1. Introduction
It has been recognized early on during the corona virus infectious disease 2019 (COVID-19) pandemic that comorbidities such as hypertension and cardiovascular disease present major risk factors for severe illness and fatal outcome. Two facts directed the spotlight of COVID-19 research to the renin-angiotensin-system (RAS): 1) It is critical to retain blood pressure homeostasis, and according to experimental and clinical data, SARS-CoV-2 infection promotes a rise in blood pressure during the acute phase of infection [
1]. 2) The RAS’s counter-regulatory process involves angiotensin-converting enzyme 2 (ACE2), the functional receptor of severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) [
2,
3].
In earlier work, we have been studying RAS components in sera of 45 hospitalized (HoP) and 26 covalescent (CoP) COVID-19 patients from our clinic with both a bradykinin (BK) degradation assay [
4] and high-definition mass spectrometry (HDMS)-based expression analysis (see publications for details [
5,
6]). BK is a target of angiotensin-converting enzyme (ACE), a critical enzyme of the RAS, and carboxypeptidase N (CPN) a member of the kallikrein-kinin system (KKS) [
7,
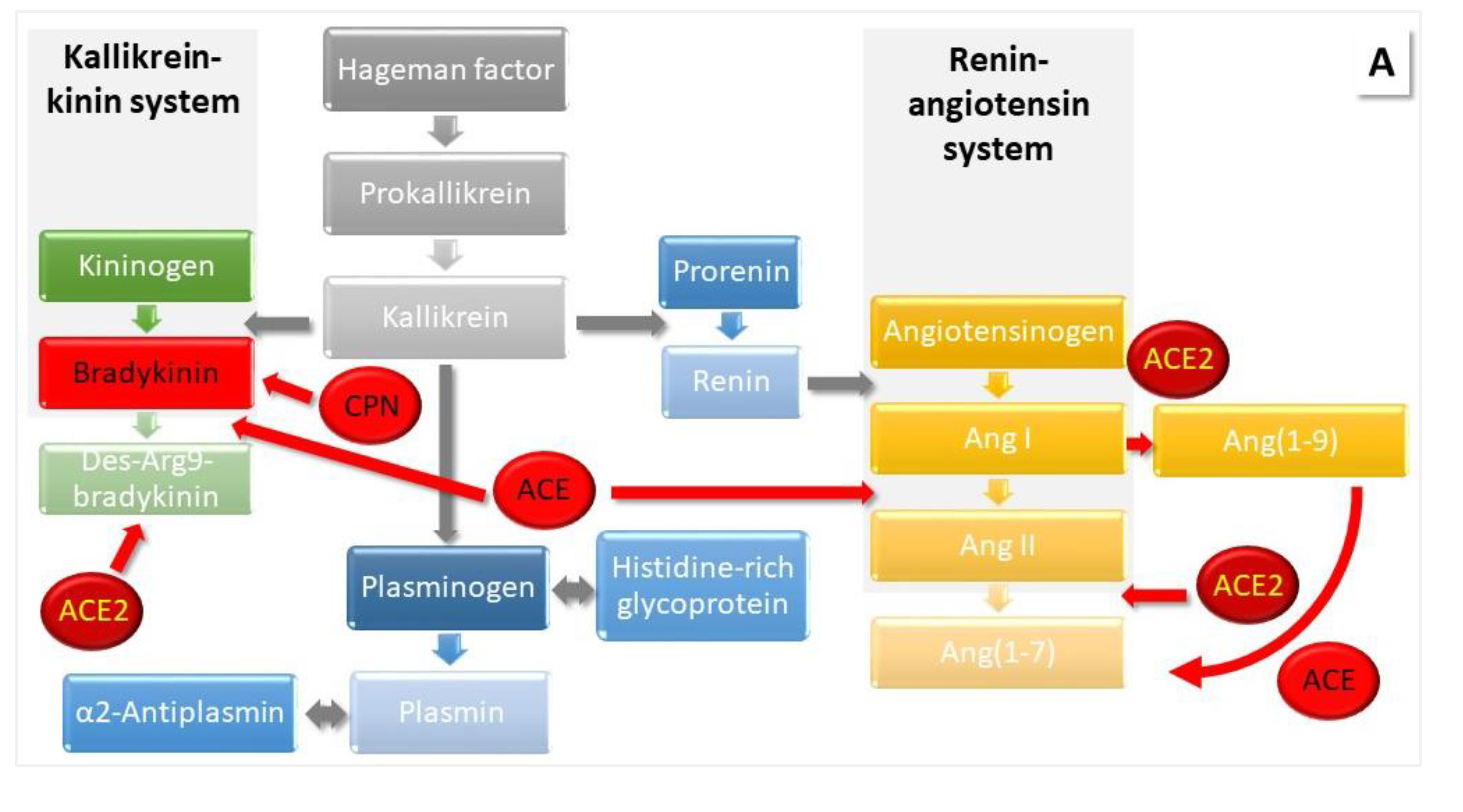
8]. RAS and KKS are interconnected by ACE activity (
Figure 1). We found that the degradation of the labelled form of BK (dabsylated BK – DBK) was generally impaired in HoP. CPN activity was significantly reduced, and the DBK cleavage product generated by ACE, fragment 1-5 (DBK1-5), was significantly increased in critically ill patients and strongly correlated with clinical heart and liver parameters [
5]. Experimental values returned to normal during convalescence in the majority of patients (CoP-NV group), but 10 of the 26 persons showed partially similar results as measured in HoP (CoP-DV group). This result had us wonder if it could be an indication for Long COVID. However, at the time of our experiments, persisting post-COVID-19 symptoms were not fully appreciated.
