. Inset shows the current values normalized to the Pd mass (specific currents) of the (a) Pd/GC, (b) Pd/Pt/GC, and (c) Pd/Au/GC catalysts.
Figure 4.
CVs obtained at the (a) Pd, (b) Pd/GC, (c) Pd/Pt/GC, and (d) Pd/Au/GC catalysts in 0.1 M NaOH solution containing 0.3 M 2-propanol. Potential scan rate: 100 mV s−1. Inset shows the current values normalized to the Pd mass (specific currents) of the (a) Pd/GC, (b) Pd/Pt/GC, and (c) Pd/Au/GC catalysts.
Figure 5.
Current transients measured at −0.4 V at the (a) Pd, (b) Pd/GC, (c) Pd/Pt/GC, and (d) Pd/Au/GC catalysts in 0.1 M NaOH solution containing 0.3 M 2-propanol.
Figure 5.
Current transients measured at −0.4 V at the (a) Pd, (b) Pd/GC, (c) Pd/Pt/GC, and (d) Pd/Au/GC catalysts in 0.1 M NaOH solution containing 0.3 M 2-propanol.
Figure 6.
Nyquist plots obtained recorded at AC potential amplitude of −0.4 V obtained at the (a) Pd, (b) Pd/GC, (c) Pd/Pt/GC, and (d) Pd/Au/GC catalysts in 0.1 M NaOH solution containing 0.3 M 2-propanol. Frequency ranged from 10 mHz to 100 kHz.
Figure 6.
Nyquist plots obtained recorded at AC potential amplitude of −0.4 V obtained at the (a) Pd, (b) Pd/GC, (c) Pd/Pt/GC, and (d) Pd/Au/GC catalysts in 0.1 M NaOH solution containing 0.3 M 2-propanol. Frequency ranged from 10 mHz to 100 kHz.
Figure 7.
The linear correlation between Ip and NaOH concentration at the (A) Pd, (B) Pd/GC, (C) Pd/Pt/GC, and (D) Pd/Au/GC catalysts. The CVs measured for 2POR at 100 mV s1 in NaOH with different concentrations containing 0.3 M methanol (pH = 3.5).
Figure 7.
The linear correlation between Ip and NaOH concentration at the (A) Pd, (B) Pd/GC, (C) Pd/Pt/GC, and (D) Pd/Au/GC catalysts. The CVs measured for 2POR at 100 mV s1 in NaOH with different concentrations containing 0.3 M methanol (pH = 3.5).
Figure 8.
The linear correlation between Ip and the square root of the potential scan rate at the (A) Pd, (B) Pd/GC, (C) Pd/Pt/GC, and (D) Pd/Au/GC catalysts. The CVs measured for 2POR in 0.1 M NaOH containing 0.3 M methanol (pH = 3.5).
Figure 8.
The linear correlation between Ip and the square root of the potential scan rate at the (A) Pd, (B) Pd/GC, (C) Pd/Pt/GC, and (D) Pd/Au/GC catalysts. The CVs measured for 2POR in 0.1 M NaOH containing 0.3 M methanol (pH = 3.5).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).