1. Introduction
With rapid development of society and economy, the impact of industry, agriculture and urban life on the environment has become increasingly prominent, and water pollution problems of different types and degrees are widespread all over the world [
1,
2]. In industry, harmful substances such as heavy metals, dyes, phenols and various saline wastewaters are discharged. For agricultural production, pesticides are widely used and only 20% of them was absorbed by crops; the rest is gasified into the atmosphere, while the other part penetrates into soil and groundwater through the earth surface. In addition, a large amount of garbage and drug residues produced by urban life. All these hazardous objects pose a serious threat to human health and sustainable development, and the incidence of tumors, skin diseases, allergies, malformations and nervous system diseases is greatly increased in the past decade [
3].
As the two kinds of new green solvents, ionic liquids (ILs) and deep eutectic solvents (DESs) are attracting huge attention from academic and industrial circles [
4]. Among them, the former refers to the salts that appear liquid at or near room temperature, which is completely composed of cation and anion. Their low melting point is ascribed to that the asymmetry of some substituents in their structure makes the ions unable to accumulate into crystals regularly. The most common cations are imidazole salts, while the frequently applied anions include halogen, tetrafluoroborate, and hexafluorophosphate ions, etc. As for DESs, they are composed of hydrogen bond acceptors and hydrogen bond donors with a certain stoichiometric ratio (commonly molar ratio=1:1). Most DESs are two-component mixtures, and a few eutectic solvents are three-component mixtures. Water molecules can be used as one of the components of some eutectic solvents. The most common hydrogen bond acceptor is choline chloride, and hydrogen bond donors such as urea, carboxylic acid, polyol, amino acid, sugar and so on. In separation science, more and more ILs and DESs are being successfully applied for their advantages of good selectivity, designability, stability, recyclability and so on [
5].
At present, researchers pay their great interests on the removal and detection of aforementioned hazardous substances in various forms and ways. Among them, adsorption methods based on various innovative sorbents have the most active performance [
6,
7]. From the theoretical, mechanistic, and practical perspectives, the aforementioned green solvents have enormous potential for application in this field. Especially, the immobilized ILs or DESs on some supports or related sorbents modified with them can make their use more convenient, which also save consumption and avoids residue of them [
8,
9]. For instance, a modified IL clay was prepared to remove Pb(II), Co(II) and Zn(II) ions from water [
10], and its catalytic activity after treated with heavy metal ion solutions was proved in the reduction of nitroarenes to its corresponding amines. Various metal organic frameworks combined with imidazolium, quinolinum and benzothiazolium ILs were developed for removal of three antibiotics from water [
11]. Besides, a series of natural and low-cost menthol-based hydrophobic DESs were synthesized to extract triphenylmethane (TPM) dye micropollutants from dilute aqueous solution [
12]. DES-modified mixed iron hydroxide–silica was successively used in magnetic solid-phase extraction for enrichment of organochlorine pesticides [
13]. In summary, ILs and DESs have achieved great success as extractants and sorbents together with their immobilized forms for treating related targets. However, there is no report to use these two green media simultaneously in the same sorbent, and the immobilized way of them together with separation way can be further simplified. Under these backgrounds, a unique composite sorbent disc was invented to achieve above goals, which was expected for contribution to sustainable development. The technical route of the whole work is shown as
Scheme 1.
2. Materials and Methods
2.1. Reagents and materials
Choline chloride, glacial acetic acid, methanol, absolute ethanol, potassium hydroxide, acetonitrile and standard metal salts as well as pigment were all purchased from Kelong chemical factory (Chengdu, China). β-CD (molecular weight: 1135 g/mol), N-methylimidazole, 2-bromoethylamine hydrobromate, methyl, ethyl and carboxymethyl cellulose were purchased from Aladdin chemical reagent factory (Shanghai, China). Except for chromatographic grade methanol used for quantitative analysis, all reagents and solvents were analytical pure grade and were used without further purification if not stated otherwise. All the standard hazardous substances were purchased from Aladdin and Aike chemical reagent factory (Shanghai, China), and all of their purity was not lower than 98.0%. Experimental water was prepared with Milli-Q water purification system (Millipore, Bedford, USA). Actual water samples were collected from local.
2.2. Instruments
Conductivity was detected with DDSJ-319L conductivity meter (0.000 μS/cm–3000 mS/cm, ±0.5% FS, Leici Instrumental, Shanghai, China). NMR spectra of the IL and DES were performed on AV II-400 MHz spectrometer (Bruker, Basel, Switzerland). A Spectrum Two L1600300 Spectrometer (Perkin Elmer, USA) was pplied to record Fourier transform infrared spectra (FT-IR) in the range of 4000–450 cm−1. Thermogravimetric analysis (TGA) was performed on a TGA/DSC 2 type instrument (Mettler Toledo, ImLangacher, Switzerland) with a heating rate of 10°C/min from 30℃ to 600℃ in nitrogen atmosphere. The contents of hazardous substances were determined by an AOE380 UV spectrometer (Aoyi Instrument Co., Ltd., Shanghai, China). Quantitative determination of hazardous substance was performed by UltiMate3000 high performance liquid chromatography (DIONEX Co., Sunnyvale, USA).
