1. Introduction
Inflammation is the response of the immune system to infection and injury, with complex interactions with the body, tissues and cells [
1]. If inflammation is not alleviated in time, it will develop into a chronic disease, resulting in systemic metabolic disorders, organ and tissue damage and dysfunction, and subsequently causing a large rise in chronic diseases with high mortality and disability, and playing an important role in the initiation and progression of many diseases such as cancer and cardiovascular disease [
2]. Anti-inflammatory drugs can be effective in relieving symptoms, but they are often accompanied by systemic side effects such as gastrointestinal, renal and cardiovascular side effects (e.g. NSAIDs) [
3], increased risk of serious infections and neurological adverse events (e.g. biologics) [
4], whereas targeted drug delivery systems (DDSs) can be effective in reducing off-target side effects.
Pharmaceutical excipients hold important functions such as solubilizing, shaping, acting as carriers, improving stability, controlling drug release, etc., which affects the quality, safety and functionality of the final pharmaceuticals. In the study of DDSs, some excipients as carriers play a role in determining the delivery effect. In recent years, the influence of carrier material properties on delivery effects and pharmacodynamic contribution has become the focus of research [
5]. For example, Zhang et al. explored the facilitation of autophagy induced by materials type of DDSs on cancer therapy [
6]. Dendrimers are important carrier materials commonly used in modern targeted DDSs. Shao et al. reported that polyacylthiourea dendrimers have tumor target delivery effects and could exert intrinsic anticancer activity through non-cytotoxic pathways without cytotoxic-induced side effects [
7]. In 2022, Boehnke et al. published a paper in Science titled "Massively parallel pooled screening reveals genomic determinants of nanoparticle-cell interactions", describing the impact of some common carrier materials used to construct nanoparticles on
in vivo delivery effects [
8]. The series of reports on polyethylene glycol (PEG) provides a valuable experimental basis for the appropriate choice of PEG type for long-circulation DDSs [
9,
10,
11,
12]. However, as indispensable substances other than active ingredients in pharmaceutical formulations, although many traditional non-chemically modified excipients hold long-term and extensive clinical applications, their affinity to different organs in animals has not been reported. Therefore, we speculate that adopting modern experimental techniques to accurately evaluate the delivery effects of commonly used excipients and to build a "drug-excipient unity" system with synergistic therapeutic effects on targets will provide an experimental basis and theoretical reference for the re-recognition of the delivery potential and value of more excipients, and to better guide and enrich drugs into target organs or target sites, target cells or even cell organelles, which are of great significance to clinicians and researchers.
Nanoemulsions (NEs) are translucent or transparent oil-in-water (O/W) or water-in-oil (W/O) droplets stabilized by excipients such as surfactants and co-surfactants at the oil-water interface [
13]. Due to the average spherical size ranging from 100 to 500 nm, NEs hold high thermodynamic and kinetic stability, and have been widely used as effective drug delivery carriers in biomedical industry [
14,
15]. In the early stages, the structure, physicochemical properties and toxicity of various excipients forming nanoemulsions were extensively studied [
16,
17]. Among them, oil phase, as a common excipient in NEs, has the advantages of assisting the formation of emulsions, loading lipophilic drugs, thermodynamic stability, wide sources, low price and easy availability, etc. Some of these oil phases could also inhibit serum systemic and vascular inflammation markers [
18,
19]. etc. The oil phase in NEs was mainly screened from the aspects of solubility, stability, simple internal structure of the preparation, and adverse reactions in vivo [
20]. However, the influences of different oil phases on the construction and biological performance of NEs, such as cellular uptake, targeting performance and delivery efficiency to different organs in vivo, have not been fully investigated.
Herein, we screened out the commonly used pharmaceutical excipients, Tween 80 as emulsifier, ethyl alcohol as co-emulsifier, and selected three different oil phases including medium-chain triglyceride (MCT), oleic acid (OA), and ethyl oleate (EO) to prepare three oil phases-based nanoemulsion delivery platform (named as MCT@NEs, OA@NEs, and EO@NEs
Scheme 1). After extruding by the extruder, three NEs with similar sizes and zeta potentials were obtained, and their morphology was spherical, with good storage stability and serum stability. The MTT assay showed that the three NEs exhibited high biosafety on human umbilical vein endothelial cells (HUVECs), as demonstrated by no growth inhibitory effect even at concentrations up to 1 mol L
−1. Confocal laser scanning microscopy showed that the three NEs had good cellular uptake capacity and mitochondrial localization performance. These three NEs could effectively enter the lipopolysaccharide (LPS)-damaged HUVEC cells, and the uptake efficiency of EO@NEs was significantly higher than that of MCT@NEs and OA@NEs, suggesting that EO@NEs has a significant advantage in delivering lipid-soluble components into inflammatory cells. In the LPS-induced mouse and zebrafish larvae inflammation models, the
in vivo distribution results revealed that the three NEs have the ability to target brain, lung, heart and thoracic aorta, and could be used as an effective delivery platform for the treatment of diseases related to these organs.
2. Materials and methods
2.1. Materials
Medium chain triglyceride (MCT) was purchased from Liaoning Xinxing Pharmaceutical Co., Ltd. (Liaoning, China). Oleic acid (OA) and PEG400 were purchased from Xilong Scientific Co., Ltd. (Liaoning, China). Ethyl oleate (EO) was supplied by Aladdin Co., Ltd. Tween 80, dimethylsulfoxide (DMSO), methyl thiazolyl tetrazolium (MTT) and phosphate buffered solution (PBS) were purchased from Solarbio Co., Ltd. (Beijing, China). Polyoxyl 15 Hydroxystearate (HS15) was purchased from Shanghai Xintian Co., Ltd. (Shanghai, China). Glycerinum and Pluronic™ F-68 (F68) were purchased from Xi'an Tianzheng Pharmaceutical Accessories Co., Ltd. (Shanxi, China). KBr was purchased from Acmec Biochemistry Shanghai Co., Ltd. (Shanghai, China). Deuterium reagent was purchased from Qingdao Tenglong Microwave Technology Co., Ltd. (Shandong, China). Coumarin 6 was purchased from Shanghai Tixiai Chemical Industry Development Co., Ltd. (Shanghai, China). Fetal bovine serum and penicillin streptomycin were purchased from WISENT INC. Roswell Park Memorial Institute (RPMI-1640) medium and trypsin-EDTA were purchased from Gibco Invitrogen (Carlsbad, CA, USA). Lipopolysaccharide (LPS) was provided by Sigma, St. Louis (MO, USA). Xeno Light DiR dye was purchased from PerkinElmer (Germany). Methylene blue was purchased from Sigma (USA). Distilled water was used for the construction of phase diagrams. All other reagents and chemicals used were analytical grade and above. Human umbilical vein endothelial cell (HUVEC, CRL-1730) was obtained from the Chinese Academy of Science Cell Bank for Type Culture Collection (Shanghai, China). Kunming mice (male, 20 ± 2 g) were provided by Experimental Animal Center of Guizhou Medical University, license number: SCXK (Guizhou) 2018-0001. Wild-type AB/Tübingen zebrafish was provided by National Joint Local Engineering Laboratory for Cell Engineering and Biomedicine Technique of Guizhou Medical University. All procedures followed the guidelines outlined in the “Principles of Laboratory Animal Care” (NIH) and were approved by the local Animal Care and Use Committee (Approval No.2000664).
