1. Introduction
(1S,2E,4R,6R,7E,11 E)-2,7,11-Cembratriene-4,6-diol (4R) is a cyclic diterpenoid and the second most abundant tobacco cembranoid found in
Nicotiana tabacum. 4R crosses the blood-brain barrier (BBB) within minutes and reaches a higher concentration in the brain than in plasma [
1]. The liver rapidly metabolizes the remaining 4R in the blood and excretes it by the kidney as a soluble metabolite with no signs of toxicity [
1,
2]. As an alpha-seven nicotinic acetylcholine receptor (α7 nAChR) antagonist, 4R has been reported to rescue the functionality of hippocampal neurons after exposure to the excitotoxic effects of N-methyl-D-aspartate (NMDA) and organophosphates (paraoxon and diisopropyl fluorophosphate (DFP)) by triggering an antiapoptotic mechanism mediated by PI3K/Akt [
3,
4,
5,
6].
In vivo, 4R decreased brain degeneration caused by the analog of sarin, DFP, and improved the behavior in the 6-hydroxydopamine-induced Parkinson’s disease mouse model (6,7).
In oxygen-glucose deprivation (OGD) models, 4R recovers neuronal activity in rat acute hippocampal slices by Akt phosphorylation, showing a common mechanism of neuroprotection despite different insults [
7]. In murine brain-derived endothelial cells, 4R suppresses the expression of the intercellular cell adhesion molecule 1 (ICAM-1) [
7], demonstrating that its effects extend to cells different from neurons. Lastly, 4R promoted the polarization of the protective M2 microglia while attenuating the pro-inflammatory M1 phenotype in N9 cells (microglia cell line) following OGD treatment. In addition, the conditioned medium of 4R-treated N9 cells post-OGD increased neuronal viability by 54.5%, due in part to a decrease of TNF-α and IL-6 cytokine release [
8], suggesting that 4R has the potential to become a therapy for ischemic stroke.
Ischemic stroke is the second leading cause of death worldwide (5.5 million annually), accounting for 50% of chronic disability of its survivors and contributing to high expenditure costs [
9,
10,
11]. The only FDA-approved treatment for stroke (intravenous injection of recombinant tissue plasminogen activator (rtPA) has proven effective in dissolving blood clots [
12]. However, only 7% of stroke patients are eligible for rtPA after stroke onset due to its timeframe limitation (4.5 hours) [
13] and a mandatory CT scan by an experienced medical team before the drug administration [
14]. Additionally, electrophysiological techniques such as electroencephalogram (EEG), somatosensory evoked potentials (SSEP), and transcranial evoked motor potential (TcMEP) allow for the early detection of ischemic events and for timely intervention [
15,
16,
17,
18]. While SSEP and TcMEP provide immediate feedback regarding the integrity of the central and peripheral nervous systems, the EEG assesses the brain’s status. Neuronal inflammation is a characteristic feature in the stroke-affected region(s) as a consequence of oxygen and glucose deprivation [
19]. Stroke patients with systemic inflammation exhibit clinically poorer outcomes [
20,
21]. Treating brain inflammation and protecting the brain while waiting in the emergency room can be vital to recovering damaged tissue after hypoxia [
22]. 4R has been shown to reduce inflammation and improve neurological outcomes in rat and murine models of ischemic stroke. Animals subjected to temporary and permanent middle cerebral artery occlusion (tMCAO and pMCAO) had more than a 50% reduction in the infarction size after one single dose of 4R (6mg/Kg) compared to vehicle-treated animals [
7].
Despite the proven beneficial effect in rat and murine models with different insults [
5,
7,
23,
24] and lack of toxicity in Sprague Dawley rats [
2], 4R safety was never studied in a species close to humans. Nonhuman primates (NHPs) share structural and functional similarities to the human brain, liver, and kidney [
25,
26,
27] and are the most important model in various biomedical research fields, including neurodegenerative disorders [
28,
29]. This study aimed to determine the safety of a repeated i.v. dose of 4R given to male rhesus monkeys for 11 consecutive days. To gain insight into the effects of 4R in brain activity, we assessed the electrophysiological function of the peripheral and central nervous system. Furthermore, the safety results may support 4R use as a potential treatment against ischemic stroke and other neurodegenerative diseases with an inflammatory component involved. We also assessed the effects of 4R in the brain cortex, hippocampus, liver, and kidney by histology.
2. Materials and Methods
2.1. Test Substance (4R) and vehicle
The (1S,2E,4R,6R,7E,11E)-2,7,11-cembratriene-4,6-diol was provided by Dr. El Sayed Research Foundation, University of Louisiana–Monroe, College of Pharmacy, LA. The cembranoid was extracted from
Nicotiana tabacum leaf powder using the procedure reported before [
30]. Briefly, Virginia, Oriental, or Burley tobacco was purchased from Custom Blends, NY. The purity of 4R was >99% using the following analytical criteria: a) 1H NMR: Integration of H-6 Proton at β 4.81 versus that of the 4S epimer (δ 4.46); b) 13C NMR: C-6 in the 4R (δC 67.5) versus C-6 in the 4S (δC 66.0); c) Thin-layer chromatography (TLC): Rf value 0.42 (Silica gel, n -hexane-Ethyl acetate 1:1).
The vehicle of 4R was prepared using polyethylene glycol 400 (PEG 400) and 100% ethyl alcohol (Ethanol) obtained from VWR International, LLC (Brisbane, CA). In the following tests, 4R powder for i.v. administration was dissolved in a mixture of 10% Ethanol and 90% PEG 400. This formulation has been proven neuroprotective in vivo after the implementation of tMCAO [
7] and represented a better drug dissolution since dimethyl sulfoxide (DMSO) use in drug formulation has been restricted by the Federal Drug Administration (FDA) from the United States [
31].
2.2. Test Animals: Nonhuman primates
Ten male rhesus monkeys (Macaca mulatta, age: 36-48 months; weight 6.0 – 8.0 Kg) were housed individually at the Caribbean Primate Research Center (CPRC), Sabana Seca, PR and transferred to the Animal Resources Center (ARC) of the University of Puerto Rico, Medical Sciences Campus, San Juan, PR in a vehicle specially designated for this purpose. Transportation of the animals was performed by experienced staff in coordination with the MSC security office and following established SOPs and Institutional Policies. The animals were quarantined for 10 days. They were housed in single cages under standard conditions (a 12-h light/dark cycle with the light on from 08:00 to 20:00 h; humidity at 32% and temperature at 24 ± 2°C). All experimental and animal care procedures were carried out in accordance with the National Institute of Health (NIH) Guide for the Care and Use of Laboratory Animals. The monkeys were fed and given water ad libitum.
Animals were euthanized and perfused after 12 days; all procedures were performed under a safety hood. With the animals under deep anesthesia (Ketamine (10mg/Kg) and Xylazine (0.25mg/Kg) applied i.m.), a commercial euthanasia solution (Euthanasia III solution) at a dose of 0.22ml/Kg (86mg/Kg) was administered i.v, through the saphenous or cephalic catheter. After a terminal perfusion with chilled saline (until the corneal reflex was lost) the brain, kidney, and liver tissues were collected and fixed in buffered paraformaldehyde for further histopathological analysis.
2.3. Repeated-Dose Toxicity Study Design
The repeated-dose toxicity of 4R in monkeys was examined based on toxicity testing for Pharmaceuticals, Industry Guidelines” from the FDA [
32]. The rhesus monkeys were randomly divided into two groups, with five animals per group. 4R was administrated i.v. in the saphenous or cephalic catheterization by experienced personnel. This route of administration is typical for clinical use in humans due to its simplicity for drug delivery [
33].
Group 1 served as the control group and received only vehicle (10% Ethanol: 90% PEG 400). Treated Group 2 was injected with the test compound 4R at the dose of 1.4 mg/Kg based on the empirical approach of the dose-by-factor method to estimate the human equivalent dose compared to the rodent neuroprotective dose [
34]. The NHPs were observed for any symptoms of toxicity immediately after the injection. Observations for mortality, signs of illness, pain, injury, allergic responses, alterations in appearance, and behavioral changes, such as ataxia and hyperactivity, general stimulation, and sedation, were conducted daily. All the observations were methodically recorded.
2.4. Behavior
Assessment of the ability to self-care and neurological examinations were conducted one day before the beginning of procedures to establish the baseline behavior (day 0) and on days 2, 7, and 11, to assess the effect of the 4R or vehicle during the treatment. These evaluations were quantitated as neurological scores, with higher scores reflecting better functional status. Rhesus monkeys’ spontaneous behavioral outcome was evaluated for 30 min in the morning, and observations were recorded using the task-oriented scoring system, adapted from Mack et al. [
35] and used previously by us with
Macaca mulatta [
36]. The behavior in every session was video recorded and described by one observer who was blinded for the animals’ treatment.
2.5. Histopathology
To assess if 4R administration induces damage in the kidney, liver, and brain, the organs were fixed in 10% neutral buffered formalin containing neutral phosphate-buffered saline for at least 72 hours. Samples were trimmed, processed, embedded in paraffin, and sliced into 5-7 μm thick sections. Sections were stained with hematoxylin and eosin (H&E) according to routine histological techniques by PennVet Comparative Pathology Core (University of Pennsylvania, PA). Each lesion was listed and coded by the most specific topographic and morphologic diagnostic, severity, and distribution using the Systematized Nomenclature of Medicine (SNOMED), National Toxicology Program, and the Toxicology Data Management System (TDMS) as guides. Records of gross findings for a specimen from postmortem observations were available to the pathologist, when examining that specimen microscopically.