2.3. Preparation of IL and DES
According to current reports and our pilot experimental screening, the IL of 1-aminoethyl-3-methylimidazole bromide ([NH
2emim][Br]) and DES of choline chloride-acetic acid (1:2) were chosen for current studies. The former was synthesized through reported way [
14] as following: 0.15 mol of 2-bromoethylamine hydrobromate and equimolar N-methylimidazole were mixed and stirred with 30 mL absolute ethanol as solvent for 10 min. After thorough stirring, the radiation power of the XH-300UL multifunctional microwave synthesizer (Xianghu Technologies, Beijing, China) was set to 300 W, and the reactive duration was 20 s and three-round reaction was completed with interval of 5 min. After that, 15 mL of 0.01 mol/mL KOH aqueous solution was added into the synthesized product and stirred in ice water bath) for 5 min. At last, the system was kept at 0℃ until no more sediment formed and then filtered, the filtrate was concentrated and dried, which was further mixed with enough absolute ethanol to precipitate KBr. Yellow viscous [NH
2emim][Br] could be successfully obtained after final filtration and dryness under vacuum, and the yield of the IL was 91%.
Secondly, with choline chloride as hydrogen bond acceptor and acetic acid as hydrogen bond donor, their molar ratio was set at 1:2. In actual operation moderately modified from previous way [
15], solid choline chloride was added in liquid acetic acid and the mixture was thoroughly stirred at 60℃ for 80 min to form a colorless, uniform and transparent liquid. There is no solid precipitation after long-term storage at room temperature, which indicated the final formation of a stable DES (yield near to 100%).
2.4. Preparation of IL/DES@β-CD
It was reported that inclusion and encapsulation of an ionic liquid with β-CD [
16], which can immobilize the former and then make it more convenient and stable during application. The solubility of β-CD in 60℃ water was 7.29 mg/g, and its saturated solution at 60℃ was first prepared according to this data, and then the DES of choline chloride-acetic acid with five times the amount of β-CD was added in the solution of the latter and stirred thoroughly at 60℃ for 2 hours. After the loading was completed, the system was placed in the freezer and refrigerated for 24 hours. It could be observed that there was much crystal had been precipitated. After suction filtration, the complex of IL and CD was dried under vacuum to obtain white granular solid (DES@β-CD) for further disc pressing. Similarly, the saturation β-CD solution at 60℃ was prepared and then the IL of [NH
2emim][Br] with five times the amount of β-CD was used to form the IL@β-CD complex under stirring for 4 hours. After the crystallization and dryness, white granular solid (IL@β-CD) was obtained.
2.5. Preparation of IL/DES@β-CD discs
After thorough drying and primary sieving, the complex of IL/DES@β-CD was mixed with diluent (substituted celluloses) evenly with the mass ratio of 0.15:0, 0.125:0.025, 0.1:0.05, 0.075:0.075, 0.05:0.1, 0.025:0.125 (diluent: IL/DES@β-CD, g/g). After passing through 200 mesh sieve, the powders were pressed in a stainless steel mold with a diameter of 13 mm under the pressure of 5~20 MPa for 0~3 h. With the increasing dosage of the complex of IL/DES@β-CD and diluents at fixed mass ratio, various tablet weight of 0.1 g, 0.15 g, 0.2 g and 0.3 g could be obtained and their thickness ratio was 1:1.5:2:3. Furthermore, if it was needed to make a coin-like double-face sorbent disc, the equivalent powders of IL@β-CD and DES@β-CD were successively spread in the mold to form two layers. After pressing, the faces of IL@β-CD and DES@β-CD could be successfully formed and play respective role of selective adsorption for different targets.
2.6. Removal of hazardous substances from water
After obtaining the stable sorbent discs based on IL/DES@β-CD, they were placed in the aqueous solution of hazardous substances at the preset concentrations and actual water samples collected from local. During the adsorption process, the whole system was continuously shaken in the constant temperature shaker at R.T. After the investigated duration, appropriate volume of the supernatant was taken out and analyzed, and the adsorption efficiency (%) was determined by the ratio with the target concentration before and after adsorption, which was used to evaluate the removal performance of the new sorbent discs.
3. Results and discussion
3.1. Structural identification of the IL and DES
In order to indentify the two green solvents before their applications,
1H-NMR (400 MHz, DMSO-d
6) was firstly applied to determine their structures. For [NH
2emim][Br], the signals included 9.24 ppm (s, -N-CH=N=), 7.82 ppm (d, =N-CH=CH-N≡), 7.75 ppm (d, =N-CH=CH-N≡), 4.22 ppm (t, ≡N-CH
2-), 3.84 ppm (s, =N-CH
3) and 2.72 (m, NH
2-CH
2-). For choline chloride-acetic acid, the signals at 5.64 ppm (t, -CH
2-Cl), 3.77 ppm (t, -CH
2-N
+) and 3.39 ppm (s, -CH
3×3) originate from the hydrogen bond acceptor of choline chloride, and those at 11.90 ppm (s, -COOH) and 1.86 ppm (s, -CH
3) result from acetic acid. Compared with those before forming DES, their chemical shift was a little increased for the intermolecular hydrogen bonds. Above results proved the structure of the two green solvents as well as their successful preparation [
14,
15,
17].
