Submitted:
16 May 2023
Posted:
17 May 2023
You are already at the latest version
Abstract
Keywords:
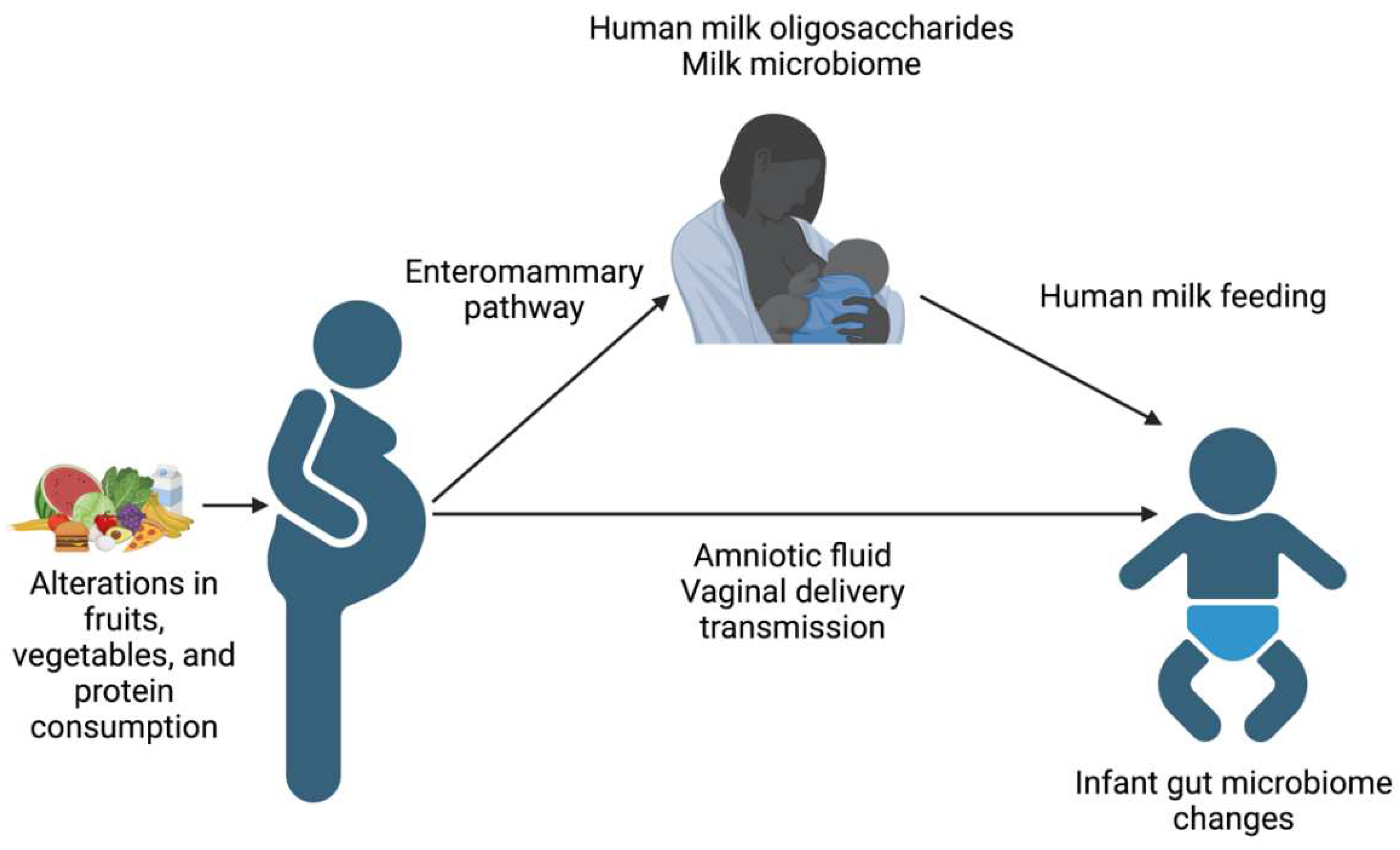
INTRODUCTION
PRE-PREGNANCY BMI AND ITS ROLE IN MICROBIAL CHANGES
OVERWEIGHT/OBESITY
UNDERWEIGHT
GESTATIONAL WEIGHT GAIN
BODY COMPOSITION
GESTATIONAL DIABETES
PARENTAL DIET AND THE MICROBIOME
FUTURE DIRECTIONS – DIETARY AND PROBIOTIC INTERVENTIONS
GAPS IN THE LITERATURE
CONCLUSIONS
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Most, J.; Dervis, S.; Haman, F.; Adamo, K.B.; Redman, L.M. Energy Intake Requirements in Pregnancy. Nutrients 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Gorczyca, K.; Obuchowska, A.; Kimber-Trojnar, Ż.; Wierzchowska-Opoka, M.; Leszczyńska-Gorzelak, B. Changes in the Gut Microbiome and Pathologies in Pregnancy. Int J Environ Res Public Health 2022, 19. [Google Scholar] [CrossRef] [PubMed]
- Sikder, S.S.; Labrique, A.B.; Shamim, A.A.; Ali, H.; Mehra, S.; Wu, L.; Shaikh, S.; West, K.P.; Christian, P. Risk factors for reported obstetric complications and near misses in rural northwest Bangladesh: analysis from a prospective cohort study. BMC Pregnancy Childbirth 2014, 14, 347. [Google Scholar] [CrossRef] [PubMed]
- Rush, D. Nutrition and parental mortality in the developing world. Am. J. Clin. Nutr. 2000, 72, 212S–240S. [Google Scholar] [CrossRef]
- Brabin, B.J.; Hakimi, M.; Pelletier, D. An analysis of anemia and pregnancy-related parental mortality. J. Nutr. 2001, 131, 604S–614S, discussion 614S-615S. [Google Scholar] [CrossRef]
- Ornoy, A. Prenatal origin of obesity and their complications: Gestational diabetes, parental overweight and the paradoxical effects of fetal growth restriction and macrosomia. Reprod Toxicol 2011, 32, 205–212. [Google Scholar] [CrossRef]
- Belkacemi, L.; Nelson, D.M.; Desai, M.; Ross, M.G. Parental undernutrition influences placental-fetal development. Biol Reprod 2010, 83, 325–331. [Google Scholar] [CrossRef]
- Isganaitis, E.; Venditti, S.; Matthews, T.J.; Lerin, C.; Demerath, E.W.; Fields, D.A. Parental obesity and the human milk metabolome: associations with infant body composition and postnatal weight gain. Am J Clin Nutr 2019, 110, 111–120. [Google Scholar] [CrossRef]
- Saben, J.L.; Sims, C.R.; Abraham, A.; Bode, L.; Andres, A. Human Milk Oligosaccharide Concentrations and Infant Intakes Are Associated with Parental Overweight and Obesity and Predict Infant Growth. Nutrients 2021, 13. [Google Scholar] [CrossRef]
- Hegar, B.; Wibowo, Y.; Basrowi, R.W.; Ranuh, R.G.; Sudarmo, S.M.; Munasir, Z.; Atthiyah, A.F.; Widodo, A.D.; Supriatmo; Kadim, M.; et al. The Role of Two Human Milk Oligosaccharides, 2'-Fucosyllactose and Lacto-N-Neotetraose, in Infant Nutrition. Pediatr Gastroenterol Hepatol Nutr 2019, 22, 330–340. [Google Scholar] [CrossRef]
- Jost, T.; Lacroix, C.; Braegger, C.; Chassard, C. Impact of human milk bacteria and oligosaccharides on neonatal gut microbiota establishment and gut health. Nutr Rev 2015, 73, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Bunesova, V.; Lacroix, C.; Schwab, C. Fucosyllactose and L-fucose utilization of infant Bifidobacterium longum and Bifidobacterium kashiwanohense. BMC Microbiol 2016, 16, 248. [Google Scholar] [CrossRef] [PubMed]
- Morita, C.; Tsuji, H.; Hata, T.; Gondo, M.; Takakura, S.; Kawai, K.; Yoshihara, K.; Ogata, K.; Nomoto, K.; Miyazaki, K.; et al. Gut Dysbiosis in Patients with Anorexia Nervosa. PLoS One 2015, 10, e0145274. [Google Scholar] [CrossRef] [PubMed]
- Cömert, T.K.; Akpinar, F.; Erkaya, S.; Durmaz, B.; Durmaz, R. The effect of pre-pregnancy obesity on gut and meconium microbiome and relationship with fetal growth. J Matern Fetal Neonatal Med 2022, 35, 10629–10637. [Google Scholar] [CrossRef] [PubMed]
