1. Introduction
Cancer is a complex and devastating illness that impacts millions of lives globally every year. Despite significant advances in cancer treatment, it remains the second leading cause of death worldwide [
1]. Phase IV clinical trials in oncology are conducted subsequent to a cancer medication or treatment being cleared for market release. The general objective of these trials is to monitor the safety and efficacy/effectiveness of the cancer treatment among a larger population and for an extended duration. Such trials are often more open and entail the accumulation of data from individuals often enrolled under less rigid eligibility criteria than phase III trials, with participation guided by the permissible indications and contra-indications of the drug in accordance with the package insert [
2]. Importantly, such studies aim to identify any latent and rarer safety concerns and focus on effectiveness, as opposed to strictly drug efficacy, characterized by less intensive monitoring and compliance, in line with real-world use of a drug/device in practice [
2].
Post-marketing surveillance (PMS) is the practice where marketed medications are monitored for potential adverse events (AEs) post-phase III clinical trials and may consist of active (randomized or observational phase IV studies, large data review) or passive (spontaneous reporting) processes [
3]. PMS studies may help to evaluate the safety and effectiveness of novel therapeutics more precisely and extensively than clinical trials, especially with respect to rare AEs [
4]. The safety profile of a novel therapeutic may not be able to be adequately investigated by the phase I-III trials due to various limitations, including small sample sizes, strict inclusion/exclusion criteria, less diverse populations, and their short duration [
5]. As such, rarer AEs or those occurring only in specific populations may often be missed or go unnoticed in earlier phases of development. In some situations, such studies may be mandated by regulatory agencies.
Importantly, roughly 20% of drugs received additional black box warnings during the post-marketing phase, while 4% of the drugs were eventually withdrawn due to safety concerns [
5]. PMS studies in oncology may provide more realistic results regarding the safety and effectiveness of novel cancer therapeutics as they take place in a more natural setting [
6]. However, there is a lack of information regarding the general characteristics, design, and attributes of phase IV clinical trials.
Clinicaltrials.gov is a database provided by the U.S. National Library of Medicine on behalf of the National Institutes of Health (NIH), which stores data concerning both privately and publicly funded clinical studies conducted around the world. It was first launched in February 2000 [
7], and starting in 2005, the International Committee of Medical Journal Editors made it mandatory for clinical trials to be registered before publication [
8]. Furthermore, under the Food and Drug Administration Amendments Act of 2007, sponsors or their representatives must register trials and report important data elements and basic trial results at ClinicalTrials.gov since 2007 [
9]. Therefore, the ClinicalTrials.gov database is widely regarded as the most comprehensive global source of clinical trial information [
5].
In the present investigation, we explored the general characteristics of registered phase IV oncology clinical trials in oncology by analyzing studies on Clinicaltrials.gov that were initiated over the last decade (2013-2022). The analysis included a thorough investigation of the various types of interventions examined by the phase IV clinical trials, the indications, their outcome measures, funding, study design, and more.
2. Materials and Methods
Design and Data Source
In this retrospective observational study, we investigated the characteristics of phase IV clinical trials in oncology that were registered with ClinicalTrials.gov with a start date during the timeframe of January 1, 2013, to December 31, 2022. To be eligible for inclusion in this analysis, a registered trial must be interventional and evaluate an anti-cancer drug or device or combination of drugs or drug/device in a defined population of cancer patients. Drugs without an anti-tumor effect, such as anesthetics or supportive therapies (ex., drugs for cytopenia), behavioral, diagnostic, prevention, or public health studies, were excluded. To facilitate our analysis, we obtained a dataset of phase IV clinical studies from ClinicalTrials.gov, which was downloaded following the search and selection process described below and secured from the website on 31 March 2023. Additionally, we designed a database to assist in our analysis, analogically to previous investigations of ClinicalTrials.gov [
5,
10,
11].
