1. Introduction
As an advanced drug controlled release profile, dual-step release (or biphasic release) has its advantages of endowing a fast therapeutic action effect and a long time period of constant blood drug concentration for the patients’ conveniences [
1,
2,
3,
4,
5,
6,
7]. In general, the first phase fast or pulsatile release of drug is able to promote the drug concentration in the blood to the therapeutic windows. Later, the second phase release is realized through a sustained or extended manner, by which the drug blood concentration is kept within the therapeutic window [
8,
9,
10,
11,
12,
13,
14,
15]. Thus their combination, on one hand, can promote the therapeutic effect. On the other hand, it can reduce the oral administration times for an increased patients’compliances [
16,
17,
18,
19].
Berberine hydrochloride (BH), a popular biomolecule having broad functional performances, is a typical poorly water-soluble drug. It can be extracted from coptis chinensis and belongs to a chemical drug purified from traditional Chinese medicine. BH has a broad antibacterial spectrum and has antibacterial effects on a variety of gram positive and negative bacteria, including hemolytic streptococci, Staphylococcus aureus, Vibrio cholera, Meningococci, Shigella dysentery bacilli, Typhoid bacilli, and Diphtheria bacilli [
20]. At low concentrations, they inhibit bacteria, while at high concentrations, they kill bacteria. BH also has certain inhibitory effects on influenza viruses, amoeba, leptospira, and certain skin fungi [
21]. In vitro experiments have confirmed that BH can enhance the phagocytic ability of white blood cells and the hepatic reticuloendothelial system. BH has a therapeutic effect on helicobacter, and can alleviate gastritis, gastric and duodenal ulcers. It is reported that BH can reduce the number of pili on the surface of the bacterial body, preventing bacteria from adhering to human cells, and thus has the therapeutic functional performance [
22]. BH has no cross resistance to penicillin, streptomycin, etc. However, the oral absorption of BH is very poor. What is more, it quickly enters various organs and tissues and distributed widely (mostly in the heart, bone, lung, and liver) after intramuscular injection, and the blood drug concentration is maintained to the level over the minimum inhibitory concentration for only a very short time period [
23]. Thus, a frequent oral administration of the commercial BH products (such as tablets, capsules, and pills) is needed for the patients. Biphasic release of BH may benefit an improved therapeutic effect after oral administration.
During the past several decades, although many new excipients (including organic materials such as polymers and lipids, inorganic materials, their composites and hybrids [
24,
25,
26,
27,
28,
29]) and new strategies have been frequently introduced into the pharmaceutical field for endowing the active ingredients a better therapeutic effect [
30,
31,
32,
33,
34], the mainstream is still the traditional pharmaceutical excipients. This is because these excipients have been demonstrated to be safe and compatible with organisms. Thus, for a certain drug, it is the material conversion methods that have played their important roles in endowing an improved functional application. Particularly in this nano era, nano fabrication methods are continuously adopted by pharmacists to convert the drug molecules and excipient molecules to medicated nanoproducts for realizing the designed therapeutic effects [
35,
36]. Numerous examples can be found in literature. One example is the electrospun nanofibers of traditional hydrophilic polymeric excipients (e.g. PVA, PVP, PEO, gelatin and so on) for fast dissolution and therapeutic action of a poorly water-soluble drug [
37,
38,
39,
40,
41]. Another example is the electrosprayed microparticles and electrospun nanofibers of conventional insoluble or biodegradable polymeric excipients (e.g. CA, EC, PLA, PCL, PAN and zein) for a designed drug sustained release profile [
42,
43,
44,
45,
46,
47].
Based on the hints from the above-mentioned studies, the present study studied a combination of electrospinning and electrospraying (both belong to the technique of electrohydrodynamic atomization, EHDA) [
48,
49,
50], by which a new types of hybrids consisting of both electrospun nanofibers and electrosprayed microparticles were fabricated for providing a biphasic release of BH. The prepared hybrids were experienced a series of characterizations including their morphology, the physical state and compatibility of the loaded components, and the in vitro drug controlled release profiles. Both the electrohydrodynamic atomization mechanism and drug biphasic release mechanism are proposed.
2. Materials and Methods
2.1. Materials
Berberine hydrochloride (BH, purity over 98%) was purchased from a local Laobaixing Drugstore (Shanghai, China). Cellulose acetate (Mw=30,000) was bought from Aldrich. Polyvinylpyrrolidone K90 (Mw=1,300,000) was purchased from Sigma-Aldrich Corp. (Shanghai, China) The solvents acetone, ethanol, Di-ChloroMethane (DCM) and N, N-Dimethylacetamide (DMAc) were obtained from Shanghai Fitst Shiji Factory (Shanghai, China). Water was double distilled just before use.
2.2. Fabrication Methods
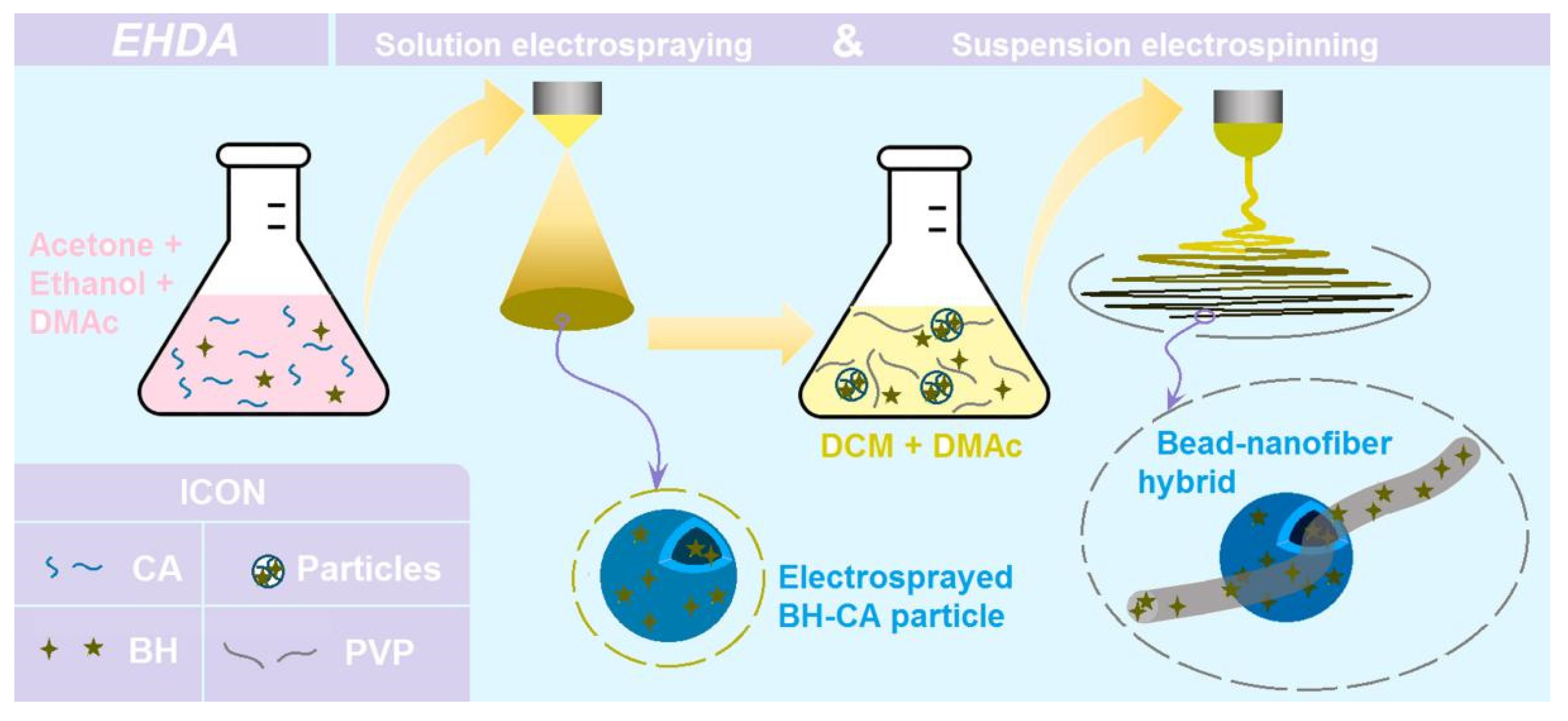
Two different EHDA processes (i.e. a single-fluid blending electrospraying and a single-fluid blending electrospinning) were arranged in a sequential manner for preparing the hybrids of electrospun nanofibers and electrosprayed microparticles.
According to literature, CA is soluble in a mixture of acetone:ethanol:DMAc with a volume ratio of 4:1:1. Meanwhile, the drug BH is soluble in DMAc, thus a co-dissolving working fluid containing CA and BH could be prepared for the single-fluid electrospraying process. After some pre-experiments, 15 g BH and 25 g CA were co-dissolved in 500 mL of the solvent mixture.
A homemade EHDA apparatus was exploited to conduct the electrospraying process. The experimental conditions include an applied voltage of 20 kV, a fluid flow rate of 1.0 mL/h, and a particle deposition distance of 20 cm from the nozzle of spinneret to the grounded collector. The environmental temperature and humidity were 21 ± 5 ℃ and 47 ± 7%, respectively.
An amount of 15.0 g microparticles E1 from electrospraying was suspended into 200 mL Fluid 2 uniformly through continuous stirring to form a suspension working fluid. During the electrospinning process, little sedimentation of the microparticles was observed, which should be attributed to the relatively lower density of electrosprayed microparticles.
2.3. Characterization
2.3.1. Morphology
The morphologies of the EHDA products were evaluated using a field-emission scanning electron microscope (SEM, Quanta FEG450, Hillsboro, USA). The SEM pictures were used to estimate the average diameters of the nanofibers in about 100 places using the ImageJ software (National Institutes of Health, Bethesda, USA). The sampling processes included fixing some powders E1 or a strip of E2 and E3 on a sample holder using a double-sided conductive adhesive, and a thin layer of Au was sprayed for 60 s before assessments under an applied voltage of 5.0 kV.
2.3.2. Physical state and compatibility among the components
X-ray diffraction (XRD) tests were carried out using the Bruker X-ray Powder diffractometer (Bruker-AXS, Karlsruhe, Germany). The raw materials and their fiber mats were measured within a 2θ angle range of 5°-60°. The applied voltage and working current were 40 kV and 30 mA, respectively. The rotation speed was 5° per minute).
Fourier transform infrared (FTIR) analyses were implemented using a PerkinElmer FTIR Spectrometer (Spectrum 100, Billerica, USA). The experiments were performed in range 500-4000 cm− 1 with a resolution of 2 cm− 1. The sampling for the solid materials included weighing 0.2 g of potassium bromide powder, grinding it with about 10 mg of the sample, pressing the mixture into solid tablets, and placing the tablets into the instrument for scanning.
2.3.3. Functional performances
The drug release profiles of the three EHDA products were assessed using the paddle method in accordance with the Chinese Pharmacopoeia (2020 Ed.). Approximately 200 mg of the EHDA products were placed into a vessel with 900 mL physiological saline. The dissolution media were maintained at 37 ± 1 ℃ and a rotation rate of 50 rpm. At predetermined time points, a 5.0 mL aliquot was withdrawn and filtered through a 0.22 μm membrane (Millipore, MA, USA). Five milliliters of fresh physiological saline was added to maintain a constant dissolution bulk volume. The amounts of BH released were measured at λmax=263 nm using a UV-vis spectrophotometer (UV-2102PC, Unico Instrument Co. Ltd., Shanghai, China). A calibration equation was pre-determined for calculating the BH concentration. The experimental results were reported as mean ± SD. All experiments were repeated six times.
3. Results and discussion
3.1. The sequential EHDA process
EHDA processes are hydrodynamic atomization procedures that are initiated by the applied high voltage, and are exploited to prepare solid products by taking advantages of the easy interactions of electrostatic energy and working fluids [
51,
52]. For electrospinning, the solid products are often nanofibers resulted from the continuous drawing of viscous polymer fluid jets [
53,
54,
55]. For electrospraying, the solid products are typically microparticles resulted from the fission and repelling of droplets [
56]. Thus, based on the capabilities of these two EHDA processes, a new types of trans-scale hybrids can be conceived as
Figure 1, and the kinds of EHDA products were fabricated according to the conditions listed in
Table 1.
Firstly, the water insoluble polymer CA and drug BH were co-dissolved into a solvent mixture containing acetone, ethanol and DMAc with a volume ratio of 4:1:1. After some pre-experiments, the solid microparticles could be prepared through a single-step and straightforward electrospraying process. Later, these microparticles were dispersed into the co-dissolving solution containing PVP and BH in a solvent mixture containing DCM and DMAc with a volume ratio of 5:1. The CA particles didn’t dissolve in the solvent. Thus, the working fluid was a suspension. After the electrospinning of the suspension, the hybrids containing both nanofibers of PVP and microparticles of CA were prepared, and the drug BH was distributed both in the nanofibers and also in the microparticles.
