1. Introduction
Mango (
Mangifera indica L.) is a tropical fruit native to Asia and introduced to the American continent in the 17th century [
1]. Today its annual global production is equivalent to 2.3 million tons [
2]. The main producing countries of this fruit are India, China, Thailand, Indonesia, Pakistan, Mexico and Brazil. Mexico ranks fourth in the world with a production of more than 476,000 tons in 2021 [
2], however, it is the world’s largest exporter.
Due to the climatic and edaphic conditions of the regions where mango is grown in Mexico, a wide diversity of shapes, aromas and flavors of this fruit have developed. However, the predominant varieties are Manila, Tommy Atkins, Haden, Irwin and Ataulfo. Ataulfo variety is native to Mexico and represents about 90% of the cultivated area in the south of the country [
3]. The first Ataulfo mango tree grew in the municipality of Tapachula, located in the Soconusco region, in the state of Chiapas at south of the country in the border with Guatemala; therefore, this variety has had a designation of origin by the Mexican government since 2003 [
4]. The positioning of this crop in national and international markets is related to the quality of the fruit, more specifically the pulp, since it has a high sugar content, low amount of fiber, aroma,
sui generis flavor and long shelf life [
5].
Mango provides both essential nutrients such as vitamins, minerals and antioxidant compounds (phenols, flavonoids, carotenoids and anthocyanins). The continuous consumption of antioxidant compounds is related to the prevention of various degenerative and cardiovascular diseases in humans [
6]. It is known that the Ataulfo mango has the highest polyphenol content [
7], however, the type and quantity of these compounds may vary according to the genotype, state of maturity, edapho-climatic conditions, agricultural practices and pre- and post-harvest handling of the fruit [
8].
It has been reported that environmental temperature is related to the flavonols content in grape leaves of the Tempranillo variety [
9]. It has also been shown that the content of phenolic compounds and carotenoids in extracts of
Crataegus azarolus L. are affected by the origin of the plant and it is also reported that in semi-arid zones there is an increase in these compounds [
10].
The current Ataulfo mango crops in Soconusco, southern Mexico are the result of the vegetative propagation of the seven original parent trees, however, even among the parent trees there is some genetic dissimilarity [
4], which can give rise to variability in the progeny. Such variability can be expressed in different phenotypic characters, including chemometric ones. On the other hand, even when genetic variability is scarce or null (identical clones), there may be variability in the content of antioxidant molecules exerted by the environment, as has been reported to occur in other fruits [
11]. In addition, to date there are no studies that report the phenotypic characteristics (including chemical composition) of fruits cultivated in all municipalities (locations) protected by the Ataulfo mango designation of origin. Therefore, the objective of this research was to quantify the antioxidant molecules of the Ataulfo mango grown in the Soconusco region, Chiapas, Mexico and of the parent trees; in addition, we analyze the possible relationship of compound content with some climatic characteristics collected from databases of this region of Mexico.
2. Results and Discussion
2.1. Total polyphenols
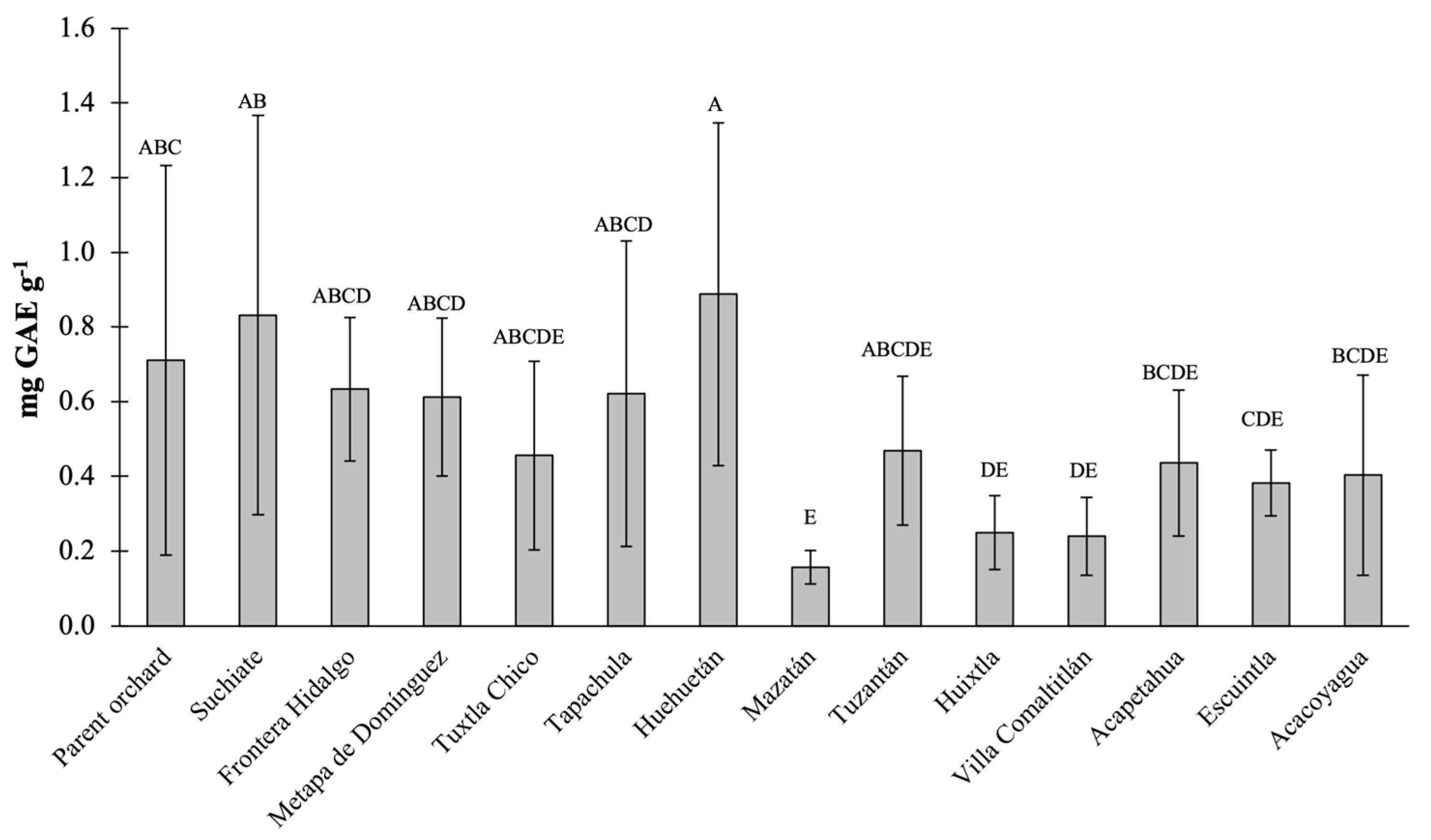
The total polyphenols content (TPC) in Ataulfo mango fruits from the different sampling sites (municipalities) are presented in
Figure 1. The highest values of these compounds were found in the fruits from Huehuetán (0.89 mg GAE g
-1), followed by fruits from Suchiate (0.83 mg GAE g
-1) and fruits from Parent orchard (0.71 mg GAE g
-1). On the contrary, the lowest concentration was recorded in the fruits from Mazatán (0.16 mg GAE g
-1). The fruits from Mazatán, Huixtla and Villa Comaltitlán were the only ones that presented significant differences (P<0.05) when contrasted with the fruits from Parent orchard. The highest values found in the present study are higher than those reported for other mango varieties from Colombia [
12] and Spain [
13], they are even slightly higher than other studies with the same Ataulfo variety where values of 0.5 mg GAE g
-1 [
8] and 0.1 mg GAE g
-1 [
14] are reported. This demonstrates that Ataulfo mangoes from Soconusco Mexico are an important source of TPC when consumed as fresh fruit.