HDMS expression analysis of undepleted patient sera revealed significantly different protein profiles of HoP and CoP patients and healthy probands as expected, but it also showed distinct subgroups in HoP [
6]. This cohort could be divided into six groups according to the concentration of their most abundant serum proteins as visualized in the heatmap. The protein profiles distinguished patients of different disease severity and pathophysiology. Thereby, group G1 represented the youngest and the least ill patient population, while G6 collected the critically ill patients, who were, on average, older than 55 and overweight. The groups could be arranged according to disease severity; a correlation with age, body mass index (BMI), SAPS II (Simplified Acute Physiology Score [
9]), and the number of deviating-from-normal laboratory parameters was then evident. The protein profiles indicated at least two major pathophysiological schemes in the groups differing in KKS/RAS activity and possibly defects in the complement alternative pathway [
6]. Likely, also seroconversion played a role, because other authors have demonstrated that it stages COVID-19 into distinct pathological states [
10] and it would influence the serum proteome. Gender was not critical in our study which consisted mostly of male probands [
5,
6], but, of note, estrogen has been shown to inhibit inflammation and immune response in COVID-19, which may be one reason for the fact that males have been more affected by severe COVID-19 progression than females [
11].
A clear distinction according to co-morbidities was not observed among the HoP sub-groups. In fact, hypertensive and antihypertensiva-taking patients were distributed across all groups. Incidently, we noticed that none of the hospitalized patients taking ACE inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) died in contrast to half of the untreated hypertonic patients [
5]. Indeed, studies have been published which provided evidence that ACEI/ARB medication is not enhancing COVID-19 symptoms and that it can even be protective [
2,
3]. As our study had not been designed to evaluate the influence of antihypertensiva and we did not have suitable patient cohorts for the purpose, no significant results were obtained in that regard. Nevertheless, we have followed the research into the topic with great interest, because this medication also has an impact on the results of our BK reporter assay [
4].
Below, we briefly summarize the current knowledge about the RAS in the light of our own findings. We have re-evaluated the data from our study in the context of recent information regarding the so-called Long COVID syndrome [
12] and demonstrate that our assay may have diagnostic potential in that respect.
1.1. The RAS
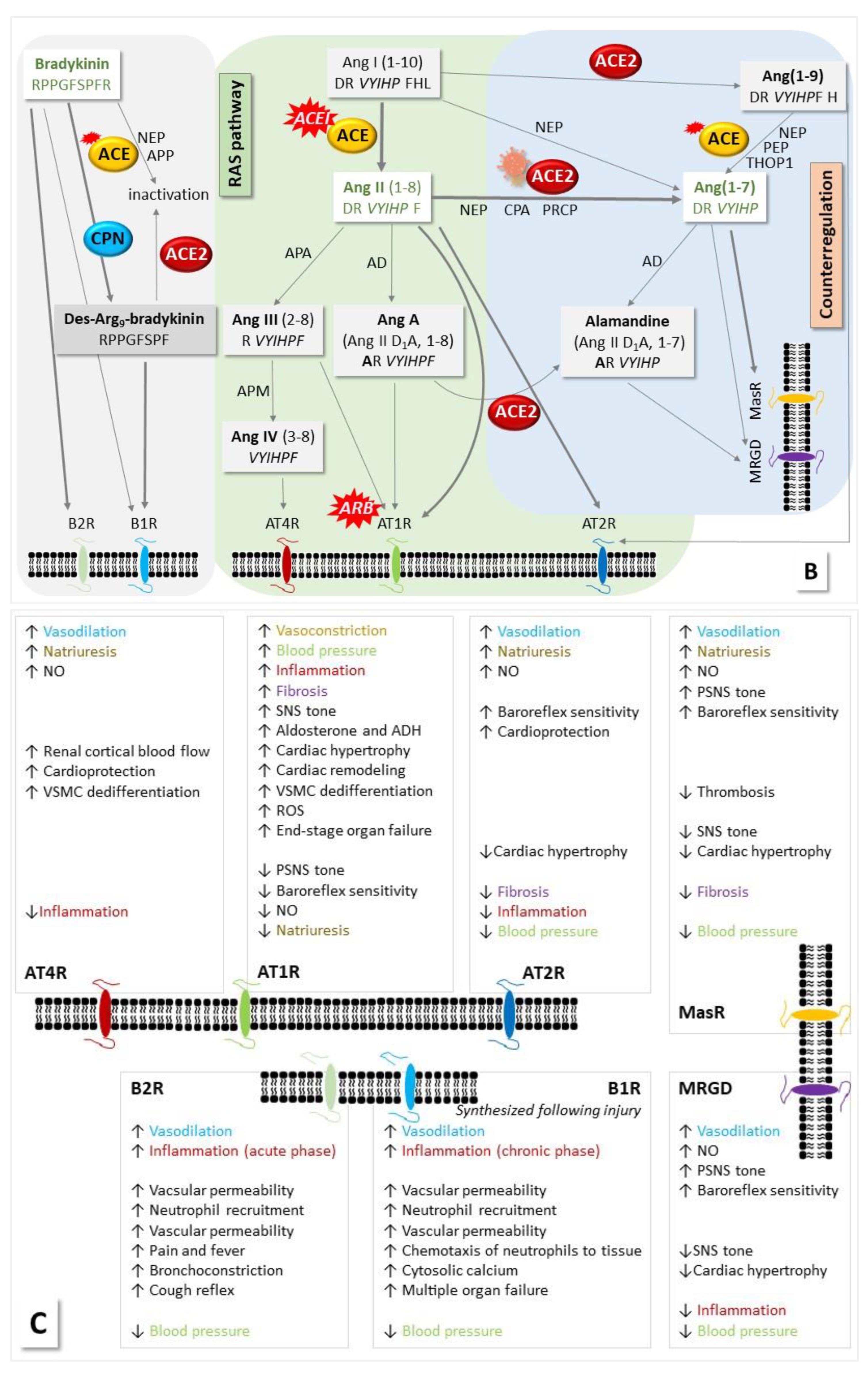
The main product of the classical RAS is angiotensin (Ang) II, which is formed from angiotensinogen (AGT) with the assistance of renin and ACE. Binding of Ang II to its receptors leads to effects such as elevated blood pressure and disturbed renal water sodium balance. The counter-regulatory arm of the RAS involves ACE2, which cleaves Ang II and generates products such as Ang(1-9), Ang(1-7) and alamandine with opposite effects to those of Ang II. ACE2 also serves as virus entrance during SARS-CoV-2 infection leading to dysregulation. Another counterbalance to the RAS is the KKS, in which BK production contributes to blood pressure decreasing effects [
7,
8]. BK is inactivated by ACE [
15]. CPN also degrades BK to des-Arg9-BK, which, in turn, can be deactivated by ACE2 [
16]. We present as a quick overview and a graphic backdrop to the discussion below a schematic highlighting the interconnection of the KKS and the RAS in
Figure 1 (for recent reviews, see [
2,
3,
13,
14]).
