1. Introduction
The position of potatoes on the table varies with the development of the region. In many developed countries, it acts as a vegetable with intakes vary from the lowest value in the UK of 102 g per capita to the maximum intake of 181 g per capita in Belarus per day for adults [
1]. On the other side, especially in some rural areas of America and in the highlands of Latin American countries, potato is consumed a day for adults as much as 5-6 times of quantities in developed countries [
2]. China ranks first in potatoes production and makes up about 24.53% of world production in 2018, which was deposited in the Food and Agriculture Organization of the United Nations database [
3]. The potatoes have gained its fame as a globally consumed food crop in qualities mostly from its tremendous yield per unit area [
4], affordability [
5] and large daily consumption. Potato functions as an antioxidant, antibacterial, anti-inflammatory, anti-obesity, anti-cancer, and anti-diabetes in human and animal clinical studies [
6]. Compounds existing in potatoes such as starch, protein, fiber, mineral, polyphenols, and carotenoids are thought to have a variety of benefits for human beings [
6], although significant differences in the nutritional profiles and contributions of different potato cultivars to the human body. For whole potato tuber, the carotenoid concentration of yellow-fleshed potatoes is higher than that of white-or purple-fleshed potatoes, while the anthocyanin concentration of purple potatoes is higher than that of red or white-fleshed potatoes. Since polyphenols are mainly concentrated in peels, colored potatoes generally have higher anthocyanin and carotenoid concentration than whole white potatoes. The beneficial properties are attributed to the presence of these nutritional compositions.
However, with the increasing concern about weight and diabetes as well as other cancers, potato behaviors as carbohydrate-rich food and are generally considered to a high glycemic index and glycemic load [
7,
8], which routinely described have something to do with the risk of type 2-diabetes [
8,
9] and weight gain [
10]. Hopefully, some studies support the prospective effects of eating potatoes on our overall health [
11], even though a small minority of them claim that the consumption does not affect weight control or diabetes [
6]. While some researches indicated that the consumption of potatoes has a direct association with an increase in hypertension [
12] since potassium supplementation has a potential prevention effect on hypertension and chronic disease [
13]. To our knowledge, hypertension is a major risk factor for cardiovascular diseases, especially coronary heart disease, stroke, and heart failure, as well as renal failure (WHO, 2004). This is the reason for the consumption of potatoes declined in the past few decades. To change the negative trend and alleviate grain shortage by eating potatoes in some areas, it is crucial to make it clear about the functions of nutritionally important components of potato tubers and what the factors should be first consideration while processed certain potatoes.
Before this review, there are a number of studies discussing the functional properties of potatoes, but they lack conclusive results about the main factors that have on those compounds. Thus, this review intends to summarize the factors that influence the changes in the main functional components of potatoes and discuss referred research focuses, then help to make it clear for further researchers to make efforts into. These recent researches have revealed possible directions for breeding programs and processed approaches to foster the functional composition of potatoes.
2. Potato starch and its functional properties
2.1. Structure of potato starch
Starch is a chief reserve carbohydrate for providing energy in potato tubers [
14], which is a semi-crystalline biopolymer and consists of two major components: amylose and amylopectin. Native potato starch contains a large proportion of amylopectin nearly up to 70%. Amylose, with approximately 0.2% α-(1→6) linkages, exists as a long linear glucan, the molecular chain turns every 6 glucosyl units to form a helical structure [
15] which can form a double helix by associating with another glucan chain. The established double helix is resistant to digestive enzymes [
16] and therefore the proportion of amylase in starch affects starch digestion. Amylopectin, with about 5% of α-(1→6) linkages, shows a complex branch structure thereby is less likely to associate with other molecular. Thus, starch with a higher ratio of amylose to amylopectin prone to from gels than starch with a lower ratio, which is usually glue-like after heat-moisture treatment [
17].
Base on the structure of two types of starch, starch with a high amount of amylase is not recommended in complementary feeding [
17], but “high amylose” crops have been widely prevailing in the health food markets because high-amylose starch can enhance colonic fermentation, acting similarly to dietary fiber [
18]. The properties of starch, to some degree, depending on the ration of amylose and amylopectin, they are also affected by the shape and size of starch granules. The utility and development of starch involve not only food processing but also papers, cosmetic, and textile industries et al [
19].
2.2. Digestive properties of potato starch
The cooking process and storage condition can greatly influence the molecular properties, functional properties and digestibility of starch. For instance, uncooked potatoes are resistant to digestion, whereas cooked potatoes can be easily digested. Cooled potatoes are less digestible than warm cooked potatoes. It is vital to know the composition of starch in potatoes and choose proper processing and storage approaches to maximize the benefits. Starch digestion relies on its susceptibility to α-amylase and α-glucosidase (maltase) [
20]. The digestion process is affected by the architecture of starch granules and the structure of glucan, as well as the interaction between starch and other compounds [
21]. Digestible starch is the major glycemic carbohydrate providing energy and is given as the first complementary nutrients in many areas [
17].
Resistant starch (RS) is a form of starch and gets the name for resisting digestion in the small intestine [
22] but can be fermented by the colonic microflora in the large intestine [
21,
23]. RS as a functional ingredient normally derives from foods like bananas, potatoes, grains, and legumes, acting as a substrate for gut microbiome and deserving a great many health benefits for human beings [
18,
23]. Indigestible starch was reported can enhance markers of bowel health, like increasing short-chain fatty acids (SCFAs) levels [
22,
24]. As Wolfsdorf and Weinstein (2003) reported granular starches (uncooked starches) can be digested slowly and have the role of adjuvant therapy of some diseases, such as glycogen storage disease.
Potato starch, a B-type starch that has a smooth surface with no visible voids or pinholes [
25], is classified as type-2 resistant starch [
28] and less likely to vulnerable to a digestive enzyme [
26]. The starch granules do not dissolve in cold water before being modified (eg, by cooking, chemical, or enzymatic modifications). Cooking with sufficient water initiates starch gelatinization, cooked starches digestion is determined by amylose content and amylopectin structure. The parameters of gelatinization vary with the source of the starch, potato starches can be gelatinized at a temperature higher than 60 °C [
17].
The glycemic index is an indicator of the digestibility or glucogenesis of starchy food. Several in vitro methods were used to predict in vivo glycemic index [
27]. A drawback of blood glucose measurement is that blood glucose is buffered by glyconeogenesis, with the result that the amount of dietary glucose generated from starch can’t be accurately reflected [
28]. To distinguish dietary glucose from total blood glucose, the isotope tracing methods of feeding 13C-labeled or 13C-enriched starchy materials were adopted [
28].
In vitro Englyst assay has been commonly applied in the food industry. The method classifies as “rapidly digestible starch (RDS),” “slowly digestible starch (SDS),” and “resistant starch (RS), which causes argument from some researchers, such as Butterworth et al, who said that all starch molecules are digested at the same rate and there is no reason for the classification [
29,
30]. Besides, the Englyst assay doesn’t take into account the interaction between starch molecules and mucosal α-glucosidases, the two have different digestion mechanisms, from that of fungal glucoamylase [
29].
Make good use of starch in potato and properly prepare starchy foods are critical to clinical application. Starchy foods, especially amylopectin and modified starch can be applied to young children’s diet as a complementary feeding [
31]. More and more recent, numerous works for health reason were proposed, like using potato peel powder as a protein/fiber source to make the cake and by observing the quality of the cake, the baking test showed that potato peel flour can significantly enhance the cake aspect and quality, especially by substituting wheat flour with 10% potato peel powder, cake with less hardness and the brighter and more saturated brown-orange color was established [
32]. The study gives us a fascinating insight and lets us find a new way out about developing fiber-rich cakes to supply the dietary fiber intake. Additionally, as carbohydrates are an important part of a post-exercise recovery meal, potatoes can be consumed to fuel glycolysis during and after activity. Moreover, potato starch can be applied to manufacture polymers. Actively, bio-friendly and natural-based materials are an innovative schedule for plastic processing plants and packaging manufacturers.
2.3. Potato starch and edible films
Starch, a natural polysaccharide, is one of the most commonly used materials for producing films or coatings. However, starch-based films are usually brittle and not resistant to water activity, which limits their application in packaging. To prepare starch-based films with good mechanical properties, water-resistant properties as well as anti-microbial properties, numerous works have been done. Especially, to protect and preserve fruits and vegetables, various starch-based composites (films or coating) were established and their anti-microbial properties were assessed. Bajer, et al. (2020) manufactured a novel biodegradable composite based on potato starch modified with chitosan, and Aloe vera gel and glycerol as plasticizers were incorporated into the system. The composites showed good transparency, flexibility as well as thermal resistance. Meanwhile, the composite turned out to be more susceptible to the bacterial (Bacillus sp.) rather than to fungal (F. culmorum) activity [
33]. Zhang, et al. (2019) suggested cooperation of mesoporous silica nanoparticles and cinnamon essential oil (CEO) into potato starch film markedly enhanced the tensile strength and physical properties of films, the films also had better antimicrobial activity against FJ09 species than against CNRMA 03.0371 strain [
34].
By investigating the bio-composite films based on potato starch-glycerol-olive oil, incorporating with zein nanoparticles (ZNP), Farajpour et al. reported both olive oil and zein nanoparticles reduced water vapor permeability, and zein nanoparticles increased the mechanical resistance of the composite films [
35]. The effects of different treatment methods including pullulanase debranching (PD), ultrasound treatment (UT), dimethyl sulfoxide heating (DSH)) on the physicochemical properties of potato starch (PS) and PS-based films were studied. The results showed that PS-LA composite films had higher tensile strength, lower elongation at break, and lower moisture permeability than native starch-based films, the films prepared by PD method had the highest tensile strength and lowest water vapor permeability among the tested films [
36].
Some other researchers use potato husk starch to prepare edible films. For example, edible films that were fabricated from by-products, prickly pear peel mucilage (PPM) and potato husk starch (PHS) showed low water permeability (WP) [
37]. On top of that, using agro-industrial wastes and exploring the applications in food packaging contributes to sustainable alternatives due to the recovery and reuse of the processing residues.
3. Potato protein as functional food ingredients
3.1. Potato starch and edible films
Potato protein is reported to have a high biological value, which is a measure of the proportion of protein from a specific food that can be used to synthesis the protein of the organism. Some indicators can exhibit the nutritional value or protein: such as the essential amino acids index (EAAI) and the chemical score (CS) [
38]. The CS means making a comparison of each essential amino acid (EAA) in a specific protein to the content of a standard protein, typically whole egg. The CS of the EAAs and the EAAI can be calculated concerning the reference protein of the joint FAO/WHO (1991).
3.2. Amino acid composition of potato protein
EAAI and CS of EAA present in potatoes of different flesh color varieties are shown in
Table 1. As shown in
Table 1, potato contains plenty of aspartic and glutamic acids and their amides, also EAAs like leucine, lysine, phenylalanine, valine, and tyrosine [
39]. Potatoes are one of the best plant sources of lysine [
40], an EAA generally absent in grains. The main fractions of proteins present in the tuber are patatin, protease inhibitors, and other high-molecular-weight proteins [
41]. They are associated with several health benefits such as lower allergic response [
42], antimicrobial effects [
43], antioxidant potential [
44], and the regulation of blood pressure and blood serum cholesterol control [
44,
45] and anticarcinogenic behavior [
46]. To compare with egg white, potato protein is of great biological and nutritional value, and it has a CS range of 57-69 [
47], changing depending on the storage time and variety [
38].
The amino acid profile in dry matter of potato was checked, saying leucine limits the quality of purple- and red-fleshed varieties while sulfur amino acids, methionine, and cysteine primarily influences yellow-fleshed cultivars [
38]. The result consistent with the data published by Eppendorfer et al. (1994) who thought that methionine, cysteine and leucine were the most limited amino acids in the studied potato tubers [
48], which is however in contrast to another report claiming that the sulfur amino acids were the limiting amino acids in all investigated potato varieties [
49].
Peksa et al. surveyed the influence of storage conditions of potato on its amino acid composition, they concluded that with the increased storage time under certain conditions, the total protein and amino acid content declined. Most parts of the amino acid content decreased from 19 to 6% and from 38 to 21% after three and six months’ storage, respectively. Nevertheless, the coagulable protein showed a rising tendency and increased by 25%. They also claimed that storage temperature did the effect on the coagulable protein content or serine, glycine, cysteine, tyrosine, and phenylalanine [
38].
3.3. Fractions of potato protein
There are two key fractions of potato protein which can be treated with high nutritive value: coagulable protein (nitrogen compounds which can be precipitated by trichloroacetic acid) and nonprotein organic compounds (such as free amino acids). In particular, the coagulable protein is more valuable since its well-balanced amino acid pattern [
50]. The total and coagulable protein content in the potatoes depended on the variety, not on the flesh color [
38]. Different extraction techniques of tuber protein like precipitation methods (thermal and acidic precipitation, salt precipitation, ethanol precipitation, ammonium sulfate precipitation, carboxymethyl cellulose complexation), ion exchange, and separation of bioactive proteins and peptides have been explored to retain functionality and associated health benefits, and in turn, improve their potential application. The recovery of native protein from potato fruit water discharged from the starch factory has been commercially produced, in 2007, the Dutch potato starch group introduced the adsorbent processing platform with proprietary mixed-mode ligand chemistry in its subsidiary of Solanic to extract high-performance proteins for the food and pharmaceutical industries [
51]. Jin, et al. [
52] studied the effect of continuous polymer and pore phase known as Amberlite XAD7HP in lab-scale and pilot-scale on the recovery of potato protein from potato fruit water, this method resulted in an extract primarily composed of a large proportion of protease inhibitors.