2.2. Methods
The emulsion sizes and zeta potential were measured using a DLS analyzer (Brookhaven Instruments Corporation, USA). The transmission electron microscope (TEM) images were photographed with a TecnaiG2F20S-TWIN instrument (FEI, USA). The cell viability was determined by MTT assay. The fluorescence imaging for cells was observed under the confocal laser scanning microscope (CLSM, Olympus, Japan). The cell uptake was detected by flow cytometry (NovoExpress, Agilent, USA). The staining of living/dead cells was observed under an inverted fluorescence microscope. The in vivo and ex vivo fluorescence images were detected with IVIS Spectrum (PerkinElmer, USA).
2.3. Screening of emulsifier
Tween 80, Polyoxyl 15 Hydroxystearate (HS15) and Pluronic™ F-68 (F68) were selected as emulsifiers, ethyl alcohol was selected as co-emulsifier, MCT, OA and EO were selected as oil phases, and pure water was selected as aqueous phase. The water titration method was adopted to draw a pseudo-ternary phase diagram, in which a mixture of emulsifiers and co-emulsifiers, the oil phase and the aqueous phase respectively represent one side of the triangle, and each corner in the phase diagram represents the content of each phase as 100%. In brief, the mass ratio (Km) of emulsifier and co-emulsifier was 2:1, and the total mass of mixed emulsifiers and oil phase was fixed at 1.0 g. The three oil phases were mixed with the mixed emulsifiers at the mass ratio of 1:9, 2:8, 3:7, 4:6, 5:5, 6:4, 7:3, 8:2, 9:1 (w/w), and then the mixture systems were titrated with aqueous phase, respectively. In this process, the amounts of water added to the mixture systems at the critical point from turbidity to clarification were recorded, and the mass percentage of each phase at this critical point was calculated. Taking the amount of oil phase, water phase and mixed emulsifiers as three vertices, the pseudo ternary phase diagram was drawn with Origin 7.5 software, and the emulsion area (M area in the figure) was taken as the investigation index. All studies were repeated three times with similar observations between replicates.
2.4. Screening of co-emulsifier
PEG-400, glycerol and ethyl alcohol were selected as co-emulsifiers. According to the experimental results of emulsifier screening, the optimal emulsifier was Tween 80, Km value was 2:1, and the mass ratio of oil phase to mixed emulsifiers was 1:9, 2:8, 3:7, 4:6, 5:5, 6:4, 7:3, 8:2, 9:1 (w/w). The amounts of water in the titration process were recorded and pseudo ternary phase diagrams were drawn as described above.
2.5. Construction of different oil phases-based nanoemulsion delivery platform
The phase transition points in the pseudo-ternary phase diagram were determined according to the existing literatures [
21]. According to the experimental results of emulsifier and co- emulsifier screening, Tween 80 and ethyl alcohol were the optimal emulsifier and co-emulsifier,
Km value was 2:1, then the oil phase and mixed emulsifiers were fixed at the total mass of 1.0 g and successively mixed at different mass ratios of 1:9, 2:8, 3:7, 4:6, 5:5, 6:4, 7:3, 8:2, and 9:1 (w/w), followed by adding dropwise pure water under a vortex state. In this process, the amounts of water added to the mixture systems at the critical point were recorded and the corresponding mass percentage of each phase was calculated, then the pseudo ternary phase diagrams were drawn as described above. Finally, the three nanoemulsions with droplet sizes kept around 300 nm through an avestin lipid extruder (Canada, USA) were used for subsequent testing.
2.6. Morphology
The appearance color and fluidity of the three nanoemulsions were observed by naked eyes after diluting to 0.75 mg mL−1. The microscopic morphology of nanoemulsions was observed under a transmission electron microscopy (TEM, JOEL, Tokyo, Japan). The TEM samples were prepared by gently dropping the samples onto a carbon-coated copper grid, stained with an aqueous solution of 2% phosphotungstic acid, dried at room temperature for 0.5 h, and then observed under TEM.
2.7. Droplet size and zeta potential changes
The average droplet sizes and zeta potentials of three nanoemulsions after dilution and storage were measured using a DLS analyzer. Briefly, the droplet sizes and zeta potentials of the three nanoemulsions in phosphate buffered saline (PBS) and (RPMI-1640) medium after being diluted 400 times and stored for 0, 2, 4, 6, 8, and 12 h were measured by a DLS analyzer.
2.8. MTT assay
The viabilities of HUVEC cells treated by different materials were investigated by MTT assay. Briefly, HUVEC cells at logarithmic growth stage were inoculated into 96-well plates with an adjusted cell density of 6 × 103/well and incubated in 37 °C, 5% CO2-saturated humidity incubator for 24 h. The cells were treated with various concentrations of Tween 80, MCT, OA, EO and three NEs (20, 1, 10−1, 10−2 and 10−3 mol/L) for 24 h. After treatment, the cells were incubated with MTT solution (5 mg/mL, 20 μL/well) for 4 h, and the medium was then substituted with dimethyl sulfoxide (DMSO, 150 μL/well). The optical density (OD) values at 490 nm were detected using a microplate reader (BioTek, Winooski, VT, USA). Cell viabilities were calculated according to the following formula: Cell viability (%) = OD490 (sample)/OD490 (control) × 100. The untreated cells were set as control group. The experiment was repeated three times.
2.9. Oxidative stress toxicity test
HUVECs cells were cultured in a 25 cm2 flask and placed in a 37 °C, 5% CO2 incubator for 24 h. The culture medium was abandoned, and the administration group was set up with single excipients group, group MCT, OA, EO, Tween 80, and preparation group MCT@NEs, OA@NEs, EO@NEs, with the concentration of 10−3 and 10−4 mol/L. Meanwhile, the control group was set up with the same amount of RPMI 1640 culture medium. After 24 h of culture, cells were collected and operated according to SOD, MDA, ROS kit instructions.
2.10. Live/dead cells staining
HUVEC cells were inoculated into 12-well plates with an adjusted cell density of 5 × 104/well and incubated in 37 °C, 5% CO2-saturated humidity incubator for 24 h. The cells were treated with LPS (1 µg/mL) for 8, 12 and 24 h, respectively. After washing with pre-cooled PBS for three times, the cells were stained with fluorescein diacetate (FDA, 20 µM) and propidium iodide (PI, 20 µM) for 30 min, and the fluorescence imaging of living and dead cells was observed under the inverted fluorescence microscope.
