Submitted:
15 May 2023
Posted:
16 May 2023
You are already at the latest version
Abstract
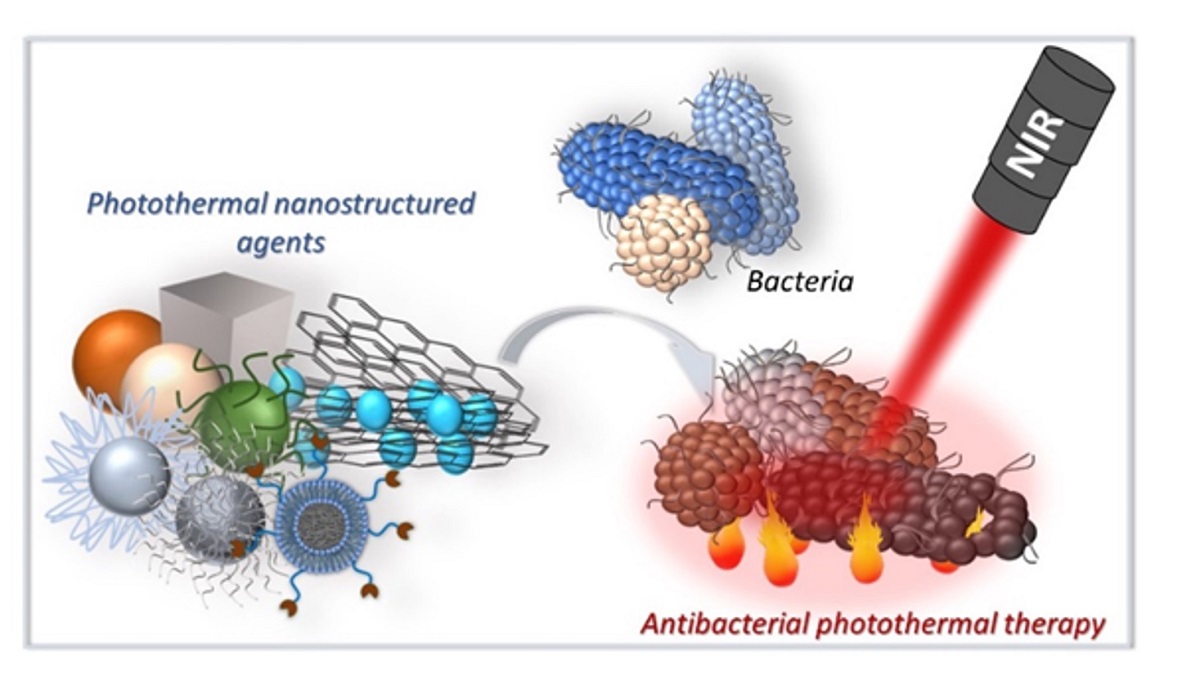
Keywords:
1. Introduction
2. Photothermal antimicrobial mechanism
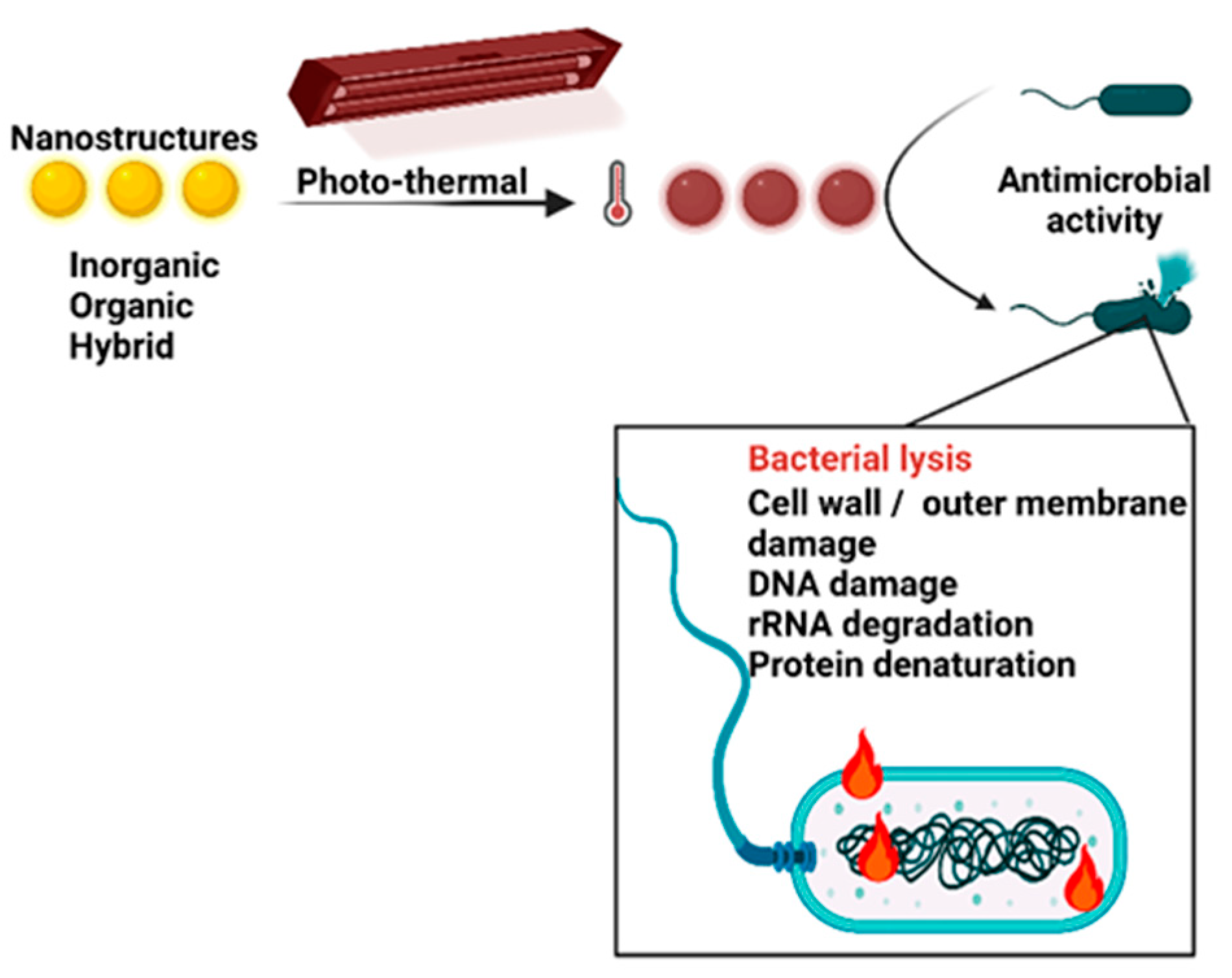
3. Photothermal antimicrobials agents
| Type of nanomaterials | Characterisation Morphology |
Tested bacteria | PTT parameters | Performance | Ref. | |
|---|---|---|---|---|---|---|
| Metalic based PTAs | Au NR | 10 × 45 nm Au NR attached to glass surfaces | S. epidermidis ATCC 35984 | LED - 850 nm, I=0.2 W·cm-2, 5 min |
AR=71% of biofilm Max- 97% |
[61] |
| Au nanaworms covered with PDA | Nanoworms with diameters of 5 ± 1.5 nm, interconnected |
E. coli S. aureus |
808 nm I=1 W·cm-2, 20 min 100 µg·mL-1 PTAs |
∆T= 30.9 °C AR=80% E. coli and AR=90% S. aureus |
[67] | |
| glycomimetic polymers decorated Au NR | AuNR- 50–100 nm long | drug-resistant P. aeruginosa | 808 nm laser, I=2 W·cm-2, 5 min 125 μg·mL−1 PTAs |
∆T= 15.4 °C AR=80% |
[71] | |
| Protease (bromelain) -conjugated AuNR | Au NR -32 nm length, 7.8 nm width |
E. coli S. aureus |
808 nm 50 μg·mL−1 PTAs |
Tmax = 66°C AR=96.8% E. coli AR=97.9% S. aureus |
[72] | |
| Peptides/neuropeptide conjugated AuNR | Au NR - 49 nm length and 11 nm width |
MRSA E. coli |
808 nm I= 2 W·cm-2 , 4 min |
Tmax ~70 ◦C stable after 4 cycles AR= 99% for MRSA AR= 96% for E. coli |
[73] | |
| AuAg yolk−shell cubic nanoframes | well-defined cubic nanoframes 10 nm Au core and frame edge length: 25- 60 nm; frame thickness: 3.8 - 6.1 nm Ag/Au ≈ 3:1, |
MRSA E. faecalis P. aeruginosa K. pneumoniae B. bacillus E. coli |
808 nm laser I=0.33 W·cm-2, 10 min |
η = 65.6% at 0.27 W·cm−2 ‘; ΔT = 23.7 °C AR=96.55%, P. aeruginosa AR=93.69% K. pneumoniae AR=92.34 % B. bacillus AR=96.73%, E. coli AR=98.08% E. faecalis |
[76] | |
| fractal-like Ag nanoaggregates in SiO2 deposited on PDMS layer | AgNPs 10-20 nm, few nm interNPs distances SiO2 =1.3-25% |
S. aureus and E. coli |
808 nm laser I=1.4 W·cm-2, 10 min; m=15.4 μg Ag/SiO2 |
η = 50% AR=100% of S. aureus biofilm (10 min) AR=100% of E. coli biofilm (5 min.) |
[74] | |
| Pd NPs | 4 nm and 41 nm in diameter |
S. aureus and E. coli. |
808 nm laser , I= 1.35 W·cm-2, 10 min, 20 mg·L−1 PTAs |
η = 33.1% AR=99.99% S. aureus AR=99.99% E.coli. |
[81] | |
| Ag /Au bimetallic NPs on Jellyfish Nanofibers scaffold |
Bimetallic Ag/AuNPs: nanospheres, nanotriangles |
B. subtilis P. aeruginosa E. coli, S. epidermidis |
808 nm NIR laser, I= 1 W·cm-2, 5 min | Tmax= 80 °C. Effective (AR=n.a.) |
[83] | |
| Pd-Cu nanoalloy NPs+ AMO in ZIF-8 | Spherical Pd-Cu nanoalloy NPs size 9.02 nm |
S. aureus P. aeruginosa |
λ = 808 nm NIR laser, I = 1 W·cm-2 , 10 min, 200 μg·mL−1 PTAs |
η = 45.8% AR=99.8% S. aureus AR=99.1% P.aeruginosa CR= 75.3% S. aureus CR= 74.8% P. aeruginosa |
[84] | |
| Sulfides | Cu7S4 nanosheets | Cu7S4 samples with (224) exposed facet, a large number of nanosheets, diameter of 30–50 nm. |
B. subtilis, E. coli drug-resistant P. aeruginosa |
808 nm laser, I= 1.5 W·cm-2, 10 min, 50 μg·mL−1 PTAs |
η = 40.52% ∆T= 29.4 °C AR= 100% E. coli AR= 100% B. Subtilis , AR> 90% P. aeruginosa |
[88] |
| CuS@GDY | graphdiyne nanowalls wrapped hollow CuS nanocubes |
MRSA and E.coli |
808-nm laser, I=0.4 W·cm-2, 10 min |
η = 48%, ∆T= 28 °C AR >99.999% MRSA AR >99.999% E.coli |
[79] | |
| CuS nanosheets with sulfur vacancies | Nanosheets: Diameters= 60–100 nm the thickness =25–30 nm |
B. subtilis and E. coli |
808 nm laser, I=1.2 W·cm-2, 10 min 50 μg·mL−1 PTAs |
η = 41.8%, ∆T= 30 °C, AR=99.999% (both) |
[85] | |
| sulfur vacancy modulated MoS2 | Nano spheres- diameter 200–300 nm | E. coli. | 808 nm laser, I=1.5 W·cm-2; 10 min 50 μg·mL−1 PTAs |
η = 45.97% ∆T 32 ◦C ≈100% killed bacteria |
[89] | |
| Cu doped MoS2 nanoflowers | Nanospheres of 50-500 nm; Cu2+ were uniformly distributed on the surface edge sites | S. aureus | 660 nm laser, I=0.898 W·cm-2, 20 min., 2 μg·mL−1 PTAs |
∆T= 30.3 °C AR=99.64% |
[90] | |
| NiS2 nanozymes | Spherical NPs- diameter of 112 nm |
E. coli, DH5α MRSA,Mu50 |
808 nm laser, I=0.75 W·cm-2, 10min, 75 μg·mL−1 PTAs |
η = 43.8% ∆T= 23.4 °C AR=E.coli 98.33% AR≈92% MRSA |
[91] | |
| selenides | SnSe | spherical particles |
E. coli and B. subtilis |
808 nm laser, I=1.5 W·cm-2, 10 min 25 μg·mL−1 PTAs |
η =41.4% Tmax =57 °C AR=99.99% E. coli and AR=99.99% B. subtilis |
[93] |
| Cu2Se NPs in PVDF membrane | 80 nm size NPs |
E. coli and |
1064 nm laser, I= 2.0 W·cm-2, 400 s 160 μg·mL−1 PTAs |
η =30.8% ∆T= 14.6 °C AR=97.52% E. coli |
[92] | |
| Oxides | Fe3O4 NPs | mesoporous hollow Fe3O4 NPs |
E. coli S. aureus |
808-nm NIR + H2O2 (1mM) I=1 W·cm-2 ,10 min; 4 cycles 1 mg·mL−1 PTAs |
η =28.5% AR=72% S. aureus and AR=100% E. coli |
[94] |
| magneto-plasmonic Fe3O4@Au core@shell |
spherical core of Fe 3O4 and Au - branched structure |
E. coli S. aureus |
980 nm laser diode, I=1.0 W·cm-2, 10 min, 50 μg·mL−1 PTAs | η = 69.9% AR=100% E. coli and AR=100% S. aureus |
[95] | |
| MXene | Ti3C2 MXene combined with Cip | Ti3C2 nanosheets monolayer with 50–200 nm lateral size |
E. coli MRSA |
808 nm, I=0.4 W·cm-2 , 15 min, 100 μg/mL Ti3C2+ 5 mg/mL Cip | Tmax =60.7 °C AR= > 99.99999% |
[99] |
| Ti3C2TX MXene-PDA functionalized +lysozyme | Ti3C2 MXene - monolayer | MRSA | 808 nm laser, I=2.0 W·cm-2, 15 min. 50 μg·mL−1 PTAs |
η = 46.88% Tmax =63.5 °C. AR>95 % MRSA |
