Submitted:
15 May 2023
Posted:
17 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
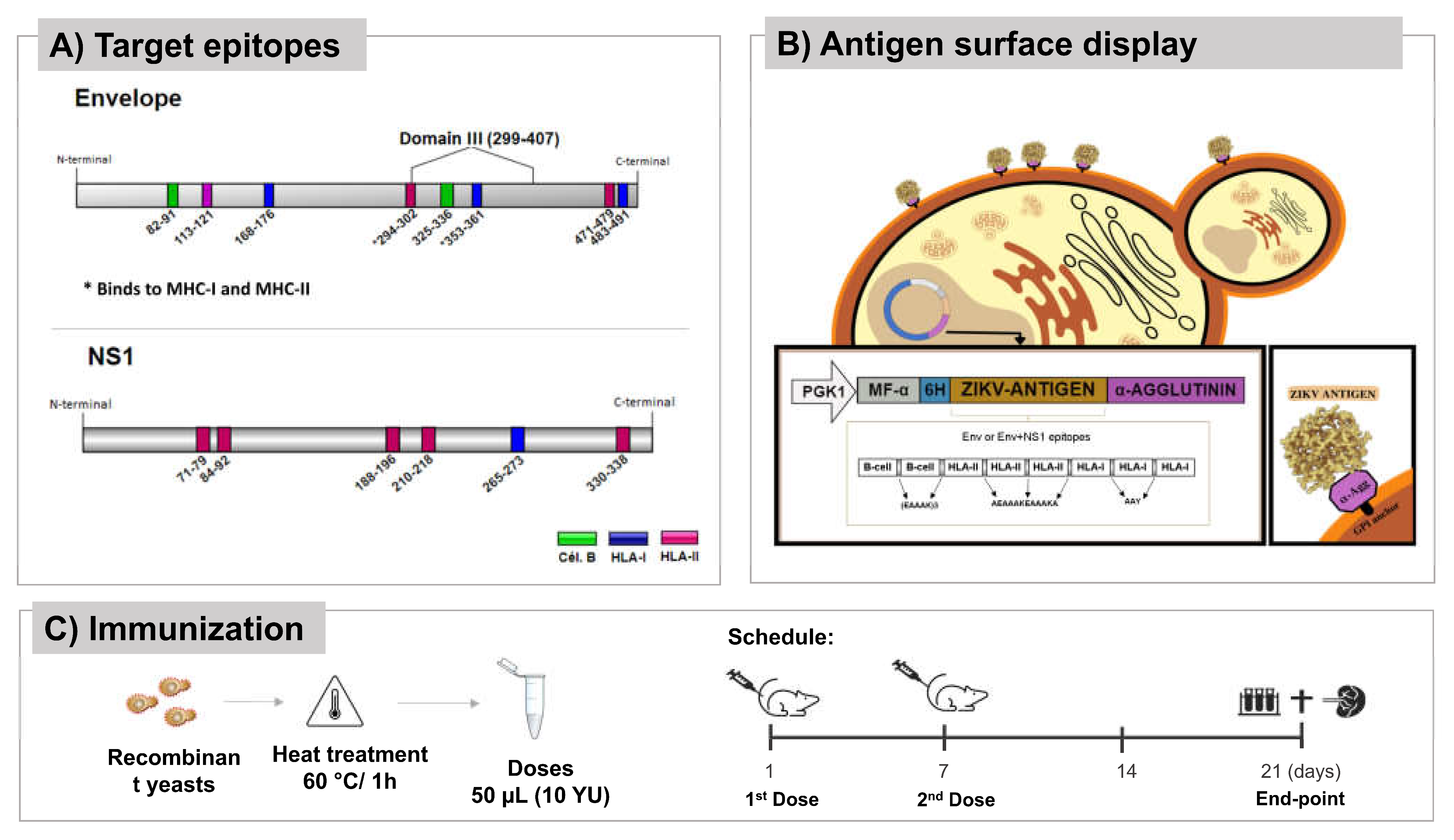
Vaccine antigens and yeast strains
Yeast preparation
Mice, ethical parameters, and immunization protocol
In vitro culture and stimulation of isolated spleen lymphocytes
Immunological analysis
Hematological and biochemical analyzes
Statistical analysis
3. Results
3.1. P. pastoris can surface display vaccine antigens
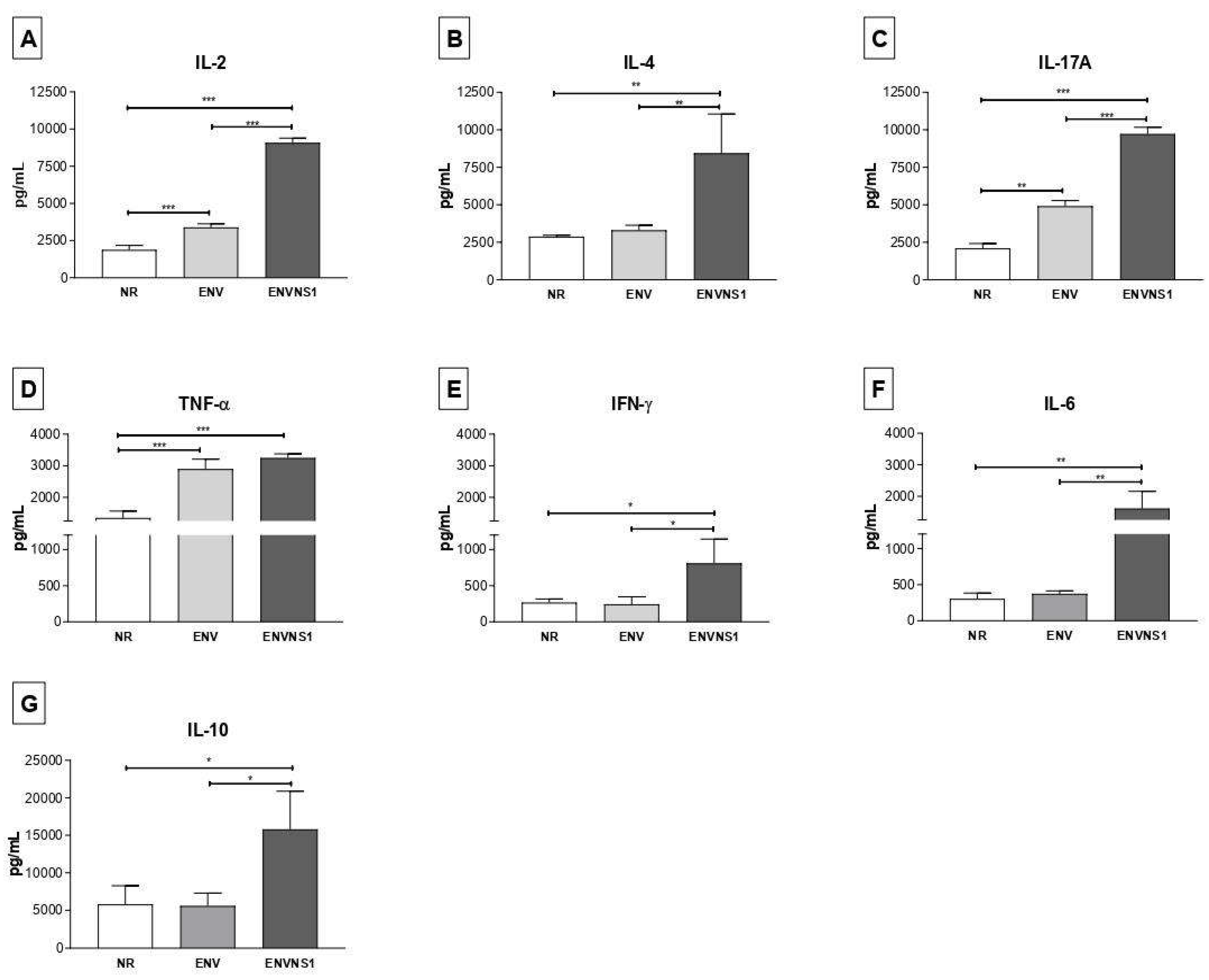
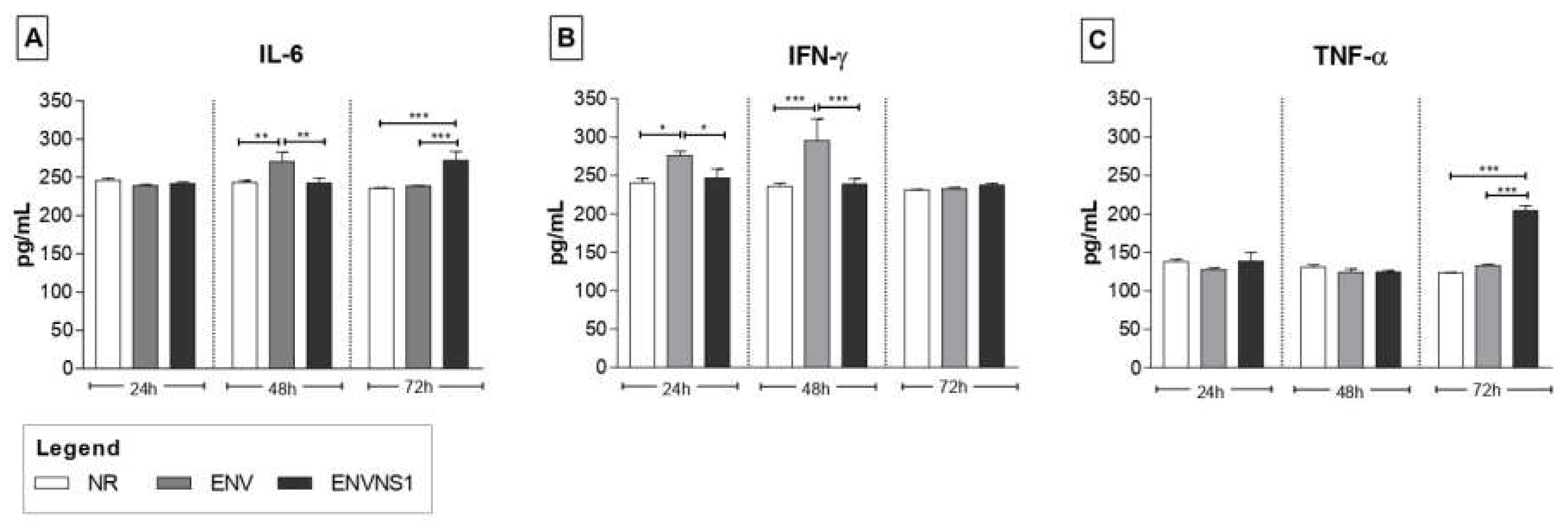
3.2. Recombinant P. pastoris strains induce increased secretion of serum and splenic cytokines
3.3. P. pastoris:ENV and P. pastoris:ENVNS1 enhance antibody production
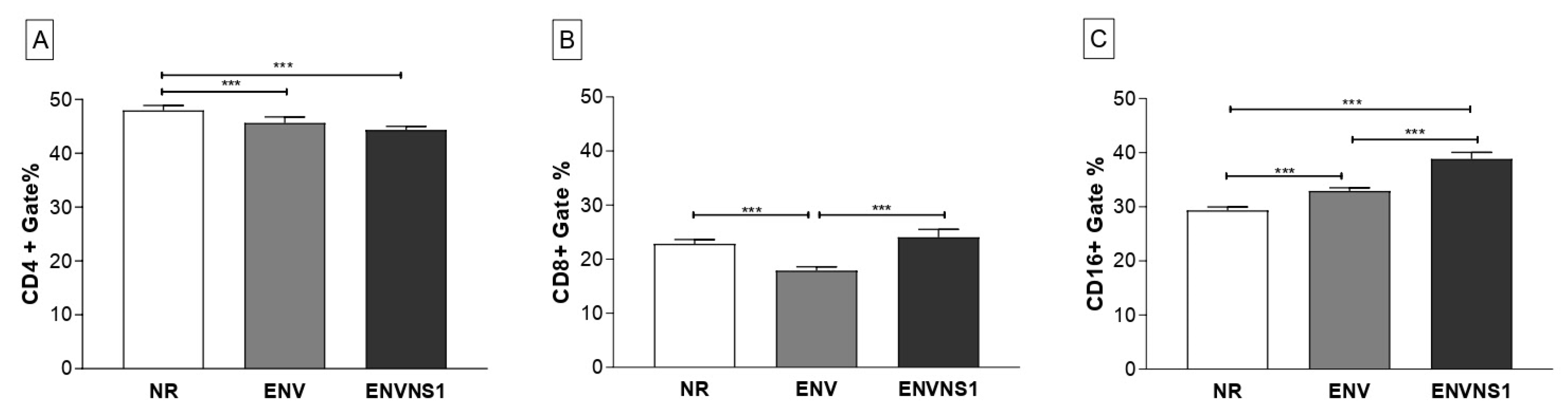
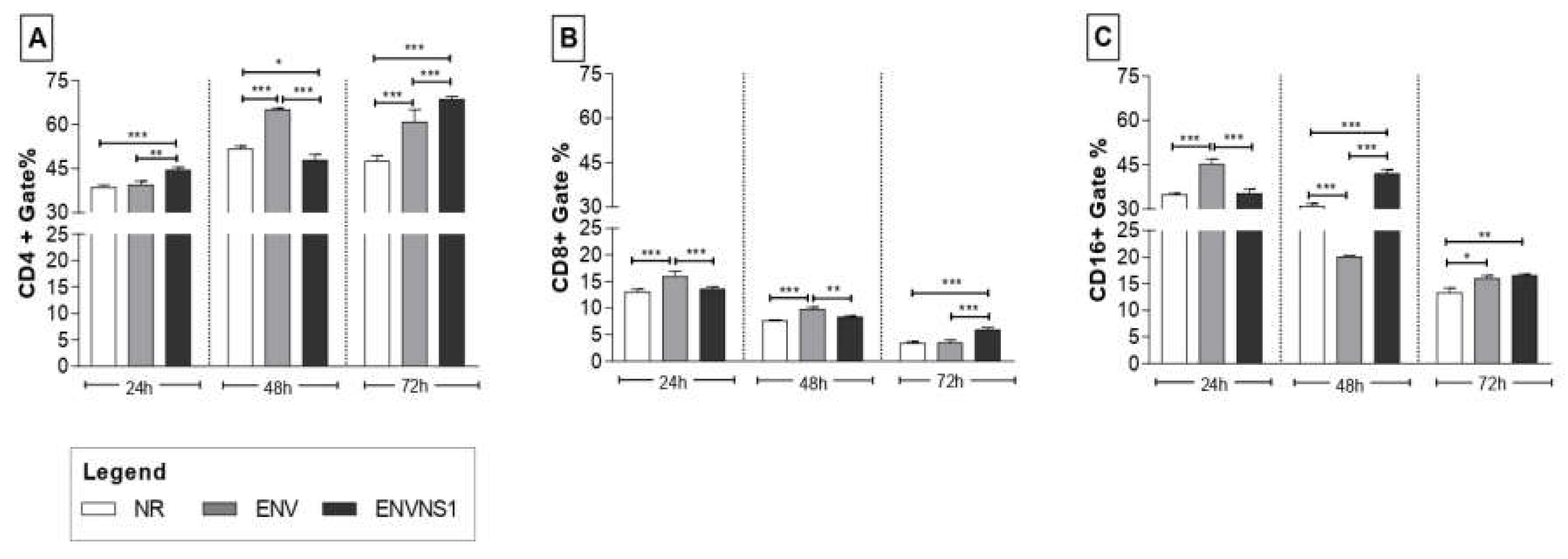
3.4. Recombinant yeasts stimulate cellular responses
3.5. The whole yeast vaccines do not cause significant side effects
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, H.; Yoo, S.J.; Kang, H.A. Yeast Synthetic Biology for the Production of Recombinant Therapeutic Proteins. FEMS Yeast Res 2014, n/a-n/a. [CrossRef]
- Stubbs, A.C.; Martin, K.S.; Coeshott, C.; Skaates, S.V.; Kuritzkes, D.R.; Bellgrau, D.; Franzusoff, A.; Duke, R.C.; Wilson, C.C. Whole Recombinant Yeast Vaccine Activates Dendritic Cells and Elicits Protective Cell-Mediated Immunity. Nat Med 2001, 7, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Vogl, T.; Hartner, F.S.; Glieder, A. New Opportunities by Synthetic Biology for Biopharmaceutical Production in Pichia Pastoris. Current Opinion in Biotechnology 2013, 24, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Kumar, P. Yeast-Based Vaccines: New Perspective in Vaccine Development and Application. FEMS Yeast Research 2019, 19. [Google Scholar] [CrossRef] [PubMed]
- Ardiani, A.; Higgins, J.P.; Hodge, J.W. Vaccines Based on Whole Recombinant Saccharomyces Cerevisiae Cells: Yeast-Based Vaccines. FEMS Yeast Research 2010, 10, 1060–1069. [Google Scholar] [CrossRef] [PubMed]