3.2. Loading of IL/DES on β-CD
In order to achieve better loading performance and kinetics, various loading methods were compared besides the aforementioned way of β-CD saturation solution in this section. Based on current studies [
18,
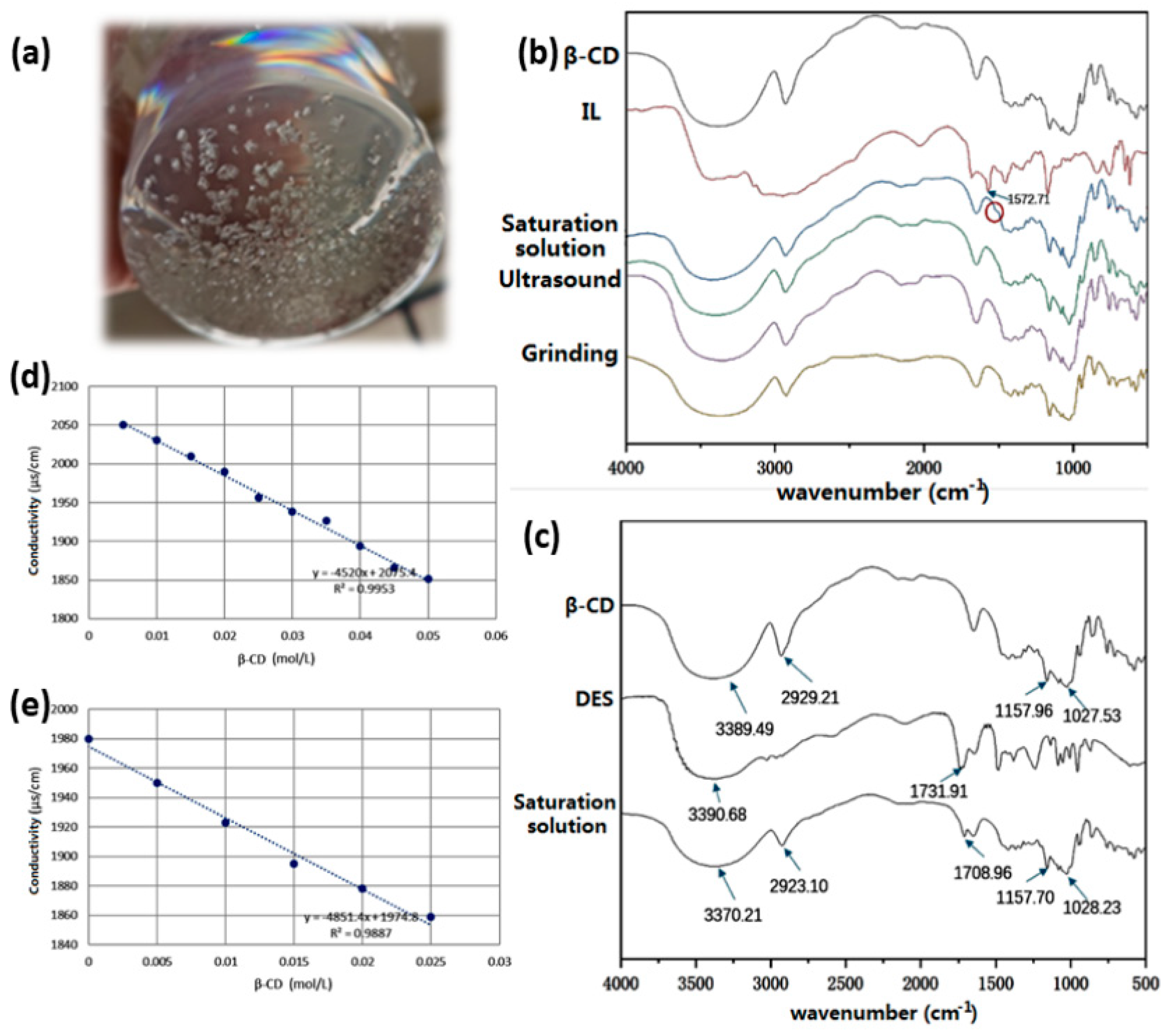
19], the other two were selected as ultrasound assistance and grinding method, respectively. In detail, ultrasonic wave was used for strengthening loading in the former method and resulted in more uniform system; after preparing 40/60℃ β-CD saturated aqueous solution, the IL/DES with five times the amount of β-CD was added in the solution and ultrasonicated (300/600 W) at 40/60℃ for 1 hour. After the loading was completed, the system was put into the freezer for refrigeration for 24 hours. When there was no more crystallization, the solid complex was collected for comparison. Compared to ultrasonic method, grinding method is mainly carried out in the dry state, and the loading process involves squeezing, shearing, and friction effects. In detail, a certain amount of dry β-CD was added in distilled water with 2 times the mass of β-CD, and the mixture was evenly ground in the mortar. Then the equimolar IL/DES was added in the system, which was continuously ground for 30 min to obtain a uniform paste. After that, the mixture was placed in the freezer and refrigerate for 24 hours. It was observed that the solid phase was white (see
Figure 1a). After suction filtration and dryness, it was obtained as the complex of IL/DES on β-CD. Taking the example of IL@β-CD, it can be found that through the comparison on corresponding IR spectra of different products obtained by above three ways (see
Figure 1b,c), the saturation solution way resulted in the expected complex because the IR absorbance peak of IL around 1570 cm
-1 was observed in related product. Therefore, this way was finally applied for loading both IL and DES on β-CD. Based on the online measurement of conductivity, the loading process (complex formation process) for them can be fit as the following equations:
where x was the concentration of β-CD (mol/L), and y was the system conductivity (μS/cm). As the result, the conductivity decreases remarkably with increasing β-CD concentration, which proves the inclusion complex formation between β-CD and IL/DES. Moreover, the conductivity curve shows a good linear relationship (R
2 > 0.98) at a concentration range of 0~0.025/0.050 mol/L β-CD (see
Figure 1d,e). Compared the loading process in the tunnels (inner diameter: 10~30 nm) of carbon nanotubes in our previous study [
20], the inner cavity size of β-cyclodextrin is in the range of 0.5~0.8 nm and immobilization method in this study is based on supramolecular interactions and inclusion effect instead of loading. Both of the two modes can result in enough stability of green solvents and are easier than the chemical modification.
