Submitted:
15 May 2023
Posted:
16 May 2023
You are already at the latest version
Abstract
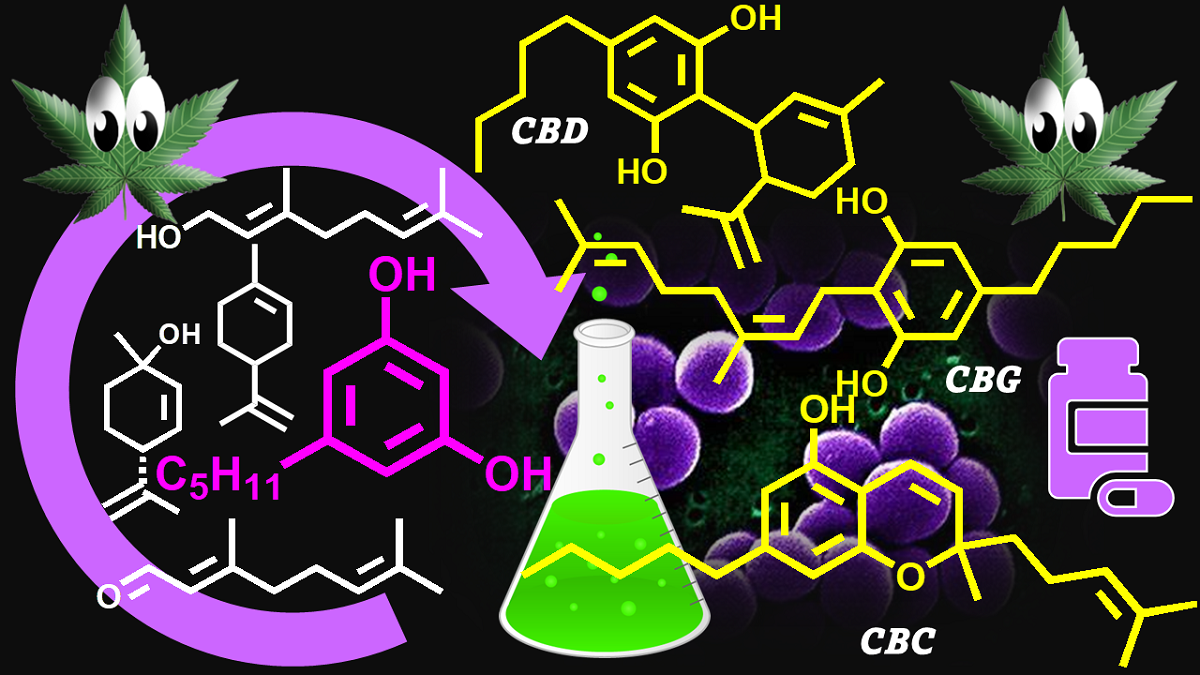
Keywords:
