Submitted:
18 May 2023
Posted:
19 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
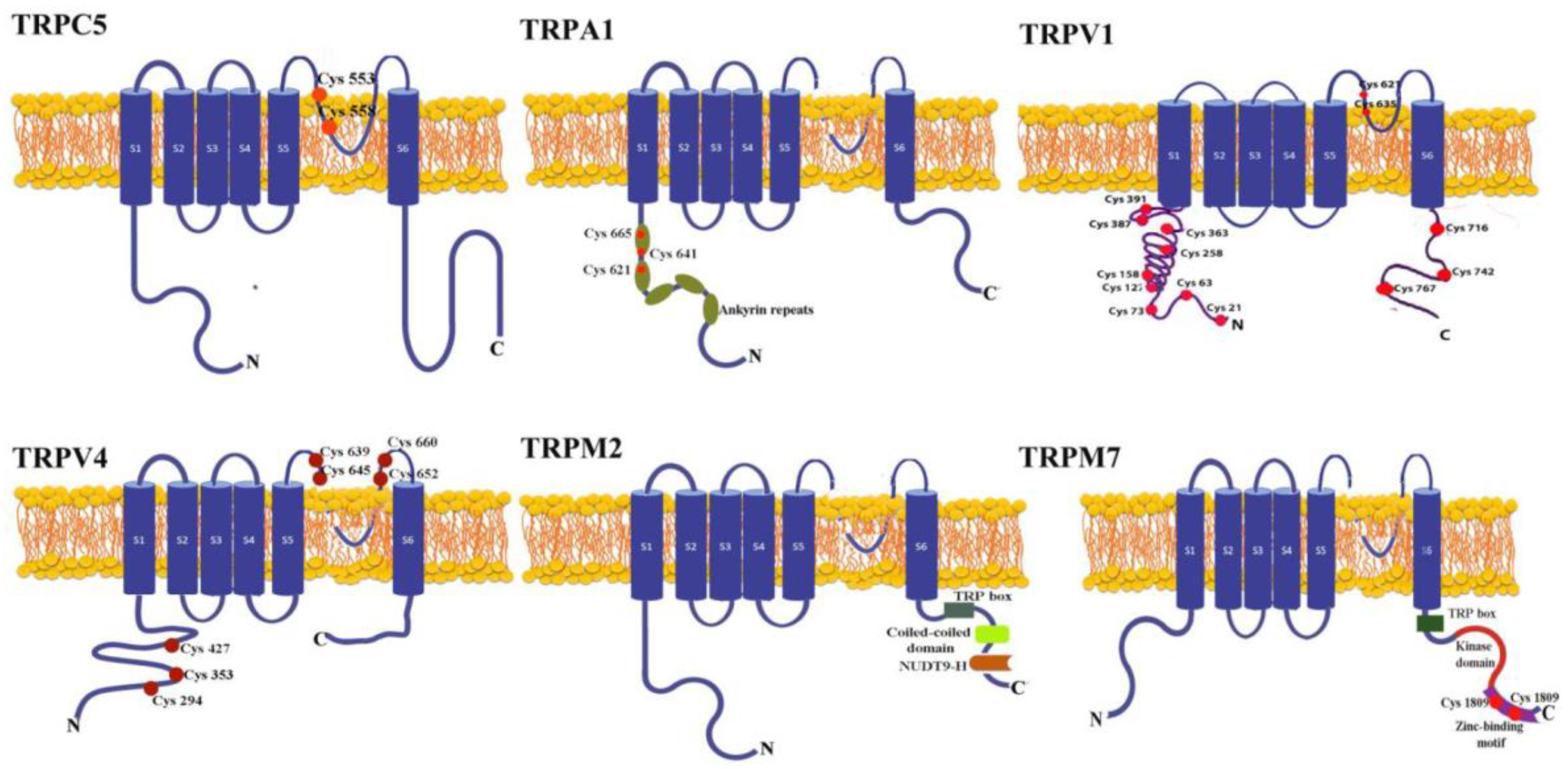
2. The Structure of the TRP Redox Channels
3. Sources of Oxidative Stress and Their Interaction with Redox TRP Channels
3.1. Role of TRP Channels in ROS-Induced DNA Damage
3.2. Role of TRP Channels in ROS-Induced DNA Damage
4. Regulation of TRP Channels by Oxidative Stress in Cancer Cell Proliferation
5. Apoptosis Induced by Modulation of Redox TRP Channels
5. TRP Channels Modulation by Oxidative Stress in Cancer Cell Migration
6. Redox TRP Channels and Inflammation
7. Conclusions
Funding
Conflicts of Interest
References
- Jardin, I.; Lopez, J.J.; Diez, R.; Sanchez-Collado, J.; Cantonero, C.; Albarran, L.; Woodard, G.E.; Redondo, P.C.; Salido, G.M.; Smani, T.; et al. TRPs in Pain Sensation. Frontiers in physiology 2017, 8, 392. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Owsianik, G. The transient receptor potential family of ion channels. Genome biology 2011, 12, 218. [Google Scholar] [CrossRef] [PubMed]
- Vennekens, R.; Hoenderop, J.G.; Prenen, J.; Stuiver, M.; Willems, P.H.; Droogmans, G.; Nilius, B.; Bindels, R.J. Permeation and gating properties of the novel epithelial Ca(2+) channel. The Journal of biological chemistry 2000, 275, 3963–3969. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Prenen, J.; Janssens, A.; Owsianik, G.; Wang, C.; Zhu, M.X.; Voets, T. The selectivity filter of the cation channel TRPM4. The Journal of biological chemistry 2005, 280, 22899–22906. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, R.; Mori, Y. Transient receptor potential (TRP) channels: Biosensors for redox environmental stimuli and cellular status. Free radical biology & medicine 2020, 146, 36–44. [Google Scholar] [CrossRef]
- Takahashi, N.; Mori, Y. TRP Channels as Sensors and Signal Integrators of Redox Status Changes. Frontiers in pharmacology 2011, 2, 58. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Inoue, R.; Morii, T.; Takahashi, N.; Yamamoto, S.; Hara, Y.; Tominaga, M.; Shimizu, S.; Sato, Y.; Mori, Y. Nitric oxide activates TRP channels by cysteine S-nitrosylation. Nature chemical biology 2006, 2, 596–607. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, N.; Kurokawa, T.; Fujiwara, K.; Polat, O.K.; Badr, H.; Takahashi, N.; Mori, Y. Functional and Structural Divergence in Human TRPV1 Channel Subunits by Oxidative Cysteine Modification. The Journal of biological chemistry 2016, 291, 4197–4210. [Google Scholar] [CrossRef]
- Vellino, S.; Oddou, C.; Rivier, P.; Boyault, C.; Hiriart-Bryant, E.; Kraut, A.; Martin, R.; Coute, Y.; Knolker, H.J.; Valverde, M.A.; et al. Cross-talk between the calcium channel TRPV4 and reactive oxygen species interlocks adhesive and degradative functions of invadosomes. The Journal of cell biology 2021, 220. [Google Scholar] [CrossRef]
- Talavera, K.; Startek, J.B.; Alvarez-Collazo, J.; Boonen, B.; Alpizar, Y.A.; Sanchez, A.; Naert, R.; Nilius, B. Mammalian Transient Receptor Potential TRPA1 Channels: From Structure to Disease. Physiological reviews 2020, 100, 725–803. [Google Scholar] [CrossRef]
- Ding, R.; Yin, Y.L.; Jiang, L.H. Reactive Oxygen Species-Induced TRPM2-Mediated Ca(2+) Signalling in Endothelial Cells. Antioxidants (Basel) 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Malko, P.; Jiang, L.H. TRPM2 channel-mediated cell death: An important mechanism linking oxidative stress-inducing pathological factors to associated pathological conditions. Redox biology 2020, 37, 101755. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Murayama, T.; Tashiro, M.; Sakurai, T.; Konishi, M. Mg(2+)- and ATP-dependent inhibition of transient receptor potential melastatin 7 by oxidative stress. Free radical biology & medicine 2014, 72, 257–266. [Google Scholar] [CrossRef]
- Inoue, H.; Murayama, T.; Kobayashi, T.; Konishi, M.; Yokoyama, U. The zinc-binding motif of TRPM7 acts as an oxidative stress sensor to regulate its channel activity. The Journal of general physiology 2021, 153. [Google Scholar] [CrossRef] [PubMed]
- Fricke, T.C.; Echtermeyer, F.; Zielke, J.; de la Roche, J.; Filipovic, M.R.; Claverol, S.; Herzog, C.; Tominaga, M.; Pumroy, R.A.; Moiseenkova-Bell, V.Y.; et al. Oxidation of methionine residues activates the high-threshold heat-sensitive ion channel TRPV2. Proceedings of the National Academy of Sciences of the United States of America 2019, 116, 24359–24365. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative medicine and cellular longevity 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. ROS Are Good. Trends in plant science 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Sinenko, S.A.; Starkova, T.Y.; Kuzmin, A.A.; Tomilin, A.N. Physiological Signaling Functions of Reactive Oxygen Species in Stem Cells: From Flies to Man. Frontiers in cell and developmental biology 2021, 9, 714370. [Google Scholar] [CrossRef]
- Canton, M.; Sanchez-Rodriguez, R.; Spera, I.; Venegas, F.C.; Favia, M.; Viola, A.; Castegna, A. Reactive Oxygen Species in Macrophages: Sources and Targets. Frontiers in immunology 2021, 12, 734229. [Google Scholar] [CrossRef]
- Nugud, A.; Sandeep, D.; El-Serafi, A.T. Two faces of the coin: Minireview for dissecting the role of reactive oxygen species in stem cell potency and lineage commitment. Journal of advanced research 2018, 14, 73–79. [Google Scholar] [CrossRef]