2.11. Hematoxylin and eosin (H&E) staining
HUVEC cells were inoculated into 12-well plates with an adjusted cell density of 5 × 104/well and incubated in 37 °C, 5% CO2-saturated humidity incubator for 24 h. The cells were treated with LPS (1 µg/mL) for 8, 12 and 24 h, respectively. After washing with pre-cooled PBS for three times, cells were fixed with 4% paraformaldehyde and washed with PBS for three times, then stained with fematoxylin for 10 min and eosin for 2 min, and permeated with xylene for 5 min. Finally, the cell damage was observed and photographed under a light microscope.
2.12. Cellular uptake
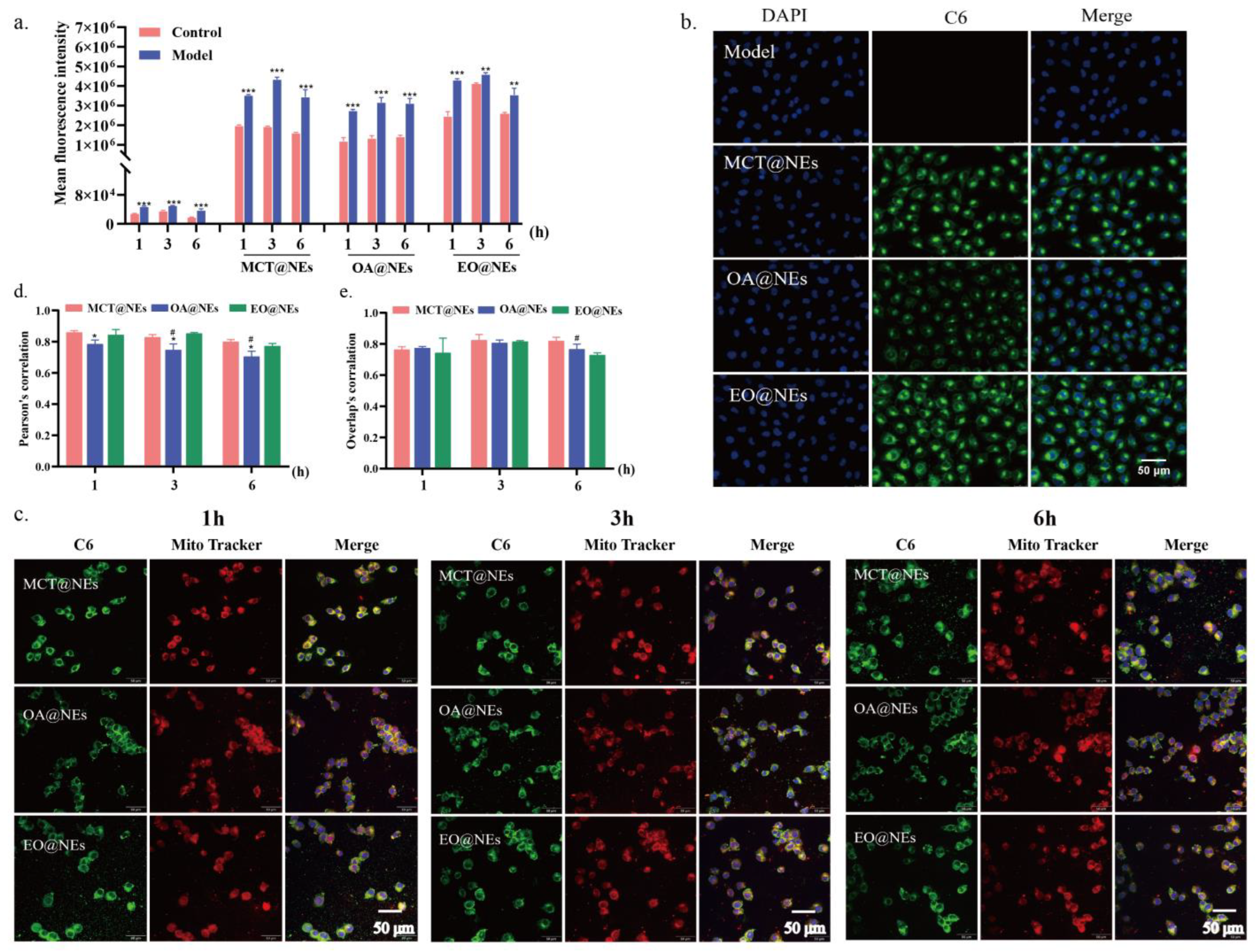
Coumarin 6 (C6) was used as a fluorescent probe to label three oil phases or NEs. HUVEC cells at logarithmic growth stage were taken, digested and re-suspended at a density of 5 × 104 cells/well in 6-well plates and incubated in 37 °C, 5% CO2-saturated humidity incubator for 24 h. Then the cells were added fresh medium containing LPS ([LPS] = 1 μg mL−1) and incubated for 24 h to build the LPS-damaged cell model. After that, C6 solution and C6-labelled NEs solutions ([C6] = 100 ng mL−1) were added to incubate for another 3 h, the untreated cells were set as controls. After washing with pre-cooled PBS for 3 times, 0.5% paraformaldehyde was added and fixed in the dark for 10 min, the fixative was discarded and the cells were washed three times with PBS. The nucleus was stained with DAPI solution (10 μg mL−1) for 8 min. the cell uptake was observed and photographed under an inverted Xds-2b fluorescence microscope (Nikon, Japan).
Meanwhile, the cellular uptake profiles of three oil phases or NEs were further quantified by flow cytometry. Briefly, HUVEC cells were adjusted to 8 × 104 cells/well and incubated in 12-well plates for 24 h, then C6 solution and C6-labelled NEs solutions ([C6] = 100 ng mL−1) were added to incubate for 1, 3 and 6 h, the untreated cells were sat as controls. Subsequently, the cells were washed with PBS for three times, digested with 0.25% trypsin, centrifuged at 1500 r/min for 5 min, and re-suspended with 1 mL PBS. Fluorescence quantitative detection was performed using a Novo Cyte flow cytometer with NovoExpress analysis software (ACEA Biosciences, San Diego, CA, USA).
2.13. Mitochondrial localization
HUVEC cells were seeded on the coverslips placed in 6-well plate with an adjusted cell density of 1 × 105 cells/well and incubated in 37 °C, 5% CO2-saturated humidity incubator for 24 h. The experiment was divided into LPS model group (1 μg mL−1, 24 h) and normal control group. After incubation with C6-labelled MCT@NEs, OA@NEs and EO@NEs for 1, 3, and 6 h, HUVEC cells were washed with PBS for three times and stained with MitoTrackerTM Red (100 nM) for 15 min and Hoechst 33342 (25 µM) for another 15 min. After that, the cells were washed three times with PBS, fixed with 4% paraformaldehyde for 10 min, and sealed. Confocal laser scanning microscopy (CLSM, Olymus spinSR10, Japan) was used to observe the intracellular distribution of the three NEs in HUVECs. The co-localization of NEs with mitochondria in each group was reflected by Pearson's coefficient calculated using Image J.
