1. Introduction
Advances in neonatology have increased the survival rates of premature infants, yet long term complications remain a major consequence of prematurity [
1]. Complications in the neonatal period, including respiratory failure, intraventricular hemorrhage, and sepsis, are closely associated with the degree of prematurity [
1,
2,
3]. Longer term complications, including neurodevelopmental disabilities and social deficits are in turn strongly associated with prior development of those short-term morbidities [
4]. Clinicians are challenged to aggressively treat neonates with potential sepsis to avoid the profound complications that can follow delayed antibiotic initiation [
5], but also avoid antibiotic use in infants without clear signs of infection to minimize antibiotic resistance or alterations in the microbiome [
6]. However, diagnosis has historically relied on nonspecific blood counts and bacterial cultures that can take days to result. For these reasons, there is demand for fast and reliable markers of neonatal infection in those where there is a desire to avoid initiating antibiotic therapy immediately upon delivery.
While several biomarkers have been investigated to clarify their roles in improving neonatal care and antibiotic stewardship, several potential markers of inflammation are of particular interest [
7]. Interleukin-6 (IL-6) is a pleiotropic cytokine produced during the acute phases of inflammation, and it modulates the inflammatory response of CD4 and CD8 T cells and B cells [
8]. C-reactive protein (CRP) is produced primarily in the liver, and its production significantly increases in response to pro-inflammatory cytokines to amplify the activation of the complement system [
9]. Monocyte chemoattractant protein-1 (MCP-1) is a chemokine prominently involved, among other roles, in the recruitment of leukocytes to areas of inflammation [
10].
Several factors have limited the widespread use of biomarkers in the diagnosis of neonatal sepsis, including assay challenges, and a lack of normative values for infants across gestational ages with variable severities of other illnesses. For example, Qiu and colleagues reported cutoff levels of IL-6 for the diagnosis of sepsis in moderately preterm infants in the context of premature rupture of membranes, yet they did not extrapolate to uncomplicated births or other maternal morbidities [
11]. While baseline hormone levels have been reported for moderately preterm and term neonates [
12,
13,
14], there are insufficient reference data for extremely preterm infants. Filling these gaps in the literature offers the potential for clinical use of biomarkers to more accurately and appropriately dictate care for this vulnerable population [
15].
The aims of this study were to investigate the relationship amongst inflammatory biomarkers in neonates within the first week of life, and further report on biomarker levels within an extremely preterm population. We hypothesized that suspected prenatal infection and the diagnosis of postnatal infection would be associated with unique biomarker signatures, and those patterns would be influenced by the degree of prematurity. In addition to providing normative values, our results reveal distinct biomarker profiles in infants that are likely or unlikely to have postnatal infection and could thus benefit from or avoid antibiotic therapy.
2. Materials and Methods
All infants without congenital anomalies born between 22- and 32-weeks gestation admitted to the University of Iowa Stead Family Children’s Hospital neonatal intensive care unit were eligible for enrollment [
16]. Infants were prospectively enrolled in three predefined birth cohorts, 22 to 25 weeks, 26 to 29 weeks, and 30 to 32 weeks. Informed parental consent was obtained prior to enrollment and institutional approval was obtained (IRB #201510835). CRP values were retroactively obtained from the electronic medical record (Epic, Verona, WI, USA) as part of the Retrospective Neonatal Research Studies (IRB #201410743). MCP-1 and IL-6 were measured on 200 microliters of blood collected daily for 7 days and then weekly until achievement of either discharge or maturation to 36 weeks postmenstrual age. Plasma was stored at −80 degrees Celsius, and samples were analyzed using a customized magnetic bead assay (Millipore Sigma, Burlington, MA, USA) on BioPlex 200 with BioPlex manager 6.1 software (Bio-Rad, Hercules, CA, USA). All clinically recorded data was stored in REDCap version 8.3.2 (Vanderbilt, TN, USA).
The cause of preterm delivery was categorized as suspected infection if there was spontaneous (non-induced) preterm labor, clinically diagnosed chorioamnionitis or otherwise unexplained fetal distress that prompted preterm delivery. In the absence of those indications, delivery as a consequence of maternal preeclampsia, abnormal placentation (e.g., accreta or previa with abruption), cervical insufficiency or maternal morbidities (e.g., cancer or heart failure) were categorized as “no suspicion of prenatal infection”. Given historically very low rates of culture-proven sepsis at our institution and a desire to enhance the clinical utility of our investigation by having it conform with our neonatal standard of care that completes a full course of antibiotics for infants with a clinical course consistent with infection even if all cultures are negative, postnatal infection was predefined clinically as the administration of 7 or more days of intravenous antibiotics.
Statistical analysis was performed using SigmaPlot version 14 (Systat Software, San Jose, CA, USA) and GraphPad Prism version 7.0 (GraphPad Software, La Jolla, CA, USA). Continuous variables were analyzed by the Kruskal-Wallis test while categorical variables were analyzed by Chi-Square tests. Linear regression was utilized to correlate biomarkers with birth weight, chronological age, or postmenstrual age. One-way ANOVA was utilized to compare biomarker levels across gestational age cohorts, sex, suspected prenatal infection, and diagnosed postnatal infection. Factors that we found significant on univariate analysis were contrasted by two-way ANOVA with the Holm-Sidak Test for multiple comparisons. Data are presented as mean plus or minus the standard error of the mean, and statistical significance is defined by P < 0.05. To minimize the risk of type 1 error, P < 0.01 was used to define statistical significance for the post-hoc subgroup analyses.
