1. Introduction
Currently, aortic valve (AV) pathology is becoming the most frequent cause of heart valve surgery [
1,
2]. AV replacement is considered to be the gold standard of treatment of patients with aortic malformation [
3,
4]. The problem of prosthesis choice has always been topical. Mechanical prostheses are durable, but there are high risks of thromboembolic complications, so lifelong anticoagulant therapy is required [
5]. Biological prostheses form a flow structure close to the physiological one, and the gradual development of dysfunction makes it possible to perform repeated surgery as planned [
6].
However, with time, they are prone to structural valve degradation (SVD), which necessitates reoperation, particularly in younger patients [
7,
8,
9]. Nevertheless, recent randomized data indicate that the long-term survival rates following biological and mechanical AVR are comparable [
10]. As a result, when choosing the type of prosthesis, the likelihood of difficulties with the valve become important. According to the National Thoracic Surgery Society database, the number of implanted bioprostheses has steadily increased over the past ten years [
11]. Furthermore, during the last decade, the use of valve-in-valve transcatheter aortic valve implantation has complemented surgical aortic valve replacement in the treatment of high-risk patients with degenerated aortic bioprostheses [
12].
Here is presented a statistics on total AVR operations (including biological valve) in 2021 in thousands: Russia – 11.2 (6.3); USA – 41.6 (23.4); Germany – 26.8(15.1); China – 18.3 (10.3).
Therefore, the purpose of this single-center study was to examine the relationship between short- and long-term problems, including mortality, and the type of aortic valve prosthesis (biologic or mechanical) placed in a relatively large cohort of patients who underwent AVR.
Previously, some single-center studies were made in Japan [
13,
14] and China [
15]. Nevertheless, it is necessary to gain the experience from all over the World to get more adequate data.
Therefore, the purpose of this single-center study was to examine the relationship between short- and long-term problems, including mortality, and the type of aortic valve prosthesis (biologic or mechanical) placed in a relatively large cohort of patients who underwent AVR.
With this study, we aimed to evaluate short- and long-term outcomes after AVR with biological prostheses at Federal Center of Cardiovascular Surgery (Perm, Russia). Furthermore, we examined how the surgery affected patients’ subjective quality of life.
2. Materials and Methods
2.1. Ethical Statement
This cohort study was conducted in Federal Center of Cardiovascular Surgery (Perm, Russia). Written informed consent was obtained from patients or their representatives. The study was approved by the Ethics Committee of Federal Center of Cardiovascular Surgery (Perm, Russia) (protocol No. 12 on 17 December 2022).
