Keywords continuous glucose monitoring; application in sports; carbohydrate management; active subjects; validation
1. Introduction
It is generally known, that substrate oxidation changes by increasing intensity of exercise with increasing contribution of muscle glycogen and blood glucose to energy provision (Jeukendrup, 2014; Loon et al., 2001; Romijn et al., 2000). Therefore, regular carbohydrate (CHO) intake during exercise which should be adapted to the overall duration of the exercise is recommended (Burke et al., 2019; Kerksick et al., 2017; Thomas et al., 2016). While free blood glucose concentration is no proper indicator for CHO availability, maintenance of blood glucose levels during exercise are assumed to reflect a successful CHO intake strategy during exercise (Bowler et al., 2023). Sensor-based continuous glucose monitoring (CGM) is a minimally-invasive technology that allows recognition of glucose flux and is rather known as a therapeutic application for people living with diabetes. In people with diabetes, it has been shown to be indirectly effective in the management of glycaemic control, the reduction of time spent in hypoglycaemia and the reduction of glycolated haemoglobin (HbA1c) concentration (Ehrhardt & Al Zaghal, 2019; Moser et al., 2020). In order to achieve the best possible performance, the use of innovative technology to support the monitoring of critical parameters in training periods and in-competition strategies has long reached professional sports. Therefore, athletes’ training plans and training sessions are accompanied by non-invasive and continuous measurements of parameters such as heart rate (HR), global positioning system (GPS), accelerometry or core temperature (Jonvik et al., 2022). Since continuous glucose monitoring (CGM) devices have become accessible to a healthy population, a considerable interest was recognised amongst athletes of endurance-based sport disciplines, aiming at optimizing physiological variables e.g. by tracing glucose. CGM devices in sports are promoted to enable CHO intake control during physical activity to enhance energy supply and, therefore, to improve athletic performance. For instance, Jan Frodeno (Triathlon, GER), Eluid Kipchoge (Marathon, KEN), Kristian Blumenfelt (Triathlon, NOR) and Sophie Power (Ultrarunning, UK) are prominent examples of endurance athletes living without diabetes but using sensor-based glucose concentration analysis. Different from conventional glucose testing which is common in capillary blood (CB) and recent performance studies and of that derived recommendations for CHO intake are based on, sensors measure glucose subcutaneously in the interstitial fluid (ISF). Recent studies in people with diabetes report a lag-time of 5 to 25 minutes depending on physiological changes during exercise, alterations in blood flow rate, body temperature and body acidity (Madden et al., 2020; Moser et al., 2020; Siegmund et al., 2017; Zaharieva et al., 2019). As also healthy people are advised to avoid hypoglycemia during exercise in order to delay fatigue, occurrence of a lag-time probably evokes delayed reaction and experiencing hypoglycemia. Despite the increasing interest and application in athletes, data in healthy active subjects are missing (Bowler et al., 2023). The authors hypothesize, that there will be a lag-time between CB and ISF in healthy subjects just as previously seen in people with diabetes. Therefore, the objective of this exploratory pilot study was to compare glucose concentration in capillary blood (CB) samples analysed by a validated method and glucose concentration measured in the ISF by CGM under different physical activity levels and different nutrition status in healthy active subjects.
2. Methods
2.1. Subjects
Healthy, non-smoking subjects between 18 and 35 years with an average training volume of > 8 hours / week were included in the study. For data analysis, data of 10 active subjects (4 females, 6 males) were considered doing the following sports on competitive level: crossfit (n=1), running (n=5), triathlon (n=3), cycling (n=1). At the beginning of the study, subjects were 26±4 years, had a bodyweight (BW) of 67±11 kg and a training volume of 11±3 h. All of them were following a training plan that they were given by their coach. Subjects were recruited from their relating sports club. Subjects gave written informed consent before their participation. The study was approved based on the Declaration of Helsinki by the university’s local ethics committee (159/2021) before the start of data collection.
2.2. Glucose monitoring devices
Glucose concentration was monitored by two different devices a) measuring glucose concentration from CB samples and b) in the ISF, respectively. Both devices are based on an enzymatic-amperometric measurement technique. (1) In a first reaction, the C1-atom of beta-D-glucose present in the sample is oxidised into D-glucono-delta-lactone by glucose oxidase (GOD). (2) At the same time, GOD-bound FAD is reduced to GOD-FADH2. (3) D-glucono-delta-lactone can be further hydrolysed into gluconic acid. (4) In a side reaction of (2), GOD-FAD is oxidised to GOD-FADH2 by donating electrons to oxygen which leads to formation of hydrogen peroxide. The amperometric potential of hydrogen peroxide or oxygen, respectively, is detected by an electrode. Because of the stochiometric relation of glucose and hydrogen peroxide or oxygen, respectively, the signal can be translated into glucose concentration (Witt et al., 2000).
a) Capillary Blood Glucose Analysis
CB samples were analysed by Biosen C-Line (EKF diagnostics Holding, Cardiff, UK). Biosen C-Line measures glucose and lactate in human blood, plasma and serum samples. The manufacturer reports a precision of ≤ 1.5% (at 12 mmol). Biosen C-Line was calibrated against a 12 mmol/L glucose solution standard each morning before the first sample array was measured. The amperometric signal of the standard forms the basis for calculation of unknown glucose concentrations. Capillary blood samples were taken at the earlobe and stored in end-to-end capillary tubes containing a haemolysis solution (EKF diagnostics Holding, Cardiff, UK) to avoid metabolic degradation of glucose. Glucose values are measured in the manner described previously. Validity and reliability of Biosen C-Line was confirmed previously (Gijzen et al., 2012; Nowotny et al., 2012). For this study, Biosen C-Line was used as reference method.
Interstitial Fluid Glucose Analysis
ISF glucose concentration was measured by Libre Sense Glucose Biosensor for Sport (Abbott Laboratories, Chicago, IL, US). For accessing crude minute-per-minute glucose concentration data, software by SuperSapiens (TT1 Products Inc.,Atlanta, GA, US) was used. Glucose sensors are equipped with a 4 mm-needle that is implanted in the dermis (thickness of 1-4 mm), which lays between epidermis (thickness of 75-150µm) and subcutaneous tissue (1-20 mm) (Madden et al., 2020). The needle carries the enzyme glucose oxidase and reacts oxygen-dependent in the manner described above (Bao et al., 2019; Teymourian et al., 2021). According to manufacturer’s guidelines, no calibration with blood glucose is needed for the sensors used in this study. Validity and reliability tests are performed at the moment, but no data has been published yet. Common body sites for sensor location are periumbilical or at the back of the forearm, respectively. In line with the manufacturer’s recommendation, in this study, the sensor was applied at the back of the upper arm of the subjects. For continuous data generation, Bluetooth connection to an NFC-enabled phone is necessary. Libre Sense Glucose Biosensor for Sport was used as comparator method.
