Submitted:
18 May 2023
Posted:
18 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Factor affecting cover crops seed germination
2.1. Abiotic factors
2.1.1. Temperature and soil moisture
2.2. Seed dormancy
3. Seed traits of main cover crop species
3.1. Fabaceae
3.1.1. Vicia spp.
3.1.2. Trifolium spp.
3.2. Poaceae
3.3. Brassicaceae
4. Solutions for enhancing cover crop seed germinability
4.1. Agronomic practices
4.1.1. Sowing time and planting geometry
4.2. Seed pretreatment
4.2.1. Seed priming
| Cover crop species | 1000-Seed weight (mg) § | Seed treatment | |
| Priming | Coating | ||
| Guizotia abyssinica | 3.3 | + [127] | unknown |
| Helianthus annuus | 48.0 | + [128] | + [129] |
| Brassica carinata | 5.0 | Unknown | unknown |
| Brassica juncea | 3.0 | + [104] | unknown |
| Brassica napus | 2.7 | + [130,131] | + [132] |
| Brassica rapa | 3.7 | + [133,134] | + [135] |
| Camelina sativa | 1.3 | + [136,137] | unknown |
| Eruca sativa | 1.3 | + [138] | unknown |
| Raphanus sativus | 13.0 | + [139] | unknown |
| Sinapis alba | 8.0 | Unknown | unknown |
| Lathyrus sativus | 176.0 | + [140] | unknown |
| Lens nigricans | 21.5 | Unknown | unknown |
| Lupinus angustifolius | 179.4 | Unknown | unknown |
| Medicago lupulina | 1.5 | Unknown | + [141] |
| Melilotus officinalis | 2.5 | Unknown | unknown |
| Onobrychis viciifolia | 23.0 | + [142] | + [141] |
| Pisum sativum | 168.8 | + [143,144] | + [145] |
| Trifolium alexandrinum | 3.0 | + [99] | unknown |
| Trifolium incarnatum | 4.7 | Unknown | unknown |
| Trifoliumhybridum | 0.83 | Unknown | |
| Trifolium resupinatum | 1.48 | Unknown | unknown |
| Trifoliumpratense | 2.04 | + [146] | + [113] |
| Trifoliumsubterraneum | 6.28 | + [147] | unknown |
| Trifoliumrepense | 075 | + [148] | + [141] |
| Trigonella foenum graecum | 16.0 | + [149] | unknown |
| Vicia faba | 359.6 | + [150] | unknown |
| Vicia sativa | 53.8 | + [151] | unknown |
| Vicia villosa | 26.7 | + [103,108] | unknown |
| Phacelia tanacetifolia | 1.8 | + [152] | unknown |
| Avena sativa | 39.4 | + [153] | + [154] |
| Lolium hybridum | 3.4 | Unknown | unknown |
| Lolium multiflorum | 2.7 | + [155] | + [156] |
| Secale cereale | 32.3 | + [157] | unknown |
| Secale multicaule | 18.8 | Unknown | unknown |
| Setaria italica | 2.2 | + [158] | unknown |
| Sorghum sudanense | 13.8 | + [159] | unknown |
| Fagopyrum esculentum | 25.0 | Unknown | unknown |
4.2.2. Seed coating
5. Conclusion
Author Contributions
Data Availability Statement
Conflicts of Interest
References
- Bond, W.; Turner, R.; Grundy, A. A review of non-chemical weed management. HDRA, the Organic Organisation, Ryton Organic Gardens, Coventry, UK 2003, 81. [Google Scholar]
- Colbach, N.; Petit, S.; Chauvel, B.; Deytieux, V.; Lechenet, M.; Munier-Jolain, N.; Cordeau, S. The Pitfalls of Relating Weeds, Herbicide Use, and Crop Yield: Don’t Fall Into the Trap! A Critical Review. Frontiers in Agronomy 2020, 2, 33. [Google Scholar]
- Travlos, I.; De Prado, R.; Chachalis, D.; Bilalis, D.J. Herbicide Resistance in Weeds: Early Detection, Mechanisms, Dispersal, New Insights and Management Issues; Frontiers Media SA: 2020.
- Albrecht, H.; Cambecèdes, J.; Lang, M.; Wagner, M. Management options for the conservation of rare arable plants in Europe. Botany Letters 2016, 163, 389–415. [Google Scholar] [CrossRef]
- Adeux, G.; Vieren, E.; Carlesi, S.; Bàrberi, P.; Munier-Jolain, N.; Cordeau, S. Mitigating crop yield losses through weed diversity. Nature Sustainability 2019, 2, 1018–1026. [Google Scholar] [CrossRef]
- Heap, I. The International Herbicide-Resistant Weed Database. Online. http://www.weedscience.org/Home.aspx. Availabe online: www.weedscience.org (accessed on 19/03/2020).