2.2. Study Population and Data Sources
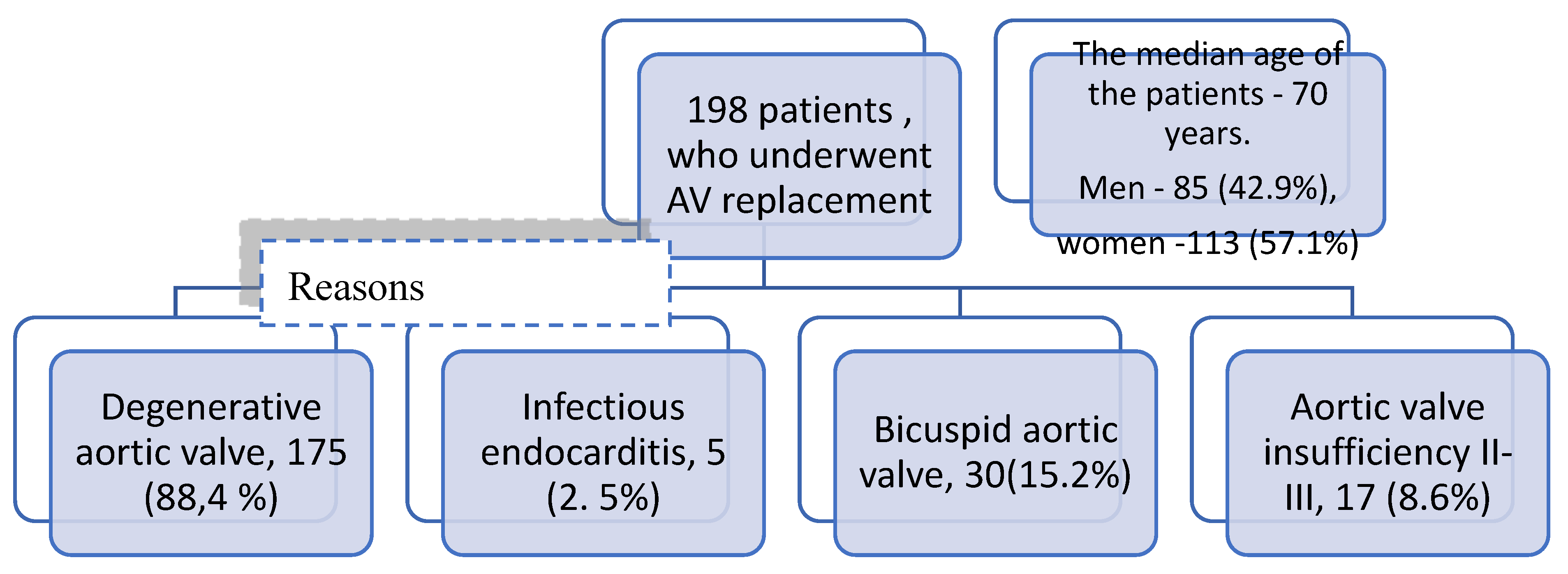
Single-center retrospective study of 198 patients (85 men and 113 women) who underwent AV replacement with biological prosthesis at Federal Center for Cardiovascular Surgery (Perm, Russia) (from 2015 to 2021) was carried out. The clinical and demographic characteristics are presented in
Table 1. The median age of the patients was 70 years (min 25 and max 80 years). Among the comorbidities, CHD was the most common in 141 patients (71.2%). NYHA III-IV class coronary artery disease occurred in 102 patients (51.5%). The causes of AV dysfunction were mainly degenerative changes of native valve in 175 patients (88.4%), insufficiency of AV II-III in 17 (8.6%), IE occurred in 5 patients (2.5%), bicuspid AV in 30 patients (15.2%). Cohort characteristics are presented in
Table 1.
2.3. Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics 26 (Chicago, IL, USA), Jamovi (Version 1.6.9) (
https://www.jamovi.org), and R (R version 4.0.5, R Foundation for Statistical Computing, Vienna, Austria). Quantitative measures were assessed for normality using the Kolmogorov-Smirnov test with Lilliefors test. Quantitative measures with a normal distribution were described with arithmetic mean (M) and standard deviation (SD), 95% confidence interval (95% CI) limits.
Figure 1.
Cohort flow chart.
Figure 1.
Cohort flow chart.
Table 1.
Clinical and population features.
Table 1.
Clinical and population features.
| Parameter |
Quantity |
| Age, Me [Q1-Q3] |
70 [66-73] |
| Sex (male/female), n(%) |
85(42.9%) / 113 (57.1%) |
| BMI M ± SD |
30 ± 5 |
| NYHA III-IV FC, n (%) |
102 (51,5) |
| Coronary heart disease, n(%) |
141 (71,2) |
| Percutaneous coronary intervention, n(%) |
16 (8,1) |
| Myocardial infarction, n(%) |
43 (21,7) |
| Diabetes mellitus, n (%) |
32 (16,2) |
| Peripheral artery lesion, n(%) |
47 (23,7) |
| Atrial fibrillation,n (%) |
53 (26,9) |
| Previous heart surgeries, n (%) |
11 (5,6) |
| Previous surgeries on the aortic valve, n (%) |
8 (4) |
| Infectious endocarditis, n(%) |
5 (2,5) |
| Bicuspid aortic valve, n(%) |
30 (15,2) |
| Degenerative aortic valve, t(%) |
175 (88,4) |
| Aortic valve insufficiency II-III , n (%) |
17 (8,6) |
| LVEF , % |
54 [47 – 58] |
| Mean pulmonary artery pressure, mmHg Me [Q1-Q3] |
35 [27 – 45] |
| Median of the fibrous ring of the aortic valve, mm Me [Q1-Q3] |
21 [20 – 23] |
Where there was no normal distribution, quantitative data were described using median (Me) and lower and upper quartiles (Q1 – Q3). Categorical data were described with absolute values and percentages. The time to the first clinical endpoints (mortality and AV reoperation) was estimated using the Kaplan-Meier method.
