1. Introduction
At present, continuous studies of nanocomposites, nanomaterials based on graphene oxide (GO) modified with cellulose, nanocellulose, including carboxymethylcellulose, make it possible to solve many problematic issues in the field of nanotechnology, information technology, medicine, and high-dielectric materials of nanoelectronics. GO can be obtained by oxidation and exfoliation of graphite using the standard Hummers method, as a result of which hydroxyl, carboxyl and epoxy groups are formed on the surface, increasing hydrophilic character of GO and further expansion of its possible functionalization, therefore, expanding its scope of practical application as a sensing element in various sensors, in biocomposite materials, also proposed for drug delivery and other biomedical applications [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19].
In the chemical structure of carboxymethyl cellulose (CMC), like GO, there are functional oxygen-containing groups, which polymerizes well with graphene oxide, which contributes to a change in the physico-mechanical, physico-chemical properties, electrical and structural characteristics. In this regard, the preparation of nanocomposites of GO modified with CMC attracts the interest of scientists and expands its field of practical application due to the excellent physical and chemical properties. The CMC is a common semi-synthetic natural polymer derived from cellulose, which is an anionic linear biopolymer in which the hydroxyl groups of cellulose are partially replaced by carboxymethyl groups [
20,
21,
22]. Along with other biopolymers, CMC has attractive properties such as water solubility, non-toxicity, hydrophilicity, film-forming ability, bioadhesiveness, pH sensitivity, non-toxicity, low immunogenicity and gel-forming properties, which are used in areas like coating liquids, binders, textiles, paper, food, drug delivery systems and cosmetics [
1,
23,
24,
25,
26]. However, biocomposites based on CMC have poor mechanical properties and low thermal stability compared to common polymers, which makes them almost impossible for practical applications. Recently, scientists have been diligently engaged in the research and production of a composite material based on GO/CMC. For example, [
27] CMC/graphene oxide nanocomposite hydrogel beads have been investigated as a drug delivery system that is highly dependent on pH. Also, [
1] researchers investigated the OG/CMC nanocomposite for dressing materials. In recent study [
28], by combining GO with CMC, a three-dimensional composite airgel was obtained to achieve additional advantages and synergistic effects. The reusable carboxymethyl cellulose/graphene oxide composite airgel with a large surface area was made by research team for methylene blue adsorption [
29].
Due to the multifunctional properties of GO and its possible functionalization with various biopolymers, and also due to the presence of functional oxygen-containing groups, it provides a huge opportunity for surface modification. In this regard, the modification of GO with CMC contributes to the production of a biocomposite membrane of a certain thickness with uniform dispersibility and a relatively flat surface with improved mechanical properties [
30,
31,
32]. Therefore, in this work, GO was modified with CMC using ultrasonic treatment and its physicochemical properties were studied.
The most important part in the production of GO and GO/CMC is the correct choice of the initial raw material, which in turn affects the quality of GO/CMC, as well as the cost of the obtained product. To obtain GO, synthetic graphite is mainly used, which is synthesized from graphitized carbon as a by-product of oil at a temperature of 3000 °C [
12,