3.3. Preparation of IL/DES@β-CD sorbent discs
In the first round of screening through pre-experiments, it was found that ethyl cellulose could obtain more ideal mechanical strength of sorbent disc than methyl cellulose and carboxymethyl cellulose at the same dose. Then a single complex of IL or DES@β-CD was ground into powders and mixed with the diluents of ethyl cellulose in different proportions. Here the pressure of 15 MPa was selected according to the pilot experiments, which is lower than that of common pressed tablets (20~410 MPa) [
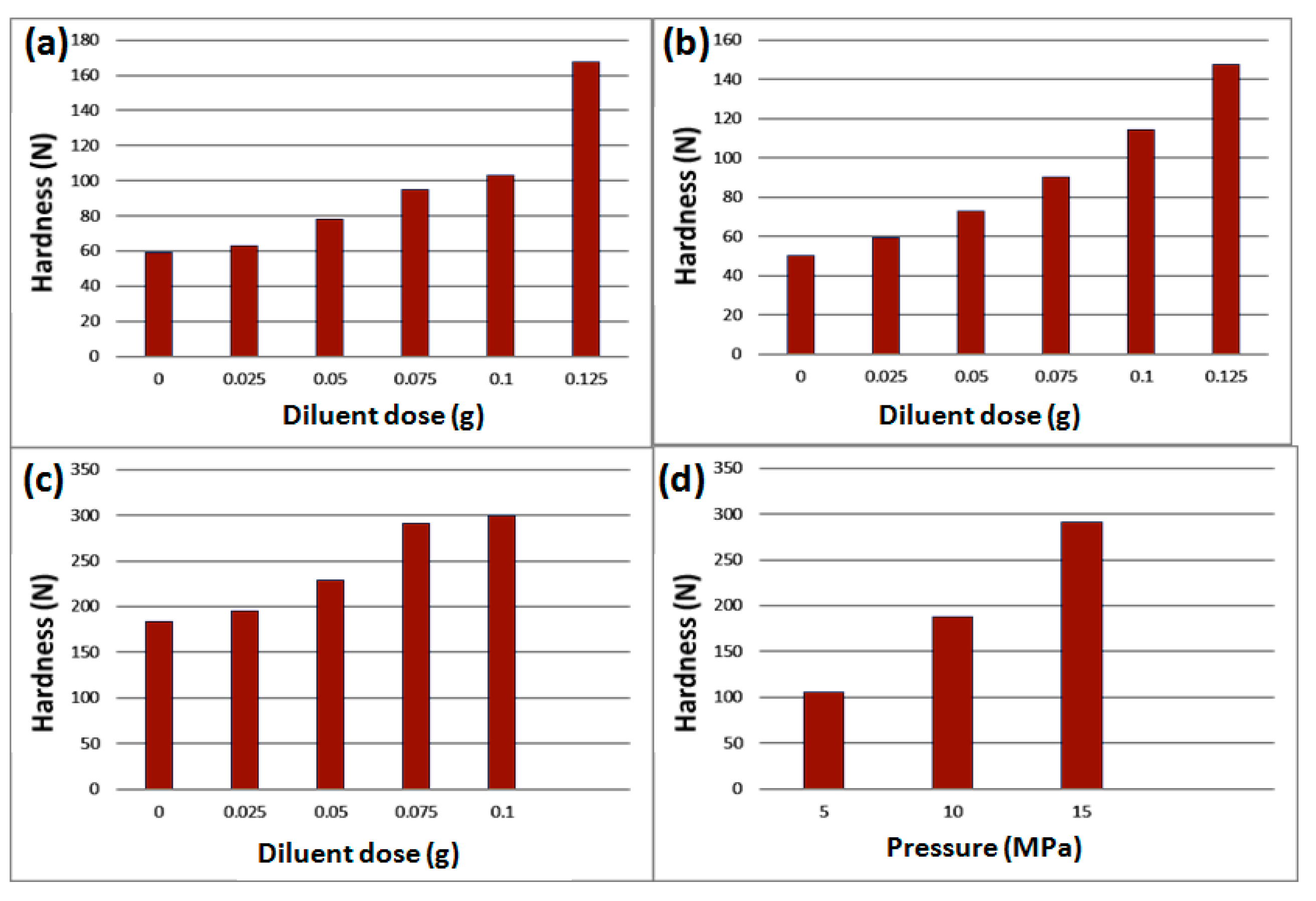
21]. After sieved with 200 mesh sieve and pressed under 15 MPa for 30 min to obtain white tablet (0.15 g), and the relationship between hardness and diluent dose was explored. As shown in
Figure 2a, it can be seen that the hardness of the IL@β-CD disc increases slowly when the diluent dosage is in the range of 0~0.1 g, and reaches the ordinary disc hardness (around 100 N) at 0.1g. When it exceeds 0.1 g, the hardness increases rapidly. For comparison, the DES@β-CD disc shows more stable growth of strength after the diluent dose was around 0.1 g (
Figure 2b); its hardness is a little higher than that of IL@β-CD disc below 0.1 g, which means higher density and tighter combination of involved components; after that it begins to become lower.