2.6. Blood collection
To compare hematological and biochemical parameters between 4R-treated and vehicle-injected animals, blood samples (5ml) were collected on days 4, 8, and 12 using Vacutainer tubes with dipotassium ethylenediaminetetraacetic acid (K2EDTA) to perform a Complete Blood Count (CBC) with the equipment XN10 (Sysmex) applying clinical parameters used for humans. No anticoagulant was used for serum chemistry samples, also known as comprehensive metabolic panel (CMP). The CMP was performed using COBAS (Roche). Animals did not fast before blood collection.
2.7. Electrophysiological assessment
SSEP, EEG, and TcMEP were recorded for up to 30 min on days 0, 4, 8, and 12 utilizing an Axon Eclipse (Medtronic, PA).
2.7.1. SSEPs were obtained by stimulating a peripheral nerve (Sciatic and Median for upper and lower extremity, respectively) following an alternating, random square electrical pulse.
These potentials were recorded from subdermal needle electrodes placed in the scalp, over the somatosensory cortex bilaterally (C3’ and C4’), as well as over the brachial plexus (position analog to the Erb’s point in humans). For stimulation parameters, we have used an intensity of 4mA, a repetition rate of 2.1Hz, and a pulse duration of 200µs. The waveforms were recorded utilizing an analog-to-digital filter with a low frequency filter (LFF) set at 30Hz, and a high-frequency filter (HFF) set at 3000Hz. The processed signal was filtered utilizing a bandpass with the LFF set at 30Hz, and the HFF set a 500 Hz for both median and sciatic nerves.
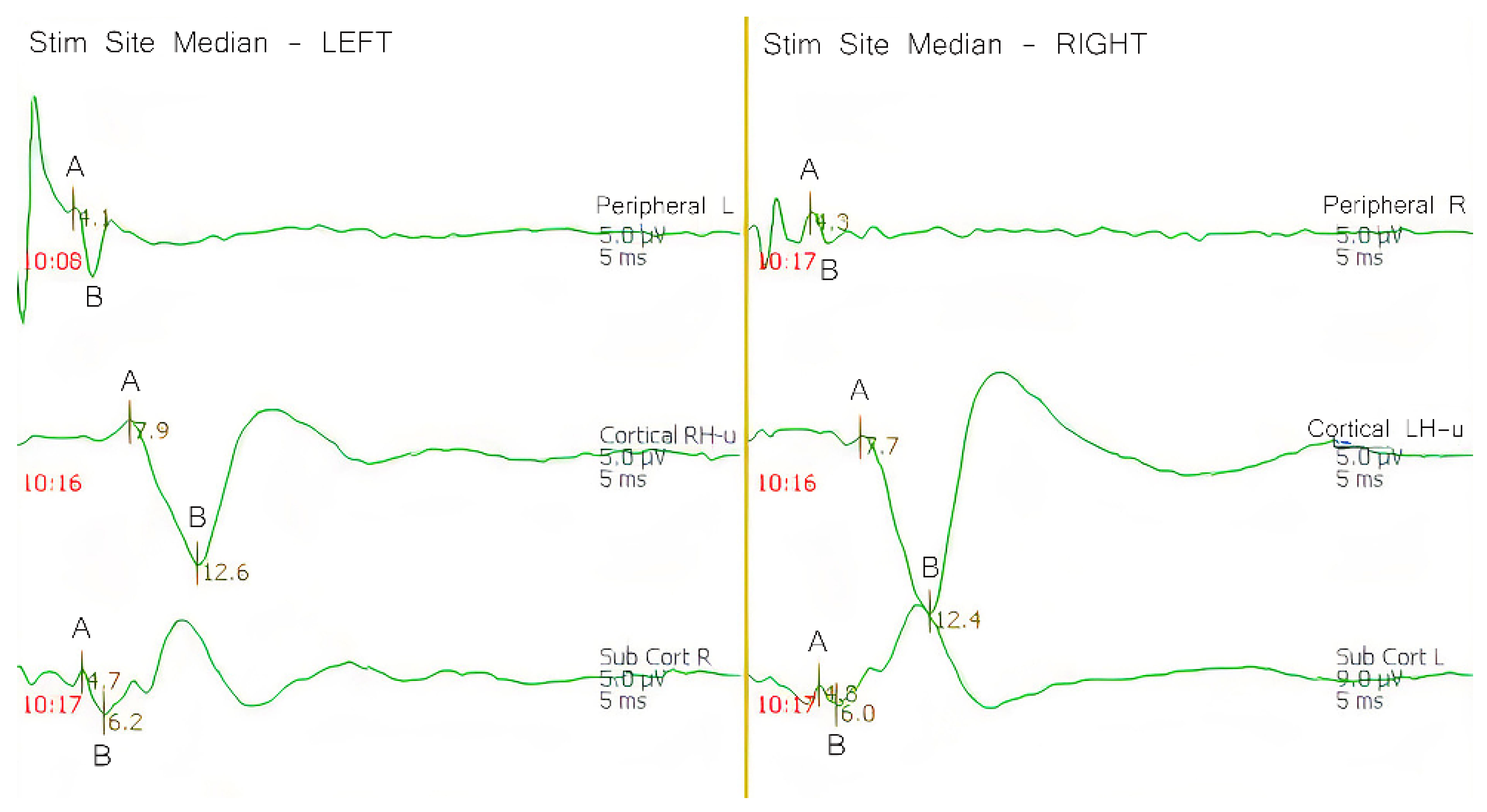
The SSEP potential originates at the stimulation site in the peripheral nerve and travels into the spinal cord through the dorsal root ganglion to reach medulla oblongata, in which the first synapse occurs. Then, these action potentials move from the medulla oblongata to the ventral posterior lateral nucleus of the thalamus, where the second synapse occurs. From the thalamus, the impulse travels to the primary sensory cortex for the third and final synapse. These action potentials, measured at different points through the somatosensory pathway, allow us to confirm the stimulation integrity, while also providing feedback of the pathway, and if treatment or vehicle are affecting the normal brain function. SSEPs were analyzed according to the peak-to-trough amplitude of the primary negative peak and its secondary positive peak for all points evaluated (peripheral, sub-cortical, and cortical) (
Figure 1). Latency, inter-latency, and amplitude were also evaluated. Latency was defined as the time (s) from the stimulation site to the appearance of the primary negative peak. Inter-latency was defined as the time between the primary negative peak and the secondary positive peak. Amplitude was defined by the vertical measurement between A and B (
Figure 1).
2.7.2. EEGs were recorded utilizing a subdermal needle over the somatosensory cortex (C3’ and C4’).
The waveforms were recorded using an analog-to-digital converter with the HFF set to 70Hz, and the LFF set to 1Hz. The gain was set to 7µV/div, and the page scrolling was set at 15mm/s. The EEG frequency and morphology can be influenced by several factors, including the stage of awareness, anesthesia, or a decrease in blood perfusion, among others. Prior studies have used the appearance or increase of theta or delta activity, suppression of alpha and beta activity of greater than 50%, or both in the raw EEG data as their definition of a significant change [
37]. In our experiments, we looked for global changes in EEG frequency on both hemispheres individually and globally: for vehicle vs. experimental, vehicle vs. vehicle, and experimental vs. experimental across the experimental timeline (day 0, 4, 8, and 12).
2.7.3. TcMEP were stimulated utilizing a subdermal needle over the motor cortex (C3 and C4).
The compound muscle action potentials (CMAPs) were recorded utilizing subdermal needles over the target muscles (recorded from extensor carpi radialis longus and extensor radialis brevis for the forelimb, and the tibialis anterior and the gastrocnemius medialis for the hindlimb) utilizing an analog to digital converter with the HFF set to 3000Hz and the LFF set to 10Hz. The motor pathway was activated utilizing a constant voltage stimulator. The stimulation parameters were a square-monophasic train pulse of stimulation (7 pulses) with an interstimulus of 3µs and a stimulatory rate of 0.5Hz. The intensity was set at 40V. The signals were acquired utilizing an analog-to-digital converter with a HFF of 3000Hz and a LFF of 30Hz. With an activated motor pathway, the action potential travels from the motor cortex into the spinal cord through the corticospinal tracts. Reaching the spinal cord, the action potential arrives at the anterior horn, where the first synapse occurs and continues to the neuromuscular junction, where the second and final synapse occurs, and a CMAP was recorded. Due to the variable nature of the activation of the motor pathway, we evaluated the recordings of each extremity individually and looked for significant changes from baseline values in control vs. experimental, control vs. control, and experimental vs. experimental across the experimental timeline (day 0, 4, 8, and 12) to determine whether there are significant changes.
2.8. Anesthesia Management
During the electrophysiological procedures, the animals were maintained under anesthesia with a combination of Ketamine (10mg/Kg) and Xylazine (0.25mg/Kg) applied intramuscularly (i.m.) using a 3ml sterile syringe with a 23-gauge needle. No paralytic agents were used after induction and intubation. A bite block was used to prevent tongue lacerations resulting from motor stimulation.
