2. Neo-Darwinism
With the emergence of Mendel’s ideas on genetics, the orthogenesis framework gave way to the modern era of biology and the Neo-Darwinian era (
Figure 1 and
Figure 2; Agosta, 2020). Besides Mendel, proponents of Neo-Darwinism were significantly influenced by the ideas of Herbert Spencer, a sociologist with strong Lamarckian tendencies (later discussed in Paul, 1988) who opposed Darwin’s original “survival of the adequate” (
Figure 2), replacing it with the famous “survival of the fittest” evolutionary perspective. Spencer’s apparently subtle change to the jargon of natural selection stiffened the evolutionary process, shifting what was characterized as a sloppy restriction of diversity to what would later be defined as the Hardened Synthesis of Neo-Darwinism (Agosta, 2020).
Darwin’s original evolutionary theory describes a dynamic where the nature of the organism is the driving force behind diversity, one which balanced with and was limited by the conditions of the environment, or conditions of existence providing counterweights and limitations (
Figure 1 and
Figure 2). Neo-Darwinian thought, on the other hand, limited diversity to a reality of maximum efficiency, envisioning the diversity of character states as a transitory condition to be replaced by the most efficient trait. Observable phenotypes were always associated with a positive or negative fitness contribution; a character could not simply be neutral or adequate [Spencer, 1852].
In terms of coevolution, Neo-Darwinism describes symbiont evolution as a 1:1 relationship in a continuous process of innovation, selection, and speciation. It envisions a scenario in which parasites become increasingly dependent on their host while the host adapts defenses against them. Orthogenesis and Neo-Darwinism, then, appear to draw similar pictures of coevolution.
Neo-Darwinism was built under the pretense of uniting Mendelian and modern biology and received contributions from authors such as Kellogg, Fahrenholz, and Eichler, whose ideas on coevolution will now be succinctly introduced.
2.1. Kellogg
Kellogg was one of the most influential proponents of the vicariant model of speciation, proposing that geographical isolation followed by differential selection on the isolated populations was a necessary precursor for divergent lineages. This classical vicariant model relies on the interruption of gene flow between subpopulations and selection that promotes character differentiation to the point of speciation (Kellog, 1907).
Kellogg posited that host-parasite interactions were characterized by two main aspects: (1) parasites are highly attuned to their host species; (2) the parasite’s environment is so uniform (host) that it becomes isolated from external selection forces, that is, the geographical isolation of hosts does not affect their parasites’ populations. Parasite evolution, then, should be buffered by their hosts’ uniformity.
With these two assumptions, Kellog then recognized three types of host-parasite interactions: 1) Sister parasite species occurring in sister host species in disjunct regions, the commonness of genealogy scenario. 2) A single parasite species occurring in sister host species that are in disjunct regions. In this case, the isolation of both symbionts would lead to the speciation of the host but not of the parasite due to a buffer-like effect of the host on the evolution of parasites – the presumed uniformity of environment provided by the host would block parasite selection.
The third type of host-parasite interaction describes parasite species associated with two or more phylogenetically distant host species that occur in the same geographical region. Such a scenario is not contemplated in orthogenesis models, and represents an important case in geographical isolation studies.
In order to address the third scenario that bothered the general view of Neo-Darwinian parasite evolution, Kellogg addresses three possible factors that could enable or disable the generalist behavior observed in such cases: (1) co-occurrence of hosts; (2) differential speciation of hosts and parasites and (3) inherent incapacity to expand in host use. These hypotheses were not explored any further, but Kellogg’s idea of the right adaptation for each environment remained a key component of the Neo-Darwinian school of thought.
2.2. Fahrenholz
Fahrenholz also made important contributions to the Neo-Darwinian conception of host-parasite interactions, proposing that the presence of parasites could be used as a tool to infer hosts’ phylogenetic relatedness. The presence of the same or closely related parasite species in different host groups should allow researchers to infer the relatedness of different host groups regardless of their geographical context. Parasites are assumed to be as dependent on their hosts as free-living organisms are on their environment. Under this view, members of the same host species would be utilized by individuals of the same parasite species, while members of different species would be utilized by parasites whose phylogenetic relationship reflected the hosts’, including the degree of divergence between them [Hopkins, 1942].
According to Fahrenholz’s rule [Brooks, 2019; Eichler, 1948, 1966], if parasites produce a new species in response to their environment – as is the case for every biological system – and the host is its environment, they must follow the new host species after a speciation event, and the parasite’s phylogeny must reflect the host’s. The author does not address Kellogg’s third scenario of multiple hosts for a parasite species, holding out cospeciation as the most satisfactory explanation for parasite evolution.
2.3. Eichler
Eichler agrees with the cospeciation view suggested by Fahrenholz’s rule (Eichler, 1948), presenting the so-called Divergence rule, where one should expect – based on the diversity of parasites occurring in a single host – that host species that are more phylogenetically isolated should carry fewer parasites, while when finding a great number of parasite groups in one host species, one can expect that the host group it belongs to is also great in diversity (Eichler, 1948). The evidence of asymmetry between host and parasite lineages is simply overlooked, since Eichler’s main aim was to use parasites as a tool to study host evolution.
Eichler also discusses the continuous specialization of parasites to hosts, going as far as discussing Szidat’s rule – basal parasite lineages utilize basal host groups, while diversified parasites should inhabit diversified hosts (Eichler, 1948). Neo-Darwinian thought kept the cospeciation perspective of symbiont evolution, incorporating all the previously mentioned ideas.