3.4. Potential applications of potato protein
Cardiovascular disease (CVD), as a major global crisis, has been arousing wide interests around the world. The schedule of control hypertension and prevent CVD is considered to use the angiotensin-converting enzyme (ACE) inhibitory to regulate blood pressure and cardiovascular function currently can be very promising [
53]. Nowadays, even though several ACE inhibitors are available, we cannot ignore some side effects of them, like dry cough and angioedema [
54]. Thus, nutraceuticals such as bioactive peptides will be a choice to replace those drugs, which can reduce the cost of drug therapy and are more safety [
55,
56]. Although few doubts remain, some food-derived peptides have been shown to have ACE inhibitory activity in vitro and many of them performed significant anti-hypertensive activity in rat studies [
57]. The arguments arise because of different study designs and diverse methods of blood pressure, as well as a variety of genetic backgrounds of the study population [
58]. Mäkinen et al. designed a schedule to check the effects of potato peptides (PP) and rapeseed peptides (RP) on blood pressure in vivo in the Goldblatt rat model of hypertension and showed that both the two peptides had the anti-hypertensive effects due to synergistic effects of several different ACE inhibitory peptide sequences in the samples [
59]. According to current knowledge, more extensive researches focus on the practical application of food-derived bioactive peptides and on solving their not enough effective issue for replacing drug therapy in the actual treatment of hypertension. While other functional qualities of potato protein such as emulsification and foaming abilities should be taken into account since they can help broaden the application of potato in the food industry.
Potato protease inhibitors have several potential applications, such as treatment for weight loss, periananl dermatitis, infections, thrombotic disease, and cancer. Previous research showed that potato protease inhibitors can elevate plasma cholecystokinin levels which influences food intake [
60,
61]. This gastrointestinal hormone is involved in satiety and food intake regulation as well as blood glucose control in humans, and its increased levels in plasma delay gastric emptying, including feelings of fullness and reduce food intake. Most noteworthy, the Slendesta (a natural potato protein with 5% protease inhibitor P12) was found that can enhance the feeling of fullness without any side effect. When food is eaten, a factor appears in the gut that is absorbed by the small intestine. The small intestine then releases a signal molecule called CCK into the bloodstream. CCK travels through the bloodstream to various digestive organs and to the vagus nerve which signals the brain to produce feelings of fullness and satisfaction. A clinical study showed it can help people manage hunger and then achieve the goal of healthy weight management. Consuming 300 mg Slendesta (contain 15mg P12) before diets have a-day effect on dietary intake since P12 helps to increase CCK level and causing, prolong feelings of fullness [
62,
63,
64]. Slendasta has been produced by an American company KEMIN, as a successful commercial product of controlling weight provides new insight into solving obesity. Due to the potential allergenicity of animal protein in susceptible subjects, it has gained much attention that using plant-derived proteins as a wine fining agent.
Patatin P is the name of a family of glycoproteins that can be recovered from potato aqueous by-product. It has been proved that Patatin is a suitable alternative to animal proteins used as a fining agent since it can reduce the total phenolics and tannins, as well as it can diminish astringency and the content of red wine phenolics able to react with salivary proteins [
65].
Potato tuber proteins also can inhibit human fecal proteolytic activity efficiently, which opens up the possibility of using potato protease inhibitors to prevent and treat the disease. Potato carboxypeptidase inhibitor has been seen as a potential fibrinolytic agent for treatment and prevention of thrombotic disease for it can inhibit plasma carboxypeptidase activated thrombin-activatable fibrinolysis inhibitor [
66,
67,
68]. Besides, Blanco-Aparicio et al (1998) showed that potato carboxypeptidase inhibitor competes with epidermal growth factor for binding to the receptor and has antitumoral properties; the epidermal growth factor receptor signal transduction pathway plays a prominent role in the development of carcinomas [
69]. Potato tuber protease inhibitors also interfere with hydrogen peroxide formation [
70], and processes resulting from solar UV radiation [
71]. A series of antimicrobial Kunitz-type serine protease inhibitors (AFP-J, PT-1, Potide-G) have recently been isolated from potato tubers. As showed that AFP-J has potent antifungal activity against human fungal pathogens, eg., Candida albicans, which is the most common cause of candidiasis [
72]. PT-1strongly inhibited pathogenic microbial strains, including C. Albicans, the plant pathogenic fungus Rhizoctoniasolani, and the plant pathogenic bacterium Clavibacter michiganese [
73]. Potide-G potently inhibited the growth of various human or plant pathogenic bacterial (Staphylococcus aureus, Listeria monocytogenes, Escherichia coli, C. michiganense) and fungal (C. Albicans, R. solani) strains, and has also potent antiviral activity against potato virus Y infection [
74]. According to current knowledge, more extensive researches focus on the practical application of food-derived bioactive peptides and on solving the not enough effective issue for replacing drug therapy in the actual treatment of hypertension. While other functional qualities of potato protein such as emulsification and foaming abilities should be taken into account since they can help broaden the application of potato in the food industry. Potato protease inhibitors have several potential applications involving treatment for weight loss, peri-ananl dermatitis, infections, thrombotic disease, and cancer. Previous research showed that potato protease inhibitors can elevate plasma cholecystokinin levels which influences food intake [
60,
61]. This gastrointestinal hormone is involved in satiety and food intake regulation as well as blood glucose control in humans, and its increased levels in plasma delay gastric emptying, including feelings of fullness and reduce food intake.
4. Potato phytochemicals and nutritional potential
4.1. Functional phytochemicals in potato
Potatoes are an excellent source of antioxidants, which mainly including carotenoids, anthocyanin, phenolic compounds, and vitamin C. Nutritionally, these compounds play a role in preventing cancer and heart attacks with the potent antioxidative properties [
75]. Carotenoids accumulate in many plants, giving yellow, orange, and red colors. The color of yellow potatoes is due to carotenoids, which present high concentration in yellow cultivars, while in red- or purple-flesh potatoes where the color may be masked by anthocyanins. Carotenoids are primarily lutein, zeaxanthin, and violaxanthin, all of which are xanthophylls, presenting in the flesh of potatoes. The composition of tuber carotenoid varies with cultivars, however, violaxanthin and lutein usually are the most abundant proportions.
4.2. Carotenoids and health benefits
The quantities of total carotenoid in vegetables vary among cultivars and range from 0.038 (potato) to 17.31 (spinach) [
76], mg/100 g FW, whereas in potato, the value range from 0.038 to 2.00 mg/100 g FW [
77,
78,
79]. Among all the fresh fruits and vegetables analyzed, some certain potatoes (2.00 mg/100 g FW) [
80] have a comparable value with other vegetables, like cabbage (0.25 to 0.43 mg/100 g FW) [
76], strawberry (0.96 to 3.30 mg/100 g FW) [
81] and tomato (1.63 to 8.57 mg/100 g FW) [
82,
83].
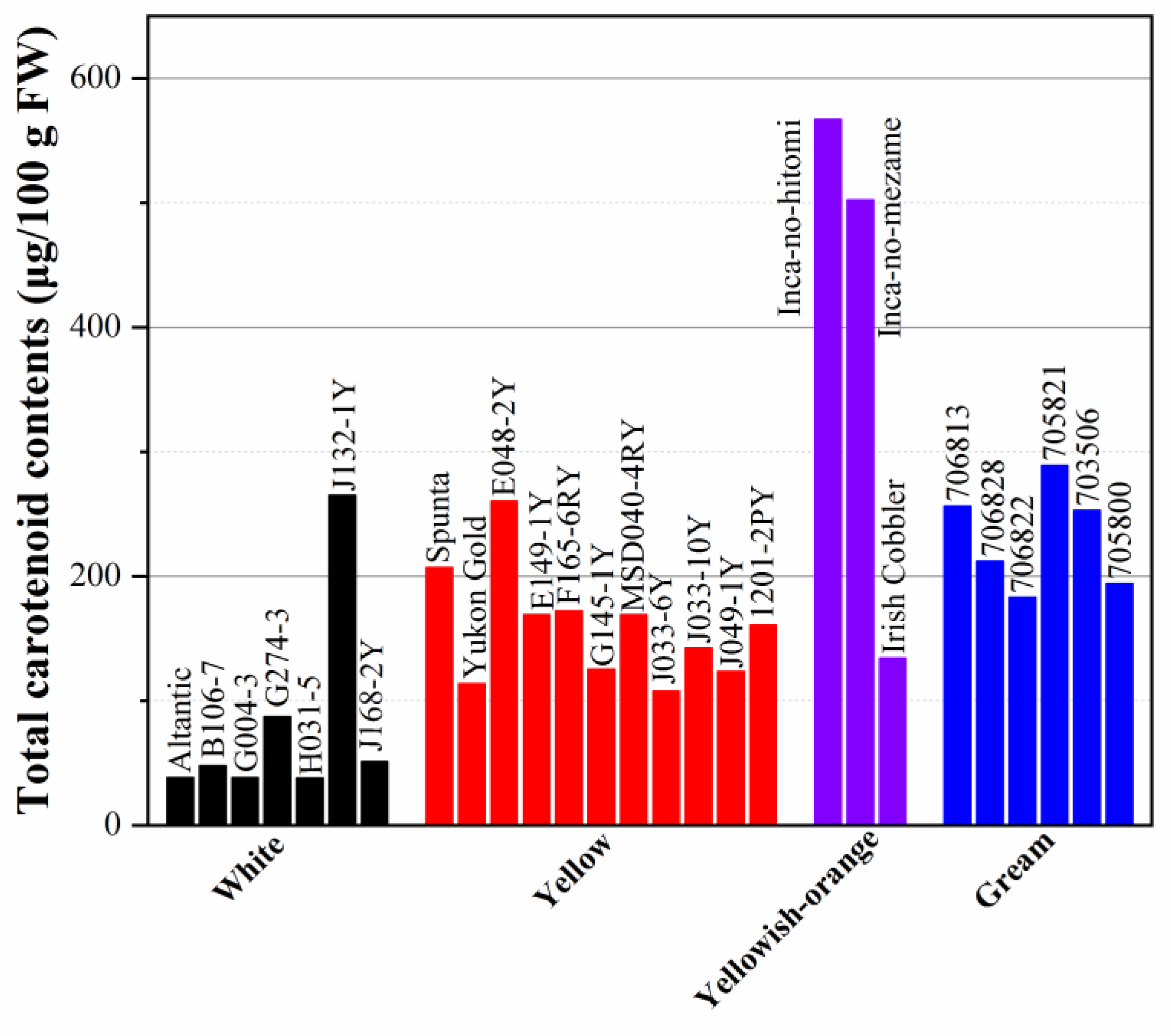
The carotenoids concentration in colored flesh potatoes is shown in
Figure 1. The content of carotenoids presents 38.1-265μg /100 g FW in white-fleshed varieties and 107.5-260.3μg /100g FW in yellow-fleshed cultivars to 567μg /100g FW in yellowish-orange cultivars, some dark yellow-fleshed cultivars even have a carotenoids content as high as 2000μg /100g FW (As shown in
Figure 1). Among all the various colored fresh cultivars, yellow-fresh varieties have the highest carotenoid content, followed by cream and white. Besides, as shown that over 100 cultivars grown in Ireland and Spain had carotenoid content from trace amounts to 28 μg/g DW in the skin and 9 μg/g DW in the flesh [
84,
85]. The lipophilic extract of potato with total carotenoids ranging from 35 to 795 per 100 g FW flesh shows 4.6-15.3 nmoles α-tocopherol equivalents per 100 g FW of oxygen radical absorbance capacity (ORAC) values [
86].
Furthermore, potato as a staple food is most commonly consumed and has the most intake every day than other selected vegetables. That is to say, potato can be a key and more accessible carotenoid supplement in our daily life. Potato is not an origin of pro-vitamin. However, carotenoids can have provitamin A activity and thus can decrease the risk of several diseases [
87,
88], age-related macular degeneration, and the onset of cataracts [
89,
90,
91]. The process of carotenoid accumulation is the result of biosynthesis, degradation, and stable storage of synthetic products [
92,
93].
Multiple factors control the broad diversity of carotenoid composition and content in storage tissue. Regulation of the catalytic activity of carotenoid biosynthesis can be a key and practical control for the final carotenoid accumulation. Phytoene synthase (PSY) is the rate-limiting step in the carotenoid biosynthetic pathway and manipulation of PSY expression in many plants has been demonstrated to enhance carotenoid synthesis by directing metabolic flux into the carotenoid biosynthetic pathway [
94,
95,
96].