2.9. Statistical Analysis
2.9.1. Blood Biochemistry
Means and standard deviations were used to describe the results of the biochemistry tests in two study groups (experimental vs. vehicle). We compared the average test measurements of these biochemical variables between the experimental and control groups using the Mann-Whitney test at each study timepoint, beginning at baseline and continuing at each follow-up (day 4, 8) until day 12. The False Discovery Rate (FDR) of Benjamini-Hochberg was used to correct for multiple comparisons [
38]. All statistical analyses were performed using STATA SE 16 (Stata Corp., Texas, USA) at a 0.05 level of statistical significance.
2.9.2. Electrophysiological recordings
The authors working with the electrophysiological recordings and the statistical analysis were blind to the conditions of the experiment. Linear mixed-effect regression models were used for all continuous outcomes (amplitude, latency, and inter-latency) to explore the differences between the groups, accounting for changes over time and stratifying by brain hemisphere, extremity, and recording location. To evaluate the potential interactions between the groups and covariates, the “main effects only” models were compared with the full models (which included interactions between the intervention group and observation time, hemisphere or extremity, and location of the measurement), using a likelihood ratio (LR) test. For outcome variables for which the LR test was not statistically significant, the “main effects only” model was preferred. For the outcomes with significant interactions, each interaction between the experimental group and a covariate was evaluated individually based on their Wald-type test p-values; the final model presented in the results included only significant interactions. A similar analysis was made to describe measurements of all electrophysiological recordings evaluated in this study. Regression coefficients and their 95% confidence intervals for all fixed and random effects were presented.
3. Results
3.1. Electrophysiological recordings
Baseline recordings of SSEP, TcMEP, and EEG were recorded on day 0 before the administration of 4R and vehicle (
Figure 2). All subsequent recordings were then compared against the values of the baseline recording. Variability among intact subjects is normal and expected, as each subject may have different comorbidities or genetic differences that may influence the amplitude and conduction velocity. Other parameters, such as the effects of anesthesia and physiological differences, can also affect these recordings. Our statistical approach was designed to adjust for these differences.
3.1.1. SSEP recordings
We compared the values of latency and amplitude for each of the generators (peripheral, subcortical, and cortical) during the experimental days against the results obtained at baseline (day 0). No statistically significant differences were observed between the 4R- and vehicle- treated animals (
Table 1). Variance is explained differently for each outcome. The estimated intraclass correlation coefficients for the outcome variables ranged from 0.06 to 0.25. This means that 6% to 25% of the variance observed in the measurements can be attributed to the physiological differences between the NHPs.
3.1.2. EEG recordings
The observed frequency was analyzed while the subject was under anesthesia, and those values were compared between the vehicle and 4R. Also, the effects of each variable, vehicle, and 4R were evaluated individually against their own baseline values. No statistically significant difference was observed when comparing global frequencies (left and right hemispheres together) or when evaluating the average of each hemisphere individually between 4R experimental and vehicle (
Table 2).
However, subjects exhibited an increase in frequency on days 8 and 12 when compared to their own baseline values on the left hemisphere. Measurements of EGG-Hz obtained on day 8 after the trial started higher by 2.20 Hz [95%CI: 0.72; 3.68] compared to baseline measures. On day 12, the measures decreased; however, they were still significantly higher than the baseline measurements of 1.63 Hz [95%CI: 0.15; 3.11]. Nevertheless, an EEG increase in the single digits is not biologically relevant. In addition, no significant increase in frequency was observed in the right hemisphere (
Table 3).
3.1.3. TcMEP recordings
We evaluated the phases or compound muscle action potentials observed in the target muscles for all four extremities following the activation of the motor cortex and compared the experimental values against the control values. Since the left side of the body is represented in the right side of the brain, while the left side of the body is represented on the right side of the brain, we did not observe any significant statistical difference when activating the right hemisphere motor cortex and recorded responses on the left side of the body. However, a statistically significant difference was found when activating the left hemisphere motor cortex (responses were recorded on the right side of the body) for both the upper and lower extremities (
Table 4).
3.2. Behavior evaluation of rhesus macaque monkeys before and after 4R administration
The primary purpose of the behavioral observations was to determine whether 4R or its vehicle (10% Ethanol: 90% PEG 400) affected spontaneous behaviors in the rhesus macaques.
Table 5 represents the data obtained by using the task-oriented neurologic scale [
7]. During the four observation time points on days 0, 2, 7, and 11, no impairment was observed in movement, interaction with surroundings, and self-care between the experimental and control groups. Additionally, no significant difference was detected in the categories of vocalization, response to noise, and aggressive defensive behavior between the control and 4R-experimental groups.
3.3. Histopathological analysis of rhesus macaque monkeys after 4R administration
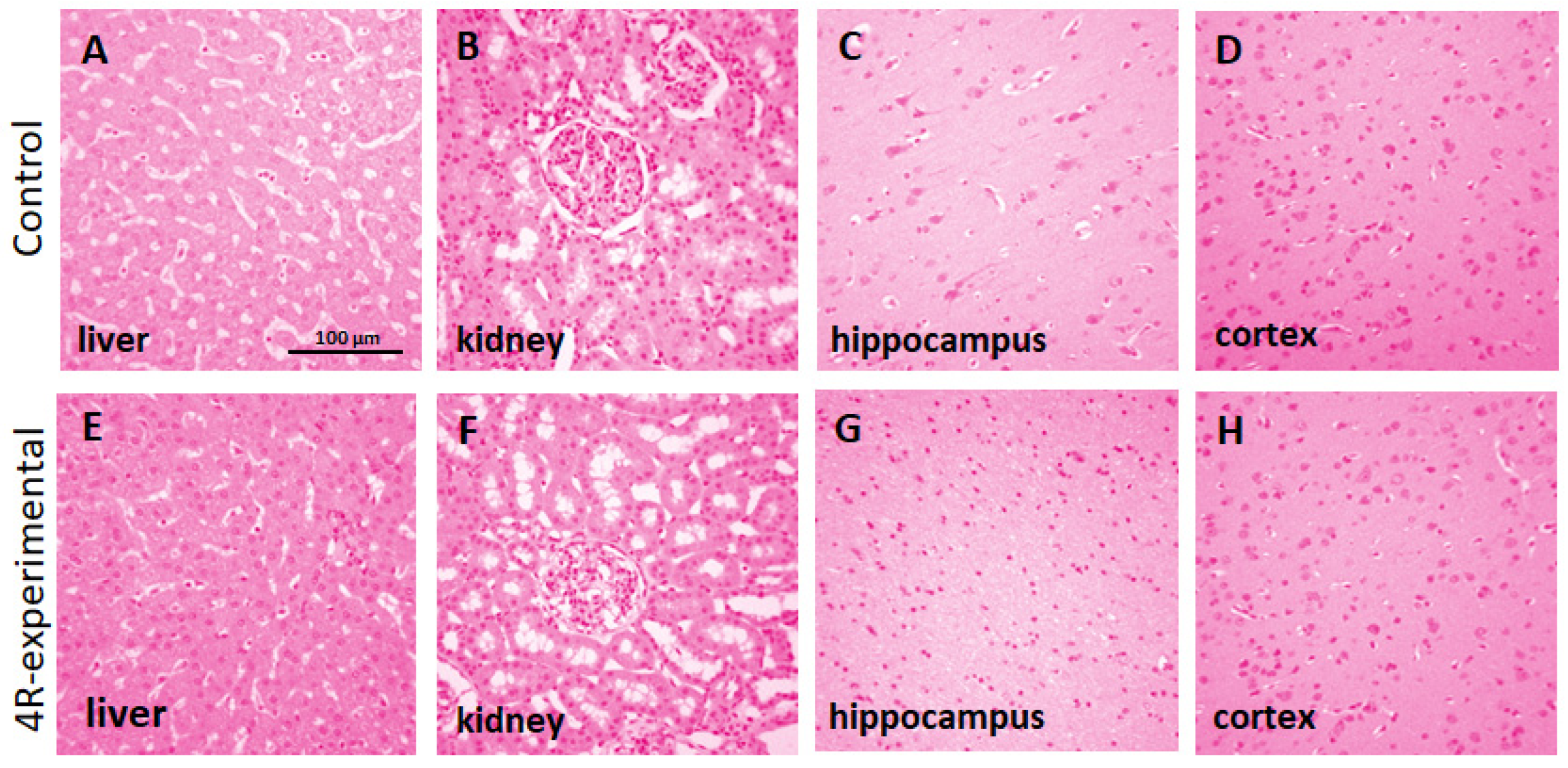
Histopathological observation of the liver, kidney, cortex, and hippocampus showed no pathological lesions after administering 4R. As shown in
Figure 3, the brain and kidney sections were within normal parameters in 4R- and vehicle-treated animals. There was no apparent trend in the prevalence or severity of the histological findings between the groups. The histological changes, including perivascular cuffing (inflammation around blood vessels) in the brain, inflammatory infiltrates in the liver and kidney, and mineralization in the brain, were compatible with incidental background changes.
