1. Introduction
Pseudomyxoma peritonei (PMP) is a rare syndrome characterized by the buildup of mucin in the peritoneal cavity, often resulting from ruptured appendiceal mucinous neoplasms. While ovarian involvement is common in this condition, it is usually metastatic in nature. Ovarian cystic teratoma is the only tumor of ovarian origin identified as a likely cause of PMP. Although less common, gastrointestinal mucinous adenocarcinomas and urachal cancer have also been identified as potential origins of PMP [
1].
According to the Peritoneal Surface Oncology Group International (PSOGI) classification, Acellular mucin is characterized by an absence of tumor epithelial cells. In contrast, PMP containing neoplastic epithelial cells in the mucin can be classified into three types based on histopathologic features and volume of tumor cells [
2]:
Low-grade mucinous carcinoma peritonei: characterized by low-grade cytology, few mitoses, and scant mucinous tumor epithelium (< 20% of tumor volume).
High-grade mucinous carcinoma peritonei: characterized by the presence of at least one of the following features: high-grade cytology, infiltration of adjacent tissues, invasion of vascular lymphatic vessels or surrounding nerves, cribriform growth, or extensive mucinous tumor epithelium (> 20% of tumor volume).
High-grade mucinous carcinoma peritonei with signet ring cells: characterized by the presence of neoplastic signet ring cells (signet ring cells ≥ 10%).
Furthermore, the Ki-67 proliferation index, has recently been proposed as a tool for stratifying high-grade PMP and predicting prognosis [
3].
While the classification and prognosis of patients with PMP depends on the aforementioned histopathologic features, mucin itself has unique characteristics that warrant further study. Despite the current treatment option of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC), tumor recurrence and progression are frequent, with high mortality rates. Therefore, identifying new therapeutic targets is crucial.
Our research aims to investigate the proteomic and biological characteristics of mucinous material in PMP as well as its metagenomic features (i.e., microbiome). We believe that a fuller understanding of mucin may provide insight into the development and progression of the disease, potentially leading to new treatment options for patients.
2. Materials and Methods
Patients: We obtained mucin samples from patients diagnosed with PMP who underwent surgery at Fundación Jiménez Díaz University Hospital from April 2016 to July 2020. All patients received information on the study and provided written consent to participate. The study protocol was approved by the Ethics Committee for Clinical Research of Fundación Jiménez Díaz University Hospital (PIC 75/2016_FJD). The animal study protocol was approved by the Committee on Animal Ethics and Welfare of the Fundación Jiménez Díaz University Hospital Research Institute (PIC 63/2016_FJD). The study sample comprised nine patients who received surgical cytoreduction combined with HIPEC. In eight cases, the origin of the PMP was a low-grade appendiceal mucinous neoplasm, while in one case a mucinous adenocarcinoma of the colon was the source. Following the postoperative histopathologic study, five of the PMP cases were diagnosed as acellular pseudomyxoma, and four were diagnosed as low-grade peritoneal mucinous carcinoma.
Subsequently, all histopathologic analyses were repeated to detect neoplastic cells in mucin samples. Six consecutive patients were included in the final sample: three with acellular mucin and three with neoplastic cells in the mucin. The multi-step process followed to characterize mucin is described below.
Mucin degradation: The viscosity of the mucinous component in PMP is related to such characteristics of mucin as protein concentration or cellularity (higher cellularity and protein concentration, greater sclerosis) and other external factors related to the microenvironment such as hyperosmolarity, pH <4, or the existence of trefoil factors (soluble peptides secreted by goblet cells of the digestive tract that promote mucin viscosity) [
4]. Mucolytics such as bromelain and N-acetylcysteine can be used to digest soft mucin, which is easily degradable, and hard mucin (
Figure 1), which exhibits greater sclerosis and an increased resistance to degradation [
5].
Soft mucin was digested with a solution consisting of 0.3 mg/ml bromelain and 2% N-acetylcysteine and left to incubate for 90-120 minutes at 37 °C. Hard mucin required a longer incubation time to degrade (≤ 240 minutes).
Proteomic analysis: Following mucin digestion with bromelain and N-acetylcysteine, proteins were extracted using RIPA lysis buffer [Tris-HCl (50 nM), NaCl (150 mM), EDTA (1 mM), Nonidet P-40 (1%), DOC (0.5%), and SDS (80.1%)]. The proteins were then quantified by Coomassie Brilliant blue R-250, running the gel at 100 V for 1.15 h. Finally, 20 yL per well was loaded into a precast gel (Mini-Protean TGx 4-15%, Bio-Rad) using 4× Laemmli sample buffer (Bio-Rad) and 5% β-mercaptoethanol as loading buffer, according to manufacturer recommendations. Subsequently, each membrane was incubated overnight with specific antibodies against the different mucins [MUC 1/Proteintech), 2 (ABCore), 3 (SantaCruz), 5AC (Cloud-Clone Corp), 13 (SantaCruz), and 16 (SantaCruz)] at 4 °C and washed 4× with TTBS under gentle agitation for 15 min; goat anti-mouse secondary antibody (Southern Biotech) was added (MUC 2, 3, 13, and 16) as well as goat anti-rabbit (Southern Biotech) (MUC 1, 5AC), incubating for 1 h at room temperature under agitation. The membranes were washed with TTBS for 15 minutes and analyzed in an iBright system (Thermo Fisher).