1. Introduction
2. Phytocannabinois (PCs), Endocannabinoids (ECs) and Synthetic Cannabinoids (SCs)
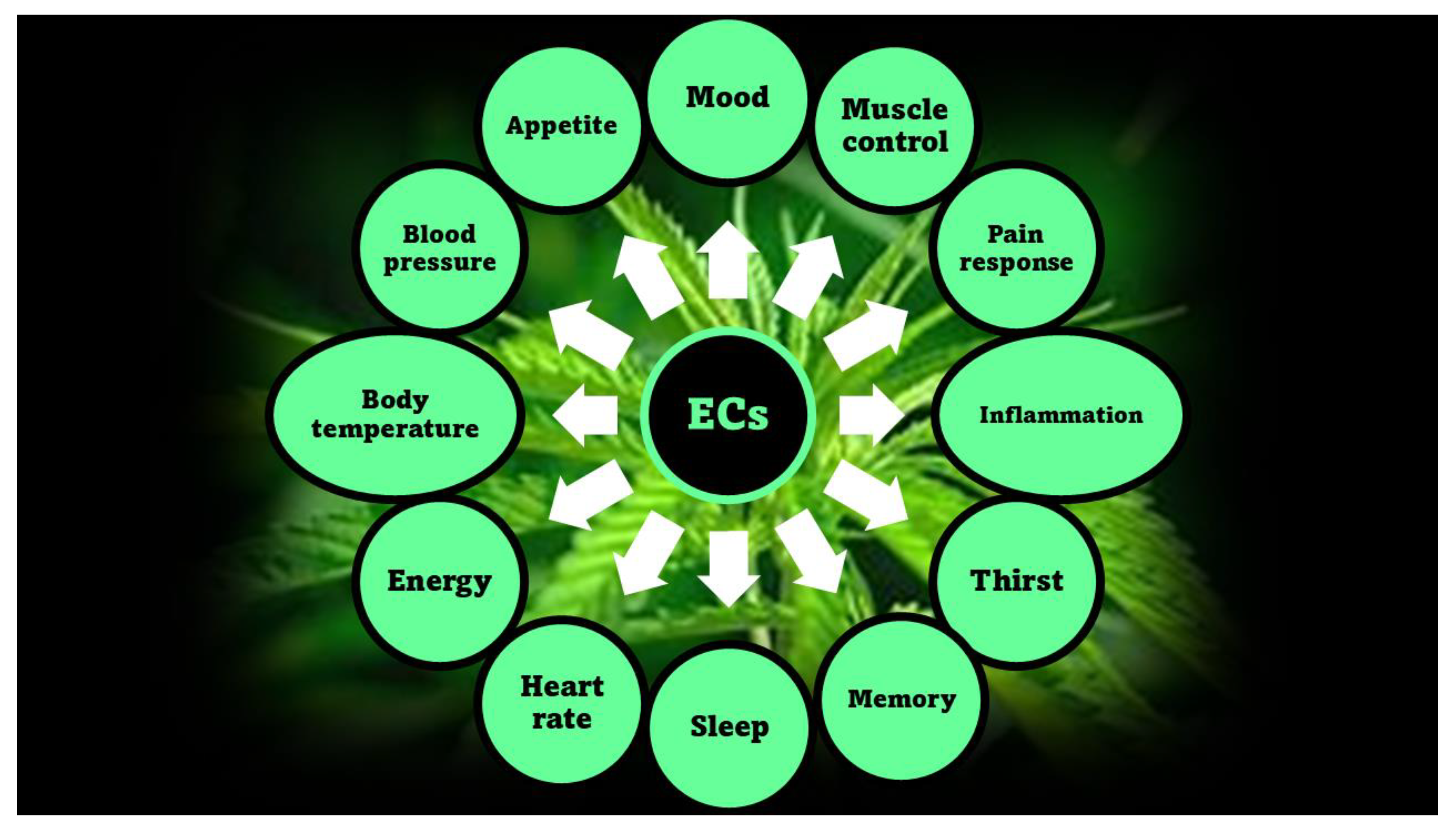
2.1. Phytocannabinoids (PCs) and Endocannabinoids (ECs)
2.2. Synthetic Cannabinoids (SCs)
2.3. Cannabinoids Clinically Approved
3. Phytocannibinoids: Polyfunctional Molecules Promising to Develop Novel Antibiotics
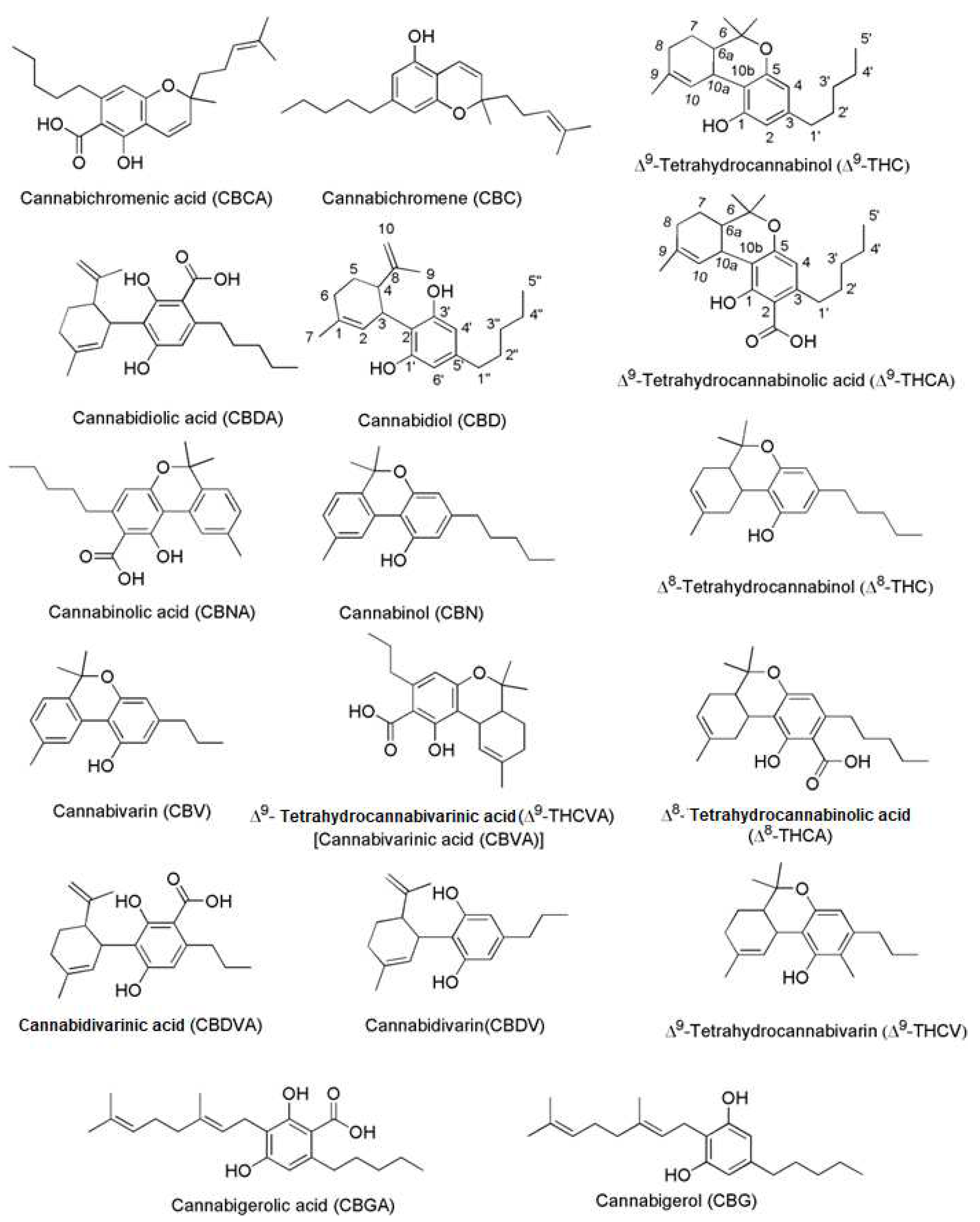
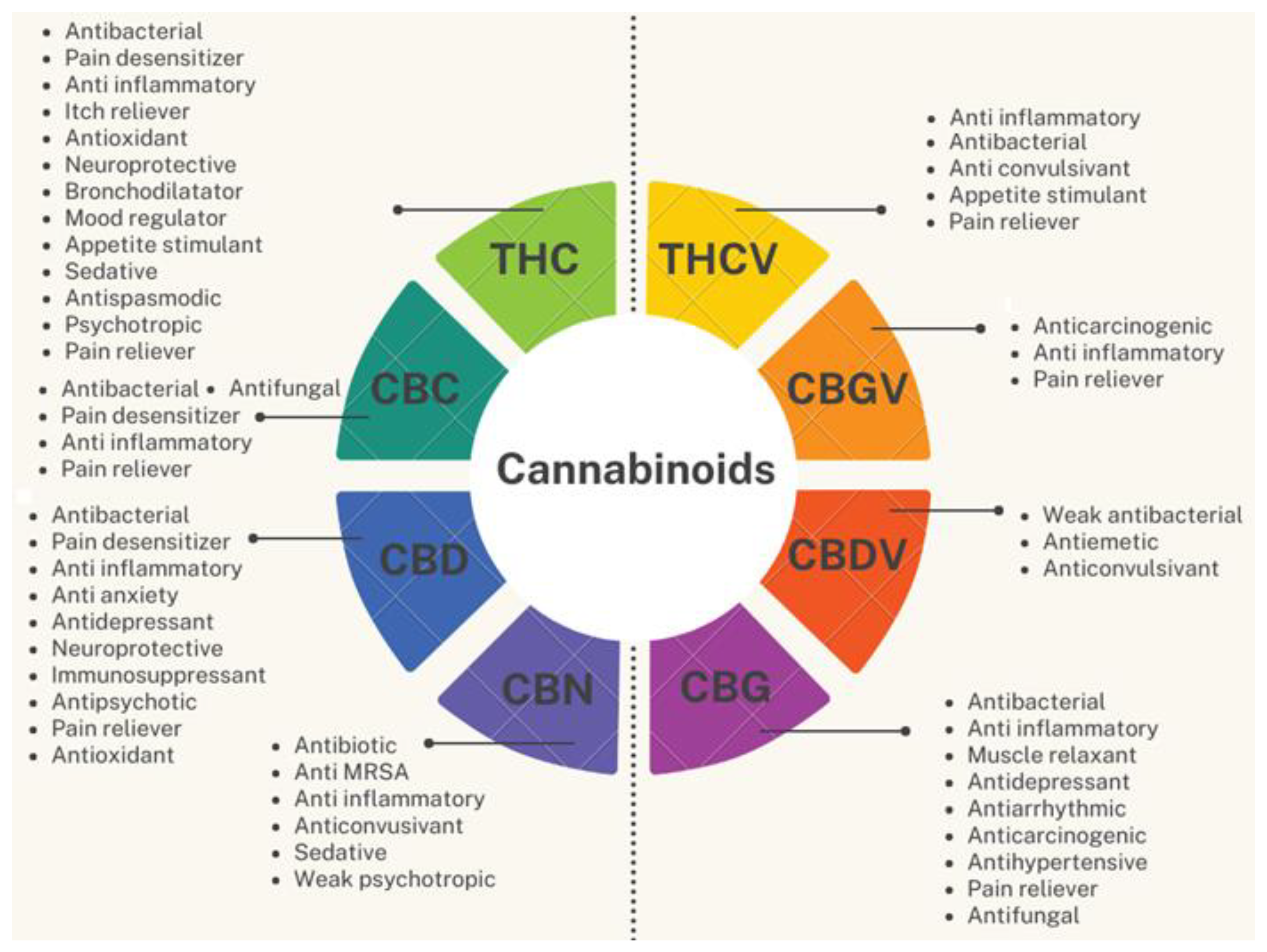
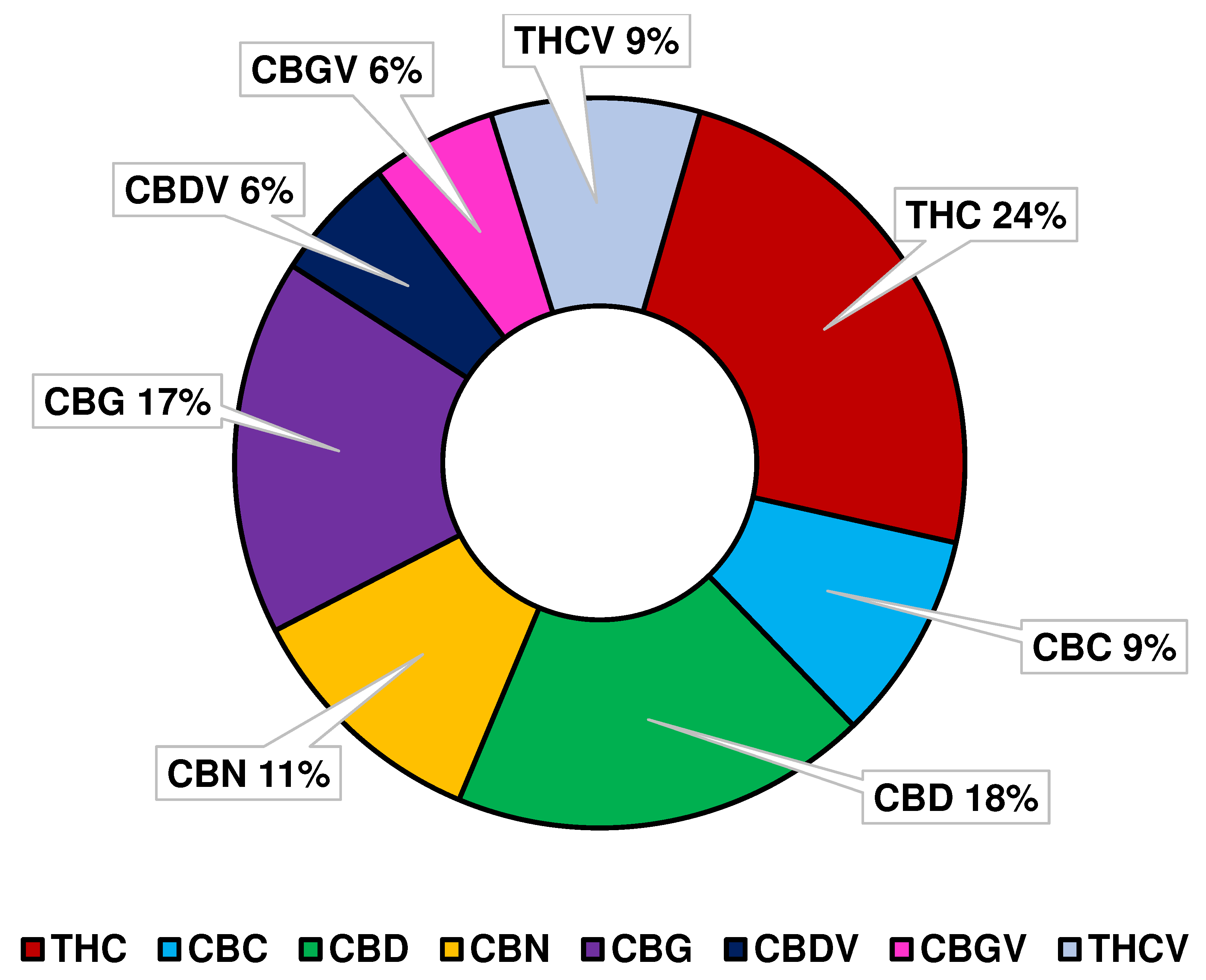
3.1. Not only THC and CBD
3.2. Much more Beyond the Psychotropic Effect of THC
3.3. Antimicrobial Cannabinoids
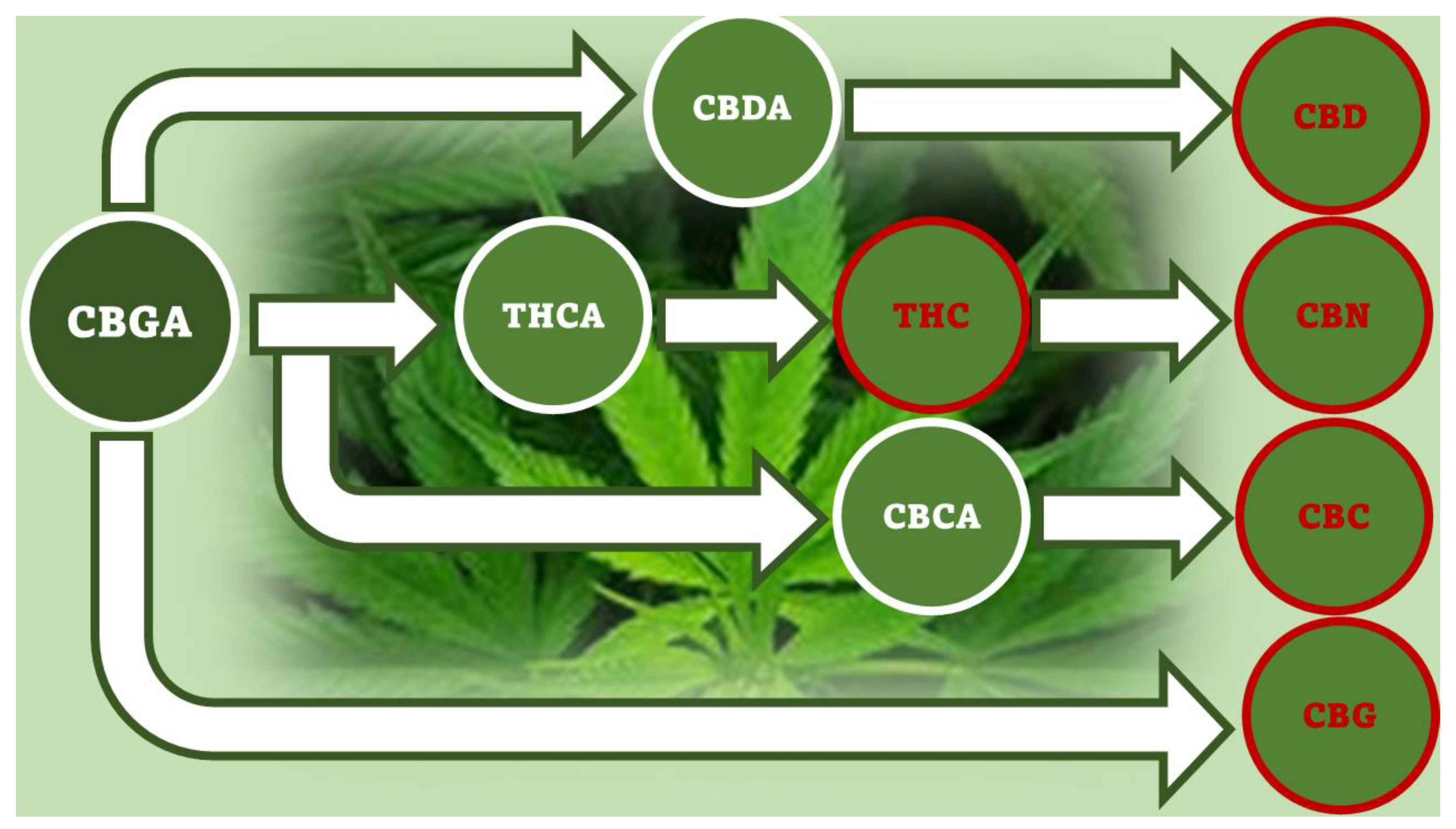
4. Production of Phytocannabinois: From Biosynthesis to Synthetic Procedures
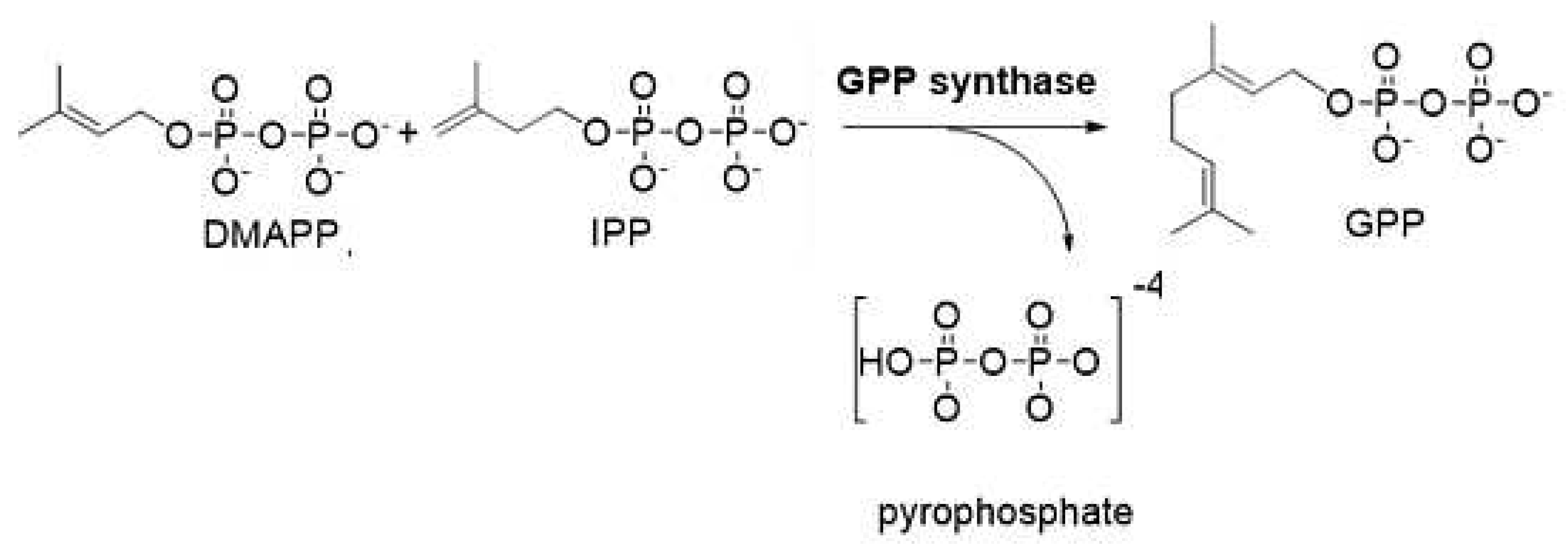
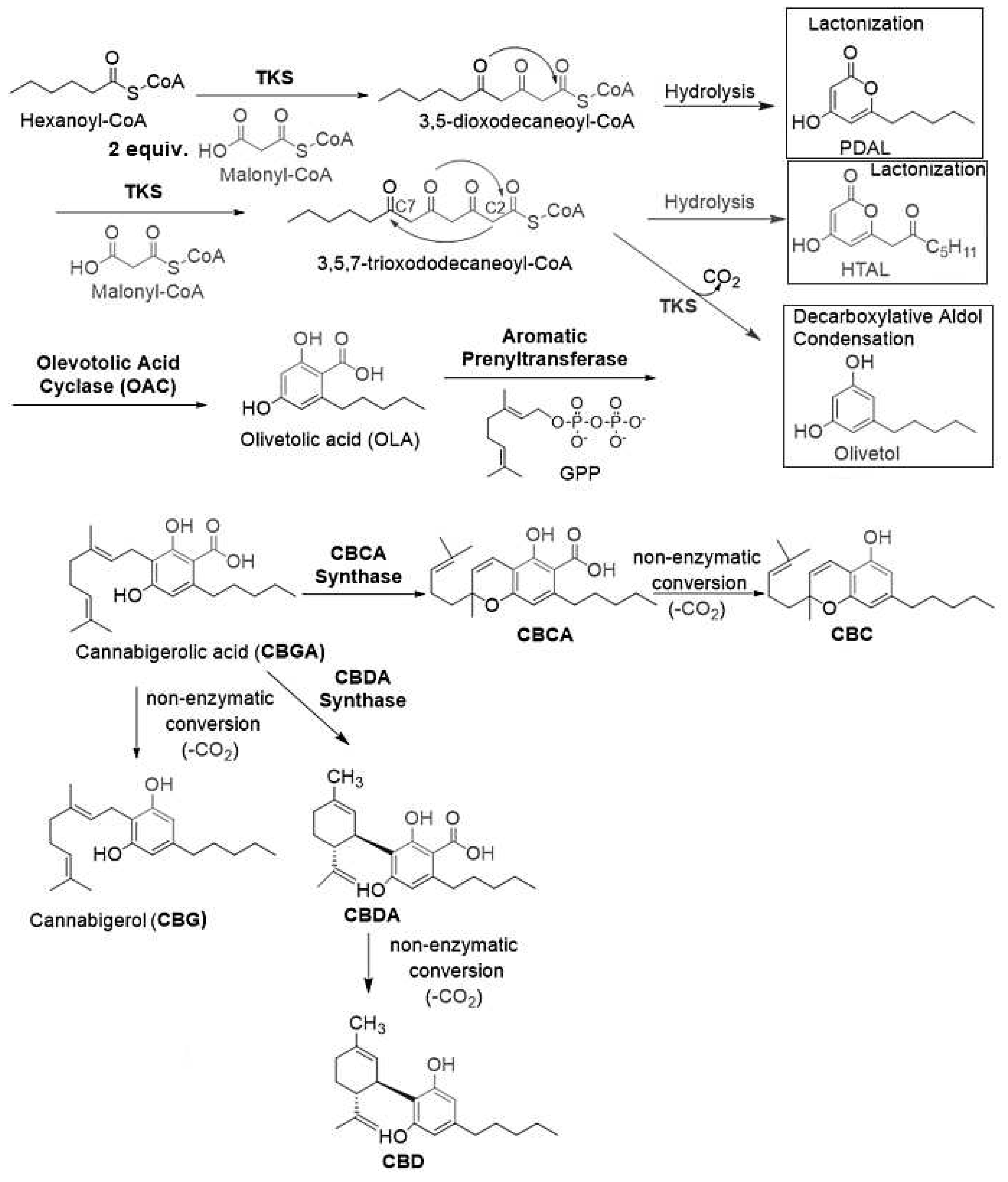
4.1. Biosynthesis of Not Psychotropic Cannabinoids (CBC, CBG and CBD).
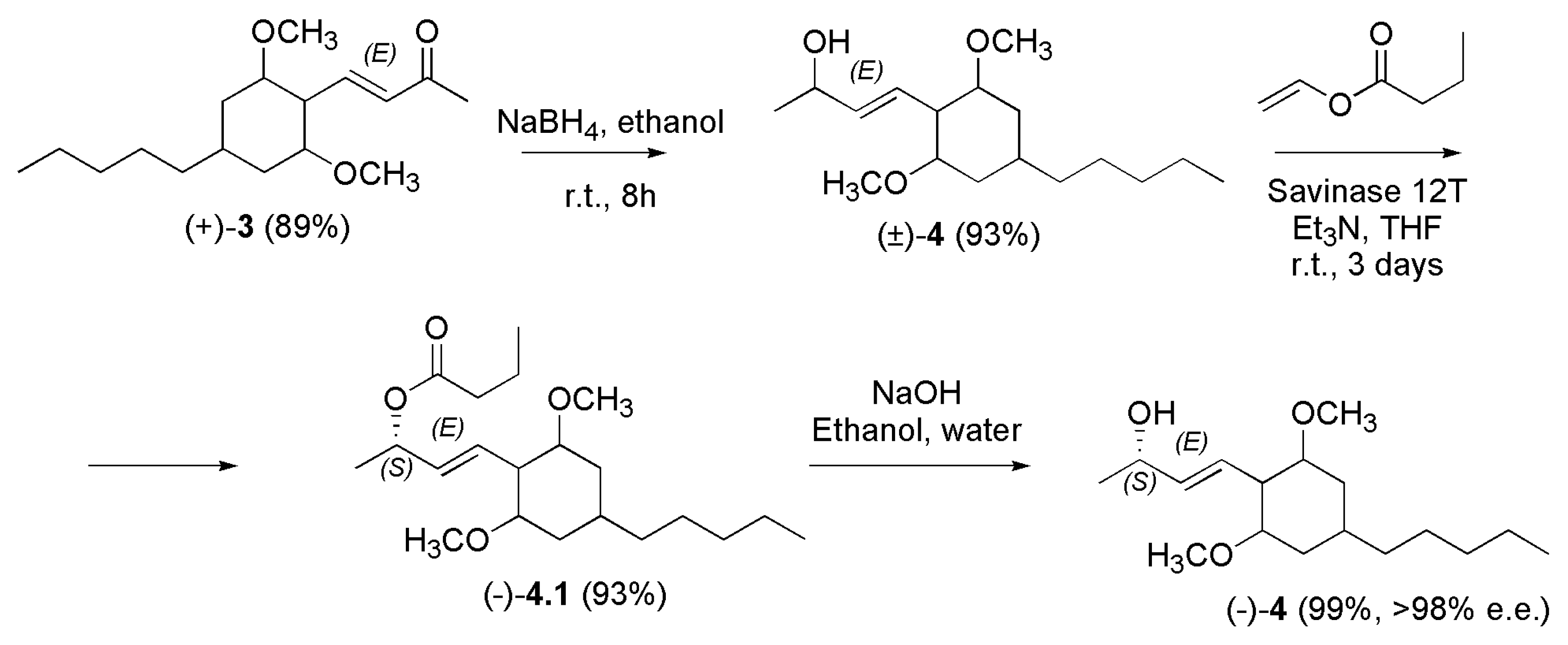
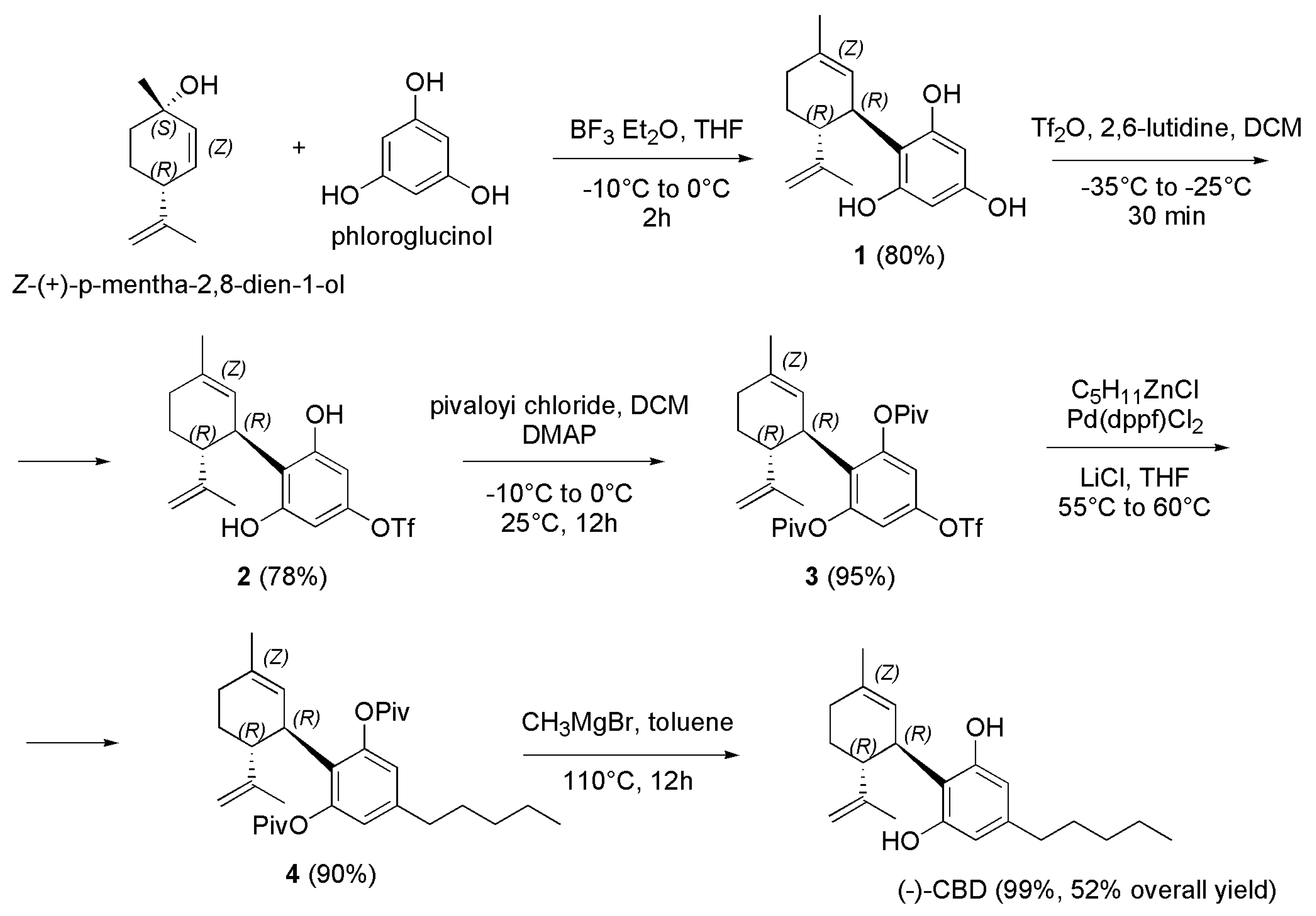
4.2. Synthetic Procedures to Prepare Not Psychotropic Cannabinoids CBC, CBG and (-)-CBD.
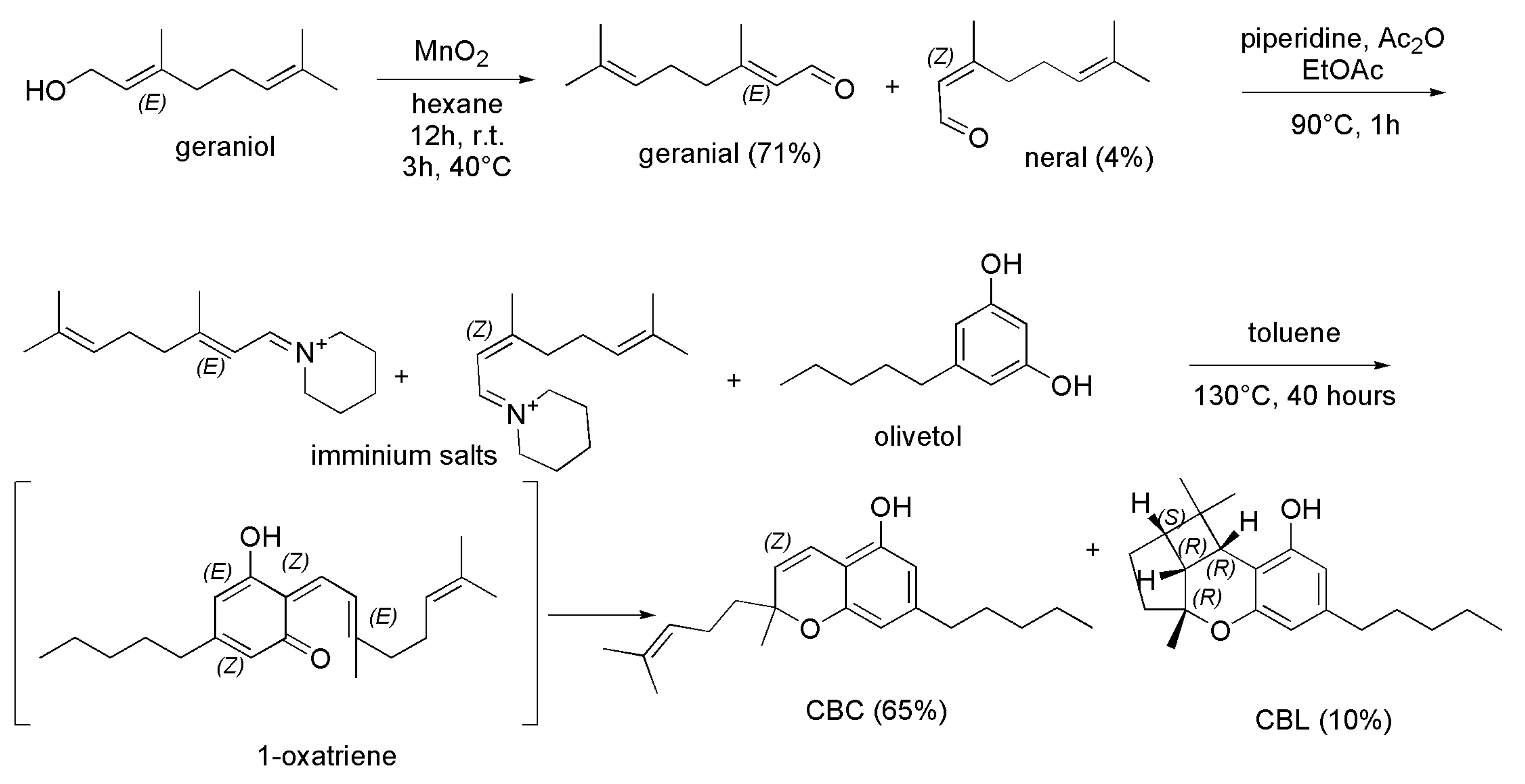
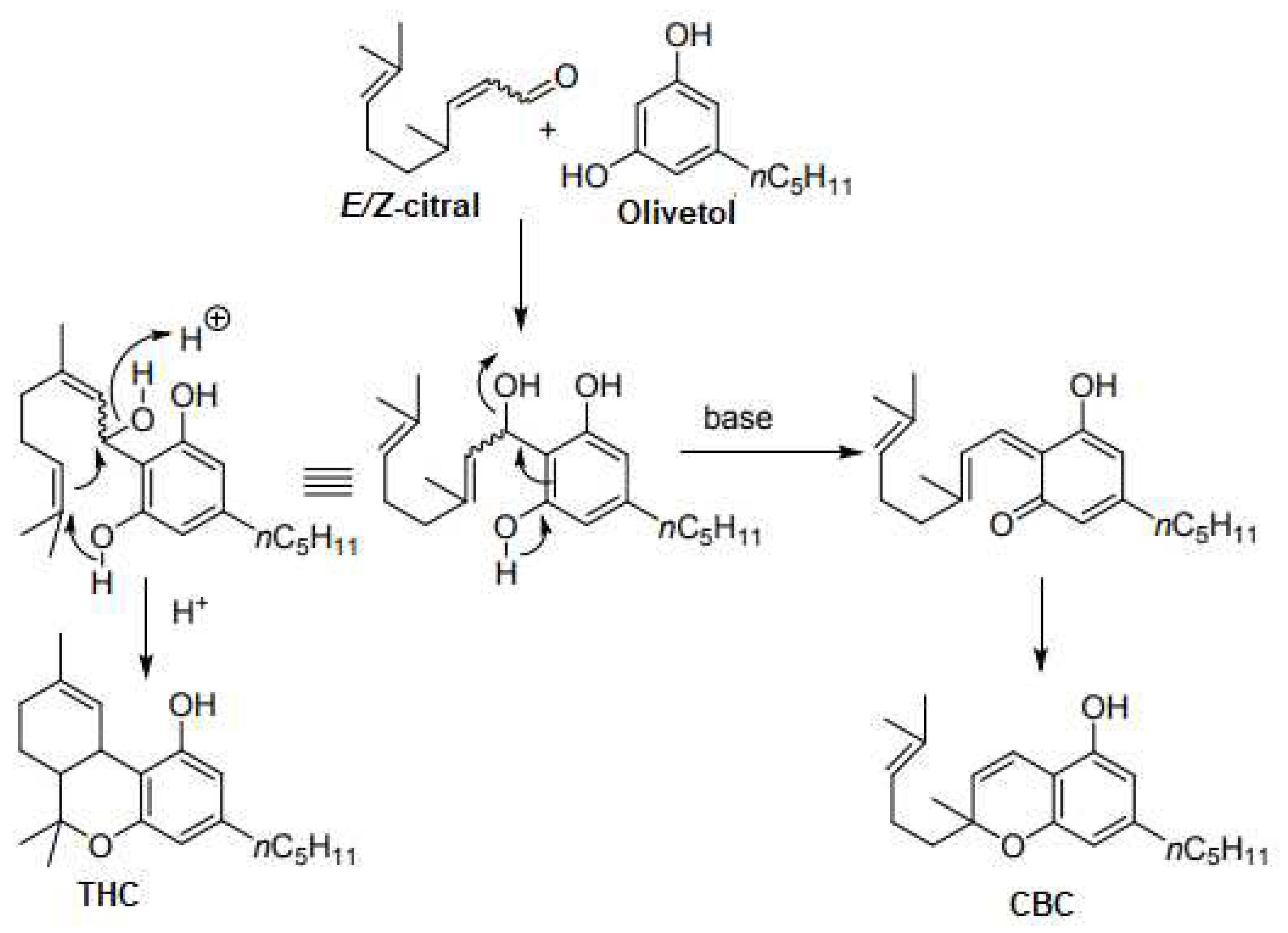
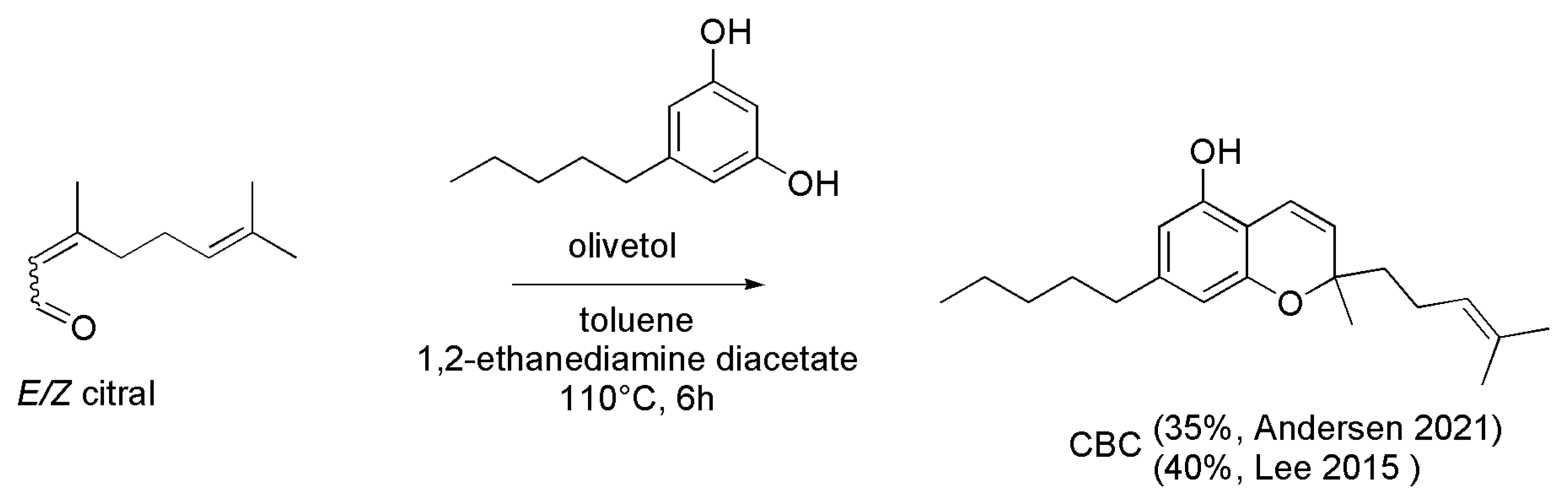
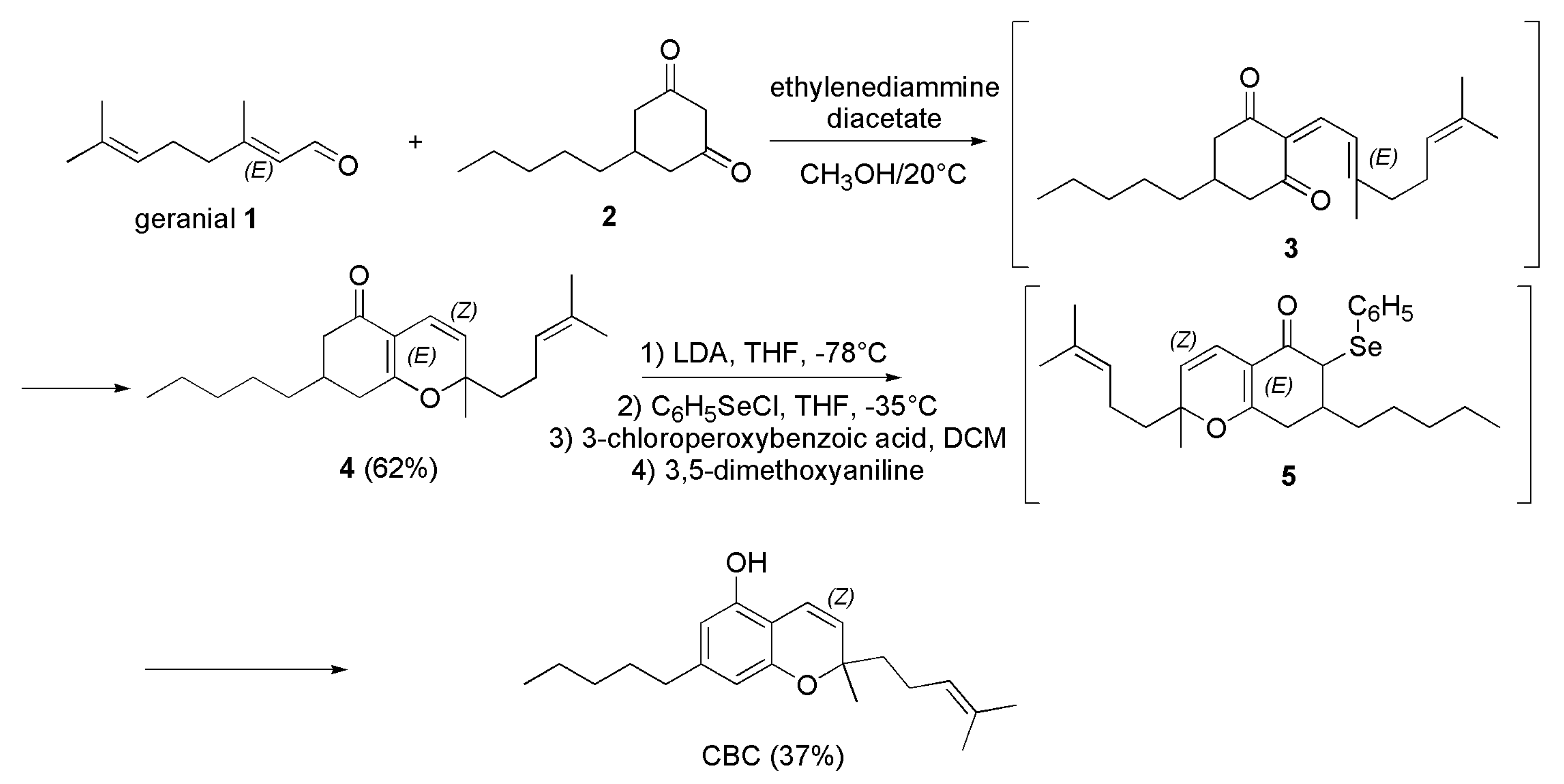
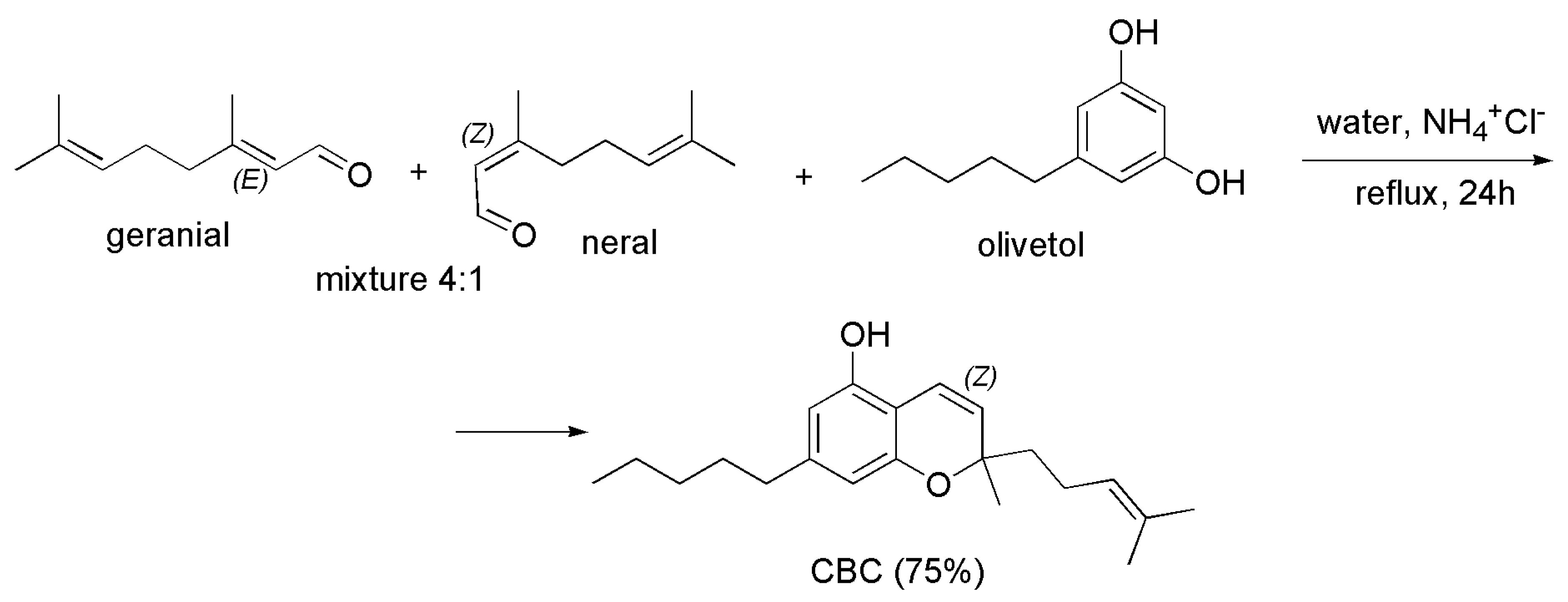
4.2.1. Syntheses of CBC
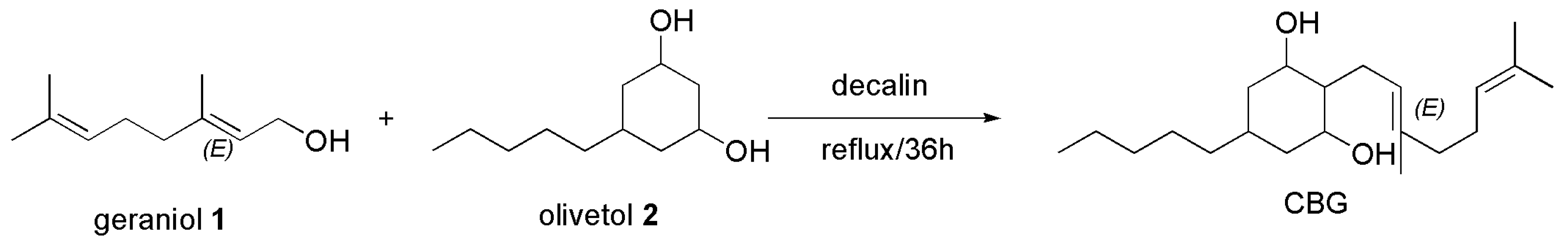
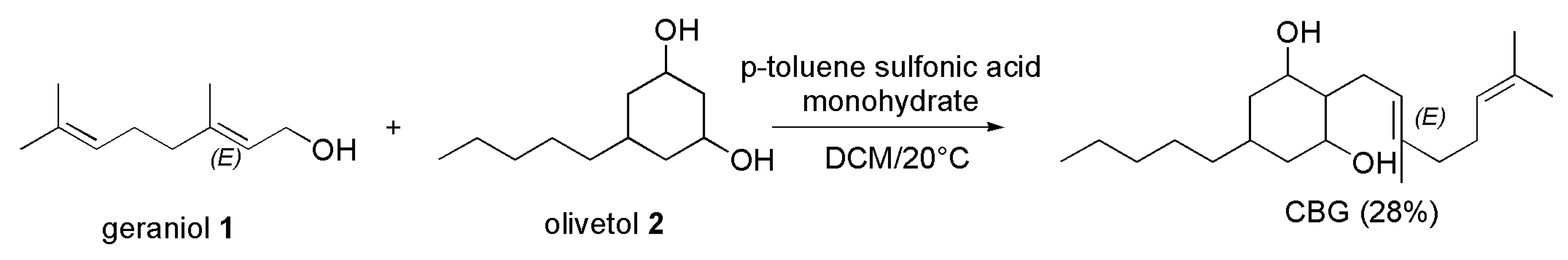
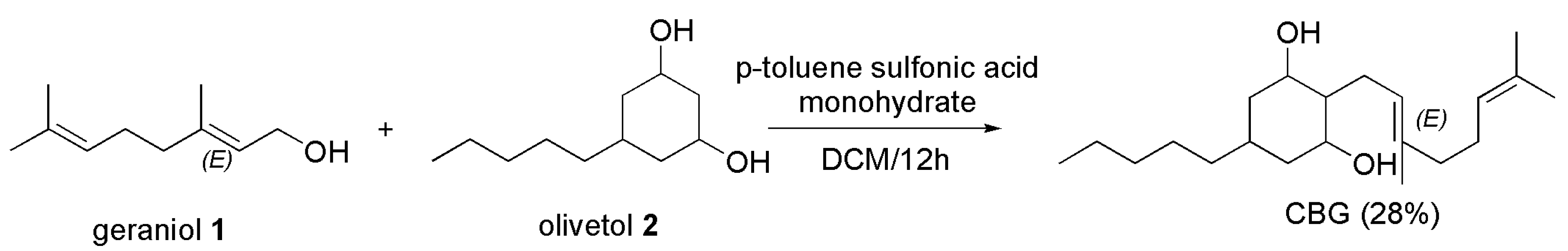
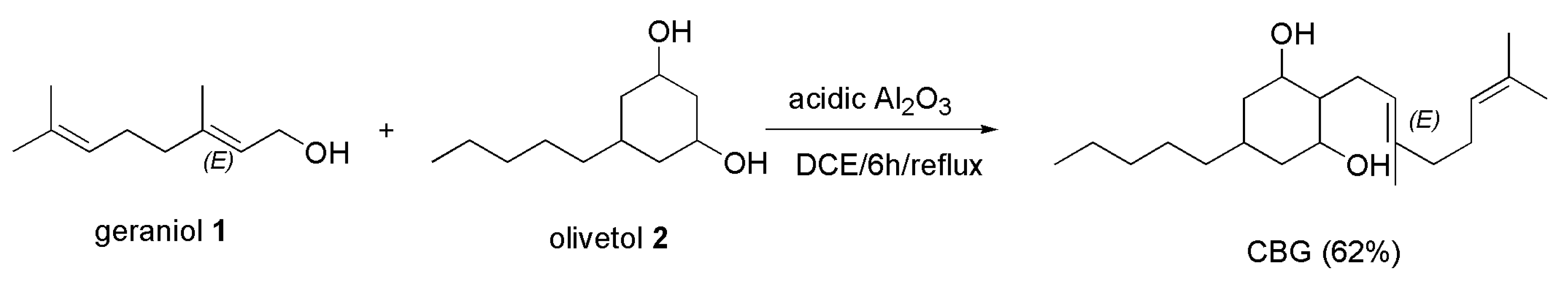
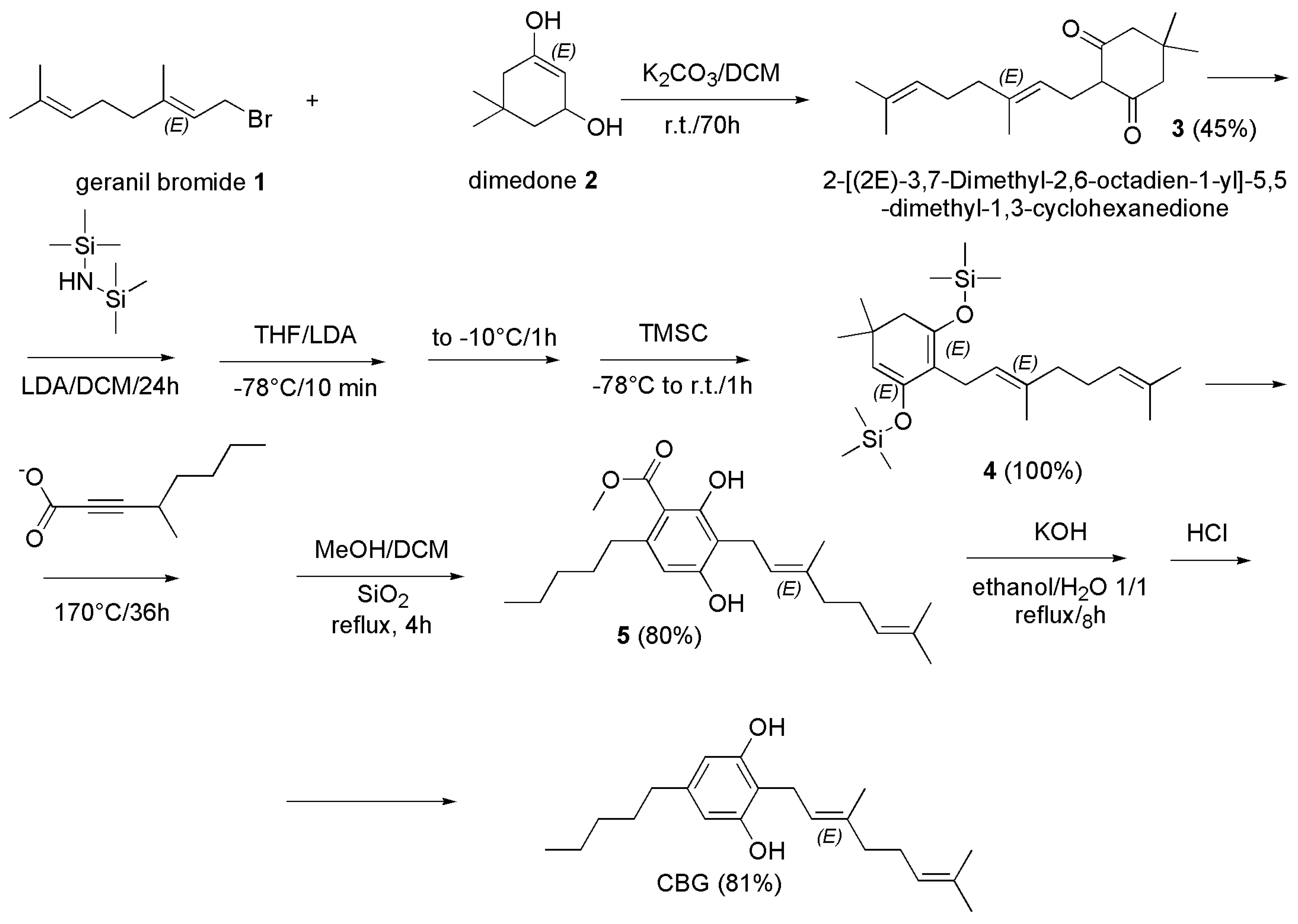
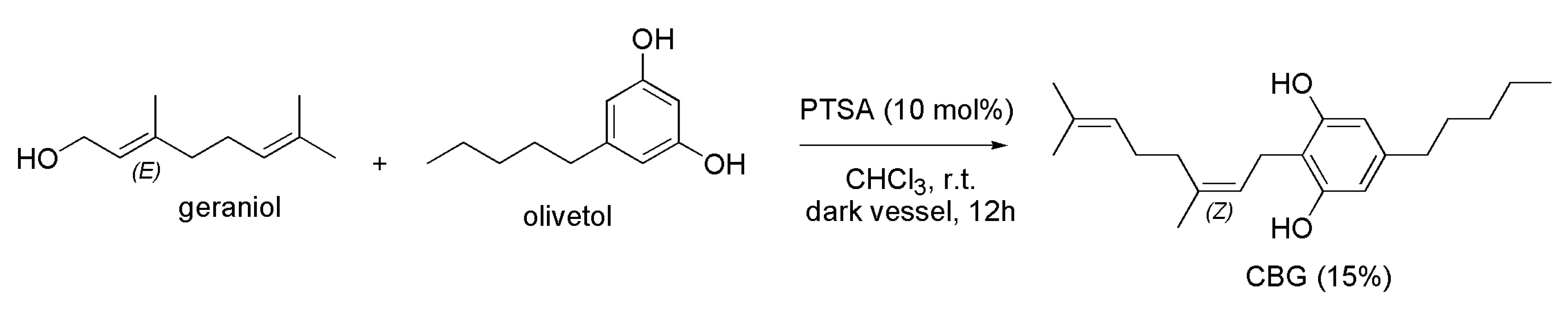