13], which, in turn, is very expensive. The wide-scale application of commercial graphite in many industries, as well as in the battery industry, contributes to an increase in the price and demand for the product and other supply problems. Therefore, one of the best ways to solve the problem of using graphite as a raw material for GO and GO/CMC is to use natural graphite from local deposits of the Republic of Kazakhstan. Based on the above, the main goal and novelty of this work is the use of local Kazakh graphite “Ognevsky” as a raw material for the production of GO and GO/CMC and the study of their physicochemical properties. The location of the raw material of graphite is in the Ognevsk ore field (Ognevka mine), Kalbinsk ridge, Ulan district, East Kazakhstan region.
2. Experimental section
2.1. Materials
In this study next materials were used: potassium permanganate (KMnO4, 99%), sodium nitrate (NaNO3, 99%), hydrochloric acid (HCl, 35%), graphite was obtained from a Ognevsk ore field (Ognevka mine) located in Ulan district, East Kazakhstan region, Hydrogen peroxide (H2O2, 31%), glacial acetic acid (CH3COOH, ≥85%), ethanol (C2H5OH, 96%), Sulfuric acid (H2SO4, 98%), sodium hydroxide NaOH, ≥99%), trichloroacetic acid (Cl3CHCOOH, 99%) were obtained from Alita LLP (Kazakhstan) and deionized water. All other reagents were of analytical grade and were used without additional purification.
2.2. Methods
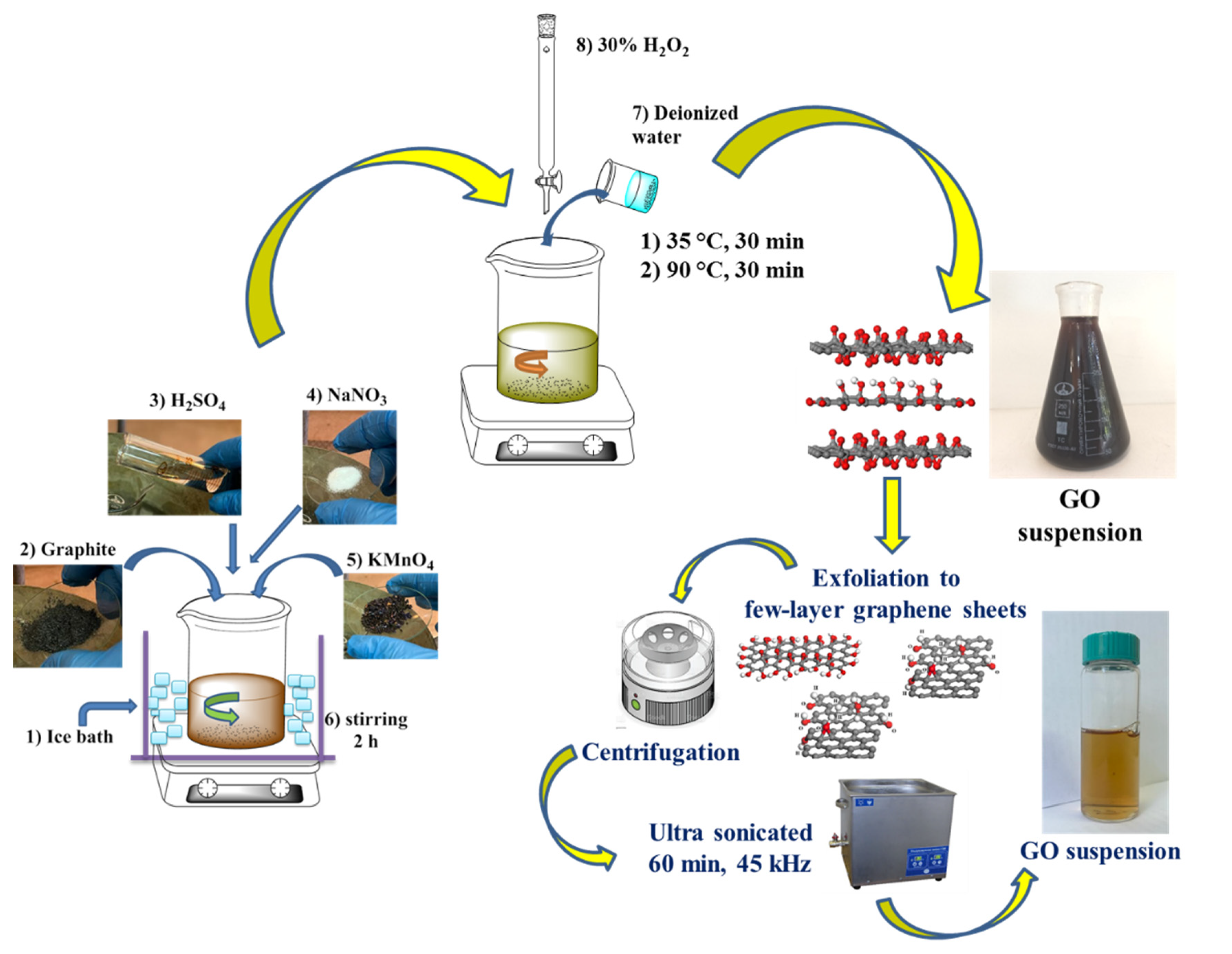
2.2.1. Synthesis of GO
GO was obtained using the Hummers method, in which the synthesis was a two-stage oxidation of graphite and exfoliation of graphite oxide. Graphite oxidation included a three-phase procedure with low, medium and high temperature reactions, each of them occurred separately in time. In the low-temperature reaction procedure, a beaker with 1 g of graphite was placed in a container filled with ice at a temperature of 0 ºC. Then, keeping the sequence, the following reagents were gradually added: 94% H
2SO
4; NaNO
3 (0.5g); KMnO
4 (3g) after adding each reagent, the mixture was stirred for 15 min and left for 2 hours for continuous stirring on a magnetic stirrer. In order to carry out the medium temperature reaction, the temperature was raised to 35° C, and held for 30 minutes. Then, after adding deionized water, the temperature was raised to 90°C and stirring was continued for 30 minutes. Finally, 30% hydrogen peroxide was added until the color of the mixture changed to bright yellow and gas evolution was detected. The product was then filtered through filter paper and washed several times with 5% hydrochloric acid to remove residual metal ions, and at last was neutralized with deionized water in a centrifuge (Centrifuge 5427R Eppendorf) to a pH of 7 (
Figure 1).
At the stage of delamination of graphite oxide into few-layer graphene sheets, centrifugation and ultrasonic treatment were used, as a result of which graphite oxide having functional oxygen-containing groups was delaminated into multilayer sheets, even few-layer and single-layer sheets of GO were present. The GO suspension was treated with a centrifuge at a rotation speed of 5000 rpm, then processed by ultrasound at a frequency of 45 kHz (UZTA-0.15 / 22-0, Russia) at a temperature of 25 °C for 60 minutes (
Figure 1).
2.2.2. Synthesis of CMC
For the synthesis of CMC, 5 g of cellulose and 100 mL of 95% ethanol and 10 mL of 45% NaOH solution were poured into a flask and mixed with a magnetic stirrer at a speed of 750 rpm at room temperature for 60 min. After that, 5 mL of trichloroacetic acid was added to the mixture, stirring in a water bath at a temperature of 600°C for 60 minutes, heating and cooling to room temperature, it was neutralized with glacial acetic acid until the pH was 6-7. The prepared mixture was filtered with filter paper and washed with 500 mL of 80% ethyl alcohol in a soxhlet for 3 hours. The prepared CMC was dried at room temperature.
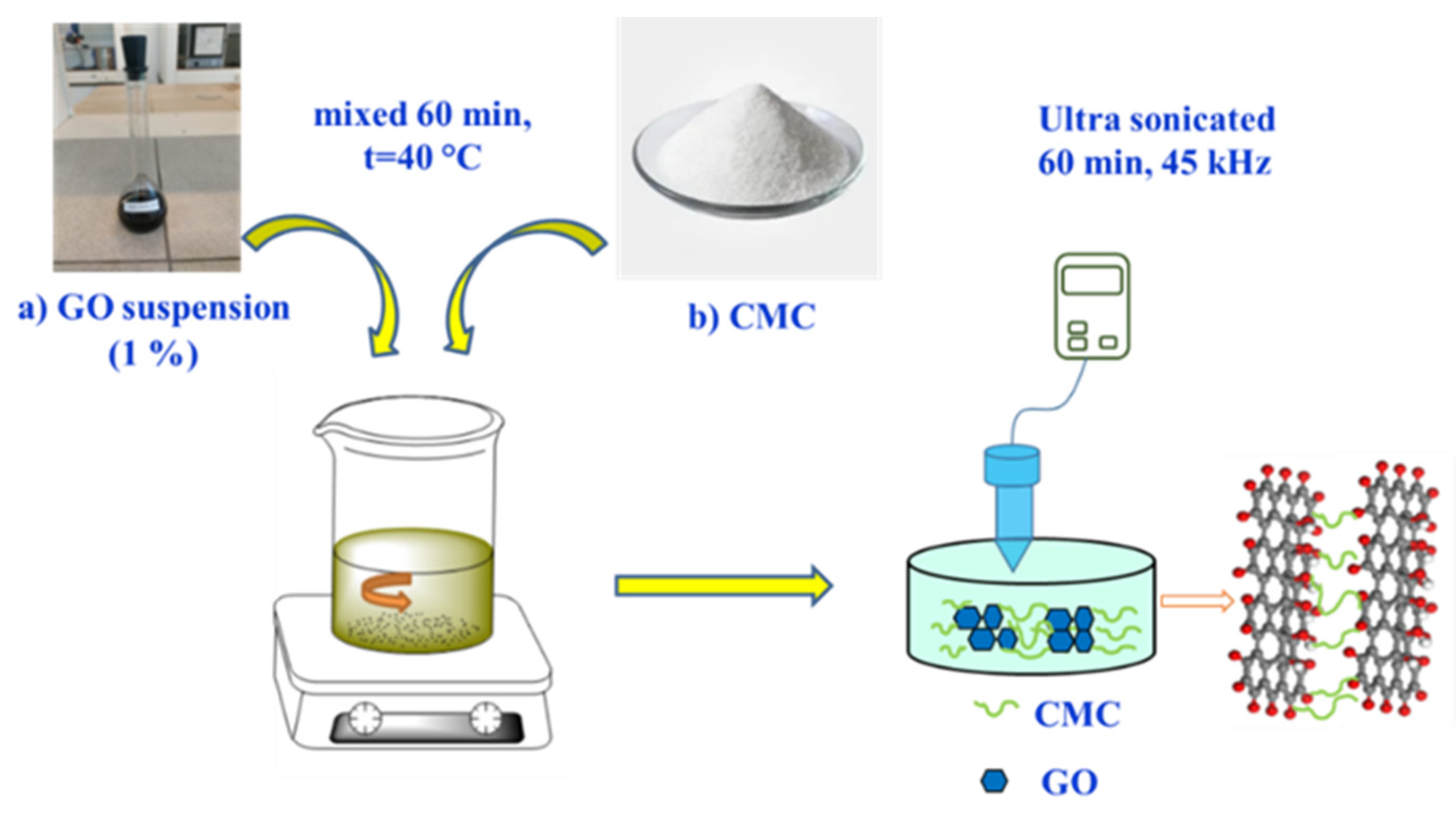
2.2.3. Synthesis of composite membrane based on GO/CMC
In order to obtain a GO/CMC membrane, 1% GO was continuously stirred for an hour with a crushed solid mass of CMC (0.03 g; 0.06 g; 0.15 g) on a magnetic stirrer (
Figure 2). The GO/CMC suspensions were treated at 800 rpm by magnetic stirrer (ES-6120 with heated, Russia) in a temperature of 40 °C for 60 minutes. Then suspensions were processed by ultrasound at a frequency of 45 kHz (UZTA-0.15/22-0, Russia) at a temperature of 30 °C for 60 minutes.