2.3. Test protocol
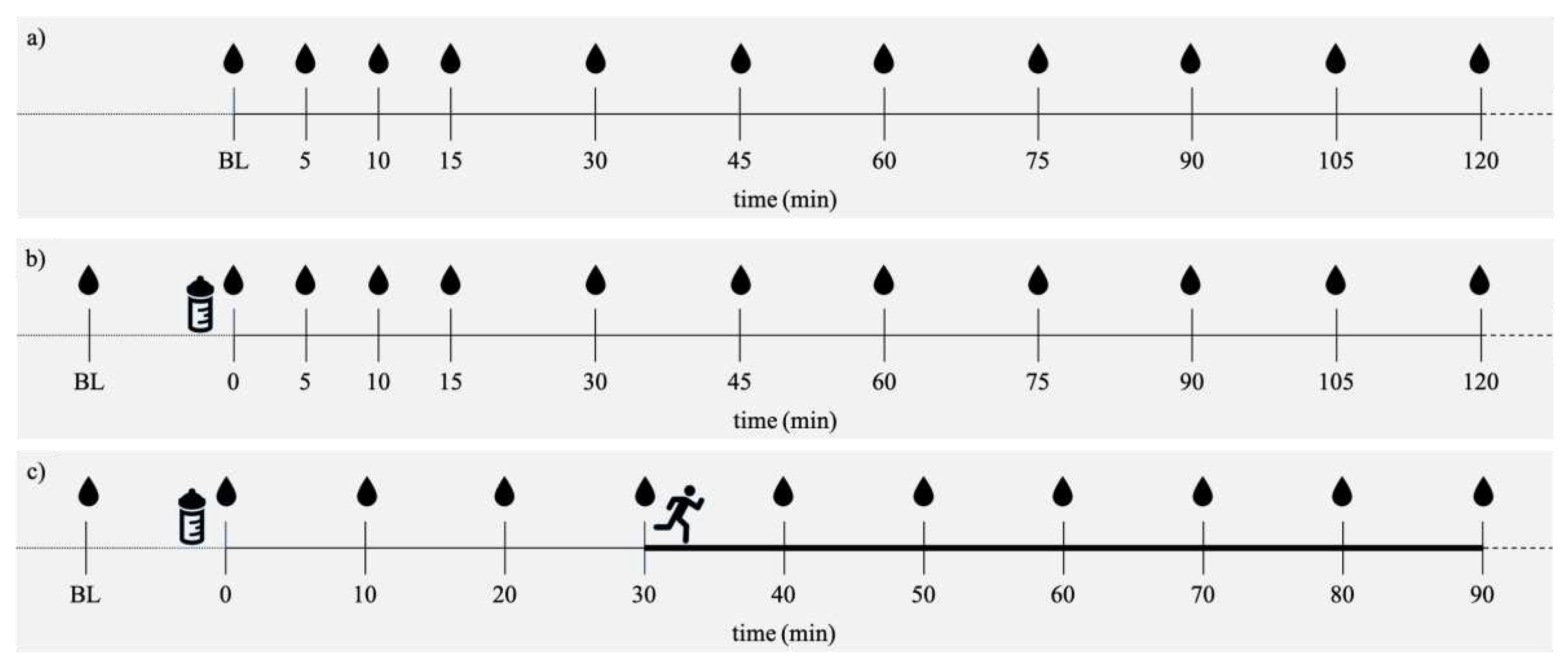
Sensor durability is limited to 14 days. Therefore, subjects underwent six tests at the laboratory within 14 days after sensor application plus three further tests that are not presented in the current article. As recommended by the manufacturer, no tests were conducted within the first 24 hours due to sensor calibration. Subjects arrived at the laboratory after an overnight. Before the test started, subjects were instructed to sit relaxed for 30 minutes. Two tests were performed under resting conditions in a fasted state (Rest/Fast). CB samples were taken at baseline (BL) and after 5, 10, 15, 30, 45, 60, 75, 90, 105 and 120 minutes. Two tests were performed under resting conditions in postprandial state (Rest/Glc). After BL measurement, subjects ingested 1 g Glc / kg BW, followed by resting for 120 minutes. CB samples were taken at BL, right after glucose ingestion, after 5, 10, 15, 30, 45, 60, 75, 90, 105 and 120 minutes. Two tests were performed in a postprandial state under exercising conditions. After BL measurement, subjects ingested 1 g Glc / kg BW, followed by resting for 30 minutes first and then starting the exercise program that consisted of running for 60 minutes at a constant moderate (ModExerc/Glc) and intensive (IntExerc/Glc) load, respectively. Total time of data collection for the tests including exercise was 90 minutes. Running tests were performed at the university’s 400m-tartan track in January and February 2022. Sampling was performed always in the same place. One researcher per subject was present to guarantee time accuracy in CB sampling. CB samples were taken at BL, right after glucose ingestion, after 10, 20, 30 (start of running), 40, 50, 60, 70, 80 and 90 minutes. Due to COVID-19 restrictions, lab-based performance diagnostics for determination of HR
max could not be performed. Instead, age-derived equation by Fox et al. (1968) was used to estimate HR
max by calculating the difference of 220 minus the subject’s age (Fox et al., 1968). By that, subjects’ 65% and 85% HRmax reflecting moderate and intensive exercise load, respectively, was calculated. This method was considered the most practical possible under given circumstances. As CB and ISF data of the same person were matched and their relating glucose kinetics should be analysed in the first place, this method is not considered a full limitation of the study. Subjects were instructed to have their last meal the day before their test at least 9 hours before the first blood sampling. Dinner prior to each test morning was standardised according to each subject’s BW. Before one of the two Rest/Fast and Rest/Glc measurements, subjects received dinner high in CHO (2 g/kg BW, HC). Before the other Rest/Fast and Rest/Glc measurement, subjects were provided with dinner low in CHO (0.5 g/kg BW, LC). Those tests were done as we wanted to see if the CHO load of the dinner has an influence on glucose values in the different media in the resting fasted condition and resting condition with glucose intake, respectively. Before exercising conditions, subjects’ dinner was moderate in CHO (1.5 g/kg BW) to guarantee sufficient CHO intake before one hour of exercise the next morning. An overview of each test protocol is given in
Figure 1. Dinner recipes were isocaloric, but differed in macronutrient distribution (
Table 1). As all of our subjects followed a structured training plan, the test schedule was not randomised but individually planned to exclude intensive training sessions the day before test day. Exercising conditions were separated by at least 48 hours. All subjects received their individual test schedule and BW-related recipes as a pdf-document.
2.4. Data access and statistical analysis
Analysis included 519 data pairs (R/Fast: 164, R/Glc: 179, 65/Glc: 94, 85/Glc: 82). For analysis, 75% (R/Fast), 75% (R/Glc), 85% (65/Glc) and 75% (85/Glc) of possible total paired data were available. Lacking data pairs were mainly due to sensor errors or Bluetooth connection failure. CB glucose data were accessed via Biosen C-Line printout after measurement of each subject’s array. Time of CB sample collection was documented during data collection to match continuous glucose data. Statistics were performed using Excel 2019 (Microsoft Corp., Redmond, WA, USA) and Statistical Package for Social Sciences 28 (IBM SPSS Statistics, Chicago, IL, USA). Statistical difference of BL, maximum value (PEAK), area under the curve (AUC) between HC_R/Fast and LC__R/Fast such as HC_R/Glc and LC_R/Glc was analysed by paired parametric or non-parametric tests. Parametric test was performed after normal distribution of residuals was confirmed by graphical analysis (histogram and QQ-plot) and Shapiro-Wilk test. Wilcoxon test was performed for non-parametric analysis. For comparison of ISF and CB glucose values, data were analysed by linear regression analysis and Pearson’s correlation coefficient, systematic measurement difference (bias) (Bland & Altman, 1986) and mean absolute relative deviation (MARD). For systematic measurement difference, mean of paired ISF and CB data was plotted against their difference. Difference between ISF and CB data was analysed for normal distribution by graphical analysis (histogram and QQ-plot) and Shapiro-Wilk test. MARD was calculated by |(GLCISF – GLCCB)|/GLCCB(%) (Freckmann et al., 2019). For all statistical tests, alpha was set at 5%. Effect size was calculated post hoc by Cohen’s d = | (Cohen, 1988; Lakens, 2013). Study design, data analysis, reporting of results and interpretation was conducted under consideration of CHecklist for statistical Assessment of Medical Papers (CHAMP) (Mansournia et al., 2021).
3. Results
3.1. Resting fasted condition
For residuals of BLCB, BLISF, AUCCB, AUCISF, normal distribution was confirmed. Parametric tests for BLCB and BLISF, respectively did not reveal any statistical differences between HC and LC dinner. Parametric tests of AUCCB and AUCISF, respectively showed a significant difference for AUCISF (t(3) = 4.683, 95%CI[549;2879], p < .018, d = 1.62) between HC and LC. Therefore, data of test protocols HC_R/Fast and LC_R/Fast were analysed separately. As glucose fluctuations under resting and fasted conditions are low, detailed data of parametric and non-parametric test results such as MARD calculation, glucose curves reflecting mean ± standard deviation and Bland-Altman plot can be found in supplementary material, Suppl. 1, Suppl. 2, Suppl. 3 and Suppl. 4
3.2. Resting postprandial condition
For residuals of BLCB, BLISF, PEAKCB, PEAKISF, AUCISF, normal distribution was confirmed. Parametric tests for BL and PEAK such as non-parametric test for AUC did not reveal any statistical differences between HC and LC dinner (detailed data of parametric and non-parametric test results in supplementary material, Suppl. 5).