2.4. Primary and Secondary End-Points
The primary endpoint was hospital mortality. The secondary endpoints assessed were: surgery duration time, artificial circulation, aortic constriction, period of hospital stay, postoperative complications (stroke, sternal infection, acute kidney injury (AKI), arrhythmia requiring pacemaker implantation, sepsis, reoperation for bleeding), peak transvalvular pressure gradient after surgery, mid-term outcomes (three-year overall survival and freedom from AV reoperation)
3. Results
3.1. Surgical Technique
The access to the heart was a median sternotomy in 181 patients (91.4%), in 17 (8.6%) - J-shaped upper mini-sternotomy. The operation was carried out under the conditions of cardiopulmonary bypass, myocardium protection during aortic clamping was carried out by using blood cardioplegia technique. The median artificial circulation time was 78 (65 – 96) minutes; aortic constriction time was 59 (48 – 70) minutes. Neocor Uniline - 170 patients (85.9%), Tiara prosthesis - 17 patients (8.6%), Medtronic Hancock - 3 patients (1.5%), St.Jude Biocor - 3 patients (1.5%), Biolab - 5 patients (2.5%) were used as bioprosthesis. The percentage of combined surgical interventions was 46% - 91 patients, including 70 patients underwent coronary artery bypass grafting (35.4%), 8 patients underwent cardiac ablation + left atrial appendage intervention (4%), 3 patients underwent mitral valve intervention (1.5%), 3 patients underwent aortic root dilation (1.5%) (
