Introduction & Background
The field of artificial intelligence (A.I) within medicine has witnessed a remarkable surge in publications. However, quantifying the clinical advantages of incorporating AI-assisted tools in patient care remained elusive until recently [
1]. A.I, characterized by its capacity to discern meaningful patterns from data sets and make decisions based on these patterns, offers valuable support in clinical decision-making processes [
2]. Machine learning (ML), an A.I subdomain, utilizes algorithms to empirically learn data patterns [
3]. Unlike conventional statistics, ML algorithms detect non-linear relationships, high-order interactions among multiple variables, and more subtle patterns [
3]. A.I, often perceived as human-like intelligence exhibited by a machine, encompasses systems based on ML, expert systems, and robotics. ML, in contrast, comprises a set of algorithms enabling a computer to learn a specific task through numerous examples.
Machine learning algorithms have their foundation in traditional statistics [
4]. The most basic ML model can utilize logistic regression [
4]. Nevertheless, more sophisticated methods, such as decision trees, support vector machines, random forests, or neural networks, provide the capability to handle intricate and non-linear relationships within data, circumventing improper dichotomization [
5]. Recently developed techniques encompass deep neural networks, commonly referred to as deep learning. These algorithms facilitate rapid advancements in image recognition, natural language processing, speech recognition, and are extensively employed in cutting-edge medical research [
6,
7]. Deep learning (DL) has evolved, necessitating large datasets and recent enhancements in computational power to make efficient decisions on intricate data [
3]. ML and DL have demonstrated success across diverse disciplines, including language processing, gaming, computer vision, engineering, industrial, and scientific fields, showcasing promise at multiple levels [
3].
Neural networks, artificial computational models designed to mimic biological neural networks, hierarchically transmit data through nodes in each layer [
8]. These networks have proven effective in pattern recognition tasks and can be implemented in various decision-making phases, delivering information, diagnoses, and even comprehensive clinical assessments based on medical history, clinical findings, laboratory results, and other relevant factors [
9,
10,
11,
12,
13,
14,
15]. Diverse data types can serve as input; however, human observational data may introduce interobserver variability, potentially reducing accuracy, particularly when utilized outside the system's development setting [
9]. Networks directly accepting data inputs, such as electrocardiographic signals, maintain higher accuracy since they provide the same precise data regardless of location [
10,
11,
12,
13]. Furthermore, Bio-signal data-based A.I tools are more prevalent than those based on clinical data [
1].
The increasing availability of digital health data and recent advancements in A.I technology (particularly in machine learning and deep neural networks) have enabled the rapid and accurate processing and analysis of vast data volumes, paving the way for A.I applications in cardiology. Additionally, cardiology represents a research-intensive, rapidly advancing, and dynamic discipline, with cardiologists, regularly making high-risk, high-reward decisions, thereby suggesting that A.I will play a crucial role in cardiology's future. With this context in mind, this review aims to examine the literature reporting the latest developments and implications of A.I in cardiovascular medicine.
Review
Artificial intelligence: A brief overview
Prior to delving into the various applications of artificial intelligence in cardiovascular medicine, this paper aims to provide clarity on technical terms that may be unfamiliar to readers within the context of A.I.
A.I definition and subfields:
There are several types of learning models, each with its own strengths and weaknesses, and suited to different types of tasks and datasets.
