Submitted:
17 May 2023
Posted:
18 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Focus on Bio-Based Building Materials and Their Specificities
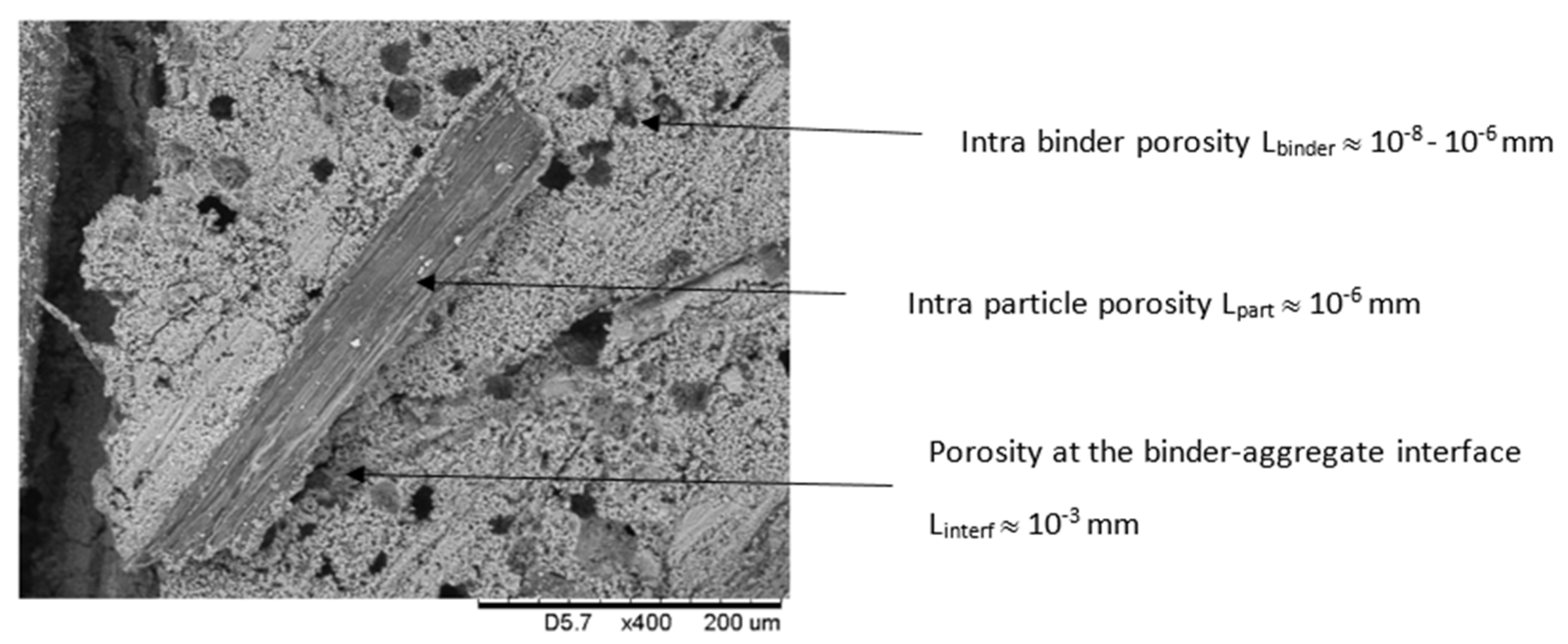
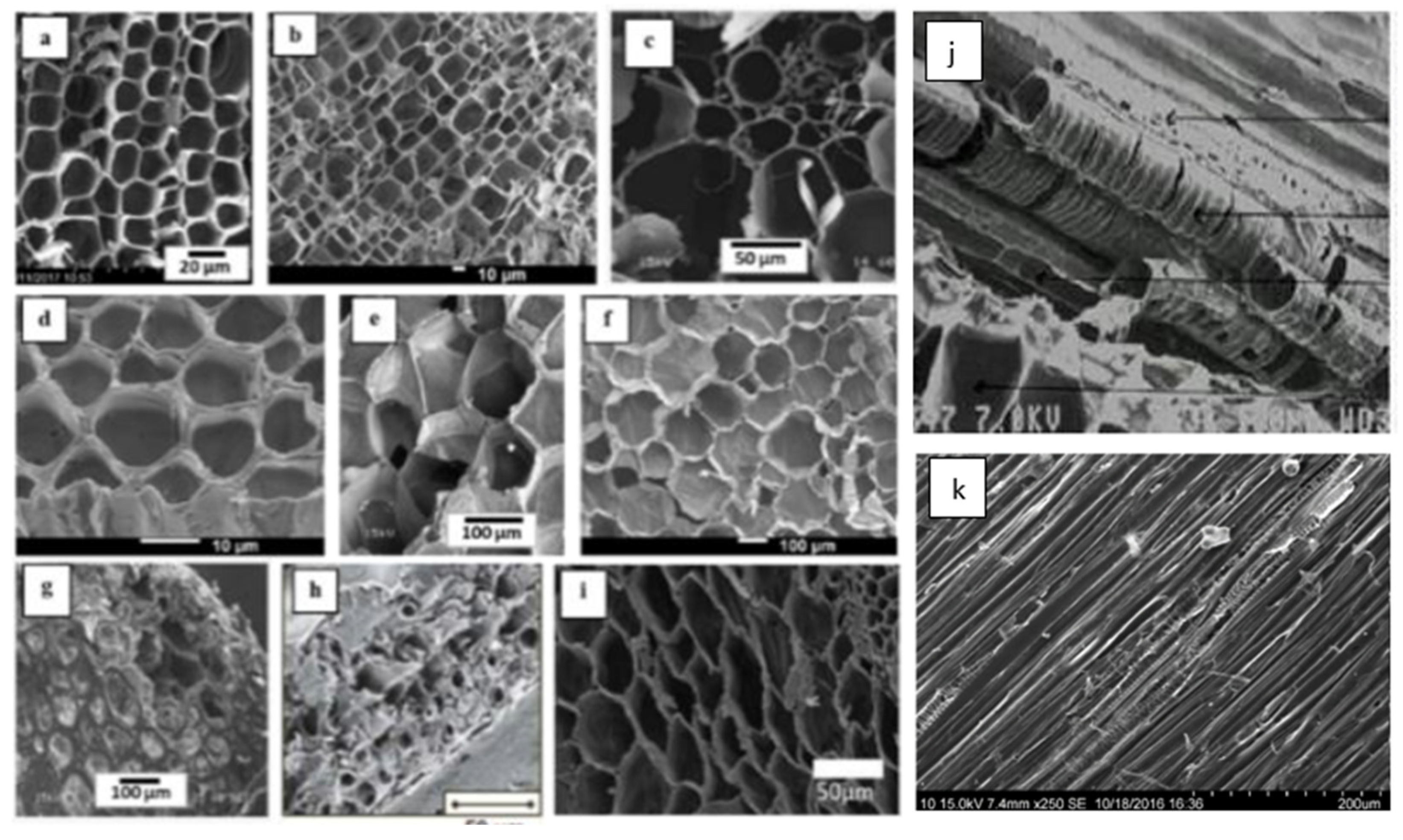
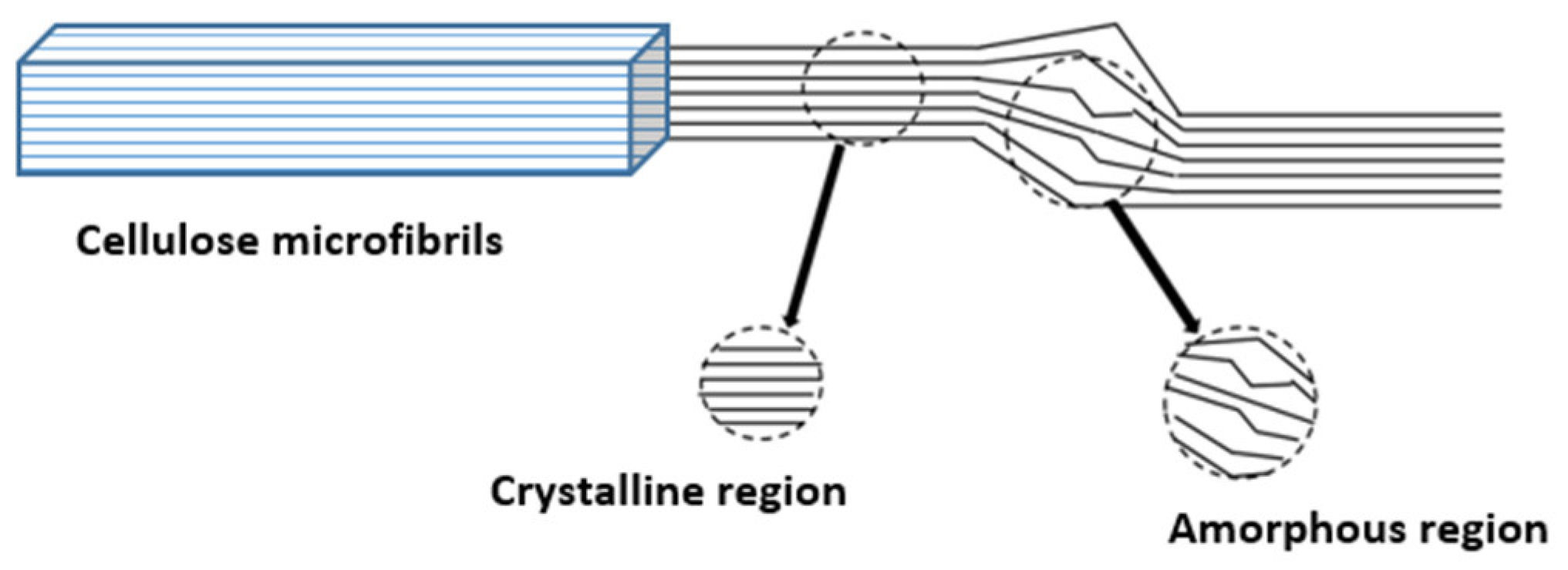
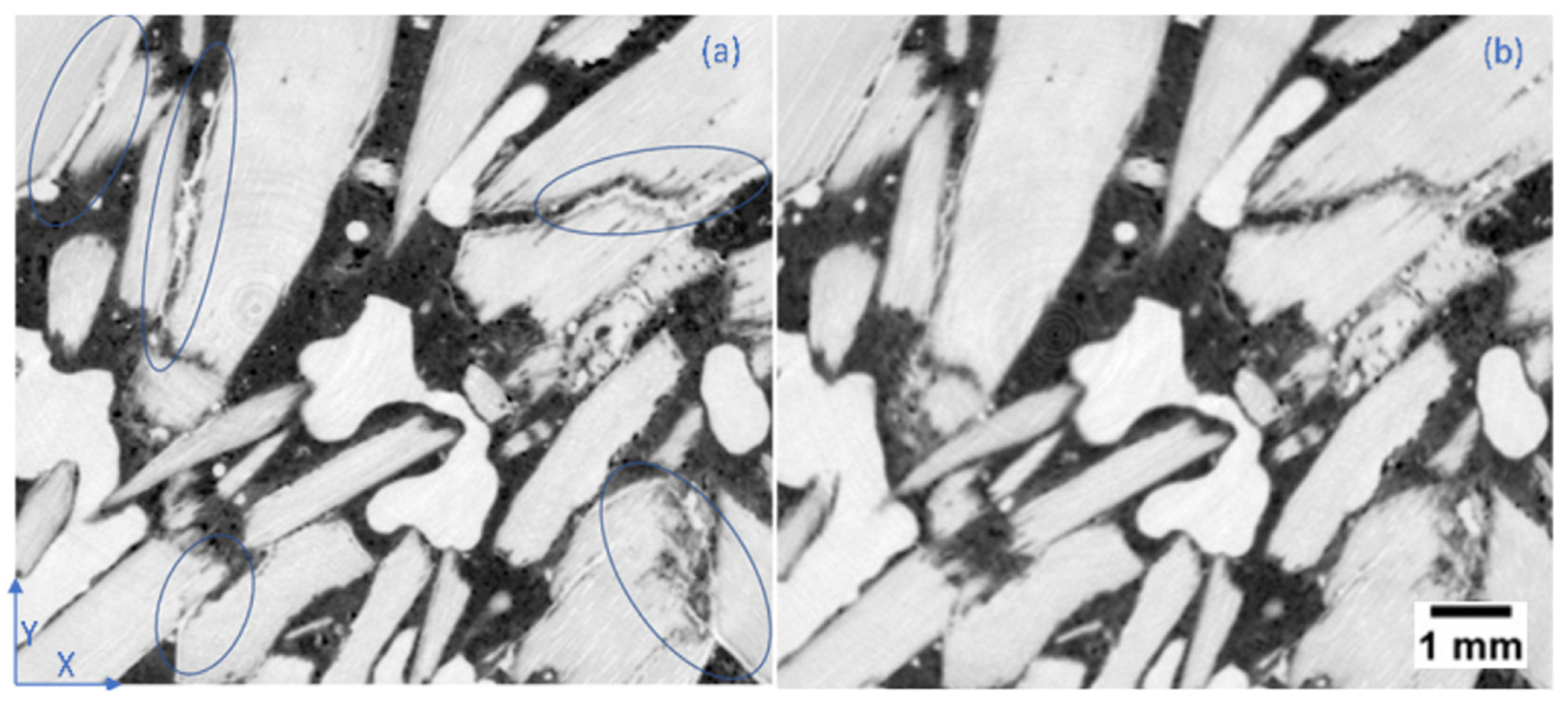
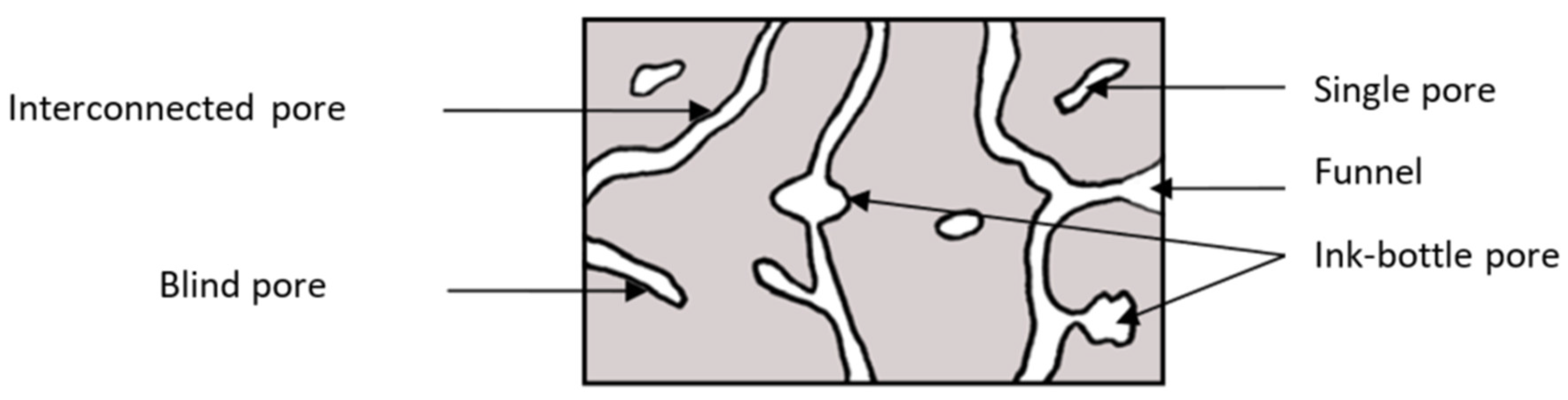
2.1. Microstructure
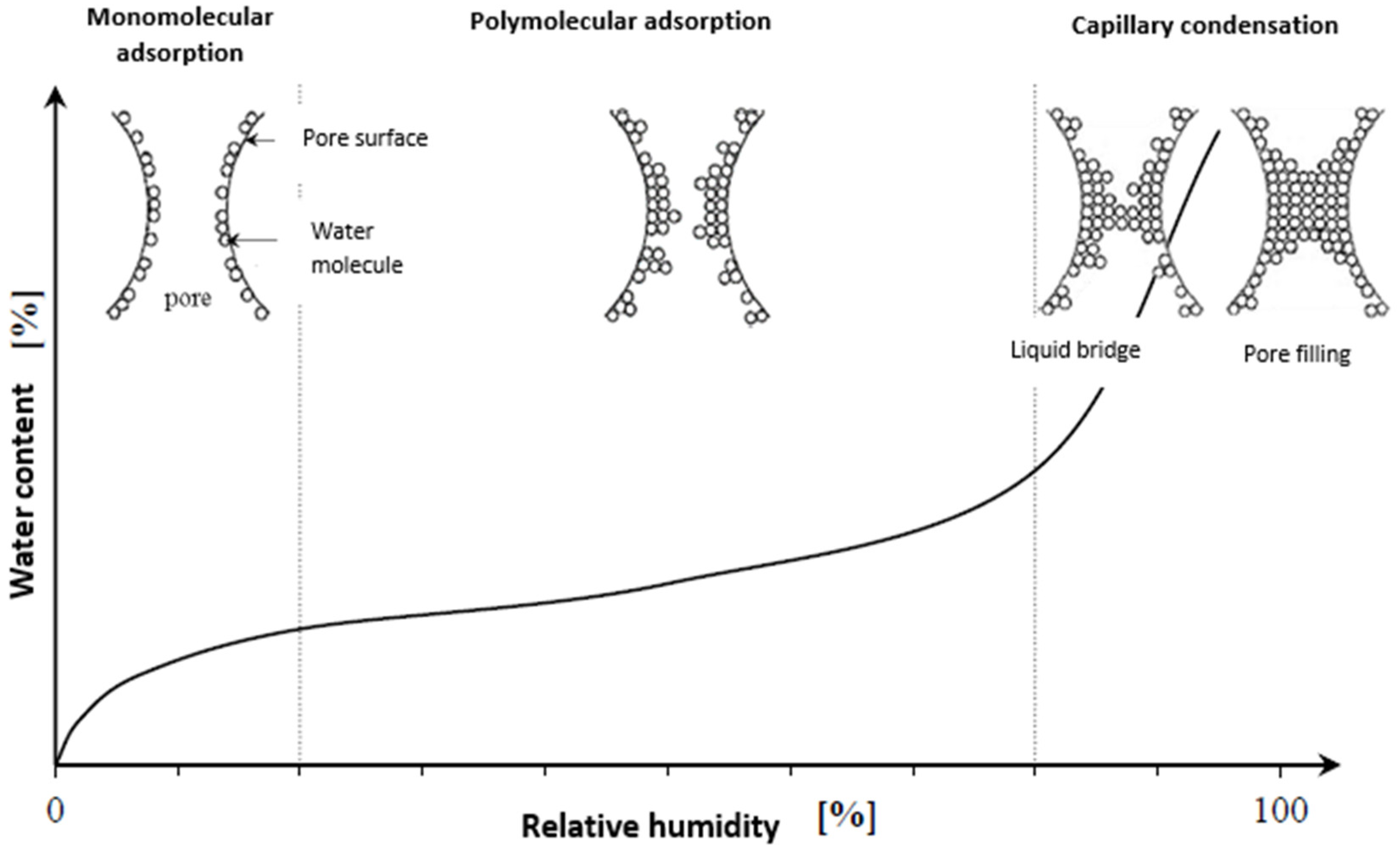
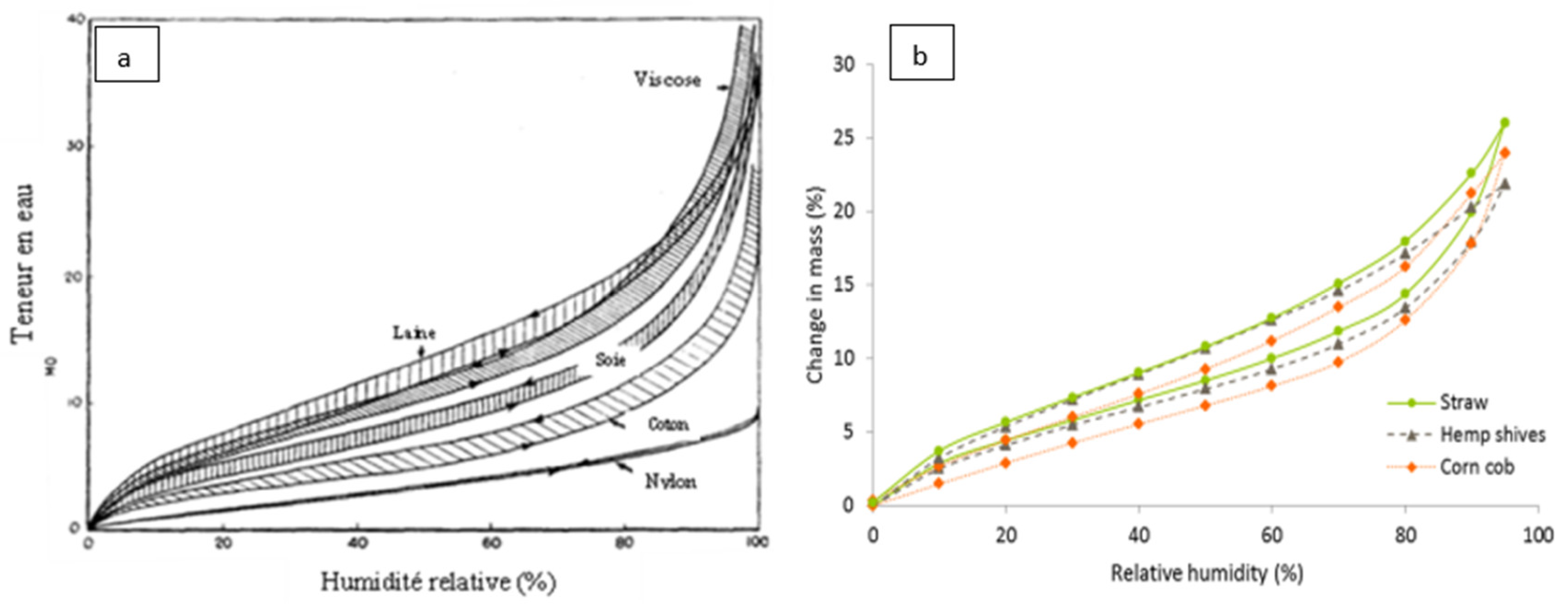
2.2. Hygroscopic properties
2.3. Chemical Composition
2.4. Swelling and Shrinkage
2.5. Functional Properties with Age
2.6. Temperature Effects
2.7. Local Kinetic Sorption
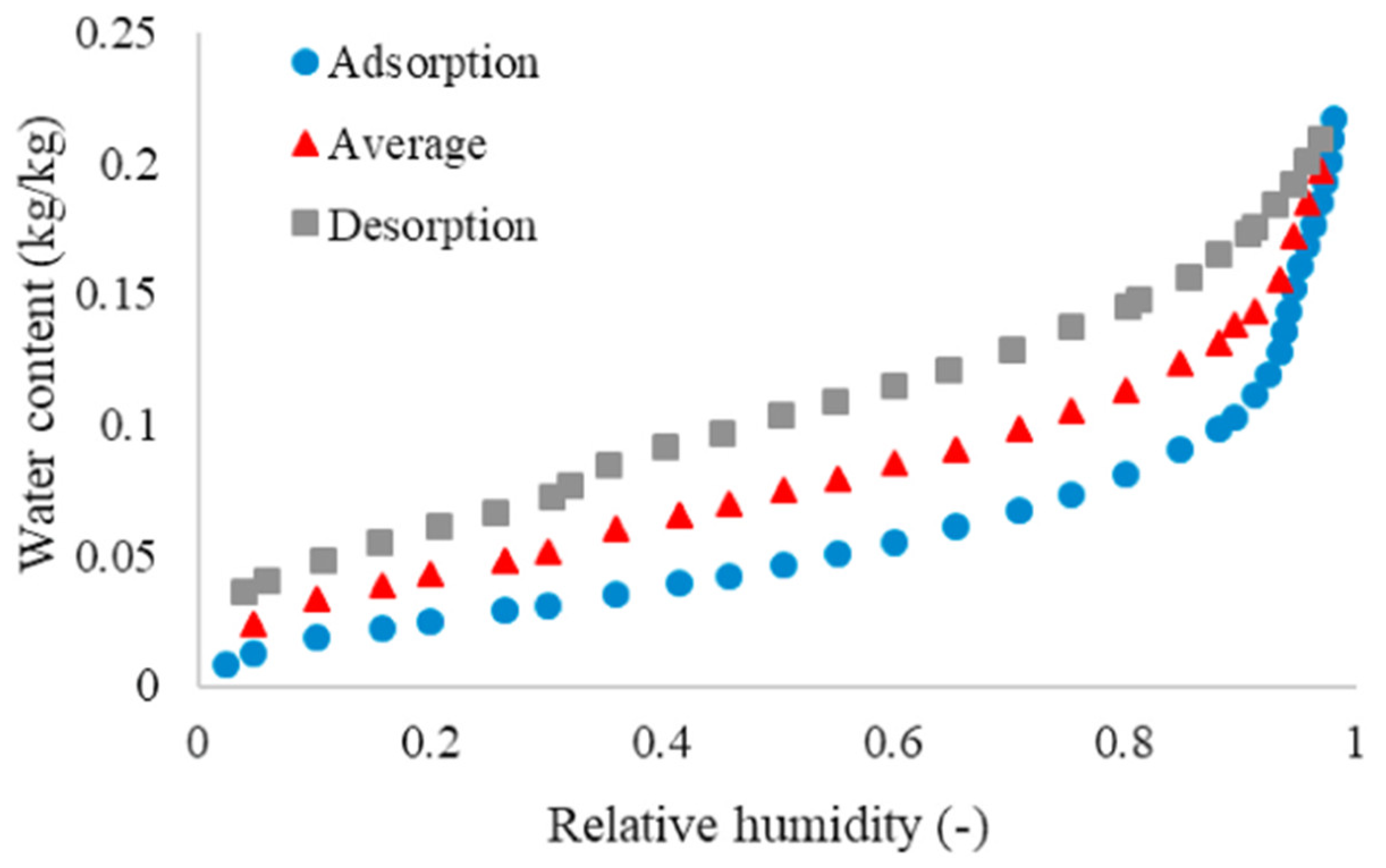
2.8. Sorption Hysteresis
- The sorption mechanism is reversible since the original state is obtained at a dry state [56]
- Aging reduces the rate of adsorption and desorption for hemp concrete [59]
