Submitted:
17 May 2023
Posted:
17 May 2023
You are already at the latest version
Abstract
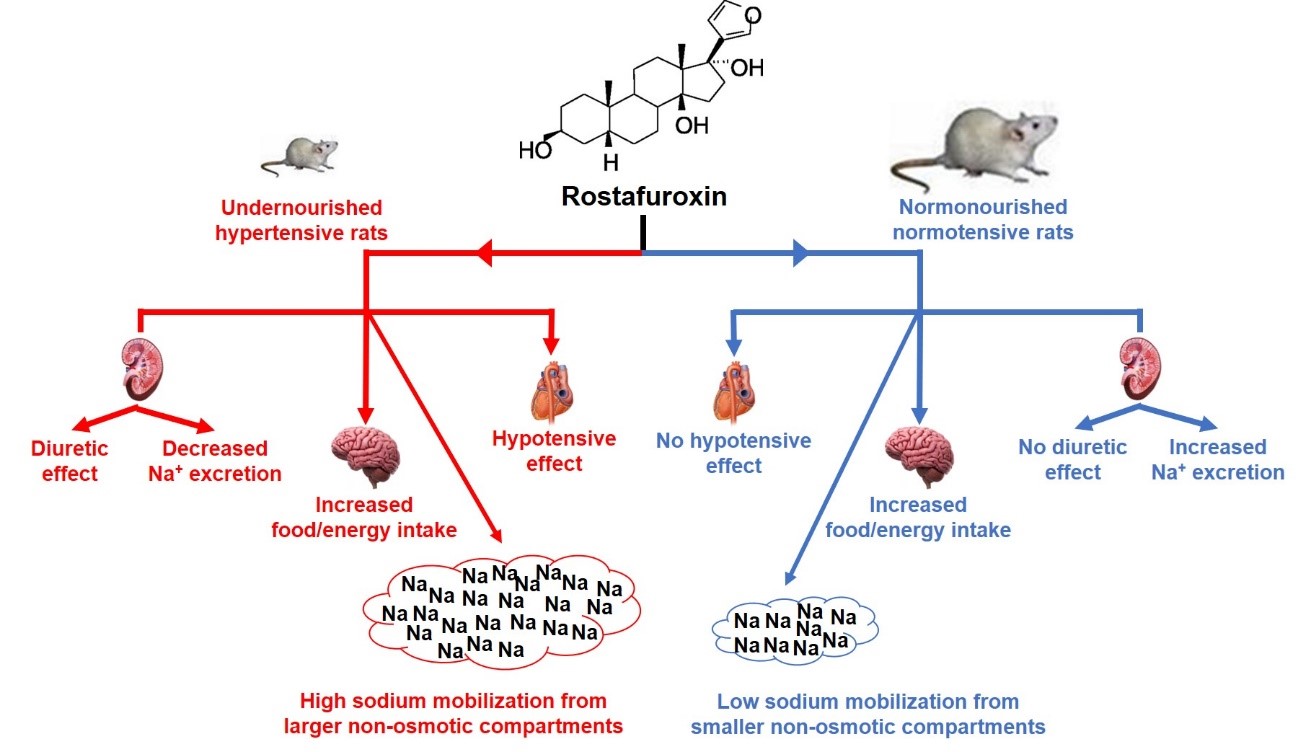
Keywords:
1. Introduction
2. Results
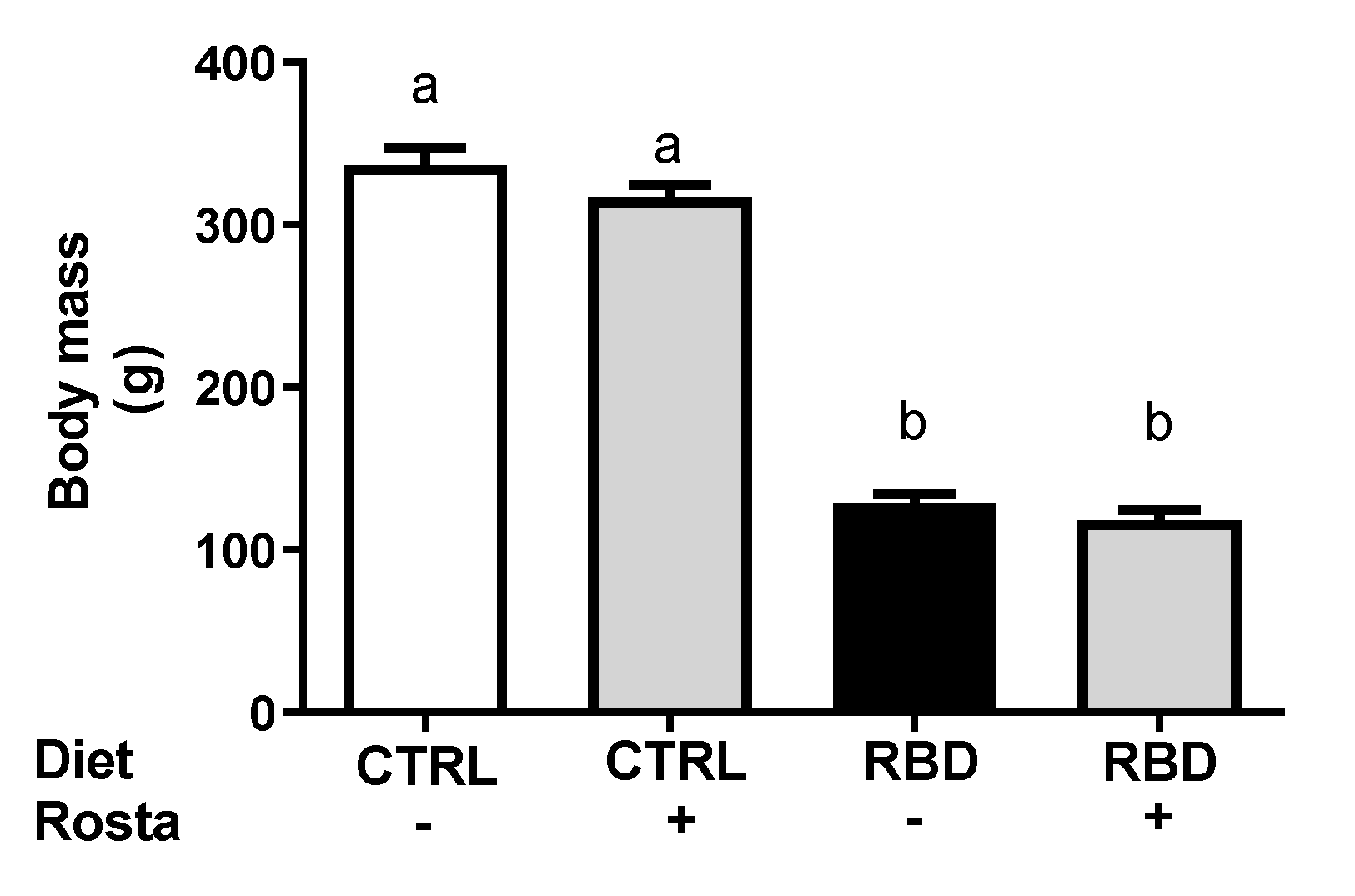
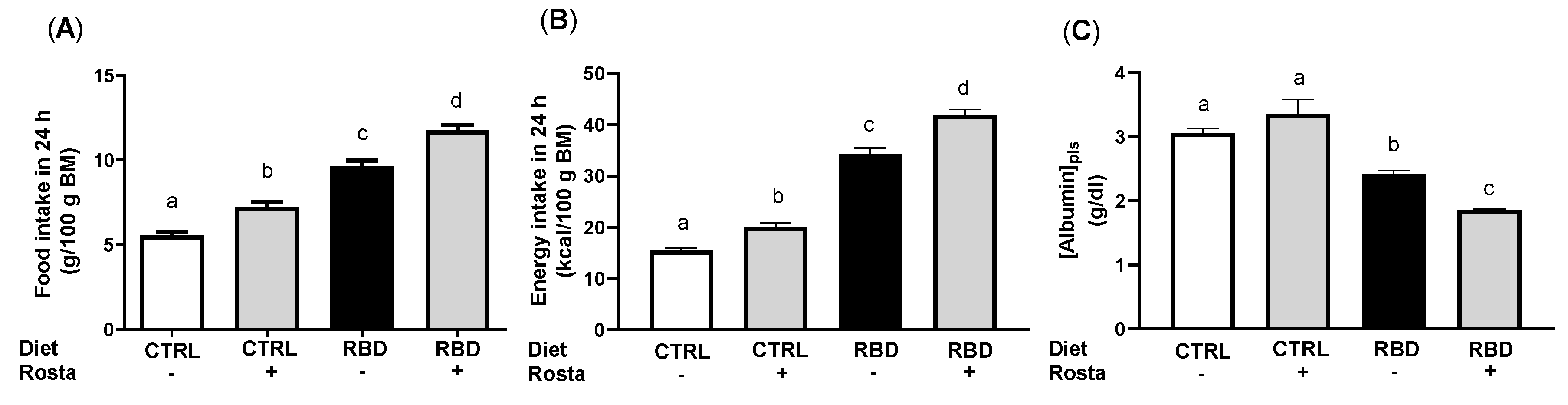
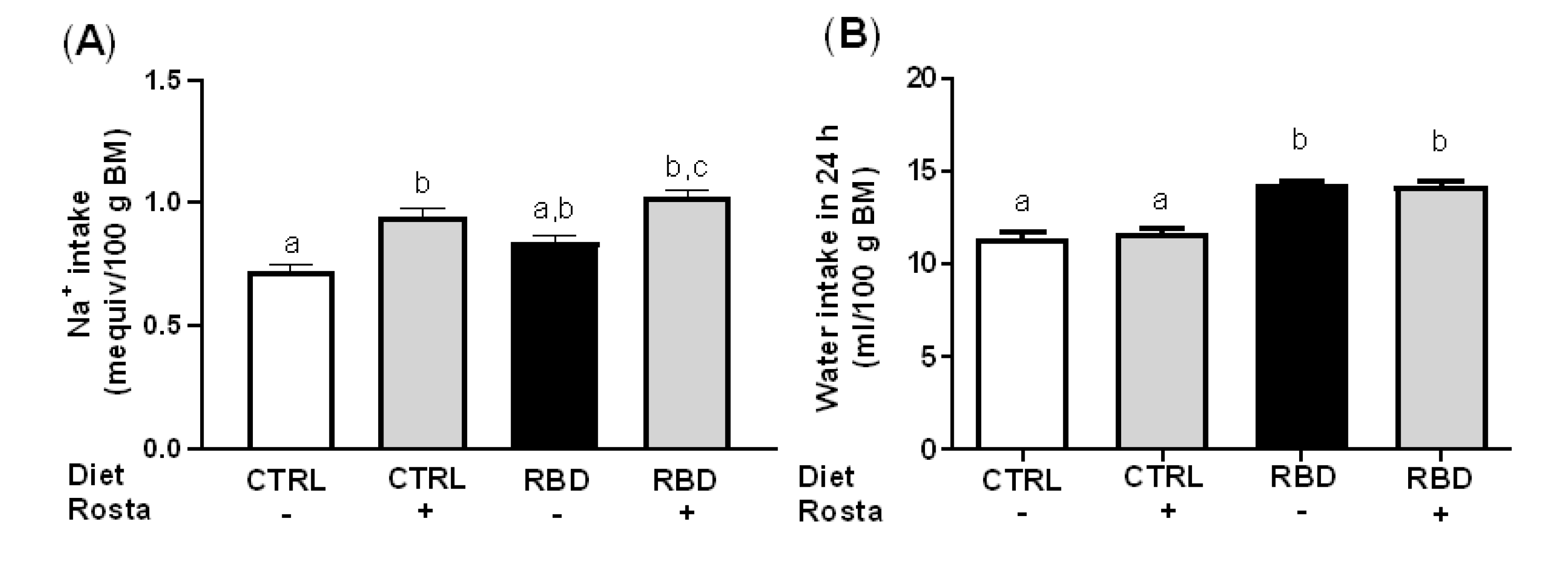
2.1. Body Mass, Food, Energy, Na+, and Water Intake in Undernourished Rats: Effects of Rostafuroxin Administration
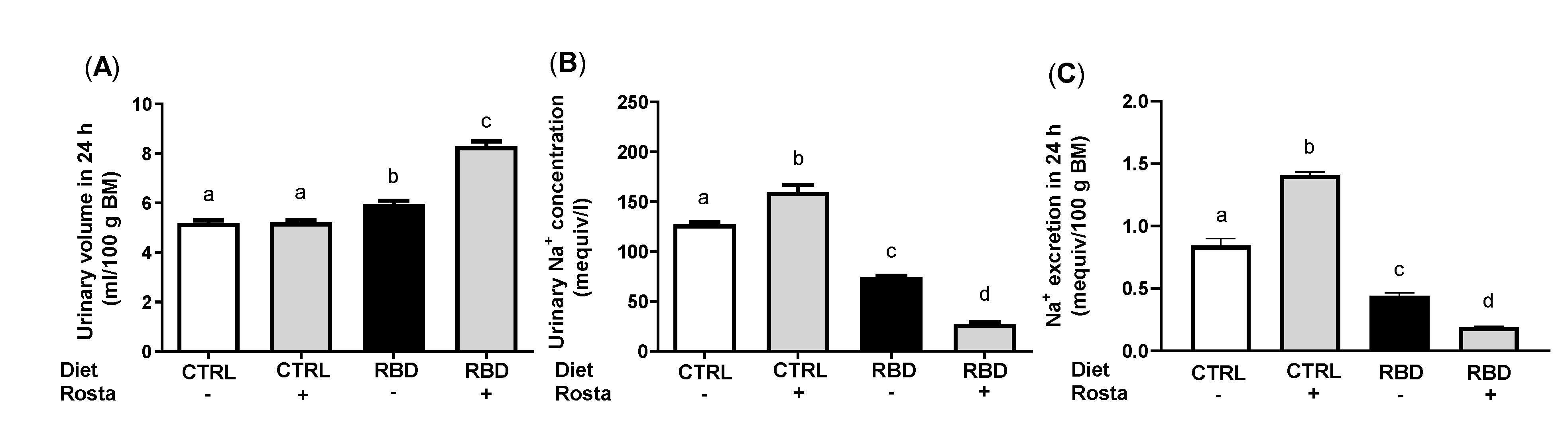
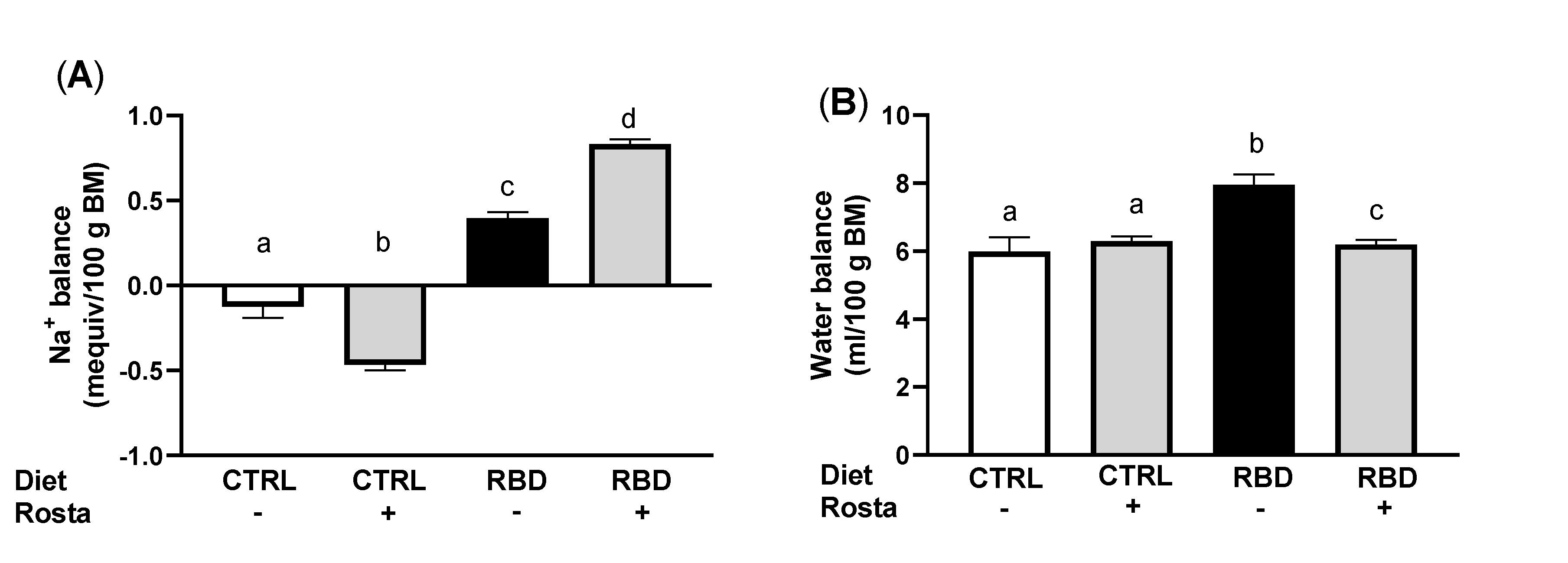
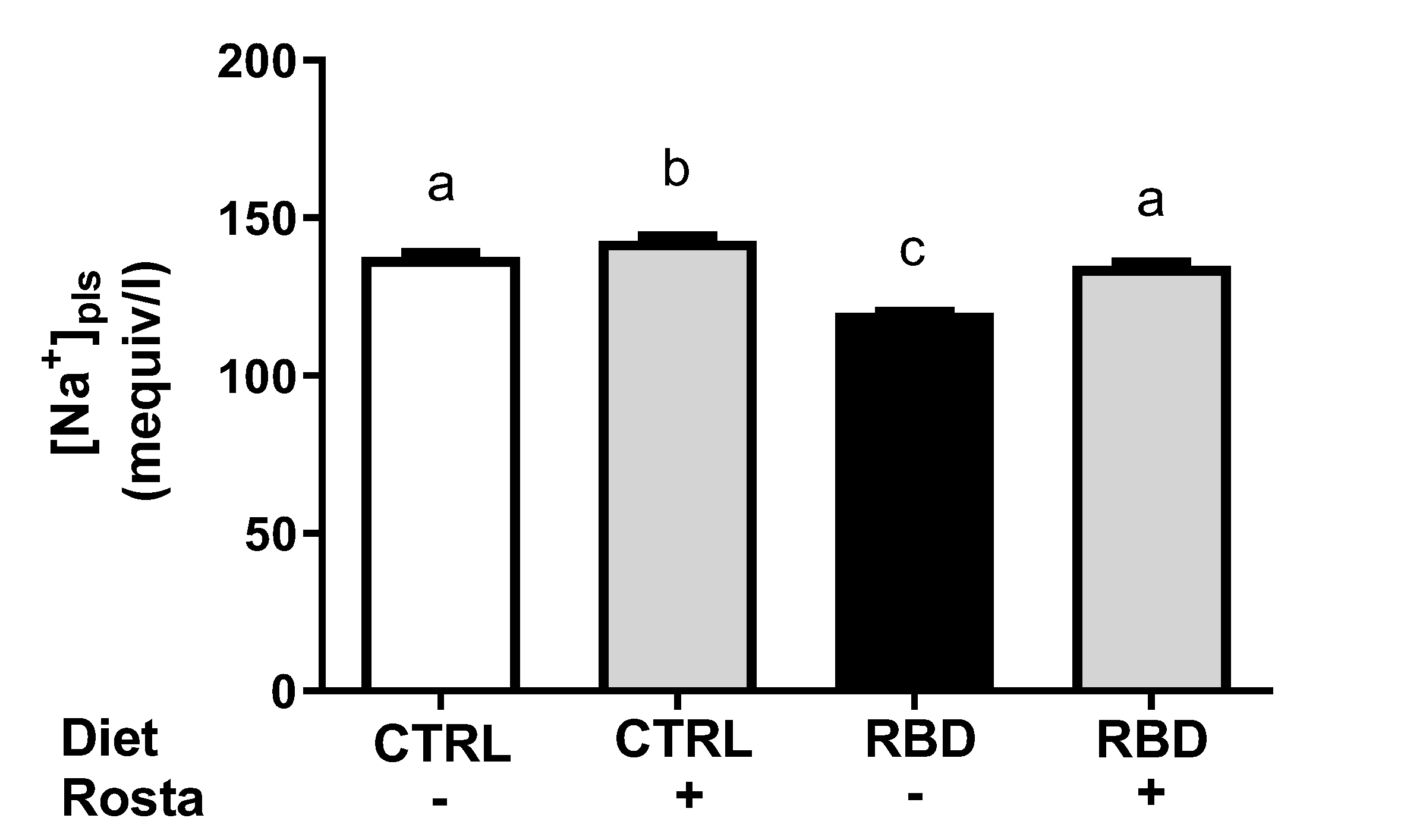
2.2. Urinary Na+ Excretion, Urinary Volume, Na+ Balance, Water Balance, and Plasma Na+ Concentration in Undernourished Rats: Effects of Rostafuroxin
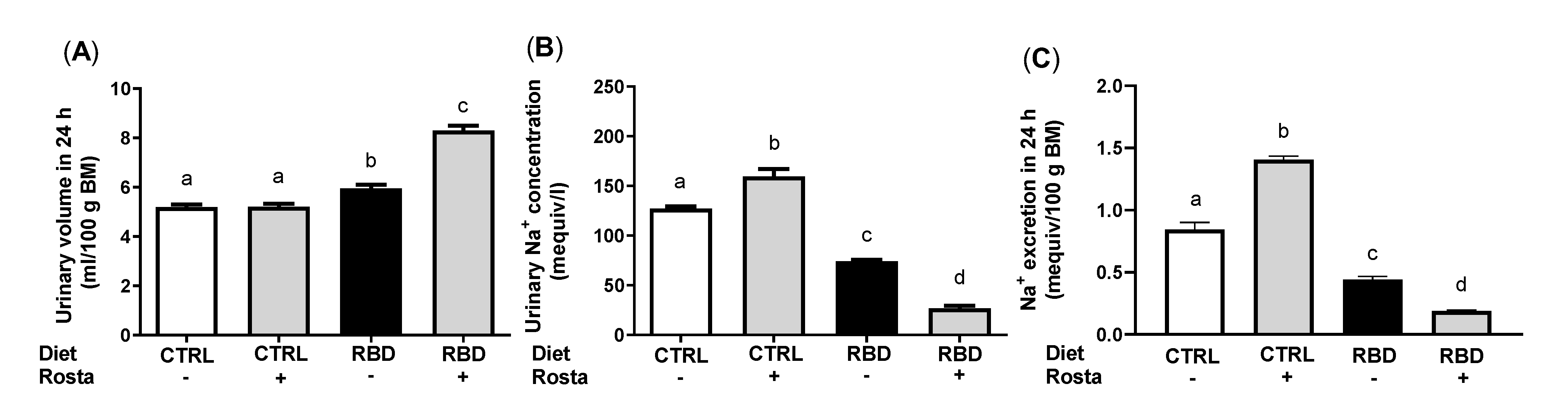
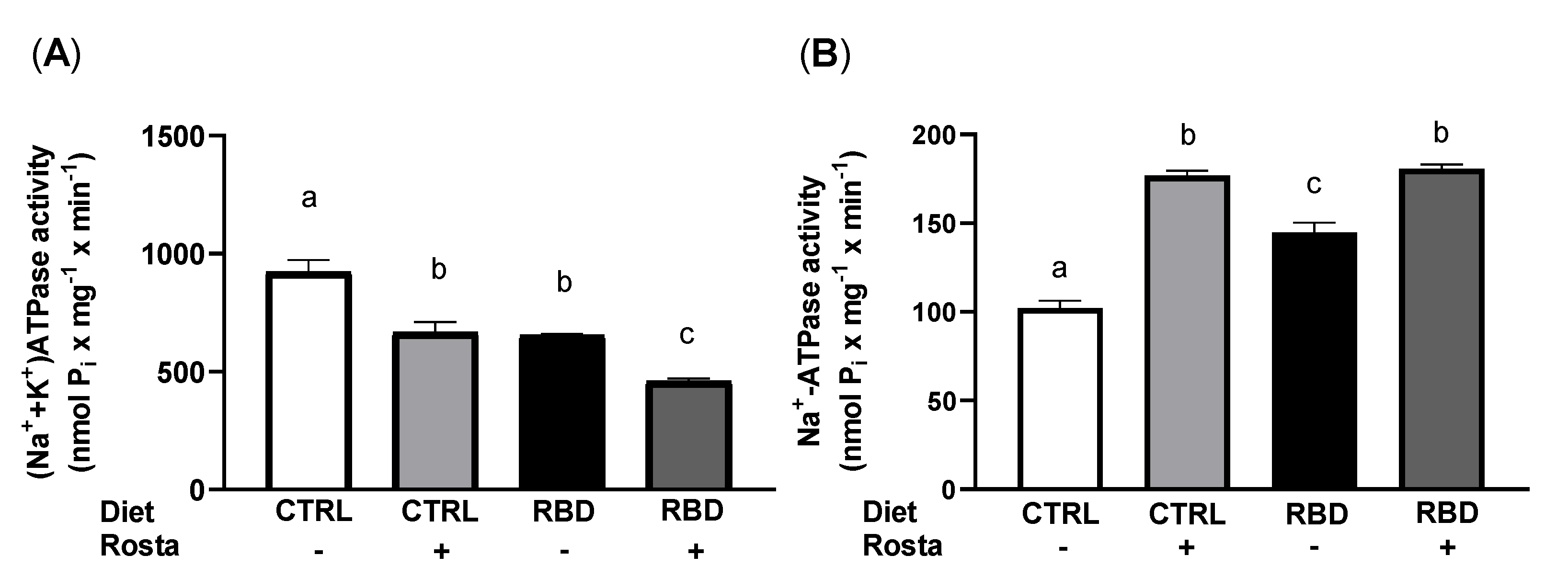
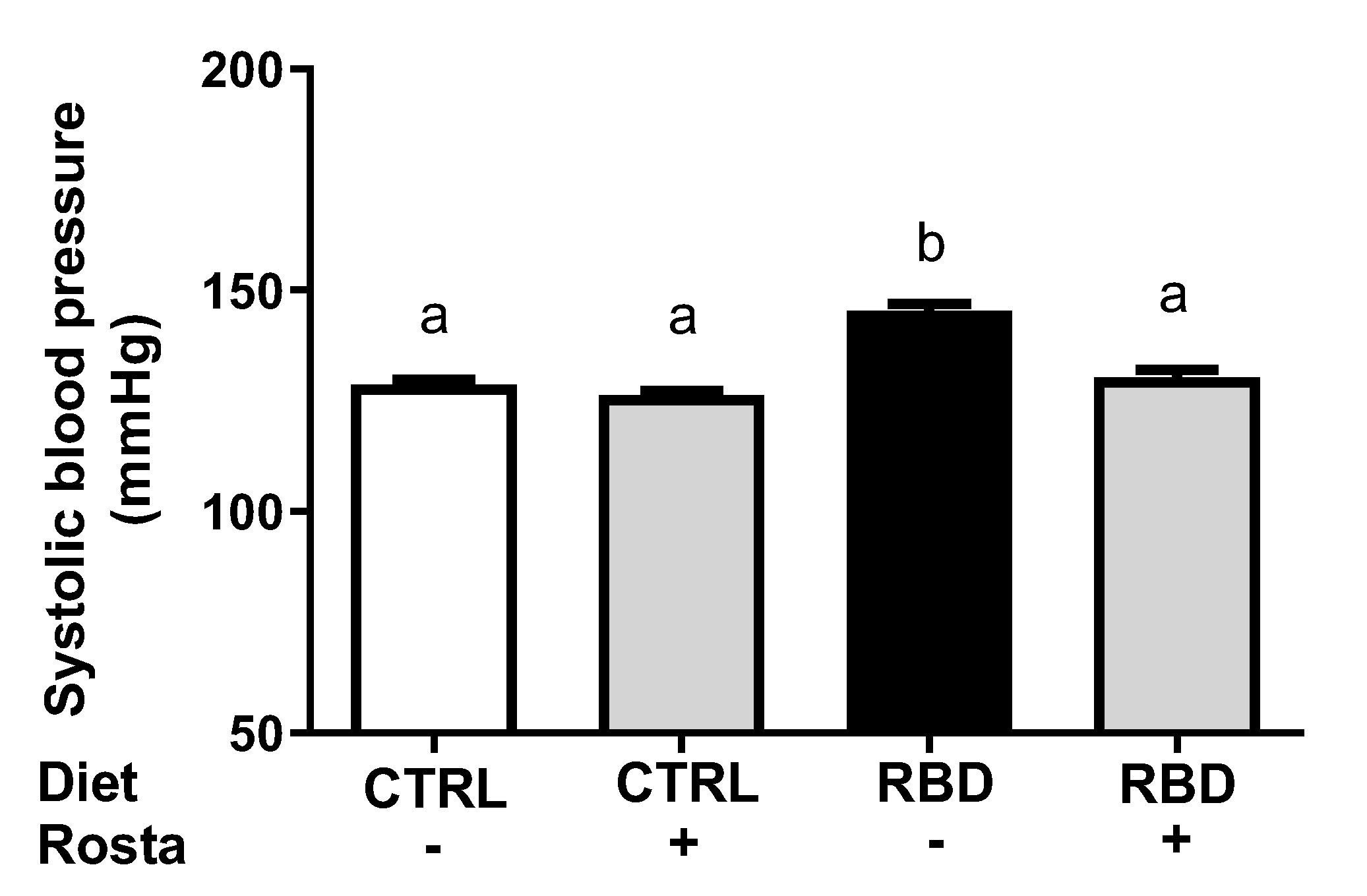
2.3. Na+-Transporting ATPases from Proximal Tubule Cells and Systolic Blood Pressure: Effects of Rostafuroxin
3. Discussion
4. Materials and Methods
4.1. Ethical Statement
4.2. Diets
4.3. Experimental Groups
4.4. Measurement of Blood Pressure
4.5. Preparation of Plasma Membrane-Enriched Fraction from Kidney Proximal Tubule Cells
4.6. Determination of Na+ in Urine and Plasma Samples
4.7. Determination of Albumin in Plasma Samples
4.8. Determination of the Activities of Na+-Transporting ATPases
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferrari, P.; Ferrandi, M.; Valentini, G.; Bianchi, G. Rostafuroxin: An Ouabain Antagonist That Corrects Renal and Vascular Na+-K+- ATPase Alterations in Ouabain and Adducin-Dependent Hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R529–R535. [Google Scholar] [CrossRef] [PubMed]
- Ferrandi, M.; Barassi, P.; Minotti, E.; Duzzi, L.; Molinari, I.; Bianchi, G.; Ferrari, P. PST 2238: A New Antihypertensive Compound That Modulates Renal Na-K Pump Function without Diuretic Activity in Milan Hypertensive Rats. J. Cardiovasc. Pharmacol. 2002, 40, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Schoner, W.; Scheiner-Bobis, G. Endogenous and Exogenous Cardiac Glycosides: Their Roles in Hypertension, Salt Metabolism, and Cell Growth. Am. J. Physiol. Cell Physiol. 2007, 293, C509–C536. [Google Scholar] [CrossRef] [PubMed]