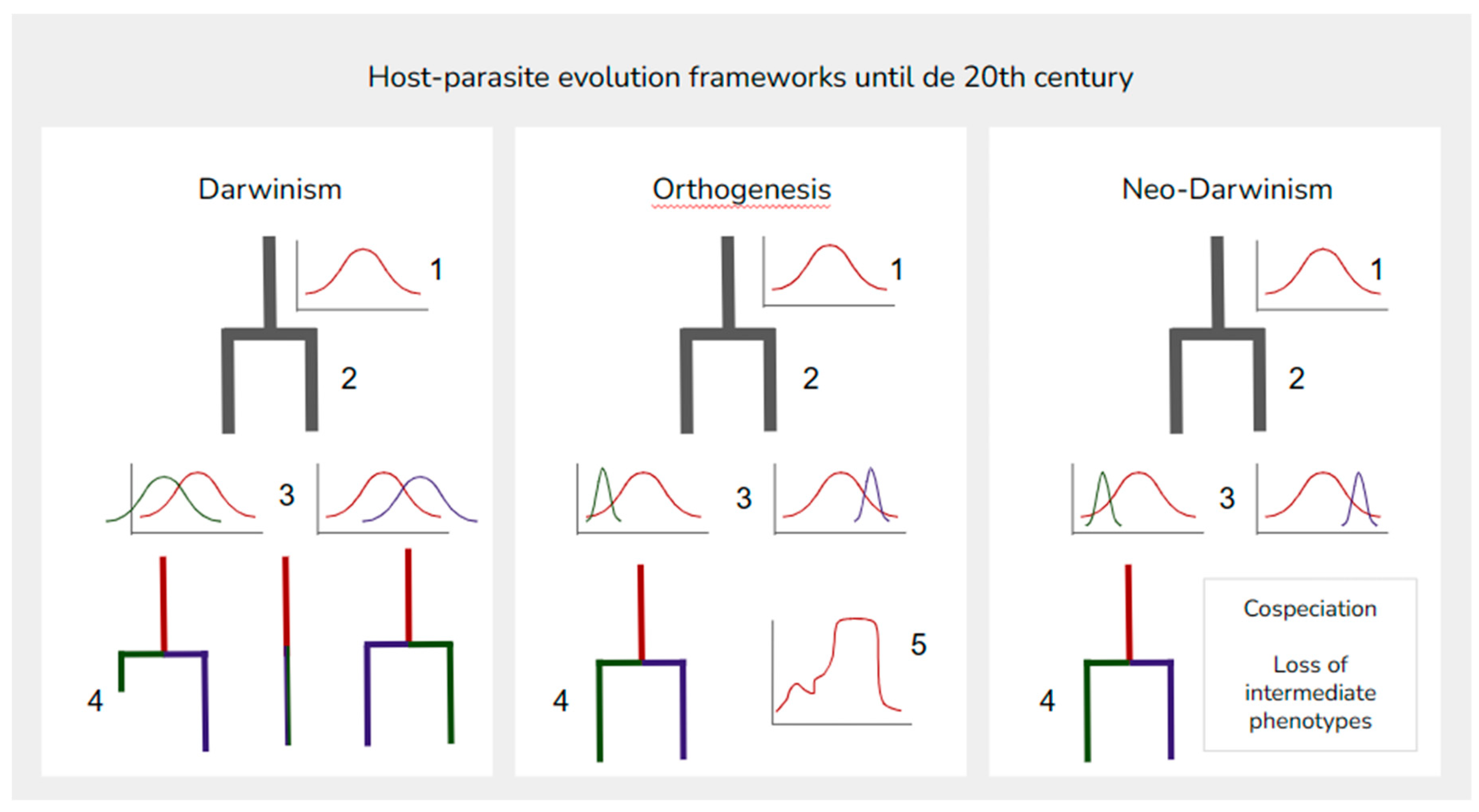
Figure 2. Graphic representation of pre-21st century theory where 1 represents the general distributions of an ancestral population’s phenotypes, where the y-axis depicts the frequency of a phenotype whereas the x-axis describes the phenotypic value of an organism; 2 schematic representations of a host’s lineage before and after a speciation event; 3 schematic representation of the parasite’s phenotypic distributions after the speciation event of the host. In Darwinism, speciation could lead to a differentiation in the phenotypic distribution of the parasite population, which adjusts toward the optimum phenotype imposed by the host; in orthogenesis and Neo-Darwinism, the speciation of the host should result in rapid differentiation of the parasite population, with an acute change in phenotype distribution. 4 The phenotypic differentiation associated with host change — in the Darwinian view — could lead to the speciation of the parasite, but not inevitably so; according to orthogenesis, the acute change in parasite’s phenotypic distribution should lead to a rapid specialization of parasites, usually accompanied by loss of structures or complexity, and with that, speciation. This pattern is expected by the Neo-Darwinian perspective, where the phenotypic specialization would lead to the loss of phenotypes with sub-optimal fitness to host’s selection. 5 Simple representation of the self-imposed extinction described by orthogenesis. In this case, the change in the host’s natural selection pattern would lead to a fluctuation in the population size, with an initial oscillation with positive growth tendencies (selection of specialist parasite), followed by a period of maximum population size (optimum exploitation of resource), and a final decrease in population size, where the parasites become so specialized to that specific selection pressure that any change would lead to inaptitude to access the resources provided by the host, leading to parasite extinction.
Figure 2.