- Hysteresis increases while crystallinity decreases [60]
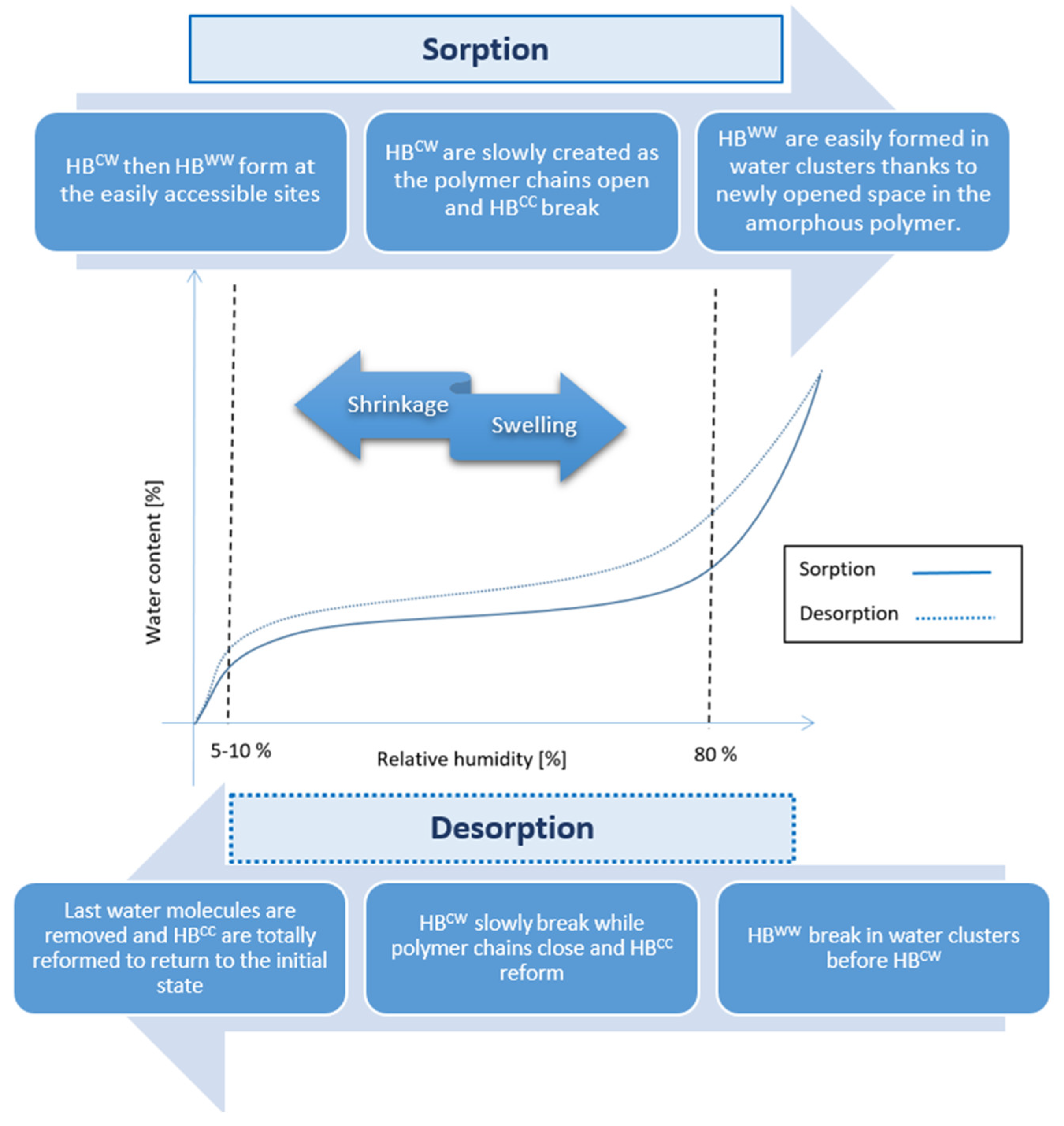
- Water content is always higher in the desorption than in the adsorption phase for the same relative humidity.
3. From Hydrogen Bonding to Hysteresis
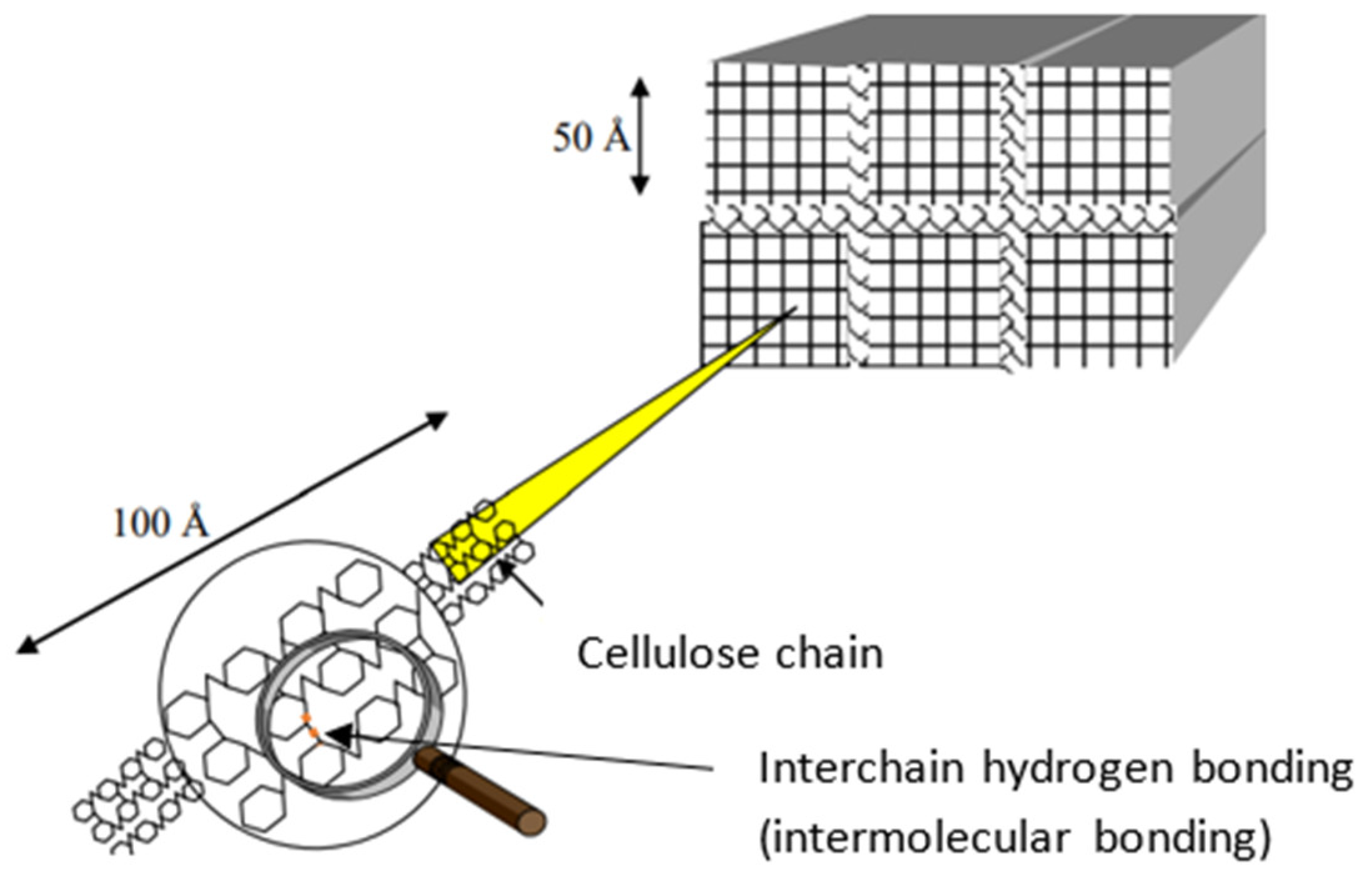
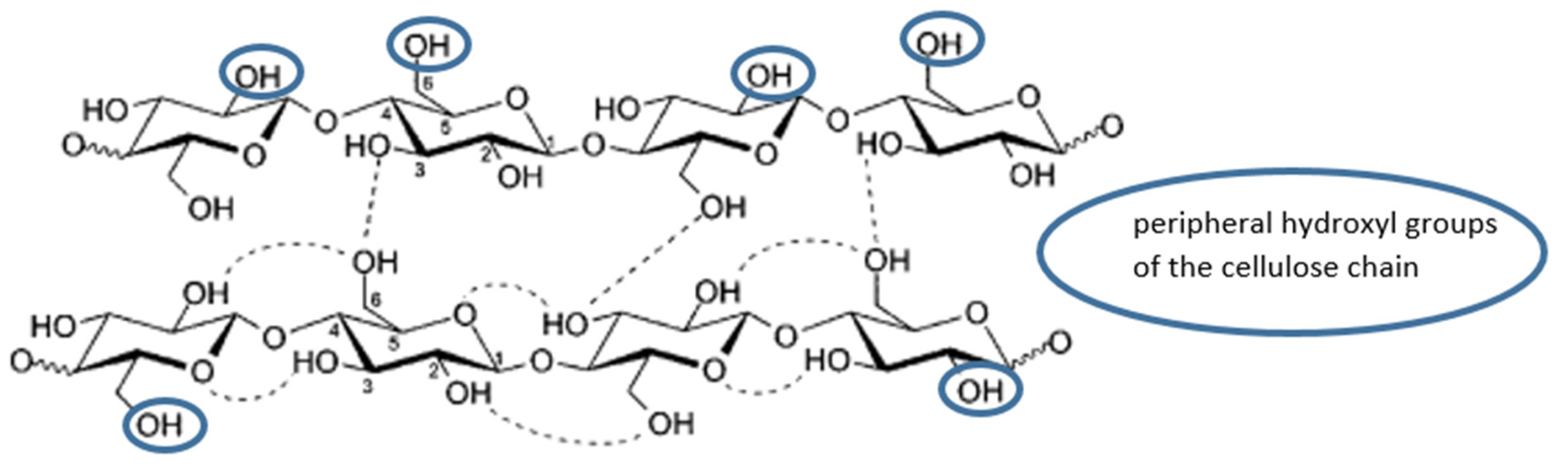
3.1. Hydrogen Bonding
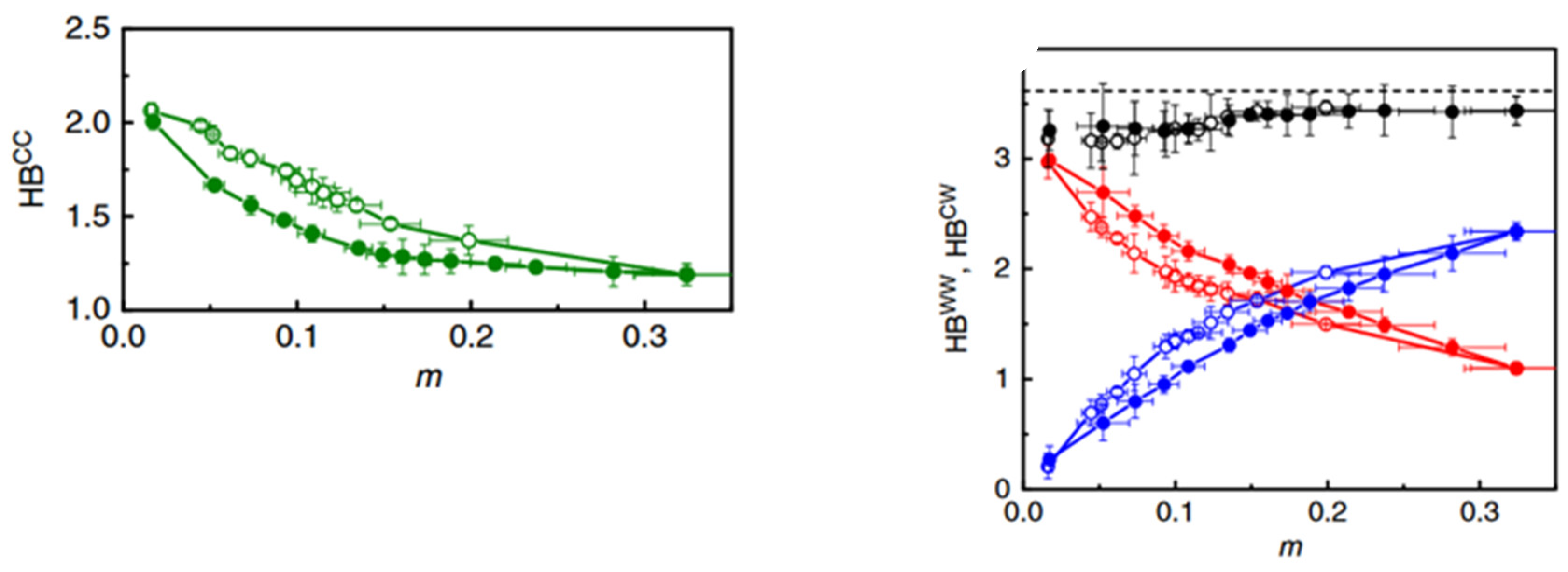
3.2. Microscopic Understanding of Hysteresis
- Inter molecular hydrogen bonds between water molecules (HBWW)
- Inter molecular hydrogen bond between water molecules and cellulose (HBCW)
- Inter chain Hydrogen bonds in cellulose (HBCC)
4. Discussion: New Insights into Hysteresis in Bio-Sourced Materials
4.1. A Necessary New Approach
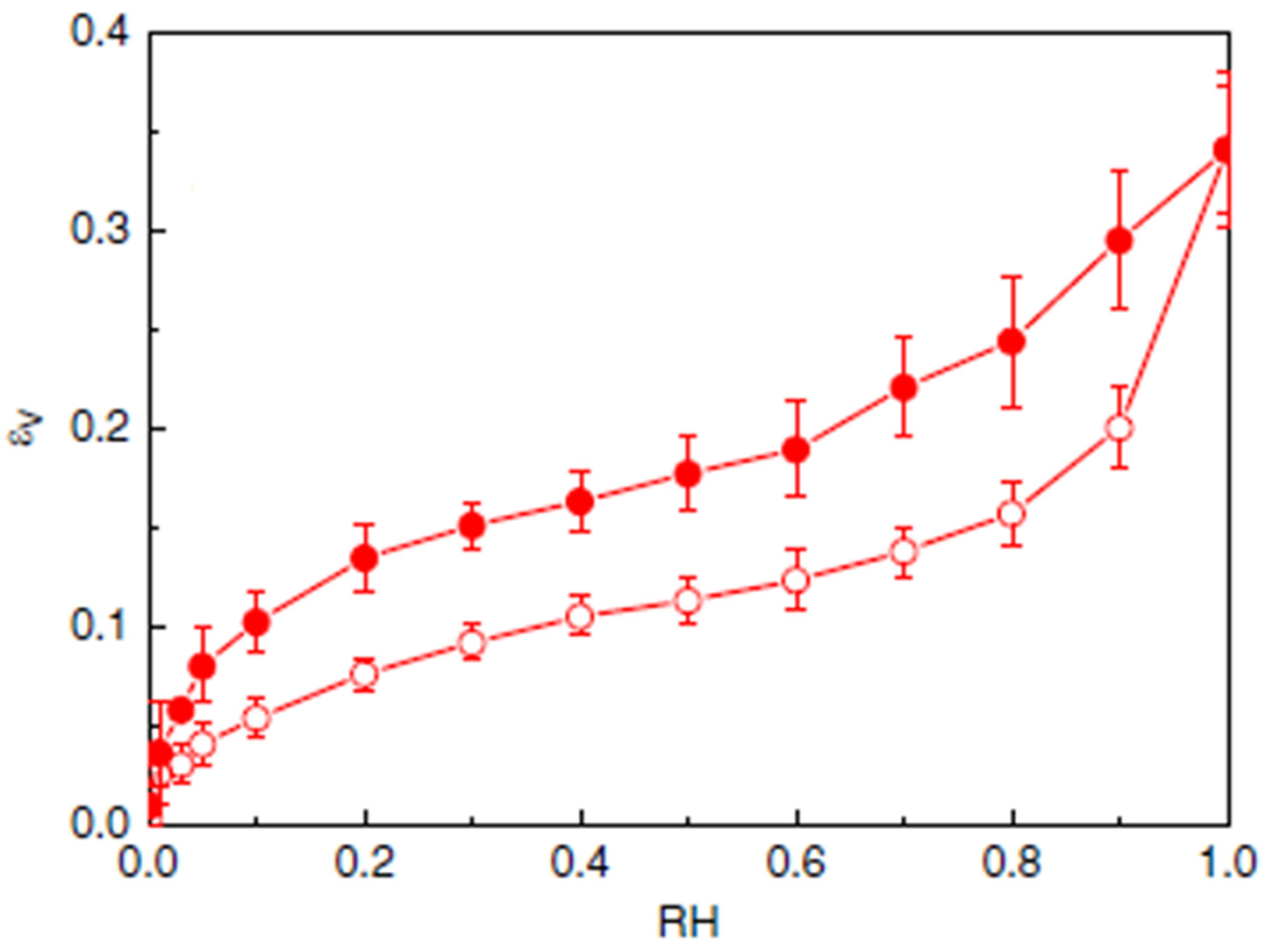
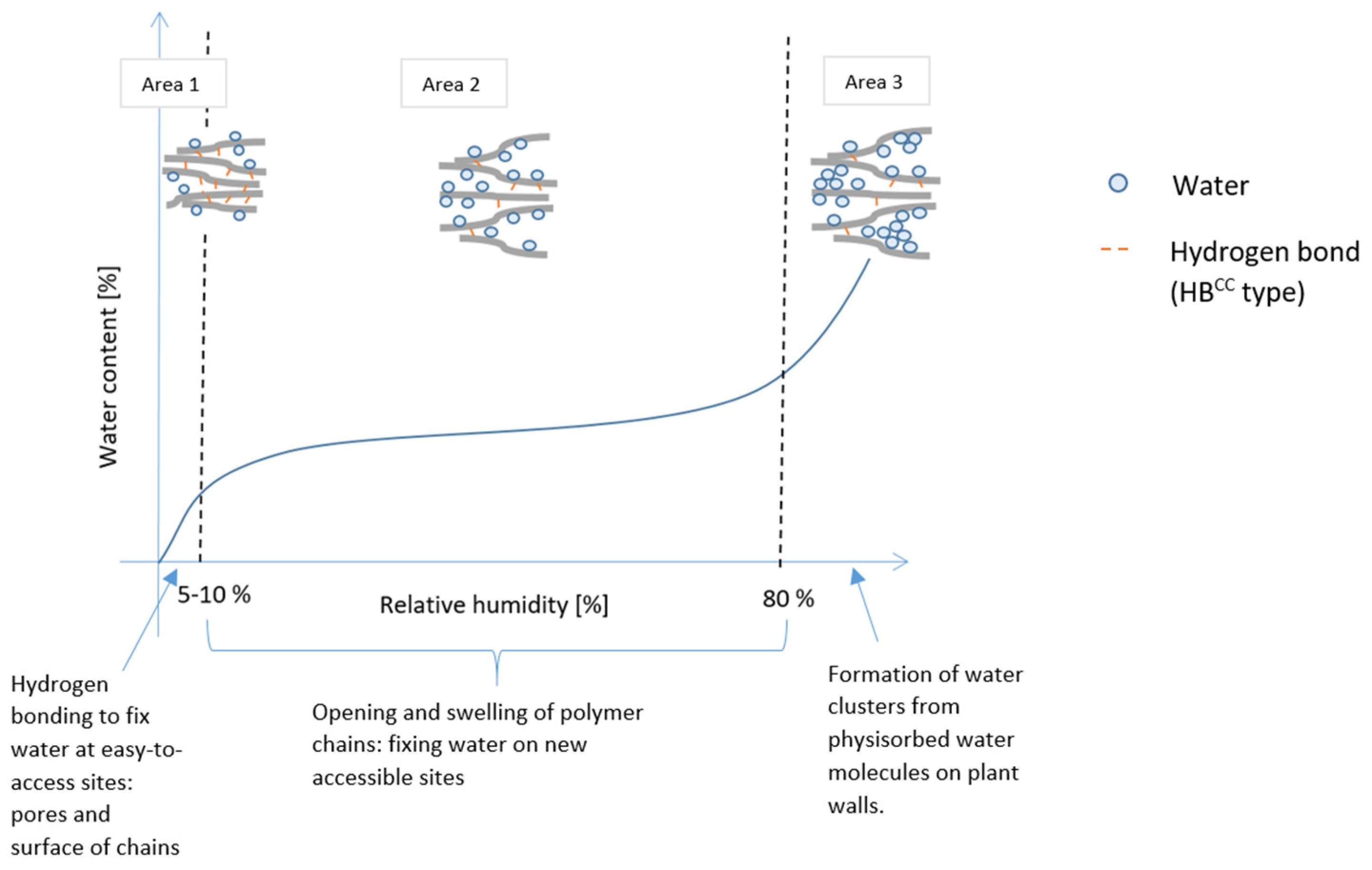
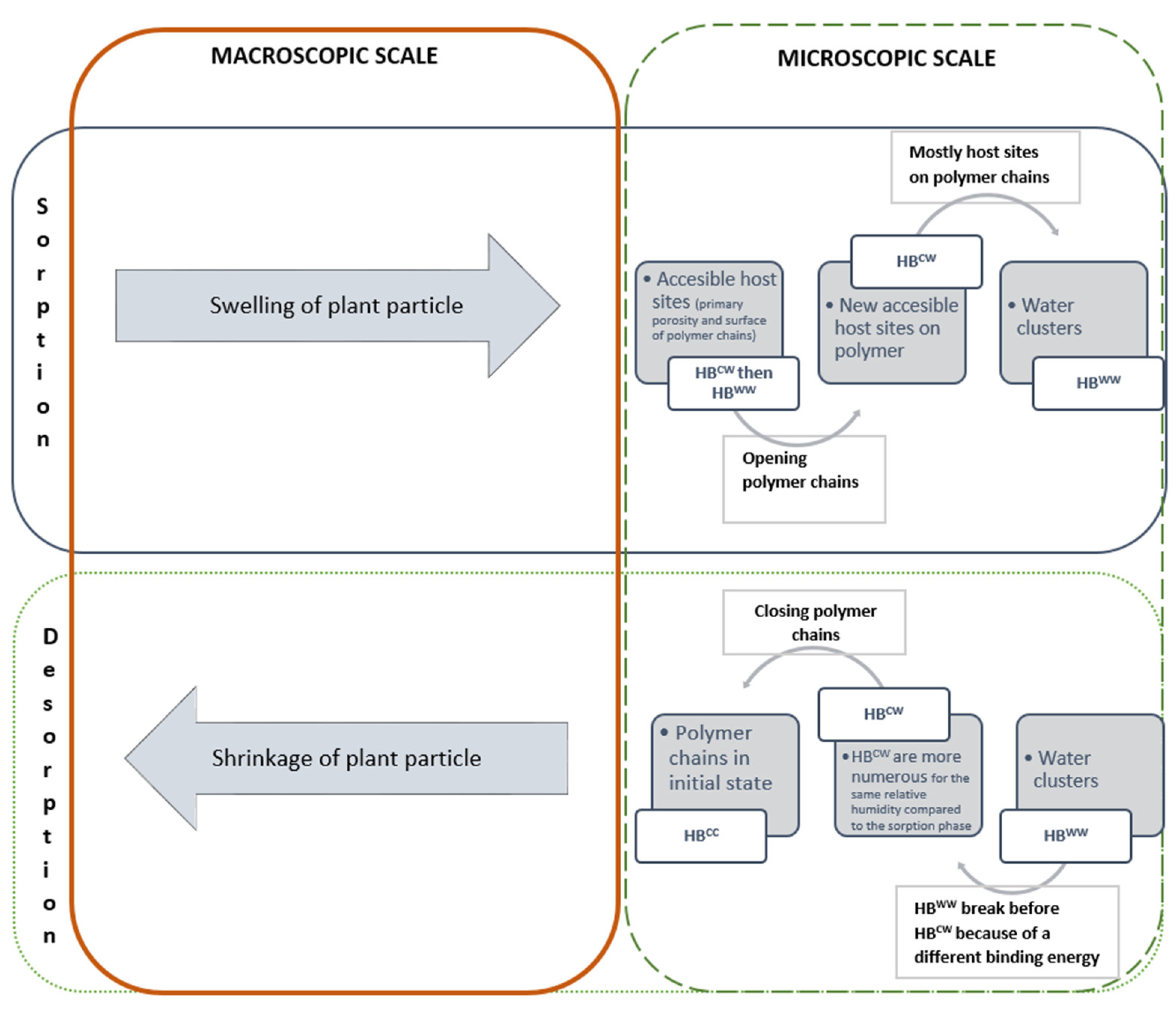