- Ringer, S. Regarding the Influence of the Organic Constituents of the Blood on the Contractility of the Ventricle. J. Physiol. 1885, 6, 361–481. [Google Scholar] [CrossRef] [PubMed]
- Schoner, W. Endogenous Cardiac Glycosides, a New Class of Steroid Hormones. Eur. J. Biochem. 2002, 269, 2440–2448. [Google Scholar] [CrossRef] [PubMed]
- Glynn, I.M. A Hundred Years of Sodium Pumping. Annu. Rev. Physiol. 2002, 64, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Askari, A. Na+/K+-ATPase as a Signal Transducer. Eur. J. Biochem. 2002, 269, 2434–2439. [Google Scholar] [CrossRef]
- Bagrov, A.Y.; Shapiro, J.I.; Fedorova, O.V. Endogenous Cardiotonic Steroids: Physiology, Pharmacology, and Novel Therapeutic Targets. Pharmacol. Rev. 2009, 61, 9–38. [Google Scholar] [CrossRef]
- Swinburn, B.A.; Kraak, V.I.; Allender, S.; Atkins, V.J.; Baker, P.I.; Bogard, J.R.; Brinsden, H.; Calvillo, A.; De Schutter, O.; Devarajan, R.; et al. The Global Syndemic of Obesity, Undernutrition, and Climate Change: The Lancet Commission Report. Lancet Lond. Engl. 2019, 393, 791–846. [Google Scholar] [CrossRef]
- McLaren, D.S.; Pellett, P.L. Nutrition in the Middle East. World. Rev. Nutr. Diet 1970, 12, 43–127. [Google Scholar] [CrossRef]
- Murillo, B.; Cabezas, M.T.; Bressani, R. Influence of Energy Density on the Use of Protein in Diets Prepared from Corn and Beans (Article in Spanish). Arch. Latinoam. Nutr. 1974, 24, 223–241. [Google Scholar]
- Pak, N.; Araya, H. Potentiality of legume-cereal mixture to cover the safe level of protein intake. Arch. Latinoam. Nutr. 1977, 27, 495–504. [Google Scholar] [PubMed]
- Ramos-Aliaga, R. Biochemical and nutritional aspects in growing rats receiving proteins of 2 dietary regimens of the Peruvian Andes. Arch. Latinoam. Nutr. 1978, 28, 378–400. [Google Scholar] [PubMed]