[98] | |
| Bi2S3NR/Ti3C2Tx MXene | Ti3C2Tx Mxene few-layer nanosheets |
E. coli S. aureus |
808 nm light, I= 0.7 W·cm-2 , 10 min | η = 35.43% Tmax =65 °C RA=99.86% S. aureus RA=99.92% E. coli |
[100] | |
| Other | BPs@cationicCDs | few-layer or monolayer BPs with a flat structure CDs (8–13 nm) grew in situ by BPs |
E. coli S. aureus |
660 nm + 808 nm laser, I=1.5 W·cm-2, 5 min , 200 μg·mL−1 PTAs | η = 34.1% ∆T= 28.2 °C RA≈99 % S. aureus and E. coli |
[106] |
| BPQDs@NH | BP quantum dots (BPQDs) of 3 nm encapsulated in hydrogel |
MRSA Amp r E. coli |
808 nm laser, I = 1 W·cm-2, 5 min, 200 μg·mL−1 PTAs | η = 42.9% ∆T= 35 °C RA= 90% MRSA RA= 90% Ampr E. coli |
[112] | |
3.2. Organic based PTAs
|
Matrix/ material |
Light (nm) and power | Temperature reached | Antibacterial mechanism | In vitro biological performances | Ref | ||
| Type of bacteria | Efficacity | ||||||
| Carbon-based nanomaterials | rGO/AuNP | 808 nm; 3.0 W/cm2 |
73.5 °C | PTT |
S. aureus E. coli |
100% | [120] |
| MWNT/DTTC | 808 nm; 1.0 W/cm2 |
92 °C, 120 °C |
PTT | P. aeruginosa | 77% -100% | [121] | |
| GO/Ag | 808 nm; 1.5 W/cm2 |
24.6 °C | PTT & Ag+ release | MDR E. coli | ̴ 96% | [123] | |
| rGO/Ag | 808 nm; 0.30 W/cm2 |
Higher with ̴ 20 °C | PTT &Ag+ release |
E. coli, K. pneumonia |
100% | [122] | |
| AgNPs PVP@rGO | Visible-light | - | PTT & Ag+ release & physical wall demolition | E. coli | Effective | [125] | |
| Fe3O4@GO-QCS | 808 nm; 3.0 W/cm2 |
≥50 °C | Bacteria capture & PTT & Magnetic Recycle |
S. aureus E. coli |
̴ 100% | [141] | |
| Fe3O4-CNT-PNIPAM | 808 nm; 3.0 W/cm2 |
- | Bacteria capture & PTT & Magnetic Recycle |
S. aureus E. coli |
̴ 100% | [142] | |
| CP | PTDBD | 808 nm; 1.0 W/cm2 |
66 °C | PTT |
S. aureus E. coli C. albicans |
Effective | [128] |
| PDTPBT | 808 nm; 1.0 W/cm2 |
57 °C | PTT |
E. coli MRSA |
Effective | [84] | |
| PEDOT:PSS/agarose | 808 nm; 2.0 W/cm2 |
24.5 °C | PTT |
S. aureus E. coli |
̴ 100% | [129] | |
| PDPP3T | 808 nm; 0.50 W/cm2 |
̴ 45°C | PTT | E. coli | ̴ 100% | [143] | |
| DMCPNs | 808 nm; 0.50 W/cm2 |
62.4 °C | PTT & PDT | E. coli | 93% | [144] | |
| Polymer functionalized nanomaterials | MagI-PEG@PDA NPs | 808 nm; 2.0 W/cm2 |
45 °C | PTT | E. coli | 99.99% | [131] |
| GO–IO–CS nanocomposite | 808 nm; 2.0 W/cm2 |
̴ 25°C | PTT & capture bacteria & aggregation |
S. aureus E. coli |
̴ 80% | [132] | |
| CPNs-Tat | 808 nm; 2.0 W/cm2 |
55.3 ºC | PTT |
E. coli S. aureus C. albicans |
̴ 100% | [134] | |
| SF-CS-PDA cryogels | 808 nm; 2.0 W/cm2 |
̴ 45 °C | PTT & ROS-scavenging capacity, tissue affinity |
S. aureus E. coli |
Effective | [145] | |
| COFs | TP-Por-CON@BNN6 | 635 nm | - | PTT & PDT & gaseous therapy |
S. aureus E. coli |
Effective | [139] |
| TAPP-BDP | 808 nm | 65 °C | PTT & PDT & ROS |
S. aureus E. coli |
Effective | [140] | |
| CTCS | 660 nm 0.4 W/cm2 |
̴ 54 °C | PTT & PDT |
S. aureus E. coli |
> 98.5% | [136] | |
3.3. Hybrid photothermal antimicrobials and inorganic-organic nanocomposites
4. Applications
4.1. Anti-bacterial biofilms
4.2. Synergistic photodynamic effects-based antibacterial systems
4.3. Cutaneous wounds
5. Conclusions, challenges, and perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- https://www.cdc.gov/drugresistance/ar-lab-networks/global.html. Available online: (accessed on.
- Ventola, C.L. The antibiotic resistance crisis: part 1: causes and threats. Pharmacy and therapeutics 2015, 40, 277. [Google Scholar] [PubMed]
- https://www.cdc.gov/drugresistance/biggest-threats.html. Available online: (accessed on.
- https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance. Available online: (accessed on.
- Razzaque, M.S. Commentary: microbial resistance movements: an overview of global public health threats posed by antimicrobial resistance, and how best to counter. Frontiers in Public Health 2021, 8, 629120. [Google Scholar] [CrossRef] [PubMed]
- Blair, J.M.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J. Molecular mechanisms of antibiotic resistance. Nature reviews microbiology 2015, 13, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.D.; Sutherland, A.D. New strategies for combating multidrug-resistant bacteria. Trends in molecular medicine 2007, 13, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Separovic, F.; O'Brien-Simpson, N.M.; Wade, J.D. Chemically modified and conjugated antimicrobial peptides against superbugs. Chemical Society Reviews 2021, 50, 4932–4973. [Google Scholar] [CrossRef] [PubMed]
- Neu, H.C. The crisis in antibiotic resistance. Science 1992, 257, 1064–1073. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: a common cause of persistent infections. science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Chen, M.; Yu, Q.; Sun, H. Novel strategies for the prevention and treatment of biofilm related infections. International journal of molecular sciences 2013, 14, 18488–18501. [Google Scholar] [CrossRef]
- Hetta, H.F.; Ramadan, Y.N.; Al-Harbi, A.I.; A. Ahmed, E.; Battah, B.; Abd Ellah, N.H.; Zanetti, S.; Donadu, M.G. Nanotechnology as a Promising Approach to Combat Multidrug Resistant Bacteria: A Comprehensive Review and Future Perspectives. Biomedicines 2023, 11, 413. [Google Scholar] [CrossRef]
- Kwon, H.-Y.; Godman, B. Pharmaceutical policy, impact and health outcomes; Frontiers Media SA: 2023; Volume 16648714.