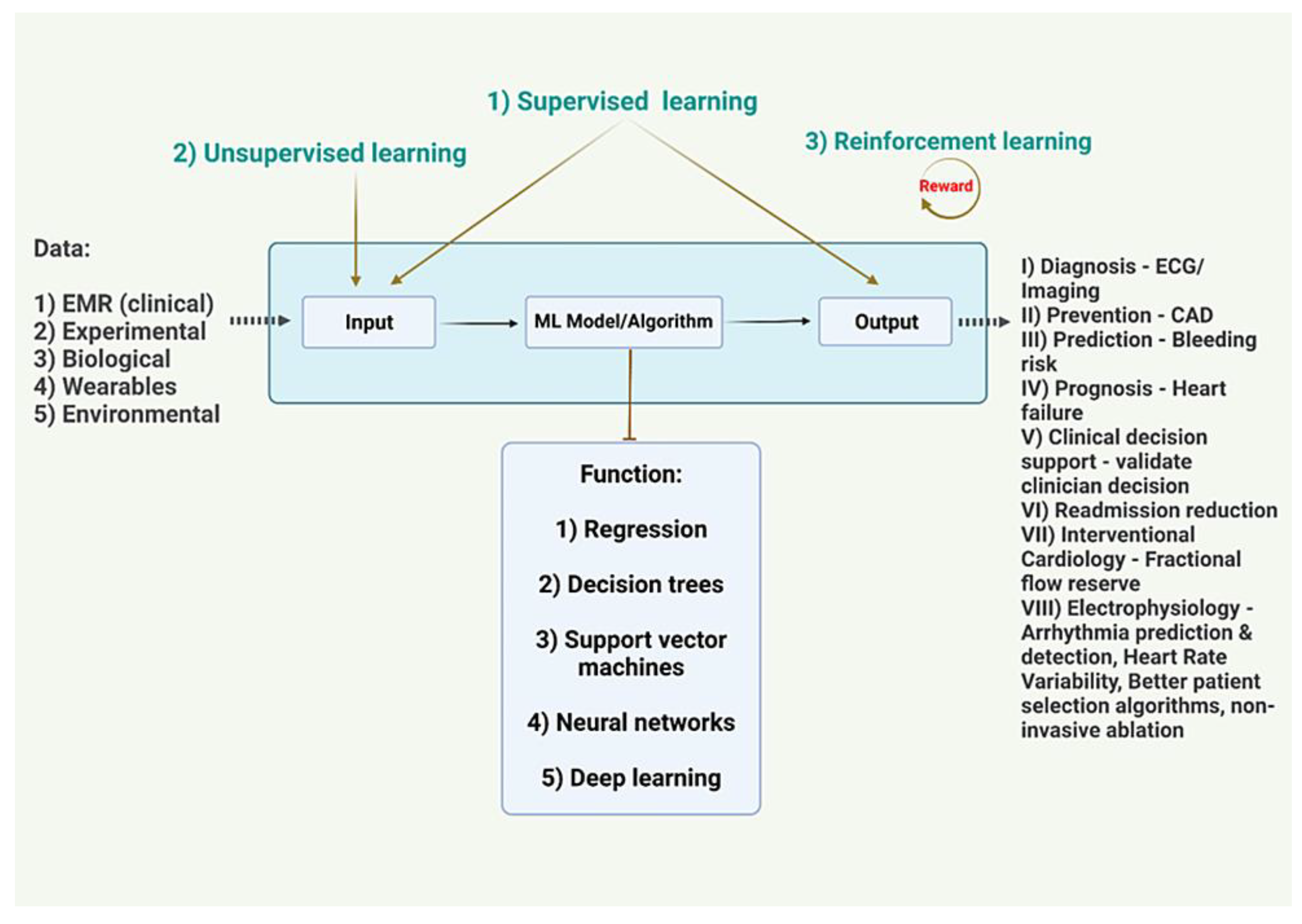
Figure 1.
EKG - Electrocardiogram
CAD - Coronary Artery Disease
EMR - Electronic Medical Record
Image Credits - Dr. Vijaya Durga Pradeep Ganipineni and Dr. Sai Dheeraj Gutlapalli
I) Supervised learning models:
These models are trained using labeled data, where the input data is paired with corresponding output or target values. Examples of supervised learning models include linear regression model, logistic regression, decision trees, random forests, support vector machines (SVMs), and artificial neural networks (ANNs) [
16,
17,
18,
19].
II) Unsupervised learning models:
These models are used when the data does not have any predefined labels or targets. The goal of these models is to identify patterns and relationships within the data. Examples of unsupervised learning models include clustering algorithms, such as K-means clustering, and dimensionality reduction techniques, such as principal component analysis (PCA) [
16,
17,
18,
19].
III) Semi-supervised learning models:
These models are used when only a small portion of the data is labeled. The goal is to use the labeled data to guide the learning of the model on the unlabeled data. Examples of semi-supervised learning models include generative models, such as variational autoencoders (VAEs), and self-training algorithms [
16,
17,
18,
19].