Study selection
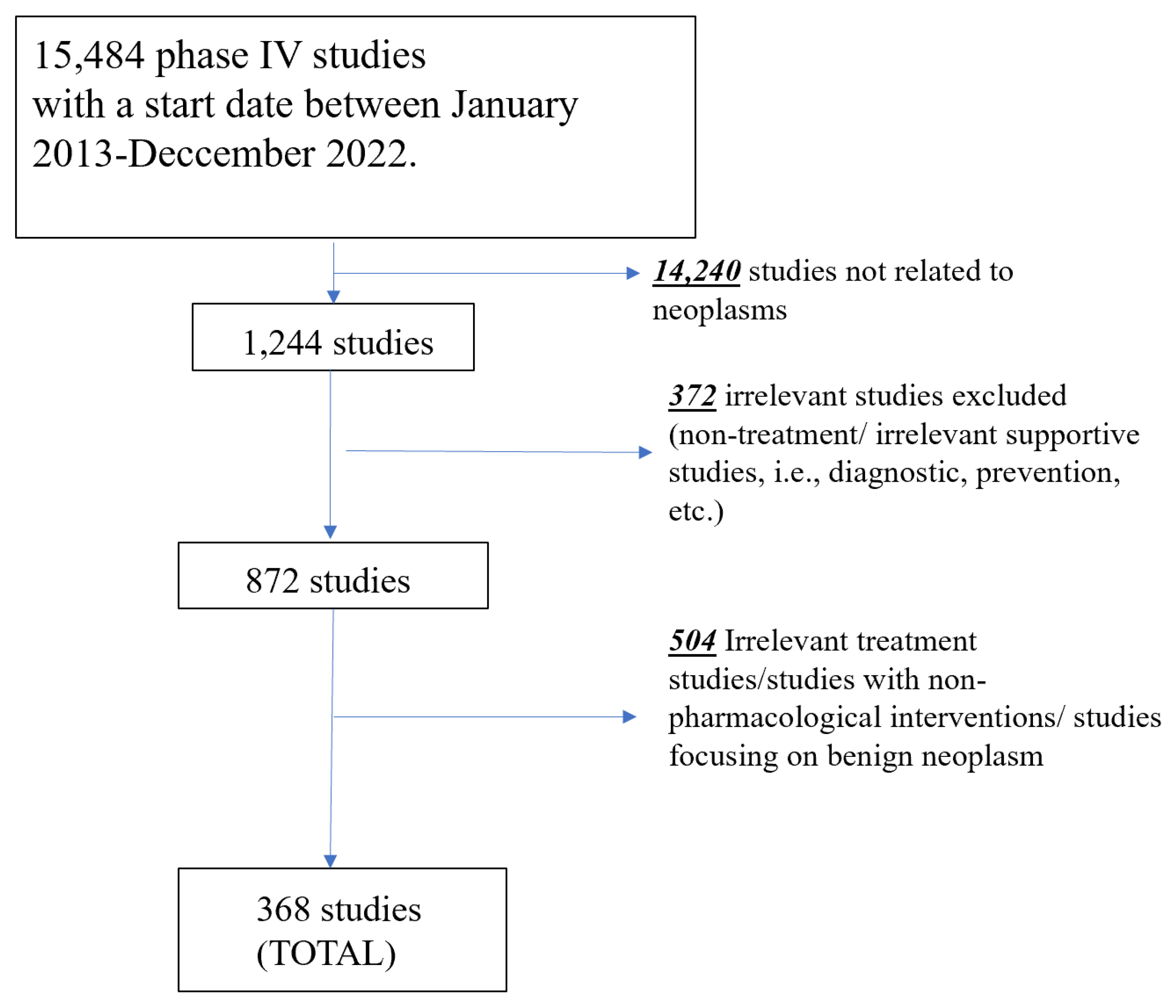
An initial search of ClinicalTrials.gov for phase IV studies was conducted with a start date between January 1, 2013, to December 31, 2022, registered by 31 March 2023. The study selection process is presented in
Figure 1. A total of 15,484 phase IV studies were initially identified. Following the use of a filter term, “neoplasms” (which includes terms “cancer” and “tumor”), in line with the terminology of the registry topic selection filter, 14,240 studies were excluded, leaving 1,244 potentially eligible studies. Next, studies were excluded by their primary purpose, which can be found in the “study design” section in ClinicalTrials.gov. All non-treatment studies (i.e., prevention, diagnostic, supportive care, health services, basic science) were excluded (n=372). Next, the remaining 872 studies were reviewed independently by two authors (PO, AN to exclude all studies focusing on non-oncological pathologies (i.e., rheumatoid arthritis, etc.) that were not excluded using the above filters and studies focused on broad cohorts with various indications (not specific to cancer patients). Moreover, if studies were not exclusive to or strictly focused on therapeutic agents (defined as drugs or devices) as a treatment for cancer or were non-pharmacologic studies (surgery or radiotherapy), they were excluded as per the a priori eligibility criteria (n=504). After the eligibility review process, a total of 368 studies remained eligible and were included in this investigation.
Data collection
The ClinicalTrials.gov registry requires the sponsors or investigators of a clinical trial to report key trial data, which should include a set of data elements describing the study's conditions, study design, sponsor, location, enrollment, eligibility criteria, and other protocol information. This data, which describes the general characteristics of the included studies, was extracted and analyzed. The said data included dates of the study, study design, outcome measures, cancer indication, age, sex, size of the cohorts, funding, location, and use of a data monitoring committee (DMC).
