Submitted:
15 May 2023
Posted:
16 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Case Presentation
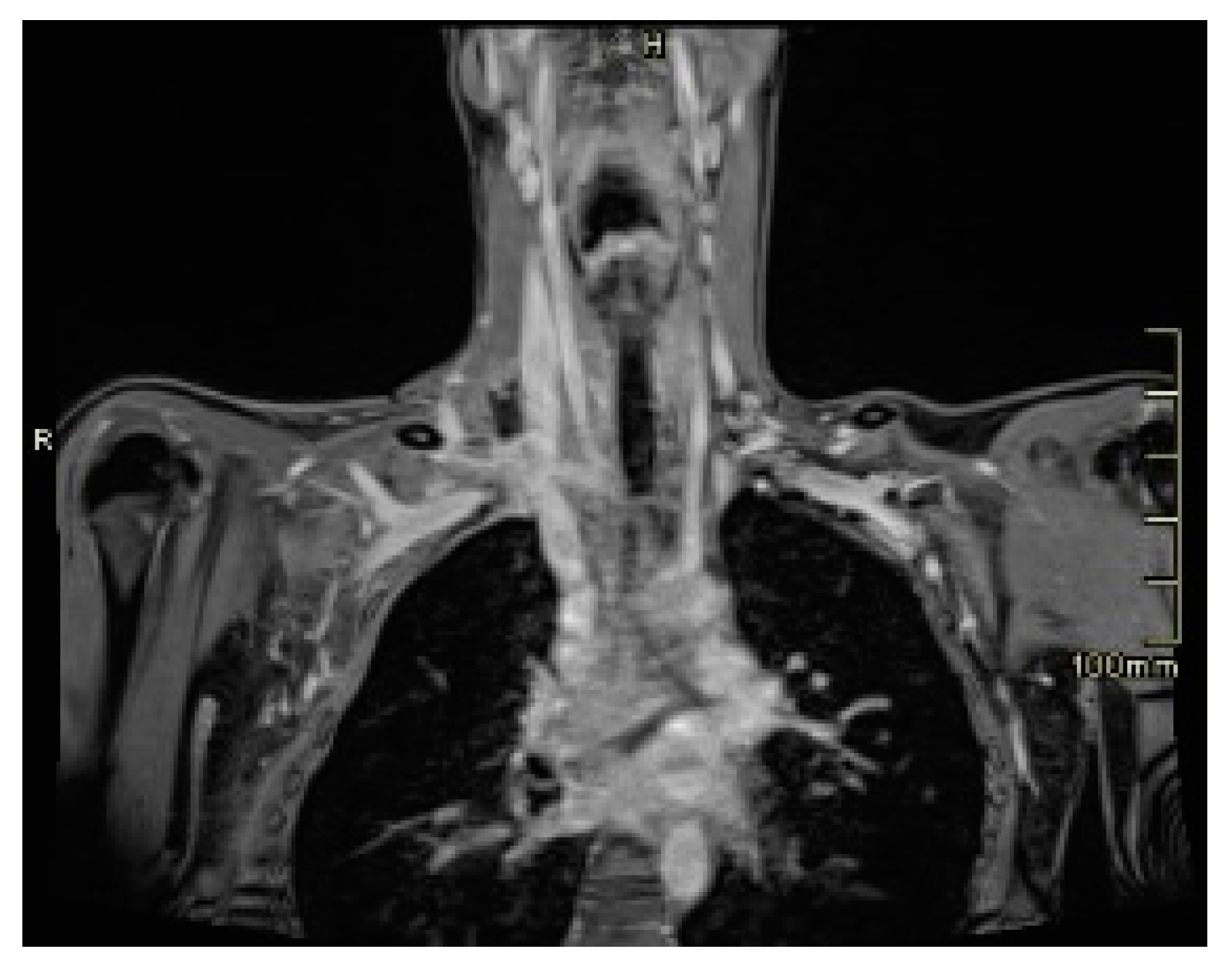
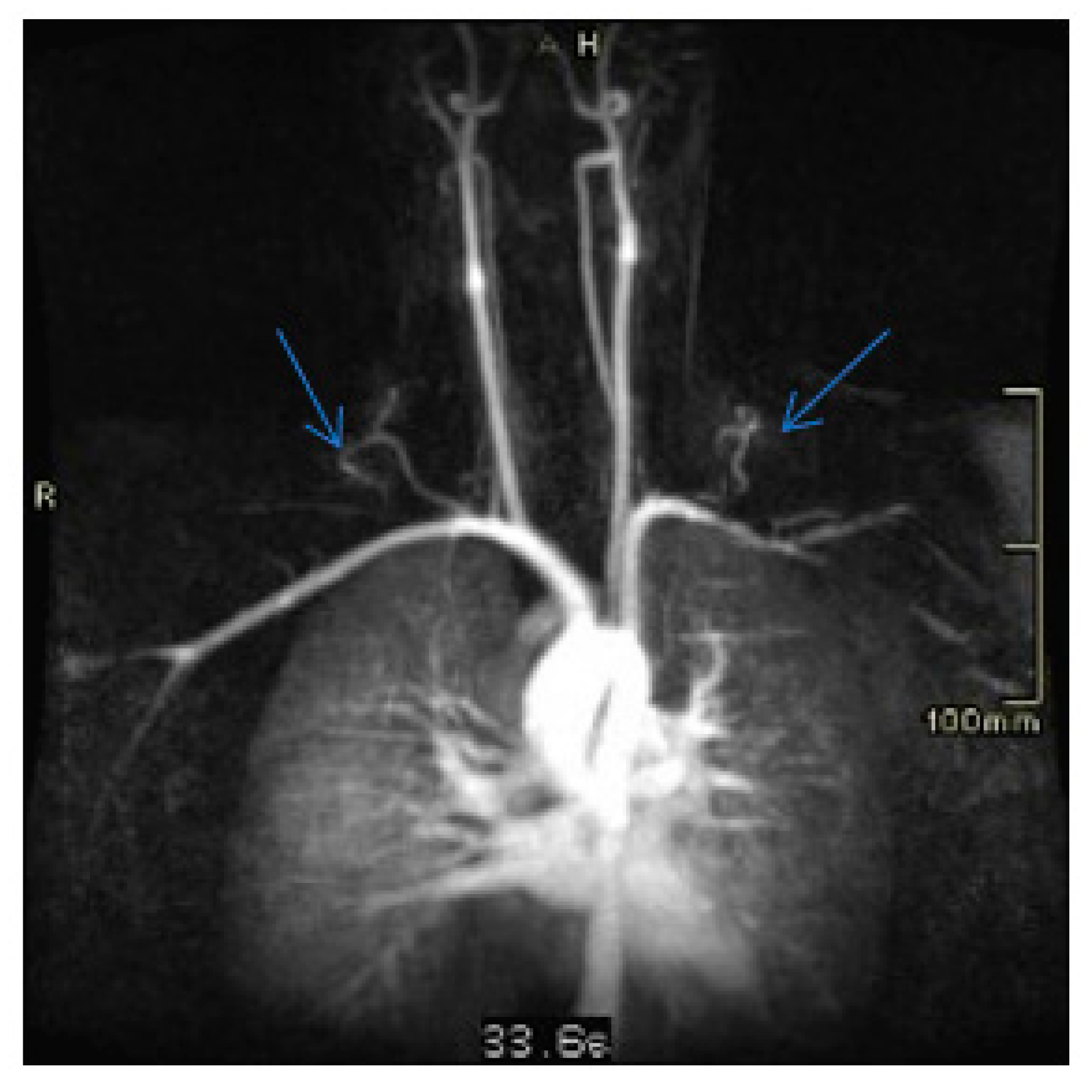
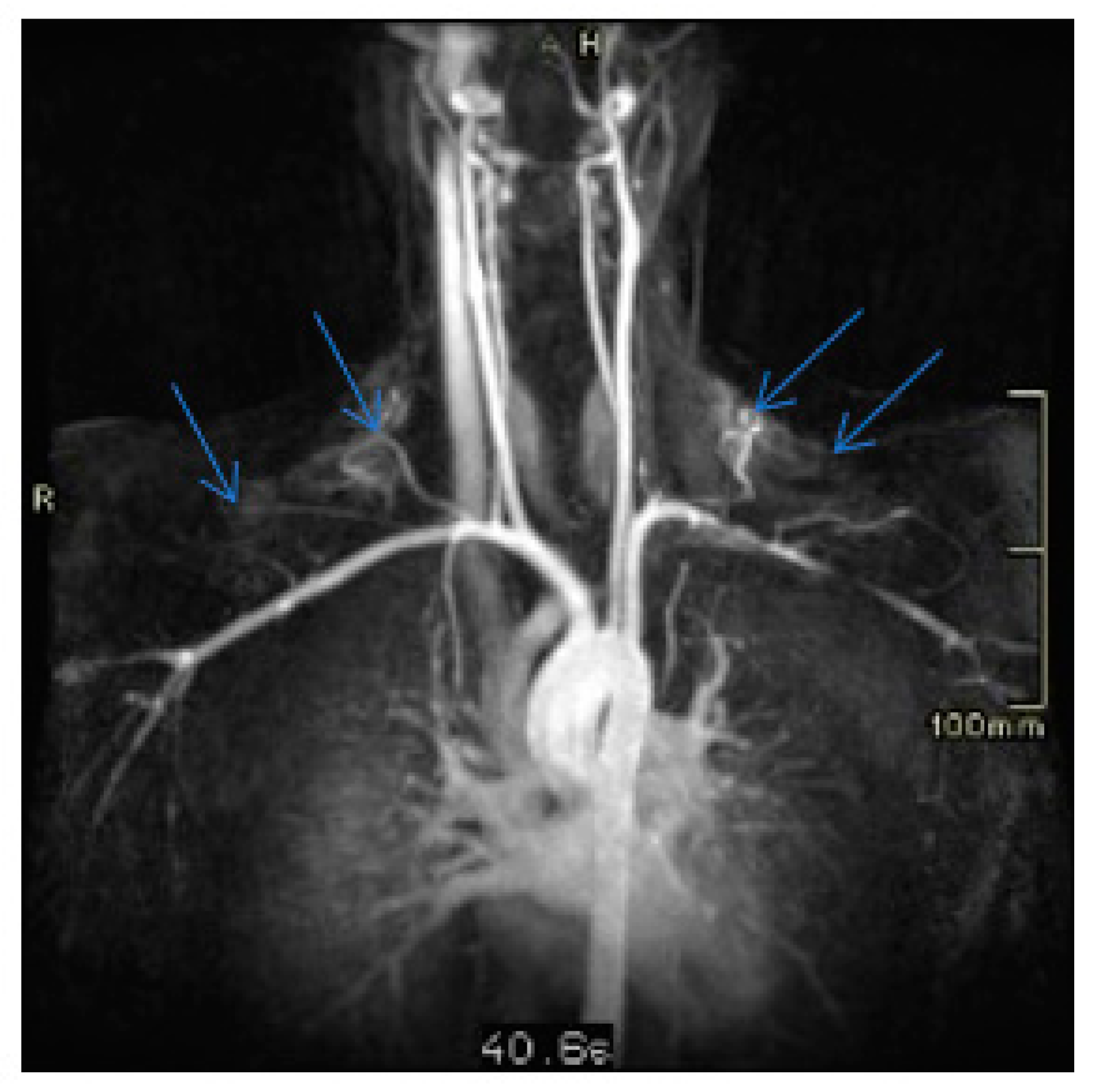
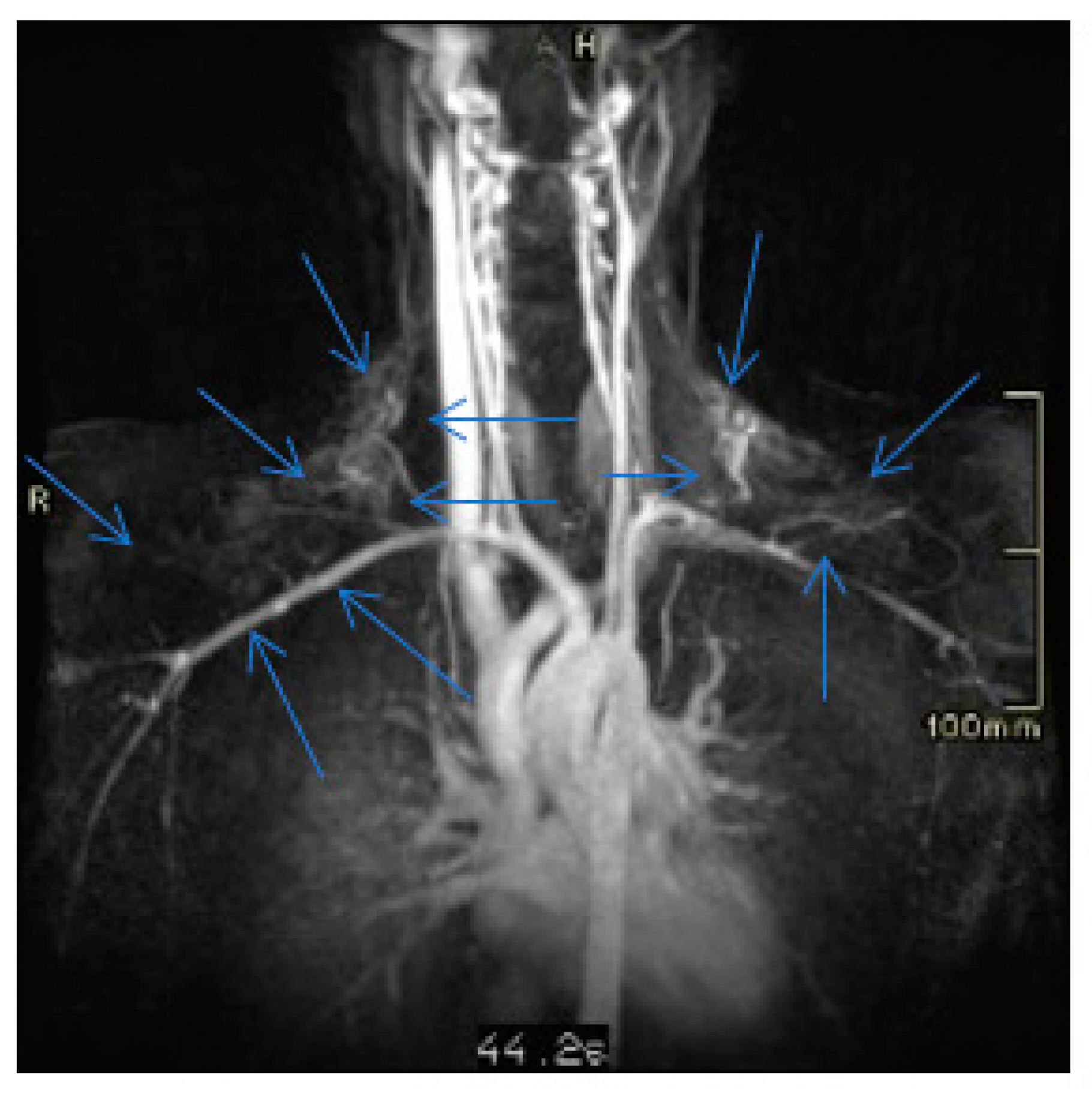
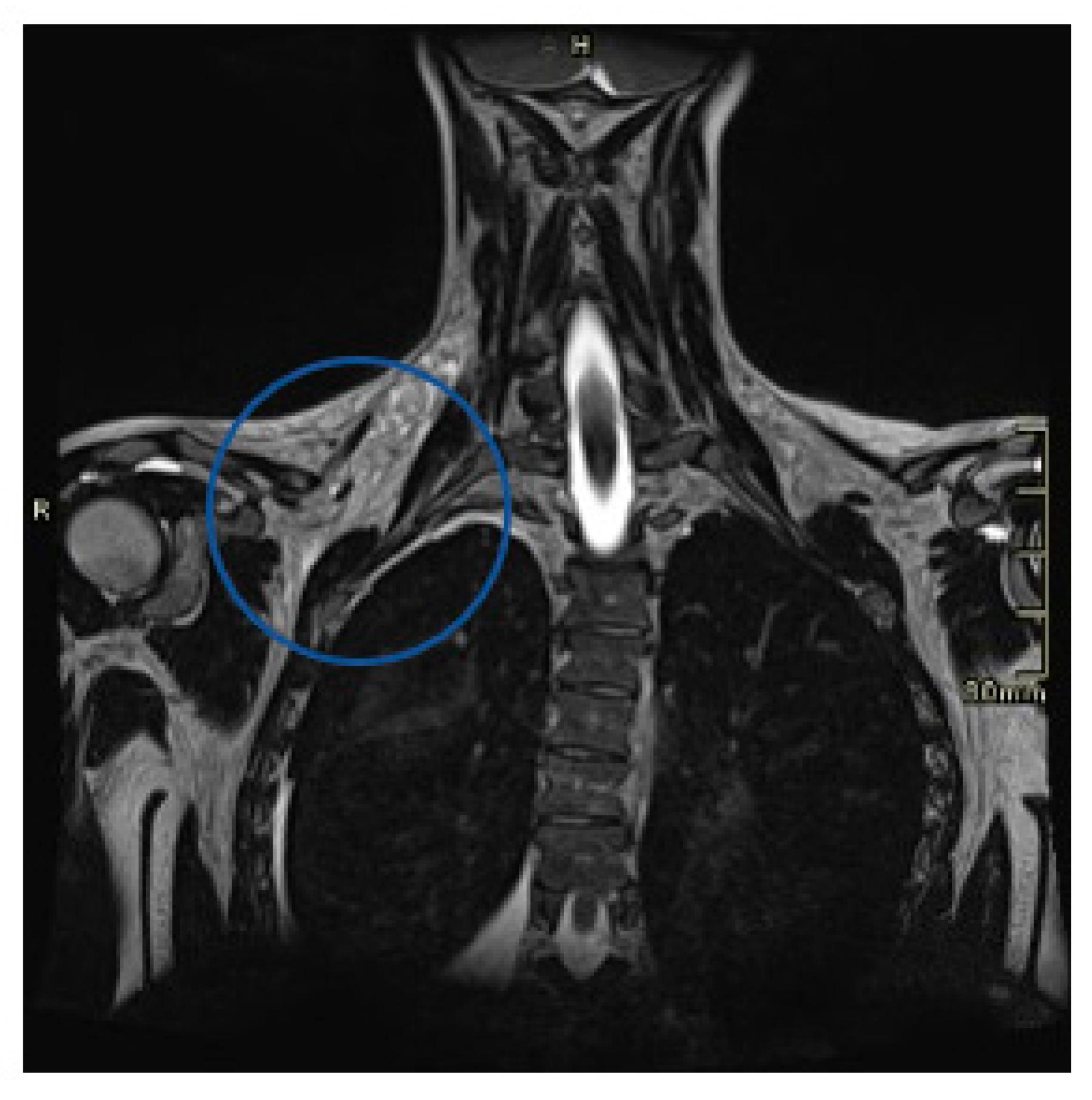
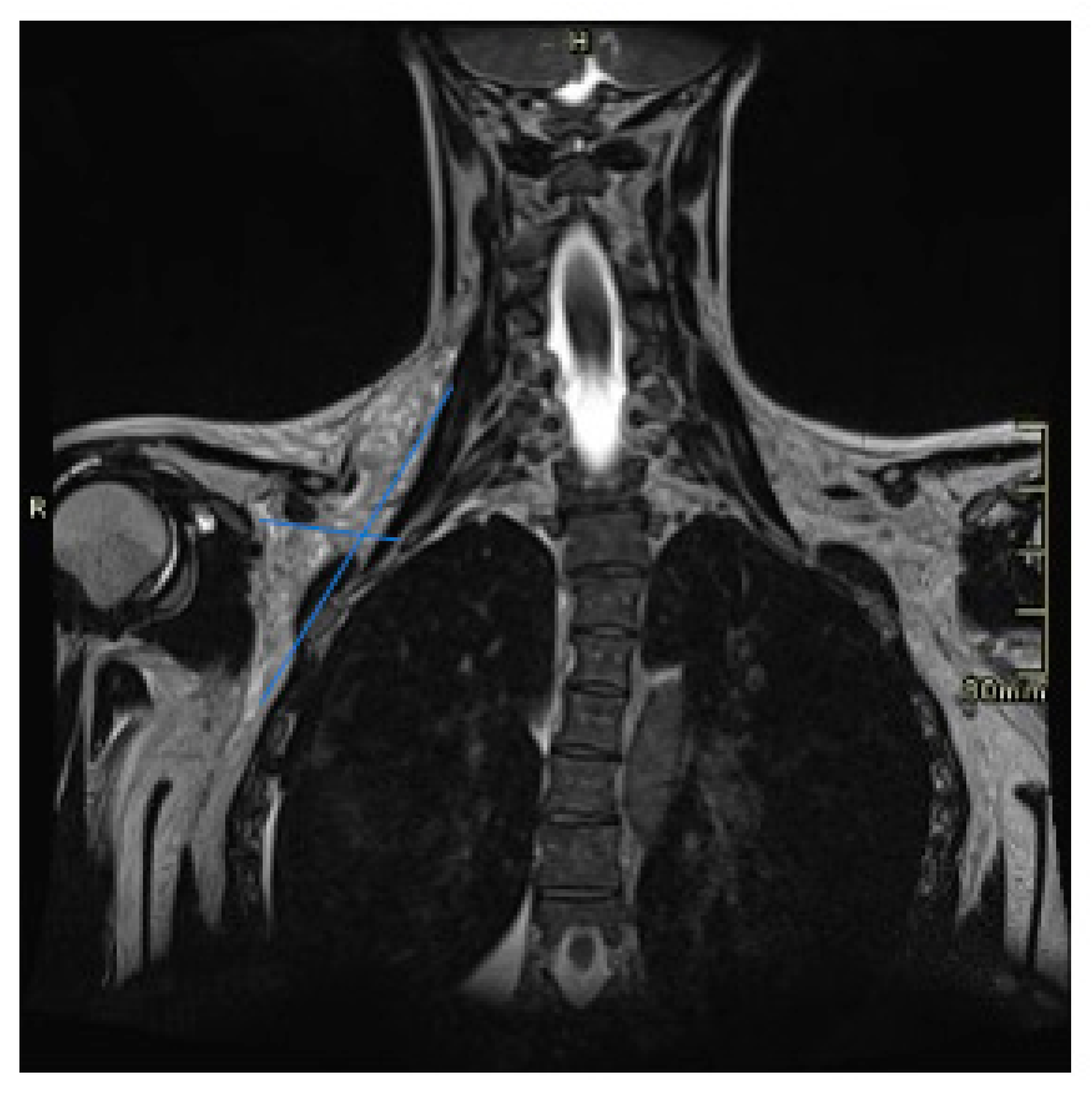
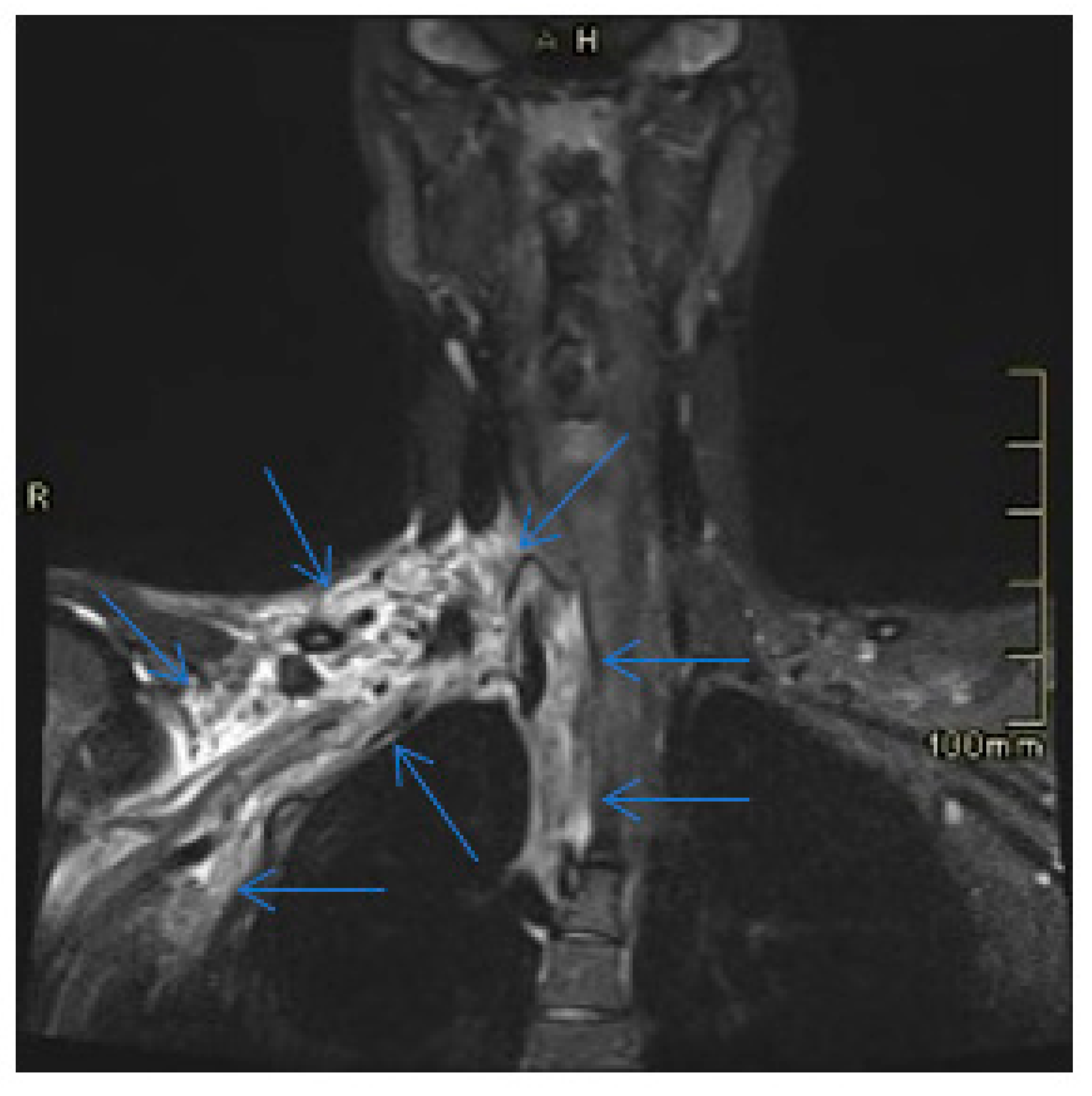
