1. Introduction
Biocatalysts are used in various industries such as medicine, pharmaceuticals, cosmetics, and agriculture due to their substrate specificity, optical selectivity, and involvement in various chemical reactions within the body [
1,
2,
3,
4,
5,
6,
7].
Candida antarctica lipase B (CalB) is a biocatalyst that belongs to the class of enzymes involved in catalyzing reactions. It has high activity and regioselectivity. Depending on reaction conditions, CalB can promote reactions such as hydrolysis and acidolysis [
8,
9,
10,
11,
12]. CalB is expected to be a non-toxic and renewable energy source. It has the highest catalytic activity at neutral pH and 45 ℃ [
13,
14,
15]. However, CalB has limitations that hinder its widespread commercial use. CalB is water-soluble, which makes it highly sensitive to organic solvents, leading to reduced reaction efficiency [
16,
17]. Furthermore, it is difficult to store CalB for a long time. Its storage life is short, resulting in high costs in production and processing stages. In particular, it is difficult to maintain the structural stability of proteins during biochemical reactions, which can affect biological activity the most. Therefore, research on enzyme immobilization, which can complement shortcomings of CalB, is being actively conducted [
18,
19,
20,
21,
22,
23,
24,
25].
Levan, known as a fructose polymer, is a natural bio-polymer found in small amounts in some plants and microorganisms. Levan has a cationic property in an aqueous solution due to carboxyl and hydroxyl groups of its constituent substance fructose, contributing to its very stable shape. Levan is a functional material that is used in various industries such as functional cosmetics, agriculture, and industry because it is a water-soluble fructose polymer that melts not only at high temperatures, but also at low temperatures [
26,
27,
28]. Therefore, levan encapsulated enzymes or proteins have been studied and utilized to solve problems of reduced activities of proteinaceous substances such as enzymes. In addition, they are resistant to organic solvents [
29,
30,
31,
32]. Furthermore, we developed carboxymethyl levan (CML)-based nanocomposites dubbed ‘nanofructosome (NF)’ and demonstrated effects of NF particles by entrapping lipase [
33]. However, these levan encapsulated enzyme complexes are still inherently denatured during long-term storage with poor acid and heat resistance, resulting in reduced catalytic activity. In addition, because it is a water-soluble fructose polymer that melts even at low temperatures, it is difficult to use it in continuous processes as an industrial catalyst. To overcome these limitations, more advanced immobilization techniques are needed.
Silica encapsulation technology is widely known as a method that can efficiently embed materials for industrial purposes [
34,
35,
36]. The material encapsulated with silica is subordinated to the insoluble support, allowing multiple uses in a continuous process. As a result, it is acid-resistant and heat-resistant, reducing sensitivity to environmental factors and contributing to high efficiency. In addition, it enables long-term storage even at room temperature, effectively reducing the cost of industrial processes. Encapsulation technology using silica has recently been proven to be an effective method for enzyme encapsulation [
37,
38,
39,
40,
41]. Therefore, it is expected that silica immobilization technology can also be applied to protein-levan complexes. Research is needed to effectively multi-use water-soluble enzymes in organic solvents.
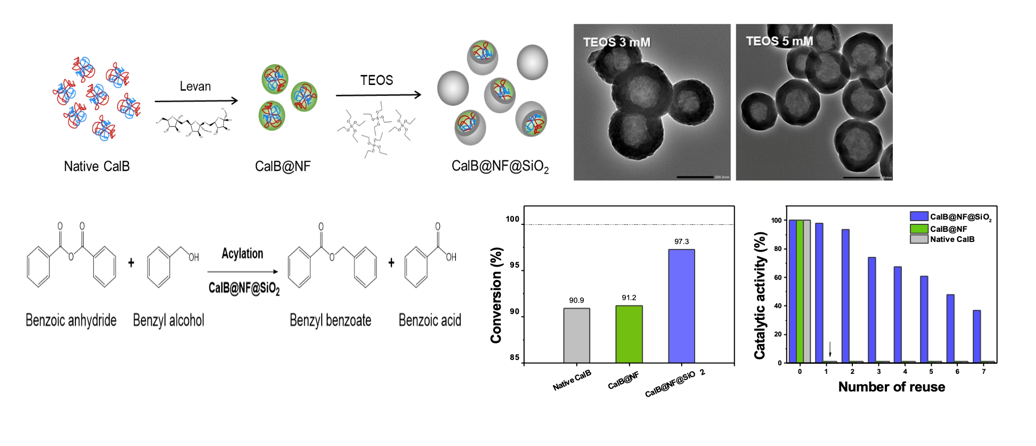
In this study, the enzyme activity of native CalB immobilized via levan (Nano-fructose CalB, CalB@NF) to be used in organic solvents was stabilized under various environmental factors by encapsulating it using tetraethyl orthosilicate (TEOS), a silica precursor. CalB@NF@SiO2 particles were produced as a function of TEOS concentration ranging from 3-100 mM. The encapsulation morphology of CalB@NF@SiO2 was confirmed through TEM. Its catalytic activity and reusability were evaluated at various pH and temperature ranges compared to native CalB and CalB@NF. The enzyme amount of CalB@NF@SiO2 was confirmed through Bradford assay and TGA analysis. Catalytic parameters such as Km, Vmax, and Kcat were calculated using Michaelis-Menten equation and Line-weaver Burk plot and confirmed through enzymatic hydrolysis using p-nitrophenyl butyrate (PNPB). Enzymatic synthesis was conducted through synthesis of benzyl benzoate using benzoic anhydride. The efficiency of converting benzoic anhydride to benzyl benzoate was calculated.
