Submitted:
15 May 2023
Posted:
15 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of the Samples
2.2.2. Preparation of the Acrylic Testing Block
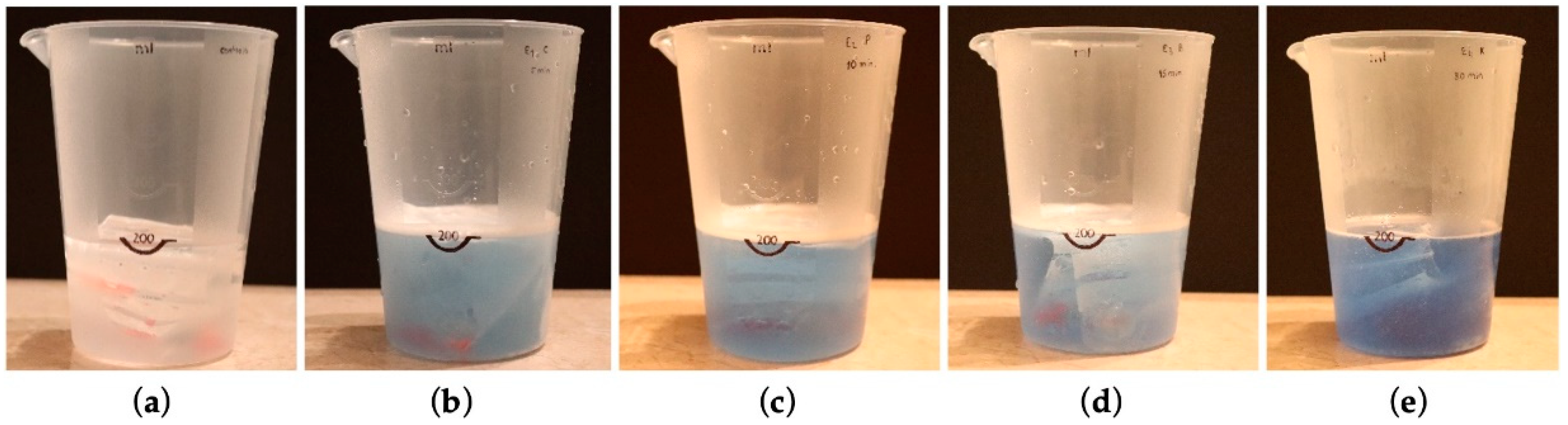
2.2.3. Protocol for Immersion in Cleaning Solutions
2.2.4. Dynamic Fatigue Test
2.3. Statistical Analysis
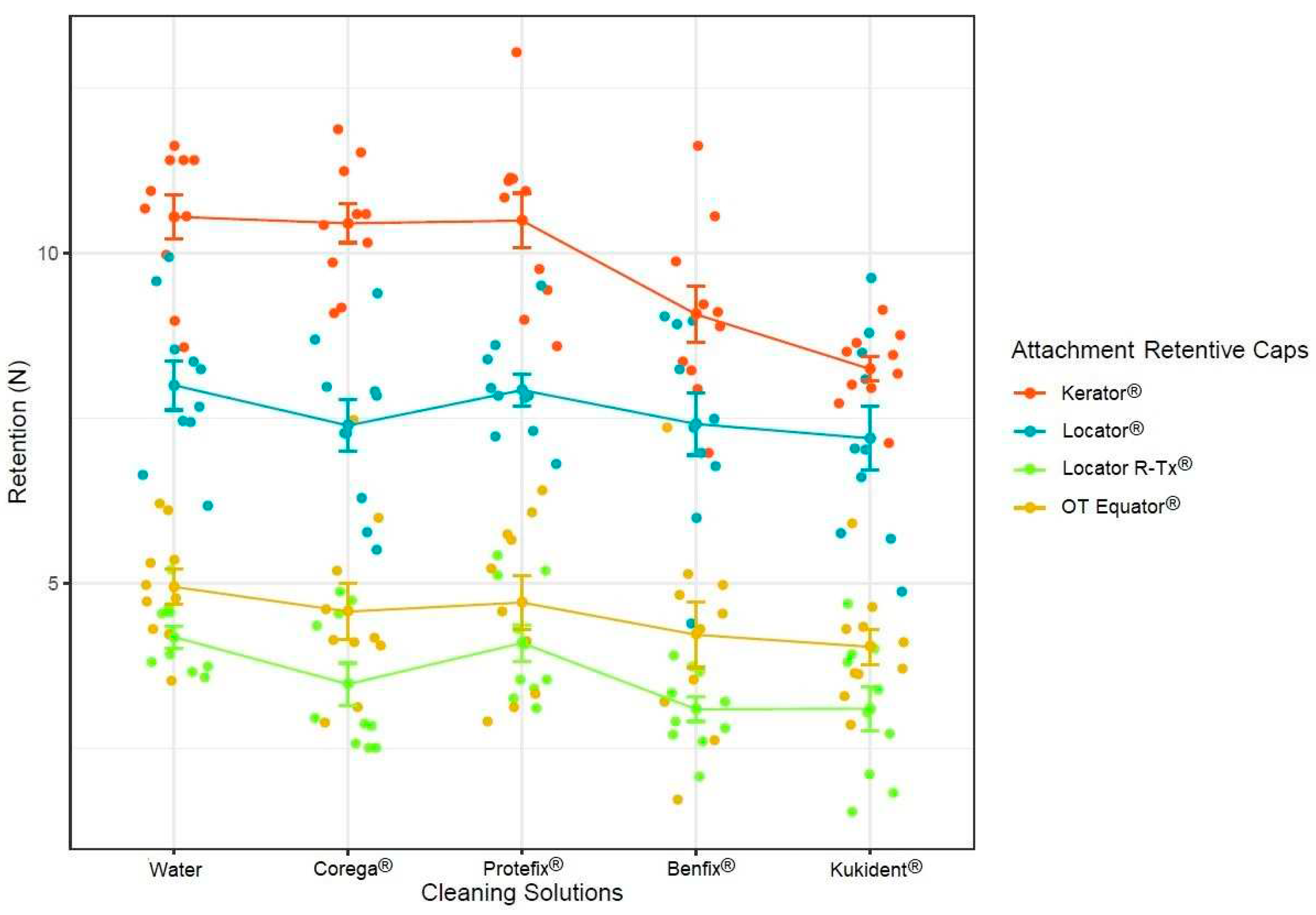
3. Results
4. Discussion
5. Conclusions
- There were no significant statistical differences between Corega®, Protefix®, and tap water, despite the retention decreasing in all three solutions.
- The only statistically different results found were between the Kukident® and Benfix® cleaning solutions groups, suggesting that the amount of time required for the cleaning solution to work could influence the attachment and cap degradation.
- There were significant statistical differences between the different manufacturers in terms of the retention forces of the attachment retentive caps, despite the fact that the caps are made of the same material. There were different components that caused each one to have a different elasticity, resulting in retention differences, and explaining the variation between the initial retentive forces from all of the groups.
- Further studies are necessary to analyze whether the percentage of different material elements used to make the attachment influence or accelerate the attachment retentive cap’s degradation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. World Report on Ageing and Health; World Health Organization: Geneva, Switzerland, 2015.
- Nitschke, I.; Wendland, A.; Weber, S.; Jockusch, J.; Lethaus, B.; Hahnel, S. Considerations for the Prosthetic Dental Treatment of Geriatric Patients in Germany. J. Clin. Med. 2021, 10, 304. [CrossRef]
- Gray, D.; Patel, J. Implant-supported overdentures: Part 1. Br. Dent. J. 2021, 231, 94–100. [CrossRef]
- Vahidi, F.; Pinto-Sinai, G. Complications Associated with Implant-Retained Removable Prostheses. Dent. Clin. N. Am. 2015, 59, 215–226. [CrossRef]
- Prasad, D.K.; Prasad, D.A.; Buch, M. Selection of attachment systems in fabricating an implant supported overdenture. J. Dent. Implant. 2014, 4, 176.