- Teodósio, N.R.; Lago, E.S.; Romani, S.A.; Guedes, R.C. A Regional Basic Diet from Northeast Brazil as a Dietary Model of Experimental Malnutrition. Arch. Latinoam. Nutr. 1990, 40, 533–547. [Google Scholar] [PubMed]
- Jannuzzi, L.B.; Pereira-Acacio, A.; Ferreira, B.S.N.; Silva-Pereira, D.; Veloso-Santos, J.P.M.; Alves-Bezerra, D.S.; Lopes, J.A.; Costa-Sarmento, G.; Lara, L.S.; Vieira, L.D.; et al. Undernutrition - Thirty Years of the Regional Basic Diet: The Legacy of Naíde Teodósio in Different Fields of Knowledge. Nutr. Neurosci. 2022, 25, 1973–1994. [Google Scholar] [CrossRef]
- Silva, P.A.; Monnerat-Cahli, G.; Pereira-Acácio, A.; Luzardo, R.; Sampaio, L.S.; Luna-Leite, M.A.; Lara, L.S.; Einicker-Lamas, M.; Panizzutti, R.; Madeira, C.; et al. Mechanisms Involving Ang II and MAPK/ERK1/2 Signaling Pathways Underlie Cardiac and Renal Alterations during Chronic Undernutrition. PloS One 2014, 9, e100410. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Acácio, A.; Veloso-Santos, J.P.M.; Nossar, L.F.; Costa-Sarmento, G.; Muzi-Filho, H.; Vieyra, A. Angiotensin-(3-4) Normalizes the Elevated Arterial Blood Pressure and Abnormal Na+/Energy Handling Associated with Chronic Undernutrition by Counteracting the Effects Mediated by Type 1 Angiotensin II Receptors. PloS One 2022, 17, e0273385. [Google Scholar] [CrossRef]
- McDonough, A.A. Mechanisms of Proximal Tubule Sodium Transport Regulation That Link Extracellular Fluid Volume and Blood Pressure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R851–861. [Google Scholar] [CrossRef]
- Zhuo, J.L.; Li, X.C. Proximal Nephron. Compr. Physiol. 2013, 3, 1079–1123. [Google Scholar] [CrossRef]
- Agoston, D.V. How to Translate Time? The Temporal Aspect of Human and Rodent Biology. Front. Neurol. 2017, 8, 92. [Google Scholar] [CrossRef]
- Schwartz, M.W.; Woods, S.C.; Porte, D.; Seeley, R.J.; Baskin, D.G. Central Nervous System Control of Food Intake. Nature 2000, 404, 661–671. [Google Scholar] [CrossRef]