IV) Reinforcement learning models:
These models are used to train agents to make decisions based on a series of actions and rewards. The goal is to learn a policy that maximizes the cumulative reward over time. Examples of reinforcement learning models include Q-learning and deep reinforcement learning algorithms [
16,
17,
18,
19].
A model can be used to make predictions, classify data, or generate new data based on the patterns and relationships learned from a given dataset [
20,
21].
The goal of building a model is to create a generalizable representation of the underlying process or system that can be used to make predictions or decisions in real-world applications. The performance of a model is typically evaluated using metrics such as accuracy, precision, recall, and F1 score [
22].
The parameters in a deep learning model determine the multiple weights and biases of the connections between the layers of the network, allowing it to learn complex features and patterns in the input data [
22]. The use of large amounts of parametric data is a key feature of deep learning, as it enables the models to learn and generalize to new data [
23,
24].
Healthcare data:
The application of A.I in cardiovascular medicine has been fueled by the rapid growth and availability of healthcare data [
5]. Electronic health records (EHRs) are a valuable source of information, providing structured and unstructured data on patients' demographics, medical histories, diagnoses, treatments, and outcomes [
25]. The widespread adoption of EHRs has resulted in the generation of vast amounts of data that can be analyzed to improve patient care [
26].
Imaging data plays a critical role in cardiovascular medicine, including modalities such as echocardiography, magnetic resonance imaging (MRI), and computed tomography (CT) scans [
27]. These imaging techniques generate a plethora of data that can be used to identify disease patterns, monitor disease progression, and guide treatment decisions [
28]. A.I algorithms, particularly deep learning methods, have demonstrated remarkable success in interpreting and analyzing medical imaging data, enabling more accurate and faster diagnosis of cardiovascular diseases [
6].
Wearable devices and mobile health (mHealth) technologies have further expanded the available data for cardiovascular medicine [
29]. These devices continuously monitor physiological parameters such as heart rate, blood pressure, and physical activity, generating large volumes of real-time data [
30]. By incorporating this data into A.I algorithms, it is possible to create personalized treatment plans, predict disease progression, and improve patient outcomes [