4.2. Hysteresis: From the Aggregate Scale to the Material One
- Area 1: Water fixation on a pore surface is relatively fast because the host sites are easily accessible: HBCW bonds form on polymer surface chains or in pores of an amorphous region then HBWW form easily until the initial pores are filled.
- Area 2: Polymer chains open up, freeing new host sites to create HBCW bonds. In parallel, HBCC bonds break.
- Area 3: At high relative humidity, hydrogen bonds mostly form between water molecules because many host sites are occupied on polymer chains. This leads to water clusters in the new pore spaces created by the swelling of the polymer chains. Because host sites are very accessible, the associated kinetics is quite fast, as in area 1.
4.3. Macroscopic Effects
5. Conclusions
- (i)
- A better understanding of macroscopic swelling makes it possible to anticipate and to predict. It is important to leave a corresponding gap in the wall to avoid any disorder. In addition, swelling effects impact the porosity of the material and therefore probably affect its mechanical properties and durability.
- (ii)
- It would be interesting to investigate whether, as in the case of hysteresis in electromagnetism, the area of the hysteresis curve gives additional information but, here, on the sorption/desorption phenomenon.
- (iii)
- To conclude, this work combines the fields of chemistry, civil engineering and applied physics. It underlines the interest of conducting interdisciplinary studies to understand the full complexity of bio-based materials.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- M. Lagouin, C. Magniont, Sénéchal, Moonen, J.