It has previously been reported that within the same variety it is possible to find differences (intravarietal) both in the amount and in the composition of TPC. This may be due to differences in the external stimuli presented by the plant or directly by the fruit. It has been reported that geographic origin impacts the content of phenolic compounds in fruits [
15], as well as pre- and post-harvest handling and fertilization, among other factors [
16,
17,
18]. Although there were variations in the contents of TPC among the municipalities, only a few found significant differences (P<0.05), which would be revealing a low effect of environmental conditions and pre and postharvest management on the content of TPC. The standard deviations found in each set of determinations (municipalities) and in the fruits of the parent trees were also high, except for the municipality of Mazatán (
Figure 1). This may support the idea that these molecules are highly variable and that the environmental conditions of all the sites where they were sampled are not enough to differentiate the populations in terms of TPC.
2.2. Antioxidant capacity
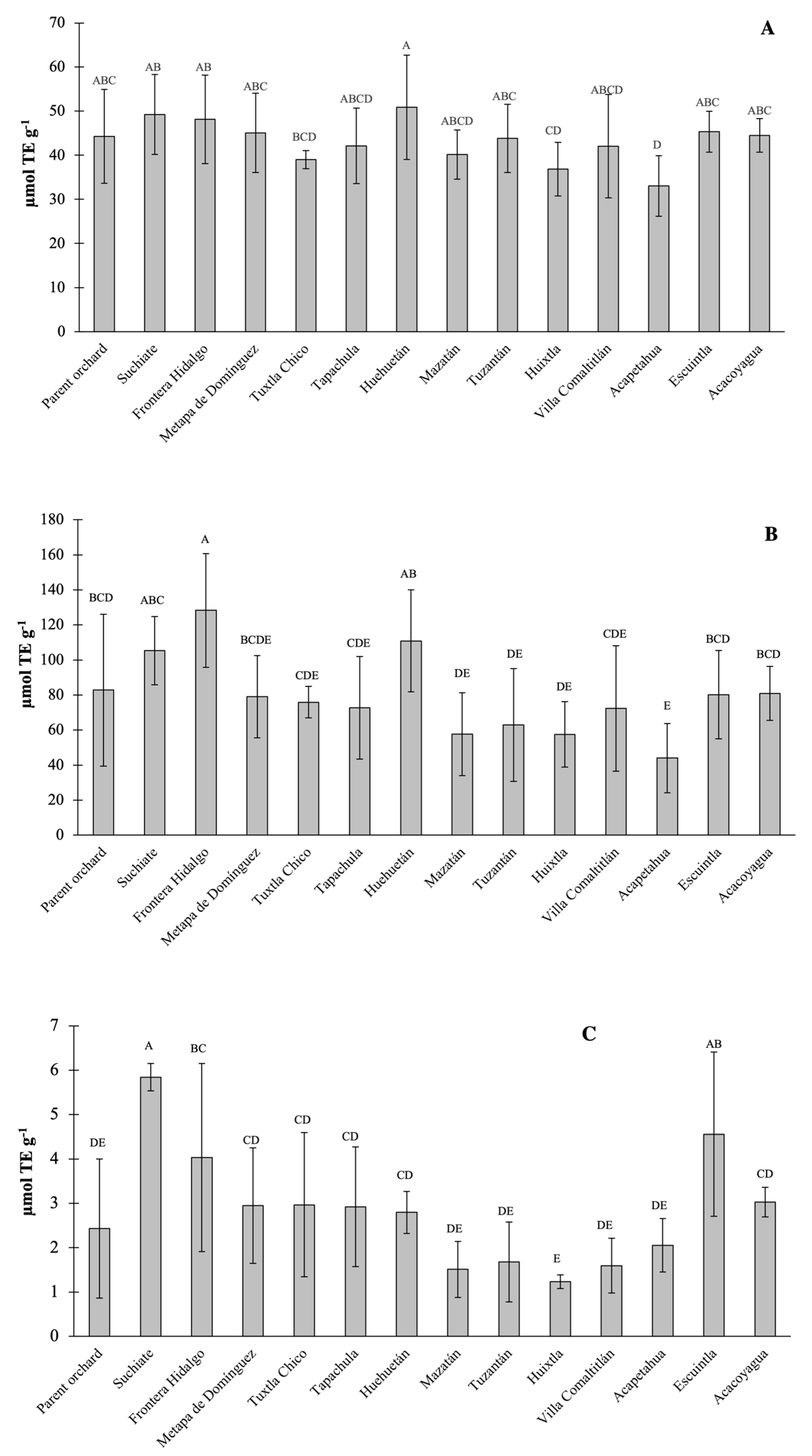
Due to the complexity of the oxidation processes and consequently of the antioxidant compounds, there is no single method that completely determines the antioxidant profile of a sample and, therefore, it is convenient to evaluate such capacity with different methods to guarantee that the results they approximate the total antioxidant capacity [
13,
15]. The results of the antioxidant capacity (AC) of the Ataulfo mango fruits sampled in the municipalities of Soconusco are shown in
Figure 2. Consistent with the nature of the methods, the highest AC values were detected by the ABTS method, followed by the DPPH method, while the lowest concentrations were recorded in the FRAP assay (
Figure 2). This is due to the fact that the tests detect different antioxidant molecules [
19].
The highest AC values in the ABTS procedure (128.29 and 110.86 µmol TE g
-1) were from the fruits of the municipalities of Frontera Hidalgo and Huehuetán, respectively. The fruits of this last municipality, followed by those of Suchiate and Frontera Hidalgo, had the highest AC values determined by the DPPH method (50.88; 49.26 and 48.16 µmol TE g
-1, respectively). With the FRAP assay, the fruits from the municipalities of Suchiate, Escuintla and Frontera Hidalgo obtained the highest values in the ferric ion reduction capacity (5.84; 4.56 and 4.03 µmol TE g
-1, respectively). The fruits from the municipality of Acapetahua reported the lowest values in AC for the DPPH and ABTS assays (33.02 and 44.02 µmol TE g
-1, respectively) while the fruits belonging to Huixtla reported the lowest values in FRAP (1.24 µmol TE g
-1). Like what happened for the phenolic compounds, high standard deviation values were found in the three AC assays, which possibly contributed to the fact that in most cases, no significant differences (P>0.05) were found between the municipalities. Only some localities were significantly different, mainly in the FRAP method (
Figure 2), where the fruits from the Parent orchard had a lower value and significantly different (P<0.05) from the values of the Suchiate fruits (
Figure 2C).