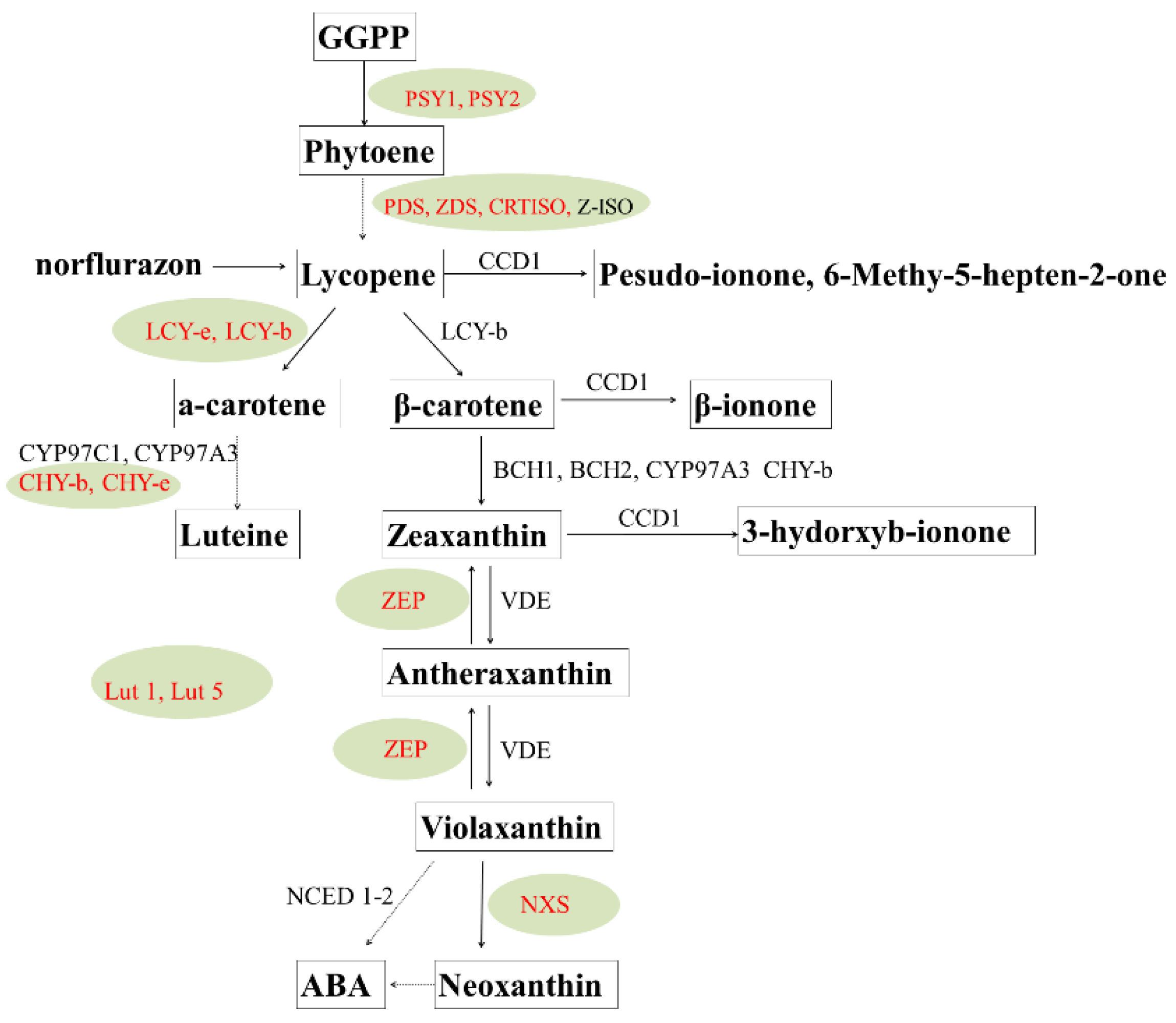
Carotenoid cleavage dioxygenases (CCDs) catabolizes enzymatic degradation of carotenoids. Expression of these genes inversely regulates carotenoid accumulation [
97,
98]. LCY-b and LCY-e can manipulate the synthesis of α-carotene and β-carotene respectively. The tissue-specific expression of carotenoid biosynthesis genes in potato is marked in red color in
Figure 2. Some measures like traditional breeding and metabolic engineering approaches have been used to prove the tuber carotenoid. Traditional breeding can increase carotenoids based on broad-sense heritability of carotenoids [
99]. Y locus can largely determine the tuber fresh color and zeaxanthin synthesis, encoding a β-carotene hydroxylase [
100,
101]. Molecular analysis identified a QTL on chromosome 3 that accounted for up to 71% of the carotenoid variation that was probably an allele of β–carotene hydroxylase, and several additional alleles affecting carotenoid amounts have been identified [
102,
103]. Multiple transgenic approaches have achieved a big success in increasing tuber carotenoids. Overexpressing bacterial phytoene synthase increased total carotenoids from 5.6 to35 μg/g DW, lutein increased 19-fold and β-carotene increased from trace amounts to 11 μg/g DW [
96]. Furthermore, a study showed that the overexpression of three bacterial genes Desiree caused a 3600-fold increase in β-carotene to 47 μg/g DW, a 30-fold increase in lutein and a 20-fold increase in total carotenoids, producing a “golden potato” [
104]. Manipulating the vitamin A pathway can fulfill 42% of the daily requirement for vitamin A (retinal activity equivalents) and 34% of the vitamin E daily requirement by consuming a modest 150 serving of boiled potatoes [
105].
4.3. Phenolic compounds and antioxidant activities
Potato supplies considerable phenolic compounds, which are concentrated in the peel and adjoining tissues [
106]. The predominant one is chlorogenic acid (CGA) and consists of about 80% of the total phenolic acids. Red- and purple-flesh potatoes typically contain greater amounts of CGA than white potatoes. CGA may have a potential effect on lowering the risk of type 2-diabetes and slowing the entry of glucose into the bloodstream [
107]. CGA in potatoes is synthesized via hydroxycinnamoyl CoA: quinatehydroxycinnamoyltransferase [
108,
109]. The R2R3 transcription factor StAN1 appears to mediate CGA expression, in addition to regulating anthocyanins [
110]. Phenylpropanoid (phenolic acids, flavonols, and anthocyanins) content varies markedly among cultivars, somehow has a relationship with the genetic diversity [
111]. Andean potato landraces have about an 11-fold variation in phenolic acids and flavan-3-ols, and a high correlation between phenolics and total antioxidant capacity [
112,
113,
114]. Chilean landraces had 8- and 11-fold more phenylpropanoids than Desiree or Shepody, two common cultivars [
115]. Phenolic content extracted from potato peel has been reported to have an antioxidant-mediated protective effect in erythrocytes against oxidative damage. However, polyphenols preservation is one of the keys to the quality of potato, concerning their flavor induction (astringency) and capacity to cause discolorations, such as enzymatic browning reactions [
106]. Since most potato phenolics except anthocyanins are colorless, thus they present in white- and yellow-fleshed cultivars, which are desirable culinary materials in many countries. Total phenols/ Total phenolic content (TPC) in a variety of plant cultivars as obtained from Folin-Ciocalteau reagent (FCR) or HPLC was shown in
Table 2.
By reviewing plenty of researches, we found it hard to make a comparison between various vegetables since different methods were used previously. Thus, the review summarizes phenolic content in various plants measured by commonly used methods of Folin-Ciocalteau reagent (FCR) or HPLC and expressed with fresh weight and dry weight to provide a reference for next further study. By checking total phenols of plants determined according to the Folin-Ciocalteu (F-C) colorimetric method with expressing as a gram of gallic acid equivalents (GAE) per kilogram of fresh weight basis(g GAE/ kg FW), Potato (0.31 to 8.83 g GAE/ kg FW) offers comparable value to some certain vegetables and fruits, such as carrot (0.16 to 10.29 g GAE/ kg FW), blueberry( 2.20 to 7.53 g GAE/ kg FW), and is superior to some cultivars, such as cauliflower( 0.57 to 2.55 g GAE/ kg FW), cabbage( 1.70 to 2.53 g GAE/ kg FW) as well as strawberry(0.99 to 3.05 g GAE/ kg FW). Besides, as expressed by a dry weight basis, potato (4.48 to 11.19 g GAE/ kg DW) can provide almost the same quantity of total phenols as spinach. According to the maximum total phenols intake (based on a gram of gallic acid equivalents per kilogram of fresh weight) from plant cultivars, if 200g potato were consumed every day, the total phenols that potato ensures should be provided by 700g cabbage or cauliflower, 170g carrot, 580 g strawberry or 970 g mushrooms, if 200g potato were consumed every day, the total phenols that potato ensures should be provided by 700g cabbage or cauliflower, 170g carrot, 580 g strawberry or 970 g mushroom. Otherwise, according to the data obtained from the HPLC method, total phenols of potato range from 23.2 to 67.4 mg/100g FW or260-2852 mg/100g DW, are similar to the green bean (17.10 to 66.3 mg/100g FW). While according to data of the Singapore Chinese population aged 45-74 years were obtained from Singapore Chinese Health study from 1993 to 1998, daily intake of potato is 6.9 g with 4.1 mg GAE/ day of TPC. In conclusion, the potato should be a lower-cost alternative for daily total phenols intake than other vegetables and fruits, especially, in some development areas, it can be the first consideration for antioxidants supplies.
4.4. Flavonoids and healthcare functions
The flavonoids, containing two leading constitutes-catechin and epicatechin, in the flesh of white potato is as high as 30 μg /100 g FW, almost twice of red- and purple-fleshed potatoes [
86]. One group suggested that flavonols increased in fresh-cut tubers up to 14mg/100g FW and though they can be a valuable dietary source because of the large number of potatoes consumed [
116]. The content of flavonols varies by more than 30-fold among different potato genotypes and there is even sizable variation within the same genotypes. Interestingly, potato flowers can synthesize a 1000-fold higher amount of flavonols than tubers which contain only micrograms per gram amount of flavonols [
117] Several studies show that quercetin and related flavonols have multiple health-promoting effects, including reduced risk of heart disease; lower risk of certain respiratory diseases, such as asthma, bronchitis, and reduced risk of some cancers including prostate and lung cancer [
118].
Colored potatoes are rich in anthocyanins that heavily exist in the skin of the potato, or partially or entirely derived from the flesh. Anthocyanins are natural colorants belonging to the flavonoid family [
119]. The compounds are responsible for all the visible colors ranging from red to blue of fruits, vegetables, flowers, and roots. The most common anthocyanidins in plants are delphinidin, cyanidin, petunidin, peonidin, pelargonidin, and malvidin [
120]. Anthocyanin-rich foods have proved an important role in the prevention of several types of human cancer, such as colon, breast, prostate, oral and stomach cancers [
121], while showing no toxic effects on normal human cells [
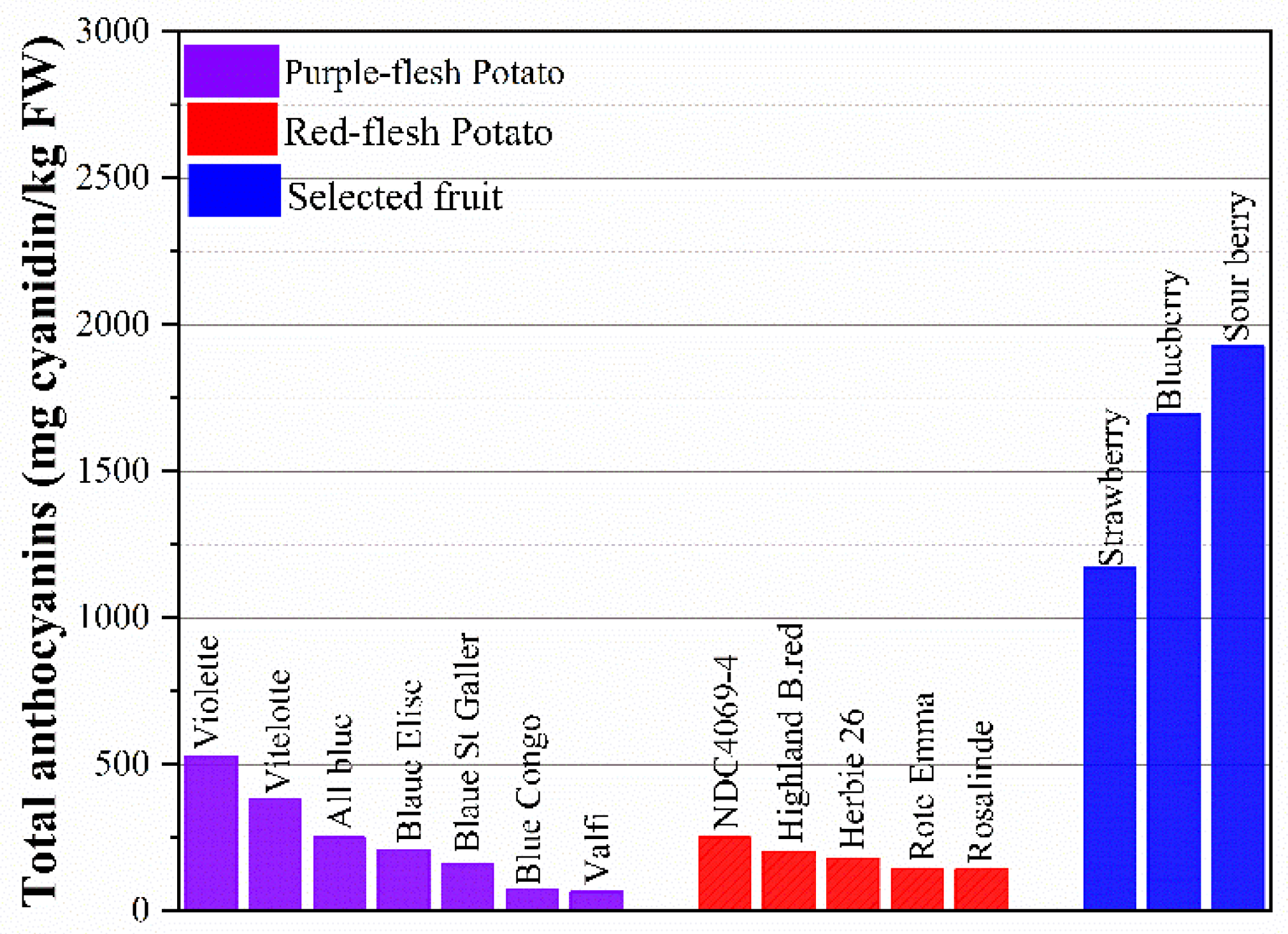
122]. Total anthocyanins of selected fruits and potatoes are shown in
Figure 3. Unpeeled potato with totally pigmented the flesh can collect up to 527.4 mg/ kg FW of total anthocyanins. Red- or purple- flesh with skin can be a profitable source of anthocyanins, is similar to cranberries, and is superior to red cabbage [
38]. As shown in
Figure 3, the purple-flesh potato has a higher total anthocyanin content than red-flesh cultivars, ranging from 65.5 to 527.4 mg cyanidin /kg FW, while the value is 142-250 mg cyanidin /kg FW in red-flesh potato. Compare to blueberry (1694 mg cyanidin /kg FW) and strawberry (1171 mg cyanidin /kg FW), which are known as high-anthocyanin food, some varieties of potatoes are hopeful and have the potential to meet the demand of anthocyanins in daily life. What most noteworthy is that potato is a more accessible food and is consumed largely than strawberry and blueberry since the high cost and season limitation in eating those fruits. Trough investigating over 50 colored-fleshed cultivars, researchers found that the anthocyanins vary in the range of 0.5-7mg/FW in the skin and up to 2mg/g FW in the flesh [
123].
The issue that arises to our attention is that high anthocyanin potatoes, usually with colored skin, are not as widely consumed as white or yellow potatoes. Tuber anthocyanin in the periderm is regulated by at least three loci, D, P, and R [
124,
125,
126]. Biochemical and expression analysis identified an AN1 transcription factor complex involved in potato anthocyanin synthesis [
127,
128], and global expression studies using microarray and RNA-seq analysis of white and purple potatoes have identified various transcription factor variants, including a ten amino acid C-terminalmotifonAN1 required for optimal anthocyanin synthesis [
129,
130,
131]. SSR markers for anthocyanin biosynthesis can be a technique for potato breeding programs [
132]. Purple-flesh potatoes known as a high-phenolic cultivar has been proved to benefit health with anti-cancer properties [
133,
134,
135,
136] and amelioration of chromium toxicity [
137]. Observed by a mouse model, anthocyanins from purple potatoes showed an effect of attenuating alcohol-induced hepatic injury [
138]. In a human feeding study adults fed 150g of purple potatoes a day for six weeks, results showed that inflammation and DNA damage decrease [
139]. Another small human trail suggested people who have an average of 54 years old consumed purple potatoes and had a significant drop in blood pressure and without weight gained [
140]. Also, postprandial glycemia and insulinemia were observed to have a downward trend in males fed purple potatoes [
140]. A previous study demonstrated that potato with high polyphenol content was inversely associated with their glycemic index [
141]. For rats fed an obesity-promoting diet, purple potatoes promised metabolic and cardiovascular benefits [
142].