- Vieira, H.L.; Alves, P.M.; Vercelli, A. Modulation of neuronal stem cell differentiation by hypoxia and reactive oxygen species. Progress in neurobiology 2011, 93, 444–455. [Google Scholar] [CrossRef] [PubMed]
- Circu, M.L.; Aw, T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free radical biology & medicine 2010, 48, 749–762. [Google Scholar] [CrossRef]
- Catala, A.; Diaz, M. Editorial: Impact of Lipid Peroxidation on the Physiology and Pathophysiology of Cell Membranes. Frontiers in physiology 2016, 7, 423. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Lei, G.; Zhuang, L.; Gan, B. Targeting ferroptosis as a vulnerability in cancer. Nature reviews. Cancer 2022, 22, 381–396. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Green, M.; Choi, J.E.; Gijon, M.; Kennedy, P.D.; Johnson, J.K.; Liao, P.; Lang, X.; Kryczek, I.; Sell, A.; et al. CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy. Nature 2019, 569, 270–274. [Google Scholar] [CrossRef]
- Li, M.; Zheng, J.; Wu, T.; He, Y.; Guo, J.; Xu, J.; Gao, C.; Qu, S.; Zhang, Q.; Zhao, J.; et al. Activation of TRPV4 Induces Exocytosis and Ferroptosis in Human Melanoma Cells. International journal of molecular sciences 2022, 23. [Google Scholar] [CrossRef]
- Yahya, F.; Mohd Bakri, M.; Hossain, M.Z.; Syed Abdul Rahman, S.N.; Mohammed Alabsi, A.; Ramanathan, A. Combination Treatment of TRPV4 Agonist with Cisplatin Promotes Vessel Normalization in an Animal Model of Oral Squamous Cell Carcinoma. Medicina (Kaunas) 2022, 58. [Google Scholar] [CrossRef]
- Fujii, S.; Tajiri, Y.; Hasegawa, K.; Matsumoto, S.; Yoshimoto, R.U.; Wada, H.; Kishida, S.; Kido, M.A.; Yoshikawa, H.; Ozeki, S.; et al. The TRPV4-AKT axis promotes oral squamous cell carcinoma cell proliferation via CaMKII activation. Laboratory investigation; a journal of technical methods and pathology 2020, 100, 311–323. [Google Scholar] [CrossRef]
- Kang, D.H.; Park, C.K.; Chung, C.; Oh, I.J.; Kim, Y.C.; Park, D.; Kim, J.; Kwon, G.C.; Kwon, I.; Sun, P.; et al. Baseline Serum Interleukin-6 Levels Predict the Response of Patients with Advanced Non-small Cell Lung Cancer to PD-1/PD-L1 Inhibitors. Immune network 2020, 20, e27. [Google Scholar] [CrossRef]
- Shang, G.S.; Liu, L.; Qin, Y.W. IL-6 and TNF-alpha promote metastasis of lung cancer by inducing epithelial-mesenchymal transition. Oncology letters 2017, 13, 4657–4660. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Li, S.; Yang, Y.; Bai, Z. Interleukin-6 promotes ferroptosis in bronchial epithelial cells by inducing reactive oxygen species-dependent lipid peroxidation and disrupting iron homeostasis. Bioengineered 2021, 12, 5279–5288. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Jin, S.; Zhang, Z.; Ma, H.; Yang, X. Interleukin-6 facilitates tumor progression by inducing ferroptosis resistance in head and neck squamous cell carcinoma. Cancer letters 2022, 527, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Uz, U.; Eskiizmir, G. Association Between Interleukin-6 and Head and Neck Squamous Cell Carcinoma: A Systematic Review. Clinical and experimental otorhinolaryngology 2021, 14, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Gasparovic, A.C.; Milkovic, L.; Sunjic, S.B.; Zarkovic, N. Cancer growth regulation by 4-hydroxynonenal. Free radical biology & medicine 2017, 111, 226–234. [Google Scholar] [CrossRef]
- DelloStritto, D.J.; Sinharoy, P.; Connell, P.J.; Fahmy, J.N.; Cappelli, H.C.; Thodeti, C.K.; Geldenhuys, W.J.; Damron, D.S.; Bratz, I.N. 4-Hydroxynonenal dependent alteration of TRPV1-mediated coronary microvascular signaling. Free radical biology & medicine 2016, 101, 10–19. [Google Scholar] [CrossRef]
- Di, Y.; Xu, T.; Tian, Y.; Ma, T.; Qu, D.; Wang, Y.; Lin, Y.; Bao, D.; Yu, L.; Liu, S.; et al. Ursolic acid protects against cisplatin-induced ototoxicity by inhibiting oxidative stress and TRPV1-mediated Ca2+-signaling. International journal of molecular medicine 2020, 46, 806–816. [Google Scholar] [CrossRef]
- Rawat, P.S.; Jaiswal, A.; Khurana, A.; Bhatti, J.S.; Navik, U. Doxorubicin-induced cardiotoxicity: An update on the molecular mechanism and novel therapeutic strategies for effective management. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 2021, 139, 111708. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, M.; Liu, J.; Ye, J.; Jiang, H.; Xu, Y.; Ye, D.; Wan, J. Inhibition of TRPA1 Attenuates Doxorubicin-Induced Acute Cardiotoxicity by Suppressing Oxidative Stress, the Inflammatory Response, and Endoplasmic Reticulum Stress. Oxidative medicine and cellular longevity 2018, 2018, 5179468. [Google Scholar] [CrossRef]
- Tai, Y.K.; Chan, K.K.W.; Fong, C.H.H.; Ramanan, S.; Yap, J.L.Y.; Yin, J.N.; Yip, Y.S.; Tan, W.R.; Koh, A.P.F.; Tan, N.S.; et al. Modulated TRPC1 Expression Predicts Sensitivity of Breast Cancer to Doxorubicin and Magnetic Field Therapy: Segue Towards a Precision Medicine Approach. Frontiers in oncology 2021, 11, 783803. [Google Scholar] [CrossRef]
- Nishiyama, K.; Numaga-Tomita, T.; Fujimoto, Y.; Tanaka, T.; Toyama, C.; Nishimura, A.; Yamashita, T.; Matsunaga, N.; Koyanagi, S.; Azuma, Y.T.; et al. Ibudilast attenuates doxorubicin-induced cytotoxicity by suppressing formation of TRPC3 channel and NADPH oxidase 2 protein complexes. British journal of pharmacology 2019, 176, 3723–3738. [Google Scholar] [CrossRef]