Table 6 represents the pathological report of the histological findings for every animal. Considering the nature of these changes and the lack of any trend between the two groups, we concluded that the findings are unlikely to be related to the administration of 4R.
3.4. Biochemical and hematological analyses
Most of the biochemical results concerning the male NHPs did not display significant changes caused by the 4R treatment (
Table 7). Although the aspartate aminotransferase (AST) was significantly different in the experimental compared to the control group at day 12 (81.2±40.81vs. 44.2±12.98). However, when multiple test corrections were performed for False Discovery Rate (FDR) the significance was lost. Thus, we did not consider it as an indication of hepatic damage since we detected AST levels at 68.2±50.94 in the control group at day 8 since on day 12 AST vehicle control group spontaneously normalized to 44.2±12.98. Additionally, the 4R dose did not affect the activity of the liver enzymes: aspartate alanine aminotransferase (ALT) and alkaline phosphatase (ALP), which supports the notion that 4R is not hepatotoxic. Considering the minor changes in mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean platelet volume (MPV) on day 4, we concluded that 4R does not contribute to anemia or the development of cardiovascular diseases and stroke (
Table 7).
4. Discussion
Neurological diseases comprise 19% of all disability-adjusted life years and add to the immense public health burden in the United States and worldwide [
39]. This is partially due to the fact that therapeutic strategies are not only missing timely diagnosis but have limited treatment modality, such as the first 4.5 h after a stroke begins [
40] or lack of disease-modifying treatment in the case of Alzheimer’s and Parkinson’s disease [
41]. In the past couple of decades, a small diterpenoid found in tobacco and octocorals (4R) has emerged as a potential neuroprotective and anti-inflammatory therapeutic candidate [
42]. Although 4R has proven safe at a dose of up to 98 mg/kg in Sprague Dawley rats [
2], there are no reports of its action in more evolved specie such as NHPs. NHPs research has a more significant clinical translation due to their complex cognitive and motor functions and highly developed neuroanatomy. This is the first report in which cembranoid safety was evaluated in NHPs. No clinically related adverse effects were detected during electrophysiological central and peripheral nervous system monitoring, spontaneous behavior observations, and hematological and histological analyses.
Developing therapeutics that effectively treat CNS pathologies is challenging because most tested drugs do not cross the blood-brain barrier into the brain parenchyma. However, 4R reaches the brain in 1.5 hours independently of the administration route with concentrations higher than in plasma (brain to plasma ratios of 2.49 (i.m.) and 2.48 (s.c.) at 1.5 hours) [
1]. 4R has an estimated therapeutic index of at least 16 with an in vivo effective dose of 6 mg/kg [
5,
7,
23,
24]. Based on the literature, 4R complemented classical OP poisoning antidotes [
5] and protected against brain damage after stroke [
7] and Parkinson’s disease [
23]. 4R promoted M2 phenotype while attenuating the M1 phenotype in a microglial cell line [
8] and restored cognitive decline during an LPS-induced systemic inflammation by activating pro-survival pathways in the hippocampus [
24]. 4R presents good absorption, distribution, metabolism, and excretion (ADME) characteristics. These features and its neuroprotective capability indicate that this molecule exhibits drug-like properties and could become an efficacious therapeutic agent for neurodegenerative diseases [
3,
5,
6,
7,
23,
24].
Electrophysiological recordings are well-known tools for evaluating nervous system integrity, discriminating against different pathologies, or detecting evolving injuries. To assess whether 4R administration interferes with the electrophysiological activity of the peripheral and central nervous system, we employed SEEP, EEG, and TcMEP. EEG assessment is considered a standard of care for individuals suffering from ischemia since it allows for the noninvasive identification of stroke [
43,
44]. EEGs are also included as part of the monitoring paradigm for patients undergoing carotid endarterectomy surgery, as well as brain and thoracic aneurysm repair surgeries [
45,
46]. SSEP and TcMEP are also standard care during vascular surgeries. Used along with EEG to monitor the integrity of the nervous system, SSEP and TcMEP allow the detection of ischemic events efficiently and quickly during surgical intervention [
15,
16,
17,
18]. While the scalp EEG can provide a global idea of the brain status, SSEP and TcMEP provide immediate feedback regarding the integrity of both the central and peripheral nervous systems. To evaluate the effects of 4R and vehicle over these neurophysiological parameters, we established a baseline recording on day 0 and monitored for any changes on days 4, 8, and 12 after the 4R or vehicle treatment. We did not observe any significant changes in the neurophysiological recordings, except for the increase in the EEG frequency on the left hemisphere and the increase in action potentials recorded from the right upper and lower extremities. This increase in frequency in the left hemisphere that is observed in 4R-treated subjects on days 8 and 12, along with the resulting increase in compound muscle action potentials observed in the right upper and lower extremity, is a finding that will require further investigation.
Since we did not observe any electrophysiological readings indicative of injury to the nervous system between experimental and control groups, we can suggest that 4R application is safe at the tested dose. It has been shown that in the presence of ischemia, 4R not only decreased brain inflammation but also saved more than 50% of the affected brain tissue compared to the untreated group [
7,
8]. In the future, this drug could be administered after a stroke or during surgery with a predicted risk for ischemia, potentially minimizing brain damage.
Several blood chemistry parameters and hematology findings were statistically different in subjects treated with 4R compared to vehicle controls. However, those differences were not considered clinically relevant since the initial baseline values differed between the groups (
Table 4). Biomarkers such as mean platelet volume (MPV) and platelet count (PLT) could be considered potential risk factors for stroke occurrence and a bad prognosis for stroke outcome [
47,
48]. However, our study’s MPV, MCH, and MCV demonstrated only temporary changes in the beginning and normalized by the end of 4R administration. Additionally, PLT mean values remained constant within the groups, suggesting that this finding, although noteworthy, was not critical. Changes in BUN and CREA levels are usually attributed to reduced ingestion of food or severe hepatotoxicity [
49]. Nonetheless, BUN and CREA remained constant throughout the treatment.
Additionally, measurement of alanine aminotransferase (ALT) and alanine aminotransferase (AST) enzymatic activity in circulation is still the most commonly used biochemistry test in clinical practice, when evaluating putative liver injury [
50]. We detected significant elevation of the AST levels on day 12 in the 4R-experimental group compared to the controls. However, when multiple test corrections were performed the significance was lost. Although it has been reported that the normal range of AST in rhesus monkeys is approximately 25.7 ± 10.3 U/l [
51], we detected an AST baseline of 53.80 ± 25.87 U/l which varied to 68.2±50.94 throughout the study in the vehicle controls. Moreover, the levels of liver enzymes (ALT, ALP), bilirubin, and albumin remained in the normal range, supporting the safety of 4R for no signs of hepatotoxicity. Finally, the histopathologic evaluation of the liver revealed minimal scattered lymphohistiocytic portal infiltrates but those findings were attributed to 4R since both controls and vehicles possessed similar alterations.
5. Summary and Conclusions
The main objective of this study was to determine whether 4R is safe for NHPs, not causing acute toxicity or allergic reactions when administrated repetitively. Our results showed that 1.4 mg/kg of 4R injected intravenously for 11 days does not cause behavior and brain tissue abnormalities. Blood biochemistry and histology in the liver and kidney confirmed that 4R does not contribute to hepatotoxicity or nephrotoxicity in NHPs. Additionally, 4R did not interfere with electrophysiological central and peripheral nervous system assessments (EEG, TcMEP, and SSEP). These results suggest that 4R is safe in NHP, and in the future, 4R could be employed as a neuroprotective treatment in humans with neuroinflammatory diseases.
Author Contributions
The following statements should be used “Conceptualization, AHM, PA, VAE; methodology, WC, SRT, NPR; validation, WC, AHM, DSM, and PAF; formal analysis, SRT⊀ investigation, AHM, NS, WC; resources, AHM; data curation, AHM, YFA, NS; writing—original draft preparation, NS, YFA, AHM, SRT, NPR; writing—review and editing, NS, YFA, WC, DSM, AHM.; visualization, AHM.; supervision, AHM; project administration, AHM.; funding acquisition, AHM. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Hispanic Alliance for Clinical and Translational Research (Alliance) is supported by the National Institute of General Medical Sciences (NIGMS) National Institutes of Health under the Award Number U54GM133807; PR-INBRE program supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM103475; Support of Competitive Research (SCORE) SC2NS119144 from National Institute of Neurological Disorders and Stroke (NINDS). THE CONTENT IS SOLELY THE RESPONSIBILITY OF THE AUTHORS AND DOES NOT NECESSARILY REPRESENT THE OFFICIAL VIEWS OF THE NATIONAL INSTITUTES OF HEALTH”.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Puerto Rico-Medical Sciences Campus (protocol code A330118 and August 31, 2019) for studies involving animals.