3. Results
A total of 142 neonates were enrolled in the study across three predefined gestational age cohorts with 20% born at 22-25 weeks gestation, 36% born at 26-29 weeks gestation, and 44% born at 30-32 weeks (
Table 1). The median gestational age at delivery was 29 weeks, the median birth weight was 1.2 kg, and 53% of the infants were male. Infants born at gestational ages of 22 to 25 weeks were more likely than the other cohorts to have suspected prenatal infection (P < 0.01) or clinically diagnosed postnatal infection (P < 0.01). In contrast, preeclampsia and other maternal morbidities were most common in the cohort born at 30-32 weeks gestation (P < 0.01).
Of 142 blood cultures obtained in the first 7 days following delivery, only one was positive. In that case, the blood culture from admission grew Escherichia coli. Two additional infants had positive blood cultures after day 7; one infant had consecutive blood cultures with Staphylococcus epidermidis, and the other had a single blood culture with Klebsiella pneumonia. For each of the 3 infants that had positive blood cultures, prenatal infection had been suspected prior to admission based on unexpected preterm labor despite no sign of chorioamnionitis, and postnatal infection was diagnosed for all three based on their clinical status. Placental pathology ultimately showed chorioamnionitis and funisitis for the infant with a positive blood culture on admission, but placental pathology was normal for the two infants with positive blood cultures after day 7.
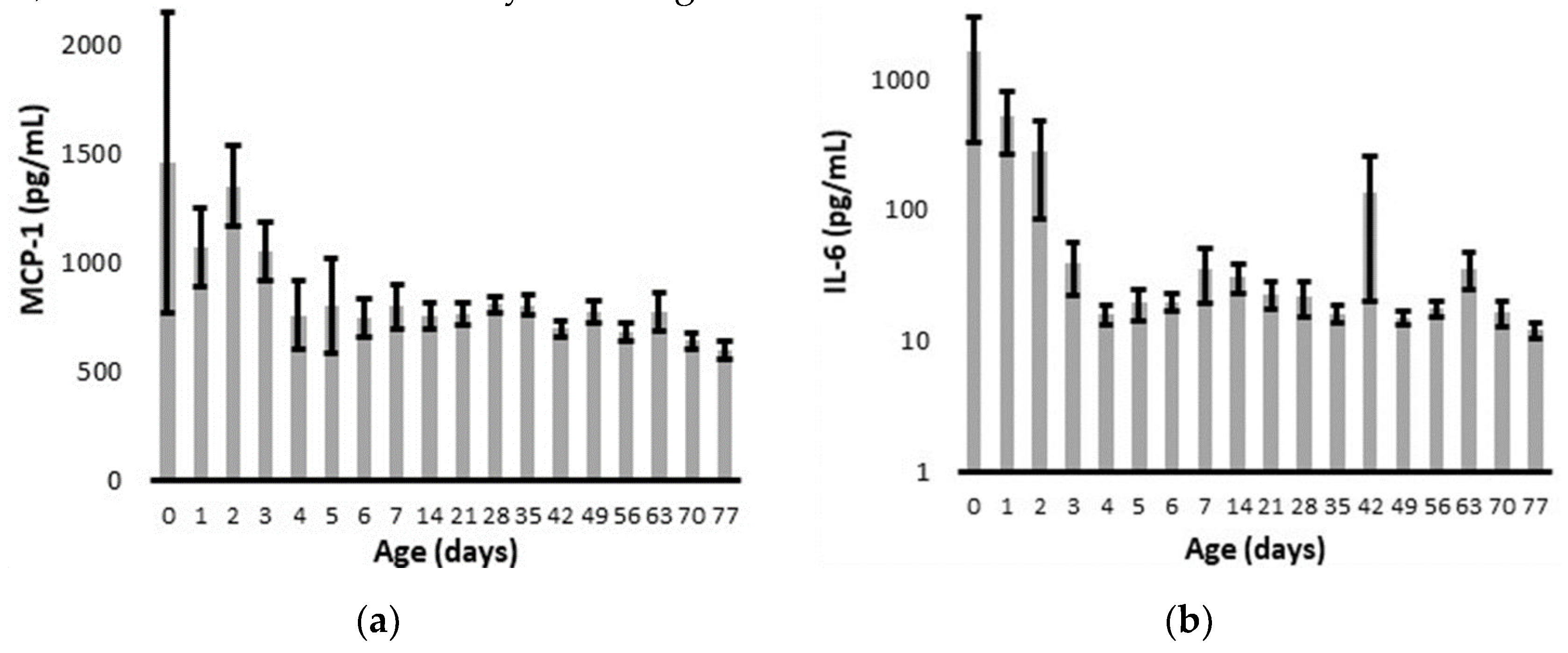
Overall, MCP-1 and IL-6 levels declined to relatively stable values within four days of delivery (
Figure 1). The infant with a positive blood culture for
E.coli on admission had a peak IL-6 of 3887 pg/ml and MCP-1 of only 273 pg/ml on day 1. The CRP on admission was 3.2 mg/dl, and it increased to a peak of 14.7 on day 2. The infant with late onset
K. pneumonia sepsis had a protracted course of multi-organ failure with a peak MCP-1 of 3395 pg/ml, IL-6 of 10424 pg/ml, and CRP of 13.2 mg/dl. The infant with
S. epidermidis sepsis had MCP-1 levels of 655 +/- 115 pg/ml, IL-6 levels of 16 +/- 3 pg/ml, and CRP levels were always < 0.5 mg/dl.