For a successful electrospinning process, the working fluid must be electrospinnable [
57,
58]. Thus a relatively high polymer concentration is needed to keep enough physical entanglements of polymeric molecules in the working fluid, by which the electrostatic drawing can be resisted for elongating the fluid jets till the formation of nanofibers [
59,
60,
61]. However, for a successful electrospraying process, the working fluid needs only to be solidifiable, i.e. the effective removement of organic solvents [
62]. Thus the polymer concentration is often lower than the electrospinnable one. Showed in
Figure 2a is the typical digital picture taken from the electrospraying processes for fabricating the microparticles E1. At the top of Taylor cone, an opposite cone can be observed for the Coulombic expansion. The “white” section region, indicated by a red arrow, was formed by the fast moving speed of the fast splitted droplets. As the droplets splitted and reduced their sizes and weights, their movings were decelerated, which can be obviously recorded by the digital camera (30 frames per second). The PVP-BH solution had very fine electrospinnability, a typical working process in given in
Figure 2b, by which the composite nanofibers E2 were fabricated.
Figure 2c exhibited the typical suspension electrospinning process for producing hybrids of E3.
The comparison between
Figure 2b,c can tell the influence of the added CA microparticles on the electrospinning processes. Although under the same experimental conditions (a fixed applied voltage, flow rate and a collected distance of 12 kV, 2.0 mL/h, and 20 cm, respectively), the three sections (Taylor cone, straight fluid jet, and bending and whipping region [
63,
64,
65,
66]) have significant differences. The Taylor cone and straight fluid jet of the treated suspension had a larger volume and a longer size than those of treated PVP solution. Apparently, the “large” weight of microparticles had played their role in enlarging the Taylor cone and elongating the straight fluid jet. Furthermore, in the bending and whipping process of the unstable regions, it is clear that the bright dots, formed by the microparticles, are always there from the end of straight fluid jet to the deposition just above the collector. Meanwhile, the suspension jets movings showed the unsmooth polygonal lines, different with the smooth and continuous lines of solution jets movement trajectory. Still, the microparticles clung on the jets had resulted in these complex phenomena. During the electrospinning of suspension for fabricating hybrids E3, the fluid jets were more easily separated when a small elevation of the applied voltage to 15 kV. A typical record is shown in
Figure 2d. After separation into two branches, the branced contained more microparticles mainly moved downwards owing to the “heavy” microparticles. Meanwhile, the easy aggregation of electric charges on the surface of microparticles should be also a reason for easy separation under a higher voltage. During all the processes, clogging of spinneret was seldom observed.
3.2. The morphologies of the resultant products
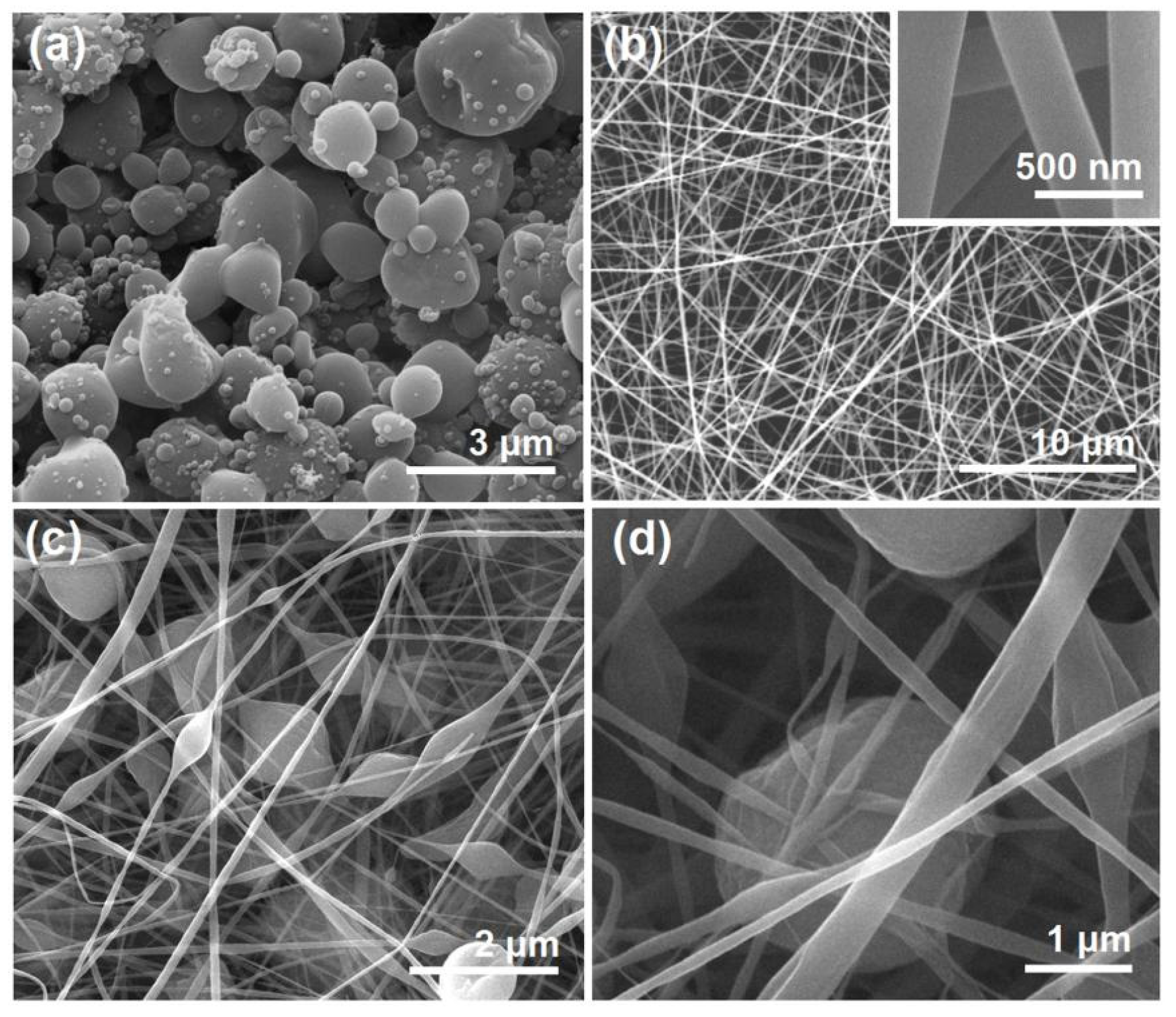
The morphologies of the different EHDA products are included in
Figure 3. Interestingly, there are many satellites around the electrosprayed microparticles E1 (
Figure 3a). For the application of drug sustained release, these satellites may be a negative factor due to the extremely small size and correspondingly the short routes for the loaded drug molecules diffusion from them to the bulk solutions. Just as anticipated, the electrospun BH-PVP nanofibers E2 have fine linear morphology without any discerned beads or spindles (
Figure 3b). Meanwhile, an enlarged image in the up-right inset of
Figure 3b indicates that these nanofibers have a very smooth surface without the possible drug particles formed by phase separation during the storage process. The electrospun hybrids from the suspensions exhibited a typical “mixture” of beads or spindles and nanofibers, as indicated by
Figure 3c,d.
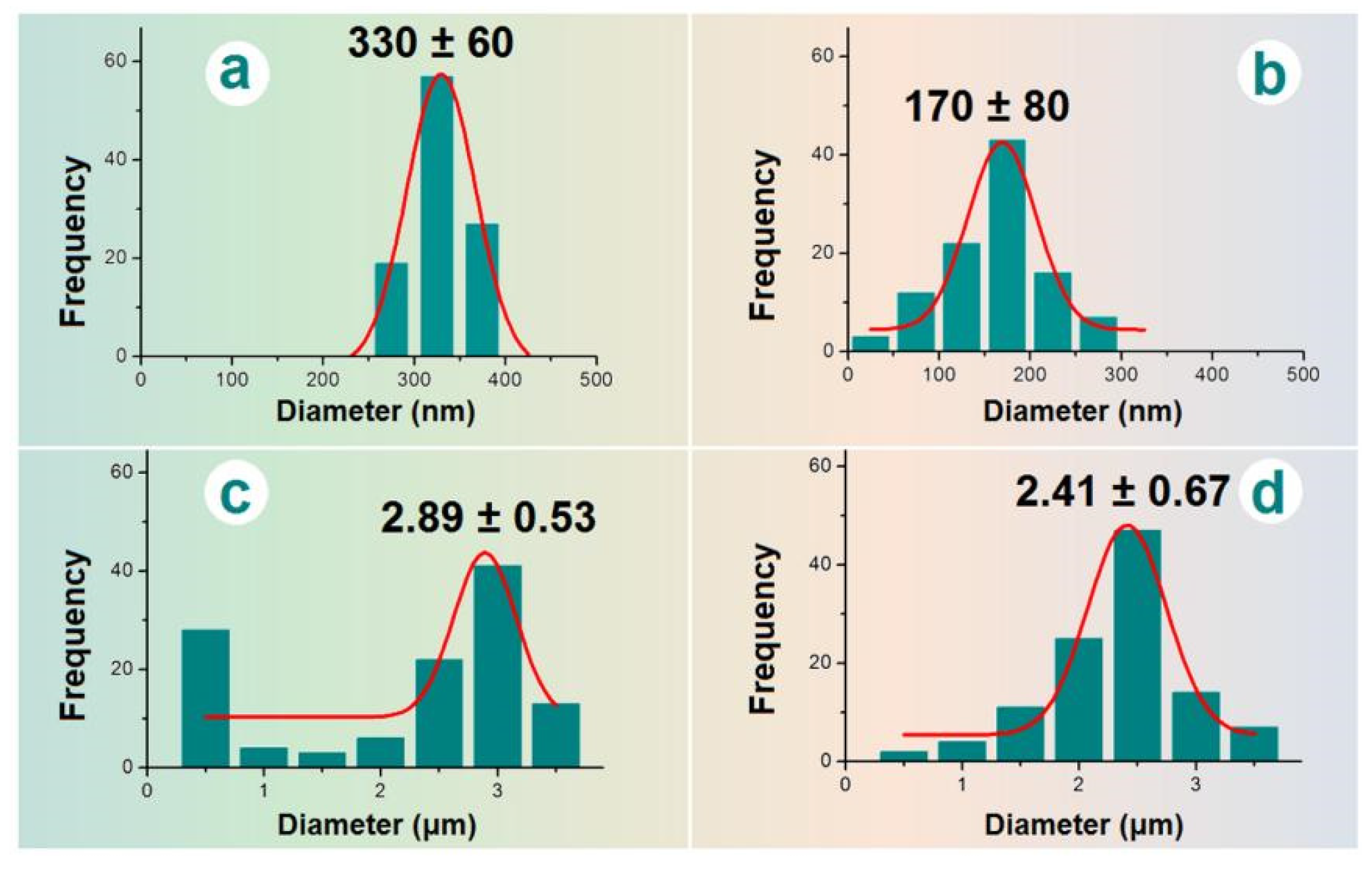
The sizes and size distributions of these EHDA products were estimated using Image J software. All the results are concluded in
Figure 4. The average diameters of nanofibers E2 were 330 ± 60 nm (
Figure 4a). In sharp contrast, the diameters of the nanofiber sections in the electrospun hybrids E3 were only 170 ± 80 nm (
Figure 4b). The difference was a direct result of the added microparticles, whose presence should increase the drawing effects on the moving fluid jets, and in turn a significant reduction of the nanofibers’ diameter. The electrosprayed microparticles E2 have an average diameter of 2.89 ± 0.53 μm (
Figure 4c). In contrast, the diameter of particular sections in electrospun hybrids E3 was 2.41 ± 0.67 μm (
Figure 4d), a slightly reduction of the average size. This indicates that the re-dispersing of microparticles E1 in the solvent mixture of DCM and DMAc for preparing the suspension working fluid may result in a little influence on their final morphology and size. Although CA is insoluble in DCM and DMAc, the drug BH distributed on the surface of microparticles may re-dissolve into the suspensions and build a dynamic balance between the surfaces of microparticles and the bulk suspensions.