Data Availability Statement
All raw data is available upon request following the regulations of MDPI and internal regulations of the University of Puerto Rico.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Velez-Carrasco W, Green CE, Catz P, Furimsky A, O’Loughlin K, Eterovic VA, Ferchmin PA: Pharmacokinetics and Metabolism of 4R-Cembranoid. PLoS One 2015, 10(3):e0121540. [CrossRef]
- Sabeva N, Pagan OR, Ferrer-Acosta Y, Eterovic VA, Ferchmin PA: In Vivo Evaluation of the Acute Systemic Toxicity of (1S,2E,4R,6R,7E,11E)-Cembratriene-4,6-diol (4R) in Sprague Dawley Rats. Nutraceuticals (Basel) 2022, 2(2):60-70. [CrossRef]
- Ferchmin PA, Perez D, Cuadrado BL, Carrasco M, Martins AH, Eterovic VA: Neuroprotection Against Diisopropylfluorophosphate in Acute Hippocampal Slices. Neurochemical research 2015, 40(10):2143-2151. [CrossRef]
- Berrios VO, Boukli NM, Rodriguez JW, Negraes PD, Schwindt TT, Trujillo CA, Oliveira SL, Cubano LA, Ferchmin PA, Eterovic VA et al.: Paraoxon and Pyridostigmine Interfere with Neural Stem Cell Differentiation. Neurochemical research 2015, 40(10):2091-2101. [CrossRef]
- Ferchmin PA, Andino M, Reyes Salaman R, Alves J, Velez-Roman J, Cuadrado B, Carrasco M, Torres-Rivera W, Segarra A, Martins AH et al.: 4R-cembranoid protects against diisopropylfluorophosphate-mediated neurodegeneration. Neurotoxicology 2014, 44:80-90. [CrossRef]
- Ferchmin PA, Hao J, Perez D, Penzo M, Maldonado HM, Gonzalez MT, Rodriguez AD, de Vellis J: Tobacco cembranoids protect the function of acute hippocampal slices against NMDA by a mechanism mediated by alpha4beta2 nicotinic receptors. Journal of neuroscience research 2005, 82(5):631-641. [CrossRef]
- Martins AH, Hu J, Xu Z, Mu C, Alvarez P, Ford BD, El Sayed K, Eterovic VA, Ferchmin PA, Hao J: Neuroprotective activity of (1S,2E,4R,6R,-7E,11E)-2,7,11-cembratriene-4,6-diol (4R) in vitro and in vivo in rodent models of brain ischemia. Neuroscience 2015, 291:250-259. [CrossRef]
- Fu H, Wang J, Wang J, Liu L, Jiang J, Hao J: 4R-cembranoid protects neuronal cells from oxygen-glucose deprivation by modulating microglial cell activation. Brain Res Bull 2022, 179:74-82. [CrossRef]
- Feigin V, Norrving B, Sudlow CLM, Sacco RL: Updated Criteria for Population-Based Stroke and Transient Ischemic Attack Incidence Studies for the 21st Century. Stroke 2018, 49(9):2248-2255. [CrossRef]
- Collaborators GBDS: Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol 2021, 20(10):795-820. [CrossRef]
- The L: 21st century management and prevention of stroke. Lancet 2018, 392(10154):1167. [CrossRef]
- Herpich F, Rincon F: Management of Acute Ischemic Stroke. Crit Care Med 2020, 48(11):1654-1663. [CrossRef]
- Del Zoppo GJ, Saver JL, Jauch EC, Adams HP, Jr., American Heart Association Stroke C: Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: a science advisory from the American Heart Association/American Stroke Association. Stroke 2009, 40(8):2945-2948. [CrossRef]
- Provost C, Soudant M, Legrand L, Ben Hassen W, Xie Y, Soize S, Bourcier R, Benzakoun J, Edjlali M, Boulouis G et al.: Magnetic Resonance Imaging or Computed Tomography Before Treatment in Acute Ischemic Stroke. Stroke 2019, 50(3):659-664. [CrossRef]
- Branston NM, Ladds A, Symon L, Wang AD: Comparison of the effects of ischaemia on early components of the somatosensory evoked potential in brainstem, thalamus, and cerebral cortex. J Cereb Blood Flow Metab 1984, 4(1):68-81. [CrossRef]
- Branston NM, Ladds A, Symon L, Wang AD, Vajda J: Somatosensory evoked potentials in experimental brain ischemia. Prog Brain Res 1984, 62:185-199. [CrossRef]
- Quinones-Hinojosa A, Alam M, Lyon R, Yingling CD, Lawton MT: Transcranial motor evoked potentials during basilar artery aneurysm surgery: technique application for 30 consecutive patients. Neurosurgery 2004, 54(4):916-924; discussion 924. [CrossRef]
- Stulin ID, Savchenko AY, Smyalovskii VE, Musin RS, Stryuk GV, Priz IL, Bagir VN, Semenova EN: Use of transcranial magnetic stimulation with measurement of motor evoked potentials in the acute period of hemispheric ischemic stroke. Neurosci Behav Physiol 2003, 33(5):425-429. [CrossRef]
- Kamel H, Iadecola C: Brain-immune interactions and ischemic stroke: clinical implications. Arch Neurol 2012, 69(5):576-581. [CrossRef]
- McColl BW, Allan SM, Rothwell NJ: Systemic inflammation and stroke: aetiology, pathology and targets for therapy. Biochem Soc Trans 2007, 35(Pt 5):1163-1165. [CrossRef]
- Elkind MS, Cheng J, Rundek T, Boden-Albala B, Sacco RL: Leukocyte count predicts outcome after ischemic stroke: the Northern Manhattan Stroke Study. J Stroke Cerebrovasc Dis 2004, 13(5):220-227. [CrossRef]
- Yin NS, Benavides S, Starkman S, Liebeskind DS, Saver JA, Salamon N, Jahan R, Duckwiler GR, Tateshima S, Vinuela F et al.: Autopsy findings after intracranial thrombectomy for acute ischemic stroke: a clinicopathologic study of 5 patients. Stroke 2010, 41(5):938-947. [CrossRef]
- Hu J, Ferchmin PA, Hemmerle AM, Seroogy KB, Eterovic VA, Hao J: 4R-Cembranoid Improves Outcomes after 6-Hydroxydopamine Challenge in Both In vitro and In vivo Models of Parkinson’s Disease. Frontiers in neuroscience 2017, 11:272. [CrossRef]
- Rojas-Colon LA, Dash PK, Morales-Vias FA, Lebron-Davila M, Ferchmin PA, Redell JB, Maldonado-Martinez G, Velez-Torres WI: 4R-cembranoid confers neuroprotection against LPS-induced hippocampal inflammation in mice. J Neuroinflammation 2021, 18(1):95. [CrossRef]
- D’Arceuil HE, Duggan M, He J, Pryor J, de Crespigny A: Middle cerebral artery occlusion in Macaca fascicularis: acute and chronic stroke evolution. J Med Primatol 2006, 35(2):78-86. [CrossRef]
- Li Y, Zhou Y, Si N, Han L, Ren W, Xin S, Wang H, Zuo R, Wei X, Yang J et al.: Comparative Metabolism Study of Five Protoberberine Alkaloids in Liver Microsomes from Rat, Rhesus Monkey, and Human. Planta Med 2017, 83(16):1281-1288. [CrossRef]
- C JJ, Consortium TM, Wood JS, Moreno A, Garcia A, Galinski MR: Case Report: Severe and Complicated Cynomolgi Malaria in a Rhesus Macaque Resulted in Similar Histopathological Changes as Those Seen in Human Malaria. Am J Trop Med Hyg 2017, 97(2):548-555. [CrossRef]
- Yeo HG, Lee Y, Jeon CY, Jeong KJ, Jin YB, Kang P, Kim SU, Kim JS, Huh JW, Kim YH et al.: Characterization of Cerebral Damage in a Monkey Model of Alzheimer’s Disease Induced by Intracerebroventricular Injection of Streptozotocin. J Alzheimers Dis 2015, 46(4):989-1005. [CrossRef]
- Yi KS, Choi CH, Lee SR, Lee HJ, Lee Y, Jeong KJ, Hwang J, Chang KT, Cha SH: Sustained diffusion reversal with in-bore reperfusion in monkey stroke models: Confirmed by prospective magnetic resonance imaging. J Cereb Blood Flow Metab 2017, 37(6):2002-2012. [CrossRef]
- Baraka HN, Khanfar MA, Williams JC, El-Giar EM, El Sayed KA: Bioactive natural, biocatalytic, and semisynthetic tobacco cembranoids. Planta Med 2011, 77(5):467-476. [CrossRef]
- Swanson BN: Medical use of dimethyl sulfoxide (DMSO). Rev Clin Basic Pharm 1985, 5(1-2):1-33.
- Food, Drug Administration HHS: International Conference on Harmonisation; Guidance on M3(R2) Nonclinical Safety Studies for the Conduct of Human Clinical Trials and Marketing Authorization for Pharmaceuticals; availability. Notice. Fed Regist 2010, 75(13):3471-3472.
- Bauman MD, Lesh TA, Rowland DJ, Schumann CM, Smucny J, Kukis DL, Cherry SR, McAllister AK, Carter CS: Preliminary evidence of increased striatal dopamine in a nonhuman primate model of maternal immune activation. Transl Psychiatry 2019, 9(1):135. [CrossRef]
- Nair AB, Jacob S: A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm 2016, 7(2):27-31. [CrossRef]
- Mack WJ, King RG, Hoh DJ, Coon AL, Ducruet AF, Huang J, Mocco J, Winfree CJ, D’Ambrosio AL, Nair MN et al.: An improved functional neurological examination for use in nonhuman primate studies of focal reperfused cerebral ischemia. Neurol Res 2003, 25(3):280-284. [CrossRef]
- Rodriguez-Mercado R, Ford GD, Xu Z, Kraiselburd EN, Martinez MI, Eterovic VA, Colon E, Rodriguez IV, Portilla P, Ferchmin PA et al.: Acute neuronal injury and blood genomic profiles in a nonhuman primate model for ischemic stroke. Comp Med 2012, 62(5):427-438.