By linear regression across the first 7 days, chronologic age was significantly correlated with both MCP-1 (R = 0.16, P = 0.03) and IL-6 (R = 0.17, P = 0.01). The biomarkers did not vary between male and female infants or across gestational age cohorts, although the difference between gestational age cohorts did approach statistical significance for IL-6 (P =0.06) (
Table 2). By univariate analysis, MCP-1 and CRP levels were increased in infants born without suspected prenatal infection while IL-6 and CRP levels were increased in infants diagnosed with postnatal infection (
Table 2).
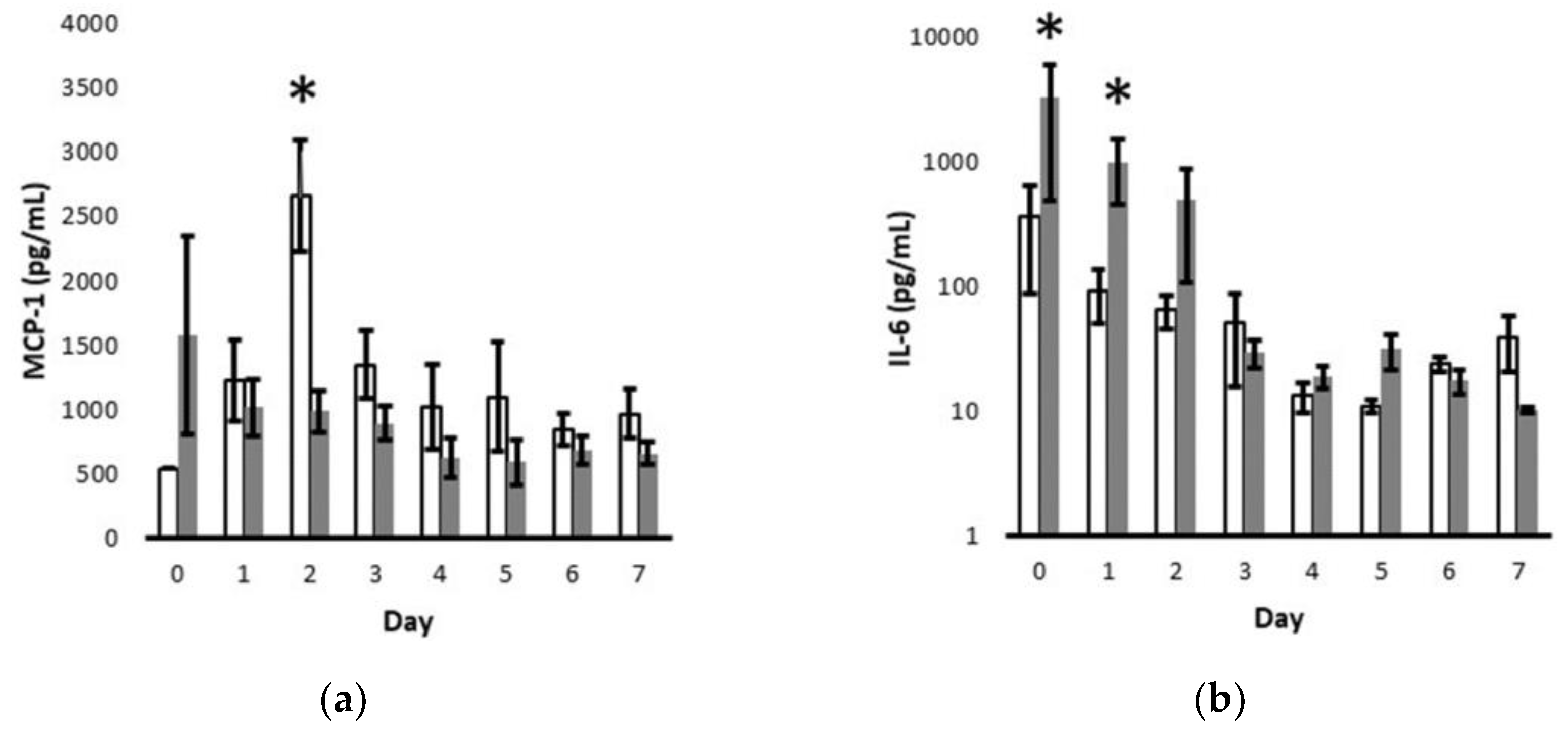
Given the sharp decline in cytokine levels over the critical first 7 days after delivery (
Figure 1), we further interrogated the temporal association of MCP-1 values with clinically suspected prenatal infection and IL-6 values with clinically diagnosed postnatal infection (
Figure 2). By multivariate analysis, the MCP-1 levels were significantly increased on day 2 for those without suspected prenatal infection and IL-6 levels were significantly increased on postnatal days 0 and 1 for those diagnosed with postnatal infection.