-E. Aubert, and A. Laborel-Préneron. Influence of types of binder and plant aggregates on hygrothermal and mechanical properties of vegetal concretes. Constr. Build. Mater. 2019, 222, 852–871. [Google Scholar] [CrossRef]
- G. Delannoy et al. Performances des bétons de chanvre : Des propriétés microscopiques aux propriétés fonctionnelles. Acad. J. Civ. Eng. 2017, 35. [Google Scholar] [CrossRef]
- M. S. Abbas, E. Gourdon, Glé, F. McGregor, M. Y. Ferroukhi, and A. Fabbri. Relationship between hygrothermal and acoustical behavior of hemp and sunflower composites. Build. Environ. 2021, 188, 107462. [Google Scholar] [CrossRef]
- F. Collet, J. Chamoin, S. Pretot, and C. Lanos. Comparison of the hygric behaviour of three hemp concretes. Energy Build. 2013, 62, 294–303. [Google Scholar] [CrossRef]
- M. Chabannes, V. Nozahic, and S. Amziane. Design and multi-physical properties of a new insulating concrete using sunflower stem aggregates and eco-friendly binders. Mater. Struct. 2015, 48, 1815–1829. [Google Scholar] [CrossRef]
- F. Benmahiddine, F. Bennai, R. Cherif, R. Belarbi, A. Tahakourt, and K. Abahri. Experimental investigation on the influence of immersion/drying cycles on the hygrothermal and mechanical properties of hemp concrete. J. Build. Eng. 2020, 32, 101758. [Google Scholar] [CrossRef]
- M. Maaroufi, F. Bennai, R. Belarbi, and K. Abahri. Experimental and numerical highlighting of water vapor sorption hysteresis in the coupled heat and moisture transfers. J. Build. Eng. 2021, 40, 102321. [Google Scholar] [CrossRef]
- N. Issaadi. Effets de la variabilité des propriétés de matériaux cimentaires sur les transferts hygrothermiques : Développement d’une approche probabiliste. Ph.D. Thesis, La Rochelle, 2015. Available online: https://www.theses.fr/2015LAROS028 (accessed on 14 April 2023).
