1. Introduction
Interleukin-31 (IL-31), a possible mediator of itching, induces severe pruritus and dermatitis in mice [
1]. Elevated cutaneous IL-31 expression levels have been observed in lesional skin atopic dermatitis [
2,
3]. Moreover, repeated administration of IL-31 causes itch-associated scratching behavior, which is significantly increased with increased expression of IL-31 receptor A (IL-31RA) in the dorsal root ganglia (DRG) [
4]. The interaction between cutaneous IL-31 and neuronal DRG IL-31RA causes severe itch-associated scratching behavior (long-lasting scratching, LLS) [
5]. Many endogenous pruritogens, such as amines, proteases, growth factors, neuropeptides, and cytokines, are locally pruritogenic when injected into the skin [
6,
7,
8,
9,
10]. However, except for IL-31 or morphine, no other drug treatment [
11].
The central and peripheral nervous systems (CNS and PNS, respectively) are well-known sites of action for analgesia. Morphine, an opioid, induces analgesia by acting on both the CNS and PNS [
12]. It inhibits the release of neurotransmitters from the primary afferent terminals in the spinal cord and activates the descending inhibitory controls in the midbrain [
13,
14]. However, the site of action of morphine in the PNS and its role in regulating pain transmission remain unclear. The administration of morphine increases the scratch counts within 10 min, followed by a return to basal levels approximately 90 min after administration. A close correlation has been observed between time-course changes in morphine-induced LLS counts and antinociceptive activity [
11]. Moreover, IL-31 causes characteristic LLS upon administration [
5]. In IL-31RA deficient (
IL-31 receptor AtLacZ/+ knock-in) mice, the administration of morphine results in the disappearance of LLS and the partial disappearance of antinociceptive activity [
11]. Additionally, recent findings have indicated that IL-31 is partially involved in the peripheral analgesic mechanism. As IL-31 and IL-31RA are not expressed in the CNS, the endogenous opioid system is activated under pathological conditions. Moreover, morphine-induced antinociceptive activity in the peripheral site is better than that in the central site of morphine action because central untoward adverse effects, such as respiratory depression, somnolence, and addiction, are avoided [
12,
15]. Although non-steroidal anti-inflammatory drugs (NSAIDs) also mediate a peripheral analgesic effect, they inhibit peripheral inflammation. In contrast, morphine has no anti-inflammatory action on the peripheral site.
Hot-plate test using mice is simple and easy to perform [
16] and is generally used for strong-acting analgesics, such as opioids, but not for peripherally acting drugs. Currently, a few experimental model can evaluate peripherally acting analgesic drugs in mice. A well-known model for analgesic action for NSAIDs is paw inflammation induced by injection of Freund’s complete adjuvant [
17] or carrageenin [
18] and subsequent hyperalgesia assessment in rats [
19,
20]. However, it is substantially difficult to perform long-term tests in mice. Moreover, although these methods cause inflammation, they can be used to evaluate even weak analgesics, such as NSAIDs and acetaminophen, if the pain threshold is lowered. Acute contact dermatitis is induced after a single application of hapten, dinitrochrolbenzen (DNCB) [
21]. A modified hot-plate test on the DNCB derivative 2,4,6-trinitrochlorobenzene (TNCB) has been previously performed [
22]. TNCB was applied to the limbs of mice to reduce the pain threshold, which allowed the evaluation of antinociceptive activity at a temperature lower than that used in the hot-plate test [
23]. Additionally, it was possible to evaluate the antinociceptive activity of NSAIDs and IL-31 using TNCB.
Itching elicits a strong desire to scratch; therefore, scratching behavior count is a useful index to evaluate itching [
24]. Mice exhibit two types of scratching behavior, long-lasting scratching (LLS, scratching behavior lasting more than 1.0 s) and short-lasting scratching (SLS, scratching behavior lasting from 0.3 to 1.0 s). Current studies on itching are based on the human perceptive sense, and these nociceptive stimuli have no discernible differences. However, the sensory perception of a foreign substance and true itching could be differentiated by dividing the scratching behavior of mice into LLS and SLS [
6]. The number of spontaneous scratches can be automatically detected and objectively evaluated via a computer. Based on our evaluation standard, we have previously found that histamine is not a pruritogen [
25].
Capsaicin acts on the transient receptor potential cation channel vanilloid subfamily V member 1 (TRPV1) which modulates nociceptive inputs to the spinal cord and brain stem centers integrating diverse painful stimuli [
26,
27]. The sensation of itching can be reduced by the painful sensations caused by scratching [
28]. The inhibition of itching by painful stimuli has been experimentally demonstrated using various painful stimuli. NC/Nga mice, an animal model of atopic dermatitis with chronic itching, spontaneously develop skin lesions [
29]. We have previously demonstrated that cutaneous prostaglandins (PGs) levels are significantly elevated upon scratching the mouse skin with a stainless-steel wire brush (mechanical scratching), and these PGs suppressed LLS in skin-lesioned NC/Nga mice [
28,
30,
31]. Because PGs are associated with inflammation, their administration enhances pain [
32]. Notably, although pain-induced suppression of itching is temporary, the effect of capsaicin lasts more than 72 h, suggesting that this effect may not only be due to pain but also another action of capsaicin. The partial activation of TRPV1 by capsaicin was results in pain, which in turn may partially suppress itching sensations [
33]. Paradoxically, the application of capsaicin produces transient burning pain and induces analgesia for neuropathic pain. The mechanisms underlying these opposing actions of capsaicin on the onset of pain and induction of analgesia have not yet been explained. The application of capsaicin causes desensitization of TRPV1 [
34], inhibition of nociceptor firing [
35], and a decrease in mechanotransduction [
36]. These early effects of capsaicin on the function of primary afferents might contribute to the analgesic effects immediately after capsaicin application. However, these effects may be unrelated to suppressing itching sensations because there are no reports of an analgesic action inhibiting itching for more than 72 h following capsaicin application. Recently, we reported that capsaicin suppresses LLS and SLS for more than 72 h; at this point, the expression of DRG IL-31RA mRNA is significantly decreased, whereas that of cutaneous IL-31RA shows no significant change. Thus, capsaicin may suppress LLS by inhibiting IL-31RA mRNA expression in the DRG [
37].
Our previous report suggested the partial involvement of IL-31 in the antinociceptive action of morphine [
11]. Moreover, we investigated the effects of pretreatment with IL-31 on morphine-induced antinociceptive activity using a modified hot-plate test and found a close correlation between morphine-induced LLS and antinociceptive effect. We also investigated LLS as an indicator of itching by morphine-induced itching and found that repeated administration of IL-31 gradually promoted LLS in a dose-dependent manner and increased DRG neuronal IL-31RA expression. These data show that cutaneously-injected IL-31 induces LLS and promotes DRG IL-31RA expression [
4].
The present study aimed to reveal the mechanism of action of IL-31 on TNCB- or capsaicin-induced pain models, and assess the long-term physiological effects of its application. Our results provide important insights into the development of a new type of algesic based on the antinociceptive mechanism of IL-31.
3. Discussion
The IL-31-caused scratching behavior is distinct from other pruritogens (e.g., histamine, serotonin)-induced scratching. A single intradermal or other administration rote (e.g., intravenously) injection of IL-31 elicits LLS but not SLS, with LLS manifesting gradually approximately 1 h after injection and persisting over 24 h [
8]. However, other pruritogens, injected only via an intradermal route, elicits SLS but not LLS, with SLS manifesting immediately after the intradermal injection and persisting for at least 30 min [
8]. IL-31 is a neuronal transmitter that causes aloknesis but is not a pruritogens, indicating that it causes alloknesis-induced itching [
38,
39]. In a previous study, we showed that repeated administration of IL-31 gradually increased the LLS counts. Our results indicated that IL-31-induced LLS counts depend on the interaction of blood IL-31 concentration, DRG neuronal IL-31RA expression induces LLS, and IL-31 promotes the onset of DRG neuronal IL-31RA [
5]. In this study, a single pretreatment with IL-31 (50 μg/kg, intrapretoneal) showed no significant antinociceptive activity whereas repeated pretreatment with IL-31 showed significant antinociceptive activity on TNCB applied hot-plate test at 45°C. The repeated administration of IL-31 enhanced the action of IL-31-induced increase in DRG neuronal IL-31RA expression. Moreover, a single large dose of IL-31 (1 mg/kg, intraperitoneal) elicited clear LLS, and TNCB-induced pain inhibited the IL-31-induced LLS. These results suggest that IL-31-induced itch inhibits TNCB-induced pain. Although it is well-known that pain inhibits itching [
40], the reverse phenomenon that an itch inhibits pain is controversial. Our results show that IL-31-induced alloknesis inhibits pain.
In the present study, repeated pretreatment of IL-31 decreased latency; however, this result was insignificant in the conventional hot-plate test (51 °C). This result suggest that IL-31-induced itching is caused by heat stimulation; therefore pain and itching actions are indistinguishable. However, morphine-induced antinociception was enhanced with pretreatment with IL-31, suggesting that the increased analgesic action was caused by the IL-31-induced peripheral action of morphine and its interaction with the central analgesic action of morphine. Morphine produces analgesia by acting on both the CNS and PNS, and it is a one of the few drugs to induce LLS counts and cause alloknesis simultaneously [
41,
42]. In addition, morphine-induced LLS and antinociceptive effects are closely correlated in mice; morphine-induced LLS and antinociceptive effects were completely and partially inhibited in IL-31RAKI mice, respectively [
11]. Previously, we reported that IL-31 might play a significant role in the modulation of peripheral morphine-induced antinociception by sensory neurons in IL-31RAKI mice compared to wild-type mice [
11]. In the present study, we demonstrated that repeated pretreatment with IL-31 showed antinociceptive activity. These results suggest that the antinociceptive activity of IL-31 partially contributes to the antinociceptive action of morphine.
In the present study, loxoprofen, a PGs inhibitor, significantly increased latency and total antinociceptive activity in the TNCB (1%)-applied hot plate (45 °C) test. The application of 3% TNCB will be shows almost the same result. Repeated pretreatment with IL-31 also increased latency and total nociception; however, the combined effect of loxoprofen and IL-31 was not significantly enhanced. These results suggest that the antinociceptive activity of IL-31 was approximately as effective as that of loxoprofen (NSAIDs). An enhanced action was not observed because the compound characteristics have the same peripheral action site. Inflammatory reactions increased tissue PGs (PGD2, PGE2, and PGI2) biosynthesis and are characterized by redness (arterial extension), fever (increased body temperature), and edema (swelling). Therefore, we examined the comparative effects of IL-31 and loxoprofen on TNCB-induced fever (surface body temperature) and swelling (cutaneous weight) in mice. TNCB significantly increased the cutaneous temperature and skin tissue weight 24 h after application, whereas IL-31 did not change the cutaneous temperature and swelling. In contrast, loxoprofen significantly decreased TNCB-induced fever and swelling. These results show that the main action of IL-31 is not to inhibit PGs biosynthesis. Moreover, even if they share the same peripheral analgesic sites of action, NSAIDs and IL-31 have different mechanisms of action; NSAIDs suppress inflammation by inhibiting the biosynthesis of these PGs. However, these inflammatory reactions are spontaneous defense mechanisms of the body; particularly, fever and swelling are involved in the reproduction of the wound tissue by vasodilatation. Pain is also a part of the defense mechanism; however, the reaction to stressful psychological damage is more serious than the reaction that induces fever and swelling. Therefore, NSAIDs, such as loxoprofen (PGs inhibitor) are used to treat different inflammatory diseases. As the substance that specifically inhibits pain, IL-31 may be a more suitable analgesic drug for patients with various inflammatory diseases.