Considering these main processes involving the inverse relationship between ACE and ACE2, the use of ACEIs/ARBs has been proposed to counteract the inflammatory effects of COVID-19 infection by shifting metabolic activity away from the AT1 receptor pathway toward the AT2R/MasR pathways, but these inhibitors may potentially also increase ACE2 activity [
17]. The corresponding pathophysiological processes upon viral infection are far from clear, yet. Higher levels of ACE2 may increase susceptibility to COVID-19 by allowing more virus into cells, but having more ACE2 could also be organ-protective [
18].
1.2. ACEI/ARB and COVID-19
Both ACE2 and the RAS are involved in SARS-CoV-2 infection and many papers appeared suggesting that an imbalanced RAS may be associated with severe COVID-19 progression (see references [
2,
3,
20,
21,
22] and an extensive overview of scientific results up to the year 2021 in the Supplement to reference [
5]). There were controversial discussions in the general public early on concerning potential dangers in the use of antihypertensive medication in COVID-19 [
20,
23] possibly due to initial worries resulting from studies which associated higher in-hospital mortality or a higher risk of hospital admission with the use of ACEIs/ARBs [
24,
25,
26]. However, by now, there is consensus that ACEI/ARB treatment should be continued in the presence of SARS-CoV-2 infection [
2,
3,
17,
24]. Retrospective analyses found no evidence for a positive correlation of the use of ACEIs/ARBs with COVID-19 severity [
27] and some even reported an apparent reduction in the risk of mortality [
21,
28]. A review of 7 938 123 health records from the Providence Health System (2020-2021) [
17] detected an association between a reduced risk of COVID-19 infection/complications and ACEI/ARB use. Another study on hypertensive patients specified a connection of the use of ACEIs/ARBs prior to intensive care and a reduced risk of in-hospital mortality, but measured a prolonged hospital stay [
29]. A 2023 meta-analysis among East-Asian patients reported reduced mortality and, in contrast, a shorter duration of hospital stay especially for females [
30]. Authors of a meta-analysis involving 1 351 633 hypertensive people also associated the use of ACEIs/ARBs with a lower risk of hospitalisation, intubation or death, but they did raise serious concerns about quality and bias of clinical studies regarding COVID-19 patients [
31].
Meanwhile, single-cell sequencing of airway samples showed that patients with hypertension had a distinct inflammatory predisposition of immune cells that correlated with critical COVID-19 progression [
32]. The study found that both ACEIs and ARBs reduced the risk for severe illness, but ACEIs much more than ARBs: ACEI treatment seemed to dampen hyperinflammation and increase cell intrinsic antiviral responses and almost abolished the risk of severe disease. ARBs, however, only slightly reduced it. The authors explained their observations with the decrease of Ang(1-7) upon SARS-CoV-2 infection, which could be restored by ACEI, but not ARB treatment [
32] (for inhibitor points-of-attack within the RAS, see
Figure 1B). The notion that ARB might be superior to ACEI for treatment of hypertensive COVID-19 patients was based on the ideas that ACEIs do not inhibit non-ACE-mediated Ang II production, that ACE-induced BK may instigate acute respiratory disease syndrome, and that ARB alleviates sputa production and inflammation and attenuates lung fibrosis [
15,
33], has thus been disproven experimentally. In summary, a substantial amount of COVID-19 research is currently directed towards the counterregulatory RAS and in particular the Ang(1-7)-MasR axis, intellectual property has been filed and several clinical trials are underway [
2,
3,
34].
1.3. Long COVID
It became apparent during the pandemic that some patients did not recover well from the disease. In fact, at least 10% of SARS-CoV-2 infections continued with symptoms on multiple organs (heart, lung, immune system, pancreas, gastrointestinal tract, neurological system, kidney, spleen, liver, blood vessels, reproductive system, skin) with an immense impact both on personal wellbeing and the healthcare system [
35]. Through machine learning analysis of over 137 symptoms and conditions from electronic health record data from the national Patient-Centered Clinical Research Network, four subphenotypes were identified involving cardiac and renal (~33%); respiratory, sleep and anxiety (~33%); musculoskeletal and nervous system (~24%); and digestive and respiratory system (~10%) sequelae [
36]. Symptoms such as myalgic encephalomyelitis/chronic fatigue syndrome and postural orthostatic tachycardia syndrome were already known from other viral-onset illnesses [
35]. Long COVID affects both adults and children and predominates in non-hospitalized patients with mild acute illness, but symptoms are typically resolved within one year according to a recent study from Israel [
37]. For a comprehensive overview on Long COVID major findings, mechanisms and recommendations, the interested reader is directed to a timely 2023 review on the topic [
35].