- F. Bennai, C. El Hachem, K. Abahri, and R. Belarbi. Microscopic hydric characterization of hemp concrete by X-ray microtomography and digital volume correlation. Constr. Build. Mater. 2018, 188, 983–994. [Google Scholar] [CrossRef]
- Laborel-Préneron, C. Magniont, and J.-E. Aubert. Characterization of Barley Straw, Hemp Shiv and Corn Cob as Resources for Bioaggregate Based Building Materials. Waste Biomass Valorization 2018, 9, 1095–1112. [Google Scholar] [CrossRef]
- Y. Aït Oumeziane, S. Moissette, M. Bart, and C. Lanos. Influence of temperature on sorption process in hemp concrete. Constr. Build. Mater. 2016, 106, 600–607. [Google Scholar] [CrossRef]
- M. Chen, B. Coasne, R. Guyer, D. Derome, and J. Carmeliet. Role of hydrogen bonding in hysteresis observed in sorption-induced swelling of soft nanoporous polymers. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef]
- T. Jami, S. R. Karade, and L. P. Singh. A review of the properties of hemp concrete for green building applications. J. Clean. Prod. 2019, 239, 117852. [Google Scholar] [CrossRef]
- M. Maaroufi. Modélisation des transferts hygrothermiques dans les matériaux de construction : Incidence de l’hystérésis. Ph.D. Thesis, La Rochelle, 2019. Available online: https://www.theses.fr/2019LAROS028 (accessed on 1 October 2022).
- B. Mazhoud, F. Collet, S. Prétot, and C. Lanos. Effect of hemp content and clay stabilization on hygric and thermal properties of hemp-clay composites. Constr. Build. Mater. 2021, 300, 123878. [Google Scholar] [CrossRef]
- M. Babaee and A. Castel. Water vapor sorption isotherms, pore structure, and moisture transport characteristics of alkali-activated and Portland cement-based binders. Cem. Concr. Res. 2018, 113, 99–120. [Google Scholar] [CrossRef]
- G. Promis, L. Freitas Dutra, O. Douzane, A. D. Tran Le, and T. Langlet. Temperature-dependent sorption models for mass transfer throughout bio-based building materials. Constr. Build. Mater. 2019, 197, 513–525. [Google Scholar] [CrossRef]
- F. Collet. Caracterisation Hydrique Et Thermique De Materiaux De Genie Civil A Faibles Impacts Environnementaux. 2004.
- C. Magniont. Contribution à la formulation et à la caractérisation d’un écomatériau de construction à base d’agroressources. Ph.D. Thesis, Toulouse 3, 2010. Available online: https://www.theses.fr/2010TOU30101 (accessed on 4 October 2022).
- M. Le Troëdec et al. Influence of chemical treatments on adhesion properties of hemp fibres. J. Colloid Interface Sci. 2011, 356, 303–310. [Google Scholar] [CrossRef]
- F. Benmahiddine. Études des transferts couplés de chaleur, d’air et d’humidité par des techniques de changement d’échelle (microscopique-macroscopique) : Application à l’évaluation de la performance énergétique et la durabilité des matériaux de construction. Ph.D. Thesis, La Rochelle, 2020. Available online: https://www.theses.fr/2020LAROS029 (accessed on 1 October 2022).
- M. Lagouin. Caractérisation et optimisation multiphysiques d’une paroi bicouche bio et géosourcée. PhD thesis, Toulouse 3, 2020. Available online: https://www.theses.fr/2020TOU30258 (accessed on 1 October 2022).
- C. Magniont and G. Escadeillas. Chemical Composition of Bio-aggregates and Their Interactions with Mineral Binders. In Bio-aggregates Based Building Materials: State-of-the-Art Report of the RILEM Technical Committee 236-BBM, S. Amziane and F. Collet, Eds.; in RILEM State-of-the-Art Reports; Springer: Dordrecht, The Netherlands, 2017; pp. 1–37. [Google Scholar] [CrossRef]
- H. H. Ratsimbazafy, A. Laborel-Preneron, C. Magniont, and P. Evon. Comprehensive Characterization of Agricultural By-Products for Bio-Aggregate Based Concrete. Constr. Technol. Archit. 2022, 1, 77–84. [Google Scholar]
- N. Stevulova, J. Cigasova, I. Schwarzova, A. Sicakova, and J. Junak. Sustainable Bio-Aggregate-Based Composites Containing Hemp Hurds and Alternative Binder. Buildings 2018, 8, 2. [Google Scholar] [CrossRef]
- S. Amziane and F. Collet, Bio-aggregates Based Building Materials: State-of-the-Art Report of the RILEM Technical Committee 236-BBM; Springer: 2017.
- N. Dujardin. Un Materiau Biosource De Choix: Les Fibres Naturelles. Caractérisations Et Applications. presented at the 25èmes Journées Scientifiques de l’Environnement—L’économie verte en question, Feb. 2014. Available online: https://hal.science/hal-00978360 (accessed on 12 April 2023).
- F. Gehring. Étude du comportement mécanique et de l’endommagement de composites thermoplastiques renforcés de fibres courtes de chanvre: Approche expérimentale et modélisation. 2013.
- K. H. Gardner and J. Blackwell. The hydrogen bonding in native cellulose. Biochim. Biophys. Acta BBA Gen. Subj. 1974, 343, 232–237. [Google Scholar] [CrossRef]
- S. Perez. Structure et morphologie de la cellulose. Jan. 2000.
- T. T. Teeri. Crystalline cellulose degradation: New insight into the function of cellobiohydrolases. Trends Biotechnol. 1997, 15, 160–167. [Google Scholar] [CrossRef]
- Sofiane and, A. Laurent, Les bétons de granulats d’origine végétale: Application au béton de chanvre. Lavoisier, 2013.
- G. B. Mitra and P. S. Mukherjee. X-ray diffraction study of fibrous polymers. I. Degree of paracrystallinity—A new parameter for characterizing fibrous polymers. Polymer 1980, 21, 1403–1409. [Google Scholar] [CrossRef]
- E. Frollini, A. 34. E. Frollini, A. Leao, L. Mattoso, R. Rowell, J. Han, and J. Rowell. Characterization and Factors Effecting Fiber Properties. Nat. Polym. Agrofibers Compos.
- J. Rebiere. Nouvelle méthodologie pour la caractérisation de distributions de masses molaires d’échantillons cellulosiques complexes. 2017.
- Pinkert, K. Marsh, S. Pang, and M. Staiger. Ionic Liquids and Their Interaction with Cellulose. Chem. Rev. 2009, 109, 6712–28. [Google Scholar] [CrossRef]
- D. Rabideau and A. E. Ismail. Mechanisms of hydrogen bond formation between ionic liquids and cellulose and the influence of water content. Phys. Chem. Chem. Phys. 2015, 17, 5767–5775. [Google Scholar] [CrossRef] [PubMed]
- V. Troncoso, N. Kouta, G. Escadeillas, and V. H. G. Barragan. Réactivité des cendres volcaniques équatoriennes dans les liants minéraux. presented at the NOMAD 2022—4e conférence internationale francophone Nouveaux Matériaux et Durabilité, Nov. 2022. Available online: https://hal.science/hal-03879708 (accessed on 12 April 2023).
- K. A. J. Ouedraogo. Stabilisation de matériaux de construction durables et écologiques à base de terre crue par des liants organiques et/ou minéraux à faibles impacts environnementaux. Ph.D. Thesis, Toulouse 3, 2019. Available online: https://www.theses.fr/2019TOU30199 (accessed on 1 October 2022).
- “Guide des bonnes pratiques de la construction en terre crue. Réhabilitation Bâti Ancien—CREBA, Jan. 01, 2018. Available online: https://www.rehabilitation-bati-ancien.fr/espace-documentaire/guide-des-bonnes-pratiques-la-construction-en-terre-crue (accessed on 12 April 2023).
- S. Garcia-Boivin. Retrait au jeune age du beton: Developpement d’une methode experimentale et contribution a l’analyse physique du retrait endogene. THESE Present. POUR OBTENIR TITRE Dr. ECOLE Natl. PONTS CHAUSSEES—Spec. Struct. Mater. 1999. Available online: https://trid.trb.org/view/960738 (accessed on 12 April 2023).
- M. S. Matthieu. Potentiel des fibres végétales courtes dans l’amélioration du comportement mécanique des mortiers. Ph.D. Thesis, INSA de Toulouse, 2022. Available: https://theses.hal.science/tel-03772550. (accessed on 12 April 2023).
- C. Achour, S. Remond, and N. Belayachi. Mesure du gonflement-retrait des granulats végétaux par analyse d’image. Acad. J. Civ. Eng. 2022, 40, 1. [Google Scholar] [CrossRef]
- S. Marceau et al. Impact de vieillissements accélérés sur les propriétés de bétons de chanvre. Acad. J. Civ. Eng. 2016, 34, 1. [Google Scholar] [CrossRef]
- G. Delannoy. Durabilité du béton de chanvre soumis à des cycles d’humidification-séchage. Acad. J. Civ. Eng. 2018, 36, 1. [Google Scholar] [CrossRef]
- G. Delannoy et al. Durability of hemp concretes exposed to accelerated environmental aging. Constr. Build. Mater. 2020, 252, 119043. [Google Scholar] [CrossRef]
- et al. Characterization of a hemp-based agro-material: Influence of starch ratio and hemp shive size on physical, mechanical, and hygrothermal properties. Energy Build. 2017, 153, 501–512. [Google Scholar] [CrossRef]
- Rode and C., O. Clorius. Modeling of Moisture Transport in Wood with Hysteresis and Temperature-Dependent Sorption Characteristics.