2. Experimental
2.1. Materials
Candida antarctica lipase B (Native CalB) and CalB@NF were obtained from Korea Research Institute of Bioscience and Biotechnology (33 kDa, Korea) used in encapsulation. Tetraethyl orthosilicate (99%, TEOS), Oleic acid, Iepal CO-520, p-nitrophenyl butyrate (98%, PNPB), bovine serum albumin (BSA), Coomassie brilliant blue G-250, phosphoric acid, tris base, phosphate buffer saline (PBS), sodium acetate, glacial acetic acid, benzoic anhydride, and benzyl alcohol were purchased from Sigma-Aldrich (Korea). Cyclohexane, ammonia solution (28-30%), ethanol (99%), methanol, hydrochloric acid, and n-hexane were purchased from Deaejung (Korea). Ethyl ether was provided by J.T.Baker (U.S).
2.2. Preparation of silica encapsulated CalB@NF (CalB@NF@SiO2)
CalB@NF@SiO
2 particles were prepared with a sol-gel method [
41]. Oleic acid (2 mL, solution A), Igepal CO-520 (24 g, solution B), and CalB@NF (90 mg, solution C) were mixed with cyclohexane (100, 300, and 20 mL, respectively). After adding solution A and solution C to solution B, TEOS of various concentrations (3-100 mM) was added drop-wise and stirred for 1 hr. Then 163 mL of ammonia solution was added and stirred for 20 hr. After stirring was complete, 325 mL of methanol was added. After confirming precipitate was formed, the supernatant was then removed. The precipitate was washed three times with n-hexane and completely vacuum dried at room temperature.
2.3. Quantification of enzymes in native CalB, CalB@NF, and CalB@NF@SiO2
The amount of entrapped enzyme was determined by Bradford assay using a protein reagent [
42,
43]. The protein reagent was prepared as follows. First, add 50 mL of ethanol and 100 mg of Coomassie brilliant blue G-250 into a 1 L volumetric flask. After adding 100 mL of 85% (w/v) phosphoric acid, distilled water was added to have a final volume of 1 L. The enzyme content was evaluated based on standard solutions and estimated by comparing concentration differences. BSA was used as a standard. The absorbance was measured at 595 nm by UV-Visible spectrophotometry.
2.3. pH and thermal stability ofnative CalB, CalB@NF, and CalB@NF@SiO2
Effects of pH and temperature on stability of native CalB, CalB@NF, and CalB@NF@SiO2 were determined based on their catalytic activities using PNPB at various pH (0.1 M of sodium acetate for pH 5-6 and 0.1 M of tris-HCl for pH 7-9) and temperature (5-65 ℃) conditions. After 0.1 mg/mL of native CalB, CalB@NF, or CalB@NF@SiO2 was added to the substrate solution, catalytic activities were calculated at optimum pH and temperature conditions.
2.5. Reusability of native CalB, CalB@NF, and CalB@NF@SiO2
Reusability of native CalB, CalB@NF, and CalB@NF@SiO2 was evaluated in repeated cycles with PNPB. First, 0.1 mg/mL of native CalB, CalB@NF, or CalB@NF@SiO2 was added into the substrate solution. Nnative CalB, CalB@NF, and CalB@NF@SiO2 were then recovered from reaction medium by centrifugation at 15,000 rpm for 1 min and washed with 0.1 M of tris-HCl buffer (pH 7.4) to remove any residual substrate. The process was evaluated up to 7 cycles to examine reusability of each catalyst. The absorbance was measured at 400 nm by UV-Visible spectrophotometry.
2.6. Enzymatic hydrolysis for p-nitrophenyl butyrate with native CalB, CalB@NF, and CalB@NF@SiO2
Enzymatic hydrolysis against PNPB was achieved with native CalB, CalB@NF, or CalB@NF@SiO2. PNPB solution at 0.1 M was prepared and diluted to different concentrations (0.0625-1.0 mM) with 0.1 M of tris-HCl buffer (pH 7.4). After 0.1 mg/mL of native CalB, CalB@NF, or CalB@NF@SiO2 was added into substrate solution, the mixture was centrifuged at 15,000 rpm for 1 min. The absorbance of the product, p-nitrophenol (PNP), was measured at 400 nm by UV-Visible spectrophotometry. Catalytic parameters such as Michaelis-Menten constant (Km), maximum reaction velocity (Vmax), and turnover value (Kcat) were calculated for native CalB, CalB@NF, and CalB@NF@SiO2.
2.7. Enzymatic synthesis for benzyl benzoate with native CalB, CalB@NF, and CalB@NF@SiO2
Enzymatic synthesis of benzyl benzoate proceeded through an acylation reaction by mixing 10 mL of benzoic anhydride and 2.26 g of benzyl alcohol. After 0.5 g of native CalB, CalB@NF, or CalB@NF@SiO2 was added, the mixture was incubated at 50 °C for 24 h. After the reaction was completed, the supernatant was separated by centrifugation at 2,500 rpm for 5 min. Separated supernatants were then subjected to column chromatography. The solvent was evaporated using a rotary evaporator after thin layer chromatography (TLC) analysis. The production of benzyl benzoate was confirmed by measuring absorbance at 229 nm using UV-Visible spectrophotometry.