4.5. Vitamins and nutritional potential
4.5.1. Vitamin C
When it comes to the bioactive compounds in potato, we cannot ignore the vitamin C, which has received the most attention. Vitamin C can be synthesized in the tuber part of the potato, and also be transported from leaves and stems and then be accumulated here [
143,
144]. Potato generally contains 20 mg /100 g FW of vitamin C, which may account for up to 13 % of the total antioxidant capacity. The vitamin C recommended intake per day for women (18-60 years old) is 60 mg. Potato (16.10 to 34.80 mg/100 g FW) [
145,
146] is a much better vitamin C source than cabbage (5.27 to 23.50 mg/100 g FW) [
147,
148] and even some kinds of tomato (8.26 to 22.54 mg/100 g FW) [
149]. It should not be ignored as a crucial nutrient supplement based on vitamin C intake. Plus, in terms of the consumption rate and economical concern, as well as the storage condition for vegetables, the potato could be the best choice for humans. As deficiency of iron has been a global problem, the consumption of potato with high Vitamin C content might be a way to solve the problem.
Based on some researches, the content of Vitamin C is not only influenced by the varieties, but also by area and time of planting [
150,
151]. Many efforts should be focused on maintaining Vitamin C level in cold storage since loses of up to 60% have been observed after cold storage [
152,
153]. By examining the Vitamin C content in 12 potato genotypes after storing for two, four, and seven months, a substantial loss after four-month storage occurred, but several showed no significant loss after two months [
154]. While this contrast markedly with a report about Vitamin C increased as many as several-fold in 11 Indian potato varieties after storage [
155]. It is highly desirable that finding a cultivar show no loss after at least two months of storage. Other studies mentioned that compare with the effect of storage temperature on Vitamin C, atmospheric oxygen levels function greater [
155].
Cares should be seriously taken during processing because almost half the Vitamin C was lost during pre-freezing, which can be actually avoided [
156]. Therefore, to optimize processing and choose proper methods to preserve crops is a practical measure to reduce the loss of vitamin C. Also, crop management plays an important role in maximizing vitamin C, say utility of high nitrogen fertilization result in reducing vitamin C amounts, followed with a more rapid loss when storage of the cut product [
157]. Consumers can choose potatoes with the peel to maximize phytonutrient intake. This theory proposes challenges for the food industry about how to improve the appearance and taste of the potatoes so that they can accept widely, also about how to use the waste generating during processing and get more natural products, including antioxidants [
158,
159,
160].
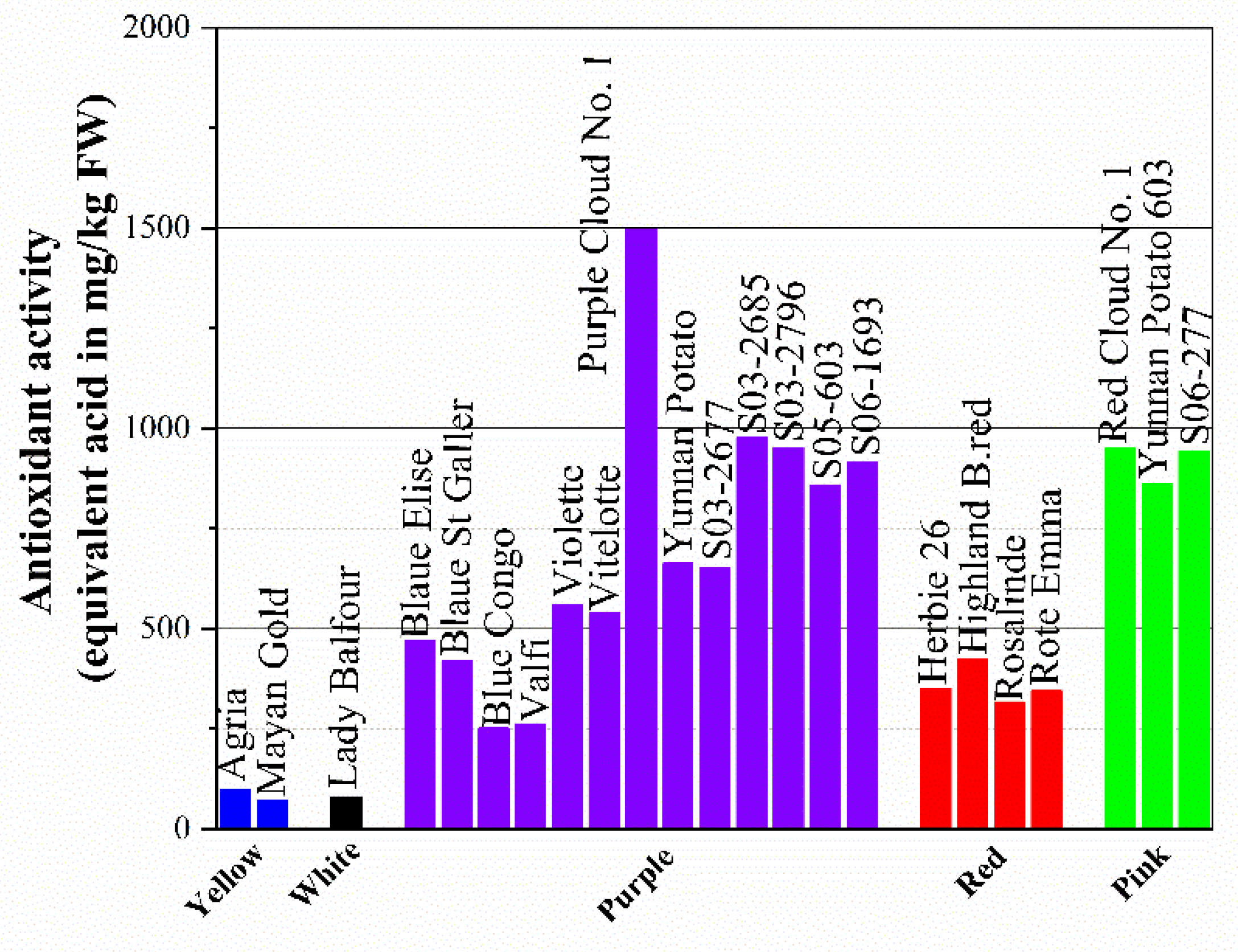
According to some studies, the hydrophilic antioxidant activity of totally pigmented red or purple potato is comparable to brussels sprouts or spinach. The total anthocyanins range from 9 to 38 mg/100 g FW and ORAC varies from 7.6 and 14.2 μmole/g FW of Trolox equivalents. Meanwhile, potato generally contains 20 mg /100 g FW of vitamin C, which may account for up to 13 % of the total antioxidant capacity. The total antioxidant activity (TAA) of potatoes was estimated using the ABTS radical cation method and the DPPH assay is presented in
Figure 4. It can be concluded from the figure that purple-fleshed potato has the highest total antioxidant activity among the selected colors with a range of 251 to 1497.6 equivalent ascorbic acid in mg/kg FW, following by pink (860.3-948.6 equivalent ascorbic acid in mg/kg FW) and red (316.9-424.4 equivalent ascorbic acid in mg/kg FW). Which means colorless cultivars, including white-fleshed ones and light yellow-fleshed potato generally have a relatively low antioxidant activity, might be responsible for low vitamin C and anthocyanins and even phenolic compounds content.
Some other studies demonstrated a reverse effect of phytochemicals on various diseases (e.g., chronic inflammation, cardiovascular diseases, cancer, and diabetes) [
161]. Many of the compounds discussed above are present in higher concentrations in immature potatoes. This is because certain tuber nutrition contents decrease as the growth of the tuber. Baby potatoes (with golf ball size) have the amounts of phenylpropanoids as many as 3-fold of mature potatoes of the same cultivar, and higher amounts of carotenoids and various other phytonutrients [
162]. CGA content decreased 39-72% during development and varied among cultivars [
162].
By investigating the effects of domestic cooking methods (boiling, baking, steaming, microwaving, frying, stir-frying, and air flying) on the composition of phytochemicals (phenolics, anthocyanins, and carotenoids) in purple-fleshed potatoes, reduction of the vitamin C, total phenolic, anthocyanin, and carotenoid contents were observed after cooking. Among these changes, the decrease of antioxidant activity was responsible for a reduction in the total phenolic content. The loss of vitamin C and phytochemicals was caused by frying methods but not alter the antioxidant activity, which is likely due to the prevention of by-products of the Maillard reactions. It should be noted that steaming and microwaving have the potential to be used as a measure to retain phytochemicals and antioxidant activity [
163]. Xu, et al. (2009) also conclude that all cooking methods (boiling, baking, and microwaving) caused a decrease in antioxidant activity and phytochemical concentration in potato [
164]. While according to the report of Blessington, et al. (2010), baking, frying, and microwaving can significantly increase the total phenolic content, chlorogenic acid content, and antioxidant activity in potatoes [
165]. And Faller and Fialho (2009) showed that despite a significant increase in the total phenolic content, the antioxidant activity of potatoes decreased [
166], whereas Burgos, et al. proposed that boiling has a positive effect on enhancing the total phenolic content and antioxidant activity and has an obvious reverse effect on the total anthocyanin content. The difference might be attributed to the different cultivars and pretreatment methods and cooking conditions, as well as the nonuniformity of analytical methods used [
167]. More importantly, anthocyanin usually is unstable and can be significantly influenced by different pH [
168]. Therefore, more systematic studies of the effects of processing and cooking methods on phytochemicals, especially the function such phytochemical compounds have on various diseases.
4.5.2. Vitamin B9
Folate (Vitamin B9) deficiency is a worldwide concern that has a connection with birth defects [
169]. Potatoes can be a source of dietary folate, providing 7%–12% of the total folate in Dutch, Finish, and Norwegian diets [
170]. Increased consumption of potatoes can help to reduce the risk of low serum folate concentrations [
171]. Folate concentrations in potatoes have been examined, the content is significantly different, and with a range of 12 and 41 μg/100g FW and 0.5–1.4 μg/g DW among cultivars. Some wild species, like S. Boliviense, contain 115 μg/100 g FW of Folate [
172,
173,
174].
4.5.3. Vitamin B6
Vitamin B6 is indispensable in a wide range of metabolic, physiological, and developmental processes and shows a high concentration in potatoes. The USDA’s Supertracker website released a medium potato that can provide 48% of the supplement of Vitamin B6. Vitamin B6 deficiency can contribute to numerous health issues, including diabetes, neurological, and skin disorders. The maturity of the potato has something to do with the degree of Vitamin B6. According to the report by Mooney et al, vitamin B6 ranged from 16 to 27 μg/g DW in immature and mature tubers [
175]. Also, there is a small proportion of thiamine (vitamin B1) in potatoes with a concentration of 0.06–0.23 μg/ 100 g FW [
173,
176]. Thiamine content as a moderate broad-sense heritability, suggesting that breeding program can be the first consideration for increasing the level [
177].
5. Potato Minerals and health benefits
5.1. Potassium
Potato is also well known to be a provider of potassium, copper, phosphorous, iron, zinc, magnesium, and manganese. This makes itself an indispensable candidate for contributing to the alleviation of mineral deficiencies caused by dietary constraints. Especially, for potassium supplement, potato behaves extremely well. The content of potassium in potatoes is higher than bananas, a well-known potassium source (SR28 2016). Potassium content increases during the entire growing season of potato [
178]. The range of potassium content in potato is about 3.0–8.2 mg/g FW [
14,
179,
180]. Contents (mg/100g) of certain macro- and microelements in edible parts of a variety of plant cultivars in comparison to recommended intakes are presented in
Table 3. As we conclude, potato as a potassium source containing potassium in range of 104-540 mg/100g FW and is superior to partial vegetables, even has a comparable quantity with banana which is known as a high-potassium food. Consumed potato together with cabbage, cauliflower, spinach, or even cow milk can promise the recommended daily intake of potassium which is about 3.1g for an 18-60 years old woman. In a study carried out in the Pacific Northwest, the potassium content for breeding lines and established varieties was between 19-25mg/g DW. Broad-sense heritabilities (H) were 0.33 and 0.81 for the Tri-State russet skin varieties and Regional Red/Specialty varieties [
181]. A survey of wild tuber-bearing Solanum species in the Commonwealth Potato Collection showed a range of 15–27 mg potassium /g DW [
182].
The interaction between sodium and potassium can maintain the balance of blood pressure, reduce the risk of heart disease, strokes, and kidney stones, and guarantee normal heart rhythms. The potassium in potatoes is also vital for helping muscles contract. Meanwhile, a study demonstrated that potassium deficiency correlates with the hardening of the arteries [
183]. Some estimate claims that only 2% of Americans intake enough potassium. Potassium deficiency has been proposed as a public health concern in the 2015–2020 Dietary Guidelines for Americans. The average adult intake is about half of the Institute of Medicine’s recommendation of 4700 mg/day [
184]. Storey and Anderson (2016) surveyed average nutrient intakes as well as total vegetable and white potato (WP) consumption among children aged 1–3 years using day 1 dietary data from the NHANES 2009–2012 and the Food Patterns Equivalents Database 2009–2012 and concluded that average intakes of most nutrients, including calcium, were exceeded Dietary Reference Intakes (DRIs), but the average intakes of potassium, dietary fiber (DF), and vitamin D were 67%, 55%, and 49% of DRIs, respectively. Looking for excellent sources of potassium and DF, such as potatoes, is an urgent need today [
185]. The final content of most minerals of potato relies on whether removing the skin, and the way of processing, preparation, and cooking such a material [
14,
186,
187,
188]. The USDA nutrient database typically lists baked potatoes within 5.5mg/ g FW and a potato that contains 8.2mg/g FW would provide ~50% more potassium. Selecting the fine genetic capacity of producing higher amounts of potassium is expected to be on the agenda. The potassium in potato tuber increased throughout the growing season as a report reveals [
189]. Some factors, like the environment as well as the crop management, can affect the tuber potassium content, for example, drought condition was found to increase K about 12% [
190].