- Hopkins, M.M.; Feng, X.; Liu, M.; Parker, L.P.; Koh, D.W. Inhibition of the transient receptor potential melastatin-2 channel causes increased DNA damage and decreased proliferation in breast adenocarcinoma cells. International journal of oncology 2015, 46, 2267–2276. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.J.; Bao, L.; Keefer, K.; Shanmughapriya, S.; Chen, L.; Lee, J.; Wang, J.; Zhang, X.Q.; Hirschler-Laszkiewicz, I.; Merali, S.; et al. Transient receptor potential ion channel TRPM2 promotes AML proliferation and survival through modulation of mitochondrial function, ROS, and autophagy. Cell death & disease 2020, 11, 247. [Google Scholar] [CrossRef]
- Bao, L.; Festa, F.; Freet, C.S.; Lee, J.P.; Hirschler-Laszkiewicz, I.M.; Chen, S.J.; Keefer, K.A.; Wang, H.G.; Patterson, A.D.; Cheung, J.Y.; et al. The Human Transient Receptor Potential Melastatin 2 Ion Channel Modulates ROS Through Nrf2. Scientific reports 2019, 9, 14132. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bu, F.; Ma, S.; Cananzi, F.; Zhao, Y.; Xiao, M.; Min, L.; Luo, C. The Janus-faced role of TRPM2-S in retroperitoneal liposarcoma via increasing ROS levels. Cell communication and signaling : CCS 2022, 20, 128. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Wei, X.; Wang, M.M.; Liu, Y.; Sui, Z.; Wang, X.; Zhang, Y.; Fei, Y.H.; Jiang, Y.; Lu, C.; et al. Stimulating TRPM7 suppresses cancer cell proliferation and metastasis by inhibiting autophagy. Cancer letters 2022, 525, 179–197. [Google Scholar] [CrossRef] [PubMed]
- Berrout, J.; Kyriakopoulou, E.; Moparthi, L.; Hogea, A.S.; Berrout, L.; Ivan, C.; Lorger, M.; Boyle, J.; Peers, C.; Muench, S.; et al. TRPA1-FGFR2 binding event is a regulatory oncogenic driver modulated by miRNA-142-3p. Nature communications 2017, 8, 947. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Luo, J.; He, L.; Montell, C.; Perrimon, N. Oxidative stress induces stem cell proliferation via TRPA1/RyR-mediated Ca(2+) signaling in the Drosophila midgut. eLife 2017, 6. [Google Scholar] [CrossRef]
- Nie, Y.; Feng, F.; Luo, W.; Sanders, A.J.; Zhang, Y.; Liang, J.; Chen, C.; Feng, W.; Gu, W.; Liao, W.; et al. Overexpressed transient receptor potential vanilloid 1 (TRPV1) in lung adenocarcinoma harbours a new opportunity for therapeutic targeting. Cancer gene therapy 2022, 29, 1405–1417. [Google Scholar] [CrossRef]
- Li, L.; Chen, C.; Chiang, C.; Xiao, T.; Chen, Y.; Zhao, Y.; Zheng, D. The Impact of TRPV1 on Cancer Pathogenesis and Therapy: A Systematic Review. International journal of biological sciences 2021, 17, 2034–2049. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: the bright side of the moon. Experimental & molecular medicine 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Moreno-Celis, U.; Garcia-Gasca, T.; Mejia, C. Apoptosis-Induced Compensatory Proliferation in Cancer. In Metastasis, Sergi, C.M., Ed.; Brisbane (AU), 2022.
- Diwanji, N.; Bergmann, A. An unexpected friend - ROS in apoptosis-induced compensatory proliferation: Implications for regeneration and cancer. Seminars in cell & developmental biology 2018, 80, 74–82. [Google Scholar] [CrossRef]
- Yang, D.; Kim, J. Emerging role of transient receptor potential (TRP) channels in cancer progression. BMB reports 2020, 53, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Chen, H.Y.; Harris, I.S.; Stover, D.G.; Selfors, L.M.; Bronson, R.T.; Deraedt, T.; Cichowski, K.; Welm, A.L.; Mori, Y.; et al. Cancer Cells Co-opt the Neuronal Redox-Sensing Channel TRPA1 to Promote Oxidative-Stress Tolerance. Cancer cell 2018, 33, 985–1003. [Google Scholar] [CrossRef] [PubMed]
- Cojocaru, F.; Selescu, T.; Domocos, D.; Marutescu, L.; Chiritoiu, G.; Chelaru, N.R.; Dima, S.; Mihailescu, D.; Babes, A.; Cucu, D. Publisher Correction: Functional expression of the transient receptor potential ankyrin type 1 channel in pancreatic adenocarcinoma cells. Scientific reports 2021, 11, 8853. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, C.; Hu, H.; Zhang, B. Activated TRPA1 plays a therapeutic role in TMZ resistance in glioblastoma by altering mitochondrial dynamics. BMC molecular and cell biology 2022, 23, 38. [Google Scholar] [CrossRef]
- Naziroglu, M.; Cig, B.; Blum, W.; Vizler, C.; Buhala, A.; Marton, A.; Katona, R.; Josvay, K.; Schwaller, B.; Olah, Z.; et al. Targeting breast cancer cells by MRS1477, a positive allosteric modulator of TRPV1 channels. PloS one 2017, 12, e0179950. [Google Scholar] [CrossRef]
- Sung, B.; Prasad, S.; Ravindran, J.; Yadav, V.R.; Aggarwal, B.B. Capsazepine, a TRPV1 antagonist, sensitizes colorectal cancer cells to apoptosis by TRAIL through ROS-JNK-CHOP-mediated upregulation of death receptors. Free radical biology & medicine 2012, 53, 1977–1987. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, J.; Liu, X. TRPV4 induces apoptosis via p38 MAPK in human lung cancer cells. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas 2021, 54, e10867. [Google Scholar] [CrossRef]
- Zhao, L.Y.; Xu, W.L.; Xu, Z.Q.; Qi, C.; Li, Y.; Cheng, J.; Liu, L.K.; Wu, Y.N.; Gao, J.; Ye, J.H. The overexpressed functional transient receptor potential channel TRPM2 in oral squamous cell carcinoma. Scientific reports 2016, 6, 38471. [Google Scholar] [CrossRef]
- Klumpp, D.; Misovic, M.; Szteyn, K.; Shumilina, E.; Rudner, J.; Huber, S.M. Targeting TRPM2 Channels Impairs Radiation-Induced Cell Cycle Arrest and Fosters Cell Death of T Cell Leukemia Cells in a Bcl-2-Dependent Manner. Oxidative medicine and cellular longevity 2016, 2016, 8026702. [Google Scholar] [CrossRef] [PubMed]