- Wang, Z.; Koirala, B.; Hernandez, Y.; Zimmerman, M.; Park, S.; Perlin, D.S.; Brady, S.F. A naturally inspired antibiotic to target multidrug-resistant pathogens. Nature 2022, 601, 606–611. [Google Scholar] [CrossRef]
- Su, Y.; Yrastorza, J.T.; Matis, M.; Cusick, J.; Zhao, S.; Wang, G.; Xie, J. Biofilms: Formation, Research Models, Potential Targets, and Methods for Prevention and Treatment. Advanced Science 2022, 9, 2203291. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Joo, J.; Kang, J.; Kim, B.; Braun, G.B.; She, Z.-G.; Kim, D.; Mann, A.P.; Mölder, T.; Teesalu, T. Antibiotic-loaded nanoparticles targeted to the site of infection enhance antibacterial efficacy. Nature biomedical engineering 2018, 2, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, Y.; Shi, Y.; Song, H.; Yu, C. Antibiotic-free antibacterial strategies enabled by nanomaterials: progress and perspectives. Advanced Materials 2020, 32, 1904106. [Google Scholar] [CrossRef] [PubMed]
- Maillard, J.-Y.; Hartemann, P. Silver as an antimicrobial: facts and gaps in knowledge. Critical reviews in microbiology 2013, 39, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.-H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.-Y. Antimicrobial effects of silver nanoparticles. Nanomedicine: Nanotechnology, biology and medicine 2007, 3, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Lansdown, A.B. Silver in health care: antimicrobial effects and safety in use. Biofunctional textiles and the skin 2006, 33, 17–34. [Google Scholar]
- Liao, C.; Li, Y.; Tjong, S.C. Bactericidal and cytotoxic properties of silver nanoparticles. International journal of molecular sciences 2019, 20, 449. [Google Scholar] [CrossRef]
- Kumar, R.; Umar, A.; Kumar, G.; Nalwa, H.S. Antimicrobial properties of ZnO nanomaterials: A review. Ceramics International 2017, 43, 3940–3961. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A. Metal-based nanoparticles as antimicrobial agents: an overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef]
- Dediu, V.; Busila, M.; Tucureanu, V.; Bucur, F.I.; Iliescu, F.S.; Brincoveanu, O.; Iliescu, C. Synthesis of ZnO/Au Nanocomposite for Antibacterial Applications. Nanomaterials 2022, 12, 3832. [Google Scholar] [CrossRef]
- Tian, E.-K.; Wang, Y.; Ren, R.; Zheng, W.; Liao, W. Gold nanoparticle: Recent progress on its antibacterial applications and mechanisms. Journal of Nanomaterials 2021, 2021, 1–18. [Google Scholar] [CrossRef]
- Zheng, K.; Setyawati, M.I.; Leong, D.T.; Xie, J. Observing antimicrobial process with traceable gold nanoclusters. Nano Research 2021, 14, 1026–1033. [Google Scholar] [CrossRef]
- Zhang, Y.; Shareena Dasari, T.P.; Deng, H.; Yu, H. Antimicrobial activity of gold nanoparticles and ionic gold. Journal of Environmental Science and Health, Part C 2015, 33, 286–327. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Vary, P.S.; Lin, C.-T. Anatase TiO2 nanocomposites for antimicrobial coatings. The journal of physical chemistry B 2005, 109, 8889–8898. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, C.; La Parola, V.; Testa, M.L.; Liotta, L.F. Antifouling and antimicrobial activity of Ag, Cu and Fe nanoparticles supported on silica and titania. Inorganica Chimica Acta 2022, 529, 120636. [Google Scholar] [CrossRef]
- Ren, G.; Hu, D.; Cheng, E.W.; Vargas-Reus, M.A.; Reip, P.; Allaker, R.P. Characterisation of copper oxide nanoparticles for antimicrobial applications. International journal of antimicrobial agents 2009, 33, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.H.; Al Abboud, M.A.; Alawlaqi, M.M.; Abdelghany, T.M.; Hasanin, M. Synthesis of nanocapsules based on biosynthesized nickel nanoparticles and potato starch: Antimicrobial, antioxidant, and anticancer activity. Starch-Stärke 2022, 74, 2100165. [Google Scholar] [CrossRef]
- Filipović, N.; Ušjak, D.; Milenković, M.T.; Zheng, K.; Liverani, L.; Boccaccini, A.R.; Stevanović, M.M. Comparative study of the antimicrobial activity of selenium nanoparticles with different surface chemistry and structure. Frontiers in bioengineering and biotechnology 2021, 8, 624621. [Google Scholar] [CrossRef]
- Jung, H.S.; Verwilst, P.; Sharma, A.; Shin, J.; Sessler, J.L.; Kim, J.S. Organic molecule-based photothermal agents: an expanding photothermal therapy universe. Chemical Society Reviews 2018, 47, 2280–2297. [Google Scholar] [CrossRef]
- Parisi, O.I.; Scrivano, L.; Sinicropi, M.S.; Puoci, F. Polymeric nanoparticle constructs as devices for antibacterial therapy. Current Opinion in Pharmacology 2017, 36, 72–77. [Google Scholar] [CrossRef]
- Khalid, M.; El-Sawy, H.S. Polymeric nanoparticles: Promising platform for drug delivery. International journal of pharmaceutics 2017, 528, 675–691. [Google Scholar]
- Zhao, Y.; Jiang, X. Multiple strategies to activate gold nanoparticles as antibiotics. Nanoscale 2013, 5, 8340–8350. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.; Alruwaili, N.K.; Imam, S.S.; Alsaidan, O.A.; Ahmed, M.M.; Yasir, M.; Warsi, M.H.; Alquraini, A.; Ghoneim, M.M.; Alshehri, S. Development and optimization of hybrid polymeric nanoparticles of apigenin: Physicochemical characterization, antioxidant activity and cytotoxicity evaluation. Sensors 2022, 22, 1364. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xiao, Y.; Cao, Y.; Guo, Z.; Li, F.; Wang, L. Construction of chitosan-based hydrogel incorporated with antimonene nanosheets for rapid capture and elimination of bacteria. Advanced Functional Materials 2020, 30, 2003196. [Google Scholar] [CrossRef]
- Qiu, Y.; Yu, S.; Wang, Y.; Xiao, L.; Pei, L.; Pu, Y.; Zhang, Y. Photothermal therapy may be a double-edged sword by inducing the formation of bacterial antibiotic tolerance. Biomaterials Science 2022, 10, 1995–2005. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Y.; Wang, J.; Wang, Y.; Chen, A.; Wang, C.; Mo, W.; Li, Y.; Yuan, Q.; Zhang, Y. Photon-responsive antibacterial nanoplatform for synergistic photothermal-/pharmaco-therapy of skin infection. ACS applied materials & interfaces 2018, 11, 300–310. [Google Scholar]
- Wang, B.; Xu, Y.; Shao, D.; Li, L.; Ma, Y.; Li, Y.; Zhu, J.; Shi, X.; Li, W. Inorganic nanomaterials for intelligent photothermal antibacterial applications. Pharmaceutical Materials for Tumor Imaging and Therapy 2023, 16648714, 79. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.; Ovais, M.; Atiq, A.; Ansari, T.M.; Xing, R.; Spruijt, E.; Yan, X. Tailoring supramolecular short peptide nanomaterials for antibacterial applications. Coordination Chemistry Reviews 2022, 460, 214481. [Google Scholar] [CrossRef]
- Hamblin, M.R. Antimicrobial photodynamic inactivation: a bright new technique to kill resistant microbes. Current opinion in microbiology 2016, 33, 67–73. [Google Scholar] [CrossRef]
- Chen, T.; Gu, T.; Cheng, L.; Li, X.; Han, G.; Liu, Z. Porous Pt nanoparticles loaded with doxorubicin to enable synergistic Chemo-/Electrodynamic Therapy. Biomaterials 2020, 255, 120202. [Google Scholar] [CrossRef]
- Krieg, A.M.; Wagner, H. Causing a commotion in the blood: immunotherapy progresses from bacteria to bacterial DNA. Immunology today 2000, 21, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Jauffred, L.; Samadi, A.; Klingberg, H.; Bendix, P.M.; Oddershede, L.B. Plasmonic heating of nanostructures. Chemical reviews 2019, 119, 8087–8130. [Google Scholar] [CrossRef] [PubMed]
- Beyth, N.; Houri-Haddad, Y.; Domb, A.; Khan, W.; Hazan, R. Alternative antimicrobial approach: nano-antimicrobial materials. Evidence-based complementary and alternative medicine 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Ju, Q.; Chen, X.; Ai, F.; Peng, D.; Lin, X.; Kong, W.; Shi, P.; Zhu, G.; Wang, F. An upconversion nanoprobe operating in the first biological window. Journal of Materials Chemistry B 2015, 3, 3548–3555. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-W.; Yao, K.; Xu, Z.-K. Nanomaterials with a photothermal effect for antibacterial activities: an overview. Nanoscale 2019, 11, 8680–8691. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Yang, Y.; Zheng, W.; Huang, Y.; Xu, F.; Bao, Z. Synergistic Photothermal Antibacterial Therapy Enabled by Multifunctional Nanomaterials: Progress and Perspectives. Materials Chemistry Frontiers 2023. [Google Scholar] [CrossRef]
- Yao, W.; Deng, T.; Huang, A.; Zhang, Y.; Li, Q.; Li, Z. Promoting photothermal antibacterial activity through an excited-state intramolecular proton transfer process. Journal of Materials Chemistry B 2023. [Google Scholar] [CrossRef]
- Xu, D.; Li, Z.; Li, L.; Wang, J. Insights into the photothermal conversion of 2D MXene nanomaterials: synthesis, mechanism, and applications. Advanced Functional Materials 2020, 30, 2000712. [Google Scholar] [CrossRef]
- Wandelt, K. Encyclopedia of interfacial chemistry: surface science and electrochemistry; Elsevier: 2018.
- Tee, S.Y.; Win, K.Y.; Goh, S.S.; Teng, C.P.; Tang, K.Y.; Regulacio, M.D.; Li, Z.; Ye, E. Introduction to Photothermal Nanomaterials. 2022.