The AC measured by the ABTS method was much higher than that reported for mangoes of several varieties from other regions of Mexico, including Ataulfo variety where it reports 0.0456 µmol TE g
-1 [
6]. Contrary to what was reported by Vázquez-Olivo et al. [
6] but in agreement with several other studies [
7,
20], the highest AC values were observed in the ABTS assay, which could indicate that this method it is more efficient and more sensitive to quantify antioxidants in fruit pulp as suggested [
21]. The content of total polyphenols was found to be related to the AC only in some of the municipalities, the fruits of Huehuetán presented the highest content of polyphenols and the highest AC reported by DPPH and ABTS. It is common to find a correlation between the content of and AC; however, in Ataulfo mango it has already been reported that there is no total correlation between both characteristics [
5,
7]. The nature and proportion of each of the phenolic compounds is also decisive in the correlation or not between TPC and AC, since certain types of TPC (chlorogenic acid, gallic acid, vinylic acid and others) exert greater AC and these could be found in different concentration in the fruits of the different municipalities as it has been reported occurs in mango fruits grown in China, as well as various varieties of fruits [
17,
22,
23].
2.3. Total anthocyanins
The content of total anthocyanins (TA) in the different sampling sites expressed in µg equivalents of cyanidin-3-glucoside per g of pulp are shown in the
Figure 3. The fruits from the municipality of Tuzantán presented the highest values in the concentration of TA (39.11 µg g
-1 pulp) while the lowest concentrations were found in the Acapetahua fruits (1.50 µg g
-1 pulp). These contents are low compared to those found in the peels of other mango varieties [
24], this may be since the level of anthocyanins is concentrated in the peel of the fruits and is lower in the pulp of mangoes [
15]. However, when compared with the content of these pigments in the pulp, the results of the present study are superior to a variety of fruits studied in northern Brazil [
23] and in the skin of mangoes of different varieties [
25]. Significant differences (P<0.05) were observed between municipalities, being the fruits of Tuzantán different from the rest of the municipalities, even the fruits of the Parent orchard. The fruits of the municipalities that did not present significant differences between them were those that had the lowest concentration, i.e., Suchiate, Tapachula, Mazatán, Acapetahua and Acacoyagua (
Figure 3). It has been reported that in mango the major groups of anthocyanin compounds include cyanidin, peonidin, petunidin, delphinidin and pelargonidin and that some of them accumulate in response to environmental stimuli such as the amount of irradiation, water, pests [
25]. In the present study, the amount of sunlight received by the plants of all localities was very similar (
Table 1) and it is not possible to explain the differences in TA from this perspective.
2.4. Ascorbic acid
Ascorbic acid (AA) is considered one of the substances with high antioxidant potential. The highest levels were observed in the fruits from Huehuetán (0.25 mg g
-1 pulp), while the lowest values of AA were detected in the fruits from Huixtla (0.01 mg g
-1 pulp). The mango fruits of the Parent orchard did not present significant differences (P>0.05) with most of the fruits of the municipalities analyzed; except for those with the lowest concentration sampled in the municipalities of Metapa de Domínguez, Tapachula, Huixtla and Villa Comaltitlán (
Figure 4). The values found in the present study are very similar to others reports with different varieties of mango [
12,
26] and are slightly higher for mangoes of the Ataulfo variety grown in the Mexican state of Guerrero [
8]. It is notorious that for this variable, differences were found between municipalities, which may be a direct indication that AA is influenced to a greater extent by environmental conditions or the agronomic treatment of the plants and the pre- and post-harvest handling of the fruits. It has been reported that different systems for the accumulation of AA are expressed in fruits of different varieties [
27] and although in this study all the fruits are of the same variety, it is feasible to think that something similar could occur with fruits collected from different production sites.
2.5. Weather data
To evaluating the possible influence of environmental factors on the accumulation of antioxidant substances, environment data were obtained (collected) in the period from June 2018 to June 2019 (13 data for each variable) to each of the 13 sampling municipalities of the Soconusco region, Chiapas, Mexico.