- Bian, G.; Cheng, Y.; Wang, Z.; Hu, Y.; Zhang, X.; Wu, M.; Chen, Z.; Shi, B.; Sun, S.; Shen, Y.; et al. Whole Recombinant Hansenula Polymorpha Expressing Hepatitis B Virus Surface Antigen (Yeast-HBsAg) Induces Potent HBsAg-Specific Th1 and Th2 Immune Responses. Vaccine 2009, 28, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Bazan, S.B.; Geginat, G.; Breinig, T.; Schmitt, M.J.; Breinig, F. Uptake of Various Yeast Genera by Antigen-Presenting Cells and Influence of Subcellular Antigen Localization on the Activation of Ovalbumin-Specific CD8 T Lymphocytes. Vaccine 2011, 29, 8165–8173. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Jin, S.; Karlsson, E.; Schultz-Cherry, S.; Ye, K. Yeast Surface-Displayed H5N1 Avian Influenza Vaccines. Journal of Immunology Research 2016, 2016, 1–12. [Google Scholar] [CrossRef]
- Jacob, D.; Ruffie, C.; Dubois, M.; Combredet, C.; Amino, R.; Formaglio, P.; Gorgette, O.; Pehau-Arnaudet, G.; Guery, C.; Puijalon, O.; et al. Whole Pichia Pastoris Yeast Expressing Measles Virus Nucleoprotein as a Production and Delivery System to Multimerize Plasmodium Antigens. PLoS ONE 2014, 9, e86658. [Google Scholar] [CrossRef]
- Sun, H.; Wang, L.; Wang, T.; Zhang, J.; Liu, Q.; Chen, P.; Chen, Z.; Wang, F.; Li, H.; Xiao, Y.; et al. Display of Eimeria Tenella EtMic2 Protein on the Surface of Saccharomyces Cerevisiae as a Potential Oral Vaccine against Chicken Coccidiosis. Vaccine 2014, 32, 1869–1876. [Google Scholar] [CrossRef]
- Gebauer, M.; Hürlimann, H.C.; Behrens, M.; Wolff, T.; Behrens, S.-E. Subunit Vaccines Based on Recombinant Yeast Protect against Influenza A Virus in a One-Shot Vaccination Scheme. Vaccine 2019, 37, 5578–5587. [Google Scholar] [CrossRef]
