1. Introduction
Symptomatic Pelvic Congestion Syndrome (PCS) accounts for 30-40% of female chronic pelvic pain cases in the United States, affecting as much as 40% of the female population during their lifetime [
1]–[
3]. Although awareness of PCS has continued to rise, several factors have led to a high incidence of underdiagnosis. Traditionally, the most common symptoms include pelvic pain/heaviness, dysmenorrhea, dyspareunia, bladder irritability and/or urgency, lower extremity varices, back and/or leg pain, worsening of symptoms during/after pregnancy, and presence of visible or non-visible pelvic varices. No one symptom has been shown to accurately predict diagnosis. Treatment includes embolization of the gonadal or hypogastric veins, however, many question the effectiveness due to high reoccurrence rates.
A new framework focused on the positional nature of symptoms has previously been proposed [
4]. Positional symptoms include pain worsening after long periods of standing, walking, sitting, or exacerbated by activity [
5]. For those with pelvic varicose veins, pelvic venous reflux severity correlates with pelvic pain. Moderate to severe reflux in the parametrial, uterine, and gonadal is a predictor of severe pelvic pain [
6]. For diagnostic purposes, the anatomy of the female pelvic area can be divided into the anterior pelvic region (APR) and the posterior pelvic region (PPR). Structures of the APR include the urinary bladder, uterus, ovaries, vulva, groin, gonadal vein, and left renal vein (LRV). Pathologies stemming from this region likely cause anterior symptoms (urinary frequency, dyspareunia, dysmenorrhea). Structures of the PPR include the levator ani, psoas, spinal canal, piriformis, and hip muscles, the rectum, the sciatic nerve, and the bilateral common iliac veins and their tributaries. Pathologies in this region include leg/hip pain, pelvic or upper thigh pain, or pain in the lower back.
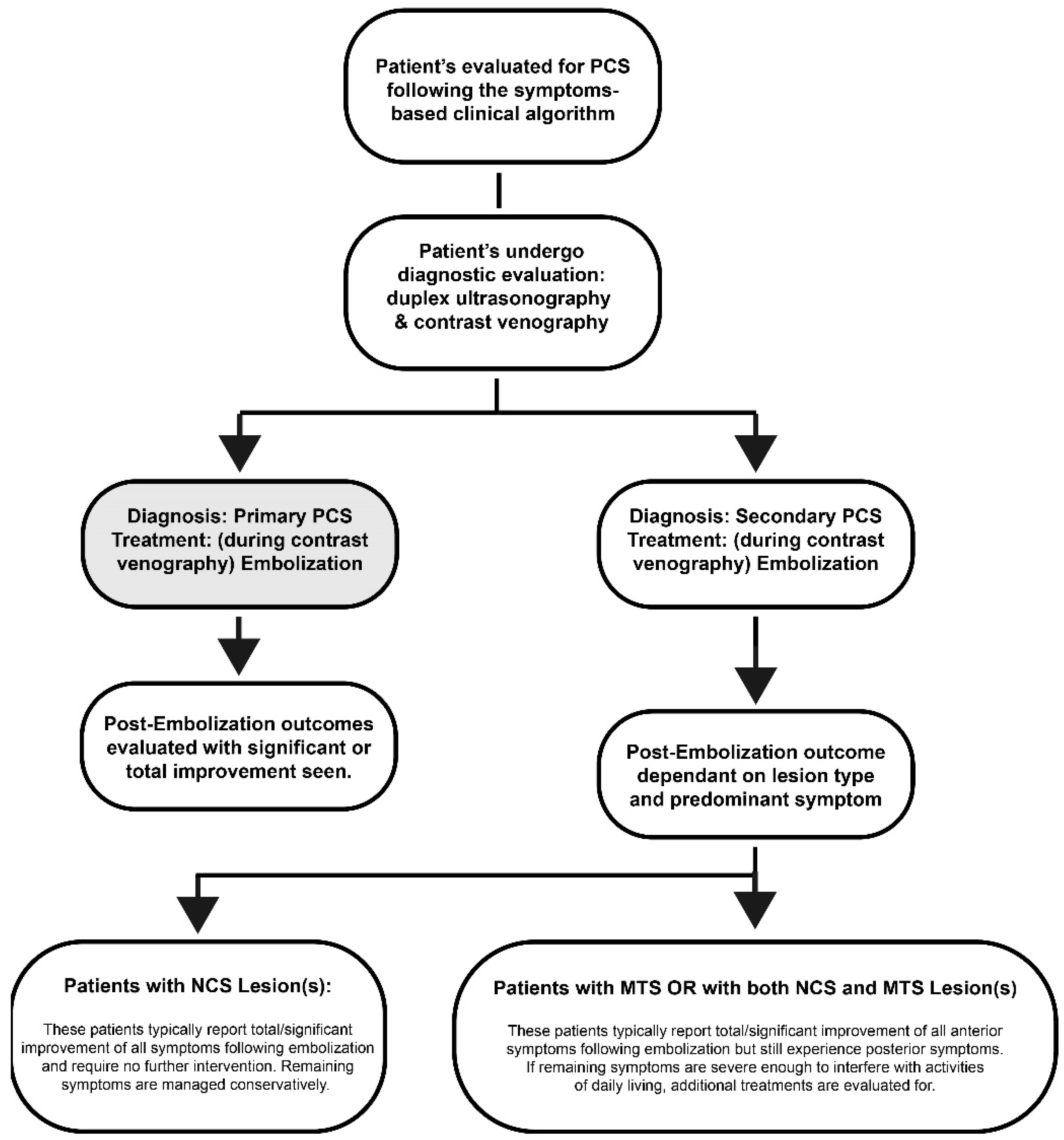
When treating patients, it is important to differentiate between different types of PCS. Type I PCS, known as “Primary Pelvic Congestion Syndrome” or “Pelvic Venous Insufficiency”, is defined by the presence of incompetent ovarian and/or internal iliac veins [
1], [
7]. The cause is often hormonal. Type I PCS is almost exclusively seen in women of child-bearing age. Type II PCS, described as “Secondary Pelvic Congestion Syndrome,” is caused by obstructed outflow due to compression of the pelvic veins. Its presentation is related to the type and location of the associated lesion(s). For example, in PCS secondary to Nutcracker Syndrome (NCS), compression of the LRV can cause retrograde filling of the left gonadal vein leading patients to experience anterior symptoms although differences in opinion and in the data exist [
6], [
8], [
9]. In PCS secondary to May-Thurner Syndrome (MTS), iliac vein compression can cause retrograde filling of the internal iliac veins (IIVs) [
10]. Tributaries of the IIVs stem from both anterior and posterior pelvic structures, causing patients with PCS secondary to MTS to present with anterior symptoms, posterior symptoms, or a combination of the two [
11].
Patients presenting with symptoms of PCS are often sent for pelvic DUS with utilization of the Valsalva’s maneuver to accentuate venous filling. Diagnostic criteria includes tortuous pelvic veins measuring >6mm in diameter, reverse caudal or retrograde blood flow, slow blood flow <3cm/s, and dilated arcuate veins crossing the myometrium and communicating with bilateral pelvic varicosities [