- Blume WT, Ferguson GG, McNeill DK: Significance of EEG changes at carotid endarterectomy. Stroke 1986, 17(5):891-897. [CrossRef]
- Newson RB: Frequentist q-values for multiple-test procedures. The Stata Journal 2010, 10(4):568–584. [CrossRef]
- Vigo DV, Kestel D, Pendakur K, Thornicroft G, Atun R: Disease burden and government spending on mental, neurological, and substance use disorders, and self-harm: cross-sectional, ecological study of health system response in the Americas. Lancet Public Health 2019, 4(2):e89-e96. [CrossRef]
- Jin C, Huang RJ, Peterson ED, Laskowitz DT, Hernandez AF, Federspiel JJ, Schwamm LH, Bhatt DL, Smith EE, Fonarow GC et al.: Intravenous tPA (Tissue-Type Plasminogen Activator) in Patients With Acute Ischemic Stroke Taking Non-Vitamin K Antagonist Oral Anticoagulants Preceding Stroke. Stroke 2018, 49(9):2237-2240. [CrossRef]
- Emborg ME: Nonhuman Primate Models of Neurodegenerative Disorders. ILAR J 2017, 58(2):190-201. [CrossRef]
- Yan N, Du Y, Liu X, Zhang H, Liu Y, Zhang Z: A Review on Bioactivities of Tobacco Cembranoid Diterpenes. Biomolecules 2019, 9(1). [CrossRef]
- Sutcliffe L, Lumley H, Shaw L, Francis R, Price CI: Surface electroencephalography (EEG) during the acute phase of stroke to assist with diagnosis and prediction of prognosis: a scoping review. BMC Emerg Med 2022, 22(1):29. [CrossRef]
- Wilkinson CM, Burrell JI, Kuziek JWP, Thirunavukkarasu S, Buck BH, Mathewson KE: Predicting stroke severity with a 3-min recording from the Muse portable EEG system for rapid diagnosis of stroke. Sci Rep 2020, 10(1):18465. [CrossRef]
- Lam AM, Manninen PH, Ferguson GG, Nantau W: Monitoring electrophysiologic function during carotid endarterectomy: a comparison of somatosensory evoked potentials and conventional electroencephalogram. Anesthesiology 1991, 75(1):15-21. [CrossRef]
- Ferguson GG: Intra-operative monitoring and internal shunts: are they necessary in carotid endarterectomy? Stroke 1982, 13(3):287-289. [CrossRef]
- Gasparyan AY, Ayvazyan L, Mikhailidis DP, Kitas GD: Mean platelet volume: a link between thrombosis and inflammation? Curr Pharm Des 2011, 17(1):47-58. [CrossRef]
- Slavka G, Perkmann T, Haslacher H, Greisenegger S, Marsik C, Wagner OF, Endler G: Mean platelet volume may represent a predictive parameter for overall vascular mortality and ischemic heart disease. Arterioscler Thromb Vasc Biol 2011, 31(5):1215-1218. [CrossRef]
- Hosten AO: BUN and Creatinine. In: Clinical Methods: The History, Physical, and Laboratory Examinations. Edited by Walker HK, Hall WD, Hurst JW, 3rd edn. Boston: Butterworths; 1990.
- Sherman KE: Alanine aminotransferase in clinical practice. A review. Arch Intern Med 1991, 151(2):260-265.
- Koo BS, Lee DH, Kang P, Jeong KJ, Lee S, Kim K, Lee Y, Huh JW, Kim YH, Park SJ et al.: Reference values of hematological and biochemical parameters in young-adult cynomolgus monkey (Macaca fascicularis) and rhesus monkey (Macaca mulatta) anesthetized with ketamine hydrochloride. Lab Anim Res 2019, 35:7. [CrossRef]
Figure 1.
Peaks identified with A depict the primary negative peak. Peaks identified with B depict the secondary positive peak. The first peak confirms the integrity of the conduction velocity of the pathway, while the amplitude determines the overall integrity of the potential generator. In an ischemic event, these two parameters are most sensitive to detect changes and pathway integrity, which can be translated to brain tissue viability.
Figure 1.
Peaks identified with A depict the primary negative peak. Peaks identified with B depict the secondary positive peak. The first peak confirms the integrity of the conduction velocity of the pathway, while the amplitude determines the overall integrity of the potential generator. In an ischemic event, these two parameters are most sensitive to detect changes and pathway integrity, which can be translated to brain tissue viability.
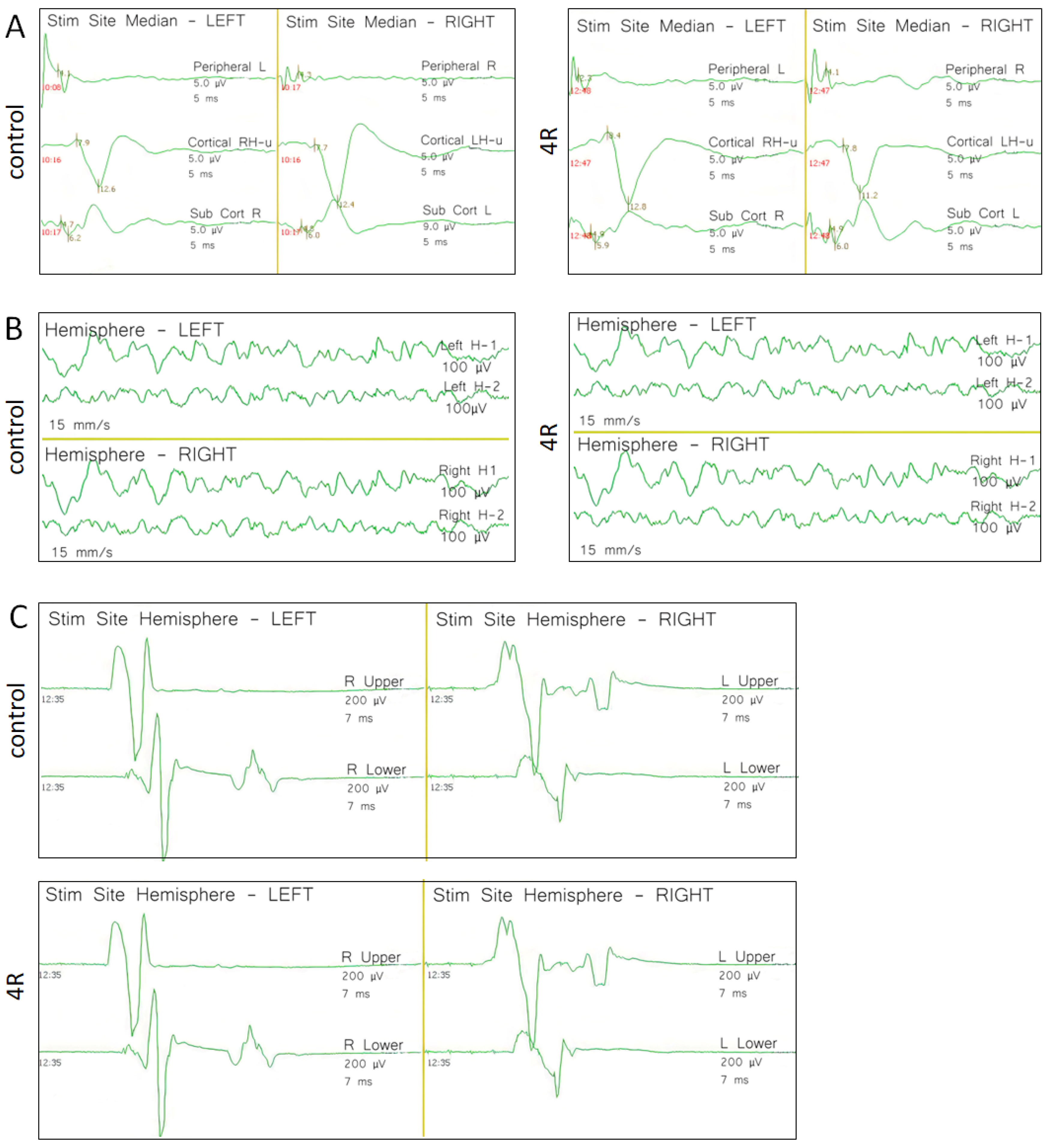
Figure 2.
Representative baseline (day 0) neurophysiological recordings on vehicle control and 4R-experimental groups. A) SSEP, B) EEG, and C) TcMEP recordings.
Figure 2.
Representative baseline (day 0) neurophysiological recordings on vehicle control and 4R-experimental groups. A) SSEP, B) EEG, and C) TcMEP recordings.
Figure 3.