- Akpinar, O.; Ozsimsek, A.; Guzel, M.; Naziroglu, M. Clostridium botulinum neurotoxin A induces apoptosis and mitochondrial oxidative stress via activation of TRPM2 channel signaling pathway in neuroblastoma and glioblastoma tumor cells. Journal of receptor and signal transduction research 2020, 40, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Le Gal, K.; Ibrahim, M.X.; Wiel, C.; Sayin, V.I.; Akula, M.K.; Karlsson, C.; Dalin, M.G.; Akyurek, L.M.; Lindahl, P.; Nilsson, J.; et al. Antioxidants can increase melanoma metastasis in mice. Science translational medicine 2015, 7, 308re308. [Google Scholar] [CrossRef] [PubMed]
- Meyskens, F.L., Jr.; McNulty, S.E.; Buckmeier, J.A.; Tohidian, N.B.; Spillane, T.J.; Kahlon, R.S.; Gonzalez, R.I. Aberrant redox regulation in human metastatic melanoma cells compared to normal melanocytes. Free radical biology & medicine 2001, 31, 799–808. [Google Scholar] [CrossRef]
- Ma, L.; Zhu, W.Z.; Liu, T.T.; Fu, H.L.; Liu, Z.J.; Yang, B.W.; Song, T.Y.; Li, G.R. H2O2 inhibits proliferation and mediates suppression of migration via DLC1/RhoA signaling in cancer cells. Asian Pacific journal of cancer prevention : APJCP 2015, 16, 1637–1642. [Google Scholar] [CrossRef] [PubMed]
- Savino, L.; Di Marcantonio, M.C.; Moscatello, C.; Cotellese, R.; Centurione, L.; Muraro, R.; Aceto, G.M.; Mincione, G. Effects of H(2)O(2) Treatment Combined With PI3K Inhibitor and MEK Inhibitor in AGS Cells: Oxidative Stress Outcomes in a Model of Gastric Cancer. Frontiers in oncology 2022, 12, 860760. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Hsu, J.Y.; Chu, C.T.; Chang, Y.W.; Fan, J.R.; Yang, M.H.; Chen, H.C. Loss of cell-cell adhesion triggers cell migration through Rac1-dependent ROS generation. Life science alliance 2023, 6. [Google Scholar] [CrossRef]
- Yao, X.; Kwan, H.Y.; Huang, Y. Regulation of TRP channels by phosphorylation. Neuro-Signals 2005, 14, 273–280. [Google Scholar] [CrossRef]
- Xu, H.; Zhao, H.; Tian, W.; Yoshida, K.; Roullet, J.B.; Cohen, D.M. Regulation of a transient receptor potential (TRP) channel by tyrosine phosphorylation. SRC family kinase-dependent tyrosine phosphorylation of TRPV4 on TYR-253 mediates its response to hypotonic stress. The Journal of biological chemistry 2003, 278, 11520–11527. [Google Scholar] [CrossRef]
- Li, X.; Cheng, Y.; Wang, Z.; Zhou, J.; Jia, Y.; He, X.; Zhao, L.; Dong, Y.; Fan, Y.; Yang, X.; et al. Calcium and TRPV4 promote metastasis by regulating cytoskeleton through the RhoA/ROCK1 pathway in endometrial cancer. Cell death & disease 2020, 11, 1009. [Google Scholar] [CrossRef]
- Huang, S.; Yu, S.; Deng, R.; Liu, H.; Ding, Y.; Sun, Y.; Chen, W.; Wang, A.; Wei, Z.; Lu, Y. TRPV4 Promotes Metastasis in Melanoma by Regulating Cell Motility through Cytoskeletal Rearrangement. International journal of molecular sciences 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Cai, N.; Bai, Z.; Nanda, V.; Runnels, L.W. Mass Spectrometric Analysis of TRPM6 and TRPM7 Phosphorylation Reveals Regulatory Mechanisms of the Channel-Kinases. Scientific reports 2017, 7, 42739. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Chun, S.Y.; Kim, B.; Yoon, B.H.; Lee, J.N.; Kim, B.S.; Yoo, E.S.; Lee, S.; Song, P.H.; Kwon, T.G.; et al. Knockdown of TRPM7 prevents tumor growth, migration, and invasion through the Src, Akt, and JNK pathway in bladder cancer. BMC urology 2020, 20, 145. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.; Middelbeek, J.; Morrice, N.A.; Figdor, C.G.; Lasonder, E.; van Leeuwen, F.N. Massive autophosphorylation of the Ser/Thr-rich domain controls protein kinase activity of TRPM6 and TRPM7. PloS one 2008, 3, e1876. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Tong, Q.; Conrad, K.; Wozney, J.; Cheung, J.Y.; Miller, B.A. Regulation of TRP channel TRPM2 by the tyrosine phosphatase PTPL1. American journal of physiology. Cell physiology 2007, 292, C1746–C1758. [Google Scholar] [CrossRef] [PubMed]
- Almasi, S.; Sterea, A.M.; Fernando, W.; Clements, D.R.; Marcato, P.; Hoskin, D.W.; Gujar, S.; El Hiani, Y. TRPM2 ion channel promotes gastric cancer migration, invasion and tumor growth through the AKT signaling pathway. Scientific reports 2019, 9, 4182. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Festa, F.; Hirschler-Laszkiewicz, I.; Keefer, K.; Wang, H.G.; Cheung, J.Y.; Miller, B.A. The human ion channel TRPM2 modulates migration and invasion in neuroblastoma through regulation of integrin expression. Scientific reports 2022, 12, 20544. [Google Scholar] [CrossRef]
- Manolache, A.; Selescu, T.; Maier, G.L.; Mentel, M.; Ionescu, A.E.; Neacsu, C.; Babes, A.; Szedlacsek, S.E. Regulation of TRPM8 channel activity by Src-mediated tyrosine phosphorylation. Journal of cellular physiology 2020, 235, 5192–5203. [Google Scholar] [CrossRef]
- Arfin, S.; Jha, N.K.; Jha, S.K.; Kesari, K.K.; Ruokolainen, J.; Roychoudhury, S.; Rathi, B.; Kumar, D. Oxidative Stress in Cancer Cell Metabolism. Antioxidants (Basel) 2021, 10. [Google Scholar] [CrossRef]
- Tan, Z.; Xue, H.; Sun, Y.; Zhang, C.; Song, Y.; Qi, Y. The Role of Tumor Inflammatory Microenvironment in Lung Cancer. Frontiers in pharmacology 2021, 12, 688625. [Google Scholar] [CrossRef]