12]–[
14]. The strongest indicator of diagnosis includes dilated ovarian veins and the presence of reverse caudal blood flow on DUS [
15], [
16]. DUS remains the safest, most cost-effective method without the use of radiation, however several other non-invasive modalities may suggest the presence of PCS including Computed Tomography (CT), magnetic resonance imaging (MRI), and magnetic resonance venography (MRV) [
17]. DUS is recommended over transvaginal ultrasound (TVS) due to the invasive nature of TVS, limited ability to follow ovarian vein pathways, and challenge in identifying possible compressions/obstructions in the renal or iliac veins [
18]. Despite concerns of increased bowel gas or incomplete penetration of ultrasound waves in patients who have a high body mass index (BMI), DUS remains a useful first-line test for patients with suspicion of PCS.
For confirmatory diagnosis, contrast venography is the diagnostic gold-standard for PCS. Access can be gained through the femoral, jugular, or saphenous veins. Contrast is used as the diagnostic medium under fluoroscopy. The vessels evaluated include the inferior vena cava (IVC), bilateral internal and external iliac systems, bilateral gonadal veins, and the left renal vein. While there is some variation in the diagnostic criteria for PCS, most studies include one or more of the following to indicate confirmatory diagnosis: moderate-severe engorgement of the ovarian plexus, incompetent pelvic veins with a diameter >5-10mm, venous reflux indicated by (slow) proximal injection into ovarian veins with distal filling of ovarian venous plexus, filling of vulvar or thigh varicosities, filling of veins across the midline [
7], [
19]–[
21]. Treatment involves transcatheter embolization, often occurring during diagnostic venography, with variability in rates of long-term success [
19]. To date, there has yet to be randomized or high-quality placebo-controlled trials although studies comparing different forms of embolization exist [
7], [
22]. Studies report between 47%-100% of patients experience significant symptom relief 18 months to 5 years post-treatment [
23]–[
27]. Despite the high variability of results, transcatheter embolization during diagnostic contrast venography remains the most reliable treatment.
In this study, we evaluate the validity of a new diagnostic algorithm focused on symptoms and the efficacy of a secondary treatment algorithm focused on the type of PCS present. The incorporation of these algorithms into current practice can improve diagnosis, treatment, and outcomes for women suffering from chronic pelvic pain secondary to pelvic congestion syndrome.
2. Materials and Methods
The present study is a retrospective, single-institution, multi-site analysis of female patients who presented with pain symptoms suspicious of PCS between December 2016 and November 2019. Data was collected by a full review of patient documentation, including history and presentations, objective findings, duplex ultrasonography reports, contrast venography documentation, patient satisfaction forms, and patient-reported pre-operative and post-operative symptom surveys. This study was granted Institutional Review Board (IRB) exemption status following initial IRB review.