- Vélez-Cordero, J.R.; Hernandez-Cordero, J. Heat generation and conduction in PDMS-carbon nanoparticle membranes irradiated with optical fibers. International Journal of Thermal Sciences 2015, 96, 12–22. [Google Scholar] [CrossRef]
- Ye, L.; Cao, Z.; Liu, X.; Cui, Z.; Li, Z.; Liang, Y.; Zhu, S.; Wu, S. Noble metal-based nanomaterials as antibacterial agents. Journal of Alloys and Compounds 2022, 164091. [Google Scholar] [CrossRef]
- Vines, J.B.; Yoon, J.-H.; Ryu, N.-E.; Lim, D.-J.; Park, H. Gold nanoparticles for photothermal cancer therapy. Frontiers in chemistry 2019, 7, 167. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Chen, J.; Li, Z.-Y.; Au, L.; Hartland, G.V.; Li, X.; Marquez, M.; Xia, Y. Gold nanostructures: engineering their plasmonic properties for biomedical applications. Chemical Society Reviews 2006, 35, 1084–1094. [Google Scholar] [CrossRef] [PubMed]
- Link, S.; El-Sayed, M.A. Size and temperature dependence of the plasmon absorption of colloidal gold nanoparticles. The Journal of Physical Chemistry B 1999, 103, 4212–4217. [Google Scholar] [CrossRef]
- Zharov, V.P.; Mercer, K.E.; Galitovskaya, E.N.; Smeltzer, M.S. Photothermal nanotherapeutics and nanodiagnostics for selective killing of bacteria targeted with gold nanoparticles. Biophysical journal 2006, 90, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Pihl, M.; Bruzell, E.; Andersson, M. Bacterial biofilm elimination using gold nanorod localised surface plasmon resonance generated heat. Materials Science and Engineering: C 2017, 80, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Win, K.Y.; Yao, X.; Yang, W.; Han, M.Y. Plasmonically modulated gold nanostructures for photothermal ablation of bacteria. Advanced Healthcare Materials 2021, 10, 2001158. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, L.; Zhang, J.; Aslan, H.; Dong, M. Photoactive antimicrobial nanomaterials. Journal of Materials Chemistry B 2017, 5, 8631–8652. [Google Scholar] [CrossRef]
- Yougbaré, S.; Chou, H.-L.; Yang, C.-H.; Krisnawati, D.I.; Jazidie, A.; Nuh, M.; Kuo, T.-R. Facet-dependent gold nanocrystals for effective photothermal killing of bacteria. Journal of Hazardous Materials 2021, 407, 124617. [Google Scholar] [CrossRef]
- Link, S.; Wang, Z.L.; El-Sayed, M.A. How does a gold nanorod melt? The Journal of Physical Chemistry B 2000, 104, 7867–7870. [Google Scholar] [CrossRef]
- Yan, J.; Zhu, D.; Xie, J.; Shao, Y.; Xiao, W. Light tailoring of internal atomic structure of gold nanorods. Small 2020, 16, 2001101. [Google Scholar] [CrossRef]
- Liao, Z.; Zhang, W.; Qiao, Z.; Luo, J.; Erpuding, A.; Niwaer, A.; Meng, X.; Wang, H.; Li, X.; Zuo, F. Dopamine-assisted one-pot synthesis of gold nanoworms and their application as photothermal agents. Journal of colloid and interface science 2020, 562, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Li, H.; Wang, B.; Ye, Z.; Lei, W.; Jia, F.; Jin, Q.; Ren, K.-F.; Ji, J. Surface-adaptive gold nanoparticles with effective adherence and enhanced photothermal ablation of methicillin-resistant Staphylococcus aureus biofilm. ACS nano 2017, 11, 9330–9339. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.; Yao, Y.; Song, S.; Yin, M.; Yang, M.; Yan, D.; Yang, L.; Luo, J. Gold nanorods with surface charge-switchable activities for enhanced photothermal killing of bacteria and eradication of biofilm. Journal of materials chemistry B 2020, 8, 3138–3149. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, N.N.; Alkilany, A.M.; Khalil, E.A.; Al-Bakri, A.G. Nano-photothermal ablation effect of hydrophilic and hydrophobic functionalized gold nanorods on Staphylococcus aureus and Propionibacterium acnes. Scientific Reports 2018, 8, 6881. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Guo, Q.; Dai, X.; Wei, X.; Yu, Y.; Chen, X.; Li, C.; Cao, Z.; Zhang, X. A biomimetic non-antibiotic approach to eradicate drug-resistant infections. Advanced Materials 2019, 31, 1806024. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Geng, X.; Liu, D.; Li, Z. Near-infrared light-enhanced protease-conjugated gold nanorods as a photothermal antimicrobial agent for elimination of exotoxin and biofilms. International Journal of Nanomedicine 2019, 8047–8058. [Google Scholar] [CrossRef]
- Sankari, S.S.; Dahms, H.-U.; Tsai, M.-F.; Lo, Y.-L.; Wang, L.-F. Comparative study of an antimicrobial peptide and a neuropeptide conjugated with gold nanorods for the targeted photothermal killing of bacteria. Colloids and Surfaces B: Biointerfaces 2021, 208, 112117. [Google Scholar] [CrossRef]
- Merkl, P.; Zhou, S.; Zaganiaris, A.; Shahata, M.; Eleftheraki, A.; Thersleff, T.; Sotiriou, G.A. Plasmonic coupling in silver nanoparticle aggregates and their polymer composite films for near-infrared photothermal biofilm eradication. ACS Applied Nano Materials 2021, 4, 5330–5339. [Google Scholar] [CrossRef]
- Liu, Y.; Li, F.; Guo, Z.; Xiao, Y.; Zhang, Y.; Sun, X.; Zhe, T.; Cao, Y.; Wang, L.; Lu, Q. Silver nanoparticle-embedded hydrogel as a photothermal platform for combating bacterial infections. Chemical Engineering Journal 2020, 382, 122990. [Google Scholar] [CrossRef]
- Zhang, H.; Tan, J.; Yang, X.; Ma, Y.; Zou, H.; Wang, Y.; Zhang, P.; Zheng, Y. Size-Tunable Yolk–Shell Gold–Silver Nanostructures for Photothermal Treatment of Multidrug-Resistant Bacteria. ACS Applied Nano Materials 2022, 5, 10818–10828. [Google Scholar] [CrossRef]
- Deng, F.; Wu, P.; Qian, G.; Shuai, Y.; Zhang, L.; Peng, S.; Shuai, C.; Wang, G. Silver-decorated black phosphorus: a synergistic antibacterial strategy. Nanotechnology 2022, 33, 245708. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yu, S.; Wu, S.; Xu, M.; Gao, T.; Wu, Q.; Xu, H.; Liu, Y. Rational design of silver NPs-incorporated quaternized chitin nanomicelle with combinational antibacterial capability for infected wound healing. International Journal of Biological Macromolecules 2023, 224, 1206–1216. [Google Scholar] [CrossRef] [PubMed]
- Bai, Q.; Liang, M.; Wu, W.; Zhang, C.; Li, X.; Liu, M.; Yang, D.; Yu, W.W.; Hu, Q.; Wang, L. Plasmonic nanozyme of graphdiyne nanowalls wrapped hollow copper sulfide nanocubes for rapid bacteria-killing. Advanced Functional Materials 2022, 32, 2112683. [Google Scholar] [CrossRef]
- Wang, X.; Shi, Q.; Zha, Z.; Zhu, D.; Zheng, L.; Shi, L.; Wei, X.; Lian, L.; Wu, K.; Cheng, L. Copper single-atom catalysts with photothermal performance and enhanced nanozyme activity for bacteria-infected wound therapy. Bioactive materials 2021, 6, 4389–4401. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, Z.; Yang, D.; Zhu, L.; Liang, Z.; Pang, Y.; Zhou, L. Novel Microbial Palladium Nanoparticles with a High Photothermal Effect for Antibacterial Applications. ACS omega 2022. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Fan, Y.; Lin, J.; Yu, P.; Wang, Z.; Ning, C. Near-Infrared Light-Activatable Bismuth-Based Nanomaterials for Antibacterial and Antitumor Treatment. Advanced Therapeutics 2022, 5, 2200027. [Google Scholar] [CrossRef]
- Nudelman, R.; Gavriely, S.; Bychenko, D.; Barzilay, M.; Gulakhmedova, T.; Gazit, E.; Richter, S. Bio-assisted synthesis of bimetallic nanoparticles featuring antibacterial and photothermal properties for the removal of biofilms. Journal of Nanobiotechnology 2021, 19, 1–10. [Google Scholar] [CrossRef]
- Wang, Z.; Peng, Y.; Zhou, Y.; Zhang, S.; Tan, J.; Li, H.; He, D.; Deng, L. Pd-Cu nanoalloy for dual stimuli-responsive chemo-photothermal therapy against pathogenic biofilm bacteria. Acta Biomaterialia 2022, 137, 276–289. [Google Scholar] [CrossRef]
- Mo, S.; Song, Y.; Lin, M.; Wang, J.; Zhang, Z.; Sun, J.; Guo, D.; Liu, L. Near-infrared responsive sulfur vacancy-rich CuS nanosheets for efficient antibacterial activity via synergistic photothermal and photodynamic pathways. Journal of Colloid and Interface Science 2022, 608, 2896–2906. [Google Scholar] [CrossRef]
- Zhang, Z.; Wen, J.; Zhang, J.; Guo, D.; Zhang, Q. Vacancy-Modulated of CuS for Highly Antibacterial Efficiency via Photothermal/Photodynamic Synergetic Therapy. Advanced Healthcare Materials 2023, 12, 2201746. [Google Scholar] [CrossRef]
- Chen, J.; Qi, C.; Zhang, Y.; Zhang, Q.; Tu, J. Photothermal/lysozyme-catalyzed hydrolysis dual-modality therapy via halloysite nanotube-based platform for effective bacterial eradication. International Journal of Biological Macromolecules 2023, 124530. [Google Scholar] [CrossRef] [PubMed]
- Mo, S.; Zhao, Y.; Wen, J.; Sun, J.; Zhang, Z.; Yu, Q.; Wang, G.; Chen, X.; Liu, M. Efficient photothermal and photodynamic synergistic antibacterial therapy of Cu7S4 nanosheets regulated by facet engineering. Journal of Hazardous Materials 2022, 432, 128662. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Wu, Z.; Liu, L.; Cui, W.; Du, D.; Xue, Y. NIR light responsive MoS2 nanomaterials for rapid sterilization: Optimum photothermal effect via sulfur vacancy modulation. Chemical Engineering Journal 2022, 427, 132007. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Liu, X.; Cui, Z.; Chen, D.-F.; Li, Z.; Liang, Y.; Zhu, S.; Wu, S. The rapid photoresponsive bacteria-killing of Cu-doped MoS 2. Biomaterials Science 2020, 8, 4216–4224. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fan, L.; Cheng, L.; Sun, Y.; Wang, X.; Zhong, X.; Shi, Q.; Gong, F.; Yang, Y.; Ma, Y. Biodegradable nickel disulfide nanozymes with GSH-depleting function for high-efficiency photothermal-catalytic antibacterial therapy. Iscience 2020, 23, 101281. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-M.; Huang, L.; Wang, Y.-J.; Xuan, L.; Li, W.-W.; Tian, L.-J. Highly efficient near-infrared photothermal antibacterial membrane with incorporated biogenic CuSe nanoparticles. Chemical Engineering Journal 2021, 405, 126711. [Google Scholar] [CrossRef]
- Kim, J.; Yun, H.; Ri, K.; Sun, J.; Kim, H.; Liu, L. NIR-driven SnSe particles for rapid and effective bacteria sterilization. Journal of Environmental Chemical Engineering 2023, 11, 109109. [Google Scholar] [CrossRef]
- Guo, J.; Wei, W.; Zhao, Y.; Dai, H. Iron oxide nanoparticles with photothermal performance and enhanced nanozyme activity for bacteria-infected wound therapy. Regenerative Biomaterials 2022, 9. [Google Scholar] [CrossRef]
- Lv, X.; Fang, Z.; Sun, Y.; Yang, Y.; Wang, X.; Chen, Y.; Qin, Y.; Li, N.; Li, C.; Xu, J. Interfacial preparation of multi-branched magneto-plasmonic Fe3O4@ Au core@ shell nanocomposites as efficient photothermal agents for antibacterial application. Journal of Alloys and Compounds 2023, 932, 167712. [Google Scholar] [CrossRef]