Although no general theory for instructing the implementation of EHDA processes, there are abundant suggested mechanisms in literature for the treatment of a certain working fluid, both for electrospinning and electrospraying. Those mechanisms are important for the continuous and robust production and duplication of the EHDA products. Meanwhile, as more and more EHDA products are going into the commercial markets, these mechanisms for optimizing the production processes, and other related issues such as energy-saving, safety implementation, and environmental friendliness and projecting their places for the final social benefits and a better people life [
67,
68,
69,
70]. In this study, the mechanism for strange phenomenon of many satellites is diagrammed in
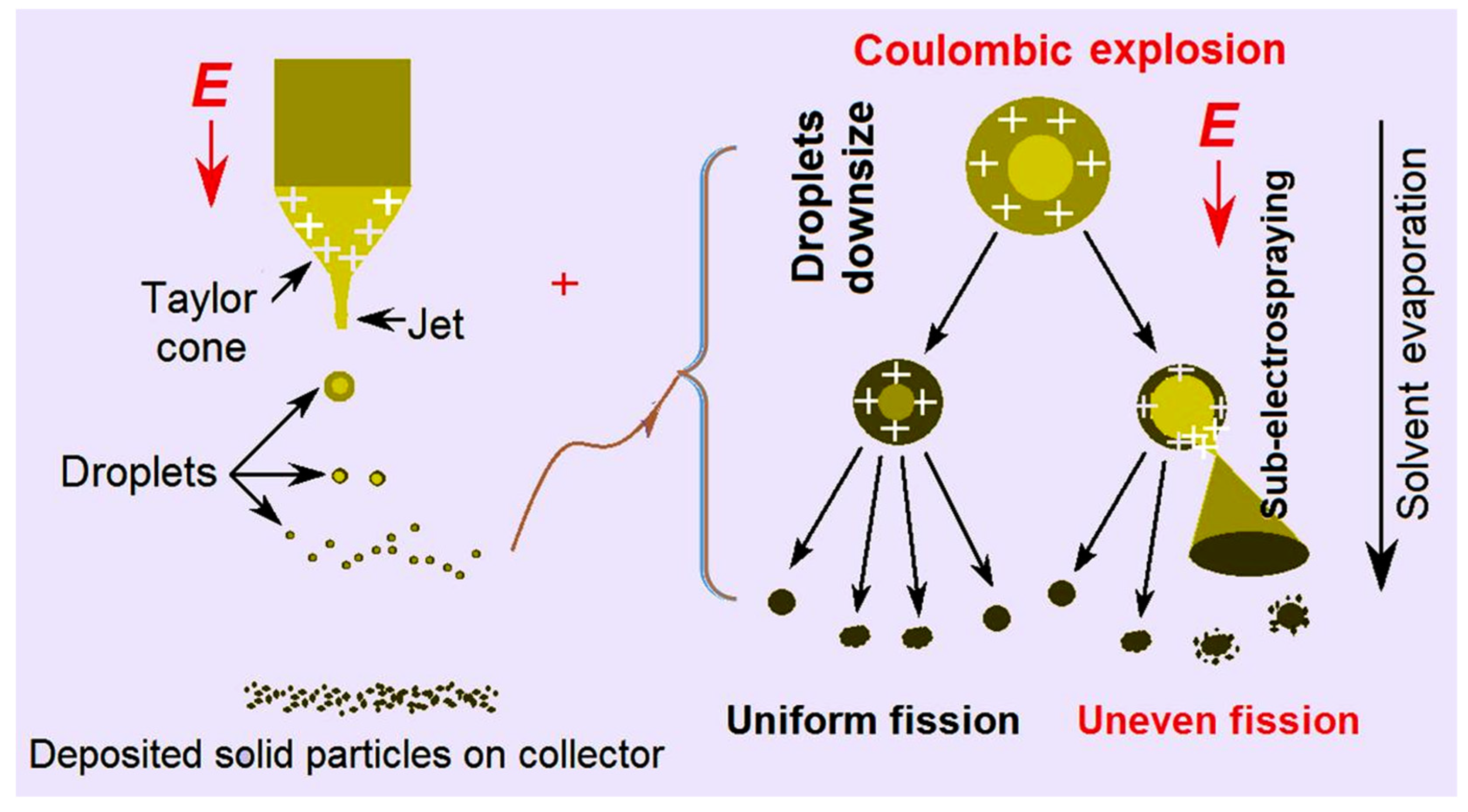
Figure 5. In the left part, a whole electrospraying process is sketched, i.e. a Taylor one, to a convergent point, and later to the Coulombic explosion region, in which the droplets are continuously splitted and reduced, until the formation and deposition of solid particles on the collector.
In the right part of
Figure 5, two different fission processes are sketched. One kind is the uniform fission, by which the final electrosprayed particles were generated, as indicated in
Figure 3a where the surfaces of microparticles are smooth. The other kind is uneven fission, by which many satellites are formed during the electrospraying processes and are around the electrosprayed microparticles (
Figure 3a). Their diameters are estimated to be several decades nanometers. In the electrospraying solution, the three solvents acetone, ethanol and DMAc have their own’s main uses. CA is soluble in acetone and BH is soluble in DMAc. Although ethanol is a non-solvent for both BH and CA, it is useful for keeping the stretching state of CA molecules, and in turn for promoting a stable and robust EHDA process. However, these solvents have different boiling points ( 56 ℃, 78 ℃, and 164 ℃ for acetone, ethanol and DMAc, respectively) and different volatility. During the Coulombic explosion processes, it is possible that some droplets have more DMAc and keep a longer time period of the fluid state. Meanwhile, the surface charges may have different densities. Under these conditions, some sub-electrospraying processes may occur, by which the satellites are formed around the microparticles.
3.3. The physical state and compatibility
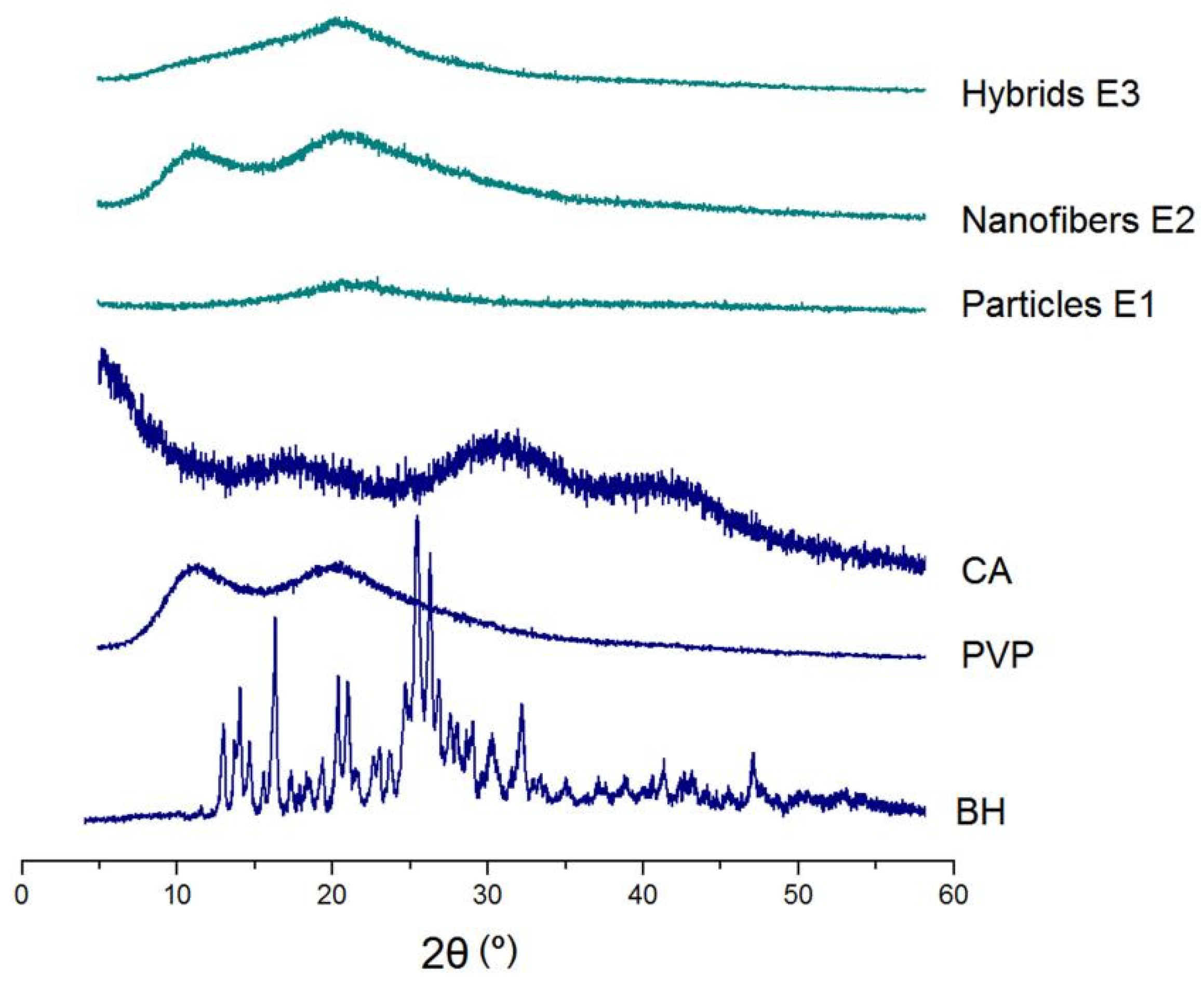
XRD patterns of the raw materials (CA, PVP and BH) and their EHDA products (Hybrids E3, nanofibers E2 and their combinations) are included in
Figure 6. Just as anticipated, BH has many sharp brag peaks in its pattern due to the crystalline state of the original powders. In contrast, the hydrophilic polymer PVP K90 and insoluble polymer CA are all amorphous materials and thus only humps on their XRD patterns. The three EHDA products, i.e. the electrosprayed microparticles E1, the electrospun nanofibers E2 and the electrospun hybrids E3, show no any sharp peaks in their patterns. These phenomena suggested that the drug BH has been converted into amorphous composites in all of them after the electrospraying or electrospinning processes. An amorphous state of the drug is favorable for its dissolution and the manipulation of a certain controlled release profile from the matrices [
71,
72].
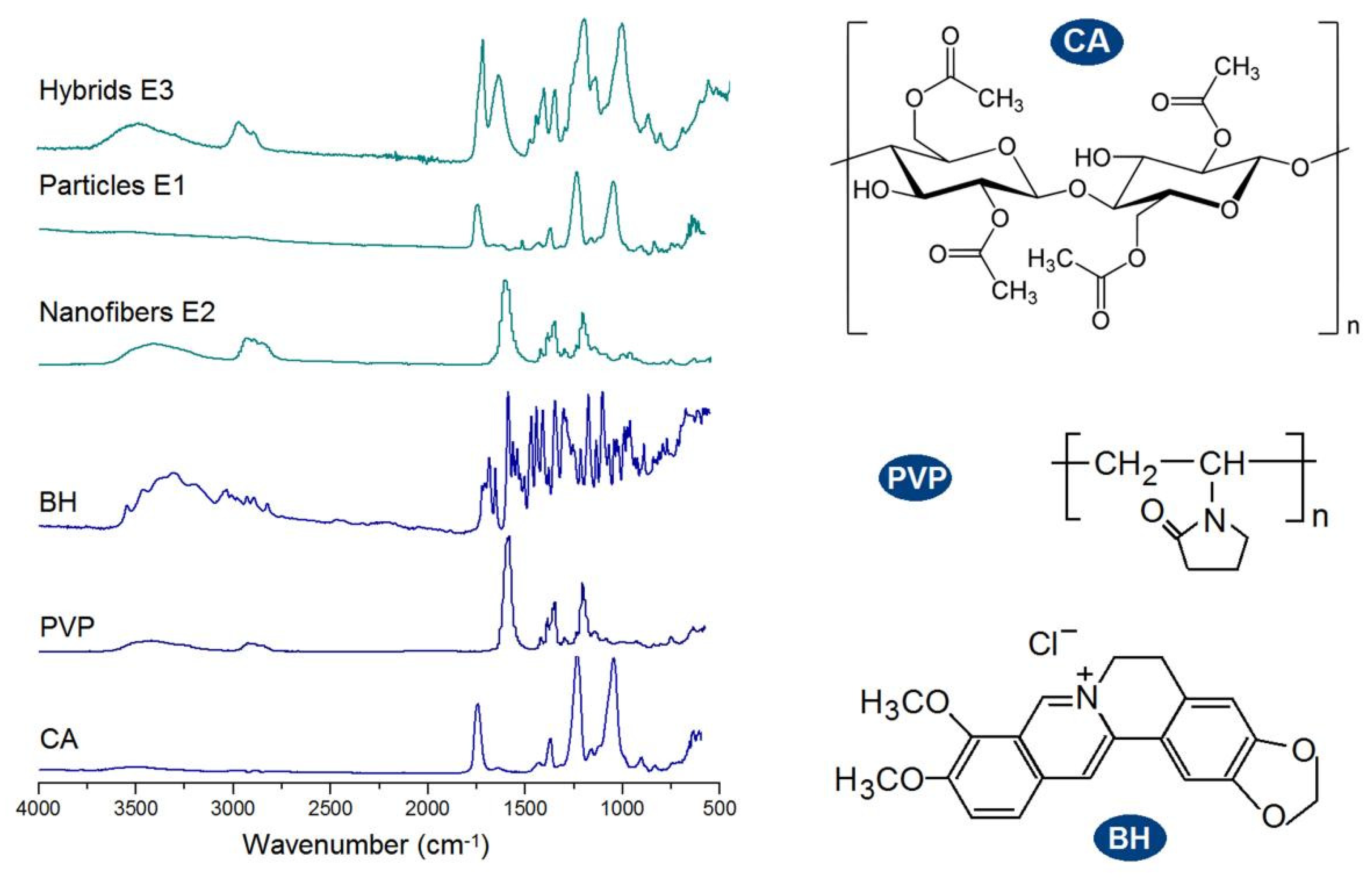
FTIR spectra of the raw materials (CA, PVP and BH) and their EHDA products are concluded in the left part of
Figure 7. And the molecular formats of CA, PVP and BH are shown in the right part of
Figure 7. CA has its characteristic peaks at 1724, 1376, 1236, and 1051 cm
-1. PVP has its characteristic peaks at 1662, 1423, and 1291 cm
-1. BH has the typical absorbance of 1740, 1598 and 1504 cm
-1 owing to the three benzene rings in one BH molecule. Compared with the raw materials, the spectra of microparticles E1 are almost the same as CA with little hints from BH, suggesting E1 particles are composites. Similarly, the spectra of electrospun nanofibers E2 are similar to PVP, giving a hint that BH formed composites with PVP. In spectra of E1 and E2, the substantial decrease and even disappearance in the intensities of characteristic peaks and peaks in the finger regions of BHC should be attributed to the secondary interactions between the drug BH and the polymeric carriers. These secondary interactions include hydrogen bonding, hydrophobic interactions, and electrostatic interactions, which favor the compatibility between the drug and its carrier and are beneficial to the stability of formed binary composites [
73,
74]. Compared with spectra of E1 and E2, the hybrids E3’s spectra, on one hand, has also no BH sharp peaks and thus suggest an amorphous state of BH in them. On the other hand, E3’s spectra is a superimposition of the spectra of E1 and E2 to a certain extent, suggesting that the ternary EHDA products E3 are hybrids of the two binary composites.