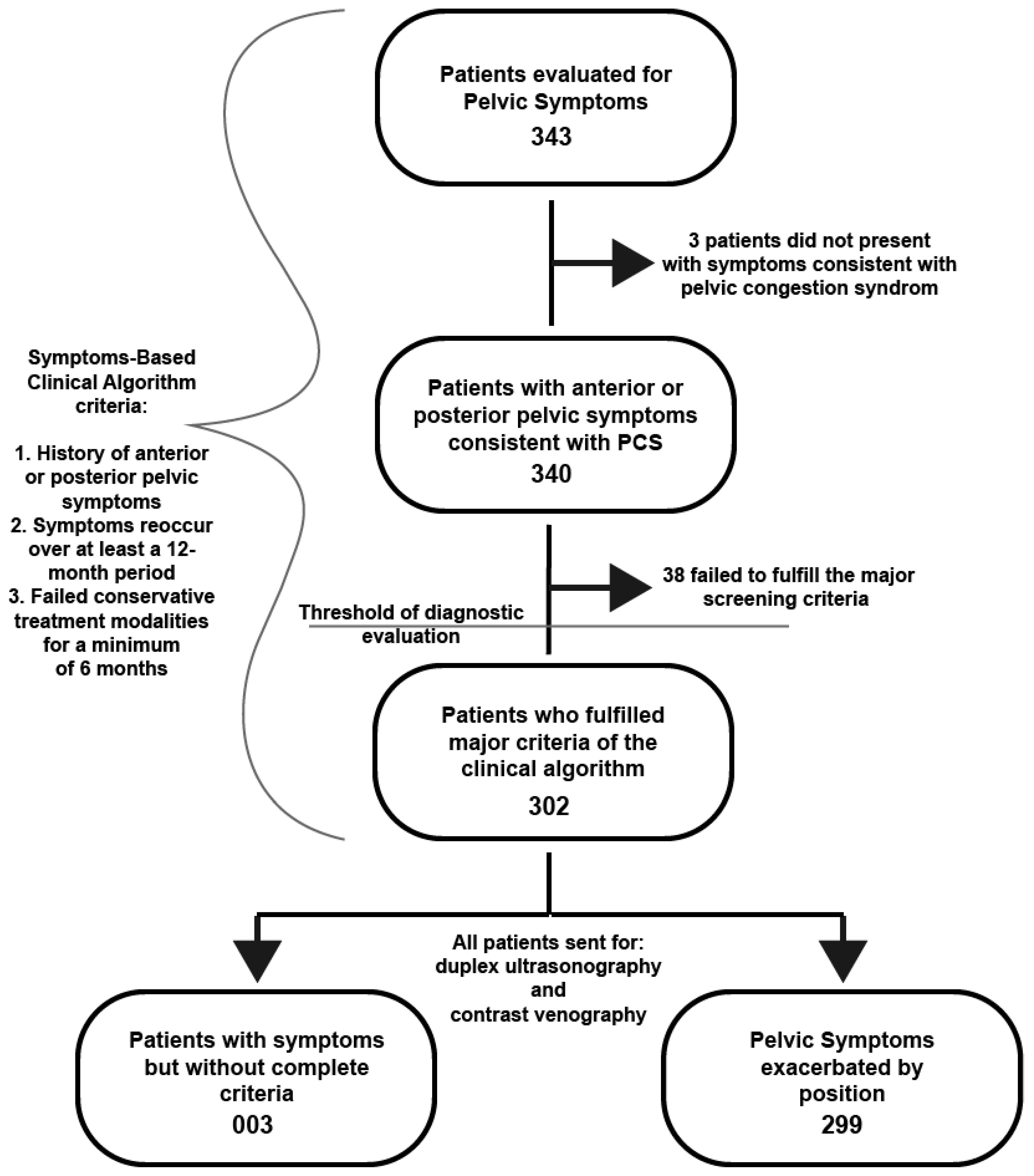
Patient Selection: During a 3-year period, 343 women presented with pelvic pain symptoms suspicious for PCS and were included for initial review. Patient’s subjective symptoms and clinical objective findings were assessed using a Symptoms-Based Clinical Algorithm (SBCA) comprised of four criteria. First criteria included female patients with a history of anterior or posterior pelvic symptoms that commonly accompany PCS (pelvic pain/heaviness, dysmenorrhea, dyspareunia, bladder irritability and/or urgency, lower extremity varices, back and/or leg pain, worsening of symptoms during/after pregnancy, and presence of visible or non-visible pelvic varices). The second criteria required that patients’ pelvic symptoms reoccurred multiple times over a minimum of a 12-month period. The third criteria required that patients had failed conservative treatment for a minimum period of 6 months. Patients that fulfilled the first, second, and third criteria met the threshold for diagnostic evaluation including duplex ultrasonography and contrast venography. Lastly, the fourth criteria included patients whose pelvic symptoms were positional in nature, i.e., exacerbated with long periods of standing, walking, sitting, or exacerbated by activity. Additional minor criteria included accurate completion of a pre-operative conservative management and symptom evaluation form, accurate completion of a post-operative patient satisfaction and symptom evaluation form during the time of their initial follow-up visit (approximately 1-2 weeks after procedure), accurate completion of a post-operative patient satisfaction and symptom evaluation form at the time of their 1-2 week, and 6-month follow-up visits.
After careful evaluation of the initial 343 patients, 41 patients were eliminated from participation, as they did not meet all the necessary inclusion criteria and were treated for disease processes not consistent with PCS or did not meet screening for PCS due to the length of their symptoms without conservative management attempts. 302 participants meet the criteria for complete PCS workup and diagnostic evaluation.