- Krajmalnik-Brown, R.; Ilhan, Z.E.; Kang, D.W.; DiBaise, J.K. Effects of gut microbes on nutrient absorption and energy regulation. Nutr. Clin. Pract. 2012, 27, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Isolauri, E.; Laitinen, K.; Salminen, S. Distinct composition of gut microbiota during pregnancy in overweight and normal-weight women. Am. J. Clin. Nutr. 2008, 88, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Ruebel, M.L.; Gilley, S.P.; Sims, C.R.; Zhong, Y.; Turner, D.; Chintapalli, S.V.; Piccolo, B.D.; Andres, A.; Shankar, K. Associations between Parental Diet, Body Composition and Gut Microbial Ecology in Pregnancy. Nutrients 2021, 13. [Google Scholar] [CrossRef]
- Griffee, M.B. An update on endodontics and other oral microbiology. J Indiana Dent Assoc 1986, 65, 27–28. [Google Scholar]
- Cao, B.; Stout, M.J.; Lee, I.; Mysorekar, I.U. Placental Microbiome and Its Role in Preterm Birth. Neoreviews 2014, 15, e537–e545. [Google Scholar] [CrossRef]
- Faucher, M.A.; Greathouse, K.L.; Hastings-Tolsma, M.; Padgett, R.N.; Sakovich, K.; Choudhury, A.; Sheikh, A.; Ajami, N.J.; Petrosino, J.F. Exploration of the Vaginal and Gut Microbiome in African American Women by Body Mass Index, Class of Obesity, and Gestational Weight Gain: A Pilot Study. Am J Perinatol 2020, 37, 1160–1172. [Google Scholar] [CrossRef]
- Dall'Asta, M.; Laghi, L.; Morselli, S.; Re, M.C.; Zagonari, S.; Patuelli, G.; Foschi, C.; Pedna, M.F.; Sambri, V.; Marangoni, A.; et al. Pre-Pregnancy Diet and Vaginal Environment in Caucasian Pregnant Women: An Exploratory Study. Front Mol Biosci 2021, 8, 702370. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Yan, Y.; Ji, F.; Zhou, H. Parental Obesity Increases Oxidative Stress in Placenta and It Is Associated With Intestinal Microbiota. Front Cell Infect Microbiol 2021, 11, 671347. [Google Scholar] [CrossRef] [PubMed]
- Antony, K.M.; Ma, J.; Mitchell, K.B.; Racusin, D.A.; Versalovic, J.; Aagaard, K. The preterm placental microbiome varies in association with excess parental gestational weight gain. Am J Obstet Gynecol 2015, 212, 653.e651–616. [Google Scholar] [CrossRef]
- Benny, P.A.; Al-Akwaa, F.M.; Dirkx, C.; Schlueter, R.J.; Wolfgruber, T.K.; Chern, I.Y.; Hoops, S.; Knights, D.; Garmire, L.X. Placentas delivered by pre-pregnant obese women have reduced abundance and diversity in the microbiome. FASEB J 2021, 35, e21524. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Rubio, R.; Collado, M.C.; Laitinen, K.; Salminen, S.; Isolauri, E.; Mira, A. The human milk microbiome changes over lactation and is shaped by parental weight and mode of delivery. Am J Clin Nutr 2012, 96, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Laitinen, K.; Salminen, S.; Isolauri, E. Parental weight and excessive weight gain during pregnancy modify the immunomodulatory potential of breast milk. Pediatr Res 2012, 72, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Qi, C.; Yang, Z.; Jiang, S.; Bi, Y.; Lai, J.; Sun, J. Geographical location specific composition of cultured microbiota and Lactobacillus occurrence in human breast milk in China. Food Funct 2019, 10, 554–564. [Google Scholar] [CrossRef]
- LeMay-Nedjelski, L.; Butcher, J.; Ley, S.H.; Asbury, M.R.; Hanley, A.J.; Kiss, A.; Unger, S.; Copeland, J.K.; Wang, P.W.; Zinman, B.; et al. Examining the relationship between parental body size, gestational glucose tolerance status, mode of delivery and ethnicity on human milk microbiota at three months post-partum. BMC Microbiol 2020, 20, 219. [Google Scholar] [CrossRef]
- Williams, J.E.; Carrothers, J.M.; Lackey, K.A.; Beatty, N.F.; York, M.A.; Brooker, S.L.; Shafii, B.; Price, W.J.; Settles, M.L.; McGuire, M.A.; et al. Human Milk Microbial Community Structure Is Relatively Stable and Related to Variations in Macronutrient and Micronutrient Intakes in Healthy Lactating Women. J Nutr 2017, 147, 1739–1748. [Google Scholar] [CrossRef]