2.14. Biodistribution of NEs in LPS-induced mice inflammation model
Firstly, the inflammation mice model was successfully established after 12 h injection of LPS (1 μg mL−1) into the Kunming mice via tail vein. Then the mice were divided into three groups (DIR-labelled MCT@NEs, OA@NEs and EO@NEs, n = 3) and injected corresponding nanoemulsion ([NEs] = 2.5 µL/g) at various times (1, 6, 12, 24 and 36 h). The whole thoracic aorta and main tissues (i.e., heart, liver, lung, spleen, kidneys and brain) were then dissected and visualized under IVIS Spectrum (PerkinElmer, USA). The total radiant efficiency (TRE) values of the ROIs were measured.
2.15. Zebrafish maintenance
The adult zebrafish were bred in our fish facility and housed under standard laboratory conditions with 15 fish per tank (ZebTec 3.5 L tanks) at 28 °C on a 14-hour light and 10-hour dark cycle. Fishes were fed twice daily with dry food and brine shrimp. Before the experiment, zebrafish were intercrossed in a male-to-female ratio of 1:2, and their embryos were incubated in egg water containing 0.003% 1-phenyl-2-thiourea (PTU).
2.16. Biodistribution of NEs in LPS-induced zebrafish larvae inflammation model
Injection needles were produced using borosilicate capillaries (Beijing, China) and a pipet puller (Narishige, Japan). The needles were loaded with an appropriate sample before being connected to an Eppendorf Femtojet Express pump and mounted onto a Narishige MN-153 micromanipulator that allowed movement in the x, y and z planes. Zebrafish embryos (3 dpf) were anesthetized using a solution of buffered tricaine (120 μg/mL). MCT@NEs, OA@NEs and EO@NEs at the dosages of 15 or 30 mg mL−1 of oil phase were injected into zebrafish larvae via tail vein using a femtojet (Harvard) and a stereomicroscope (Nikon SMZ645), the toxicity of three NEs was observed by stereoscopic microscopy, and the number of deaths was counted.
The inflammation zebrafish larvae model was successfully established after 12 h injection of LPS (0.5 mg mL−1) via tail vein. Then the zebrafish larvae were divided into three groups (C6-labelled MCT@NEs, OA@NEs and EO@NEs, n = 5) and injected corresponding nanoemulsion at the dosage of 15 mg mL−1 of oil phase. At 0.5, 1, 3, 6, 12, 24 and 48 h after injection, the larvae were anesthetized and placed onto an agarose support for in vivo imaging using stereoscopic fluorescence microscopy (Nikon, Japan).
2.17. Immunofluorescence
HUVEC cells were seeded on the coverslips placed in 6-well plate with an adjusted cell density of 1 × 105 cells/well and incubated in 37 °C, 5% CO2-saturated humidity incubator for 24 h. The experiment was divided into LPS model group (1 μg mL−1, 8 h) and normal control group. After incubation MCT@NEs, OA@NEs and EO@NEs for 24 h, 4% paraformaldehyde was fixed for 15 min, 0.2% Triton-100 was permeabilized for 10 min, and the cells were blocked for 30 min with the primary antibody added dropwise and incubated at 4 °C overnight. The next day, the primary antibody was recovered and washed twice with PBS, and the secondary antibody with fluorescence was added uniformly into each well under light-proof conditions and incubated for 1 h at room temperature.
2.18. Statistical analysis
Data was analyzed by Graph Pad Prism 7.0 software (Graph Pad Software, San Diego, CA, USA). Measurement data were expressed as the mean ± standard deviation (SD) for at least 3 independent experiments. Differences between two groups were analyzed using a two-tailed Student’s t-test, and differences between multiple groups were analyzed using one-way ANOVA followed. p < 0.05, the difference was statistically significant.
2.19. Data availability
The data that support the plots within this paper and other findings of this study are available from the corresponding author on reasonable request.
Author Contributions
Ying Chen, Conceptualization, Methodology, Funding acquisition, Writing—original draft. Writing—review & editing. Huanhuan Liu, Methodology, Investigation, Visualization. Sibu Wang, Methodology, Investigation. Yongyu Tang, Methodology, Investigation. Lidan Zhang, Investigation, Visualization. Xingjie Wu, Formal analysis, Software. Qianqian Guo, Formal analysis, Software. Yu-e Wang, Supervision, Validation. Yang Ding, Supervision, Validation. Maochen Wei, Supervision. Xiangchun Shen, Supervision, Validation, Resources. Ling Tao, Conceptualization, Resources, Writing—review & editing, Project administration, Funding acquisition.
Scheme 1.
(a) Construction schematic and (b) distribution evaluation of different oil phases-based nanoemulsion delivery platform.
Scheme 1.
(a) Construction schematic and (b) distribution evaluation of different oil phases-based nanoemulsion delivery platform.
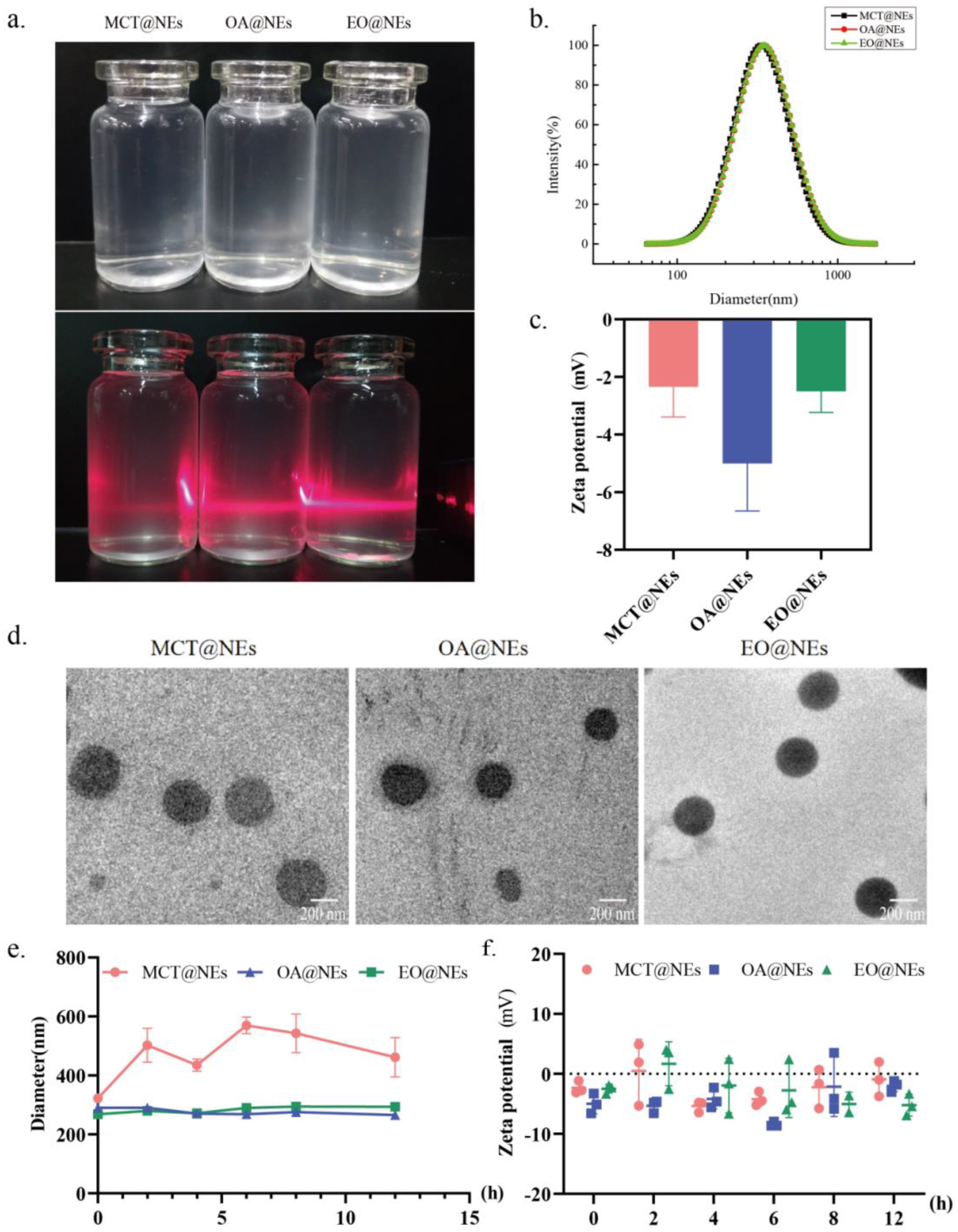
Figure 1.