Table 1 shows the average annual values for the variables included. Average values may seem like an unorthodox measure to present information on the climatic conditions of an environment; however, derived from the individualized multivariate analysis (PCA and AHC) of these data (not shown), it could be inferred that only a few values contribute to the total variance of the new components of the principal component analysis and that the grouping into new variables (PC1 and PC2) does not differ when all the data are used, the selected ones (which contribute significantly to the variance) and the averages for each municipality shown in
Table 1. This is because the variations between sites is very small and only visual for some variables. The average annual rainfall was slightly higher in Mazatán, as well as the highest average maximum temperature occurred in Acacoyagua.
2.6. Multivariate analysis
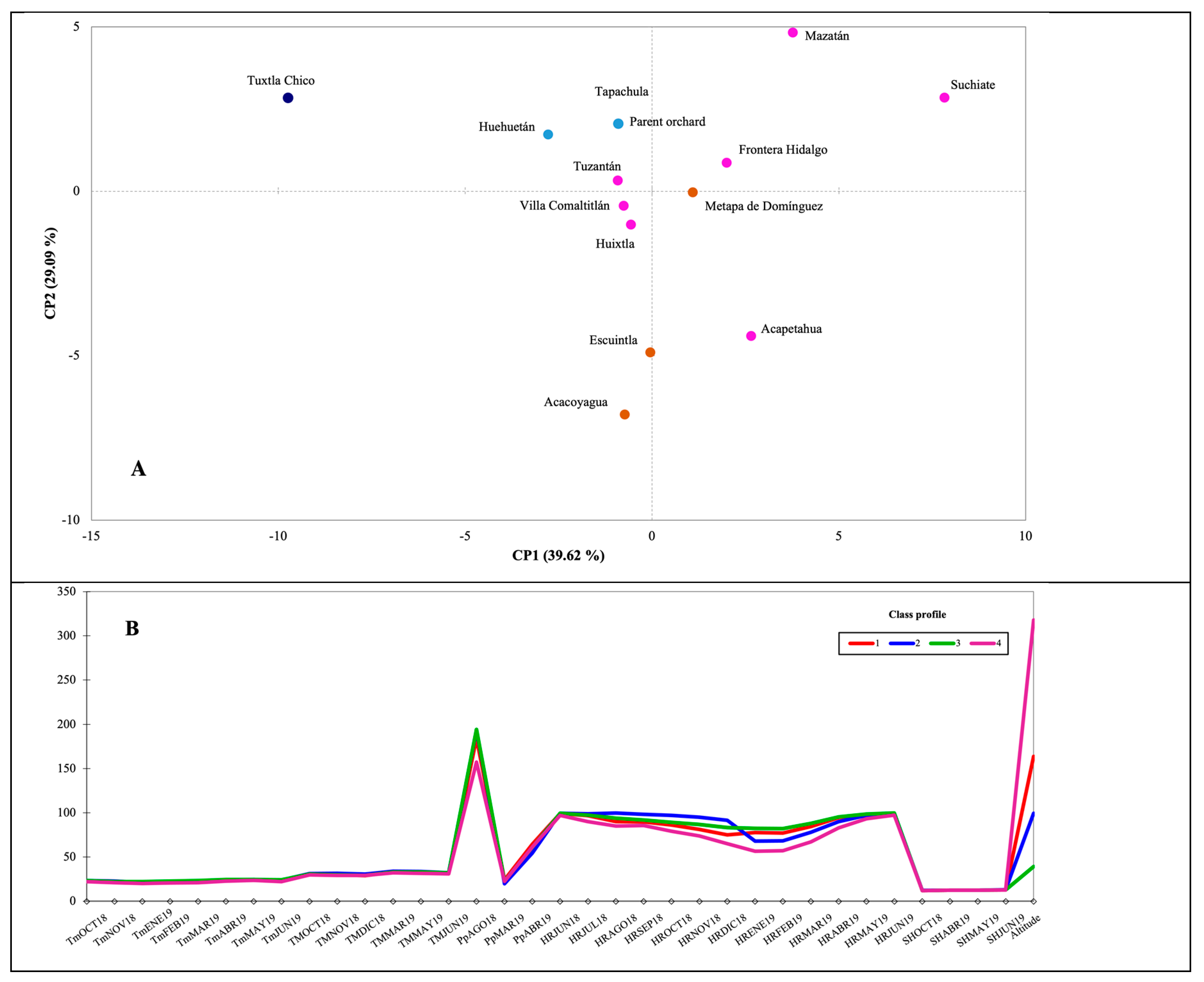
As mentioned in the previous section, the PCA and AHC with the monthly averages of all the climatological data corresponding to the period Jun2018-Jun2019 (not shown) revealed that only a select group of climatological data influences the formation of the new principal components (PC1 and PC2) and that when these are analyzed they do not differ from when the average annual values are analyzed. Contrary to what was expected, it was found that the main environmental data that influence the composition of the fruits are relative humidity (13 of 13 values), minimum temperature (8 of 13 values), maximum temperature (6 of 13 values) and the altitude. On the contrary, only three variables of rainfall and four of sunlight hours (
Figure 5B) show an effect on the composition of the new PC and consequently do explain the grouping of sampling sites (municipalities) and of the antioxidant composition of Ataulfo del Soconusco mangoes.
For this reason, a second PCA and AHC were run with the selected data (values not shown) and the distribution in the PCA plane, and the grouping of sampling sites shown in
Figure 5A were obtained. The two new PC explain almost 70% of the total variance and show the formation of four clusters or groups between the thirteen municipalities and the Parent orchard, which are mainly due to altitude (
Figure 5A), and as mentioned before, to a lesser extent to RH, rainfall (only august 2018) and temperature (
Figure 5B).
The municipality of Tuxtla Chico appears separated from the rest of the municipalities exclusively by its altitude (318 masl); however, considering the other factors and the values of AC and TPC (
Figure 1 and
Figure 2); Ataulfo mango fruits from Suchiate, which has the lowest altitude (5 masl) and one of the highest HR values, exhibited one of the highest values in TPC and AC. The foregoing, although it was not reflected in the univariate analysis, gives a trend that higher values of RH and temperatures, as well as lower altitudes, would contribute to the accumulation of certain antioxidant substances in Ataulfo mango fruits grown in the Soconusco region, Mexico. This phenomenon, although with other molecules, was reported by Andola et al. [
28] who reported that berberine (alkaloid) contents were increased in plant populations from the western Himalayas growing at low altitudes and higher humidity values. Other authors have also demonstrated the effect of these conditions and of the soil characteristics on the accumulation of antioxidants in fruits [
16] or
Aloe vera extracts [
17]. Confirming what happened in Ataulfo mango, in wild blueberries it has been reported [
18] that sunlight is the most determining factor in the increase of phenolic compounds and antioxidants, since at a lower altitude, the amount of sunlight is greater.
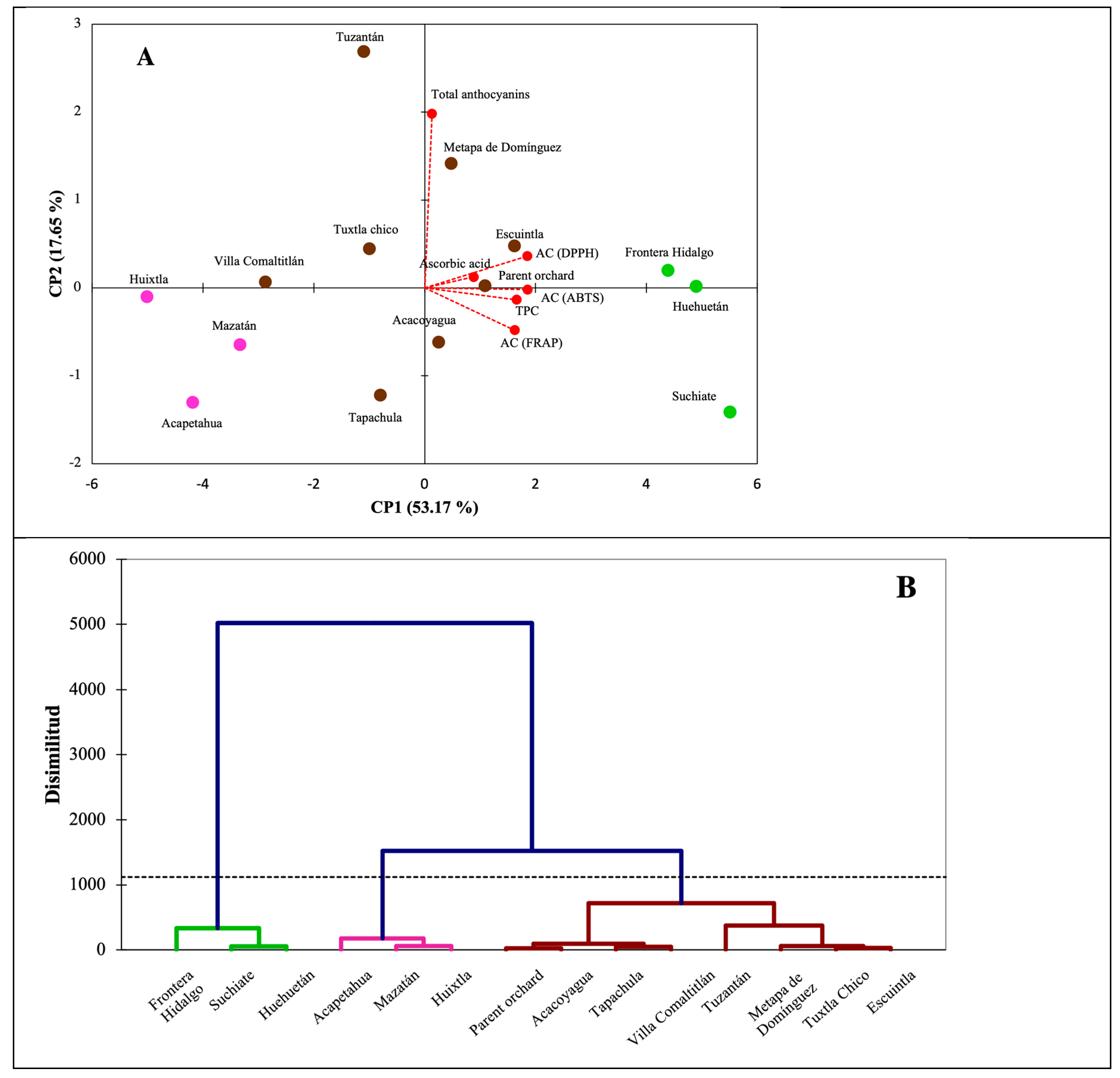
Lastly,
Figure 6 shows the associations between antioxidant compounds and collection sites in the Soconusco region. The variation explained by the PC1 and PC2 components in this PCA is around 70%. Component 1 groups all the variables of the antioxidant molecules in the positive quadrant, which shows a positive association between the presence of these compounds and the municipalities mainly marked in green (
Figure 6A). These municipalities, in turn, are the most dissimilar to the rest of the municipalities (
Figure 6B) when the chemical variables are analyzed together through the AHC, which forms three groups. In the same way, a strong association can be seen between the TPC, and the AC values measured by the three methods (
Figure 6A). The intermediate values in AC and low values in AA and TA are the main explanation for the negative (opposite) association between the municipalities located in the negative quadrant of PC1 and the molecules evaluated. The fruits from seven municipalities (brown color,
Figure 6A,B) showed a very close grouping in terms of chemical and antioxidant composition with the fruits of the Parent orchard.