In the present study, the application of TNCB and capsaicin caused pain and decreased latency of the hot-plate test (45 °C) immediately or within 6 h after treatment. However, the onset of pain caused by the application of these materials was different. TNCB caused tissue injury and inflammation. Alternatively, the responses to noxious stimuli may be enhanced (hyperalgesia), or normally innocuous stimuli may produce pain (allodynia). PGs influence inflammation, and their administration induces the major signs of inflammation, including augmented pain [
32,
43]. However, the topical application of PGD
2, PGE
2 and PGI
2 significantly suppresses LLS in skin-lesioned NC/Nga mice, and their inhibitory activities are in the order of PGD
2 > PGI
2 > PGE
2 [
31]. Moreover, PGs significantly enhance nociceptive activity on hot-plate tests in mice [
43]; PGD
2, PGE
2, and PGI
2 significantly increase the nociceptive effec in the order of PGD
2 > PGI
2 > PGE
2, which is the same as the order of their anti-pruritic activities. Although the scratching behavior strips off the epidermis and removes the alien substance invading the epidermis, it is a physiological reaction to directly control itching. In other words, scratching increases epidermal PGD
2 biosynthesis, and PGD
2-induced pain inhibits itching [
31]. These previous and present findings suggest that cutaneous PGD
2 could be mainly produced in response to pain and play a critical role in regulating the sensation of pain [
44]. The significant individual differences in cutaneous IL-31 expression may be a reaction to TNCB-induced pain. Cutaneous IL-31 expression is unstable and intermittent; however, DRG neuronal IL-31RA expression always shows a stable increase by stimulation [
5]. This phenomenon may be a physiological reaction to inhibit pain.
In the present study, capsaicin did not affect cutaneous PGD
2 contents but significantly decreased DRG neuronal IL-31RA expression. These results indicate that the activation of TRPV1 and inhibition of IL-31RA mRNA expression in the DRG may mediate the antipruritic effects of capsaicin. The decrease in TRPV1 mRNA expression in the DRG triggered by capsaicin is hypothesized to respond to the onset of TRPV1-stimulated pain [
37]. Therefore, we examined the effect of repeated administration of IL-31 on PGD
2- or capsaicin-induced hyperalgesia on the modified hot-plate (45 °C) test. Repeated pretreatment with IL-31 inhibited PGD
2-induced pain but not capsaicin-induced pain in the modified hot-plate test, suggesting that the site of action of IL-31 was after the PGs of the peripheral sensory nervous system. Furthermore, we assessed the effect of several analgesics, such as morphine, loxoprofen, and acetaminophen, on TNCB or capsaicin-applied hot-plate (45 or 35 °C) test to determine the effect of the IL-31-induced antinociceptive activity in wild-type and IL-31RAKI mice.
Morphine (3 mg/kg, subcutaneous) showed significant antinociception on the TNCB- or wild-type mice on the TNCB-applied hot-plate (45 °C) test but not in capsaicin- and IL-31RAKI mice. However, this is partial inhibitory action as morphine-induced antinociceptive activity recovered in high doses (10 mg/kg, subcutaneously). Loxoprofen (15 mg/kg, oral) and acetaminophen (300 mg/kg, oral) showed significant antinociception on TNCB- or wild-type mice on TNCB-applied hot-plate (35 °C) test; however, these antinociceptive activities were also completely inhibited by capsaicin- and IL-31RAKI mice. Moreover, acetaminophen does not inhibit PG biosynthesis [
45]. Although our present study suggested that the site of action of acetaminophen is the CNS, these collective results indicate that acetaminophen functions on the PNS, suggesting that IL-31 participates in the antinociceptive activity of these drugs. In addition, some antinociceptive activities remained in the morphine (opioid) group but not, in the loxoprofen and acetaminophen groups. Chronic neuropathic pain shows strong resistance to treatment with opioids and NSAIDs [
46]. This reaction is similar to drug action in chronic neuropathic pain. Therefore, the decrease in DRG neuronal IL-31RA may play a role in the onset of chronic neuropathic pain (
Figure 10).
In the present study, we objectively evaluated the distinction between pain and itch by a wave pattern of scratching behavior by the movement of the hind leg of the mouse [
6]. When the mouse skin is in normal condition, e.g., histamine injected intradermally, the mouse elicits 0.3–1.0 s lasting scratching behavior (SLS) rising suggesting that the mouse felt something on the epidermis. When mice experience an itch, e.g., when IL-31 is injected intradermally, they elicit over 1.0 s lasting scratching behavior (LLS). This itching strips off the epidermis and removes an alien substance. For example, when a mite irritates the skin, IL-31 is highly expressed in that region, and an itch sensation is produced in response to various kinds of cutaneous stimulation [
38]. If this stimulation continues for a long time, it may become atopic dermatitis due to overexpression of DRG neuronal IL-31RA [
39] (
Figure 10). In contrast, when the mouse skin is experiencing a painful condition, e.g., TNCB (PGs inducer) application in dorsal skin, the mouse elicits ultra-short-lasting scratching behavior (USLS) rising (less than 0.3 s) or no scratching behavior. Therefore, in the present study, we used a modified hot-plate test for a real pain evaluation. When the mouse was experiencing pain, it did not touch the skin, if possible. PGs is produced in response to inflammation, such as that in response to a burn, and may be involved in the onset of pain in response to various kinds of cutaneous stimulation. If this stimulation continues for a long time, it may induce chronic neuropathic pain due to decreased expression of DRG neuronal IL-31RA (
Figure 10).
In this study, we evaluated the effects of TNCB- or capsaicin-induced pain and itch after 24 h of this application on a modified hot-plate (45 °C) test. TNCB and capsaicin-induced pain and inhibited itch after the application in a similar manner until 6 h, but they showed different reactions 24 h later. TNCB application led to the development of pain torpor, which was not observed in IL-31RAKI mice. Therefore, this reaction was considered a result of endogenous IL-31. The itch (LLS counts) increase corresponded with the increase in the DRG neuronal IL-31RA expression. This reaction might be the cause of itching during the wound recovery period. The itch observed 24 h after TNCB application may be due to an increase in DRG neuronal IL-31RA expression after the restoration of the wound. In contrast, the application of capsaicin induced decreased latency 24 h after application and a significant decrease 6 days after. Strong pain is sometimes observed after the application of capsaicin cream when an individual takes a warm water shower. This pain can be explained by a decrease in DRG neuronal IL-31RA expression after capsaicin application. Although decreased latency caused by capsaicin was restored after 72 h of application, IL-31-induced (1 mg/kg, intravenously) LLS counts and DRG neuronal IL-31RA expression significantly decreased 144 h (6 days) after capsaicin application. IL-31RAKI mice lack a peripheral analgesic mechanism; therefore, the recovery from this latency involves the participation of the central analgesic mechanism.
These findings suggest that IL-31 may cause alloknesis; it alters the non-selective irritant stimulation into itch stimulation in mouse skin [
47,
48]. Therefore, it is possible that pain and itch are transmitted on the same nerve fibers, and a sensation is perceived as pain or itch depending on the operation of IL-31. However, PGD
2 decreased LLS counts induced by the cutaneous injection of pruritogens or algogens in IL-31-induced itchy skin. These results indicate that PGD
2 improves IL-31-induced alloknesis, it alters the non-selective irritant stimulation into pain stimulation in mouse skin [
32]. Collectively, these data suggest that the sensation of pain and itch may be regulated by PGD
2 (allodynia-inducer) [
49] and/or IL-31 (alloknesis-inducer) [
38], through their functional antagonism (
Figure 10.).
The itch can be produced by a gentle touch, pressure, vibration, thermal, and electrical stimuli, such as transcutaneous or direct nerve stimulation. While itching may have different causes, it is conceived similarly by our senses. Our study indicates that the sensation of itch and pain depends on the type of stimulant of the peripheral sensory nerve ending. A strong pain will be felt, even if it is an itch if the burnt part is gently touched, suggesting that the sense of itch and pain are not stimulated from the outside, but from the inside. This study indicates that the analgesic action of IL-31 involves the PNS, which directly affects sensory nerve, providing a basis for developing novel analgesics using this mechanism.
Figure 1.
Effects of IL-31 on TNCB-applied hot-plate test in BALB/c mice. (a) Effect of repeated pretreatment of IL-31 (50 μg/kg, intraperitoneally, every 12 h for three days) on TNCB (3%, 0.04 mL/each limb, total 0.16 mL/site) applied hot-plate (45 °C) test. The blue line indicates phosphate-buffered saline (PBS) + vehicle (acetone-ethanol mixed liquor, AEM) treated group; the yellow line indicates IL-31 + AEM-treated group; the red line indicates PBS + TNCB-treated group; the green line indicates IL-31 + TNCB-treated group. *p < 0.05, **p < 0.01, ***p < 0.001 compared with the corresponding values in the PBS + TNCB treated group; # p < 0.05, ##, ### p < 0.001 compared with the corresponding values in the PBS + AEM-treated group (students t-test with Bonferroni correction). (b) Total antinociceptive index of IL-31 after 0.5 to 6 h (AUC 0.5–6 h). The blue column indicates the PBS-treated group; the red column indicates the IL-31-treated group. *p < 0.05, ***p < 0.001 compared with the corresponding values in the PBS + TNCB-treated group (student’s t-test). (c) Time-course change of itch-associated scratching behavior (LLS, counts/h) after the application of TNCB (3%, 0.2 mL/dorsal site). The blue line indicates the IL-31 + AEM-treated group; the red line indicates IL-31 + TNCB-treated group. The lateral axis indicates the clock hour, and the shaded area represents nighttime (dark phase, 7:00 pm to 7:00 am). (d) Total LLS counts for 24 h. The blue column indicates the IL-31 + AEM-treated group; the red column indicates the IL-31 + TNCB-treated group. The red arrow indicates the AEM or TNCB application point. The blue arrow indicates the IL-31 (1 mg/kg, intraperitoneally) administration point. Each value represents the mean ± standard error (S.E.) from 6 mice (total 36 mice). ***p < 0.001 compared with the vehicle (PBS) + TNCB-treated group (students t-test ).
Figure 1.