- Xia, Y.; Shen, S.; Verma, I.M. NF-kappaB, an active player in human cancers. Cancer immunology research 2014, 2, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Maeda, S.; Kamata, H.; Luo, J.L.; Leffert, H.; Karin, M. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell 2005, 121, 977–990. [Google Scholar] [CrossRef] [PubMed]
- Dajee, M.; Lazarov, M.; Zhang, J.Y.; Cai, T.; Green, C.L.; Russell, A.J.; Marinkovich, M.P.; Tao, S.; Lin, Q.; Kubo, Y.; et al. NF-kappaB blockade and oncogenic Ras trigger invasive human epidermal neoplasia. Nature 2003, 421, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Ralph, S.J.; Pritchard, R.; Rodriguez-Enriquez, S.; Moreno-Sanchez, R.; Ralph, R.K. Hitting the Bull's-Eye in Metastatic Cancers-NSAIDs Elevate ROS in Mitochondria, Inducing Malignant Cell Death. Pharmaceuticals (Basel) 2015, 8, 62–106. [Google Scholar] [CrossRef] [PubMed]
- Sevinç, S.K.; Orun, O.; Tiber, P.M.; Çıkla-Süzgün, P.; Küçükgüzel, Ş.G. Anti-Cancer Acitivity of Etodolac and Its Derivatives on Prostate and Colorectal Cancer Cell Lines. Proceedings 2018, 2, 1573. [Google Scholar]
- Tsagareli, M.G.; Nozadze, I.; Tsiklauri, N.; Gurtskaia, G. Non-steroidal anti-inflammatory drugs attenuate agonist-evoked activation of transient receptor potential channels. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 2018, 97, 745–751. [Google Scholar] [CrossRef]
- Muller, C.; Morales, P.; Reggio, P.H. Cannabinoid Ligands Targeting TRP Channels. Frontiers in molecular neuroscience 2018, 11, 487. [Google Scholar] [CrossRef]
- Li, D.; Ilnytskyy, Y.; Ghasemi Gojani, E.; Kovalchuk, O.; Kovalchuk, I. Analysis of Anti-Cancer and Anti-Inflammatory Properties of 25 High-THC Cannabis Extracts. Molecules 2022, 27. [Google Scholar] [CrossRef]
- Dogru, A.; Naziroglu, M.; Cig, B. Modulator role of infliximab and methotrexate through the transient receptor potential melastatin 2 (TRPM2) channel in neutrophils of patients with rheumatoid arthritis: a pilot study. Archives of medical science : AMS 2019, 15, 1415–1424. [Google Scholar] [CrossRef]
- Sharan, M.; Jha, M.; Chandel, R.; Syeda, S.; Mathur, R.; Jha, N.K.; Jha, S.K.; Goel, H.; Shrivastava, A.; Chauhan, S.; et al. Demethylation of CADM1 and SOCS1 using capsaicin in cervical cancer cell line. Naunyn-Schmiedeberg's archives of pharmacology 2023, 396, 649–657. [Google Scholar] [CrossRef]
- Pawar, J.S.; Mustafa, S.; Ghosh, I. Chrysin and Capsaicin induces premature senescence and apoptosis via mitochondrial dysfunction and p53 elevation in Cervical cancer cells. Saudi journal of biological sciences 2022, 29, 3838–3847. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Dong, J.; Tian, W.; Qiao, S.; Wang, H. Role of TRPV1 ion channel in cervical squamous cell carcinoma genesis. Frontiers in molecular biosciences 2022, 9, 980262. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.H.; Jung, S.H.; Sethi, G.; Ahn, K.S. Pleiotropic Pharmacological Actions of Capsazepine, a Synthetic Analogue of Capsaicin, against Various Cancers and Inflammatory Diseases. Molecules 2019, 24. [Google Scholar] [CrossRef] [PubMed]
- Bautista, D.M.; Pellegrino, M.; Tsunozaki, M. TRPA1: A gatekeeper for inflammation. Annual review of physiology 2013, 75, 181–200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lin, C.; Liu, Z.; Sun, Y.; Chen, M.; Guo, Y.; Liu, W.; Zhang, C.; Chen, W.; Sun, J.; et al. Cancer cells co-opt nociceptive nerves to thrive in nutrient-poor environments and upon nutrient-starvation therapies. Cell metabolism 2022, 34, 1999–2017. [Google Scholar] [CrossRef] [PubMed]
- De Logu, F.; Souza Monteiro de Araujo, D.; Ugolini, F.; Iannone, L.F.; Vannucchi, M.; Portelli, F.; Landini, L.; Titiz, M.; De Giorgi, V.; Geppetti, P.; et al. The TRPA1 Channel Amplifies the Oxidative Stress Signal in Melanoma. Cells 2021, 10. [Google Scholar] [CrossRef]
- Yao, K.; Dou, B.; Zhang, Y.; Chen, Z.; Li, Y.; Fan, Z.; Ma, Y.; Du, S.; Wang, J.; Xu, Z.; et al. Inflammation-the role of TRPA1 channel. Frontiers in physiology 2023, 14, 1093925. [Google Scholar] [CrossRef]
- Lee, K.I.; Lee, H.T.; Lin, H.C.; Tsay, H.J.; Tsai, F.C.; Shyue, S.K.; Lee, T.S. Role of transient receptor potential ankyrin 1 channels in Alzheimer's disease. Journal of neuroinflammation 2016, 13, 92. [Google Scholar] [CrossRef]
- Maki-Opas, I.; Hamalainen, M.; Moilanen, L.J.; Haavikko, R.; Ahonen, T.J.; Alakurtti, S.; Moreira, V.M.; Muraki, K.; Yli-Kauhaluoma, J.; Moilanen, E. Pyrazine-Fused Triterpenoids Block the TRPA1 Ion Channel in Vitro and Inhibit TRPA1-Mediated Acute Inflammation in Vivo. ACS chemical neuroscience 2019, 10, 2848–2857. [Google Scholar] [CrossRef]
- Vinuesa, A.G.; Sancho, R.; Garcia-Limones, C.; Behrens, A.; ten Dijke, P.; Calzado, M.A.; Munoz, E. Vanilloid receptor-1 regulates neurogenic inflammation in colon and protects mice from colon cancer. Cancer research 2012, 72, 1705–1716. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Yang, H.; Shao, D.; Zhao, X.; Zhang, G. Intraperitoneal injection of 4-hydroxynonenal (4-HNE), a lipid peroxidation product, exacerbates colonic inflammation through activation of Toll-like receptor 4 signaling. Free radical biology & medicine 2019, 131, 237–242. [Google Scholar] [CrossRef]