- Thomason, J.M.; Kelly, S.A.M.; Bendkowski, A.; Ellis, J.S. Two implant retained overdentures––A review of the literature supporting the McGill and York consensus statements. J. Dent. 2012, 40, 22–34. [CrossRef]
- Stilwell, C. Mandibular Implant Overdentures: Treatment and Medico-Legal Considerations. Prim. Dent. J. 2017, 6, 28–35. [CrossRef]
- Melescanu Imre, M.; Marin, M.; Preoteasa, E.; Tancu, A.M.; Preoteasa, C.T. Two implant overdenture–the first alternative treatment for patients with complete edentulous mandible. J. Med. Life 2011, 4, 207.
- Silva, A.S.; Martins, D.; Sá, J.; Mendes, J.M. Clinical evaluation of the implant survival rate in patients subjected to immediate implant loading protocols. Dent. Med. Probl. 2021, 58, 61–68. [CrossRef]
- Zhang, L.; Lyu, C.; Shang, Z.; Niu, A.; Liang, X. Quality of Life of Implant-Supported Overdenture and Conventional Complete Denture in Restoring the Edentulous Mandible: A Systematic Review. Implant Dent. 2017, 26, 945–950. [CrossRef]
- Kutkut, A.; Bertoli, E.; Frazer, R.; Pinto-Sinai, G.; Hidalgo, R.F.; Studts, J. A systematic review of studies comparing conventional complete denture and implant retained overdenture. J. Prosthodont. Res. 2018, 62, 1–9. [CrossRef]
- Sun, X.; Zhai, J.-J.; Liao, J.; Teng, M.-H.; Tian, A.; Liang, X. Masticatory efficiency and oral health-related quality of life with implant-retained mandibular overdentures. Saudi Med. J. 2014, 35, 1195–1202.
- Renvert, S.; Aghazadeh, A.; Hallström, H.; Persson, G.R. Factors related to peri-implantitis—A retrospective study. Clin. Oral Implants Res. 2014, 25, 522–529. [CrossRef]
- Serino, G.; Ström, C. Peri-implantitis in partially edentulous patients: Association with inadequate plaque control. Clin. Oral Implants Res. 2009, 20, 169–174. [CrossRef]
- de Araújo Nobre, M.; Mano Azul, A.; Rocha, E.; Maló, P. Risk factors of peri-implant pathology. Eur. J. Oral Sci. 2015, 123, 131–139. [CrossRef]
- Felton, D.; Cooper, L.; Duqum, I.; Minsley, G.; Guckes, A.; Haug, S.; Meredith, P.; Solie, C.; Avery, D.; Chandler, N.D. Evidence-Based Guidelines for the Care and Maintenance of Complete Dentures: A Publication of the American College of Prosthodontists. J. Prosthodont. 2011, 20, S1–S12. [CrossRef]
- NNishi, Y.; Seto, K.; Kamashita, Y.; Kaji, A.; Kurono, A.; Nagaoka, E. Survival of microorganisms on complete dentures following ultrasonic cleaning combined with immersion in peroxide-based cleanser solution. Gerodontology 2012, 31, 202–209. [CrossRef]
- Budtz-Jørgensen, E. Materials and methods for cleaning dentures. J. Prosthet. Dent. 1979, 42, 619–623. [CrossRef]
- Mohammed, H.S.; Singh, S.; Hari, P.A.; Amarnath, G.S.; Kundapur, V.; Pasha, N.; Anand, M. Evaluate the Effect of Commercially Available Denture Cleansers on Surface Hardness and Roughness of Denture Liners at Various Time Intervals. Int. J. Biomed. Sci. 2016, 12, 130–142. [CrossRef]
- Valentini-Mioso, F.; Maske, T.T.; Cenci, M.S.; Boscato, N.; Pereira-Cenci, T. Chemical hygiene protocols for complete dentures: A crossover randomized clinical trial. J. Prosthet. Dent. 2019, 121, 83–89. [CrossRef]
- Axe, A.S.; Varghese, R.; Bosma, M.; Kitson, N.; Bradshaw, D.J. Dental health professional recommendation and consumer habits in denture cleansing. J. Prosthet. Dent. 2015, 115, 183–188. [CrossRef]
- Hayran, Y.; Sarikaya, I.; Aydin, A.; Tekin, Y.H. Determination of the effective anticandidal concentration of denture cleanser tablets on some denture base resins. J. Appl. Oral Sci. 2018, 26, e20170077. [CrossRef]
- Papadiochou, S.; Polyzois, G. Hygiene practices in removable prosthodontics: A systematic review. Int. J. Dent. Hyg. 2018, 16, 179–201. [CrossRef]
- Verhaeghe, T.V.; Wyatt, C.C.; Mostafa, N.Z. The effect of overnight storage conditions on complete denture colonization by Candida albicans and dimensional stability: A systematic review. J. Prosthet. Dent. 2020, 124, 176–182. [CrossRef]
- Williams, B.H.; Ochiai, K.T.; Hojo, S.; Nishimura, R.; Caputo, A.A. Retention of maxillary implant overdenture bars of different designs. J. Prosthet. Dent. 2001, 86, 603–607. [CrossRef]
- Warreth, A.; Alkadhimi, A.; Sultan, A.; Byrne, C.; Woods, E. Mandibular implant-supported overdentures: Attachment systems, and number and locations of implants—Part I. J. Ir. Dent. Assoc. 2015, 61, 93–97.