3.4. In vitro drug release profiles
The pre-determined calibration equation for BH was
A = 0.0688*
C − 0.0047 (R=0.9999, and a linear range of 0.5 to 50 μg/mL), where
A and
C represent absorbance and BH concentration in μg/mL, respectively. The
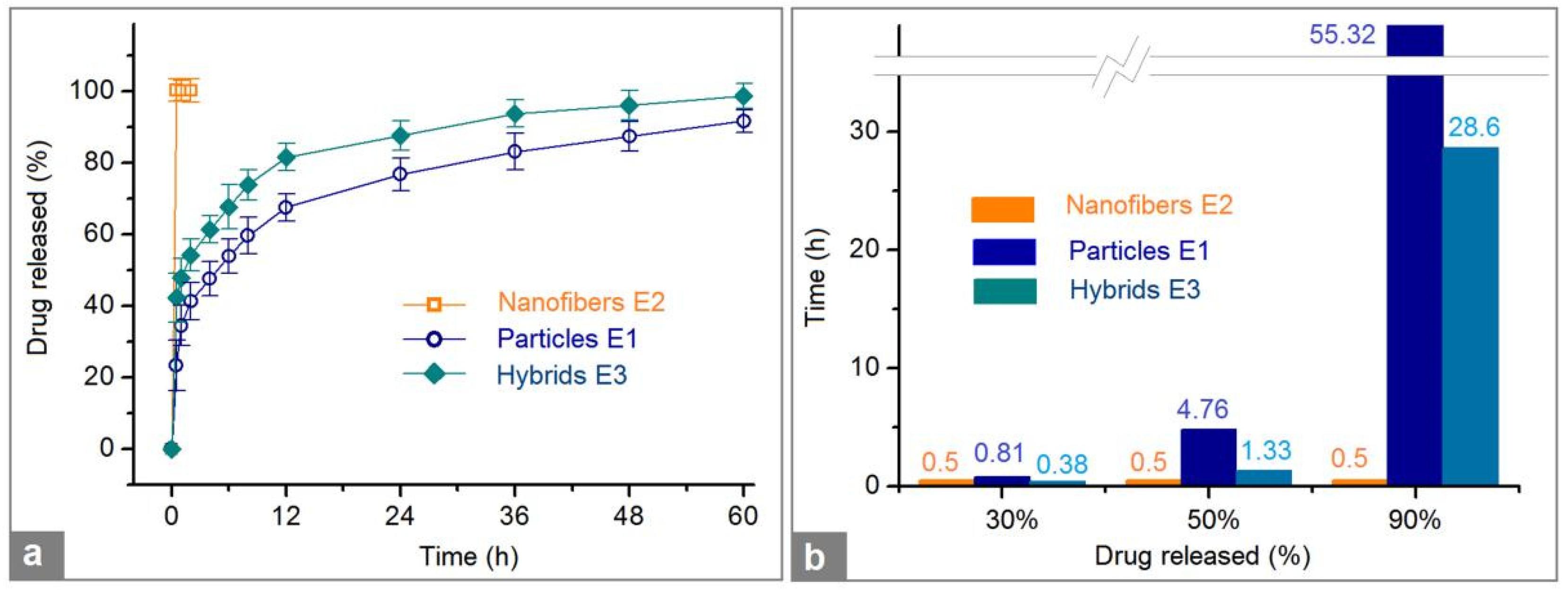
in vitro dissolution test results of the three types of EHDA products are concluded in
Figure 8. In
Figure 8a, the lines were drawn according to the drug accumulative release percentage (%) vs sampling time point (h), and in
Figure 8b, the results are expressed according to the estimated durations vs a certain percentage of BH (30%, 50%, and 90%).
The first pre-determined time point for sampling was 0.5 h after the samples were placed into the dissolution media. The electrospun nanofibers E2 had released all the loaded BH through an erosion mechanism, i.e. the drug BH and the polymeric matrix PVP were co-dissolved into the dissolution media. In
Figure 8a, the release profile shows a straight line, and in
Figure 8b, all the times for releasing 30%, 50%, and 90% BH are indiscriminate 0.5 h.
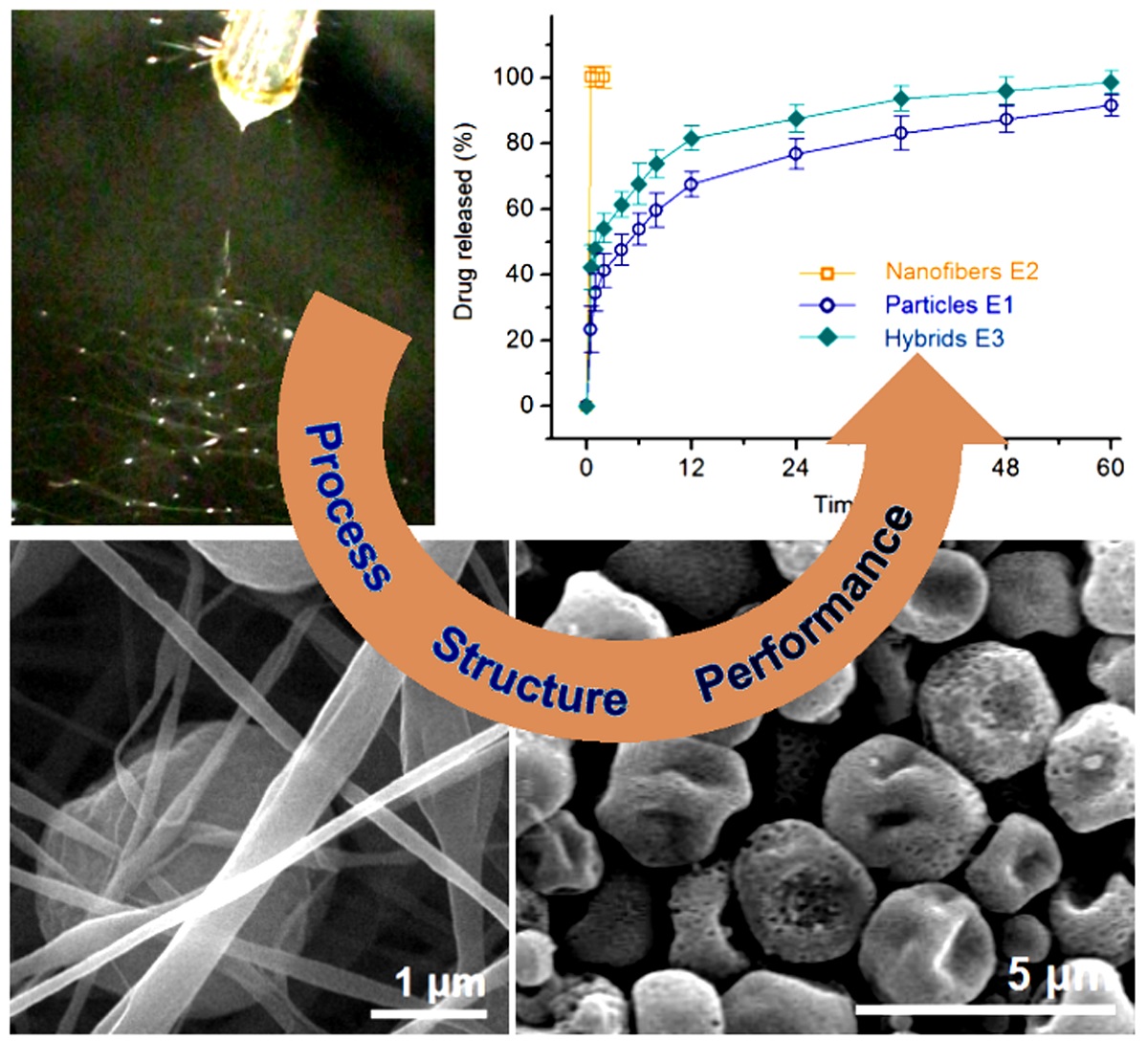
Compared with a 100% percentage release in 0.5 and 1 h, the electrosprayed microparticles E2 and electrospun hybrids E3 released 23.4 ± 7.1% and 42.3 ± 6.8% after 0.5 h dissolution; and 34.5 ± 5.6 % and 47.9 ± 5.3 % within one-hour dissolution, respectively. After a time period of 60 h dissolution, microparticles E2 and hybrids E3 released 91.7 ± 3.2% and 98.6 ± 3.5%, respectively. These data suggested that the hybrids E3 could provide a typical biphasic release profile with a release amount of 42.3% at the first phase in a pulsatile manner and 56.3% (98.6%-42.3%) at the second phase in a sustained manner. It seems that the electrosprayed particles E1 also furnished a biphasic release, i.e. 34.5% and 57.2% (91.7%-34.5%) at the first and second phases, respectively. However, the release contents at different phases from hybrids E3 are intentionally tailored in a relatively accurate manner. Whereas, the release contents at different phases from the microparticles E1 are random and often uncontrollable. Thus, in pharmaceutics, this case is regarded as an abnormal phenomenon to drug sustained release, i.e. initial burst effect.
The drug controlled release advantages of electrospun hybrids E3 over the electrosprayed microparticles E1 can be further projected from
Figure 8b. For a 30% release of the loaded BH, 0.38 h and 0.81 h are needed for the electrospun hybrids E3 and electrosprayed microparticles E1, respectively. Meanwhile, for a 50% release of the loaded BH, 1.33 h and 4.76 h are needed for the electrospun hybrids E3 and electrosprayed microparticles E1, respectively. For fast reaching a therapeutic blood drug concentration, the faster the dosage forms can provide, the better effectiveness and compliance the patients have. From this standpoint, the hybrids E3 are apparently better than the microparticles E1.
When for a 90% release of the loaded BH, 28.6 h and 55.32 h are needed for the electrospun hybrids E3 and electrosprayed microparticles E1, respectively. In drug sustained release, an abnormal phenomenon is a tailing-off release, in which the drug is exhausted very slowly from its carrier and can’t keep an effective therapeutic blood drug concentration. The release percentage between 90% to 100% often falls within this abnormal region and should be avoided. From this standpoint, hybrids E3 are better than microparticle E1 due to a smaller tailing-off release and also a terminal release amount (98.7 ± 3.5% and 91.8 ± 3.2% after 60 h for E3 and E1, respectively).