Microbiome analysis: The prokaryotic 16S ribosomal RNA (rRNA) gene is frequently used in metagenomic surveys of microbial populations due to its conserved and variable regions, which facilitate sequencing and phylogenetic classification. The microbiota in human and mouse biospecimens can be effectively studied through targeted amplification of bacterial 16S rRNA genes [
6]. To identify bacteria present in cellular and acellular mucin specimens, we performed 16S gene sequencing on the DNA extracted from these samples. Subsequently, we inoculated the mucin samples into both immunocompetent and immunocompromised mice to study the behavior of the microbiome in these experimental models.
Generation of 16S amplicons and amplicon sequencing was performed using the Illumina Miseq platform in the Genomics Unit of the Madrid Scientific Park. An initial PCR was performed with the Q5® Hot Start High-Fidelity DNA Polymerase enzyme (New England Biolabs) using 300 pg of DNA. The primers used amplify the V3-V4 region of 16S and add extra sequences on which the second PCR is performed: 5'-ACACTGACGACATGGTTCTACA CCTACGGGNGGCWGCAG-3' and 5'- TACGGTAGCAGAGACTTGGTCTGACTACHVGG GTATCTAAT CC-3'. Cycling of the first PCR was performed as follows: 1 × 98 °C 30 sec; 23 × (98 °C 10 sec, 50 °C 20 sec, 72 °C 20 sec); and 1 × 72 °C 2 min.
We performed a second PCR on the amplification products of the first PCR using the Q5® Hot Start High-Fidelity DNA Polymerase enzyme, with the following primers (5'-AATGATACGGCGACCACCGA GATCTACACTGACGACATGGTTCTACA-3' and 5'-CAAGCAGAAGACGGCATACGAGAT - [10 nucleotides] -TACGGTAGCAGAGACTTGGTCT-3 ') from Fluidigm (Illumina Sequencers). Cycling of this PCR was as follows: 1 × 98 °C 30 sec; 14 × (98 °C 10 sec, 60 °C 20 sec, 72 °C 20 sec); and 1 × 72 °C 2 min.
The final products were quantified by Bioanalyzer (Agilent) to prepare an equimolecular pool that was subsequently purified by selecting the band of interest in an agarose gel with SYBR Gold (Thermo Fisher). After the pool of amplicons was quantified by qPCR using the Kapa SYBR FAST qPCR kit for Light Cycler 480 master mix and a reference library belonging to the Genomics Unit of the Madrid Scientific Park.