- The Glossary of Prosthodontic Terms: Ninth Edition. J. Prosthet. Dent. 2017, 117, e1–e105.
- Daou, E. Biomaterial aspects: A key factor in the longevity of implant overdenture attachment systems. J. Int. Soc. Prev. Community Dent. 2015, 5, 255–262. [CrossRef]
- Al-Zubeidi, M.I.; Alsabeeha, N.H.; Thomson, W.M.; Payne, A.G. Patient Satisfaction and Dissatisfaction with Mandibular Two-Implant Overdentures Using Different Attachment Systems: 5-Year Outcomes. Clin. Implant. Dent. Relat. Res. 2010, 14, 696–707. [CrossRef]
- Passia, N.; Ghazal, M.; Kern, M. Long-term retention behaviour of resin matrix attachment systems for overdentures. J. Mech. Behav. Biomed. Mater. 2016, 57, 88–94. [CrossRef]
- Campos, M.R.d.; Marcondes Agnelli, J.A.; Cândido dos Reis, A. Factors influencing retention and durability of attachments for overdentures—adverse effects of cleansings, pH, and temperature: A systematic review. Heliyon 2022, 8, e12411. [CrossRef]
- Silva, A.S.; Aroso, C.; Ustrell, R.; Braga, A.C.; Mendes, J.M.; Escuin, T. The influence of saliva pH value on the retention and durability of bar-clip attachments. J. Adv. Prosthodont. 2015, 7, 32–38. [CrossRef]
- Ozyilmaz, O.Y.; Kara, O.; Akin, C. Evaluation of various denture cleansers on color stability and surface topography of polyetherketoneketone, polyamide, and polymethylmethacrylate. Microsc. Res. Tech. 2020, 84, 3–11. [CrossRef]
- You, W.; Masri, R.; Romberg, E.; Driscoll, C.F.; You, T. The Effect of Denture Cleansing Solutions on the Retention of Pink Locator Attachments after Multiple Pulls: An In Vitro Study. J. Prosthodont. 2011, 20, 464–469. [CrossRef]
- Ayyıldız, S.; Şahin, C.; Emir, F.; Ersu, B. Effect of Denture Cleansing Solutions on the Retention of Locator Attachments Over Time. J. Prosthodont. 2020, 29, 237–242. [CrossRef]
- Derafshi, R.; Mohaghegh, M.; Saki, M.; Safari, A.; Haghighi, M.R. The Effects of Denture Cleansing Solutions on the Retention of Attachments of Implant Supported Overdentures. J. Dent. 2015, 16 (Suppl. 1), 68–72.
