Submitted:
16 May 2023
Posted:
17 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Method
2.1. NAFLD: Definition and prevalence, Diagnosis, Physiopathology and Therapeutical approach
2.1.1. Definition and prevalence
2.1.2. Risk factors
2.1.3. Diagnosis of NAFLD
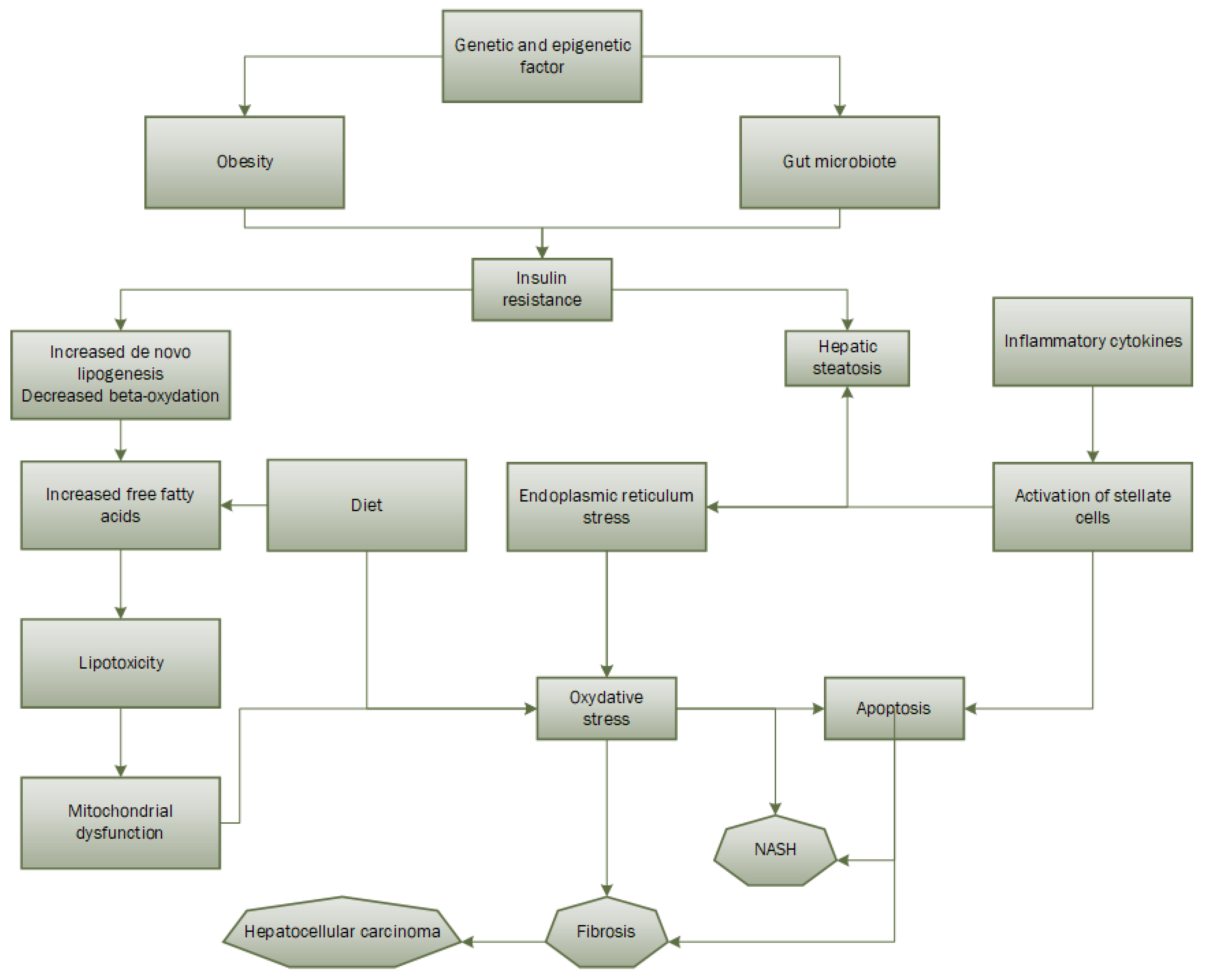
2.1.4. Physiopathology
2.1.5. Therapeutical approach
2.2. Oxidative stress
2.2.1. Definition
2.2.2. Oxidative stress in NAFLD development
2.3. Carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1)
2.3.1. Definition and Generalities
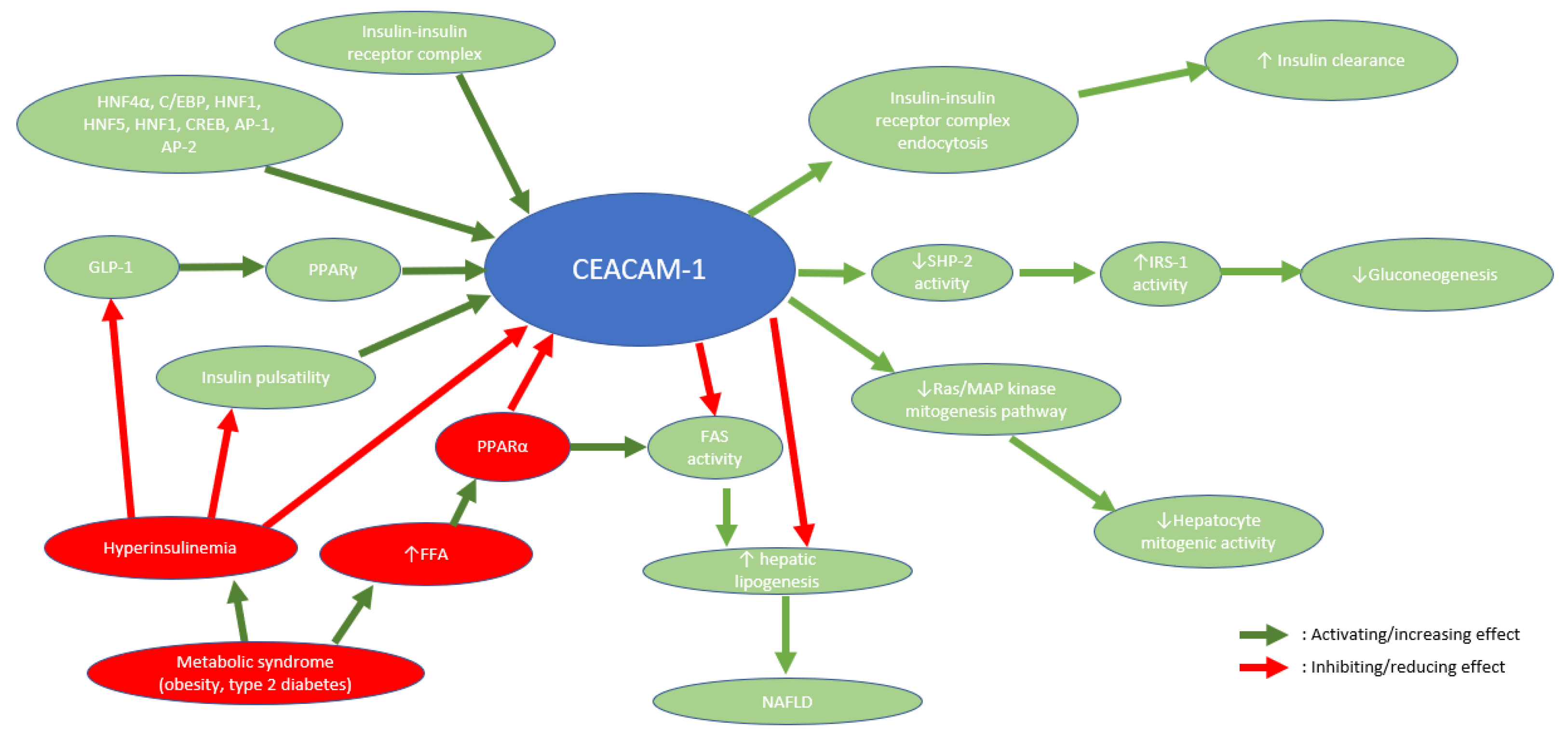
2.3.2. CEACAM1 role in metabolic balance and NAFLD/NASH
3. Discussion
4. Conclusion
References
- Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Powell EE, Wong VWS, Rinella M. Nonalcoholic fatty liver disease. The Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef] [PubMed]
- Michelotti GA, Machado MV, Diehl AM. NAFLD, NASH and liver cancer. Nat Rev Gastroenterol Hepatol. 2013, 10, 656–665. [Google Scholar] [CrossRef]
- Younossi ZM, Gramlich T, Matteoni CA, Boparai N, McCullough AJ. Nonalcoholic fatty liver disease in patients with type 2 diabetes. Clin Gastroenterol Hepatol. 2004, 2, 262–265. [Google Scholar] [CrossRef]
- Berzigotti A, Tsochatzis E, Boursier J, Castera L, Cazzagon N, Friedrich-Rust M, et al. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis – 2021 update. J Hepatol. 2021, 75, 659–689. [Google Scholar] [CrossRef] [PubMed]
- Marjot T, Moolla A, Cobbold JF, Hodson L, Tomlinson JW. Nonalcoholic Fatty Liver Disease in Adults: Current Concepts in Etiology, Outcomes, and Management. Endocr Rev. 2020, 41, 66–117. [Google Scholar] [CrossRef] [PubMed]
- Zhou JH, Cai JJ, She ZG, Li HL. Noninvasive evaluation of nonalcoholic fatty liver disease: Current evidence and practice. World J Gastroenterol. 2019, 25, 1307–1326. [Google Scholar] [CrossRef]
- Brunt EM, Janney CG, Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999, 94, 2467–2474. [Google Scholar] [CrossRef]
- Rich NE, Oji S, Mufti AR, Browning JD, Parikh ND, Odewole M, et al. Racial and Ethnic Disparities in Nonalcoholic Fatty Liver Disease Prevalence, Severity, and Outcomes in the United States: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2018, 16, 198–210. [Google Scholar] [CrossRef]