2.8. Characterizations
Transmitted morphological details of the prepared CalB@NF@SiO2 according to TEOS concentration were evaluated with a JEM-2100Plus transmission electron microscope (TEM) (JEOL, Japan) at an accelerating voltage of 200 kV. Overall morphological details were analyzed with a TESCAN MIRA3 field emission scanning electron microscopy (FE-SEM) (TESCAN, Czech) operated at 2 kV. To evaluate the amount of enzyme encapsulated in CalB@NF@SiO2, thermogravimetric analysis (TGA) at 700 °C (10 °C/min) in a nitrogen atmosphere was performed with a Q600 TA instrument (Waters, USA). Fourier transform infrared (FT-IR) was performed with the KBr method using Frontier (Perkinelmer, US) for characterization of CalB@NF@SiO2. UV-Visible spectrophotometry was performed using a Mega 900 (SCINCO, Korea) instrument at 190–500 nm. Column chromatography was performed on silica gel with a pore size of 60 Å and a particle size of 32-63 nm. After separation, the reaction was confirmed by thin chromatography (TLC) visualized with 240 nm UV light on Merck silica gel 60 F254 plates.
3. Results and discussion
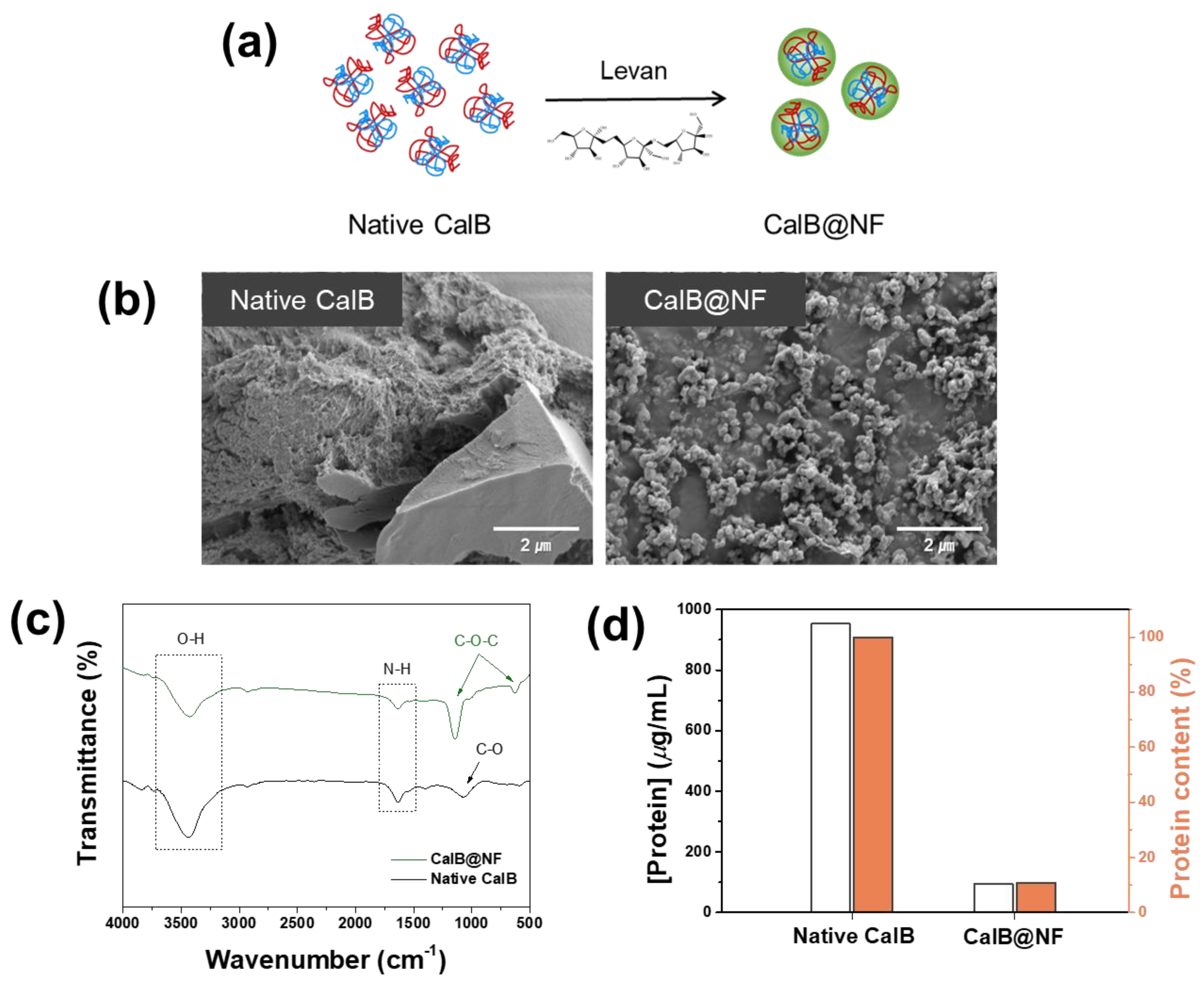
Figure 1 shows a comparison of native CalB and CalB@NF. CalB@NF was inserted into levan, a fructose polymer, as shown in
Figure 1(a). CalB@NF was a complex formed by trapping protein inside a nanoparticle. The protein was simply trapped without forming a chemical bond with the levan nanoparticle. Morphological details of native CalB and CalB@NF are shown in
Figure 1(b). Native CalB had no specific shape, while CalB@NF particles were spherical in shape with a mean size of 150 nm.