2.2.4. SEM analysis
The surface morphology of GO/CMC membranes were studied by scanning electron microscope JSM-6390LV (Jeol, Japan). Measurements were carried out in a high vacuum mode using a secondary electron detector at an accelerating voltage of 15 kV. The surface of the nano starch film was coated with gold to improve the transfer of electrons. The specimens were mounted on aluminum pins with carbon tape.
2.2.5. FTIR analysis
FTIR analysis of chemical structures of GO and GO/CMC membranes were performed on a spectrometer FT-801 (Simex, Russia), with resolution 1 cm-1. Measurements were conducted in the region between 450–4700 cm-1 according to the standard method using a single-use universal full internal and mirror-diffuse reflection with the upper position of the model, at a temperature 25 ºC.
2.2.6. X-ray diffraction
The crystal structures of GO and GO/CMC membranes were studied via X-ray diffraction on a X’PertPROdiffractometer (Malvern Panalytical Empyrean, Netherlands) using monochromatized copper (CuKα), at a scan speed of 0.05º for 10 s, with a K-Alpha1 wavelength of 1.54187 Å. The measurement in reflection mode, using an aluminium rectangular multi-purpose sample holder (PW1172/01), was performed at a diffraction angle 2θ between 10° and 40°, with the X-ray tube voltage at 45 kV, current intensity at 30 mA, and a measurement time of each step 0.5 s.
2.2.7. Electrical Characterization
The electrical properties of GO and GO/CMC membranes were investigated by four-point probes method. Electrical measurements were made using the traditional four-probe method, with current measured on a Keithley 6485 (USA) and voltage applied with a Tektronix PWS2326.
3. Results and discussion
3.2.1. SEM analysis
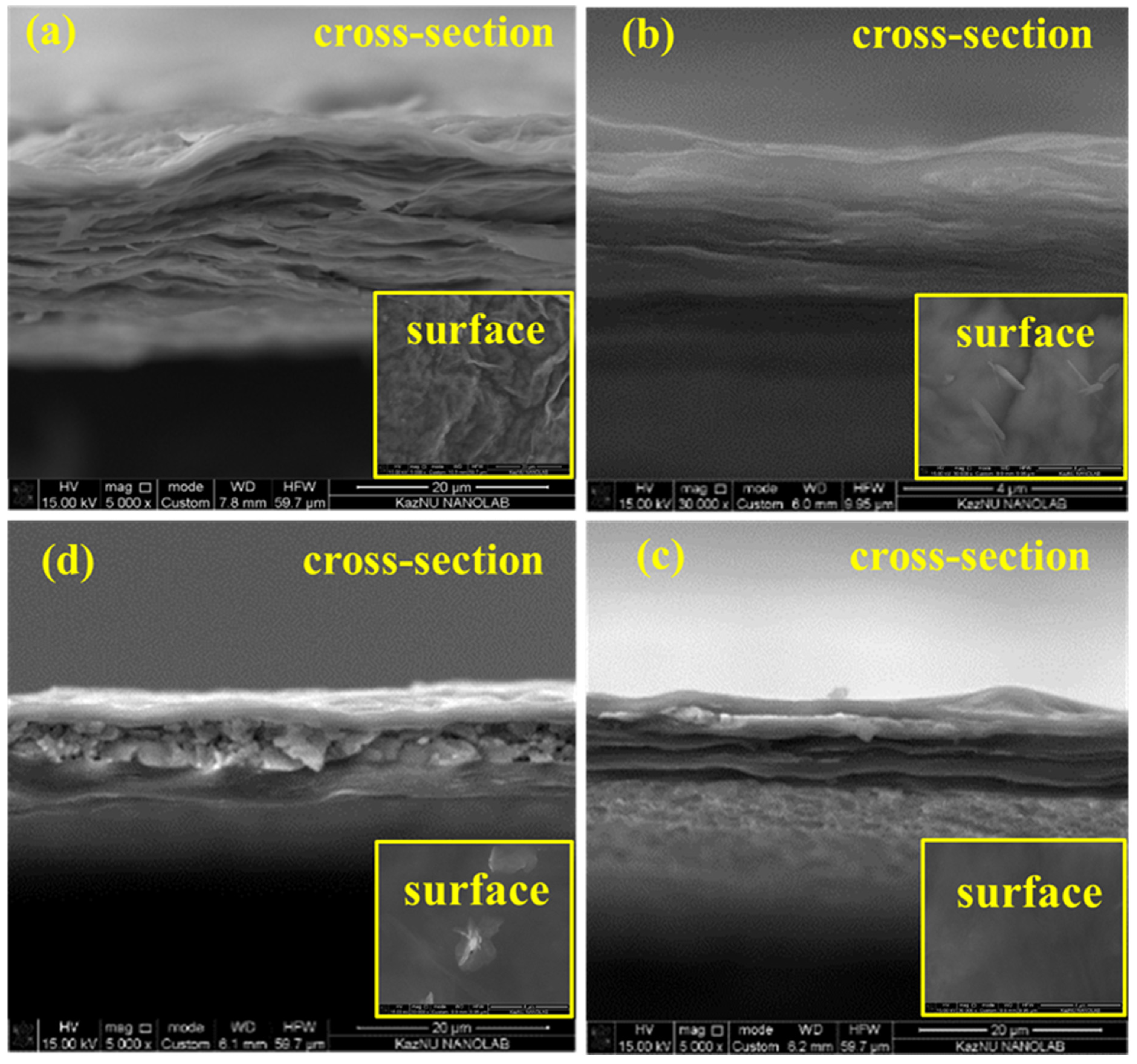
The obtained SEM results of GO/CMC (0.03 g; 0.06 g; 0.15 g) demonstrated that CMC particles are effectively dispersed in the GO matrix, almost in all samples (
Figure 3). In some images of OG/CMC (0.03 g; 0.06 g; 0.15 g) individual CMC clusters can be seen depending on the mass of added CMC particles of composite membranes. In general, the surface morphology in comparison with the initial GO in all GO/CMC samples is smooth, only in single samples with an increase in the mass of added CMC, small particles of CMC and slight wrinkles on the surfaces are observed.