Graphic representation of pre-21st century theory where 1 represents the general distributions of an ancestral population’s phenotypes, where the y-axis depicts the frequency of a phenotype whereas the x-axis describes the phenotypic value of an organism; 2 schematic representations of a host’s lineage before and after a speciation event; 3 schematic representation of the parasite’s phenotypic distributions after the speciation event of the host. In Darwinism, speciation could lead to a differentiation in the phenotypic distribution of the parasite population, which adjusts toward the optimum phenotype imposed by the host; in orthogenesis and Neo-Darwinism, the speciation of the host should result in rapid differentiation of the parasite population, with an acute change in phenotype distribution. 4 The phenotypic differentiation associated with host change — in the Darwinian view — could lead to the speciation of the parasite, but not inevitably so; according to orthogenesis, the acute change in parasite’s phenotypic distribution should lead to a rapid specialization of parasites, usually accompanied by loss of structures or complexity, and with that, speciation. This pattern is expected by the Neo-Darwinian perspective, where the phenotypic specialization would lead to the loss of phenotypes with sub-optimal fitness to host’s selection. 5 Simple representation of the self-imposed extinction described by orthogenesis. In this case, the change in the host’s natural selection pattern would lead to a fluctuation in the population size, with an initial oscillation with positive growth tendencies (selection of specialist parasite), followed by a period of maximum population size (optimum exploitation of resource), and a final decrease in population size, where the parasites become so specialized to that specific selection pressure that any change would lead to inaptitude to access the resources provided by the host, leading to parasite extinction.
Figure 2.
Graphic representation of pre-21st century theory where 1 represents the general distributions of an ancestral population’s phenotypes, where the y-axis depicts the frequency of a phenotype whereas the x-axis describes the phenotypic value of an organism; 2 schematic representations of a host’s lineage before and after a speciation event; 3 schematic representation of the parasite’s phenotypic distributions after the speciation event of the host. In Darwinism, speciation could lead to a differentiation in the phenotypic distribution of the parasite population, which adjusts toward the optimum phenotype imposed by the host; in orthogenesis and Neo-Darwinism, the speciation of the host should result in rapid differentiation of the parasite population, with an acute change in phenotype distribution. 4 The phenotypic differentiation associated with host change — in the Darwinian view — could lead to the speciation of the parasite, but not inevitably so; according to orthogenesis, the acute change in parasite’s phenotypic distribution should lead to a rapid specialization of parasites, usually accompanied by loss of structures or complexity, and with that, speciation. This pattern is expected by the Neo-Darwinian perspective, where the phenotypic specialization would lead to the loss of phenotypes with sub-optimal fitness to host’s selection. 5 Simple representation of the self-imposed extinction described by orthogenesis. In this case, the change in the host’s natural selection pattern would lead to a fluctuation in the population size, with an initial oscillation with positive growth tendencies (selection of specialist parasite), followed by a period of maximum population size (optimum exploitation of resource), and a final decrease in population size, where the parasites become so specialized to that specific selection pressure that any change would lead to inaptitude to access the resources provided by the host, leading to parasite extinction.
5. Modern Evolutionary Theory – Post Neo-Darwinism – later 20th-century evolutionary theory
5.1. Charles Mode
Host-parasite coevolution, in the sense that both host and parasite influence each other’s evolutionary path, was first introduced by Charles Mode (Mode, 1958), representing the first proposal of symbiont evolution that diverges from Neo-Darwinian cospeciation. Although Mode’s work continues to frame the process as dual evolution, disregarding the complexity of interactions, it is one of the first studies to actually address the parasite’s influence on host selection instead of envisioning parasites as playing a passive role in symbiont evolution.
5.2. Ehrlich and Raven
Ehrlich and Raven went on to argue that plant-insect diversification must be driven by a complex interaction between both lineages, where both parts of the association would be influenced by the symbiosis that, in time, could leave phylogenetic marks, though not necessarily in a mirrored manner. The authors do not explore in detail the evolutionary implications of coevolution in the final topology of the lineages, focusing instead, on the flexibility of coevolution and diverging from the general Neo-Darwinian view (Ehrich & Raven, 1964).
By exploring the diversification of butterflies and their plant groups, Ehrlich and Raven structured a series of questions on coevolutionary thinking, with the first authors in community evolution who express discomfort in their current perspective of coevolution — to assume a state of neutrality for one of the symbionts in diversification (Ehrlich & Raven, 1964). In their perspective, coevolution should involve studying groups of organisms with a close ecological relationship instead of the traditional 1:1 exploration of lineages. Although initially simple, this perspective enables the understanding of complexity in coevolution. Instead of a strict symbiosis, it embraces events such as host-switching and generalization (i.e., host-repertoire expansion) (Braga, 2020) and an escape from ideas like co-extinction in community evolution.
By the 1960s, the coevolutionary perspective described by Ehrlich and Raven gained supporters as they evidenced more “mistakes” in symbiont utilization, both in host-parasite interactions and in plant-insect investigations (Ehrlich, 1948; Ehrlich & Raven,1964, Verschaffelt, 1910). The new perspective was accompanied by the essential works of Bigelow and Hennig (Bigelow 1956), who enabled community evolution to investigate the phylogenetic history of symbionts, which had lacked method and robustness to that point (Hennig, 1965).
By the end of the twentieth century, evolutionary biology stopped searching for deterministic rules in diversification and coevolution and began looking for general evolution guidelines. Mode’s and Ehrlich and Raven’s ideas, therefore, took a more central stage in coevolutionary thinking due to their less strict views on diversification and selection. This scenario was later met with discussions on climate change, general biological reassembly, and the emergent diseases crisis, reported by authors such as Elton (1958) and Carson (1962).
5.3. Stockholm Paradigm
As opposed to the Extended Synthesis, the Stockholm Paradigm aims to recover Darwinian ideas in order to discuss evolution, claiming that the historical construction of Neo-Darwinism misses essential information from Darwin’s original proposal for the study of the diversification of life (Brooks, 2019). The authors discuss coevolution as a process that entails the synergy of capacity and opportunity in determining biological interactions. They define capacity as the general ability of an organism (whether free-living or symbionts) to utilize resources available to it through the Information Space comprised of inherited information (genetic, epigenetic, and developmental) as well as information acquired by other means, such as education. Opportunity, however, is defined as the co-occurrence of resource availability (either environmental or in hosts) in time and space, or in other words, performance capacity is conditioned by opportunity.