3. Materials and Methods
3.1. Mango fruits and sampling
To obtain the representative number of trees grown in the entire Soconusco region (Chiapas, Mexico), a completely randomized sampling design was established to estimate the sample size, based on the equation 1:
In the equation; n= sample size, N= population size, P= proportion of the sample size or 0.5, Z α2= 2 confidence intervals and e= experimental sampling error. The largest possible area was covered in the 13 municipalities of this region, which make up the area protected by the denomination of origin (
Table 2). From each selected tree, an average of 10 fruits at mature green and homogeneous size (ranged between 214-354 g) were sampled, as specified in NOM-188-SCFI-2012 for Ataulfo mangoes for export [
29]. They were transferred immediately after cutting to the laboratory, fruits without visual defects and healthy were selected, for their subsequent washing with tap water. The fruits were stored in a room at 25 °C until full ripe. When full maturity was reached, an average of three (range 2-4) fruits were selected from each tree; those that during maturation remained healthy and without apparent defects.
3.2. Polyphenol content
A total of 100 mg of mango pulp was macerated in 1 mL of 80% v/v methanol and subsequently stirred at 200 rpm for 24 h at room temperature. The suspension was centrifuged at 2,626 g for 25 min. The supernatant was recovered and stored at –25 °C. The precipitate was subjected to a second extraction as previously described. Both methanolic extracts were combined and stored at –25 °C until use to quantify the phenolic compounds. The determination of polyphenols was performed by the colorimetric Folin-Ciocalteu method as described previously [
6]. An aliquot (20 µL) of the extraction supernatant was taken, to which 80 µL of methanol (80% v/v), 500 µL of Folin-Ciocalteu reagent (1:10) and 400 µL of Na
2CO
3 were added, followed by let stand 60 min in the dark. The absorbance at 765 nm was then measured in a spectrophotometer (Microplate reader MR-96A). A calibration curve (0-10 mg L
-1) was performed using gallic acid as a standard. The results were expressed as mg gallic acid equivalents (GAE) per g of pulp (mg GAE g
-1).
3.3. Measurement of antioxidants capacity
To extract antioxidant compounds, mango pulp (100 mg) was macerated in 1 mL of a methanol-acetic acid-water (64:2:34) solution. Samples were homogenized on a rotary shaker (200 rpm) for 24 h at room temperature. The suspension was centrifuged at 2,626 g for 25 min. The supernatant was separated and stored at –20 °C. The precipitate was extracted again as previously described. Both extracts were combined, stored at –20 °C [
30] and then used to estimate the antioxidant capacity.
3.3.1. Antioxidant capacity by the DPPH method
The DPPH (2,2-diphenyl-1-picrylhydrazyl) assay [
31] was carried out adapting volumes for the reading of absorbances in microplate. An amount of 2.3 mg of DPPH in 100 mL of 80% methanol was used as a standard. The solution was adjusted to 1.0 ± 0.02 absorbance units at 520 nm. The calibration curve was established using 20 μL Trolox solution (0–80 mM in 80% methanol) and 280 μL DPPH stock solution. The homogenized mixture was kept in the dark for 60 min. A blank solution (methanol: acetic acid: water, 64:2:34) was used. Absorbances at 520 nm were read in a microplate reader (Mindray MR-96A, Shenzhen Mindray Biomedical Electronics, China). Antioxidant capacity was measured using 20 μL extract and 280 μL of the DPPH radical. Results were expressed as antioxidant capacity in Trolox equivalents per gram of mango pulp (μmol TE g
-1).
3.3.2. Antioxidant capacity by the ABTS method
The ABTS (2,2-azino-bis-3-ethylbenzothiazoline-6- sulfonic acid) assay [
32] was carried out according with adaptation in volumes for the reading of absorbances in microplate. ABTS
•+ radical was produced by the reaction of 5 mL ABTS (7 mM in 70% ethanol) with 88 μL of 140 mM K
2S
2O
8; the reaction was left to stand in the dark at 25 °C for 12 h. Absorbance (734 nm) of the solution was adjusted to 0.800 ±0.03 with 7% ethanol, using a microplate reader (Mindray MR-96A, Shenzhen Mindray Biomedical Electronics, China). This value was considered as the absorbance of the blank. For preparing the calibration standard curve, 280 μL of the ABTS
•+ diluted solution was used and 0-20 μL of Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2- carboxylic acid) to reach different concentrations (0-800 μM in 80% methanol) were added; after 6 min the absorbance was determined. For mango samples, the same procedure was repeated using 20 μL of the previously extracted samples. Results were expressed as Trolox equivalent per gram of fresh pulp (μmol TE g
-1).
3.3.3. Ferric reducing antioxidant power (FRAP)
The FRAP assay [
33] was carried out with adaptation in volumes for the reading of absorbances in microplate. Solutions A (300 mM sodium acetate buffer, pH 3.6), B (10 mM TPTZ, 2,4,6-tripyridyl-s-triazine in 40 mM HCl), and C (20 mM FeCl
36H
2O) were prepared and stored in the dark until use (no more than three days). To obtain the FRAP reagent, 25 mL of solution A, 2.5 mL of solution B, and 2.5 mL of solution C were gently mixed. To carry out the calibration curve, 280 µL of FRAP reagent were mixed with 20 µL of Trolox solution (0-30 µM), left to rest for 4 min in the dark for subsequent microplate reading at 630 nm using a microplate reader (Mindray MR-96A, Shenzhen Mindray Biomedical Electronics, China). For the mango samples, 20 µL of extract and 280 µL of the FRAP reagent were mixed and the absorbances were read. Results were expressed in Trolox equivalents per gram of fresh pulp weight (µmol ET g
-1).