According to the obtained cross section SEM images of the GO/CMC, depending on the mass of the added CMC particles, all composite membranes have compact lamellar structures compared to the original GO membrane, only some samples have slight coarse structures (
Figure 3 (a, b, c, d)). In addition, according to literature data, the formation of a compact lamellar structure and a stable bond to impart a certain mechanical strength between GO/CMC is promoted by the presence of a large number of carboxymethyl groups in CMC and various functional oxygen-containing groups in GO, as well as hydrogen bonds between composite membranes [
16,
33,
34,
35,
36].
3.2.2. X-ray diffraction
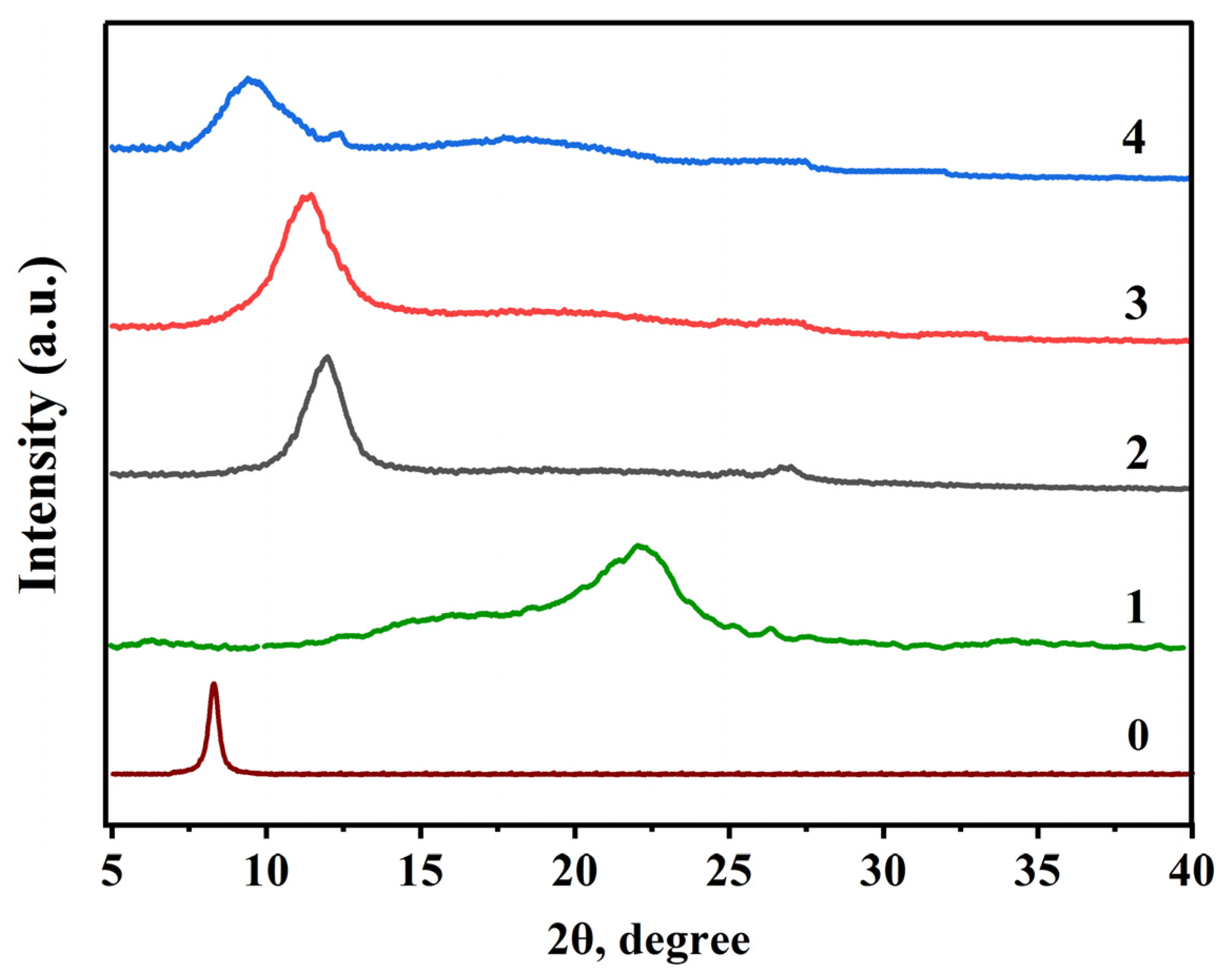
The X-ray phase analysis of the obtained membrane from a liquid suspension of GO with 1% concentration and GO/CMC membranes, was carried out in order to assess their crystallinity (0.03 g; 0.06 g; 0.15 g) (
Figure 4). According to the obtained XRD spectrum of GO and GO/CMC the following peaks were found: 2θ=7.06° (GO); 2θ=11.97° (GO/CMC-0.03 g); 2θ=11.22° (GO/CMC-0.06 g) and 2θ=9.45° (GO/CMC-0.15 g).
The intense peak characteristic of GO was equal to 2θ=7.06° (002), inter-layer distance d= 10.2 Å (
Figure 4 (0)), due to the presence of oxygen-containing functional groups attached to both sides of the graphene sheet, and atomic scale roughness arising from structural defects (sp
3 bond) formed on the initially atomically flat graphene sheet. In the diffractogram of CMC, one peak characteristic of amorphous carbon was recorded at 2θ=22.2° (
Figure 4(1)) [
37], which indicates that the hydrogen bonds in the cellulose molecule were completely broken during the synthesis of CMC, and the diffractogram of the obtained material corresponds to CMC [
38,
39].