18].
Omics data, including genomics, transcriptomics, proteomics, and metabolomics, have also been increasingly integrated into cardiovascular medicine [
31]. By leveraging A.I techniques to analyze these complex datasets, researchers have identified novel biomarkers, therapeutic targets, and insights into the underlying mechanisms of cardiovascular diseases [
32].
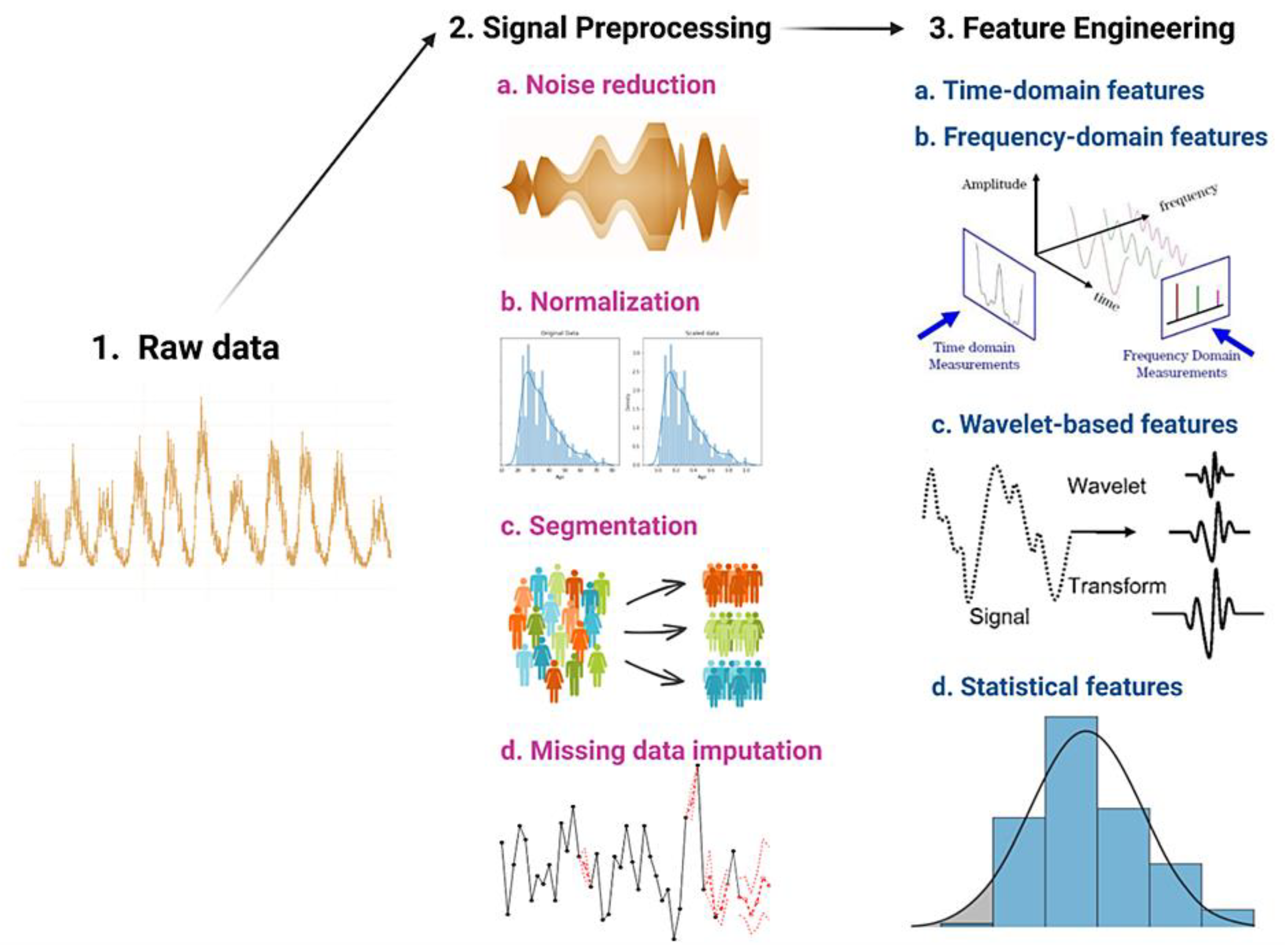
Raw health data once selected for input undergoes signal preprocessing and feature engineering to transform it into a analyzable format that can be used to develop machine learning models for various healthcare applications, such as disease diagnosis, personalized treatment, and health monitoring [
8]. Signal preprocessing involves filtering, noise removal, normalization, and other techniques to enhance the quality of the data, while feature engineering involves selecting and extracting relevant features or characteristics from the data to represent important aspects of the health condition or process being studied [
8]. These steps are crucial for ensuring the accuracy and reliability of the results obtained from machine learning algorithms trained on health data
Figure 2.
Despite the potential benefits of A.I in cardiovascular medicine, challenges remain in utilizing healthcare data effectively. Data privacy, security, and interoperability are key concerns that must to be addressed to ensure the safe and efficient use of healthcare data in A.I applications [
33]. Additionally, the quality and representativeness of healthcare data are crucial for ensuring that A.I models are accurate and unbiased [
34].
The assertion by John McCarthy who coined the term "artificial intelligence" that the quality of the input determines the effectiveness of A.I serves as a basis for an in-depth analysis of the crucial role played by robust data feeding and validation processes in the development of A.I systems and the associated limitations.
Methods of A.I application in cardiology:
Although the concept of A.I was first proposed in the 1950s, its application in the healthcare sector has only recently expanded. This expansion is due to A.I's ability to process a significant volume of data, making it useful for risk prediction, cardiovascular imaging, and electrophysiology in cardiology. In the near future, A.I is expected to have a tremendous impact on the diagnosis and management of cardiovascular diseases, leading to a paradigm shift. However, it is crucial to note that as powerful as A.I techniques can be, they must be motivated by clinical problems and applied to assist clinical practice ultimately [
17,
19,
35].