The post-acute sequelae of the infection are persistent, exacerbated or newly incident and thus difficult to diagnose and treat. Standard tests in routine patient care often come back normal and it takes symptom-specific testing of which many providers are not aware [
35]. As a result of the high diversity of Long COVID sequelae, the knowledge of specialists (cardiologists, neurologists, dermatologists, etc.) is required (for a list of available diagnostic tools and treatment options, see reference [
35]). Therefore, the increasingly established treatment centers for Long COVID patients take an interdisciplinary approach.
2. Results and Discussion
The fact that among our covalescent patients were some, whose sera degraded DBK as poorly as the HoP group had baffled us, but at the time of our experiments, there was still little scientific data regarding the symptom complex Long COVID. We have thus looked at our data again, re-testing the hypothesis that the DBK assay could indicate Long COVID candidates. Unfortunately, we did not have follow-up data for the patient cohort regarding their symptoms months after the acute disease (other than those published before [
5,
6]) so that we could not substantiate our hypothesis. Nevertheless, the fact that BK metabolism seems to be compromised in some covalescent patients may assist research into Long COVID pathophysiology. Moreover, if our low-tech assay, requiring a drop of capillary blood only [
38], would indicate Long-COVID candidates, it might lead more quickly to the application of special test procedures, reduce uncertainty, and shorten the patient’s Odyssey through the doctor’s offices.
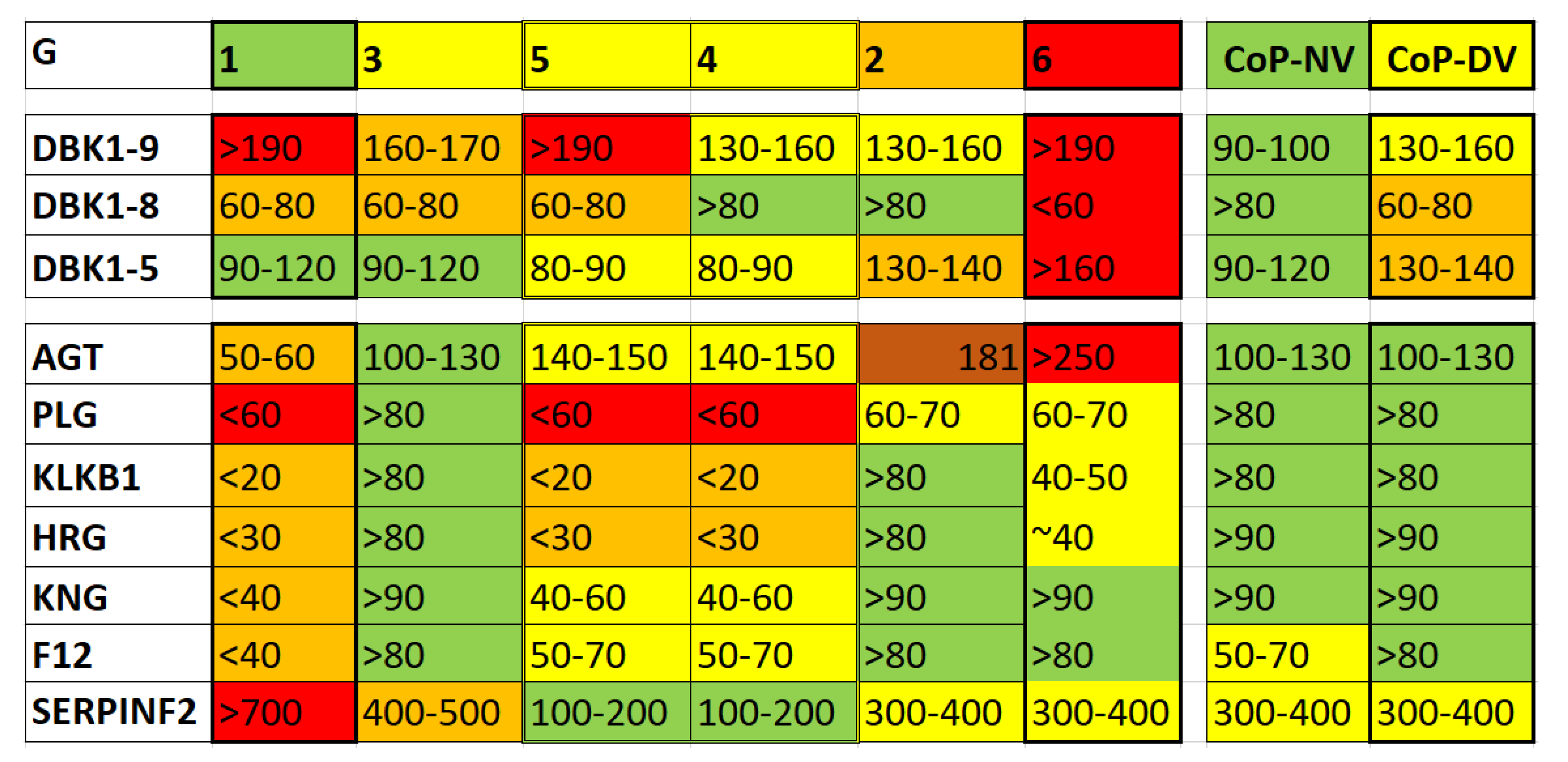
In
Figure 2, we set experimental data (DBK degradation assay results and selected protein intensities as obtained by HDMS expression analysis [
5,
6]) for the HoP and CoP groups in relation to those of healthy controls in order to demonstrate differences among the HoP subgroups on the one hand, and CoP-NV and CoP-DV on the other hand. DBK serum degradation capacity and DBK1-8 formation were lower than normal in HoP with no significant differences among groups. The lowest serum DBK degradation capacity and the least formation of fragment DBK1-8 were detected in the critically ill patients (G6) along with excessive formation of DBK1-5. G1, the group comprising the least ill of the hospitalized patients, also showed poor values for DBK cleavage and only 60-80% of DBK1-8 formation compared to healthy controls, but values for DBK1-5 were not conspicuous. These results indicated that in G6 - among other multi-system failures - also both the RAS and the KKS were seriously compromised while in G1 ACE (as expressed in DBK1-5 formation) was unaffected. G2 to G5 showed variations of these observations with G4 and G2 coming closest to normal values in DBK degradation and DBK1-8 formation (KKS axis). G2 consisted of also very ill patients next to G6 and presented increased DBK1-5 indicating dysregulation in the RAS.