- Morton, G.J.; Cummings, D.E.; Baskin, D.G.; Barsh, G.S.; Schwartz, M.W. Central Nervous System Control of Food Intake and Body Weight. Nature 2006, 443, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Farr, O.M.; Li, C.-S.R.; Mantzoros, C.S. Central Nervous System Regulation of Eating: Insights from Human Brain Imaging. Metabolism 2016, 65, 699–713. [Google Scholar] [CrossRef]
- Kurita, H.; Xu, K.Y.; Maejima, Y.; Nakata, M.; Dezaki, K.; Santoso, P.; Yang, Y.; Arai, T.; Gantulga, D.; Muroya, S.; et al. Arcuate Na+,K+-ATPase Senses Systemic Energy States and Regulates Feeding Behavior through Glucose-Inhibited Neurons. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E320–E333. [Google Scholar] [CrossRef] [PubMed]
- Otero-Rodiño, C.; Conde-Sieira, M.; Comesaña, S.; Álvarez-Otero, R.; López-Patiño, M.A.; Míguez, J.M.; Soengas, J.L. Na+/K+-ATPase Is Involved in the Regulation of Food Intake in Rainbow Trout but Apparently Not through Brain Glucosensing Mechanisms. Physiol. Behav. 2019, 209, 112617. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, D. Endogenous Cardiotonic Steroids and Cardiovascular Disease, Where to Next? Cell Calcium 2020, 86, 102156. [Google Scholar] [CrossRef] [PubMed]
- Titze, J.; Krause, H.; Hecht, H.; Dietsch, P.; Rittweger, J.; Lang, R.; Kirsch, K.A.; Hilgers, K.F. Reduced Osmotically Inactive Na Storage Capacity and Hypertension in the Dahl Model. Am. J. Physiol. Renal Physiol. 2002, 283, F134–F141. [Google Scholar] [CrossRef]
- Titze, J.; Lang, R.; Ilies, C.; Schwind, K.H.; Kirsch, K.A.; Dietsch, P.; Luft, F.C.; Hilgers, K.F. Osmotically Inactive Skin Na+ Storage in Rats. Am. J. Physiol. Renal Physiol. 2003, 285, F1108–F1117. [Google Scholar] [CrossRef]
- Canaud, B.; Kooman, J.; Selby, N.M.; Taal, M.; Francis, S.; Kopperschmidt, P.; Maierhofer, A.; Kotanko, P.; Titze, J. Sodium and Water Handling during Hemodialysis: New Pathophysiologic Insights and Management Approaches for Improving Outcomes in End-Stage Kidney Disease. Kidney Int. 2019, 95, 296–309. [Google Scholar] [CrossRef]
- Tordoff, M.G. Effect of Chronic Ouabain Infusion on Food, Water, and NaCl Intake, Body Composition, and Plasma Hormones of Sprague-Dawley Rats. Physiol. Behav. 1996, 59, 87–92. [Google Scholar] [CrossRef]