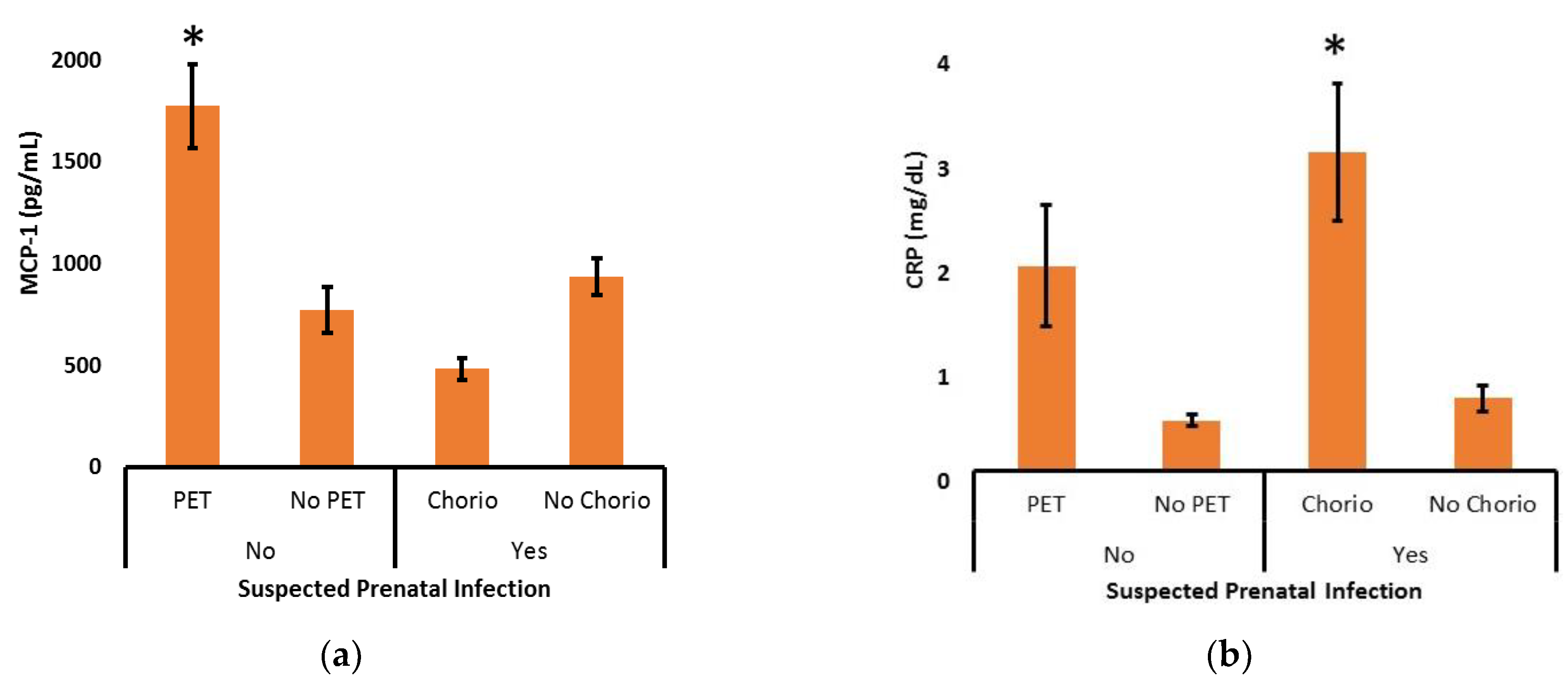
To further explore the relationship between suspected prenatal infection and the levels of MCP-1 or CRP across the first 7 days after delivery, post-hoc subgroup analyses were performed (
Figure 3). In the absence of suspected prenatal infection, the increase in MCP-1 was strongly associated with the diagnosis of maternal preeclampsia (P <0.001). Likewise, CRP tended to be high in the presence of maternal preeclampsia, but that did not reach statistical significance (P = 0.06). In the presence of suspected prenatal infection, a decrease in MCP-1 was seen in those diagnosed with chorioamnionitis, but that did not reach statistical significance (P = 0.09). In sharp contrast, postnatal CRP levels were significantly increased in the context of maternal chorioamnionitis (P < 0.001).