4.2.2. Synthesis of CBG
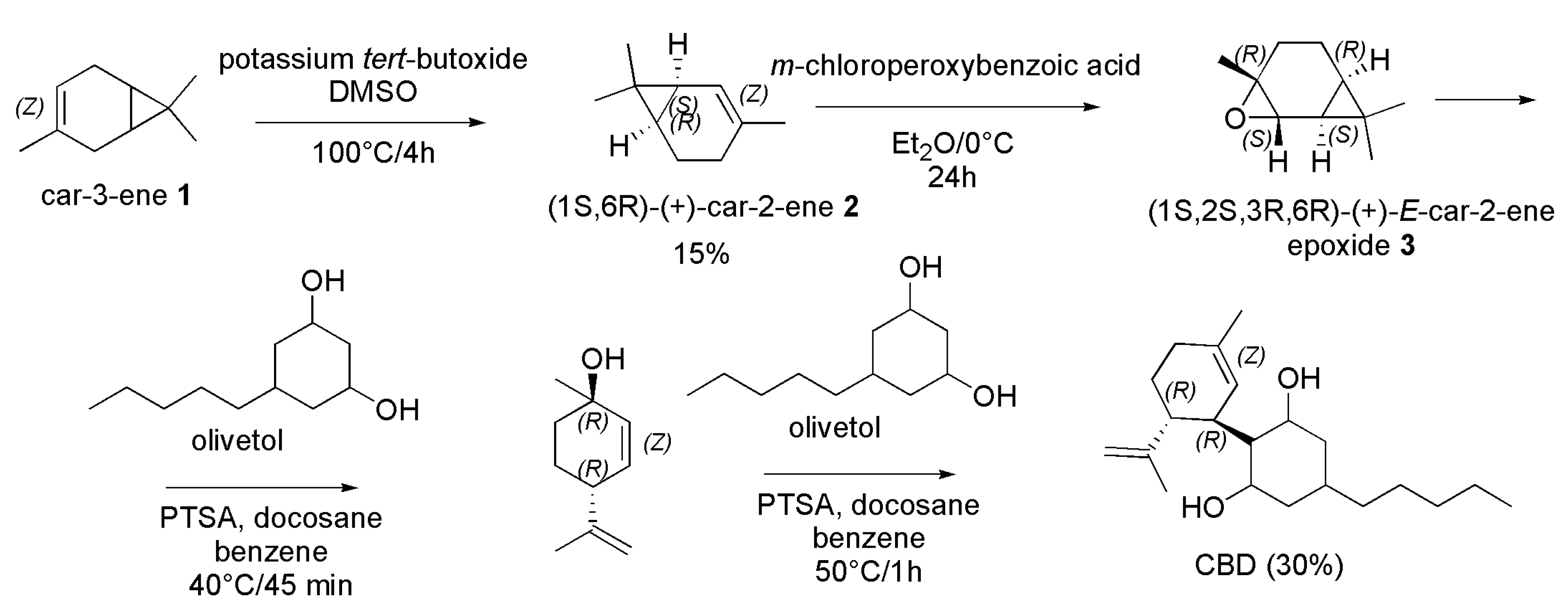
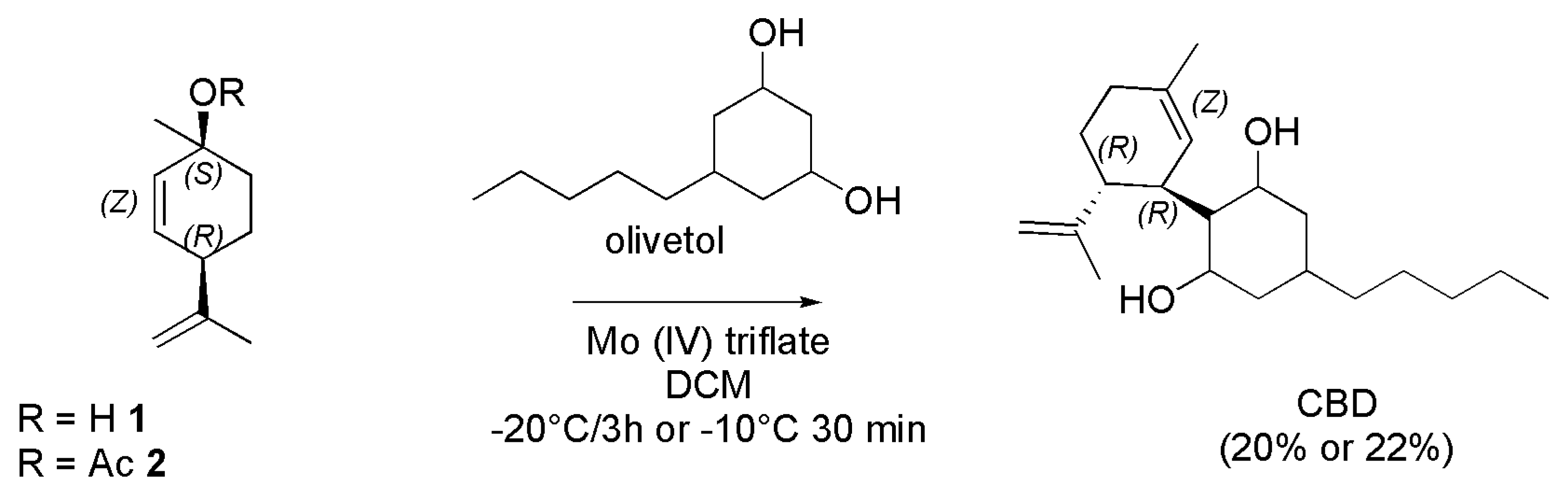
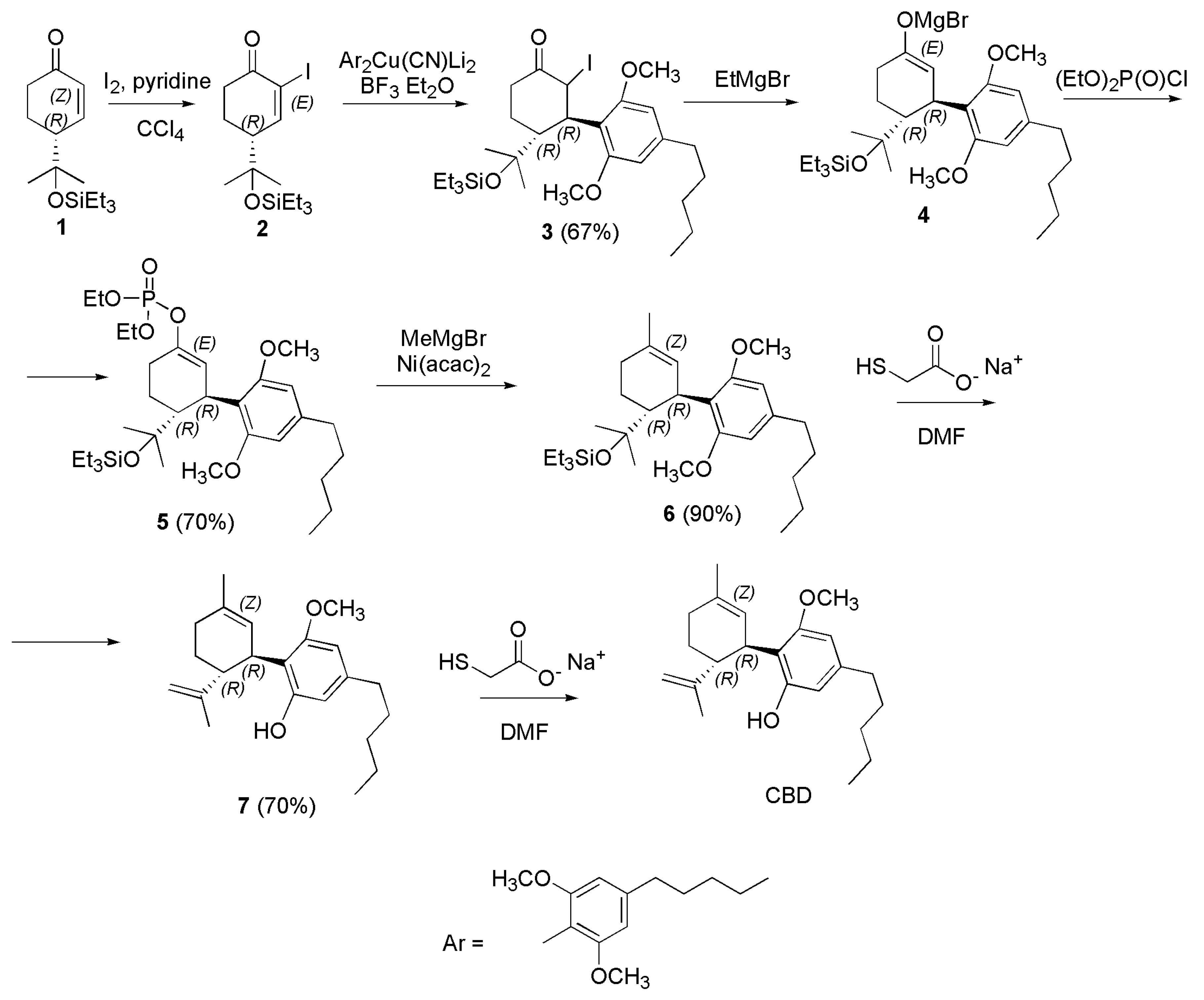
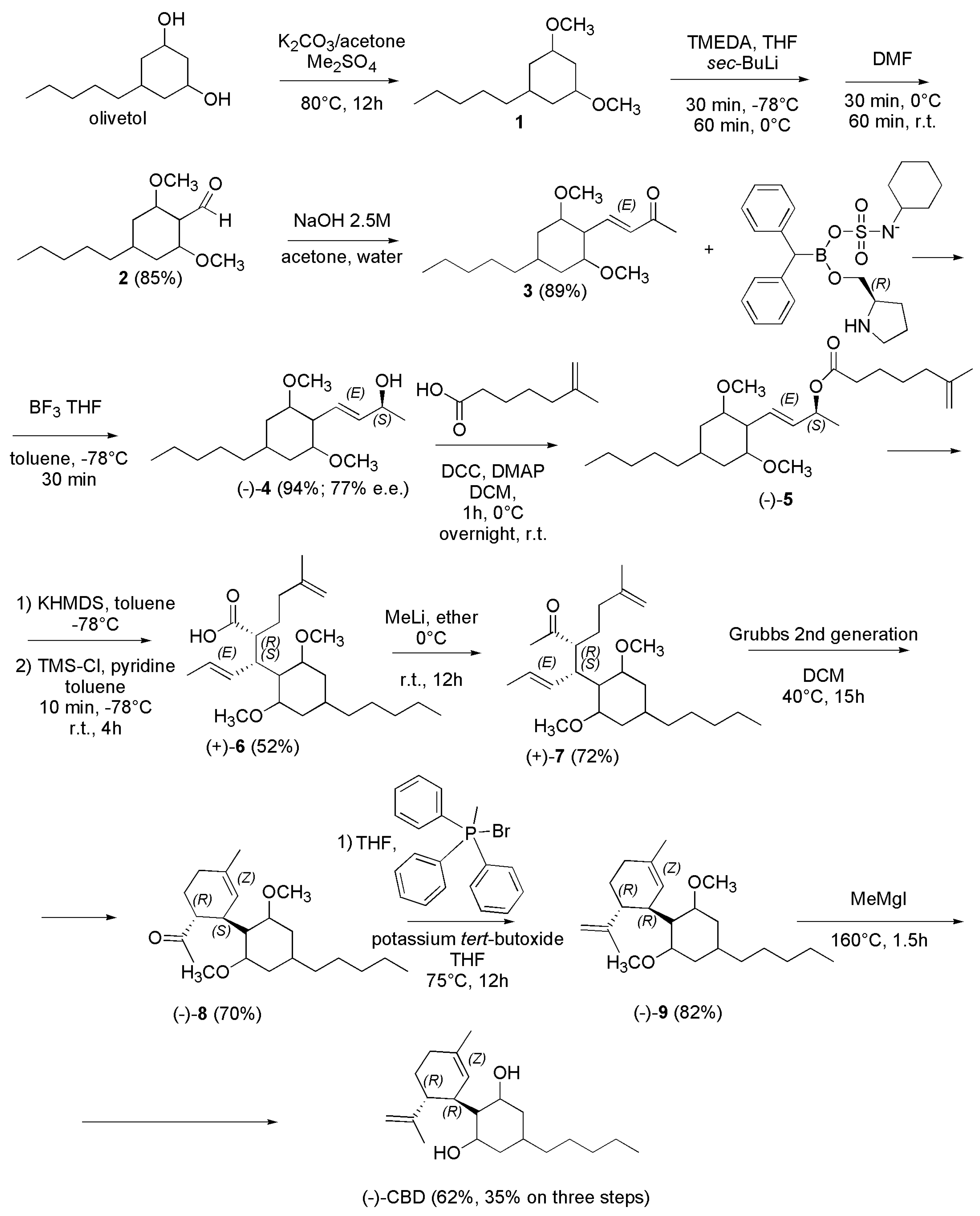
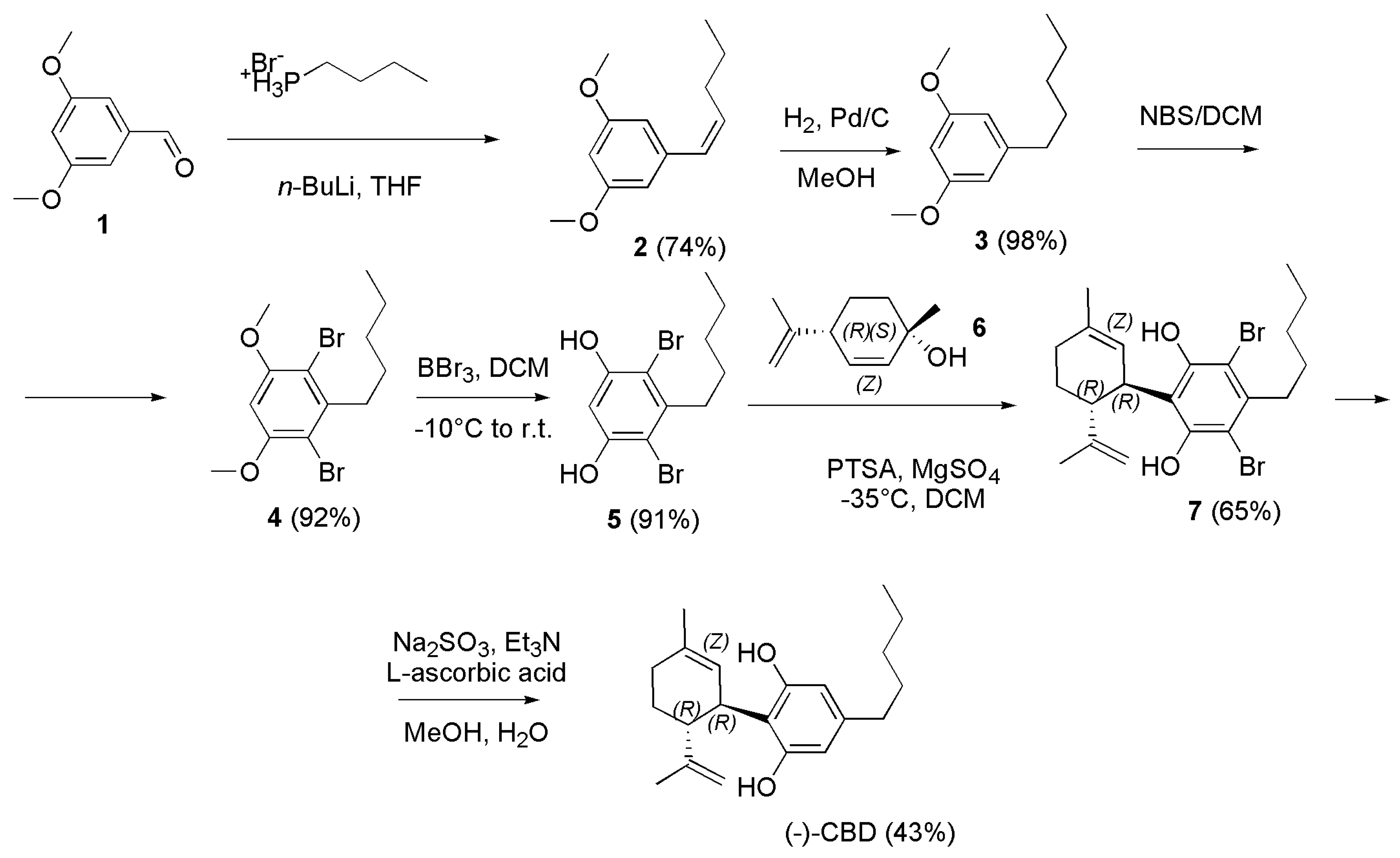
4.2.3. Synthesis of (-)-CBD
5. Conclusions and Future Perspective
Author Contributions
Conflicts of Interest
References
- Vivas, R.; Barbosa, A.A.T.; Dolabela, S.S.; Jain, S. Multidrug-Resistant Bacteria and Alternative Methods to Control Them: An Overview. Microbial drug resistance (Larchmont, N.Y.), 2019, 25, 890–908. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial Antibiotic Resistance: The Most Critical Pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef] [PubMed]
- WHO. Antimicrobial resistance. Available online at https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 03 May 2023).
- Breijyeh, Z.; Karaman, R. Design and Synthesis of Novel Antimicrobial Agents. Antibiotics 2023, 12, 628. [Google Scholar] [CrossRef] [PubMed]
- Chancey, S.T.; Zahner, D.; Stephens, D.S. Acquired inducible antimicrobial resistance in Gram-positive bacteria. Future Microbiol. 2012, 7, 959–978, Mahon, C.R.; Lehman, D.C. Textbook of Diagnostic Microbiology—E-Book; Elsevier Health Sciences: USA, 2022. [Google Scholar] [CrossRef] [PubMed]
- Spengler, G.; Kincses, A.; Gajdacs, M.; Amaral, L. New Roads Leading to Old Destinations: Efflux Pumps as Targets to Reverse Multidrug Resistance in Bacteria. Molecules 2017, 22, 468, Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4, 481–511. [Google Scholar] [CrossRef]