The extracted characteristics of the clinical trials were later divided into more specific categories by a team of authors (PO, AN), with selected records checked for agreement by a third author (BMH). The outcome measures were divided into three categories; (1) safety, (2) efficacy, and (3) efficacy and safety in accordance with the outcome measures recorded in the registry record. Due to limited data available, we did not attempt to distinguish between efficacy and effectiveness, thus using the term efficacy henceforth. The interventions were classified by type of anti-cancer therapeutics: (1), chemotherapy, which included alkylating agents, antimetabolites, antimicrotubule agents (mitotic inhibitors), topoisomerase inhibitors; and miscellaneous antineoplastics, (2) immunotherapy, which included cytokines, vaccine therapies, immunomodulatory drugs, and differentiating agents, (3) hormonal therapy, which consisted of antiestrogens, antiandrogens, aromatase inhibitors, luteinizing hormone-releasing hormone agonists, androgen biosynthesis inhibitors, androgens, corticosteroids, somatostatin analogs, and more, (4) targeted therapy, which included monoclonal antibodies, kinase inhibitors (targeting anaplastic lymphoma kinase, BRAF serine-threonine kinase, amongst others), proteasome inhibitors, mammalian target of rapamycin (MTORS), and more, (5) other anti-cancer therapy, (6 ) herbal/traditional medicine, (7) devices, (8) other therapies. The indication for treatment (i.e., type(s) of cancers) was also extracted and analyzed according to system organ class. The included studies were also classified into sample size groups by total number of participants: (1) <300, (2) 300-599, (3) 600-2999, (4) ≥ 3000, and (5) missing. Same-size groups were determined based on the fact that per binomial and Poisson distributions, enrolment must be greater than 300, 600, or 3000 to have a 95% chance of observing at least one adverse event (AEs) with a probability of occurrence of 1%, 0.5%, or 0.1%, respectively (
Table 1) [
5,
10].
In cases where a data field was incomplete (such as in the case of missing data concerning the allocation of the trial), we conducted a manual search (ClinicalTrials.gov) to attempt to retrieve the missing information. If the information was still unavailable on the website, we labeled the field as NA (not applicable) or missing. If a study presented an interventional model involving a single group and did not provide information on allocation and blinding, we made the assumption that allocation was non-randomized and blinding was open. Nonetheless, if the single-arm studies were registered as randomized or blind, allocation or blinding was reported as "Missing/Uncertain/N.A" [
5].
Statistical Analysis
Results were analyzed using descriptive statistics, with categorical data reported as absolute number (n) and relative frequency (%) and continuous variables reported as the median and interquartile range (IQR). Differences in frequencies of a variable between studies grouped by outcome measures were compared using the Chi-square test. Statistical analyses were carried out with Prism 8 (GraphPad Software, San Diego, CA, USA) with statistical significance set at p < 0.05.
This study was conducted in compliance with the Declaration of Helsinki and within the terms of local legislation. No ethical committee approval was required for performing this retrospective analysis of publicly available clinical trial registry data.