- Bril F, Cusi K. Nonalcoholic Fatty Liver Disease. Endocrinol Metab Clin North Am. 2016, 45, 765–781. [Google Scholar] [CrossRef]
- Williamson RM, Price JF, Glancy S, Perry E, Nee LD, Hayes PC, et al. Prevalence of and Risk Factors for Hepatic Steatosis and Nonalcoholic Fatty Liver Disease in People With Type 2 Diabetes: the Edinburgh Type 2 Diabetes Study. Diabetes Care. 2011, 34, 1139–1144. [Google Scholar] [CrossRef]
- El-serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology 2004, 126, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Perumpail BJ, Khan MA, Yoo ER, Cholankeril G, Kim D, Ahmed A. Clinical epidemiology and disease burden of nonalcoholic fatty liver disease. World J Gastroenterol. 2017, 23, 8263–8276. [Google Scholar] [CrossRef] [PubMed]
- Pallayova M, Taheri S. Nonalcoholic fatty liver disease in obese adults: clinical aspects and current management strategies: Nonalcoholic fatty liver disease in obese adults. Clin Obes. 2014, 4, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli L, Beaugrand M. Transient elastography in nonalcoholic fatty liver disease. Ann Hepatol. 2012, 11, 172–178. [Google Scholar] [CrossRef]
- Jin S, Jiang S, Hu A. Association between obstructive sleep apnea and nonalcoholic fatty liver disease: a systematic review and meta-analysis. Sleep Breath 2018, 22, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Kalafati IP, Borsa D, Dimitriou M, Revenas K, Kokkinos A, Dedoussis GV. Dietary patterns and nonalcoholic fatty liver disease in a Greek case–control study. Nutrition. 2019, 61, 105–110. [Google Scholar] [CrossRef]
- Yang CQ, Shu L, Wang S, Wang JJ, Zhou Y, Xuan YJ, et al. Dietary Patterns Modulate the Risk of Nonalcoholic Fatty Liver Disease in Chinese Adults. Nutrients. 2015, 7, 4778–4791. [Google Scholar] [CrossRef]
- de Vries M, Westerink J, Kaasjager KHAH, de Valk HW. Prevalence of Nonalcoholic Fatty Liver Disease (NAFLD) in Patients With Type 1 Diabetes Mellitus: A Systematic Review and Meta-Analysis. J Clin Endocrinol Metab. 2020, 105, 3842–3853. [Google Scholar] [CrossRef]