- Schaenzer, A.J.; Wright, G.D. Antibiotic Resistance by Enzymatic Modification of Antibiotic Targets. Trends Mol. Med. 2020, 26, 768–782. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.N.; Hauryliuk, V.; Atkinson, G.C.; O’Neill, A.J. Target protection as a key antibiotic resistance mechanism. Nat. Rev. Microbiol. 2020, 18, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Finley, R.L.; Collignon, P.; Larsson, D.G.; McEwen, S.A.; Li, X.Z.; Gaze, W.H.; Reid-Smith, R.; Timinouni, M.; Graham, D.W.; Topp, E. The scourge of antibiotic resistance: The important role of the environment. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2013, 57, 704–710. [Google Scholar] [CrossRef]
- Guetiya Wadoum, R.E.; Zambou, N.F.; Anyangwe, F.F.; Njimou, J.R.; Coman, M.M.; Verdenelli, M.C.; Cecchini, C.; Silvi, S.; Orpianesi, C.; Cresci, A.; et al. Abusive use of antibiotics in poultry farming in Cameroon and the public health implications. Br. Poult. Sci. 2016, 57, 483–493. [Google Scholar] [CrossRef]
- Baynes, R.E.; Dedonder, K.; Kissell, L.; Mzyk, D.; Marmulak, T.; Smith, G.; Tell, L.; Gehring, R.; Davis, J.; Riviere, J.E. Health concerns and management of select veterinary drug residues. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2016, 88, 112–122. [Google Scholar] [CrossRef]
- Radwan, M.M.; Chandra, S.; Gul, S.; ElSohly, M.A. Cannabinoids, Phenolics, Terpenes and Alkaloids of Cannabis. Molecules 2021, 26, 2774. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.N.; Shahbazi, F.; Rondeau-Gagné, S. et al. The biosynthesis of the cannabinoids. J. Cannabis. Res. 2021, 3, 7 (2021). [CrossRef]