5.2. Iron
Nearly two billion people worldwide have been suffering iron deficiency and are one of the most widespread nutritional deficiencies of all according to the World Health Organization (WHO), which calls it a “public health condition of epidemic proportions” [
191]. Potatoes contain modest amounts of iron, which should be readily bioavailable because of its lower phytic acid content than other crops. Considering the high broad-sense heritability estimates up to 0.73 for potato, developing new cultivars with higher amounts of iron should be achievable [
192,
193]. A range of 17-62 μg / g DW was presented in an investigation about potatoes grown in the Pacific Northwest, Colorado, and Texas [
192]. Some varieties were reported to have a rather wider range of 0.3-2.3 mg Fe/g FW [
194]. As shown in
Table 3, the amount is 0.34-1.80 mg/100g FW in potato. The iron content of some Andean germplasm is comparable to amounts in rice, maize, and wheat [
195].
5.3. Magnesium
Magnesium is another mineral necessary for the human body. Couples of studies showed that increased magnesium intake may help to inhibit diabetics, one of the most widespread and costly diseases [
196]. The RDA for adults ranges from 310 to 420 mg. It is estimated that nearly 70% of American adults lack Mg, and the other 20% of them consume less than half of the recommended amount of magnesium. Mg deficiency is associated with higher levels of inflammation and cardiovascular risk [
197]. Potato magnesium levels were reported to range from 142 to370 μg/g FW [
198,
199] and 0.9–1.1 mg/g DW. However, the magnesium content in potato showed in
Table 3 is 13.7-41.9 mg/100g FW. The content is almost equal to the quantities in cabbage. The cultivated area of potato could be a factor that decides the content of Mg. In the Pacific Northwest, Colorado, and Texas, the magnesium content of potatoes ranged from 787 to 1089 μg /g DW [
200]. In the Tri-State and Western Regional russet skin trials and the Red/Specialty trials, the broad heritability (H) was 0.57, 0, and 0.72 respectively. Crop nutrient management was reported to have different effects on Mg. As showed it can affect Mg rather than K [
201], while another study found little effect of fertilizer on Mg. In addition to the minerals discussed above, some other minerals in potato were also examined [
202,
203,
204].
5.4. Phosphorus
Other than potassium, there is another main mineral present in the tuber-phosphorus. It plays a crucial role in the human body. Inadequate phosphorous intake results in abnormally low serum phosphate levels, which result in loss of appetite, anemia, muscle, and tingling of extremities, and difficulty walking. According to the daily requirement of phosphorus is 800-1000mg. While in potatoes, phosphorus ranges from ~1300 to 6000 µg/g DW [
178,
203,
205] and 30-60 mg/100g FW (shown in
Table 3). A study informed us that the fertilizer P applied together with nitrogen resulted in the phosphorus increase in potato parts, but without a significant impact on the yield of tubers [
206]. However, potato combines a high P-demand with low P uptake [
207], thus a large amount of P needed to plant potatoes. Apart from improved management practices aiming at the reduction of P losses to the environment and closing nutrient cycles, new cultivars with higher P efficiencies may contribute to more sustainable use of P resources.
6. Conclusion
As the story of potatoes in the human diet continues to evolve, new and advanced techniques will promise significant opportunities to further explore the role of potatoes and their functional properties. Both in Asia and Africa, the potato production has boomed, especially in China where the potato production is by far the largest and has made potatoes as its forward-looking food security strategy, whereas the production in the West has declined. With the characteristics of ease of cultivation, minimum transportation and storage condition, and rich in carbohydrate, vitamin, and antioxidants, potato as a stable food still has much space to be examined. Remarkably, on the other hand, potato contributes only 440 calories per pound, which makes it a promising healthy food source to meet today’s requirements of dieters. Potato starch has some unique properties compared with cereal starches. The most significant ones involve long amylopectin chains forming hydrated and ordered B-type crystallites of tuberous starch. To date, certain details regarding the structure of potato starch remain unclear. Thus, more efforts should focus on the actual model of the interconnection of the cluster units of the amylopectin component and the connection between the proposed superhelical structure and the blocklet structures in the granules. And genetic engineering can be a possible solution to help to control the synthesis and development of potato starches with altering structures and functionality. Owing to high consumption, potato proteins are of particular interest, nutritionally, they are superior to animal protein Iysozyme. Different extraction techniques of tuber protein like precipitation methods (thermal and acidic precipitation, salt precipitation, ethanol precipitation, ammonium sulfate precipitation, carboxymethyl cellulose complexation), ion exchange, and separation of bioactive proteins and peptides have been explored to retain functionality and associated health benefits, and in turn improve their potential application. Moreover, the potato has a complex chemical composition that includes important amounts of minerals, vitamins, and phytonutrients. Fight against with the assertion of some scientists that the carbonates in potato are the primary contributor to obesity, the constant research work should be done on the next step. While considering increasing the phytonutrient content of potato, advance in crop management works together with traditional breeding and molecular breeding like TALEN and CRISPR can be a potential outlet. The strong incentive for quality control and safety of final products calls for applicable methods to analyze biologically active alkaloids and glycoalkaloids in potato. Also, accurate analyses of these controversial components will benefit the grower, processor, researcher, and consumers. Further studies like studies of toxicities and beneficial health effects as well as investigation of the biosynthesis of the secondary metabolites are needed. Additionally, getting to know the impact of climate change, genetics and reproductive dynamics of potato as well as purification of waste streams (both the fruit and the pulp) will be an essential topic for researchers around the world. Advances in genomics, proteomics, metabolomics, and bioinformations will open a door to produce new generations of advanced germplasm for emerging priorities or special needs. In recent years, there has been an increasing interest in finding functional food, like the natural antioxidants which can protect the human body from radicals and retard the process of many chronic diseases, and special protein as well as fiber function with healthy and industrial additives.
Author Contributions
Conceptualization, J.X. and F.Z.; investigation, Y.L., J.X. and F.Z.; writing—original draft preparation, Y.L.; writing—review and editing, J.X., L.K. and J.S.; visualization, L.K. and J.S.; supervision, J.X.; project administration, F.Z.; funding acquisition, F.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Special Fund Project for High-Tech Industrialization of Science and Technology Cooperation Between Jilin Province and Chinese Academy of Sciences (Grant No. 2021SYHZ0005), China Agriculture Research System (Grant No. CARS-9), Key Research and Development Plan of Ningxia Hui Autonomous Region (Grant No. 2020BBF03018), and the Government-sponsored study abroad Program of the Chinese Academy of Sciences (Liufangxijizi [2018] No. 35). The authors would also like to thank the SFAT and Ridder CoRE for hosting Fan-kui Zeng and funding the study.
Data Availability Statement
Experimental data associated with this research are available from the authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- A. Cesari, A.L. Falcinelli, J.R. Mendieta, M.R. Pagano, N. Mucci, G.R. Daleo, M.G. Guevara, Potato aspartic proteases (StAPs) exert cytotoxic activity on bovine and human spermatozoa, Fertility and sterility 2007, 88, 1248-1255.
- G. Burgos, T.Z. Felde, C. Andre, S. Kubow, The Potato and Its Contribution to the Human Diet and Health, The Potato Crop, 2020, 5, 37-74.
- C.M. Rommens, H. Yan, K. Swords, C. Richael, J. Ye, Low-acrylamide French fries and potato chips, Plant Biotechnology Journal 2008, 6, 843-853.
- W. Kaguongo, C. Lungaho, D. Borus, D. Kipkoech, N. Ng’Ang’A, A policymakers’ guide to crop diversification: The case of the potato in Kenya, Food and Agriculture Organization of the United Nations Rome 2013.
- M.E. Camire, S. Kubow, D.J. Jcrifs. Donnelly, Nutrition, Potatoes and Human Health, 2009, 49, 823-840.
- R. Visvanathan, C. Jayathilake, B. Chaminda Jayawardana, R. Liyanage, Health-beneficial properties of potato and compounds of interest, J Sci Food Agric 2016, 96, 4850-4860.
- F.S. Atkinson, K. Foster-Powell, J. Cjdc, Brand-Miller, International Tables of Glycemic Index and Glycemic Load Values: 2008, 31, 2281-2283.
- H. Farhadnejad, F. Teymoori, G. Asghari, P. Mirmiran, F. Azizi, The association of potato intake with risk for incident type 2 diabetes in adults, Can J Diabetes 2018, 42, 613-618.
- H. Khosravi-Boroujeni, N. Mohammadifard, N. Sarrafzadegan, F. Sajjadi, M. Maghroun, A. Khosravi, H. Alikhasi, M. Rafieian, L. Ajijof, Potato consumption and cardiovascular disease risk factors among Iranian population, International journal of food sciences and nutrition 2012, 63, 913-920.
- D. Mozaffarian, T. Hao, E.B. Rimm, W.C. Willett, F.B.J.N.E.J.M. Hu, Changes in diet and lifestyle and long-term weight gain in women and men, The New England Journal of Medicine 2011, 364, 2392-2404.
- J.C. King, J.L.J.A.i.N. Slavin, White Potatoes, Human Health, and Dietary Guidance, Advances in Nutrition 2013, 4, 393-401.
- M. Huang, P. Zhuang, J. Jiao, J. Wang, X. Chen, Y. Zhang, Potato consumption is prospectively associated with risk of hypertension: an 11.3-year longitudinal cohort study, Clin Nutr 2019, 38, 1936-1944.
- N.J. Aburto, S. Hanson, H. Gutierrez, L. Hooper, P. Elliott, F.P. Cappuccio, Effect of increased potassium intake on cardiovascular risk factors and disease: systematic review and meta-analyses, British Medical Journal 2013, 346, 1378-1385.
- C.V. Niekerk, H. Schönfeldt, N. Hall, B. Pretorius, The role of biodiversity in food security and nutrition: a potato cultivar case study, Food & Nutrition Sciences, 2016, 7, 371-382.
- R.L. Whistler, J.R. Daniel, Chapter 5-Molecular Structure of Starch, Strach: Chemistry and Technology 1984, 153-182.
- M. Osawa, N. Inoue, Studies on the in vitro starch digestibility and the glycemic index in rice cultivars differing in amylose content, Japanese Journal of Crop Science 2007, 76, 410-415.
- Lin, A. Hui-Mei, Structure and digestion of common complementary food starches, Journal of Pediatric Gastroenterology & Nutrition 2018, 66, 35-38.
- A. Hall, J. Versalovic, Microbial metabolism in the mammalian gut: molecular mechanisms and clinical implications, Journal of Pediatric Gastroenterology & Nutrition 2018, 66, 72-79.
- Tan, Xiaoyan, Huang, Jidong, Li, Lin, Xie, Fengwei, Xiaoxi, Chen, Effect of heat-moisture treatment on multi-scale structures and physicochemical properties of breadfruit starch, Carbohydrate Polymers Scientific & Technological Aspects of Industrially Important Polysaccharides 2017.
- H.M. Lin, B.H. Lee, B.L. Nichols, R. Quezada-Calvillo, B.R. Hamaker, Starch source influences dietary glucose generation at the mucosal-glucosidase level, Journal of Biological Chemistry 2012, 287, 36917-36921.
- B. Sawicka, Resistant starch in potato, Acta Scientiarum Polonorum Technologia Alimentaria 2018, 17, 1-17.
- S. Lockyer, A.P. Nugent, Health effects of resistant starch, Nutrition Bulletin 2017, 42, 10-41.
- B. Brahma, N. Sit, Physicochemical properties, and digestibility of heat moisture–treated potato starches for different treatment conditions, Potato Research 2020, 63, 367-383.
- C. Shortt, O. Hasselwander, A. Meynier, A. Nauta, E.N. Fernández, P. Putz, I. Rowland, J. Swann, J. Türk, J. Vermeiren, Systematic review of the effects of the intestinal microbiota on selected nutrients and non-nutrients, European Journal of Nutrition 2017, 57, 25-49.
- Jane, Jay-lin, Starch-structural features of starch granules II, 2009, 193-236.
- H.M. Lin, Y.H. Chang, J.H. Lin, J.L. Jane, M.J. Sheu, T.J. Lu, Heterogeneity of lotus rhizome starch granules as revealed by α-amylase degradation, Carbohydrate Polymers 2006, 66, 528-536.
- G. Zhang, B.R. Hamaker, Slowly digestible starch: concept, mechanism, and proposed extended glycemic index, Crit Rev Food Sci Nutr 2009, 49, 852-867.
- M. Diaz-Sotomayor, R. Quezada-Calvillo, S.E. Avery, S.K. Chacko, L.K. Yan, H.M. Lin, Z.H. Ao, B.R. Hamaker, B.L. Nichols, Maltase-glucoamylase modulates gluconeogenesis and sucrase- isomaltase dominates starch digestion glucogenesis, Journal of Pediatric Gastroenterology & Nutrition 2013, 57, 704-712.
- A.H.-M. Lin, B.-H. Lee, W.-J. Chang, Small intestine mucosal alpha-glucosidase: A missing feature of in vitro starch digestibility, Food Hydrocolloids 2016, 53, 163-171.
- P.J. Butterworth, F.J. Warren, T. Grassby, H. Patel, P.R. Ellis, Analysis of starch amylolysis using plots for first-order kinetics, Carbohydrate Polymers 2012, 87, 2189-2197.
- H.M. Amy, Lin, Buford, L., Nichols, The digestion of complementary feeding starches in the young child, Starch Strke 2017, 69, 170-178.
- K. Ben Jeddou, F. Bouaziz, S. Zouari-Ellouzi, F. Chaari, S. Ellouz-Chaabouni, R. Ellouz-Ghorbel, O. Nouri-Ellouz, Improvement of texture and sensory properties of cakes by addition of potato peel powder with high level of dietary fiber and protein, Food Chem 2017, 217, 668-677.
- D. Bajer, K. Janczak, K. Bajer, Novel starch/chitosan/aloe vera composites as promising biopackaging materials, Journal of Polymers and the Environment 2020, 28, 1021-1039.