- Stoate, C.; Baldi, A.; Beja, P.; Boatman, N.; Herzon, I.; Van Doorn, A.; De Snoo, G.; Rakosy, L.; Ramwell, C. Ecological impacts of early 21st century agricultural change in Europe–a review. Journal of environmental management 2009, 91, 22–46. [Google Scholar]
- Bond, W.; Grundy, A. Non-chemical weed management in organic farming systems. Weed research 2001, 41, 383–405. [Google Scholar]
- Weber, J.F.; Kunz, C.; Peteinatos, G.G.; Zikeli, S.; Gerhards, R. Weed control using conventional tillage, reduced tillage, no-tillage, and cover crops in organic soybean. Agriculture 2017, 7, 43. [Google Scholar] [CrossRef]
- Petit, S.; Cordeau, S.; Chauvel, B.; Bohan, D.; Guillemin, J.-P.; Steinberg, C. Biodiversity-based options for arable weed management. A review. Agronomy for Sustainable Development 2018, 38, 1–21. [Google Scholar]
- Nosratti, I.; Sabeti, P.; Chaghamirzaee, G.; Heidari, H. Weed problems, challenges, and opportunities in Iran. Crop Protection 2020, 134, 104371. [Google Scholar]
- Bhaskar, V.; Westbrook, A.S.; Bellinder, R.R.; DiTommaso, A. Integrated management of living mulches for weed control: A review. Weed Technology 2021, 35, 856–868. [Google Scholar] [CrossRef]
- Gaba, S.; Lescourret, F.; Boudsocq, S.; Enjalbert, J.; Hinsinger, P.; Journet, E.-P.; Navas, M.-L.; Wery, J.; Louarn, G.; Malézieux, E. Multiple cropping systems as drivers for providing multiple ecosystem services: from concepts to design. Agronomy for sustainable development 2015, 35, 607–623. [Google Scholar]
- Kumar, V.; Obour, A.; Jha, P.; Liu, R.; Manuchehri, M.R.; Dille, J.A.; Holman, J.; Stahlman, P.W. Integrating cover crops for weed management in the semiarid US Great Plains: opportunities and challenges. Weed Science 2020, 68, 311–323. [Google Scholar]
- Teasdale, J.; Brandsaeter, L.; Calegari, A.; Neto, F.S.; Upadhyaya, M.; Blackshaw, R. Cover crops and weed management. Non chemical weed management principles. Concepts and Technology, CABI, Wallingford, UK 2007, 49–64. [Google Scholar]
- Teasdale, J.; Hatfield, J.; Buhler, D.; Stewart, B. Cover crops, smother plants, and weed management. Integrated weed and soil management 1998. [Google Scholar]
- Tursun, N.; Işık, D.; Demir, Z.; Jabran, K. Use of Living, Mowed, and Soil-Incorporated Cover Crops for Weed Control in Apricot Orchards. Agronomy 2018, 8, 150. [Google Scholar] [CrossRef]
- Ward, M.J.; Ryan, M.R.; Curran, W.S.; Barbercheck, M.E.; Mortensen, D.A. Cover crops and disturbance influence activity-density of weed seed predators Amara aenea and Harpalus pensylvanicus (Coleoptera: Carabidae). Weed science 2011, 59, 76–81. [Google Scholar] [CrossRef]
- Schipanski, M.E.; Barbercheck, M.; Douglas, M.R.; Finney, D.M.; Haider, K.; Kaye, J.P.; Kemanian, A.R.; Mortensen, D.A.; Ryan, M.R.; Tooker, J. A framework for evaluating ecosystem services provided by cover crops in agroecosystems. Agricultural Systems 2014, 125, 12–22. [Google Scholar] [CrossRef]
- Moonen, A.; Barberi, P. Size and composition of the weed seedbank after 7 years of different cover-crop-maize management systems. Weed Research 2004, 44, 163–177. [Google Scholar] [CrossRef]
- Mahé, I.; Chauvel, B.; Colbach, N.; Cordeau, S.; Gfeller, A.; Reiss, A.; Moreau, D. Deciphering field-based evidences for crop allelopathy in weed regulation. A review. Agron. Sustainable Dev. In press.
- Mahé, I.; Chauvel, B.; Colbach, N.; Cordeau, S.; Gfeller, A.; Reiss, A.; Moreau, D. Deciphering field-based evidences for crop allelopathy in weed regulation. A review. Agronomy for Sustainable Development 2022, 42, 1–20. [Google Scholar]
- Osipitan, O.A.; Dille, J.A.; Assefa, Y.; Knezevic, S.Z. Cover crop for early season weed suppression in crops: Systematic review and meta-analysis. Agronomy Journal 2018, 110, 2211–2221. [Google Scholar] [CrossRef]
- Lawley, Y.E.; Teasdale, J.R.; Weil, R.R. The mechanism for weed suppression by a forage radish cover crop. Agronomy journal 2012, 104, 205–214. [Google Scholar] [CrossRef]
- Johnson, G.A.; Defelice, M.S.; Helsel, Z.R. Cover crop management and weed control in corn (Zea mays). Weed Technology 1993, 425–430. [Google Scholar] [CrossRef]
- Alonso-Ayuso, M.; Gabriel, J.L.; García-González, I.; Del Monte, J.P.; Quemada, M. Weed density and diversity in a long-term cover crop experiment background. Crop Protection 2018, 112, 103–111. [Google Scholar] [CrossRef]
- Büchi, L.; Wendling, M.; Amossé, C.; Jeangros, B.; Charles, R. Cover crops to secure weed control strategies in a maize crop with reduced tillage. Field Crops Research 2020, 247, 107583. [Google Scholar] [CrossRef]
- Smith, R.G.; Warren, N.D.; Cordeau, S. Are cover crop mixtures better at suppressing weeds than cover crop monocultures? Weed Science 2020, 68, 186–194. [Google Scholar] [CrossRef]
- Cordeau, S.; Smith, R.G.; Gallandt, E.R.; Brown, B.; Salon, P.; DiTommaso, A.; Ryan, M.R. Timing of tillage as a driver of weed communities. Weed Sci 2017, 65, 504–514. [Google Scholar] [CrossRef]