- Moossavi, S.; Sepehri, S.; Robertson, B.; Bode, L.; Goruk, S.; Field, C.J.; Lix, L.M.; de Souza, R.J.; Becker, A.B.; Mandhane, P.J.; et al. Composition and Variation of the Human Milk Microbiota Are Influenced by Parental and Early-Life Factors. Cell Host Microbe 2019, 25, 324–335.e324. [Google Scholar] [CrossRef]
- Li, S.W.; Watanabe, K.; Hsu, C.C.; Chao, S.H.; Yang, Z.H.; Lin, Y.J.; Chen, C.C.; Cao, Y.M.; Huang, H.C.; Chang, C.H.; et al. Bacterial Composition and Diversity in Breast Milk Samples from Mothers Living in Taiwan and Mainland China. Front Microbiol 2017, 8, 965. [Google Scholar] [CrossRef] [PubMed]
- Larsson, M.W.; Lind, M.V.; Laursen, R.P.; Yonemitsu, C.; Larnkjær, A.; Mølgaard, C.; Michaelsen, K.F.; Bode, L. Human Milk Oligosaccharide Composition Is Associated With Excessive Weight Gain During Exclusive Breastfeeding-An Explorative Study. Front Pediatr 2019, 7, 297. [Google Scholar] [CrossRef] [PubMed]
- Turroni, F.; Milani, C.; Duranti, S.; Ferrario, C.; Lugli, G.A.; Mancabelli, L.; van Sinderen, D.; Ventura, M. Bifidobacteria and the infant gut: an example of co-evolution and natural selection. Cell. Mol. Life Sci. 2018, 75, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Gilley, S.P.; Ruebel, M.L.; Sims, C.; Zhong, Y.; Turner, D.; Lan, R.S.; Pack, L.M.; Piccolo, B.D.; Chintapalli, S.V.; Abraham, A.; et al. Associations between parental obesity and offspring gut microbiome in the first year of life. Pediatr Obes 2022, 17, e12921. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.B.; Madan, J.; Coker, M.; Hoen, A.; Baker, E.R.; Karagas, M.R.; Mueller, N.T. Does birth mode modify associations of parental pre-pregnancy BMI and gestational weight gain with the infant gut microbiome? Int J Obes (Lond) 2020, 44, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Miquel, S.; Martin, R.; Rossi, O.; Bermudez-Humaran, L.G.; Chatel, J.M.; Sokol, H.; Thomas, M.; Wells, J.M.; Langella, P. Faecalibacterium prausnitzii and human intestinal health. Curr. Opin. Microbiol. 2013, 16, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Hummel, G.; Woodruff, K.; Austin, K.; Knuth, R.; Lake, S.; Cunningham-Hollinger, H. Late Gestation Parental Feed Restriction Decreases Microbial Diversity of the Placenta While Mineral Supplementation Improves Richness of the Fetal Gut Microbiome in Cattle. Animals (Basel) 2021, 11. [Google Scholar] [CrossRef]
- Xue, Y.; Lin, L.; Hu, F.; Zhu, W.; Mao, S. Disruption of ruminal homeostasis by malnutrition involved in systemic ruminal microbiota-host interactions in a pregnant sheep model. Microbiome 2020, 8, 138. [Google Scholar] [CrossRef]
- Xue, Y.; Hu, F.; Guo, C.; Mei, S.; Xie, F.; Zeng, H.; Mao, S. Undernutrition shifted colonic fermentation and digest-associated bacterial communities in pregnant ewes. Appl Microbiol Biotechnol 2020, 104, 5973–5984. [Google Scholar] [CrossRef]
- Villasenor-Aranguren, M.; Roses, C.; Riezu-Boj, J.I.; Lopez-Yoldi, M.; Ramos-Lopez, O.; Barcelo, A.M.; Milagro, F.I. Association of the Gut Microbiota with the Host's Health through an Analysis of Biochemical Markers, Dietary Estimation, and Microbial Composition. Nutrients 2022, 14. [Google Scholar] [CrossRef]
- Ze, X.; Duncan, S.H.; Louis, P.; Flint, H.J. Ruminococcus bromii is a keystone species for the degradation of resistant starch in the human colon. ISME J 2012, 6, 1535–1543. [Google Scholar] [CrossRef] [PubMed]
- Morgan, X.C.; Tickle, T.L.; Sokol, H.; Gevers, D.; Devaney, K.L.; Ward, D.V.; Reyes, J.A.; Shah, S.A.; LeLeiko, N.; Snapper, S.B.; et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012, 13, R79. [Google Scholar] [CrossRef] [PubMed]