Data Collection: Patient age/date of birth, presence of symptoms, symptom type (anterior symptoms or posterior symptoms), and positional nature of symptoms were collected from physician reported history and physical. Severity of symptoms were collected from patient reported pre-operative forms filled out at the time of their initial consultation. Patients were asked to rate their symptoms on a scale from 0-10 (0 = least severe symptoms, 10 = most severe symptoms), the amount of time (in years) that they have been experiencing symptoms, and the amount of time (in months) that they have tried conservative management (non-steroidal anti-inflammatory drugs, leg elevation, walking/avoidance of prolonged standing, weight reduction, daily exercise i.e.: walking, biking, stairs, gym, etc., and prescription-strength compression stockings) for reduction of symptom severity. Operative reports were evaluated to obtain diagnosis (including PCS Type).
Endpoints: Success of treatment was measured using patient-reported satisfaction forms at the time of their post-operative follow-up examination approximately 1 week after treatment and during their follow-up visit at approximately 6 months following their procedure. Participants were asked to rate their post-operative symptoms on a scale of 0 - 10 (0 = least severe, 10 = most severe). They were also asked to rate their satisfaction with various aspects of their treatment (before, during, and after their procedure.) These aspects were rated on a scale from 1-5 (1 = least satisfied, 5 = most satisfied). Treatment satisfaction scores were averaged to obtain an “overall satisfaction” rating.
After evaluation including data from all patients, patients were separated into the following 4 groups to evaluate the effectiveness of treatment by PCS type/subtype: Type I - no lesions, Type II – Nutcracker syndrome (NCS) lesion(s), Type II – May-Thurner syndrome (MTS) lesion(s), Type II - NCS & MTS lesions.
Analysis: Statistical analysis was performed using GraphPad Prism Version 9.1.1 (San Diego, California) and Microsoft Excel Version 16.55 (Redmond, Washington). Data is percent as raw numbers or as a percent with the 95% confidence interval (CI) for quantitative variables. Odds ratio, likelihood ratio, sensitivity, specific, and predictive value significance was generated using Fisher’s exact test with P value cutoff of 0.05. Confidence Intervals for the Odds ratio was calculated with the Woolf logit method the Wilson/Brown method for the confidence interval of sensitivity and specificity. Individual groups were compared using either a paired or unpaired t-Test with an assumption of parametric Gaussian distribution.
3. Results
Patients presented over a 3-year period for evaluation of pelvic symptoms suspicious for PCS. Inclusion criteria required patients were women who had symptoms that reoccurred multiple times over a minimum of a 12-month period and who failed conservative treatment for a minimum of six months,
Figure 1.
343 patients met the above criteria for review, mean age of 44.6. Anterior or posterior pelvic symptoms were reported by 340 of the patients consistent with possible PCS, including urinary frequency, dyspareunia, dysmenorrhea, leg/hip pain, pelvic or upper thigh pain, or pain in the lower back. 302 patients were sent for PCS diagnostic evaluation including duplex ultrasonography and contrast venography. 299 reported positional symptoms defined as symptoms worsening after long periods of standing, walking, sitting, or exacerbated by activity,
Table 1.
Of those patients with positional symptoms who met criteria, 259 yielded positive DUS results, 8 yielded negative DUS results, and 35 had inconclusive DUS results. The confirmative diagnosis of PCS was made with contrast venography, of which 293 of the 299 patients received a positive diagnosis through venography. Of the 302 patients sent for diagnostic evaluation, 296 were diagnosed with PCS through venography. The three patients who did not have positional symptoms who were screened with duplex ultrasonography and contrast venography were positively diagnosed with PCS. The odds of someone testing positive for PCS with contrast venography after a positive PCS evaluation with ultrasonography was 6.4 with a 95% CI of 1.248-32.82 with a likelihood ratio of 1.730 (p = 0.0396 Fisher’s exact test; sensitivity 86.49%; specificity 50.0%),
Table 2. Three patients who were screened, who reported pelvic pain symptoms, whose symptoms were not positional, yielded a positive diagnosis by contrast venography.
From the 302 participants who underwent contrast venography, 296 had confirmatory diagnoses of PCS. The likelihood ratio of someone with positional PCS-like symptoms and testing positive for PCS through venography was 1.485 (p = 0.0003 Fischer’s exact test; OR 48.83 with 95% 8.130 to 293.3). Presence of positional symptoms alone yielded a positive predictive value (PPV) of 97.99%, confirmed by the gold-standard of PCS diagnosis, contrast venography, and a negative predictive value of 50.00% (Sensitivity 98.99% and specificity 33.33%). In combination, both positional symptoms and a positive DUS, the likelihood of PCS diagnosis was 1.709 with an OR of 5.884 (p = 0.475 Fisher’s exact test; PPV 98.83% with a sensitivity of 85.47%). It is interesting to note that positional symptoms alone, without DUS resulted in a higher sensitivity than with DUS alone.