- Janion, K.; Szczepanska, E.; Nowakowska-Zajdel, E.; Walkiewicz, K.; Strzelczyk, J. Lipid peroxidation and total oxidant/antioxidant status in colorectal cancer patients. Journal of biological regulators and homeostatic agents 2020, 34, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhu, Y.; Dong, Y.; Zhang, P.; Han, X.; Jin, J.; Ma, X. Overexpression of TrpC5 promotes tumor metastasis via the HIF-1alpha-Twist signaling pathway in colon cancer. Clin Sci (Lond) 2017, 131, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Park, M.K.; Choi, B.Y.; Kho, A.R.; Lee, S.H.; Hong, D.K.; Jeong, J.H.; Kang, D.H.; Kang, B.S.; Suh, S.W. Effects of Transient Receptor Potential Cation 5 (TRPC5) Inhibitor, NU6027, on Hippocampal Neuronal Death after Traumatic Brain Injury. International journal of molecular sciences 2020, 21. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Jin, L.; Lei, K.; Liu, H.; Yang, Y. Fibulin-5 contributes to colorectal cancer cell apoptosis via the ROS/MAPK and Akt signal pathways by downregulating transient receptor potential cation channel subfamily V member 1. Journal of cellular biochemistry 2019, 120, 17838–17846. [Google Scholar] [CrossRef]
- Wang, Z.; Li, S.; Cao, Y.; Tian, X.; Zeng, R.; Liao, D.F.; Cao, D. Oxidative Stress and Carbonyl Lesions in Ulcerative Colitis and Associated Colorectal Cancer. Oxidative medicine and cellular longevity 2016, 2016, 9875298. [Google Scholar] [CrossRef]
- Matsumoto, K.; Deguchi, A.; Motoyoshi, A.; Morita, A.; Maebashi, U.; Nakamoto, T.; Kawanishi, S.; Sueyoshi, M.; Nishimura, K.; Takata, K.; et al. Role of transient receptor potential vanilloid subtype 4 in the regulation of azoymethane/dextran sulphate sodium-induced colitis-associated cancer in mice. European journal of pharmacology 2020, 867, 172853. [Google Scholar] [CrossRef]
- Kaya, İ.; Dağ, S.; Kaya, M.M.; Tanrıverdi, E.A.; Beşeren, H.; Aşasın, G. Modulatory effect of pomegranate extract on TRPA1, TRPM2 and caspase-3 expressions in colorectal cancer induction of mice. Turkish Journal of Biochemistry 2022, 47, 612–619. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Wu, K.; Liu, Z. Expression of 4-hydroxynonenal in esophageal squamous cell carcinoma. Oncology letters 2017, 14, 35–40. [Google Scholar] [CrossRef]
- Nakashima, S.; Shiozaki, A.; Ichikawa, D.; Hikami, S.; Kosuga, T.; Konishi, H.; Komatsu, S.; Fujiwara, H.; Okamoto, K.; Kishimoto, M.; et al. Transient Receptor Potential Melastatin 7 as an Independent Prognostic Factor in Human Esophageal Squamous Cell Carcinoma. Anticancer research 2017, 37, 1161–1167. [Google Scholar] [CrossRef]
- Jakovcevic, A.; Zarkovic, K.; Jakovcevic, D.; Rakusic, Z.; Prgomet, D.; Waeg, G.; Sunjic, S.B.; Zarkovic, N. The Appearance of 4-Hydroxy-2-Nonenal (HNE) in Squamous Cell Carcinoma of the Oropharynx. Molecules 2020, 25. [Google Scholar] [CrossRef] [PubMed]
 TRPM2 activates mitochondria and the transcription factors: HIF-1/2α, Nrf2, ATF4, and CREB; induces the proliferation of acute myeloid leukemia (AML) cells. The pathway is indicated with magenta connectors.
TRPM2 activates mitochondria and the transcription factors: HIF-1/2α, Nrf2, ATF4, and CREB; induces the proliferation of acute myeloid leukemia (AML) cells. The pathway is indicated with magenta connectors.  A pathway illustrated in blue, indicates that Ca2+ uptake by TRPM2 promotes survival of neuroblastoma cells. Nrf2 activates the antioxidant response and cofactors GSH, NADPH, and NADH.
A pathway illustrated in blue, indicates that Ca2+ uptake by TRPM2 promotes survival of neuroblastoma cells. Nrf2 activates the antioxidant response and cofactors GSH, NADPH, and NADH.  TRPM2-S is activated by ROS and consecutively increases apoptosis and decreases proliferation in retroperitoneal liposarcoma (RPLS) cells, through AKT/MAPK pathways as depicted in scarlet. agonists of TRPM7 stimulate ROS production and minimize mitochondrial turnover. The connections between TRPM7, ROS production, and consecutive reduction in autophagy and proliferation are displayed in green.
TRPM2-S is activated by ROS and consecutively increases apoptosis and decreases proliferation in retroperitoneal liposarcoma (RPLS) cells, through AKT/MAPK pathways as depicted in scarlet. agonists of TRPM7 stimulate ROS production and minimize mitochondrial turnover. The connections between TRPM7, ROS production, and consecutive reduction in autophagy and proliferation are displayed in green.
 TRPM2 activates mitochondria and the transcription factors: HIF-1/2α, Nrf2, ATF4, and CREB; induces the proliferation of acute myeloid leukemia (AML) cells. The pathway is indicated with magenta connectors.
TRPM2 activates mitochondria and the transcription factors: HIF-1/2α, Nrf2, ATF4, and CREB; induces the proliferation of acute myeloid leukemia (AML) cells. The pathway is indicated with magenta connectors.  A pathway illustrated in blue, indicates that Ca2+ uptake by TRPM2 promotes survival of neuroblastoma cells. Nrf2 activates the antioxidant response and cofactors GSH, NADPH, and NADH.
A pathway illustrated in blue, indicates that Ca2+ uptake by TRPM2 promotes survival of neuroblastoma cells. Nrf2 activates the antioxidant response and cofactors GSH, NADPH, and NADH.  TRPM2-S is activated by ROS and consecutively increases apoptosis and decreases proliferation in retroperitoneal liposarcoma (RPLS) cells, through AKT/MAPK pathways as depicted in scarlet. agonists of TRPM7 stimulate ROS production and minimize mitochondrial turnover. The connections between TRPM7, ROS production, and consecutive reduction in autophagy and proliferation are displayed in green.