For double-faced discs composed of DES@β-CD and IL@β-CD simultaneously, the mixture powders of 1.5 g DES@β-CD and ethyl cellulose was firstly spread in the mold as a thin layer (bottom side), and the same amount of IL@β-CD and ethyl cellulose mixed powders was spread on this layer as another layer (top side). After pressed at 15 MPa for 5 min to obtain white double-faced discs, the relationship between its hardness and diluents dose was also explored (
Figure 2c). Interestingly, it can be found that the hardness of the double-sided disc formed by the combination of the two complexes is much higher than that of the single-component disc with the same amount of diluent. When it is above 0.075 g, the hardness increase will become very slow; and the obtained double-sided disc can be kept in the shaker (500 rpm) for more than 5 h without any disintegration, which can ensure its stable work in the adsorption process. When the amount of diluent reached 0.125 g, the force sensor was overloaded, indicating the hardness was excessive high. In the investigation on the pressure on the hardness (see the results in
Figure 2d), the mixture powders composed with complex and diluents (1:1) were pressed under different pressures for 5 min, and the hardness of the discs was measured. It can be found 15 MPa is enough for preparing stable disc with the hardness near to 300 N. When the pressure is higher than 15 MPa, the force sensor will be overloaded. Moreover, excessively tight matrix is not beneficial for mass transfer [
22], which will make the duration is very long to reach adsorption equilibrium.
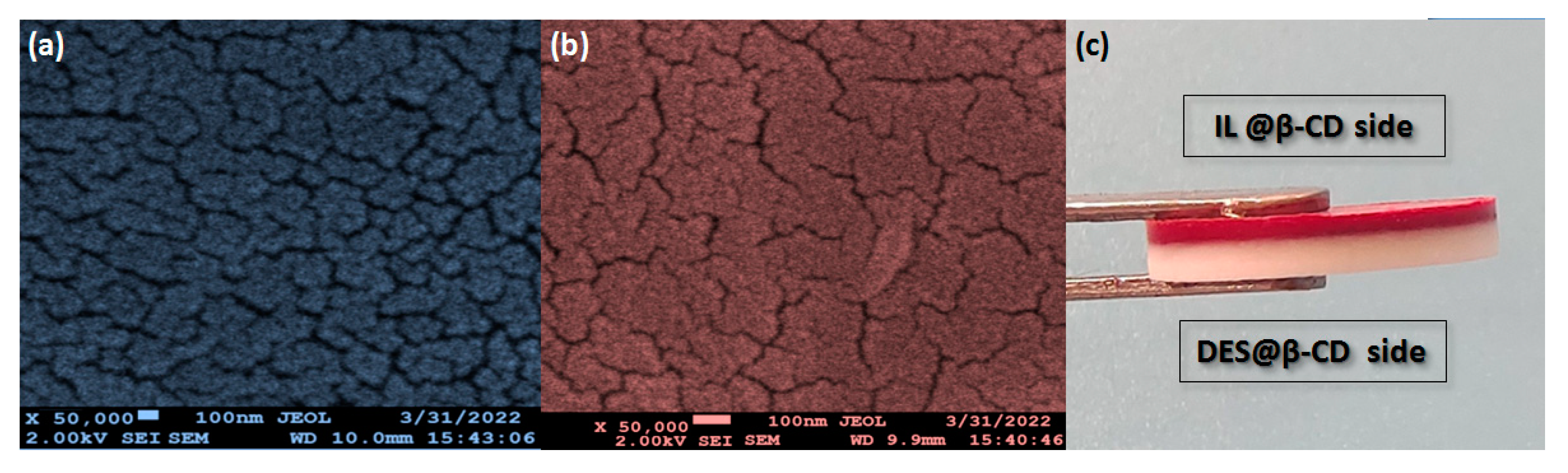
Moreover,
Figure 3a–c provide the micro morphology of IL@β-CD and DES@β-CD layer sections together with appearance of double-sided disc, respectively. Obviously, no apparent pores, cavities, and uneven color areas can be found; moreover, the DES@β-CD layer shows fewer cracks at the same magnification, which accords with the aforementioned results of strength measurement. That is, higher density and tighter combination of involved components was achieved in the DES@β-CD layer than in the IL@β-CD layer. As seen in
Figure 3c, two layers can form a clear and smooth boundary through the simple stacking and pressurization process, which is easy to enlarge and repeat than other preparation methods of current sorbents. Finally, the current adsorption tablets were continuously stirred (300 rpm) in water for 72 hours without any disintegration phenomenon, indicating its good stability in possible working environments during applied for separation.