3. Discussion
- Capillary: it is made up of multiple fine lymphatic ducts, less than 1mm, located in the skin and mucous membranes. Generally located in the subcutaneous tissue.
- Cavernous: it is formed by dilated lymphatic ducts, with thin walls, with cysts smaller than 5 mm. Located on the tongue and mouth.
- Cystic: it is composed of cystic spaces lined by flat epithelium, which contain a clear liquid. They occur most often in the neck.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Luis Hernández J, Rojas Crespo KL, Artazkoz del Toro JJ, de Serdio-Arias JL. Linfangioma cervical en un adulto (Cervical lymphangioma in an adult). Med Clin (Barc). 2014 Jun 16;142(12):e23. Spanish. [CrossRef] [PubMed]
- Basurto-Kuba EO, Hurtado-Lopez LM, Campos-Castillo C, Buitrón Garcia-Figueroa R, Figueroa-Tentori D, Pulido-Cejudo A. Linfangioma de cuello en el adulto. Reporte de 2 casos (Cervical lymphangioma in the adult. A report of 2 cases). Cir Cir. 2016 Jul-Aug;84(4):313-7. Spanish. [CrossRef] [PubMed]
- Valenzuela Martínez MJ, Santero MP, Arribas MD, Córdoba E, Martínez F. Linfangioma quístico cervical en el adulto (Cervical cystic lymphangioma in adults). Cir Esp. 2010 Feb;87(2):122-3. Spanish. [CrossRef] [PubMed]
- Peral Cagigal B, Serrat Soto A, Calero H, Verrier Hernández A. OK-432 como tratamiento del linfangioma cervicofacial en el adulto (OK-432 therapy for cervicofacial lymphangioma in adults). Acta Otorrinolaringol Esp. 2007 May;58(5):222-4. Spanish. [PubMed]
- V.T. Kandakure, G.V. Thakur, A.R. Thote, A.J. Kausar. Cervical lymphangioma in adult. Case report. Otorhinolaringology Clin Inter J, 4 (3) (2012), pp. 147-150.
- Aydin S, Demir MG, Selek A. A Giant Lymphangioma on the Neck. J Craniofac Surg. 2015 Jun;26(4):e323-5. [CrossRef] [PubMed]
- Acta Otorrinolaringológica Gallega Caso clínico Higroma quístico del adulto Adult cystic hygroma Mohamad Al Rifai Al Masri, Celia Lendoiro Otero, Socorro Tedín García, Manuel Blanco Labrador.