- Pagano, C.; Navarra, G.; Coppola, L.; Avilia, G.; Bifulco, M.; Laezza, C. Cannabinoids: Therapeutic Use in Clinical Practice. Int. J. Mol. Sci. 2022, 23, 3344. [Google Scholar] [CrossRef]
- Palomares, B.; Ruiz-Pino, F.; Garrido-Rodriguez, M.; Eugenia Prados, M.; Sánchez-Garrido, M.A.; Velasco, I.; Vazquez, M.J.; Nadal, X.; Ferreiro-Vera, C.; Morrugares, R.; et al. Tetrahydrocannabinolic Acid A (THCA-A) Reduces Adiposity and Prevents Metabolic Disease Caused by Diet-Induced Obesity. Biochemical Pharmacology 2020, 171, 113693. [Google Scholar] [CrossRef] [PubMed]
- Pisanti, S.; Malfitano, A.M.; Ciaglia, E.; Lamberti, A.; Ranieri, R.; Cuomo, G.; Abate, M.; Faggiana, G.; Proto, M.C.; Fiore, D.; et al. Cannabidiol: State of the Art and New Challenges for Therapeutic Applications. Pharmacology & Therapeutics 2017, 175, 133–150. [Google Scholar] [CrossRef]
- Farha, M.A.; El-Halfawy, O.M.; Gale, R.T.; MacNair, C.R.; Carfrae, L.A.; Zhang, X.; Jentsch, N.G.; Magolan, J.; Brown, E.D. Uncovering the Hidden Antibiotic Potential of Cannabis. ACS Infect. Dis. 2020, 6, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Breijyeh, Z.; Karaman, R. Design and Synthesis of Novel Antimicrobial Agents. Antibiotics 2023, 12, 628. [Google Scholar] [CrossRef] [PubMed]