- Joossens, M.; Huys, G.; Cnockaert, M.; De Preter, V.; Verbeke, K.; Rutgeerts, P.; Vandamme, P.; Vermeire, S. Dysbiosis of the faecal microbiota in patients with Crohn's disease and their unaffected relatives. Gut 2011, 60, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Sim, J.X.Y.; Lee, W.L.; Cui, L.; Chan, Y.F.Z.; Chang, E.D.; Teh, Y.E.; Zhang, A.N.; Armas, F.; Chandra, F.; et al. Gut Ruminococcaceae levels at baseline correlate with risk of antibiotic-associated diarrhea. iScience 2022, 25, 103644. [Google Scholar] [CrossRef] [PubMed]
- Mörkl, S.; Lackner, S.; Müller, W.; Gorkiewicz, G.; Kashofer, K.; Oberascher, A.; Painold, A.; Holl, A.; Holzer, P.; Meinitzer, A.; et al. Gut microbiota and body composition in anorexia nervosa inpatients in comparison to athletes, overweight, obese, and normal weight controls. Int J Eat Disord 2017, 50, 1421–1431. [Google Scholar] [CrossRef] [PubMed]
- Jumpertz, R.; Le, D.S.; Turnbaugh, P.J.; Trinidad, C.; Bogardus, C.; Gordon, J.I.; Krakoff, J. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am J Clin Nutr 2011, 94, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.I.; Yatsunenko, T.; Manary, M.J.; Trehan, I.; Mkakosya, R.; Cheng, J.; Kau, A.L.; Rich, S.S.; Concannon, P.; Mychaleckyj, J.C.; et al. Gut microbiomes of Malawian twin pairs discordant for kwashiorkor. Science 2013, 339, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Monira, S.; Nakamura, S.; Gotoh, K.; Izutsu, K.; Watanabe, H.; Alam, N.H.; Endtz, H.P.; Cravioto, A.; Ali, S.I.; Nakaya, T.; et al. Gut microbiota of healthy and malnourished children in bangladesh. Front Microbiol 2011, 2, 228. [Google Scholar] [CrossRef]
- Gough, E.K.; Stephens, D.A.; Moodie, E.E.; Prendergast, A.J.; Stoltzfus, R.J.; Humphrey, J.H.; Manges, A.R. Linear growth faltering in infants is associated with Acidaminococcus sp. and community-level changes in the gut microbiota. Microbiome 2015, 3, 24. [Google Scholar] [CrossRef]
- Force, U.S.P.S.T.; Davidson, K.W.; Barry, M.J.; Mangione, C.M.; Cabana, M.; Caughey, A.B.; Davis, E.M.; Donahue, K.E.; Doubeni, C.A.; Krist, A.H.; et al. Behavioral Counseling Interventions for Healthy Weight and Weight Gain in Pregnancy: US Preventive Services Task Force Recommendation Statement. JAMA 2021, 325, 2087–2093. [Google Scholar] [CrossRef]
- Cömert, T.K.; Akpinar, F.; Erkaya, S.; Durmaz, B.; Durmaz, R. The effect of gestational weight gain on serum total oxidative stress, total antioxidant capacity and gut microbiota. Biosci Microbiota Food Health 2022, 41, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, S.N.; Madan, J.C.; Karagas, M.R.; Morrison, H.G.; Hoen, A.G.; Christensen, B.C. Microbial Communities in Human Milk Relate to Measures of Parental Weight. Front Microbiol 2019, 10, 2886. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Macías, E.; Selma-Royo, M.; Martínez-Costa, C.; Collado, M.C. Breastfeeding Practices Influence the Breast Milk Microbiota Depending on Pre-Gestational Parental BMI and Weight Gain over Pregnancy. Nutrients 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Daiy, K.; Harries, V.; Nyhan, K.; Marcinkowska, U.M. Parental weight status and the composition of the human milk microbiome: A scoping review. PLoS One 2022, 17, e0274950. [Google Scholar] [CrossRef] [PubMed]
- Aagaard, K.; Ma, J.; Antony, K.M.; Ganu, R.; Petrosino, J.; Versalovic, J. The placenta harbors a unique microbiome. Sci Transl Med 2014, 6, 237ra265. [Google Scholar] [CrossRef] [PubMed]
- Baumann-Dudenhoeffer, A.M.; D'Souza, A.W.; Tarr, P.I.; Warner, B.B.; Dantas, G. Infant diet and parental gestational weight gain predict early metabolic maturation of gut microbiomes. Nat Med 2018, 24, 1822–1829. [Google Scholar] [CrossRef]