3.3. Resting postprandial condition – High carbohydrate dinner before test morning
Mean glucose concentrations including SD and regression analysis are displayed in
Figure 2a.i and
Figure 2a.ii. There is a positive linear correlation (r = .88, p < .001) between CB and ISF glucose concentration measurements. Systematic measurement difference shows 95% limits of agreement between -13 and 52 mg/dL (∆LOA 65 mg/dL) (Bland & Altman, 1986). Mean difference between CB and ISF glucose values is 20 mg/dL (
Figure 3a). MARD ± SD is 17±10%. Dispersion of absolute relative deviation (ARD) as recommended by the Food and Drug Administration is displayed in
Figure 4. (FDA, 2014).
3.4. Resting postprandial condition – Low carbohydrate dinner before test morning
Mean glucose concentrations including SD and regression analysis are displayed in
Figure 2b.i and Figure2b.ii. There is a positive linear correlation (r = 0.9, p < .001) between CB and ISF glucose concentration measurements. Systematic measurement difference shows 95% limits of agreement between -7 and 47 mg/dL (∆LOA 54 mg/dL) (Bland & Altman, 1986). Mean difference between CB and ISF glucose values is 20 mg/dL (
Figure 3b).MARD ± SD is 17±9%. Dispersion of absolute relative deviation (ARD) as recommended by the Food and Drug Administration is displayed in
Figure 4. (FDA, 2014).
3.5. Exercise at 65% HRmax
Mean glucose concentrations including SD and regression analysis are displayed in
Figure 2c.i and
Figure 2c.ii. There is a positive linear correlation (r = 0.60, p < .001) between CB and ISF glucose concentration measurements. Systematic measurement difference shows 95% limits of agreement between -58 and 67 mg/dL (∆LOA 125 mg/dL) (Bland & Altman, 1986). Mean difference between CB and ISF glucose values is 4 mg/dL(
Figure 3c). MARD ± SD is 22±24%. Dispersion of ARD is displayed in
Figure 4.
3.6. Exercise at 85% HRmax
Mean glucose concentrations including SD and regression analysis are displayed in
Figure 2d.i and d.ii. There is a positive linear correlation (r = 0.70, p < .001) between CB and ISF glucose concentration measurements. Systematic measurement difference shows 95% limits of agreement between -48 and 52 mg/dL (∆LOA 100 mg/dL) (Bland & Altman, 1986). Mean difference between CB and ISF glucose values is 2 mg/dL (
Figure 3d).MARD ± SD is 18±17%. Dispersion of ARD is displayed in
Figure 4.
4. Discussion
4.1. Impact of physical activity
To our knowledge, to date, there has been only one study published comparing ISF and CB data systematically in subjects without diabetes investigating different protocols including the assessment of validity postprandially after different breakfasts, pre-, during and post-exercise (Clavel et al., 2022). In our study, systematic measurement difference shows a smaller mean bias under exercising compared to resting conditions (65/Glc: 4 mg/dL; 85/Glc: 2 mg/dL vs. HC_R/Glc: 20 mg/dL; LC_R/Glc: 20 mg/dL)., However, their 95% CI is higher under exercising conditions compared to resting conditions (∆LOA: 65/Glc: 125 mg/dL; 85/Glc: 102 mg/dL vs. HC_R/Glc: 65 mg/dL; LC_R/Glc: 55 mg/dL). Clavel et al. (2022) compared the CGM system (Freestyle Libre, Abbott, France) with a finger prick system (FreeStyle Optimum, Abbott, France). The FreeStyle Optimum is self-moniroting blood glucose device, which is different from our comparator lab-based device. Eight subjects received standardised isocaloric breakfasts with different macronutrient distribution which was either CHO loaded or protein and fat loaded. Irrespectively of breakfast, authors reported a mean bias of -2.99 mg/dL and ∆LAO of 58.36 mg/dL within 60 minutes post-breakfast, a mean bias of -1.67 mg/dL and a ∆LAO of 36.02 within the 60 minutes after the post-breakfast period, that the authors claimed as pre-exercise. The exercise protocol started with a 10 minutes low-intensity run that was followed by high-intensity intermittent training which was different from ours. Mean bias during 40 minutes of exercise was 12.25 mg/dL (∆LOA [45.60 mg/dL]). Within 30 minutes post-exercise, mean bias was 4.18 mg/dL (∆LOA [58.8 mg/dL]). Findings by (Clavel et al., 2022) were different from our findings, revealing the highest mean bias during exercising protocols and a similar ∆LAO between all conditions concluding that accuracy is negatively influenced by physical activity. However, we found a lower mean bias during exercise but a higher ∆LOA indicating a higher variability of ISF and CB glucose values. Studies that were performed in subjects with type 1 diabetes confirm our findings and the findings of Clavel et al. (2022) showing a higher deviation of ISF and CB values under exercising conditions compared to resting and/or fasted conditions (Aberer et al., 2017; Adolfsson et al., 2011; Bally et al., 2016; Giani et al., 2018; Moser et al., 2019; Schierbauer et al., 2022). Moser et al. (2018) assume physiological changes during exercise, such as alterations in blood flow rate, body temperature and body acidity, can theoretically have an impact on interstitial glucosesensing accuracy.
4.2. Impact of CHO intake before testing
Based on our findings showing a significant difference under resting fasted conditions related to CHO load of the dinner at least 9 hours before test morning, we assume changes in glucose flux from CB into the ISF dependent on glucose availability. As some recommend arterial or arterialised venous blood glucose as the gold standard for glucose measurement (Brouns et al., 2005), Siegmund et al. (2017) suggest, that glucose measured in the ISF might be the more relevant physiological parameter than blood glucose values, as the ISF might be more sensitive towards changes in glucose concentrations than blood. Siegmund et al. (2017) further hypothesise, that ISF glucose concentration might better reflect glucose status than CB does due to homeostatic regulation of glucose concentration in blood. There are several studies trying to estimate glucose flux in healthy people (Basu et al., 2013) and people living with diabetes (Basu et al., 2015; Rebrin et al., 1999) under resting conditions. Taken from our and previous studies, varying glucose flux from CB into the ISF due to chronic or acute nutritional status and physical activity is one major factor influencing the evaluation of CGM devices when comparing to blood glucose analysers (Bally et al., 2016; Muñoz Fabra et al., 2021; Schierbauer et al., 2022; Zaharieva et al., 2019). Also different body sites of sensor application show slight but no statistical differences (Faccioli et al., 2017; Steineck et al., 2019). Taken from our findings that show a different reaction of the CGM device during resting and fasted condition between a different CHO load of the dinner before test day, we want to encourage further studies to consider nutritional status and overall CHO intake. As most studies were performed in subjects with diabetes controlling for glycemia or insulinemia in order to keep subjects away from a serious hypo- or hyperglycaemic event, more data in healthy subjects are needed.
4.3. Conclusion for practical application
As people living with diabetes are using CGM systems in order to manage their CHO intake and insulin doses, correct handling of CGM devices and data is essential. Therefore, the application of CGM systems in patients with diabetes is accompanied with medical and/or nutritional experts’ advice. People without diabetes, including athletes, are not necessarily professionally advised before starting to use CGM devices as they are freely available in the market. Due to the fact that the reaction of the ISF towards changes in glucose concentration data are generally less known and, depending on the situation, are not transferable into CB glucose values, especially under exercising conditions, referring to ISF glucose data can lead to CHO intake mismanagement. If the accompanied use of digital applications connected to the CGM sensor can replace professional advice and educate the user on how to interpret data and how to react under different circumstances is a question for future research. Also, as stated by Moser et al. (2018), CGM devices pose a risk for skin irritation (Feig et al., 2017; Hilliard et al., 2019), sleep disruption due to discomfort of wearing the sensor (Messer et al., 2018) and an overload of information for people with diabetes and cannot be excluded for application by people without diabetes. While applying the sensor at the backarm or any other body site (periumbilical or at the upper leg), an insight into the software still shows longer periods of data lacks. Also, sensors tended to show errors that required a replacement of the sensor with a new one. Especially during swimming, sensors seem to have problems with minute-by-minute data production. Further, some subjects reported that sensors were torn off during or after their swimming session. On the other hand, CGM devices pose a chance to raise athletes’ awareness for increasing their CHO intake as several studies found an insufficient daily carbohydrate intake as indicated by a meta-analysis by (Steffl et al., 2019). Spronk et al. (2014) assume a lack of nutritional knowledge to be one factor for insufficient nutritional status. Also internal, yet unpublished data show an insufficient CHO intake four hours before, during and within two hours after exercise. If the application of CGM devices can support nutritional consultation as another tool in the practitioner’s toolbox, should be investigated in future research.