The authors discuss at length the framework that this paradigm entails, all of which is built on past Darwinian proposals as a conglomerate of thought that opposes the general Neo-Darwinian view described earlier. It relies on three general pillars: (1) Ecological Fitting; (2) Oscillation Hypothesis; (3) Taxon Pulse, where Ecological Fitting addresses the initial stages of an interaction, describing the interaction in terms of realization of capacity limited by opportunity, selection of phenotypic variations, and establishment of an interaction.
Oscillation Hypothesis and Taxon Pulse, on the other hand, have to do with the phylogenetic and phylogeographic aspects of evolution, the first exploring the alternating aspect of specialism and generalism, while the second describes the spatiotemporal reality of evolution and how the exploration of hosts by parasites, for example, relies on cycles of stability and disturbance, or better yet, on opportunistic events. This paradigm aims, therefore, to present evolution from the populational to the phylogeographic perspectives.
6. Applied evolution in host-virus interactions
The absorption of evolutionary ideas by medicine can be roughly divided into two distinct conceptual periods: (1) Medical Darwinism, which gained traction between 1881 and the 1940s, and (2) the proposal of Darwinian Medicine in 1991 by Williams & Nesse, both of which will now be succinctly described.
6.1. Medical Darwinism (1881 to the 1940s)
Medicine and evolution have been described as two interdisciplinary yet profoundly different fields (Zampieri, 2009): the first does not aim to deeply understand the theories behind the scientific data but rather seeks to apply the findings to societal issues of pathogens and human diseases. Therefore, medicine has not closely followed the changes in evolutionary theory laid out above, although it has been invested in the specific subjects of the evolution of infectious diseases and immunology (Zampieri 2009) within the framework of Medical Darwinism (Zampieri 2009) from 1881 to the 1940s. However, Medical Darwinism’s embrace of toward eugenic ideals, which reached a peak during World War 2, led to it falling out of favor among many research groups.
The primary focus of Medical Darwinism was a typology of human types that used internal characteristics to define humans along the lines of dominance of diathesis, that is, a general temperament such as lymphatic, choleric, and sanguine. According to this view, different groups of humans would be subject to imbalances in their organs and systems, which would result in group-specific capabilities and susceptibility to disease (Waller, 2002; Zampieri, 2009).
The theory of diathesis, or human types, relied on the evolution of human variation. The concept of human types as drivers of particular diseases came into opposition with the emerging germ theory, supported by Pasteur’s experiments in microbiology (Pasteur, 1881). Under the latter view, human diseases were actually caused by microorganisms that invade the organism. The decline of Medical Darwinism was accelerated by the expansion of medical research based on experimentation as well as the manipulation of Medical Darwinism by eugenic propaganda and religious opposition. It also diverged from original Darwinian ideas by replacing the fundamental idea of population — and individual variation — with the utility of types, simplifying the evolution of diseases to a phenomenon with one or few causes (Zampieri, 2009). Diseases were then approached as norms or universal types instead of individual pathogens or an inconsequential variation of the pure form (Nesse & Williams, 1995).
From a constitutionalist perspective of medicine, diseases are identified by first defining a type to be considered as the ideal form (the healthy), with diseases envisioned as variations from the norm. This conception of an ideal type was then explained by Natural Selection without considering the reality of variation that is inherent to nature. This conceptualization of types led to deviations from the “norm” being seen as a lesser, negative variations. Natural selection, then, must be the driving force enabling the conservation of higher types and elimination of lower forms. It is not difficult to see how eugenic groups readily endorsed this viewpoint in World War 2 (Tracy, 1992; Méthod, 2015).
Medical Darwinism, then, was founded on the duality of a rejection of the populational perspective of species and pathogens on the one hand and a view of healthy and sick as two isolated and incompatible entities on the other. The idea of hereditary diseases was extensively discussed, and its incongruence with the dominant view Darwinism — Neo-Darwinism — was explained away by arguing that civilization and medicine blocked the elimination of such traits (Zampieri, 2009).
Regarding diseases caused by pathogens, which is our focus here, one can see how the definition of types and the elimination of the less fit variants influence the current view of pathogen evolution. The severity of natural selection described by Medical Darwinism was described as why it was then replaced by Darwinian Medicine, although it still seems to influence the medical conception of pathogens in different ways (Nesse & Williams, 1991).
6.2. Darwinian Medicine
Proposed in 1991 by Williams and Nesse, this approach aimed to replace the typological view with a populational one that was based on the advances in evolutionary biology, especially the discovery of genetic polymorphisms and the non-directionality of evolution, as well as an improved understanding of genetic variation. The concept of trade-offs became paramount to understanding pathogenesis and disease, since genes become more prevalent if they lead to higher reproductive success, not necessarily to better health. Concepts like the linkage of characters and trade-offs were then used to explain the persistence of hereditary diseases, which is currently described in evolution as the genetic load (Wallace, 1991).
Darwinian Medicine focuses on the origin of vulnerability to disease rather than the evolution of disease per se, since natural selection must shape vulnerability or the malfunction of traits leading to disease vulnerability and not the disease itself. This vulnerability varies between host groups or populations due to their phenotypic variation, which does not necessarily escape selection, but may be maintained by it due to trade-off phenomena (Williams & Nesse, 1991; Wallace, 1991).