Table 2).
3.2. Post-Operative Outcome
Postoperative data are shown in
Table 3. In-hospital mortality was 4.5% in 9 patients. The cause of mortality was multiple organ failure syndrome, in 1 case peritonitis. Rhythm disturbance as AF occurred in 14 patients (7.1%), total AV blockade - in 9 (4.5%), therefore, permanent pacemaker was implanted. Four patients (2%) underwent resternotomy for the control of bleeding. Two patients (1%) underwent pericardiocentesis. Eight (4%) patients had a stroke. No wound complications were detected. The median ICU stay was 1 (1-2) days. Echocardiographic data in the early postoperative period are shown in
Table 4. The median number of days spent in hospital was 17 (14-21) days.
3.3. Mid-Term Outcome
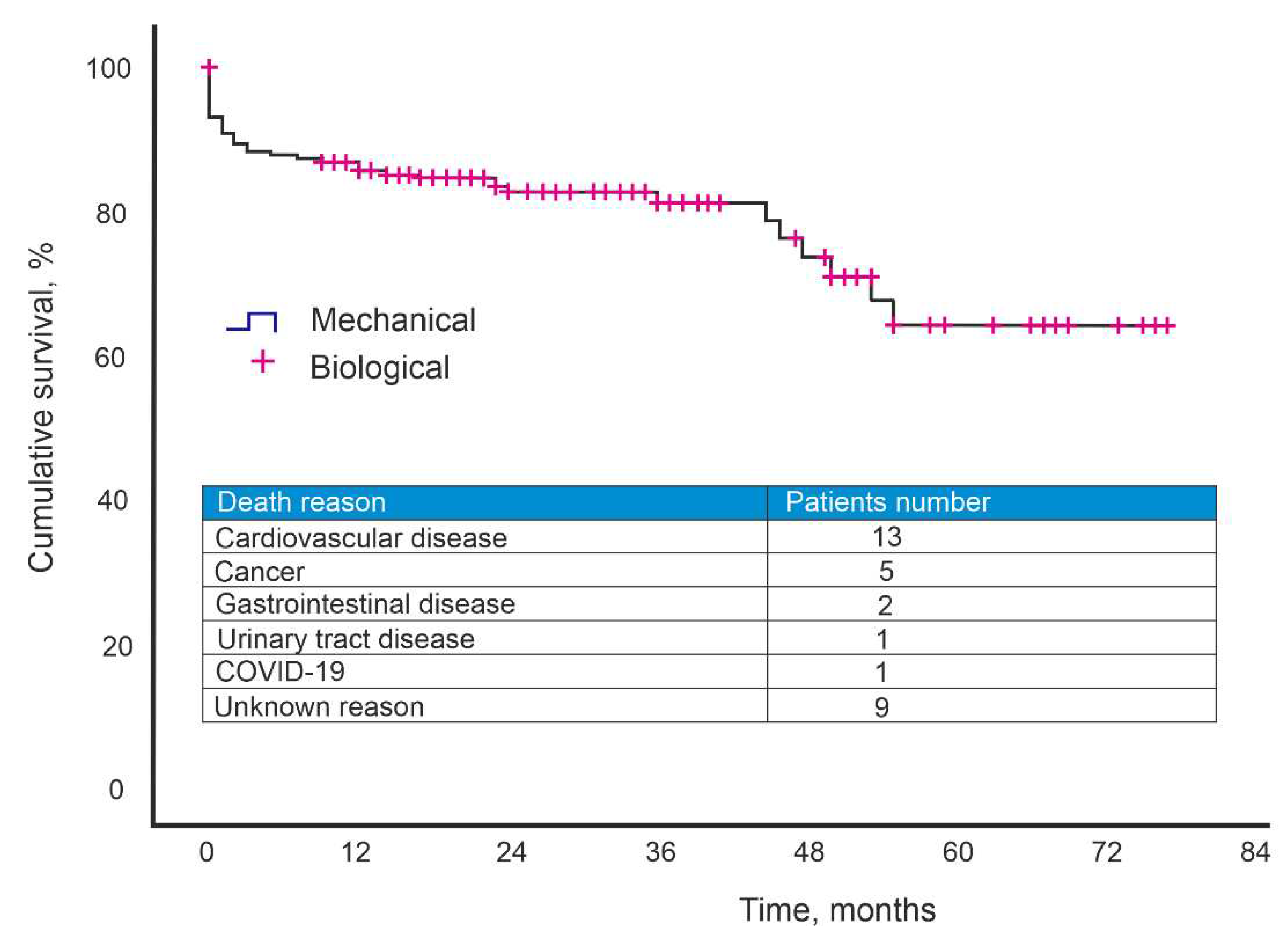
The follow-up period was 26.3±1.4 months. During the observation period, 31 patients died. The causes of mortality were: cardiovascular disease - 13 patients, malignant neoplasm - 5 patients, gastrointestinal disease - 2 patients, urinary tract disease - 1 patient, COVID-19- 1 patient, unknown cause - 9 patients. The five-year survival rate after surgery was 63% (
Figure 1).
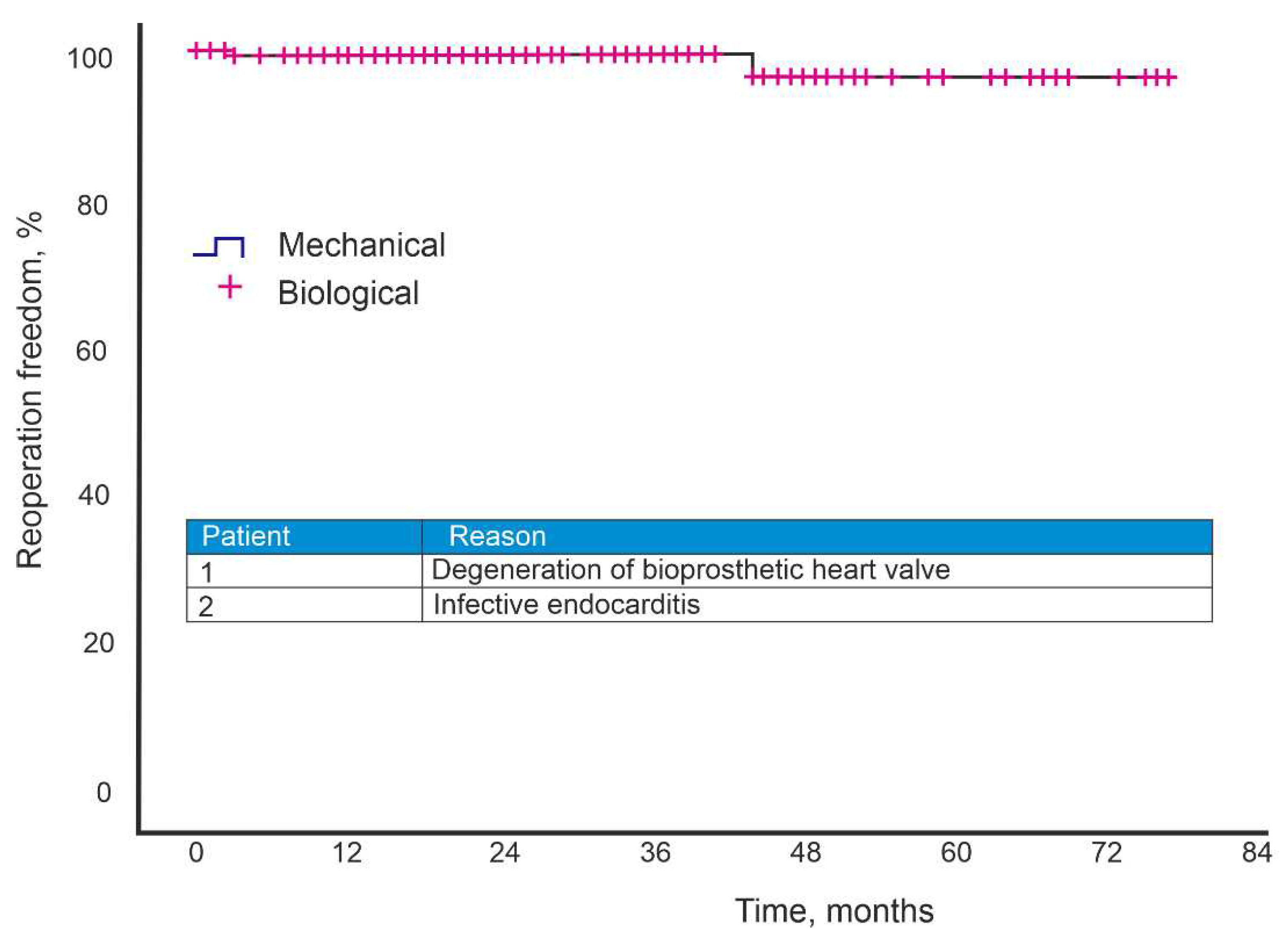