5. Limitations
Data collection was conducted during COVID-19 restrictions in our institute. Therefore, all tests under exercising conditions were performed outdoors, where standardisation of environmental issues (change in temperature, rain) was not possible. Performance diagnostics before the start of the study were not feasible as time spent in the institute needed to be as short as possible. Therefore, HRmax, that we used in order to standardise subject’s running intensity was calculated by 220 minus age, which is generally considered to be inaccurate. As we consider newer concepts and equations to estimate HRmax to be limited, e.g. by small sample size and specific characteristics of sample (Nikolaidis, 2015; Papadopoulou et al., 2019; Tanaka et al., 2021), we chose the most established equation by Fox et al. (1968) despite the criticism. Due to the focus of this study to compare paired ISF and CB data for estimating the application in practice, we rate this limitation to only have a low impact on results. Further, there is some criticism about statistical tests to evaluate CGM devices. As most concepts were developed for the evaluation of self-monitoring blood-glucose devices measuring in the same media as lab-method analyser (FDA, 2014; Freckmann et al., 2019; Heinemann et al., 2020; Villena Gonzales et al., 2019), due to natural physiological differences, applicability for CGM devices is debatable (Færch et al., 2021; Siegmund et al., 2017).
6. Conclusions
In summary, the herein presented results suggest that ISF glucose concentrations reflect CB glucose concentrations under resting conditions better than under exercising conditions in healthy subjects, which is in line with previous studies in subjects with diabetes. As lag-time under exercising conditions, data sensor-derived data could lead to misinterpretation by athletes. Therefore, application of CGM should be accompanied by medical and/or nutritional experts’ advise. Practitioners should evaluate risks and chances of the sensor considering the individual athlete.
Author Contributions
Author 1 conceived the study, conducted the statistical analysis and wrote the original draft of the manuscript. Author 2 supported data collection and data analysis. Author 3 and Author 4 reviewed and edited the final manuscript. (Authors are blinded for review).
Funding
The authors have not declared received a specific grant for this research.
Data Sharing
Data are available on request.
Patient Consent for Publication
Subjects gave written informed consent for anonymous publication of data.
Ethics Approval
The study was approved based on the Declaration of Helsinki by the university’s local ethics committee (number is known, but blinded for review).
Acknowledgments
The authors express appreciation to SuperSapiens and EKF diagnostics for providing devices for the purpose of this study. Further, the authors wish to acknowledge support from Manfred-Donike Society for Doping Analysis (Cologne, Germany).
Competing Interests
None declared.
References
- Aberer, F., Hajnsek, M., Rumpler, M., Zenz, S., Baumann, P. M., Elsayed, H., Puffing, A., Treiber, G., Pieber, T. R., Sourij, H., & Mader, J. K. (2017). Evaluation of subcutaneous glucose monitoring systems under routine environmental conditions in patients with type 1 diabetes: ABERER et al. Diabetes, Obesity and Metabolism, 19(7), 1051–1055. [CrossRef]
- Adolfsson, P., Nilsson, S., & Lindblad, B. (2011). Continuous glucose monitoring system during physical exercise in adolescents with type 1 diabetes: Physical exercise and diabetes. Acta Paediatrica, 100(12), 1603–1609. [CrossRef]
- Bally, L., Zueger, T., Pasi, N., Carlos, C., Paganini, D., & Stettler, C. (2016). Accuracy of continuous glucose monitoring during differing exercise conditions. Diabetes Research and Clinical Practice, 112, 1–5. [CrossRef]
- Bao, Y., Chen, L., Chen, L., Dou, J., Gao, Z., Gao, L., Guo, L., Guo, X., Ji, L., Ji, Q., Jia, W., Kuang, H., Li, Q., Li, Q., Li, X., Li, Y., Li, L., Liu, J., Ma, J., … On behalf of Chinese Diabetes Society. (2019). Chinese clinical guidelines for continuous glucose monitoring (2018 edition). Diabetes/Metabolism Research and Reviews, 35(6). [CrossRef]
- Basu, A., Dube, S., Slama, M., Errazuriz, I., Amezcua, J. C., Kudva, Y. C., Peyser, T., Carter, R. E., Cobelli, C., & Basu, R. (2013). Time Lag of Glucose From Intravascular to Interstitial Compartment in Humans. Diabetes, 62(12), 4083–4087. 12. [CrossRef]
- Basu, A., Dube, S., Veettil, S., Slama, M., Kudva, Y. C., Peyser, T., Carter, R. E., Cobelli, C., & Basu, R. (2015). Time Lag of Glucose From Intravascular to Interstitial Compartment in Type 1 Diabetes. Journal of Diabetes Science and Technology, 9(1), 63–68. 1. [CrossRef]
- Bland, J. Bland, J., & Altman, D. (1986). Statistical methods for assessing agreement between two methods of clinical measurement. The Lancet, 8476(327), 307–310. 327. [CrossRef]
- Bowler, A.-L. M., Whitfield, J., Marshall, L., Coffey, V. G., Burke, L. M., & Cox, G. R. (2023). The Use of Continuous Glucose Monitors in Sport: Possible Applications and Considerations. International Journal of Sport Nutrition and Exercise Metabolism, 33(2), 121–132. [CrossRef]
- Brouns, F., Bjorck, I., Frayn, K. N., Gibbs, A. L., Lang, V., Slama, G., & Wolever, T. M. S. (2005). Glycaemic index methodology. Nutrition Research Reviews, 18(1), 145–171. 1. [CrossRef]
- Burke, L. M., Castell, L. M., Casa, D. J., Close, G. L., Costa, R. J. S., Desbrow, B., Halson, S. L., Lis, D. M., Melin, A. K., Peeling, P., Saunders, P. U., Slater, G. J., Sygo, J., Witard, O. C., Bermon, S., & Stellingwerff, T. (2019). International Association of Athletics Federations Consensus Statement 2019: Nutrition for Athletics. International Journal of Sport Nutrition and Exercise Metabolism, 29(2), 73–84. [CrossRef]
- Clavel, P., Tiollier, E., Leduc, C., Fabre, M., Lacome, M., & Buchheit, M. (2022). Concurrent Validity of a Continuous Glucose-Monitoring System at Rest and During and Following a High-Intensity Interval Training Session. International Journal of Sports Physiology and Performance, 17(4), 627–633. [CrossRef]
- Cohen, J. (1988). Statistical power analysis for the behavioral sciences (2. ed., reprint). Psychology Press.
- Ehrhardt, N., & Al Zaghal, E. (2019). Behavior Modification in Prediabetes and Diabetes: Potential Use of Real-Time Continuous Glucose Monitoring. Journal of Diabetes Science and Technology, 13(2), 271–275. [CrossRef]
- Faccioli, S., Del Favero, S., Visentin, R., Bonfanti, R., Iafusco, D., Rabbone, I., Marigliano, M., Schiaffini, R., Bruttomesso, D., Cobelli, C., & on behalf of the PedArPan Study Group. (2017). Accuracy of a CGM Sensor in Pediatric Subjects With Type 1 Diabetes. Comparison of Three Insertion Sites: Arm, Abdomen, and Gluteus. Journal of Diabetes Science and Technology, 11(6), 1147–1154. [CrossRef]
- Færch, K., Amadid, H., Bruhn, L., Clemmensen, K. K. B., Hulman, A., Ried-Larsen, M., Blond, M. B., Jørgensen, M. E., & Vistisen, D. (2021). Discordance Between Glucose Levels Measured in Interstitial Fluid vs in Venous Plasma After Oral Glucose Administration: A Post-Hoc Analysis From the Randomised Controlled PRE-D Trial. Frontiers in Endocrinology, 12, 753810. [CrossRef]
- FDA. (2014). Self-monitoring Blood Glucose Test Systems for Over-the-Counter Use. 1–38.