It is interesting to note that disease vulnerability (external origin) is described by the authors as dependent on six principles: 1) the response to natural selection can be slow relative to the rate of environmental change, causing a mismatch between design and environment; 2) natural selection can be slower in the host than in the pathogen, which is especially crucial in competition with a pathogen that reproduces more quickly than humans; 3) natural selection cannot solve some problems on any time horizon, since trade-offs force compromises or because there are constraints peculiar to living systems (see Gerhart and Kirschner 1997; Minelli 2003); 4) we misunderstand what selection shapes, since traits may be maintained if they increase reproductive success at the cost of disease vulnerability, and finally 6) we may misunderstand what selection shapes, as defenses can be readily mistaken for diseases.
Williams & Nesse went on to describe the evolutionary perspective of humans and their diseases in a less strict way, where human traits are not the evolutionary construction of a perfect design but that of the best possible reproducer. Darwinian Medicine does not see selection as a process that maximizes strength, health, and longevity (Williams & Nesse, 1991). The authors describe infectious disease dynamics by considering the different changes infections induce in the host’s health — (1) direct damage; (2) impairment of function; (3) repair by the host; (4) compensatory adjustments; (5) hygienic measures; (6) host defenses that expel, destroy or sequester pathogens; (7) evasion of host defenses by parasites; (8) attack on host defenses by parasite; (9) trophic mechanisms by the parasite; (10) dispersal mechanisms – transmission dynamics, and (11) manipulation of host’s behavior or metabolism by the parasite.
In terms of pathogen evolution, Darwinian Medicine highlights the role of the coevolutionary arms race, where pathogens evolve in the course of the infection through mechanisms such as developing resistance to medication. Coevolution is also discussed regarding the development of novelties through host and parasite generations. Virulence is another pathogen trait that is commonly discussed in this scenario, with the authors describing the avirulent hypothesis (Dobsanksy, 1951) as defective since it considers pathogen evolution as a slow and gradual process; the authors also note that some of the parasite’s success must rely on some level of virulence (factors such as 6, 10, and 11).
The current evolutionary perspective of parasite evolution has generally rejected the avirulence hypothesis, since it supposes an evolutionary equilibrium in parasites, where virulence and other parasite traits should not vary unless ecological circumstances change rapidly and extremely. Ewald (1987 and 1991) lengthily describes and predicts virulence as a process that varies according to transmission characteristics, where in indirect transmission scenarios (human host and insect vector), virulence should be high in humans and low in vectors, whereas in direct transmission virulence should be lower in order to enable contact between hosts.
Another prediction presented by Ewald and Schubert (1989) is that diseases transmitted by contact with inanimate vectors (i.e., water) should be more virulent since the high density of the pathogen population in a host must be released into the media and overcome dilution. This problem does not emerge in diseases that involve only hosts. Another mode of transmission the authors discuss that could lead to changes in virulence is alterations in the mode of transmission, from person-person to transmission through water, which is expected to increase virulence.
It is important to note that the variation in virulence due to host-switching events or changes in transmission dynamics presume, necessarily, the evolution of such virulence as a novel character that arose from these disruption events. The emergence of such novelties is explained by the rapid evolutionary rate of pathogens due to their short life cycle and high fertility rate, with mutation and multiple infections enabling recombination events, great sources of novelty. Ewald discusses many other scenarios of virulence evolution, which are not relevant here (Williams & Nesse, 1991).
The second event of merging evolutionary thinking into medicine has led to important changes in the practical aspect of evolution and how to understand and handle emerging infectious diseases. However, one can see how Darwinian Medicine has kept a more Neo-Darwinian view of evolution, where the evolution of pathogens implies the directional and opportunistic emergence of evolutionary novelties instead of admitting evolutionary processes such as plasticity and conservatism to pathogen evolution. In this perspective, increases in virulence or host-switching events are associated with rapid evolution of a novelty that allows host infection disregarding the possibility of a preexisting capacity. The six principles described by their perspective are interesting in order to comprehend the selective pressures in host-parasite interactions, but their implications for host and parasite diversification remain unexplored.
The understanding of evolution in these terms seems to be consistent with the general understanding of viral evolution, with host-switching events seen as rare and “unexpected,” as observed in many articles describing the evolution of emerging and re-emerging pathogens (Lai, 2003; Benvenuto, 432 2020; Wu, 2020). The general strategy when handling “new” viruses usually involves exploring closely related species that are better understood by the scientific community and, through the concept of homology and phylogenetic conservatism, developing and testing antiviral drugs and vaccines even before knowledge of the current variant remains unavailable (Lai, 2003; Adler, 2022). Such an approach is evolutionarily sound and agrees with modern evolution.
The traditional understanding of emerging infectious diseases highlights the origin of novel capacity in the viral lineage, where mutations and recombination events in the new strain are frequently described as the causative agent of the species jump – as described in Darwinian Medicine — without discussing the possibility of preexisting capacity. The Stockholm Paradigm, meanwhile, proposes that the emergence of an important capacity that conveniently enables the realization of a host-switching event should be less probable than the existence of capacity that was already part of the pathogen’s information space, which becomes expressed due to an opportunity arising (Brooks, 2019; Feronato, 2021; Boeger, 2022).
Such a congruence of capacity between emergent and non-emergent strains should be clearly borne out by phylogenetic analysis. When the phylogeny portrays shows that a “new” emergent disease is similar to its sister taxa through small branch lengths or close monophyly, for example, these topological measures indicate that their capacity spaces, or general information spaces, are more similar than different. When a host-switching event can be easily associated with few mutations, these mutations often end up receiving greater importance than the overall genetic similarity between sister viruses.
The discussion on the overestimation of mutations in emerging diseases is accompanied by the empirical evidence of the great frequency of host-switching events based on data from helminth host and insect-plant coevolutionary analyses, which are also presented by the authors of the Stockholm Paradigm (Brooks, 2019). Phylogenetic models that analyze coevolutionary processes seem to maximize cospeciation between symbiont species, further confirming the rarity of species jumps.