3. Results
A total of 368 phase IV oncology studies started between January 2013-December 2022 were included in our analysis, 35.9% of which were currently reported as active as of March 31, 2023.
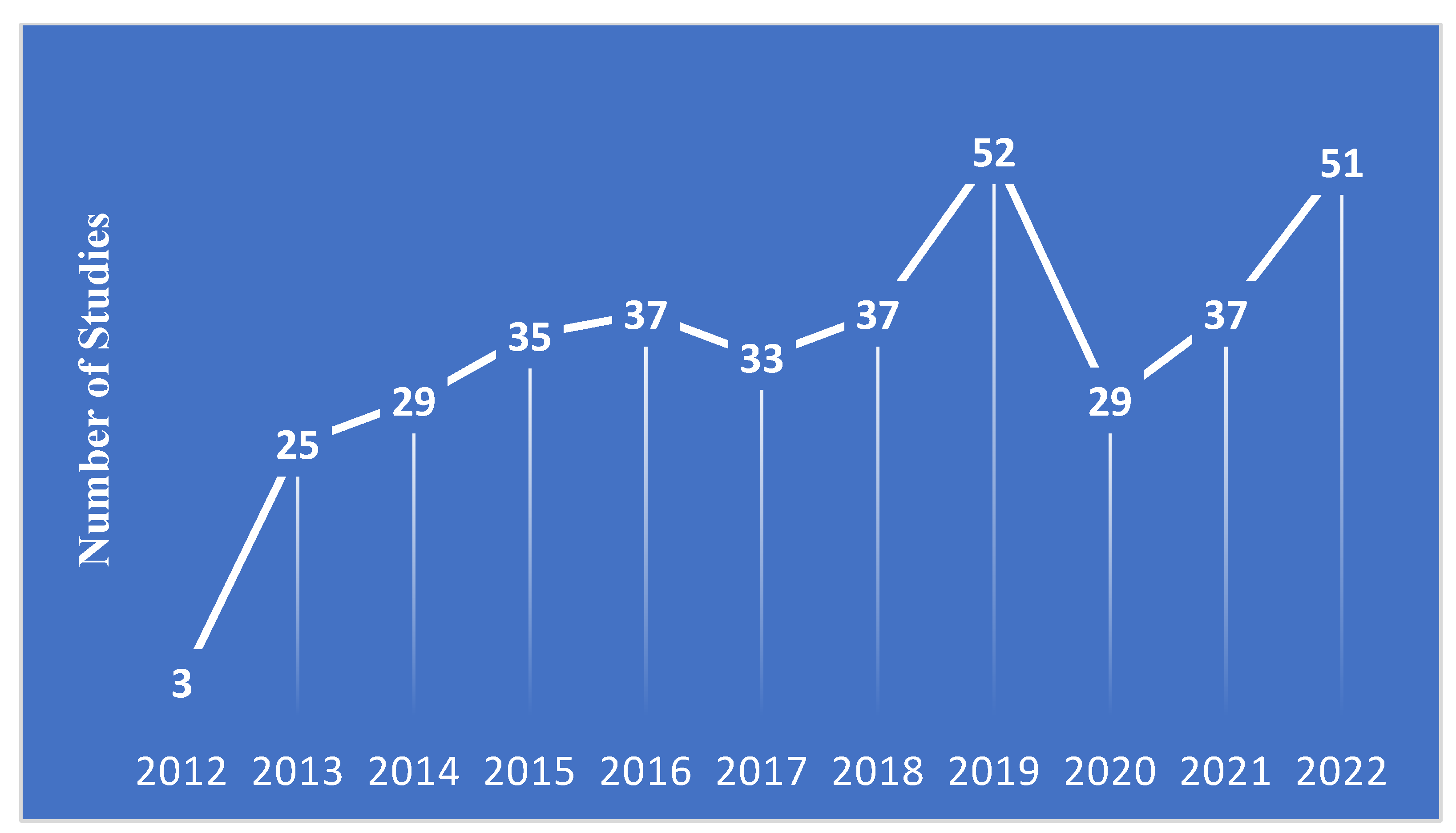
Figure 2 shows the number of studies in the registry by start year. The full characteristics of the included studies are presented in
Table 2. Among the included studies, only 19.8% were limited to a single-sex. The majority of studies (86.1%) did not include children (<18 years), while only 6.8% excluded elderly (>65 years) participants. Most of the included phase IV studies were focused on solid tumors (71.2%).
The sample size of included studies ranged from 0 to 5000. The majority of included studies were small in size, with a median of 90 (IQR: 40 – 200), with 83.2% of studies consisting of <300 participants. Only one study (0.3%) was powered to detect AEs occurring at a rate of 0.1%, while only 21 (5.7%) and 32 (8.7%) studies were powered to detect Aes occurring at a rate of 0.5% and 1%, respectively.
With respect to trial design, single-group assignment accounted for 50.0% of studies, while parallel assignment accounted for 47.0% of studies. All but 8.7% of included studies were open-label. The majority of studies were funded by industry (37.3%), followed closely by universities (or a similar institution) (34.5%) and hospitals (or a similar institution) (30.7%). Nearly half of all studies employed a Data Monitoring Committee (DMC; 49.5%). Over one-third of studies (39.4%) had started before submission and registration on ClincialTrials.gov.
Exploring geographical distribution, a large majority of studies were conducted in Asia and Pacific regions (52.72%), which participated in more of the included studies than the next two largest regions, North America (17.9%) and Europe (17.1%) combined. Limited studies were performed in Africa (1.6%), Central and South America (3.3%), and the Middle East (1.4%).
Looking at the reported outcome measures, exactly half of the studies looked at both safety and efficacy, whilst 43.5% reported only efficacy outcome measures and 6.5% reported only safety outcome measures. The majority of safety-only studies were small in size, with 75% having <300 participants and thus not powered to detect Aes occurring at a rate of 1 in 100 participants with 95% confidence. Safety studies were not found to frequently have larger sample sizes (i.e.,>300 participants) compared to efficacy-only studies (25.0% vs. 13.4%; p=0.178). No statistically significant differences were observed between safety-only vs. efficacy-only studies (p>0.05 for all comparisons, data not shown).
Table 3 shows the characteristics of studies by type of pharmacologic therapy. Targeted therapies (53.5%) accounted for the majority of included studies, followed by chemotherapy (43.0%) and hormonal therapies (17.7%), and immunotherapies (6.0%). Only 14 (3.8%) studies utilized herbal/traditional medications, and 2 (0.5%) studies utilized devices. Key study characteristics were similar between types of pharmacologic therapy. Unsurprisingly, trials using hormonal therapies more frequently included only a single-sex (49.2%) compared to other types of interventions (0-20.3%). Of note, 14 (3.8%) trials combined a drug with surgical or radiotherapy intervention, while 64 (17.4%) trials were drug-drug combination studies.