Effects of IL-31 on TNCB-applied hot-plate test in BALB/c mice. (a) Effect of repeated pretreatment of IL-31 (50 μg/kg, intraperitoneally, every 12 h for three days) on TNCB (3%, 0.04 mL/each limb, total 0.16 mL/site) applied hot-plate (45 °C) test. The blue line indicates phosphate-buffered saline (PBS) + vehicle (acetone-ethanol mixed liquor, AEM) treated group; the yellow line indicates IL-31 + AEM-treated group; the red line indicates PBS + TNCB-treated group; the green line indicates IL-31 + TNCB-treated group. *p < 0.05, **p < 0.01, ***p < 0.001 compared with the corresponding values in the PBS + TNCB treated group; # p < 0.05, ##, ### p < 0.001 compared with the corresponding values in the PBS + AEM-treated group (students t-test with Bonferroni correction). (b) Total antinociceptive index of IL-31 after 0.5 to 6 h (AUC 0.5–6 h). The blue column indicates the PBS-treated group; the red column indicates the IL-31-treated group. *p < 0.05, ***p < 0.001 compared with the corresponding values in the PBS + TNCB-treated group (student’s t-test). (c) Time-course change of itch-associated scratching behavior (LLS, counts/h) after the application of TNCB (3%, 0.2 mL/dorsal site). The blue line indicates the IL-31 + AEM-treated group; the red line indicates IL-31 + TNCB-treated group. The lateral axis indicates the clock hour, and the shaded area represents nighttime (dark phase, 7:00 pm to 7:00 am). (d) Total LLS counts for 24 h. The blue column indicates the IL-31 + AEM-treated group; the red column indicates the IL-31 + TNCB-treated group. The red arrow indicates the AEM or TNCB application point. The blue arrow indicates the IL-31 (1 mg/kg, intraperitoneally) administration point. Each value represents the mean ± standard error (S.E.) from 6 mice (total 36 mice). ***p < 0.001 compared with the vehicle (PBS) + TNCB-treated group (students t-test ).
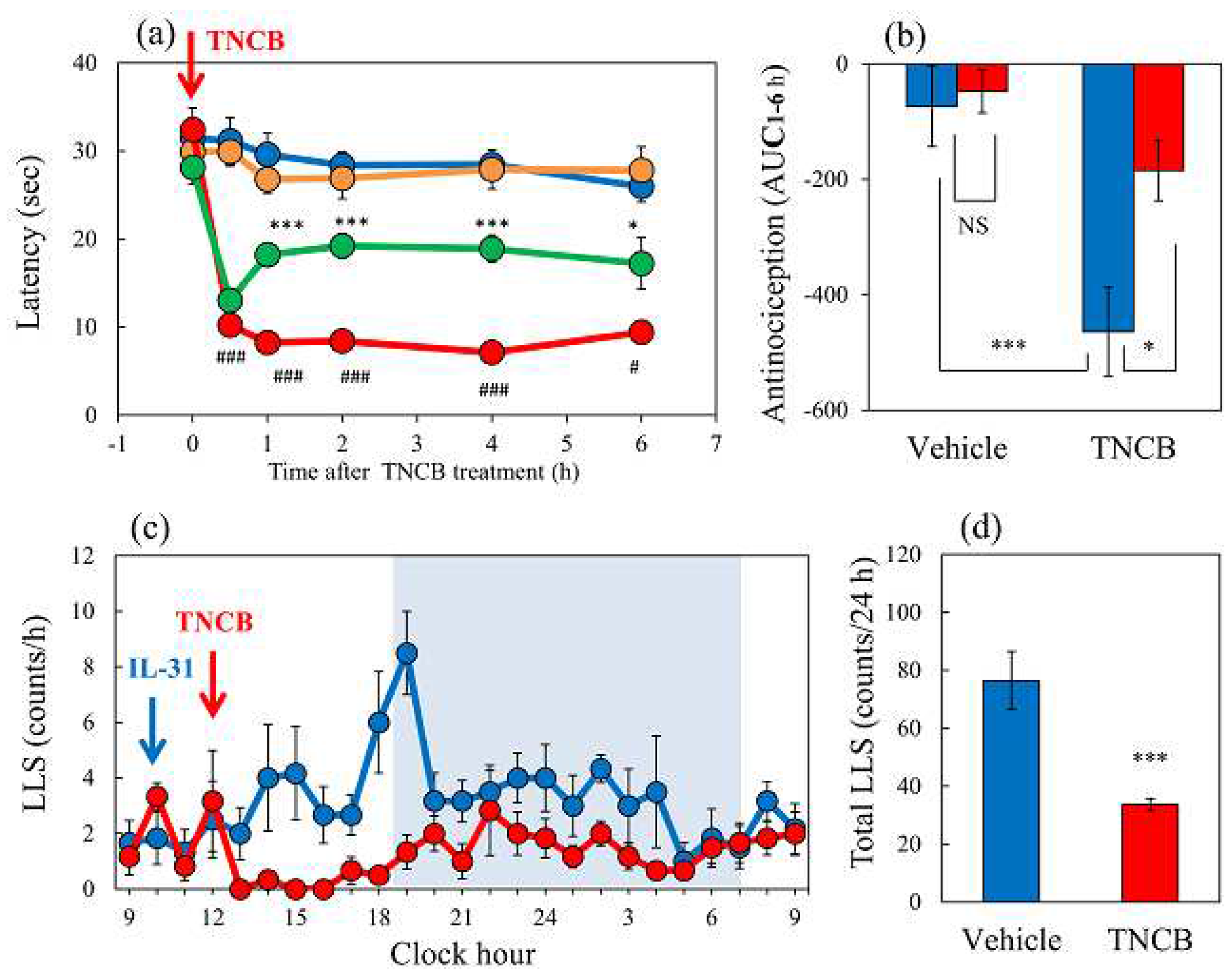
Figure 2.
Effects of IL-31 and morphine on conventional hot-plate (51°C) test in BALB/c mice. (a) Effect of repeated pretreatment of IL-31 (50 μg/kg, intraperitoneally, every 12 h for three days) and a single dose of morphine (3 mg/kg, subcutaneously) on conventional hot-plate (51 °C) test. The blue line indicates the phosphate-buffered saline (PBS)-treated group; the red line indicates the IL-31 + saline-treated group; the green line indicates PBS + morphine-treated group; the yellow line indicates IL-31 + morphine-treated group. Each value represents the mean ± standard error (S.E.) from 6 mice (24 mice). *p < 0.05, **p < 0.01, ***p < 0.001 compared with the corresponding values in the PBS + saline-treated group (student’s t-test with Bonferroni correction). (b) Total antinociceptive index of morphine after 15 to 120 min (AUC15–120 min). The blue column indicates PBS + saline-treated group; the green column indicates PBS + morphine-treated group. The red column indicates the IL-31 + saline-treated group; the yellow column indicates IL-31 + morphine-treated group. Each value represents the mean ± standard error (S.E.) from 6 mice (total 24 mice). *p < 0.05, **p < 0.01, ***p < 0.001 compared with each group (Turkey’s test).
Figure 2.
Effects of IL-31 and morphine on conventional hot-plate (51°C) test in BALB/c mice. (a) Effect of repeated pretreatment of IL-31 (50 μg/kg, intraperitoneally, every 12 h for three days) and a single dose of morphine (3 mg/kg, subcutaneously) on conventional hot-plate (51 °C) test. The blue line indicates the phosphate-buffered saline (PBS)-treated group; the red line indicates the IL-31 + saline-treated group; the green line indicates PBS + morphine-treated group; the yellow line indicates IL-31 + morphine-treated group. Each value represents the mean ± standard error (S.E.) from 6 mice (24 mice). *p < 0.05, **p < 0.01, ***p < 0.001 compared with the corresponding values in the PBS + saline-treated group (student’s t-test with Bonferroni correction). (b) Total antinociceptive index of morphine after 15 to 120 min (AUC15–120 min). The blue column indicates PBS + saline-treated group; the green column indicates PBS + morphine-treated group. The red column indicates the IL-31 + saline-treated group; the yellow column indicates IL-31 + morphine-treated group. Each value represents the mean ± standard error (S.E.) from 6 mice (total 24 mice). *p < 0.05, **p < 0.01, ***p < 0.001 compared with each group (Turkey’s test).
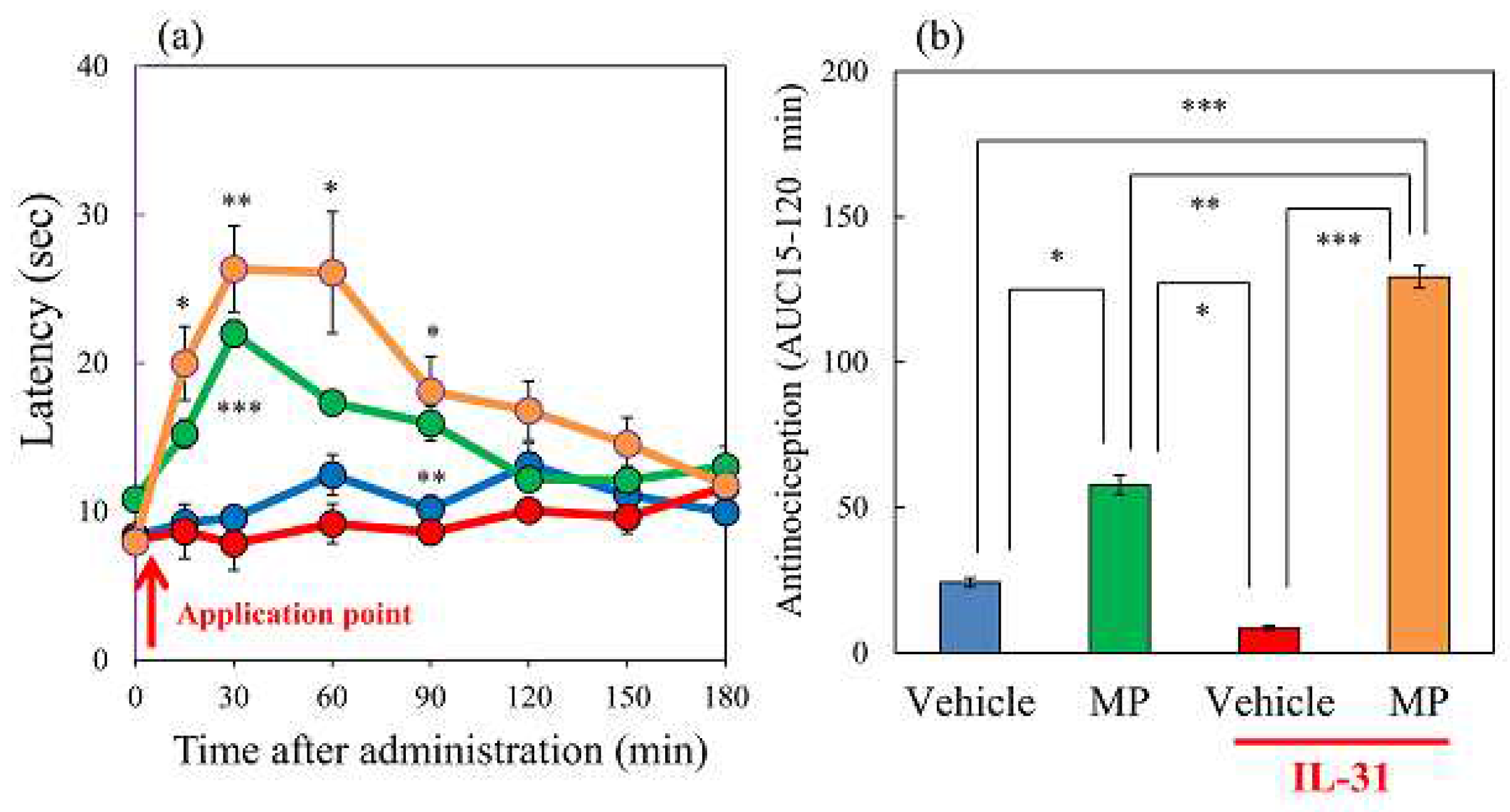
Figure 3.