Characterization of three oil phases-based NEs. (a) The digital photographs of MCT@NEs, OA@NEs and EO@NEs under daylight and irradiation with a 630 ± 10 nm continuous laser. (b) Distribution of the hydrodynamic diameter of MCT@NEs, OA@NEs and EO@NEs in water determined by DLS. (c) Zeta potentials of MCT@NEs, OA@NEs and EO@NEs in PBS solution at pH 7.4. (d) TEM micrographs of MCT@NEs, OA@NEs and EO@NEs. (e) Particle size and (f) zeta potential changes of three NEs diluted 400 times with PBS within 12 hours.
Figure 1.
Characterization of three oil phases-based NEs. (a) The digital photographs of MCT@NEs, OA@NEs and EO@NEs under daylight and irradiation with a 630 ± 10 nm continuous laser. (b) Distribution of the hydrodynamic diameter of MCT@NEs, OA@NEs and EO@NEs in water determined by DLS. (c) Zeta potentials of MCT@NEs, OA@NEs and EO@NEs in PBS solution at pH 7.4. (d) TEM micrographs of MCT@NEs, OA@NEs and EO@NEs. (e) Particle size and (f) zeta potential changes of three NEs diluted 400 times with PBS within 12 hours.
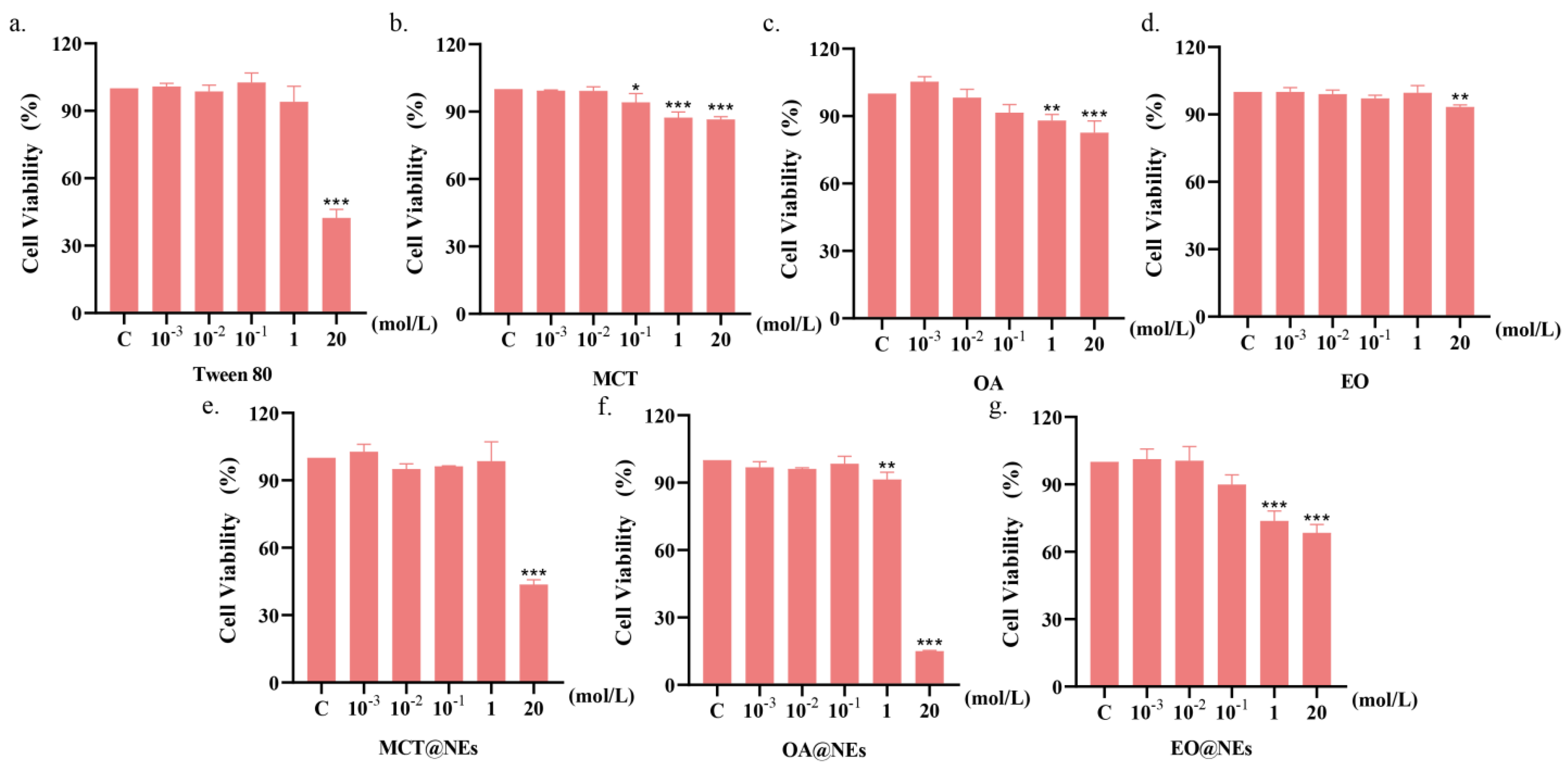
Figure 2.
Cell viability of Tween 80 (a), MCT (b), OA (c), EO (d), MCT@NEs (e), OA@NEs (f) and EO@NEs (g) against HUVEC cells after incubation for 24 h with a series of concentrations. * p < 0.05, ** p < 0.001, *** p < 0.0001.
Figure 2.