- Freitas, R.G.B.O.; Vasques, A.C.J.; Fernandes, G.R.; Ribeiro, F.B.; Solar, I.; Barbosa, M.G.; Almeida-Pititto, B.; Geloneze, B.; Ferreira, S.R.G. Gestational weight gain and visceral adiposity in adult offspring: Is there a link with the fecal abundance of Acidaminococcus genus? Eur J Clin Nutr 2022, 76, 1705–1712. [Google Scholar] [CrossRef]
- Flegal, K.M.; Shepherd, J.A.; Looker, A.C.; Graubard, B.I.; Borrud, L.G.; Ogden, C.L.; Harris, T.B.; Everhart, J.E.; Schenker, N. Comparisons of percentage body fat, body mass index, waist circumference, and waist-stature ratio in adults. Am. J. Clin. Nutr. 2009, 89, 500–508. [Google Scholar] [CrossRef]
- Hung, W.C.; Hung, W.W.; Tsai, H.J.; Chang, C.C.; Chiu, Y.W.; Hwang, S.J.; Kuo, M.C.; Chen, S.C.; Dai, C.Y.; Tsai, Y.C. The Association of Targeted Gut Microbiota with Body Composition in Type 2 Diabetes Mellitus. Int J Med Sci 2021, 18, 511–519. [Google Scholar] [CrossRef]
- Chleilat, F.; Schick, A.; Deleemans, J.M.; Ma, K.; Alukic, E.; Wong, J.; Noye Tuplin, E.W.; Nettleton, J.E.; Reimer, R.A. Paternal high protein diet modulates body composition, insulin sensitivity, epigenetics, and gut microbiota intergenerationally in rats. FASEB J 2021, 35, e21847. [Google Scholar] [CrossRef]
- Natividad, J.M.; Lamas, B.; Pham, H.P.; Michel, M.L.; Rainteau, D.; Bridonneau, C.; da Costa, G.; van Hylckama Vlieg, J.; Sovran, B.; Chamignon, C.; et al. Bilophila wadsworthia aggravates high fat diet induced metabolic dysfunctions in mice. Nat Commun 2018, 9, 2802. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, H.D.; Catalano, P.; Zhang, C.; Desoye, G.; Mathiesen, E.R.; Damm, P. Gestational diabetes mellitus. Nat Rev Dis Primers 2019, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Hasain, Z.; Mokhtar, N.M.; Kamaruddin, N.A.; Mohamed Ismail, N.A.; Razalli, N.H.; Gnanou, J.V.; Raja Ali, R.A. Gut Microbiota and Gestational Diabetes Mellitus: A Review of Host-Gut Microbiota Interactions and Their Therapeutic Potential. Front Cell Infect Microbiol 2020, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Thonusin, C.; Chattipakorn, N.; Chattipakorn, S.C. Impacts of gut microbiota on gestational diabetes mellitus: a comprehensive review. Eur J Nutr 2021, 60, 2343–2360. [Google Scholar] [CrossRef] [PubMed]
- Mijatovic-Vukas, J.; Capling, L.; Cheng, S.; Stamatakis, E.; Louie, J.; Cheung, N.W.; Markovic, T.; Ross, G.; Senior, A.; Brand-Miller, J.C.; et al. Associations of Diet and Physical Activity with Risk for Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. Nutrients 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zheng, J.; Shi, W.; Du, N.; Xu, X.; Zhang, Y.; Ji, P.; Zhang, F.; Jia, Z.; Wang, Y.; et al. Dysbiosis of parental and neonatal microbiota associated with gestational diabetes mellitus. Gut 2018, 67, 1614–1625. [Google Scholar] [CrossRef] [PubMed]
- Rafat, D.; Singh, S.; Nawab, T.; Khan, F.; Khan, A.U.; Khalid, S. Association of vaginal dysbiosis and gestational diabetes mellitus with adverse perinatal outcomes. Int J Gynaecol Obstet 2022, 158, 70–78. [Google Scholar] [CrossRef]
- Mokkala, K.; Houttu, N.; Vahlberg, T.; Munukka, E.; Rönnemaa, T.; Laitinen, K. Gut microbiota aberrations precede diagnosis of gestational diabetes mellitus. Acta Diabetol 2017, 54, 1147–1149. [Google Scholar] [CrossRef]
- Koren, O.; Goodrich, J.K.; Cullender, T.C.; Spor, A.; Laitinen, K.; Bäckhed, H.K.; Gonzalez, A.; Werner, J.J.; Angenent, L.T.; Knight, R.; et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 2012, 150, 470–480. [Google Scholar] [CrossRef]
- Dualib, P.M.; Taddei, C.R.; Fernandes, G.; Carvalho, C.R.S.; Sparvoli, L.G.; Silva, I.T.; Mattar, R.; Ferreira, S.R.G.; Dib, S.A.; Almeida-Pititto, B. Gut Microbiota across Normal Gestation and Gestational Diabetes Mellitus: A Cohort Analysis. Metabolites 2022, 12. [Google Scholar] [CrossRef]