- Kim, J.-M.; Jung, D.-I.; Eom, Y.J.; Park, S.-M.; Yoo, H.-S.; Jang, Y.-S.; Yang, M.-S.; Kim, D.-H. Surface-Displayed Expression of a Neutralizing Epitope of ApxIIA Exotoxin in Saccharomyces Cerevisiae and Oral Administration of It for Protective Immune Responses against Challenge by Actinobacillus Pleuropneumoniae. Bioscience, Biotechnology, and Biochemistry 2010, 74, 1362–1367. [Google Scholar] [CrossRef] [PubMed]
- King, T.H.; Kemmler, C.B.; Guo, Z.; Mann, D.; Lu, Y.; Coeshott, C.; Gehring, A.J.; Bertoletti, A.; Ho, Z.Z.; Delaney, W.; et al. A Whole Recombinant Yeast-Based Therapeutic Vaccine Elicits HBV X, S and Core Specific T Cells in Mice and Activates Human T Cells Recognizing Epitopes Linked to Viral Clearance. PLoS ONE 2014, 9, e101904. [Google Scholar] [CrossRef]
- Wasilenko, J.L.; Sarmento, L.; Spatz, S.; Pantin-Jackwood, M. Cell Surface Display of Highly Pathogenic Avian Influenza Virus Hemagglutinin on the Surface of Pichia Pastoris Cells Using α-Agglutinin for Production of Oral Vaccines. Biotechnol Progress 2009, NA–NA. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, S.; Maeda, H.; Ueda, M. Molecular Display Technology Using Yeast—Arming Technology—. Anal. Sci. 2009, 25, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Ananphongmanee, V.; Srisala, J.; Sritunyalucksana, K.; Boonchird, C. Yeast Surface Display of Two Proteins Previously Shown to Be Protective Against White Spot Syndrome Virus (WSSV) in Shrimp. PLoS ONE 2015, 10, e0128764. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, K.; Ueda, M. Cell Surface Engineering of Yeast for Applications in White Biotechnology. Biotechnol Lett 2011, 33, 1–9. [Google Scholar] [CrossRef]
- Garcia Serpa Osorio-de-Castro, C.; Silva Miranda, E.; Machado de Freitas, C.; Rochel de Camargo, K.; Cranmer, H.H. The Zika Virus Outbreak in Brazil: Knowledge Gaps and Challenges for Risk Reduction. Am J Public Health 2017, 107, 960–965. [Google Scholar] [CrossRef]