- Nguyen, C.T.; Masri, R.; Driscoll, C.F.; Romberg, E. The Effect of Denture Cleansing Solutions on the Retention of Pink Locator Attachments: An in Vitro Study. J. Prosthodont. 2010, 19, 226–230. [CrossRef]
- Mariotto, L.; Valente, M.; de Castro, D.; dos Reis, A. Effects of Denture Cleansing Solutions on Different Materials Used for Fabrication of Polymer Attachment Components. Int. J. Prosthodont. 2020, 33, 74–80. [CrossRef]
- Varghese, R.M.; Masri, R.; Driscoll, C.F.; Romberg, E. The Effect of Denture Cleansing Solutions on the Retention of Yellow Hader Clips: An In Vitro Study. J. Prosthodont. 2007, 16, 165–171. [CrossRef]
- Berger, D.; Rakhamimova, A.; Pollack, A.; Loewy, Z. Oral Biofilms: Development, Control, and Analysis. High-Throughput 2018, 7, 24. [CrossRef]
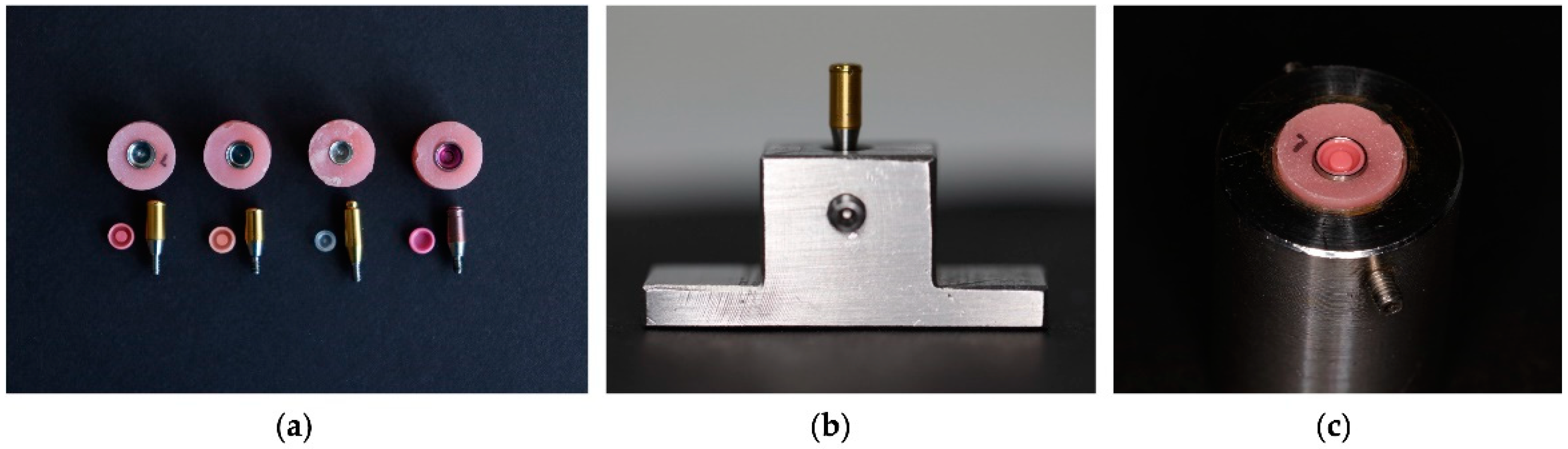
| Brand | Color | Force |
|---|---|---|
| Locator® | Pink 
|
1360 g |
| OT Equator® | Clear 
|
1300 g |
| Kerator® | Pink 
|
1088 g |
| Locator R-Tx® | Pink 
|
907 g |
| Locator® | OT Equator® | Kerator® | Locator R-Tx® | |
|---|---|---|---|---|
| Corega® (1460 tablets) | 10 | 10 | 10 | 10 |
| Benfix® (1460 tablets) | 10 | 10 | 10 | 10 |
| Protefix® (1460 tablets) | 10 | 10 | 10 | 10 |
| Kukident® (1460 tablets) | 10 | 10 | 10 | 10 |
| Control | 10 | 10 | 10 | 10 |
| Total | 50 | 50 | 50 | 50 |
| Daily Hygiene (1 Day) |
One Year (365 Days) |
|
|---|---|---|
| Corega® | 5 min | 1825 min |
| Protefix® | 10 min | 3650 min |
| Benfix® | 15 min | 5475 min |
| Kukident® | 30 min | 10,950 min |
| Corega®. | Dissolve one Corega Cleanser® tablet in warm (not hot) water to cover the denture. | For an antifungal action, leave it submerged for 5 min. You can also leave it overnight. | Rinse the denture with plenty of running water before putting it in your mouth. |
|---|---|---|---|
| Protefix® | Dissolve one Protefix Active Cleanser® tablet in a glass of lukewarm water (100–200 mL, about 35 °C). | Clean and fresh in 3 min, disinfected in 10 min. Cleaning is also possible overnight. | Rinse the dental prosthesis well with running water before putting it in the mouth. |
| Benfix® | Introduce a single cleaning tablet in a glass of warm water. | Let the product act for a minimum of 15 min. For deep cleaning, you can leave your denture in the cup overnight. | Rinse with plenty of water to eliminate possible product residue. |