- Cotter TG, Rinella M. Nonalcoholic Fatty Liver Disease 2020: The State of the Disease. Gastroenterology 2020, 158, 1851–1864. [Google Scholar] [CrossRef]
- Mertens J, Van Gaal LF, Francque SM, De Block C. NAFLD in type 1 diabetes: overrated or underappreciated? Ther Adv Endocrinol Metab. 2021, 12, 204201882110555. [Google Scholar] [CrossRef] [PubMed]
- Kinner S, Reeder SB, Yokoo T. Quantitative Imaging Biomarkers of NAFLD. Dig Dis Sci. 2016, 61, 1337–1347. [Google Scholar] [CrossRef] [PubMed]
- Mertens J, De Block C, Spinhoven M, Driessen A, Francque SM, Kwanten WJ. Hepatopathy Associated With Type 1 Diabetes: Distinguishing Nonalcoholic Fatty Liver Disease From Glycogenic Hepatopathy. Front Pharmacol. 2021, 12, 768576. [Google Scholar] [CrossRef] [PubMed]
- Gariani K, Philippe J, Jornayvaz FR. Nonalcoholic fatty liver disease and insulin resistance: From bench to bedside. Diabetes Metab. 2013, 39, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Gariani K, Jornayvaz FR. NAFLD: From Mechanisms to Therapeutic Approaches. Biomedicines 2022, 10, 1747. [Google Scholar]
- Asrih M, Jornayvaz FR. Metabolic syndrome and nonalcoholic fatty liver disease: Is insulin resistance the link? Mol Cell Endocrinol. 2015, 418, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Asrih M, Jornayvaz FR. Inflammation as a potential link between nonalcoholic fatty liver disease and insulin resistance. J Endocrinol. 2013, 218, R25–R36. [Google Scholar] [CrossRef]
- Nascè A, Gariani K, Jornayvaz FR, Szanto I. NADPH Oxidases Connecting Fatty Liver Disease, Insulin Resistance and Type 2 Diabetes: Current Knowledge and Therapeutic Outlook. Antioxidants 2022, 11, 1131. [Google Scholar] [CrossRef]
- Delli Bovi AP, Marciano F, Mandato C, Siano MA, Savoia M, Vajro P. Oxidative Stress in Nonalcoholic Fatty Liver Disease. An Updated Mini Review. Front Med. 2021, 8, 595371. [Google Scholar] [CrossRef]
- Shabalala SC, Dludla PV, Mabasa L, Kappo AP, Basson AK, Pheiffer C, et al. The effect of adiponectin in the pathogenesis of nonalcoholic fatty liver disease (NAFLD) and the potential role of polyphenols in the modulation of adiponectin signaling. Biomed Pharmacother. 2020, 131, 110785. [Google Scholar]