3.4. Performance validation of the sorbent discs to capture hazardous substances
Heavy metal wastewater is considered one of the industrial wastewater that causes serious environmental damage and poses significant harm to humans [
23]. Unlike other organic compounds, heavy metal elements in water cannot be degraded and have enrichment properties. Therefore, it was first selected as our adsorption object. Because if the existence of –NH
2 in the structure of the IL, the single IL@β-CD disc showed wide removal effect for heavy metal ions in our study, which included Cu
2+, Cd
2+, Ni
2+, As
2+, Co
2+, and Pb
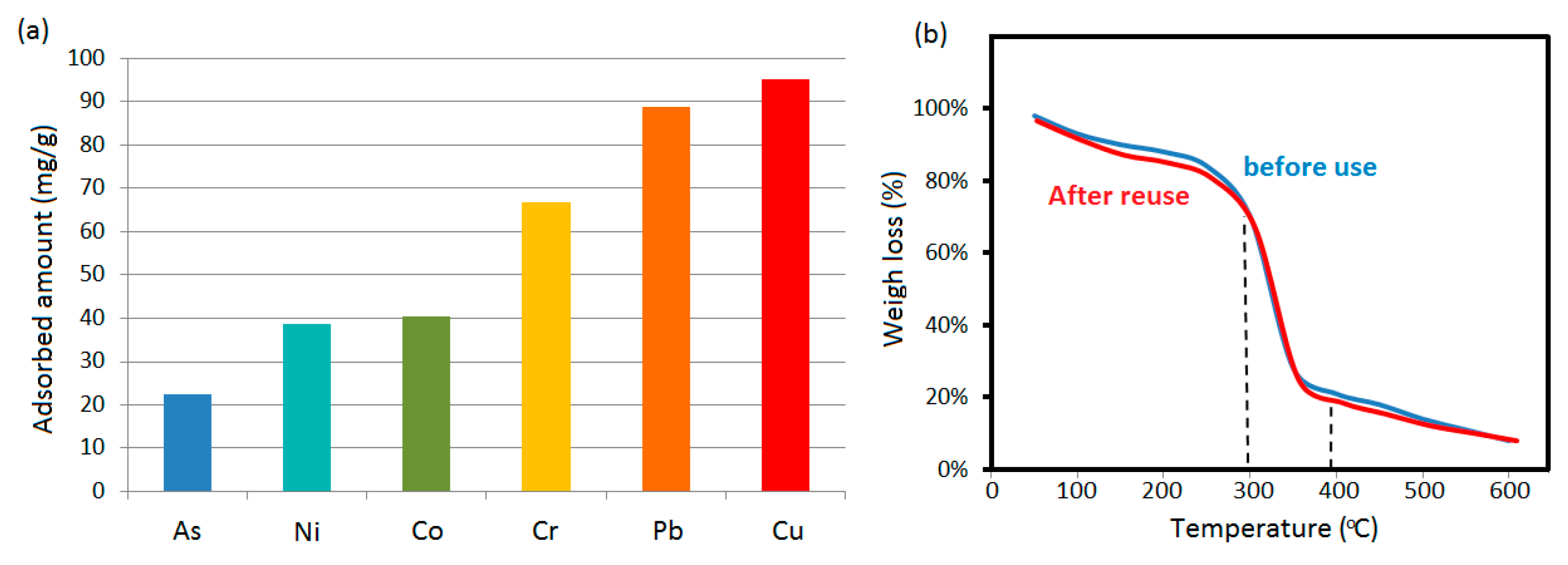
2+. The amino groups can form stable chelates with these target ions, so as to make the disc play the role of adsorption. Among them, the removal efficiency of Cu
2+ and Pb
2+ was the highest two (experimental conditions: a 0.15 g disc, 1 mg/mL ion concentration, 10 mL solution, 300 rpm, unadjusted pH, adsorption until the solution color no longer became lighter, R.T.), which reached 95.2 and 88.7 mg/g (see
Figure 4a) according to previous quantitative method [
20,
24]. The further adsorption improvement can be realized by adding the loading IL amount and optimization on the separation conditions. As for the adsorption of kinetics of Cu
2+, it was found that the pseudo second-order model has higher correlation coefficient (R
2=0.9902,
k2=3.09×10
-2 g/mg min) than pseudo first-order kinetic models (R
2=0.9712,
k1=0.028 /min), indicating overall rate process is influenced by both intraparticle diffusion and external mass transfer. Moreover, chemisorption is the rate-limiting step during adsorption [
25].
On the other hand, cationic dyes are widely used in textile, dyeing and finishing industries. Once they appear in water environment, they have a significant impact on the water chromaticity. Therefore, we chosen alkaline red 18 (C.I.11085) here as the target of the investigation for the single DES@β-CD disc in this section; although it is easier to be adsorbed by acidic adsorbents from the perspective of its structure, here it was aimed to investigate whether DES of choline chloride-acetic acid would also play a certain role. After the adsorption experiments (experimental conditions: a 0.15 g disc, 1 mg/mL ibuprofen concentration, 10 mL solution, 300 rpm, unadjusted pH, 24 h, R.T.), the adsorption efficiency of alkaline red was 60.8 mg/g according to the developed quantitative way [
26], which was in the reported range from over ten to hundred of mg/g in current studies of similar sorbents [
27]. Based on all above performance, the double-face disc of two complexes was used to adsorb these substances with its two sides simultaneously, and the actual water sample was collected from Mingyuan Lake in Jiangan campus of Sichuan University, Chengdu. Before adsorption experiment, it was spiked with standard Cu
2+ and alkaline red 18 to the concentration of 100 μg/L according to the previous way [
28]. As the result, 96.5% Cu
2+ and 94.9% pigment were adsorbed by the double-face sorbent disc. After the desorption of 0.12 M HCl solution, the disc can be regenerated for the recycling use. As shown in
Figure 4b, the thermogravimetric analysis results indicate that there is no significant change in the composition of the double-layer composite disc before and after reuse. Because the polysaccharides including cyclodextrin and substituted cellulose were the main components in the tablet, the main weigh loss around 300~400℃ corresponds to their decomposition [
29,
30]. The two green solvents are not as stable as them and begin to degrade before this temperature range. If there is a decrease in adsorption performance after multiple uses, it is suggested to crush the tablet and employ ethyl acetate to remove ethyl cellulose from the powders; then the remaining complexes of green solvents and β-CD were further washed with HCl and UP water again. After drying, the resulted powders were mixed with clean diluent in preset proportion for repressing the new tablet.