- Adeux, G.; Cordeau, S.; Antichi, D.; Carlesi, S.; Mazzoncini, M.; Munier-Jolain, N.; Bàrberi, P. Cover crops promote crop productivity but do not enhance weed management in tillage-based cropping systems. European Journal of Agronomy 2021, 123, 126221. [Google Scholar] [CrossRef]
- Derrouch, D.; Chauvel, B.; Felten, E.; Dessaint, F. Weed Management in the Transition to Conservation Agriculture: Farmers’ Response. Agronomy 2020, 10, 843. [Google Scholar] [CrossRef]
- Barzman, M.; Bàrberi, P.; Birch, A.N.E.; Boonekamp, P.; Dachbrodt-Saaydeh, S.; Graf, B.; Hommel, B.; Jensen, J.E.; Kiss, J.; Kudsk, P. Eight principles of integrated pest management. Agronomy for sustainable development 2015, 35, 1199–1215. [Google Scholar] [CrossRef]
- Osipitan, O.A.; Dille, J.A.; Assefa, Y.; Radicetti, E.; Ayeni, A.; Knezevic, S.Z. Impact of cover crop management on level of weed suppression: a meta-analysis. Crop Science 2019, 59, 833–842. [Google Scholar] [CrossRef]
- Mennan, H.; Jabran, K.; Zandstra, B.H.; Pala, F. Non-chemical weed management in vegetables by using cover crops: A review. Agronomy 2020, 10, 257. [Google Scholar] [CrossRef]
- Grünwald, N.J.; Hu, S.; van Bruggen, A.H.C. Short-term Cover Crop Decomposition in Organic and Conventional Soils: Characterization of Soil C, N, Microbial and Plant Pathogen Dynamics. European Journal of Plant Pathology 2000, 106, 37–50. [Google Scholar] [CrossRef]
- Constantin, J.; Le Bas, C.; Justes, E. Large-scale assessment of optimal emergence and destruction dates for cover crops to reduce nitrate leaching in temperate conditions using the STICS soil–crop model. European Journal of Agronomy 2015, 69, 75–87. [Google Scholar] [CrossRef]
- Bradford, K.J. A water relations analysis of seed germination rates. Plant Physiology 1990, 94, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Kropff, M.J.; McLaren, G.; Visperas, R.M. A nonlinear model for crop development as a function of temperature. Agricultural and Forest Meteorology 1995, 77, 1–16. [Google Scholar] [CrossRef]
- Constantin, J.; Dürr, C.; Tribouillois, H.; Justes, E. Catch crop emergence success depends on weather and soil seedbed conditions in interaction with sowing date: A simulation study using the SIMPLE emergence model. Field Crops Research 2015, 176, 22–33. [Google Scholar] [CrossRef]
- Brisson, N.; Gary, C.; Justes, E.; Roche, R.; Mary, B.; Ripoche, D.; Zimmer, D.; Sierra, J.; Bertuzzi, P.; Burger, P.; et al. An overview of the crop model stics. European Journal of Agronomy 2003, 18, 309–332. [Google Scholar] [CrossRef]
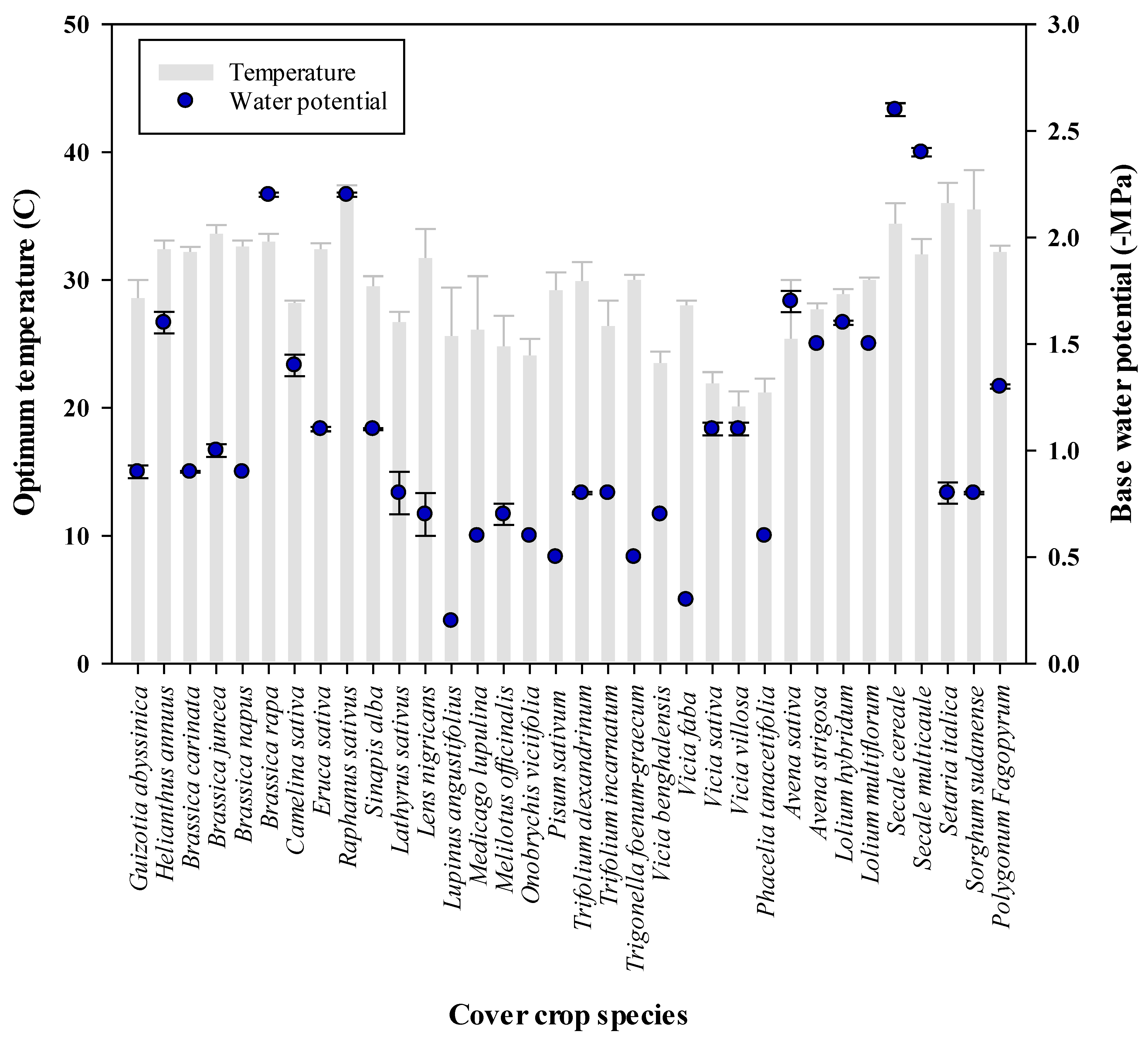
- Tribouillois, H.; Dürr, C.; Demilly, D.; Wagner, M.H.; Justes, E. Determination of Germination Response to Temperature and Water Potential for a Wide Range of Cover Crop Species and Related Functional Groups. PLoS One 2016, 11, e0161185. [Google Scholar] [CrossRef]
- Bewley, J.D. Seed germination and dormancy. The plant cell 1997, 9, 1055. [Google Scholar] [CrossRef]
- Nosratti, I.; Almaleki, S.; Chauhan, B.S. Seed Germination Ecology of Soldier Thistle (<i>Picnomon acarna</i>): An Invasive Weed of Rainfed Crops in Iran. Weed Science 2019, 67, 261–266. [Google Scholar]
- Payamani, R.; Nosratti, I.; Amerian, M. Variations in the germination characteristics in response to environmental factors between the hairy and spiny seeds of hedge parsley (Torilis arvensis Huds.). Weed Biology and Management 2018, 18, 176–183. [Google Scholar] [CrossRef]
- Fuller, D.Q.; Allaby, R.G.; Stevens, C. Domestication as innovation: the entanglement of techniques, technology and chance in the domestication of cereal crops. World archaeology 2010, 42, 13–28. [Google Scholar] [CrossRef]
- Kissing Kucek, L.; Riday, H.; Rufener, B.P.; Burke, A.N.; Eagen, S.S.; Ehlke, N.; Krogman, S.; Mirsky, S.B.; Reberg-Horton, C.; Ryan, M.R. Pod Dehiscence in Hairy Vetch (Vicia villosa Roth). Frontiers in plant science 2020, 11, 82. [Google Scholar] [CrossRef]
- Parker, T.A.; y Teran, J.C.B.M.; Palkovic, A.; Jernstedt, J.; Gepts, P. Genetic control of pod dehiscence in domesticated common bean: Associations with range expansion and local aridity conditions. biorxiv 2019, 517516. [Google Scholar]
- Nosratti, I.; Soltanabadi, S.; Honarmand, S.J.; Chauhan, B.S. Environmental factors affect seed germination and seedling emergence of invasive Centaurea balsamita. Crop and Pasture Science 2017, 68, 583–589. [Google Scholar] [CrossRef]
- Dürr, C.; Dickie, J.B.; Yang, X.Y.; Pritchard, H.W. Ranges of critical temperature and water potential values for the germination of species worldwide: Contribution to a seed trait database. Agricultural and Forest Meteorology 2015, 200, 222–232. [Google Scholar] [CrossRef]
- Mischler, R.; Duiker, S.W.; Curran, W.S.; Wilson, D. Hairy vetch management for no-till organic corn production. Agronomy Journal 2010, 102, 355–362. [Google Scholar] [CrossRef]