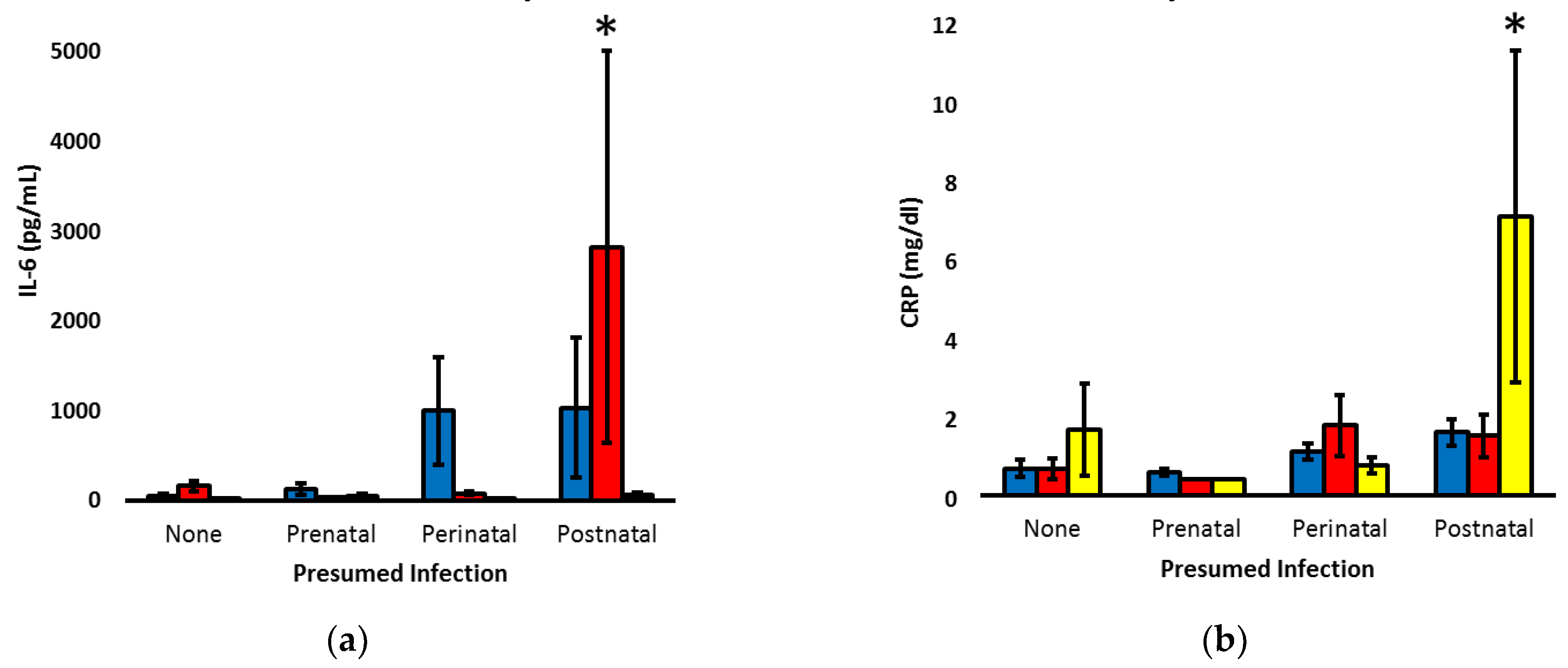
To explore the temporal patterns of CRP and IL-6, the markers significantly associated with postnatal infection, infants were re-classified as having no concern for infection, only suspected prenatal infection, suspected prenatal and postnatal infection (perinatal infection) or only postnatal infection. Two-way ANOVA was utilized to assess the patterns on days 1 to 3, when clinicians are most often debating the need to initiate or discontinue antibiotic therapy in the context of prenatal and postnatal clinical status (
Figure 4). Compared to the other cohorts, infants with isolated postnatal infection had increased IL-6 on day 2 (P < 0.001) and increased CRP on day 3 (P < 0.01).
Although our primary analysis was based on real-time clinical decisions regarding the likelihood of infection, we performed secondary analysis of biomarker levels based on final placental pathology reports. Of 142 infants, 112 (79%) had placental pathology and 51 (46%) of those placentas had chorioamnionitis, while 23 (45%) of the placentas with chorioamnionitis also had funisitis. Chorioamnionitis was seen on pathology for all infants that had previously been clinically diagnosed with chorioamnionitis. By univariate analysis, MCP-1 was decreased (P < 0.01) and CRP was increased (P < 0.05) in infants with signs of chorioamnionitis on pathology (
Table 3). The increase in CRP was specifically associated with the presence of funisitis in addition to chorioamnionitis (P < 0.01). Only one mother with preeclampsia also had chorioamnionitis, and the chorioamnionitis was only diagnosed retrospectively, on placental pathology, and it was not associated with funisitis. That infant had the second-highest MCP-1 levels within the chorioamnionitis-positive cohort (mean of 2078 mg/dl) as well as elevated IL-6 and CRP values on day 1 (2946 pg/ml and 5.2 mg/dl, respectively).
4. Discussion
Premature infants are at heightened risk of neonatal complications, and this has contributed to an ongoing demand for new diagnostic methods specific to this early phase of life [
15]. To help address this, the aim of our study was to analyze inflammation biomarkers levels across gestational ages with a focus on the correlations of IL-6 and MCP-1 with clinically diagnosed infections. While we hypothesized that the degree of prematurity would influence the biomarker levels, univariate analysis of GA cohorts did not show significance for IL-6, MCP-1, or CRP. This varies from some other hormones, including the adipokine leptin that is produced by specific tissues and affected if tissue development is not complete at the time of delivery [
16]. Instead of differences based on degree of prematurity, the chronological age of neonates had a significant inverse relationship with IL-6 and MCP-1 levels over the first week after delivery. In the process of defining the potential role of these markers in the clinical diagnosis of infection among infants born as early as 22 weeks gestation, our investigations extend the existing literature by contextualizing biomarker levels based on chronological age, maternal preeclampsia and the clinical suspicion of infection.