By the way, the suspension fluid had an amount of 15.0 g microparticles E1 from electrospraying and 200 mL Fluid 2. The BH weight in the microparticles was 15.0×20%=3.0 g. The BH weight in the nanofibers was 4.0 × (200 mL/400 mL)=2.0 g. Thus in theory, the drug released in the first phase should be 2.0/(2.0+3.0)×100% = 40%. The released BH from hybrids E3 after 0.5 and 1 h were 42.3 ± 6.8% and 47.9 ± 5.3%, respectively. The values are larger than the theoretically calculated value of 40%. This case should be attributed to both the preparation of working suspensions for creating hybrids E3 and drug release from the particles. Some surface BH on the surface of electrosprayed microparticles E1 should re-dissolve into the suspensions, making a little higher drug concentration in the hydrophilic PVP. Further studies may be designed to improve the accuracy of drug release contents at different phases, e.g. a blank CA coating on the microparticles through coaxial electrospraying.
3.5. Drug release mechanism
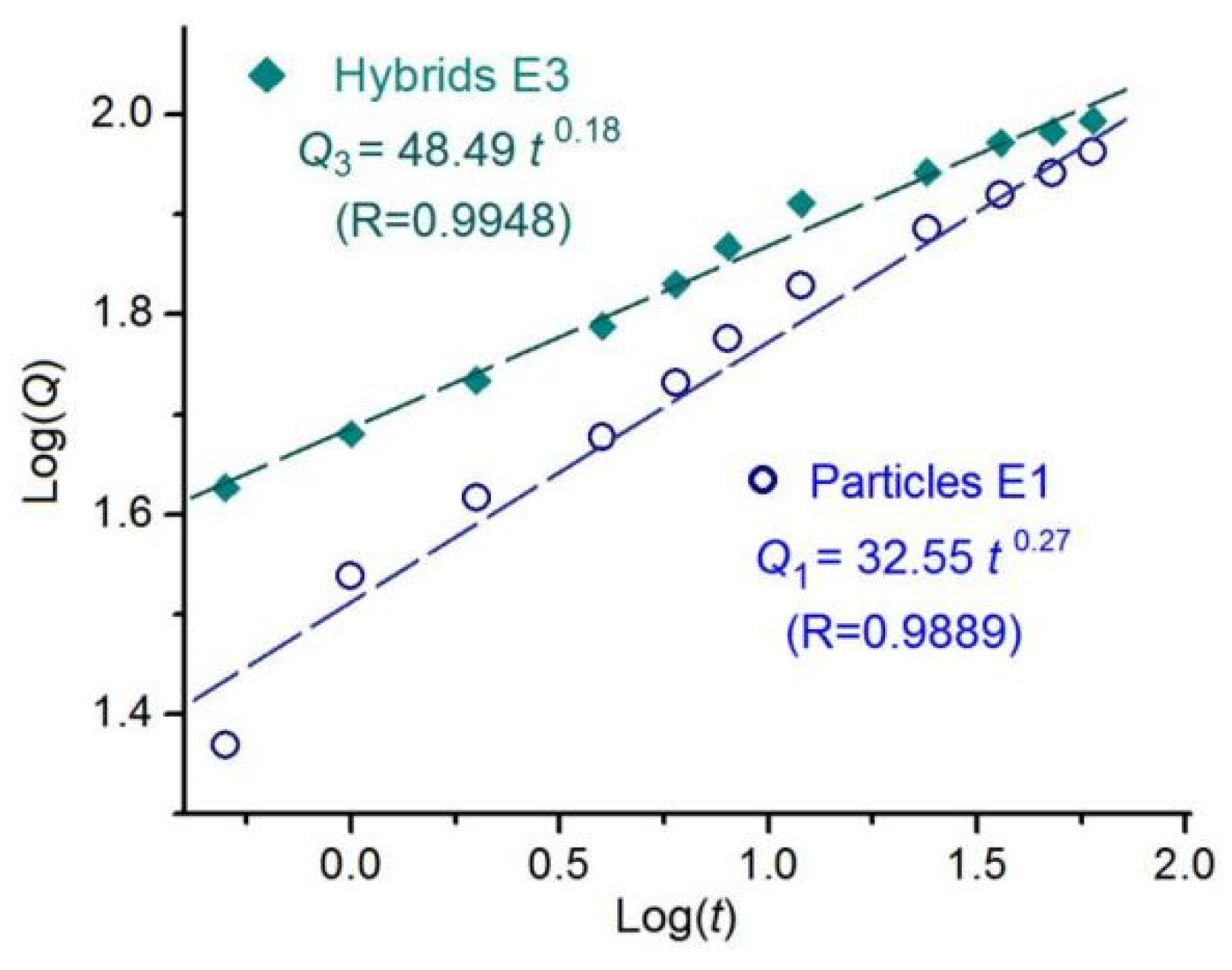
To disclose the drug release mechanism, Peppas equation (Q=k
tn, where Q is the drug release content, k is a constant, and t is an indicator of drug release behaviors [
75]) is exploited to regress the BH release data achieved during the in vitro dissolution tests. The results for the electrosprayed microparticles E1 and electrospun hybrids E3 are exhibited in
Figure 9. For particles E1, the regressed equation is Log
Q1= 1.51 + 0.27 Log
t ( or
Q1=32.55 t
0.27, R=0.9889). For the second phase of hybrids E3, the regressed equation is Log
Q3= 1.69 + 0.18 Log
t ( or
Q1=48.49 t
0.18, R=0.9948). Both EHDA products had an n value smaller than the critical judgment value of 0.45, suggesting that BH was released from the CA microparticles through a typical Fickian diffusion mechanism, regardless of a sole state of microparticles E2 or a co-existing state with hydrophilic polymer PVP.
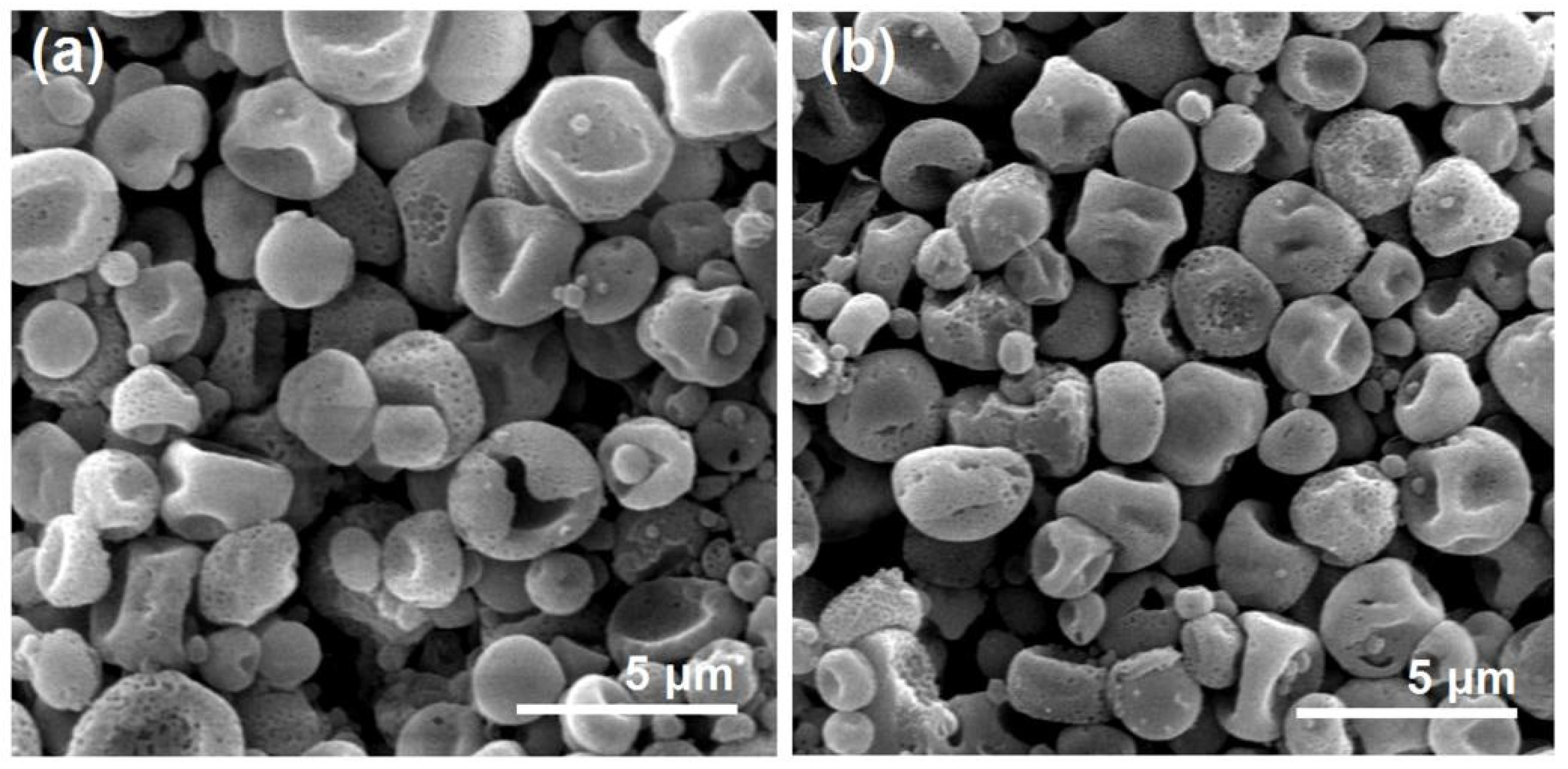
After a full time period of 60 h dissolution, the residue particles were taken out from the in vitro dissolution vessels and naturally dried. These particles experienced SEM evaluations. Their images are exhibited in
Figure 10. Compared with the previous images before dissolution, both microparticles E1 (
Figure 10a) particles in hybrids E3 (
Figure 10b) lost their original smooth surface and solid state, but exhibited a porous surface and a more concave morphology. Apparently, those surface holes and deformed surface morphology (resulted from a void inner) are direct outcomes owing to the removement of loaded BH molecules, providing an intuitive clue of a drug diffusion mechanism from the insoluble CA matrix.
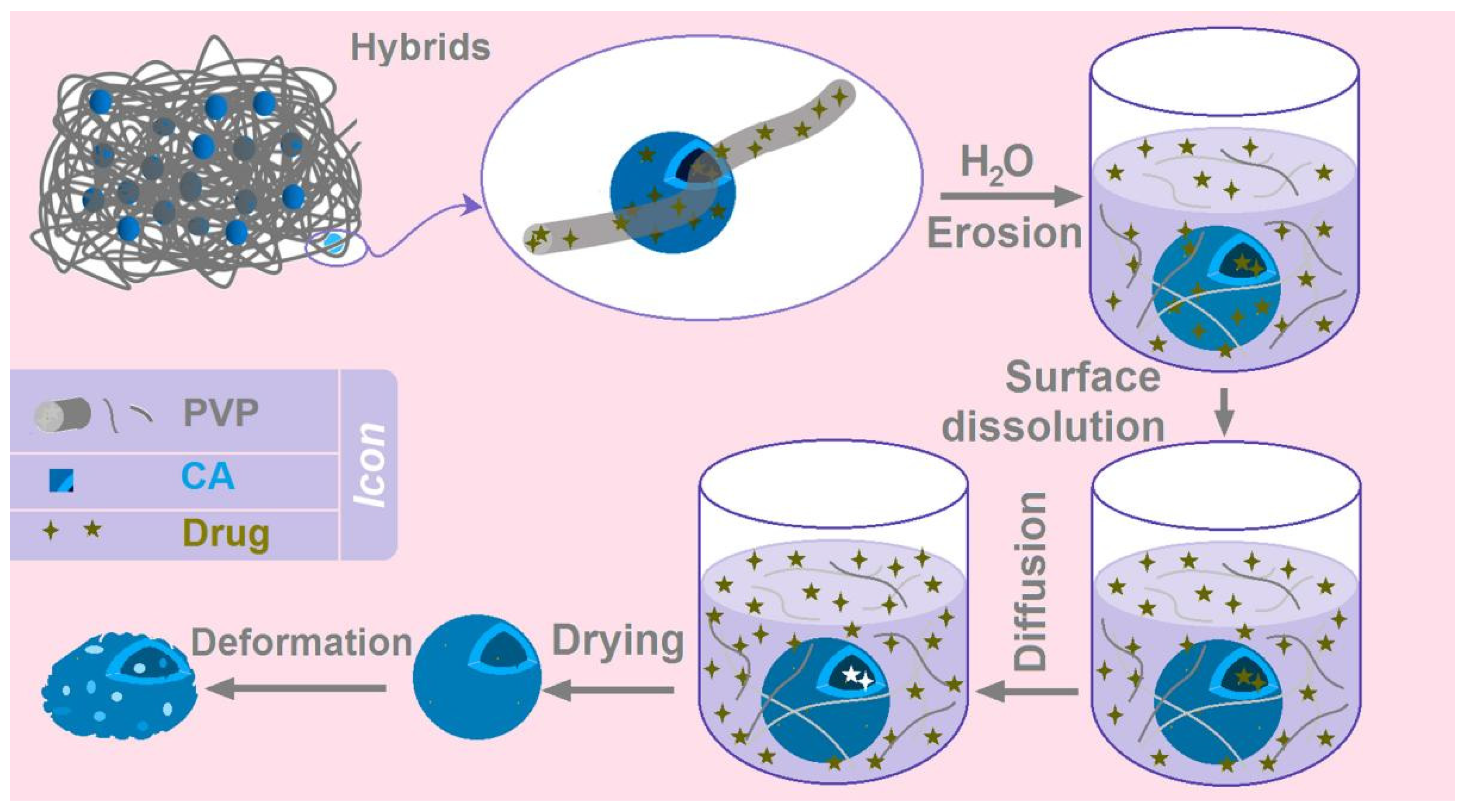
Based on the above-mentioned analyses, a diagram is shown in
Figure 11. The combined mechanism of the BH biphasic release from the hybrids E3 containing electrospun PVP nanofibers and electrosprayed CA microparticles is clear. When the hybrids E3 are placed into the dissolution media, the BH-PVP nanofibers will be fast dissolved due to a series of synergistic actions, including the amorphous state of BH, the small diameter of PVP nanofibers, the large surface area of nanofibers, the big porosity of nanofibers, and the highly hydrophilic and soluble properties of PVP. This is typical erosion process for the fast controlled release of BH. Later, the BH molecules distributed or absorbed on the surface of CA microparticles would dissolve into the dissolution media, by which the routes for water molecules’ penetration into the inner sections of CA particles gradually open. Along with the penetration of water molecules from surface to the core of CA microparticles, the loaded drug BH molecules would be free and inversely diffused from the CA particles to the bulk solution. During all the processes, the CA skeleton is insoluble and keeps the routes for diffusions and exchanges of both water and BH molecules. In theory, the diffusion process will not be terminated until a uniform BH distribution all over the bulk solution and a dynamic absorbance balance between the dissolution media and solid CA skeleton. After the CA skeletons were fetched out and dried, it is inevitable for them to experience some deformations.