According to the results of XRD patterns, compared with the initial GO, after the addition of CMC with a mass of 0.03 g, a shift of 2θ from 7.06° to 11.97° is observed, then with an increase in the added mass of CMC (0.03 g; 0.06 g; 0.15 g), a shift in peak positions is observed GO/CMC from 11.97° towards lower values of 2θ = 11.22° and 9.45° respectively. This is explained by the fact that due to the hydrogen bond formed between GO and CMC, the space between GO layers (sheet graphene) is saturated with CMC molecules, disrupting the order of arrangement of graphene layers (sheet graphene), resulting in a shift of the diffraction peak to the right and an increase in the peak area [
40,
41]. The existence of a hydrogen bond between GO and SMS is confirmed by the IR spectrum. Also, the reason for the shift of 2θ value in GO/CMC 0.06 g and 0.15 g samples to the initial GO value is explained by the fact that as the mass of CMC increases, the hydrogen bond between GO and CMC becomes more saturated and has a negative effect on the bond strength [
42]. The obtained results are consistent with the results of the study [
34,
41,
43,
44,
45,
46].
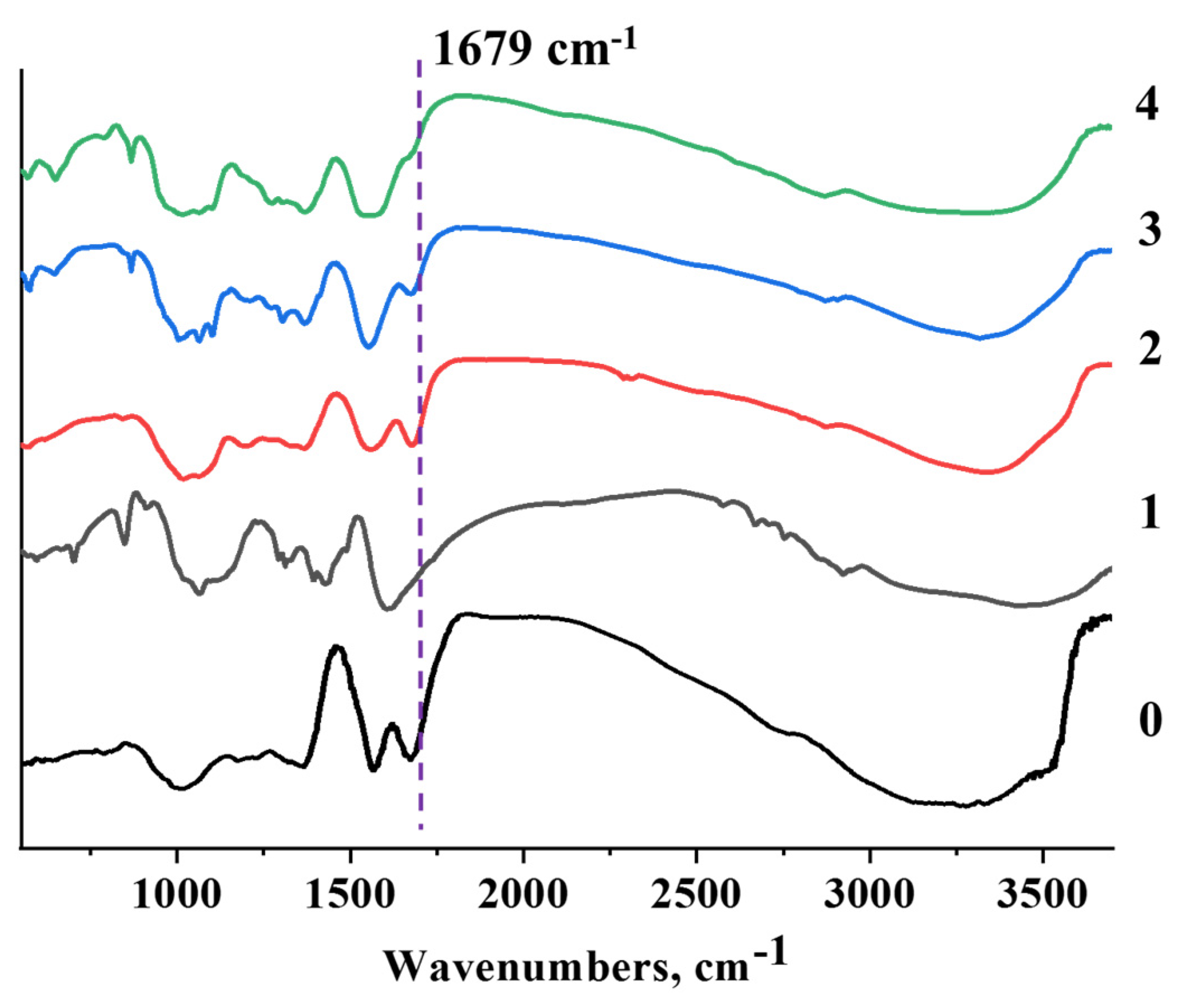
3.2.3. FTIR analysis
According to the result of IR spectroscopy of GO, the following signals can be seen in the absorption region: 3600–3000 cm
-1 O-H, aromatic ring 1585 cm
-1 C=C, epoxy functional groups 1249 cm
-1 C-O, alkoxy groups 1054 cm
−1 C–O (
Figure 5 (0)) [
10].
According to the IR spectrum, CMC are observed at wavelengths hydroxyl group (stretching –OH) at 3200–3600 cm
–1, C–H stretching vibration at 3000 cm
–1, carbonyl group (stretching C=O) at 1680 cm
–1, hydrocarbon groups (cleavage –CH
2) at 1450 cm
–1 and ester groups (stretch –O–) at 1000–1200 cm
–1 (
Figure 5 (1)) [
34,
36,
37,
38,
39,
40,
41,
42,
43,
44].
Figure 5 (2; 3; 4) shows the IR spectrum of the GO membrane with CMC [
34,
35,
36,
37,
38,
39,
40,
41,
42,
43,
44,
45]. The results of IR spectroscopy of the GO membrane with CMC show the following signals in the wavelength range: 3600–3000 cm
-1 O-H, aromatic ring 1585 cm
-1 C=C, epoxy functional groups 1249 cm
-1 C-O, alkoxy groups 1054 cm
−1 C–O (
Figure 5 (2; 3; 4)). At wavelength, an decrease of intensity at a wavelength of 1679 cm
-1 denotes an ether bond (O=C-O) between hydroxyl groups (-OH) from GO and carboxyl groups from CMC. In the IR spectra, new ether bonds –C=O were found at a wavelength of 1679 cm
-1.