Figure 1(c) shows FT-IR spectra of native CalB and CalB@NF. Both samples showed common O-H stretching vibration at 3500 cm
−1 and N-H bending at 1635 cm
−1. In addition, anti-symmetric stretching of C-O-C and C-O-C aromatics bending was observed at 1150 cm
−1 and 630 cm
−1 for CalB@NF due to the chemical bond of levan. These results indicated that native CalB was well inserted into levan in CalB@NF by C-O-C bending of levan.
Figure 1(d) shows amounts of CalB enzyme in native CalB and CalB@NF determined by the Bradford assay. Amounts of CalB enzyme for native CalB and CalB@NF at the same weight were 953.5
μg/mL and 94.7
μg/mL, respectively. Therefore, CalB@NF contained 10 times less protein than native CalB. To enable CalB@NF, in which the CalB enzyme was inserted into levan, to be used efficiently in industrial processes under various environmental conditions and for long-term storage at room temperature, silica encapsulation was performed to maintain a stable catalytic activity.
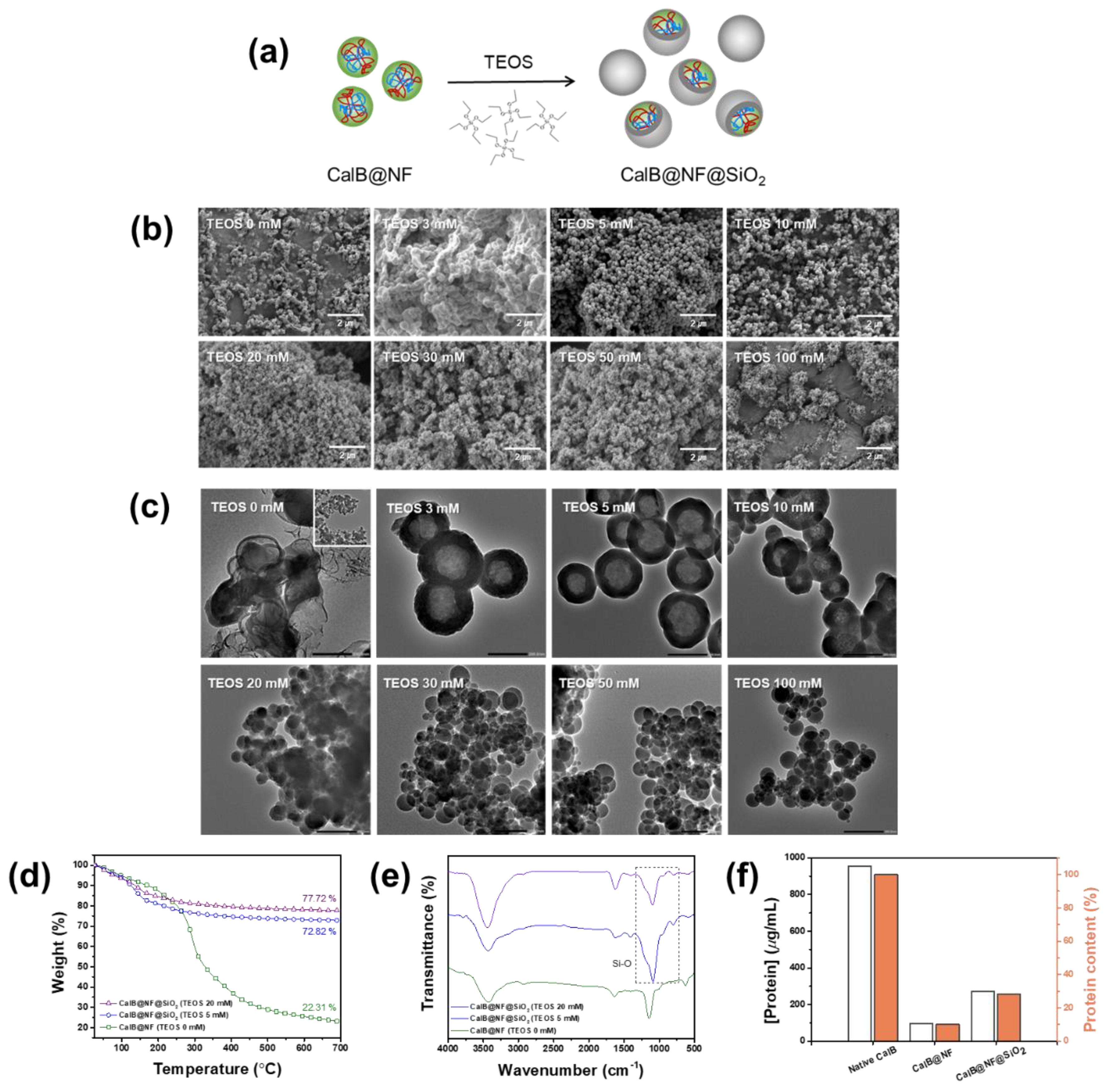
CalB@NF@SiO
2 was prepared by adjusting the TEOS concentration for CalB@NF in the range of 3-100 mM as shown in
Figure 2(a). The morphology of the prepared CalB@NF@SiO
2 was confirmed by FE-SEM and TEM for each TEOS concentration as shown in Figs. 2(b) and 2(c). CalB@NF@SiO
2 had a spherical shape. It became more uniform as the concentration of TEOS increased. Average sizes of particles prepared with TEOS concentrations of 0, 3, 5, 10, 20, 30, 50, and 100 mM were 150, 308, 186, 149, 35, 47, 52, and 66 nm, respectively. As shown in
Figure 2(c), a silica shell was obtained at TEOS concentrations of 3, 5, and 10 mM, but not obtained at TEOS concentration of 0 mM, proving successful encapsulation. Moreover, only normal silica was observed at TEOS concentration of 20 mM or higher. As shown in
Figure 2(b), when TEOS was 20 mM or more, the average particle size was much smaller than that when TEOS concentration was 0 mM, indicating that CalB@NF was not encapsulated. This result was because excessive TEOS concentration in the sol-gel reaction could cause silanols generated by hydrolysis to undergo a condensation reaction.