All 296 patients diagnosed with PCS underwent treatment including venous embolization of the gonadal and/or hypogastric veins. 8 patients were found to have Type I, primary, pelvic congestion syndrome. All 8 patients with Type I PCS reported the presence of anterior pelvic symptoms, with one patient also complaining of posterior pelvic symptoms. Following embolization, the 7 patients who only experienced anterior symptoms reported total symptom relief and required no further treatment,
Table 3. The remaining patient reported total relief of anterior pelvic symptoms following embolization and ≥80% symptom relief of posterior pelvic symptoms. The remaining symptoms were managed conservatively with no further procedures required.
Of the remaining 288 patients, all underwent subsequent embolization during contrast venography for the treatment of Type II PCS. 46 patients reported total or significant (≥80%) symptom relief at the time of their follow-up visits requiring no additional intervention. Among the remaining 242 participants, 226 (93.39%) reported cessation of their anterior symptoms, and 16 (6.61%) individuals reported incomplete resolution of symptoms. The majority reported residual posterior symptoms,
Table 4. These patients went on to have symptomatic venous pathology evaluated and treated with placement of venous stents. After full treatment of Type II PCS, 95.83% of patients reported at least some symptom relief, 88.54% reported ≥50% symptom relief, and 59.03% reported ≥80% symptom relief.
After following our treatment algorithm for both Type I and Type II PCS
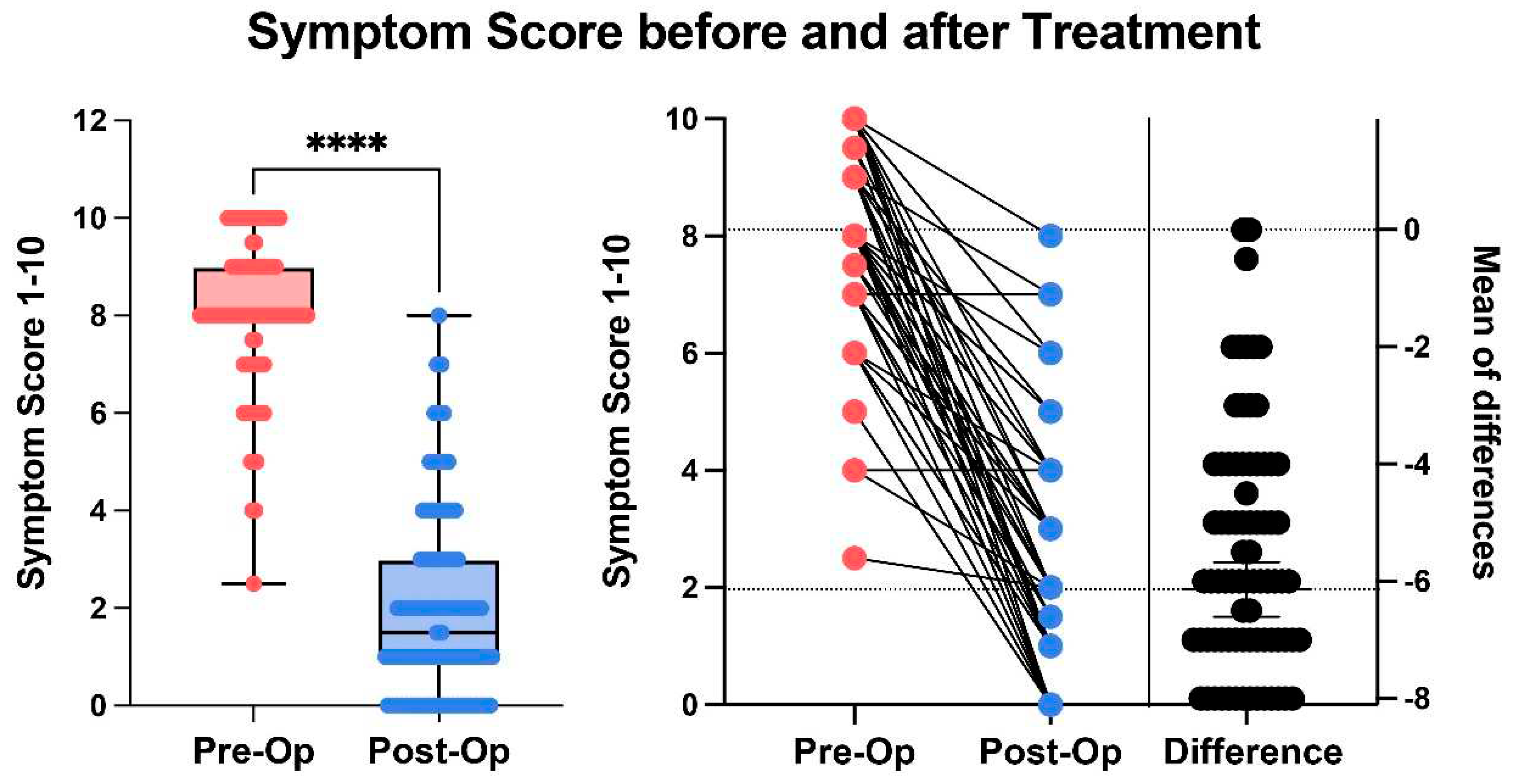
, 95.95% of patients reported some symptom relief, 88.85% reported ≥50% symptom relief, and 59.80% reported ≥80% symptom relief 6-months following their last procedure. Collectively, there was a significant improvement in post-op symptom score compared to pre-op (p < 0.0001 Paired parametric t-test),
Figure 2. Correction of symptomatic venous pathology resulted in nearly all patients being satisfied with both their treatment and independently satisfied with their diagnostic/therapeutic process, as evidenced with a mean “overall satisfaction” score of 96.07%. Overall, there was a significant difference between Type I PCS and Type II PCS and thus a tailored treatment protocol is recommended,
Figure 3.