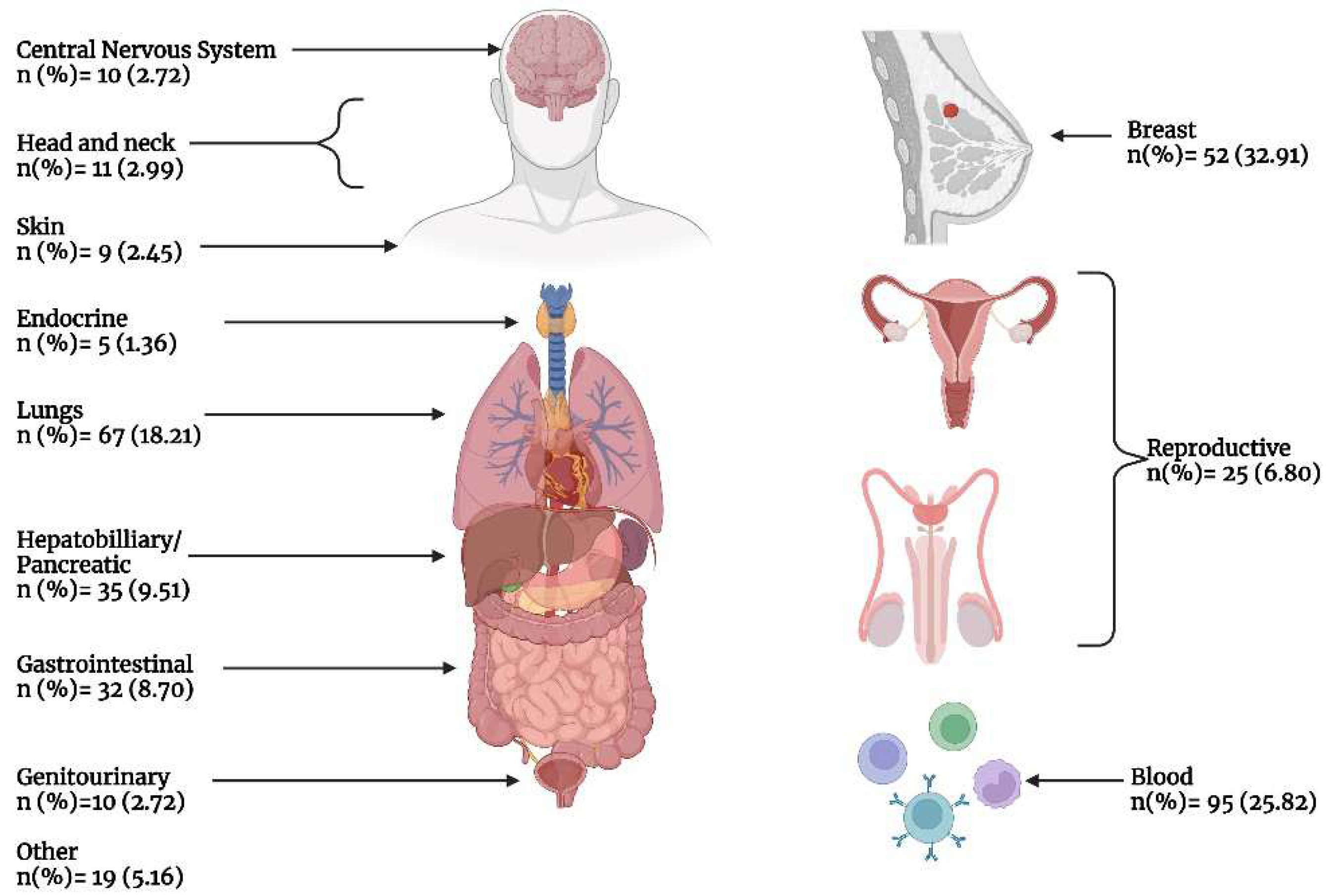
Figure 3 presents the number of included phase IV studies by primary cancer indication. Breast cancer accounted for nearly one-third (32.9%) of all studies, while hematologic malignancies accounted for slightly over a quarter (25.8%) of all studies, and lung cancer accounted for 18.21% of all studies. All other cancers accounted for <10% of all studies.