We selected proteins from the HDMS experiments, which are part of the extended KKS/RAS networks (
Figure 1), to shed more light on the results from the assay [
6]. These eight proteins were by no means sufficient to describe all involved protein cascades, but they provided a suitable entry for further investigations. ACE itself was not detected in undepleted sera, but the ion intensities of the precursor protein in the RAS, AGT, increased from G1 to G6; in G1, AGT was downregulated, in G3, values came close to normal. The AGT concentration correlated with disease severity in HoP indicating that Ang II-stimulated signaling was then dysregulated. The general protein profiles of G2 and G6 were similar and shared, e.g., comparatively normal values for Hageman factor (complement factor 12, F12) and kininogen (KNG), 3- to 4-fold higher values for α2-antiplasmin (SERPINF2), and about the same reduction of plasminogen (PLG). For G3, the results for this choice of proteins came close to those for healthy controls apart from SERPINF2 levels, which were about 4 to 5-fold higher. Incidently, SERPINF2 serum concentration was more than 3-fold increased compared to normal in all groups including CoP except for G4 and G5, where it came close to normal. This serine protease inhibitor is responsible for inactivating plasmin, which is important in fibrinolysis [
39]. G4 and G5 differed considerably in their protein profiles to the other groups. The contact activation system did not seem to play the same dominant role as in G2, 3 and 6, and KKS members KNG and KLKB1 were present at reduced concentration. In general, the either very low or excessive levels of some members of the extended RAS and KKS network distinguished the HoP groups and indicated a different impact of these pathways in disease progression. Thereby, we could not associate any particular co-morbidity; groups were heterogenous in that respect, and sub-analyses did not have sufficient power. [
5,
6]
The observation that DBK degradation and DBK1-8 formation differed significantly between CoP-NV and CoP-DV [
2] was not reflected in the concentration of the selected proteins as most did not change much between those two groups (
Figure 2). SERPINF2 was generally increased in CoP, and PLG, HRG, KNG1, and F12 showed considerable fluctuation, which may be part of enzyme re-balancing during the healing process. Nevertheless, results of the BK assay indicated significant differences in BK degradation capacity between CoP-NV and CoP-DV. These could result from both impaired serum CPN and dysregulated (ACE) activity [
40,
41]. In our earlier publication [
6], we hypothesized that the reduced CPN activity could be due to reduced CPN synthesis in the liver following organ impairment due to COVID-19. Possibly, this condition lasts much longer than the acute disease phase indicating that, what we observe, is not a predictor of Long COVID but a sign of liver problems. The excessive DBK1-5 values, however, point to overactive ACE in CoP-DV and dysregulation of the RAS as observed in COVID-19.
3. Conclusion
Using BK degradation for testing the protease (CPN, ACE) activity in patient sera, we noted a general reduction in hospitalized COVID-19 patients, but also in some convalescent patients. CPN activity was decreased and contributed to a large extent to the reduced serum BK degradation capacity. Increased values for the BK fragment generated by ACE, DBK1-5, were, however, also detected. These observations in CoP-DV raised the question, if the assay results could be indicative of Long COVID, in particular, because the DBK1-5 level significantly correlated with COVID-19 severity in HoP. This question can only be answered by dedicated clinical studies. They have potential, because our BK assay is a low-cost/low-tech procedure based on thin-layer chromatography (TLC) [
4] that can be performed in any laboratory and that requires only a drop of serum or capillary blood [
38]. If its data truly indicate Long COVID candidates, it might be a valuable tool to speed up patient diagnosis and therapy. Other components of the RAS such as Ang II and Ang(1-7) for which assays are available, should be tested alongside in these studies to obtain a deeper knowledge about the underlying pathophysiology.
When designing these clinical studies, it is important to skillfully assemble patient cohorts with regard to co-morbidities. Diseases like the metabolic syndrome, in particular hypertension, directly rely on the RAS and they are known risk factors in this virus infection. Care must be taken to have sufficiently large cohorts of untreated, ACEI- and ARB- (or otherwise-) treated hypertensive patients to be able to draw significant conclusions, because the medication impacts on the assay results.