- Promrat K, Kleiner DE, Niemeier HM, Jackvony E, Kearns M, Wands JR, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology 2010, 51, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Mummadi RR, Kasturi KS, Chennareddygari S, Sood GK. Effect of Bariatric Surgery on Nonalcoholic Fatty Liver Disease: Systematic Review and Meta-Analysis. Clin Gastroenterol Hepatol. 2008, 6, 1396–402. [Google Scholar] [CrossRef] [PubMed]
- Li Y, Liu L, Wang B, Wang J, Chen D. Metformin in nonalcoholic fatty liver disease: A systematic review and meta-analysis. Biomed Rep. 2013, 1, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, et al. Pioglitazone, Vitamin E, or Placebo for Nonalcoholic Steatohepatitis. N Engl J Med. 2010, 362, 1675–1685. [Google Scholar] [CrossRef] [PubMed]
- Gupta NA, Mells J, Dunham RM, Grakoui A, Handy J, Saxena NK, et al. Glucagon-like peptide-1 receptor is present on human hepatocytes and has a direct role in decreasing hepatic steatosis in vitro by modulating elements of the insulin signaling pathway. Hepatology 2010, 51, 1584–1592. [Google Scholar] [CrossRef]
- Armstrong MJ, Hull D, Guo K, Barton D, Hazlehurst JM, Gathercole LL, et al. Glucagon-like peptide 1 decreases lipotoxicity in nonalcoholic steatohepatitis. J Hepatol. 2016, 64, 399–408. [Google Scholar] [CrossRef]
- Gastaldelli A, Gaggini M, Daniele G, Ciociaro D, Cersosimo E, Tripathy D, et al. Exenatide improves both hepatic and adipose tissue insulin resistance: A dynamic positron emission tomography study. Hepatology 2016, 64, 2028–2037. [Google Scholar] [CrossRef]
- Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, et al. Liraglutide safety and efficacy in patients with nonalcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. The Lancet 2016, 387, 679–690. [Google Scholar] [CrossRef]
- Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N Engl J Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef]
- Newsome PN, Allison ME, Andrews PA, Auzinger G, Day CP, Ferguson JW, et al. Guidelines for liver transplantation for patients with nonalcoholic steatohepatitis. Gut. 2012, 61, 484–500. [Google Scholar] [CrossRef]