4. Discussion
Our research suggests that while screening for PCS, positional nature of symptoms holds a greater significance than their exact location. A focused history of patients presenting with positional symptoms combined with positive DUS has shown to be a reliable way to select candidates to undergo contrast venography for confirmatory diagnosis. In the presence of positional symptoms, inconclusive DUS results should not deter progress to venography, as indicated by the positive predictive value and sensitivity for positional symptoms combined with DUS results. This protocol is not only accurate as indicated by a high predictive value, but it also eliminates the necessity for additional, expensive diagnostic modalities. Computed tomography angiography and/or magnetic resonance angiography were not needed for the diagnosis or treatment of PCS. With appropriate inclusion criteria, venography reduces the volume and cost of diagnostic testing, allowing for concrete confirmatory diagnosis and possible intervention with curative intent in the same outpatient procedure.
We found that the key to selecting the most effective treatment for PCS is identifying the type and presence of symptoms. Rather than using a “one-size-fits-all” approach, it is important to distinguish between different types of PCS and use the predominant symptom type as a guide to the best method for treatment. If we consider the different types and subtypes, we can better understand the atypical presentation of symptoms and, thus, select an appropriate treatment plan. Only one of the 8 participants with Type I, and 16 of the 288 participants with Type II PCS experienced remaining anterior symptoms post-embolization. Since Type I and Type II: NCS typically involve only structures of the APR, all of these patients experienced total/significant symptom relief following embolization and did not require any further treatment.
Patients with Type II PCS returned after embolization to treat their remaining posterior symptoms with vascular stent placement, which is necessary to perform a full evaluation of patients using contrast venography [
28]. Although some may argue that the number of patients who returned for stent placement indicates therapeutic failure, it is important to remember that while posterior symptoms remained, embolization successfully eliminated the most predominant symptom in 93.39% of these participants. While venous stent placement is the most reliable method for treatment of vascular lesions, not all lesions of the renal and iliac veins are symptomatic. 17 participants within our study with Type II: MTS or Type II: NCS & MTS reported ≥80% symptom relief following embolization and did not require further treatment. Of all patients in the study with PCS secondary to stenotic lesions, 15.97% experienced total/significant relief of symptoms post-embolization. These results suggest that embolization of the gonadal and/or hypogastric veins is not only effective in the treatment of anterior symptoms but also ensures that no patients will undergo unnecessary procedures.
Limitations of this study include the study design with the retrospective aspect of it. Future research should focus on prospective methods and patient selection. Patient bias is of concern as most patients presenting to our clinic have heard of our services and treatment modalities by word of mouth. In the future, we hope to validate this research with a prospective study and to expand the study to multi-institutional, multi-center, multi-site locations to validate our methods with other populations.
We conclude that coupled with increased understanding, utilization of this simple and accurate approach to the diagnosis and treatment of PCS will help to improve detection of a grossly underdiagnosed source of pelvic pain for women who have been suffering for years without relief.
5. Conclusions
A single-institution, multi-site, retrospective chart review of 343 patients found that selection of candidates to further screen for Pelvic Congestion Syndrome (PCS) may be determined using a focused symptom-based clinical algorithm combined with pelvic venous duplex and that after full treatment, 95.95% of patients experienced at least some relief of pelvic pain. Increased awareness combined with a standard diagnosis and treatment algorithm will increase detection and reduce the number of women suffering from PCS.
Author Contributions
Conceptualization, F.A.C. and D.A.G.; methodology, F.A.C., M.C.; validation, N.C.; formal analysis, F.A.C., M.C. and D.A.G.; resources, N.G., N.C. and A.T.; data curation, F.A.C., M.C., N.C. and N.G.; writing—draft preparation, F.A.C.; writing—review and editing, F.A.C., A.T. and D.A.G.; visualization, F.A.C. and D.A.G.; supervision, A.T. and D.A.G.; project administration, N.C., N.G. and A.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The ethics committee of NYC Surgical Associates gave ethical approval for this work. This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of NYC Surgical Associates, IORG0009136, Protocol NYCSA1012 of 02/01/2021. All authors use of protected health information complied with HIPAA and patient identifiers were not collected.
Informed Consent Statement
Consent was obtained from each patient for treatment and publication of this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article or are readily available upon request. Inquiries can be directed to the corresponding author.
Acknowledgments
We thank ultrasound technologists Christa Ziffer, Christine Mitchell, and Adriana Vaglica for their hard work, and for preforming the duplex ultrasound exams used in this study. We also thank Danielle De Marco, Karem Bortecen, Arno Rotgans, and Renard Ruiz for initial work prepared as a preprint.