New methods for human health are always highly desired [
76,
77,
78,
79,
80,
81]. Today, on one hand, numerous strategies are reported in literature to create novel functional ingredients on a molecular scale with chemical reactions as the fundamental supports [
82,
83,
84,
85,
86,
87,
88]. On the other hand, new methods are bloomed in manipulating the molecules into nano aggregates from both “top-down” manner and “bottom-up” way [
89,
90,
91,
92,
93]. In this study, a new concept is demonstrated that a trans-dimensional strategy is explored to generate functional hybrid materials through a combination of nanoproducts and products at microscale for an improved final functional performance. Based on the protocols reported here, there are a wide variety of possibilities for conceiving novel functional materials in the future.
4. Conclusions
In this study, a sequential EHDA process was successfully developed for creating a new kind of medicated hybrids E3. The hybrids E3 contained both BH-loaded hydrophilic PVP nanofibers and insoluble BH-loaded CA microparticles. The key element was that the electrosprayed BH-CA microparticles were insoluble in the solvent mixture of DCM and DMAc (with a volume ratio of 9:1) and thus an electrospinnable suspension was prepared, and in turn, the nanofiber-microparticle hybrids E3 were achieved through the single-fluid electrospinning process. The routine characterization results indicated that the hybrids E3 were a mixture of particles and nanofibers with BH distributed in the PVP and CA matrices in an amorphous state. In vitro dissolution tests demonstrated that the hybrids E3 were able to furnish the designed biphasic release profile, with a 42.3% drug release at the first immediate release phase and a 56.3% drug release at the second phase in a sustained manner. The BH molecule release was manipulated through a combination of molecular erosion mechanism and the typical molecular Fickian diffusion mechanism. This study paves a new way for developing functional materials through organizing materials at different scale levels and with different outer shapes.
Author Contributions
Conceptualization, J.Z. and D.Y.; methodology, J.Z., D.Y., J.F., and C.Y.; writing-original draft preparation, J.Z., and D.G.Y.; writing-review and editing, D.-G.Y., and T.Y.; visualization, J.F., and C.Y.; supervision, D.-G.Y.; project administration, D.-G.Y.; funding acquisition, D.-G.Y. and T.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This investigation was financially supported by the Shanghai Natural Science Foundation (No. 20ZR1439000), the Macao Polytechnic University Research Fund (RP/FCSD-01/2023), and the innovation projects for USST students to J.F. and C.Y. (Nos. SH2022225 & SH2022229).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting the findings of this manuscript are available from the corresponding authors upon reasonable.
Acknowledgments
Wanli He is appreciated for his helps on studies.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, G.; Kong, P.; Jiang, A.; Wang, X.; Sun, Y.; Yu, T.; Chi, H.; Song, C.; Zhang, H.; Subedi, D.; et al. A Modular Programmed Biphasic Dual-Delivery System on 3D Ceramic Scaffolds for Osteogenesis in Vitro and in Vivo. J. Mater. Chem. B 2020, 8, 9697–9717.
- Ejeta, F.; Gabriel, T.; Joseph, N.M.; Belete, A. Formulation, Optimization and In Vitro Evaluation of Fast Disintegrating Tablets of Salbutamol Sulphate Using a Combination of Superdisintegrant and Subliming Agent. Curr. Drug Deliv. 2022, 19, 129–141. [CrossRef]
- Kose, M.D.; Ungun, N.; Bayraktar, O. Eggshell Membrane Based Turmeric Extract Loaded Orally Disintegrating Films. Curr. Drug Deliv. 2022, 19, 547–559. [CrossRef]
- Phaechamud, T.; Choncheewa, C. Double-Layered Matrix of Shellac Wax-Lutrol in Controlled Dual Drug Release. AAPS PharmSciTech 2016, 17, 1326–1335. [CrossRef]
- Beugeling, M.; Grasmeijer, N.; Born, P.A.; van der Meulen, M.; van der Kooij, R.S.; Schwengle, K.; Baert, L.; Amssoms, K.; Frijlink, H.W.; Hinrichs, W.L.J. The Mechanism behind the Biphasic Pulsatile Drug Release from Physically Mixed Poly(DL-Lactic(-Co-Glycolic) Acid)-Based Compacts. Int. J. Pharm. 2018, 551, 195–202. [CrossRef]
- Zhou J, Wang P, Yu DG, Zhu Y. Biphasic Drug Release from Electrospun Structures. Exp. Opin. Drug Del. 2023. [CrossRef]
- Chen, Y.; Yu, W.; Qian, X.; Li, X.; Wang, Y.; Ji, J. Dissolving Microneedles with a Biphasic Release of Antibacterial Agent and Growth Factor to Promote Wound Healing. Biomater. Sci. 2022, 10, 2409–2416. [CrossRef]
- Tung, N.-T.; Dong, T.-H.-Y.; Tran, C.-S.; Nguyen, T.-K.-T.; Chi, S.-C.; Dao, D.-S.; Nguyen, D.-H. Integration of Lornoxicam Nanocrystals into Hydroxypropyl Methylcellulose-Based Sustained Release Matrix to Form a Novel Biphasic Release System. Int. J. Biol. Macromol. 2022, 209, 441–451. [CrossRef]
- Adala, I.; Ramis, J.; Ntone Moussinga, C.; Janowski, I.; Amer, M.H.; Bennett, A.J.; Alexander, C.; Rose, F.R.A.J. Mixed Polymer and Bioconjugate Core/Shell Electrospun Fibres for Biphasic Protein Release. J. Mat. Chem. B 2021, 9, 4120–4133. [CrossRef]
- Conceicao, J.; Adeoye, O.; Cabral-Marques, H.; Concheiro, A.; Alvarez-Lorenzo, C.; Sousa Lobo, J.M. Carbamazepine Bilayer Tablets Combining Hydrophilic and Hydrophobic Cyclodextrins as a Quick/Slow Biphasic Release System. J. Drug Deliv. Sci. Technol. 2020, 57, 101611. [CrossRef]
- Yang, B.; Dong, Y.; Shen, Y.; Hou, A.; Quan, G.; Pan, X.; Wu, C. Bilayer Dissolving Microneedle Array Containing 5-Fluorouracil and Triamcinolone with Biphasic Release Profile for Hypertrophic Scar Therapy. Bioact. Mater. 2021, 6, 2400–2411. [CrossRef]
- Chen, Y.; Yu, W.; Qian, X.; Li, X.; Wang, Y.; Ji, J. Dissolving Microneedles with a Biphasic Release of Antibacterial Agent and Growth Factor to Promote Wound Healing. Biomater. Sci. 2022, 10, 2409–2416. [CrossRef]
- Tabakoglu, S.; Kolbuk, D.; Sajkiewicz, P. Multifluid Electrospinning for Multi-Drug Delivery Systems: Pros and Cons, Challenges, and Future Directions. Biomater. Sci. 2022, 11, 37–61. [CrossRef]
- Hameed, A.; Rehman, T.U.; Rehan, Z.A.; Noreen, R.; Iqbal, S.; Batool, S.; Qayyum, M.A.; Ahmed, T.; Farooq, T. Development of Polymeric Nanofibers Blended with Extract of Neem (Azadirachta Indica), for Potential Biomedical Applications. Front. Mater. 2022, 9, 1042304. [CrossRef]
- Brimo, N.; Serdaroglu, D.C.; Uysal, B. Comparing Antibiotic Pastes with Electrospun Nanofibers as Modern Drug Delivery Systems for Regenerative Endodontics. Curr. Drug Deliv. 2022, 19, 904–917. [CrossRef]
- Kuang, G.; Zhang, Z.; Liu, S.; Zhou, D.; Lu, X.; Jing, X.; Huang, Y. Biphasic Drug Release from Electrospun Polyblend Nanofibers for Optimized Local Cancer Treatment. Biomater. Sci. 2018, 6, 324–331. [CrossRef]
- Yang, S.; Li, X.; Liu, P.; Zhang, M.; Wang, C.; Zhang, B. Multifunctional Chitosan/Polycaprolactone Nanofiber Scaffolds with Varied Dual-Drug Release for Wound-Healing Applications. ACS Biomater. Sci. Eng. 2020, 6, 4666–4676. [CrossRef]
- Lee, H.; Xu, G.; Kharaghani, D.; Nishino, M.; Song, K.H.; Lee, J.S.; Kim, I.S. Electrospun Tri-Layered Zein/PVP-GO/Zein Nanofiber Mats for Providing Biphasic Drug Release Profiles. Int. J. Pharm. 2017, 531, 101–107. [CrossRef]
- Khalek, M.A.A.; Gaber, S.A.A.; El-Domany, R.A.; El-Kemary, M.A. Photoactive Electrospun Cellulose Acetate/Polyethylene Oxide/Methylene Blue and Trilayered Cellulose Acetate/Polyethylene Oxide/Silk Fibroin/ Ciprofloxacin Nanofibers for Chronic Wound Healing. Int. J. Biol. Macromol. 2021, 193, 1752–1766. [CrossRef]
- Zhou, H.Y.; Tong, J.N.; Ren, L.J.; Hao, P.Y.; Zheng, H.J.; Guo, X.M.; Chen, Y.W.; Li, J.B.; Park, H.J. Preparation and Performance of Chitosan/Cyclodextrin-g-Glutamic Acid Thermosensitive Hydrogel. J. Drug Deliv. Sci. Technol. 2022, 74, 103504. [CrossRef]
- Peng, L.; Kang, S.; Yin, Z.; Jia, R.; Song, X.; Li, L.; Li, Z.; Zou, Y.; Liang, X.; Li, L.; et al. Antibacterial Activity and Mechanism of Berberine against Streptococcus Agalactiae. Int J Clin Exp Pathol 2015, 8, 5217–5223.