The resulting gel network was composed only of silica, forming spherical silica [
44]. Therefore, the particle shape of CalB@NF@SiO
2 had a confirmed silica shell and a uniform size, with 5 mM being the most optimal concentration of TEOS.
Figure 2(d) shows amounts of CalB@NF in CalB@NF@SiO
2 at various TEOS concentrations to investigate the amount of CalB@NF for each morphology change via TGA within a temperature range of 25-700 ℃. Amounts of CalB@NF at TEOS 5 mM and 20 mM were 50.51% and 55.41%, respectively, compared to that at TEOS 0 mM.
Figure 2(e) shows FT-IR spectra of CalB@NF@SiO
2. Characteristic vibrations of CalB@NF were observed during silica encapsulation. Asymmetric and symmetric stretching vibrations of Si-O-Si bonds were observed at 1080 cm
−1, 970 cm
−1, and 800 cm
−1, indicating that CalB@NF was encapsulated by silica precursor TEOS.
Figure 2(f) shows amounts of CalB enzyme in native CalB, CalB@NF, and CalB@NF@SiO
2 (TEOS 5 mM) according to the Bradford assay. At the same weight, amounts of CalB enzyme for native CalB, CalB@NF, and CalB@NF@SiO
2 (TEOS 5 mM) were 953.5
μg/mL, 94.7
μg/mL, and 270.5
μg/mL, respectively. These results revealed that the amount of encapsulated CalB enzyme in CalB@NF@SiO
2 was three times higher than that of CalB@NF. This result was supported by
Figure 2(d), where amounts of CalB@NF and CalB@NF@SiO
2 (TEOS 5 mM) were 22.31% and 72.82%, respectively, at 700 °C, with a ratio of approximately 1 : 3, which confirmed its reliability. Therefore, the ratio of native CalB : CalB@NF : CalB@NF@SiO
2 (TEOS 5 mM) containing protein was 10 : 1 : 3.
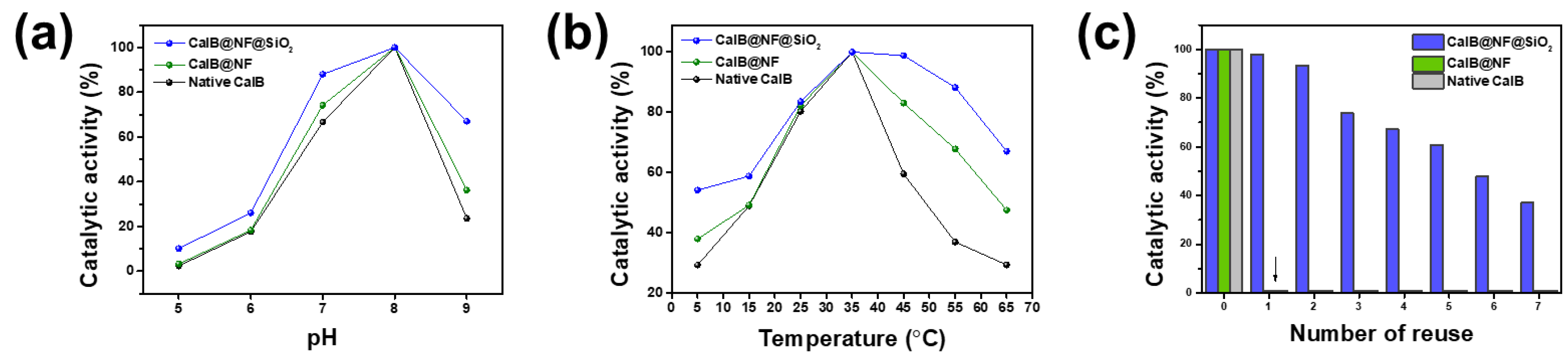
Figure 3 shows effects of encapsulation on catalytic activity, pH, thermal stability, and reusability. Catalytic activities of native CalB, CalB@NF, and CalB@NF@SiO
2 (TEOS 5 mM) were evaluated through hydrolysis of PNPB.
Figure 3(a) displays pH dependence of catalytic activities of native CalB, CalB@NF, and CalB@NF@SiO
2 (TEOS 5 mM). All three samples exhibited optimum catalytic activities at pH 8, with reduced activities at other pH values. These results indicate that the catalytic activity is dependent on the enzyme’s active state at specific pH values. However, CalB@NF@SiO
2 (TEOS 5 mM) exhibited 10-30% higher catalytic activity than native CalB and CalB@NF under all pH conditions, with pH stability provided by silica encapsulation.
Figure 3(b) shows thermal stability of native CalB, CalB@NF, and CalB@NF@SiO
2 (TEOS 5 mM). All three samples exhibited optimum catalytic activities at 35 ℃, with reduced activities at other temperatures.