- Morabito, K.M.; Graham, B.S. Zika Virus Vaccine Development. The Journal of Infectious Diseases 2017, 216, S957–S963. [Google Scholar] [CrossRef]
- Musso, D.; Ko, A.I.; Baud, D. Zika Virus Infection — After the Pandemic. N Engl J Med 2019, 381, 1444–1457. [Google Scholar] [CrossRef]
- Zhou, K.; Li, C.; Shi, W.; Hu, X.; Nandakumar, K.S.; Jiang, S.; Zhang, N. Current Progress in the Development of Zika Virus Vaccines. Vaccines 2021, 9, 1004. [Google Scholar] [CrossRef]
- Hassert, M.; Wolf, K.J.; Schwetye, K.E.; DiPaolo, R.J.; Brien, J.D.; Pinto, A.K. CD4+T Cells Mediate Protection against Zika Associated Severe Disease in a Mouse Model of Infection. PLoS Pathog 2018, 14, e1007237. [Google Scholar] [CrossRef] [PubMed]
- Elong Ngono, A.; Young, M.P.; Bunz, M.; Xu, Z.; Hattakam, S.; Vizcarra, E.; Regla-Nava, J.A.; Tang, W.W.; Yamabhai, M.; Wen, J.; et al. CD4+ T Cells Promote Humoral Immunity and Viral Control during Zika Virus Infection. PLoS Pathog 2019, 15, e1007474. [Google Scholar] [CrossRef]
- Antonelli, A.C.B.; Almeida, V.P.; de Castro, F.O.F.; Silva, J.M.; Pfrimer, I.A.H.; Cunha-Neto, E.; Maranhão, A.Q.; Brígido, M.M.; Resende, R.O.; Bocca, A.L.; et al. In Silico Construction of a Multiepitope Zika Virus Vaccine Using Immunoinformatics Tools. Sci Rep 2022, 12, 53. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.J.D.; Jesus, A.L.S.; Leal, L.R.S.; Silva, G.A.S.; Melo, C.M.L.; Freitas, A.C. Pichia Pastoris Displaying ZIKV Protein Epitopes from the Envelope and NS1 Induce in Vitro Immune Activation. Vaccine 2021, 39, 2545–2554. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, J.R.M.; de Moraes, L.M.P.; Torres, F.A.G. Molecular Characterization of the 3-Phosphoglycerate Kinase Gene (PGK1) from the Methylotrophic YeastPichia Pastoris. Yeast 2005, 22, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Gu, L.; Zheng, P.; Chen, Z.; Dou, X.; Qin, Q.; Cai, X. Improvement of Cell Counting Method for Neubauer Counting Chamber. J Clin Lab Anal 2020, 34. [Google Scholar] [CrossRef] [PubMed]