In fact, as we have learned from the serum proteomics experiments, multiple parameters other than those typically considered during the assembly of patient cohorts (BMI, age, gender, co-morbidities) may influence the results. We detected, by way of example, six distinct groups among the hospitalized patients, which were clearly distinguished by their serum protein profiles. These groups could be arranged according to disease severity, BMI and age, and seemed to represent subsets of different pathophysiology (e.g., different levels of RAS and KKS involvement); a correlation with co-morbidities such as hypertension or diabetes could, however, not be found. In fact, hypertensive patients were present in all groups. These observations may be one reason for bias seen in COVID-19 research [
31] and they beg the question how to best assemble study cohorts. More often than not, researchers are limited by the availability of eligible patients. An increase in participant numbers and the formation of sufficiently large cohorts can help so that sub-groups can be evaluated later based on the experimental data. Depending on the experiments performed and their sensitivity, this can become very important, because, as we demonstrated for the serum protein profiles, it is not useful and advisable to treat sample sets as a single groups, which clearly differ in their measured properties. Such results would only show the most striking differences among the major groups of probands and subtle differences such as distinct protein profiles as in our case would go unnoticed.
In summary, above, we have taken a second look at earlier [
5,
6] proteomics data and results on serum protease activity obtained with BK as reporter peptide from hospitalized and covalescent COVID-19 patients. In the light of the general acceptance of Long COVID, we would like to direct the attention of the reader to two insights:
- (1)
Sensitive technology such as omics methods might provide unexpected significant differences within the pre-defined patient groups of a clinical study. Those can only be explored with sufficient power, if the cohorts are large enough and properly matched with respect to the parameters known beforehand (e.g., age, gender, co-morbidities).
- (2)
The TLC-based BK-reporter serum protease activity assay singled out covalescent patients with experimental values similar to those measured in the HoP group. The assay may thus be indicative of persisting liver problems and/or potentially of Long COVID. Clinical studies are required to test this hypothesis.
4. Materials and Methods
This paper is a re-evaluation of data generated in two earlier studies [
5,
6] of 45 hospitalized patients (HoP) with laboratory-confirmed SARS-CoV-2 infection, admitted to the University Hospital Münster and Marien-Hospital Steinfurt in Germany between March and June 2020, 26 individuals with laboratory-confirmed SARS-CoV-2 infection, who had recovered (CoP), and 8 healthy volunteers. The Ethics Committee of Münster University approved the study (AZ 2020-220-f-S and AZ 2020-210-f-S), and the procedures were in accordance with the Helsinki Declaration of 1975 as revised in 1983. For all patient and measurement details, see our earlier publications [
5,
6]. Selected results from this prior work were set in perspective to data from healthy probands using Excel tools and discussed with regard to the role of the RAS in Long COVID, here.
Author Contributions
Conceptualization, Methodology, Formal Analysis, Writing – Original Draft Preparation, S.K.; Writing – Review & Editing, S.K., P.-R.T., R.V.
Funding
This research received no funding.
Institutional Review Board Statement
The Ethics Committee of Münster University approved the current study (AZ 2020-220-f-S and AZ 2020-210-f-S), and the procedures were in accordance with the Helsinki Declaration of 1975 as revised in 1983.
Informed Consent Statement
Informed written consent was obtained from all subjects involved in the study as published before [
5,
6].
Data Availability Statement
Data have been made available in earlier publications [
5,
6].
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
ACE - angiotensin-converting enzyme, ACEI - ACE inhibitor, AD – aspartate decarboxylase, AGT – angiotensinogen, Ang – angiotensin, APA – aminopeptidase A, APN – alanyl aminopeptidase N, ARB - angiotensin receptor blocker, AT1R/AT2R – Ang II receptors, BK – bradykinin, BMI - body mass index, B1R/B2R – bradykinin receptors, CoP - covalescent patients, CoP-NV – group with normal assay values during convalescence, CoP-DV - with not normal assay values during convalescence, COVID-19 - corona virus infectious disease 2019, CPA – carboxypeptidase A, CPN - carboxypeptidase N, DBK – dabsylated bradykinin, DBK1-5/DBK1-8 – bradykinin fragments, F12 - Hageman factor/complement factor 12, G – group, HoP - hospitalized patients, HDMS - high-definition mass spectrometry, HRG - histidine-rich glycoprotein, KLKB1 – kallikrein, KKS - kallikrein-kinin system, KNG – kininogen, MasR - Mas receptor, MRGD – Mas-related G protein-coupled receptor member D, NEP - neutral endopeptidase/neprylisin, NO – nitric oxide, PEP – prolyl endopeptidase, PLG – plasminogen, PSNS – parasynthetic nervous system, RAS - renin-angiotensin-system, ROS – reactive oxygen species, SAPS II - Simplified Acute Physiology Score, SARS-CoV-2 - severe acute respiratory syndrome coronavirus type 2, SERPINF2 - α2-antiplasmin, SNS – sympathic nervous system, TLC - thin-layer chromatography, THOP1 – thiocyanate oligopeptidase, VSMC – vascular smooth muscle cells.