However, CalB@NF@SiO
2 (TEOS 5 mM) exhibited up to 40% higher catalytic activity than native CalB and CalB@NF under all temperature conditions, with greater differences at higher temperatures. These results were due to silica interfering with protein heat transfer and the superior catalytic activity with thermal stability provided by insoluble silica encapsulation in an aqueous solution.
Figure 3(c) presents reusability of native CalB, CalB@NF, and CalB@NF@SiO
2 (TEOS 5 mM). Native CalB and CalB@NF could not be recovered for evaluation after one use. However, CalB@NF@SiO
2 (TEOS 5 mM) could be recovered and used repeatedly, with reduced catalytic activity after each use. Therefore, enzyme immobilized in silica can be reused repeatedly, increasing process efficiency and reducing costs by continuous use in industrial processes.
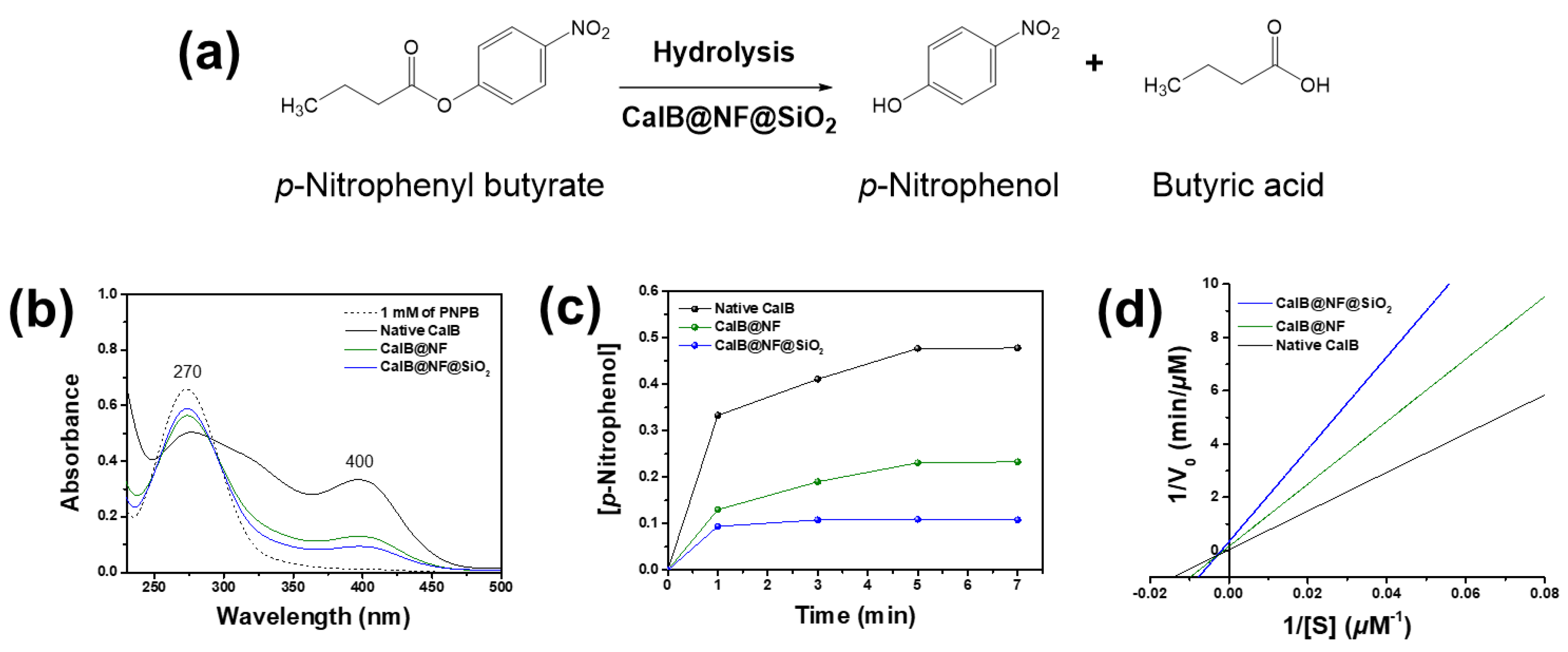
Enzymatic hydrolysis was confirmed using native CalB, CalB@NF, and CalB@NF@SiO
2 (TEOS 5 mM) with PNPB as shown in
Figure 4(a). PNPB was hydrolyzed by the enzyme to PNP and butyric acid.
Figure 4(b) shows hydrolysis results of PNPB using native CalB, CalB@NF, and CalB@NF@SiO
2 (TEOS 5 mM) with the same weight and reaction time. PNPB as a reactant exhibited an absorption spectrum at 270 nm, while PNP, the product of PNPB hydrolysis, exhibited an absorption spectrum at 400 nm. Absorbance spectra at 270 nm decreased while those at 400 nm increased for all three samples when using 1 mM of PNPB as a reference.
These results demonstrate that native CalB, CalB@NF, and CalB@NF@SiO
2 (TEOS 5 mM) can participate in PNPB hydrolysis.
Figure 4(c) shows concentrations of PNP generated during PNPB hydrolysis over time using native CalB, CalB@NF, and CalB@NF@SiO
2 (TEOS 5 mM). Concentrations of PNP for all three samples did not increase after 5 minutes of reaction time, with an order of native CalB > CalB@NF > CalB@NF@SiO
2 (TEOS 5 mM). A high concentration of PNP, the product of PNPB hydrolysis, implied that the reactant PNPB reacted more quickly or extensively with CalB in the catalyst. Thus, the concentration of the product varied depending on the amount and concentration of CalB in the catalyst.