Finally, the pool of amplicons was sequenced with the Illumina Miseq platform following the manufacturer's instructions, in a paired-end (2×300 bp) sequencing run using MiSeq reagent kit v3 (600 cycles).
3. Results
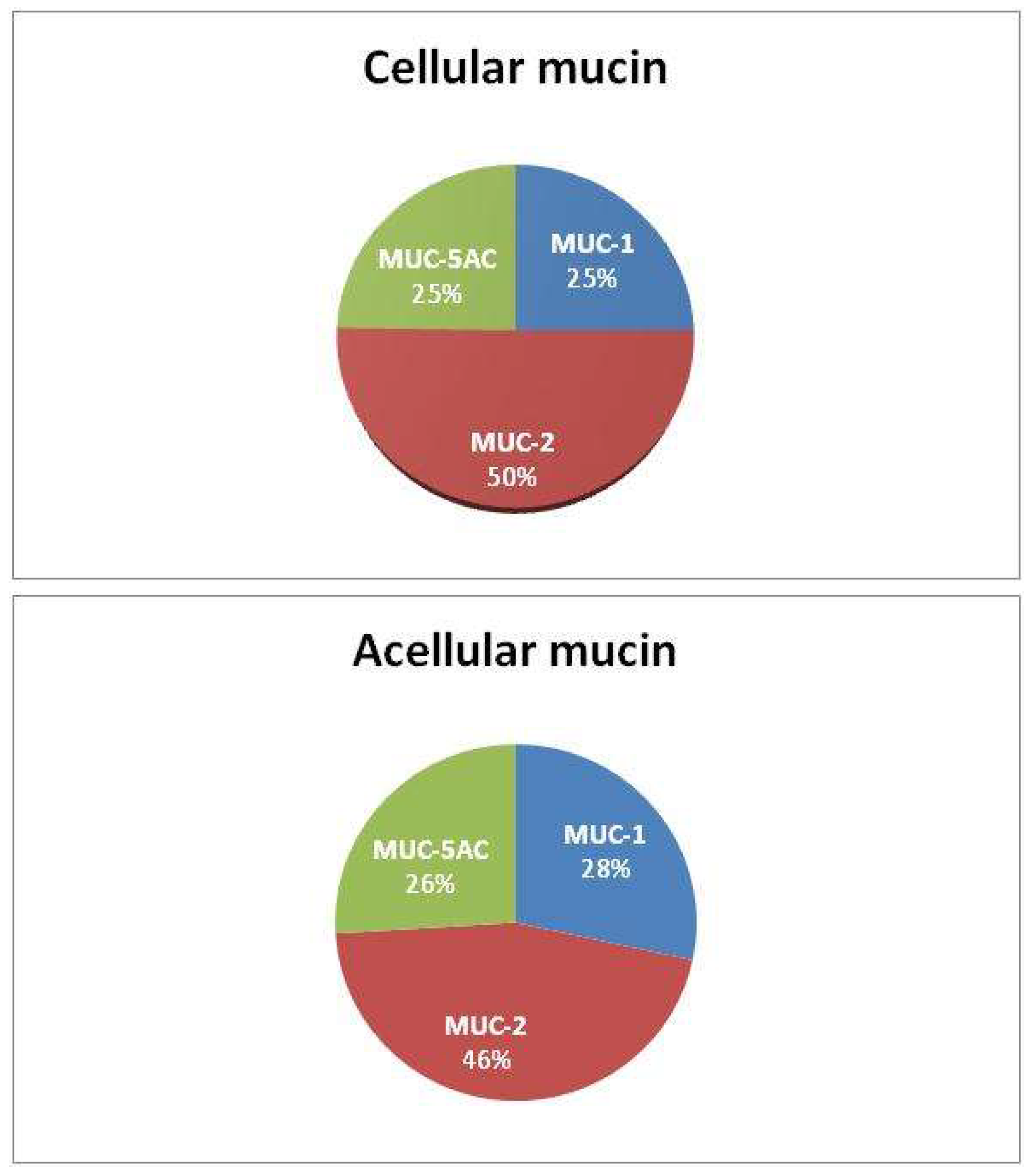
Proteomic analysis revealed the presence of secretory mucins MUC-2 and MUC-5AC, as well as the membrane mucin MUC-1 in all samples analyzed, predominantly MUC-2 (
Table 1). We further detected the MUC-1 protein in mucin for the first time (MUC-1 overexpression was previously described only in tumor tissue). No other mucin types were identified, and there were no significant differences in the composition or distribution of mucin types between acellular and cellular samples (
Figure 2).
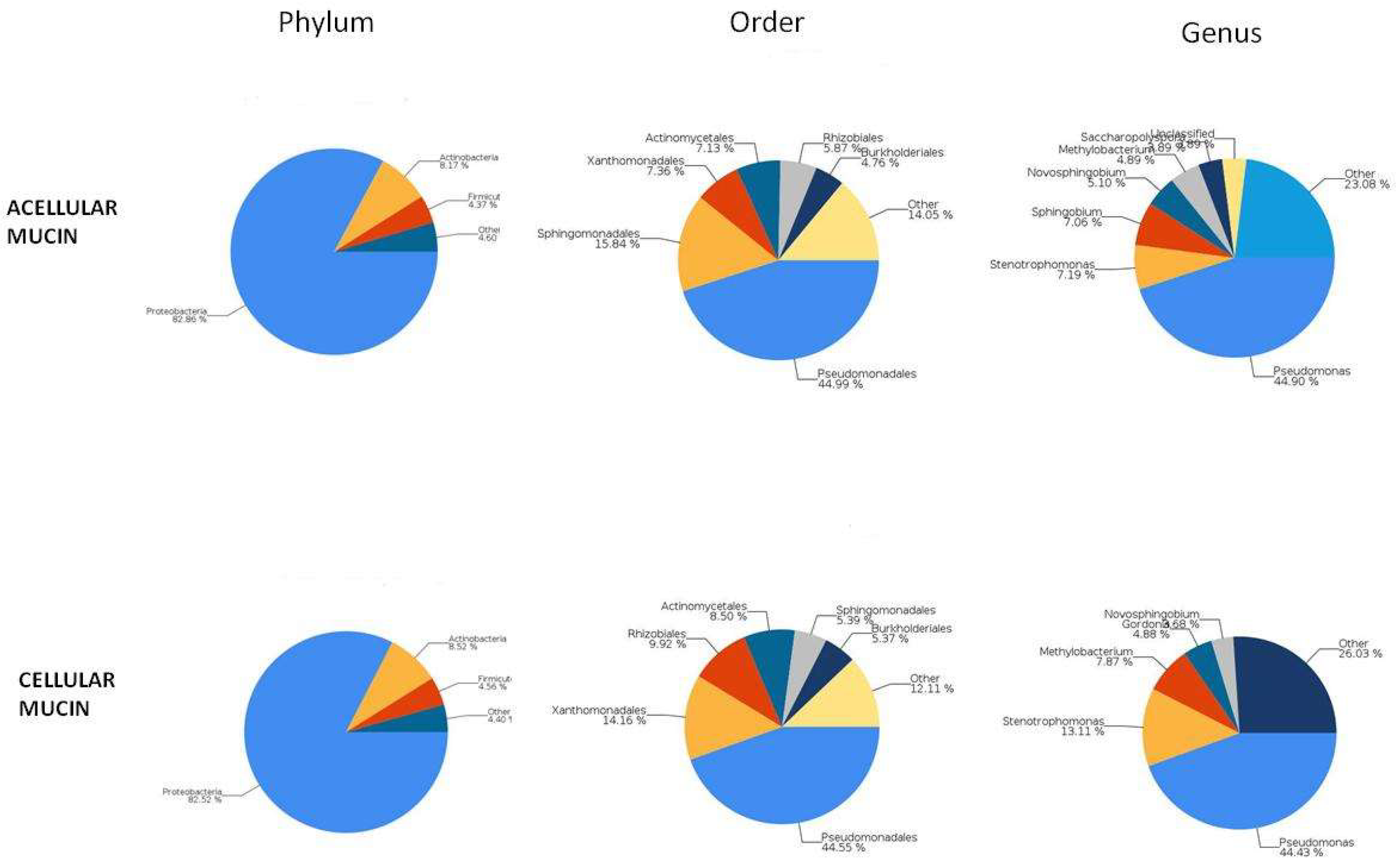