- Hao, S.; Han, H.; Yang, Z.; Chen, M.; Jiang, Y.; Lu, G.; Dong, L.; Wen, H.; Li, H.; Liu, J. Recent advancements on photothermal conversion and antibacterial applications over MXenes-based materials. Nano-Micro Letters 2022, 14, 178. [Google Scholar] [CrossRef]
- Rasool, K.; Helal, M.; Ali, A.; Ren, C.E.; Gogotsi, Y.; Mahmoud, K.A. Antibacterial activity of Ti3C2T x MXene. ACS nano 2016, 10, 3674–3684. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Huang, L.; Sun, D.-W.; Pu, H.; Wei, Q. Bio-interface engineering of MXene nanosheets with immobilized lysozyme for light-enhanced enzymatic inactivation of methicillin-resistant Staphylococcus aureus. Chemical Engineering Journal 2023, 452, 139078. [Google Scholar] [CrossRef]
- Zheng, Y.; Yan, Y.; Lin, L.; He, Q.; Hu, H.; Luo, R.; Xian, D.; Wu, J.; Shi, Y.; Zeng, F. Titanium carbide MXene-based hybrid hydrogel for chemo-photothermal combinational treatment of localized bacterial infection. Acta Biomaterialia 2022, 142, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Z.; Liu, X.; Li, C.; Zheng, Y.; Yeung, K.W.K.; Cui, Z.; Liang, Y.; Zhu, S.; Hu, W. Interfacial engineering of Bi2S3/Ti3C2T x MXene based on work function for rapid photo-excited bacteria-killing. Nature Communications 2021, 12, 1224. [Google Scholar] [CrossRef]
- Naskar, A.; Kim, K.-s. Black phosphorus nanomaterials as multi-potent and emerging platforms against bacterial infections. Microbial pathogenesis 2019, 137, 103800. [Google Scholar] [CrossRef]
- Choi, J.R.; Yong, K.W.; Choi, J.Y.; Nilghaz, A.; Lin, Y.; Xu, J.; Lu, X. Black phosphorus and its biomedical applications. Theranostics 2018, 8, 1005. [Google Scholar] [CrossRef]
- Guo, T.; Zhuang, S.; Qiu, H.; Guo, Y.; Wang, L.; Jin, G.; Lin, W.; Huang, G.; Yang, H. Black phosphorus nanosheets for killing bacteria through nanoknife effect. Particle & Particle Systems Characterization 2020, 37, 2000169. [Google Scholar]
- Shi, Z.; Ren, X.; Qiao, H.; Cao, R.; Zhang, Y.; Qi, X.; Zhang, H. Recent insights into the robustness of two-dimensional black phosphorous in optoelectronic applications. Journal of Photochemistry and Photobiology C: Photochemistry Reviews 2020, 43, 100354. [Google Scholar] [CrossRef]
- Aksoy, I.; Kucukkececi, H.; Sevgi, F.; Metin, O.; Hatay Patir, I. Photothermal antibacterial and antibiofilm activity of black phosphorus/gold nanocomposites against pathogenic bacteria. ACS applied materials & interfaces 2020, 12, 26822–26831. [Google Scholar]
- Zhang, P.; Sun, B.; Wu, F.; Zhang, Q.; Chu, X.; Ge, M.; Zhou, N.; Shen, J. Wound healing acceleration by antibacterial biodegradable black phosphorus nanosheets loaded with cationic carbon dots. Journal of Materials Science 2021, 56, 6411–6426. [Google Scholar] [CrossRef]
- Naskar, A.; Cho, H.; Kim, K.-s. Black phosphorus-based CuS nanoplatform: Near-infrared-responsive and reactive oxygen species-generating agent against environmental bacterial pathogens. Journal of Environmental Chemical Engineering 2022, 10, 108226. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Y.; Tian, C.; Liu, Z.; Wu, K.; Zhang, C.; Han, X. Construction of antibacterial photothermal PCL/AgNPs/BP nanofibers for infected wound healing. Materials & Design 2023, 226, 111670. [Google Scholar]
- Çekceoğlu, İ.A.; Eroglu, Z.; Küçükkeçeci, H.; Sevgi, F.; Ersoz, M.; Patir, I.H.; Metin, Ö. A NIR-light-driven Black Phosphorus Based Nanocomposite for Combating Bacteria. ChemistrySelect 2022, 7, e202104137. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, X.; Tan, L.; Cui, Z.; Li, Z.; Liang, Y.; Zhu, S.; Yeung, K.; Zheng, Y.; Wu, S. An UV to NIR-driven platform based on red phosphorus/graphene oxide film for rapid microbial inactivation. Chemical Engineering Journal 2020, 383, 123088. [Google Scholar] [CrossRef]
- Lv, J.; Qi, Y.; Tian, Y.; Wang, G.; Shi, L.; Ning, G.; Ye, J. Functionalized boron nanosheets with near-infrared-triggered photothermal and nitric oxide release activities for efficient antibacterial treatment and wound healing promotion. Biomaterials Science 2022, 10, 3747–3756. [Google Scholar] [CrossRef]
- Liu, B.; Su, Y.; Wu, S.; Shen, J. Local photothermal/photodynamic synergistic antibacterial therapy based on two-dimensional BP@ CQDs triggered by single NIR light source. Photodiagnosis and Photodynamic Therapy 2022, 39, 102905. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gao, Y.; Chen, Y.; Liu, L.; Mo, A.; Peng, Q. Nanomaterials-based photothermal therapy and its potentials in antibacterial treatment. Journal of Controlled Release 2020, 328, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Saleem, J.; Wang, L.; Chen, C. Carbon-based nanomaterials for cancer therapy via targeting tumor microenvironment. Advanced healthcare materials 2018, 7, 1800525. [Google Scholar] [CrossRef]
- Khan, A.A.P.; Khan, A.; Rahman, M.M.; Asiri, A.M.; Oves, M. Lead sensors development and antimicrobial activities based on graphene oxide/carbon nanotube/poly (O-toluidine) nanocomposite. International journal of biological macromolecules 2016, 89, 198–205. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS nano 2010, 4, 5731–5736. [Google Scholar] [CrossRef]
- Kang, S.; Herzberg, M.; Rodrigues, D.F.; Elimelech, M. Antibacterial effects of carbon nanotubes: size does matter! Langmuir 2008, 24, 6409–6413. [Google Scholar] [CrossRef] [PubMed]
- Catanio, A.T.; Bergmann, E.V.; Kimura, N.M.; Petrucci, T.; Freitas, C.F.; Herculano, L.S.; Malacarne, L.C.; Astrath, N.G. Spectroscopic and photothermal characterization of graphene quantum dots for antimicrobial applications. Journal of Applied Physics 2022, 131, 155102. [Google Scholar] [CrossRef]
- Qie, X.; Zan, M.; Gui, P.; Chen, H.; Wang, J.; Lin, K.; Mei, Q.; Ge, M.; Zhang, Z.; Tang, Y. Design, synthesis, and application of carbon dots with synergistic antibacterial activity. Frontiers in Bioengineering and Biotechnology 2022, 10. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, Q.; Yin, Q.; Pan, G.; Tu, Z.; Liu, L. Reduced graphene oxide functionalized with gold nanostar nanocomposites for synergistically killing bacteria through intrinsic antimicrobial activity and photothermal ablation. ACS Applied Bio Materials 2019, 2, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Oruc, B.; Unal, H. Fluorophore-decorated carbon nanotubes with enhanced photothermal activity as antimicrobial nanomaterials. ACS Omega 2019, 4, 5556–5564. [Google Scholar] [CrossRef]
- Tan, S.; Wu, X.; Xing, Y.; Lilak, S.; Wu, M.; Zhao, J.X. Enhanced synergetic antibacterial activity by a reduce graphene oxide/Ag nanocomposite through the photothermal effect. Colloids and Surfaces B: Biointerfaces 2020, 185, 110616. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, W.; Xu, Z.; Jiang, C.; Han, S.; Ruan, J.; Wang, Y. Photothermal-assisted antibacterial application of graphene oxide-Ag nanocomposites against clinically isolated multi-drug resistant Escherichia coli. Royal Society Open Science 2020, 7, 192019. [Google Scholar] [CrossRef]
- Yang, Y.; Yin, H.; Li, H.; Zou, Q.; Zhang, Z.; Pei, W.; Luo, L.; Huo, Y.; Li, H. Synergistic photocatalytic-photothermal contribution to antibacterial activity in BiOI-graphene oxide nanocomposites. ACS Applied Bio Materials 2018, 1, 2141–2152. [Google Scholar] [CrossRef]
- Lv, Y.-k.; Mei, L.; Zhang, L.-x.; Yang, D.-h.; Yin, Z.-y. Multifunctional graphene-based nanocomposites for simultaneous enhanced photocatalytic degradation and photothermal antibacterial activity by visible light. Environmental Science and Pollution Research 2021, 28, 49880–49888. [Google Scholar] [CrossRef]
- Lin, F.; Duan, Q.-Y.; Wu, F.-G. Conjugated polymer-based photothermal therapy for killing microorganisms. ACS Applied Polymer Materials 2020, 2, 4331–4344. [Google Scholar] [CrossRef]
- Pham, T.-T.D.; Phan, L.M.T.; Cho, S.; Park, J. Enhancement approaches for photothermal conversion of donor–acceptor conjugated polymer for photothermal therapy: a review. Science and Technology of Advanced Materials 2022, 23, 707–734. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, Z.; Wang, Y.; Feng, L. Near-infrared light-triggered synergistic phototherapy for antimicrobial therapy. ACS Applied Bio Materials 2020, 3, 1730–1737. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.; Kim, J.; Jeong, H.Y.; Kwon, G.; Kim, D.; Ku, M.; Yang, J.; Yamauchi, Y.; Kim, H.-Y.; Lee, C. Antibacterial poly (3, 4-ethylenedioxythiophene): poly (styrene-sulfonate)/agarose nanocomposite hydrogels with thermo-processability and self-healing. Carbohydrate polymers 2019, 203, 26–34. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, L.; Gu, Z.; Dai, L. Carbon nanomaterials for phototherapy. Nanophotonics 2022, 11, 4955–4976. [Google Scholar] [CrossRef]
- Fan, X.-L.; Li, H.-Y.; Ye, W.-Y.; Zhao, M.-Q.; Huang, D.-n.; Fang, Y.; Zhou, B.-Q.; Ren, K.-F.; Ji, J.; Fu, G.-S. Magainin-modified polydopamine nanoparticles for photothermal killing of bacteria at low temperature. Colloids and Surfaces B: Biointerfaces 2019, 183, 110423. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Ahmad, I.; Yang, R.; Wang, C. Versatile graphene-based photothermal nanocomposites for effectively capturing and killing bacteria, and for destroying bacterial biofilms. Journal of Materials Chemistry B 2017, 5, 2459–2467. [Google Scholar] [CrossRef] [PubMed]
- Korupalli, C.; Huang, C.-C.; Lin, W.-C.; Pan, W.-Y.; Lin, P.-Y.; Wan, W.-L.; Li, M.-J.; Chang, Y.; Sung, H.-W. Acidity-triggered charge-convertible nanoparticles that can cause bacterium-specific aggregation in situ to enhance photothermal ablation of focal infection. Biomaterials 2017, 116, 1–9. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Liu, L.; Feng, L. Photothermal-responsive conjugated polymer nanoparticles for the rapid and effective killing of bacteria. ACS Applied Bio Materials 2018, 1, 27–32. [Google Scholar] [CrossRef]
- Mohajer, F.; Ziarani, G.M.; Badiei, A.; Iravani, S.; Varma, R.S. Recent advances in covalent organic frameworks (COFs) for wound healing and antimicrobial applications. RSC advances 2023, 13, 8136–8152. [Google Scholar] [CrossRef]
- Li, P.; Li, B.; Wang, C.; Zhao, X.; Zheng, Y.; Wu, S.; Shen, J.; Zhang, Y.; Liu, X. In situ fabrication of co-coordinated TCPP-Cur donor-acceptor-type covalent organic framework-like photocatalytic hydrogel for rapid therapy of bacteria-infected wounds. Composites Part B: Engineering 2023, 110506.