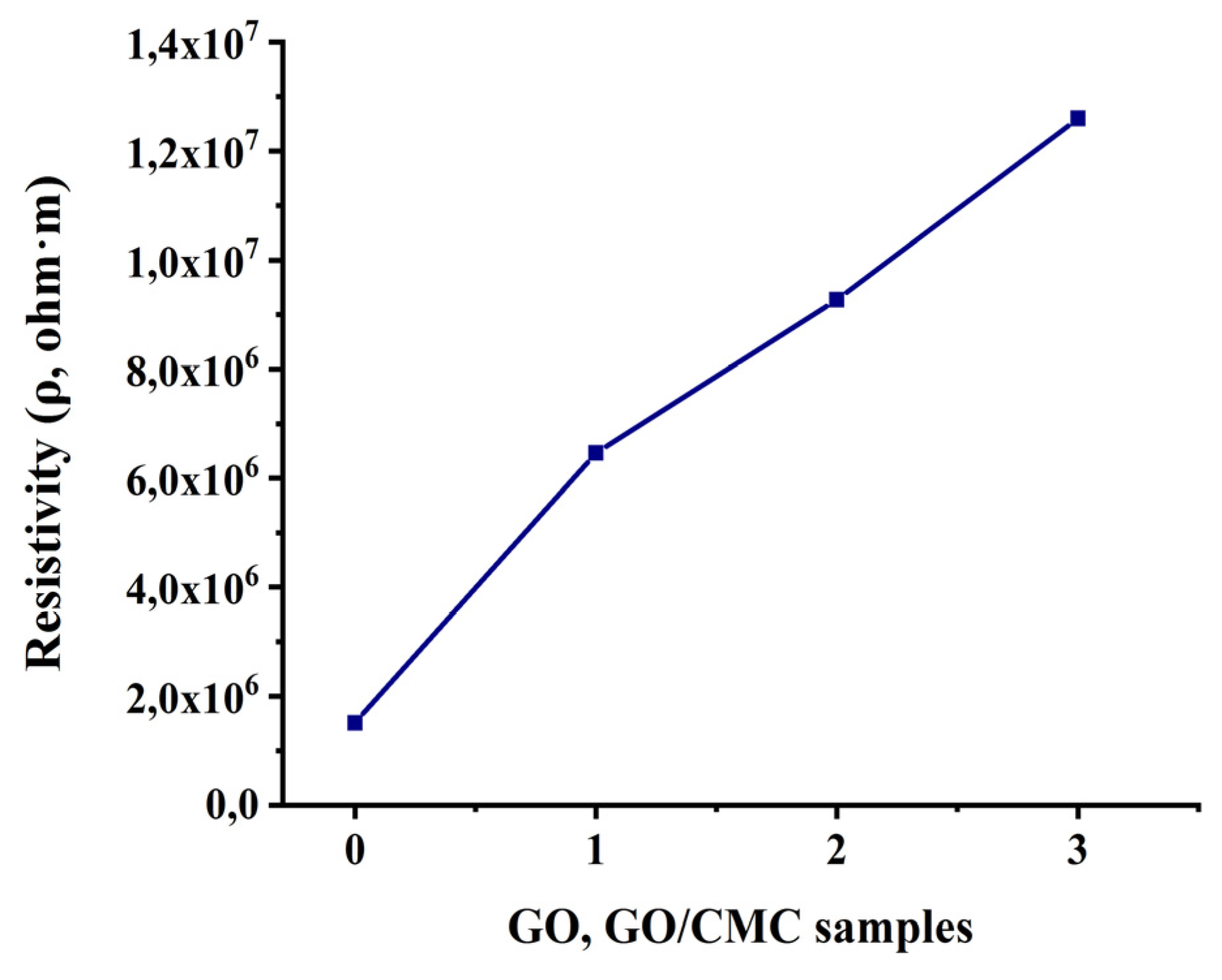
3.2.5. Electrical Characterization
The electrical characterization of the GO and GO/CMC membrane was carried out using Keithley picoammeter (Model 6485) by four-point probes method. Resistivity of the GO/CMC membrane increases from 1,51•10
6 to 1.26•10
7 Ohm•m compared to the initial GO with an increasing in amount of CMC (0.03 g; 0.06 g; 0.15 g) (
Figure 6 (0; 1; 2; 3)).
The value of the resistivity of the initial GO was equal to 1,51•10
6, and for the GO/CMC sample (0.03 g) it showed the following value 6.46
·10
6 (
Figure 6 (1)). On the following GO samples of added CMC solid masses (0.06 g; 0.15 g) there is an increase from 9,27 ·10
6 to 1,26 ·10
7 (
Figure 6 (2; 3)).
In general, according to the obtained results of the specific resistance of the GO of the added CMC particles (0.03 g; 0.06 g; 0.15 g) it is observed an increasing in resistivity compared to the initial sample. According to researches, an increasing in the permittivity of the composite with GO is associated with interfacial polarization that occurs at the CMC-GO interface [
47,
48]. Moreover, the increase in the dielectric constant was affected by the presence of polar functional groups of GO, such as –OH, –CHO, –CO, –COOH providing good adhesion and corresponding local electrical contacts that can lead to strong interfacial polarization. In the case of a pure GO sample, this polarization can be due to the immiscible phase of the polymer matrix. Spatial polarization due to trap states leads to the accumulation of charge carriers and to an increase in the value of the permittivity [
47,
49].
4. Conclusion
Composite GO/CMC membranes by adding solid mass of CMC were obtained (0.03 g; 0.06 g; 0.15 g), on 1 % of GO suspension. For the production of GO and its membrane with carboxymethylcellulose (CMC), local Kazakhstan “Ognevsky” graphite was used as the initial raw material. SEM images of the surface and side section of GO/CMC showed that the obtained composite membranes have a smooth surface and a compact lamellar structure compared to the initial GO membrane, and only in some samples small particles of CMC were found on the surfaces, as well as a slightly rough structure according to side section images. Analysis of XRD spectra showed that after the intercalation of solid masses of CMC (0.03 g; 0.06 g; 0.15 g) to the sheet layers GO, peaks were detected 2θ=34,92º (GO/CMC) that confirms crystal planes (041) CMC. In the IR spectra after the modification of GO with CMC, new ether bonds –C=O were found at a 1679 cm-1 wavelength. Resistivity of composite membrane GO/CMC with increasing CMC mass (0,03 g; 0,06 g; 0,15 g), which were added to the GO increased from 1,51•106 to 1,26•107 Ohm•m compared to the initial GO. These changes in resistivity values are presumably related to the interfacial polarization that occurs at the CMC-GO interface, and it shows that obtained membrane can be a composite material, which has dielectric properties.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by TK, ZhS, EZh, KA, BK, NG, ZhT, NK and BK. The first draft of the manuscript was written by TK and all authors commented on the previous versions of the manuscript. All authors read and approved the final manuscript.
Acknowledgments
This research has been funded by the Science Committee of the Ministry of Science and Higher Education of the Republic of Kazakhstan within the framework of Grant No. AP09058548 «Development of a sensitive humidity sensor based on a graphene oxide membrane obtained from activated carbon for military needs» (2021–2023).
Conflicts of Interest
The authors declare no conflict of interest.