Conflicts of Interest
F.A.C., M.C., N.C., N.G. declare no conflicts of interest or competing financial interests. A.T. and D.A.G. own and manage NYC Surgical Associates.
References
- C. Borghi and L. Dell’Atti, “Pelvic congestion syndrome: the current state of the literature,” Arch. Gynecol. Obstet., vol. 293, no. 2, pp. 291–301, Feb. 2016. https://doi.org/10.1007/s00404-015-3895-7. [CrossRef]
- N. Rane, J. J. Leyon, T. Littlehales, A. Ganeshan, P. Crowe, and R. Uberoi, “Pelvic congestion syndrome,” Curr. Probl. Diagn. Radiol., vol. 42, no. 4, pp. 135–140, Aug. 2013. https://doi.org/10.1067/j.cpradiol.2012.11.002. [CrossRef]
- R. K. N. Santoshi, S. Lakhanpal, V. Satwah, G. Lakhanpal, M. Malone, and P. J. Pappas, “Iliac vein stenosis is an underdiagnosed cause of pelvic venous insufficiency,” J. Vasc. Surg. Venous Lymphat. Disord., vol. 6, no. 2, pp. 202–211, Mar. 2018. https://doi.org/10.1016/j.jvsv.2017.09.007. [CrossRef]
- Demarco, Danielle; Greuner, David A., “Current Clinical Management of Pelvic Congestion Syndrome,” Vasc. Dis. Manag., vol. 17, no. 2, pp. 23–28, Feb. 2020.
- J. D. Durham and L. Machan, “Pelvic congestion syndrome,” Semin. Interv. Radiol., vol. 30, no. 4, pp. 372–380, Dec. 2013. https://doi.org/10.1055/s-0033-1359731. [CrossRef]
- S. Gavrilov, Y. P. Moskalenko, N. Y. Mishakina, O. I. Efremova, V. M. Kulikov, and A. S. Grishenkova, “Stratification of pelvic venous reflux in patients with pelvic varicose veins,” J. Vasc. Surg. Venous Lymphat. Disord., vol. 9, no. 6, pp. 1417–1424, Nov. 2021. https://doi.org/10.1016/j.jvsv.2021.04.019. [CrossRef]
- O. Mahmoud et al., “Efficacy of endovascular treatment for pelvic congestion syndrome,” J. Vasc. Surg. Venous Lymphat. Disord., vol. 4, no. 3, pp. 355–370, Jul. 2016. https://doi.org/10.1016/j.jvsv.2016.01.002. [CrossRef]
- M. T. O’Brien and D. L. Gillespie, “Diagnosis and treatment of the pelvic congestion syndrome,” J. Vasc. Surg. Venous Lymphat. Disord., vol. 3, no. 1, pp. 96–106, Jan. 2015. https://doi.org/10.1016/j.jvsv.2014.05.007. [CrossRef]
- M. S. Whiteley, “Pelvic venous pain due to pelvic congestion syndrome is becoming a primary diagnosis,” J. Vasc. Surg. Venous Lymphat. Disord., vol. 9, no. 6, p. 1425, Nov. 2021. https://doi.org/10.1016/j.jvsv.2021.05.003. [CrossRef]
- S. F. Daugherty, “Nonthrombotic Venous Obstructions Cause Pelvic Congestion Syndrome,” J. Vasc. Surg. Venous Lymphat. Disord., vol. 3, no. 1, pp. 117–118, Jan. 2015. https://doi.org/10.1016/j.jvsv.2014.10.008. [CrossRef]
- T. A. Khan, K. P. Rudolph, T. S. Huber, and J. Fatima, “May-Thurner syndrome presenting as pelvic congestion syndrome and vulvar varicosities in a nonpregnant adolescent,” J. Vasc. Surg. Cases Innov. Tech., vol. 5, no. 3, pp. 252–254, Sep. 2019. https://doi.org/10.1016/j.jvscit.2019.02.008. [CrossRef]
- M. P. Steenbeek, C. J. M. van der Vleuten, L. J. Schultze Kool, and T. E. Nieboer, “Noninvasive diagnostic tools for pelvic congestion syndrome: a systematic review,” Acta Obstet. Gynecol. Scand., vol. 97, no. 7, pp. 776–786, Jul. 2018. https://doi.org/10.1111/aogs.13311. [CrossRef]
- Osman MW, Nikolopoulos I, Jayaprakasan K, and Raine-Fenning N, “Pelvic congestion syndrome,” Obstet Gynaecol, vol. 15, pp. 151–157, 2013.