Cell viability of Tween 80 (a), MCT (b), OA (c), EO (d), MCT@NEs (e), OA@NEs (f) and EO@NEs (g) against HUVEC cells after incubation for 24 h with a series of concentrations. * p < 0.05, ** p < 0.001, *** p < 0.0001.
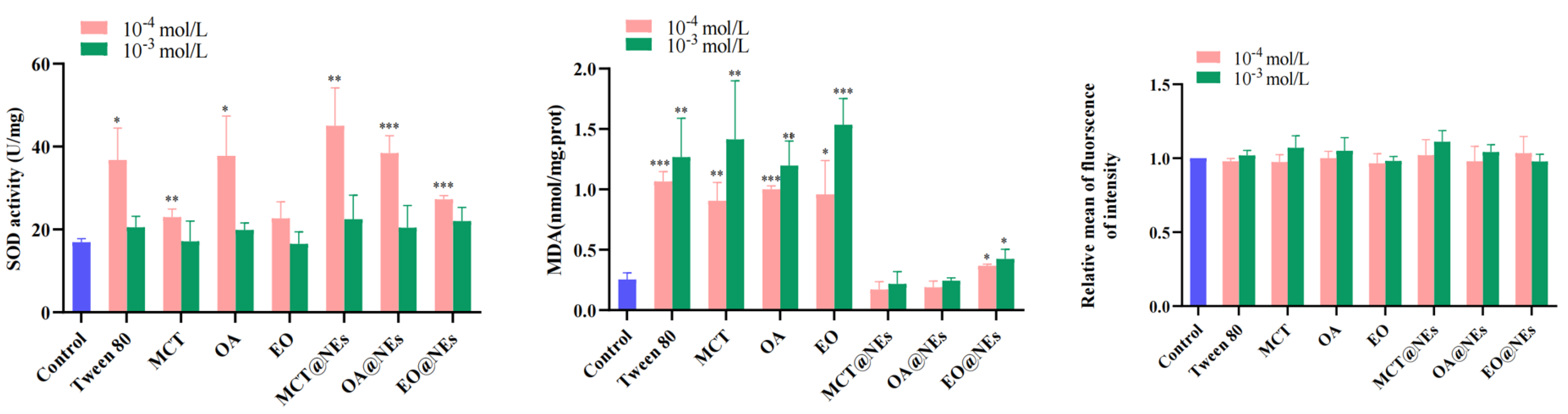
Figure 3.
Cell viability of Tween 80, three oleic phases and NEs on SOD, MDA, ROS of HUVECs cells (compared with normal group, * p < 0.05, ** p < 0.001, *** p < 0.0001.)
Figure 3.
Cell viability of Tween 80, three oleic phases and NEs on SOD, MDA, ROS of HUVECs cells (compared with normal group, * p < 0.05, ** p < 0.001, *** p < 0.0001.)
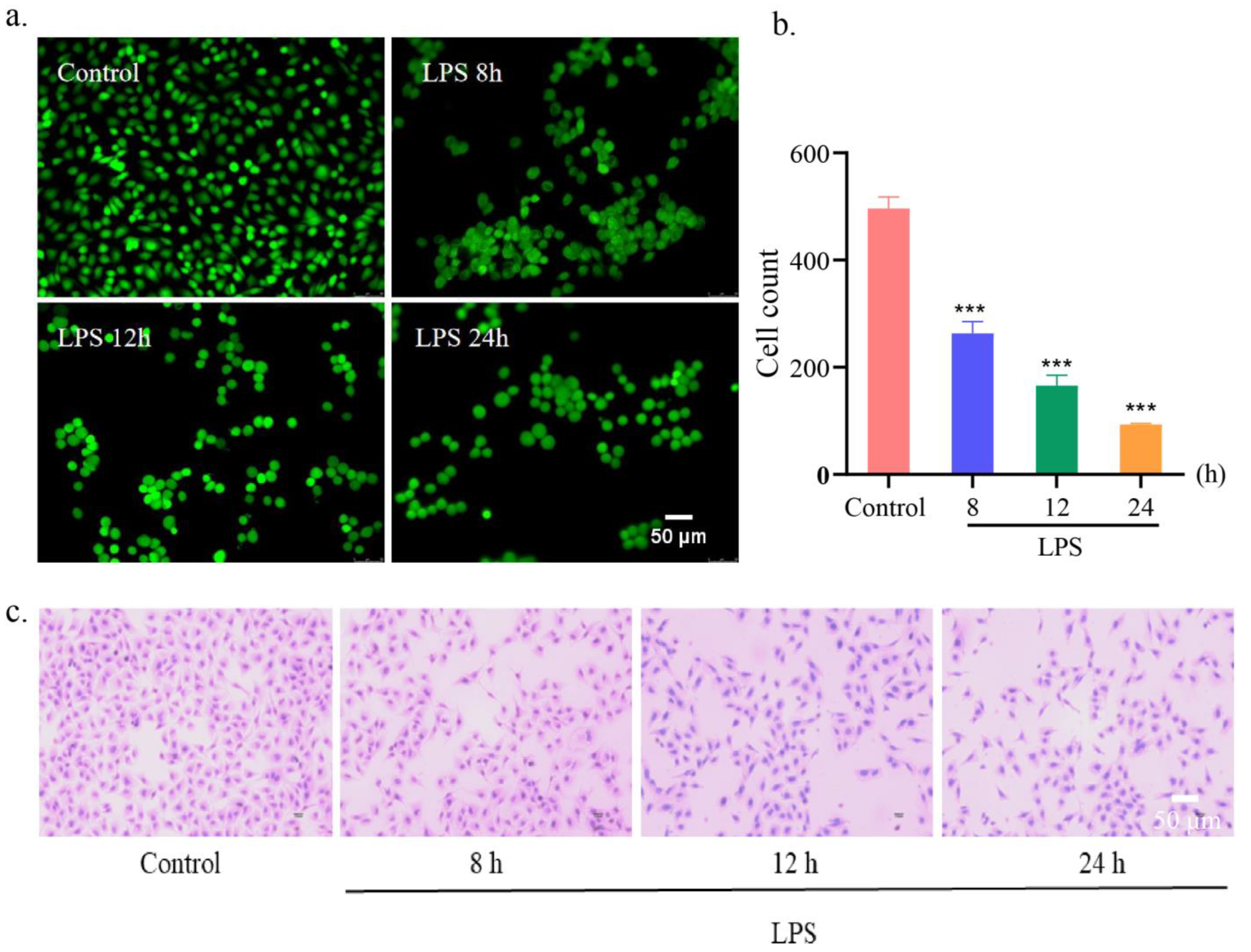
Figure 4.
Establishment of LPS-induced HUVEC injury model. (a) Fluorescent images and (b) cell count of the living HUVEC cells after incubation with LPS for different time and staining with FDA. Scar bar: 50 μm. n = 3. ***p < 0.001. (c) The H&E-stained images of HUVEC cells after incubation with LPS for different time. Scar bar: 50 μm.