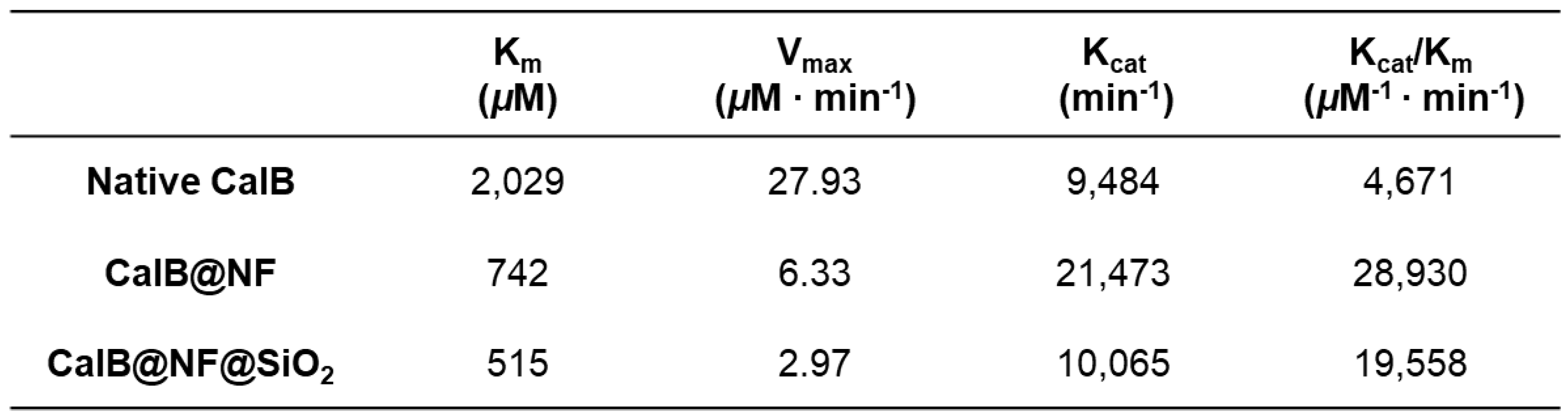
Figure 4(d) shows PNPB enzyme kinetics for native CalB, CalB@NF, and CalB@NF@SiO
2 (TEOS 5 mM) obtained from Lineweaver-Burk plot and Michaelis-Menten kinetics. Enzyme kinetic parameters were calculated. Results are shown in
Table 1. The Michaelis-Menten constant (K
m) represents an enzyme’s affinity for its substrate, with lower values indicating higher affinity and better binding between the enzyme and substrate. The turnover number (K
cat) is a measure of an enzyme’s ability to convert substrate into product per unit time. The K
cat/K
m ratio has been used as a measure of enzyme performance. K
m values were observed in the order of native CalB > CalB@NF > CalB@NF@SiO
2, with CalB@NF@SiO
2 showing the highest substrate affinity. V
max values were observed in the order of CalB@NF@SiO
2 > CalB@NF > native CalB, in contrast to K
m results. In general, the reaction between a substrate and an enzyme proceeds faster when the enzyme has a higher affinity for the substrate. However, in this study, the high substrate affinity did not result in a correspondingly high reaction rate due to the number of hydroxyl groups on each catalyst surface and in the encapsulated layer. As the hydrolysis reaction occurred in an aqueous solution, the substrate was highly intimate with hydroxyl groups. Therefore, the surface of the silica-encapsulated CalB@NF@SiO
2 synthesized using the sol-gel method contained a large number of hydroxyl groups. CalB@NF was produced using levan, which contained many hydroxyl groups, while native CalB only possessed hydroxyl groups that it originally had. Consequently, the number of hydroxyl groups on the surface of each catalyst was in the order of CalB@NF@SiO
2 > CalB@NF > native CalB. Substrate affinity was also observed in the same order. Moreover, as the CalB@NF@SiO
2 catalyst had an encapsulated layer that did not dissolve in the aqueous solution, the active site that had to directly bind to the substrate was obscured. Although CalB@NF was encapsulated with levan, which dissolved in the aqueous solution, it reacted with the substrate only to the extent that it possessed protein. Therefore, according to results shown in
Figure 2(f), the reaction rate was the highest for native CalB, which had the most protein, and the lowest for CalB@NF@SiO
2, despite having a higher protein content than CalB@NF. This result was also confirmed in
Figure 4(c). The K
cat and K
cat/K
m ratio related to the enzyme’s ability and efficiency were highest for CalB@NF, followed by CalB@NF@SiO
2 and then native CalB. This result was calculated based on K
m and V
max values. CalB@NF, which had a high affinity for the substrate due to levan and reacted well with the substrate in the aqueous solution, was the most excellent. Therefore, CalB@NF was the best for enzymatic hydrolysis with the substrate. However, according to results shown in
Figure 3, CalB@NF@SiO
2 was the most convenient for use in harsh environments or continuous processes.