- Fojon Martinez Reboredo, S. [Modification of the Fonio method of counting platelets and other components of the blood]. Laboratorio 1958, 26, 511–517. [Google Scholar]
- Almeida, A.S.; Faleiros, A.C.G.; Teixeira, D.N.S.; Cota, U.A.; Chica, J.E.L. Valores de Referência de Parâmetros Bioquímicos No Sangue de Duas Linhagens de Camundongos. J. Bras. Patol. Med. Lab. 2008, 44, 429–432. [Google Scholar] [CrossRef]
- Barbosa, B.D.S.; Praxedes, É.A.; Lima, M.A.; Pimentel, M.M.L.; Santos, F.A.; Brito, P.D.; Lelis, I.C.N.G.; Macedo, M.F. de; Bezerra, M.B. Haematological and Biochemical Profile of Balb-c Mice. Acta Scientiae. Vet. 2017, 45, 5. [Google Scholar] [CrossRef]
- Silva-Santana, G.; Bax, J.C.; Fernandes, D.C.S.; Bacellar, D.T.L.; Hooper, C.; Dias, A.A.S.O.; Silva, C.B.; Souza, A.M.; Ramos, S.; Santos, R.A.; et al. Clinical Hematological and Biochemical Parameters in Swiss, BALB/c, C57BL/6 and B6D2F1 Mus Musculus. Anim. Models Exp. Med. 2020, 3, 304–315. [Google Scholar] [CrossRef]
- Reynolds, J.M.; Angkasekwinai, P.; Dong, C. IL-17 Family Member Cytokines: Regulation and Function in Innate Immunity. Cytokine & Growth Factor Reviews 2010, 21, 413–423. [Google Scholar] [CrossRef]
- Lima, N.S.; Rolland, M.; Modjarrad, K.; Trautmann, L. T Cell Immunity and Zika Virus Vaccine Development. Trends in Immunology 2017, 38, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Pardy, R.D.; Rajah, M.M.; Condotta, S.A.; Taylor, N.G.; Sagan, S.M.; Richer, M.J. Analysis of the T Cell Response to Zika Virus and Identification of a Novel CD8+ T Cell Epitope in Immunocompetent Mice. PLoS Pathog 2017, 13, e1006184. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Tang, J.; Liu, Z.; Liu, Y.; Huang, Y.; Xu, Y.; Hao, P.; Yin, Z.; Zhong, J.; Ye, L.; et al. ZIKV Infection Induces Robust Th1-like Tfh Cell and Long-Term Protective Antibody Responses in Immunocompetent Mice. Nat Commun 2019, 10, 3859. [Google Scholar] [CrossRef] [PubMed]