- Feig, D. S., Donovan, L. E., Corcoy, R., Murphy, K. E., Amiel, S. A., Hunt, K. F., Asztalos, E., Barrett, J. F. R., Sanchez, J. J., de Leiva, A., Hod, M., Jovanovic, L., Keely, E., McManus, R., Hutton, E. K., Meek, C. L., Stewart, Z. A., Wysocki, T., O’Brien, R., … Pragnell, M. (2017). Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): A multicentre international randomised controlled trial. The Lancet, 390(10110), 2347–2359. [CrossRef]
- Fox, L., Beck, R., & Xing, D. (2010). Variation of Interstitial Glucose Measurements Assessed by Continuous Glucose Monitors in Healthy, Nondiabetic Individuals. Diabetes Care, 33(6), 1297–1299. 6. [CrossRef]
- Fox, S. (3rd), Haskell, W., & Naughton, J. (1968). Physical activity and the prevention of coronary heart disease. Preventive Medicine, 8(44), 950–967. 44. [CrossRef]
- Freckmann, G., Hagenlocher, S., Baumstark, A., Jendrike, N., Gillen, R. C., Rössner, K., & Haug, C. (2007). Continuous Glucose Profiles in Healthy Subjects under Everyday Life Conditions and after Different Meals. Journal of Diabetes Science and Technology, 1(5), 695–703. 5. [CrossRef]
- Freckmann, G., Pleus, S., Grady, M., Setford, S., & Levy, B. (2019). Measures of Accuracy for Continuous Glucose Monitoring and Blood Glucose Monitoring Devices. Journal of Diabetes Science and Technology, 13(3), 575–583. 13, 3, 575–583. [CrossRef]
- Giani, E., Macedoni, M., Barilli, A., Petitti, A., Mameli, C., Bosetti, A., Cristiano, A., Radovanovic, D., Santus, P., & Zuccotti, G. V. (2018). Performance of the Flash Glucose Monitoring System during exercise in youth with Type 1 diabetes. Diabetes Research and Clinical Practice, 146, 321–329. [CrossRef]
- Gijzen, K., Moolenaar, D. L. J., Weusten, J. J. A. M., Pluim, H. J., & Demir, A. Y. (2012). Is there a suitable point-of-care glucose meter for tight glycemic control? Evaluation of one home-use and four hospital-use meters in an intensive care unit. Clinical Chemistry and Laboratory Medicine (CCLM), 50(11), 1985–1992. [CrossRef]
- Heinemann, L., Schoemaker, M., Schmelzeisen-Redecker, G., Hinzmann, R., Kassab, A., Freckmann, G., Reiterer, F., & Re, L. D. (2020). Benefits and Limitations of MARD as a Performance Parameter for Continuous Glucose Monitoring in the Interstitial Space. Journal of Diabetes Science and Technology, 14(1), 135–150. [CrossRef]
- Hilliard, M. E., Levy, W., Anderson, B. J., Whitehouse, A. L., Commissariat, P. V., Harrington, K. R., Laffel, L. M., Miller, K. M., Van Name, M., Tamborlane, W. V., DeSalvo, D. J., & DiMeglio, L. A. (2019). Benefits and Barriers of Continuous Glucose Monitoring in Young Children with Type 1 Diabetes. Diabetes Technology & Therapeutics, 21(9), 493–498.
- Jeukendrup, A. (2014). A Step Towards Personalized Sports Nutrition: Carbohydrate Intake During Exercise. Sports Medicine, 44(S1), 25–33. [CrossRef]
- Jonvik, K. L., King, M., Rollo, I., Stellingwerff, T., & Pitsiladis, Y. (2022). New Opportunities to Advance the Field of Sports Nutrition. Frontiers in Sports and Active Living, 4, 852230. [CrossRef]
- Kerksick, C. M., Arent, S., Schoenfeld, B. J., Stout, J. R., Campbell, B., Wilborn, C. D., Taylor, L., Kalman, D., Smith-Ryan, A. E., Kreider, R. B., Willoughby, D., Arciero, P. J., VanDusseldorp, T. A., Ormsbee, M. J., Wildman, R., Greenwood, M., Ziegenfuss, T. N., Aragon, A. A., & Antonio, J. (2017). International society of sports nutrition position stand: Nutrient timing. Journal of the International Society of Sports Nutrition, 14(1), 33. [CrossRef]
- Lakens, D. (2013). Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Frontiers in Psychology, 4. [CrossRef]
- Loon, L. J. C., Greenhaff, P. L., Constantin-Teodosiu, D., Saris, W. H. M., & Wagenmakers, A. J. M. (2001). The effects of increasing exercise intensity on muscle fuel utilisation in humans. The Journal of Physiology, 536(1), 295–304. [CrossRef]
- Madden, J., O’Mahony, C., Thompson, M., O’Riordan, A., & Galvin, P. (2020). Biosensing in dermal interstitial fluid using microneedle based electrochemical devices. Sensing and Bio-Sensing Research, 29, 100348. [CrossRef]
- Mansournia, M. A., Collins, G. S., Nielsen, R. O., Nazemipour, M., Jewell, N. P., Altman, D. G., & Campbell, M. J. (2021). A CHecklist for statistical Assessment of Medical Papers (the CHAMP statement): Explanation and elaboration. British Journal of Sports Medicine, 55(18), 1009–1017. [CrossRef]
- Messer, L. H., Johnson, R., Driscoll, K. A., & Jones, J. (2018). Best friend or spy: A qualitative meta-synthesis on the impact of continuous glucose monitoring on life with Type 1 diabetes. Diabetic Medicine, 35(4), 409–418. [CrossRef]
- Moser, O., Eckstein, M. L., Mueller, A., Birnbaumer, P., Aberer, F., Koehler, G., Sourij, C., Kojzar, H., Holler, P., Simi, H., Pferschy, P., Dietz, P., Bracken, R. M., Hofmann, P., & Sourij, H. (2019). Impact of physical exercise on sensor performance of the FreeStyle Libre intermittently viewed continuous glucose monitoring system in people with Type 1 diabetes: A randomized crossover trial. Diabetic Medicine, 36(5), 606–611. [CrossRef]
- Moser, O., Riddell, M. C., Eckstein, M. L., Adolfsson, P., Rabasa-Lhoret, R., van den Boom, L., Gillard, P., Nørgaard, K., Oliver, N. S., Zaharieva, D. P., Battelino, T., de Beaufort, C., Bergenstal, R. M., Buckingham, B., Cengiz, E., Deeb, A., Heise, T., Heller, S., Kowalski, A. J., … Mader, J. K. (2020). Glucose management for exercise using continuous glucose monitoring (CGM) and intermittently scanned CGM (isCGM) systems in type 1 diabetes: Position statement of the European Association for the Study of Diabetes (EASD) and of the International Society for Pediatric and Adolescent Diabetes (ISPAD) endorsed by JDRF and supported by the American Diabetes Association (ADA). Diabetologia, 63(12), 2501–2520. [CrossRef]
- Moser, O., Yardley, J., & Bracken, R. (2018). Interstitial Glucose and Physical Exercise in Type 1 Diabetes: Integrative Physiology, Technology, and the Gap In-Between. Nutrients, 10(1), 93. [CrossRef]
- Muñoz Fabra, E. Muñoz Fabra, E., Díez, J.-L., Bondia, J., & Laguna Sanz, A. J. (2021). A Comprehensive Review of Continuous Glucose Monitoring Accuracy during Exercise Periods. Sensors, 21(2), 479. 2. [CrossRef]
- Nikolaidis, P. (2015). Maximal heart rate in soccer players: Measured versus age-predicted. Biomedical Journal, 38(1), 84. [CrossRef]
- Nowotny, B., Nowotny, P. J., Strassburger, K., & Roden, M. (2012). Precision and accuracy of blood glucose measurements using three different instruments: Validity of blood glucose measurement instruments. Diabetic Medicine, 29(2), 260–265. [CrossRef]
- Papadopoulou, S. D., Papadopoulou, S. K., Alipasali, F., Hatzimanouil, D., Rosemann, T., Knechtle, B., & Nikolaidis, P. T. (2019). Validity of Prediction Equations of Maximal Heart Rate in Physically Active Female Adolescents and the Role of Maturation. Medicina, 55(11), 735. [CrossRef]
- Rebrin, K., Steil, G. M., van Antwerp, W. P., & Mastrototaro, J. J. (1999). Subcutaneous glucose predicts plasma glucose independent of insulin: Implications for continuous monitoring. American Journal of Physiology-Endocrinology and Metabolism, 277(3), E561–E571. [CrossRef]