- Blaskovich, M.A.T.; Kavanagh, A.M.; Elliott, A.G.; Zhang, B.; Ramu, S.; Amado, M.; Lowe, G.J.; Hinton, A.O.; Pham, D.M.T.; Zuegg, J.; et al. The antimicrobial potential of cannabidiol. Commun. Biol. 2021, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Kosgodage, U.S.; Matewele, P.; Awamaria, B.; Kraev, I.; Warde, P.; Mastroianni, G.; Nunn, A.V.; Guy, G.W.; Bell, J.D.; Inal, J.M.; et al. Cannabidiol Is a Novel Modulator of Bacterial Membrane Vesicles. Front. Cell. Infect. Microbiol. 2019, 9, 324. [Google Scholar] [CrossRef]
- Luz-Veiga, M.; Amorim, M.; Pinto-Ribeiro, I.; Oliveira, A.L.S.; Silva, S.; Pimentel, L.L.; Rodríguez-Alcalá, L.M.; Madureira, R.; Pintado, M.; Azevedo-Silva, J.; Fernandes, J. Cannabidiol and Cannabigerol Exert Antimicrobial Activity without Compromising Skin Microbiota. Int. J. Mol. Sci. 2023, 24, 2389. [Google Scholar] [CrossRef]
- Wu, Q.; Guo, M.; Zou, L.; Wang, Q.; Xia, Y. 8,9-Dihydrocannabidiol, an Alternative of Cannabidiol, Its Preparation, Antibacterial and Antioxidant Ability. Molecules 2023, 28, 445. [Google Scholar] [CrossRef]
- Gildea, L.; Ayariga, J.A.; Xu, J.; Villafane, R.; Robertson, B.K.; Samuel-Foo, M.; Ajayi, O.S. Cannabis sativa CBD Extract Exhibits Synergy with Broad-Spectrum Antibiotics against Salmonella enterica subsp. Enterica serovar typhimurium. Microorganisms 2022, 10, 2360. [Google Scholar] [CrossRef] [PubMed]
- CBG: The mother of all Cannabinoids with broad antibacterial activity. Available online at https://www.openaccessgovernment.org/cbg-the-mother-of-all-cannabinoids-with-broad-antibacterial-activity/95824/ (accessed on 03 May 2023).