- R. Zhang, M. Cheng, X. Wang, J. Wang, Bioactive mesoporous nano-silica/potato starch films against molds commonly found in post-harvest white mushrooms, Food Hydrocolloids 2019, 95, 517-525.
- R. Farajpour, Z.E. Djomeh, S. Moeini, H. Tavakolipour, S. Safayan, Structural and physico-mechanical properties of potato starch-olive oil edible films reinforced with zein nanoparticles, International Journal of Biological Macromolecules 2020, 149, 941-950.
- R. Wang, P. Liu, B. Cui, X. Kang, B.J Yu, Effects of different treatment methods on properties of potato starch-lauric acid complex and potato starch-based films, International Journal of Biological Macromolecules 2019, 124, 34-40.
- E. Ayquipa-Cuellar, L. Salcedo-Sucasaca, J.A. Azamar-Barrios, G. Chaquilla-Quilca, Assessment of prickly pear peel mucilage and potato husk starch for edible films production for food packaging industries, Waste and Biomass Valorization. 2020, 9, 649-656.
- A. Peksa, J. Miedzianka, A. Nems, Amino acid composition of flesh-coloured potatoes as affected by storage conditions, Food Chem 2018, 266, 335-342.
- R. Pranaitiene, H. Danilcenko, E. Jariene, Z. Dabkevicius, The effect of inhibitors on the changes of potato tuber quality during the storage period, Journal of Food Agriculture & Environment 2008, 6, 231-235.
- V. Bartova, J. Barta, Chemical composition and nutritional value of protein concentrates isolated from potato (Solanum tuberosum L.) fruit juice by precipitation with ethanol or ferric chloride, J Agric Food Chem 2009, 57, 9028-9034.
- A. Waglay, S. Karboune, Chapter 4 - potato proteins: functional food ingredients, advances in potato chemistry and technology, Academic Press, San Diego, 2016, 75-104.
- T.-J. Fu, U.R. Abbott, C. Hatzos, Digestibility of food allergens and nonallergenic proteins in simulated gastric fluid and simulated intestinal fluid comparative study, Journal of Agricultural & Food Chemistry, 2002, 50, 7154-7160.
- J.Y. Kim, S.C. Park, M.H. Kim, H.T. Lim, Y. Park, K.S. Hahm, Antimicrobial activity studies on a trypsin–chymotrypsin protease inhibitor obtained from potato, Biochemical & Biophysical Research Communications 2005, 330, 920-927.
- A. Pihlanto, S. Akkanen, H.J. Korhonen, ACE-inhibitory and antioxidant properties of potato (Solanum tuberosum), Food Chemistry 2008, 109, 104-112.
- R. Liyanage, K.H. Han, S. Watanabe, K.I. Shimada, M. Sekikawa, K. Ohba, Y. Tokuji, M. Ohnishi, S. Shibayama, T. Nakamori, Potato and soy peptide diets modulate lipid metabolism in rats, Journal of the Agricultural Chemical Society of Japan 2008, 72, 943-950.
- C. Blanco-Aparicio, M.A. Molina, E. Fernandez-Salas, M.L. Frazier, J.M. Mas, E. Querol, F.X. Aviles, R. De Llorens, Potato carboxypeptidase inhibitor, a T-knot protein, is an epidermal growth factor antagonist that inhibits tumor cell growth, Journal of Biological Chemistry 1998, 273, 12370-12377.
- J. Mitrus, C. Stankiewicz, E. Steć, M. Kamecki, J. Starczewski, The influence of selected cultivation on the content of total protein and amino acids in the potato tubers, Plant Soil & Environment 2003, 3, 49-55.
- W.H. Eppendorfer, B.O. Eggum, Effects of sulphur, nitrogen, phosphorus, potassium, and water stress on dietary fibre fractions, starch, amino acids and on the biological value of potato protein, Plant Foods for Human Nutrition 1994, 45, 299-313.
- A. Sotelo, E. Contreras, H. Sousa, V. Hernandez, Nutrient composition and toxic factor content of four wild species of mexican potato, Journal of Agricultural & Food Chemistry 1998, 46, 1355-1358.
- W.M Gelder, C.R.V. Vonk, Amino acid composition of coagulable protein from tubers of 34 potato varieties and its relationship with protein content, Potato Research 1980, 23, 427-434.
- S.O. Kärenlampi, P.J. White, Chapter 5 - potato proteins, lipids, and minerals, advances in potato chemistry and technology, Academic Press, San Diego, 2009, 99-125.
- C.Y. Jin, F.K. Zeng, G. Liu, Recovery of protease inhibitors from potato fruit water by expanded bed adsorption chromatography in pilot scale, American Journal of Potato Research 2017, 95, 1-8.
- J.L. Lavoie, C.D. Sigmund, Minireview: overview of the renin-angiotensin system - an endocrine and paracrine system, Endocrinology 2003, 6, 236-242.
- H. Miyamoto, K. Ito, K. Ito, S. Wakabayashi, H. Suzaka, H. Matsuo, T. Iga, Y. Sawada, Comparative study of effects of angiotensin II receptor antagonist, KD3-671, and angiotensin converting enzyme inhibitor, enalaprilat, on cough reflex in guinea pig, European Journal of Drug Metabolism & Pharmacokinetics 2001, 26, 47-52.
- F. Shahidi, Y. Zhong, Bioactive Peptides, Journal of Aoac International 2010, 91, 914-931.
- C. Guang, R.D. Phillips, B. Jiang, F. Milani, Three key proteases angiotensin-I-converting enzyme (ACE), ACE2 and renin within and beyond the renin-angiotensin system, Archives of Cardiovascular Diseases 2012, 105, 373-385.
- C.C. Udenigwe, R.E.J.J.o.F.S. Aluko, Food protein-derived bioactive peptides: production, processing, Potential Health Benefits, 2012, 77, 11-24.
- A.F.G. Cicero, B. Gerocarni, L. Laghi, C. Borghi, Blood pressure lowering effect of lactotripeptides assumed as functional foods: a meta-analysis of current available clinical trials, Journal of Human Hypertension 2011, 25, 425-436.
- S. Mäkinen, T. Streng, L.B. Larsen, A. Laine, A. Pihlanto, Angiotensin I-converting enzyme inhibitory and antihypertensive properties of potato and rapeseed protein-derived peptides, Journal of Functional Foods, 2016, 25, 160-173.
- A.J. Hill, S.R. Peikin, C.A. Ryan, J.E.J.P. Blundell, Behavior, Oral administration of proteinase inhibitor II from potatoes reduces energy intake in man, 1990, 48, 241-246.
- H. Jiang, B. Edmondson, J. Radosevich, Potato proteinase inhibitor II exhibits activity in elevating fasting plasma cholecystokinin concentrations, United States Patent Application 2006, 20060204567.
- C.A. Ryan, G.M. Hass, R.W. Kuhn, Purification and properties of a carboxypeptidase inhibitor from potatoes, Journal of Biological Chemistry 1974, 249, 5495-5499.
- J. Bryant, T.R. Green, T. Gurusaddaiah, C.A.J.B. Ryan, Proteinase inhibitor II from potatoes: isolation and characterization of its protomer components, Biocehmistry 1976, 15, 3418-3424.
- I.H Barrette, K.S. K. Ng, M.M Cherney, G.J Pearce, Chemistry unbound form of tomato inhibitor reveals interdomain flexibility, conformational variability in the reactive site loops, The Journal of biological chemistry 2003, 278, 31391-31400.
- A. Gambuti, A. Rinaldi, L. Moio, Use of patatin, a protein extracted from potato, as alternative to animal proteins in fining of red wine, Eur Food Res Technol 2012, 235, 753-765.
- M. Nagashima, M. Werner, M. Wang, L. Zhao, P.J Verhallen, An inhibitor of activated thrombin-activatable fibrinolysis inhibitor potentiates tissue-type plasminogen activator-induced thrombolysis in a rabbit jugular vein thrombolysis model, Thrombosis Research 2000, 98, 333-342.
- M. Schneider, M. Nesheim, Haemostasis, Reversible inhibitors of TAFIa can both promote and inhibit fibrinolysis, Journal of Thrombosis and Haemostasis 2003, 1, 147-54.
- W. John B, H. Bernadette, J. Ian, H. Peter, K. Cornelis, B. Laszlo, Stabilization versus inhibition of TAFIa by competitive inhibitors in vitro, Journal of Biological Chemistry 2003, 278, 8913-8921.
- C. Blanco-Aparicio, M.A. Molina, E. Fernandez-Salas, M.L. Frazier, J.M. Mas, E. Querol, F.X. Aviles, R. Llorens, Potato carboxypeptidase inhibitor, a T-knot protein, is an epidermal growth factor antagonist that inhibits tumor cell growth, Journal of Biological Chemistry 1998, 273, 12370-12377.
- K. Frenkel, K. Chrzan, C.A. Ryan, R. Wiesner, W.J.C. Troll, Chymotrypsin-specific protease inhibitors decrease H2O2 formation by activated human polymorphonuclear leukocytes, Carcinogenesis 1987, 8, 1207-1212.
- C. Huang, W.Y. Ma, C.A.J.P.N.A.S.U.S.A. Ryan, Proteinase inhibitors I and II from potatoes specifically block UV-induced activator protein-1 activation through a pathway that is independent of extracellular signal-regulated kinases, c-Jun N-terminal kinases, and P38 kinase, Proceedings of the National Academy of Science of the United States of America 1997, 94, 11957-11962.
- Y. Park, B.H. Choi, J.S. Kwak, C.W. Kang, H.T. Lim, H.S. Cheong, K.S. Hahm, Kunitz-type serine protease inhibitor from potato, Journal of Agricultural and Food Chemistry 2005, 53, 6491-6496.
- J.Y. Kim, S.C. Park, M.H. Kim, H.T. Lim, Y. Park, K.S.J.B. Hahm, B.R. Communications, Antimicrobial activity studies on a trypsin–chymotrypsin protease inhibitor obtained from potato, Biochemical and biophysical research communications 2005, 330, 920-927.
- G.R. Tripathi, J. Park, Y. Park, I. Hwang, H. Cheong, Potide-G derived from potato (solanum tuberosum L.) is active against potato virus infection, Journal of agricultural and food chemistry 2006, 54, 8437-8443.
- T. Albishi, J.A. John, A.S. Al-Khalifa, F. Shahidi, Antioxidant, anti-inflammatory and DNA scission inhibitory activities of phenolic compounds in selected onion and potato varieties, Journal of Functional Foods 2013, 5, 930-939.
- H. Müller, Determination of the carotenoid content in selected vegetables and fruit by HPLC and photodiode array detection, Lebensm Unters Forsch A 1997, 204, 88-94.
- S. Nesterenko, K.C. Sink, Carotenoid Profiles of Potato Breeding Lines and Selected Cultivars, Hort Science 2003,38, 1173-1177.
- G. Burgos, E. Salas, W. Amoros, M. Auqui, L. MuOa, M. Kimura, M. Bonierbale, Total and individual carotenoid profiles in Solanum phureja of cultivated potatoes: I. Concentrations and relationships as determined by spectrophotometry and HPLC, Journal of Food Composition and Analysis 2009, 22, 503-508.
- A. Kobayashi, A. Ohara-Takada, S. Tsuda, C. Matsuura-Endo, M. Mori, Breeding of potato variety "Inca-no-hitomi" with a very high carotenoid content, Breeding Science 2008, 58, 77-82.
- H. Müller, Determination of the carotenoid content in selected vegetables and fruit by HPLC and photodiode array detection, Zeitschrift für Lebensmitteluntersuchung und-Forschung A 1997, 204, 88-94.
- A. Hossain, P. Begum, M.S. Zannat, M.H. Rahman, M. Ahsan, S.N. Islam, Nutrient composition of strawberry genotypes cultivated in a horticulture farm, Food Chemistry 2016, 199, 648-652.
- K. Venkatachalam, R. Rangasamy, V. Krishnan, Total antioxidant activity and radical scavenging capacity of selected fruits and vegetables from south India, International Food Research Journal 2014, 21, 1003-1007.
- A.W. Chassy, L. Bui, E.N.C. Renaud, M. Van Horn, A.E. Mitchell, Three-year comparison of the content of antioxidant microconstituents and several quality characteristics in organic and conventionally managed tomatoes and bell peppers, Journal of Agricultural Food Chemistry 2006, 54, 8244-8252.
- J. Valcarcel, K. Reilly, M. Gaffney, N. O’Brien, Total carotenoids and l-ascorbic acid content in 60 varieties of potato (solanum tuberosum l.) grown in Ireland, Potato Research 2014, 58, 29-41.
- R. Fernandez-Orozco, L. Gallardo-Guerrero, D. Hornero-Mendez, Carotenoid profiling in tubers of different potato {Solarium sp) cultivars: Accumulation of carotenoids mediated by xanthophyll esterification, Food Chemistry 2013, 141, 2864-2872.
- C.R. Brown, Antioxidants in potato, American Journal of potato Resewarch 2005, 82, 163-172.
- P.D. Fraser, P.M. Bramley, The biosynthesis and nutritional uses of carotenoids, Progress in Lipid Research 2004, 43, 228-265.
- M.A. Gammone, G. Riccioni, N. D'Orazio, Carotenoids: potential allies of cardiovascular health, Food Nutr Res 2015, 59, 26762-26771.
- S.M. Abdel-Aalel, H. Akhtar, K. Zaheer, R. Ali, Dietary sources of lutein and zeaxanthin carotenoids and their role in eye health, Nutrients 2013, 5, 1169-1185.
- A.J. Chucair, N.P. Rotstein, J.P. Sangiovanni, A. During, E.Y. Chew, L.E. Politi, lutein and zeaxanthin protect photoreceptors from apoptosis induced by oxidative stress: relation with docosahexaenoic acid, Invest Ophthalmol Vis Sci 2007, 48, 5168-5177.
- J.S.L. Tan, J.W. Jie, V. Flood, E. Rochtchina, P. Mitchell, Dietary antioxidants and the long-term incidence of age-related macular degeneration. The Blue Mountains Eye Study, Ophthalmology 2008, 115, 334-341.