- Lewis, L.K.; Yandle, T.G.; Hilton, P.J.; Jensen, B.P.; Begg, E.J.; Nicholls, M.G. Endogenous Ouabain Is Not Ouabain. Hypertension 2014, 64, 680–683. [Google Scholar] [CrossRef] [PubMed]
- Simonini, M.; Casanova, P.; Citterio, L.; Messaggio, E.; Lanzani, C.; Manunta, P. Endogenous Ouabain and Related Genes in the Translation from Hypertension to Renal Diseases. Int. J. Mol. Sci. 2018, 19, 1948. [Google Scholar] [CrossRef] [PubMed]
- Torres, S.J.; Nowson, C.A. Relationship between Stress, Eating Behavior, and Obesity. Nutrition 2007, 23, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.M.; Jastreboff, A.M.; White, M.A.; Grilo, C.M.; Sinha, R. Stress, Cortisol, and Other Appetite-Related Hormones: Prospective Prediction of 6-Month Changes in Food Cravings and Weight. Obesity (Silver Spring) 2017, 25, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Viollet, B.; Foretz, M.; Guigas, B.; Horman, S.; Dentin, R.; Bertrand, L.; Hue, L.; Andreelli, F. Activation of AMP-Activated Protein Kinase in the Liver: A New Strategy for the Management of Metabolic Hepatic Disorders. J. Physiol. 2006, 574, 41–53. [Google Scholar] [CrossRef]
- Foretz, M.; Viollet, B. Measurement of AMPK-Induced Inhibition of Lipid Synthesis Flux in Cultured Cells. Methods Mol. Biol. 2018, 1732, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Nesher, M.; Dvela, M.; Igbokwe, V.U.; Rosen, H.; Lichtstein, D. Physiological Roles of Endogenous Ouabain in Normal Rats. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H2026–H2034. [Google Scholar] [CrossRef] [PubMed]
- Citterio, L.; Bianchi, G.; Scioli, G.A.; Glorioso, N.; Bigazzi, R.; Cusi, D.; Staessen, J.A.; Cavuto, S.; Ferrandi, M.; Lanzani, C.; et al. Antihypertensive Treatment Guided by Genetics: PEARL-HT, the Randomized Proof-of-Concept Trial Comparing Rostafuroxin with Losartan. Pharmacogenomics J. 2021, 21, 346–358. [Google Scholar] [CrossRef]