- Taylor RS, Taylor RJ, Bayliss S, Hagström H, Nasr P, Schattenberg JM, et al. Association Between Fibrosis Stage and Outcomes of Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Gastroenterology 2020, 158, 1611–1625. [Google Scholar] [CrossRef] [PubMed]
- Robertson G, Leclercq I, Farrell GC. II. Cytochrome P -450 enzymes and oxidative stress. Am J Physiol-Gastrointest Liver Physiol. 2001, 281, G1135–G1139. [Google Scholar] [CrossRef] [PubMed]
- Świderska M, Maciejczyk M, Zalewska A, Pogorzelska J, Flisiak R, Chabowski A. Oxidative stress biomarkers in the serum and plasma of patients with nonalcoholic fatty liver disease (NAFLD). Can plasma AGE be a marker of NAFLD? Oxidative stress biomarkers in NAFLD patients. Free Radic Res. 2019, 53, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Irie M, Sohda T, Iwata K, Kunimoto H, Fukunaga A, Kuno S, et al. Levels of the Oxidative Stress Marker γ-Glutamyltranspeptidase at Different Stages of Nonalcoholic Fatty Liver Disease. J Int Med Res. 2012, 40, 924–933. [Google Scholar] [CrossRef] [PubMed]
- Yesilova Z, Yaman H, Oktenli C, Ozcan A, Uygun A, Cakir E, et al. Systemic Markers of Lipid Peroxidation and Antioxidants in Patients with Nonalcoholic Fatty Liver Disease. Am J Gastroenterol. 2005, 100, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Videla LA, Rodrigo R, Araya J, Poniachik J. Oxidative stress and depletion of hepatic long-chain polyunsaturated fatty acids may contribute to nonalcoholic fatty liver disease. Free Radic Biol Med. 2004, 37, 1499–1507. [Google Scholar] [CrossRef] [PubMed]
- Liu S, Shi W, Li G, Jin B, Chen Y, Hu H, et al. Plasma reactive carbonyl species levels and risk of nonalcoholic fatty liver disease: Reactive carbonyl species and NAFLD. J Gastroenterol Hepatol. 2011, 26, 1010–1015. [Google Scholar] [CrossRef] [PubMed]
- Loguercio C, De Girolamo V, de Sio I, Tuccillo C, Ascione A, Baldi F, et al. Nonalcoholic fatty liver disease in an area of southern Italy: main clinical, histological, and pathophysiological aspects. J Hepatol. 2001, 35, 568–574.