We observed a sharp decrease in MCP-1 within days of delivery, and that temporal pattern is consistent with the results seen in 30-week to 32-week gestation infants by Lusyati and colleagues [
13]. In our study, infants that were born following pregnancies complicated by suspected infection or proven chorioamnionitis had the lowest MCP-1 levels. This contrasts with previous reports showing elevation of MCP-1 in older pediatric patients with sepsis [
17], but it is consistent with the chorioamnionitis-induced immune hypo-responsiveness that has been described in several studies [
18,
19]. Beyond a potential for infection-related suppression in MCP-1 production, the decreased level of MCP-1 in pregnancies with suspected infection was linked with the increase in MCP-1 levels among infants born to mothers with preeclampsia, our most common cause for preterm delivery in the absence of preterm labor.
Investigations have identified increased maternal MCP-1 levels in the presence of preeclampsia [
20,
21], but ours is the first investigation we are aware of that has identified increased MCP-1 levels in the offspring of mothers with preeclampsia. MCP-1 is overexpressed in preeclamptic placentas [
22], and while it is also known that MCP-1 levels quickly fall in the newborn’s circulation [
13], it is not known whether the placenta is a source for neonatal MCP-1. Alternatively, a common humoral or genetic factor might drive increased MCP-1 expression in both mother and offspring. For example, heritable MCP-1 gene variants are associated with increased MCP-1 expression and preeclampsia [
23,
24], and that could explain some of the increased risk of cardiovascular disease and preeclampsia in the offspring of preeclamptic mothers [
25,
26]. Ultimately, for the purposes of our investigations, the association of MCP-1 levels with both infectious and non-infections complications of pregnancy suggests poor specificity for its use in the clinical diagnosis of infection [
27].
It is notable that although the increase in CRP levels we observed in infants born to mothers with preeclampsia did not reach statistical significance, the directionality of the observation paralleled the significant increase in MCP-1 associated with preeclampsia. This could again reflect a common etiology, such as an increased inflammatory process. In that regard, multiple investigations have demonstrated increased oxidative stress during preeclamptic pregnancies and there is also the potential for ischemia-reperfusion related changes after delivery has occurred [
28]. With the notable exception of pregnancies complicated by chorioamnionitis, the CRP levels we obtained generally paralleled MCP-1 levels. The marked increase in CRP following the diagnosis of chorioamnionitis with funisitis is consistent with an extensive body of literature and likely reflects the intense inflammation that contributes to the features that are hallmarks of chorioamnionitis [
29]. Beyond chorioamnionitis, prenatal infection was not associated with an increase in biomarker levels, but that is perhaps not surprising given that over half of the patients with suspected prenatal infection had preterm labor as the index of suspicion, and preterm labor is clearly a multifactorial process. Regarding antenatal infection surveillance and confirmation, based on our results, CRP has value in support of the clinical diagnosis of chorioamnionitis.
Both CRP and IL-6 are established biomarkers for postnatal infection, but their relative utility and specificity are debated [
30]. In our investigation, early postnatal CRP levels were increased in association with not just postnatal infection, but also with chorioamnionitis. In those with only postnatal infection, the increase in CRP was not observed until day 3. In contrast, IL-6 was significantly increased on the day of birth in the presence of suspected postnatal infection, and marked elevations were seen on day 2 among infants with isolated concern for postnatal infection. The increase in IL-6 prior to the elevation of CRP follows previous literature on the diagnostic capability of IL-6 and CRP together as reported by Tessema and others [
31,
32,
33], it is also consistent with the well-described effect of IL-6 on subsequent CRP production [
34], and it is very reminiscent of the results seen in late-onset culture proven sepsis [
35].
Our study does have some limitations. Our population consisted of 142 neonates from a single institution. While the diagnosis of clinical sepsis might vary across providers and institutions, our study was designed to reflect upon and inform the current management of infection at an institution with historically high rates of periviable infant survival. Because CRP values were obtained retrospectively, there were more values available for infants undergoing higher scrutiny for possible infection, but this again reflects current clinical practice.
To our knowledge, we are the first group to report on these three biomarkers and their associations with maternal preeclampsia, chorioamnionitis and postnatal infections among a cohort of very preterm infants. Independent of gestational age at delivery, our data suggest a role for expanded biomarker screening, including early postnatal IL-6 levels, to potentially allow earlier and more reliable detection of sepsis to promote proper antibiotic use and earlier discontinuation of antibiotics when they are not needed. Future investigations are needed to improve our understanding of the relationship of preeclampsia and chorioamnionitis with postnatal MCP-1 levels. It is paramount to further investigate the longitudinal biomarker levels of neonates, and the impact of clinical interpretation and utilization of those levels on prospective short-term and long-term clinical outcomes of very preterm infants.
Author Contributions
Conceptualization, B.S., J.B., T.C., M.S. and R.R.; methodology, B.S.; formal analysis, J.E., B.S. and R.R.; investigation, B.S.; resources, D.S. and R.R.; data curation, J.E.; writing—original draft preparation, J.E.; writing—review and editing, B.S., J.B., D.S., T.C., M.S. and R.R.; supervision, J.B., T.C. and R.R.; funding acquisition, B.S. and R.R. All authors have read and agreed to the published version of the manuscript.