To examine the microbiota in mucin samples, we conducted 16S sequencing and identified different bacterial taxa. The most frequently detected phylum was genomic DNA from Proteobacteria in both acellular (82.86%) and cellular (82.52%) mucin, followed by Actinobacteria (8.17% and 8.52%, respectively). The most common bacterial order was Pseudomonadales, comprising 44.99% of the microbiome in acellular mucin and 44.55% in cellular mucin. The predominant genus was Pseudomonas, accounting for about 45% of the germs detected in both mucin groups (
Figure 3).
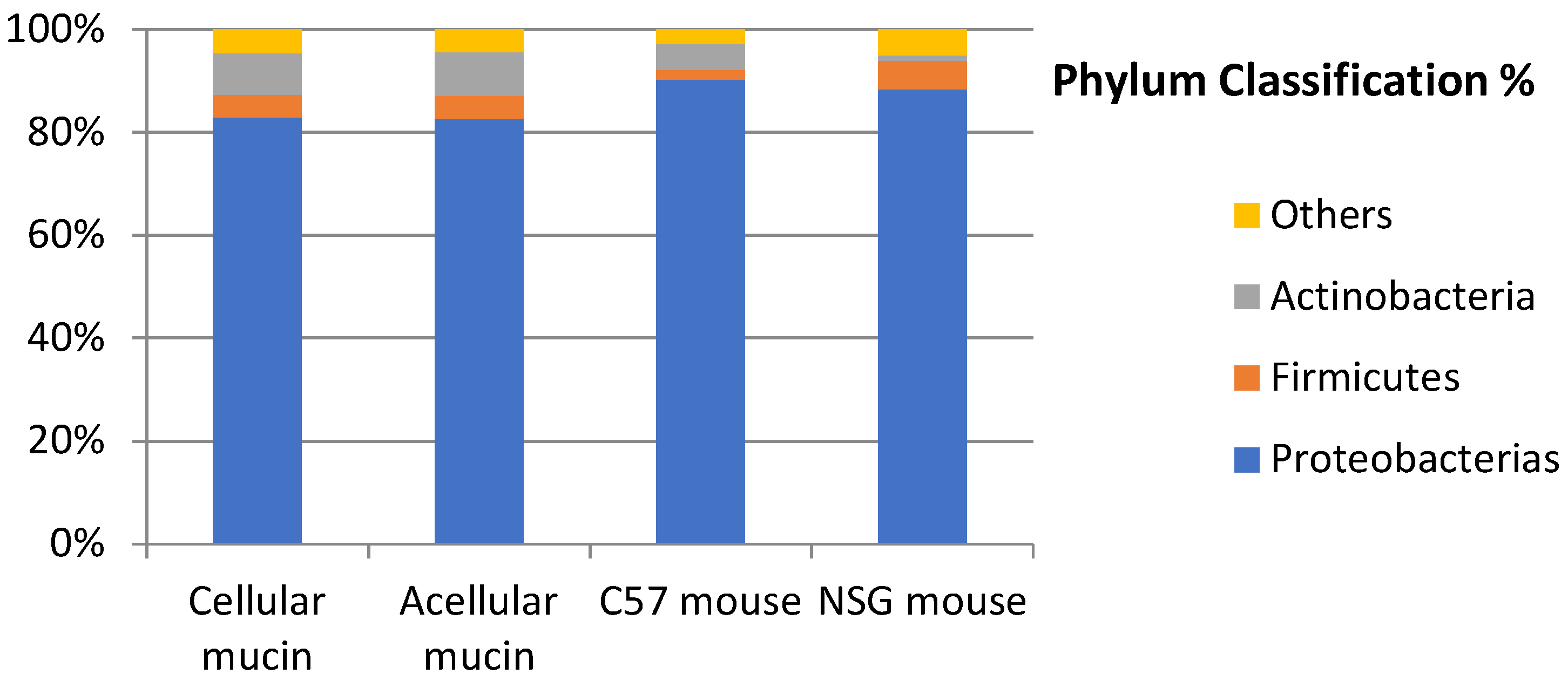
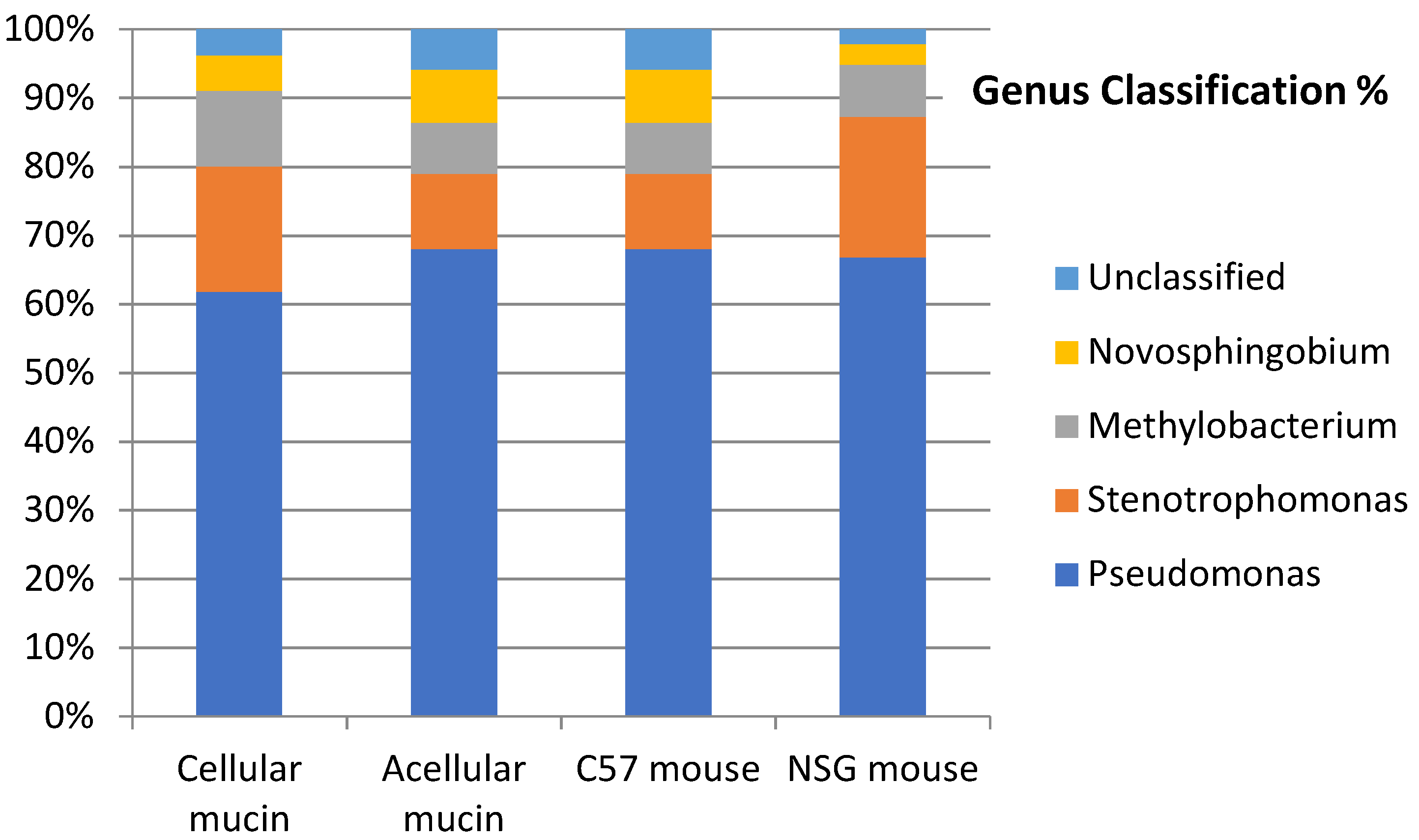
Interestingly, the microbiota of patients with PMP and acellular mucin was almost identical to that of patients with cellular mucin. This suggests that germ colonization of accumulated mucus in PMP, primarily from Pseudomonas, is a specific feature of mucin independent of the patient and tumor histopathology. To confirm this hypothesis, we inoculated mucin samples into immunocompetent (C57) and immunosuppressed (NSG) mice and found that the microbiota was maintained regardless of host species and immune status (
Figure 4).