Viral evolution, therefore, could be more complex than is currently believed. The current SARS-CoV-2 pandemic, for example, has been extensively analyzed molecularly, phylogenetically, and ecologically, and has laid bare the incongruences between phylogenetic divergence and the general understanding of the viral group. We will now compare the phylogenetic data from four current host switching events: the SARS-CoV-2 virus, dengue virus, pmd H1N1 (2009–2010), and the new monkeypox virus (MPXV – 2022), and raise the question of previous capacity space, in opposition to the emergence of a key novelty for species jumps leading to differential host utilization.
7. Discourse on current viruses of epidemiological importance
7.1. Monkeypox
Monkeypox virus (MPXV) is a member of the family Poxviridae and the genus Orthopoxvirus, which also contains smallpox, camelpox, and cowpox, as well as other important human and animal pathogens. Like all orthopoxviruses, monkeypox is a double-stranded DNA virus and is considered to be a sister to the smallpox virus, varying in genome size, organization, and open reading frame (ORF) content. Its identity with the two main Variola strains is 84.6% (with VARV – India) and 84.5% (VARV – Gar), with evidence of duplication of four ORFs in terminal regions of the genome, making MPXV genome slightly larger than that of VARV. The higher variation of monkeypox relative to smallpox is observed in the terminal regions of the genome, the structural ORFs being the most conserved portions of the DNA (Shchelkunov, 2005).
The terminal regions of the orthopoxvirus genome contain the majority of the virulence, immunomodulatory, and host cell infection genes previously investigated in VARV and camelpox (CPXV). In MPXV, two interferon resistance genes have been affected, resulting in lower efficiency of virus transmission via aerosol (Massung, 1995). Central African MPXV immunomodulatory response has been previously described as less efficient than VARV, leading to a stronger host inflammatory response (Shchelkunov et al., 1998). Lastly, the MPXV genome, although not the shortest, has one of the lowest number of immune evasion genes of any orthopoxvirus — after two variola viruses — with camelpox being the species with the greatest number of ORFs (18) (Shchelkunov, 2005; Kugelman, 2014). These and other investigations point to lower transmission efficiency and human-to-human transmission of the monkeypox virus, supporting the cessation of vaccination in the 1980s (Shchelkunov, 2005; Fine, 1988).
Monkeypox was first described in 1958 in a Danish laboratory, first studied in monkey hosts. The first human outbreak of monkeypox occurred in 1970, identified in a 9-month-old infant, with subsequent spread throughout the Democratic Republic of Congo in 1970. Cases have since been associated with close contact with wild hosts: monkeys, or, more recently, rodents (Giulio, 2004). Previous American introductions of the disease have been linked to close contact with infected prairie dogs, raising concerns of monkeypox spread in the country’s rodent population.
By 2000, there had been a clear increase in monkeypox reports, most of which were suspected rather than confirmed. Between January and September 2020, more than 4,000 suspected cases were reported in Nigeria (183 confirmed cases, two of which were diagnosed in Israel and Singapore) (Bunge, 2022).
The virus strains have been previously genetically divided into two large clades, the Central and West African strains, named for the regions where the disease is endemic. The recent disease cases in other regions are described as monkeypox exportation. The African case fatality rate is considered low, being 3.6% in the Western clade and 10.6% in the Central African (Bunge, 2022). The largest epidemic spread of the disease before the year 2000 was documented in the Democratic Republic of Congo in 1996, with 520 confirmed cases of the Central African clade (Bunge, 2022).
Only in 2003 were monkeypox cases identified outside of the African continent, with an outbreak in the USA that was later associated with infection by close contact with prairie dogs. The rodents appeared to have been transported with infected hosts from Ghana before being sold in Wisconsin (Reed, 2004; Bunge, 2022). All 47 cases were linked to exposure to the same shipment of prairie dogs; the FDA and CDC banned any importation of rodents from Africa following the outbreak (Wisconsin Department of Health and Family Services).
Contemporary cases of the disease have been seen as a consequence of the cessation of smallpox vaccination campaigns after its eradication in 1980 in many countries, and its omission from routine vaccination programs in many regions (Yong, 2020; Alankunle, 2020). The traditional smallpox vaccine, designed with Vaccinia strains (Orthopoxvirus), also provided 85% protection against monkeypox (Fine, 1988). Unfortunately, although Fine’s work identified the potential growth of monkeypox cases in the future as a consequence of the cessation of vaccination, it ultimately agreed with the Global Commission for the Certification of Smallpox Eradication to discontinue vaccination based on the reduced transmissibility of monkeypox compared to smallpox (Parker, 2007).
The reemergence of the disease identified in May 2022 (ECDC, 2022) was, from its beginning, characterized by two main types of infections: (1) those associated with close contact with infected wild hosts and (2) human-to-human infections through contact with large droplets of saliva, contaminated objects, and skin lesions (Velavan, 2022). The 2003 USA outbreak, as well as the UK epidemic of 2018–2019, were traced back to West African MXPV based on genome identity.
Monkeypox outbreaks outside of its endemic region are also usually linked to travel-related events, which enables the international spread of the disease, both among humans and its establishment in wild populations, especially rodents (Velavan, 2022). The most recent reports of the disease in several countries are yet to be linked to a source infection, suggesting human-human transmission. However, the emergence of monkeypox in humans needs to be better characterized according to the context that precluded the host-switch from the wild host to the human population.