- Earlenbaugh, E. What are cannabinoids? Available online at https://cannigma.com/plant/cannabinoids-and-their-effects/#easy-footnote-bottom-10-283 (accessed on 03 May 2023).
- Vučković, S.; Srebro, D.; Vujović, K.S.; Vučetić, Č.; Prostran, M. Cannabinoids and Pain: New Insights From Old Molecules. Frontiers in pharmacology, 2018, 9, 1259. [Google Scholar] [CrossRef] [PubMed]
- Lafaye, G.; Karila, L.; Blecha, L.; Benyamina, A. Cannabis, Cannabinoids, and Health. Dialogues in Clinical Neuroscience 2017, 19, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Berman, P.; Futoran, K.; Lewitus, G.M.; Mukha, D.; Benami, M.; Shlomi, T.; Meiri, D. A New ESI-LC/MS Approach for Comprehensive Metabolic Profiling of Phytocannabinoids in Cannabis. Scientific Reports 2018, 8, 14280. [Google Scholar] [CrossRef] [PubMed]
- Fraguas-Sánchez, A.I.; Fernández-Carballido, A.; Torres-Suárez, A.I. Phyto-, Endo- and Synthetic Cannabinoids: Promising Chemotherapeutic Agents in the Treatment of Breast and Prostate Carcinomas. Expert Opinion on Investigational Drugs 2016, 25, 1311–1323. [Google Scholar] [CrossRef]
- GERTSCH, J.; RADUNER, S.; ALTMANN, K.-H. New Natural Noncannabinoid Ligands for Cannabinoid Type-2 (CB2) Receptors. Journal of Receptors and Signal Transduction 2006, 26, 709–730. [Google Scholar] [CrossRef]
- Li, X.; Chang, H.; Bouma, J.; de Paus, L.V.; Mukhopadhyay, P.; Paloczi, J.; Mustafa, M.; van der Horst, C.; Kumar, S.S.; Wu, L.; et al. Structural Basis of Selective Cannabinoid CB2 Receptor Activation. Nature Communications 2023, 14, 1447. [Google Scholar] [CrossRef]
- Lambert, D.M. Pharmacologic Targeting of the CB2 Cannabinoid Receptor for Application in Centrally-Mediated Chronic Pain. Text, 2019. University of British Columbia. Retrieved from https://open.library.ubc.ca/collections/ubctheses/24/items/1.0376050.
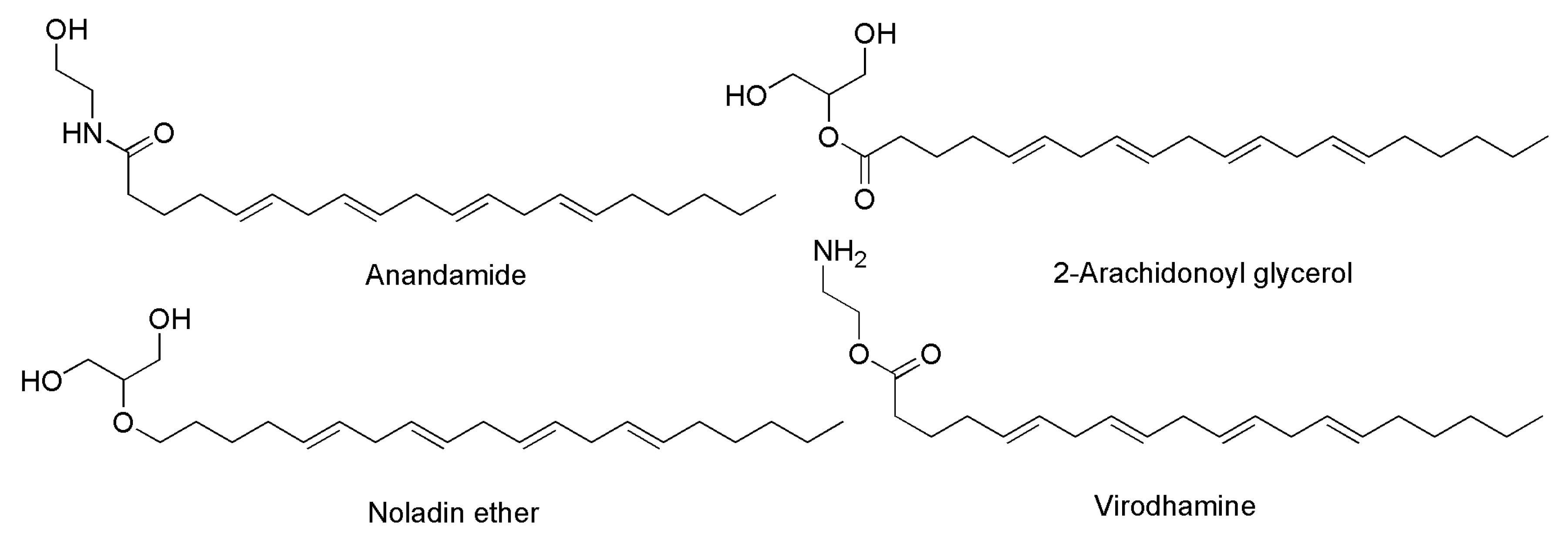
- Fezza, F.; Bari, M.; Florio, R.; Talamonti, E.; Feole, M.; Maccarrone, M. Endocannabinoids, Related Compounds and Their Metabolic Routes. Molecules 2014, 19, 17078–17106. [Google Scholar] [CrossRef]
- What Is Cannabicyclolic Acid? Available online at https://leafwell.com/blog/cannabicyclolic-acid/ (accessed on 03 May 2023).
- Cannabicyclol. Available online at https://en.wikipedia.org/wiki/Cannabicyclol (accessed on 03 May 2023).
- Schwilke, E.W.; Schwope, D.M.; Karschner, E.L.; Lowe, R.H.; Darwin, W.D.; Kelly, D.L.; Goodwin, R.S.; Gorelick, D.A.; Huestis, M.A. Δ9-Tetrahydrocannabinol (THC), 11-Hydroxy-THC, and 11-Nor-9-Carboxy-THC Plasma Pharmacokinetics during and after Continuous High-Dose Oral THC. Clinical Chemistry 2009, 55, 2180–2189. [Google Scholar] [CrossRef]
- Dronabinol. Available online at https://en.wikipedia.org/wiki/Dronabinol (accessed on 03 May 2023).
- (R)-(+)-Methanandamide. Available online at https://www.tocris.com/products/r-methanandamide_1121 (accessed on 03 May 2023).
- Cannabinor. Available online at https://en.wikipedia.org/wiki/Cannabinor. (accessed on 03 May 2023).
- HU-2010. Available online at https://en.wikipedia.org/wiki/HU-210. (accessed on 03 May 2023).
- L-759,656. Available online at https://www.chemspider.com/Chemical-Structure.4470735.html. (accessed on 03 May 2023).
- WIN 55,212-2. Available online at https://en.wikipedia.org/wiki/WIN_55,212-2. (accessed on 03 May 2023).
- JHW-015. Available online at https://www.chemspider.com/Chemical-Structure.3480676.html?rid=d64c760a-20a4-46ff-8e28-1de7539e1125. (accessed on 03 May 2023).
- Pravadoline. Available online at https://en.wikipedia.org/wiki/Pravadoline. (accessed on 03 May 2023).
- Abadji, V.; Lin, S.; Taha, G.; Griffin, G.; Stevenson, L.A.; Pertwee, R.G.; Makriyannis, A. (R)-Methanandamide: A Chiral Novel Anandamide Possessing Higher Potency and Metabolic Stability. Journal of Medicinal Chemistry 1994, 37(12), 1889–1893. [Google Scholar] [CrossRef]
- WIN 55212-2. Available online at https://pubchem.ncbi.nlm.nih.gov/compound/5311501. (accessed on 03 May 2023).
- JWH-133. Available online at https://pubchem.ncbi.nlm.nih.gov/compound/6918505. (accessed on 03 May 2023).
- Delta-11-Tetrahydrocannabinol. Available online at https://en.wikipedia.org/wiki/Delta-11-Tetrahydrocannabinol#:~:text=Delta-11-Tetrahydrocannabinol%20%28Delta-11-THC%2C%20%CE%9411-THC%2C%20%CE%949%20%2811%29-THC%2C%20exo-Tetrahydrocannabinol%29%20is%20a,though%20only%20the%20%286aR%2C%2010aR%29%20enantiomer%20is%20known. (accessed on 03 May 2023).
- Engels, F.K.; de Jong, F.A.; Mathijssen, R.H.J.; Erkens, J.A.; Herings, R.M.; Verweij, J. Medicinal Cannabis in Oncology. European Journal of Cancer 2007, 43, 2638–2644. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.J.; McAllister, S.D.; Kawamura, R.; Murase, R.; Neelakantan, H.; Walker, E.A. Cannabidiol Inhibits Paclitaxel-Induced Neuropathic Pain through 5-HT1A Receptors without Diminishing Nervous System Function or Chemotherapy Efficacy. British Journal of Pharmacology 2014, 171, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Sativex spray. Available online at https://www.my-personaltrainer.it/Foglietti-illustrativi/Sativex.html. (accessed on 03 May 2023).
- Reddy, D.S.; Golub, M.V. The Pharmacological Basis of Cannabis Therapy for Epilepsy. J Pharmacol Exp Ther 2016, 357, 45. [Google Scholar] [CrossRef] [PubMed]
- Navarro, G.; Gonzalez, A.; Sánchez-Morales, A.; Casajuana-Martin, N.; Gómez-Ventura, M.; Cordomí, A.; Busqué, F.; Alibés, R.; Pardo, L.; Franco, R. Design of Negative and Positive Allosteric Modulators of the Cannabinoid CB2 Receptor Derived from the Natural Product Cannabidiol. J. Med. Chem. 2021, 64, 9354–9364. [Google Scholar] [CrossRef] [PubMed]
- Rimonabant. Available online at https://it.wikipedia.org/wiki/Rimonabant. (accessed on 03 May 2023).
- Karas, J.A.; Wong, L.J.M.; Paulin, O.K.A.; Mazeh, A.C.; Hussein, M.H.; Li, J.; Velkov, T. The Antimicrobial Activity of Cannabinoids. Antibiotics 2020, 9, 406. [Google Scholar] [CrossRef] [PubMed]
- Stone, N.L.; Murphy, A.J.; England, T.J.; O’Sullivan, S.E. A Systematic Review of Minor Phytocannabinoids with Promising Neuroprotective Potential. British Journal of Pharmacology 2020, 177, 4330–4352. [Google Scholar] [CrossRef] [PubMed]
- Walsh, K.B.; McKinney, A.E.; Holmes, A.E. Minor Cannabinoids: Biosynthesis, Molecular Pharmacology and Potential Therapeutic Uses. Frontiers in Pharmacology 2021, 12. [Google Scholar] [CrossRef]
- van Klingeren, B.; ten Ham, M. Antibacterial Activity of Δ9-Tetrahydrocannabinol and Cannabidiol. Antonie van Leeuwenhoek 1976, 42, 9–12. [Google Scholar] [CrossRef]
- Appendino, G.; Gibbons, S.; Giana, A.; Pagani, A.; Grassi, G.; Stavri, M.; Smith, E.; Rahman, M.M. Antibacterial cannabinoids from Cannabis sativa: A structure-activity study. J. Nat. Prod. 2008, 71, 1427–1430. [Google Scholar] [CrossRef]
- Martinenghi, L.D.; Jønsson, R.; Lund, T.; Jenssen, H. Isolation, purification, and antimicrobial characterization of cannabidiolic acid and cannabidiol from Cannabis sativa L. Biomolecules 2020, 10, 900. [Google Scholar] [CrossRef]
- Wu, Q.; Guo, M.; Zou, L.; Wang, Q.; Xia, Y. 8,9-Dihydrocannabidiol, an Alternative of Cannabidiol, Its Preparation, Antibacterial and Antioxidant Ability. Molecules 2023, 28, 445. [Google Scholar] [CrossRef] [PubMed]
- Wassmann, C.S.; Højrup, P.; Klitgaard, J.K. Cannabidiol Is an Effective Helper Compound in Combination with Bacitracin to Kill Gram-Positive Bacteria. Scientific Reports 2020, 10, 4112. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.E.; Elsohly,M. A. Biological activity of cannabichromene, its homologs and isomers. J. Clin. Pharmacol. 1981, 21, 283s–291s. [Google Scholar] [CrossRef] [PubMed]
- Aqawi, M.; Sionov, R.V.; Gallily, R.; Friedman, M.; Steinberg, D. Anti-Bacterial Properties of Cannabigerol Toward Streptococcus Mutans. Frontiers in Microbiology 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Elsohly, H.N.; Turner, C.E.; Clark, A.M.; Elsohly, M.A. Synthesis and Antimicrobial Activities of Certain Cannabichromene and Cannabigerol Related Compounds. Journal of Pharmaceutical Sciences 1982, 71, 1319–1323. [Google Scholar] [CrossRef] [PubMed]
- Feldman, M.; Smoum, R.; Mechoulam, R.; Steinberg, D. Antimicrobial potential of endocannabinoid and endocannabinoid-like compounds against methicillin-resistant Staphylococcus aureus. Sci. Rep. 2018, 8, 17696. [Google Scholar] [CrossRef]
- CLSI. Available online at https://clsi.org/. (accessed on 03 May 2023).
- Kosgodage, U.S.; Matewele, P.; Awamaria, B.; Kraev, I.; Warde, P.; Mastroianni, G.; Nunn, A.V.; Guy, G.W.; Bell, J.D.; Inal, J.M.; et al. Cannabidiol Is a novel modulator of bacterial membrane vesicles. Front. Cell. Infect. Microbiol. 2019, 9, 324. [Google Scholar] [CrossRef]
- Seccamani, P.; Franco, C.; Protti, S.; Porta, A.; Profumo, A.; Caprioglio, D.; Salamone, S.; Mannucci, B.; Merli, D. Photochemistry of Cannabidiol (CBD) Revised. A Combined Preparative and Spectrometric Investigation. J. Nat. Prod. 2021, 84, 2858–2865. [Google Scholar] [CrossRef]
- Luo, G.-Y.W. , Hao; Tang, Yu; Li, Hui; Yeom, Hyun-Suk; Yang, Ka; Hsung, Richard P. A Total Synthesis of (±)-Rhododaurichromanic Acid A via an Oxa-[3+3] Annulation of Resorcinols. Synthesis 2015, 47, 2713–2720. [Google Scholar] [CrossRef]
- Lodewyk, M.W.; Lui, V.G.; Tantillo, D.J. Synthesis of (Sulfonyl)Methylphosphonate Analogs of Prenyl Diphosphates. Tetrahedron Letters 2010, 51, 170–173. [Google Scholar] [CrossRef]
- Yeom, H.-S.; Li, H.; Tang, Y.; Hsung, R.P. Total Syntheses of Cannabicyclol, Clusiacyclol A and B, Iso-Eriobrucinol A and B, and Eriobrucinol. Org. Lett. 2013, 15, 3130–3133. [Google Scholar] [CrossRef] [PubMed]
- Crombie, L.; Ponsford, R.; Shani, A.; Yagnitinsky, B.; Mechoulam, R. Hashish Components. Photochemical Production of Cannabicyclol from Cannabichromene. Tetrahedron Letters 1968, 9, 5771–5772. [Google Scholar] [CrossRef] [PubMed]
- Schafroth, M.A.; Mazzoccanti, G.; Reynoso-Moreno, I.; Erni, R.; Pollastro, F.; Caprioglio, D.; Botta, B.; Allegrone, G.; Grassi, G.; Chicca, A.; et al. Δ9-Cis-Tetrahydrocannabinol: Natural Occurrence, Chirality, and Pharmacology. J. Nat. Prod. 2021, 84, 2502–2510. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.L.; Ametovski, A.; Lin Luo, J.; Everett-Morgan, D.; McGregor, I.S.; Banister, S.D.; Arnold, J.C. Cannabichromene, Related Phytocannabinoids, and 5-Fluoro-Cannabichromene Have Anticonvulsant Properties in a Mouse Model of Dravet Syndrome. ACS Chem. Neurosci. 2021, 12, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.R.; Wang, X. Concise Synthesis of Biologically Interesting (′)-Cannabichromene, (′)-Cannabichromenic Acid, and (′)-Daurichromenic Acid. Bulletin of The Korean Chemical Society 2005, 26, 1933–1936. [Google Scholar] [CrossRef]
- Tietze, L.-F.V.K. , Günter; Berger, Bernhard A New Method of Aromatization of Cyclohexenone Derivatives; Synthesis of Cannabichromene. Synthesis 1982, 8, 683–684. [Google Scholar] [CrossRef]
- Eisohly, M.A.; Boeren, E.G.; Turner, C.E. Constituents of Cannabis Sativa L. An Improved Method for the Synthesis of Dl-Cannabichromene. Journal of Heterocyclic Chemistry 1978, 15, 699–700. [Google Scholar] [CrossRef]
- Quílez del Moral, J.F.; Ruiz Martínez, C.; Pérez del Pulgar, H.; Martín González, J.E.; Fernández, I.; López-Pérez, J.L.; Fernández-Arteaga, A.; Barrero, A.F. Synthesis of Cannabinoids: “In Water” and “On Water” Approaches: Influence of SDS Micelles. J. Org. Chem. 2021, 86, 3344–3355. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Shouji, N.; Kuroda, K. A New Approach to Dl-Cannabichromene. BCSJ 1995, 68, 305–308. [Google Scholar] [CrossRef]
- Gaoni, Y.; Mechoulam, R. The Structure and Synthesis of Cannabigerol, a New Hashish Constituent. Proceedings of the Chemical Society, 1964, March, 82.