- Schlachter, A.; Asselin, P.; Harvey, P.D. Porphyrin-containing MOFs and COFs as heterogeneous photosensitizers for singlet oxygen-based antimicrobial nanodevices. ACS Applied Materials & Interfaces 2021, 13, 26651–26672. [Google Scholar]
- Zhang, L.; Wang, S.; Zhou, Y.; Wang, C.; Zhang, X.Z.; Deng, H. Covalent organic frameworks as favorable constructs for photodynamic therapy. Angewandte Chemie International Edition 2019, 58, 14213–14218. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Ye, Z.; Zhang, M.; Song, Q.; Chu, X.; Gao, S.; Zhang, Q.; Jiang, C.; Zhou, N.; Yao, C. Light-activated biodegradable covalent organic framework-integrated heterojunction for photodynamic, photothermal, and gaseous therapy of chronic wound infection. ACS Applied Materials & Interfaces 2021, 13, 42396–42410. [Google Scholar]
- Yang, G.-P.; Meng, X.-L.; Xiao, S.-J.; Zheng, Q.-Q.; Tan, Q.-G.; Liang, R.-P.; Zhang, L.; Zhang, P.; Qiu, J.-D. Construction of D–A-Conjugated Covalent Organic Frameworks with Enhanced Photodynamic, Photothermal, and Nanozymatic Activities for Efficient Bacterial Inhibition. ACS Applied Materials & Interfaces 2022, 14, 28289–28300. [Google Scholar]
- Yang, F.; Feng, Y.; Fan, X.; Zhang, M.; Wang, C.; Zhao, W.; Zhao, C. Biocompatible graphene-based nanoagent with NIR and magnetism dual-responses for effective bacterial killing and removal. Colloids and Surfaces B: Biointerfaces 2019, 173, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ma, L.; Cheng, C.; Deng, Y.; Huang, J.; Fan, X.; Nie, C.; Zhao, W.; Zhao, C. Nonchemotherapic and robust dual-responsive nanoagents with on-demand bacterial trapping, ablation, and release for efficient wound disinfection. Advanced Functional Materials 2018, 28, 1705708. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, S.; Chong, K.C.; Wang, S.; Liu, B. Near-infrared light-induced shape memory, self-healable and anti-bacterial elastomers prepared by incorporation of a diketopyrrolopyrrole-based conjugated polymer. Materials Chemistry Frontiers 2019, 3, 836–841. [Google Scholar] [CrossRef]
- Zhang, H.; Liang, Y.; Zhao, H.; Qi, R.; Chen, Z.; Yuan, H.; Liang, H.; Wang, L. Dual-Mode Antibacterial Conjugated Polymer Nanoparticles for Photothermal and Photodynamic Therapy. Macromolecular Bioscience 2020, 20, 1900301. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Li, P.; Tang, P.; Wang, X.; Zhou, T.; Wang, K.; Ren, F.; Guo, T.; Lu, X. Mussel-inspired cryogels for promoting wound regeneration through photobiostimulation, modulating inflammatory responses and suppressing bacterial invasion. Nanoscale 2019, 11, 15846–15861. [Google Scholar] [CrossRef]
- Wang, X.; Sun, X.; Bu, T.; Wang, Q.; Jia, P.; Dong, M.; Wang, L. In situ fabrication of metal-organic framework derived hybrid nanozymes for enhanced nanozyme-photothermal therapy of bacteria-infected wounds. Composites Part B: Engineering 2022, 229, 109465. [Google Scholar] [CrossRef]
- Xu, X.; Liu, Y.; Fu, W.; Yao, M.; Ding, Z.; Xuan, J.; Li, D.; Wang, S.; Xia, Y.; Cao, M. Poly (N-isopropylacrylamide)-based thermoresponsive composite hydrogels for biomedical applications. Polymers 2020, 12, 580. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, X.; Yu, B.; Zhao, N.; Zhang, C.; Xu, F.-J. Rough carbon–iron oxide nanohybrids for near-infrared-II light-responsive synergistic antibacterial therapy. ACS nano 2021, 15, 7482–7490. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, K.; Zhao, S.; Xiong, Q.; Liu, G.; Li, Y.; Fang, Q.; Gong, X.; Xuan, S. Rough surface NiFe2O4@ Au/Polydopamine with a magnetic field enhanced photothermal antibacterial effect. Chemical Engineering Journal 2022, 437, 135282. [Google Scholar] [CrossRef]
- Zheng, H.; Li, H.; Deng, H.; Fang, W.; Huang, X.; Qiao, J.; Tong, Y. Near infrared light-responsive and drug-loaded black phosphorus nanosheets for antibacterial applications. Colloids and Surfaces B: Biointerfaces 2022, 214, 112433. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Zhang, B.; Zhang, Y.; Su, R.; Li, P.; Su, W. Fluorescent carbon dot–curcumin nanocomposites for remarkable antibacterial activity with synergistic photodynamic and photothermal abilities. ACS Applied Bio Materials 2021, 4, 6703–6718. [Google Scholar] [CrossRef] [PubMed]
- Moorcroft, S.C.; Roach, L.; Jayne, D.G.; Ong, Z.Y.; Evans, S.D. Nanoparticle-loaded hydrogel for the light-activated release and photothermal enhancement of antimicrobial peptides. ACS applied materials & interfaces 2020, 12, 24544–24554. [Google Scholar]
- Dong, X.; Ye, J.; Chen, Y.; Tanziela, T.; Jiang, H.; Wang, X. Intelligent peptide-nanorods against drug-resistant bacterial infection and promote wound healing by mild-temperature photothermal therapy. Chemical Engineering Journal 2022, 432, 134061. [Google Scholar] [CrossRef]
- Huang, S.; Xu, S.; Hu, Y.; Zhao, X.; Chang, L.; Chen, Z.; Mei, X. Preparation of NIR-responsive, ROS-generating and antibacterial black phosphorus quantum dots for promoting the MRSA-infected wound healing in diabetic rats. Acta biomaterialia 2022, 137, 199–217. [Google Scholar] [CrossRef]
- Cao, W.; Yue, L.; Khan, I.M.; Wang, Z. Polyethylenimine modified MoS2 nanocomposite with high stability and enhanced photothermal antibacterial activity. Journal of Photochemistry and Photobiology A: Chemistry 2020, 401, 112762. [Google Scholar] [CrossRef]
- Li, H.; Gong, M.; Xiao, J.; Hai, L.; Luo, Y.; He, L.; Wang, Z.; Deng, L.; He, D. Photothermally activated multifunctional MoS2 bactericidal nanoplatform for combined chemo/photothermal/photodynamic triple-mode therapy of bacterial and biofilm infections. Chemical Engineering Journal 2022, 429, 132600. [Google Scholar] [CrossRef]
- Xu, M.; Hu, Y.; Xiao, Y.; Zhang, Y.; Sun, K.; Wu, T.; Lv, N.; Wang, W.; Ding, W.; Li, F. Near-infrared-controlled nanoplatform exploiting photothermal promotion of peroxidase-like and OXD-like activities for potent antibacterial and anti-biofilm therapies. ACS Applied Materials & Interfaces 2020, 12, 50260–50274. [Google Scholar]
- Ziesmer, J.; Larsson, J.V.; Sotiriou, G.A. Hybrid microneedle arrays for antibiotic and near-IR photothermal synergistic antimicrobial effect against Methicillin-Resistant Staphylococcus aureus. Chemical Engineering Journal 2023, 462, 142127. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Lin, C.; Zhu, H.; Cheng, X.; Liu, C.; Shi, J. An injectable self-healing CS/PDA–AgNPs hybrid hydrogel for mild and highly-efficient photothermal sterilization. New Journal of Chemistry 2022, 46, 8043–8052. [Google Scholar] [CrossRef]
- Prinz Setter, O.; Snoyman, I.; Shalash, G.; Segal, E. Gold Nanorod-Incorporated Halloysite Nanotubes Functionalized with Antibody for Superior Antibacterial Photothermal Treatment. Pharmaceutics 2022, 14, 2094. [Google Scholar] [CrossRef] [PubMed]
- Abee, T.; Kovács, Á.T.; Kuipers, O.P.; Van der Veen, S. Biofilm formation and dispersal in Gram-positive bacteria. Current opinion in biotechnology 2011, 22, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; van Hullebusch, E.D.; Neu, T.R.; Nielsen, P.H.; Seviour, T.; Stoodley, P.; Wingender, J.; Wuertz, S. The biofilm matrix: Multitasking in a shared space. Nature Reviews Microbiology 2023, 21, 70–86. [Google Scholar] [CrossRef]
- Ikuma, K.; Decho, A.W.; Lau, B.L. When nanoparticles meet biofilms—interactions guiding the environmental fate and accumulation of nanoparticles. Frontiers in microbiology 2015, 6, 591. [Google Scholar] [CrossRef]
- Makabenta, J.M.V.; Nabawy, A.; Li, C.-H.; Schmidt-Malan, S.; Patel, R.; Rotello, V.M. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nature Reviews Microbiology 2021, 19, 23–36. [Google Scholar] [CrossRef]
- Qi, Z.; Bharate, P.; Lai, C.-H.; Ziem, B.; Böttcher, C.; Schulz, A.; Beckert, F.; Hatting, B.; Mülhaupt, R.; Seeberger, P.H. Multivalency at interfaces: supramolecular carbohydrate-functionalized graphene derivatives for bacterial capture, release, and disinfection. Nano letters 2015, 15, 6051–6057. [Google Scholar] [CrossRef]
- Teng, W.; Zhang, Z.; Wang, Y.; Ye, Y.; Yinwang, E.; Liu, A.; Zhou, X.; Xu, J.; Zhou, C.; Sun, H. Iodine Immobilized Metal–Organic Framework for NIR-Triggered Antibacterial Therapy on Orthopedic Implants. Small 2021, 17, 2102315. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilm formation: a clinically relevant microbiological process. Clinical infectious diseases 2001, 33, 1387–1392. [Google Scholar] [CrossRef]
- Elbourne, A.; Cheeseman, S.; Atkin, P.; Truong, N.P.; Syed, N.; Zavabeti, A.; Mohiuddin, M.; Esrafilzadeh, D.; Cozzolino, D.; McConville, C.F. Antibacterial liquid metals: biofilm treatment via magnetic activation. ACS nano 2020, 14, 802–817. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Song, L.; Fan, Y.; Tian, L.; Luan, S.; Niu, S.; Ren, L.; Ming, W.; Zhao, J. Synergistic photodynamic and photothermal antibacterial nanocomposite membrane triggered by single NIR light source. ACS applied materials & interfaces 2019, 11, 26581–26589. [Google Scholar]
- Xu, Y.; Cai, Y.; Xia, Y.; Wu, Q.; Li, M.; Guo, N.; Tu, Y.; Yang, B.; Liu, Y. Photothermal nanoagent for anti-inflammation through macrophage repolarization following antibacterial therapy. European Polymer Journal 2023, 111840. [Google Scholar] [CrossRef]
- Shariati, A.; Hosseini, S.M.; Chegini, Z.; Seifalian, A.; Arabestani, M.R. Graphene-Based Materials for Inhibition of Wound Infection and Accelerating Wound Healing. Biomedicine & Pharmacotherapy 2023, 158, 114184. [Google Scholar]