- Zeng, M.Y.; Inohara, N.; Nunez, G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol. 2017, 10, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Qin, Y.; Chen, M.; Zhang, Y.; Wang, X.; Dong, T.; Chen, G.; Sun, X.; Lu, T.; White, R.A.; et al. Gestational diabetes mellitus is associated with the neonatal gut microbiota and metabolome. BMC Med 2021, 19, 120. [Google Scholar] [CrossRef]
- Crusell, M.K.W.; Hansen, T.H.; Nielsen, T.; Allin, K.H.; Rühlemann, M.C.; Damm, P.; Vestergaard, H.; Rørbye, C.; Jørgensen, N.R.; Christiansen, O.B.; et al. Gestational diabetes is associated with change in the gut microbiota composition in third trimester of pregnancy and postpartum. Microbiome 2018, 6, 89. [Google Scholar] [CrossRef]
- Hu, J.; Nomura, Y.; Bashir, A.; Fernandez-Hernandez, H.; Itzkowitz, S.; Pei, Z.; Stone, J.; Loudon, H.; Peter, I. Diversified microbiota of meconium is affected by parental diabetes status. PLoS One 2013, 8, e78257. [Google Scholar] [CrossRef] [PubMed]
- Soderborg, T.K.; Carpenter, C.M.; Janssen, R.C.; Weir, T.L.; Robertson, C.E.; Ir, D.; Young, B.E.; Krebs, N.F.; Hernandez, T.L.; Barbour, L.A.; et al. Gestational Diabetes Is Uniquely Associated With Altered Early Seeding of the Infant Gut Microbiota. Front Endocrinol (Lausanne) 2020, 11, 603021. [Google Scholar] [CrossRef]
- Selma-Royo, M.; García-Mantrana, I.; Calatayud, M.; Parra-Llorca, A.; Martínez-Costa, C.; Collado, M.C. Parental diet during pregnancy and intestinal markers are associated with early gut microbiota. Eur J Nutr 2021, 60, 1429–1442. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, A.Z.; Moore, S.E.; Okala, S.G. Impact of Parental Nutritional Supplementation during Pregnancy and Lactation on the Infant Gut or Breastmilk Microbiota: A Systematic Review. Nutrients 2021, 13. [Google Scholar] [CrossRef]
- Chu, D.M.; Antony, K.M.; Ma, J.; Prince, A.L.; Showalter, L.; Moller, M.; Aagaard, K.M. The early infant gut microbiome varies in association with a parental high-fat diet. Genome Med 2016, 8, 77. [Google Scholar] [CrossRef]
- Savage, J.H.; Lee-Sarwar, K.A.; Sordillo, J.E.; Lange, N.E.; Zhou, Y.; O'Connor, G.T.; Sandel, M.; Bacharier, L.B.; Zeiger, R.; Sodergren, E.; et al. Diet during Pregnancy and Infancy and the Infant Intestinal Microbiome. J Pediatr 2018, 203, 47–54.e44. [Google Scholar] [CrossRef]
- Garcia-Mantrana, I.; Selma-Royo, M.; Gonzalez, S.; Parra-Llorca, A.; Martinez-Costa, C.; Collado, M.C. Distinct parental microbiota clusters are associated with diet during pregnancy: impact on neonatal microbiota and infant growth during the first 18 months of life. Gut Microbes 2020, 11, 962–978. [Google Scholar] [CrossRef]
- Barrett, H.L.; Gomez-Arango, L.F.; Wilkinson, S.A.; McIntyre, H.D.; Callaway, L.K.; Morrison, M.; Dekker Nitert, M. A Vegetarian Diet Is a Major Determinant of Gut Microbiota Composition in Early Pregnancy. Nutrients 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.B.; Benny, P.; Riel, J.; Boushey, C.; Perez, R.; Khadka, V.; Qin, Y.; Maunakea, A.K.; Lee, M.J. Adherence to Mediterranean diet impacts gastrointestinal microbial diversity throughout pregnancy. BMC Pregnancy Childbirth 2021, 21, 558. [Google Scholar] [CrossRef] [PubMed]
- Shively, C.A.; Register, T.C.; Appt, S.E.; Clarkson, T.B.; Uberseder, B.; Clear, K.Y.J.; Wilson, A.S.; Chiba, A.; Tooze, J.A.; Cook, K.L. Consumption of Mediterranean versus Western Diet Leads to Distinct Mammary Gland Microbiome Populations. Cell Rep 2018, 25, 47–56.e43. [Google Scholar] [CrossRef] [PubMed]