Histopathological staining of tissues using hematoxylin and eosin (H&E) in male rhesus macaque monkeys after daily injection of a 4R dose (1.4 mg/kg) for 11 days. A, E: liver; B, F: kidney; C, G: hippocampus and D, H: cerebral cortex. 100X magnification. Scale bar: 100 µm.
Figure 3.
Histopathological staining of tissues using hematoxylin and eosin (H&E) in male rhesus macaque monkeys after daily injection of a 4R dose (1.4 mg/kg) for 11 days. A, E: liver; B, F: kidney; C, G: hippocampus and D, H: cerebral cortex. 100X magnification. Scale bar: 100 µm.
Table 1.
Amplitude and latency for each generator (peripheral, subcortical, and cortical) in somatosensory evoked potentials (SSEP) in a linear mixed model of selected neuroanatomy characteristics stratified for extremity, brain, and hemisphere location.
Table 1.
Amplitude and latency for each generator (peripheral, subcortical, and cortical) in somatosensory evoked potentials (SSEP) in a linear mixed model of selected neuroanatomy characteristics stratified for extremity, brain, and hemisphere location.
| Recording location |
Group |
Amplitude |
Latency |
| |
|
Estimated
Coefficient (95% CI) |
Estimated
Coefficient (95% CI) |
| Left Upper Extremity, Peripheral |
vehicle
experimental |
REFERENCE
0.26 (-0.37;0.89) |
REFERENCE
-0.46(-1.00;0.08) |
| Left Upper Extremity, Subcortical |
vehicle
experimental |
REFERENCE
0.48 (-0.15; 1.11) |
REFERENCE
-0.10 (-0.97; 0.78) |
| Left Upper Extremity, Cortical |
vehicle
experimental |
REFERENCE
1.17 (-1.03; 3.37) |
REFERENCE
0.21 (-0.80; 1.22) |
| Left Lower Extremity, Cortical |
vehicle
experimental |
REFERENCE
-2.60 (-5.61; 0.41) |
REFERENCE
0.83 (-2.10; 0.45) |
| Right Upper Extremity, Peripheral |
vehicle
experimental |
REFERENCE
0.33 (-0.18; 0.83) |
REFERENCE
-0.40 (-0.82; 0.01) |
| Right Upper Extremity, Subcortical |
vehicle
experimental |
REFERENCE
0.05 (-0.38; 0.48) |
REFERENCE
-0.07 (-0.75; 0.62) |
| Right Upper Extremity, Cortical |
vehicle
experimental |
REFERENCE
0.03 (-0.99; 1.06) |
REFERENCE
-0.30 (-1.08; 1.02) |
| Right Lower Extremity, Cortical |
vehicle
experimental |
REFERENCE
-0.60 (-3.09; 1.89) |
REFERENCE
-0.92 (-2.53; 0.68) |
Table 2.
Differences between the experimental and vehicle group in terms of average EEG measurements, stratified by hemisphere.
Table 2.
Differences between the experimental and vehicle group in terms of average EEG measurements, stratified by hemisphere.
| Recording location |
Group |
Average Frequency |
| |
|
Estimated Coefficient (95% CI) |
| Left Hemisphere |
vehicle
experimental |
REFERENCE
-0.27 (-2.24; 1.70) |
| Right Hemisphere |
vehicle
experimental |
REFERENCE
-0.43 (-2.65; 1.79) |
Table 3.
Changes in average EEG measurements over time, for each hemisphere.
Table 3.
Changes in average EEG measurements over time, for each hemisphere.
Recorded from the Left Hemisphere
Time (in days)
|
| baseline |
REFERENCE |
| 4th day |
1.49 (0.01; 2.97) |
| 8th day |
2.20 (0.72; 3.68) * |
| 12th day |
1.63 (0.15; 3.11) * |
Recorded from the Right Hemisphere
Time (in days)
|
| baseline |
REFERENCE |
| 4th day |
1.06 (-0.36; 2.47) |
| 8th day |
-0.06 (-1.48; 2.47) |
| 12th day |
-0.27 (-1.69; 1.14) |
Table 4.
Changes in average TcMEP measurements over time, for each hemisphere.
Table 4.
Changes in average TcMEP measurements over time, for each hemisphere.
| Recording location |
Group |
Compound Muscle Action Potential |
| |
|
Estimated Coefficient (95% CI) |
| Left upper extremity |
control
experimental |
REFERENCE
-0.56 (-2.08; 0.96) |
| Right upper extremity |
control
experimental |
REFERENCE
-1.53 (-2.56; - 0.50) * |
| Left lower extremity |
control
experimental |
REFERENCE
0.79 (-0.51; 2.09) |
| Right lower extremity |
control
experimental |
REFERENCE
-1.53 (-2.56; - 0.50) * |
Table 5.
Behavioral observations in male rhesus monkeys before and after repeated-dose administration of vehicle control or 4R experimental treatment.
Table 5.
Behavioral observations in male rhesus monkeys before and after repeated-dose administration of vehicle control or 4R experimental treatment.
| |
Vehicle |
4R-experimental |
| |
Day |
| Parameters |
0 |
2 |
7 |
11 |
0 |
2 |
7 |
11 |
| Slowly moves |
|
|
|
|
|
|
|
|
| Yes |
4 (80.0) |
4 (80.0) |
5 (100.0) |
5 (100.0) |
4 (80.0) |
4 (80.0) |
5 (100.0) |
5 (100.0) |
| No |
1 (20.0) |
1 (20.0) |
0 |
0 |
1 (20.0) |
(80.0) |
0 |
0 |
| Aggressive defensive behavior |
|
|
|
|
|
|
|
|
| Yes |
5 (100.0) |
4 (80.0) |
3 (60.0) |
5 (100.0) |
5 (100.0) |
4 (80.0) |
3 (60.0) |
5 (100.0) |
| No |
0 |
1 (20.0) |
2 (40.0) |
0 |
0 |
1 (20.0) |
2 (40.0) |
0 |
| Vocalizes |
|
|
|
|
|
|
|
|
| Yes |
1 (20.0) |
5 (100.0) |
1 (20.0) |
2 (40.0) |
3 (60.0) |
5 (100.0) |
0 |
3 (60.0) |
| No |
4 (80.0) |
0 |
4 (80.0) |
3 (60.0) |
2 (40.0) |
0 |
5 (100.0) |
2 (40.0) |
| Interacts with toys |
|
|
|
|
|
|
|
|
| Yes |
4 (80.0) |
2 (40.0) |
3 (60.0) |
4 (80.0) |
4 (80.0) |
3 (60.0) |
1 (20.0) |
3 (60.0) |
| No |
1 (20.0) |
3 (60.0) |
2 (40.0) |
1 (20.0) |
1 (20.0) |
2 (40.0) |
3 (60.0) |
2 (40.0) |
| missing |
|
|
|
|
|
|
1 (10) |
|
| Grooms, scratches |
|
|
|
|
|
|
|
|
| Yes |
3 (60.0) |
5 (100.0) |
4 (80.0) |
5 (100.0) |
2 (40.0) |
5 (100.0) |
3 (60.0) |
5 (100.0) |
| No |
2 (40.0) |
0 |
1 (20.0) |
0 |
3 (60.0) |
0 |
2 (40.0) |
0 |
| Responds to a noise |
|
|
|
|
|
|
|
|
| Yes |
4 (80.0) |
4 (80.0) |
3 (60.0) |
5 (100.0) |
5 (100.0) |
4 (80.0) |
2 (40.0) |
4 (80.0) |
| No |
1 (20.0) |
1 (20.0) |
2 (40.0) |
0 |
0 (0.00) |
1 (20.0) |
3 (60.0) |
1 (20.0) |
Table 6.
Histopathological evaluation of liver, kidney, hippocampus, and cerebral cortex in rhesus monkeys after daily injection of a 4R dose (1.4 mg/kg) for 11 days.
Table 6.
Histopathological evaluation of liver, kidney, hippocampus, and cerebral cortex in rhesus monkeys after daily injection of a 4R dose (1.4 mg/kg) for 11 days.
| Individuals |
Liver |
Kidney |
Hippocampus |
Cortex |
| Vehicle |
|
|
|
|
| 5U8 |
Minimal scattered lymphohistiocytic portal infiltrates. |
No lesions. |
No lesions. |
No lesions. |
| 1V0 |
No lesions. |
No lesions. |
Focal perivascular lymphocytic infiltrate. |
No lesions. |
| CF89 |
Minimal scattered lymphohistiocytic portal infiltrates. |
No lesions |
No lesions. |
No lesions |
| CF75 |
Minimal scattered lymphohistiocytic portal infiltrates. |
No lesions. |
Not examined. |
No lesions. |
| 2T3 |
Minimal scattered lymphohistiocytic portal infiltrates. |
Minimal focal lymphocytic
interstitial infiltrate. |
No lesions. |
No lesions. |
| Experimental |
|
|
|
|
| 2W4 |
No lesions. |
No lesions. |
No lesions. |
No lesions. |
| MA286 |
Minimal scattered lymphohistiocytic portal infiltrates. |
No lesions. |
No lesions. |
No lesions. |
| MA327 |
Minimal scattered lymphohistiocytic portal infiltrates. |
Minimal multifocal lymphocytic interstitial infiltrates. |
No lesions. |
No lesions. |
| CF90 |
Minimal scattered lymphohistiocytic portal infiltrates. |
No lesions. |
Minimal focal
mineralization. |
No lesions. |
| 1X8 |
Minimal scattered lymphohistiocytic portal infiltrates. |
No lesions. |
Not examined. |
No lesions |
Table 7.