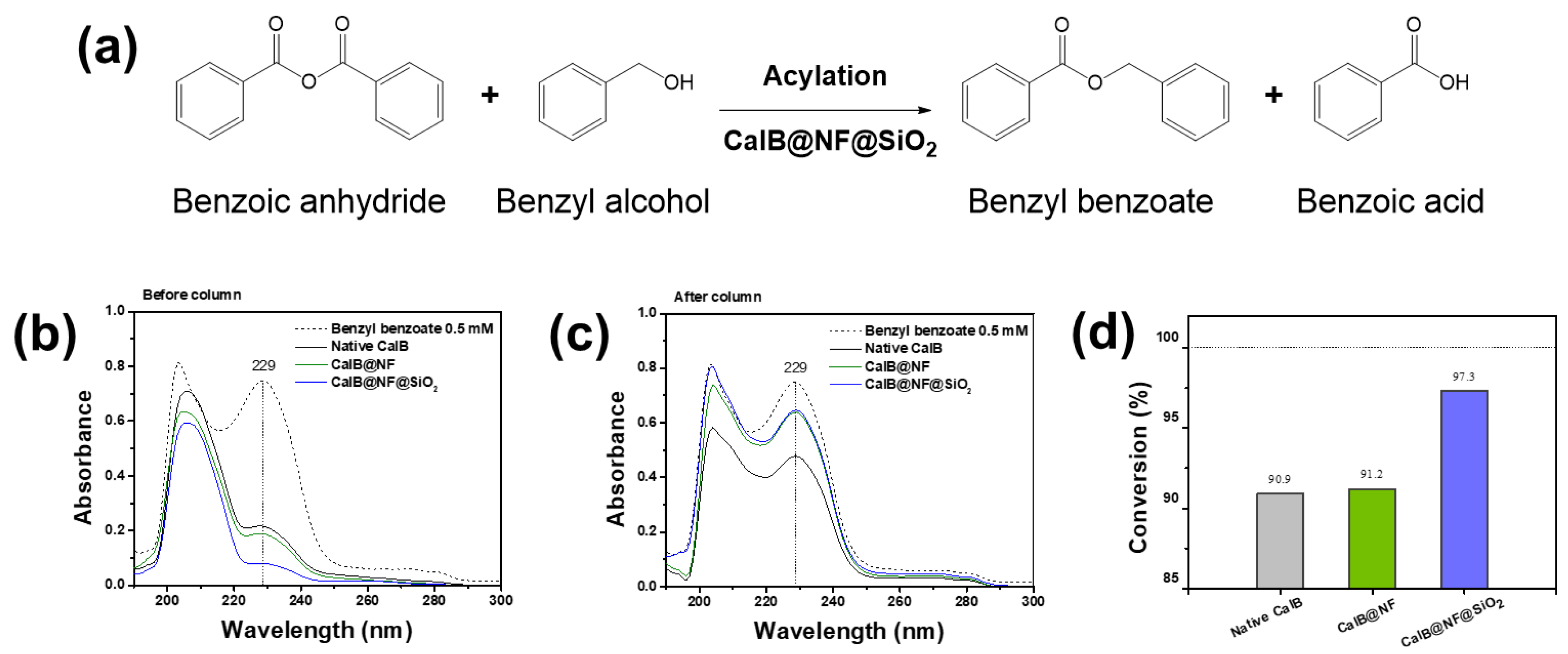
Figure 5 shows the process of synthesizing benzyl benzoate through enzymatic synthesis and its conversion efficiency. As shown in
Figure 5(a), enzymatic synthesis of benzyl benzoate using CalB@NF@SiO
2 (TEOS 5 mM) involves reactions using benzoic anhydride as a reactant. In the reaction, benzoic anhydride has two acyl donors, leading to the generation of not only benzyl benzoate (first acyl donor), but also benzoic acid (second acyl donor) when reacting with benzyl alcohol [
37,46]. Therefore, benzoic anhydride is suitable for synthesizing benzyl benzoate. According to this phenomenon, benzyl benzoate conversion efficiency was evaluated using native CalB, CalB@NF, and CalB@NF@SiO
2 (TEOS 5 mM).
Figure 5(b) shows results of UV-vis analysis of benzyl benzoate synthesized. Benzyl benzoate showed absorbance at 229 nm. However, it was difficult to distinguish because it was hindered by benzyl alcohol or benzoic acid remaining in the synthesis process. Therefore, it was separated and purified through column chromatography and analyzed for UV-vis. (
Figure 5(c)). As a result, native CalB, CalB@NF, and CalB@NF@SiO
2 (TEOS 5 mM) all showed benzyl benzoate at 229 nm. Therefore, native CalB, CalB@NF, and CalB@NF@SiO
2 (TEOS 5 mM) were demonstrated for enzymatic synthesis of benzyl benzoate in this process.
Figure 5(d) shows conversion efficiency of benzoic anhydride to benzyl benzoate using native CalB, CalB@NF, and CalB@NF@SiO
2 (TEOS 5 mM). The conversion efficiency was calculated according to the following equation:
In this equation, Ci and Cf were initial and final concentrations of benzoic anhydride before and after enzymatic synthesis, respectively. The conversion efficiency from benzoic anhydride to benzyl benzoate was calculated to be 90.9%, 91.2%, and 97.3% for native CalB, CalB@NF, and CalB@NF@SiO2 (TEOS 5 mM), respectively. Although conversion efficiencies of native CalB and CalB@NF were similar, that of CalB@NF@SiO2 (TEOS 5 mM) was found to be almost 100%. This result suggests that silica-encapsulated CalB@NF@SiO2 (TEOS 5 mM) can prevent a decrease in catalytic activity in organic solvents. Therefore, protein immobilization through silica provides higher stability in organic solvents, resulting in increased catalytic efficiency.