I) Clinical Practice:
Artificial intelligence algorithms are being employed to improve the accuracy and efficiency of cardiovascular disease diagnosis and treatment selection. Machine learning models are utilized to analyze medical data such as ECGs, echocardiograms, and medical imaging for early diagnosis and risk stratification [
5]. By incorporating patient-specific data, including medical history, genetic information, and lifestyle factors, AI can assist clinicians in tailoring treatment strategies to optimize patient response and minimize adverse effects [
5].
II) Research and Development:
AI-powered drug discovery is being used to accelerate the identification and development of novel therapeutic agents for cardiovascular diseases. Machine learning algorithms analyze large datasets of molecular structures, biological targets, and drug interactions to identify potential drug candidates more efficiently than traditional methods [
14]. Precision disease stratification enables healthcare professionals to identify patient-specific risk factors, understand disease progression, and predict response to therapy [
5]. A.I has the potential to integrate multi-omic data, including genomics, transcriptomics, proteomics, and metabolomics, to provide a comprehensive understanding of disease mechanisms and identify potential therapeutic targets [
5].
III) Population Health:
A.I can assist healthcare providers and policymakers in allocating resources more effectively by identifying areas of greatest need and predicting the demand for healthcare services. AI-driven tools can augment physician decision-making by providing real-time clinical decision support and reducing the cognitive load on healthcare professionals. AI-powered remote monitoring technologies, such as wearable devices and telemedicine platforms, can enable continuous patient monitoring outside of the clinical setting, facilitating early detection of cardiovascular events and empowering patients to take a more proactive role in managing their health [
5].
Challenges and limitations of A.I in cardiology:
Despite the many benefits of A.I in cardiology, limitations exist. One significant concern revolves around the "black box" problem where A.I systems mechanisms and processes are not readily understood by observers or users [
3,
8,
21]. This issue restricts the technology's usefulness in research and innovation since understanding how models generate their outcomes is challenging and critical in refining or improving them.
Explainability, Uncertainty, Robustness in A.I:
Explainability, also known as interpretability, is a crucial aspect of artificial intelligence applications in cardiovascular medicine. As A.I models become more complex, it becomes increasingly challenging to understand the rationale behind their predictions and decisions [
36]. Explainable A.I (XAI) aims to provide insights into the inner workings of A.I models, facilitating the understanding of the relationships between input features and output predictions [
37]. This is particularly important in healthcare, where clinicians must trust the A.I models' recommendations to make informed treatment decisions. Incorporating XAI techniques, such as LIME (Local Interpretable Model-agnostic Explanations) and SHAP (SHapley Additive exPlanations), helps to bridge the gap between model predictions and clinical decision-making, ensuring transparency and fostering trust in AI solutions [
38].
Uncertainty quantification is another critical aspect of A.I applications in cardiovascular medicine. Inherent uncertainty in healthcare data and the stochastic nature of A.I models can lead to predictions with varying degrees of confidence [
39]. Estimating and conveying this uncertainty is essential for clinicians to gauge the reliability of A.I model predictions and make informed decisions [
40]. Bayesian methods, such as Monte Carlo Dropout and Bayesian Neural Networks, provide a principled way to estimate uncertainty in A.I models, allowing clinicians to better understand the limitations of A.I-based predictions and avoid overreliance on potentially erroneous recommendations [
41].
Robustness is the ability of A.I models to maintain their performance in the face of perturbations, such as noisy data, missing values, or adversarial attacks [
42]. In the context of cardiovascular medicine, A.I models must be robust to variations in data quality, acquisition protocols, and patient populations to ensure accurate and reliable predictions across diverse clinical settings [
43]. Techniques such as data augmentation, transfer learning, and adversarial training help improve the robustness of A.I models, making them more resilient to changes in input data and potential attacks [
44]. Ensuring robustness in A.I applications is crucial to maintain patient safety and enable the widespread adoption of A.I solutions in cardiovascular medicine.
In decision-making areas within cardiology, such as the diagnosis and treatment of patients, A.I can assist physicians in making more informed choices. However, legal, and ethical challenges emerge when A.I is used to make decisions that affect patients lives. In such cases, the workings of A.I systems must be fully transparent and accountable individuals can assess these systems fairness and accuracy. Another limitation to A.I in cardiology is the interpretability of complex models. Although such models can predict patient outcomes effectively, they can be difficult to explain to physicians or patients. This interpretability concern may hinder the commitment to and acceptance of A.I in cardiology. Furthermore, A.I's reliance on large data sets can also serve as hurdles in smaller, resource-limited clinical settings. A.I models that necessitate extensive amounts of data to produce accurate predictions would be insignificant in such settings.