- D. T. Le, D. Samri, M. Rahim, O. Douzane, G. Promis, and T. Langlet. Effect of Temperature-dependent Sorption Characteristics on The Hygrothermal Behavior of Hemp Concrete. Energy Procedia 2015, 78, 1449–1454. [Google Scholar] [CrossRef]
- N. Reuge, S. Moissette, M. Bart, F. Collet, and C. Lanos. Modèle de cinétique locale de sorption couplé au phénomène d’hystérésis pour les matériaux biosourcés. Acad. J. Civ. Eng. 2018, 36, 1. [Google Scholar] [CrossRef]
- N. Reuge, F. N. Reuge, F. Collet, S. Pretot, S. Moissette, M. Bart, and C. Lanos, Cinétique locale de sorption: Modélisation d’une paroi biosourcée. 2021. [CrossRef]
- Yacine, M. Bart, S. Moissette, C. Lanos, F. Collet, and S. Pretot, Hysteresis phenomenon in hemp concrete. 2015.
- M. Nouri. Développement d’éléments en biocomposite à base de fibre végétale pour la réhabilitation énergétique des bâtiments. Ph.D. Thesis, Ecole centrale de Nantes, 2020. Available online: https://www.theses.fr/2020ECDN0038 (accessed on 1 October 2022).
- M. S. Abbas. Caractérisations multi-physiques des mortiers bio-sourcés isolants et modélisation de leurs impacts sur les transferts hygrothermiques à l’échelle des parois : Application aux bétons de moelles végétales. Ph.D. Thesis, Lyon, 2021. Available online: https://www.theses.fr/2021LYSET003 (accessed on 1 October 2022).
- D. Lelièvre. Simulation numérique des transferts de chaleur et d’humidité dans une paroi multicouche de bâtiment en matériaux biosourcés. Ph.D. Thesis, Lorient, 2015. Available online: https://www.theses.fr/2015LORIS359 (accessed on 31 March 2023).
- Maalouf, T. Moussa, B. S. Umurigirwa, and T. H. Mai. Etude des isothermes de sorption d’un agromatériau à base de chanvre-amidon”.
- M. S. Abbas, F. McGregor, A. Fabbri, and M. Y. Ferroukhi. The use of pith in the formulation of lightweight bio-based composites: Impact on mechanical and hygrothermal properties. Constr. Build. Mater. 2020, 259, 120573. [Google Scholar] [CrossRef]
- M. S. Abbas, F. McGregor, A. Fabbri, and M. Y. Ferroukhi. The use of pith in the formulation of lightweight bio-based composites: Impact on mechanical and hygrothermal properties. Constr. Build. Mater. 2020, 259, 120573. [Google Scholar] [CrossRef]
- F. Bennai, N. Issaadi, K. Abahri, R. Belarbi, and A. Tahakourt. Experimental characterization of thermal and hygric properties of hemp concrete with consideration of the material age evolution. Heat Mass Transf. 2018, 54, 1189–1197. [Google Scholar] [CrossRef]
- N. Bhouri. Comportement thermodynamique et dimensionnel des matériaux textiles soumis à des variations des conditions climatiques. PhD thesis, Nancy 1, 2009. Available online: https://www.theses.fr/2009NAN10120 (accessed on 14 April 2023).
- Fabbri and, F. McGregor. Impact of the determination of the sorption-desorption curves on the prediction of the hemp concrete hygrothermal behaviour. Constr. Build. Mater. 2017, 157, 108–116. [Google Scholar] [CrossRef]
- L. Soudani. Modelling and experimental validation of the hygrothermal performances of earth as a building material. Ph.D. Thesis, Lyon, 2016. Available online: https://www.theses.fr/2016LYSET011 (accessed on 14 April 2023).
- F. Champiré. Étude expérimentale du comportement hydro-mécanique de la terre crue compactée pour la construction. Ph.D. Thesis, Lyon, 2017. Available online: https://www.theses.fr/2017LYSET007 (accessed on 14 April 2023).
- Z. Zhang. Modelling of sorption hysteresis and its effect on moisture transport within cementitious materials. Ph.D. Thesis, Université Paris-Est, 2014. Available online: https://theses.hal.science/tel-01127302 (accessed on 20 April 2023).
- Y. A. Oumeziane. Evaluation des performances hygrothermiques d’une paroi par simulation numérique : Application aux parois en béton de chanvre. Ph.D. Thesis, INSA de Rennes, 2013. Available online: https://theses.hal.science/tel-00871004 (accessed on 14 April 2023).
- H. Derluyn, D. Derome, J. Carmeliet, E. Stora, and R. Barbarulo. Hysteretic moisture behavior of concrete: Modeling and analysis. Cem. Concr. Res. 2012, 42, 1379–1388. [Google Scholar] [CrossRef]
- F. Cavillon. Caractérisation de la liaison hydrogène dans des systèmes moléculaires d’intérêt biologique par diffusion de neutrons. PhD thesis, Université des Sciences et Technologie de Lille—Lille I, 2004. Available online: https://theses.hal.science/tel-00286946 (accessed on 17 April 2023).
- T. Steiner and G. R. Desiraju. Distinction between the weak hydrogen bond and the van der Waals interaction. Chem. Commun. 1998, 891–892. [Google Scholar] [CrossRef]
- A. D. Buckingham, J. E. Del Bene, and S. A. C. McDowell. The hydrogen bond. Chem. Phys. Lett. 2008, 463, 1–10. [Google Scholar] [CrossRef]
- M. Spinu. Evaluation des paramètres physiques et physico-chimiques qui influencent l’accessibilité de la cellulose. Ph.D. Thesis, École Nationale Supérieure des Mines de Paris, 2010. Available online: https://pastel.archives-ouvertes.fr/pastel-00612881 (accessed on 17 April 2023).
- G. Mouchaham. Architectures supramoléculaires à structures ouvertes fondées sur la liaison hydrogène : Élaboration, caractérisation structurale et propriétés de sorption. Ph.D. Thesis, Toulouse 3, 2012. Available online: https://www.theses.fr/2012TOU30097 (accessed on 14 April 2023).
- S. K. Kannam, D. P. Oehme, M. S. Doblin, M. J. Gidley, A. Bacic, and M. T. Downton. Hydrogen bonds and twist in cellulose microfibrils. Carbohydr. Polym. 2017, 175, 433–439. [Google Scholar] [CrossRef]
- H. H. Ratsimbazafy. Évaluation du potentiel de co-produits agricoles locaux valorisables dans le domaine des matériaux de construction (PALOMAC). Ph.D. Thesis, Toulouse 3, 2022. Available online: https://www.theses.fr/2022TOU30005 (accessed on 1 October 2022).
- E. T. Engelund, L. G. Thygesen, S. Svensson, and C. A. S. Hill. A critical discussion of the physics of wood–water interactions. Wood Sci. Technol. 2013, 47, 141–161. [Google Scholar] [CrossRef]
- K. Gogoli. Contribution à l’étude des faisceaux de fibres de lin: Analyse des relations morphologie-comportement mécanique-ultrastructure.
| Type of bonding | Bonding energy [kJ.mol-1] |
|---|---|
| Covalent | ≈ 100 |
| Hydrogen | ≈ 10 |
| Van der Waals | ≈ 1 |
| Observation | Origin | Explanation |
|---|---|---|
| Sorption mechanism is reversible. | sorption-swelling coupling at molecular scale in vegetalaggregate | Hydrogen bonds form and break easily, even at ambient temperature, due to their low binding energy. |
| Hysteresis is more pronounced for plant-based concrete materials than for aggregates. | Because of additional origins of hysteresis in material than in aggregate. Effects are cumulative. | |
| Aging reduces the rate of adsorption and desorption. | Residual water masks “host sites“: they are no longer accessible, as inhibited by the first sorption phase. | |
| Swelling of the plant particles or fibers during hysteresis is irreversible. | Returning to a dry state allows the last physisorbed water to be extracted. The intercellulosic chains seem to return to their original state. In any case, there is no (or negligible) macroscopic manifestation of swelling. | |
| Hysteresis increases while crystallinity decreases | The more amorphous the cellulose is, the more important is sorption-swelling coupling. Interchain bonds cannot open in crystalline regions due to high stability. | |
| Swelling and shrinkage is observed at a young age or after a period of accelerated aging. | Swelling and shrinkage are possible as soon as HBCW replaces HBCC. This potential decreases with age (inhibited sites) but remains possible given the large number of host sites in the plant aggregate. | |
| Temperature dependence of sorption curves. | Hydrogen bonding is temperature dependent. | |
| Relevance of considering local sorption kinetics in bio-based materials, especially when coupled with hysteresis. | The opening/closing of the cellulose chains is probably a rather slow process that needs a kinetic factor to be taken into account, both in sorption and desorption phases. | |
| Swelling is observed between dry state and 80 % RH | The opening of the cellulose occurs from 5-10 % to 80 % RH ( cf. Figure 13) | |
| Water content is always higher in desorption than in sorption phase for the same relative humidity | More water molecules are physisorbed during desorption because they do not have the same chemical potential. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