Two patients underwent AV reoperation during the follow-up period. During the observation period, an AV reoperation was performed on two patients. The biological valve's leaflet degradation and the bioprosthesis's were the reasons for the second operation. Aortic valve replacement with mechanical prosthesis, mitral valve replacement with mechanical prosthesis, closure of the interventricular septal defect, and aortic root and aortic left atrial fistula plasty with xenopericardial patch were all performed on the patient with leaflet degeneration who had undergone AV valve replantation with biological prosthesis. The five-year freedom from reoperation was 97.1% (
Figure 2).
Figure 1.
Kaplan–Meier curves for survival after AV implantation.
Figure 1.
Kaplan–Meier curves for survival after AV implantation.
4. Discussion
The surgical repair of the aortic valve is a significant heart procedure that ranks second in cardiac surgery and among the top heart treatments. Depending on the patient's features, a biological, mechanical, or autopericardium replacement may be chosen [
16]. Depending on the surgeon's discretion, there are various implantation procedures for aortic valve repair, including U-shaped sutures on spacers, an interrupted sew technique for thin aortic valve rings, and continuous sutures [
17].
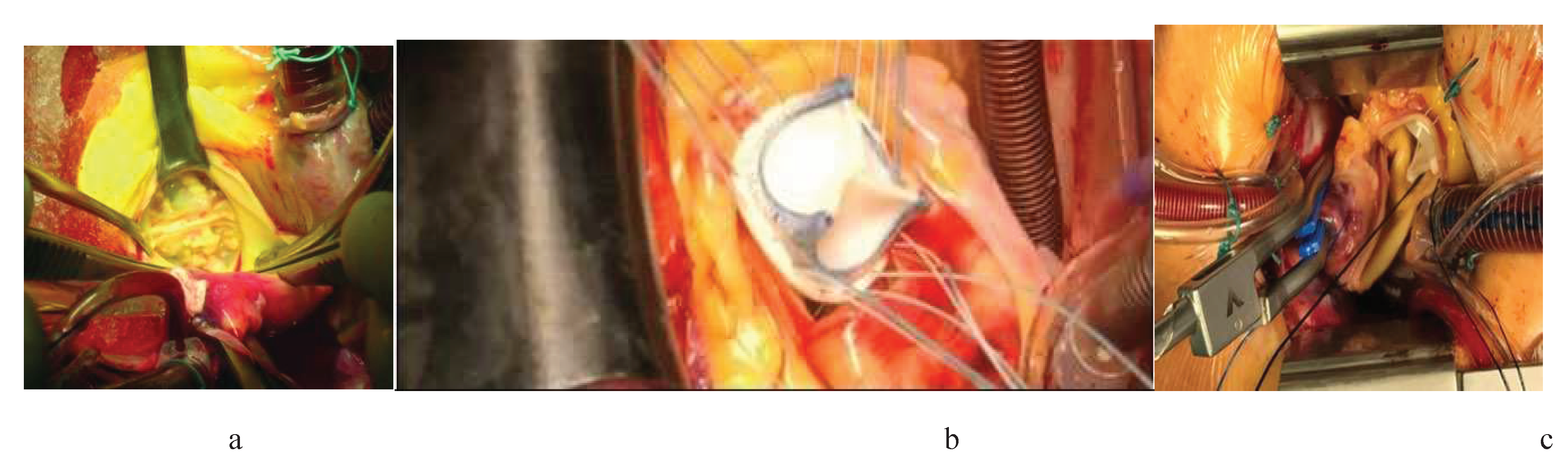
Figure 3.