- Brea-Álvarez B, Roldán-Hidalgo A. Quistes en el triángulo cervical posterior en adultos (Cysts in the posterior triangle of the neck in adults). Acta Otorrinolaringol Esp. 2015 Mar-Apr;66(2):106-10. Spanish. [CrossRef] [PubMed]
- Rodríguez-Montes JA, Collantes-Bellido E, Marín-Serrano E, Prieto-Nieto I, Pérez-Robledo JP. Linfangioma esplénico. Un tumor raro. Presentación de 3 casos y revisión de la bibliografía (Splenic lymphangioma. A rare tumour. Presentation of 3 cases and a literature review). Cir Cir. 2016 Mar-Apr;84(2):154-9. Spanish. [CrossRef] [PubMed]
- García-Vico A, Cañete-Gómez J, Gómez-Sotelo AI, Parra-Membrives P. Retroperitoneal cystic lymphangioma as an incidental finding in a patient with pancreatitis symptoms. Cir Esp (Engl Ed). 2019 Dec;97(10):594. English, Spanish. [CrossRef] [PubMed]
- Palomeque Jiménez A, Herrera Fernández FA, Calzado Baeza S, Reyes Moreno M. Linfangioma quístico mesentérico gigante como hallazgo incidental en adulto joven (Giant mesenteric cystic lymphangioma as an incidental finding in a young adult). Gastroenterol Hepatol. 2014 Aug-Sep;37(7):416-7. Spanish. [CrossRef] [PubMed]
- Guruprasad, Y. , Chauhan, D.S. Cervical Cystic Hygroma. J. Maxillofac. Oral Surg. 11, 333–336 (2012). [CrossRef]
- Rev. Soc. Otorrinolaringol. Castilla Leon Cantab. La Rioja 2013 Dic. 4 (24): 194-200 Linfangioma quístico supraclavicular derecho. Presentación inusual en adulto Torres-Morientes LM et al.
- Mathew M, Dil SK. Adult lymphangioma – a rare entity: a report of two cases. Turk Patoloji Derg. 2012; 28 80-82.
- Gleason TJ, Yuh WT, Tali ET, Harris KG, Mueller DP. Traumatic cervical cystic lymphangioma in an adult. Ann Otol Rhinol Laryngol. 1993 Jul;102(7):564-6. [CrossRef] [PubMed]
- Miceli A, Stewart KM. Lymphangioma. 2022 Aug 8. In: StatPearls (Internet). Treasure Island (FL): StatPearls Publishing; 2023 Jan–. [PubMed]
- Liu X, Cheng C, Chen K, Wu Y, Wu Z. Recent Progress in Lymphangioma. Front Pediatr. 2021 Dec 15;9:735832. [CrossRef]
- Torres-Palomino G, Juárez-Domínguez G, Guerrero-Hernández M, Méndez-Sánchez L. Obstrucción de la vía aérea por higroma quístico en un recién nacido (Airway obstruction due to cystic hygroma in a newborn). Bol Med Hosp Infant Mex. 2014 Jul-Aug;71(4):233-237. Spanish. [CrossRef] [PubMed]
- Berrada O, Beghdad M, El Krimi Z, Oukessou Y, Rouadi S, LarbiAbada R, Roubal M, Mahtar M. Cervicofacial cystic lymphangiomas in 17 childrens: A case series. Ann Med Surg (Lond). 2022 May 19;78:103835. [CrossRef]
- L. Gow, R. Gulati, A. Khan, F. Mihaimeed. Adult-onset cystic hygroma: A case report and review of management. Gran Rounds, 11 (2011), pp. 5-11.
- Q. Zhou, J.W. Zheng, H.M. Mai, Q.F. Luo, X.D. Fan, L.X. Su, et al. Treatment guidelines of lymphatic malformations of the head and neck. Oral Oncol, 47 (12) (2011), pp. 1105-1109.
- 22. Lerat J, Mounayer C, Scomparin A, Orsel S, Bessede JP, Aubry K. Head and neck lymphatic malformation and treatment: Clinical study of 23 cases. Eur Ann Otorhinolaryngol Head Neck Dis 2016; 133: 393-396.
- 23. Molino J, Guillén G, Peiró J, García-Vaquero J, et al. Linfangioma quístico cervical: todavía un reto. Cir Pediatr 2010; 23: 147-152.
- 24. Tucci FM, De Vincentiis GC, Sitzia E, Giuzio L, Trozzi M, Bottero S. Head and neck vascular anomalies in children. Int J Pediatr Otorhinolaryngol 2009; 73 Suppl 1:S71-6.
- Kaira V, Kaira P, Agarawal T. Cervical Cystic Lymphangiomas in Adults: A Case Series of a Rare Entity with Literature Review. Head Neck Pathol. 2021 Jun;15(2):503-508. [CrossRef]
- López Uriarte B, Frías Vargas M, Rivera Teijido M, Montes Belloso E, García Martínez G. Linfangioma axilar en el adulto. A propósito de un caso: La ecografía clínica como herramienta de orientación diagnóstica (Axillary lymphangioma in the adult: Clinical ultrasound as a diagnostic tool). Semergen. 2021 Jul-Aug;47(5):350-352. Spanish. [CrossRef] [PubMed]
- Woo EK, Connor SE. Computed tomography and magnetic resonance imaging appearances of cystic lesions in the suprahyoid neck: a pictorial review. Dentomaxillofac Radiol. 2007 Dec;36(8):451-8. [CrossRef] [PubMed]
- 28. Gaddikeri S, Vattoth S, Gaddikeri RS, et al. Congenital cystic neck masses: embryology and imaging appearances, with clinicopathological correlation. Curr Probl Diagn Radiol 2014; 43: 55-67.