Pseudomonas plecoglossicida was the most frequently identified bacterial species among all the samples analyzed, representing 11-21% of the total bacterial population.
4. Discussion
Overexpression of genes encoding different proteins of the mucin family has been described in the primary and metastatic tumor tissues of PMP. These proteins include mucin-2 (MUC-2), mucin-5AC (MUC-5AC), mucin-5B (MUC-5B), mucin-4 (MUC-4), and mucin-1 (MUC-1). The MUC-2, MUC-5AC, and MUC-5B proteins are secreted gel-forming mucins, while the MUC-1 and MUC-4 proteins are membrane-associated mucins. While there is extensive research on overexpression of mucin in tumor tissues, investigations focusing on mucin itself is considerably more limited (See
Table 2 for an overview).
No reports to date have described the protein composition of acellular mucin in PMP. Our results show that the mucus in acellular mucin has a makeup that resembles the mucin of other types of PMP.
Secreted MUC-2 and MUC-5AC are the main components of mucus in PMP. MUC-5AC is expressed in the goblet cells of the gastrointestinal and respiratory epithelium as well as ovarian epithelial cells, whereas MUC-2 expression is specific to goblet cells of the intestinal epithelium [
7]. MUC-2 is the only protein that has been consistently described in studies involving PMP, both those performed directly on the protein composition of mucin as well as research using tumor tissues. This pattern of mucin expression explains the appendicular origin of most cases of PMP. MUC-2 is characterized by extensive glycosylation and has been associated with mucus sclerosis and even with patient prognosis in PMP [
4].
With respect to the microbiome of patients with PMP, it must be noted that the peritoneal cavity is an aseptic anatomical region. Therefore, bacterial contamination of mucin should originate from the intestine, secondary to perforation of appendicular mucinous neoplasms. However, the microbiota found in our study is not native to the gastrointestinal tract.
Gilbreath et al. [
15] were the first to describe the microbiota associated with PMP and to suggest its potential impact on the pathogenesis of this disease. Different methods have been used to study the microbiota of PMP, such as cultures, in situ hybridization
, and 16S sequencing. The dominant phylum described is Proteobacteria, with a predominance of the Pseudomonas genus [
16], which coincides with our results.
No previous studies have specifically characterized the microbiota of acellular mucin. The results of the present analysis reveal that it has microbiota that closely resembles that of other types of PMP. Although the presence of a microbiome having these features is not characteristic of the gastrointestinal tract, it is frequently found in the mucin within the respiratory epithelium of patients with cystic fibrosis disease. Therefore, the existence of abundant mucin and a predominance of Pseudomonas in the microbiome are common to PMP and cystic fibrosis. Another shared finding between these two conditions is MUC-2 overexpression, despite the fact that MUC-2 expression is specific to goblet cells of the intestinal epithelium and is not present in the respiratory epithelium under normal conditions [
17]. An association between mucus hyperproduction and infection by the genus Pseudomonas has been described, as well as a direct relationship between the overexpression of MUC-2 and MUC-5AC and the lipopolysaccharides of this bacterial family [
18,
19].
Previous studies by our group demonstrated that inoculating acellular mucin in an experimental murine model could be used to reproduce acellular PMP [
20]. With these findings in mind, we set out to explore the mechanism by which tumor cell-free mucin can reproduce and grow. Based on the results of the present research, and taking into account the results of Dohrman A et al [
18] and Ben Mohamed F et al [
19], we can hypothesize that mucin overproduction may be related to colonization by bacteria belonging to the Pseudomonas family. To advance our understanding of PMP and improve patient outcomes, future research should investigate the relationship between the mucin microbiome and the aggressiveness and prognosis of the disease.
Findings from this and other research indicate that the microbiota may be a new therapeutic target. Preliminary results from studies that added antibiotics to the standard treatment approach for PMP (i.e., cytoreduction and HIPEC) were inconclusive, although a phase II clinical trial (NCT 02387203) is currently underway to analyze the long-term results of antibiotic administration in patients with PMP [
16,
21]
.