Animal-human transmission is knowingly present in the endemic regions of the disease (Kugelman, 2014; Fuller, 2011), either by monkeys during the first identification of the virus (Giulio, 539 2004) or more recently, and especially in the American emergence of the disease, by rodents such as squirrels and prairie dogs (Guarner, 2004; Reed, 2004). In these cases, infection has been traced back to content with a sick wild host. The human-to-human cases, however, have been understood as phenomena where the specific MPXV genotype has suffered gene losses or frame-shifting mutations leading to its establishment in the human population. Gene losses and other mutations have been documented in Central African viruses (Kugelman, 2014; Vaughan, 2020), where the authors associate the genetic changes to higher transmission capacity in human hosts, but the USA and UK cases do not seem to present such evidence.
Several phylogenetic analyses have been performed to better understand MPXV evolution, distinguishing two large clades of viruses endemic to Central and Western Africa. The most recent publications on the recent outbreaks outside of Africa that also include the May 2022 case from Portugal (Isidro, 2022) trace all the international strains as part of the Western MPX clade. It is important to note that the data shows a distinct separation of the 2003 USA strain from the 2020 Singapore 2020 cases, 2018 Israel cases, and 2022 European strains, since almost all cases of the 2003 USA epidemic were associated with a shipment of infected prairie dogs to be kept as pets (Reed, 2004). In contrast, the other cases of 2018–2022 were not traced back to contact with a wild host but were associated with human-human transmission through sexual activity (Thornhill, 2022).
Finally, it seems that the emergence of monkeypox disease in non-endemic regions since 2018 is associated with a genomic change in the transmission dynamics of a subclade within the West African monkeypox. This well-determined phylogenetic difference (Isidro, 2022) points to a general change in capacity involving more than one monkeypox gene instead of an individual change in surface proteins that enable host-cell entrance, as has been discussed in the SARS-CoV-2 reemergence of 2020 (Jain, 2020).
7.2. SARS-CoV-2 (Coronavirus)
SARS-CoV-2 is a member of the family Coronaviridae, subfamily Orthocoronavirinae, and genus Coronavirus. Like all members of the genus, it is characterized as a positive-stranded RNA virus whose genome size is around 28kb and is enveloped by a crown-like capsid [Gonzalez, 2003]. Its sister genus is Torovirus, which has a significant phylogenetic distance from Coronavirus. The genus has also been divided into four groups based on antigenic and phylogenetic divergence (Helmy, 2020): Alphacoronavirus [Woo, 2014], which includes transmissible gastroenteritis virus (TGEV), feline coronavirus (FeCoV), canine coronavirus (CCoV), human coronavirus 229E (HCoV-229E) and porcine epidemic diarrhea virus (PEDV); Betacoronavirus [Woo, 2014], includes murine hepatitis virus (MHV), bovine coronavirus (BCoV), human coronavirus OC43 (HCoV-OC43) - pandemic Coronavirus, porcine hemagglutinating encephalomyelitis virus (HEV), rat coronavirus (RtCoV), and equine coronavirus (ECoV); Gammacoronavirus [Woo, 2014] is comprised by avian infectious bronchitis virus (IBV), beluga whale coronavirus (BWCoV-SW1), and Deltacoronavirus, with avian (WiCoV-HKU20 and BuCoV-HKU11) and porcine (PoCoV-HKU15) coronaviruses (Helmy, 2020).
Gonzales et al. (2003) revised the Coronaviridae taxonomy, considering both structural and sequence identity between six viral proteins (S, E, M, N, polymerase, and helicase) for Coronavirus, Torovirus, and other representative Nidovirales species. Their phylogenetic analyses support the previous clustering of the Coronavirus members, with overall branch significance. It is important to note that before the 2019 pandemic, other works such as Woo (2014) and Benvenuto (2020) had highlighted the molecular evidence that HCoV-OC43 is sister to the Bat coronavirus species in the genus Betacoronavirus.
Most studies involving Coronavirus species emerged during the 2019 SARS-CoV 2 pandemic, with unprecedented speed, ranging from viral infection and replication cycle, evolution, and pathogenesis [Benvenuto, 2020; Naqvi, 2020, among others]. With a general consensus by the scientific community, SARS-CoV-2 was defined as a distinct coronavirus species, distant from the first SARS-CoV, more closely related to a Bat coronavirus, both diverging from all other Coronavirus species, considering several amino acid residue substitutions in the S (spike), and N (nucleocapsid) proteins, considered key proteins involved in viral infection and pathogenicity, respectively (Benvenuto, 2020), although less pathogenic than the first SARS coronavirus.
Recent studies discuss other mechanisms involved in inter-species jumps, such as homologous recombination of the S glycoprotein (Ji, 2020) in bat coronaviruses using snake hosts. The authors hypothesize that the identified recombination suggests that SARS-CoV-2 uses snakes as reservoirs based on relative synonymous codon usage (RSCU) bias. The RSCU bias identified with the new coronavirus is more similar to Bungarus multicinctus bias than any other host species, including bats (Ji, 2020).
In summary, the current literature on SARS-CoV-2 evolution suggests that the virus emerged from an initial animal-human infection identified in Wuhan’s wild animal markets through patient reports (Hui, 2020; Lu, 2020), a species jump enabled by important S and N protein substitutions, that are assumed to impact on viral infection and pathogenicity. At the same time, the phylogenetic analyses show that the SARS-CoV-2 species, together with the Bat SARS-like virus of 2015 (Benvenuto, 2020), is phylogenetically distant from all other coronaviruses, reflecting possible differences beyond those characterized by the protein-centered works.
Coronaviruses are exceedingly diverse, with a wide host range. Their diversity is mostly explained by the nature of their genome, as well as by their random template-switching mechanism that emerges during RNA replication, leading to events such as homologous recombination of protein segments. Both molecular phenomena are coupled with their large genome, one of the largest RNA viruses (Woo, 2014). Woo et al. (2014) extensively explore the taxonomy and phylogenetic analysis of the Betacoronavirus. Additionally, their study on host coevolution elegantly depicts the viral diversity and host range, both factors enabled by the host-host contact (allowing interspecies jumps), along with the highly flexible nature of the virus. The essential role of bat hosts in host range expansion, also influencing the diversification of the four Coronavirus groups (Woo, 2006; Woo, 2014), must also impact the group’s diversity.