References
- L.S. Maria, Graphene Oxide Carboxymethylcellulose Nanocomposite for Dressing Materials. J. Materials. 13(8) (2020) 1980. [CrossRef]
- A. Maio, D. Giallombardo, R. Scaaro, P.A. Palumbo, I. Pibiri: Synthesis of a fluorinated grapheme oxide–silica nanohybrid: Improving oxygen a_nity. J. RSC Adv. 6(52) (2016). 46037–46047.
- Z.A. Mansurov, J.M. Jandosov, A.R. Kerimkulova, S. Azat, A.A. Zhubanova, I.E. Digel, I.S. Savitskaya, N.S. Akimbekov, A.S. Kistaubaeva, Nanostructured carbon materials for biomedical use. J. Eurasian chemico-tecnological. 15(3) 2(2013) 09-217. [CrossRef]
- S. Azat, B. Rosa, V.V. Pavlenko, A.R. Kerimkulova, L.D. Raymond, Z.A. Mansurov, Applications of activated carbon sorbents based on greek walnut. J Applied Mechanics and Materials. 467 (2014) 49-51. [CrossRef]
- S. Azat, E. Arkhangelsky, T. Papathanasiou, A.A. Zorpas, A. Abirov, V.J. Inglezakis, Synthesis of biosourced silica-Ag nanocomposites and amalgamation reaction with mercury in aqueous solutions. J. Comptes Rendus Chimie. 23(1) (2020) 77–92. [CrossRef]
- T.K. Kuanyshbekov, A.M.Ilyin, G.W. Beall, N.R. Guseinov, Creation of a Humidity Sensor Based on Functionalized Graphene Nanostructures. J. Sensors & Transducers. 229 (2019) 39-46.
- A.M. Ilyin, N.R. Guseinov, T.K. Kuanyshbekov, G.W. Beall, M.A. Tulegenova, Computer Simulation and First Principles Study of Ga-Doped Graphene Nanostructures. Journal of Computational and Theoretical Nanoscience. 16 (2019) 39-41. [CrossRef]
- M.A. Tulegenova, A.M. Ilyin, N.R. Guseinov, T.K. Kuanyshbekov, , Beall, G.W., Computer Simulation of the Effect of Structural Defects on the Effectiveness of the Graphene’s Protective Properties. Journal of Computational and Theoretical Nanoscience. 16 (2019) 351–354. [CrossRef]
- T.K. Kuanyshbekov, K. Akatan, S.K. Kabdrakhmanova, R. Nemkaeva, M. Aitzhanov, A. Imasheva, E. Kairatuly, Synthesis of Graphene Oxide from Graphite by the Hummers Method. J. Oxidation Communications. 44(2) (2021) 356–365.
- T. Kuanyshbekov, S. Kabdrakhmanova, A. Imasheva, A. Battalova, R. Abylkalykova, A. Nasyrova, Z. Ibraeva, Synthesis of nanocomposite material through modification of graphene oxide by nanocellulose. J. Chemical Bulletin of Kazakh National University. 102(3) (2021) 14-20. [CrossRef]
- K. Akatan, S. Kabdrakhmanova, T. Kuanyshbekov, A. Battalova, K.S. Joshy, S. Thomas, Highly-efficient isolation of microcrystalline cellulose and nanocellulose from sunflower seed waste via environmentally benign method. J. Cellulose. 29(7) (2022) 3787–3802. [CrossRef]
- A. Maio, R. Scaaro, L. Lentini, Palumbo, A. Piccionello, I. Pibiri, Perfluorocarbons–graphene oxide nanoplatforms as biocompatible oxygen reservoirs. J. Anal Chim Acta. 1037 (2018) 1–380. [CrossRef]
- R. Scaaro, F. Lopresti, A. Maio, L.Botta, S. Rigogliuso, G. Ghersi, Electrospun PCL/GO-g-PEG structures: Processing-morphology-properties relationships. J. Compos Part S Appl Sci Manuf. 92 (2017) 97–107. [CrossRef]
- R. Scaaro, A. Maio, F. Lo Presti, D. Giallombardo, L. Botta, M.L. Bondì, S. Agnello, Synthesis and self-assembly of a PEGylated-graphene aerogel. J. Compos Sci Technol. 128 (2016) 193–200. [CrossRef]
- Y. Wang, Z. Li, J. Wang, J. Li, Y. Lin, Graphene and graphene oxide: Biofunctionalization and applications in biotechnology. J. Trends Biotechnol. 29 (2011) 205–212. [CrossRef]
- H. Zhang, D. Zhai, Y. He, Graphene oxide/polyacrylamide/carboxymethyl cellulose sodium nanocomposite hydrogel with enhanced mechanical strength: Preparation, characterization and the swelling behavior. J. RSC Adv. 4 (2014) 44600–44609. [CrossRef]
- X. Li, F. Li, Z. Gao, L. Fang, Toxicology of graphene oxide nanosheets against paecilomyces catenlannulatus. J. Bull Environ Contam Toxicol. 95 (2015) 25–30. [CrossRef]
- S. Liu, T.H. Zeng, M. Hofmann, E. Burcombe, J. Wei, , R.Jiang, , Kong, J., Chen, Y.: Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: Membrane and oxidative stress. J. ACS Nano. 5 (2011) 6971–6980. [CrossRef]
- Z. Song, Y. Xu, W. Yang, L. Cui, J. Zhang, J. Liu, Graphene/tri-block copolymer composites prepared via RAFT polymerizations for dual controlled drug delivery via pH stimulation and biodegradation. J. Eur Polym. 69 (2015) 559–572. [CrossRef]
- Jiao Zepeng, Carboxymethyl cellulose-grafted graphene oxide for efficient antitumor drug delivery. J. Nanotechnol Rev. 7(4) (2018) 291–301. [CrossRef]
- Jutamas Ampaiwong.: Reduced Graphene Oxide/Carboxymethyl Cellulose Nanocomposites Novel Conductive Films. J. Nanosci. Nanotechnol. 19(6) (2019) 3544-3550. [CrossRef]
- Son Yeong-Rae, Yop Rhee Kyong., Park Soo-Jin, Influence of reduced graphene oxide on mechanical behaviors of sodium carboxymethyl cellulose. J. Composites Part B: Engineering. 83 (2015) 36-42. [CrossRef]
- J.R. Potts, R.D. Dreyer, C.W. Bielawski, R.S. Ruoff, Graphene-based polymer nanocomposites. J. Polymer. 52(1) (2011) 5-25. [CrossRef]
- G.H. Li, K. Liu, Z.W. Ma, Flexible Solid-State Supercapacitors Based on Carbon Nanoparticles/MnO2 Nanorods Hybrid Structure. J. ACS Nano. 6 (2012) 656–661. [CrossRef]
- F.G. Torres, S. Commeaux, O.P. Troncoso, Starch-based Biomaterials For Wound-dressing Applications. J Starch. 65 (2013) 543-551. [CrossRef]
- S. Javanbakht, A. Shaabani, Carboxymethyl cellulose-based oral delivery systems. J. Biol Macromol. 13 (2019) 21–29. [CrossRef]