- Leib, D.E.; Zimmerman, C.A.; Knight, Z.A. Thirst. Curr. Biol. 2016, 26, R1260–R1265. [Google Scholar] [CrossRef]
- Bergmann, F.; Chaimovitz, M.; Costin, A.; Gutman, Y.; Ginath, Y. Water Intake of Rats after Implantation of Ouabain into the Hypothalamus. Am. J. Physiol. 1967, 213, 328–332. [Google Scholar] [CrossRef]
- Gonzalez-Vicente, A.; Hong, N.J.; Yang, N.; Cabral, P.D.; Berthiaume, J.M.; Dominici, F.P.; Garvin, J.L. Dietary Fructose Increases the Sensitivity of Proximal Tubules to Angiotensin II in Rats Fed High-Salt Diets. Nutrients 2018, 10, 1244. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.C.; Jeong, H.S.; Park, J.S.; Shin, J.H.; Kook, Y.J. Renal Functional Responses to Centrally Administered Ouabain in Anesthetized Rabbits. Methods Find. Exp. Clin. Pharmacol. 2000, 22, 573–579. [Google Scholar] [CrossRef]
- Ferrandi, M.; Molinari, I.; Barassi, P.; Minotti, E.; Bianchi, G.; Ferrari, P. Organ Hypertrophic Signaling within Caveolae Membrane Subdomains Triggered by Ouabain and Antagonized by PST 2238. J. Biol. Chem. 2004, 279, 33306–33314. [Google Scholar] [CrossRef] [PubMed]
- Brensilver, J.M.; Daniels, F.H.; Lefavour, G.S.; Malseptic, R.M.; Lorch, J.A.; Ponte, M.L.; Cortell, S. Effect of Variations in Dietary Sodium Intake on Sodium Excretion in Mature Rats. Kidney Int. 1985, 27, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Titze, J.; Dahlmann, A.; Lerchl, K.; Kopp, C.; Rakova, N.; Schröder, A.; Luft, F.C. Spooky Sodium Balance. Kidney Int. 2014, 85, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Eaton, D.C.; Pooler, J.P. Vander’s Renal Physiology, 9 ed.; McGraw-Hill Education: New York, NY, USA, 2018. [Google Scholar]
- Vieyra, A.; Silva, P.A.; Muzi-Filho, H.; Dick, C.F.; Araujo-dos-Santos, A.L.; Dias, J.; Vieira-Filho, L.D.; Paixão, A.D.O. The Role of the Second Na+ Pump in Mammals and Parasites. In Regulation of Membrane Na+-K+ ATPase; Chakraborti, S., Dhalla, N.S., Eds.; Springer International Publishing: Cham, 2016; ISBN 978-3-319-24750-2. [Google Scholar]