- Furet, J.P.; Firmesse, O.; Gourmelon, M.; Bridonneau, C.; Tap, J.; Mondot, S.; Dore, J.; Corthier, G. Comparative assessment of human and farm animal faecal microbiota using real-time quantitative PCR. FEMS Microbiol. Ecol. 2009, 68, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Ozato, N.; Yamaguchi, T.; Mori, K.; Katashima, M.; Kumagai, M.; Murashita, K.; Katsuragi, Y.; Tamada, Y.; Kakuta, M.; Imoto, S.; et al. Two Blautia Species Associated with Visceral Fat Accumulation: A One-Year Longitudinal Study. Biology (Basel) 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.Y.; Tung, Y.T.; Yang, Y.S.H.; Hsu, J.B.; Lee, C.Y.; Chang, T.H.; Su, E.C.; Hsieh, R.H.; Chen, Y.C. Parental Vegetable and Fruit Consumption during Pregnancy and Its Effects on Infant Gut Microbiome. Nutrients 2021, 13. [Google Scholar] [CrossRef]
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L.; et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014, 20, 159–166. [Google Scholar] [CrossRef]
- Lundgren, S.N.; Madan, J.C.; Emond, J.A.; Morrison, H.G.; Christensen, B.C.; Karagas, M.R.; Hoen, A.G. Parental diet during pregnancy is related with the infant stool microbiome in a delivery mode-dependent manner. Microbiome 2018, 6, 109. [Google Scholar] [CrossRef]
- Won, T.J.; Kim, B.; Oh, E.S.; Bang, J.S.; Lee, Y.J.; Yoo, J.S.; Yu, H.; Yoon, J.; Hyung, K.E.; Park, S.Y.; et al. Immunomodulatory activity of Lactobacillus strains isolated from fermented vegetables and infant stool. Can. J. Physiol. Pharmacol. 2011, 89, 429–434. [Google Scholar] [CrossRef]
- Sun, M.; Luo, J.; Liu, H.; Xi, Y.; Lin, Q. Can Mixed Strains of Lactobacillus and Bifidobacterium Reduce Eczema in Infants under Three Years of Age? A Meta-Analysis. Nutrients 2021, 13. [Google Scholar] [CrossRef]
- Warner, B.B.; Deych, E.; Zhou, Y.; Hall-Moore, C.; Weinstock, G.M.; Sodergren, E.; Shaikh, N.; Hoffmann, J.A.; Linneman, L.A.; Hamvas, A.; et al. Gut bacteria dysbiosis and necrotising enterocolitis in very low birthweight infants: a prospective case-control study. Lancet 2016, 387, 1928–1936. [Google Scholar] [CrossRef] [PubMed]
- Claud, E.C.; Keegan, K.P.; Brulc, J.M.; Lu, L.; Bartels, D.; Glass, E.; Chang, E.B.; Meyer, F.; Antonopoulos, D.A. Bacterial community structure and functional contributions to emergence of health or necrotizing enterocolitis in preterm infants. Microbiome 2013, 1, 20. [Google Scholar] [CrossRef] [PubMed]
- de la Cochetiere, M.F.; Piloquet, H.; des Robert, C.; Darmaun, D.; Galmiche, J.P.; Roze, J.C. Early intestinal bacterial colonization and necrotizing enterocolitis in premature infants: the putative role of Clostridium. Pediatr. Res. 2004, 56, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Heida, F.H.; Harmsen, H.J.; Timmer, A.; Kooi, E.M.; Bos, A.F.; Hulscher, J.B. Identification of bacterial invasion in necrotizing enterocolitis specimens using fluorescent in situ hybridization. J. Perinatol. 2017, 37, 67–72. [Google Scholar] [CrossRef]
- Davidson, S.J.; Barrett, H.L.; Price, S.A.; Callaway, L.K.; Dekker Nitert, M. Probiotics for preventing gestational diabetes. Cochrane Database Syst Rev 2021, 4, CD009951. [Google Scholar] [CrossRef] [PubMed]
- Pellonperä, O.; Vahlberg, T.; Mokkala, K.; Houttu, N.; Koivuniemi, E.; Tertti, K.; Rönnemaa, T.; Laitinen, K. Weight gain and body composition during pregnancy: a randomised pilot trial with probiotics and/or fish oil. Br J Nutr 2021, 126, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Houttu, N.; Mokkala, K.; Saleem, W.T.; Virtanen, S.; Juhila, J.; Koivuniemi, E.; Pellonperä, O.; Tertti, K.; Luokola, P.; Sorsa, T.; et al. Potential pathobionts in vaginal microbiota are affected by fish oil and/or probiotics intervention in overweight and obese pregnant women. Biomed Pharmacother 2022, 149, 112841. [Google Scholar] [CrossRef]
- Xu, Y.P.; Hu, J.M.; Huang, Y.Q.; Shi, L.P. Parental Ureaplasma exposure during pregnancy and the risk of preterm birth and BPD: a meta-analysis. Arch. Gynecol. Obstet. 2022, 306, 1863–1872. [Google Scholar] [CrossRef]
- Dawe, J.P.; McCowan, L.M.E.; Wilson, J.; Okesene-Gafa, K.A.M.; Serlachius, A.S. Probiotics and Parental Mental Health: A Randomised Controlled Trial among Pregnant Women with Obesity. Sci Rep 2020, 10, 1291. [Google Scholar] [CrossRef]