Funding
Children’s Miracle Network and the National Center for Advancing Translational Sciences of the National Institutes of Health (UL1TR002537).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and it was approved by the Institutional Review Board of the University of Iowa (IRB numbers 201510835 and 201410743).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available to protect patient confidentiality.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Stoll, B.J.; Hansen, N.I.; Bell, E.F.; Walsh, M.C.; Carlo, W.A.; Shankaran, S.; Laptook, A.R.; Sánchez, P.J.; Van Meurs, K.P.; Wyckoff, M.; Das, A.; Hale, E.C.; Ball, M.B.; Newman, N.S.; Schibler, K.; Poindexter, B.B.; Kennedy, K.A.; Cotten, C.M.; Watterberg, K.L.; D'Angio, C.T.; DeMauro, S.B.; Truog, W.E.; Devaskar, U.; Higgins, R.D. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA 2015, 314, 1039–51. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.; Weitkamp, J.H.; Wynn, J.L. Why are preterm newborns at increased risk of infection? Arch Dis Child Fetal Neonatal Ed 2018, 103, F391–F394. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.M.; Beachy, J.C. Neonatal complications following preterm birth. BJOG 2003, 110, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Moster, D.; Lie, R.T.; Markestad, T. Long-term medical and social consequences of preterm birth. N Engl J Med 2008, 359, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Schmatz, M.; Srinivasan, L.; Grundmeier, R.W.; Elci, O.U.; Weiss, S.L.; Masino, A.J.; Tremoglie, M.; Ostapenko, S.; Harris, M.C. Surviving sepsis in a referral neonatal intensive care unit: association between time to antibiotic administration and in-hospital outcomes. J Pediatr 2020, 217, 59–65.e1. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Claud, E.C. Connection between gut microbiome and brain development in preterm infants. Dev Psychobiol 2019, 61, 739–51. [Google Scholar] [CrossRef]
- Chiesa, C.; Panero, A.; Osborn, J.F.; Simonetti, A.F.; Pacifico, L. Diagnosis of neonatal sepsis: a clinical and laboratory challenge. Clin Chem 2004, 50, 279–87. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol 2014, 6, a016295. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ashworth, J.J. Role of C-reactive protein at sites of inflammation and infection. Front Immunol 2018, 9, 754. [Google Scholar] [CrossRef]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res 2009, 29, 313–26. [Google Scholar] [CrossRef]
- Qiu, X.; Zhang, L.; Tong, Y.; Qu, Y.; Wang, H.; Mu, D. Interleukin-6 for early diagnosis of neonatal sepsis with premature rupture of the membranes: A meta-analysis. Medicine (Baltimore) 2018, 97, e13146. [Google Scholar] [CrossRef] [PubMed]
- Leal, Y.A.; Álvarez-Nemegyei, J.; Lavadores-May, A.I.; Girón-Carrillo, J.L.; Cedillo-Rivera, R.; Velazquez, J.R. Cytokine profile as diagnostic and prognostic factor in neonatal sepsis. J Matern Fetal Neonatal Med 2019, 32, 2830–6. [Google Scholar] [CrossRef] [PubMed]
- Lusyati, S.; Hulzebos, C.V.; Zandvoort, J.; Sauer, P.J. Levels of 25 cytokines in the first seven days of life in newborn infants. BMC Res Notes 2013, 6, 547. [Google Scholar] [CrossRef] [PubMed]
- Tasci, Y.; Dilbaz, B.; Uzmez Onal, B.; Caliskan, E. , Dilbaz S.; Doganci L.; Han U. The value of cord blood interleukin-6 levels for predicting chorioamnionitis, funisitis and neonatal infection in term premature rupture of membranes. Eur J Obstet Gynecol Reprod Biol 2006, 128, 34–9. [Google Scholar] [CrossRef] [PubMed]
- Iroh Tam P., Y.; Bendel C., M. Diagnostics for neonatal sepsis: current approaches and future directions. Pediatr Res 2017, 82, 574–83. [Google Scholar] [CrossRef]
- Steinbrekera, B.; Colaizy, T.T.; Vasilakos, L.K.; Johnson, K.J.; Santillan, D.A.; Haskell, S.E. , Roghair R.D. Origins of neonatal leptin deficiency in preterm infants. Pediatr Res 2019, 85, 1016–23. [Google Scholar] [CrossRef] [PubMed]
- Hassuna, N.A.; Elgezawy, E.; Mousa, S.O.; AbdelAziz, R.A.; Ibrahem, R.A.; Wahed, W.Y.A; Nasif, K.A.; Hefzy, E.M. Diagnostic value of monocyte chemoattractant protein-1, soluble mannose receptor, presepsin, and procalcitonin in critically ill children admitted with suspected sepsis. BMC Pediatr 2021, 21, 458. [Google Scholar] [CrossRef]
- Bermick, J.; Gallagher, K.; denDekker, A.; Kunkel, S.; Lukacs, N.; Schaller, M. Chorioamnionitis exposure remodels the unique histone modification landscape of neonatal monocytes and alters the expression of immune pathway genes. FEBS J 2019, 286, 82–109. [Google Scholar] [CrossRef]