| Kukident® | Put the tablet in enough warm water to cover the denture. | Place the denture in the solution and let it sit for 30 mor overnight. | Remove the dentures and rinse in plenty of running water. |
| Locator® | Kerator® | OT Equator® | Locator R-Tx® | |||||
|---|---|---|---|---|---|---|---|---|
| Time | Solution | Time | Solution | Time | Solution | Time | Solution | |
| Control (water) | - | - | - | - | - | - | - | - |
| Experiment 1 | 5 min | Corega | 5 min | Corega | 5 min | Corega | 5 min | Corega |
| Experiment 2 | 10 min | Protefix | 10 min | Protefix | 10 min | Protefix | 10 min | Protefix |
| Experiment 3 | 15 min | Benfix | 15 min | Benfix | 15 min | Benfix | 15 min | Benfix |
| Experiment 4 | 30 min | Kukident | 30 min | Kukident | 30 min | Kukident | 30 min | Kukident |
| Df | Sum Sq | Mean Sq | F Value | Pr(>F) | |
|---|---|---|---|---|---|
| Cleaning solutions | 4 | 48.1 | 12.0 | 9.616 | 4.15 × 10−7 *** |
| Attachment retentive caps | 3 | 1208.6 | 402.9 | 322.066 | <2 × 10−16 *** |
| Residuals | 192 | 240.2 | 1.3 | 10 |
| Cleaning Solution Attachment System |
Water (Control) | |
|---|---|---|
| Mean | SD | |
| Locator® | 8.00 N | 1.18 N |
| Kerator® | 10.6 N | 1.07 N |
| OT Equator® | 4.95 N | 0.834 N |
| Locator R-Tx® | 4.19 N | 0.534 N |
|
Cleaning Solution Attachment System |
Corega® | |
| Mean | SD | |
| Locator® | 7.39 N | 1.24 N |
| Kerator® | 10.5 N | 0.926 N |
| OT Equator® | 4.58 N | 1.35 N |
| Locator R-Tx® | 3.48 N | 1.01 N |
|
Cleaning Solution Attachment System |
Protefix® | |
| Mean | SD | |
| Locator® | 7.93 N | 0.769 N |
| Kerator® | 10.5 N | 1.31 N |
| OT Equator® | 4.71 N | 1.29 N |
| Locator R-Tx® | 4.10 N | 0.871 N |
|
Cleaning Solution Attachment System |
Benfix® | |
| Mean | SD | |
| Locator® | 7.42 N | 1.49 N |
| Kerator® | 9.07 N | 1.34 N |
| OT Equator® | 4.23 N | 1.56 N |
| Locator R-Tx® | 3.10 N | 0.580 N |
|
Cleaning Solution Attachment System |
Kukident® | |
| Mean | SD | |
| Locator® | 7.20 N | 1.53 N |
| Kerator® | 8.25 N | 0.578 N |
| OT Equator® | 4.05 N | 0.843 N |
| Locator R-Tx® | 3.11 N | 1.04 N |
| Fmax (Mean ± SD) | Multiple Comparison Results | |
|---|---|---|
| 1. Kerator® | 9.76 ± 1.40 N | 1 vs. 2 (<0.001) 1 vs. 3 (<0.001) 1 vs. 4 (<0.001) |
| 2. Locator® | 7.59 ± 1.26 N | 2 vs. 3 (<0.001) 2 vs. 4 (<0.001) |
| 3. OT Equator® | 4.50 ± 1.21 N | 3 vs. 4 (<0.001) |
| 4. Locator R-Tx® | 3.60 ± 0.93 N |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