- Tappe, D.; Pérez-Girón, J.V.; Zammarchi, L.; Rissland, J.; Ferreira, D.F.; Jaenisch, T.; Gómez-Medina, S.; Günther, S.; Bartoloni, A.; Muñoz-Fontela, C.; et al. Cytokine Kinetics of Zika Virus-Infected Patients from Acute to Reconvalescent Phase. Med Microbiol Immunol 2016, 205, 269–273. [Google Scholar] [CrossRef]
- da Silva, M.H.M.; Moises, R.N.C.; Alves, B.E.B.; Pereira, H.W.B.; de Paiva, A.A.P.; Morais, I.C.; Nascimento, Y.M.; Monteiro, J.D.; de Souto, J.T.; Nascimento, M.S.L.; et al. Innate Immune Response in Patients with Acute Zika Virus Infection. Med Microbiol Immunol 2019, 208, 703–714. [Google Scholar] [CrossRef]
- Huang, H.; Li, S.; Zhang, Y.; Han, X.; Jia, B.; Liu, H.; Liu, D.; Tan, S.; Wang, Q.; Bi, Y.; et al. CD8 + T Cell Immune Response in Immunocompetent Mice during Zika Virus Infection. J. Virol. 2017, 91, e00900–17. [Google Scholar] [CrossRef]
- Li, G.; Teleki, C.; Wang, T. Memory T Cells in Flavivirus Vaccination. Vaccines 2018, 6, 73. [Google Scholar] [CrossRef]
- Flaxman, A.; Ewer, K. Methods for Measuring T-Cell Memory to Vaccination: From Mouse to Man. Vaccines 2018, 6, 43. [Google Scholar] [CrossRef]
- Grubor-Bauk, B.; Wijesundara, D.K.; Masavuli, M.; Abbink, P.; Peterson, R.L.; Prow, N.A.; Larocca, R.A.; Mekonnen, Z.A.; Shrestha, A.; Eyre, N.S.; et al. NS1 DNA Vaccination Protects against Zika Infection through T Cell–Mediated Immunity in Immunocompetent Mice. Sci. Adv. 2019, 5, eaax2388. [Google Scholar] [CrossRef]
- Lucas, C.G.O.; Kitoko, J.Z.; Ferreira, F.M.; Suzart, V.G.; Papa, M.P.; Coelho, S.V.A.; Cavazzoni, C.B.; Paula-Neto, H.A.; Olsen, P.C.; Iwasaki, A.; et al. Critical Role of CD4+ T Cells and IFNγ Signaling in Antibody-Mediated Resistance to Zika Virus Infection. Nat Commun 2018, 9, 3136. [Google Scholar] [CrossRef] [PubMed]