Effects of IL-31 and loxoprofen (LXP) on TNCB-applied the hot-plate test in BALB/c mice. (a) Effects of IL-31 and LXP on TNCB-applied hot-plate (45 °C) test in mice. The black line and column indicate the non-TNCB-treated group; the blue line indicates phosphate-buffered saline (PBS) + vehicle (carboxymethyl cellulose sodium, CMC, 10 mL/kg, oral)-treated group; the red line indicates IL-31 + CMC-treated group; the green line indicates PBS + LXP-treated group; the yellow line indicate IL-31 + LXP treated group. The red arrow indicates the AEM or TNCB application point. # p < 0.05, ##P, ### p < 0.001 compared with the corresponding values in the saline + vehicle-treated group (student’s t-test with Bonferroni correction). (b) Total antinociceptive index of LXP after 30–120 min (AUC30–120 min). The black line and column indicate the non-treated group; the blue column indicates the PBS + CMC-treated group; the green column indicates the PBS + LXP-treated group. The red column indicates the IL-31 + CMC treated-group; the yellow column indicates the IL-31 + LXP-treated group. Each value represents the mean ± standard error (S.E.) from 6 mice (total 54 mice). ### p < 0.001 compared with the corresponding values in the saline + vehicle-treated group (student’s t-test). *p < 0.05, *** p < 0.001 compared with the corresponding values in the vehicle + TNCB treated group (Turkey’s test) ; (c) Effects of IL-31 and LXP on TNCB-induced increasing cutaneous temperature (fever) after 24 h application of TNCB. (d) Effects of IL-31 and LXP on TNCB-induced increasing cutaneous weight (swelling) after 24 h application of TNCB. The blue column indicates saline + vehicle-treated group; the red column indicates vehicle + TNCB treated group; the green column indicates IL-31 + TNCB treated group; the yellow column indicates LXP + TNCB treated group. Each value represents the mean ± S.E. from 6 mice (total 24 mice), NS, not significant, #p < 0.05, ##p < 0.01 compared with the non-TNCB-treated group (student’s t-test). * p < 0.05 when compared with each group (Turkey’s test).
Figure 3.
Effects of IL-31 and loxoprofen (LXP) on TNCB-applied the hot-plate test in BALB/c mice. (a) Effects of IL-31 and LXP on TNCB-applied hot-plate (45 °C) test in mice. The black line and column indicate the non-TNCB-treated group; the blue line indicates phosphate-buffered saline (PBS) + vehicle (carboxymethyl cellulose sodium, CMC, 10 mL/kg, oral)-treated group; the red line indicates IL-31 + CMC-treated group; the green line indicates PBS + LXP-treated group; the yellow line indicate IL-31 + LXP treated group. The red arrow indicates the AEM or TNCB application point. # p < 0.05, ##P, ### p < 0.001 compared with the corresponding values in the saline + vehicle-treated group (student’s t-test with Bonferroni correction). (b) Total antinociceptive index of LXP after 30–120 min (AUC30–120 min). The black line and column indicate the non-treated group; the blue column indicates the PBS + CMC-treated group; the green column indicates the PBS + LXP-treated group. The red column indicates the IL-31 + CMC treated-group; the yellow column indicates the IL-31 + LXP-treated group. Each value represents the mean ± standard error (S.E.) from 6 mice (total 54 mice). ### p < 0.001 compared with the corresponding values in the saline + vehicle-treated group (student’s t-test). *p < 0.05, *** p < 0.001 compared with the corresponding values in the vehicle + TNCB treated group (Turkey’s test) ; (c) Effects of IL-31 and LXP on TNCB-induced increasing cutaneous temperature (fever) after 24 h application of TNCB. (d) Effects of IL-31 and LXP on TNCB-induced increasing cutaneous weight (swelling) after 24 h application of TNCB. The blue column indicates saline + vehicle-treated group; the red column indicates vehicle + TNCB treated group; the green column indicates IL-31 + TNCB treated group; the yellow column indicates LXP + TNCB treated group. Each value represents the mean ± S.E. from 6 mice (total 24 mice), NS, not significant, #p < 0.05, ##p < 0.01 compared with the non-TNCB-treated group (student’s t-test). * p < 0.05 when compared with each group (Turkey’s test).
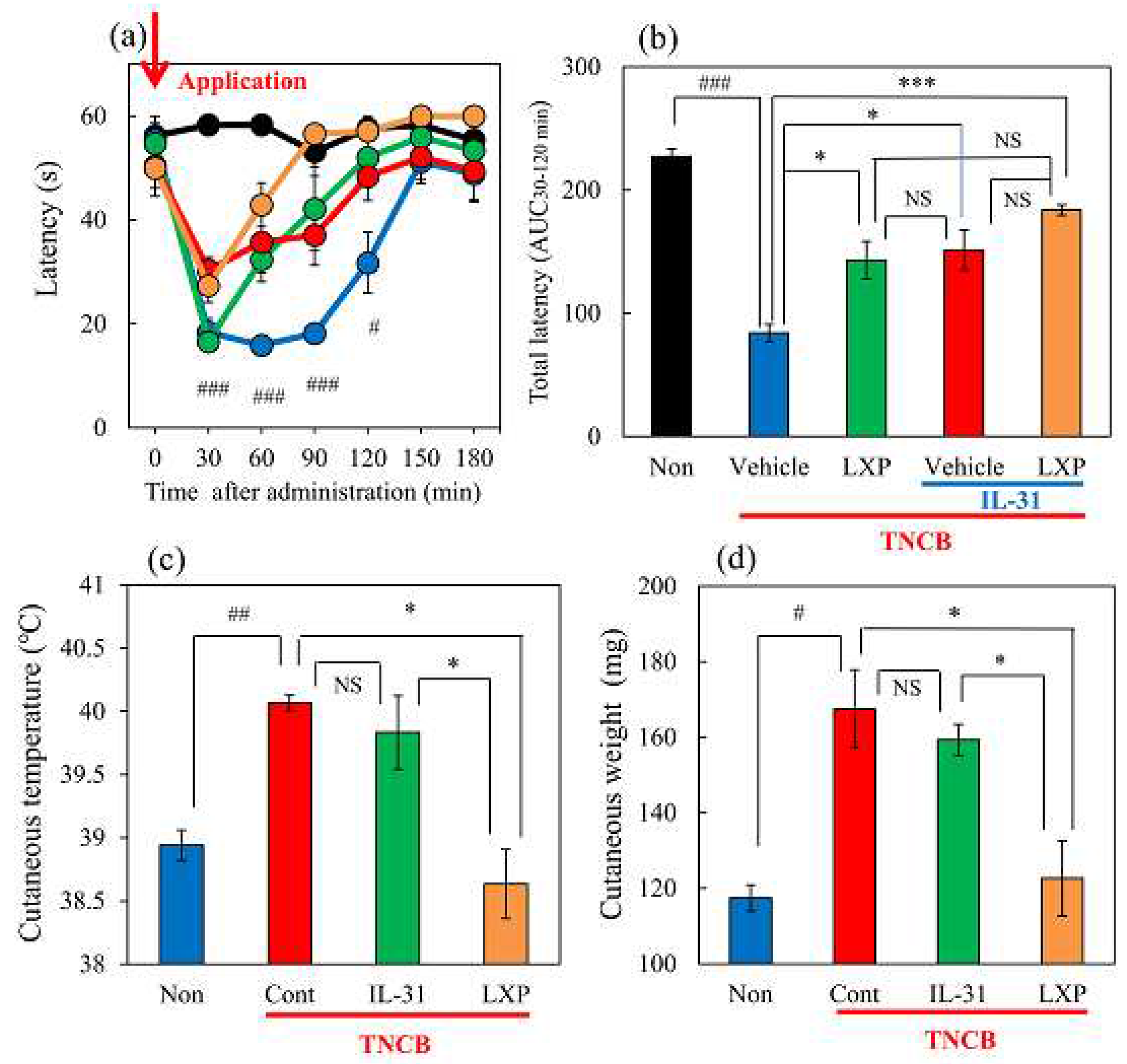
Figure 4.
Comparative effects of TNCB or capsaicin on latency in modified hot-plate test and several pain-related parameters in BALB/c mice. (a) Time-course changes of latency caused by topical application of TNCB (3%) or capsaicin (1%) in modified hot-plate (45°C) test. The red arrow indicates the vehicle (acetone-ethanol mixed liquor, AEM, 0.2 mL/site) or TNCB or capsaicin application point. The values represent the means ± standard error (S.E.) from 6 mice. ***p < 0.001 compared with the respective value of the AEM-treated group (student’s t-test with Bonferroni correction). (b) Effects of TNCB and capsaicin on cutaneous PGD2 contents 1 h after application. (c) Effects of TNCB or capsaicin on DRG neuronal IL-31RA mRNA expression 6 h after application. (d) Effects of TNCB or capsaicin on cutaneous IL-31 mRNA expression 1 h after application. (e) Effects of TNCB or capsaicin on DRG neuronal TRPV1 mRNA expression 6 h after application. The values represent the means ± standard error (S.E.) from 6 mice. (total 42 mice). NS, not significant, *p < 0.05, **p < 0.01, ***p < 0.001 compared with the respective value of the AEM-treated group (student’s t-test).
Figure 4.
Comparative effects of TNCB or capsaicin on latency in modified hot-plate test and several pain-related parameters in BALB/c mice. (a) Time-course changes of latency caused by topical application of TNCB (3%) or capsaicin (1%) in modified hot-plate (45°C) test. The red arrow indicates the vehicle (acetone-ethanol mixed liquor, AEM, 0.2 mL/site) or TNCB or capsaicin application point. The values represent the means ± standard error (S.E.) from 6 mice. ***p < 0.001 compared with the respective value of the AEM-treated group (student’s t-test with Bonferroni correction). (b) Effects of TNCB and capsaicin on cutaneous PGD2 contents 1 h after application. (c) Effects of TNCB or capsaicin on DRG neuronal IL-31RA mRNA expression 6 h after application. (d) Effects of TNCB or capsaicin on cutaneous IL-31 mRNA expression 1 h after application. (e) Effects of TNCB or capsaicin on DRG neuronal TRPV1 mRNA expression 6 h after application. The values represent the means ± standard error (S.E.) from 6 mice. (total 42 mice). NS, not significant, *p < 0.05, **p < 0.01, ***p < 0.001 compared with the respective value of the AEM-treated group (student’s t-test).