- Gambino R, Musso G, Cassader M. Redox Balance in the Pathogenesis of Nonalcoholic Fatty Liver Disease: Mechanisms and Therapeutic Opportunities. Antioxid Redox Signal. 2011, 15, 1325–1365. [Google Scholar] [CrossRef]
- Lavoie JM, Gauthier MS. Regulation of fat metabolism in the liver: link to nonalcoholic hepatic steatosis and impact of physical exercise. Cell Mol Life Sci. 2006, 63, 1393–1409. [Google Scholar] [CrossRef]
- Tessari P, Coracina A, Cosma A, Tiengo A. Hepatic lipid metabolism and nonalcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2009, 19, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Parola M, Marra F. Adipokines and Redox Signaling: Impact on Fatty Liver Disease. Antioxid Redox Signal. 2011, 15, 461–483. [Google Scholar] [CrossRef] [PubMed]
- Larter CZ, Chitturi S, Heydet D, Farrell GC. A fresh look at NASH pathogenesis. Part 1: The metabolic movers. J Gastroenterol Hepatol. 2010, 25, 672–690. [Google Scholar] [CrossRef] [PubMed]
- Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005, 115, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Sanyal AJ, Campbell–Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, et al. Nonalcoholic steatohepatitis: Association of insulin resistance and mitochondrial abnormalities. Gastroenterology 2001, 120, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Chitturi S, Farrell GC. Etiopathogenesis of Nonalcoholic Steatohepatitis. Semin Liver Dis. 2001, 21, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Seki S, Kitada T, Yamada T, Sakaguchi H, Nakatani K, Wakasa K. In situ detection of lipid peroxidation and oxidative DNA damage in nonalcoholic fatty liver diseases. J Hepatol. 2002, 37, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Marchesini G, Marzocchi R, Agostini F, Bugianesi E. Nonalcoholic fatty liver disease and the metabolic syndrome. Curr Opin Lipidol. 2005, 16, 421–427. [Google Scholar] [CrossRef]
- Feldstein AE, Werneburg NW, Li Z, Bronk SF, Gores GJ. Bax inhibition protects against free fatty acid-induced lysosomal permeabilization. Am J Physiol-Gastrointest Liver Physiol. 2006, 290, G1339–G1346.