Figure 4.
Establishment of LPS-induced HUVEC injury model. (a) Fluorescent images and (b) cell count of the living HUVEC cells after incubation with LPS for different time and staining with FDA. Scar bar: 50 μm. n = 3. ***p < 0.001. (c) The H&E-stained images of HUVEC cells after incubation with LPS for different time. Scar bar: 50 μm.
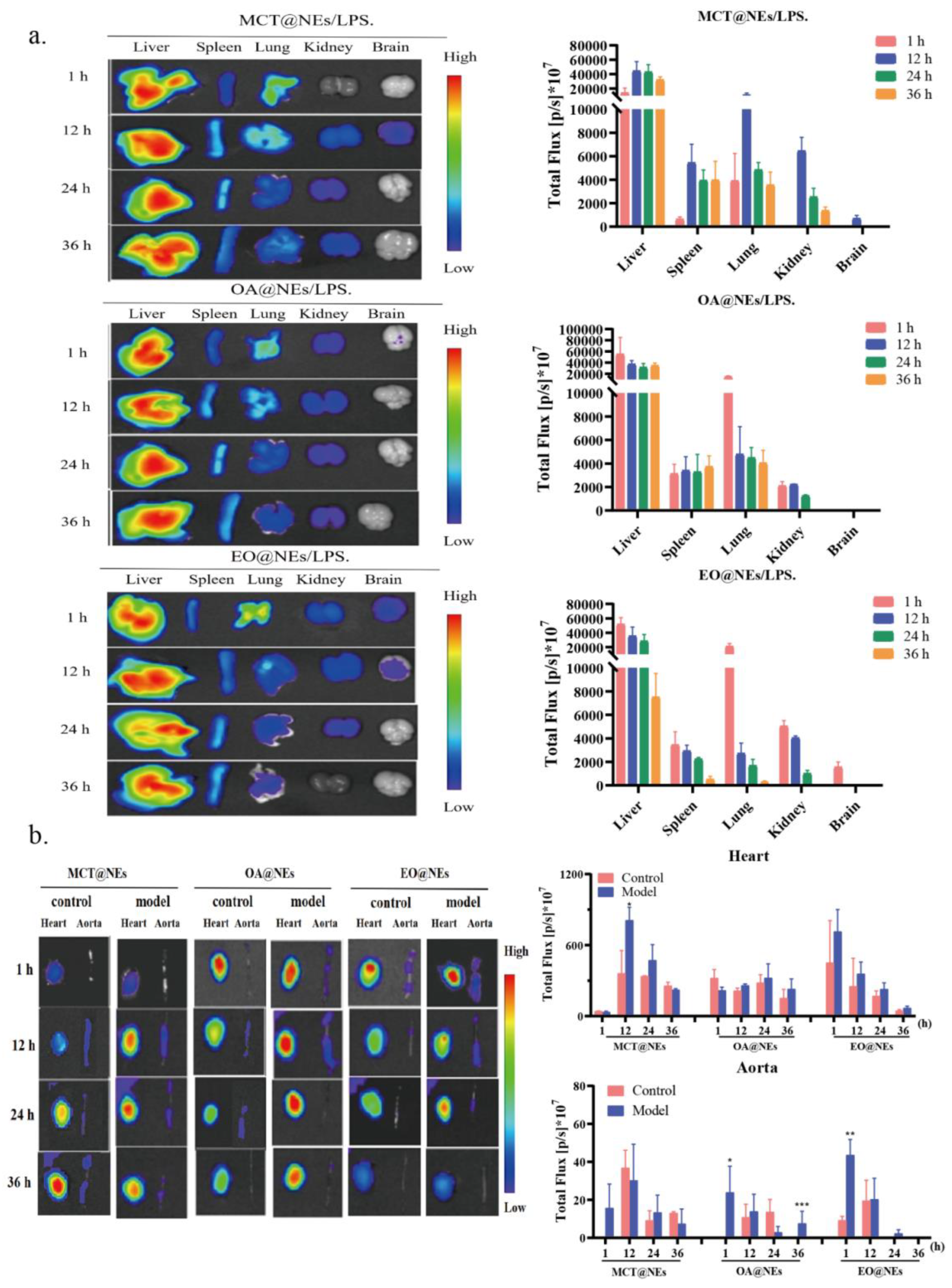
Figure 6.
Biodistribution of MCT@NEs, OA@NEs and EO@NEs in mice. (a) Ex vivo fluorescence images of liver, spleen, lung, kidney, brain and corresponding mean fluorescence intensity (MFI) in LPS-induced mice inflammation model. (b) Ex vivo fluorescence images of heart, thoracic aorta and corresponding MFI in normal mice and LPS-induced mice inflammation model. n = 3. Data present as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001.
Figure 6.
Biodistribution of MCT@NEs, OA@NEs and EO@NEs in mice. (a) Ex vivo fluorescence images of liver, spleen, lung, kidney, brain and corresponding mean fluorescence intensity (MFI) in LPS-induced mice inflammation model. (b) Ex vivo fluorescence images of heart, thoracic aorta and corresponding MFI in normal mice and LPS-induced mice inflammation model. n = 3. Data present as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001.