- Teoh, C.M.; Cooper, A.; Renteria, K.M.; Lane, M.; Zhu, J.; Koh, G.Y. Supplementation of Methyl-Donor Nutrients to a High-Fat, High-Sucrose Diet during Pregnancy and Lactation Normalizes Circulating 25-Dihydroxycholecalciferol Levels and Alleviates Inflammation in Offspring. Metabolites 2022, 12. [Google Scholar] [CrossRef]
- Antoun, E.; Kitaba, N.T.; Titcombe, P.; Dalrymple, K.V.; Garratt, E.S.; Barton, S.J.; Murray, R.; Seed, P.T.; Holbrook, J.D.; Kobor, M.S.; et al. Parental dysglycaemia, changes in the infant's epigenome modified with a diet and physical activity intervention in pregnancy: Secondary analysis of a randomised control trial. PLoS Med 2020, 17, e1003229. [Google Scholar] [CrossRef] [PubMed]
- Sandborg, J.; Söderström, E.; Henriksson, P.; Bendtsen, M.; Henström, M.; Leppänen, M.H.; Maddison, R.; Migueles, J.H.; Blomberg, M.; Löf, M. Effectiveness of a Smartphone App to Promote Healthy Weight Gain, Diet, and Physical Activity During Pregnancy (HealthyMoms): Randomized Controlled Trial. JMIR Mhealth Uhealth 2021, 9, e26091. [Google Scholar] [CrossRef] [PubMed]
| Maternal Factor | Diversity | Increased Abundance | Decreased Abundance |
|---|---|---|---|
| Elevated pre-pregnancy BMI | Potentially decreased diversity [13] | Firmicutes [14], Bacteroides [16], Clostridium[16], S. aureus [16] Biolphila [17], Roseburia [17], Dialster [17], | Proteobacteria [14], Phascolarctobacterium [17] |
| Underweight | Potentially decreased [35,43,46] | Acidaminococcus [47] | Firmicutes [44], Bacteroidetes [44] |
| Excessive Gestational Weight Gain | Prevotella [17], Dialister [17], Firmicutes [49], Bacteroidetes [49] | Bifidobacterium [16] | |
| Gestational Diabetes | Ruminococcaceae family [66], Faecalibacterium [67], Eubacterium [67], Streptococcus [67], Enterobacteriaceae family [67], Bacteroides [68], | ||
| Fat Intake | Increased Simpson diversity [17] | Ruminococcus [16,17], Paraprevotella [17] | Bacteroidetes, Firmicutes [74] |
| Vegetable Intake | Roseburia [79], Lachnospiraceae [79] | Collinsella [79], Holdemania, Eubacterium [79] | |
| Animal Protein Intake | Increased Shannon diversity [17] | Collinsella [17] | |
| Carbohydrate Intake | Bacteroidetes [74] |
| Maternal Factor | Infant Gut Microbiome Diversity | Infant Gut Microbiome Increased Abundance | Infant Gut Microbiome Decreased Abundance | Infant Gut Microbiome Functional Roles |
|---|---|---|---|---|
| Elevated Pre-pregnancy BMI | Increased [34] | Proteobacteria [14] Vaginal delivery infants [35]: Bacteroides fragilis, Escherichia coli, Veillonella dispar, Staphylococcus, Enterococcus |
Firmicutes [14] | Decreased butyrate production [36] |
| Gestational Weight Gain | Increased [34] | Akkermansia [34] | Enrichment of glucose and glycogen degradation pathways, increased phenylalanine, cysteine/serine, folate, thiamin, biotin, and pyridoxine synthesis pathways [56] | |
| Gestational Diabetes | Decreased [72] | Clostridium [73], Veillonella [73], Firmicutes [72], Streptooccus [66] | Proteobacteria [72], Lactobacillus [66,75], Flavonifractor [75], Erysipelotrichaceae [75], Gammoproteobacteria [75], Bacteroides [66] | |
| Maternal Fat Intake | Firmicutes [76] | Proteobacteria [76], Bacteroides [78] | ||
| Maternal Fruit and Vegetable Intake | Lactobacillus [79], Propionibacteriales [86], Priopionibacteriaceae [86], Cutibacterium [86], Tannerellaceae [86], Parabacteroides [86], Lactococcus [86] | Coprococcus [76], Blautia [76], Roseburia [76], Rumiococcaceae [76], Lachnospiracea [76] | ||
| Maternal Animal Protein | Veillonella [76], Escherichia/Shigella [76], Klebsiella [76], and Clostridium [76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