- Keene, C.; Curran, W.; Wallace, J.; Ryan, M.; Mirsky, S.; VanGessel, M.; Barbercheck, M. Cover crop termination timing is critical in organic rotational no-till systems. Agronomy Journal 2017, 109, 272–282. [Google Scholar] [CrossRef]
- Bekker, R.; Bakker, J.; Grandin, U.; Kalamees, R.; Milberg, P.; Poschlod, P.; Thompson, K.; Willems, J. Seed size, shape and vertical distribution in the soil: indicators of seed longevity. Functional Ecology 1998, 12, 834–842. [Google Scholar] [CrossRef]
- Cordeau, S.; Wayman, S.; Reibel, C.; Strbik, F.; Chauvel, B.; Guillemin, J.P. Effects of drought on weed emergence and growth vary with the seed burial depth and presence of a cover crop. Weed Biology and Management 2018, 18, 12–25. [Google Scholar] [CrossRef]
- Petit, S.; Cordeau, S.; Chauvel, B.; Bohan, D.; Guillemin, J.-P.; Steinberg, C. Biodiversity-based options for arable weed management. A review. Agronomy for Sustainable Development 2018, 38, 48. [Google Scholar] [CrossRef]
- Teasdale, J.R.; Devine, T.E.; Mosjidis, J.A.; Bellinder, R.R.; Beste, C.E. Growth and development of hairy vetch cultivars in the northeastern United States as influenced by planting and harvesting date. Agronomy Journal 2004, 96, 1266–1271. [Google Scholar] [CrossRef]
- Sainju, U.M.; Singh, B.P. Nitrogen storage with cover crops and nitrogen fertilization in tilled and nontilled soils. Agronomy journal 2008, 100, 619–627. [Google Scholar] [CrossRef]
- Hyvönen, T.; Ketoja, E.; Salonen, J.; Jalli, H.; Tiainen, J. Weed species diversity and community composition in organic and conventional cropping of spring cereals. Agriculture, Ecosystems & Environment 2003, 97, 131–149. [Google Scholar]
- Renzi, J.P.; Chantre, G.R.; Cantamutto, M.A. Development of a thermal-time model for combinational dormancy release of hairy vetch (Vicia villosa ssp. villosa). Crop and Pasture Science 2014, 65, 470–478. [Google Scholar] [CrossRef]
- Dabney, S.; Delgado, J.; Reeves, D. Using winter cover crops to improve soil and water quality. Commun Soil Sci Plant 32 (7–8): 1221–1250. 2001.
- Van Assche, J.A.; Debucquoy, K.L.; Rommens, W.A. Seasonal cycles in the germination capacity of buried seeds of some Leguminosae (Fabaceae). New Phytologist 2003, 158, 315–323. [Google Scholar] [CrossRef]
- Zhang, Q.; Tu, B.; Liu, C.; Liu, X. Pod anatomy, morphology and dehiscing forces in pod dehiscence of soybean (Glycine max (L.) Merrill). Flora 2018, 248, 48–53. [Google Scholar] [CrossRef]
- Volesky, J.; Mowrey, D.; Smith, G. Performance of rose clover and hairy vetch interseeded into Old World bluestem. Rangeland Ecology & Management/Journal of Range Management Archives 1996, 49, 448–451. [Google Scholar]
- Andersen, B.J.; Samarappuli, D.P.; Wick, A.; Berti, M.T. Faba bean and pea can provide late-fall forage grazing without affecting maize yield the following season. Agronomy 2020, 10, 80. [Google Scholar] [CrossRef]
- Vann, R.; Reberg-Horton, S.; Castillo, M.; McGee, R.; Mirsky, S. Winter Pea, Crimson Clover, and Hairy Vetch Planted in Mixture with Small Grains in the Southeast United States. Agronomy Journal 2019, 111, 805–815. [Google Scholar] [CrossRef]
- Scavo, A.; Restuccia, A.; Lombardo, S.; Fontanazza, S.; Abbate, C.; Pandino, G.; Anastasi, U.; Onofri, A.; Mauromicale, G. Improving soil health, weed management and nitrogen dynamics by Trifolium subterraneum cover cropping. Agronomy for Sustainable Development 2020, 40. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, R.; Yan, Z.; Jin, H.; Cui, H.; Lu, L.; Zhang, D.; Qin, B. Phytotoxic allelochemicals from roots and root exudates of Trifolium pratense. Journal of agricultural and food chemistry 2013, 61, 6321–6327. [Google Scholar] [CrossRef] [PubMed]
- Wyngaarden, S.L.; Gaudin, A.; Deen, W.; Martin, R.C. Expanding red clover (Trifolium pratense) usage in the corn–soy–wheat rotation. Sustainability 2015, 7, 15487–15509. [Google Scholar] [CrossRef]
- Ross, S.M.; King, J.R.; Izaurralde, R.C.; O'Donovan, J.T. Weed suppression by seven clover species. Agron. J. 2001, 93, 820–827. [Google Scholar] [CrossRef]
- Nichols, P.; Foster, K.; Piano, E.; Pecetti, L.; Kaur, P.; Ghamkhar, K.; Collins, W. Genetic improvement of subterranean clover (Trifolium subterraneum L.). 1. Germplasm, traits and future prospects. Crop and Pasture Science 2013, 64, 312–346. [Google Scholar] [CrossRef]
- Baresel, J.P.; Nichols, P.; Charrois, A.; Schmidhalter, U. Adaptation of ecotypes and cultivars of subterranean clover (Trifolium subterraneum L.) to German environmental conditions and its suitability as living mulch. Genetic Resources and Crop Evolution 2018, 65, 2057–2068. [Google Scholar] [CrossRef]
- Balfourier, F.; Imbert, C.; Charmet, G. Evidence for phylogeographic structure in Lolium species related to the spread of agriculture in Europe. A cpDNA study. Theoretical and Applied Genetics 2000, 101, 131–138. [Google Scholar] [CrossRef]
- Sebastian, J.; Dinneny, J.R. Setaria viridis: a model for understanding panicoid grass root systems. In Genetics and Genomics of Setaria, Springer: 2017; pp. 177-193.