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for Combating Bacterial Biofilms: A Focus on Anti-Biofilm Agents and Their Mechanisms of Action. Virulence 2018, 9, 522–554. [CrossRef]
- Tan, X.-S.; Ma, J.-Y.; Feng, R.; Ma, C.; Chen, W.-J.; Sun, Y.-P.; Fu, J.; Huang, M.; He, C.-Y.; Shou, J.-W.; et al. Tissue Distribution of Berberine and Its Metabolites after Oral Administration in Rats. PLoS ONE 2013, 8, e77969. [CrossRef]
- Wang, X.; Feng, C. Chiral Fiber Supramolecular Hydrogels for Tissue Engineering. Wiley Interdiscip. Rev.-Nanomed. Nanobiotechnol. 2023, e1847. [CrossRef]
- Esquivel, S.V.; Bhatt, H.N.; Diwan, R.; Habib, A.; Lee, W.-Y.; Khatun, Z.; Nurunnabi, M. β-Glucan and Fatty Acid Based Mucoadhesive Carrier for Gastrointestinal Tract Specific Local and Sustained Drug Delivery. Biomolecules 2023, 13, 768. [CrossRef]
- Yu, D.-G.; Zhao, P. The Key Elements for Biomolecules to Biomaterials and to Bioapplications. Biomolecules 2022, 12, 1234. [CrossRef]
- Kramar, A.; González-Benito, J. Preparation of Cellulose Acetate Film with Dual Hydrophobic-Hydrophilic Properties Using Solution Blow Spinning. Mater. Des. 2023, 227, 111788. [CrossRef]
- Song, W.; Zhang, Y.; Tran, C.H.; Choi, H.K.; Yu, D.G.; Kim, I. Porous Organic Polymers with Defined Morphologies: Synthesis, Assembly, and Emerging Applications. Prog. Polym. Sci. 2023. [CrossRef]
- Huang, J.; Feng, C. Aniline Dimers Serving as Stable and Efficient Transfer Units for Intermolecular Charge-Carrier Transmission. Iscience 2023, 26, 105762. [CrossRef]
- Zhang, Y.; Liu, X.; Geng, C.; Shen, H.; Zhang, Q.; Miao, Y.; Wu, J.; Ouyang, R.; Zhou, S. Two Hawks with One Arrow: A Review on Bifunctional Scaffolds for Photothermal Therapy and Bone Regeneration. Nanomaterials 2023, 13, 551. [CrossRef]
- Qi, Q.; Wang, Q.; Li, Y.; Silva, D.Z.; Ruiz, M.E.L.; Ouyang, R.; Liu, B.; Miao, Y. Recent Development of Rhenium-Based Materials in the Application of Diagnosis and Tumor Therapy. Molecules 2023, 28, 2733. [CrossRef]
- Wang, L.; Ahmad, Z.; Huang, J.; Li, J.-S.; Chang, M.-W. Multi-Compartment Centrifugal Electrospinning Based Composite Fibers. Chem. Eng. J. 2017, 330, 541-549. [CrossRef]
- Sivan, M.; Madheswaran, D.; Hauzerova, S.; Novotny, V.; Hedvicakova, V.; Jencova, V.; Kostakova, E.K.; Schindler, M.; Lukas, D. AC Electrospinning: Impact of High Voltage and Solvent on the Electrospinnability and Productivity of Polycaprolactone Electrospun Nanofibrous Scaffolds. Mater. Today Chem. 2022, 26, 101025. [CrossRef]
- Zhou, Y.; Wang, M.; Yan, C.; Liu, H.; Yu, D.-G. Advances in the Application of Electrospun Drug-Loaded Nanofibers in the Treatment of Oral Ulcers. Biomolecules 2022, 12, 1254. [CrossRef]
- Wang, X.; Peng, Y.; Wu, Y.; Cao, S.; Deng, H.; Cao, Z. Chitosan/Silk Fibroin Composite Bilayer PCL Nanofibrous Mats for Bone Regeneration with Enhanced Antibacterial Properties and Improved Osteogenic Potential. Int. J. Biolog. Macromol. 2023, 230, 123265. [CrossRef]
- Wang, Q.; Liu, Q.; Gao, J.; He, J.; Zhang, H.; Ding, J. Stereo Coverage and Overall Stiffness of Biomaterial Arrays Underly Parts of Topography Effects on Cell Adhesion. ACS Appl. Mater. Interfaces 2023, 15, 6142–6155. [CrossRef]
- Chen, W.; Zhao, P.; Yang, Y.; Yu, D. Electrospun Beads-on-the-String Nanoproducts: Preparation and Drug Delivery Application. Current Drug Delivery 2022, 19, 1224-1240. [CrossRef]
- Huang, H.; Song, Y.; Zhang, Y.; Li, Y.; Li, J.; Lu, X.; Wang, C. Electrospun Nanofibers: Current Progress and Applications in Food Systems. J. Agric. Food Chem. 2022, 70, 1391–1409. [CrossRef]
- Wang, Y.; Yu, D.-G.; Liu, Y.; Liu, Y.-N. Progress of Electrospun Nanofibrous Carriers for Modifications to Drug Release Profiles. J. Func. Biomater. 2022, 13, 289. [CrossRef]
- Shen, S.-F.; Zhu, L.-F.; Liu, J.; Ali, A.; Zaman, A.; Ahmad, Z.; Chen, X.; Chang, M.-W. Novel Core-Shell Fiber Delivery System for Synergistic Treatment of Cervical Cancer. J. Drug Del. Sci. Technol. 2020, 59, 101865. [CrossRef]
- Yildiz, Z.I.; Topuz, F.; Kilic, M.E.; Durgun, E.; Uyar, T. Encapsulation of Antioxidant Beta-carotene by Cyclodextrin Complex Electrospun Nanofibers: Solubilization and Stabilization of Beta-Carotene by Cyclodextrins. Food Chem. 2023, 423, 136284. [CrossRef]
- Guler, E.; Nur Hazar-Yavuz, A.; Tatar, E.; Morid Haidari, M.; Sinemcan Ozcan, G.; Duruksu, G.; Graça, M.P.F.; Kalaskar, D.M.; Gunduz, O.; Emin Cam, M. Oral Empagliflozin-Loaded Tri-Layer Core-Sheath Fibers Fabricated Using Tri-Axial Electrospinning: Enhanced in Vitro and in Vivo Antidiabetic Performance. Int. J. Pharm. 2023, 635, 122716. [CrossRef]
- Afshar, S.K.; Abdorashidi, M.; Dorkoosh, F.A.; Akbari Javar, H. Electrospun Fibers: Versatile Approaches for Controlled Release Applications. Int. J. Polym. Sci. 2022, 2022, 9116168. [CrossRef]
- Shen, Y.; Yu, X.; Cui, J.; Yu, F.; Liu, M.; Chen, Y.; Wu, J.; Sun, B.; Mo, X. Development of Biodegradable Polymeric Stents for the Treatment of Cardiovascular Diseases. Biomolecules 2022, 12, 1245. [CrossRef]
- Wang, M.; Ge, R.; Zhao, P.; Williams, G.R.; Yu, D.-G.; Bligh, S.W.A. Exploring Wettability Difference-Driven Wetting by Utilizing Electrospun Chimeric Janus Microfiber Comprising Cellulose Acetate and Polyvinylpyrrolidone. Mater. Design 2023, 226, 111652. [CrossRef]
- Wang, M.; Ge, R.L.; Zhang F, Yu DG, Liu ZP, Li X, Shen H, Williams GR. Electrospun fibers with blank surface and inner drug gradient for improving sustained release. Biomater. Adv. 2023, 150, 213404. [CrossRef]
- Yao, Z.-C.; Zhang, C.; Ahmad, Z.; Huang, J.; Li, J-S.; Chang, M.-W. Designer Fibers from 2D to 3D-Simultaneous and Controlled Engineering of Morphology, Shape and Size. Chem. Eng. J. 2018, 334, 89-98. [CrossRef]
- Lv, H.; Liu, Y.; Bai, Y.; Shi, H.; Zhou, W.; Chen, Y.; Liu, Y.; Yu, D-G. Recent Combinations of Electrospinning with Photocatalytic technology for Treating Polluted Water. Catalysts 2023, 13, 758. [CrossRef]
- Feng, Z.; Zhao, Z.; Liu, Y.; Liu, Y.; Cao, X.; Yu, D.G.; Wang, K. Piezoelectric Effect Polyvinylidene Fluoride (PVDF): From Energy Harvester to Smart Skin and Electronic Textiles. Adv. Mater. Technol. 2023. [CrossRef]
- Wang, P.; Lv, H.; Cao, X.; Liu, Y.; Yu, D.-G. Recent Progress of the Preparation and Application of Electrospun Porous Nanofibers. Polymers 2023, 15, 921. [CrossRef]
- Du, Y.; Yu, D.-G.; Yi, T. Electrospun Nanofibers as Chemosensors for Detecting Environmental Pollutants: A Review. Chemosensors 2023, 11, 208. [CrossRef]
- Guler, E.; Hazar-Yavuz, A.N.; Tatar, E.; Haidari, M.M.; Ozcan, G.S.; Duruksu, G.; Cam, M.E. Oral Empagliflozin-loaded Tri-Layer Core-Sheath Fibers Fabricated Using Tri-Axial Electrospinning: Enhanced in vitro and in vivo Antidiabetic Performance. Int. J. Pharm. 2023, 635, 122716. [CrossRef]
- Liu, H.; Bai, Y.; Huang, C.; Wang, Y.; Ji, Y.; Du, Y.; Xu, L.; Yu, D.-G.; Bligh, S.W.A. Recent Progress of Electrospun Herbal Medicine Nanofibers. Biomolecules 2023, 13, 184. [CrossRef]
- Wang, M.-L.; Yu, D.-G.; Bligh, S.W.A. Progress in Preparing Electrospun Janus Fibers and Their Applications. Appl. Mater. Today 2023, 31, 101766. [CrossRef]
- Yao, L.; Sun, C.; Lin, H.; Li, G.; Lian, Z.; Song, R.; Zhuang, S.; Zhang, D. Electrospun Bi-Decorated BixTiyOz/TiO2 Flexible Carbon Nanofibers and Their Applications on Degradating of Organic Pollutants under Solar Radiation. J. Mater. Sci. Technol. 2023, 150, 114–123. [CrossRef]
- Yao, Z.-C.; Yuan, Q.; Ahmad, Z.; Huang, J.; Li, J.-S.; Chang, M.-W. Controlled Morphing of Microbubbles to Beaded Nanofibers via Electrically Forced Thin Film Stretching. Polymers. 2017, 9, 265. [CrossRef]
- Song, W.; Tang, Y.; Qian, C.; Kim, B.J.; Liao, Y.; Yu, D.-G. Electrospinning Spinneret: A Bridge between the Visible World and the Invisible Nanostructures. Innovation 2023, 4, 100381. [CrossRef]
- Yao, L.; Sun, C.; Lin, H.; Li, G.; Lian, Z.; Song, R.; Zhuang, S.; Zhang, D. Enhancement of AFB1 Removal Efficiency via Adsorption/Photocatalysis Synergy Using Surface-Modified Electrospun PCL-g-C3N4/CQDs Membranes. Biomolecules 2023, 13, 550. [CrossRef]
- Li, H.; Zhang, Z.; Ren, Z.; Chen, Y.; Huang, J.; Lei, Z.; Qian, X.; Lai, Y.; Zhang, S. A Quadruple Biomimetic Hydrophilic/Hydrophobic Janus Composite Material Integrating Cu(OH)(2) Micro-Needles and Embedded Bead-on-String Nanofiber Membrane for Efficient Fog Harvesting. Chem. Eng. J. 2023, 455, 140863. [CrossRef]
- Yu, D.G.; Xu, L. Impact Evaluations of Articles in Current Drug Delivery Based on Web of Science. Curr. Drug Del. 2023, 20. [CrossRef]
- Xu, J.; Zhong, M.; Song, N.; Wang, C.; Lu, X. General Synthesis of Pt and Ni Co-Doped Porous Carbon Nanofibers to Boost HER Performance in Both Acidic and Alkaline Solutions. Chin. Chem. Lett. 2023, 34, 107359. [CrossRef]
- Yang, Y.; Chen, W.; Wang, M.; Shen, J.; Tang, Z.; Qin, Y.; Yu, D.-G. Engineered Shellac Beads-On-The-String Fibers Using Triaxial Electrospinning for Improved Colon-Targeted Drug Delivery. Polymers 2023, 15, 2237. [CrossRef]
- Lv, H.; Liu, Y.; Zhao, P.; Bai, Y.; Cui, W.; Shen, S.; Liu, Y.; Wang, Z.; Yu, D.-G. Insight into the Superior Piezophotocatalytic Performance of BaTiO3//ZnO Janus Nanofibrous Heterostructures in the Treatment of Multi-Pollutants from Water. Appl. Catal. B-Environ 2023, 330, 122623. [CrossRef]
- Cao, X.; Chen, W.; Zhao, P.; Yang, Y.; Yu, D.-G. Electrospun Porous Nanofibers: Pore-Forming Mechanisms and Applications for Photocatalytic Degradation of Organic Pollutants in Wastewater. Polymers 2022, 14, 3990. [CrossRef]
- Bai, Y.; Liu, Y.; Lv, H.; Shi, H.; Zhou, W.; Liu, Y.; Yu, D.-G. Processes of Electrospun Polyvinylidene Fluoride-Based Nanofibers, Their Piezoelectric Properties, and Several Fantastic Applications. Polymers 2022, 14, 4311. [CrossRef]
- Zhu, M.; Yu, J.; Li, Z.; Ding, B. Self-Healing Fibrous Membranes. Angew. Chem.-Int. Edit. 2022, 61, e202208949. [CrossRef]
- Yao, Z.-C.; Gao, Y.; Chang, M.-W.; Ahmad, Z.; Li, J.-S. Regulating Polycaprolactone Fiber Characteristics In-Situ Dduring One-Step Coaxial Electrospinning via Enveloping Liquids. Mater. Lett. 2016, 183, 202-206. [CrossRef]
- Yu, D.-G.; Du, Y.; Chen, J.; Song, W.; Zhou, T. A Correlation Analysis between Undergraduate Students’ Safety Behaviors in the Laboratory and Their Learning Efficiencies. Behav. Sci. 2023, 13, 127. [CrossRef]
- Kang, S.; Hou, S.; Chen, X.; Yu, D.-G.; Wang, L.; Li, X.; Williams, R.G. Energy-Saving Electrospinning with a Concentric Teflon-Core Rod Spinneret to Create Medicated Nanofibers. Polymers 2020, 12, 2421. [CrossRef]
- Han, W.; Wang, L.; Li, Q.; Ma, B.; He, C.; Guo, X.; Nie, J.; Ma, G. A Review: Current Status and Emerging Developments on Natural Polymer-Based Electrospun Fibers. Macromol. Rapid Commun. 2022, 43, 2200456. [CrossRef]
- Wang, H.; Lu, H.; Yang, H.; Yu, D.G.; Lu, H. The Influence of the Ultrasonic Treatment of Working Fluids on Electrospun Amorphous Solid Dispersions. Front. Mol. Biosci. 2023, 10, 1184767. [CrossRef]
- Wang, M.; Hou, J.; Yu, D.-G.; Li, S.; Zhu, J.; Chen, Z. Electrospun Tri-Layer Nanodepots for Sustained Release of Acyclovir. J. Alloy. Compd. 2020, 846, 156471. [CrossRef]
- Huang, X.; Jiang, W.; Zhou, J.; Yu, D.-G.; Liu, H. The Applications of Ferulic-Acid-Loaded Fibrous Films for Fruit Preservation. Polymers 2022, 14, 4947. [CrossRef]
- Jiang, W.; Zhang, X.; Liu, P.; Zhang, Y.; Song, W.; Yu, D.-G.; Lu, X. Electrospun Healthcare Nanofibers from Medicinal Liquor of Phellinus Igniarius. Adv. Compos. Hybrid Mater. 2022, 5, 3045–3056. [CrossRef]
- Peppas, N.A. Analysis of Fickian and Non-Fickian Drug Release from Polymers. Pharm Acta Helv 1985, 60, 110–111.