- M. Rasoulzadeh, H. Namazi, Carboxymethyl cellulose/graphene oxide bio-nanocomposite hydrogel beads as anticancer drug carrier agent. J. Carbohydr. Polym. 168 (2017) 320–326. [CrossRef]
- X. Ge, Y. Shan, L.Wu, X. Mu, H. Peng, Y. Jiang, High-strength and morphology-controlled aerogel based on carboxymethyl cellulose and graphene oxide. J. Carbohydr Polym. 197 (2018) 277–283. [CrossRef]
- W. Zhu, X. Jiang, K. Jiang, F. Liu, F. You, C. Yao, Fabrication of Reusable Carboxymethyl Cellulose/Graphene Oxide Composite Aerogel with Large Surface Area for Adsorption of Methylene Blue. J. Nanomaterials. 11, (2021) 1609. [CrossRef]
- F. Seidi, H. Salimi, A.A. Shamsabadi, M. Shabanian, Synthesis of hybrid materials using graft copolymerization on non-cellulosic polysaccharides via homogenous ATRP. J. Prog Polym Sci. 76 (2018) 1-39. [CrossRef]
- F. Chivrac, E. Pollet, L. Avérous, Progress in nano-biocomposites based on polysaccharides and nanoclays. J. Mater Sci Eng R Rep. 67 (2009) 1-17. [CrossRef]
- B. Imre, L. García, D. Puglia, F. Vilaplana, Reactive compatibilization of plant polysaccharides and biobased polymers: Review on current strategies, expectations and reality. J. Carbohydr Polym. 209 (2019) 20-37. [CrossRef]
- Chen. Junjun, Li. Hailong, Zhang. Lihui, Du. Chao, Fang. Tao, Hu. Jian, Direct Reduction of Graphene Oxide/Nanofbrillated Cellulose Composite Film and its Electrical Conductivity Research. J. Scientific Reports. 10 (2020) 124. [CrossRef]
- Zhao. Mengke, Zhang. Sufeng, Fang. Guigan, Huang. Chen, Wu. Ting, Directionally-Grown Carboxymethyl Cellulose/Reduced Graphene Oxide Aerogel with Excellent Structure Stability and Adsorption Capacity. J. Polymers. 12 (2020) 2219. [CrossRef]
- D.E. Ciolacu, D.M. Suflet, Cellulose-Based Hydrogels for Medical/Pharmaceutical Applications In Biomass as Renewable Raw Material to Obtain Bioproducts of High-Tech Value. The Netherlands Amsterdam: Elsevier. (2018). [CrossRef]
- H. Yu, H.J. Hong, M. Kim, C. Ko, H.S. Jeong, Mechanically enhanced graphene oxide/carboxymethyl cellulose nanofibril composite fiber as a scalable adsorbent for heavy metal removal. J. Carbohydr. Polym. 240 (2020) 116348. [CrossRef]
- L. Stobinski, B. Lesiak, A. Malolepszy, M. Mazurkiewicz, B. Mierzwa, J. Zemek, P. Jiricek, I. Bieloshapka, Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J. Electron Spectrosc. Relat. Phenom. 195 (2014) 145–154. [CrossRef]
- Adamu Abdulhameed., M. Mbuvi. Harun, O. Changamu. Evans, Francis M. Maingi, Microwave synthesis of Carboxymethylcellulose (CMC) from Rice Husk. IOSR Journal of Applied Chemistry. 12(12) (2019) 33-42.
- B. Kumar, F. Deeba, R. Priyadarshi, Development of novel cross-linked carboxymethyl cellulose/poly(potassium 1-hydroxy acrylate): Synthesis, characterization and properties. J. Polym. Bull. 77 (2020) 4555–4570. [CrossRef]
- A.A. Mekkaoui, H. Orfi, K. Bejtka, Carboxymethyl cellulose nanocolloids anchored Pd(0) nanoparticles (CMC@Pd NPs): Synthesis, characterization, and catalytic application in transfer hydrogenation. Environ Sci Pollut Res (2022). [CrossRef]
- Y. Miao, X. Wang, Y. Liu, Z. Liu, W. Chen, Preparation of Graphene Oxide/Cellulose Composites with Microcrystalline Cellulose Acid Hydrolysis Using the Waste Acids Generated by the Hummers Method of Graphene Oxide Synthesis. J. Polymers. 13 (2021) 4453. [CrossRef]
- X.Y. Wang, K. Wan, P.B. Xie, Y.Y. Miao, Z.B. Liu, Ultralight, High Capacitance, Mechanically Strong Graphene-Cellulose Aerogels. J. Molecules 26 (2021) 4891. [CrossRef]
- Y.Y. Miao, C.Y. Zhang, D.J. Huang, L. Tian, T.J. Zhao, X.Y. Zhai, Z.B. Liu, Preparation of graphene oxide /cellulose composite in mixed acid solution derived from Hummers method. J. For. Eng. 3 (2018) 97–102. [CrossRef]
- V.N. Priya, M. Rajkumar, J. Mobika, Adsorption of As (V) ions from aqueous solution by carboxymethyl cellulose incorporated layered double hydroxide/reduced graphene oxide nanocomposites: Isotherm and kinetic studies. J. Environmental Technology & Innovation. 26 (2022) 102268. [CrossRef]
- Jitendra, Nayak., Anu, Vashishtha, Synthesis, Characterization and Preparation of Nanocellulose and different Cellulose/Nanocellulose/Graphene Composites from Cassava root and its Potential Application for Pesticide Adsorption from water. J. IJRAR. 5(4) (2018) 513-524.
- Marcos, Mariano., Nadia, El Kissi., Alain, Dufresne, Cellulose nanocrystals and related nanocomposites: Review of some properties and challenges. J. Polymer Science Part B: Polymer Physics. 52(12) (2014) 791-806. [CrossRef]
- S. He, G.S. Wang, C. Lu, X. Luo, Wen, B., Guo L., Cao M.S, Enhanced wave absorption of nanocomposites based on the synthesized complex symmetrical CuS nanostructure and poly (vinylidene fluoride). J. Mater Chem A. 1 (2013) 4685-4692. [CrossRef]
- W.L. Song, M.S. Cao, M.M. Lu, J.Liu, J. Yuan, L.Z. Fan, Improved dielectric properties and highly efficient and broadened bandwidth electromagnetic attenuation of thickness-decreased carbon nanosheet/wax composites. J. Mater Chem C. 1 (2013) 1846-1854. [CrossRef]
- J. Zhang, X. Zhao, Conducting polymers directly coated on reduced grapheme oxide sheets as high-performance supercapacitor electrodes. J Phys Chem C. 116(9) (2012) 5420–5426. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).