- Jiménez-Alfaro, B.; Hernández-González, M.; Fernández-Pascual, E.; Toorop, P.; Frischie, S.; Gálvez-Ramírez, C. Germination ecology of winter annual grasses in Mediterranean climates: Applications for soil cover in olive groves. Agriculture, Ecosystems & Environment 2018, 262, 29–35. [Google Scholar]
- Bakker, J. Seeds, ecology, biogeography and evolution of dormancy, and germination. CC Baskin & JM Baskin. Plant Ecology 2001, 152, 204. [Google Scholar]
- Wilson, M.L.; Baker, J.M.; Allan, D.L. Factors Affecting Successful Establishment of Aerially Seeded Winter Rye. Agronomy Journal 2013, 105, 1868–1877. [Google Scholar] [CrossRef]
- Miedaner, T. Breeding wheat and rye for resistance to Fusarium diseases. Plant Breeding 1997, 116, 201–220. [Google Scholar] [CrossRef]
- Nosratti, I.; Sabeti, P.; Chaghamirzaee, G.; Heidari, H. Weed problems, challenges, and opportunities in Iran. Crop Protection 2017, 104371. [Google Scholar] [CrossRef]
- Sarwar, M.; Kirkegaard, J. Biofumigation potential of brassicas: II. Effect of environment and ontogeny on glucosinolate production and implications for screening. Plant and Soil 1998, 91–101. [Google Scholar] [CrossRef]
- Haramoto, E.R.; Gallandt, E.R. Brassica cover cropping: I. Effects on weed and crop establishment. Weed Science 2005, 695–701. [Google Scholar] [CrossRef]
- Krato, C.; Petersen, J. Competitiveness and yield impact of volunteer oilseed rape (Brassica napus) in winter and spring wheat (Triticum aestivum). Journal of Plant Diseases and Protection 2012, 119, 74–82. [Google Scholar] [CrossRef]
- Gruber, S.; Pekrun, C.; Claupein, W. Population dynamics of volunteer oilseed rape (Brassica napus L.) affected by tillage. European Journal of agronomy 2004, 20, 351–361. [Google Scholar] [CrossRef]
- Baraibar, B.; Mortensen, D.A.; Hunter, M.C.; Barbercheck, M.E.; Kaye, J.P.; Finney, D.M.; Curran, W.S.; Bunchek, J.; White, C.M. Growing degree days and cover crop type explain weed biomass in winter cover crops. Agronomy for Sustainable Development 2018, 38, 65. [Google Scholar] [CrossRef]
- Tribouillois, H.; Constantin, J.; Justes, E. Analysis and modeling of cover crop emergence: Accuracy of a static model and the dynamic STICS soil-crop model. European Journal of Agronomy 2018, 93, 73–81. [Google Scholar] [CrossRef]
- Cordeau, S.; Guillemin, J.P.; Reibel, C.; Chauvel, B. Weed species differ in their ability to emerge in no-till systems that include cover crops. Annals of Applied Biology 2015, 166, 444–455. [Google Scholar] [CrossRef]
- Fisher, K.; Momen, B.; Kratochvil, R. Is broadcasting seed an effective winter cover crop planting method? Agronomy Journal 2011, 103, 472–478. [Google Scholar] [CrossRef]
- Haramoto, E.R. Species, seeding rate, and planting method influence cover crop services prior to soybean. Agronomy Journal 2019, 111, 1068–1078. [Google Scholar] [CrossRef]
- Brar, G.; Gomez, J.; McMichael, B.; Matches, A.; Taylor, H. Germination of twenty forage legumes as influenced by temperature. Agronomy journal 1991, 83, 173–175. [Google Scholar] [CrossRef]
- Mirsky, S.B.; Ryan, M.R.; Teasdale, J.R.; Curran, W.S.; Reberg-Horton, C.S.; Spargo, J.T.; Wells, M.S.; Keene, C.L.; Moyer, J.W. Overcoming weed management challenges in cover crop–based organic rotational no-till soybean production in the eastern United States. Weed Technology 2013, 27, 193–203. [Google Scholar] [CrossRef]
- Noland, R.L.; Wells, M.S.; Sheaffer, C.C.; Baker, J.M.; Martinson, K.L.; Coulter, J.A. Establishment and function of cover crops interseeded into corn. Crop Science 2018, 58, 863–873. [Google Scholar] [CrossRef]
- Koehler-Cole, K.; Elmore, R.W. Seeding Rates and Productivity of Broadcast Interseeded Cover Crops. Agronomy 2020, 10, 1723. [Google Scholar] [CrossRef]
- Mirsky, S.B.; Wallace, J.M.; Curran, W.S.; Crockett, B.C. Hairy vetch seedbank persistence and implications for cover crop management. Agronomy Journal 2015, 107, 2391–2400. [Google Scholar] [CrossRef]
- Sauer, T.J.; Hatfield, J.L.; Prueger, J.H. Corn Residue Age and Placement Effects on Evaporation and Soil Thermal Regime. Soil Science Society of America Journal 1996, 60, 1558–1564. [Google Scholar] [CrossRef]
- Blanco-Canqui, H.; Wortmann, C. Crop residue removal and soil erosion by wind. Journal of Soil and Water Conservation 2017, 72, 97A–104A. [Google Scholar] [CrossRef]
- Boyd, N.S.; Brennan, E.B.; Smith, R.F.; Yokota, R. Effect of Seeding Rate and Planting Arrangement on Rye Cover Crop and Weed Growth. Agronomy Journal 2009, 101, 47–51. [Google Scholar] [CrossRef]
- Gaba, S.; Perronne, R.; Fried, G.; Gardarin, A.; Bretagnolle, F.; Biju-Duval, L.; Colbach, N.; Cordeau, S.; Fernández-Aparicio, M.; Gauvrit, C. Response and effect traits of arable weeds in agro-ecosystems: a review of current knowledge. Weed Research 2017, 57, 123–147. [Google Scholar] [CrossRef]
- Mahé, I.; Cordeau, S.; Bohan, D.A.; Derrouch, D.; Dessaint, F.; Millot, D.; Chauvel, B. Soil seedbank: Old methods for new challenges in agroecology? Annals of Applied Biology 2021, 178, 23–38. [Google Scholar] [CrossRef]
- Ibrahim, E.A. Seed priming to alleviate salinity stress in germinating seeds. Journal of Plant Physiology 2016, 192, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Karssen, C.M.; Haigh, A.; Van der Toorn, P.; Weges, R. Physiological mechanisms involved in seed priming. In Recent advances in the development and germination of seeds, Springer: 1989; pp. 269-280.