- S. Lu, L. Li, Carotenoid metabolism: biosynthesis, regulation, and beyond, Journal of Integrative Plant Biology 2008, 50, 778-785.
- C.I. Cazzonelli, B.J. Pogson, Source to sink: regulation of carotenoid biosynthesis in plants, Trends Plant Sci 2010, 15, 266-274.
- C.K. Shewmaker, J.A. Sheehy, M. Daley, S. Colburn, D.Y. Ke, Seed-specific overexpression of phytoene synthase: increase in carotenoids and other metabolic effects, Plant Journal 1999, 20, 401-412.
- P.D. Fraser, S. Romer, C.A. Shipton, P.B. Mills, J.W. Kiano, N. Misawa, R.G. Drake, W. Schuch, P.M. Bramley, Evaluation of transgenic tomato plants expressing an additional phytoene synthase in a fruit-specific manner, Proceedings of the National Academy of Sciences of the United States of America 2001, 99, 1092-1097.
- L.J. Ducreux, W. Morris, L. Hedley, P.E. Shepherd, Metabolic engineering of high carotenoid potato tubers containing enhanced levels of b-carotene and lutein, Journal of Experimental Botany 2005, 56, 81-89.
- M. Kato, H. Matsumoto, Y. Ikoma, H. Okuda, A.M. Yano, The role of carotenoid cleavage dioxygenases in the regulation of carotenoid profiles during maturation in citrus fruit, Journal of Experimental Botany 2006, 57, 2153-2164.
- R. Campbell, L.J.M. Ducreux, W.L. Morris, J.A. Morris, J.C. Suttle, G. Ramsay, G.J. Bryan, P.E. Hedley, M.A. Taylor, The Metabolic and developmental roles of carotenoid cleavage dioxygenase 4 from potato, Plant Physiology 2010, 154, 656-664.
- K.G. Haynes, B.A. Clevidence, D. Rao, B.T. Vinyard, J.M. White, Genotype environment interactions for potato tuber carotenoid content, Journal of the American Society for Horticultural Science American Society for Horticultural Science 2010, 135, 250-258.
- C.R. Brown, T.S. Kim, Z. Ganga, K. Haynes, D.D. Jong, M. Jahn, I. Paran, W.D. Jong, Segregation of total carotenoid in high level potato germplasm and its relationship to beta-carotene hydroxylase polymorphism, American Journal of Potato Research 2006, 83, 365-372.
- A.M.A. Wolters, J.G Uitdewilligen, B.A. Kloosterman, R.C.B. Hutten, R.G.F. Visser, H.J.V. Eck, Identification of alleles of carotenoid pathway genes important for zeaxanthin accumulation in potato tubers, Plant Molecular Biology 2010, 73, 659-671.
- R. Campbell, S.D.A. Pont, J.A. Morri, Genome-wide and bulked transcriptomic analysis reveals new candidate genes for the control of tuber carotenoid content in potato (Solanum tuberosum L.), Theoretical & Applied Genetics 2014, 127, 1917-1933.
- M. Sulli, G. Mandolino, M. Sturaro, C. Onofri, G. Giuliano, Molecular and biochemical characterization of a potato collection with contrasting tuber carotenoid content, Plos One 2017, 12, 184-193.
- G. Diretto, S. Al-Babili, R. Tavazza, V. Papacchioli, P. Beyer, G. Giuliano, H. El-Shemy, Metabolic engineering of potato carotenoid content through tuber-specific overexpression of a bacterial mini-pathway, Plos One 2007, 2, 350-361.
- C. Chitchumroonchokchai, G. Diretto, B. Parisi, G. Giuliano, M.L. Failla, Potential of golden potatoes to improve vitamin A and vitamin E status in developing countries, Plos One 2017, 12, 187-195.
- H.W. Im, B.S. Suh, S.U. Lee, N. Kozukue, M. Ohnisi-Kameyama, C.E. Levin, M. Friedman, Analysis of phenolic compounds by high-performance liquid chromatography and liquid chromatography/mass spectrometry in potato plant flowers, leaves, stems, and tubers and in home-processed potatoes, Journal of Agricultural & Food Chemistry 2008, 56, 3341-3349.
- B.K. Bassoli, P. Cassolla, G.R. Borba-Murad, J. Constantin, C.L. Salgueiro-Pagadigorria, R.B. Bazotte, R.S. Silva, H.M.D. Souza, Chlorogenic acid reduces the plasma glucose peak in the oral glucose tolerance test: effects on hepatic glucose release and glycaemia, Cell Biochemistry & Function 2010, 26, 320-328.
- R. Niggeweg, A.J. Michael, C. Martin, Engineering plants with increased levels of the antioxidant chlorogenic acid, Nature biotechnology 2004, 22, 746-753.
- R.S. Payyavula, R. Shakya, V.G. Sengoda, J.E. Munyaneza, P. Swamy, D.A. Navarre, Synthesis and regulation of chlorogenic acid in potato: Rerouting phenylpropanoid flux in silenced lines, Plant Biotechnology Journal 2015, 13, 551-564.
- R.S. Payyavula, R.K. Singh, D.A. Navarre, Transcription factors, sucrose, and sucrose metabolic genes interact to regulate potato phenylpropanoid metabolism, Journal of Experimental Botany 2013, 163-170.
- D.A. Navarre, C.R. Brown, V.R. Sathuvalli, Potato Vitamins, Minerals and Phytonutrients from a Plant Biology Perspective, American Journal of Potato Research 2019, 96, 111-126.
- C.M. Andre, M. Ghislain, P. Bertin, M. Oufir, D. Evers, Andean potato cultivars (Solanum tuberosum L.) as a source of antioxidant and mineral micronutrients, J Agric Food Chem 2007, 55, 366-378.
- H. Ayvaz, A. Bozdogan, M.M. Giusti, M. Mortas, R. Gomez, L.E. Rodriguez-Saona, Improving the screening of potato breeding lines for specific nutritional traits using portable mid-infrared spectroscopy and multivariate analysis, Food Chemistry 2016, 211, 374-382.
- M. Ariel Valinas, M. Luciana Lanteri, A. Ten Have, A. Balbina Andreu, Chlorogenic acid, anthocyanin and flavan-3-ol biosynthesis in flesh and skin of Andean potato tubers (Solanum tuberosum subsp andigena), Food Chemistry 2017, 229, 837-846.
- A.H. Kong, F. Carolina, H. Susan, C. Andrés, V.G. Antonio, L.M. Roberto, Antioxidant capacity and total phenolic compounds of twelve selected potato landrace clones grown in Southern Chile, Chilean Journal of Agricultural Research 2012, 72, 3-9.
- J.A. Tudela, E. Cantos, J.C. Espín, F.A. Tomás-Barberán, M.I.J.J.o.A. Gil, F. Chemistry, induction of antioxidant flavonol biosynthesis in fresh-cut potatoes. Effect of Domestic Cooking 2002, 50, 5925-5931.
- R.S. Payyavula, R. Shakya, V.G. Sengoda, J.E. Munyaneza, P. Swamy, Synthesis and regulation of chlorogenic acid in potato: Rerouting phenylpropanoid flux in HQ: silenced lines, Plant Biotechnology Journal 2015, 13, 551-564.
- K. Kawabata, R. Mukai, I.J. Akari, Food, Function, Quercetin and related polyphenols: new insights and implications for their bioactivity and bioavailability, Food and Function 2015, 6, 1399-1417.
- S. Yıldırım, A. Kadıoğlu, A. Sağlam, A. Yaşar, H.E. Sellitepe, Fast determination of anthocyanins and free pelargonidin in fruits, fruit juices, and fruit wines by high-performance liquid chromatography using a core-shell column, Journal of Separation Science 2016,39, 3927-3935.
- X. Wu, Aglycones and sugar moieties alter anthocyanin absorption and metabolism after berry consumption in weanling pigs, Journal of Nutrition 2005, 135, 2417-2424.
- S. Li, J. Li, Y. Sun, Y. Huang, J. He, Z. Zhu, Transport of flavanolic monomers and procyanidin dimer A2 across human adenocarcinoma stomach cells (MKN-28), Journal of Agricultural & Food Chemistry 2019, 67, 3354-3362.
- M.E. Camire, S. Kubow, D.J. Donnelly, Potatoes and Human Health, Critical Reviews in Food Science & Nutrition 2009, 49, 823-840.
- G. Jansen, W. Flamme, Coloured potatoes (Solanum TuberosumL.) -anthocyanin content and tuber quality, Genetic Resources & Crop Evolution 2006, 53, 1321-1331.
- C.S. Jung, H.M. Griffiths, D.M.D. Jong, S. Cheng, M. Bodis, W.S.D. Jong, The potato P locus codes for flavonoid 3′,5′-hydroxylase, Theoretical & Applied Genetics 2005, 110, 269-275.
- C.S. Jung, H.M. Griffiths, D.M.D. Jong, S. Cheng, M. Bodis, T.S. Kim, W.S.D. Jong, The potato developer (D) locus encodes an MYB transcription factor that regulates expression of multiple anthocyanin structural genes in tuber skin, Theoretical and Applied Genetics 2009, 120, 45-57.
- Y. Zhang, S. Cheng, D.D. Jong, H. Griffiths, R. Halitschke, W.D. Jong, The potato R locus codes for dihydroflavonol 4-reductase, Tag. theoretical & Applied Genetics. Theoretische Und Angewandte Genetik 2009, 119, 931-937.
- R.S. Payyavula, D.A. Navarre, J. Kuhl, A. Pantoja, Developmental effects on phenolic, flavonol, anthocyanin, and carotenoid metabolites and gene expression in potatoes, Journal of Agricultural & Food Chemistry 2013, 61, 7357-7365.
- H. Zhang, B. Yang, J. Liu, D. Guo, J. Hou, S. Chen, B. Song, C. Xie, Analysis of structural genes and key transcription factors related to anthocyanin biosynthesis in potato tubers, Scientia Horticulture 2017, 225, 310-316.
- C. Kyoungwon, C. Kwang-Soo, S. Hwang-Bae, H.I. Jin, H. Su-Young, L. Hyerim, K. Young-Mi, N.M. Hee, Network analysis of the metabolome and transcriptome reveals novel regulation of potato pigmentation, Journal of Experimental Botany 2016, 67, 1515-1533.
- Y. Liu, K. Lin-Wang, C. Deng, B. Warran, L. Wang, B. Yu, H. Yang, J. Wang, R.V. Espley, J. Zhang, Comparative transcriptome analysis of white and purple potato to identify genes involved in anthocyanin biosynthesis, Plos One 2015, 10, 129-138.
- C. Stushnoff, L.J. Ducreux, R.D. Hancock, P.E. Hedley, D.G. Holm, G.J. McDougall, J.W. McNicol, J. Morris, W.L. Morris, J.A. Sungurtas, S.R. Verrall, T. Zuber, M.A. Taylor, Flavonoid profiling and transcriptome analysis reveals new gene-metabolite correlations in tubers of Solanum tuberosum L, J Exp Bot 2010, 61, 1225-38.
- R. Tierno, R.D.G. Ignacio, Jose, Characterization of high anthocyanin-producing tetraploid potato cultivars selected for breeding using morphological traits and microsatellite markers, Plant Genetic Resources 2015, 1-10.
- V. Charepalli, L. Reddivari, S. Radhakrishnan, R. Vadde, R. Agarwal, J.K.P. Vanamala, Anthocyanin-containing purple-fleshed potatoes suppress colon tumorigenesis via elimination of colon cancer stem cells, Journal of Nutritional Biochemistry 2015, 150-162.
- M.N. Ombra, F. Fratianni, T. Granese, F. Cardinale, A. Cozzolino, F. Nazzaro, In vitro antioxidant, antimicrobial and anti-proliferative activities of purple potato extracts (Solanum tuberosum cv Vitelotte noire) following simulated gastro-intestinal digestion, Natural Product Research, 29, 1087-1091.
- A. Sido, S. Radhakrishnan, S.W. Kim, E. Eriksson, F. Shen, Q. Li, V. Bhat, L. Reddivari, J.K.P. Vanamala, A food-based approach that targets interleukin-6, a key regulator of chronic intestinal inflammation and colon carcinogenesis, Journal of Nutritional Biochemistry 2017, 43, 11-17.
- L.R. Safe, J.C.M. Jr, Anthocyanin fraction from potato extracts is cytotoxic to prostate cancer cells through activation of caspase-dependent and caspase-independent pathways, Carcinogenesis 2007, 28, 2227-2235.
- X. Zhao, F. Sheng, J. Zheng, R. Liu, Composition and stability of anthocyanins from purple solanum tuberosum and their protective influence on Cr (VI) targeted to bovine serum albumin, Journal of Agricultural & Food Chemistry 59(14) (2011) 7902-7909.
- Z. Jiang, C. Chen, J. Wang, W. Xie, M. Wang, X. Li, X. Zhang, Purple potato (Solanum tuberosum L.) anthocyanins attenuate alcohol-induced hepatic injury by enhancing antioxidant defense, J Nat Med 2016, 70, 45-53.
- K.L. Kaspar, J.S. Park, C.R. Brown, B.D. Mathison, D.A. Navarre, B.P. Chew, Pigmented potato consumption alters oxidative stress and inflammatory damage in men, Journal of Nutrition 2011, 141, 108-116.
- J.A. Vinson, C.A. Demkosky, D.A. Navarre, M.A. Smyda, High-antioxidant potatoes: acute in vivo antioxidant source and hypotensive agent in humans after supplementation to hypertensive subjects, J Agric Food Chem 2012, 60, 6749-6754.
- D.D. Ramdath, E. Padhi, A. Hawke, T. Sivaramalingam, T. Rong, The glycemic index of pigmented potatoes is related to their polyphenol content, Food & Function 2014, 5, 909-915.
- H.M. Ayoub, M.R. Mcdonald, J.A. Sullivan, R. Tsao, M. Platt, J. Simpson, K.A. Meckling, The effect of anthocyanin-rich purple vegetable diets on metabolic syndrome in obese zucker rats, Journal of Medicinal Food 2017, 20, 1240-1249.
- R. Viola, D. Vreugdenhil, H.V. Davies, L. Sommerville, Accumulation of L-ascorbic acid in tuberising stolon tips of potato (Solanum tuberosum L.), Journal of Plant Physiology 1998, 152, 58-63.