- Cao, F.; Ju, E.; Zhang, Y.; Wang, Z.; Liu, C.; Li, W.; Huang, Y.; Dong, K.; Ren, J.; Qu, X. An efficient and benign antimicrobial depot based on silver-infused MoS2. ACS nano 2017, 11, 4651–4659. [Google Scholar] [CrossRef]
- Chernousova, S.; Epple, M. Silver as antibacterial agent: ion, nanoparticle, and metal. Angewandte Chemie International Edition 2013, 52, 1636–1653. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; He, D.; Yang, T.; Liu, W.; Mao, L.; Zhu, Y.; Wu, J.; Luo, G.; Deng, J. An efficient antimicrobial depot for infectious site-targeted chemo-photothermal therapy. Journal of nanobiotechnology 2018, 16, 1–20. [Google Scholar] [CrossRef]
- AshaRani, P.; Low Kah Mun, G.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS nano 2009, 3, 279–290. [Google Scholar] [CrossRef]
- Rtimi, S.; Dionysiou, D.D.; Pillai, S.C.; Kiwi, J. Advances in catalytic/photocatalytic bacterial inactivation by nano Ag and Cu coated surfaces and medical devices. Applied Catalysis B: Environmental 2019, 240, 291–318. [Google Scholar] [CrossRef]
- Cao, J.; Sun, Q.; Shen, A.-G.; Fan, B.; Hu, J.-M. Nano Au@ Cu2-xS with near-infrared photothermal and peroxidase catalytic activities redefines efficient antibiofilm-oriented root canal therapy. Chemical Engineering Journal 2021, 422, 130090. [Google Scholar] [CrossRef]
- Bao, X.; Zheng, S.; Zhang, L.; Shen, A.; Zhang, G.; Liu, S.; Hu, J. Nitric-Oxide-Releasing aza-BODIPY: A New Near-Infrared J-Aggregate with Multiple Antibacterial Modalities. Angewandte Chemie International Edition 2022, 61, e202207250. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Lin, C.; He, Y.; Tao, B.; Chen, M.; Zhang, J.; Liu, P.; Cai, K. Near-infrared light-triggered nitric-oxide-enhanced photodynamic therapy and low-temperature photothermal therapy for biofilm elimination. ACS nano 2020, 14, 3546–3562. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhao, J.; Fan, D. A dissolving microneedle patch for antibiotic/enzymolysis/photothermal triple therapy against bacteria and their biofilms. Chemical Engineering Journal 2022, 437, 135475. [Google Scholar] [CrossRef]
- Yuan, Z.; Lin, C.; Dai, L.; He, Y.; Hu, J.; Xu, K.; Tao, B.; Liu, P.; Cai, K. Near-infrared light-activatable dual-action nanoparticle combats the established biofilms of methicillin-resistant Staphylococcus aureus and its accompanying inflammation. Small 2021, 17, 2007522. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, X.; Li, B.; Zheng, Y.; Han, Y.; Chen, D.-f.; Yeung, K.W.K.; Cui, Z.; Liang, Y.; Li, Z. Near-infrared light triggered phototherapy and immunotherapy for elimination of methicillin-resistant Staphylococcus aureus biofilm infection on bone implant. ACS nano 2020, 14, 8157–8170. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-Q.; Sun, Y.; Zhang, Y.; Ding, X.; Zhao, N.; Yu, B.; Zhao, H.; Duan, S.; Xu, F.-J. Well-defined gold nanorod/polymer hybrid coating with inherent antifouling and photothermal bactericidal properties for treating an infected hernia. Acs Nano 2020, 14, 2265–2275. [Google Scholar] [CrossRef] [PubMed]
- Pircalabioru, G.G.; Chifiriuc, M.-C. Nanoparticulate drug-delivery systems for fighting microbial biofilms: From bench to bedside. Future Microbiology 2020, 15, 679–698. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Luo, Y.; Li, Z.; Tan, L.; Liu, X.; Li, C.; Zheng, Y.; Cui, Z.; Yeung, K.W.K.; Liang, Y. Antibacterial hybrid hydrogels. Macromolecular Bioscience 2021, 21, 2000252. [Google Scholar] [CrossRef]
- Xu, M.; Li, L.; Hu, Q. The recent progress in photothermal-triggered bacterial eradication. Biomaterials Science 2021, 9, 1995–2008. [Google Scholar] [CrossRef]
- Xie, C.; Zhang, Q.; Li, Z.; Ge, S.; Ma, B. Sustained and microenvironment-accelerated release of minocycline from alginate injectable hydrogel for bacteria-infected wound healing. Polymers 2022, 14, 1816. [Google Scholar] [CrossRef]
- Guo, C.; Wu, Y.; Li, W.; Wang, Y.; Kong, Q. Development of a microenvironment-responsive hydrogel promoting chronically infected diabetic wound healing through sequential hemostatic, antibacterial, and angiogenic activities. ACS Applied Materials & Interfaces 2022, 14, 30480–30492. [Google Scholar]
- Zhu, Y.; Matsumura, Y.; Velayutham, M.; Foley, L.M.; Hitchens, T.K.; Wagner, W.R. Reactive oxygen species scavenging with a biodegradable, thermally responsive hydrogel compatible with soft tissue injection. Biomaterials 2018, 177, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Yang, J.; Wu, J.; Su, H.; Sun, G.; Ni, Y.; Sun, W. Antibacterial carbon dots/iron oxychloride nanoplatform for chemodynamic and photothermal therapy. Colloid and Interface Science Communications 2021, 45, 100552. [Google Scholar] [CrossRef]
- Lin, L.S.; Song, J.; Song, L.; Ke, K.; Liu, Y.; Zhou, Z.; Shen, Z.; Li, J.; Yang, Z.; Tang, W. Simultaneous Fenton-like ion delivery and glutathione depletion by MnO2-based nanoagent to enhance chemodynamic therapy. Angewandte Chemie 2018, 130, 4996–5000. [Google Scholar] [CrossRef]
- Sai, D.L.; Lee, J.; Nguyen, D.L.; Kim, Y.-P. Tailoring photosensitive ROS for advanced photodynamic therapy. Experimental & Molecular Medicine 2021, 53, 495–504. [Google Scholar]
- Han, H.; Xu, X.; Kan, H.; Tang, Y.; Liu, C.; Wen, H.; Wu, L.; Jiang, Y.; Wang, Z.; Liu, J. Synergistic photodynamic/photothermal bacterial inactivation over heterogeneous quaternized chitosan/silver/cobalt phosphide nanocomposites. Journal of Colloid and Interface Science 2022, 616, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Zhao, Y.; Yu, Y.; Chen, X.; Wei, X.; Zhang, X.; Li, C. All-in-one NIR-activated nanoplatforms for enhanced bacterial biofilm eradication. Nanoscale 2018, 10, 18520–18530. [Google Scholar] [CrossRef] [PubMed]
- Syama, S.; Mohanan, P. Comprehensive application of graphene: emphasis on biomedical concerns. Nano-micro letters 2019, 11, 1–31. [Google Scholar] [CrossRef]
- Chu, X.; Zhang, P.; Wang, Y.; Sun, B.; Liu, Y.; Zhang, Q.; Feng, W.; Li, Z.; Li, K.; Zhou, N. Near-infrared carbon dot-based platform for bioimaging and photothermal/photodynamic/quaternary ammonium triple synergistic sterilization triggered by single NIR light source. Carbon 2021, 176, 126–138. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, H.; Tang, J.; Li, P.; Su, R.; Zhong, H.; Su, W. Dual-mode antibacterial core-shell gold nanorod@ mesoporous-silica/curcumin nanocomplexes for efficient photothermal and photodynamic therapy. Journal of Photochemistry and Photobiology A: Chemistry 2022, 425, 113722. [Google Scholar] [CrossRef]
- Dong, M.; Sun, X.; Bu, T.; Zhang, H.; Wang, J.; He, K.; Li, L.; Li, Z.; Wang, L. 3D/2D TMSs/TiO2 nanofibers heterojunctions for photodynamic-photothermal and oxidase-like synergistic antibacterial therapy co-driven by VIS and NIR biowindows. Composites Part B: Engineering 2022, 230, 109498. [Google Scholar] [CrossRef]
- Xu, Z.; Qiu, Z.; Liu, Q.; Huang, Y.; Li, D.; Shen, X.; Fan, K.; Xi, J.; Gu, Y.; Tang, Y. Converting organosulfur compounds to inorganic polysulfides against resistant bacterial infections. Nature communications 2018, 9, 3713. [Google Scholar] [CrossRef]
- Mao, C.; Xiang, Y.; Liu, X.; Zheng, Y.; Yeung, K.W.K.; Cui, Z.; Yang, X.; Li, Z.; Liang, Y.; Zhu, S. Local photothermal/photodynamic synergistic therapy by disrupting bacterial membrane to accelerate reactive oxygen species permeation and protein leakage. ACS applied materials & interfaces 2019, 11, 17902–17914. [Google Scholar]
- Chang, R.; Zhao, D.; Zhang, C.; Liu, K.; He, Y.; Guan, F.; Yao, M. Nanocomposite multifunctional hyaluronic acid hydrogel with photothermal antibacterial and antioxidant properties for infected wound healing. International Journal of Biological Macromolecules 2023, 226, 870–884. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhang, X.; Wei, Y.; Gu, Y.; Xu, H.; Liao, Z.; Zhao, L.; Du, J.; Hu, Y.; Lian, X. Nanocatalytic Hydrogel with Rapid Photodisinfection and Robust Adhesion for Fortified Cutaneous Regeneration. ACS Applied Materials & Interfaces 2023, 15, 6354–6370. [Google Scholar]
- Hu, H.; Wang, H.; Yang, Y.; Xu, J.F.; Zhang, X. A Bacteria-Responsive Porphyrin for Adaptable Photodynamic/Photothermal Therapy. Angewandte Chemie 2022, 134, e202200799. [Google Scholar] [CrossRef]
- Lv, R.; Liang, Y.-Q.; Li, Z.-Y.; Zhu, S.-L.; Cui, Z.-D.; Wu, S.-L. Flower-like CuS/graphene oxide with photothermal and enhanced photocatalytic effect for rapid bacteria-killing using visible light. Rare Metals 2022, 41, 639–649. [Google Scholar] [CrossRef]
- Rong, F.; Tang, Y.; Wang, T.; Feng, T.; Song, J.; Li, P.; Huang, W. Nitric oxide-releasing polymeric materials for antimicrobial applications: a review. Antioxidants 2019, 8, 556. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, H.; Dong, W.; Cheng, S.; Li, H.; Tan, J.; Zhou, J.; He, W.; Li, L.; Zhang, J. A multifunctional platform with single-NIR-laser-triggered photothermal and NO release for synergistic therapy against multidrug-resistant Gram-negative bacteria and their biofilms. Journal of Nanobiotechnology 2020, 18, 1–25. [Google Scholar]
- Yu, S.; Li, G.; Liu, R.; Ma, D.; Xue, W. Dendritic Fe3O4@ poly (dopamine)@ PAMAM nanocomposite as controllable NO-releasing material: a synergistic photothermal and NO antibacterial study. Advanced Functional Materials 2018, 28, 1707440. [Google Scholar] [CrossRef]