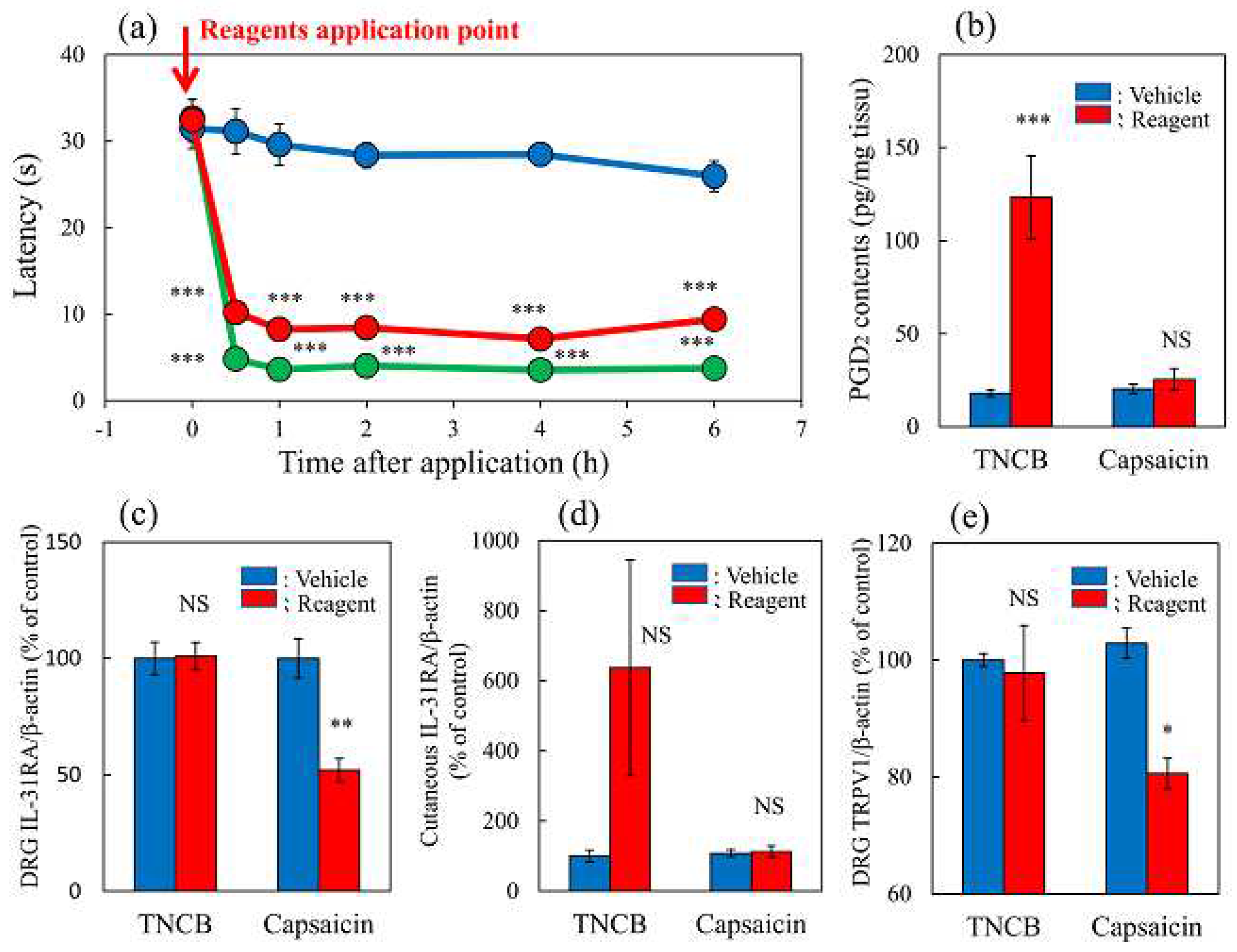
Figure 5.
Effects of IL-31 on PGD2- or capsaicin-applied hot-plate test in BALB/c mice. (a) Effect of IL-31 on PGD2-induced decreasing latency on hot-plate (45 °C) test. **p < 0.01, ***p < 0.001 compared with the corresponding values in the vehicle (ethanol, 0.2 mL/site)-treated group; # p < 0.05, ## p < 0.01, ### p < 0.001 compared with the corresponding values in the vehicle-treated group (students t-test with Bonferroni correction). (b) Total antinociceptive index from 30 to 120 min (AUC30–120 min) after PGD2 application. NS, not significant, *p < 0.05 compared with the corresponding values in the vehicle-treated group (students t-test). (c) Effect of IL-31 on capsaicin-induced decreasing latency on modified hot-plate (45 °C) test. ### p < 0.001 compared with the corresponding values in the vehicle-treated group (students t-test with Bonferroni correction). (d) After capsaicin application, the total antinociceptive index changes from 30 to 120 min (AUC30–120 min). The blue line indicates the vehicle (phosphate-buffered saline, PBS, intraperitoneal, every 12 h for 3 days) pretreated group; the red line indicates the IL-31-pretreated (50 μg/kg, intraperitoneal, every 12 h for 3 days) group; the green line indicates PGD2 (0.1%, 0.04 mL/each limb) or capsaicin (3%, 0.04 mL/each limb) applied group; the yellow line indicates IL-31 + PGD2 or capsaicin-treated group. The red arrow indicates the vehicle (ethanol, EtOH) or PGD2 or capsaicin application point. Values represent the mean ± S.E. from 6 mice (total of 48 mice). NS, not significant, compared with the respective values of the vehicle-treated group (students t-test).
Figure 5.
Effects of IL-31 on PGD2- or capsaicin-applied hot-plate test in BALB/c mice. (a) Effect of IL-31 on PGD2-induced decreasing latency on hot-plate (45 °C) test. **p < 0.01, ***p < 0.001 compared with the corresponding values in the vehicle (ethanol, 0.2 mL/site)-treated group; # p < 0.05, ## p < 0.01, ### p < 0.001 compared with the corresponding values in the vehicle-treated group (students t-test with Bonferroni correction). (b) Total antinociceptive index from 30 to 120 min (AUC30–120 min) after PGD2 application. NS, not significant, *p < 0.05 compared with the corresponding values in the vehicle-treated group (students t-test). (c) Effect of IL-31 on capsaicin-induced decreasing latency on modified hot-plate (45 °C) test. ### p < 0.001 compared with the corresponding values in the vehicle-treated group (students t-test with Bonferroni correction). (d) After capsaicin application, the total antinociceptive index changes from 30 to 120 min (AUC30–120 min). The blue line indicates the vehicle (phosphate-buffered saline, PBS, intraperitoneal, every 12 h for 3 days) pretreated group; the red line indicates the IL-31-pretreated (50 μg/kg, intraperitoneal, every 12 h for 3 days) group; the green line indicates PGD2 (0.1%, 0.04 mL/each limb) or capsaicin (3%, 0.04 mL/each limb) applied group; the yellow line indicates IL-31 + PGD2 or capsaicin-treated group. The red arrow indicates the vehicle (ethanol, EtOH) or PGD2 or capsaicin application point. Values represent the mean ± S.E. from 6 mice (total of 48 mice). NS, not significant, compared with the respective values of the vehicle-treated group (students t-test).
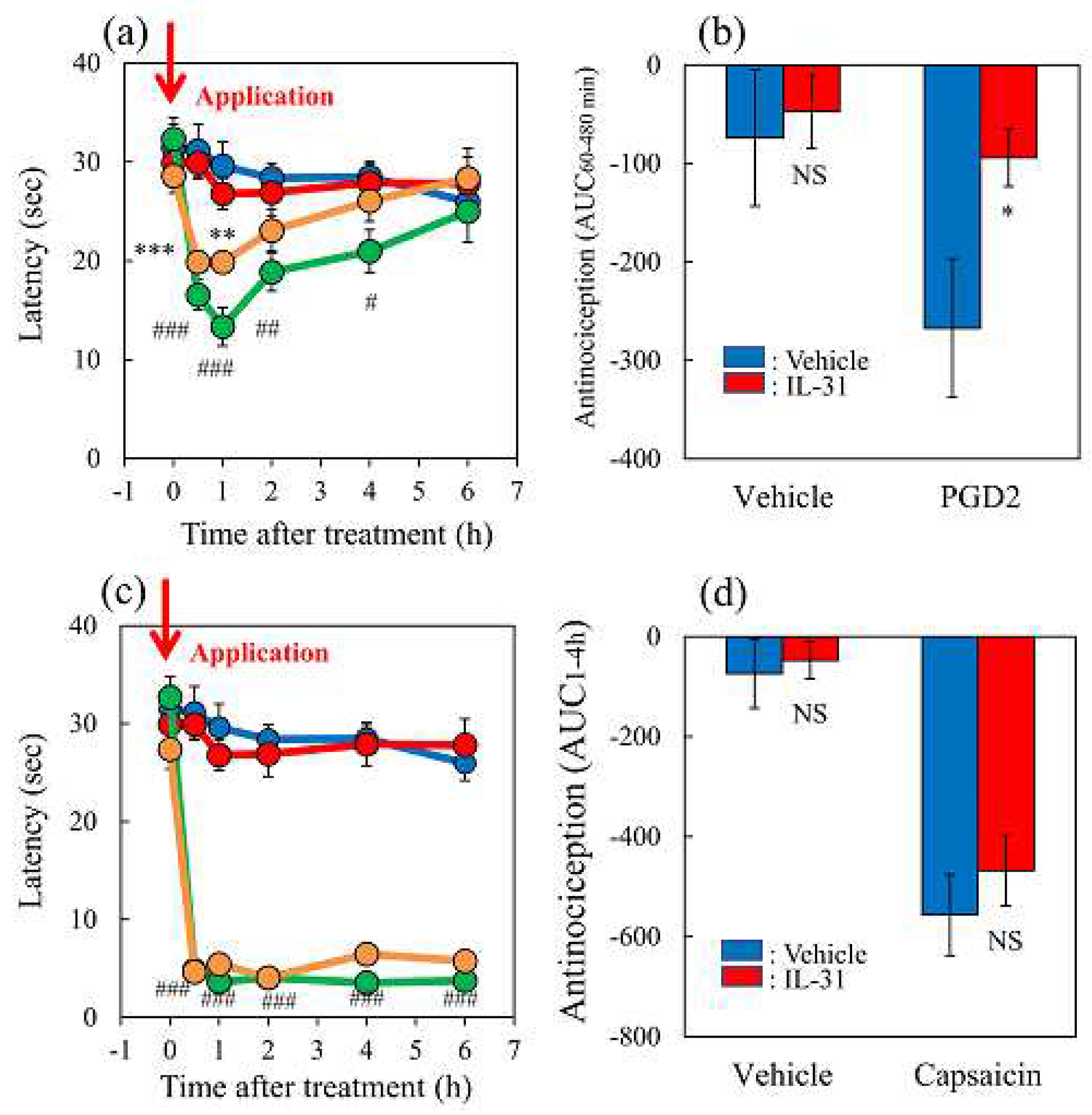
Figure 6.
Effects of morphine on TNCB or capsaicin-applied hot-plate test in wild-type or IL-31RAKI mice. (a) Effect of morphine (3 mg/kg, subcutaneous) on TNCB- or capsaicin-applied hot-plate (45 °C ) test in BALB/c mice. Each value represents the mean ± standard error (S.E.) from 6 mice *p < 0.05 compared with the respective values in vehicle (saline)-treated mice (student’s t-test with Bonferroni correction). (b) Total antinociceptive index of morphine after 30 to 120 min (AUC30–120 min) as per the TNCB or capsaicin-applied hot-plate test. NS, not significant, *p < 0.05 compared with the respective values in vehicle-treated mice (student’s t-test).(c) Effect of morphine on conventional hot-plate (51 °C ) test in wild-type (C57BL/6) and IL-31RAKI (C57BL/6 genetic background) mice. *p < 0.05, and ***p < 0.001 compared with the respective values in vehicle-treated mice (student’s t-test with Bonferroni correction). (d) Total antinociceptive index of morphine after 30 to 120 min (A UC30–120 min) of wild-type and IL-31RAKI mice. The blue line indicates the TNCB + vehicle (saline) treated group; the red line indicates the TNCB + morphine-treated group; the green line indicates the capsaicin + saline-treatd group; the yellow line indicates the capsaicin + morphine-treated group. The red arrow indicates the TNCB or capsaicin application point. Wild, C57BL/6 mice; IL-31RAKI, IL-31 receptor A deficient mice (C57BL/6 genetic background). Each value represents the mean ± standard error (S.E.) from 6 mice (total 48 mice). ***p < 0.001 compared with the respective values in vehicle-treated mice (student’s t-test).