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG Subclasses and Allotypes: From Structure to Effector Functions. Front. Immunol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Ximenes, R.A. de A.; Miranda-Filho, D. de B.; Brickley, E.B.; Montarroyos, U.R.; Martelli, C.M.T.; Araújo, T.V.B. de; Rodrigues, L.C.; de Albuquerque, M. de F.P.M.; de Souza, W.V.; Castanha, P.M. da S.; et al. Zika Virus Infection in Pregnancy: Establishing a Case Definition for Clinical Research on Pregnant Women with Rash in an Active Transmission Setting. PLoS Negl Trop Dis 2019, 13, e0007763. [Google Scholar] [CrossRef]
- Smith-Norowitz, T.A.; Wong, D.; Kusonruksa, M.; Norowitz, K.B.; Joks, R.; Durkin, H.G.; Bluth, M.H. Long Term Persistence of IgE Anti-Influenza Virus Antibodies in Pediatric and Adult Serum Post Vaccination with Influenza Virus Vaccine. Int. J. Med. Sci. 2011, 8, 239–244. [Google Scholar] [CrossRef]
- Warnecke, J.M.; Lattwein, E.; Saschenbrecker, S.; Stöcker, W.; Schlumberger, W.; Steinhagen, K. Added Value of IgA Antibodies against Zika Virus Non-Structural Protein 1 in the Diagnosis of Acute Zika Virus Infections. Journal of Virological Methods 2019, 267, 8–15. [Google Scholar] [CrossRef]
- Vono, M.; Lin, A.; Norrby-Teglund, A.; Koup, R.A.; Liang, F.; Loré, K. Neutrophils Acquire the Capacity for Antigen Presentation to Memory CD4+ T Cells in Vitro and Ex Vivo. Blood 2017, 129, 1991–2001. [Google Scholar] [CrossRef]
- Muralidharan, A.; Reid, S.P. Complex Roles of Neutrophils during Arboviral Infections. Cells 2021, 10, 1324. [Google Scholar] [CrossRef]
- Cibulski, S.; Varela, A.P.M.; Teixeira, T.F.; Cancela, M.P.; Sesterheim, P.; Souza, D.O.; Roehe, P.M.; Silveira, F. Zika Virus Envelope Domain III Recombinant Protein Delivered With Saponin-Based Nanoadjuvant From Quillaja Brasiliensis Enhances Anti-Zika Immune Responses, Including Neutralizing Antibodies and Splenocyte Proliferation. Front. Immunol. 2021, 12, 632714. [Google Scholar] [CrossRef]