- Romijn, J. A., Coyle, E. F., Sidossis, L. S., Rosenblatt, J., & Wolfe, R. R. (2000). Substrate metabolism during different exercise intensities in endurance-trained women. Journal of Applied Physiology, 88(5), 1707–1714. [CrossRef]
- Schierbauer, J. R., Günther, S., Haupt, S., Zimmer, R. T., Zunner, B. E. M., Zimmermann, P., Wachsmuth, N. B., Eckstein, M. L., Aberer, F., Sourij, H., & Moser, O. (2022). Accuracy of Real Time Continuous Glucose Monitoring during Different Liquid Solution Challenges in Healthy Adults: A Randomized Controlled Cross-Over Trial. Sensors, 22(9), 3104. [CrossRef]
- Shah, V. N., DuBose, S. N., Li, Z., Beck, R. W., Peters, A. L., Weinstock, R. S., Kruger, D., Tansey, M., Sparling, D., Woerner, S., Vendrame, F., Bergenstal, R., Tamborlane, W. V., Watson, S. E., & Sherr, J. (2019). Continuous Glucose Monitoring Profiles in Healthy Nondiabetic Participants: A Multicenter Prospective Study. The Journal of Clinical Endocrinology & Metabolism, 104(10), 4356–4364. [CrossRef]
- Siegmund, T. Siegmund, T., Heinemann, L., Kolassa, R., & Thomas, A. (2017). Discrepancies Between Blood Glucose and Interstitial Glucose—Technological Artifacts or Physiology: Implications for Selection of the Appropriate Therapeutic Target. Journal of Diabetes Science and Technology, 11(4), 766–772. [CrossRef]
- Spronk, I., Kullen, C., Burdon, C., & O’Connor, H. (2014). Relationship between nutrition knowledge and dietary intake. British Journal of Nutrition, 111(10), 1713–1726. 10. [CrossRef]
- Steffl, M., Kinkorova, I., Kokstejn, J., & Petr, M. (2019). Macronutrient Intake in Soccer Players—A Meta-Analysis. Nutrients, 11(6), 1305. [CrossRef]
- Steineck, I. I. K., Mahmoudi, Z., Ranjan, A., Schmidt, S., Jørgensen, J. B., & Nørgaard, K. (2019). Comparison of Continuous Glucose Monitoring Accuracy Between Abdominal and Upper Arm Insertion Sites. Diabetes Technology & Therapeutics, 21(5), 295–302. [CrossRef]
- Tanaka, Y., Ogata, H., Park, I., Ando, A., Ishihara, A., Kayaba, M., Yajima, K., Suzuki, C., Araki, A., Osumi, H., Zhang, S., Seol, J., Takahashi, K., Nabekura, Y., Satoh, M., & Tokuyama, K. (2021). Effect of a single bout of morning or afternoon exercise on glucose fluctuation in young healthy men. Physiological Reports, 9(7). [CrossRef]
- Teymourian, H., Tehrani, F., Mahato, K., & Wang, J. (2021). Lab under the Skin: Microneedle Based Wearable Devices. Advanced Healthcare Materials, 10(17), 2002255. [CrossRef]
- Thomas, T., Erdman, K. A., & Burke, L. M. (2016). Nutrition and Athletic Performance. Medicine & Science in Sports & Exercise, 48(3), 543–568. [CrossRef]
- Villena Gonzales, W., Mobashsher, A., & Abbosh, A. (2019). The Progress of Glucose Monitoring—A Review of Invasive to Minimally and Non-Invasive Techniques, Devices and Sensors. Sensors, 19(4), 800. [CrossRef]
- Witt, S., Wohlfahrt, G., & Schomburg, D. (2000). Conserved arginine-516 of Penicillium amagasakiense glucose oxidase is essential for the efficient binding of β-D-glucose. 7.
- Zaharieva, D. P. Zaharieva, D. P., Turksoy, K., McGaugh, S. M., Pooni, R., Vienneau, T., Ly, T., & Riddell, M. C. (2019). Lag Time Remains with Newer Real-Time Continuous Glucose Monitoring Technology During Aerobic Exercise in Adults Living with Type 1 Diabetes. Diabetes Technology & Therapeutics, 21(6), 313–321. [CrossRef]
Figure 1.
a) Fasted test protocol for resting conditions after high carbohydrate (CHO) dinner (HC_R/Fast) or a low CHO dinner (LC_R/Fast) b) Postprandial test protocol for resting conditions after a high CHO dinner (HC_R/Glc) or a low CHO dinner c) Postprandial test protocol for running at 65% HFmax or 85% HFmax after a moderate CHO dinner (65/Glc, 85/Glc). BLOOD SPOT = CB sample collection, BOTTLE = ingestion of 1 g Glc/kg BW, RUNNING PERSON = Start of activity.
Figure 1.
a) Fasted test protocol for resting conditions after high carbohydrate (CHO) dinner (HC_R/Fast) or a low CHO dinner (LC_R/Fast) b) Postprandial test protocol for resting conditions after a high CHO dinner (HC_R/Glc) or a low CHO dinner c) Postprandial test protocol for running at 65% HFmax or 85% HFmax after a moderate CHO dinner (65/Glc, 85/Glc). BLOOD SPOT = CB sample collection, BOTTLE = ingestion of 1 g Glc/kg BW, RUNNING PERSON = Start of activity.
Figure 2.
a.i-c.i)Mean capillary blood (CB) and interstitial fluid (ISF) glucose concentrations including standard deviation (SD) (CB = solid line and positive error indication reflecting SD ; ISF = dashed line and negative error indication reflecting SD). a.ii-c.ii) Linear regression analysis of CB and ISF glucose concentration; spots reflecting paired individual data points. a) results of high carbohydrate dinner (2 g/kg BW) before an overnight fast, that separated dinner and sample collection under resting conditions after an intake of a 1 g glucose / kg BW the next morning by at least 9 hours (HC_R/Glc) b) results of low carbohydrate dinner (<0.5 g/kg BW) before an overnight fast, that separated dinner and sample collection under resting conditions after an intake of a 1 g glucose / kg BW the next morning by at least 9 hours (LC_R/Glc) c) results of moderate carbohydrate dinner (1.5 g/kg BW) before an overnight fast, that separated dinner and sample collection under running for 60 minutes at a constant load of 65% of their maximal heart rate after an intake of a 1 g glucose / kg BW the next morning by at least 9 hours (65/Glc), running started 30 minutes after glucose intake d) the results of moderate carbohydrate dinner (1.5 g/kg BW) before an overnight fast, that separated dinner and sample collection under running for 60 minutes at a constant load of 85% of their maximal heart rate after an intake of a 1 g glucose / kg BW the next morning by at least 9 hours (85/Glc), running started 30 minutes after glucose intake.
Figure 2.
a.i-c.i)Mean capillary blood (CB) and interstitial fluid (ISF) glucose concentrations including standard deviation (SD) (CB = solid line and positive error indication reflecting SD ; ISF = dashed line and negative error indication reflecting SD). a.ii-c.ii) Linear regression analysis of CB and ISF glucose concentration; spots reflecting paired individual data points. a) results of high carbohydrate dinner (2 g/kg BW) before an overnight fast, that separated dinner and sample collection under resting conditions after an intake of a 1 g glucose / kg BW the next morning by at least 9 hours (HC_R/Glc) b) results of low carbohydrate dinner (<0.5 g/kg BW) before an overnight fast, that separated dinner and sample collection under resting conditions after an intake of a 1 g glucose / kg BW the next morning by at least 9 hours (LC_R/Glc) c) results of moderate carbohydrate dinner (1.5 g/kg BW) before an overnight fast, that separated dinner and sample collection under running for 60 minutes at a constant load of 65% of their maximal heart rate after an intake of a 1 g glucose / kg BW the next morning by at least 9 hours (65/Glc), running started 30 minutes after glucose intake d) the results of moderate carbohydrate dinner (1.5 g/kg BW) before an overnight fast, that separated dinner and sample collection under running for 60 minutes at a constant load of 85% of their maximal heart rate after an intake of a 1 g glucose / kg BW the next morning by at least 9 hours (85/Glc), running started 30 minutes after glucose intake.