Addressing these limitations will prove critical in fully realizing the potential of A.I in cardiology. Researchers and technicians concerted effort in promoting transparency, explainability, and interpretability of A.I algorithms can help advance the understanding of how A.I systems work and allow for more accurate predictions and better patient care.
Future directions of A.I in cardiology:
The future implications of A.I in cardiology are vast and promising. One of the most significant benefits is the potential to accelerate cardiovascular research. With the ability to process extremely large amounts of data much more quickly than humans can, A.I can analyze patient and population data to identify new patterns in medication and drug interactions. This process could lead to the development of more effective treatments and drug therapies. A.I also has implications for CRISPR models, where it can facilitate the prediction of gene targeting and increase the accuracy of gene editing. Bioprinting, another emerging field in cardiology, could be made even more precise with the use of A.I. By integrating biomechanics with A.I, we could produce custom-made, semisynthetic heart valves that mimic the function of natural valves more closely.
Athletes could benefit from A.I-based cardiac bio-enhancement technology, which would help detect and track cardiac function changes and optimize training programs. A.I technology can help predict stent longevity, and improve the placement and deployment of these devices. In addition, A.I could be used to develop nanoparticle-mediated thrombolysis and removal of plaques in a targeted and efficient manner. Another important development would be identifying novel biomarkers that can help with the very early detection and diagnosis of cardiovascular disease. Finally, in the future, robotic PCI could become more advanced with the use of A.I, making cardiac surgery safer and more precise.
The potential for using A.I in cardiac electrophysiology is still being studied. Further research is needed to explore how A.I can be used to investigate genomic and proteomic data, histological characterization, and drug discovery for treating arrhythmia.One significant advantage of A.I is that it can combine different types of patient data to develop more accurate diagnoses. Currently, most A.I research uses data independently collected from institutions, which makes it difficult to combine data across different centers. Collecting, labeling, and standardizing clinical data and outcomes would make it easier to generate more reliable and meaningful results. Making harmonized data accessible in public repositories, such as the EKG databases available from PhysioNet, would make it easier to develop AI for cardiac electrophysiology. However, there is still a significant need to increase accessibility to various other types of essential data [
3].The next challenge is developing a framework for incorporating A.I into clinical practice while considering patient safety, privacy, data transfer, and access. Clinical and technical experts must partner to validate prospective clinical studies to ensure the reliability and benefit of A.I. Additionally, commercial establishment, FDA approval, and reimbursement mechanisms must be addressed when implementing A.I into clinical practice [
3]. Overall, the future looks very bright for A.I in cardiology, and its potential applications could have a significant impact on the clinical diagnosis, treatment, and prevention of cardiovascular disease.
Author Contributions
1st Author - GV, 2nd Author - GSD, 3rd Author - PJ, 4th Author - OI, 5th Author - UD, 6th Author - FM, 7th Author - FD, 8th Author - KT, 9th Author - PG, 10th Author - IASK, 11th Author - AZ, 12th Author - AM, 13th Author - GS, 14th Author - NJ, 15th Author – RF. Concept, design and writing of the abstract was done by FD & OI and reviewed by GSD, GV, GS, RF & NJ. The Concept of the study and the overall structure and framework of the paper was concieved by GSD, GS, GV, NJ and discussed with and approved by AM, AZ, IASK, FD, OI, PJ, KT, & UD. The General discussion was structured and written by PJ,KT,AZ & AM and was edited and finalized by GSD, GS, GV & NJ. The Conclusion was written by PG & UD. The conclusion was screened by all of the authors as well chiefly GSD, GS, GV, NJ and final approval was based on unanimous consensus. Images and Figures - GSD, GV and Edited by FM, DS, OI, PJ, KT, UD, AM, IASK. Each of the authors has made substantial contributions to the concept, design, analysis and worked with the data. They have drafted and revised the article and put in their own intellectual inputs, each of them have approved the final form of the manuscript for Publication