Stages of biological valve implantation: a – calcified aortic valve, b – implantation and suturing, c – cannulation technique.
Figure 3.
Stages of biological valve implantation: a – calcified aortic valve, b – implantation and suturing, c – cannulation technique.
Mortality and fatal complications in AV repair reaches 4-8% [
18]. Cardiac problems vary by roughly 7%, and occasionally can reach 13% in the group of senior patients, depending on risk variables such the patient's age and somatic status [
19,
20].
Milewski et al. [
21] presented an 18-year experience with AV replacement. The study compared the immediate and long-term results of AV replacement with biological and mechanical prostheses in young people. The main criterion of the study was the freedom from reoperation. Experience has shown that there is no difference in the reoperation rate.
According to a retrospective study by Schnittman et al. [
22], after AV replacement with bioprosthesis the incidence of stroke was lower (5.4% [95% CI, 3.8%-7.2%] vs 8.1% (after AC replacement with mechanical prosthesis) [95% CI, 6.3%-10.2%], the incidence of bleeding was lower (4.2% [95% CI 3.0-5.6%] versus 8.4% [95% CI, 6.6-10.4%]), but reoperation rates were higher ( 24.5% [95% CI, 21.3%-27.8%] versus 9.3% [95% CI, 7.2%-11.7%]) after 15 years compared with mechanical valve replacement. No interaction between age and prosthesis choice on survival was found (P interaction = 0.16).
Lund and Bland [
23] performed a meta-analysis including 17,439 patients. The aim of the study was to compare long-term mortality. When analysing the data, they found that the mean age of patients treated with mechanical implants was 58 years and that of bioprosthesis patients was 68.8 years. The authors then excluded all risk factors, such as NYHA III and IV and CABG. The final results showed that the mortality rates did not differ between the two groups.
In the past, the main reported disadvantage of the bioprosthetic valve has been its shorter durability, hence the propensity for valve wear or reoperation. However, long-term follow-up of patients after AC replacement with a biologic valve has shown otherwise.
Johnston et al. [
8] followed 12 569 patients who underwent Carpentier-Edwards Perimount (CEP) aortic valve replacement for 20 years. The results, published in 2015, show that valve retreading due to valve leaflet degeneration is rare (overall 15%). The longevity of the CEP valve in young patients has also been confirmed in a cohort study involving patients under 60 years of age using a CEP bioprosthesis for 20 years [
11]. It reported a low incidence of bioprosthesis flap degeneration of only 37.2% ± 5.4% and an expected durability value of 17.6 years.
Ruel et al. [
24] analysed the long-term outcomes of patients aged 18-50 years who underwent aortic valve replacement. Their study included 309 patients. The follow-up period was 15 years. The results of the study showed that survival rates did not differ between mechanical and biological valves, irrespective of the frequency of reoperation or valve degeneration. The 15-year survival rate of patients with a mechanical prosthesis was 78.9%, while that of patients with a bioprosthesis was 79.2%. Meanwhile, it was suggested that bleeding, both intracranial and extracranial, is more likely to occur in patients with a mechanical prosthesis, due to warfarin administration.
According to our data, the 5-year survival rate was 63%. When considering the causes of mortality, oncological diseases, gastrointestinal diseases, diseases of the urinary system and COVID-19 are among them. Also noteworthy is the average age of the patients - 70 years. The five-year freedom from reoperation was 97.1%.
According to the data, biological prosthesis represent one of the treatment options of choice for some patient populations, including elderly patients, those with infected endocarditis, those with narrow aortic roots, and women who are of reproductive age. Second- and third-generation biological prostheses produce good outcomes both immediately and over time, with reduced calcification and structural degradation. In various clinical conditions, choosing a prosthesis enables gratifying short-term, medium-term, and long-term outcomes. The improvement of prostheses with less propensity to calcify is a goal of further technological advancement in prosthesis fabrication and analysis of short- and long-term outcomes with concurrent advancement in cardiac surgical treatment. This is a promising direction for contemporary cardiovascular surgery.