Figure 6.
Effects of morphine on TNCB or capsaicin-applied hot-plate test in wild-type or IL-31RAKI mice. (a) Effect of morphine (3 mg/kg, subcutaneous) on TNCB- or capsaicin-applied hot-plate (45 °C ) test in BALB/c mice. Each value represents the mean ± standard error (S.E.) from 6 mice *p < 0.05 compared with the respective values in vehicle (saline)-treated mice (student’s t-test with Bonferroni correction). (b) Total antinociceptive index of morphine after 30 to 120 min (AUC30–120 min) as per the TNCB or capsaicin-applied hot-plate test. NS, not significant, *p < 0.05 compared with the respective values in vehicle-treated mice (student’s t-test).(c) Effect of morphine on conventional hot-plate (51 °C ) test in wild-type (C57BL/6) and IL-31RAKI (C57BL/6 genetic background) mice. *p < 0.05, and ***p < 0.001 compared with the respective values in vehicle-treated mice (student’s t-test with Bonferroni correction). (d) Total antinociceptive index of morphine after 30 to 120 min (A UC30–120 min) of wild-type and IL-31RAKI mice. The blue line indicates the TNCB + vehicle (saline) treated group; the red line indicates the TNCB + morphine-treated group; the green line indicates the capsaicin + saline-treatd group; the yellow line indicates the capsaicin + morphine-treated group. The red arrow indicates the TNCB or capsaicin application point. Wild, C57BL/6 mice; IL-31RAKI, IL-31 receptor A deficient mice (C57BL/6 genetic background). Each value represents the mean ± standard error (S.E.) from 6 mice (total 48 mice). ***p < 0.001 compared with the respective values in vehicle-treated mice (student’s t-test).
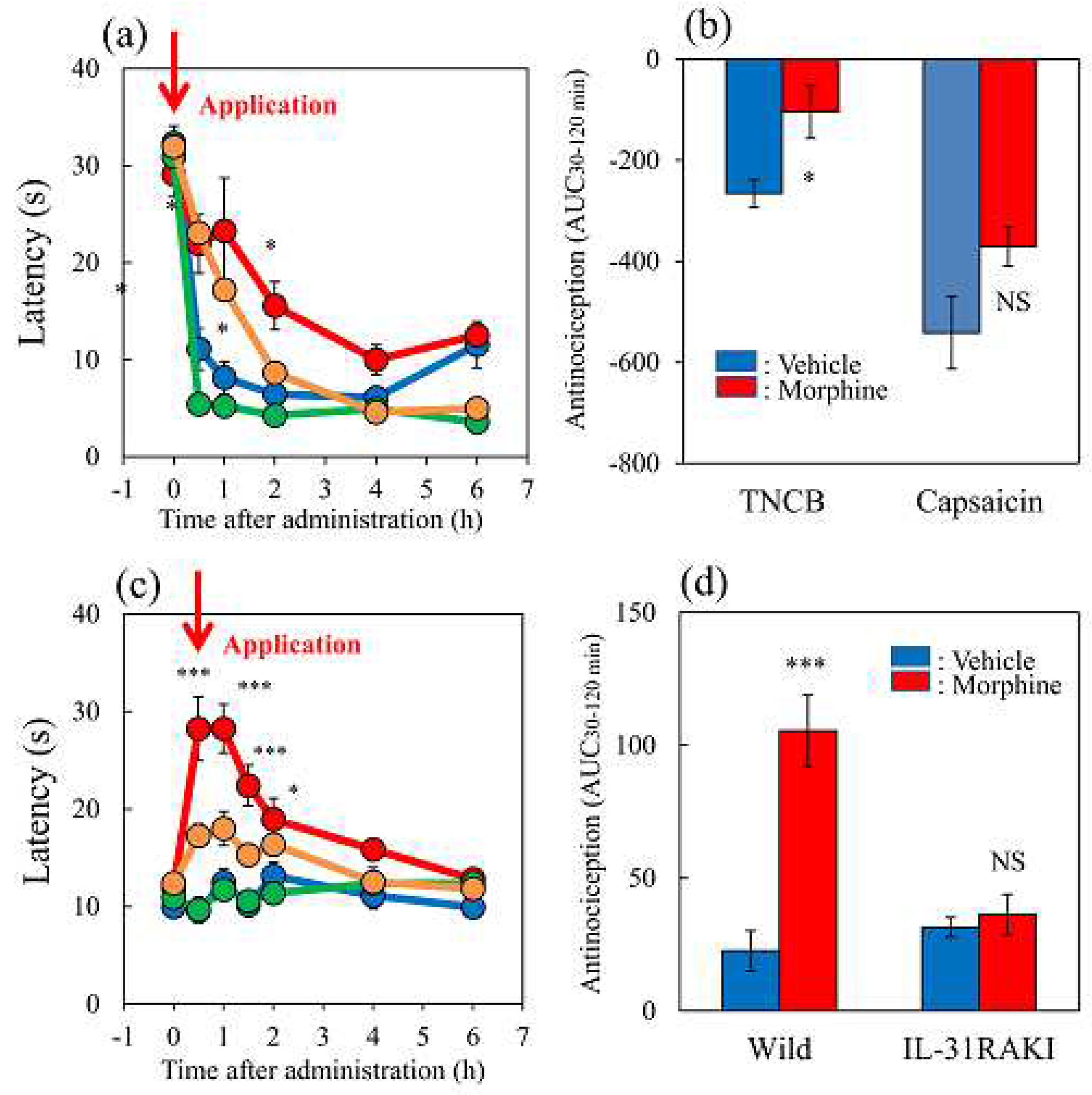
Figure 7.
Effects of loxoprofen on TNCB or capsaicin-applied the hot-plate test in wild-type and IL-31RAKI mice. (a) Effect of loxoprofen on TNCB or capsaicin-applied hot-plate (45 °C ) test in BALB/c mice. Each value represents the mean ± standard error (S.E.). *p < 0.05 compared with the respective values in the vehicle (carboxymethyl cellulose sodium saline, CMC, 10 mL/kg, oral)-treated mice (student’s t-test with Bonferroni correction). (b) Total antinociceptive index of loxoprofen after 0.5 to 6 h (AUC0.5–6 h) as per the TNCB or capsaicin-applied hot-plate test in BALB/c mice. Each value represents the mean ± standard error (S.E.) from 6 mice. NS, not significant, *p < 0.05 compared with the respective values in the CMC-treated mice (student’s t-test). (c) Effect of loxoprofen on TNCB-applied hot-plate (45 °C) test in wild-type (C57BL/6) and IL-31RAKI (C57BL/6 genetic background) mice. Each value represents the mean ± standard error (S.E.). **p < 0.01 compared with the respective values in the CMC-treated mice (student’s t-test with Bonferroni correction). (d) Total antinociceptive index of loxoprofen (AUC0.5–6 h) of wild-type and IL-31RAKI mice. *p < 0.001 compared with the respective values in vehicle-treated mice (student’s t-test). The blue line indicates TNCB + CMC-treated group; the red line indicates TNCB + loxoprofen treated (15 mg/kg, oral) group; the green line indicates capsaicin + CMC-treated group; the yellow line indicates capsaicin + loxoprofen treated group. The red arrow indicates the TNCB or capsaicin application point. Wild, C57BL/6 mice; IL-31RAKI, IL-31 receptor A deficient mice (C57BL/6 genetic background). Each value represents the mean ± standard error (S.E.) from 6 mice (total 48 mice).
Figure 7.
Effects of loxoprofen on TNCB or capsaicin-applied the hot-plate test in wild-type and IL-31RAKI mice. (a) Effect of loxoprofen on TNCB or capsaicin-applied hot-plate (45 °C ) test in BALB/c mice. Each value represents the mean ± standard error (S.E.). *p < 0.05 compared with the respective values in the vehicle (carboxymethyl cellulose sodium saline, CMC, 10 mL/kg, oral)-treated mice (student’s t-test with Bonferroni correction). (b) Total antinociceptive index of loxoprofen after 0.5 to 6 h (AUC0.5–6 h) as per the TNCB or capsaicin-applied hot-plate test in BALB/c mice. Each value represents the mean ± standard error (S.E.) from 6 mice. NS, not significant, *p < 0.05 compared with the respective values in the CMC-treated mice (student’s t-test). (c) Effect of loxoprofen on TNCB-applied hot-plate (45 °C) test in wild-type (C57BL/6) and IL-31RAKI (C57BL/6 genetic background) mice. Each value represents the mean ± standard error (S.E.). **p < 0.01 compared with the respective values in the CMC-treated mice (student’s t-test with Bonferroni correction). (d) Total antinociceptive index of loxoprofen (AUC0.5–6 h) of wild-type and IL-31RAKI mice. *p < 0.001 compared with the respective values in vehicle-treated mice (student’s t-test). The blue line indicates TNCB + CMC-treated group; the red line indicates TNCB + loxoprofen treated (15 mg/kg, oral) group; the green line indicates capsaicin + CMC-treated group; the yellow line indicates capsaicin + loxoprofen treated group. The red arrow indicates the TNCB or capsaicin application point. Wild, C57BL/6 mice; IL-31RAKI, IL-31 receptor A deficient mice (C57BL/6 genetic background). Each value represents the mean ± standard error (S.E.) from 6 mice (total 48 mice).