- Li, C.-Q.; Wang, Y.-C.; Shen, S.-Q.; Zhang, Y.-L.; Zhao, J.-Q.; Zou, W.-B.; Ge, R.-L. Effects of Exercise by Type and Duration on Quality of Life in Patients with Digestive System Cancers: A Systematic Review and Network Meta-Analysis. J. Sport. Health Sci 2022, 1–10. [CrossRef]
- Li, C.; Wang, J.; Deng, C.; Wang, R.; Zhang, H. Protocol for Atmospheric Water Harvesting Using in Situ Polymerization Honeycomb Hygroscopic Polymers. STAR Protocols 2022, 3, 101780. [CrossRef]
- Xie, D.; Zhou, X.; Xiao, B.; Duan, L.; Zhu, Z. Mucus-Penetrating Silk Fibroin-Based Nanotherapeutics for Efficient Treatment of Ulcerative Colitis. Biomolecules 2022, 12, 1263. [CrossRef]
- Castillo-Henríquez, L.; Castro-Alpízar, J.; Lopretti-Correa, M.; Vega-Baudrit, J. Exploration of Bioengineered Scaffolds Composed of Thermo-Responsive Polymers for Drug Delivery in Wound Healing. Int. J. Mol. Sci 2021, 22, 1408. [CrossRef]
- Sivan, M.; Madheswaran, D.; Valtera, J.; Kostakova, E.K.; Lukas, D. Alternating Current Electrospinning: The Impacts of Various High-Voltage Signal Shapes and Frequencies on the Spinnability and Productivity of Polycaprolactone Nanofibers. Mater. Des. 2022, 213, 110308. [CrossRef]
- Liu, H.; Dai, Y.; , Li J.; Liu P.; Zhou, W.; Yu, DG, Ge R. Safe, Fast and Convenient Delivery of Fluidextracts Liquorice through Electrospun Core-Shell Nanohybrids. Front. Bioeng. Biotechnol. 2023, 11, 1172133. [CrossRef]
- Hsiung, EMY; Celebioglu, A; Kilic, ME; Durgun, E ; Uyar, T. Fast-Disintegrating Nanofibrous Web of Pullulan/ Griseofulvin-Cyclodextrin Inclusion Complexes. Mol. Pharm. 2023. [CrossRef]
- Wu, Y.; Li, Y.; Lv, G.; Bu, W. Redox Dyshomeostasis Strategy for Tumor Therapy Based on Nanomaterials Chemistry. Chem. Sci. 2022, 13, 2202–2217. [CrossRef]
- Zhao, P.; Li, H.; Bu, W. A Forward Vision for Chemodynamic Therapy: Issues and Opportunities. Angew. Chem.-Int. Edit. 2023. [CrossRef]
- Meng, Y.; Chen, L.; Chen, Y.; Shi, J.; Zhang, Z.; Wang, Y.; Wu, F.; Jiang, X.; Yang, W.; Zhang, L.; et al. Reactive Metal Boride Nanoparticles Trap Lipopolysaccharide and Peptidoglycan for Bacteria-Infected Wound Healing. Nat Commun 2022, 13, 7353. [CrossRef]
- Wu, Y.; Li, Y.; Lv, G.; Bu, W. Redox Dyshomeostasis Strategy for Tumor Therapy Based on Nanomaterials Chemistry. Chem. Sci 2022, 13, 2202–2217. [CrossRef]
- Chen, L.; Jiang, X.; Lv, M.; Wang, X.; Zhao, P.; Zhang, M.; Lv, G.; Wu, J.; Liu, Y.; Yang, Y.; et al. Reductive-Damage-Induced Intracellular Maladaptation for Cancer Electronic Interference Therapy. Chem 2022, 8, 866–879. [CrossRef]
- Tang, Z.; Wu, S.; Zhao, P.; Wang, H.; Ni, D.; Li, H.; Jiang, X.; Wu, Y.; Meng, Y.; Yao, Z.; et al. Chemical Factory-Guaranteed Enhanced Chemodynamic Therapy for Orthotopic Liver Cancer. Adv. Sci. 2022, 9, 2201232. [CrossRef]
- Ge, R.; Ji, Y.; Ding, Y.; Huang, C.; He, H.; Yu, D.-G. Electrospun Self-Emulsifying Core-Shell Nanofibers for Effective Delivery of Paclitaxel. Front. Bioeng. Biotech 2023, 11, 1112338. [CrossRef]
- Abdul Hameed, M.M.; Mohamed Khan, S.A.P.; Thamer, B.M.; Rajkumar, N.; El-Hamshary, H.; El-Newehy, M. Electrospun Nanofibers for Drug Delivery Applications: Methods and Mechanism. Polym. Advan. Technol. 2023, 34, 6–23. [CrossRef]
- Du, Y.; Yang, Z.; Kang, S.; Yu, D.-G.; Chen, X.; Shao, J. A Sequential Electrospinning of a Coaxial and Blending Process for Creating Double-Layer Hybrid Films to Sense Glucose. Sensors 2023, 23, 3685. [CrossRef]
- Feng, Z.; Wang, K.; Liu, Y.; Han, B.; Yu, D.-G. Piezoelectric Enhancement of Piezoceramic Nanoparticle-Doped PVDF/PCL Core-Sheath Fibers. Nanomaterials 2023, 13, 1243. [CrossRef]
- Isaacoff, B.P.; Brown, K.A. Progress in Top-Down Control of Bottom-Up Assembly. Nano Lett. 2017, 17, 6508–6510. [CrossRef]
Figure 1.
A diagram showing the fabrication procedures for fabricating the hybrids composed of the electrospun nanofibers and electrosprayed microparticles.
Figure 1.
A diagram showing the fabrication procedures for fabricating the hybrids composed of the electrospun nanofibers and electrosprayed microparticles.
Figure 2.
Digital pictures taken from the different EHDA working processes: (a) a typical electrospraying process for fabricating the microparticles E1; (b) a typical solution electrospinning process for creating the nanofibers E2; (c) a typical suspension electrospinning process for producing hybrids of E3; (d) an abnormal EHDA process when treating the suspension under a super high applied voltage.
Figure 2.
Digital pictures taken from the different EHDA working processes: (a) a typical electrospraying process for fabricating the microparticles E1; (b) a typical solution electrospinning process for creating the nanofibers E2; (c) a typical suspension electrospinning process for producing hybrids of E3; (d) an abnormal EHDA process when treating the suspension under a super high applied voltage.
Figure 3.
SEM images of the resultant products: (a) microparticles E1; (b) nanofibers E2, the up-right inset shows an enlarged image; (c) hybrids E3; (d) An enlarged image of hybrids E3.
Figure 3.
SEM images of the resultant products: (a) microparticles E1; (b) nanofibers E2, the up-right inset shows an enlarged image; (c) hybrids E3; (d) An enlarged image of hybrids E3.
Figure 4.
The average diameters of EHDA products: (a) nanofibers E2; (b) the nanofibers of hybrids E3; (c) microparticles E1; (d) the microparticles of hybrids E3.
Figure 4.
The average diameters of EHDA products: (a) nanofibers E2; (b) the nanofibers of hybrids E3; (c) microparticles E1; (d) the microparticles of hybrids E3.
Figure 5.
The EHDA mechanism for the formation of satellites around the electrosprayed microparticles.
Figure 5.
The EHDA mechanism for the formation of satellites around the electrosprayed microparticles.
Figure 6.
XRD patterns of the raw materials (CA, PVP and BH) and their EHDA products (Hybrids E3, nanofibers E2 and their combinations).
Figure 6.
XRD patterns of the raw materials (CA, PVP and BH) and their EHDA products (Hybrids E3, nanofibers E2 and their combinations).
Figure 7.
Flourier Transform infrared spectra of the raw materials (CA, PVP and BH) and their EHDA products, and the molecular formats of the components within the electrosprayed products (CA, PVP and BH).
Figure 7.
Flourier Transform infrared spectra of the raw materials (CA, PVP and BH) and their EHDA products, and the molecular formats of the components within the electrosprayed products (CA, PVP and BH).
Figure 8.
The in vitro dissolution test results: (a) drug accumulative release percentage (%) vs sampling time point (h); and (b) estimated durations vs a certain percentage of BH (30%, 50%, and 90%) that was released from the three EHDA products.
Figure 8.
The in vitro dissolution test results: (a) drug accumulative release percentage (%) vs sampling time point (h); and (b) estimated durations vs a certain percentage of BH (30%, 50%, and 90%) that was released from the three EHDA products.
Figure 9.
The regressed equations drawn from the in vitro BH release data of hybrids E3 and particles E1.
Figure 9.
The regressed equations drawn from the in vitro BH release data of hybrids E3 and particles E1.
Figure 10.
The SEM images of the residue microparticles after the exhaustion of BH molecules from the microparticles E1 (a) and the hybrids E3 (b).
Figure 10.
The SEM images of the residue microparticles after the exhaustion of BH molecules from the microparticles E1 (a) and the hybrids E3 (b).
Figure 11.
The mechanism about the biphasic release of BH from the hybrids of electrospun PVP nanofibers and electrosprayed CA microparticles.
Figure 11.
The mechanism about the biphasic release of BH from the hybrids of electrospun PVP nanofibers and electrosprayed CA microparticles.
Table 1.
Parameters for the EHDA processes.
Table 1.
Parameters for the EHDA processes.
| No. |
EHDA process |
Working fluid |
Experimental conditions |
Drug contents |
Morpho-
logy |
| V (kV) |
F (mL/h) |
D (cm) |
| E1 |
Electrospraying |
Fluid 1 a
|
20 |
1.0 |
20 |
20.0% |
Particles |
| E2 |
Electrospinning |
Fluid 2 b |
8 |
2.0 |
20 |
10.0% |
Fibers |
| E3 |
Sequential EHDA process |
Fluid 3 c |
12 |
2.0 |
20 |
14.3% |
Hybrids |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).