References
- Angeli, F.; Zappa, M.; Reboldi, G.; Gentile, G.; Trapasso, M.; Spanevello, A.; Verdecchia, P. The spike effect of acute respiratory syndrome coronavirus 2 and coronavirus disease 2019 vaccines on blood pressure. European Journal of Internal Medicine 2023, 109, 12–21. [Google Scholar] [CrossRef]
- Paz Ocaranza, M.; Riquelme, J. A.; García, L.; Jalil, J. E.; Chiong, M.; Santos, R. A. S.; Lavandero, S. Counter-regulatory renin-angiotensin system in cardiovascular disease. Nature Reviews. Cardiology 2020, 17, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Peng, J.; Wang, T.; Wen, J.; Chen, S.; Huang, Y.; Zhang, Y. Counter-regulatory renin-angiotensin system in hypertension: Review and update in the era of COVID-19 pandemic. Biochemical Pharmacology 2023, 208, 115370. [Google Scholar] [CrossRef] [PubMed]
- Bayer, M.; König, S. A vote for robustness: Monitoring serum enzyme activity by thin-layer chromatography of dabsylated bradykinin products. J Pharmaceut Biomed Anal 2017, 143, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Tepasse, P.-R.; Vollenberg, R.; Steinebrey, N.; König, S. High angiotensin-converting enzyme and low carboxypeptidase N serum activity correlate with disease severity in COVID-19 patients. J Personalized Med. 2022, 12, 406. [Google Scholar] [CrossRef] [PubMed]
- Tepasse, P.-R.; Vollenberg, R.; Steinebrey, N.; König, S. The dysregulation of the renin-angiotensin-system in COVID-19 studied by serum proteomics: Angiotensinogen increases with disease severity. Molecules 2022, 27, 2495. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, A.; Iqbal, M. S.; Sultan, S.; Alhuthali, R. A.; Alshubaili, D. I.; Sayyam, R. S.; Abyad, L. M.; Qasem, A. H.; Arbaeen, A. F. Dysregulated bradykinin: Mystery in the pathogenesis of COVID-19. Mediators of Inflammation 2022, 7423537. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, P. R.; Sirois, P.; Fernandes, P. D. The role of kallikrein-kinin and renin-angiotensin systems in COVID-19 infection. Peptides 2021, 135, 170428. [Google Scholar] [CrossRef]
- Lucena, J.F.; Alegre, F.; Martinez-Urbistondo, D.; Landecho, M.F.; Huerta, A.; Garcia-Mouriz, A.; Garcia, N.; Quiroga, J. Performance of SAPS II and SAPS 3 in intermediate care. PLoS ONE 2013, 8, e77229. [Google Scholar] [CrossRef]
- Galbraith, M. D.; Kinning, K. T.; Sullivan, K. D.; Baxter, R.; Araya, P.; Jordan, K. R.; Russell, S.; Smith, K. P.; Granrath, R. E.; Shaw, J.; Dzieciatkowska, M.; Ghosh, T.; Monte, A. A.; D'Alessandro, A.; Hansen, K. C.; Bennett, T. D.; Hsieh, E. W. Y.; Espinosa, J. M. Seroconversion stages COVID19 into distinct pathophysiological states. eLife 2021, 10, e65508. [Google Scholar] [CrossRef]
- Li, F.; Boon, A.C.M.; Michelson, A.P.; et al. Estrogen hormone is an essential sex factor inhibiting inflammation and immune response in COVID-19. Sci Rep 2022, 12, 9462. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Ning, Q.; Wu, D.; Wang, X.; Xi, D.; Chen, T.; Chen, G.; Wang, H.; Lu, H.; Wang, M.; Zhu, L.; Hu, J.; Liu, T.; Ma, K.; Han, M.; Luo, X. The mechanism underlying extrapulmonary complications of the coronavirus disease 2019 and its therapeutic implication. Signal Transduction and Targeted Therapy 2022, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Lawal, I. O.; Kgatle, M. M.; Mokoala, K.; Farate, A.; Sathekge, M. M. Cardiovascular disturbances in COVID-19: an updated review of the pathophysiology and clinical evidence of cardiovascular damage induced by SARS-CoV-2. BMC Cardiovascular Disorders 2022, 22, 93. [Google Scholar] [CrossRef]
- Schieffer, E.; Schieffer, B. The race for ACE: Targeting angiotensin-converting enzymes (ACE) in SARS-CoV-2 infection. Journal of the Renin-Angiotensin-Aldosterone System: JRAAS, 2022; 2549063. [Google Scholar]
- Matthews, K. W.; Mueller-Ortiz, S. L.; Wetsel, R. A. Carboxypeptidase N: a pleiotropic regulator of inflammation. Molecular Immunology 2004, 40, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Armato, J.; DeFronzo, R. A.; Chiu, S. T.; Rider, D.; Ruby, R. Are angiotensin-converting enzyme inhibitors/angiotensin receptor blockers associated with reduced severe acute respiratory syndrome coronavirus 2 infections and improved outcomes, and does race matter? Diabetes, Obesity & Metabolism 2022, 24, 2465–2468. [Google Scholar]
- Kuba, K.; Imai, Y.; Rao, S.; Gao, H.; Guo, F.; Guan, B. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005, 11, 875–879. [Google Scholar] [CrossRef] [PubMed]
- Hrenak, J.; Paulis, L.; Simko, F. Angiotensin A/alamandine/MrgD axis: Another clue to understanding cardiovascular pathophysiology. International Journal of Molecular Sciences 2016, 17, 1098. [Google Scholar] [CrossRef]
- Arnold, R.G. COVID-19 – Does this disease kill due to imbalance of the renin angiotensin system (RAS) caused by genetic and gender differences in the response to viral ACE2 attack? Heart, Lung Circ. 2020, 29, 964–972. [Google Scholar] [CrossRef]
- Biswas, M.; Kali, M.S.K. Association of angiotensin-converting enzyme inhibitors and angiotensin-receptor blockers with risk of mortality, severity or SARS-CoV-2 test positivity in COVID-19 patients: meta-analysis. Sci. Rep. 2021, 11, 5012. [Google Scholar] [CrossRef]