- D. D. Kies and H. S. Kim, “Pelvic congestion syndrome: a review of current diagnostic and minimally invasive treatment modalities,” Phlebology, vol. 27 Suppl 1, pp. 52–57, Mar. 2012. https://doi.org/10.1258/phleb.2012.012s27. [CrossRef]
- K. Sharma, M. K. Bora, J. Varghese, G. Malik, and R. Kuruvilla, “Role of trans vaginal ultrasound and Doppler in diagnosis of pelvic congestion syndrome,” J. Clin. Diagn. Res. JCDR, vol. 8, no. 7, pp. OD05-07, Jul. 2014. https://doi.org/10.7860/JCDR/2014/8106.4570. [CrossRef]
- S. J. Park et al., “Diagnosis of pelvic congestion syndrome using transabdominal and transvaginal sonography,” AJR Am. J. Roentgenol., vol. 182, no. 3, pp. 683–688, Mar. 2004. https://doi.org/10.2214/ajr.182.3.1820683. [CrossRef]
- R. D. Malgor, D. Adrahtas, G. Spentzouris, A. P. Gasparis, A. K. Tassiopoulos, and N. Labropoulos, “The role of duplex ultrasound in the workup of pelvic congestion syndrome,” J. Vasc. Surg. Venous Lymphat. Disord., vol. 2, no. 1, pp. 34–38, Jan. 2014. https://doi.org/10.1016/j.jvsv.2013.06.004. [CrossRef]
- N. Labropoulos, P. T. Jasinski, D. Adrahtas, A. P. Gasparis, and M. H. Meissner, “A standardized ultrasound approach to pelvic congestion syndrome,” Phlebology, vol. 32, no. 9, pp. 608–619, Oct. 2017. https://doi.org/10.1177/0268355516677135. [CrossRef]
- A. Thors, M. J. Haurani, T. K. Gregio, and M. R. Go, “Endovascular intervention for pelvic congestion syndrome is justified for chronic pelvic pain relief and patient satisfaction,” J. Vasc. Surg. Venous Lymphat. Disord., vol. 2, no. 3, pp. 268–273, Jul. 2014. https://doi.org/10.1016/j.jvsv.2013.12.002. [CrossRef]
- A. J. Lopez, “Female Pelvic Vein Embolization: Indications, Techniques, and Outcomes,” Cardiovasc. Intervent. Radiol., vol. 38, no. 4, pp. 806–820, Aug. 2015. https://doi.org/10.1007/s00270-015-1074-7. [CrossRef]
- D. Phillips, A. R. Deipolyi, R. L. Hesketh, M. Midia, and R. Oklu, “Pelvic congestion syndrome: etiology of pain, diagnosis, and clinical management,” J. Vasc. Interv. Radiol. JVIR, vol. 25, no. 5, pp. 725–733, May 2014. https://doi.org/10.1016/j.jvir.2014.01.030. [CrossRef]
- J. A. Guirola, M. Sánchez-Ballestin, S. Sierre, C. Lahuerta, V. Mayoral, and M. A. De Gregorio, “A Randomized Trial of Endovascular Embolization Treatment in Pelvic Congestion Syndrome: Fibered Platinum Coils versus Vascular Plugs with 1-Year Clinical Outcomes,” J. Vasc. Interv. Radiol. JVIR, vol. 29, no. 1, pp. 45–53, Jan. 2018. https://doi.org/10.1016/j.jvir.2017.09.011. [CrossRef]
- M. S. Abd El-Galil, A. M. Bassiouny, K. A. Abd El-Tawab, and H. A. E.-K. Moursy, “Role of Trans-catheter Ovarian Vein Embolization in The Management of Symptomatic Chronic Pelvic Congestion in Females,” Egypt. J. Hosp. Med., vol. 68, no. 2, pp. 1224–1228, Jul. 2017. https://doi.org/10.12816/0039053. [CrossRef]
- H. Abdelsalam, “Clinical outcome of ovarian vein embolization in pelvic congestion syndrome,” Alex. J. Med., vol. 53, no. 1, pp. 15–20, Mar. 2017. https://doi.org/10.1016/j.ajme.2016.01.006. [CrossRef]
- A. Laborda, J. Medrano, I. de Blas, I. Urtiaga, F. C. Carnevale, and M. A. de Gregorio, “Endovascular treatment of pelvic congestion syndrome: visual analog scale (VAS) long-term follow-up clinical evaluation in 202 patients,” Cardiovasc. Intervent. Radiol., vol. 36, no. 4, pp. 1006–1014, Aug. 2013. https://doi.org/10.1007/s00270-013-0586-2. [CrossRef]
- G. Asciutto, K. C. Asciutto, A. Mumme, and B. Geier, “Pelvic venous incompetence: reflux patterns and treatment results,” Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg., vol. 38, no. 3, pp. 381–386, Sep. 2009. https://doi.org/10.1016/j.ejvs.2009.05.023. [CrossRef]
- A. C. Venbrux and D. L. Lambert, “Embolization of the ovarian veins as a treatment for patients with chronic pelvic pain caused by pelvic venous incompetence (pelvic congestion syndrome),” Curr. Opin. Obstet. Gynecol., vol. 11, no. 4, pp. 395–399, Aug. 1999. https://doi.org/10.1097/00001703-199908000-00006. [CrossRef]
- S. G. Gavrilov, A. V. Vasilyev, G. V. Krasavin, Y. P. Moskalenko, and N. Y. Mishakina, “Endovascular interventions in the treatment of pelvic congestion syndrome caused by May-Thurner syndrome,” J. Vasc. Surg. Venous Lymphat. Disord., vol. 8, no. 6, pp. 1049–1057, Nov. 2020. https://doi.org/10.1016/j.jvsv.2020.02.012. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).