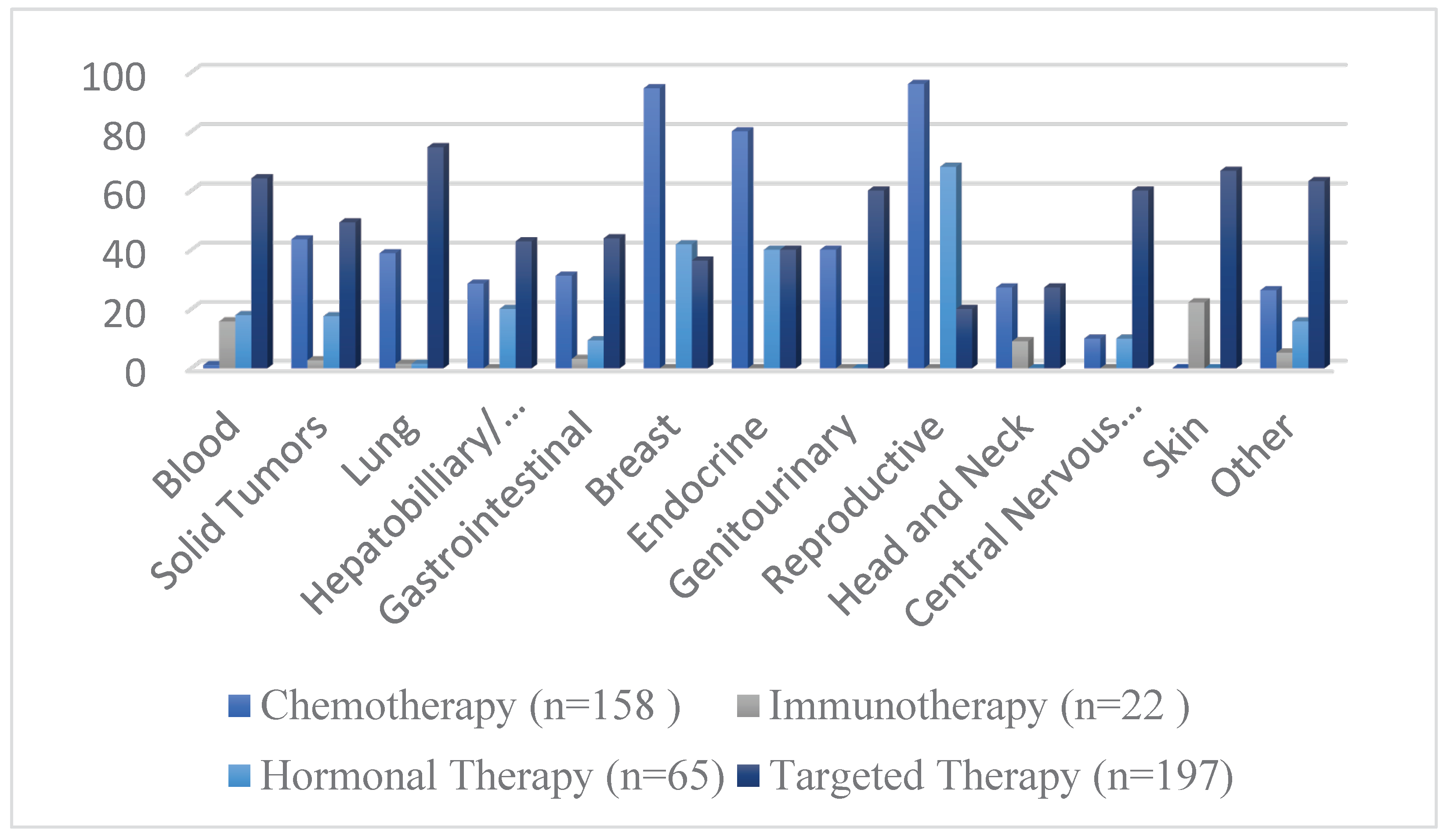
Figure 4 demonstrated the frequency of different interventions by cancer indication, while the distribution of intervention by cancer type is presented in
Table 4.
4. Discussion
The present study aimed to demonstrate the characteristics of phase IV clinical trials in oncology over the last decade, using data obtained from the ClinicalTrials.gov registry. This analysis examined various aspects of the trials, including their outcome measures, interventions, sample sizes, and study design. The study also looked at the distribution of phase IV trials across different cancer types and geographic regions. By analyzing these factors, this study aimed to identify areas of strengths and weaknesses in design and implementation of phase IV trials, with the ultimate goal of improving the quality and effectiveness of these trials in the future.
The present retrospective observational analysis included a total of 368 phase IV oncology studies. A recent analysis conducted by Zhang et al. [
5] found 251 phase IV studies between 2004-2014 using similar parameters as the presented study (in congruence with recent advances in oncology, especially targeted therapies in the last decade). The rapid rise in new anti-cancer therapeutics, such as immunotherapies and targeted therapies, necessitates the need for more phase IV clinical trials to be conducted for proper post-market safety control [
12]. Olivier et al. [
12] conducted a cross-sectional study where they analyzed the approval rate of anticancer drugs based on their mechanism of action. Their analysis included a total of 332 approvals, and between 2009 and 2020, there was an increase in the total number of approvals from 8 to 57. Furthermore, according to their findings, when considering all tumor types combined, 16% of drug approvals were based on a novel mechanism of action. However, when looking at each individual tumor type separately, the percentage of drug approvals based on a new mechanism of action increased to 37%. However, our analysis demonstrates that despite the rapid rise in approved therapeutics, post-market safety remains dependent on passive processes, including spontaneous surveillance systems, such as FDA adverse event reporting system (FAERS). However, these spontaneous reporting programs, unfortunately, encounter numerous limitations in detecting AEs, including poor health significant under-reporting, limited participation, incomplete reports, and bias (i.e., Weber Effect), and thus are likely underutilized, hampering the full assessment of patient safety [
6]. While some of this may be overcome in the future with utilization of big data approaches (such as analysis of electronic medical records), in order to fully address the problem of under-reporting, healthcare providers need to be educated and trained on pharmacovigilance and adverse drug reaction (ADR) reporting systems. Simple and accessible channels for reporting ADRs should be established, such as phone hotlines and user-friendly computer tools designed for reporting ADRs. This will encourage health care providers to report any ADRs they encounter during their busy work schedules. Pharmacovigilance centers can incentivize healthcare providers to report by recognizing their efforts, providing feedback on reported cases or pharmacovigilance activities, and offering support, such as clinical advice [
13]. Moreover, further patient education and participation in the spontaneous reporting processes should be encouraged.