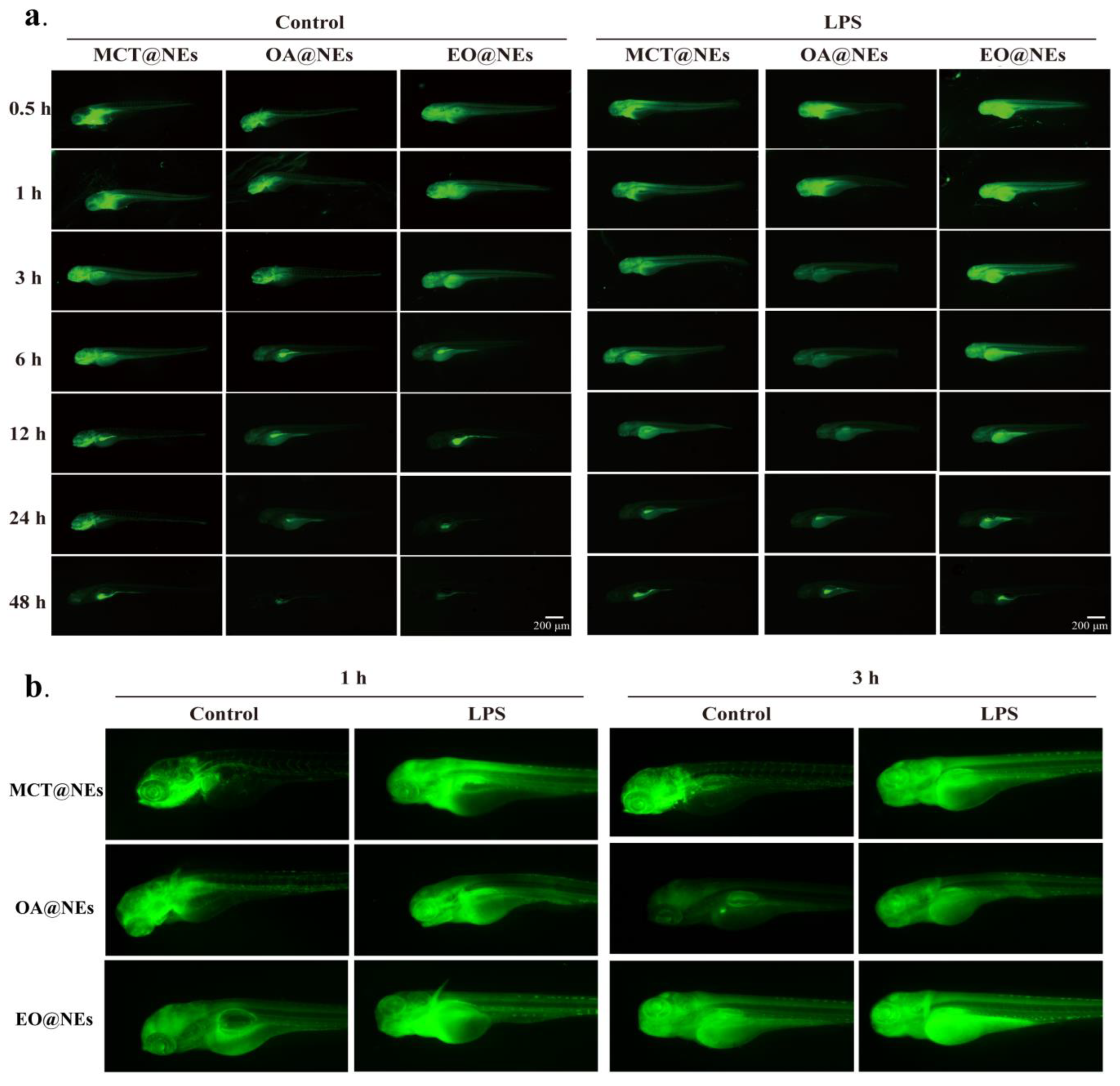
Figure 7.
Biodistribution of MCT@NEs, OA@NEs and EO@NEs in zebrafish larvae. (a) in vivo fluorescence images of LPS-induced zebrafish larvae inflammation model. (b) Representative amplified biodistribution images at 1 and 3 h in (a).
Figure 7.
Biodistribution of MCT@NEs, OA@NEs and EO@NEs in zebrafish larvae. (a) in vivo fluorescence images of LPS-induced zebrafish larvae inflammation model. (b) Representative amplified biodistribution images at 1 and 3 h in (a).
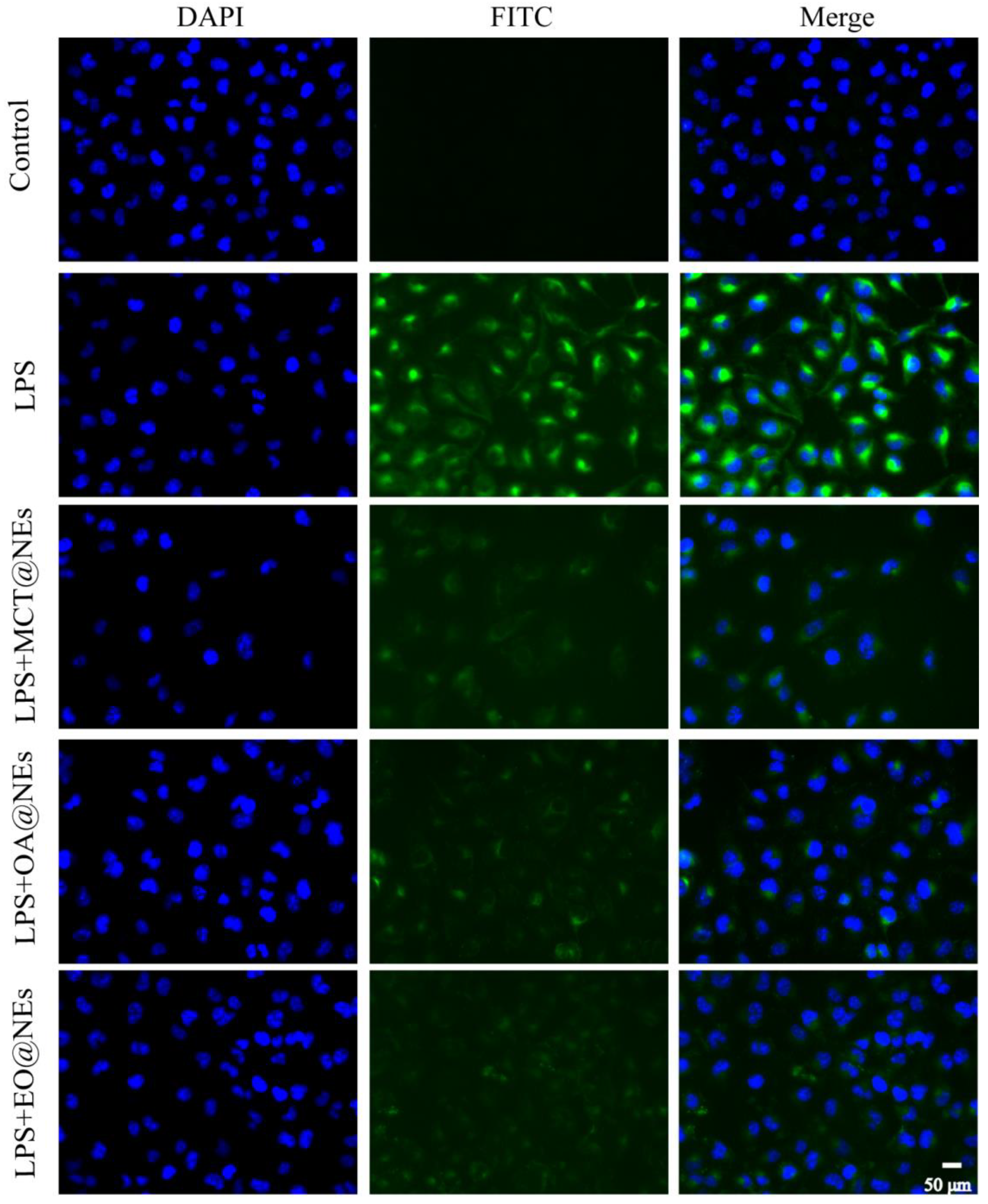
Figure 8.
Immunofluorescence of the LPS-injured HUVEC cells after incubation with MCT@NEs, OA@NEs, and EO@NEs for 24 h. Nuclei were stained with DAPI (blue), cytoplasm was stained by VCAM-1 protein (green). Scale bar: 50 μm.
Figure 8.
Immunofluorescence of the LPS-injured HUVEC cells after incubation with MCT@NEs, OA@NEs, and EO@NEs for 24 h. Nuclei were stained with DAPI (blue), cytoplasm was stained by VCAM-1 protein (green). Scale bar: 50 μm.