- Pagliassotti, MJ. Endoplasmic Reticulum Stress in Nonalcoholic Fatty Liver Disease. Annu Rev Nutr. 2012, 32, 17–33. [Google Scholar] [CrossRef]
- Smirne C, Croce E, Di Benedetto D, Cantaluppi V, Comi C, Sainaghi PP, et al. Oxidative Stress in Nonalcoholic Fatty Liver Disease. Livers 2022, 2, 30–76. [Google Scholar] [CrossRef]
- Sozio M, Liangpunsakul S, Crabb D. The Role of Lipid Metabolism in the Pathogenesis of Alcoholic and Nonalcoholic Hepatic Steatosis. Semin Liver Dis. 2010, 30, 378–90. [Google Scholar] [CrossRef] [PubMed]
- Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991, 11, 81–128. [Google Scholar] [CrossRef] [PubMed]
- Gardner, HW. Oxygen radical chemistry of polyunsaturated fatty acids. Free Radic Biol Med. 1989, 7, 65–86. [Google Scholar] [CrossRef] [PubMed]
- Takaki A, Kawai D, Yamamoto K. Multiple Hits, Including Oxidative Stress, as Pathogenesis and Treatment Target in Nonalcoholic Steatohepatitis (NASH). Int J Mol Sci. 2013, 14, 20704–20728. [Google Scholar] [CrossRef] [PubMed]
- Meex RCR, Blaak EE. Mitochondrial Dysfunction is a Key Pathway that Links Saturated Fat Intake to the Development and Progression of NAFLD. Mol Nutr Food Res. 2021, 65, 1900942. [Google Scholar] [CrossRef]
- Erhardt A, Stahl W, Sies H, Lirussi F, Donner A, Häussinger D. Plasma levels of vitamin E and carotenoids are decreased in patients with nonalcoholic steatohepatitis (NASH). Eur J Med Res. 2011, 16, 76. [Google Scholar] [CrossRef]
- Ashraf NU, Sheikh TA. Endoplasmic reticulum stress and Oxidative stress in the pathogenesis of Nonalcoholic fatty liver disease. Free Radic Res. 2015, 49, 1405–1418. [Google Scholar] [CrossRef]
- Chen Z, Tian R, She Z, Cai J, Li H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic Biol Med. 2020, 152, 116–41. [Google Scholar] [CrossRef]
- Reccia I, Kumar J, Akladios C, Virdis F, Pai M, Habib N, et al. Nonalcoholic fatty liver disease: A sign of systemic disease. Metabolism. 2017, 72, 94–108. [Google Scholar] [CrossRef]
- Horst A, Najjar S, Wagener C, Tiegs G. CEACAM1 in Liver Injury, Metabolic and Immune Regulation. Int J Mol Sci. 2018, 19, 3110. [Google Scholar] [CrossRef] [PubMed]
- Yates AD, Achuthan P, Akanni W, Allen J, Allen J, Alvarez-Jarreta J, et al. Ensembl 2020. Nucleic Acids Res. 2019, gkz966. [Google Scholar]
- Kammerer R, Zimmermann W. Coevolution of activating and inhibitory receptors within mammalian carcinoembryonic antigen families. BMC Biol. 2010, 8, 12. [Google Scholar]
- Odin P, Öbrink B. Dynamic expression of the cell adhesion molecule cell-CAM 105 in fetal and regenerating rat liver. Exp Cell Res. 1986, 164, 103–14. [Google Scholar] [CrossRef] [PubMed]
- Najjar SM, Boisclair YR, Nabih ZT, Philippe N, Imai Y, Suzuki Y, et al. Cloning and Characterization of a Functional Promoter of the Rat pp120 Gene, Encoding a Substrate of the Insulin Receptor Tyrosine Kinase. J Biol Chem. 1996, 271, 8809–17. [Google Scholar] [CrossRef] [PubMed]
- Najjar SM, Caprio S, Gastaldelli A. Insulin Clearance in Health and Disease. Annu Rev Physiol. 2023, 85, 363–81. [Google Scholar] [CrossRef] [PubMed]
- Poy MN, Ruch RJ, Fernström MA, Okabayashi Y, Najjar SM. Shc and CEACAM1 Interact to Regulate the Mitogenic Action of Insulin. J Biol Chem. 2002, 277, 1076–84. [Google Scholar] [CrossRef]
- Boucher J, Kleinridders A, Kahn CR. Insulin Receptor Signaling in Normal and Insulin-Resistant States. Cold Spring Harb Perspect Biol. 2014, 6, a009191–a009191. [Google Scholar] [CrossRef]