- Jisha, K.; Vijayakumari, K.; Puthur, J.T. Seed priming for abiotic stress tolerance: an overview. Acta Physiologiae Plantarum 2013, 35, 1381–1396. [Google Scholar] [CrossRef]
- Willenborg, C.J.; Wildeman, J.C.; Miller, A.K.; Rossnagel, B.G.; Shirtliffe, S.J. Oat germination characteristics differ among genotypes, seed sizes, and osmotic potentials. Crop Science 2005, 45, 2023–2029. [Google Scholar] [CrossRef]
- Eriksson, O. Seed size variation and its effect on germination and seedling performance in the clonal herb Convallaria majalis. Acta Oecologica 1999, 20, 61–66. [Google Scholar] [CrossRef]
- Giri, G.S.; Schillinger, W.F. Seed priming winter wheat for germination, emergence, and yield. Crop science 2003, 43, 2135–2141. [Google Scholar] [CrossRef]
- Yusefi-Tanha, E.; Fallah, S.; Pessarakli, M. Effects of seed priming on growth and antioxidant components of hairy vetch (Vicia villosa) seedlings under chilling stress. Journal of Plant Nutrition 2019, 42, 428–443. [Google Scholar] [CrossRef]
- Snapp, S.; Price, R.; Morton, M. Seed priming of winter annual cover crops improves germination and emergence. Agronomy journal 2008, 100, 1506–1510. [Google Scholar] [CrossRef]
- Parera, C.A.; Cantliffe, D.J. Presowing seed priming. Horticultural reviews 1994, 16, 109–141. [Google Scholar]
- Dutta, P. Seed priming: new vistas and contemporary perspectives. In Advances in seed priming, Springer: 2018; pp. 3-22.
- Nawaz, J.; Hussain, M.; Jabbar, A.; Nadeem, G.A.; Sajid, M.; Subtain, M.U.; Shabbir, I. Seed priming a technique. International Journal of Agriculture and Crop Sciences 2013, 6, 1373. [Google Scholar]
- Kalsa, K.K.; Abebie, B. Influence of seed priming on seed germination and vigor traits of Vicia villosa ssp. dasycarpa (Ten.). African Journal of Agricultural Research 2012, 7, 3202–3208. [Google Scholar]
- Pedrini, S.; Merritt, D.J.; Stevens, J.; Dixon, K. Seed coating: science or marketing spin? Trends in plant science 2017, 22, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Amirkhani, M.; Netravali, A.N.; Huang, W.; Taylor, A.G. Investigation of soy protein–based biostimulant seed coating for broccoli seedling and plant growth enhancement. HortScience 2016, 51, 1121–1126. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Synergistic biostimulatory action: Designing the next generation of plant biostimulants for sustainable agriculture. Frontiers in Plant Science 2018, 9, 1655. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant and soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Qiu, Y.; Amirkhani, M.; Mayton, H.; Chen, Z.; Taylor, A.G. Biostimulant seed coating treatments to improve cover crop germination and seedling growth. Agronomy 2020, 10, 154. [Google Scholar] [CrossRef]
- De Morais, L.F.; Deminicis, B.B.; de Pádua, F.T.; Morenz, M.J.; Araujo, R.P.; de Nepomuceno, D.D. Methods for breaking dormancy of seeds of tropical forage legumes. American Journal of Plant Sciences 2014, 2014. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. A classification system for seed dormancy. Seed science research 2004, 14, 1–16. [Google Scholar] [CrossRef]
- Smýkal, P.; Vernoud, V.; Blair, M.W.; Soukup, A.; Thompson, R.D. The role of the testa during development and in establishment of dormancy of the legume seed. Frontiers in Plant Science 2014, 5, 351. [Google Scholar]
- Soltani, E.; Gruber, S.; Oveisi, M.; Salehi, N.; Alahdadi, I.; Javid, M.G. Water stress, temperature regimes and light control induction, and loss of secondary dormancy in Brassica napus L. seeds. Seed Science Research 2017, 27, 217–230. [Google Scholar] [CrossRef]
- Huang, S.; Gruber, S.; Stockmann, F.; Claupein, W. Dynamics of dormancy during seed development of oilseed rape (Brassica napus L.). Seed Science Research 2016, 26, 245–253. [Google Scholar] [CrossRef]
- Gorecki, M.; Long, R.; Flematti, G.; Stevens, J. Parental environment changes the dormancy state and karrikinolide response of Brassica tournefortii seeds. Annals of Botany 2012, 109, 1369–1378. [Google Scholar] [CrossRef]