- 29. Kadom N, Lee EY. Neck masses in children: current imaging guidelines and imaging findings. Semin Roentgenol 2012; 47:7-20.
- Luján Martínez DM, Candel Arenas MF, Ruiz Marín M, Parra Baños PA, Albarracín Marín-Blázquez A. Utility of conservative treatment in cystic lymphangioma. Cir Esp. 2016 Oct;94(8):485-7. English, Spanish. [CrossRef] [PubMed]
- Díaz Rodríguez D, Benítez Del Rosario JJ, Valido Quintana M, Sánchez Tudela AT. Spontaneous regression of a cervical giant cystic lymphangioma. Acta Otorrinolaringol Esp (Engl Ed). 2021 May-Jun;72(3):195-197. English, Spanish. [CrossRef] [PubMed]
- Kennedy, T. L. , Whitaker, M., Pellitteri, P., & Wood, W. E. (2001). Cystic hygroma/lymphangioma: a rational approach to management. The laryngoscope 111(11), 1929-1937.
- Creger PE, Harper C 3rd, Curry C, Kramer A. Resection of an Asymptomatic Lymphangioma in a 76-Year-Old Male. Cureus. 2021 Jun 10;13(6):e15577. [CrossRef]
- Liu Q, Fu J, Yu Q, Gong W, Li P, Guo X. Laparoscopic surgery of intra-abdominal lymphatic malformation in children. Exp Ther Med. 2022 Jul 19;24(3):581. [CrossRef]
- Curran, A. , Malik, N., McShane, D., & Timon, C. (1996). Surgical management of lymphangiomas in adults. The Journal of Laryngology & Otology, 110 (6), 586-589. [CrossRef]
- Cirugía y Cirujanos. Volume 84, Issue 4, July–16, Pages 313-317. 20 August.
- Tamilselvi R, Tang IP, Linger S, Mohd Soffian MS. Dilemma in management of cervico-facial cystic hygroma. Med J Malaysia. 2019 Oct;74(5):450-451. [PubMed]
- 38. Perkins JA, Manning SC, Tempero RM, et al. Lymphatic malformations: review of current treatment. Otolaryngol Head Neck Surg 2010;142:795-803.
- 39. Colbert SD, Seager L, Haider F, Evans BT, Anand R, Brennan PA. Lymphatic malformations of the head and neck-current concepts in management. Br J Oral Maxillofac Surg 2013;51:98-102.
- Niramis, R. , Watanatittan, S., & Rattanasuwan, T. (2010). Treatment of cystic hygroma by intralesional bleomycin injection: experience in 70 patients. European journal of pediatric surgery 20(03), 178-182.
- Vaid, L. , Gupta, M., Gupta, N., & Singh, P. P. (2010). Bleomycin sclerotherapy in a rare case of adult-onset cervical lymphangioma. ENT: Ear, Nose & Throat Journal 89(1).
- Fasching G, Dollinger C, Spendel S, Tepeneu NF. Treatment of lymphangiomas by means of sclerotherapy with OK-432 (Picibanil®) is safe and effective - A retrospective case series. Ann Med Surg (Lond). 2022 Sep 2;81:104531. [CrossRef]
- Manzini M, Schweiger C, Manica D, Kuhl G. Response to OK-432 sclerotherapy in the treatment of cervical lymphangioma with submucosal extension to the airway. Braz J Otorhinolaryngol. 2020 Jan-Feb;86(1):127-129. [CrossRef]
- Alonso J, Barbier L, Alvarez J, Romo L, Martín JC, Arteagoitia I, Santamaría J. OK432 (picibanil) efficacy in an adult with cystic cervical lymphangioma. A case report. Med Oral Patol Oral Cir Bucal. 2005 Aug-Oct; 10(4): 362-6. English, Spanish. PMID: 16056191. [Google Scholar]
- 45. Strychowsky JE, Rahbar R, O'Hare MJ, Irace AL, Padua H, Trenor CC 3rd. Sirolimus as treatment for 19 patients with refractory cervicofacial lymphatic malformation. Laryngoscope 2018;128:269-276.
- 46. Triana P, Dore M, Cerezo VN, et al. Sirolimus in the Treatment of Vascular Anomalies. Eur J Pediatr Surg 2017;27:86-90.
- Khanwalkar A, Valika T, Maddalozzo J. Long-term symptom control following resection of cervical lymphatic malformations: a case series. J Otolaryngol Head Neck Surg. 2020 Apr 19;49(1):19. [CrossRef]
- 48. Colbert SD, Seager L, Haider F, Evans BT, Anand R, Brennan PA. Lymphatic malformations of the head and neck-current concepts in management. Br J Oral Maxillofac Surg 2013;51:98-102.
- Wang J, Yang Y, Guo J, Yao Y, Dong L, Mou Y, Zhang Y, Song X. Cervical lymphangioma in adults: A report of seven cases and review of the literature. Laryngoscope Investig Otolaryngol. 2022 Apr 22;7(3):751-756. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