No previous publications have described Pseudomonas plecoglossicida as the most abundant Pseudomonas species in the mucin of PMP. This bacterium has been identified as the cause of hemorrhagic ascites in ayu fish [
22], although it has never been found in the human microbiome.
5. Conclusions
Sufficient evidence points to a direct relationship between the dominant microbiome of the pseudomyxoma and the production of mucus in PMP.
Author Contributions
Conceptualization: DGO, MGA, and PVC. Methodology: DGO, MGA. HGL and PVC. Software: SQ.Validation: MGA. Investigation: DGO, MGA, HGL. Resources: SJG, IG, MJF, SQ. Data curation: VDP, JFVP. Writing—original draft preparation: PVC, MGA, DGO. Writing—review and editing: All authors. Funding acquisition: PVC, SJG. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by grants from the ISCIII-FEDER (Instituto de Salud Carlos III-Fondo Europeo de Desarrollo Regional), Spanish Ministry of Health (grant number PI20/01052 and DTS22/00048).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee for Clinical Research of Fundación Jiménez Díaz University Hospital (PIC 75/2016_FJD, 14/2/2017). The animal study protocol was approved by the Committee on Animal Ethics and Welfare of Research Institute-Fundación Jiménez Díaz University Hospital (PIC 63/2016_FJD, 31/5/2016).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, [PVC], upon reasonable request.
Acknowledgments
The authors thank Oliver Shaw for revising the manuscript for aspects related to the English language and Dr. C. Robledo Montero for proteomics studies.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Morera-Ocon, F.J.; Navarro-Campoy, C. History of pseudomyxoma peritonei from its origin to the first decades of the twenty-first century. World J Gastrointest Surg. 2019, 11, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Govaerts, K.; Lurvink, R.J.; De Hingh, I.H.J.T.; Van der Speeten, K.; Villeneuve, L.; Kusamura, S.; Kepenekian, V.; Deraco, M.; Glehen, O.; Moran, B.J.; PSOGI. Appendiceal tumours and pseudomyxoma peritonei: Literature review with PSOGI/EURACAN clinical practice guidelines for diagnosis and treatment. Eur J Surg Oncol. 2021, 47, 11–35. [Google Scholar] [CrossRef] [PubMed]
- Arjona-Sánchez, Á.; Martínez-López, A.; Valenzuela-Molina, F.; Rufián-Andújar, B.; Rufián-Peña, S.; Casado-Adam, Á.; Sánchez-Hidalgo, J.M.; Rodríguez-Ortiz, L.; Medina-Fernández, F.J.; Díaz-López, C.; Granados-Rodríguez, M.; Ortega-Salas, R.; Castaño, J.P.; Tena-Sempere, M.; Briceño-Delgado, J.; Romero-Ruíz, A. A Proposal for Modification of the PSOGI Classification According to the Ki-67 Proliferation Index in Pseudomyxoma Peritonei. Ann Surg Oncol. 2022, 29, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.L.; Li, Y. The Biological Synthesis and the Function of Mucin 2 in Pseudomyxoma Peritonei. Cancer Manag Res. 2021, 13, 7909–7917. [Google Scholar] [CrossRef] [PubMed]
- Pillai, K.; Akhter, J.; Mekkawy, A.; Chua, T.C.; Morris, D.L. Physical and chemical characteristics of mucin secreted by pseudomyxoma peritonei (PMP). Int J Med Sci. 2017, 14, 18–28. [Google Scholar] [CrossRef]
- Tong, M.; Jacobs, J.P.; McHardy, I.H.; Braun, J. Sampling of intestinal microbiota and targeted amplification of bacterial 16S rRNA genes for microbial ecologic analysis. Curr Protoc Immunol. 2014, 107, 7–41. [Google Scholar] [CrossRef]
- O'Connell, J.T.; Tomlinson, J.S.; Roberts, A.A.; McGonigle, K.F.; Barsky, S.H. Pseudomyxoma peritonei is a disease of MUC2-expressing goblet cells. Am J Pathol. 2002, 161, 551–64. [Google Scholar] [CrossRef]
- Mohamed, F.; Gething, S.; Haiba, M.; Brun, E.A.; Sugarbaker, P.H. Clinically aggressive pseudomyxoma peritonei: a variant of a histologically indolent process. J Surg Oncol. 2004, 86, 10–5. [Google Scholar] [CrossRef]
- Nonaka D, Kusamura S, Baratti D, Casali P, Younan R, Deraco M. CDX-2 expression in pseudomyxoma peritonei: a clinicopathological study of 42 cases. Histopathology. 2006, 49, 381–7. [Google Scholar] [CrossRef]