- L. Tedone, R.D. Hancock, S. Alberino, S. Haupt, R. Viola, Long-distance transport ofL-ascorbic acid in potato, Bmc Plant Biology 2004, 4, 16-20.
- A.B. Cabezas-Serrano, M.L. Amodio, R. Cornacchia, R. Rinaldi, G. Colelli, Suitability of five different potato cultivars (Solanum tuberosum L.) to be processed as fresh-cut products, Postharvest Biology and Technology 2009, 53, 138-144.
- S. Kaur, P. Aggarwal, Evaluation of antioxidant phytochemicals in different genotypes of potato, International Journal of Engineering Research Applications 2014, 4, 167-172.
- E.B. Adelanwa, J.M. Medugu, Variation in the nutrition composition of red and green cabbge (Brassica oleracea) with respect to age at harvest, Scientia Horticulturae 2015, 7, 183-189.
- E.B. Adelanwa, J.M. Medugu, Variation in the nutrition composition of red and green cabbage (Brassica oleracea) with respect to age at harvest, Journal of Applied Agricultural Research 2015, 7, 183-189.
- S.R. Bhandari, Y. Chae, J.G. Lee, Assessment of phytochemicals, quality attributes, and antioxidant activities in commercial tomato cultivars, Korean Journal of Horticultural Science & Technology 2016, 34, 677-691.
- S.L. Love, T. Salaiz, B. Shafii, W.J. Price, R.E. Thornton, stability of expression and concentration of ascorbic acid in North American potato germplasm, Hortscience A Publication of the American Society for Horticultural Science 2004, 39, 156-160.
- S.L. Love, J.J. Pavek, Positioning the potato as a primary food source of vitamin C, American Journal of Potato Research 2008, 85, 277-285.
- M.J.H. Keijbets, G. Ebbenhorst-Seller, Loss of vitamin C (L-ascorbic acid) during long-term cold storage of Dutch table potatoes, Potato Research 1990, 33, 125-130.
- M.F.B. Dale, D.W. Griffiths, D.T. Todd, Effects of genotype, environment, and postharvest storage on the total ascorbate content of potato (Solanum tuberosum) tubers, J Agric Food Chem 2003, 51, 244-248.
- O. Külen, C. Stushnoff, D.G. Holm, Effect of cold storage on total phenolics content, antioxidant activity and vitamin C level of selected potato clones, Journal of the Science of Food & Agriculture 2013, 93, 2437-2444.
- J.M. Blauer, G.N.M. Kumar, L.O. Knowles, A. Dhingra, N.R. Knowles, Changes in ascorbate and associated gene expression during development and storage of potato tubers (Solanum tuberosum L.), Postharvest Biology & Technology 2013, 78, 76-91.
- B.N. Tosun, S. Yücecan, Influence of commercial freezing and storage on vitamin C content of some vegetables, International journal of food science & technology 2008, 43, 316-321.
- F. Licciardello, S. Lombardo, V. Rizzo, I. Pitino, G. Mauromicale, Integrated agronomical and technological approach for the quality maintenance of ready-to-fry potato sticks during refrigerated storage, Postharvest Biology & Technology 2018, 136, 23-30.
- I.R. Amado, D. Franco, M. Sanchez, C. Zapata, J.A. Vazquez, Optimisation of antioxidant extraction from Solarium tuberosum potato peel waste by surface response methodology, Food Chemistry 2014, 165, 290-299.
- A. López-Cobo, A.M. Gómez-Caravaca, L. Cerretani, A. Segura-Carretero, A. Fernández- Gutiérrez, Distribution of phenolic compounds and other polar compounds in the tuber of Solanum tuberosum L. by HPLC-DAD-q-TOF and study of their antioxidant activity, Journal of Food Composition & Analysis 2014, 36, 1-11.
- K. Nara, T. Miyoshi, T. Honma, H. Koga, Antioxidative Activity of Bound-Form Phenolics in Potato Peel, Bioscience Biotechnology & Biochemistry 2006, 70, 1489-1491.
- D.J. Williams, D. Edwards, I. Hamernig, L. Jian, A.P. James, S.K. Johnson, L.C. Tapsell, Vegetables containing phytochemicals with potential anti-obesity properties: A review, Food Research International 2013, 52, 323-333.
- D.A. Navarre, R.S. Payyavula, R. Shakya, N.R. Knowles, S.S. Pillai, Changes in potato phenylpropanoid metabolism during tuber development, Plant Physiology & Biochemistry 2013, 65, 89-101.
- J. Tian, J. Chen, F. Lv, S. Chen, J. Chen, D. Liu, X. Ye, Domestic cooking methods affect the phytochemical composition and antioxidant activity of purple-fleshed potatoes, Food Chem 2016, 197, 1264-1270.
- X. Xu, W. Li, Z. Lu, T. Beta, A.W. Hydamaka, Phenolic content, composition, antioxidant activity, and their changes during domestic cooking of potatoes, J Agric Food Chem 2009, 57, 10231-10238.
- T. Blessington, M.N. Nzaramba, D.C. Scheuring, A.L. Hale, L. Reddivari, J.C. Miller, Cooking methods and storage treatments of potato: effects on carotenoids, antioxidant activity, and phenolics, American Journal of Potato Research 2010, 87, 479-491.
- A.L.K. Faller, E. Fialho, The antioxidant capacity and polyphenol content of organic and conventional retail vegetables after domestic cooking, Food Research International 2009, 42, 210-215.
- G. Burgos, Amoros, W., Munõa, L., Sosa, P., Cayhualla, E., Sanchez, C., et al, Total phenolic, total anthocyanin and phenolic acid concentrations and antioxidant activity of purple-fleshed potatoes as affected by boiling, Journal of Food Composition & Analysis 2013, 30, 6-12.
- Y. Liu, Y. Liu, C. Tao, M. Liu, Y. Pan, Z. Lv, Effect of temperature and pH on stability of anthocyanin obtained from blueberry, Journal of Food Measurement & Characterization 2018, 12, 1744-1753.
- D. Blancquaert, S. Storozhenko, K. Loizeau, H. De Steur, V. De Brouwer, J. Viaene, S. Ravanel, F. Rebeille, W. Lambert, V.D.S. Dominique, folates and folic acid: from fundamental research toward sustainable health, Critical Reviews in Plant Sciences 2010,29,14-35.
- G. Alfthan, M.S. Laurinen, L.M. Valsta, T. Pastinen, A. Aro, Folate intake, plasma folate and homocysteine status in a random Finnish population, European Journal of Clinical Nutrition 2003, 57, 81-88.
- C.M. Hatzis, G.K. Bertsias, M. Linardakis, J.M. Scott, A.G. Kafatos, Dietary and other lifestyle correlates of serum folate concentrations in a healthy adult population in Crete, Greece: a cross-sectional study, Nutrition Journal 2006, 5, 105-113.
- A. Goyer, D.A. Navarre, Determination of folate concentrations in diverse potato germplasm using a trienzyme extraction and a microbiological assay, J Agric Food Chem 2007, 55, 3523-3528.
- A. Goyer, K. Sweek, Genetic diversity of thiamin and folate in primitive cultivated and wild potato species, J Agric Food Chem 2011, 59, 13072-13080.
- D.N. Kuhn, J. Chappell, A. Boudet, K. Hahlbrock, Induction of phenylalanine ammonia-lyase and 4-coumarate: CoA ligase mRNAs in cultured plant cells by UV light or fungal elicitor, Proceedings of the National Academy of Sciences of the United States of America 1984, 81, 1102-1106.
- S. Mooney, L. Chen, C. Kühn, R. Navarre, H. Hellmann, Genotype-specific changes in vitamin B6 content and the PDX family in potato, Biomed Research International 2013, 389-397.
- B. Holland, I.D. Unwin, D.H. Buss, Vegetables, herbs and spices, Nutrition Bulletin 1991, 16, 386-414.
- A. Goyer, K.G. Haynes, Vitamin B Content in Potato: Effect of genotype, tuber enlargement, and storage, and estimation of stability and broad-sense heritability, American Journal of Potato Research 2011, 88, 374-385.
- G. Lisinska, W. Leszczynski, Potato Science and Technology, 1989. Springer. ISBN: 978-1-85166-307-1.
- J.D. Burrowes, N.J. Ramer, Changes in the potassium content of different potato varieties after cooking, Journal of Renal Nutrition 2008, 18, 249-255.
- C.P. Sanchez-Castillo, P.J.S. Dewey, A. Aguirre, J.J. Lara, W.P.T. James, The mineral content of mexican fruits and vegetables, Journal of Food Composition & Analysis 1998, 11, 340-356.
- C.R. Novy, Stability and broad-sense heritability of mineral content in potato: potassium and phosphorus, American Journal of Potato Research 2013, 90, 516-523.
- N.K. Subramanian, P.J. White, M.R. Broadley, G. Ramsay, Variation in tuber mineral concentrations among accessions of solanum species held in the commonwealth potato collection, Genetic Resources & Crop Evolution 2017, 64, 1927-1935.
- Y. Sun, H.B. Chang, Y. Yang, W.E. Bradley, Y. Chen, Dietary potassium regulates vascular calcification and arterial stiffness, Jci Insight 2017, 2, 949-956.
- M.K. Hoy, Dietary fiber intake of the U.S. population, what we eat in America, Faseb Journal 2014, 27, 2019-2025.
- M.L. Storey, P.A. Anderson, Nutrient intakes and vegetable and white potato consumption by children aged 1 to 3 years, Adv Nutr 2016, 7, 241-246.
- M.N. Lewu, P.O. Adebola, A.J. Afolayan, Comparative assessment of the nutritional value of commercially available cocoyam and potato tuber in South Africa, Journal of Food Quality 2010, 33, 461-476.
- N.K. Subramanian, P.J. White, M.R. Broadley, R. Gavin, The three-dimensional distribution of minerals in potato tubers, Annals of Botany 2011, 107, 681-691.
- A.N. Furrer, M. Chegeni, M.G. Ferruzzi, Impact of potato processing on nutrients, phytochemicals and human health, Critical Reviews in Food Science & Nutrition 2016, 168-173.
- G. Lisinska, W. Leszczynski, Potato Science and Technology, Springer Netherlands, 1989, ISBN:978-1-85166-307-1.
- C.B. Wegener, H.-U. Jurgens, and G. Jansen, Drought stress affects bioactive compounds in potatoes (Solanumtuberosum L.) relevant to non-communicable diseases, Functional Foods in Health and Disease 2017, 7, 17-35.
- WHO. Micronutrient deficiencies, 2017, https://www.who.int/nutrition/topics/vad/en/.
- C.R. Brown, K.G. Haynes, M. Moore, M.J. Pavek, D.C. Hane, S.L. Love, R.G. Novy, J.C. Miller, Stability and broad-sense heritability of mineral content in potato: iron, American Journal of Potato Research 2010, 87, 390-396.
- M. Paget, W. Amoros, E. Salas, R. Eyzaguirre, M. Bonierbale, Genetic Evaluation of micronutrient traits in diploid potato from a base population of andean landrace cultivars, Crop Science 2014, 54, 1949.
- R.H. True, J.M. Hogan, J. Augustin, S.J. Johnson, C. Teitzel, R.B. Toma, R.L. Shaw, Mineral composition of freshly harvested potatoes, American Potato Journal 1978, 55, 511-519.
- M. Scurrah, W. Amoros, G. Burgos, R. Schafleitner, M. Bonierbale, Back to the future: Millennium traits in native varieties, Acta Horticulturae 2007, 745, 369-377.
- C.H. Sales, L.D. Pedrosa, Magnesium and diabetes mellitus: Their relation, Clinical Nutrition 2006, 25, 554-562.
- D.T. Dibaba, P. Xun, K. He, Dietary magnesium intake is inversely associated with serum C-reactive protein levels: meta-analysis and systematic review, European Journal of Clinical Nutrition 2014, 68, 971-971.
- C.M. Ricardo, González, E. Rodríguez, A. Marrero, D. Carlos, Chemometric studies of chemical compounds in five cultivars of potatoes from Tenerife, Journal of agricultural and food chemistry 2002, 50, 2076-2082.
- A. Ramı́rez-Jiménez, E. Guerra-Hernández, B. Garcı́a-Villanova, Evolution of non-enzymatic browning during storage of infant rice cereal, Food Chemistry 2003, 83, 219-225.
- C. Peña, L.-P. Restrepo-Sánchez, A. Kushalappa, L.-E. Rodríguez-Molano, T. Mosquera, C.-E. Narváez-Cuenca, Nutritional contents of advanced breeding clones of Solanum tuberosum group Phureja, LWT - Food Science and Technology 2015, 62, 76-82.
- B. Tein, K. Kauer, V. Eremeev, A. Luik, A. Selge, E. Loit, Farming systems affect potato (Solanum tuberosum L.) tuber and soil quality, Field Crops Research 2014, 156, 1-11.
- D. Navarre, A. Goyer, R. Payyavula, H. Hellmann, R. Navarre, M.J. Pavek, Nutritional characteristics of potatoes, 2014, ISBN: 9781780642802.
- K.S. Randhawa, K.S. Sandhu, G. Kaur, D. Singh, Studies of the evaluation of different genotypes of potato (Solanum tuberosum L.) for yield and mineral contents, Plant Foods for Human Nutrition 1984, 34, 239-242.
- G. Tamasi, M. Cambi, N. Gaggelli, A. Autino, M. Cresti, R. Cini, The content of selected minerals and vitamin C for potatoes (Solanum tuberosum L.) from the high Tiber Valley area, southeast Tuscany, Journal of Food Composition and Analysis 2015, 41, 157-164.
- C.P. Sanchez-Castillo, P.J.S. Dewey, A. Aguirre, J.J. Lara, W.P.T. James, The mineral content of mexican fruits and vegetables, Journal of Food Composition and Analysis 1998, 11, 340-356.
- J. Potarzycki, W. Grzebisz, Trends in phosphorus concentrations in potato organs during the growing season, Journal of Elementology 2019, 24, 1777-1783.
- J.D. Pursglove, F.E. Sanders, The growth and phosphorus economy of the early potato (Solanum tuberosum), Communications in Soil Science Plant Analysis 1981, 12, 1105-1121.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).