- Vercellino, R.B.; Pandolfo, C.E.; Cerrota, A.; Cantamutto, M.; Presotto, A. The roles of light and pericarp on seed dormancy and germination in feral Raphanus sativus (Brassicaceae). Weed Research 2019, 59, 396–406. [Google Scholar] [CrossRef]
- Malik, M.S.; Norsworthy, J.K.; Riley, M.B.; Bridges, W. Temperature and light requirements for wild radish (Raphanus raphanistrum) germination over a 12-month period following maturation. Weed Science 2010, 58, 136–140. [Google Scholar] [CrossRef]
- Adkins, S.W.; Bellairs, S.M.; Loch, D.S. Seed dormancy mechanisms in warm season grass species. Euphytica 2002, 126, 13–20. [Google Scholar] [CrossRef]
- Stump, W.L.; Westra, P. The seedbank dynamics of feral rye (Secale cereale). Weed Technology 2000, 14, 7–14. [Google Scholar] [CrossRef]
- Goggin, D.E.; Steadman, K.J.; Emery, R.J.N.; Farrow, S.C.; Benech-Arnold, R.L.; Powles, S.B. ABA inhibits germination but not dormancy release in mature imbibed seeds of Lolium rigidum Gaud. Journal of Experimental Botany 2009, 60, 3387–3396. [Google Scholar] [CrossRef]
- Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol 2006, 171, 501–523. [Google Scholar]
- Poljakoff-Mayber, A.; Popilevski, I.; Belausov, E.; Ben-Tal, Y. Involvement of phytohormones in germination of dormant and non-dormant oat (Avena sativa L.) seeds. Plant Growth Regulation 2002, 37, 7–16. [Google Scholar] [CrossRef]
- Badalzadeh, A.; Shahraki, A.D. Effect of Hydro-priming and Salinity Stress on Germination Indices of Niger (Guizotia abyssinica Cass.). Acta Universitatis Agriculturae et Silviculturae Mendelianae Brunensis 2021, 69, 46. [Google Scholar] [CrossRef]
- Kaya, M.D.; Okçu, G.; Atak, M.; Cıkılı, Y.; Kolsarıcı, Ö. Seed treatments to overcome salt and drought stress during germination in sunflower (Helianthus annuus L.). European journal of agronomy 2006, 24, 291–295. [Google Scholar] [CrossRef]
- Allen, R.; Hollingsworth, L.; Thomas, J. Sunflower planting and emergence with coated seed. Transactions of the ASAE 1983, 26, 665–0668. [Google Scholar] [CrossRef]
- Stassinos, P.M.; Rossi, M.; Borromeo, I.; Capo, C.; Beninati, S.; Forni, C. Enhancement of Brassica napus Tolerance to High Saline Conditions by Seed Priming. Plants 2021, 10, 403. [Google Scholar] [CrossRef]
- Bijanzadeh, E.; Nosrati, K.; Egan, T. Influence of seed priming techniques on germination and emergence of rapeseed (Brassica napus L.). Seed Science and Technology 2010, 38, 242–247. [Google Scholar] [CrossRef]
- Willenborg, C.J.; Gulden, R.H.; Johnson, E.N.; Shirtliffe, S.J. Germination characteristics of polymer-coated canola (Brassica napus L.) seeds subjected to moisture stress at different temperatures. Agronomy journal 2004, 96, 786–791. [Google Scholar] [CrossRef]
- Begum, N.; Gul, H.; Hamayun, M.; Rahman, I.U.; Ijaz, F.; Sohail, Z.I.; Afzal, A.; Afzal, M.; Ullah, A.; Karim, S. Influence of seed priming with ZnSO and CuSO on germination. Middle-East Journal of Scientific Research 2014, 22, 879–885. [Google Scholar]
- Rao, S.; Akers, S.; Ahring, R. Priming Brassica Seed to Improve Emergence under Different Temperatures and Soil Moisture Conditions 1. Crop science 1987, 27, 1050–1053. [Google Scholar] [CrossRef]
- Chin, J.M.; Lim, Y.Y.; Ting, A.S.Y. Biopolymers for biopriming of Brassica rapa seeds: A study on coating efficacy, bioagent viability and seed germination. Journal of the Saudi Society of Agricultural Sciences 2021, 20, 198–207. [Google Scholar] [CrossRef]
- Ahmad, M.; Waraich, E.A.; Hussain, S.; Ayyub, C.M.; Ahmad, Z.; Zulfiqar, U. Improving Heat Stress Tolerance in Camelina sativa and Brassica napus Through Thiourea Seed Priming. Journal of Plant Growth Regulation 2021, 1–17. [Google Scholar] [CrossRef]
- Huang, P.; He, L.; Abbas, A.; Hussain, S.; Hussain, S.; Du, D.; Hafeez, M.B.; Balooch, S.; Zahra, N.; Ren, X. Seed priming with sorghum water extract improves the performance of camelina (camelina sativa (L.) crantz.) under salt stress. Plants 2021, 10, 749. [Google Scholar] [CrossRef]
- Pimpini, F.; Sambo, P. Seed germination of rocket (Eruca sativa Mill.) as affected by osmo-priming. Atti V Giornate Scientifiche SOI (Italy) 2000.