4. Conclusions
As two kinds of new green solvents, ILs and DESs are widely used in various fields including separation and environmental science, which are attracting huge attention from academia and industry. However, they have not been used in the same sorbent simultaneously and current forms of sorbents need to be extended and renewed. In this communication, the suitable IL and DES were selected for those potential targets and their complex with β-CD were firstly prepared by simple and effective inclusion way. After that, two kinds of complex were applied to prepare a special coin-like sorbent disc with two different sides by adding diluent and pressing for appropriate duration. As the result, the IL and DES could be stably immobilized on the different sides of the disc for selective adsorption some possible pollutants in water with the merits of easy use and simple operation. It was expected to be a useful tool for water purification and quality detection, which realize the combination of green solvents and adsorption technologies in the feasible mode.
Research Highlights:
✓ Two green solvents were immobilized in β-CD as adsorbents by Inclusion effect.
✓ The design of coin-like double-sided disc can combine the adsorption selectivity .
✓ The preparation process is proved to be simple, flexible, feasible and easily scaled up.
✓ Several hazardous substances in water sample can be captured simultaneously.
Author Contributions
Conceptualization, N.L. and Y.G.; methodology, N.L.; software, Y.G.; validation, N.L., and Y.G.; formal analysis, H.C.; investigation, H.C.; data curation, A.A.; writing—original draft preparation, A.A.; writing—review and editing, S.T.; visualization, S.T.; supervision, S.Y.; project administration, S.Y.; funding acquisition, S.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by Sichuan Science and Technology Program (No: 2021YFG0276).
Acknowledgments
All the authors’ affiliations provided the convenience for related studies, respectively. Special thanks to the Engineering Experimental Teaching Center, School of Chemical Engineering, Sichuan University for related measurements and characterizations including FT-IR, etc.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Juni, F.; Bashir, M.J.K.; Haider Jaffari, Z.; Sethupathi, S.; Wong, J.W.C.; Zhao, J. Recent advancements in the treatment of emerging contaminants using activated persulfate oxidation process. Separations 2023, 10, 154. [Google Scholar] [CrossRef]
- Liu, X.; Lu, D.; Zhang, A.; Liu, Q.; Jiang, G. Data-driven machine learning in environmental pollution: gains and problems. Environ. Sci. Technol. 2022, 56, 2124–2133. [Google Scholar] [CrossRef] [PubMed]
- Nitin, R.; Prabhat, K.S.; Nityanand, S.M. Pharmaceuticals in water as emerging pollutants for river health: A critical review under Indian conditions. Ecotox. Environ. Safe. 2022, 247, 114220. [Google Scholar]
- Jaqueline, B.; João, H.Z.D.S. Chapter 3 - Green solvents for remediation technologies, Editor(s): Inamuddin, Rajender B., Abdullah M.A, Green sustainable process for chemical and environmental engineering and science. Elsevier, Amsterdam, Netherlands, 2021, pp. 23–30.
- Feng, X.; Song, H.; Zhang, T.; Yao, S.; Wang, Y. Magnetic Technologies and green solvents in extraction and separation of bioactive molecules together with biochemical objects: Current opportunities and challenges. Separations 2022, 9, 346. [Google Scholar] [CrossRef]
- Abu, H.N.; Abdul, S.N.; Siti, F.M.N.; Syafikah, H.P.; Muhammad, L.N.; Siti, M.N.H.; Rushdan, A.I.; Norzita, N.; Aznizam, A.B.; Zuliahani, A.; Mohammad, S.A.; Wan, I.N.; Walid, N. Recent Advances in using adsorbent derived from agricultural waste for antibiotics and non-steroidal anti-inflammatory wastewater treatment: A review. Separations 2023, 10, 300. [Google Scholar]
- Kopac, T. Research progress on process-intensified water treatment applications. Separations 2022, 9, 353. [Google Scholar] [CrossRef]
- Wang, Y.; Li, W.; Luo, S.; Liu, S.; Li, J. Research advances on the applications of immobilized ionic liquids functional materials. Acta Chim. Sinica -Chinese Edition 2018, 76, 85–94. [Google Scholar] [CrossRef]
- Dowlatshah, S.; Saraji, M.; Pedersen-Bjergaard, S.; Ramos-Payán, M. Microfluidic liquid-phase microextraction based on natural deep eutectic solvents immobilized in agarose membranes. J. Chromatogr. A 2021, 1657, 462580. [Google Scholar] [CrossRef]
- Kakaei, S.; Khameneh, E.S.; Hosseini, M.H.; Moharreri, M.M. A modified ionic liquid clay to remove heavy metals from water: investigating its catalytic activity. Int. J. Environ. Sci. Tech. 2019, 17, 2043–2058. [Google Scholar] [CrossRef]