- Yousef A, Behiry E, Abd Allah W, Hussien A, Abdelmoneam A, Imam M, et al. IRS-1 genetic polymorphism (r.2963G>A) in type 2 diabetes mellitus patients associated with insulin resistance. Appl Clin Genet. 2018, 11, 99–106. [Google Scholar] [CrossRef]
- Najjar SM, Perdomo G. Hepatic Insulin Clearance: Mechanism and Physiology. Physiology 2019, 34, 198–215. [Google Scholar] [CrossRef]
- Poy MN, Yang Y, Rezaei K, Fernström MA, Lee AD, Kido Y, et al. CEACAM1 regulates insulin clearance in liver. Nat Genet. 2002, 30, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Memaj P, Jornayvaz FR. Nonalcoholic fatty liver disease in type 1 diabetes: Prevalence and pathophysiology. Front Endocrinol. 2022, 13, 1031633. [Google Scholar] [CrossRef] [PubMed]
- Matveyenko AV, Liuwantara D, Gurlo T, Kirakossian D, Dalla Man C, Cobelli C, et al. Pulsatile Portal Vein Insulin Delivery Enhances Hepatic Insulin Action and Signaling. Diabetes 2012, 61, 2269–2279. [Google Scholar] [CrossRef]
- Bril F, Lomonaco R, Orsak B, Ortiz-Lopez C, Webb A, Tio F, et al. Relationship between disease severity, hyperinsulinemia, and impaired insulin clearance in patients with nonalcoholic steatohepatitis. Hepatology 2014, 59, 2178–2187. [Google Scholar] [CrossRef] [PubMed]
- Dif N, Euthine V, Gonnet E, Laville M, Vidal H, Lefai E. Insulin activates human sterol-regulatory-element-binding protein-1c (SREBP-1c) promoter through SRE motifs. Biochem. J. 2006, 400, 179–188. [Google Scholar] [CrossRef]
- DeBose-Boyd RA, Ye J. SREBPs in Lipid Metabolism, Insulin Signaling, and Beyond. Trends Biochem Sci. 2018, 43, 358–368. [Google Scholar] [CrossRef]
- Laurenti MC, Matveyenko A, Vella A. Measurement of Pulsatile Insulin Secretion: Rationale and Methodology. Metabolites 2021, 11, 409. [Google Scholar] [CrossRef]
- Rojano-Toimil A, Rivera-Esteban J, Manzano-Nuñez R, Bañares J, Martinez Selva D, Gabriel-Medina P, et al. When Sugar Reaches the Liver: Phenotypes of Patients with Diabetes and NAFLD. J Clin Med. 2022, 11, 3286. [Google Scholar] [CrossRef]
- Najjar SM, Yang Y, Fernström MA, Lee SJ, DeAngelis AM, Rjaily GAA, et al. Insulin acutely decreases hepatic fatty acid synthase activity. Cell Metab. 2005, 2, 43–53. [Google Scholar] [CrossRef]
- Camastra S, Ferrannini E. Role of anatomical location, cellular phenotype and perfusion of adipose tissue in intermediary metabolism: A narrative review. Rev Endocr Metab Disord. 2022, 23, 43–50. [Google Scholar] [CrossRef]
- Ramakrishnan SK, Khuder SS, Al-Share QY, Russo L, Abdallah SL, Patel PR, et al. PPARα (Peroxisome Proliferator-activated Receptor α) Activation Reduces Hepatic CEACAM1 Protein Expression to Regulate Fatty Acid Oxidation during Fasting-refeeding Transition. J Biol Chem. 2016, 291, 8121–8129. [Google Scholar] [CrossRef] [PubMed]
- van Raalte DH, Li M, Pritchard PH, Wasan KM. Peroxisome Proliferator-Activated Receptor (PPAR)- : A Pharmacological Target with a Promising Future. Pharm Res. 2004, 21, 1531–1538. [Google Scholar] [CrossRef] [PubMed]
- Ghadieh HE, Muturi HT, Russo L, Marino CC, Ghanem SS, Khuder SS, et al. Exenatide induces carcinoembryonic antigen-related cell adhesion molecule 1 expression to prevent hepatic steatosis. Hepatol Commun. 2018, 2, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Salehi M, Aulinger B, Prigeon RL, D’Alessio DA. Effect of Endogenous GLP-1 on Insulin Secretion in Type 2 Diabetes. Diabetes 2010, 59, 1330–1337. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