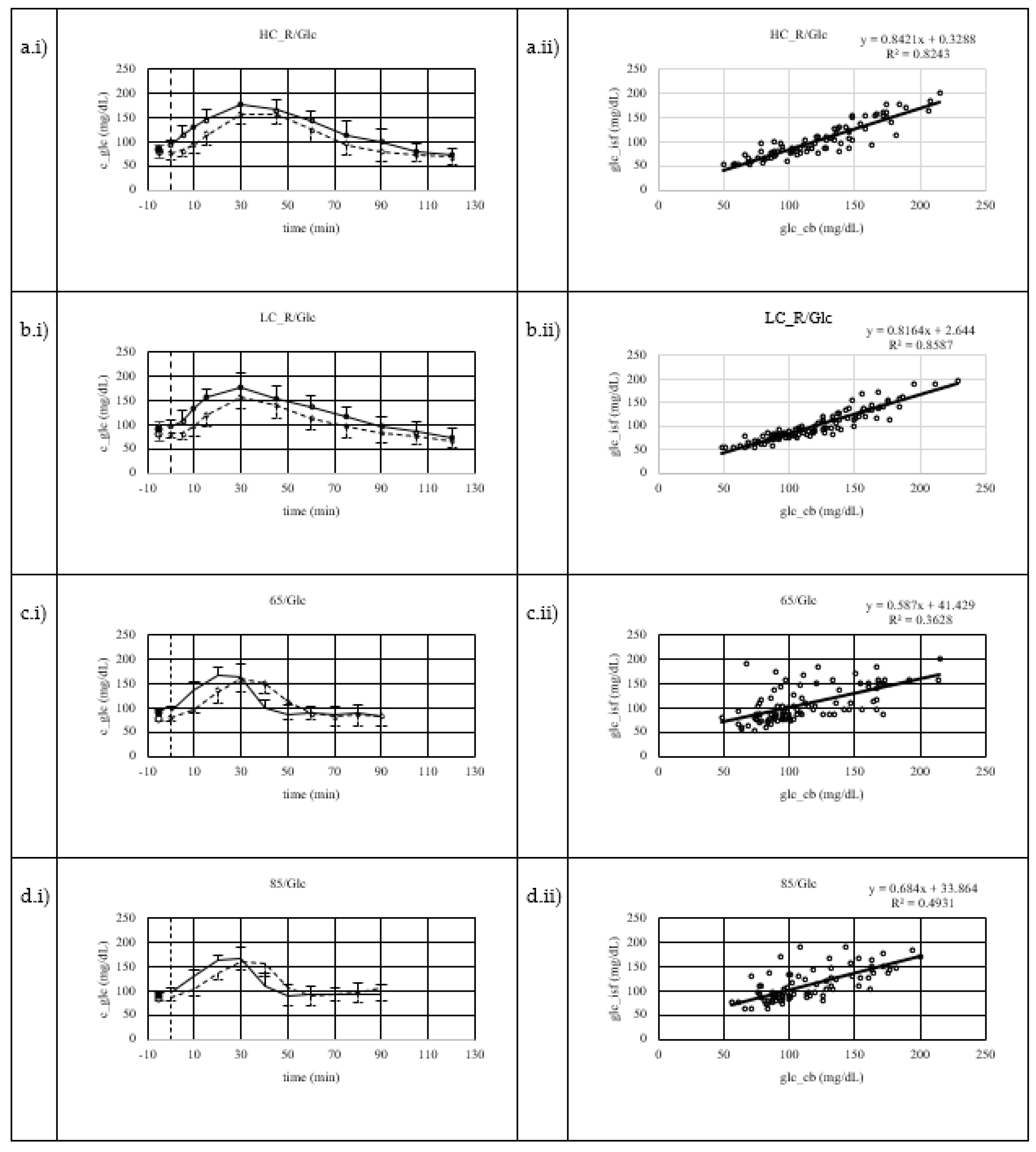
Figure 3.
Systematic measurement difference of capillary blood (CB) and interstitial fluid (ISF) glucose concentration by Bland-Altman plot showing mean difference and their 95% confidence interval as lower and upper limit of agreement. a) results of high carbohydrate dinner (2 g/kg BW) before an overnight fast, that separated dinner and sample collection under resting conditions after an intake of a 1 g glucose / kg BW the next morning by at least 9 hours (HC_R/Glc) b) results of low carbohydrate dinner (<0.5 g/kg BW) before an overnight fast, that separated dinner and sample collection under resting conditions after an intake of a 1 g glucose / kg BW the next morning by at least 9 hours (LC_R/Glc) c) results of moderate carbohydrate dinner (1.5 g/kg BW) before an overnight fast, that separated dinner and sample collection under running for 60 minutes at a constant load of 65% of their maximal heart rate after an intake of a 1 g glucose / kg BW the next morning by at least 9 hours (65/Glc), running started 30 minutes after glucose intake d) the results of moderate carbohydrate dinner (1.5 g/kg BW) before an overnight fast, that separated dinner and sample collection under running for 60 minutes at a constant load of 85% of their maximal heart rate after an intake of a 1 g glucose / kg BW the next morning by at least 9 hours (85/Glc), running started 30 minutes after glucose intake.
Figure 3.
Systematic measurement difference of capillary blood (CB) and interstitial fluid (ISF) glucose concentration by Bland-Altman plot showing mean difference and their 95% confidence interval as lower and upper limit of agreement. a) results of high carbohydrate dinner (2 g/kg BW) before an overnight fast, that separated dinner and sample collection under resting conditions after an intake of a 1 g glucose / kg BW the next morning by at least 9 hours (HC_R/Glc) b) results of low carbohydrate dinner (<0.5 g/kg BW) before an overnight fast, that separated dinner and sample collection under resting conditions after an intake of a 1 g glucose / kg BW the next morning by at least 9 hours (LC_R/Glc) c) results of moderate carbohydrate dinner (1.5 g/kg BW) before an overnight fast, that separated dinner and sample collection under running for 60 minutes at a constant load of 65% of their maximal heart rate after an intake of a 1 g glucose / kg BW the next morning by at least 9 hours (65/Glc), running started 30 minutes after glucose intake d) the results of moderate carbohydrate dinner (1.5 g/kg BW) before an overnight fast, that separated dinner and sample collection under running for 60 minutes at a constant load of 85% of their maximal heart rate after an intake of a 1 g glucose / kg BW the next morning by at least 9 hours (85/Glc), running started 30 minutes after glucose intake.
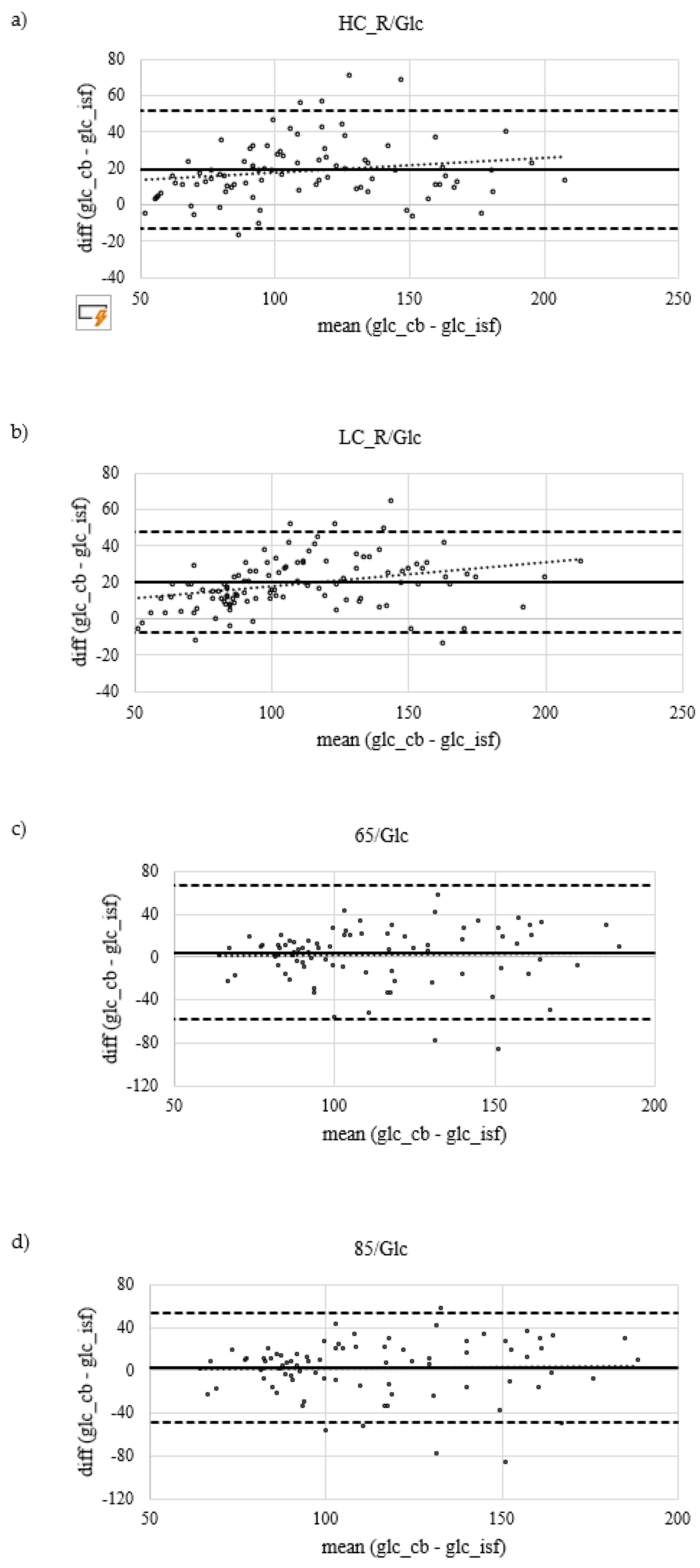
Figure 4.