5. Conclusions
Our 7-year experience with AV replacement with a biological prosthesis has shown that the quality of life of the patients has improved in the long-term period. The five-year freedom from reoperation was 97.1%. The five-year survival rate was 63% due to a history of severe comorbidities and an average patient age of 70.
Author Contributions
Conceptualization, B.K., S.E. and K.Z.; methodology, S.E.; software, A.G.K.; validation, B.K., S.E., K.Z., V.A., A.M., V.B.; investigation, B.K., S.E., K.Z. and A.G.K.; resources, B.K., S.E., K.Z., V.A.; data curation, B.K., S.E., K.Z., V.A., A.M., V.B. and A.G.K.; writing—original draft preparation, B.K., S.E., K.Z. and A.G.K.; writing—review and editing, B.K., S.E., K.Z., V.A., and A.G.K.; visualization, O.M., N.K.; funding acquisition, A.G.K. and B.K. All authors have read and agreed to the published version of the manuscript.
Funding
BK and AGK thank the Ministry of Science and Higher Education of the Russian Federation for financial assistance within the framework of the state task for performing fundamental scientific research (FSNM-2023-0003 project).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by Ethics Committee of S.G. Sukhanov Cardiovascular Center, Perm, Russia (protocol No. 12 on 17 December 2022).
Informed Consent Statement
Informed consent was obtained from parents of patients involved in the study.
Data Availability Statement
The Russian Ethics Review Authority only granted publication of aggregated data, which means that individual data cannot be shared.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fishbein, G.A.; Fishbein, M.C. Pathology of the Aortic Valve: Aortic Valve Stenosis/Aortic Regurgitation. Current Cardiology Reports 2019, 21.
- El Gamel, A. Aortic Valve Disease: State of the Art. In Advances in Complex Valvular Disease; 2021.
- Neylon, A.; Ahmed, K.; Mercanti, F.; Sharif, F.; Mylotte, D. Transcatheter Aortic Valve Implantation: Status Update. Journal of Thoracic Disease 2018, 10.
- Ruparelia, N.; Prendergast, B.D. Transcatheter Aortic Valve Implantation-What the General Physician Needs to Know. Clinical Medicine, Journal of the Royal College of Physicians of London 2015, 15. [CrossRef]
- Trzeciak, P.; Zembala, M.; Poloński, L. Major Hemorrhagic and Thromboembolic Complications in Patients with Mechanical Heart Valves Receiving Oral Anticoagulant Therapy. Heart Surgery Forum 2010, 13. [CrossRef]
- Velho, T.R.; Pereira, R.M.; Fernandes, F.; Guerra, N.C.; Ferreira, R.; Nobre, Â. Bioprosthetic Aortic Valve Degeneration: A Review from a Basic Science Perspective. Brazilian Journal of Cardiovascular Surgery 2022, 37, 239–250. [CrossRef]
- Salaun, E.; Clavel, M.A.; Rodés-Cabau, J.; Pibarot, P. Bioprosthetic Aortic Valve Durability in the Era of Transcatheter Aortic Valve Implantation. Heart 2018, 104.