- Yohannes, A.; Li, J.; Yao, S. Various metal organic frameworks combined with imidazolium, quinolinum and benzothiazolium ionic liquids for removal of three antibiotics from water. J. Mol. Liq. 2020, 318, 114304. [Google Scholar] [CrossRef]
- Fan, T.; Yan, Z.; Yang, C.; Qiu, S.; Peng, X.; Zhang, J.; Hu, L.; Chen, L. Preparation of menthol-based hydrophobic deep eutectic solvents for the extraction of triphenylmethane dyes: quantitative properties and extraction mechanism. Analyst 2021, 146, 1996–2008. [Google Scholar] [CrossRef] [PubMed]
- Phosiri, P.; Burakham, R. Deep eutectic solvent-modified mixed iron hydroxide–silica: application in magnetic solid-phase extraction for enrichment of organochlorine pesticides prior to GC-MS analysis. J. Sep. Sci. 2022, 44, 3636–3645. [Google Scholar] [CrossRef]
- Mao, X.; Liu, Y.; Chen, D.; Chen, Z.; Chunan, M.A. Electrochemical reduction of CO2 in [NH2-emim]Br/[Bmim]BF4 ionic liquid composite. CIESC Journal 2017, 68, 2027–2034. [Google Scholar]
- Sethi, O.; Singh, M.; Kang, T.S.; Sood, A.K. Volumetric and compressibility studies on aqueous mixtures of deep eutectic solvents based on choline chloride and carboxylic acids at different temperatures: experimental, theoretical and computational approach. J. Mol. Liq. 2021, 340, 117212. [Google Scholar] [CrossRef]
- Das, K.; Datta, B.; Rajbanshi, B.; Roy, M.N. Evidences for inclusion and encapsulation of an ionic liquid with β-CD and 18-C-6 in aqueous environments by physicochemical investigation. J. Phys. Chem. B 2018, 122, 1679. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lee, Y.R.; Row, K.H. Synthesis of mesoporous siliceous materials in choline chloride deep eutectic solvents and the application of these materials to high-performance size exclusion chromatography. Chromatographia 2016, 79, 375–382. [Google Scholar] [CrossRef]
- Pedro, A.Q.; Coutinho, J.A.P.; Freire, M.G. Immobilization of ionic liquids, types of materials, and applications. In: Zhang, S. (eds) Encyclopedia of ionic liquids. Springer, Singapore, 2022.
- Shah, R.V.; Pandey, A.K.; Kumar, S.J.; Paul, S.; Rao, R.M.; Jaison, P.G. One step sample treatment and loading using a deep eutectic solvent immobilized in a porous substrate for thermal ionization mass spectrometry of Pu(IV) ions. J. Anal. Atom. Spectrom. 2020, 35, 2315–2321. [Google Scholar] [CrossRef]
- Chen, C.; Feng, X.; Yao, S. Ionic liquid-multi walled carbon nanotubes composite tablet for continuous adsorption of tetracyclines and heavy metals. J. Clean. Prod. 2020, 286, 124937. [Google Scholar] [CrossRef]
- Marten, K.; Jian, X.W.; Jukka, R.; Jens, M.C.; Thomas, R.; Claudia, S.L. Multispectral UV imaging for fast and non-destructive quality control of chemical and physical tablet attributes. Eur. J. Pharm. Sci. 2016, 90, 85–95. [Google Scholar]
- Park, G.; Lee, J.H.; Kim, I.S.; Cho, J. Pharmaceutical rejection by membranes for wastewater reclamation and reuse. Water Sci, Technol. 2004, 502, 239–244. [Google Scholar] [CrossRef]
- Dietzel, B.M. Novel green technology for wastewater treatment:geo-material/geopolymer applications for heavy metal removal from aquatic media. Int. J. Sediment Res. 2023, 38, 33–48. [Google Scholar]
- Zhang, T.; Jiang, W.; Cao, Y.; Zhu, C.; Toukouki, S.; Yao, S. A facile one-pot synthesis of ionic liquid@porous organic frameworks for rapid high-capacity removal of heavy metal ions, pesticides and aflatoxin from two non-food bioactive products. Ind. Crop. Prod. 2022, 181, 114859. [Google Scholar] [CrossRef]
- Lee, S.P.; Ali, G.; Algarni, H.; Chong, K.F. Flake size-dependent adsorption of graphene oxide aerogel. J. Mol. Liq. 2019, 277, 175–180. [Google Scholar] [CrossRef]
- Wang, X.; Ying, C. UV-Vis detection-reversed- phase high performance liquid chromatography determination of basic red 1∶1, Textile Auxiliaries, 1998, 5, 35–37.
- Kopsidas, O. Pigment adsorption optimization in various low cost adsorbents. J. Environ. Sci. Eng. A 2021, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Danner, M.C.; Robertson, A.; Behrends, V.; Reiss, J. Antibiotic pollution in surface fresh waters: Occurrence and effects. Sci. Total Environ. 2019, 664, 793–804. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, L.; Zhai, X.; Liu, Q.; Sun, L.; Liu, M.; An, L.; Xian, L.; Zhang, P.; Chen, L. Study on adsorption performance of benzoic acid in cyclocarya paliurus extract by ethyl cellulose microspheres. Chemistry 2021, 3, 1113–1125. [Google Scholar] [CrossRef]
- Wang, T.; Li, B.; Feng, Y.; Guo, Q. Preparation, quantitive analysis and bacteriostasis of solid state iodine inclusion complex with β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2011, 69, 255–262. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).