3.4. Total anthocyanins by pH differential
In microtubes, 0.2 g of mango pulp were weighed and macerated dissolving in 1 mL of 96% ethanol. The tubes were placed on a rotary shaker (200 rpm) for 24 h, then the suspension was centrifuged at 2,626 g for 25 min at room temperature. For the quantification of total anthocyanins, the method described by Teng et al. [
34], with modifications in the final volume. This procedure is based on the particularity of anthocyanins to adopt different colors and structures at different pH. Two buffers of different pH were prepared: the first pH = 1.0 (0.2 N potassium chloride and 0.2 N hydrochloric acid) and the second pH=4.5 (1 M sodium acetate, 1 N hydrochloric acid). Subsequently, 0.5 mL of extract was taken and diluted with 2 mL of pH 1 and pH 4.5 buffer, respectively. The mixtures were shaken and allowed to settle for 20 min. The absorbances of the solutions were measured by means of a microplate reader (Mindray MR-96A, Shenzhen Mindray Biomedical Electronics, China) at two wavelengths (700 and 520 nm). From the data, the anthocyanin content was calculated using the formula of anthocyanin concentration (mg L
-1) = A (Mw) (DF) (1000) / (ƹ) (1). Where A= absorbance, Mw= molecular weight of cyanidin-3-glucoside, DF= dilution factor and ƹ= molar extinction coefficient of cyanidin-3-glucoside.
3.5. Ascorbic acid
One g of mango pulp was weighed, which was subsequently macerated with 5 mL of metaphosphoric acid-acetic acid (extracting solution). Following the procedure described by the AOAC method 967.21 [
35], 2.5 mL aliquots were taken for their determination, transferred to Erlenmeyer flasks and titrated with 2,6-dichloroindophenol until a light pink color was observed. The concentration is expressed as mg of ascorbic acid per gram of pulp in fresh weight.
3.6. Wheater data
Climatological data (monthly average temperature, daylight hours, altitude, monthly average rainfall, relative humidity) were collected on the Weather Spark website from June 2018 to June 2019 and the altitude was measured by means of GPS in the 13 municipalities of Soconusco region, Chiapas, Mexico.
3.7. Data analysis
All determinations were performed in triplicate. The data were subjected to univariate analysis of variance and subsequent comparison of means with Duncan's test (α=0.05) using the Infostat v. 2018. Additionally, Principal Component Analysis (PCA) and Ascending Hierarchical Classification (AHC) were carried out with the data of all the variables, both those evaluated in the fruits and those collected (climatological) from the database, using the statistical program XL-Stat v. 2012.
5. Conclusions
The antioxidant capacity and associated molecules were quantified in Ataulfo mango fruits from the 13 municipalities that demarcate the denomination of origin region in Soconusco, Chiapas, Mexico, in addition to the fruits from Parent orchard. Variations were found in the content of total phenolic compounds and in the antioxidant capacity. The fruits belonging to the municipalities of Huehuetán and Suchiate presented the highest values in the TPC content, while the highest CA was found in the fruits from Huehuetán, Suchiate and Frontera Hidalgo. Although no significant correlation was found, the results of our study show that the content of antioxidant molecules in Ataulfo mango could be slightly influenced by the environmental conditions where it is grown, since in places with higher relative humidity and lower altitude, higher content of some antioxidant molecules.
Author Contributions
Conceptualization, A.V.-O. and D.G.-L.; methodology, I.O.-G.; software, R.R.-Q.; formal analysis, A.V.-O., I.O.-G. and R.R.-Q.; writing—original draft preparation, A.V.-O. and I.O.-G.; writing—review and editing, M.S.-F., D.G.-L. and R.R.-Q.; funding acquisition, D.G.-L. and M.S.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by Consejo Nacional de Ciencia y Tecnología (CONACYT) Mexico project APN-2017/6427.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on request.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the antioxidant’s compounds are available from the authors.
References
- Warschefsky, E.J.; von Wettberg, E.J.B. Population genomic analysis of mango (Mangifera indica) suggests a complex history of domestication. New Phytol. 2019, 222, 2023–2037. [Google Scholar] [CrossRef] [PubMed]
- FAO. 2022. Major Tropical Fruits: Preliminary results 2021. Rome.
- Gálvez-López, D.; Adriano-Anaya, M.L.; Villarreal-Treviño, C.; Mayek-Pérez, N.; Salvador-Figueroa, M. Isoenzymatic diversity of mango landraces of Chiapas, Mexico. Rev. Chapingo Ser. Hortic. 2007, 13, 71–76. [Google Scholar] [CrossRef]
- Salvador-Figueroa, M.; Torres-de los Santos, R.; Ovando-Medina, I.; Vázquez-Ovando, J.A.; Adriano-Anaya, M.L. Analysis of genetic variation of orchard father of mango (Mangifera indica L.) Ataulfo variety. Quehacer Cient. Chiapas 2008, 5, 29–34. [Google Scholar]
- Cancino-Vázquez, R.; Salvador-Figueroa, M.; Hernández-Ortiz, E.; Grajales-Conesa, J.; Vázquez-Ovando, A. Gamma irradiation of mango ‘Ataulfo’at low dose: effect on texture, taste, and odor fruit. Food Sci. Technol. Res. 2020, 26, 59–64. [Google Scholar] [CrossRef]
- Vazquez-Olivo, G.; Antunes-Ricardo, M.; Gutiérrez-Uribe, J.A.; Osuna-Enciso, T.; León-Félix, J.; Basilio-Heredia, J. Cellular antioxidant activity and in vitro intestinal permeability of phenolic compounds from four varieties of mango bark (Mangifera indica L.). J. Sci. Food Agric. 2019, 99, 3481–3489. [Google Scholar] [CrossRef]
- Vázquez-Ovando, A.; Ramos-Monzón, A.; Pérez-Flores, L.J.; Hernández-Ortiz, E.; Adriano-Anaya, M.L; Salvador-Figueroa, M. Antioxidant capacity of gamma irradiated “Ataulfo” mango. Wulfenia 2017, 24, 213–224. [Google Scholar]