- Johnston, D.R.; Soltesz, E.G.; Vakil, N.; Rajeswaran, J.; Roselli, E.E.; Sabik, J.F.; Smedira, N.G.; Svensson, L.G.; Lytle, B.W.; Blackstone, E.H. Long-Term Durability of Bioprosthetic Aortic Valves: Implications from 12,569 Implants. Annals of Thoracic Surgery 2015, 99. [CrossRef]
- Bourguignon, T.; Bouquiaux-Stablo, A.L.; Candolfi, P.; Mirza, A.; Loardi, C.; May, M.A.; El-Khoury, R.; Marchand, M.; Aupart, M. Very Long-Term Outcomes of the Carpentier-Edwards Perimount Valve in Aortic Position. Annals of Thoracic Surgery 2015, 99. [CrossRef]
- Diaz, R.; Hernandez-Vaquero, D.; Alvarez-Cabo, R.; Avanzas, P.; Silva, J.; Moris, C.; Pascual, I. Long-Term Outcomes of Mechanical versus Biological Aortic Valve Prosthesis: Systematic Review and Meta-Analysis. Journal of Thoracic and Cardiovascular Surgery 2019, 158. [CrossRef]
- Bartus, K.; Litwinowicz, R.; Sadowski, J.; Filip, G.; Kowalewski, M.; Suwalski, P.; Mazur, P.; Kędziora, A.; Jasiński, M.; Deja, M.; et al. Bioprosthetic or Mechanical Heart Valves: Prosthesis Choice for Borderline Patients?-Results from 9,616 Cases Recorded in Polish National Cardiac Surgery Registry. Journal of Thoracic Disease 2020, 12. [CrossRef]
- Cekmecelioglu, D.; Preventza, O.; Dougherty, K.G.; Chatterjee, S.; Green, S.Y.; Silva, G. V.; Díez, J.G.; Coselli, J.S. Transcatheter Valve-in-Valve Implantation for Degenerated Stentless Aortic Bioroots. Annals of Cardiothoracic Surgery 2021, 10. [CrossRef]
- Fukui, S.; Yamamura, M.; Mitsuno, M.; Tanaka, H.; Ryomoto, M.; Miyamoto, Y. Aortic Valve Prosthesis Selection in Dialysis Patients Based on the Patient’s Condition. Journal of Artificial Organs 2012, 15. [CrossRef]
- Okada, K.; Inoue, Y.; Haida, H.; Suzuki, S. Aortic Valve Reconstruction Using Autologous Pericardium (Ozaki Procedure) for Active Infective Endocarditis: A Case Report. Gen Thorac Cardiovasc Surg 2018, 66, 546–548. [CrossRef]
- Zhibing, Q.; Xin, C.; Ming, X.; Lele, L.; YingShuo, J.; LiMing, W. Should Bioprostheses Be Considered the Valve of Choice for Dialysis-Dependent Patients? J Cardiothorac Surg 2013, 8.
- Jiang, Y.; Wang, S.; Bian, J.; Chen, S.; Shao, Y. Mechanical versus Bioprosthetic Aortic Valve Replacement in Middle-Aged Adults: A Systematic Review and Meta-Analysis. J Cardiovasc Dev Dis 2023, 10.
- Kitamura, T.; Edwards, J.; Miyaji, K. Continuous Suture Technique for Aortic Valve Replacement Shortens Cross-Clamp and Bypass Times. Tex Heart Inst J 2017, 44. [CrossRef]
- Stewart, S.; Hart, C.L.; Hole, D.J.; McMurray, J.J.V. A Population-Based Study of the Long-Term Risks Associated with Atrial Fibrillation: 20-Year Follow-up of the Renfrew/Paisley Study. American Journal of Medicine 2002, 113. [CrossRef]
- Ngo, H.T.; Nguyen, H.C.; Nguyen, T.T.; Le, T.N.; Camilleri, L.; Doan, H.Q. Reconstruction of Aortic Valve by Autologous Pericardium (Ozaki’s Procedure): Single Center Experience in Vietnam. Asian Cardiovasc Thorac Ann 2021, 29. [CrossRef]
- Takeda, A.; Martin, N.; Taylor, R.S.; Taylor, S.J.C. Disease Management Interventions for Heart Failure. Cochrane Database of Systematic Reviews 2019, 2019.
- Milewski, R.K.; Habertheuer, A.; Bavaria, J.E.; Fuller, S.; Desai, N.D.; Szeto, W.Y.; Korutla, V.; Vallabhajosyula, P. Selection of Prosthetic Aortic Valve and Root Replacement in Patients Younger than Age 30 Years. J Thorac Cardiovasc Surg 2019, 157, 714–725. [CrossRef]
- Schnittman, S.R.; Adams, D.H.; Itagaki, S.; Toyoda, N.; Egorova, N.N.; Chikwe, J. Bioprosthetic Aortic Valve Replacement: Revisiting Prosthesis Choice in Patients Younger than 50 Years Old. J Thorac Cardiovasc Surg 2018, 155, 539-547.e9. [CrossRef]
- Lund, O.; Bland, M. Risk-Corrected Impact of Mechanical versus Bioprosthetic Valves on Long-Term Mortality after Aortic Valve Replacement. J Thorac Cardiovasc Surg 2006, 132, 20-26.e3. [CrossRef]
- RUEL, M.; KULIK, A.; LAM, B.; RUBENS, F.; HENDRY, P.; MASTERS, R.; BEDARD, P.; MESANA, T. Long-Term Outcomes of Valve Replacement with Modern Prostheses in Young Adults. European Journal of Cardio-Thoracic Surgery 2005, 27, 425–433. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).