7.3. Influenza
Influenza viruses are generally divided into three groups — A, B, and C — which comprise half of the genera of the family Orthomyxoviridae. As described by the International Committee on Viral Taxonomy (Shaw, 2013), viruses that belong to this family are segmented, negative-sense RNA viruses. A putative Influenza D virus (Collin, 2015), as well as the Wellfleet Bay virus, recently found infecting cattle in North America (Allison, 2015), are included in the family (Suarez, 2016).
Influenza A viruses (IAVs) are the most dominant taxon of the family, with the greatest host range, with wild birds considered the primordial reservoir of Influenza A – bears 16 HA subtypes of the known 18— especially the orders Anseriformes and Charadriiformes. The centrality of wild birds as reservoirs for viruses makes these pathogens a constant and potential threat to human and animal populations.
Influenza A can be transmitted through host-switching events that either do not persist in the new species or become endemic in the new host population. In the latter case, the colonization of the new host species seems to reduce the rate of spillback, as is seen in equine, human, and swine Influenza A (Suarez, 2016). Influenza A infections that are not established in the new host population are considered sporadic, although reinfection frequently occurs.
The morphology of the virus is well known, although not well understood, since its general form varies with the medium and tissue. It can be visualized in two forms: spherical, around 80–120 nm, when in cellular culture, and filamentous, with several micrometers size when observed in clinical isolates. It comprises eight RNA packaged segments, which translate to 10 basic proteins, many of which present accessory proteins produced by alternative splicing and reading frame shift of themselves. The accessory proteins have knowingly acted as virulence factors (Chen, 2001) or immune response modulators (Jagger, 2012); however, many of them have no known function (Suarez, 2016).
The complexity of the IAV’s replication strategy has been extensively explored, and mechanisms involving host-switching have also been elegantly described. The accessory binding of the HA (hemagglutinin) protein to the surface glycoproteins containing either 2-3 or 2-6 sialic acids has been robustly described as being the determining factor for viral entry in the host cell (Suzuki, 2005). The abundance and diversity of sialic acid type by tissue and host species have so far explained the colonization of swine (both 2-3 and 2-6), human (2-6), and avian (2-3) hosts as well as the mixing vessel role of swine species, have presented in the ecology and evolution of IAVs. The pmdH1N1 fits this epidemiological scenario, as its emergence in humans, swine, turkeys, ferrets, and other species is sporadic (Pantin Jackwood, 2010; Hinshaw, 1983; Vincent, 2014).
At the same time, studies involving wild avian influenza viruses have revealed the extensive amino-acid diversity of genotypes in this group of hosts, which encompass almost all IAV subtypes, except the recently described bat IAVs. For these viruses, previous studies have revealed that, among all 16 HA subtypes, around 25% of the amino acid sequence is conserved, whereas the subtype divergence, when in a pairwise comparison, can vary between 20 and 63% (Nobusawa, 1991). Both the amino acidic and the nucleotide (Stallknecht, 1998; Suarez, 2000) sequences discuss a geographical clustering of IAV lineages among American and Eurasian groups. Meanwhile, the American/Eurasian hypothesis is met with exceptions such as the shorebird and gull H2 lineages, although they have been explained as unique subpopulations of the hemagglutinin gene associated with the migratory nature of the hosts (Makarova 1999, Suarez, 2016).
When addressing, more specifically, the host-switching event seen in the pmdH1N1, one can realize that the swine-like H1N1 IAV is a result of serial processes of reassortment between swine, avian, and human viruses that occurred in swine populations, which went undetected for decades in the population and emerged in humans in the host-switching event that occurred in Mexico in 2009 (CDC, 2009).
The complex segment interchange is accompanied by a straightforward conclusion: the pandemic strain must have been maintained in low frequencies in the original host population, and the substitutions described in the strain’s genome for the five sublineages must not have had any known, or important functional benefit that enabled human colonization (Tumpey, 2004; Garten, 2009). At the same time, previous studies have not found a single marker associated with adaptation to human hosts (mapped using 1918 H1N1 and HPIAV H5N1) (Zamarin, 2006; Jackson, 2008; Garten, 2009). The authors of these important works conclude by highlighting that the pmdH1N1 has no classical “human adaptation” substitutions, and yet successfully infected and established itself in the human population; it was also antigenically (in terms of H1 segment) similar to other Eurasian swine Influenza viruses, with no notable difference in their antigenic properties (Russell, 2008).
Experimental HIA has shown little to no cross-reactivity; however, when comparing ferret HI response to pmdH1N1 post-infection with a closely related swine H1N1 (Garten, 2009). Dunham et al. also analyzed and concluded that the low genetic novelty of pmdH1N1, compared to other swine-origin H1N1, and described the same lack of “human adaptation” substitutions (Dunham, 2009), although they did not analyze the antigenic properties of the pmdH1N1 as Garten did.
Finally, one can see how the host-switching event of pmdH1N1/2009 paints a more complicated picture than that of the previous cases discussed here. The colonization of humans has not been linked to substitutions associated with human utilization, whereas its long-term — and undetected — survival in swine hosts, along with multiple reassortments between Eurasian swine and avian Influenza, has given ample opportunity for virus transmission in Mexico. It is also important to note that the homogeneous nature of the pmdH1N1 suggests a single or few events of colonization, both suggested by Garten et al. (2009) and as predicted by the classical understanding of the Founder’s effect (Mayr, 1963).