- Dai, S.; Zhang, T.; Zhang, Y.; Wang, H.; Deng, F. Zika Virus Baculovirus-Expressed Virus-Like Particles Induce Neutralizing Antibodies in Mice. Virol. Sin. 2018, 33, 213–226. [Google Scholar] [CrossRef]
- Becerril-García, M.Á.; Flores-Maldonado, O.E.; González, G.M.; García-González, G.; Hernández-Bello, R.; Palma-Nicolás, J.P. Safety Profile of Intravenous Administration of Live Pichia Pastoris Cells in Mice. FEMS Yeast Research 2022, 22, foac023. [Google Scholar] [CrossRef]


| Analysis | NR | ENV | ENVNS1 | R.V. [29,30,31] |
|---|---|---|---|---|
| Hematological | ||||
| Red blood cells (106/mm³) | 5.27 ± 0.35 | 5.32 ± 0.49 | 4.7 3± 0.41 | 7.3 ± 2.01 |
| Hemoglobin (g.dL-1) | 15.18 ± 1.10 | 16.04 ± 1.89 | 14.05 ± 1.31 | 13.82 ± 1.07 |
| Hematocrit (%) | 47.4 ± 3.36 | 48.2 ± 5.67 | 42.25 ± 3.86 | 38,44 ± 3,93 |
| MCV (fL) | 89.82 ± 1.27 | 90.35 ± 2.43 | 89.24 ± 0.62 | 60.26 ± 18.25 |
| MCHC (%) | 33.29 ± 0.06 | 33.27 ± 0.05 | 33.27 ± 0.08 | 33.00 ± 2.60 |
| Total leukocytes (10³/mm³) | 9.38 ± 1.14 | 9.16 ± 0.58 | 9.2 ± 0.42 | 6.23 ± 2.57 |
| Neutrophils (%) | 38.8 ± 5.72 | 41 ± 6.20 | 44.25 ± 2.93 | 22.96 ± 5.54 |
| Lymphocytes (%) | 56 ± 3.16 | 56.6 ± 6.07 | 53.5 ± 2.52 | 71.76 ± 5.9 |
| Eosinophils (%) | 1.6 ± 0.89 | 1.2 ± 0.45 | 1 ± 0 | 2.16 ± 1.71 |
| Monocytes (%) | 1.6 ± 0.89 | 1.2 ± 0.45 | 1.25 ± 0.5 | 2.68 ± 1 |
| Platelets (10³/mm³) | 386 ± 39.06 | 459.2 ± 11.73 | 434 ± 37.21 | 560 ± 119 |
| Biochemical tests | ||||
| Glucose (mg.dL-1) | 71.9 ± 4.34 | 85.84 ± 5.95 | 80.57 ± 13.86 | 80.75 ± 20.25 |
| AST (UI.L-1) | 133.52 ± 5.77 | 143.8 ± 0.08 | 103.02 ± 25.98 | 239.50 ± 141.20 |
| ALT (UI.L-1) | 154.4 ± 6.57 | 145.32 ± 5.11 | 145.67 ± 17.23 | 156.70 ± 57.20 |
| ALP (UI.L-1) | 215.82 ± 8.88 | 218.16 ± 4.52 | 202.6 ± 22.93 | 362.90 ± 226.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