The most frequently analyzed oncological interventions in the last decade were therapeutics regarded as targeted therapy (53.5%), chemotherapy (42.9%), hormonal therapy (17.6%), and immunotherapy (5.9%). Moreover, the characteristics of the study design regarding these interventions were also investigated. Unsurprisingly, the majority of the aforementioned anti-cancer therapies were open-labeled trials (94.4%, 92.5%, 86.1%, and 95.4%, respectively) (
Table 3). The intervention model varied considerably between these therapeutic interventions. Our study shows that the parallel arm design was the most frequently used approach in clinical trials investigating chemotherapeutic (59.4%) and immunotherapeutic agents (45.4%). However, the single-group assignment was the most commonly utilized design in both hormonal (50.7%) and targeted therapies (63.4%). The studies which investigated chemotherapeutic treatments used most commonly subject groups with breast (32.9%) or lung (16.4%) cancer. Moreover, the hormonal therapies were mostly investigated in cohorts with breast (35.3%), reproductive (26.1%), and blood cancers (26.1%). Targeted therapies and immunotherapies were most frequently investigated in patients with blood cancers (30.9% and 68.1%, respectively).
The size of the sample used in a clinical trial is tightly related to the quality of safety surveillance of the investigated intervention [
5]. Phase IV trials of a smaller scale can be employed to assess the efficacy of a specific medication within a particular subset of patients or in unique circumstances [
5,
14]. However, there are various concerns regarding phase IV clinical trials with smaller sample sizes, particularly due to the reduced safety surveillance of the investigated intervention. The majority of the studies analyzed in our work had a low sample size, i.e., ≤299 (83.1%). Unfortunately, the majority of the said studies still had a sample size below 300 subjects (75.0%). Our results demonstrate that oncological phase IV clinical trials in the last decade have not been appropriate for safety surveillance, especially for detecting rare AEs.
Interestingly, the lead sponsor of the analyzed studies varied significantly. The majority of the studies were sponsored by either an industry-related sponsor (37.2%), a hospital (or a similar institution) (30.7%), or a university (or a similar institution) (34.5%). The presence of DMC was also investigated in the present study. Our results show that the DMC was reported in 49.4% of the clinical trials. However, it was absent in almost one-third of studies (29.1%) and not reported or missing in 21.4%. It is clear that taking advantage of DMC, even in open-label clinical trials, can help to monitor their safety, as well as assess their overall risks and benefits. Our results show that a considerable amount of clinical trials (39.4%) were started before being submitted to ClinicalTrials.gov. This could be indicative of potential bias. ClinicalTrials.gov maintains a log of any changes to the registration of a study. Thus, changes in design or study characteristics can be tracked. If a study starts before registration, there is a potential for bias, as changes could have been implemented after the study started, but these would not be captured in the registry.
Our analysis demonstrates that the majority of the clinical trials were conducted in Asia and the Pacific region (52.7%). This was also demonstrated by other studies in the past [
5]. However, a very limited amount of clinical trials were conducted in Africa (1.6%) and the Middle East (1.3%). This is a major limitation in post-market surveillance studies, as we may not gather a sufficient understanding of how these drugs work in other diverse populations. Hence, it is incredibly important to include more countries from these regions in future studies. The overall status of the clinical trials included in the present analysis was also investigated. It was established that 25.2% of the studies were still recruiting subjects, and 20.1% were completed. Interestingly, 5.4% of the clinical trials were terminated, which is higher than what was reported in a previous study conducted by Califf et al. [
10] (3.3%). However, this was lower than what the more recent analysis conducted by Zhang et al. [
5] reported (8.6%).
The present study has various limitations that need to be addressed. There is limited available data in the registry, and not all phase IV studies are registered. Hence, some ongoing clinical trials may not have been included in the present analysis. Furthermore, there is a high amount of missing/uncertain data; the sponsors/investigators should be further encouraged to fully complete all fields of the registration form. However, we made a manual screening of all the studied records; this helped address some missing data issues and improved the overall quality of our report. Lastly, as earlier mentioned, almost 1/3 of studies started before being registered in ClinicalTrials.gov; this can create a bias in both the results of the said clinical trials as well as our analysis of study attributes.