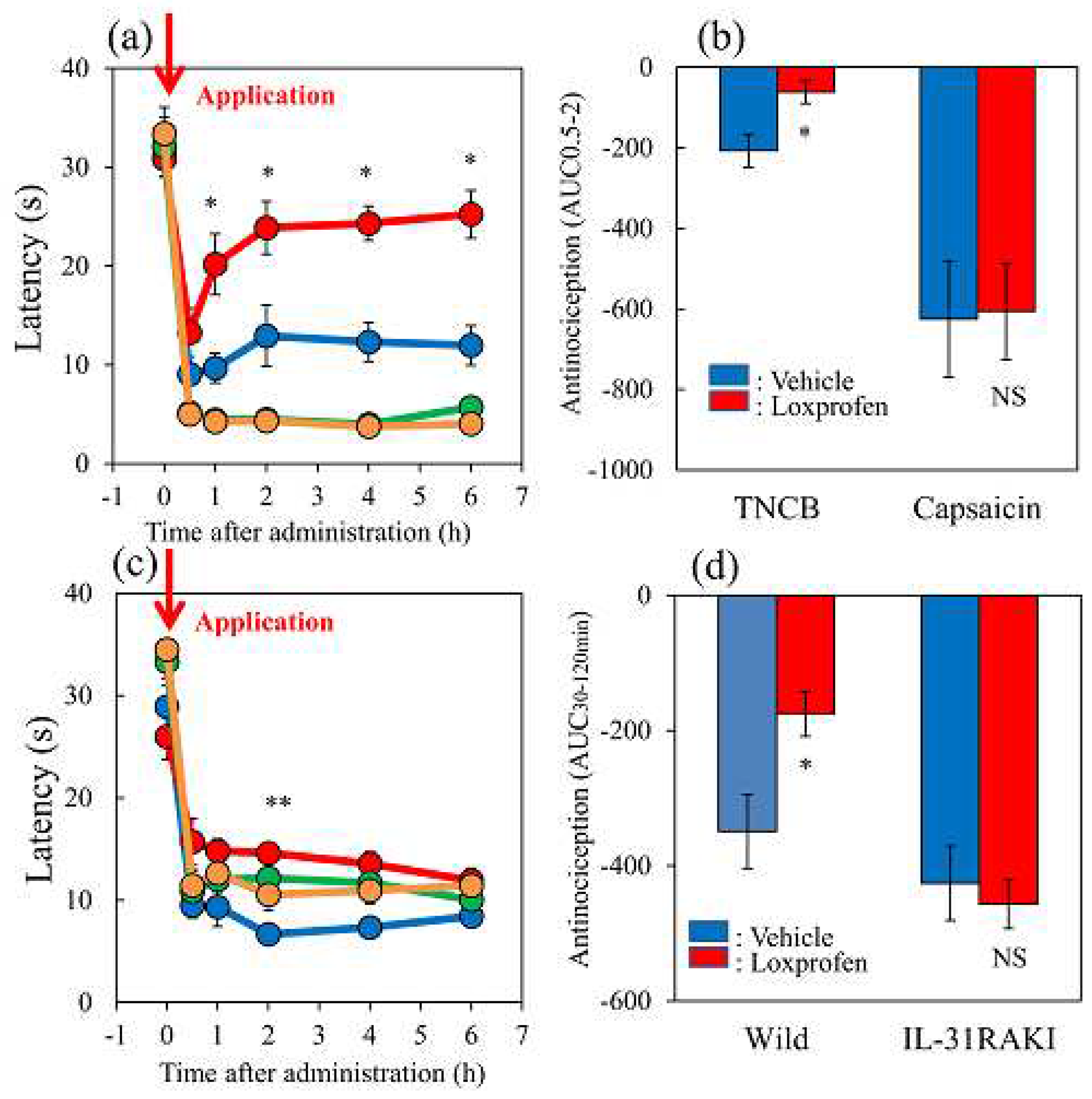
Figure 8.
Effects of acetaminophen on TNCB or capsaicin-applied hot-plate (35 °C) test in wild-type and IL-31RAKI mice. (a) Effect of acetaminophen (300 mg/kg, oral) on TNCB or capsaicin-applied hot-plate (35 °C) test in BALB/c mice. Each value represents the mean ± standard error (S.E.).*p < 0.05, **p < 0.01, and ***p < 0.001 compared with the respective values in vehicle (carboxymethyl cellulose sodium, CMC, 10 mL/kg, oral)-treated mice (student’s t-test with Bonferroni correction). (b) Total antinociceptive index of acetaminophen after 30–120 min (AUC 30–120 min) as per the TNCB or capsaicin-applied hot-plate test in BALB/c mice. Each value represents the mean ± standard error (S.E.).**p < 0.01 compared with the respective values in vehicle-treated mice (student’s t-test). (c) Effect of acetaminophen on TNCB-applied hot-plate (35 °C) test in wild-type (C57BL/6) mice and IL-31RAKI (C57BL/6 genetic background) mice. Each value represents the mean ± standard error (S.E.). *p < 0.05 compared with the respective values in the CMC-treated mice (student’s t-test with Bonferroni correction). (d) Total antinociceptive index of acetaminophen after 30–120 min (AUC30–120 min) of wild-type and IL-31RAKI mice. The blue line indicates the TNCB + CMC-treated group; the red line indicates the TNCB + acetaminophen treated group; the green line indicates the capsaicin + CMC-treated group; the yellow line indicates capsaicin + acetaminophen treated group. The red arrow indicates the TNCB or capsaicin application point. Wild, C57BL/6 mice; IL-31RAKI, IL-31 receptor A deficient mice (C57BL/6 genetic background). Each value represents the mean ± standard error (S.E.) from 6 mice (total 48 mice).
Figure 8.
Effects of acetaminophen on TNCB or capsaicin-applied hot-plate (35 °C) test in wild-type and IL-31RAKI mice. (a) Effect of acetaminophen (300 mg/kg, oral) on TNCB or capsaicin-applied hot-plate (35 °C) test in BALB/c mice. Each value represents the mean ± standard error (S.E.).*p < 0.05, **p < 0.01, and ***p < 0.001 compared with the respective values in vehicle (carboxymethyl cellulose sodium, CMC, 10 mL/kg, oral)-treated mice (student’s t-test with Bonferroni correction). (b) Total antinociceptive index of acetaminophen after 30–120 min (AUC 30–120 min) as per the TNCB or capsaicin-applied hot-plate test in BALB/c mice. Each value represents the mean ± standard error (S.E.).**p < 0.01 compared with the respective values in vehicle-treated mice (student’s t-test). (c) Effect of acetaminophen on TNCB-applied hot-plate (35 °C) test in wild-type (C57BL/6) mice and IL-31RAKI (C57BL/6 genetic background) mice. Each value represents the mean ± standard error (S.E.). *p < 0.05 compared with the respective values in the CMC-treated mice (student’s t-test with Bonferroni correction). (d) Total antinociceptive index of acetaminophen after 30–120 min (AUC30–120 min) of wild-type and IL-31RAKI mice. The blue line indicates the TNCB + CMC-treated group; the red line indicates the TNCB + acetaminophen treated group; the green line indicates the capsaicin + CMC-treated group; the yellow line indicates capsaicin + acetaminophen treated group. The red arrow indicates the TNCB or capsaicin application point. Wild, C57BL/6 mice; IL-31RAKI, IL-31 receptor A deficient mice (C57BL/6 genetic background). Each value represents the mean ± standard error (S.E.) from 6 mice (total 48 mice).
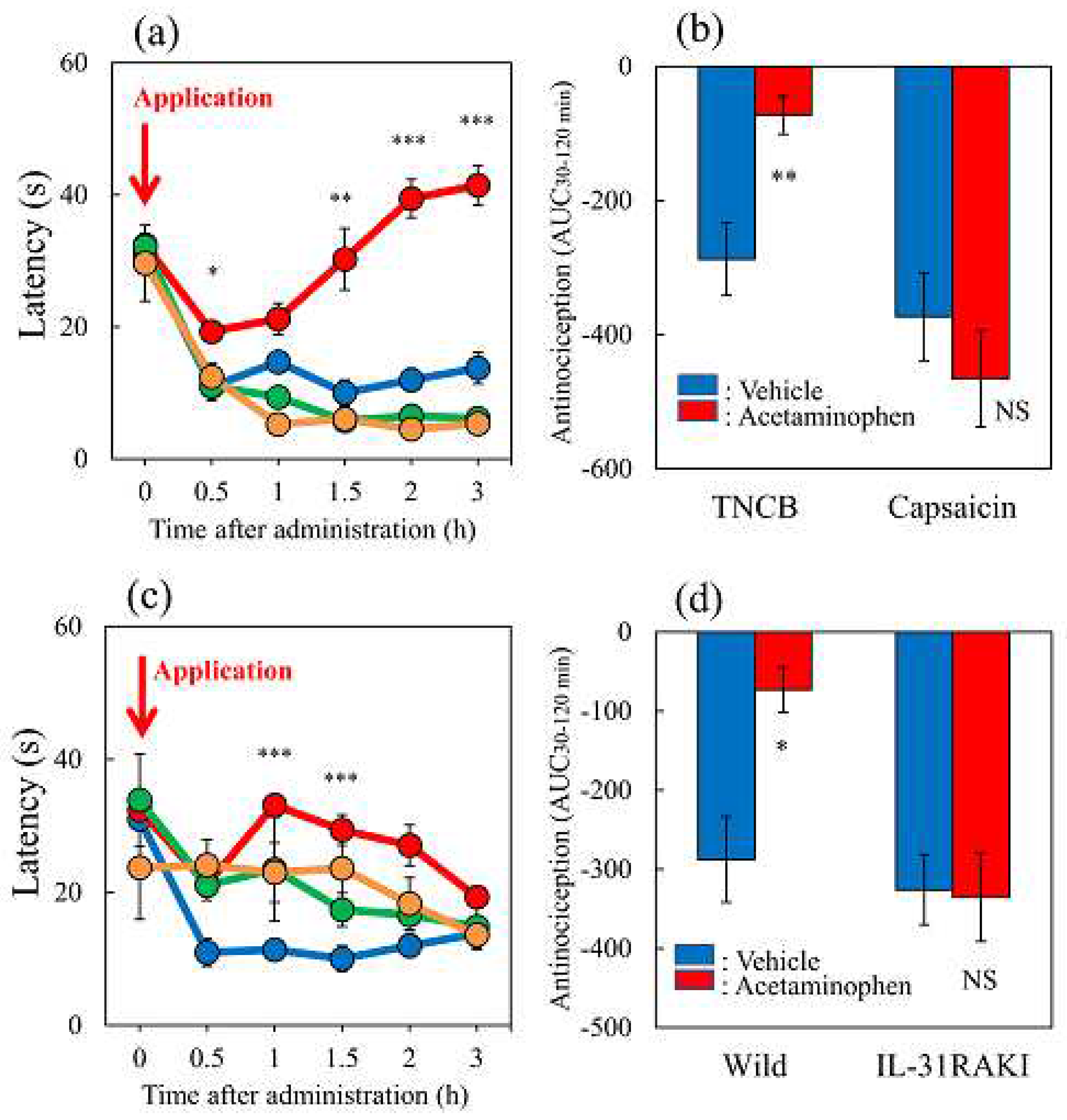
Figure 9.
Long-term changes after TNCB or capsaicin application on the sense of itch and pain. (a) Effects of topical application of TNCB or capsaicin on modified hot-plate (45 °C) test in wild-type and IL-31RAKI mice. The blue line indicates the vehicle (acetone-ethanol mixed liquor, AEM) treated mice; the red line indicates the TNCB-treated mice; the green line indicates the capsaicin-treated mice; the yellow line indicates the TNCB-treated IL-31RAKI mice. (b) Effects of topical application of TNCB or capsaicin on IL-31RA expression in the DRG. The blue line indicates AEM-treated mice; the red line indicates TNCB treated mice; the green line indicates capsaicin-treated mice (c) Effects of topical application of TNCB or capsaicin on itch-associated behavior counts (LLS counts/24 h). The blue line indicates AEM-treated mice; the red line indicates TNCB-treated mice; the green line indicates capsaicin-treated mice. The red arrow indicates the AEM or TNCB or capsaicin application point. Each value represents the mean ± standard error (S.E.) from 6 mice (total 54 mice). *p < 0.05, **p < 0.01, and ***p < 0.001 compared with the respective pretreatment values of each group (student’s t-test with Bonferroni correction). (d) Time-course change of topical application of capsaicin on IL-31-induced LLS counts. The blue arrows indicate the IL-31 (1 mg/kg, intravenous) administration point. The red arrow indicates the capsaicin (1%, 0.2 mL/site) application point. Each value represents the mean ± standard error (S.E.) from 6 mice. *p < 0.05, **p < 0.01, and ***p < 0.001 compared with IL-31-induced LLS counts before capsaicin application (student’s t-test with Bonferroni correction).
Figure 9.