- Kaymak, H.Ç.; Güvenç, İ.; Yarali, F.; Dönmez, M.F. The effects of bio-priming with PGPR on germination of radish (Raphanus sativus L.) seeds under saline conditions. Turkish Journal of Agriculture and Forestry 2009, 33, 173–179. [Google Scholar] [CrossRef]
- Gheidary, S.; Akhzari, D.; Pessarakli, M. Effects of salinity, drought, and priming treatments on seed germination and growth parameters of Lathyrus sativus L. Journal of Plant Nutrition 2017, 40, 1507–1514. [Google Scholar] [CrossRef]
- Kintl, A.; Huňady, I.; Vymyslický, T.; Ondrisková, V.; Hammerschmiedt, T.; Brtnický, M.; Elbl, J. Effect of Seed Coating and PEG-Induced Drought on the Germination Capacity of Five Clover Crops. Plants 2021, 10, 724. [Google Scholar] [CrossRef] [PubMed]
- Kavandi, A.; Jafari, A.A.; Jafarzadeh, M. Effect of seed priming on enhancement of seed germination and seedling growth of annual sainfoin (Onobrychis crista-galli (L.) Lam.) in medium and long-term collections of gene bank. Journal of Rangeland Science 2018, 8, 117–128. [Google Scholar]
- Ahmad, F.; Kamal, A.; Singh, A.; Ashfaque, F.; Alamri, S.; Siddiqui, M.; Khan, M. Seed priming with gibberellic acid induces high salinity tolerance in Pisum sativum through antioxidants, secondary metabolites and up-regulation of antiporter genes. Plant Biology 2021, 23, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Tsegay, B.A.; Andargie, M. Seed Priming with Gibberellic Acid (GA 3) Alleviates Salinity Induced Inhibition of Germination and Seedling Growth of Zea mays L., Pisum sativum Var. abyssinicum A. Braun and Lathyrus sativus L. Journal of Crop Science and Biotechnology 2018, 21, 261–267. [Google Scholar] [CrossRef]
- Skwarek, M.; Wala, M.; Kołodziejek, J.; Sieczyńska, K.; Lasoń-Rydel, M.; Ławińska, K.; Obraniak, A. Seed Coating with Biowaste Materials and Biocides—Environment-Friendly Biostimulation or Threat? Agronomy 2021, 11, 1034. [Google Scholar] [CrossRef]
- Bortolin, G.S.; Teixeira, S.B.; de Mesquita Pinheiro, R.; Ávila, G.E.; Carlos, F.S.; da Silva Pedroso, C.E.; Deuner, S. Seed priming with salicylic acid minimizes oxidative effects of aluminum on Trifolium seedlings. Journal of Soil Science and Plant Nutrition 2020, 20, 2502–2511. [Google Scholar] [CrossRef]
- Mondal, S.; Bose, B. Impact of micronutrient seed priming on germination, growth, development, nutritional status and yield aspects of plants. Journal of Plant Nutrition 2019, 42, 2577–2599. [Google Scholar] [CrossRef]
- Galhaut, L.; de Lespinay, A.; Walker, D.J.; Bernal, M.P.; Correal, E.; Lutts, S. Seed priming of Trifolium repens L. improved germination and early seedling growth on heavy metal-contaminated soil. Water, Air, & Soil Pollution 2014, 225, 1–15. [Google Scholar]
- Souguir, M.; Hassiba, F.; Hannachi, C. Effect of NaCl priming on seed germination of Tunisian fenugreek (Trigonella foenum-graecum L.) under salinity conditions. Journal of Stress Physiology & Biochemistry 2013, 9. [Google Scholar]
- Dawood, M.G.; El-Awadi, M.E. Alleviation of salinity stress on Vicia faba L. plants via seed priming with melatonin. Acta Biológica Colombiana 2015, 20, 223–235. [Google Scholar] [CrossRef]
- M'Sehli, W.; Kallala, N.; Jaleli, K.; Bouallegue, A.; Mhadhbi, H. Monopotassium phosphate (KH2PO4) and salicylic acid (SA) as seed priming in Vicia faba L. and Vicia sativa L. Bioscience Journal 2020, 36. [Google Scholar] [CrossRef]
- Tiryaki, I.; Keles, H. Reversal of the inhibitory effect of light and high temperature on germination of Phacelia tanacetifolia seeds by melatonin. Journal of Pineal Research 2012, 52, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Mao, P. Comparative Time-Course Physiological Responses and Proteomic Analysis of Melatonin Priming on Promoting Germination in Aged Oat (Avena sativa L.) Seeds. International journal of molecular sciences 2021, 22, 811. [Google Scholar] [CrossRef] [PubMed]
- Peltonen-Sainio, P.; Kontturi, M.; Peltonen, J. Phosphorus seed coating enhancement on early growth and yield components in oat. Agronomy Journal 2006, 98, 206–211. [Google Scholar] [CrossRef]
- Lee, K.-A.; Kim, Y.; Alizadeh, H.; Leung, D.W. Protection of Italian ryegrass (Lolium multiflorum L.) seedlings from salinity stress following seed priming with L-methionine and casein hydrolysate. Seed Science Research 2021, 31, 51–59. [Google Scholar] [CrossRef]
- Scott, J.; Mitchell, C.; Blair, G. Effect of nutrient seed coating on the emergence and early growth of perennial ryegrass. Australian journal of agricultural research 1985, 36, 221–231. [Google Scholar] [CrossRef]
- Khazaie, H.; Earl, H.; Sabzevari, S.; Yanegh, J.; Bannayan, M. Effects of osmo-hydropriming and drought stress on seed germination and seedling growth of rye (Secale montanum). ProEnvironment Promediu 2013, 6. [Google Scholar]
- Riazi, A.; Sharifzadeh, F.; AHMADI, A. Effect of osmopriming on seeds germination of forage millet. 2008.
- Aune, J.B.; Ousman, A. Effect of seed priming and micro-dosing of fertilizer on sorghum and pearl millet in Western Sudan. Experimental Agriculture 2011, 47, 419–430. [Google Scholar] [CrossRef]
- Den Hollander, N.; Bastiaans, L.; Kropff, M. Clover as a cover crop for weed suppression in an intercropping design: I. Characteristics of several clover species. European Journal of Agronomy 2007, 26, 92–103. [Google Scholar] [CrossRef]
- Tribouillois, H.; Dürr, C.; Demilly, D.; Wagner, M.-H.; Justes, E. Determination of germination response to temperature and water potential for a wide range of cover crop species and related functional groups. Plos One 2016, 11, e0161185. [Google Scholar] [CrossRef] [PubMed]
| Cover crop species | Dominant dormancy pattern | Main breaking dormancy method | References |
|---|---|---|---|
|
Fabaceae Vicia spp, Trifolium spp, Lathyrus sativus, Pisum sativum, Melilotus officinalis, Lupinus spp, Faba spp, and Eruca sativa) |
Physical (hard seed), physiological |
mechanical abrasion, after-ripening |
[58,114,115,116] |
|
Brassicaceae Brassica spp |
Induced secondary dormancy |
alternating temperatures + presence of light |
[117,118,119] |
| Raphanus sativus | mechanical resistance and non-leachable chemical inhibitors associated with the pericarp | dry storage | [120,121] |
|
Poaceae Sorghum spp |
Seed covering structures (mechanical, permeability and chemical barrier) |
Removal of seed coat structures |
[122] |
| Secale cereale | limited innate and induced dormancy | - | [123] |
| Lolium spp | non-deep physiological dormancy | Chilling at low temperature + dry after-ripening | [124,125] |
| Avena spp | High temperature dormancy | After-ripening in dry storage at temperatures higher than 20'C | [125,126] |
| Setaria spp | Presence of germination inhibitors in the seed coat | seed coats removed | [73] |
| Aegilops spp, Anisantha spp, Anisantha spp, Bromus spp, Hordeum spp and Trachynia spp. | non-deep physiological dormancy | high temperatures through dry-after-ripening | [73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