- de Jong, E.; Hancock, D.G.; Wells, C.; Richmond, P.; Simmer, K.; Burgner, D.; Strunk, T.; Currie, A.J. Exposure to chorioamnionitis alters the monocyte transcriptional response to the neonatal pathogen Staphylococcus epidermidis. Immunol Cell Biol 2018, 96, 792–804. [Google Scholar] [CrossRef]
- Szarka, A.; Rigó J., Jr.; Lázár, L.; Beko, G.; Molvarec, A. Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC Immunol 2010, 11, 59. [Google Scholar] [CrossRef]
- Cui, S.; Gao, Y.; Zhang, L.; Wang, Y.; Zhang, L.; Liu, P.; Liu, L.; Chen, J. Combined use of serum MCP-1/IL-10 ratio and uterine artery Doppler index significantly improves the prediction of preeclampsia. Clin Chim Acta 2017, 473, 228–36. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Shi, J.L.; Chen, M.; Zheng, Z.M.; Li, M.Q.; Shao, J. CCL2: An important cytokine in normal and pathological pregnancies: a review. Front Immunol 2023, 13, 1053457. [Google Scholar] [CrossRef] [PubMed]
- McDermott, D.H.; Yang, Q.; Kathiresan, S.; Cupples, L.A.; Massaro, J.M.; Keaney J.F., Jr.; Larson, M.G.; Vasan, R.S.; Hirschhorn, J.N.; O'Donnell, C.J.; Murphy, P.M.; Benjamin, E.J. CCL2 polymorphisms are associated with serum monocyte chemoattractant protein-1 levels and myocardial infarction in the Framingham Heart Study. Circulation 2005, 112, 1113–20. [Google Scholar] [CrossRef] [PubMed]
- Agachan, B.; Attar, R.; Isbilen, E.; Aydogan, HY.; Sozen, S.; Gurdol, F.; Isbir, T. Association of monocyte chemotactic protein-1 and CC chemokine receptor 2 gene variants with preeclampsia. J Interferon Cytokine Res 2010, 30, 673–6. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lin, R.; Yang, L.; Wang, Y.; Mao, B.; Xu, X.; Yu, J. Meta-analysis of cardiovascular risk factors in offspring of preeclampsia pregnancies. Diagnostics (Basel) 2023, 13, 812. [Google Scholar] [CrossRef] [PubMed]
- North, R.A.; McCowan, L.M.; Dekker, G.A.; Poston, L.; Chan, E.H.; Stewart, A.W.; Black, M.A.; Taylor, R.S.; Walker, J.J.; Baker, P.N.; Kenny, L.C. Clinical risk prediction for pre-eclampsia in nulliparous women: development of model in international prospective cohort. BMJ 2011, 342, d1875. [Google Scholar] [CrossRef] [PubMed]
- Iliodromiti, Z.; Anastasiadis, A.; Varras, M.; Pappa, K.I.; Siristatidis, C.; Bakoulas, V.; Mastorakos, G.; Vrachnis, N. Monocyte function in the fetus and the preterm neonate: immaturity combined with functional impairment. Mediators Inflamm 2013, 2013, 753752. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Aranguren LC, Prada CE, Riaño-Medina CE, Lopez M. Endothelial dysfunction and preeclampsia: role of oxidative stress. Front Physiol 2014, 5, 372. [Google Scholar] [CrossRef]
- Ryan, E.; Eves, D.; Menon, P.J.; Alnafisee, S.; Mooney, E.E.; Downey, P.; Culliton, M.; Murphy, J.F.A.; Vavasseur, C.; Molloy, EJ. Histological chorioamnionitis is predicted by early infant C-reactive protein in preterm infants and correlates with neonatal outcomes. Acta Paediatr 2020, 109, 720–7. [Google Scholar] [CrossRef]
- Eichberger, J.; Resch, B. Reliability of interleukin-6 alone and in combination for diagnosis of early onset neonatal sepsis: systematic review. Front Pediatr 2022, 10, 840778. [Google Scholar] [CrossRef]
- Celik, I.H.; Demirel, F.G.; Uras, N.; Oguz, S.S.; Erdeve, O.; Biyikli, Z.; Dilmen, U. What are the cut-off levels for IL-6 and CRP in neonatal sepsis? J Clin Lab Anal 2010, 24, 407–12. [Google Scholar] [CrossRef] [PubMed]
- Ebenebe, C.U.; Hesse, F.; Blohm, M.E.; Jung, R.; Kunzmann, S.; Singer, D. Diagnostic accuracy of interleukin-6 for early-onset sepsis in preterm neonates. J Matern Fetal Neonatal Med 2021, 34, 253–8. [Google Scholar] [CrossRef] [PubMed]
- Tessema, B.; Lippmann, N.; Willenberg, A.; Knüpfer, M.; Sack, U.; König, B. The diagnostic performance of interleukin-6 and C-reactive protein for early identification of neonatal sepsis. Diagnostics (Basel) 2020, 10, 978. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, P.C.; Castell, J.V.; Andus, T. Interleukin-6 and the acute phase response. Biochem J 1990, 265, 621–36. [Google Scholar] [CrossRef]
- Küster, H.; Weiss, M.; Willeitner, A.E.; Detlefsen, S.; Jeremias, I.; Zbojan, J.; Geiger, R.; Lipowsky, G.; Simbruner, G. Interleukin-1 receptor antagonist and interleukin-6 for early diagnosis of neonatal sepsis 2 days before clinical manifestation. Lancet 1998, 352, 1271–7. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).