Long-term changes after TNCB or capsaicin application on the sense of itch and pain. (a) Effects of topical application of TNCB or capsaicin on modified hot-plate (45 °C) test in wild-type and IL-31RAKI mice. The blue line indicates the vehicle (acetone-ethanol mixed liquor, AEM) treated mice; the red line indicates the TNCB-treated mice; the green line indicates the capsaicin-treated mice; the yellow line indicates the TNCB-treated IL-31RAKI mice. (b) Effects of topical application of TNCB or capsaicin on IL-31RA expression in the DRG. The blue line indicates AEM-treated mice; the red line indicates TNCB treated mice; the green line indicates capsaicin-treated mice (c) Effects of topical application of TNCB or capsaicin on itch-associated behavior counts (LLS counts/24 h). The blue line indicates AEM-treated mice; the red line indicates TNCB-treated mice; the green line indicates capsaicin-treated mice. The red arrow indicates the AEM or TNCB or capsaicin application point. Each value represents the mean ± standard error (S.E.) from 6 mice (total 54 mice). *p < 0.05, **p < 0.01, and ***p < 0.001 compared with the respective pretreatment values of each group (student’s t-test with Bonferroni correction). (d) Time-course change of topical application of capsaicin on IL-31-induced LLS counts. The blue arrows indicate the IL-31 (1 mg/kg, intravenous) administration point. The red arrow indicates the capsaicin (1%, 0.2 mL/site) application point. Each value represents the mean ± standard error (S.E.) from 6 mice. *p < 0.05, **p < 0.01, and ***p < 0.001 compared with IL-31-induced LLS counts before capsaicin application (student’s t-test with Bonferroni correction).
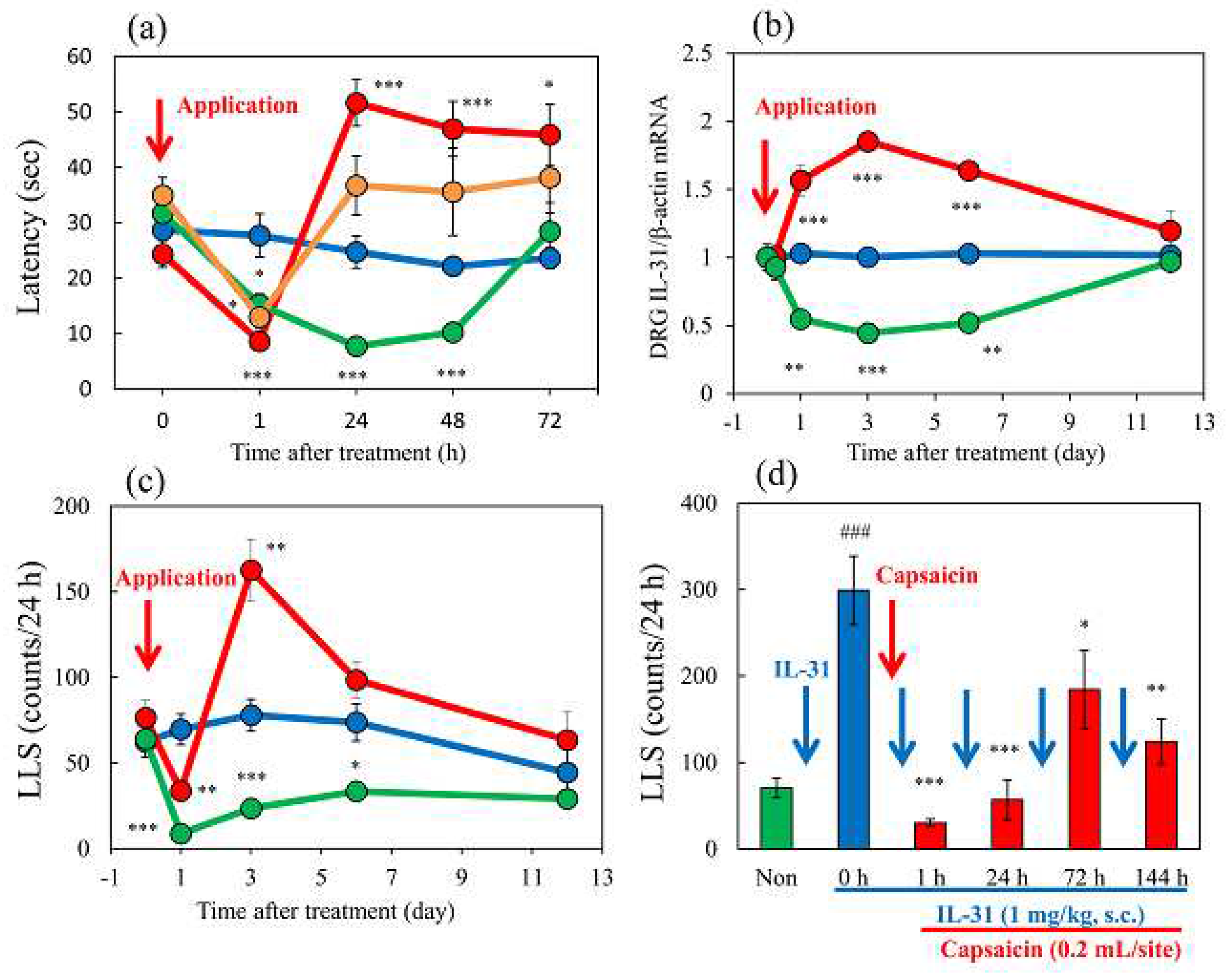
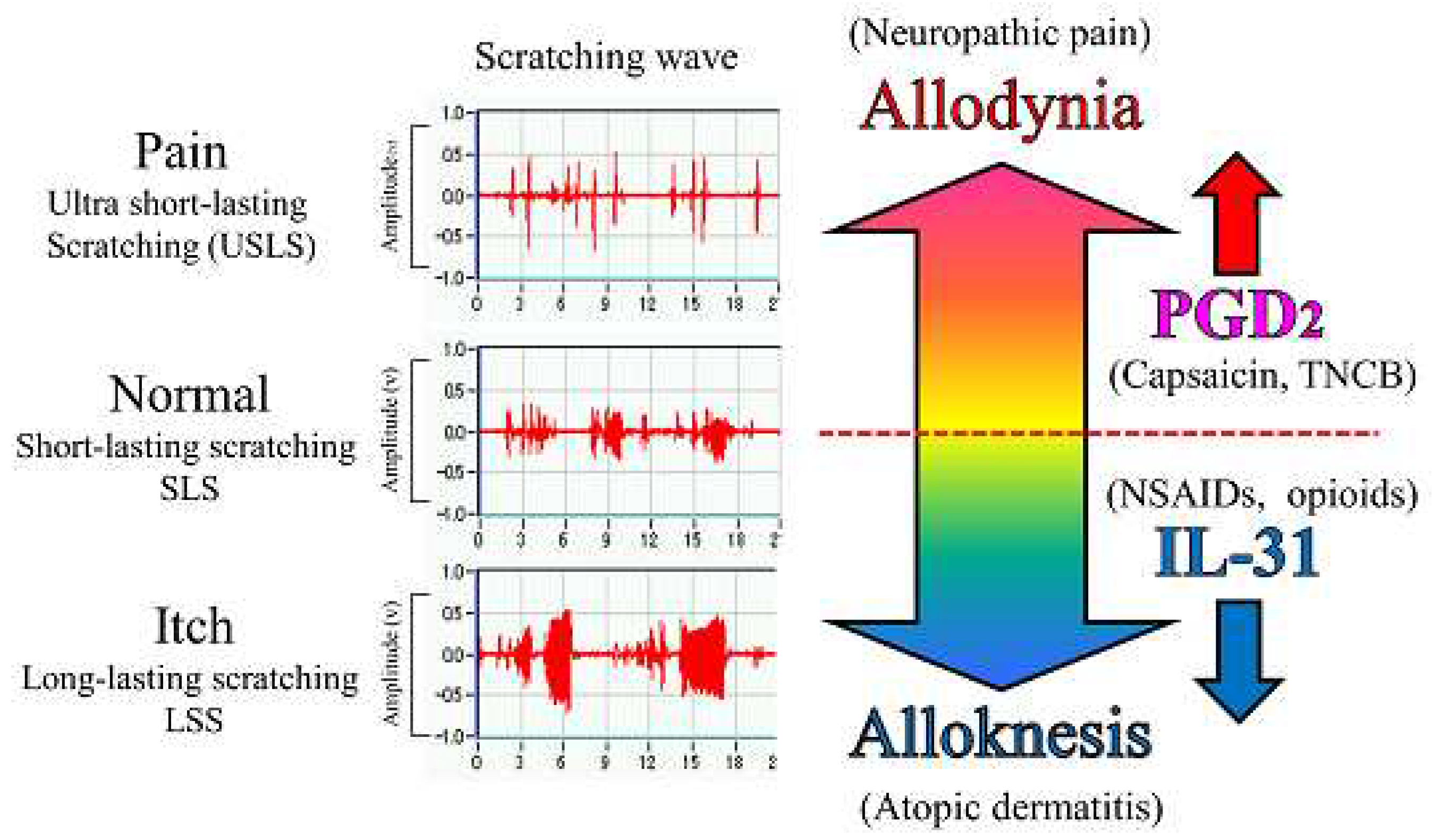
Figure 10.
Schematic diagram of the putative roles of IL-31 and PGD2 in the regulation of the sensation of cutaneous itch and pain in mice. We evaluated a wave pattern of scratching behavior by the movement of the hind leg of the mouse objectively. In normal conditions, the mouse elicits 0.3-1.0 s lasting scratching behavior (SLS) rising caused by several stimulants. Under itchy conditions, the mouse elicits over 1.0 s lasting scratching behavior (LLS) rising caused by IL-31. If this stimulation continues for a long time, it may become atopic dermatitis. Under painful conditions, the mouse elicits below 0.3 s lasting scratching behavior (USLS) rising caused by chronic inflammatory stimulant. If this stimulation continues for a long time, it may become chronic neuropathic pain. IL-31 can change non-selective stimulation into itch stimulation. In contrast, PGD2 can change non-selective stimulation into pain stimulation transmitted by the primary nerves of C-fibers and by second-order nerves and spinothalamic tract neurons in the spinal cord. This suggests that IL-31 and PGD2 regulate the perception of sense (pain or itch) through their mutual functional antagonism.
Figure 10.
Schematic diagram of the putative roles of IL-31 and PGD2 in the regulation of the sensation of cutaneous itch and pain in mice. We evaluated a wave pattern of scratching behavior by the movement of the hind leg of the mouse objectively. In normal conditions, the mouse elicits 0.3-1.0 s lasting scratching behavior (SLS) rising caused by several stimulants. Under itchy conditions, the mouse elicits over 1.0 s lasting scratching behavior (LLS) rising caused by IL-31. If this stimulation continues for a long time, it may become atopic dermatitis. Under painful conditions, the mouse elicits below 0.3 s lasting scratching behavior (USLS) rising caused by chronic inflammatory stimulant. If this stimulation continues for a long time, it may become chronic neuropathic pain. IL-31 can change non-selective stimulation into itch stimulation. In contrast, PGD2 can change non-selective stimulation into pain stimulation transmitted by the primary nerves of C-fibers and by second-order nerves and spinothalamic tract neurons in the spinal cord. This suggests that IL-31 and PGD2 regulate the perception of sense (pain or itch) through their mutual functional antagonism.