- Gao, Q.; Zhang, X.; Yin, W.; Ma, D.; Xie, C.; Zheng, L.; Dong, X.; Mei, L.; Yu, J.; Wang, C. Functionalized MoS2 nanovehicle with near-infrared laser-mediated nitric oxide release and photothermal activities for advanced bacteria-infected wound therapy. Small 2018, 14, 1802290. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Liu, W.; Wang, Z.; Zheng, P.; Liu, W.; Zhao, J.; Zhong, Y.; Zhang, Y.; Lin, J.; Xue, W. Near-infrared laser-controlled nitric oxide-releasing gold nanostar/hollow polydopamine Janus nanoparticles for synergistic elimination of methicillin-resistant Staphylococcus aureus and wound healing. Acta Biomaterialia 2022, 143, 428–444. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Liang, S.; Cai, X.; Huang, S.; Cheng, Z.; Shi, Y.; Pang, M.; Ma, P.a.; Lin, J. Yolk–shell structured Au nanostar@ metal–organic framework for synergistic chemo-photothermal therapy in the second near-infrared window. Nano Letters 2019, 19, 6772–6780. [Google Scholar] [CrossRef]
- Qing, G.; Zhao, X.; Gong, N.; Chen, J.; Li, X.; Gan, Y.; Wang, Y.; Zhang, Z.; Zhang, Y.; Guo, W. Thermo-responsive triple-function nanotransporter for efficient chemo-photothermal therapy of multidrug-resistant bacterial infection. Nature communications 2019, 10, 4336. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Sun, J.; Li, Q.; Pu, Y.; Liu, J.; Sun, R.; Wang, L.; Jiang, T. Vancomycin modified copper sulfide nanoparticles for photokilling of vancomycin-resistant enterococci bacteria. Colloids and Surfaces B: Biointerfaces 2020, 189, 110875. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Guo, H.; Cao, C.; Wang, X.; Song, X.; Wang, W.; Yang, D.; Xi, L.; Mou, X.; Dong, X. Infection microenvironment-activated nanoparticles for NIR-II photoacoustic imaging-guided photothermal/chemodynamic synergistic anti-infective therapy. Biomaterials 2021, 275, 120918. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, X.; Jia, Z.; Huo, D.; Liu, Y.; Liu, J. Cationic chitosan@ Ruthenium dioxide hybrid nanozymes for photothermal therapy enhancing ROS-mediated eradicating multidrug resistant bacterial infection. Journal of Colloid and Interface Science 2021, 603, 615–632. [Google Scholar] [CrossRef]
- Lin, X.; Fang, Y.; Hao, Z.; Wu, H.; Zhao, M.; Wang, S.; Liu, Y. Bacteria-Triggered Multifunctional Hydrogel for Localized Chemodynamic and Low-Temperature Photothermal Sterilization. Small 2021, 17, 2103303. [Google Scholar] [CrossRef]
- Li, J.; Yi, W.; Luo, Y.; Yang, K.; He, L.; Xu, C.; Deng, L.; He, D. GSH-depleting and H2O2-self-supplying hybrid nanozymes for intensive catalytic antibacterial therapy by photothermal-augmented co-catalysis. Acta Biomaterialia 2023, 155, 588–600. [Google Scholar] [CrossRef]
- Guo, N.; Xia, Y.; Duan, Y.; Wu, Q.; Xiao, L.; Shi, Y.; Yang, B.; Liu, Y. Self-enhanced photothermal-chemodynamic antibacterial agents for synergistic anti-infective therapy. Chinese Chemical Letters 2023, 34, 107542. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, L.; Luo, Y.; Cai, Z.; Zeng, H.; Wang, T.; Liu, Z.; Chen, Y.; Sheng, X.; Mandlate, A.E.d.G. Charge-Driven Self-Assembled Microspheres Hydrogel Scaffolds for Combined Drug Delivery and Photothermal Therapy of Diabetic Wounds. Advanced Functional Materials 2023, 2214036. [Google Scholar] [CrossRef]
- Sun, J.; Liu, C.; Wang, Q.; Yang, H.; Gao, X.; Deng, Y.; Yang, W.; He, M. Bimetal-Doped Nanosheet with Phototherapeutic Potential for Clearance of Bacterial Infection. Materials Letters 2023, 133884. [Google Scholar] [CrossRef]
- Huo, J.; Jia, Q.; Huang, H.; Zhang, J.; Li, P.; Dong, X.; Huang, W. Emerging photothermal-derived multimodal synergistic therapy in combating bacterial infections. Chemical Society Reviews 2021, 50, 8762–8789. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Liu, Q.; Gao, A.; Tang, N.; Zhang, Q.; Zhang, A.; Cui, D. Recent developments of sonodynamic therapy in antibacterial application. Nanoscale 2022. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Bai, Q.; Liang, M.; Yang, D.; Li, S.; Wang, L.; Liu, J.; Yu, W.W.; Sui, N.; Zhu, Z. Silver peroxide nanoparticles for combined antibacterial sonodynamic and photothermal therapy. Small 2022, 18, 2104160. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Hu, S.; Du, X.; Wen, Q.; Zhong, X.-P.; Zhou, X.; Zhou, C.; Xiong, W.; Gao, Y.; Zhang, S. Vitamin B5 reduces bacterial growth via regulating innate immunity and adaptive immunity in mice infected with Mycobacterium tuberculosis. Frontiers in immunology 2018, 365. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Wang, J.; Yin, C.; Zhang, P.; Zhang, J.; Shi, M.; Shen, K.; Xiao, Y.; Zhao, Y.; Yang, X. Near-infrared light-sensitive nano neuro-immune blocker capsule relieves pain and enhances the innate immune response for necrotizing infection. Nano letters 2019, 19, 5904–5914. [Google Scholar] [CrossRef]
- Li, Y.; Xu, X.; Liu, X.; Li, B.; Han, Y.; Zheng, Y.; Chen, D.f.; Yeung, K.W.K.; Cui, Z.; Li, Z. Photoelectrons mediating angiogenesis and immunotherapy through heterojunction film for noninvasive disinfection. Advanced science 2020, 7, 2000023. [Google Scholar] [CrossRef]
- Tian, Y.; Li, Y.; Liu, J.; Lin, Y.; Jiao, J.; Chen, B.; Wang, W.; Wu, S.; Li, C. Photothermal therapy with regulated Nrf2/NF-κB signaling pathway for treating bacteria-induced periodontitis. Bioactive Materials 2022, 9, 428–445. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, X.; Zhang, D. Electrospun chitosan/poly (vinyl alcohol)/graphene oxide nanofibrous membrane with ciprofloxacin antibiotic drug for potential wound dressing application. International journal of molecular sciences 2019, 20, 4395. [Google Scholar] [CrossRef]
- Ali, N.H.; Amin, M.C.I.M.; Ng, S.-F. Sodium carboxymethyl cellulose hydrogels containing reduced graphene oxide (rGO) as a functional antibiofilm wound dressing. Journal of Biomaterials Science, Polymer Edition 2019, 30, 629–645. [Google Scholar] [CrossRef]
- Sadat, Z.; Farrokhi-Hajiabad, F.; Lalebeigi, F.; Naderi, N.; Gorab, M.G.; Cohan, R.A.; Eivazzadeh-Keihan, R.; Maleki, A. A comprehensive review on the applications of carbon-based nanostructures in wound healing: from antibacterial aspects to cell growth stimulation. Biomaterials Science 2022, 10, 6911–6938. [Google Scholar] [CrossRef]
- He, Y.; Li, N.; Yang, S.; Tan, X.; Tang, L.; Yang, Q. Near-Infrared Molecular Photosensitizer Decorated with Quaternary Ammonium for High-Efficiency Photothermal Treatment of Bacterial Infections. Chemosensors 2023, 11, 164. [Google Scholar] [CrossRef]
- Tian, H.; Hong, J.; Li, C.; Qiu, Y.; Li, M.; Qin, Z.; Ghiladi, R.A.; Yin, X. Electrospinning membranes with Au@ carbon dots: Low toxicity and efficient antibacterial photothermal therapy. Biomaterials Advances 2022, 142, 213155. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Xu, J.; Zhang, K.; Yao, H.; Zheng, N.; Zheng, L.; Wang, J.; Wei, K.; Xiao, X.; Qin, L. Dynamic and cell-infiltratable hydrogels as injectable carrier of therapeutic cells and drugs for treating challenging bone defects. ACS central science 2019, 5, 440–450. [Google Scholar] [CrossRef]
- Dolinski, N.D.; Page, Z.A.; Callaway, E.B.; Eisenreich, F.; Garcia, R.V.; Chavez, R.; Bothman, D.P.; Hecht, S.; Zok, F.W.; Hawker, C.J. Solution mask liquid lithography (SMaLL) for one-step, multimaterial 3D printing. Advanced Materials 2018, 30, 1800364. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Yu, Y.; Yang, C.; Wu, D.; Zhao, Y. Multifunctional GO hybrid hydrogel scaffolds for wound healing. Research 2022, 2022. [Google Scholar] [CrossRef]
- Federico, S.; Catania, V.; Palumbo, F.S.; Fiorica, C.; Schillaci, D.; Pitarresi, G.; Giammona, G. Photothermal nanofibrillar membrane based on hyaluronic acid and graphene oxide to treat Staphylococcus aureus and Pseudomonas aeruginosa infected wounds. International Journal of Biological Macromolecules 2022, 214, 470–479. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, S.; Chen, C.; Zhang, D. A graphene hybrid supramolecular hydrogel with high stretchability, self-healable and photothermally responsive properties for wound healing. RSC advances 2021, 11, 6367–6373. [Google Scholar] [CrossRef]
- Rosselle, L.; Cantelmo, A.R.; Barras, A.; Skandrani, N.; Pastore, M.; Aydin, D.; Chambre, L.; Sanyal, R.; Sanyal, A.; Boukherroub, R. An ‘on-demand’photothermal antibiotic release cryogel patch: evaluation of efficacy on an ex vivo model for skin wound infection. Biomaterials Science 2020, 8, 5911–5919. [Google Scholar] [CrossRef]
- Mei, L.; Gao, X.; Shi, Y.; Cheng, C.; Shi, Z.; Jiao, M.; Cao, F.; Xu, Z.; Li, X.; Zhang, J. Augmented graphene quantum dot-light irradiation therapy for bacteria-infected wounds. ACS Applied Materials & Interfaces 2020, 12, 40153–40162. [Google Scholar]
- Sun, Z.; Song, C.; Zhou, J.; Hao, C.; Liu, W.; Liu, H.; Wang, J.; Huang, M.; He, S.; Yang, M. Rapid photothermal responsive conductive MXene nanocomposite hydrogels for soft manipulators and sensitive strain sensors. Macromolecular Rapid Communications 2021, 42, 2100499. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, W.; Zhao, L.; Yan, D.; Li, X.; Gao, Q.; Zheng, J.; Zhou, S.; Lai, S.; Feng, Y. Multifunctional AIE nanosphere-based “Nanobomb” for trimodal imaging-guided Photothermal/Photodynamic/Pharmacological therapy of drug-resistant bacterial infections. ACS nano 2023, 17, 4601–4618. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