TRPM2-S is activated by ROS and consecutively increases apoptosis and decreases proliferation in retroperitoneal liposarcoma (RPLS) cells, through AKT/MAPK pathways as depicted in scarlet. agonists of TRPM7 stimulate ROS production and minimize mitochondrial turnover. The connections between TRPM7, ROS production, and consecutive reduction in autophagy and proliferation are displayed in green.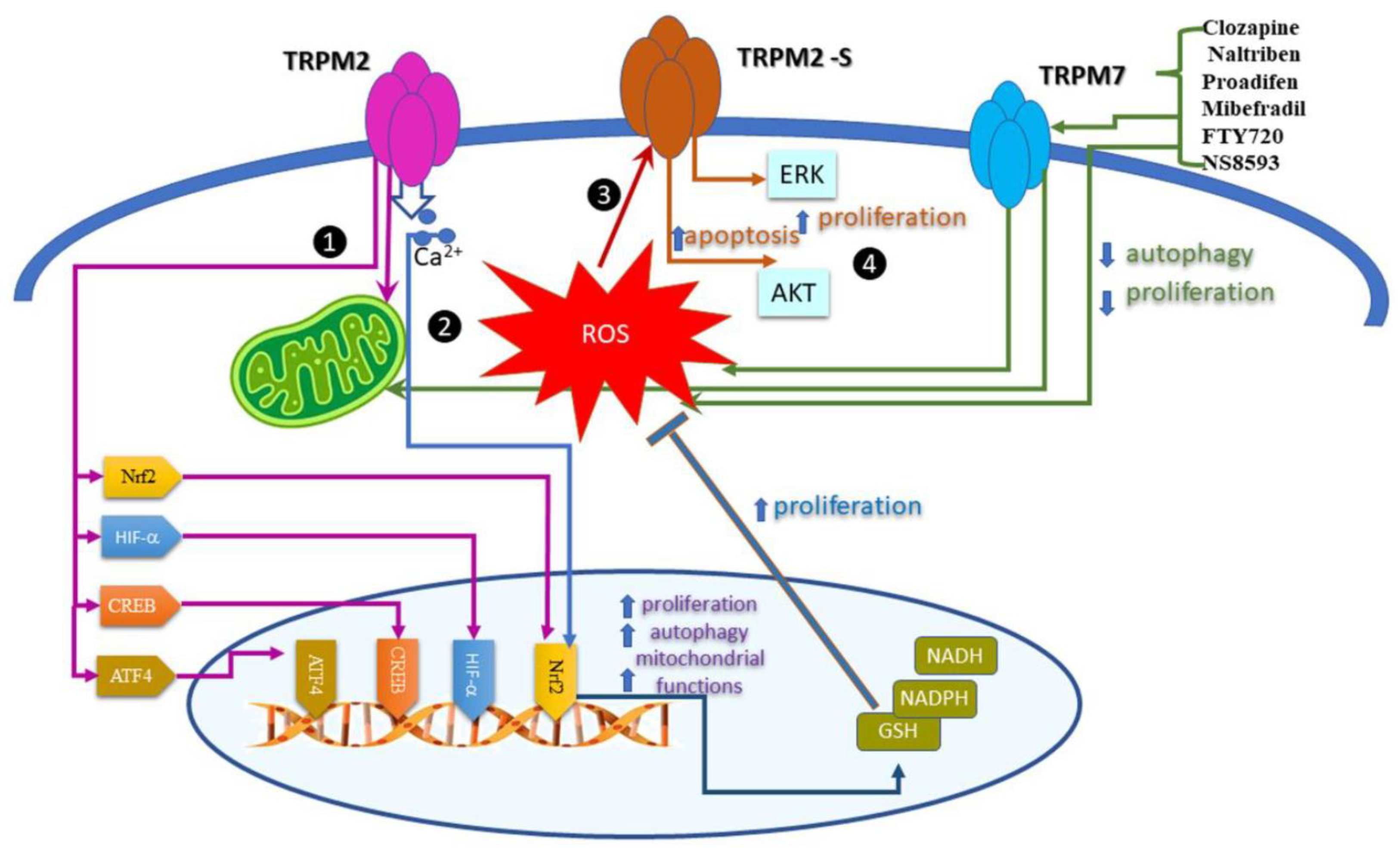
 Calcium entry through TRPA1 inhibited apoptosis upon ROS activation. The process involves the nuclear factor erythroid 2-related factor (Nrf2). The model is valid for breast cancer cells.
Calcium entry through TRPA1 inhibited apoptosis upon ROS activation. The process involves the nuclear factor erythroid 2-related factor (Nrf2). The model is valid for breast cancer cells.  In glioblastoma, temozolomide (TMZ) activates ROS, TRPA1, and apoptosis. The process is related to TMZ resistance.
In glioblastoma, temozolomide (TMZ) activates ROS, TRPA1, and apoptosis. The process is related to TMZ resistance.  Concomitant application of TRPV1 agonists (MRS1477 and capsaicin) increased apoptosis and ROS production.
Concomitant application of TRPV1 agonists (MRS1477 and capsaicin) increased apoptosis and ROS production.  capsazepin, a TRPV1 antagonist, upregulates proapoptotic proteins p53 and Bax, which augment TRAIL-induced apoptosis. The activation of TRAIL receptors triggers the ROS–JNK–CHOP pathway.
capsazepin, a TRPV1 antagonist, upregulates proapoptotic proteins p53 and Bax, which augment TRAIL-induced apoptosis. The activation of TRAIL receptors triggers the ROS–JNK–CHOP pathway.
 Calcium entry through TRPA1 inhibited apoptosis upon ROS activation. The process involves the nuclear factor erythroid 2-related factor (Nrf2). The model is valid for breast cancer cells.
Calcium entry through TRPA1 inhibited apoptosis upon ROS activation. The process involves the nuclear factor erythroid 2-related factor (Nrf2). The model is valid for breast cancer cells.  In glioblastoma, temozolomide (TMZ) activates ROS, TRPA1, and apoptosis. The process is related to TMZ resistance.
In glioblastoma, temozolomide (TMZ) activates ROS, TRPA1, and apoptosis. The process is related to TMZ resistance.  Concomitant application of TRPV1 agonists (MRS1477 and capsaicin) increased apoptosis and ROS production.
Concomitant application of TRPV1 agonists (MRS1477 and capsaicin) increased apoptosis and ROS production.  capsazepin, a TRPV1 antagonist, upregulates proapoptotic proteins p53 and Bax, which augment TRAIL-induced apoptosis. The activation of TRAIL receptors triggers the ROS–JNK–CHOP pathway.
capsazepin, a TRPV1 antagonist, upregulates proapoptotic proteins p53 and Bax, which augment TRAIL-induced apoptosis. The activation of TRAIL receptors triggers the ROS–JNK–CHOP pathway.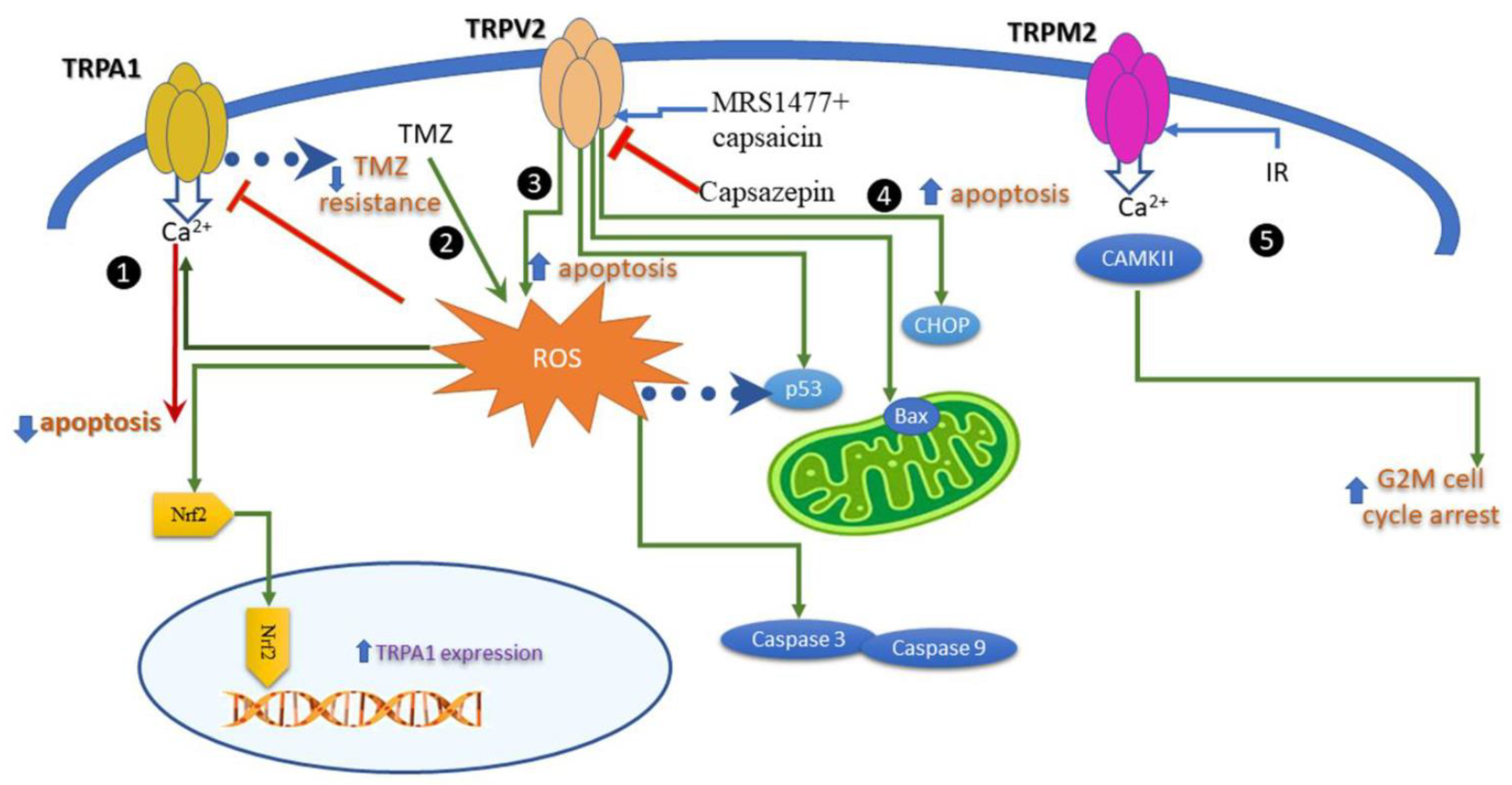
 ROS inhibits stress fibers and consecutively migration through the downregulation of the DLC1/RhoA pathway.