5. Conclusions
Our analysis demonstrated that targeted therapies (53.53%) account for the majority of all recent phase IV oncology studies, showing a rapid development of this field. Furthermore, the number of phase IV clinical trials has increased significantly over previous analyses. However, the clinical trials included in the present investigation were dominated by small sample sizes, with most of the studies enrolling less than 300 individuals (83.15%). Hence, most of the phase IV studies are not sufficiently powered to detect rare AEs and instead are focused on efficacy/effectiveness. Due to the limited number of ongoing safety studies, there is a significant requirement for additional education on and participation by both healthcare providers and patients in spontaneous reporting processes, thus ensuring that there is no gap in clinical safety data collection due to limited phase IV clinical trials. This would be especially true with respect to rare adverse events, which may not have been identified in clinical development and only observed when the drug is utilized in a broader population in routine clinical practice.
Author Contributions
Conceptualization, B.M.H., G.L., P.O. ; methodology, B.M.H., P.O..; software, B.M.H., A.N..; validation, B.M.H..; formal analysis, B.M.H., A.N., P.O.; investigation, B.M.H., A.N., P.O.; resources, B.M.H., A.N., P.O;.; data curation, B.M.H., A.N. P.O.; writing—original draft preparation, B.M.H., P.O.; writing—review and editing, B.M.H., P.O.; visualization, B.M.H., P.O. ; supervision, B.M.H., G.L.; project administration, B.M.H.; funding acquisition, Not Applicable. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used in this study is freely available from ClinicalTrials.gov.
Acknowledgments
Not Applicable.
Conflicts of Interest
The authors declare no conflict of interest. BMH is an employee of Cincinnati Children’s Hospital Medical Center and Cmed Research Inc, and a substantive patron of Youthoria, a not-for-profit Youth Research Organization.
References
- xu J, Murphy S, Kochanek K, Arias E. Mortality in the United States, 2021. Atlanta, Georgia; 2022 Dec.
- Suvarna, V. Phase IV of Drug Development. Perspect Clin Res. 2010;1:57–60.
- Vlahović-Palčevski V, Mentzer D. Postmarketing Surveillance. 2011. p. 339–51.
- Pierce CE, Bouri K, Pamer C, Proestel S, Rodriguez HW, Van Le H, et al. Evaluation of Facebook and Twitter Monitoring to Detect Safety Signals for Medical Products: An Analysis of Recent FDA Safety Alerts. Drug Saf. 2017;40:317–31. [CrossRef]
- Zhang X, Zhang Y, Ye X, Guo X, Zhang T, He J. Overview of phase IV clinical trials for postmarket drug safety surveillance: a status report from the ClinicalTrials.gov registry. BMJ Open. 2016;6:e010643. [CrossRef]
- Alomar M, Tawfiq AM, Hassan N, Palaian S. Post marketing surveillance of suspected adverse drug reactions through spontaneous reporting: current status, challenges and the future. Ther Adv Drug Saf. 2020;11:204209862093859. [CrossRef]
- Gillen JE, Tse T, Ide NC, McCray AT. Design, implementation and management of a web-based data entry system for ClinicalTrials.gov. Stud Health Technol Inform. 2004;107:1466–70. [CrossRef]
- Laine C, Horton R, DeAngelis CD, Drazen JM, Frizelle FA, Godlee F, et al. Clinical Trial Registration — Looking Back and Moving Ahead. New England Journal of Medicine. 2007;356:2734–6. [CrossRef]
- Zarin DA, Tse T. Moving Toward Transparency of Clinical Trials. Science (1979). 2008;319:1340–2. [CrossRef]
- Califf, RM. Characteristics of Clinical Trials Registered in ClinicalTrials.gov, 2007-2010. JAMA. 2012;307:1838. [CrossRef]
- Tasneem A, Aberle L, Ananth H, Chakraborty S, Chiswell K, McCourt BJ, et al. The Database for Aggregate Analysis of ClinicalTrials.gov (AACT) and Subsequent Regrouping by Clinical Specialty. PLoS One. 2012;7:e33677. [CrossRef]
- Olivier T, Haslam A, Prasad V. Anticancer Drugs Approved by the US Food and Drug Administration From 2009 to 2020 According to Their Mechanism of Action. JAMA Netw Open [Internet]. American Medical Association; 2021 [cited 2023 Apr 30];4. Available from: /pmc/articles/PMC8672232/.
- Hasford J, Goettler M, Munter KH, Müller-Oerlinghausen B. Physicians’ knowledge and attitudes regarding the spontaneous reporting system for adverse drug reactions. J Clin Epidemiol [Internet]. Elsevier; 2002 [cited 2023 Apr 30];55:945–50. Available from: http://www.jclinepi.com/article/S089543560200450X/fulltext.
- Gale EAM. Post-marketing studies of new insulins: sales or science? BMJ. 2012;344:e3974–e3974. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).