- Maldonado-Astudillo, Y.I.; Navarrete-García, H.A.; Ortiz-Morales, O.D.; Jiménez-Hernández, J.; Salazar-López, R.; Alia-Tejacal, I.; Alvarez-Fitz, P. Physical, chemical and antioxidant properties of mango varieties grown at the Guerrero coast. Rev. Fitotec. Mex. 2016, 39, 207–214. [Google Scholar]
- Torres, N.; Goicoechea, N.; Antolín, M.C. Antioxidant properties of leaves from different accessions of grapevine (Vitis vinifera L.) cv. Tempranillo after applying biotic and/or environmental modulator factors. Ind. Crops Prod. 2015, 76, 77–85. [Google Scholar] [CrossRef]
- Yahyaoui, A.; Arfaoui, M.O.; Rigane, G.; Hkir, A.; Amari, K.; Ben-Salem, R.; Ammari, Y. Investigation on the chemical composition and antioxidant capacity of extracts from Crataegus azarolus L.: Effect of growing location of an important Tunisian medicinal plant. Chem. Afr. 2019, 2, 1–5. [Google Scholar] [CrossRef]
- Chen, M.; Huang, W.; Yin, Z.; Zhang, W.; Kong, Q.; Wu, S.; Li, W.; Bai, Z. , Fernie, A.R.; Huang, X.; Yan, S. Environmentally-driven metabolite and lipid variations correspond to altered bioactivities of black wolfberry fruit. Food Chem. 2022, 372, 131342. [Google Scholar] [CrossRef]
- Arrazola, P.G.; Rojano, B.A.; Díaz, D.A. The antioxidant capacity of five mango cultivars (Mangifera indica L.) and evaluation of its performance in a food matrix. Rev. Colomb. Cienc. Hort. 2013, 7, 161–172. [Google Scholar] [CrossRef]
- Esteban-Muñoz, A.; Barea-Álvarez, M.; Oliveras-López, M.; Giménez-Martínez, R.; Henares, J.; Olalla-Herrera, M. Determination of polyphenolic compounds by ultra-performance liquid chromatography coupled to tandem mass spectrometry and antioxidant capacity of Spanish subtropical fruits. Agric. Sci. 2018, 9, 180–199. [Google Scholar] [CrossRef]
- Palafox-Carlos, H.; Yahia, E.; Islas-Osunaa, M.A.; Gutierrez-Martinez, P.; Robles-Sánchez, M.; González-Aguilar, G.A. Effect of ripeness stage of mango fruit (Mangifera indica L., cv. Ataulfo) on physiological parameters and antioxidant activity. Sci. Hortic. 2012, 135, 7–13. [Google Scholar] [CrossRef]
- Mehmood, A.A.; Guo, X.; Fu, X.; Zhou, L.; Chen, Y.; Zhu, Y.; Yan, H.; Liu, R.H. Comparative assessment of phenolic content and in vitro antioxidant capacity in the pulp and peel of mango cultivars. Int. J. Mol. Sci. 2015, 16, 13507–13527. [Google Scholar]
- Khromykh, N.O.; Lykholat, Y.V.; Kovalenko, I.M.; Kabar, A.M.; Didur, O.O.; Nedzvetska, M.I. Variability of the antioxidant properties of Berberis fruits depending on the plant species and conditions of habitat. Regul. Mech. Biosyst. 2018, 9, 56–61. [Google Scholar] [CrossRef]
- Kumar, S.; Yadav, A.; Yadav, M.; Yadav, J.P. Effect of climate change on phytochemical diversity, total phenolic content and in vitro antioxidant activity of Aloe vera (L.) Burm. f. BMC Res. Notes 2017, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Stampar, F.; Veberic, R. A comparison of fruit quality parameters of wild bilberry (Vaccinium myrtillus L.) growing at different locations. J. Sci. Food Agric., 2015, 95, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Gayosso-García, L.E.; Yahia, E.; Martínez-Téllez, M.A.; González Aguilar, G.A. Effect of maturity stage of papaya maradol on physiological and biochemical parameters. Am. J. Agric. Biol. Sci. 2010, 5, 199–208. [Google Scholar] [CrossRef]
- Castañeda-Valbuena, D.; Ayora-Talavera, T.; Luján-Hidalgo, C.; Álvarez-Gutiérrez, P.; Martínez-Galero, N.; Meza-Gordillo, R. Ultrasound extraction conditions effect on antioxidant capacity of mango by-product extracts. Food Bioprod. Process. 2021, 127, 212–224. [Google Scholar] [CrossRef]
- Kuskoski, E.M.; Asuero, A.G.; Troncoso, A.M.; Mancini-Filho, J.; Fett, R. Aplicación de diversos métodos químicos para determinar actividad antioxidante en pulpa de frutos. Food Sci. Technol. 2005, 25, 726–732. [Google Scholar] [CrossRef]
- Jurado-Teixeira, B.; Aparcana-Ataurima, I.M.; Villarreal-Inca, L.S.; Ramos-Llica, E.; Calixto-Cotos, M.R.; Hurtado-Manrique, P.E.; Acosta-Alfaro, K.M.C. Evaluation of the content of phenolic compounds and antioxidant capacity of the ethanol extracts of the fruits of aguaymanto (Physalis peruviana L.) from different locations of Peru. Rev. Soc. Quím. Perú 2016, 82, 272–279. [Google Scholar]
- Beserra-Almeida, M.M.; Machado-de Sousa, P.H.; Campos-Arriaga, A.M.; Matias-do Prado, G.; De Carvalho-Magalhães, C.E.; Arraes-Maia, G.; Gomes-de Lemos, T.L. Bioactive compounds and antioxidant activity of fresh exotic fruits from northeastern Brazil. Food Res. Int. 2011, 44, 2155–2159. [Google Scholar] [CrossRef]
- Mugwagwa, L.R.; Chimphango, A.F.A. Box-Behnken design based multi-objective optimisation of sequential extraction of pectin and anthocyanins from mango peels. Carbohydr. Polym. 2019, 219, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Ranganath, K.G.; Shivashankara, K.S.; Roy, T.K.; Dinesh, M.R.; Geetha, G.A.; Pavithra, K.C.; Ravishankar, K.V. Profiling of anthocyanins and carotenoids in fruit peel of different colored mango cultivars. J. Food Sci. Technol. 2018, 55, 4566–4577. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Ribeiro, S.M.; Queiroz, J.H.; Lopes-Ribeiro de Queiroz, M.E.; Milagres-Campos, F.; Pinheiro-Sant’Ana, H.M. Antioxidant in mango (Mangifera indica L.) pulp. Plant Foods Human Nutr. 2007, 62, 13–17. [Google Scholar] [CrossRef]
- Ifigeneia, M.; Keulemans, J.; Kanellis, A.K.; Davey, M.W. Regulation of fruit ascorbic acid concentrations during ripening in high and low vitamin C tomato cultivars. BMC Plant Biol. 2012, 12, 239. [Google Scholar]
- Andola, H.C.; Gaira, K.S.; Rawal, R.S.; Rawat, M.S.; Bhatt, I.D. Habitat-dependent variations in berberine content of Berberis asiatica Roxb. ex. DC. in Kumaon, Western Himalaya. Chem. Biodivers. 2010, 7, 415–20. [Google Scholar] [CrossRef] [PubMed]
- DOF. 2012. Norma Oficial Mexicana NOM-188-SCFI-2012. Mango Ataulfo del Soconusco, Chiapas (Mangifera caesia Jack ex Wall)-especificaciones y métodos de prueba. http://dof.gob.mx/nota-detalle.php?codigo=5280541&fecha=29/11/2012.