Dispersion of ARD data by calculation of percentages (a) within ranges of ±5, 10, 15 and 20 % (b) and visualised as Box-Whisker-Plot showing median ARD, lower and upper interquartile range, minimum and maximum values such as individual ARD values. Values differ from text as figure shows distribution of MARD in individuals. HC_R/Glc: high carbohydrate dinner (2 g/kg BW) before an overnight fast, that separated dinner and sample collection under resting conditions after an intake of a 1 g glucose / kg BW the next morning by at least 9 hours; LC_R/Glc: low carbohydrate dinner (<0.5 g/kg BW) before an overnight fast, that separated dinner and sample collection under resting conditions after an intake of a 1 g glucose / kg BW the next morning by at least 9 hours; 65/Glc: moderate carbohydrate dinner (1.5 g/kg BW) before an overnight fast, that separated dinner and sample collection under running for 60 minutes at a constant load of 65% of their maximal heart rate after an intake of a 1 g glucose / kg BW the next morning by at least 9 hours, running started 30 minutes after glucose intake; 85/Glc: moderate carbohydrate dinner (1.5 g/kg BW) before an overnight fast, that separated dinner and sample collection under running for 60 minutes at a constant load of 85% of their maximal heart rate after an intake of a 1 g glucose / kg BW the next morning by at least 9 hours, running started 30 minutes after glucose intake.
Figure 4.
Dispersion of ARD data by calculation of percentages (a) within ranges of ±5, 10, 15 and 20 % (b) and visualised as Box-Whisker-Plot showing median ARD, lower and upper interquartile range, minimum and maximum values such as individual ARD values. Values differ from text as figure shows distribution of MARD in individuals. HC_R/Glc: high carbohydrate dinner (2 g/kg BW) before an overnight fast, that separated dinner and sample collection under resting conditions after an intake of a 1 g glucose / kg BW the next morning by at least 9 hours; LC_R/Glc: low carbohydrate dinner (<0.5 g/kg BW) before an overnight fast, that separated dinner and sample collection under resting conditions after an intake of a 1 g glucose / kg BW the next morning by at least 9 hours; 65/Glc: moderate carbohydrate dinner (1.5 g/kg BW) before an overnight fast, that separated dinner and sample collection under running for 60 minutes at a constant load of 65% of their maximal heart rate after an intake of a 1 g glucose / kg BW the next morning by at least 9 hours, running started 30 minutes after glucose intake; 85/Glc: moderate carbohydrate dinner (1.5 g/kg BW) before an overnight fast, that separated dinner and sample collection under running for 60 minutes at a constant load of 85% of their maximal heart rate after an intake of a 1 g glucose / kg BW the next morning by at least 9 hours, running started 30 minutes after glucose intake.
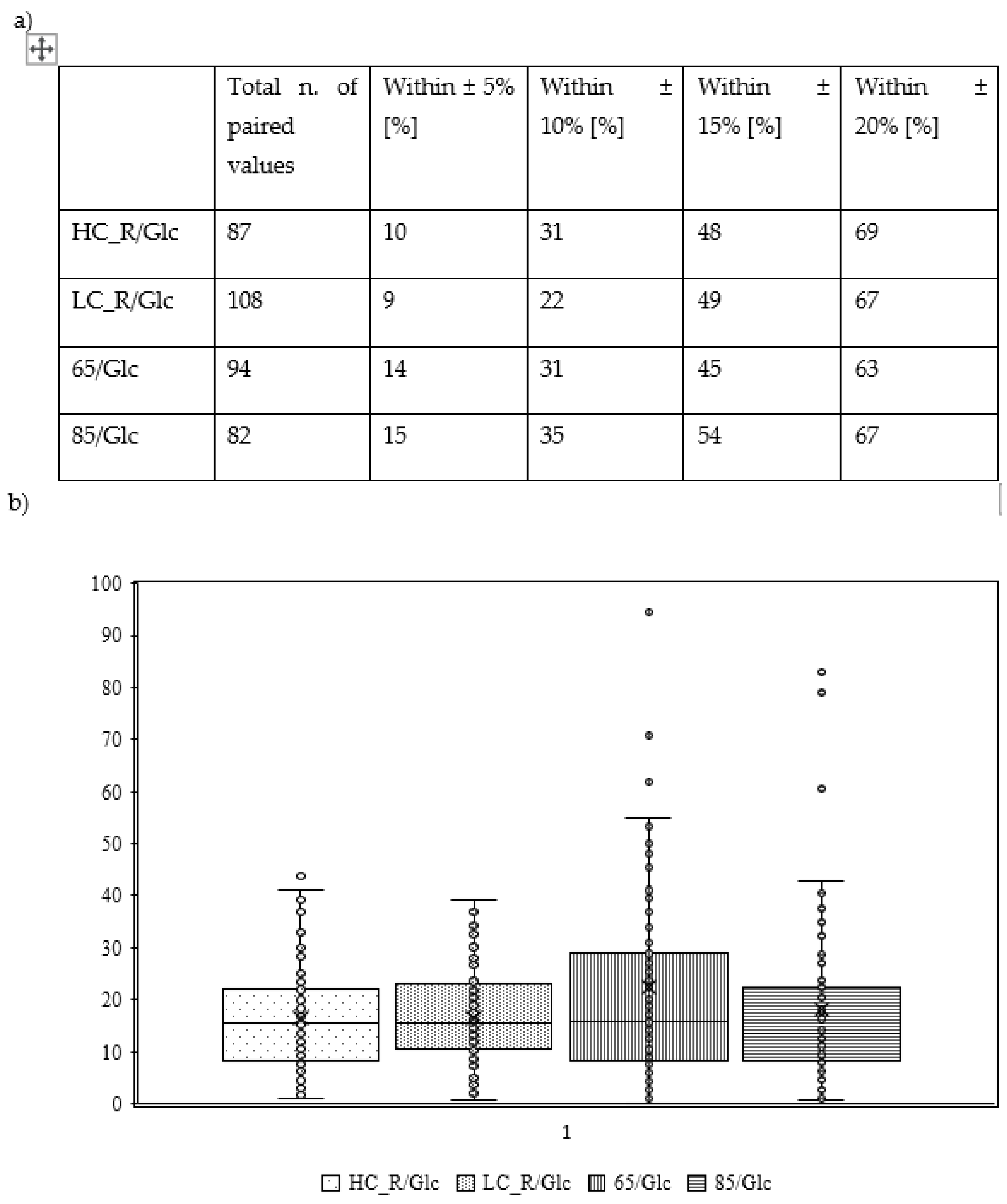
Table 1.
Constitution of standardised meals, that subjects had at least 9 hours before their test. Meals were isocaloric but differed in macronutrient distribution in order to provide different amounts of carbohydrate.
Table 1.
Constitution of standardised meals, that subjects had at least 9 hours before their test. Meals were isocaloric but differed in macronutrient distribution in order to provide different amounts of carbohydrate.
| |
HC-Dinner |
MC-Dinner |
LC-Dinner |
| Energy (kcal/kg BW) |
11.44 |
11.14 |
10.78 |
| Carbohydrate (g/kg BW) |
1.97 |
1.25 |
0.51 |
| Protein (g/kg BW) |
0.34 |
0.60 |
0.42 |
| Fat (g/kg BW) |
0.21 |
0.40 |
0.78 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).