- Bełtowski, J.; Borkowska, E.; Wójcicka, G.; Marciniak, A. Regulation of Renal Ouabain-Resistant Na+-ATPase by Leptin, Nitric Oxide, Reactive Oxygen Species, and Cyclic Nucleotides: Implications for Obesity-Associated Hypertension. Clin. Exp. Hypertens. 2007, 29, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.; Ferrão, F.M.; Axelband, F.; Carmona, A.K.; Lara, L.S.; Vieyra, A. ANG-(3-4) Inhibits Renal Na+-ATPase in Hypertensive Rats through a Mechanism That Involves Dissociation of ANG II Receptors, Heterodimers, and PKA. Am. J. Physiol. Renal. Physiol. 2014, 306, F855–F863. [Google Scholar] [CrossRef]
- Wenceslau, C.F.; Rossoni, L.V. Rostafuroxin Ameliorates Endothelial Dysfunction and Oxidative Stress in Resistance Arteries from Deoxycorticosterone Acetate-Salt Hypertensive Rats: The Role of Na+K+-ATPase/ CSRC Pathway. J Hypertens. 2014, 32, 542–554. [Google Scholar] [CrossRef]
- Reynertson, R.H.; Parmley, R.T.; Rodén, L.; Oparil, S. Proteoglycans and Hypertension. I. A Biochemical and Ultrastructural Study of Aorta Glycosaminoglycans in Spontaneously Hypertensive Rats. Coll. Relat. Res. 1986, 6, 77–101. [Google Scholar] [CrossRef]
- Olde Engberink, R.H.G.; Rorije, N.M.G.; Homan van der Heide, J.J.; van den Born, B.-J.H.; Vogt, L. Role of the Vascular Wall in Sodium Homeostasis and Salt Sensitivity. J. Am. Soc. Nephrol. 2015, 26, 777–783. [Google Scholar] [CrossRef]
- Silva, P.A.; Muzi-Filho, H.; Pereira-Acácio, A.; Dias, J.; Martins, J.F.S.; Landim-Vieira, M.; Verdoorn, K.S.; Lara, L.S.; Vieira-Filho, L.D.; Cabral, E.V.; et al. Altered Signaling Pathways Linked to Angiotensin II Underpin the Upregulation of Renal Na+-ATPase in Chronically Undernourished Rats. Biochim. Biophys. Acta 2014, 1842, 2357–2366. [Google Scholar] [CrossRef] [PubMed]
- Laredo, J.; Shah, J.R.; Lu, Z.R.; Hamilton, B.P.; Hamlyn, J.M. Angiotensin II Stimulates Secretion of Endogenous Ouabain from Bovine Adrenocortical Cells via Angiotensin Type 2 Receptors. Hypertension 1997, 29, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Dmitrieva, R.I.; Doris, P.A. Cardiotonic Steroids: Potential Endogenous Sodium Pump Ligands with Diverse Function. Exp. Biol. Med. (Maywood) 2002, 227, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Miura, S.; Matsuo, Y.; Kiya, Y.; Karnik, S.S.; Saku, K. Molecular Mechanisms of the Antagonistic Action between AT1 and AT2 Receptors. Biochem. Biophys. Res. Commun. 2010, 391, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE Guidelines 2.0: Updated Guidelines for Reporting Animal Research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Reeves, P.G. Components of the AIN-93 Diets as Improvements in the AIN-76A Diet. J. Nutr. 1997, 127, 838S–841S. [Google Scholar] [CrossRef] [PubMed]
- Sandel, E.B. Colorimetric Determinaton of Traces of Metals., 3rd ed.; Interscience Publishers: New York, NY, USA, 1959. [Google Scholar]
- Snell, F.S.; Snell, C.T. Colorimetric Methods of Analysis., 3rd ed.; Van Nostrand Reinhold Company: New York, NY, USA, 1967. [Google Scholar]
- Perkin-Elmer Corporation. Analytical Methods for Atomic Absorption Spectrometry model 303. Perkin-Elmer Corporation: Norwalk, CT, USA, 1968.
- Feng, M.; Whitesall, S.; Zhang, Y.; Beibel, M.; D’Alecy, L.; DiPetrillo, K. Validation of Volume-Pressure Recording Tail-Cuff Blood Pressure Measurements. Am. J. Hypertens. 2008, 21, 1288–1291. [Google Scholar] [CrossRef] [PubMed]
- Whittembury, G.; Proverbio, F. Two Modes of Na Extrusion in Cells from Guinea Pig Kidney Cortex Slices. Pflugers Arch. 1970, 316, 1–25. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Taussky, H.H.; Shorr, E. A Microcolorimetric Method for the Determination of Inorganic Phosphorus. J. Biol. Chem. 1953, 202, 675–685. [Google Scholar] [CrossRef]
| CTRL2 | RBD3 | |
|---|---|---|
| Protein % (w/w) | 23 | 8 |
| Carbohydrate % (w/w) | 41 | 78 |
| Lipids % (w/w) | 2.5 | 1.7 |
| Na % (w/w) | 0.34 | 0.24 |
| Fe % (w/w) | 0.018 | 0.007 |
| Ca % (w/w) | 1.8 | 0.04 |
| K % (w/w) | 0.9 | 0.3 |
| Energy supply kcal/100 g dry weight | 278 | 356 |
| Vitamin supplement | Yes | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