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- de Brito-Araújo, A.J.; da Silva, W.P.; dos Santos-Moreira, I.; Santos, N.C. Effect of drying temperature on the physicochemical characteristics, bioactive compounds, and antioxidant activity of “Palmer” mango peels. J. Food Process, Eng. 2021, 44, e13860. [Google Scholar] [CrossRef]
- Nenadis, N.; Wang, L.F.; Tsimidou, M.; Zhang, H.Y. Estimation of scavenging activity of phenolic compounds using the ABTS•+ assay. J. Agric. Food Chem. 2004, 52, 4669–4674. [Google Scholar] [CrossRef]
- Benzie, I.; Strain, J. Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurements of total antioxidant power and ascorbic acid concentration. Meth. Enzymol. 1999, 299, 15–27. [Google Scholar]
- Teng, Z.; Jiang, X.; He, F.; Bai, W. Qualitative and quantitative methods to evaluate anthocyanins. EFood, 2020, 1, 339–346. [Google Scholar] [CrossRef]
- Horwitz, W. (Ed.). Official Methods of Analysis of Association of Official Analytical Chemists. 18th ed. Washington: AOAC, 2005. Official Method 967.21.
Figure 1.
Total polyphenol content of the Ataulfo variety mango harvested from thirteen municipalities and the Parent orchard located in the Soconusco region, Chiapas, Mexico. Different letters between localities denote a significant difference (P<0.05).
Figure 1.
Total polyphenol content of the Ataulfo variety mango harvested from thirteen municipalities and the Parent orchard located in the Soconusco region, Chiapas, Mexico. Different letters between localities denote a significant difference (P<0.05).
Figure 2.
Antioxidant capacity of Ataulfo mango fruits from thirteen municipalities and Parent orchard in the Soconusco region, Chiapas, Mexico, determined by the DPPH (A), ABTS (B) and FRAP (C) methods. Different letters between municipalities denote a significant difference (P<0.05).
Figure 2.
Antioxidant capacity of Ataulfo mango fruits from thirteen municipalities and Parent orchard in the Soconusco region, Chiapas, Mexico, determined by the DPPH (A), ABTS (B) and FRAP (C) methods. Different letters between municipalities denote a significant difference (P<0.05).
Figure 3.
Total anthocyanin content of Ataulfo Mango fruits from thirteen municipalities and Parent orchard of the Soconusco region, Chiapas, Mexico. nd = not determined. Different letters between municipalities denote a significant difference (P<0.05).
Figure 3.
Total anthocyanin content of Ataulfo Mango fruits from thirteen municipalities and Parent orchard of the Soconusco region, Chiapas, Mexico. nd = not determined. Different letters between municipalities denote a significant difference (P<0.05).
Figure 4.
Ascorbic acid content of Ataulfo mango fruits grown in thirteen municipalities and the Parent orchard in the Soconusco region, Chiapas, Mexico. nd = not determined. Different letters between municipalities denote a significant difference (P<0.05).
Figure 4.
Ascorbic acid content of Ataulfo mango fruits grown in thirteen municipalities and the Parent orchard in the Soconusco region, Chiapas, Mexico. nd = not determined. Different letters between municipalities denote a significant difference (P<0.05).
Figure 5.
Biplot graph of the Principal Components Analysis (A) and dendrogram of the Ascending Hierarchical Classification (B) with selected climate data of the period Jun 2018-Jun 2019 from 13 municipalities of the Soconusco region, Chiapas, Mexico.
Figure 5.
Biplot graph of the Principal Components Analysis (A) and dendrogram of the Ascending Hierarchical Classification (B) with selected climate data of the period Jun 2018-Jun 2019 from 13 municipalities of the Soconusco region, Chiapas, Mexico.
Figure 6.
Biplot graph of the Principal Components Analysis (A) and dendrogram of the Ascending Hierarchical Classification (B) with chemical data of Ataulfo mango fruits from the 13 municipalities of the Soconusco, Chiapas, Mexico region and Parent orchard.
Figure 6.
Biplot graph of the Principal Components Analysis (A) and dendrogram of the Ascending Hierarchical Classification (B) with chemical data of Ataulfo mango fruits from the 13 municipalities of the Soconusco, Chiapas, Mexico region and Parent orchard.
Table 1.
Average annual values of environmental data in the 13 municipalities of the Soconusco region, Chiapas, Mexico.
Table 1.
Average annual values of environmental data in the 13 municipalities of the Soconusco region, Chiapas, Mexico.
| Municipality |
Tm (°C) |
TM (°C) |
Pp (mm) |
RH (%) |
SH |
Alt (msnm) |
| Parent orchard |
22.96 |
31.03 |
117.39 |
89.72 |
12.19 |
164 |
| Suchiate |
23.78 |
30.49 |
113.97 |
98.90 |
12.09 |
5 |
| Frontera Hidalgo |
22.95 |
31.34 |
113.04 |
91.97 |
12.16 |
64 |
| Metapa de Domínguez |
22.90 |
31.28 |
113.05 |
92.45 |
12.23 |
107 |
| Tuxtla Chico |
22.08 |
30.68 |
109.87 |
79.24 |
12.19 |
318 |
| Tapachula |
22.96 |
31.03 |
117.39 |
89.72 |
12.19 |
164 |
| Huehuetán |
22.82 |
32.24 |
118.08 |
85.75 |
12.09 |
164 |
| Mazatán |
23.50 |
30.88 |
125.38 |
91.72 |
12.02 |
20 |
| Tuzantán |
22.84 |
31.44 |
118.82 |
88.42 |
12.17 |
54 |
| Huixtla |
23.05 |
32.62 |
119.60 |
88.99 |
12.18 |
53 |
| Villa Comaltitlán |
23.02 |
32.02 |
117.97 |
86.82 |
11.98 |
44 |
| Acapetahua |
23.16 |
32.30 |
113.78 |
92.53 |
12.27 |
33 |
| Escuintla |
22.94 |
31.80 |
111.90 |
90.46 |
12.08 |
100 |
| Acacoyagua |
23.05 |
34.00 |
114.26 |
89.61 |
12.21 |
91 |
Table 2.
Number of trees and Ataulfo mango fruits sampled by municipality (locality) in the Soconusco region, Chiapas, Mexico, including those from Parental orchard.
Table 2.
Number of trees and Ataulfo mango fruits sampled by municipality (locality) in the Soconusco region, Chiapas, Mexico, including those from Parental orchard.
| Locality |
Number of trees |
Number of fruits |
| Tapachula |
52 |
156 |
| Suchiate |
5 |
15 |
| Frontera Hidalgo |
5 |
15 |
| Tuxtla Chico |
5 |
15 |
| Metapa de Domínguez |
5 |
15 |
| Huehuetán |
17 |
51 |
| Tuzantán |
5 |
15 |
| Mazatán |
28 |
84 |
| Huixtla |
5 |
15 |
| Villa Comaltitlán |
13 |
39 |
| Acapetahua |
5 |
15 |
| Escuintla |
5 |
15 |
| Acacoyagua |
5 |
15 |
| Total |
155 |
465 |
| Parental orchard (Tapachula) |
4 |
8 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).