- Mall AS, Chirwa N, Govender D, Lotz Z, Tyler M, Rodrigues J, Kahn D, Goldberg P. MUC2, MUC5AC and MUC5B in the mucus of a patient with pseudomyxoma peritonei: biochemical and immunohistochemical study. Pathol Int. 2007, 57, 537–47. [Google Scholar] [CrossRef]
- Ferreira, C.R.; Carvalho, J.P.; Soares, F.A.; Siqueira, S.A.; Carvalho, F.M. Mucinous ovarian tumors associated with pseudomyxoma peritonei of adenomucinosis type: immunohistochemical evidence that they are secondary tumors. Int J Gynecol Cancer. 2008, 18, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Baratti, D.; Kusamura, S.; Nonaka, D.; Cabras, A.D.; Laterza, B.; Deraco, M. Pseudomyxoma peritonei: biological features are the dominant prognostic determinants after complete cytoreduction and hyperthermic intraperitoneal chemotherapy. Ann Surg. 2009, 249, 243–9. [Google Scholar] [CrossRef] [PubMed]
- Guo, A.T.; Song, X.; Wei, L.X.; Zhao, P. Histological origin of pseudomyxoma peritonei in Chinese women: clinicopathology and immunohistochemistry. World J Gastroenterol. 2011, 17, 3531–7. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.S.; Byeon, S.J.; Yoon, S.O.; Kim, B.H.; Lee, H.S.; Kang, G.H.; Kim, W.H.; Park, K.J. Leptin, MUC2 and mTOR in appendiceal mucinous neoplasms. Pathobiology. 2012, 79, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Gilbreath, J.J.; Semino-Mora, C.; Friedline, C.J.; Liu, H.; Bodi, K.L.; McAvoy, T.J.; Francis, J.; Nieroda, C.; Sardi, A.; Dubois, A.; Lazinski, D.W.; Camilli, A.; Testerman, T.L.; Merrell, D.S. A core microbiome associated with the peritoneal tumors of pseudomyxoma peritonei. Orphanet J Rare Dis. 2013, 8, 105. [Google Scholar] [CrossRef]
- Khamzina, Y.; King, M.C.; Nieroda, C.; Merrell, D.S.; Sardi, A.; Gushchin, V. The Role of Microorganisms in Appendiceal Pseudomyxoma Peritonei: A Review. Curr Oncol. 2022, 29, 3576–3584. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; Olsson, I.; Edlund, K.; Lundberg, E.; Navani, S.; Szigyarto, C.A.; Odeberg, J.; Djureinovic, D.; Takanen, J.O.; Hober, S.; Alm, T.; Edqvist, P.H.; Berling, H.; Tegel, H.; Mulder, J.; Rockberg, J.; Nilsson, P.; Schwenk, J.M.; Hamsten, M.; von Feilitzen, K.; Forsberg, M.; Persson, L.; Johansson, F.; Zwahlen, M.; von Heijne, G.; Nielsen, J.; Pontén, F. Proteomics. Tissue-based map of the human proteome. Science. 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Dohrman, A.; Miyata, S.; Gallup, M.; Li, J.D.; Chapelin, C.; Coste, A.; Escudier, E.; Nadel, J.; Basbaum, C. Mucin gene (MUC 2 and MUC 5AC) upregulation by Gram-positive and Gram-negative bacteria. Biochim Biophys Acta. 1998, 1406, 251–9. [Google Scholar] [CrossRef]
- Ben Mohamed, F.; Garcia-Verdugo, I.; Medina, M.; Balloy, V.; Chignard, M.; Ramphal, R.; Touqui, L. A crucial role of Flagellin in the induction of airway mucus production by Pseudomonas aeruginosa. PLoS One. 2012, 7, e39888, Erratum in: PLoS One. 2012, 7. [Google Scholar] [CrossRef]
- García-Olmo, D.; Olmedillas-López, S.; Cortés-Guiral, D.; Villarejo, P.; López Rojo, I.; Guadalajara, H.; García Gómez-Heras, S.; García-Arranz, M. The role of mucin cell-free DNA detection as a new marker for the study of acellular pseudomyxoma peritonei of appendicular origin by liquid biopsy. Ther Adv Med Oncol. 2020, 12, 1758835920928233. [Google Scholar] [CrossRef]
- Semino-Mora, C.; Testerman, T.L.; Liu, H.; Whitmire, J.M.; Studeman, K.; Jia, Y.; McAvoy, T.J.; Francis, J.; Nieroda, C.; Sardi, A.; Merrell, D.S.; Dubois, A. Antibiotic treatment decreases microbial burden associated with pseudomyxoma peritonei and affects β-catenin distribution. Clin Cancer Res. 2013, 19, 3966–76. [Google Scholar] [CrossRef] [PubMed]
- Park, S.C.; Shimamura, I.; Fukunaga, M.; Mori, K.I.; Nakai, T. Isolation of bacteriophages specific to a fish pathogen, Pseudomonas plecoglossicida, as a candidate for disease control. Appl Environ Microbiol. 2000, 66, 1416–22. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).