ROS inhibits stress fibers and consecutively migration through the downregulation of the DLC1/RhoA pathway.  Migration mediated by Src kinases. ROS stimulates Src, which in turn phosphorylates TRPV4 and TRPV7. TRPV4 channel contributes to migration via Src-cofilin intracellular pathway, while the TRPV7 kinase domain modulates Src phosphorylation.
Migration mediated by Src kinases. ROS stimulates Src, which in turn phosphorylates TRPV4 and TRPV7. TRPV4 channel contributes to migration via Src-cofilin intracellular pathway, while the TRPV7 kinase domain modulates Src phosphorylation.  Ca2+ entry via TRPM2 activates transcription factors HIF-1α1, E2F1, FOXM1, and CREB, which in turn modulate the expression of α and β integrins. The proteins α and β integrin from the focal adhesion complex engage the extracellular matrix (ECM) in cell migration.
Ca2+ entry via TRPM2 activates transcription factors HIF-1α1, E2F1, FOXM1, and CREB, which in turn modulate the expression of α and β integrins. The proteins α and β integrin from the focal adhesion complex engage the extracellular matrix (ECM) in cell migration.
 ROS inhibits stress fibers and consecutively migration through the downregulation of the DLC1/RhoA pathway.
ROS inhibits stress fibers and consecutively migration through the downregulation of the DLC1/RhoA pathway.  Migration mediated by Src kinases. ROS stimulates Src, which in turn phosphorylates TRPV4 and TRPV7. TRPV4 channel contributes to migration via Src-cofilin intracellular pathway, while the TRPV7 kinase domain modulates Src phosphorylation.
Migration mediated by Src kinases. ROS stimulates Src, which in turn phosphorylates TRPV4 and TRPV7. TRPV4 channel contributes to migration via Src-cofilin intracellular pathway, while the TRPV7 kinase domain modulates Src phosphorylation.  Ca2+ entry via TRPM2 activates transcription factors HIF-1α1, E2F1, FOXM1, and CREB, which in turn modulate the expression of α and β integrins. The proteins α and β integrin from the focal adhesion complex engage the extracellular matrix (ECM) in cell migration.
Ca2+ entry via TRPM2 activates transcription factors HIF-1α1, E2F1, FOXM1, and CREB, which in turn modulate the expression of α and β integrins. The proteins α and β integrin from the focal adhesion complex engage the extracellular matrix (ECM) in cell migration.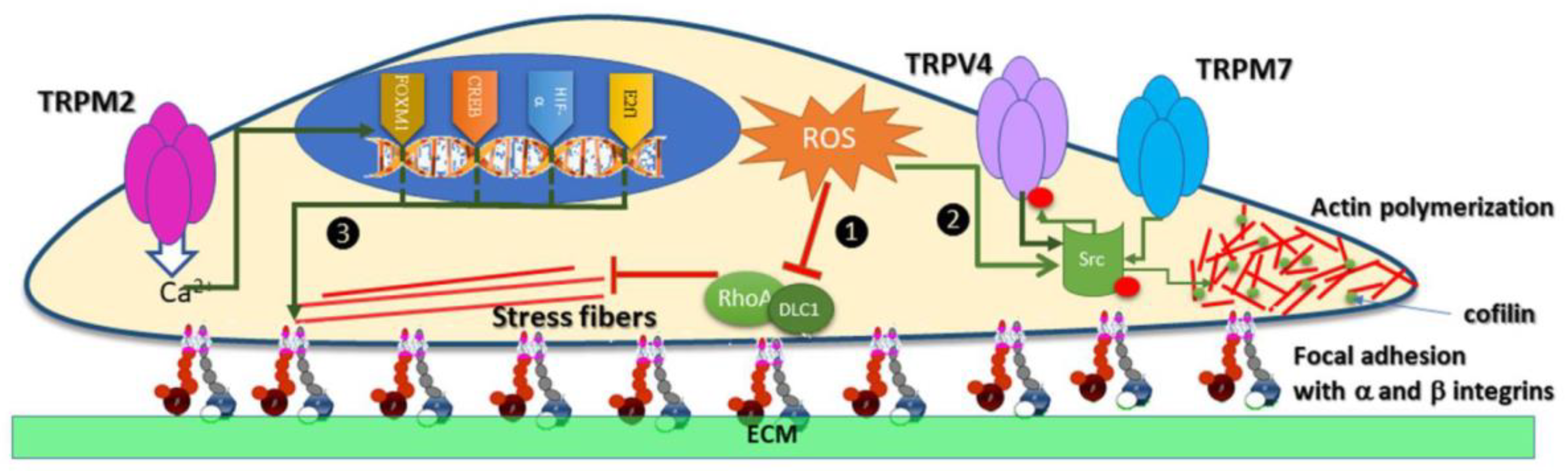
| Anti-inflammation drug | TRP channel affected | Role in tumorigenesis | Ref. |
|---|---|---|---|
| Nonsteroidal anti-inflammatory drugs (NSAIDs), e.g., aspirin and cyclooxygenase | Inhibition of TRPC4/C5 | reduces the risk of breast cancer | [13,44] [85] |
| Etodolac (Cox-2 inhibitor) | Desensitization of TRPA1 | Inhibition of proliferation of prostate and colorectal carcinoma | [86,87] |
| Cannabinoids and Cannabis Extracts | Activation of TRPV4 and TRPA1 | Inhibits growth of breast cancer cells | [88,89] |
| Methotrexate | Inhibition of TRPM2 | Chemotherapy of breast cancer, head, and neck cancer, lymphoma | [90] |
| Capsaicin | Activation of TRPV1 | Senescence and Apoptosis in cervical cancer cells | [91,92,93] |
| Capsazepine | Inhibition of TRPV1 | Inhibits growth and survival of prostate cancer, breast cancer, colorectal cancer, oral cancer, and osteosarcoma | [94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