- Skarstein Kolberg, E. ACE2, COVID19 and serum ACE as a possible biomarker to predict severity of disease. J Clin Virol. 2020, 126, 104350. [Google Scholar] [CrossRef] [PubMed]
- Kai, H.; Kai, M. Interactions of coronaviruses with ACE2, angiotensin II, and RAS inhibitors – lessons from available evidence and insights into COVID-19. Hypertension Res. 2020, 43, 648–654. [Google Scholar] [CrossRef]
- Tsampasian, V.; Corballis, N.; Vassiliou, V. S. Renin-angiotensin-aldosterone inhibitors and COVID-19 infection. Current Hypertension Reports 2022, 24, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Selçuk, M.; Çınar, T.; Keskin, M.; Çiçek, V.; Kılıç, Ş.; Kenan, B.; Doğan, S.; Asal, S.; Günay, N.; Yıldırım, E.; Keskin, Ü.; Orhan, A. L. Is the use of ACE inb/ARBs associated with higher in-hospital mortality in Covid-19 pneumonia patients? Clinical and Experimental Hypertension (New York, N.Y.: 1993), 2020; 42, 738–742. [Google Scholar]
- Mehta, N.; Kalra, A.; Nowacki, A. S.; Anjewierden, S.; Han, Z.; Bhat, P.; Carmona-Rubio, A. E.; Jacob, M.; Procop, G. W.; Harrington, S.; Milinovich, A.; Svensson, L. G.; Jehi, L.; Young, J. B.; Chung, M. K. Association of use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID-19). JAMA Cardiology 2020, 5, 1020–1026. [Google Scholar] [CrossRef] [PubMed]
- Mehra, M.R.; Desai, S.S.; Kuy, S.R.; Henry, T.D.; Patel, A.N. Cardiovascular disease, drug therapy, and mortality in Covid-19. N Engl J Med, e: 382, 2020; 382, e102. [Google Scholar]
- Duvvuri, V. R.; Baumgartner, A.; Molani, S.; Hernandez, P. V.; Yuan, D.; Roper, R. T.; Matos, W. F.; Robinson, M.; Su, Y.; Subramanian, N.; Goldman, J. D.; Heath, J. R.; Hadlock, J. J. Angiotensin-converting enzyme (ACE) inhibitors may moderate COVID-19 hyperinflammatory response: An observational study with deep immunophenotyping. Health Data Science 2022, 0002. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; White, N.; Fanning, J.P.; et al. Impact of renin–angiotensin–aldosterone system inhibition on mortality in critically ill COVID-19 patients with pre-existing hypertension: a prospective cohort study. BMC Cardiovasc Disord 2022, 22, 123. [Google Scholar] [CrossRef]
- Huang, N. X.; Yuan, Q.; Fang, F.; Yan, B. P.; Sanderson, J. E. Systematic review and meta-analysis of the clinical outcomes of ACEI/ARB in East-Asian patients with COVID-19. PloS one 2023, 18. [Google Scholar] [CrossRef]
- Loader, J.; Taylor, F. C.; Lampa, E.; Sundström, J. Renin-angiotensin aldosterone system inhibitors and COVID-19: A systematic review and meta-analysis revealing critical bias across a body of observational research. Journal of the American Heart Association 2022, 11, e025289. [Google Scholar] [CrossRef]
- Trump, S.; Lukassen, S.; Anker, M. S.; Chua, R. L.; Liebig, J.; Thürmann, L.; Corman, V. M.; Binder, M.; Loske, J.; Klasa, C.; Krieger, T.; Hennig, B. P.; Messingschlager, M.; Pott, F.; Kazmierski, J.; Twardziok, S.; Albrecht, J. P.; Eils, J.; Hadzibegovic, S.; Lena, A.; … Lehmann, I. Hypertension delays viral clearance and exacerbates airway hyperinflammation in patients with COVID-19. Nature Biotechnology 2021, 39, 705–716. [Google Scholar] [CrossRef]
- Zhao, H. J.; Li, Y.; Wang, D. Y.; Yuan, H. T. ARB might be superior to ACEI for treatment of hypertensive COVID-19 patients. Journal of Cellular and Molecular Medicine 2021, 25, 11031–11034. [Google Scholar] [CrossRef]
- Luna, P.; Fernanda Pérez, M.; Castellar-Lopez, J.; Chang, A.; Montoya, Y.; Bustamante, J.; Rosales-Rada, W.; Mendoza-Torres, E. Potential of angiotensin-(1-7) in COVID-19 treatment. Current Protein & Peptide Science 2023, 24, 89–97. [Google Scholar]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; et al. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol 2023, 21, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zang, C.; Xu, Z.; et al. Data-driven identification of post-acute SARS-CoV-2 infection subphenotypes. Nat Med 2023, 29, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, B.; Sudry, T.; Flaks-Manov, N.; Yehezkelli, Y.; Kalkstein, N.; Akiva, P.; et al. Long covid outcomes at one year after mild SARS-CoV-2 infection: nationwide cohort study. BMJ 2023, 380, e072529. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, U.; Engl, C.; Bayer, M.; König, S. Neuropeptide reporter assay for serum, capillary blood and blood cards. MethodsX 2020, 7, 100985. [Google Scholar] [CrossRef]
- Wu, G.; Quek, A. J.; Caradoc-Davies, T.T.; Ekkel, S.M.; Mazzitelli, B.; Whisstock, J.C.; Law, R.H.P. Structural studies of plasmin inhibition. Biochemical Society Transactions 2019, 47, 541–557. [Google Scholar] [CrossRef]
- Molinaro, G. , Gervais, N., Adam, A. Biochemical basis of angioedema associated with recombinant tissue plasminogen activator treatment - an in vitro experimental approach. Stroke 2002, 33, 1712–1716. [Google Scholar] [CrossRef]
- Regoli, D.; Barabe, J. Pharmacology of bradykinin and related kinins. Pharm. Rev. 1980, 32, 1–38. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).