Hematological and comprehensive metabolic panel analysis after repeated 4R administration in male rhesus monkeys (Macaca mulatta).
Table 7.
Hematological and comprehensive metabolic panel analysis after repeated 4R administration in male rhesus monkeys (Macaca mulatta).
| |
Day 0 |
Day 4 |
Day 8 |
Day 12 |
| Parameters |
control |
4R |
control |
4R |
control |
4R |
control |
4R |
| WBC (x103/µl) |
8.38±2.15 |
8.68±3.00 |
6.87±1.66 |
7.59±3.04 |
7.714±2.49 |
8.476±2.58 |
7.54±3.20 |
9.19±2.98 |
| RBC (106/µl) |
5.14±0.47 |
5.25±0.55 |
4.78±0.37 |
5.028±0.61 |
4.56±0.25 |
4.72±0.53 |
4.45±0.20 |
4.78±0.52 |
| HGB (g/dL) |
12.08±1.00 |
11.58±1.07 |
11.28±0.88 |
11.08±0.91 |
10.82±0.65 |
10.50±0.77 |
10.56±0.50 |
10.62±0.51 |
| HCT (%) |
38.18±3.37 |
37.28±3.05 |
35.82±2.50 |
35.88±3.21 |
34.58±1.56 |
33.88±2.40 |
33.90±1.40 |
34.26±2.40 |
| MCV (fL) |
74.22±0.38 |
71.12±3.29* |
74.98±1.06 |
71.62±3.24* |
75.86±1.00 |
72.06±3.32* |
76.12±1.01 |
71.86±3.27 |
| MCH (pg) |
23.50±0.33 |
22.10±1.70 |
23.60±0.41 |
22.18±1.25* |
23.74±0.27 |
22.34±1.40 |
23.70±0.70 |
22.32±1.51 |
| MCHC (gm/dl) |
31.66±0.34 |
31.04±1.10 |
31.50±0.70 |
30.90±0.55 |
31.28±0.54 |
31.00±0.73 |
31.16±0.50 |
31.06±0.74 |
| RDW (%) |
12.66±0.83 |
13.38±0.96 |
12.94±0.81 |
13.68±1.04 |
13.22±0.75 |
13.92±1.13 |
13.66±0.60 |
14.24±1.28 |
| PLT (x106/µl) |
386.4±114.47 |
270±86.43 |
393.4±56.38 |
319.2±99.30 |
444±82.63 |
361.8±93.78 |
483.60±95.5 |
334.4±97.33 |
| MPV (fL) |
10.72±0.99 |
12.06±0.52* |
10.20±0.77 |
11.40±1.05 |
10.3±0.76 |
11.42±1.08 |
10.24±0.70 |
11.50±0.94 |
| NE% |
67.04±4.59 |
60.9±10.64 |
56.32±10.49 |
56.10±5.00 |
56.94±10.07 |
57.04±5.68 |
59.78±8.30 |
60.70±3.76 |
| LY% |
26.54±4.31 |
33.06±10.71 |
37.44±10.06 |
37.52±5.80 |
37.34±10.84 |
36.44±5.40 |
33.24±7.70 |
31.50±5.68 |
| MO% |
4.26±2.17 |
4.32±1.25 |
4.26±1.33 |
5.08±0.98 |
3.94±0.75 |
4.82±1.73 |
5.60±2.30 |
5.82±2.07 |
| EO% |
1.76±1.18 |
1.38±1.33 |
1.66±1.95 |
0.86±0.33 |
1.42±1.35 |
1.36±0.85 |
0.92±0.70 |
1.56±0.51 |
| BA (numeric) |
0.10±0.07 |
0.14±0.05 |
0.12±0.13 |
0.22±0.04 |
0.14±0.05 |
0.12±0.04 |
0.16±0.10 |
0.12±0.11 |
| IG (numeric) |
0.30±0.07 |
0.20±0.10 |
0.20±0.10 |
0.22±0.15 |
0.22±0.11 |
0.22±0.13 |
0.30±0.20 |
0.30±0.12 |
| NE#(x103/µl) |
5.59±1.32 |
5.53±3.08 |
3.842±0.94 |
4.346±2.09 |
4.344±1.59 |
4.93±2.01 |
4.55±2.30 |
5.65±2.14 |
| LY# (x103/µl) |
2.25±0.84 |
2.62±0.47 |
2.59±1.06 |
2.752±0.74 |
2.9±1.38 |
2.98±0.41 |
2.45±1.11 |
2.79±0.62 |
| MO# (x103/µl) |
0.36±0.21 |
0.38±0.21 |
0.304±0.14 |
0.392±0.20 |
0.318±0.16 |
0.39±0.12 |
0.43±0.21 |
0.56±0.34 |
| EO# (x103/µl) |
0.14±0.10 |
0.10±0.11 |
0.114±0.15 |
0.072±0.06 |
0.124±0.16 |
0.13±0.12 |
0.06±0.05 |
0.14±0.06 |
| BA# (x103/µl) |
0.008±0.00 |
0.01±0.00 |
0.008±0.01 |
0.014±0.01 |
0.010±0.01 |
0.01±0.00 |
0.01±0.00 |
0.02±0.04 |
| IG# (x103/µl) |
0.026±0.01 |
0.02±0.01 |
0.014±0.01 |
0.018±0.02 |
0.018±0.01 |
0.02±0.02 |
0.02±0.026 |
0.06±0.08 |
| GLU (mg/dL) |
55.6±13.24 |
66.20±9.68 |
82.40±24.30 |
73.40±8.59 |
77.2±17.63 |
77.2±16.36 |
70.4±18.71 |
76.80±19.10 |
| BUN (mg/dL) |
17.88±4.00 |
16.90±2.03 |
16.80±3.32 |
15.74±1.60 |
18.02±5.54 |
14.92±1.78 |
17.52±3.94 |
17.52±1.43 |
| CREA (mg/dL) |
0.78±0.11 |
0.75±0.05 |
0.706±0.15 |
0.73±0.03 |
0.752±0.13 |
0.74±0.06 |
0.75±0.12 |
0.71±0.04 |
| BUN / CREAT |
22.94±5.33 |
22.46±2.10 |
23.86±1.04 |
21.60±2.36 |
23.8±5.39 |
20.12±1.12 |
23.32±3.04 |
24.84±3.70 |
| SOD (mmol/L) |
150.40±1.52 |
151.6±1.52 |
149.20±0.84 |
150.80±1.92 |
148.8±2.17 |
150.2±1.64 |
149.8±1.48 |
150.40±1.82 |
| POT (mmol/L) |
4.38±0.29 |
4.20±0.29 |
4.10±0.39 |
4.34±0.23 |
4.82±1.17 |
4.20±0.20 |
4.36±0.15 |
4.36±0.40 |
| CL(mmol/L) |
107.60±2.70 |
110.4±2.30 |
107.2±2.17 |
110.2±1.92* |
107±3.08 |
109.8±2.17 |
108.4±2.61 |
108.8±1.30 |
| CO2 (mmol/L) |
28.40±1.23 |
27.56±1.46 |
28.24±1.08 |
28.02±1.75 |
27.52±2.76 |
29.04±0.94 |
28.48±1.96 |
29.46±0.67 |
| CAL (mg/dL) |
9.92±0.18 |
9.76±0.29 |
9.78±0.49 |
9.76±0.15 |
9.98±0.25 |
9.70±0.24 |
9.72±0.22 |
9.70±0.19 |
| TPR (mg/dL) |
6.62±0.29 |
6.50±0.49 |
6.46±0.25 |
6.56±0.48 |
6.52±0.27 |
6.44±0.39 |
6.64±0.11 |
6.64±0.52 |
| ALB (x103/µl) |
4.22±0.22 |
4.06±0.29 |
4.10±0.16 |
4.10±0.25 |
4.14±0.17 |
4.08±0.27 |
4.2±0.22 |
4.14±0.29 |
| ALB / Globin |
1.78±0.19 |
1.68±0.08 |
1.76±0.25 |
1.68±0.16 |
1.76±0.18 |
1.76±0.17 |
1.74±0.27 |
1.70±0.35 |
| AST (U/L) |
53.80±25.87 |
46.60±17.95 |
49.20±12.50 |
55.20±10.99 |
68.2±50.94 |
51.20±4.32 |
44.20±12.98 |
81.20±40.81* |
| ALT (U/L) |
36.20±17.25 |
38.20±22.06 |
49.80±12.48 |
47.60±12.48 |
49±9.57 |
44.80±12.77 |
47.40±8.47 |
57.20±19.36 |
| ALP (U/L) |
509±132.07 |
452.80±139.43 |
429.80±113.2 |
424±99.66 |
431.4±112.7 |
412.2±113.2 |
422.0±108 |
400.5±107.86 |
| GLO (g/dL) |
2.40±0.20 |
2.44±0.23 |
2.36±0.25 |
2.46±0.30 |
2.38±0.20 |
2.32±0.22 |
2.44±0.27 |
2.42±0.41 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).