- Mechoulam, R.; Yagen, B. Stereoselective Cyclizations of Cannabinoid 1,5 Dienes. Tetrahedron Letters 1969, 10, 5349–5352. [Google Scholar] [CrossRef]
- Taura, F.; Morimoto, S.; Shoyama, Y. Purification and Characterization of Cannabidiolic-Acid Synthase from Cannabis Sativa L.: Biochemical analysis of a novel enzyme that catalyzes the oxidocyclization of cannabigerolic acid to cannabidiolic acid. Journal of Biological Chemistry 1996, 271, 17411–17416. [Google Scholar] [CrossRef]
- Baek, S.H.; Srebnik, M.; Mechoulam, R. Boron Trifluoride Etherate on Alumina ― a Modified Lewis Acid Reagent. An Improved Synthesis of Cannabidiol. Tetrahedron Letters 1985, 26, 1083–1086. [Google Scholar] [CrossRef]
- Baek, S.-H.; Yook, C.N.; Han, D.S. Boron trifluoride etherate on alumina - a modified Lewis acid reagent(V) a convenient single-step synthesis of cannabinoids. Bulletin of the Korean Chemical Society 1995, 16(3), 293–296. [Google Scholar]
- Baek, S.-H.; Du Han, S.; Yook, C.N.; Kim, Y.C.; Kwak, J.S. Synthesis and Antitumor Activity of Cannabigerol. Archives of Pharmacal Research 1996, 19, 228–230. [Google Scholar] [CrossRef]
- Kumano, T.; Richard, S.B.; Noel, J.P.; Nishiyama, M.; Kuzuyama, T. Chemoenzymatic Syntheses of Prenylated Aromatic Small Molecules Using Streptomyces Prenyltransferases with Relaxed Substrate Specificities. Bioorganic & Medicinal Chemistry 2008, 16, 8117–8126. [Google Scholar] [CrossRef]
- Jentsch, N.G.; Zhang, X.; Magolan, J. Efficient Synthesis of Cannabigerol, Grifolin, and Piperogalin via Alumina-Promoted Allylation. J. Nat. Prod. 2020, 83, 2587–2591. [Google Scholar] [CrossRef] [PubMed]
- Curtis, B.J.; Micikas, R.J.; Burkhardt, R.N.; Smith, R.A.; Pan, J.Y.; Jander, K.; Schroeder, F.C. Syntheses of Amorfrutins and Derivatives via Tandem Diels–Alder and Anionic Cascade Approaches. J. Org. Chem. 2021, 86, 11269–11276. [Google Scholar] [CrossRef]
- Mechoulam, R.; Gaoni, Y. A Total Synthesis of Dl-Δ1-Tetrahydrocannabinol, the Active Constituent of Hashish1. J. Am. Chem. Soc. 1965, 87, 3273–3275. [Google Scholar] [CrossRef]
- Petrzilka, T.; Haefliger, W.; Sikemeier, C.; Ohloff, G.; Eschenmoser, A. Synthese Und Chiralität Des (-)-Cannabidiols Vorläufige Mitteilung. Helvetica Chimica Acta 1967, 50, 719–723. [Google Scholar] [CrossRef]
- Petrzilka, T.; Haefliger, W.; Sikemeier, C. Synthese von Haschisch-Inhaltsstoffen. 4. Mitteilung. Helvetica Chimica Acta 1969, 52, 1102–1134. [Google Scholar] [CrossRef]
- Razdan, R.K.; Dalzell, H.C.; Handrick, G.R. Hashish. X. Simple One-Step Synthesis of (-)-.DELTA.1-Tetrahydrocannabinol (THC) from p-Mentha-2,8-Dien-1-Ol and Olivetol. J. Am. Chem. Soc. 1974, 96, 5860–5865. [Google Scholar] [CrossRef] [PubMed]
- Papahatjis, D.P.; Nikas, S.P.; Andreou, T.; Makriyannis, A. Novel 1′,1′-Chain Substituted Δ8-Tetrahydrocannabinols. Bioorganic & Medicinal Chemistry Letters 2002, 12, 3583–3586. [Google Scholar]
- Uliss, D.B.; Dalzell, H.C.; Handrick, G.R.; Howes, J.F.; Razdan, R.K. Hashish. Importance of the Phenolic Hydroxyl Group in Tetrahydrocannabinols. J. Med. Chem. 1975, 18, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Crombie, L.; Crombie, W.M.L.; Jamieson, S.V.; Palmer, C.J. Acid-Catalysed Terpenylations of Olivetol in the Synthesis of Cannabinoids. J. Chem. Soc., Perkin Trans. 1 1988, 1243–1250. [CrossRef]
- Kinney, W.A.; McDonnell, M.E.; Zhong, H.M.; Liu, C.; Yang, L.; Ling, W.; Qian, T.; Chen, Y.; Cai, Z.; Petkanas, D.; et al. Discovery of KLS-13019, a Cannabidiol-Derived Neuroprotective Agent, with Improved Potency, Safety, and Permeability. ACS Med. Chem. Lett. 2016, 7, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Villano, R.; Straker, H.; Di Marzo, V. Short and Efficient Synthesis of Alkylresorcinols: A Route for the Preparation of Cannabinoids. New J. Chem. 2022, 46, 20664–20668. [Google Scholar] [CrossRef]
- Vaillancourt, V.; Albizati, K.F. A One-Step Method for the.Alpha.-Arylation of Camphor. Synthesis of (-)-Cannabidiol and (-)-Cannabidiol Dimethyl Ether. J. Org. Chem. 1992, 57, 3627–3631. [Google Scholar] [CrossRef]
- Malkov, A.; Kocovsky, P. Tetrahydrocannabinol Revisited: Synthetic Approaches Utilizing Molybdenum Catalysts. Collect. Czech. Chem. Commun. 2001, 66, 1257−1268.
- William, A.D.; Kobayashi, Y. A Method To Accomplish a 1,4-Addition Reaction of Bulky Nucleophiles to Enones and Subsequent Formation of Reactive Enolates. Org. Lett. 2001, 3, 2017–2020. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Takeuchi, A.; Wang, Y.-G. Synthesis of Cannabidiols via Alkenylation of Cyclohexenyl Monoacetate. Org. Lett. 2006, 8, 2699–2702. [Google Scholar] [CrossRef]
- Shultz, Z.P.; Lawrence, G.A.; Jacobson, J.M.; Cruz, E.J.; Leahy, J.W. Enantioselective Total Synthesis of Cannabinoids—A Route for Analogue Development. Org. Lett. 2018, 20, 381–384. [Google Scholar] [CrossRef]
- Gong, X.; Sun, C.; Abame, M.A.; Shi, W.; Xie, Y.; Xu, W.; Zhu, F.; Zhang, Y.; Shen, J.; Aisa, H.A. Synthesis of CBD and Its Derivatives Bearing Various C4′-Side Chains with a Late-Stage Diversification Method. J. Org. Chem. 2020, 85, 2704–2715. [Google Scholar] [CrossRef]
- Chiurchiù, E.; Sampaolesi, S.; Allegrini, P.; Ciceri, D.; Ballini, R.; Palmieri, A. A Novel and Practical Continuous Flow Chemical Synthesis of Cannabidiol (CBD) and Its CBDV and CBDB Analogues. European Journal of Organic Chemistry 2021, 2021, 1286–1289. [Google Scholar] [CrossRef]
- Anand, R.; Cham, P.S.; Gannedi, V.; Sharma, S.; Kumar, M.; Singh, R.; Vishwakarma, R.A.; Singh, P.P. Stereoselective Synthesis of Nonpsychotic Natural Cannabidiol and Its Unnatural/Terpenyl/Tail-Modified Analogues. J. Org. Chem. 2022, 87, 4489–4498. [Google Scholar] [CrossRef] [PubMed]
- Grimm, J.A.A.; Zhou, H.; Properzi, R.; Leutzsch, M.; Bistoni, G.; Nienhaus, J.; List, B. Catalytic Asymmetric Synthesis of Cannabinoids and Menthol from Neral. Nature 2023, 615, 634–639. [Google Scholar] [CrossRef] [PubMed]










| Strategies for combating antibiotic resistance | |
|---|---|
| Nanotechnology | Quality by design (QbD) approach |
| Computational methods | In silico modelling |
| Fragment-based drug design (FBDD) | |
| Antibiotic alternatives | Antimicrobial peptides (AMPs) |
| Essential oils | |
| Anti-Quorum Sensing (QS) | |
| Darobactins | |
| Vitamin B6 | |
| Bacteriophages | |
| Odilorhabdins | |
| 18-β-glycyrrhetinic acid | |
| Cannabinoids | |
| Drug reproposing | Ticagrelor |
| Mitomycin C (MMC) | |
| Auranofin | |
| Pentamidine | |
| Zidovudine (AZT) | |
| Synthesis of novel antibacterial agents | Lactones |
| Piperidinol | |
| Sugar-based bactericides | |
| Isoxazole derivatives | |
| Carbazole | |
| Prodrugs | Siderophores |
| Carbapenem-oxazolidinones | |
| Oral Gyrb/ParE dual binding inhibitor | |
| AMPs prodrugs | |
| Awareness and knowledge of antibiotic prescribing | |
| Receptor type | Location | Sublocation |
|---|---|---|
| CB1 | Central nervous system (CNS) | Hippocampus, cerebellum, basal ganglia, cortical regions Olfactory areas |
| Peripheral nerve terminals Extra-neuronal sites |
Eye, vascular endothelium, adipose tissue, lungs, liver Spleen, kidneys, uterus, prostate, testis, stomach, placenta Skeletal, muscles |
|
| CB2 | Peripheral immune system tissues | Spleen, tonsils, thymus, lymph nodes |
| Peripheral immune system cells | B cells, natural killer cells, monocytes, macrophages Neutrophils, CD8+ T cells, CD4+ T cells |
|
| CNS * | Cerebellum, olfactory tubercle, striatum Thalamic nuclei (hippocampus and amygdala) |
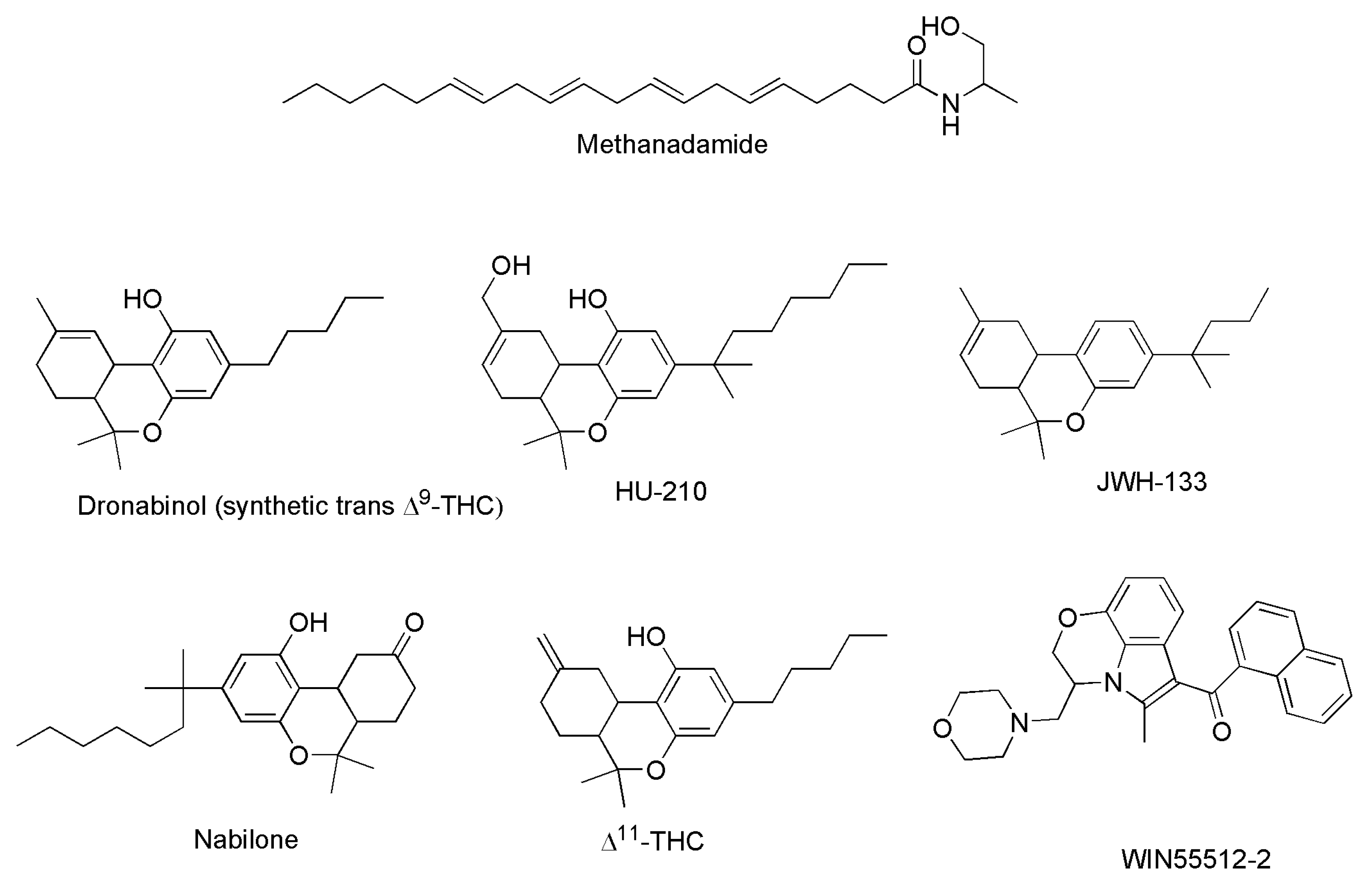
| SCs | Binding affinity | Effects | Refs. | |
|---|---|---|---|---|
| CB1 (Ki, nM) | CB2 (Ki, nM) | |||
| Dronabinol * | 15 | 51 | Appetite stimulant Psychotropic effects Analgesic ↓ Nausea Antiemetic |
[37] |
| Methanandamide (AM-356) | 20 | 815 | [38] | |
| SR144528 | 280 | 0.1 | Anti-inflammatory Analgesic ↓ Neuropathic pain |
[30] |
| Cannabinor # (PRS-211,375) |
5585 | 17.4 | [39] | |
| CP47,597 | 2.1 (Kd) | 56 | Analgesic | [30] |
| JTE907 | 490 | 2.2 | Anti-inflammatory | [30] |
| JWH133 | 680 | 3.4 | ↓↓ Neurotoxicity Anti-inflammatory ↓ Alzheimer symptoms |
[30] |
| AM1241 | 272 | 3.4 | Analgesic effects ↓ Hyperalgesia ↓ Allodynia Amyotrophic lateral sclerosis |
[30] |
| GW405,833 | 8640 | 7.2 | ↓ Hyperalgesia ↓ Allodynia |
[30] |
| L-759,633 | 1604 | 9.8 | Analgesic Antianxiety Antidepressant Anti-inflammatory ↓ Alzheimer symptoms |
[30] |
| JWH139 | 2290 | 14 | ||
| HU308 | 115000 | 23 | ||
| AM630 | 3795 | 32 | ||
| HU-210 | 0.061 | 0.52 | [40] | |
| L-759,656 | 4888 | ↑11.8 | [41] | |
| WIN 55,212-2 | 1.9 | Analgesic Anti-inflammatory ↓ Alzheimer symptoms |
[42] | |
| JWH015 | 383 | 13.8 | Analgesic Anti-inflammatory |
[43] |
| WIN 48,098 (Pravadoline) | 4.9 (IC50) | Analgesic Anti-inflammatory |
[44] | |
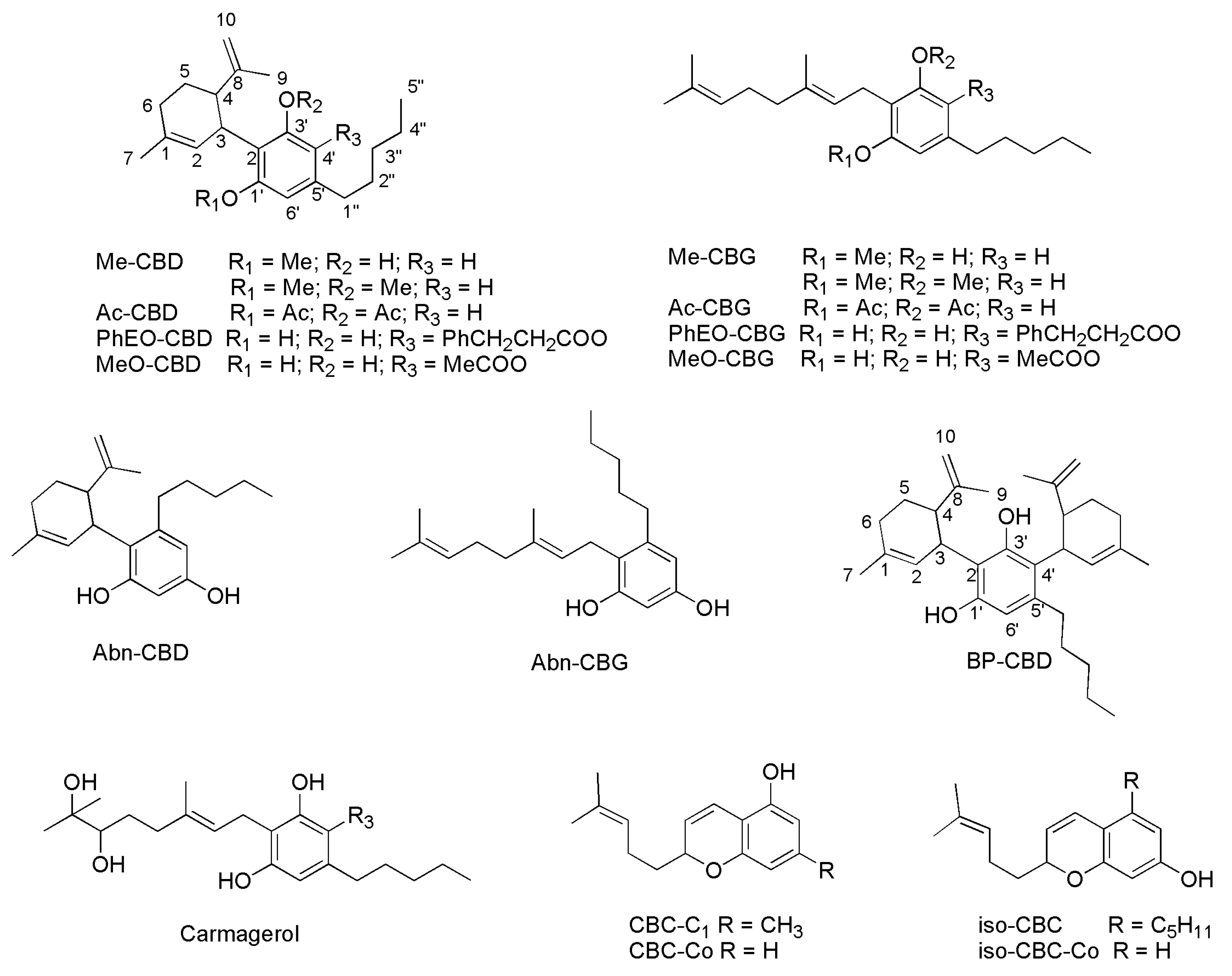
| Precannabinoids | Cannabinoids | Synthetic compounds |
|---|---|---|
| Cannabichromenic acid (CBCA) | Cannabichromene (CBC) * | Δ11-THC ** S Me-CBD Me-CBG Ac-CBD Ac-CBG PhEO-CBD PhEO-CBG MeO-CBD MeO-CBG Abn-CBD Abn-CBG Carmagerol °° BP-CBD CBC isomer (ICBC) CBC-Co CBC-C1 ICBC-Co |
| Cannabidiolic acid (CBDA) | Cannabidiol (CBD) * | |
| Cannabigerolic acid (CBGA) | Cannabigerol (CBG) * | |
| Δ9-Tetrahydrocannabinolic acid A (THCAA) | Δ9-Tetrahydrocannabinol (Δ9-THC) * | |
| Δ9-Tetrahydrocannabinolic acid B (THCAB) | ||
| Δ9-tetrahydrocannabivarin acid (THCVA) | Δ9-tetrahydrocannabivarin (THCV) * | |
| Cannabidivarinic acid (CBDVA) | Cannabidivarin (CBDV) * | |
| N.T. | Δ8-THC **N | |
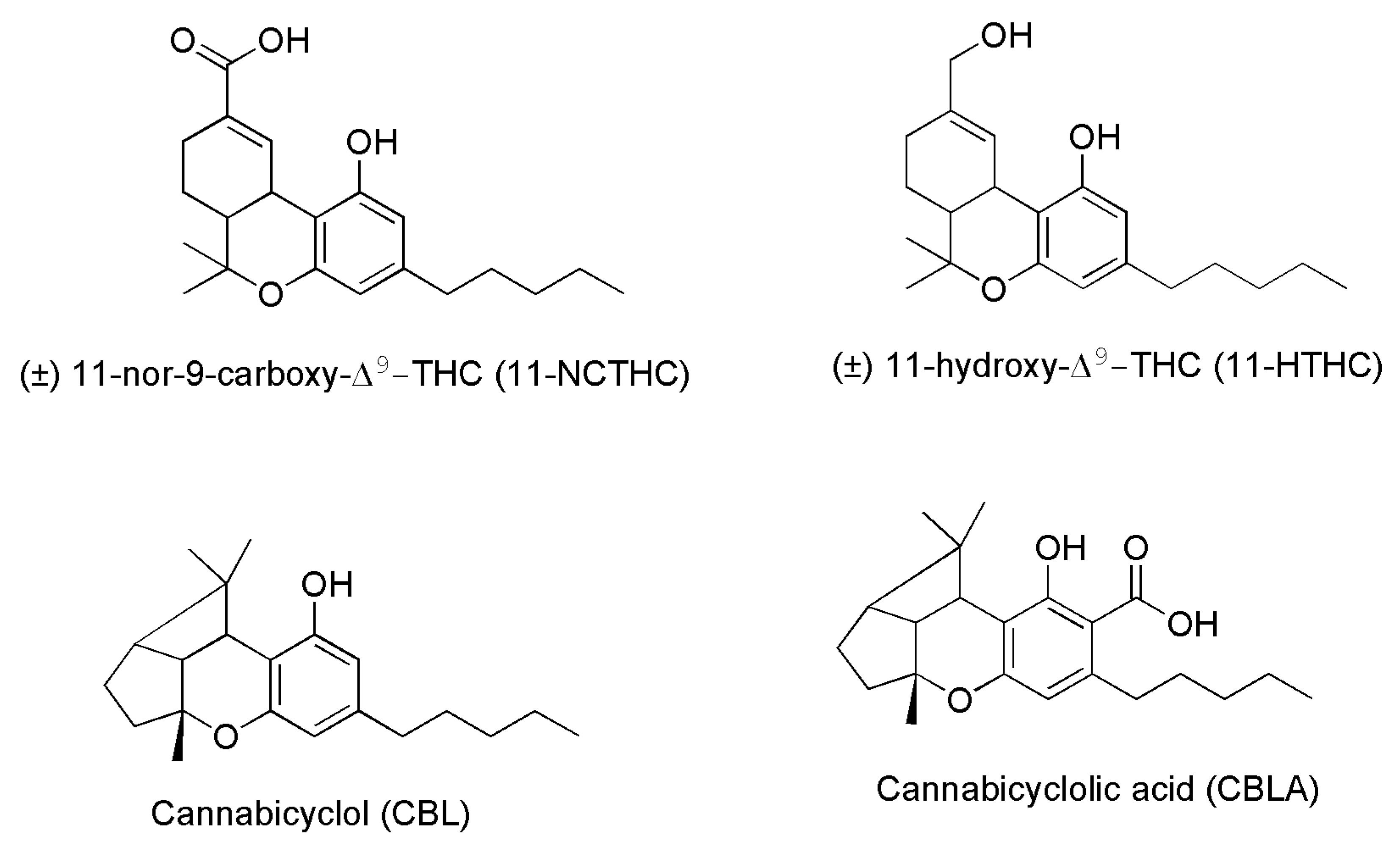
| Cannabicyclol (CBL) # | ||
| (±)11-NCTHC ° | ||
| (±)11-HTHC § | ||
| Anantamide (ANA) ***,$ | ||
| Arachidonyl serine (AraS) $ |
| Compounds | Pathogens | MIC * (µg/mL) | Ref. | Comments |
|---|---|---|---|---|
| Δ9-THC | S. aureus | 1-5 | [58] | Binding to plasma proteins ↓ Activity on Gram-negative Psychotropic |
| Streptococcus pyogenes | 5 | |||
| S. milleri | 2 | |||
| S. faecalis | 5 | |||
| S. aureus | 1 | [59] | ||
| EMRSA | 0.5-2 | |||
| S. aureus SA-1199B | 2 | |||
| S. aureus RN-4220 | 1 | |||
| S. aureus XU212 | 1 | |||
| MRSA USA300 | 2 | [17] | ||
| Δ9-THC acid A (THCAA) | MRSA USA300 | 4 | [17] | Binding to plasma proteins ↓ Activity on Gram-negative Not psychotropic ↑ Therapeutic potential ↑ Effects without the carboxylate moiety |
| S. aureus | 4 | [59] | ||
| EMRSA | 4-8 | |||
| S. aureus SA-1199B | 8 | |||
| S. aureus RN-4220 | 4 | |||
| S. aureus XU212 | 8 | |||
| Δ8-THC | MRSA USA300 | 2 | [17] | Binding to plasma proteins ↓ Activity on Gram-negative Psychotropic |
| Δ11-THC | MRSA USA300 | 2 | [17] | |
| Δ9-THCV | MRSA USA300 | 4 | [17] | Lack psychotropic effects ↑ Therapeutic potential ↓ Activity on Gram-negative ↑ Effects without the carboxylate moiety |
| THCV acid (THCVA) | MRSA USA300 | 16 | [17] | |
| CBD | S. aureus | 1 | [60] | Binding to plasma proteins ↓ Activity on Gram-negative Antiepileptic Anti-inflammatory Not psychotropic ↑ Therapeutic potential |
| 1-5 | [58] | |||
| 0.5 | [59] | |||
| 1.25 | [61] | |||
| EMRSA | 1 | [59] | ||
| S. aureus SA-1199B | ||||
| S. aureus RN-4220 | ||||
| S. aureus XU212 | ||||
| MRSA USA300 | 2 | [17] | ||
| 4 | [62] | |||
| S. epidermidis | 2 | [60] | ||
| S. pyogenes | 2 | [58] | ||
| S. milleri | 1 | |||
| S. faecalis | 5 | |||
| MRSE | 4 | [62] | ||
| Listeria monocytogenes | 4 | |||
| E. faecalis | 8 | |||
| E. coli | 1.25 | [61] | ||
| CBN | MRSA USA300 | 2 | [17] | Binding to plasma proteins ↓ Activity on Gram-negative Weakly psychotropic |
| S. aureus | 1 | [59] | ||
| EMRSA | ||||
| S. aureus SA-1199B | ||||
| S. aureus RN-4220 | ||||
| S. aureus XU212 | ||||
| Abn-CBD | S. aureus | 1 | [59] | Binding to plasma proteins ↓ Activity on Gram-negative Not psychotropic ↑ Therapeutic potential |
| EMRSA | ||||
| S. aureus SA-1199B | ||||
| S. aureus RN-4220 | ||||
| S. aureus XU212 | ||||
| CBC | B. subtilis | 0.39 | [63] | ↓ Activity on Gram-negative Not psychotropic ↑ Therapeutic potential In case of acid compounds: ↑ Effects without the carboxylate moiety |
| S. aureus | 1.56 | |||
| Mycobacterium smegmatis | 12.5 | |||
| Candida albicans | N.T. | |||
| Saccharomyces cerevisiae | 25 | |||
| Trichophyton mentagrophytes | 25 | |||
| MRSA USA300 | 8 | [17] | ||
| EMRSA | 2 | [59] | ||
| S. aureus | 2 | |||
| S. aureus SA-1199B | 2 | |||
| S. aureus RN-4220 | 2 | |||
| S. aureus XU212 | 1 | |||
| CBCA | MRSA USA300 | 2 | [17] | |
| CBC isomer (ICBC) | B. subtilis | 0.78 | [63] | |
| S. aureus | N.T. | |||
| M. smegmatis | 25 | |||
| C. albicans | 50 | |||
| S. cerevisiae | N.T. | |||
| T. mentagrophytes | N.T. | |||
| CBC-Co | B. subtilis | 6.25 | ||
| S. aureus | 12.5 | |||
| M. smegmatis | 12.5 | |||
| C. albicans | 50 | |||
| S. cerevisiae | 25 | |||
| T. mentagrophytes | 25 | |||
| CBC-C1 | B. subtilis | 3.12 | ||
| S. aureus | 3.12 | |||
| M. smegmatis | 3.12 | |||
| C. albicans | N.T. | |||
| S. cerevisiae | 6.25 | |||
| T. mentagrophytes | 6.25 | |||
| ICBC-Co | B. subtilis | 6.25 | ||
| S. aureus | 12.5 | |||
| M. smegmatis | 12.5 | |||
| C. albicans | 12.5 | |||
| S. cerevisiae | N.T. | |||
| T. mentagrophytes | 6.25 | |||
| CBDA | S. aureus | 2 | [59] | ↑ Effects without the carboxylate moiety ↓ Activity on Gram-negative Not psychotropic ↑ Therapeutic potential ↑ Effects without the carboxylate moiety |
| EMRSA | ||||
| S. aureus SA-1199B | ||||
| S. aureus RN-4220 | ||||
| S. aureus XU212 | ||||
| S. epidermidis | 4 | [60] | ||
| S. aureus | 2 | |||
| MRSA USA300 | 16 | [17] | ||
| 4 | [60] | |||
| CBGA | MRSA USA300 | 4 | [17] | Not psychotropic ↑ Therapeutic potential ↑ Effects without the carboxylate moiety ↓ Activity on Gram-negative |
| EMRSA | 2-4 | [59] | ||
| S. aureus | 4 | |||
| S. aureus SA-1199B | 4 | |||
| S. aureus RN-4220 | 2 | |||
| S. aureus XU212 | 4 | |||
| CBG | Streptococcus mutans | 2.5 | [64] | Not psychotropic ↑ Membrane permeability Cause membrane hyperpolarization ↓ Membrane fluidity Not psychotropic ↑ Therapeutic potential ↓ Activity on Gram-negative |
| S. sanguis | 1 | |||
| S. sobrinos | 5 | |||
| S. salivarius | 5 | |||
| MRSA USA300 | 2 | [17] | ||
| S. aureus | 1 | [59] | ||
| EMRSA | 1-2 | |||
| S. aureus SA-1199B | 1 | |||
| S. aureus RN-4220 | 1 | |||
| S. aureus XU212 | 1 | |||
| C. albicans | 3 a; (4)b | [65] | ||
| S. cerevisiae | 6 a; (2)b | |||
| T. mentagrophytes | 5 a; (4)b | |||
| Abn-CBG | S. aureus | 1 | [59] | |
| EMRSA | 2 | |||
| S. aureus SA-1199B | 2 | |||
| S. aureus RN-4220 | 1 | |||
| S. aureus XU212 | 0.5 | |||
| CBDV | MRSA USA300 | 8 | [17] | Not psychotropic ↑ Therapeutic potential ↓ Activity on Gram-negative |
| CBDVA | MRSA USA300 | 32 | [17] | Not psychotropic ↑ Therapeutic potential ↑ Effects without the carboxylate moiety ↓ Activity on Gram-negative |
| CBL | MRSA USA300 | >32 | [17] | Not psychotropic ↑ Therapeutic potential |
| (±) 11-NCTHC | ||||
| (±) 11-HTHC | MRSA USA300 | >32 | [17] | Psychotropic |
| ANA | MRSA | > 256 ** | [66] | Psychotropic ↓ Membrane potential in bacteria ↓ Bacteria adhesion capacity ↓ Cells aggregation capacity Not bactericidal ↓ Activity on Gram-negative |
| 64*** (51-54) # | ||||
| AraS | MRSA | 32->256** | [66] | Psychotropic Neuroprotective ↓ Activity on Gram-negative Affect membrane potential in bacteria ↓ Bacteria adhesion capacity ↓ Cells aggregation capacity Not bactericidal |
| 64*** (33-61) # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





